Prevalence of clinical mastitis and its associated risk factors among dairy cattle in mainland China during 1982–2022: a systematic review and meta-analysis
- 1Anhui Province Key Laboratory of Animal Nutritional Regulation and Health, Anhui Science and Technology University, Fengyang, China
- 2Shanxi Province Engineering & Technology Research Center of Shanbei Cashmere Goats, Yulin University, Yulin, Shanxi, China
- 3Daqing Agricultural and Rural Bureau, Daqing, Heilongjiang, China
Background: Bovine mastitis is one of the most common and prevalent diseases affecting dairy cattle worldwide. It adversely affects the quality and quantity of milk production and leads to a significant economic loss for the farmers.
Methods: This article aimed to estimate the prevalence of clinical mastitis (CM) infection in mainland China using a systematic review and meta-analysis. The research reports published during 1983–2022 in English or Chinese from databases (PubMed, Google Scholar, Cochrane Library, Science Direct, Web of Science, VIP Database for Chinese Technical Periodicals (VIP), Chinese National Knowledge Infrastructure (CNKI), and Wan Fang database) were identified after reviewing the relevant scientific literature. Based on our inclusion criteria, this study analyzed the prevalence of CM in 47 published studies prevalence extracted the total number of cattle infected with CM from the available studies, allowing us to estimate the prevalence of CM infection among these in mainland China.
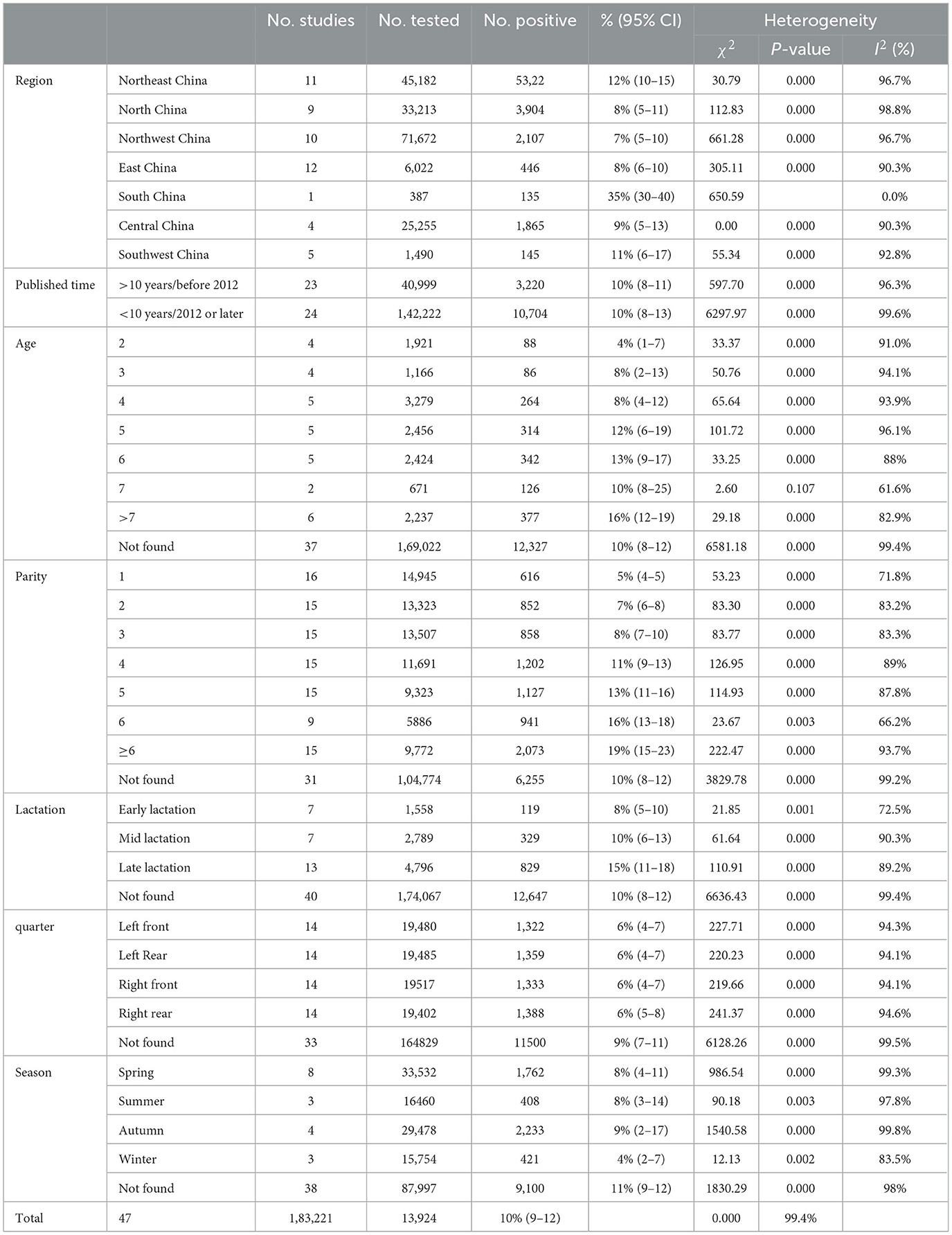
Results: The pooled prevalence with the 95% CI for the clinical mastitis was 10% (95% CI: 9.00, 12.00). The majority of CM was associated with lactation, parity, and age, with higher prevalence observed in late lactation 15% (95% CI: 11.00, 18.00) and mid-lactation 10% (95% CI: 6.00, 13.00) in comparison to early lactation 8% (95% CI: 5.00, 10.00). The incidence of CM increased significantly with the increase of parity and age, and the highest incidence rates were 19% (95% CI: 15.00, 23.00) and 16% (95% CI: 12.00, 19.00) at parity and age ≥7, respectively. Among the seasons, the highest prevalence of CM infection was found in autumn 9% (95% CI: 2.00, 17.00). Interestingly, no significant effects were evident regarding the influence of quarter on the prevalence of CM.
Conclusion: Thus, estimating the prevalence of CM among cattle in mainland China. through meta-analysis can provide adequate measures to control CM, reduce economic losses, and prevent the spread and transmission of CM in Chinese herds.
1. Introduction
Mastitis is an inflammation of the mammary gland caused mainly by an infection with a wide range of bacteria (1); it is a severe disease that can adversely affect and commonly harms the dairy sector. It can also cause substantial economic losses to the dairy industry, such as lower milk value and reduced cow milk production, thus leading to the premature culling of cows (2, 3). Depending on the infection, mastitis can present in tinct forms: clinical mastitis (CM) and subclinical mastitis (SCM). Clinical mastitis (CM) originates as a sudden onset of varying degrees of redness, swelling, heat, and pain in the diseased milk quarter, resulting in a significant reduction in lactation, thinning and yellowing of milk, symptoms of the flocculent material, and elevated body temperature (4, 5). The primary pathogens causing CM include Escherichia coli, Klebsiella sp., Staphylococcus aureus, Coagulase Negative Staphylococci (CONS), Streptococcus agalactiae, Streptococcus uberis, and other streptococci species (6). Season, Fecundity, lactation, nutritional conditions, environmental health, feeding management, and other factors have been strongly correlated with the disease (7).
According to the National Bureau of Statistics of China 2022 (https://data.stats.gov.cn), the country has 102.16 million head of cattle. Milk production is 39.32 million tons, while the prevalence of mastitis is ~60–70%, with clinical mastitis accounting for 21–23% of the total morbidity in dairy cattle, and dairy mastitis costs US$15–45 billion per year (8, 9). It has been reported that significant disparities exist between regions in China due to the lack of CM preventive and control strategies in some areas. For instance, from 1980 to 1989, the Lanzhou Institute of Chinese Veterinary Medicine and Academy of Agricultural Sciences recorded an average incidence of clinical-type mastitis of 33.41% in 32 dairy farms spread over 22 cities in China. Changchun (10), Shanxi (11), and Foshan (12) had detection rates for CM of 7.5, 11.69, and 35%, respectively. Interestingly, a study reporting 90,053 cases of clinical mastitis on 47 large dairy farms in 13 provinces in China showed that the incidence of clinical mastitis was associated with factors related to lactation period, quarter, and parity (13). These findings suggest that the incidence of CM varies considerably by region and characteristics and can cause significant economic losses and public health safety concerns; therefore, a comprehensive analysis of the prevalence and various risk variables associated with the onset of CM in Chinese cattle was conducted and this study may guide the prevention and control of the spread of the disease and may assist animal practitioners and researchers.
