A Comparison of Production Performance, Egg Quality, and Cecal Microbiota in Laying Hens Receiving Graded Levels of Vitamin B12
- 1Laboratory of Poultry Production, College of Animal Science, Shanxi Agricultural University, Taigu, China
- 2Department of Life Sciences, Luliang University, Luliang, China
The objective of the study was to investigate the effect of fortified diets with standard vs. high levels of vitamin B12 on cecal microbiota composition, production performance, and eggshell quality of laying hens. Dietary treatments consisted of a basal diet with no supplementation of vitamin B12 or supplemented with 25, 100, and 400 μg/kg vitamin B12, respectively. A total of 432 laying hens were randomly assigned to four treatments with six replicates per treatment. No significant effect of dietary treatments on the production performance of hens was detected. The shell thickness of eggs from hens fed diet supplemented with 100 μg/kg of vitamin B12 was higher (P < 0.01) than that of eggs from hens fed control diet or supplemented with 25 μg/kg vitamin B12. The shell percentage of eggs from hens fed diet supplemented with 400 μg/kg of vitamin B12 was higher (P < 0.01) than that of eggs from hens fed other treatment diets. Dietary vitamin B12 did not modulate diversity of the cecal microbiota of the layers. At genus level, the cecal content from layers fed diet with supplemental level of 100 or 400 μg/kg of vitamin B12 had higher (P < 0.01) abundance of Faecalibacterium and lower (P < 0.05) abundance of Acinetobacter compared with the cecal content from layers fed other two diets. The abundance of Lactobacillus in the cecal samples from layers fed 100 μg/kg of supplemental level of vitamin B12 was higher (P < 0.05) than that from layers fed other three diets. The abundance of Butyricicoccus was higher (P < 0.05), while Bilophila was lower (P < 0.05) in the cecal content of layers fed 400 μg/kg of vitamin B12 diet compared with those from layers fed other three diets. The results of PICRUSt analysis indicated that 10 predicted metabolic functions of the cecal microbial communities were positively correlated to dietary vitamin B12 level. Overall, dietary supplementation of 100 or 400 μg/kg of vitamin B12 had equivalent effects and caused the significant change in composition and metabolic functions of cecal microorganisms, which could positively impact eggshell quality, metabolism, and gut health of laying hens.
Introduction
Vitamin B12 is one of essential B vitamins required by humans and animals. Since it is involved in nucleic acid synthesis, carbohydrate and fat metabolism, and methyl synthesis, the deficiency of vitamin B12 will cause anemia, muscle weakness, severe neurologic problems, and many other symptoms in humans and animals (1–6). In laying hens, numerous studies indicated that vitamin B12 is required for optimal egg production, egg weight, and hen weight (7–11). Today, vitamins are commonly over fed in poultry feed to give safety margin for the deterioration of many vitamins during feed processing and storage (12–14). Also, vitamin-enriched eggs produced through supplementing extremely a high level of vitamins of interest to the layer diet for human consumption have been widely accepted (15–20). Subsequently, a significant portion of vitamins that was not absorbed in the small intestine of layers could reach the hind gut and affect the microbiota composition of the cecum. Giannella et al. reported that excess vitamin B12 in the intestine changed the gut microbiome composition and caused the overgrowth of intestinal bacterial (21). Degnan et al. found that the competition and exchange of vitamin B12 among microbes can regulate the gut microcommunity (22). It was documented that vitamin B12 and some other B vitamins changed the metabolism of microbiota, promoted bacterial colonization in the gut, and modulated bacterial virulence and the host defense to the pathogen infection (23–25). Results from in vitro and in vivo trials indicated that vitamin B12 was essential for some enteropathogens to utilize ethanolamine, which enhanced Salmonella typhimurium growth and its virulence gene expression (26–29). Xu et al. indicated that vitamin B12 supplementation changed microbial composition and increased the amount of short-chain fatty acids in an in vitro colon simulation (30).
Thus far, not much information is available for the link between high dietary vitamin B12 and the intestinal microbiota composition in poultry. Therefore, the purpose of the current study was to investigate the effect of fortified diets with standard vs. high levels of vitamin B12 on cecal microbiota composition and production performance of laying hens.
Methods and Materials
All experimental procedures were carried out in accordance with the Guidelines of the Shanxi Agricultural University Animal Experiment Ethics Committee, and the license number was SXAU-EAW-2017-002Chi.001. The experiment was performed in the Animal Production Laboratory of Shanxi Agricultural University and Xingmin Animal Husbandry Industry Cooperative located in Taigu District of Jinzhong City.