2. Methods
2.1. Basic country profile
China is located in eastern Asia, on the western coast of the Pacific Ocean. The total land area is about 9.6 million square kilometers, and the entire sea area is about 4.73 million square kilometers. The China altitude range is between 0 and 8,848, with national average precipitation of 695 mm in 2020 (http://www.gov.cn/xinwen/202102/09/content_5586383.htm).
2.2. Search strategy
We performed a systematic literature search using the following keywords: “Clinical Mastitis or Mastitis,” “prevalence” or “Epidemiology,” or “epidemiological study,” “Studies, Epidemiological,” or “Study, Epidemiological,” or “Studies, Epidemiologic,” or “Epidemiologic Study,” or “Study, Epidemiologic,” “incidence,” or “diagnosis,” or “Incidence Proportion,” or “Incidence Proportions,” or “Proportion, Incidence,” or “cows,” or “dairy cow,” or “dairy cows,” or “Cow, Bee,” or “Cow, Dairy,” “China” or “Chinese,” or “People's Republic of China,” or “Mainland China,” and variations and combinations of these terms and Systematic Review and Meta-analysis (PRISMA) guidelines for meta-analysis (14) was used to determine the overall prevalence of CM in dairy cows in China. The databases that were explored for finding the different published articles relevant to the prevalence of CM were the VIP Chinese Journal Database, China National Knowledge Infrastructure, Wanfang Data, and PubMed databases. Studies from different journal publications from 1983 to 2022 were searched for the prevalence of CM in China. We focused on articles published between January 1, 1983, and April 31, 2022, in English and Chinese by conducting a database search.
2.3. Data processing and filtration
Two investigators (HZ and SC) independently retrieved the data from the included studies using a standardized data collection form after thoroughly evaluating the prevalence of CM in the published studies. First author, year of publication, location, number of cows investigated, number of CM-positive cows, sample season, quarter or lactation or age of cows, and litter size were among the main information that was extracted from the various studies. In addition, the original articles were reviewed in detail, and references cited in the retrieved articles were searched again to trace additional relevant studies missed by the search. Any disagreements between the two researchers were resolved through discussion or arbitration with a third expert (YQ). The criteria for the selection of this study were as follows:
1. Chinese studies on the prevalence of clinical mastitis in dairy cows.
2. Sample size < 15 cows, too small to obtain reliable conclusions.
3. Sampling location within mainland China.
4. Use of animal study categories only, specifying sampling time, sample size, number of positive samples, prevalence, and study location.
2.4. Analysis of the study quality