Animals, Diets, and Experimental Design
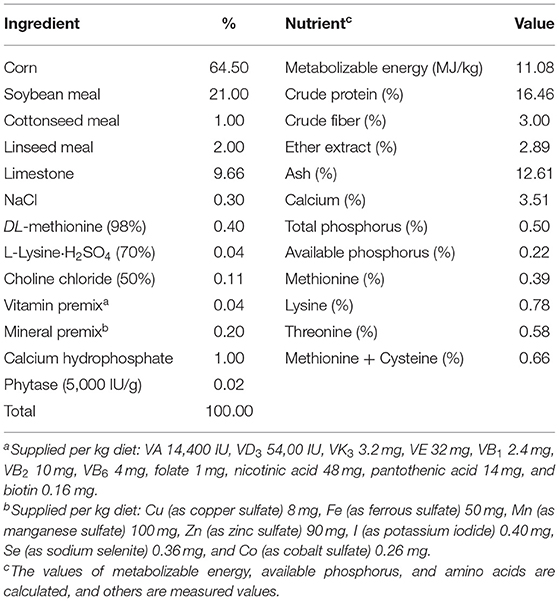
Based on a single-factor experimental design, a total of 432 healthy Jinghong No. 1 laying hens with the same initial body weight (BW) at 30 weeks of age were randomly assigned to four dietary treatments to give six replicate cages of three hens per cage. Dietary treatments included (1) corn soybean meal basal (basal) diet with no supplementation of vitamin B12 (B0), (2) basal diet supplemented with 25 μg/kg of vitamin B12 (B25), (3) basal diet supplemented with 100 μg/kg of vitamin B12 (B100), and (4) basal diet supplemented with 400 μg/kg vitamin B12 (B400). The commercial product used in this trial contained 1% vitamin B12 and was provided by Hebei Yuxing Bio-Engineering Co. Ltd. No vitamin B12 was detected in the basal diet by using high-performance liquid chromatography. This is because vitamin B12 is well-known to be the sole vitamin that is absent from plant-based feed sources. Three dietary supplemental levels of vitamin B12 included one standard level of 25 μg/kg recommended by breeders and vitamin-producing companies (31, 32), and two (100 or 400 μg/kg) high levels were used for producing vitamin B12-enriched eggs (15, 16). Other nutrient level of basal diet was designed based on the NRC recommendation (33). The composition and calculated nutrient level of basal diet are listed in Table 1.
The experimental chicken coop was completely enclosed with the stainless-steel galvanized cages of 38 cm in size. In a 14-day pretrial period, all laying hens were fed a basal diet. Then the treatment diets were provided to the laying hens for another 42 days. The layers were free to eat feed and drink water during the entire pretrial and trial periods. The daily light exposure was 16 h with an intensity not <15 lx/m2.
The Assay of Production Performance, Egg Quality, and Serum Indicators of Laying Hens
Hens in each replicate were weighed in-group at the start and at the end of the experiment to evaluate the BW change. Egg number, egg weight, and dead birds were recorded daily, whereas feed consumption was recorded weekly to calculate hen day egg production (%), feed intake (g/hen/day), egg mass (g/day), and feed conversion ratio (feed/egg mass).
A total of 18 egg samples (three eggs per replicate) were taken weekly in a 6-week trial period to do the analysis of egg weight and eggshell quality. Egg diameter was measured with a digital display vernier caliper (S102-101-101, SMCT, Shanghai). Eggshell strength was evaluated using an Egg Force Reader (EFR-01, Orka Food Technology Ltd., Israel). Eggshell thickness was measured on the large end, equatorial region, and small end, respectively, using an eggshell thickness gauge (Robotmation Co., Ltd., Tokyo, Japan), and the average value was calculated as the eggshell thickness measurement. The egg weight, egg yolk color, and Haugh unit (HU) were evaluated using an Egg Analyzer (EA-01, Orka Food Technology Ltd., Israel). Egg white and yolk were separated carefully, then the egg yolk and eggshell were weighed, and the ratio of the egg yolk, egg white, and eggshell was calculated.
Blood samples were taken from six hens per treatment (one bird per cage) to test the biochemical indicators of serum. Vitamin B12, progesterone, and estrogen levels were measured using enzyme-linked immunoassay kit (Shanghai Hushen Biological Technology Co., Ltd., China). Total cholesterol (TC) and triglyceride (TG) levels were measured using GPO-PAP (NanJing JianCheng Bioengineering Institute, China).
The Assay of Cecal Microbiome
At the end of the experiment, one bird with a BW close to the mean BW of each cage was euthanized by cervical dislocation to collect the cecal content. A total of six samples per treatment were collected. After collection, the samples were immediately frozen by liquid nitrogen and preserved at −80°C until analysis. The frozen samples were used for isolation of metagenomic DNA.
DNA Extraction
Total bacterial genomic DNA samples were extracted using the Fast DNA SPIN extraction kits (MP Biomedicals, Santa Ana, CA, USA), following the instructions of the manufacturer, and stored at −20°C prior to further analysis. The quantity and quality of extracted DNAs were measured using a NanoDrop ND-1000 spectrophotometer (Thermo Fisher scientific, Waltham, MA, USA) and agarose gel electrophoresis, respectively. 16S rDNA amplicon pyrosequencing PCR amplification of the bacterial 16S rRNA gene V3–V4 region was performed using the forward primer 338F (5′-ACTCCTACGGGAGGCAGCA-3′) and the reverse primer 806R (5′-GGACTACHVGGGTWTCTAAT-3′). Sample-specific 7-bp barcodes were incorporated into the primers for multiplex sequencing. The PCR components contained 5 μl of Q5 reaction buffer (5×), 5 μl of Q5 High-Fidelity GC buffer (5×), 0.25 μl of Q5 High-Fidelity DNA Polymerase (5 U/μl), 2 μl (2.5 mM) of dNTPs, 1 μl (10 μM) of each forward and reverse primer, 2 μl of DNA template, and 8.75 μl of ddH2O.
Thermal cycling included initial denaturation at 98°C for 2 min, followed by 25 cycles consisting of denaturation at 98°C for 15 s, annealing at 55°C for 30 s, and extension at 72°C for 30 s, with a final extension of 5 min at 72°C. PCR amplicons were purified with Agencourt AMPure Beads (Beckman Coulter, Indianapolis, IN, USA) and quantified using the PicoGreen dsDNA Assay Kit (Invitrogen, Carlsbad, CA, USA). After the individual quantification step, amplicons were pooled in equal amounts, and paired-end 2,300-bp sequencing was performed using the Illlumina MiSeq platform with MiSeq Reagent Kit v3 at Shanghai Personal Biotechnology Co., Ltd. (Shanghai, China).
Sequence Analysis
The Quantitative Insights Into Microbial Ecology (QIIME, v1.8.0) pipeline was employed to process the sequencing data, as previously described (34). Briefly, raw sequencing reads with exact matches to the barcodes were assigned to respective samples and identified as valid sequences. The low-quality sequences were filtered through following criteria (35, 36) sequences that had a length of <150 bp, sequences that had average Phred scores of <20, sequences that contained ambiguous bases, and sequences that contained mononucleotide repeats of >8 bp. Paired-end reads were assembled using FLASH (37). After chimera detection, the remaining high-quality sequences were clustered into operational taxonomic units (OTUs) at 97% sequence identity by UCLUST (38). A representative sequence was selected from each OTU using default parameters. OTU taxonomic classification was conducted by BLAST searching the representative sequences set against the Greengenes Database (39) using the best hit (40).
Bioinformatics and Statistical Analysis
Sequence data analyses were mainly performed using QIIME and R packages (v3.2.0). OTU-level alpha diversity indices, such as Chao1 richness estimator, ACE metric (Abundance-based Coverage Estimator), Shannon diversity index, and Simpson index, were calculated using the OTU table in QIIME. OTU-level ranked abundance curves were generated to compare the richness and evenness of OTUs among samples. Differences in the Unifrac distances for pairwise comparisons among groups were determined using Student's t-test and the Monte Carlo permutation test with 1,000 permutations. The taxonomy compositions and abundances were visualized using MEGAN (41) and GraPhlAn (42). Venn diagram was generated to visualize the shared and unique OTUs among samples or groups using R package “VennDiagram,” based on the occurrence of OTUs across samples/groups regardless of their relative abundance (43). Taxa abundances at the phylum and genus levels were statistically compared among samples or groups by Metastats (44). Pattern Search was used to identify correlation between the microbial composition of cecal content and dietary supplemental level of vitamin B12. Microbial functions were predicted by PICRUSt (phylogenetic investigation of communities by reconstruction of unobserved states), based on high-quality sequences (45). Correlation Heatmap was performed using the OmicStudio tools at https://www.omicstudio.cn/tool.
Data were analyzed with one-way ANOVA by using Statistical Product and Service Solutions (SPSS) 22.0 (SPSS Inc., Chicago, IL, USA). The significant difference of means among treatment groups was identified via Tukey's test. The significance was determined at P < 0.05.