The quality of the studies was evaluated according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) (15).In brief, we determined the score for each of the following items, scoring one point when the following information was provided: Clearly describe the purpose of the study, clearly indicate the test method, divide the subjects into different subgroups, describe the sampling method in detail, and accurately analyze the relevant risk factors. When overall scores in analyzed articles reached 4–5, 2–3, and 0–1 points, they were categorized as high-quality, intermediate, or low-quality studies, respectively. The quality assessment of the included studies was undertaken independently by two reviewers (HW and XC) based on the content of the articles.
2.5. Statistical analysis
We calculated the pooled prevalence of CM in the cows of the selected studies based on a meta-analysis. Because of the significant heterogeneity in the included studies, the random effects model in Stata 12.0(Stata Corp. College Station, Texas) was used to generate the forest plots. The forest plot can depict the combined estimate and the 95% confidence interval (CI). The publication bias was assessed by using the Egger test and represented graphically by a funnel plot, which was employed to determine the outliers in the CM prevalence studies. The different factors investigated by us included: the year of publication (before 2012; 2012 or later) and the geographical region (North China, East China, Southwest China, Central China, Northeast China, Northwest China, and South China). Moreover, we also analyzed the various additional risk factors, which included cow age, parity, lactation period, quarter, and the sampling season.
3. Results
3.1. Search results and eligible studies
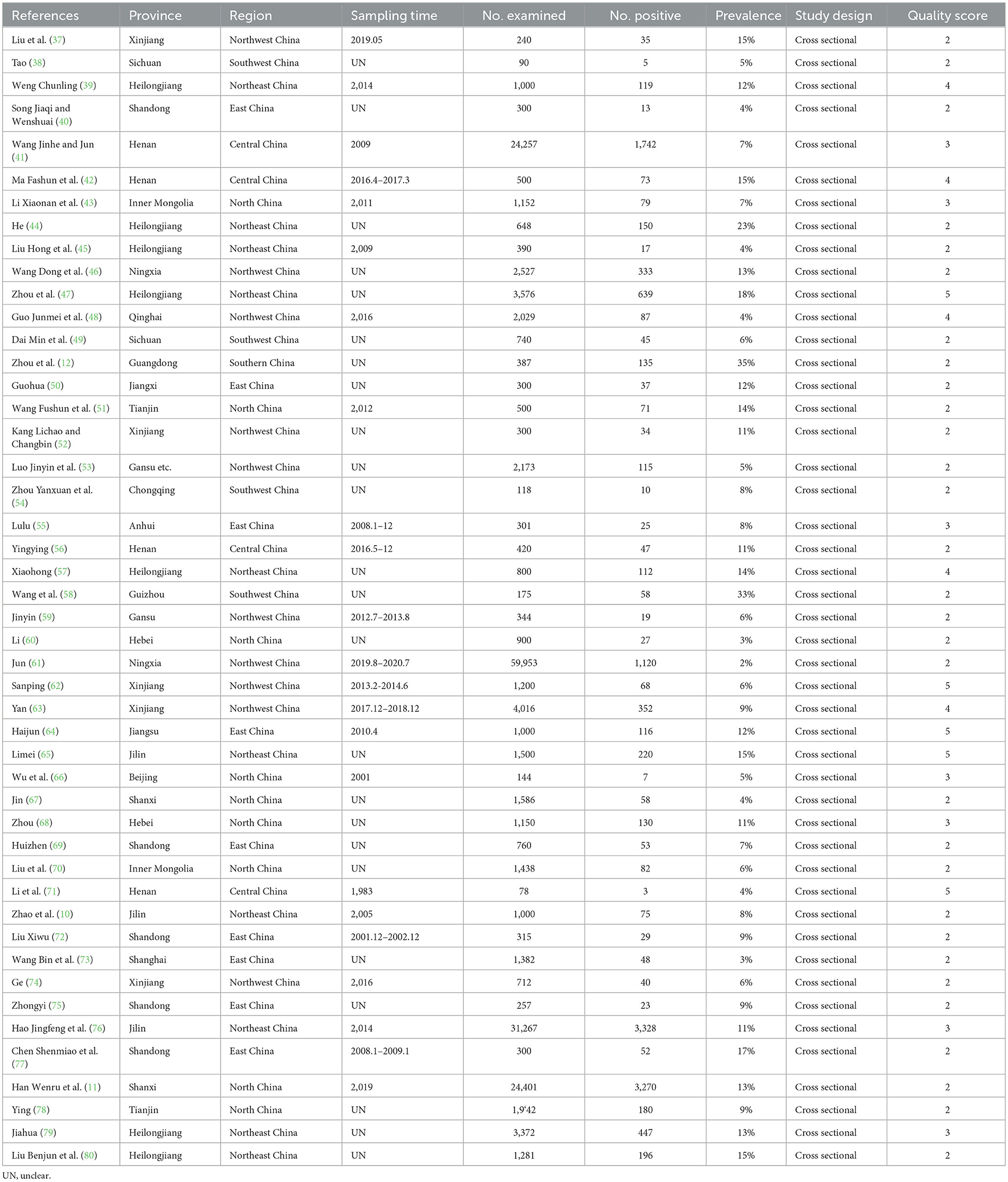
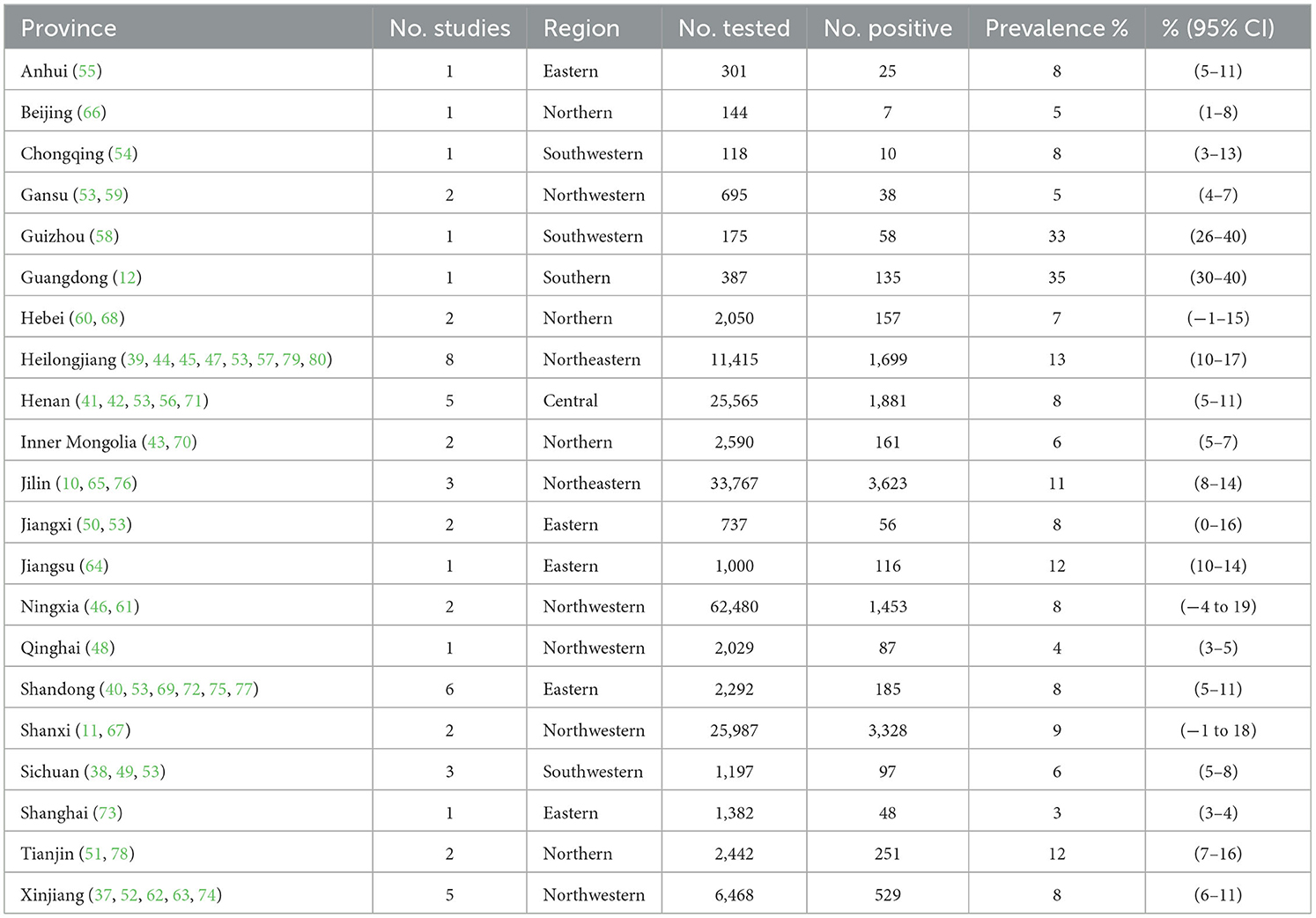
The present study aimed at employing systematic review and meta-analysis to estimate CM prevalence in the data extracted from studies related to China. The specifics used for the research included Chinese provinces, authors, years, and the reported prevalence of CM is presented (Table 1). All 47 were cross-sectional studies, 9 were of high quality (4 or 5 points), and 38 were of moderate quality (2 or 3 points). We have described in detail the procedures employed for screening articles and the reasons for exclusion, whereas articles displaying the precise prevalence of CM in China that were collected, were thoroughly examined, and considered for meta-analysis (Figure 1, Table 1). After excluding the duplicate citations and studies not relevant to this meta-analysis, a total of 1,657 full-text articles were included for screening, and a total of 47 articles met the inclusion criteria. We performed the present meta-analysis on the Chinese cattle herd and those included in the search were limited to period from 1983 to 2022 and covered 21 Chinese provinces. The meta-analysis included 13,924 CM positive cases out of 183,221 cattle (Table 2). It was evident that the Northwest region had the highest number of heads studied with 71,672; South China, was one of the seven with the lowest number of studies, nevertheless reported 135 positive cases.
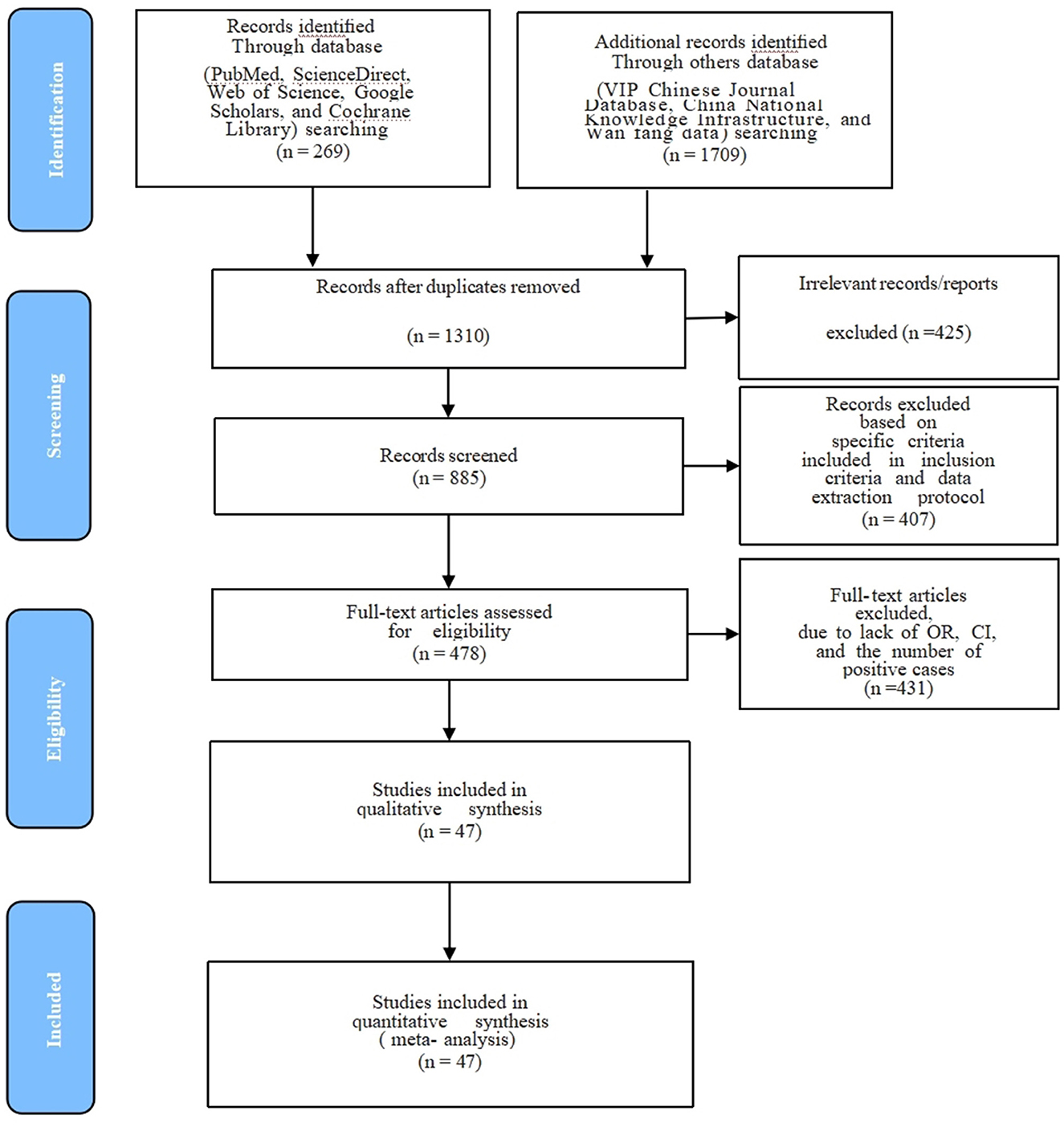
Figure 1. PRISMA flow diagram of the literature search, screening, assessing for eligibility, and selecting articles for the meta-analysis.