Results
Production Performance, Egg Quality, and Serum Indicators of Laying Hens
Effects of dietary vitamin B12 on the production performance of laying hens are shown in Table 2. There was no significant difference among all treatments for egg production, feed intake, feed conversion ratio, egg mass, and egg weight (P > 0.05).
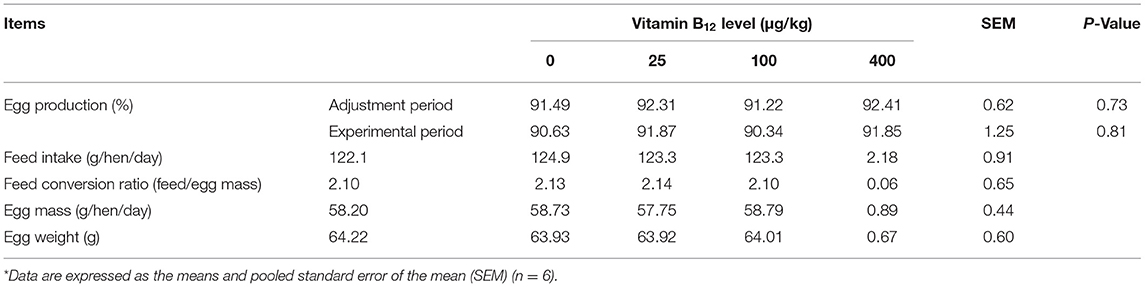
Table 2. Effects of dietary supplementation of vitamin B12 on the production performance of laying hens*.
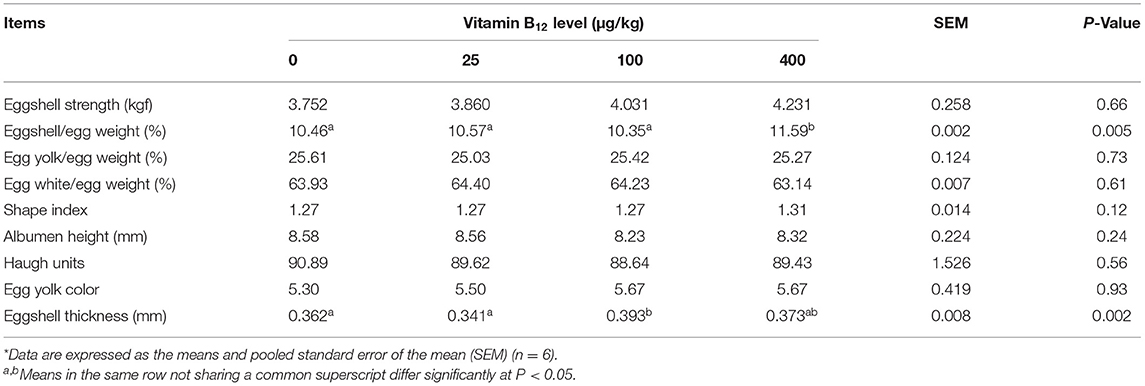
Table 3 lists the effect of dietary vitamin B12 on egg quality parameters. The shell percentage of eggs from hens fed diet supplemented with 400 μg/kg of vitamin B12 was higher (P < 0.05) than that of eggs from hens fed other treatment diets. The shell thickness of eggs from hens fed diet supplemented with 100 μg/kg of vitamin B12 was higher (P < 0.05) than that of eggs from hens fed control diet or supplemented with 25 μg/kg of vitamin B12. No significant effect of dietary treatments on other egg quality parameters was detected (P > 0.05).
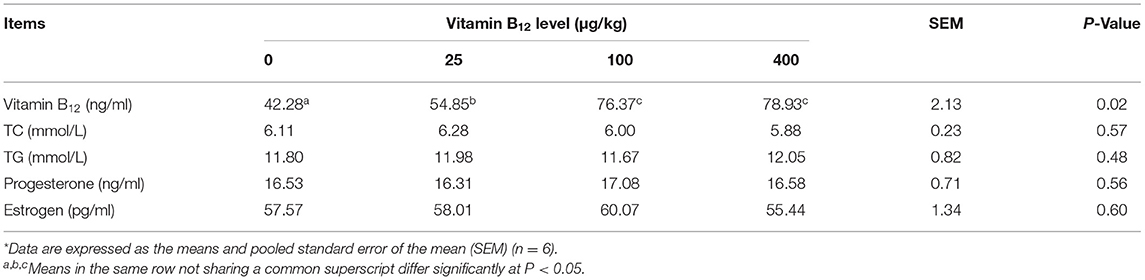
The biochemical indicators of serum are listed in Table 4. The vitamin B12 concentration of serum from hens fed diet with a supplementation of 25 μg/kg of vitamin B12 was higher (P < 0.05) than that from hens fed diet with no supplementation of vitamin B12, but lower (P < 0.05) than that from hens fed other two diets with high supplemental levels of vitamin B12. There was no significant difference among all treatments for other parameters.