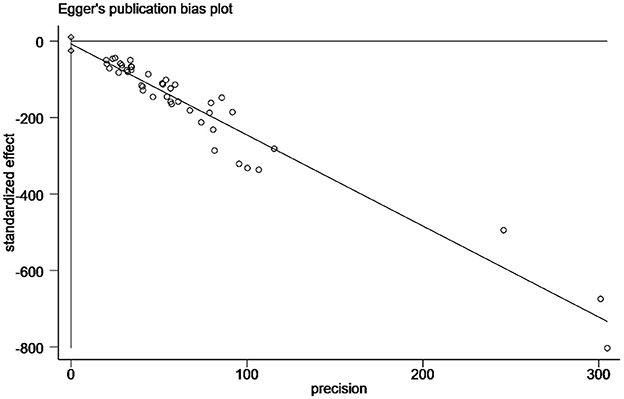
Funnel plots were used to assess potential publication bias in papers (Figure 2). Egger's test indicated a significant publication bias in this study (P < 0.05; Figure 3). However, as the finding was asymmetrical concerning overall prevalence, the results were potentially subject to publication bias.
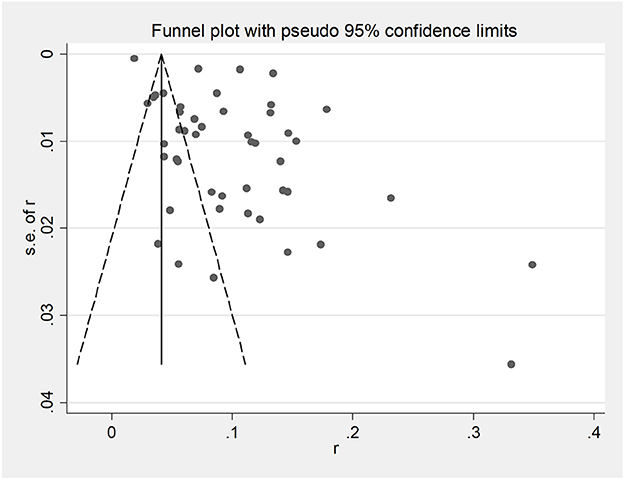
Figure 2. Funnel plot with pseudo 95% confidence limits for the analysis of potential publication bias.
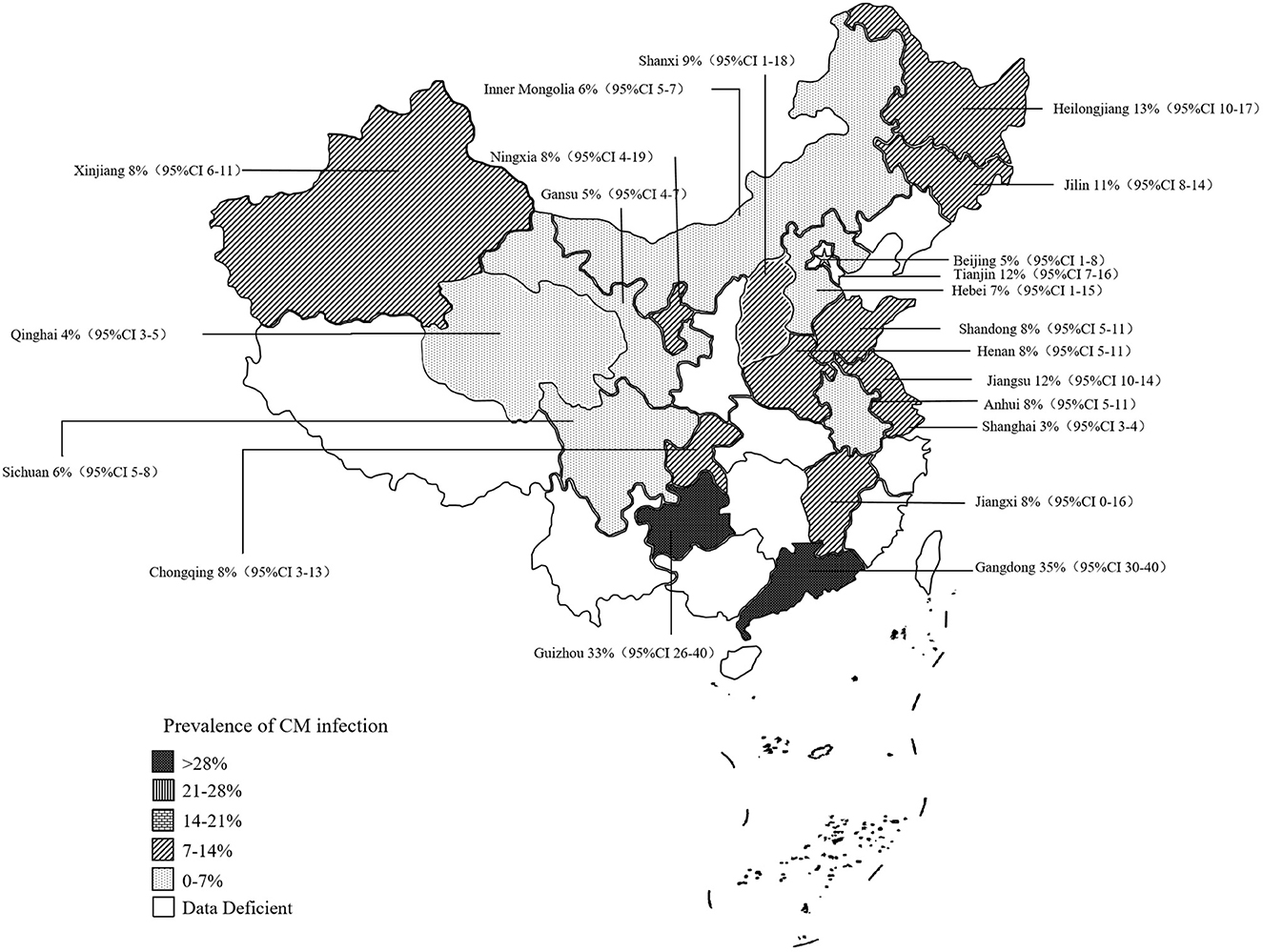
3.2. Prevalence of CM in administrative regions or provinces in China
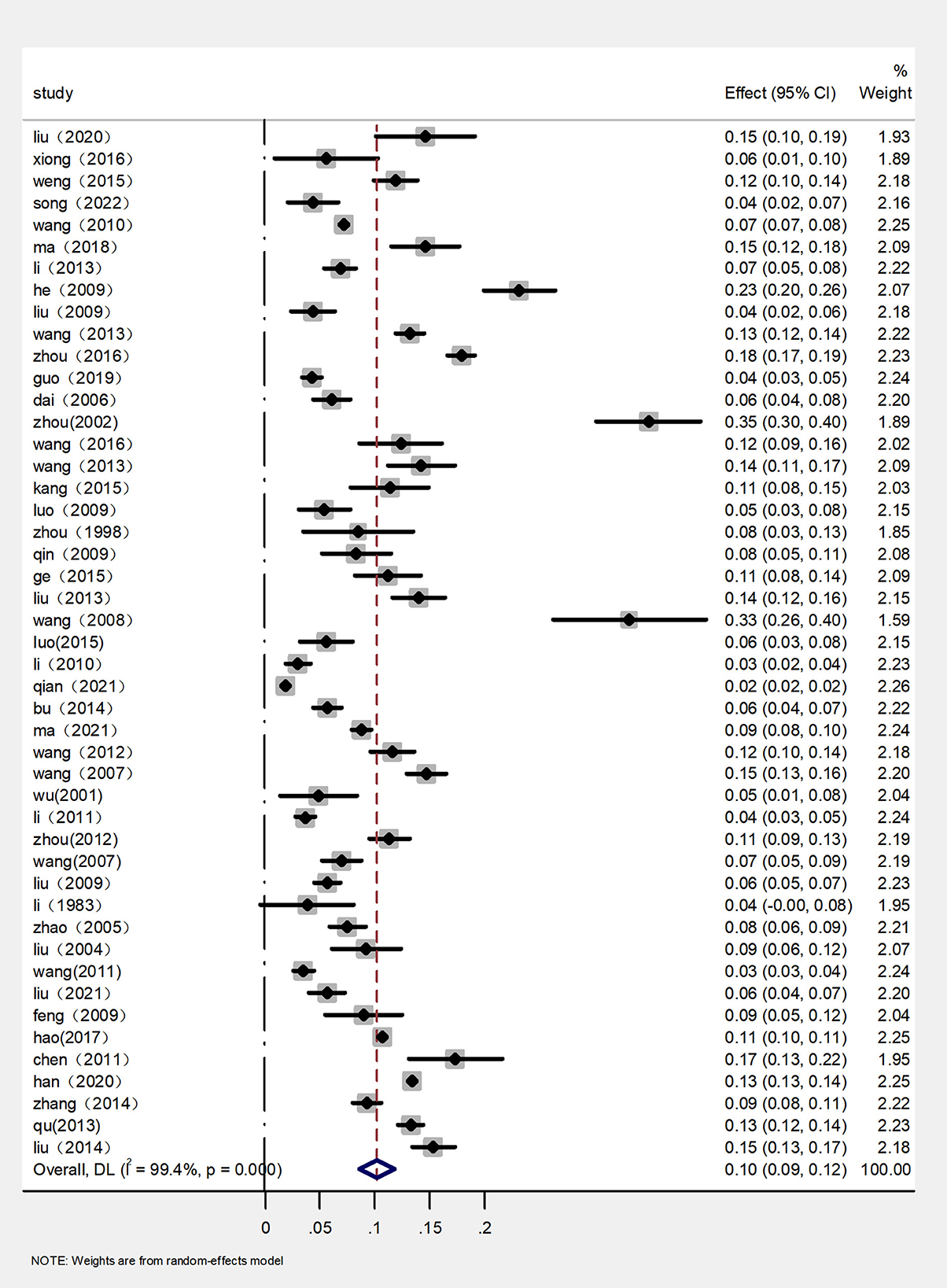
The rates of prevalence of CM among cattle ranged from 2 to 35% (Figure 4, Table 1). The estimated prevalence of CM in the different provinces and regions in China was determined from 1,83,221 samples, and the combined prevalence of CM in cattle in China was estimated to be 10% (95% CI 9–12, 13,924/1,83,221) (Figure 5, Table 2). The prevalence of CM in Northeast China, Southwest China, Central China, East China, and North China were 12% (95% CI: 10.00,15.00, 5,322/45,182), 11% (95% CI: 6.00, 17.00, 145/1,490), 9% (95% CI: 5.00, 13.00, 1,865/25,255), 8% (95% CI: 6.00, 10.00, 446/6,022), 8% (95% CI: 5.00, 11.00, 3,904/33,213).In addition, it was found that the most studies on the epidemiology of CM in dairy cattle have focused on cattle farms in the northeast and northwest regions of China (Table 2). Despite fewer studies, the prevalence of CM in south China was as high at 35% (95 % CI 30–40, 135/387), which was significantly greater than that in northwest China, which had a prevalence of just 7% (95% CI 5–10, 2,107/71,672) (Table 2).
Based on the provinces-wise breakdown shown, the highest prevalence of CM was observed in Guangdong 35% (95% CI: 30.00, 40.00), followed by Tianjin 35% (95% CI: 30.00, 40.00), Guizhou 33% (95% CI: 26.00, 40.00), Heilongjiang 13% (95% CI: 10.00, 17.00), Jiangsu 12% (95% CI: 10.00, 14.00), Jilin 11% (95% CI: 8.00, 14.00); In Shanxi 9% (95% CI: −1.00, 18.00), Anhui 8% (95% CI: 5.00, 11.00), Henan 8% (95% CI: 5.00, 11.00), Jiangxi 8% (95% CI: 0.00, 16.00), Shandong 8% (95% CI: 5.00, 11.00), Xinjiang 8% (95% CI: 6.00, 11.00), Chongqing 8% (95% CI: 3.00, 13.00), Hebei 7% (95% CI: −1.00, 15.00), Inner Mongolia 6% (95% CI: 5.00, 7.00), Sichuan 6% (95% CI: 5.00, 8.00), Beijing 5% (95% CI: 1.00, 8.00), Gansu 5% (95% CI: 4.00, 7.00), and thus the overall prevalence of CM was < 10%. The prevalence of CM in other provinces was below 5%, which included the provinces of Qinghai 4% (95% CI: 3.00, 5.00) and Shanghai 3% (95% CI: 3.00, 4.00) (Figure 5, Table 3).
3.3. Risk factors associated with the prevalence of CM
The number of cows studied, as well as age, lactation, and parity stage of cows varied considerably between the selected studies which might have contributed to the wide variation in the prevalence of CM in different regions of China. The details of the influencing factors related to the prevalence of CM were obtained by meta-analysis, and data included the time of publication, age, season, parity, lactation, and quarter (Table 2). The prevalence of CM in studies published before 2012, 2012 or later was 10% but with different confidence intervals (95% CI: 8.00, 11.00) and (95% CI: 8.00, 13.00), respectively, as seen in the publication time. The total combined prevalence of CM was 10% both before and after 2012, but the number of studies after 2012 was about 3.6 times higher than before 2012. Thus, based on the age it could be concluded that the highest prevalence of CM was 16% (95% CI: 12.00, 19.00) in cows >7 years old followed by 13% (95% CI: 9.00, 17.00) in 6-year-old CM, 12% (95% CI: 6.00, 19.00), 10% (95% CI: 8.00, 25.00) for 7-year-old CM, 8% (95% CI: 2.00, 13.00) for 3-year-old CM, also 8% (95% CI: 4.00, 12.00) for 4-year-old CM and a minimum of 4% (95% CI: 1.00, 7.00) for 2-year-old CM. The studies related to the prevalence of CM by parity showed that the prevalence of CM was 5% (95% CI: 4.00, 5.00) for 1 gestation, 7% (95% CI: 6.00, 8.00) for 2 gestations, 8% (95% CI: 7.00, 10.00) for 3 gestations, and 11% or more for 4 gestations. The prevalence of CM in early lactation was found to be 8% (95% CI: 5.00, 10.00), mid-lactation was 10% (95% CI: 6.00, 13.00), and late lactation was (95% CI:11, 18). Based on the udder regions, it could be concluded that the prevalence of CM in all four different quarters was 6%, but the confidence intervals were (95% CI: 4.00, 7.00) for left rear, right front, right rear, and (95% CI: 5.00, 8.00) for the right posterior. The season was divided into four distinct periods: spring (March to May), summer (June to August), autumn (September to November), and winter (December to February). The prevalence of CM in spring, summer, autumn, and winter was 8% (95% CI: 4.00, 11.00), 8% (95% CI: 3.00, 14.00), 9% (95% CI: 2.00, 17.00), and 4% (95% CI: 2.00, 7.00), respectively (Table 2).
4. Discussion
Bovine mastitis remains a significant epidemic disease affecting cattle, with contrasting reports from the different production systems nationwide. CM infection is a severe threat to the health of dairy cows, thereby severely restricting the healthy development of the dairy industry and causing substantial economic losses to China's dairy industry. Therefore, this study presents a systematic evaluation and meta-analysis of the prevalence of CM in Chinese bovines that can be used as potential guideline for the strategic management of dairy farms, mastitis control, and reduction of economic losses.
To our knowledge, no systematic meta-analysis of national CM prevalence estimates and potential risk factors has been published previously. This study is the first meta-analysis of the pooled prevalence of CM in dairy cows in China. In addition, prior studies have indicated that CM is widely distributed almost across most of China, with an overall prevalence of clinical mastitis in dairy cattle of 10% in this study. This finding is consistent with CM prevalence in dairy cattle in Ethiopia 12.89%, Asella 10.3%, Ecuador12% (16–18) compared to Zimbabwe 3.8%, Brazil 2.3%, Kenya 6.8% (19–21), the prevalence of CM is significantly higher. In contrast, the prevalence of CM in this study was found to be lesser than 21.9% and 18% to that reported from Japan and India, respectively (22, 23), and this variation could be attributed to the differences in agro-climatic conditions and farm management practices (24).