Cecal Microbiota Composition
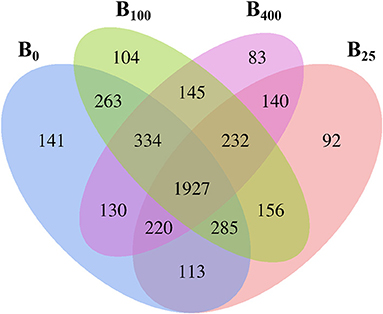
To explore the diversity of the cecal microbiota of layers after dietary vitamin B12 supplementation, the composition and species distribution of the cecal microbiota were investigated by 16S rRNA gene sequencing. After removal of the questioning sequences, a total sequencing quantity was 952,855 that was from 24 samples of cecal content with an average of 39,702 sequences per sample (range from 27,769 to 50,808) for subsequent analysis. A total of 4,386 OTUs after streamlining was characterized into different taxonomic levels including phylum, class, order, family, genus, and species based on Green gene database through QIIME with a 97% species similarity. Venn diagram analysis showed that 1,927 OTUs were shared among the four dietary treatment groups (Figure 1). There were 3,413, 3,165, 3,446, and 3,211 OTUs in treatments B0, B25, B100, and B400, respectively. These results indicated that dietary vitamin B12 did not modulate diversity of the cecal microbiota of the layers. However, there were 141, 92, 104, and 83 OUTs that were uniquely identified in four different treatments.
The rarefaction curves and species accumulation curve (Figures 2A,B) for each sample leveled off as the number of sequences increased in all four treatment groups indicating that the samples analyzed had sufficient sequence coverage to accurately describe the bacterial composition of the cecal content in this study.
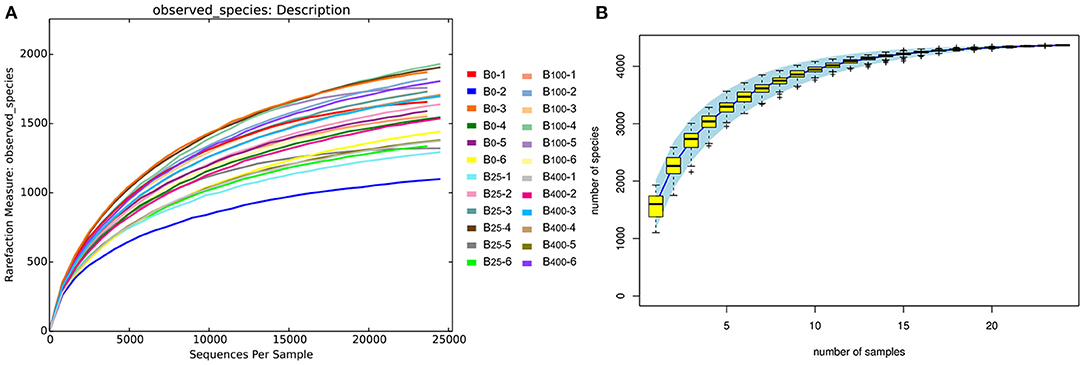
Figure 2. (A) Rarefaction curves and (B) species accumulation curve diagram analysis of proportion of the cecal microbes in different treatments.
The alpha diversity of microbiota in the cecal content, including species diversity (Shannon and Simpson indices) and richness (ACE and Chao 1 indices), is shown in Table 5. There was no significant difference among all treatments for these indices, although the group with no vitamin B12 supplementation tended to have higher values of ACE and Chao1.
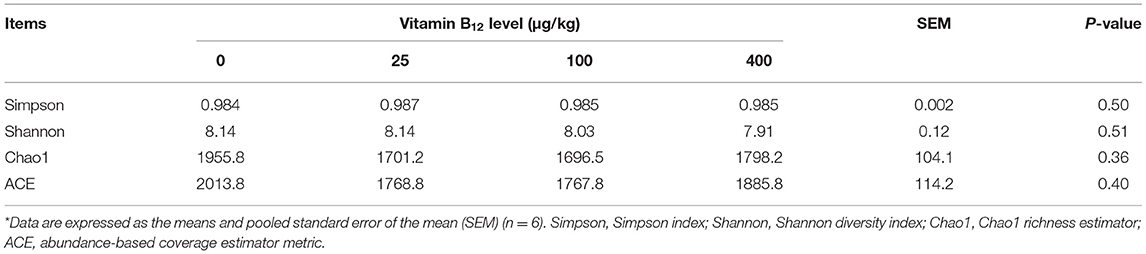
Table 5. Effects of dietary supplementation of vitamin B12 on the cecal microbial diversity in laying hens*.
Figure 3A and Supplementary Table 1 demonstrated the microbial composition of cecal content from four dietary treatments in the phylum level. The results indicated that cecal microbiota of layers was mainly composed of Firmicutes, Bacteroidetes, and Proteobacteria accounting for about 94%, with Firmicutes being the predominant phylum (>52). A numerical shift in the proportion of Bacteroidetes and Proteobacteria to Firmicutes was observed when high-level vitamin B12 (100 or 400 μg/kg) was included in the diets. The proportion of Firmicutes, Actinobacteria, Deferribacteres, WPS_2, and Spirochaetes is positively correlated, and the proportion of Elusimicrobia, Thermi, TM7, Cyanobacteria, Lentisphaerae, Planctomycetes, Proteobacteria, Fusobacteria, Verrucomicrobia, Synergistetes, and Bacteroidetes is negatively correlated with the supplemental level of vitamin B12 (Figure 3C).
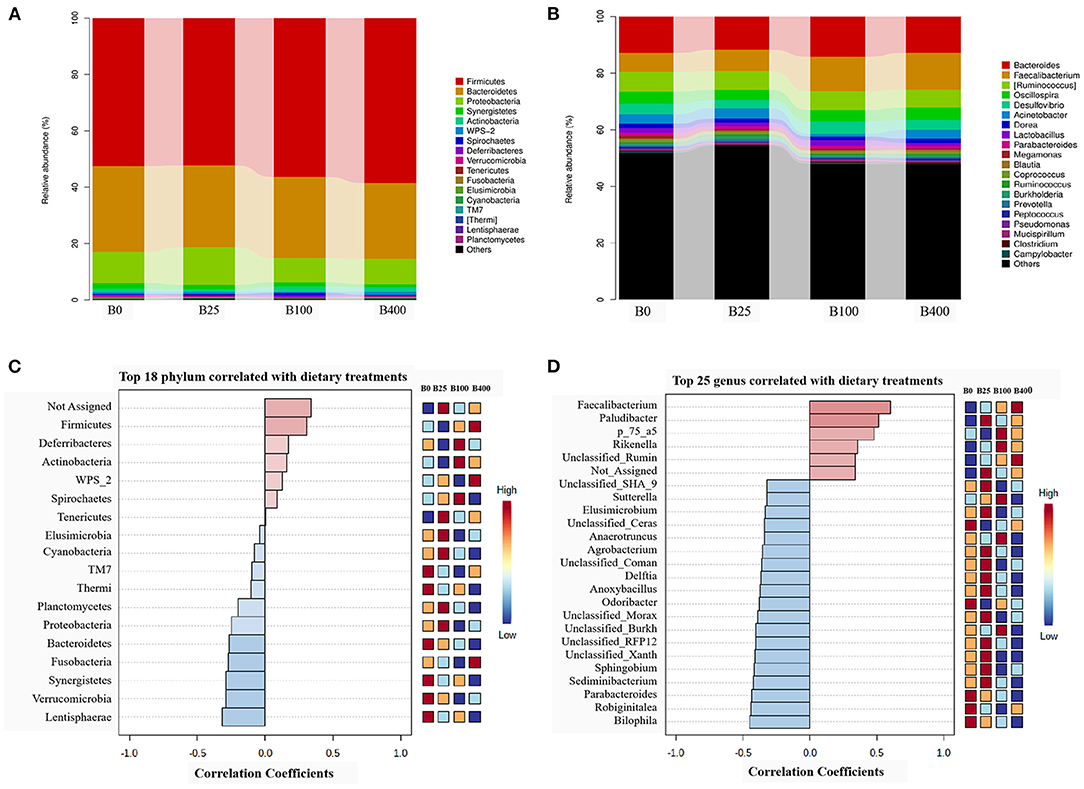
Figure 3. The relative abundance of the cecal microbial composition and correlation with the dietary vitamin B12 level at the phylum level (A,C) and the genus level (B,D).