We included studies from 7 different regions and 21 provinces in China; fewer studies were available for inclusion in a meta-analysis from South China which could be the reason for the high prevalence of CM compared to other regions. We recommend a more detailed survey of CM prevalence in these regions to provide a sound scientific basis for future prevention and control efforts. Furthermore, it was observed that the lowest preponderance was in Northwest China, where prevalence reached a minimum of around 7% among cows. According to the China Statistical Yearbook 2022 (http://www.stats.gov.cn/sj/ndsj/2022/indexch.htm), by the end of 2021, there was 21.286 million head of cattle in northwest China, thus accounting for 21.7% of the country. Thus, it could be speculated that the dairy cows in this region are kept in higher numbers in comparison to other areas and that the large-scale farms provided better preventive measures.
In this study, we found that the prevalence of CM in dairy cows was closely related to their age but showed a general increase with age. This result was consistent with the previous studies (25) and could be attributed to the fact that with age, immunity was relatively weaker, and disease-causing microorganisms are more likely to invade the cows. It is also possible that prolonged exposure to the milking apparatus leads to the loss of the normal teat terminal function and, thus, the development of CM. Therefore, farms can promptly cull the older cows to reduce the incidence of clinical mastitis.
In addition, we also observed that parity and lactation could also influence the prevalence of CM, with an increased risk of CM observed in cows with multiple parity in comparison to those with few parity and moderate parity. This finding was consistent with the previous reports published earlier (26, 27). This could potentially result from the prolonged calving and lactation of cows with multiple parity, thereby causing damage to the milking area, thus significantly increasing the chance of udder infection with pathogenic bacteria. Furthermore, the increased prevalence of CM in late lactation compared to early lactation was also consistent with the previously reported studies of mastitis (28–30), and relatively higher prevalence of mastitis in late lactation could be due to the repeated exposure to infection factors (cumulative infection) during milking lactation (31). Moreover, a number of previous studies have suggested that CM could be more prevalent in early lactation (26, 32). Consequently, teat massage and routine disinfection are recommended for multi-breed and lactating cows to significantly reduce the prevalence of CM by mitigating udder damage from prolonged milking.
Since 1983, there has been a steady stream of reports related to CM in China. We also found in this study that the highest infection rate of 9% in autumn, followed by 8% in spring and summer, respectively. Interestingly, one prior study has shown that CM infections tend to occur in July-September (33). Generally, the high CM season has been reported in the summer season because of the existence of high temperature, high humidity, and heat stress in the cows leading to loss of appetite and organism immunity, which can then accelerate excessive multiplication of disease microorganisms and result in high CM incidence (33). This difference was presumed to be due to the study's different sample areas and sampling seasons reported in different analyzed studies. Therefore, more attention should be paid to prevention during summer to impede the occurrence of CM. During the hot season, ventilation, shade, as well as dry and clean environment should be maintained in cattle sheds, occurrence of CM infection should be monitored, and infected cattle should be isolated promptly to prevent the CM onset. Owing to the meta-analysis being conducted on epidemiological surveys, microbiological investigations were not the objective of this investigation, it was not included in this analysis. However, based on the finding of the previous studies, S. agalactiae is a vital pathogen causing bovine mastitis in China (34). Notably, one prior study showed that S. aureus is a significant contributor to cow mastitis, whereas E. coli was the primary causative agent of CM (35). S. aureus is an infectious agent transmitted mainly during milking (36). We speculate that the high detection rate of large numbers of S. aureus could be due to improper milking practices resulting in the ease with which these organisms can be transmitted from infected to healthy sites via the contaminated milkers' hands, towels, or milking equipment. On the contrary, the high detection rate of E. coli could be attributed to the poor sanitary conditions in the cattle houses and playing fields, sewage sludge, fecal matter not being removed promptly, and this could be especially applicable to the cattle houses and playing fields where disinfection is not strict or not carried out, thereby resulting in bacteria entering the udder through the teat.
This meta-analysis provides an overview of CM profiles in China. The findings clearly revealed significant differences in CM prevalence across provinces and regions, but we must admit that this study has few limitations. First, 47 pieces of data reported in this study was obtained from 8 large databases, but some relevant studies may still need to be included. Secondly, the sample size of some studies was small, and limited information was included, which may lead to unstable overall estimation and subgroup analysis results. Third, only one survey or investigation was conducted in some provinces, which might not accurately represent the true prevalence of CM in these areas. Finally, due to the lack of available literature, various potential risk factors such as breed, history of previous mastitis, floor type, and breeding environment of dairy cows were not analyzed. However, this meta-analysis can provide novel insights about overall prevalence and development trend of CM infection in China during the survey period.
In conclusion, this study showed that the overall prevalence of clinical mastitis in Chinese dairy cows was 10%, with the highest and lowest prevalence of CM in South China and Northwest China being 35 and 7%, respectively, regarding the region. Parity, age, season, and lactation of Chinese cows are potential risk factors for mastitis. It is recommended that relevant practitioners improve management strategies for disease to develop appropriate prevention and control programs. In addition, monitoring of CM should be strengthened, and prevention and control programs should be adjusted promptly according to infection, which may help reduce CM's prevalence in China.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
Conceptualization: HW. Data collection: HZ, SC, and YQ. Writing—original draft preparation: SC. Data analysis: XC. Writing—review and editing: XC and YQ. Funding acquisition: JZ. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Natural Science Key Foundation of Anhui Education Department (KJ2021A0872 and KJ2020A0085) and Fund for Less Developed Regions of the National Natural Science Foundation of China (31960710).
Acknowledgments
We sincerely thank personnel from College of Anhui Province Key Laboratory of Animal Nutritional Regulation and Health, Anhui Science and Technology University and MJEditor (www.mjeditor.com) for its linguistic assistance during the preparation of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Dalanezi FM, Joaquim SF, Guimarães FF, Guerra ST, Lopes BC, Schmidt EMS, et al. Influence of pathogens causing clinical mastitis on reproductive variables of dairy cows. J Dairy Sci. (2020) 103:3648–55. doi: 10.3168/jds.2019-16841
2. Halasa T, Huijps K, Østerås O, Hogeveen H. Economic effects of bovine mastitis and mastitis management: a review. Vet Q. (2007) 29:18–31. doi: 10.1080/01652176.2007.9695224
3. Heikkila AM, Nousiainen JI, Pyorala S. Costs of clinical mastitis with special reference to premature culling. J Dairy Sci. (2012) 95:139–50. doi: 10.3168/jds.2011-4321
4. Wilson DJ, Gonzalez RN, Das HH. Bovine mastitis pathogens in New York and Pennsylvania: prevalence and effects on somatic cell count and milk production. J Dairy Sci. (1997) 80:2592–8. doi: 10.3168/jds.S0022-0302(97)76215-5
6. He W, Ma S, Lei L, He J, Li X, Tao J, et al. Prevalence, etiology, and economic impact of clinical mastitis on large dairy farms in China. Vet Microbiol. (2020) 242:108570. doi: 10.1016/j.vetmic.2019.108570
8. Cao LT, Wu JQ, Xie F, Hu SH, Mo Y. Efficacy of nisin in treatment of clinical mastitis in lactating dairy cows. J Dairy Sci. (2007) 90:3980–5. doi: 10.3168/jds.2007-0153
9. Yi S. Isolation and Identification of Pathogenic Bacteria, Virulence Gene Detection And Gene Therapy of Mastitis in Dairy Cattle. Yangzhou University (2009).
10. Lixiang Z. Investigation of the Etiology of Mastitis in Dairy Cows in Jilin Area. Jilin University (2005).
11. Han W, Yang L, Wu S, Yang Z, Dong C, Liu W, et al. A survey on the incidence of mastitis in dairy cows on large-scale cattle farms in Shanxi. Chin Dairy Cattle. (2020) 38–40. doi: 10.19305/j.cnki.11-3009/s.2020.11.009
12. Zhou QG LJ, Chen JC, Wen RJ. Investigation of the incidence of clinical-type mastitis in dairy cows in Foshan and isolation and identification of the pathogen Chin Dairy Cattle. (2002) 31–3.