At genus level, a total of 173 species of bacteria in the cecal content of laying hens was observed in this trial, among which 17 genera had the relative abundance of more than 1% (Figure 3B; Supplementary Table 2). Pattern Search results indicated that the abundance of Butyricicoccus, Faecalibacterium, Paludibacter, p_75_a5, and Unclassified_Ruminococcaceae has a positive correlation, and the abundance of the other 20 bacteria has a negative correlation with the dietary supplemental level of vitamin B12 (Figure 3D).
As shown in Figure 4, the cecal content from layers fed a diet with supplemental level of 100 or 400 μg/kg of vitamin B12 had higher (P < 0.01) abundance of Faecalibacterium and lower (P < 0.05) abundance of Acinetobacter compared with the cecal content from layers fed other two diets. The abundance of Lactobacillus in the cecal samples from layers fed 100 μg/kg of a supplemental level of vitamin B12 was higher (P < 0.05) than that from layers fed with the other three diets. The abundance of Paraprevotella, Blvii28, Perlucidibaca, and Succinatimonas in the cecal content of the layers fed diets supplemented with 25 or 100 μg/kg of vitamin B12 was higher (P < 0.05) than that from the layers fed with the other two diets. It was worth noting that Paludibacter was not detected in the cecal content of layers fed a diet with no supplementation of vitamin B12. The abundance of Butyricicoccus was significantly higher, while Bilophila was significantly lower in the cecal content of layers fed 400 μg/kg of vitamin B12 diet compared with that from layers fed with the other three diets.
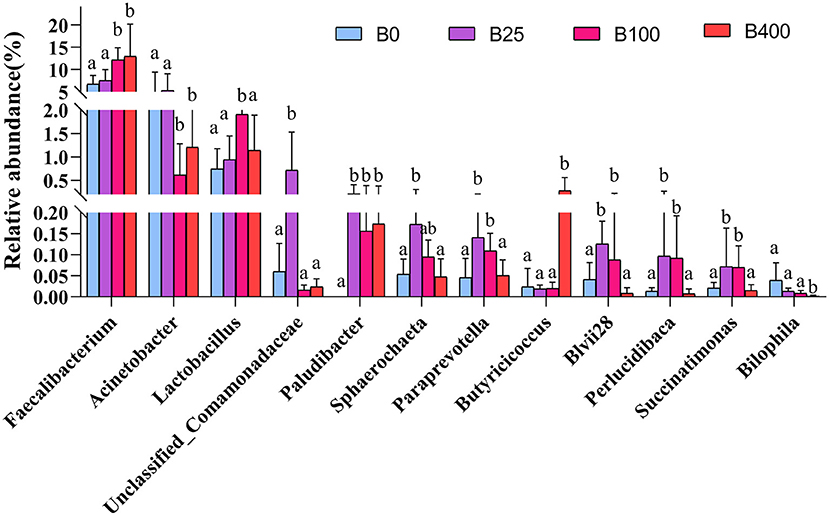
Figure 4. The relative abundance of cecal microbiota of layers fed diets with different levels of vitamin B12 at genus level only statistically significant differences are shown. Bars with different superscript letters were significantly different (P < 0.05).
Functional Prediction of the Gut Microbiota
In order to get the predicted metabolic functions of the cecal microbial communities from different treatments, PICRUSt analysis was performed. The results are shown in Figure 5. Ten predicted metabolic functions of the cecal microbial communities were positively correlated with dietary vitamin B12 level (P < 0.05). These metabolic functions included (1) carbohydrate metabolism such as starch and sucrose metabolism, pentose and glucuronate interconversions, other glycan degradation, and galactose metabolism; (2) lipid metabolism such as sphingolipid metabolism and secondary bile acid biosynthesis; (3) biosynthesis of other secondary metabolites such as phenylpropanoid biosynthesis and flavone and flavonol biosynthesis; (4) other metabolisms such as polycyclic aromatic hydrocarbon degradation and biosynthesis and biodegradation of secondary metabolites. The cecal microbiota from layers fed a diet with 400 μg/kg of vitamin B12 increased all the predicted metabolic functions except flavone and flavonol biosynthesis.