13. Zhao Lanning ZW, Yongqiang W, Shumei L, Bo H, Lijun C, Jian G. A survey report on the characteristics of clinical-type mastitis incidence in large dairy farms in China. Chin J Vet Med. (2019) 55:15–9.
14. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. (2015) 4:1. doi: 10.1186/2046-4053-4-1
15. Atkins D, Eccles M, Flottorp S, Guyatt GH, Henry D, Hill S, et al. Systems for grading the quality of evidence and the strength of recommendations I: critical appraisal of existing approaches The GRADE Working Group. BMC Health Serv Res. (2004) 4:38. doi: 10.1186/1472-6963-4-38
16. Girma A, Tamir D. Prevalence of bovine mastitis and its associated risk factors among dairy cows in Ethiopia during 2005–2022: a systematic review and meta-analysis. Vet Med Int. (2022) 2022:7775197. doi: 10.1155/2022/7775197
17. Lakew M, Tolosa T, Tigre W. Prevalence and major bacterial causes of bovine mastitis in Asella, South Eastern Ethiopia. Trop Anim Health Prod. (2009) 41:1525–30. doi: 10.1007/s11250-009-9343-6
18. Amer S, Gálvez FLA, Fukuda Y, Tada C, Jimenez IL, Valle WFM, et al. Prevalence and etiology of mastitis in dairy cattle in El Oro Province, Ecuador. J Vet Med Sci. (2018) 80:861–8. doi: 10.1292/jvms.17-0504
19. Katsande S, Matope G, Ndengu M, Pfukenyi DM. Prevalence of mastitis in dairy cows from smallholder farms in Zimbabwe. Onderstepoort J Vet Res. (2013) 80:523. doi: 10.4102/ojvr.v80i1.523
20. Silva AC, Laven R, Benites NR. Risk factors associated with mastitis in smallholder dairy farms in Southeast Brazil. Animals. (2021) 11:2089. doi: 10.3390/ani11072089
21. Mbindyo CM, Gitao GC, Mulei CM. Prevalence, etiology, and risk factors of mastitis in dairy cattle in Embu and Kajiado Counties, Kenya. Vet Med Int. (2020) 2020:8831172. doi: 10.1155/2020/8831172
22. Fukushima Y, Kino E, Furutani A, Minamino T, Mikurino Y, Horii Y, et al. Epidemiological study to investigate the incidence and prevalence of clinical mastitis, peracute mastitis, metabolic disorders and peripartum disorders, on a dairy farm in a temperate zone in Japan. BMC Vet Res. (2020) 16:389. doi: 10.1186/s12917-020-02613-y
23. Krishnamoorthy P, Goudar AL, Suresh KP, Roy P. Global and countrywide prevalence of subclinical and clinical mastitis in dairy cattle and buffaloes by systematic review and meta-analysis. Res Vet Sci. (2021) 136:561–86. doi: 10.1016/j.rvsc.2021.04.021
24. Joshi S, Gokhale S. Status of mastitis as an emerging disease in improved and periurban dairy farms in India. Ann N Y Acad Sci. (2006) 1081:74–83. doi: 10.1196/annals.1373.007
25. Schepers AJ, Lam TJ, Schukken YH, Wilmink JB, Hanekamp WJ. Estimation of variance components for somatic cell counts to determine thresholds for uninfected quarters. J Dairy Sci. (1997) 80:1833–40. doi: 10.3168/jds.S0022-0302(97)76118-6
26. Oliveira CS, Hogeveen H, Botelho AM, Maia PV, Coelho SG, Haddad JP. Cow-specific risk factors for clinical mastitis in Brazilian dairy cattle. Prev Vet Med. (2015) 121:297–305. doi: 10.1016/j.prevetmed.2015.08.001
27. Cardozo LL, Neto AT, Souza GN, Picinin LCA, Felipus NC, Reche NLM, et al. Risk factors for the occurrence of new and chronic cases of subclinical mastitis in dairy herds in southern Brazil. J Dairy Sci. (2015) 98:7675–85. doi: 10.3168/jds.2014-8913
28. Mekonnen SA, Koop G, Melkie ST, Getahun CD, Hogeveen H, Lam TJGM. Prevalence of subclinical mastitis and associated risk factors at cow and herd level in dairy farms in North-West Ethiopia. Prev Vet Med. (2017) 145:23–31. doi: 10.1016/j.prevetmed.2017.06.009
29. Ndahetuye JB, Twambazimana J, Nyman AK, Karege C, Tukei M, Ongol MP, et al. A cross sectional study of prevalence and risk factors associated with subclinical mastitis and intramammary infections, in dairy herds linked to milk collection centers in Rwanda. Prev Vet Med. (2020) 179:105007. doi: 10.1016/j.prevetmed.2020.105007
30. Iraguha B, Hamudikuwanda H, Mushonga B. Bovine mastitis prevalence and associated risk factors in dairy cows in Nyagatare District, Rwanda. J S Afr Vet Assoc. (2015) 86:1228. doi: 10.4102/jsava.v86i1.1228
31. Almaw G, Zerihun A, Asfaw Y. Bovine mastitis and its association with selected risk factors in smallholder dairy farms in and around Bahir Dar, Ethiopia. Trop Anim Health Prod. (2008) 40:427–32. doi: 10.1007/s11250-007-9115-0
32. Kivaria FM, Noordhuizen JP, Kapaga AM. Risk indicators associated with subclinical mastitis in smallholder dairy cows in Tanzania. Trop Anim Health Prod. (2004) 36:581–92. doi: 10.1023/B:TROP.0000040935.87175.bb
33. Guan W, Lei L, Cao S. Epidemiological investigation of dairy cow mastitis in south Beijing. Shandong Anim Husbandry Vet Med. (2020) 41:42–3.
34. Zhang Z, Li XP, Yang F, Luo JY, Wang XR, Liu LH, et al. Influences of season, parity, lactation, udder area, milk yield, and clinical symptoms on intramammary infection in dairy cows. J Dairy Sci. (2016) 99:6484–93. doi: 10.3168/jds.2016-10932
35. Krishnamoorthy P, Suresh KP, Jayamma KS, Shome BR, Patil SS, Amachawadi RG. An understanding of the global status of major bacterial pathogens of milk concerning bovine mastitis: a systematic review and meta-analysis (scientometrics). Pathogens. (2021) 10:545. doi: 10.3390/pathogens10050545
36. Tenhagen B-A, Hansen I, Reinecke A, Heuwieser W. Prevalence of pathogens in milk samples of dairy cows with clinical mastitis and in heifers at first parturition. J Dairy Res. (2009) 76:179–87. doi: 10.1017/S0022029908003786
37. Liu X, Ge W, Yang L, Liu X, Zheng G, Wang C. Study on the incidence pattern of mastitis in dairy cows in Changji City. Modern Anim Husbandry Vet Med. (2020) 42–6.
38. Tao X. Epidemiological survey and analysis of mastitis in dairy cows in Cuiping District. Gansu Anim Husbandry Vet Med. (2016) 46:96–7.
39. Weng Chunling WR. Epidemiological survey of mastitis in dairy cows in Daqing area. Heilongjiang Anim Husbandry Vet Med. (2015) 118–20. doi: 10.13881/j.cnki.hljxmsy.2015.1688
40. Song Jiaqi LZ, Wenshuai Y. Epidemiological investigation of mastitis in dairy cows on large-scale dairy farms. Farming Feed. (2022) 21:20–2. doi: 10.13300/j.cnki.cn42-1648/s.2022.04.049
41. Wang Jinhe SD, Jun W. A survey on the current situation of mastitis in dairy cows in Henan Province. China Agric Bull. (2010) 26:4–7.
42. Ma F, Xing Q, Song SY, Liang X. A case study of occurrence of mastitis in cows on a dairy farm. Anim Husbandry VetMed. (2018) 50:125–8.
43. Li Xiaonan ZX, Yulin D, Dong C. Survey on the incidence of mastitis in dairy cows in the Huhehaote area. Heilongjiang Anim Husbandry Vet Med. (2013) 90–1.
44. He JGY. Epidemiological survey and loss evaluation of mastitis in dairy cattle. Heilongjiang Agric Sci. (2009) 113–4.
45. Liu Hong GX, Minghe Y, Zhaoning L, Ligang W. Investigation and pathogeny analysis of bovine mastitis in dairy cattle. J Northeast Agric Univ. (2009) 40:82–6. doi: 10.19720/j.cnki.issn.1005-9369.2009.09.017
46. Wang D, Wang J, Hu D, Ding L, Shen M, Han K, et al. Investigation of mastitis in dairy cows in Ningxia region. Adv Anim Med. (2013) 34:222–7. doi: 10.16437/j.cnki.1007-5038.2013.12.051
47. Zhou QM ZY, Xu X, Feng WY, Huang J. Survey on the incidence of mastitis in dairy cows in Qiqihar City. China Vet J. (2016) 52:46–9.
48. Guo Junmei SW, Chengjuan M, ZXiaoyan. Survey on the incidence of mastitis in dairy cows in Haibei Prefecture, Qinghai Province. Adv Anim Med. (2019) 40:140–4. doi: 10.16437/j.cnki.1007-5038.2019.05.028
49. Dai M, Wang X, Luo Y, Chen X, Guo W, Liu J. Investigation and analysis of mastitis in dairy cows in Mianyang City. Northwest J Agric. (2006) 15:51–4+62.
50. Guohua W. Investigation and control of mastitis in dairy cows in Shangrao Shanqing Ranch. Rural Econ Technol. (2016) 27:58–9.
51. Wang F, Chen D, Wang D, Cai L, Jiang K, Han X, et al. Epidemiological investigation of mastitis on a dairy farm in Tianjin China Anim Quarantine. (2013) 30:30–2.
52. Kang Lichao LC, Changbin L. Isolation and identification of pathogenic bacteria of mastitis in dairy cows on the northern slope of Tianshan Mountain. Jiangsu Agric Sci. (2015) 43:271–4. doi: 10.15889/j.issn.1002-1302.2015.10.090
53. Luo Jinyin YJ, Hongsheng L, Xinpu L. A study on the incidence of mastitis in individual dairy farms in some regions of China. China Cattle Sci. (2009) 35:70–3.
54. Zhou Yanxuan MM, Yuemei L, Bo J, Jun Y. Investigation of the etiology and incidence of mastitis in lactating dairy cows in some areas of Chongqing. Chin Livestock Poultry Infect Dis. (1998) 20:27–9.
55. Lulu Q. Epidemiological Investigation of Mastitis in Dairy Cows on a Farm in Hefei and Isolation and Identification of Pathogenic Bacteria. Anhui Agricultural University (2009).
56. Yingying G. Investigation on the Incidence of Bovine Mastitis in Henan Province. Nanjing Agricultural University (2017).
57. Xiaohong L. Epidemiological Survey of Food-and-Mouth Disease and Mastitis in Dairy Cows in Part Areas of Heilongjiang Province. Yanbian University (2013).
58. Wang HM LG, Li RJ, Wang M, Wu LF. Investigation on mastitis of dairy cows in large-scale dairy farm. Guizhou Anim Husbandry Vet Med. (2008) 32:11–2.
59. Jinyin L. Investigation of epidemiology and pathogenic bacteria of mastitis in dairy cows from the Lanzhou area. Heilongjiang Anim Sci Vet Med. (2015) 11:164–6. doi: 10.13881/j.cnki.hljxmsy.2015.1886
60. Li Y. Milk Cow Mastitis Epidemiological Investigation and Prevention Research in Tang Shan City. Hebei Agricultural University (2010).
61. Jun Q. Epidemiological Investigation of Mastitis in Dairy Cows in Wuzhong Area and Application of Teat Sealant During dry Milking Period. Ningxia University(2021).
62. Sanping B. Investigation on the Etiology of Mastitis in Dairy Cows on Large-Scale Cattle Farms in the Bazhou Region of Xinjiang. Shihezi University(2014).
63. Yan M. Investigation of Dairy COW Mastitis Epidemiological in Yanqi County of Bayinguoleng Mongol Autonomous Prefecture Region from Xinjiang. Xinjiang Agricultural University (2021).
64. Haijun W. Epidemiological Investigation and Control of Mastitis in Dairy Cows in Xuzhou City. Nanjing Agricultural University (2012).
65. Limei W. Epidemiological Investigation of Mastitis in Dairy Cows in Changchun Area. Chinese Academy of Agricultural Sciences (2007).
66. Wu GJ ZC, Shen H, Kong G. A study on the positive rate and occurrence pattern of mastitis in dairy cows in Beijing. J Beijing Agric Coll. (2001) 16:43–6. doi: 10.13473/j.cnki.issn.1002-3186.2001.03.009
67. Jin L. Epidemiological Survey of Mastitis in Dairy Cows in Taiyuan Area and the in Vitro Effect of Compound Chinese Herbs on Mastitis Pathogenic Bacteria in Dairy Cows Study on the Antibacterial Effect of Chinese Herbs on Mastitis Pathogens in Dairy Cows. Shanxi Agricultural University (2014).
68. Zhou C. Clinical investigation of mastitis in dairy cows in Langfang area. Heilongjiang Anim Husbandry Vet Med. (2012) 88–9. doi: 10.13881/j.cnki.hljxmsy.2012.04.033
69. Huizhen W. Clinical investigation of mastitis in dairy cattle. Shandong Anim Husbandry Vet Med. (2007) 29:11–2.
70. Liu Xin LT, Yuqi Z, Mingqi Y. A survey on the incidence of mastitis in dairy cows in the Baotou area. Adv Anim Med. (2009) 30:107–12. doi: 10.16437/j.cnki.1007-5038.2009.07.001
71. Li Yanling CB, Minze P, Li F, Xianxun Z, Iuxin W, Guoqing W, et al. An investigation report on mastitis in dairy cows in Zhengzhou breeding farm, Henan Province. J Henan Agric Coll. (1983) 68–73.
72. Liu Xiwu MB. Investigation report of mastitis in dairy cattle. Vet Med Feed Addit. (2004) 9:27–8.
73. Wang Bin ZZ, Ruihua Z, Kechun Z, Kehe H. Investigation on the incidence of mastitis in a dairy farm in Shanghai Shanghai Anim Husbandry Vet Commun. (2011) 2–4.
74. Ge L. Survey on the prevalence of mastitis in a large-scale dairy farm in Shihezi region, Xinjiang. China Dairy Industry. (2021) 94–9.
75. Zhongyi F. Investigation of the Incidence Pattern of Mastitis in Dairy Cows in Tai'an, Identification of Pathogen Isolation and Treatment Measures. Shandong Agricultural University (2009).
76. Hao Jingfeng LJ, Yuhang Z, Boshuang Y, Lianjun F, Yan H, Xinwei L, et al. Survey on the incidence of mastitis in dairy cows in Jilin Province. Chin J Vet Med. (2017) 53:47–9.
77. Chen Shenmiao MJ, Jinsheng W, Houshun Z. Investigation and analysis of mastitis in dairy cows on a dairy farm in Shandong Province. China Dairy Farm. (2011) 50–3.
78. Ying Z. Epidemiological survey of mastitis in dairy cows in Tianjin. China Dairy Cattle. (2014) 60–2.
79. Jiahua Q. Investigation on the incidence of mastitis in dairy cows in Qiqihar City. Farming Technol Advisor. (2013) 9:103.
Keywords: dairy cows, clinical mastitis, meta-analysis, prevalence, systematic review
Citation: Chen S, Zhang H, Zhai J, Wang H, Chen X and Qi Y (2023) Prevalence of clinical mastitis and its associated risk factors among dairy cattle in mainland China during 1982–2022: a systematic review and meta-analysis. Front. Vet. Sci. 10:1185995. doi: 10.3389/fvets.2023.1185995
Received: 14 March 2023; Accepted: 28 April 2023;
Published: 18 May 2023.
Edited by:
Roswitha Merle, Freie Universität Berlin, GermanyReviewed by:
Chaiyapas Thamrongyoswittayakul, Khon Kaen University, ThailandAbayeneh Girma, Mekdela Amba University, Ethiopia
Copyright © 2023 Chen, Zhang, Zhai, Wang, Chen and Qi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuelong Chen, cxlandqyp@163.com; Yanping Qi, qiyanping2018@vip.163.com
†These authors have contributed equally to this work
 Shuiyun Chen
Shuiyun Chen Huiying Zhang1†
Huiying Zhang1†  Xuelong Chen
Xuelong Chen