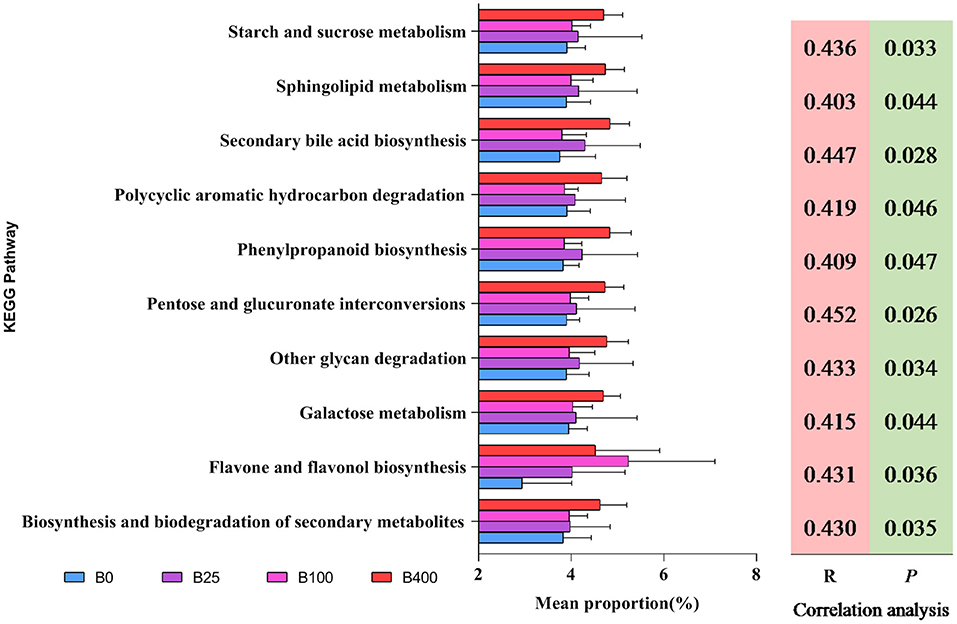
Figure 5. The predicted metabolic attributes of cecal microbiota at genus level from different treatments.
Based on the results of Spearman's analysis (Figure 6), a positive correlation (P < 0.05) between the abundance of Acinetobacter in the cecal content and the biosynthesis and biodegradation of secondary metabolites and polycyclic aromatic hydrocarbon degradation was found. The abundance of Butyricicoccus and Paludibacter in the cecal content was positively correlated (P < 0.05) with carbohydrate and lipid metabolism. A negative correlation (P < 0.05) between the abundance of Bilophila in the cecal content and biosynthesis and biodegradation of secondary metabolites was observed. The abundance of Lactobacillus was positively correlated (P < 0.05) with flavone and flavonol biosynthesis.
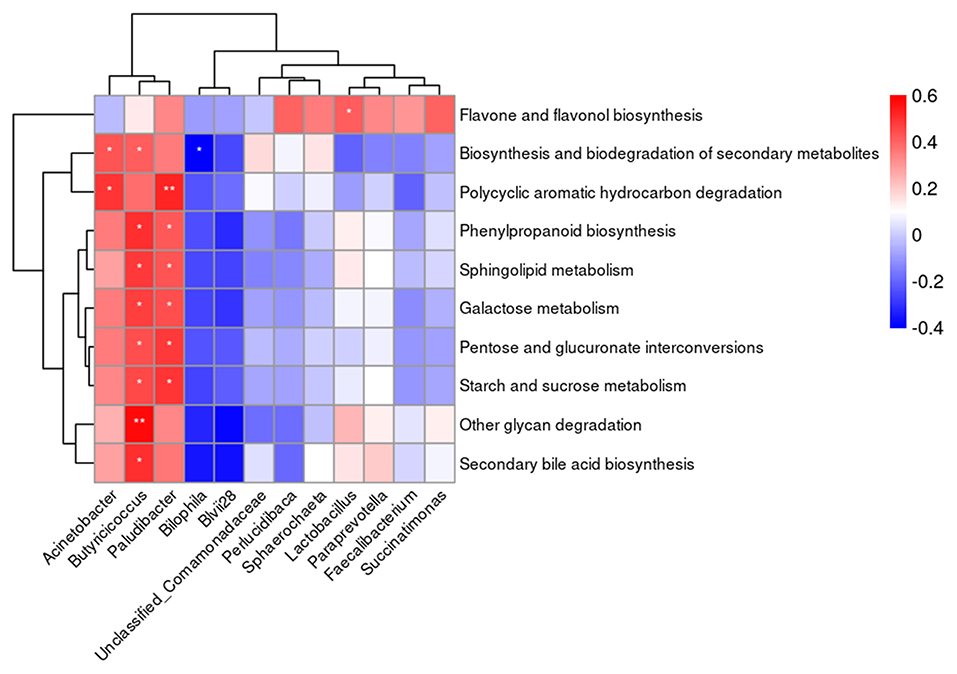
Figure 6. The correlation heatmap between the abundance of microbiota at genus level in the cecal content and the predicted metabolic functions. *P < 0.05, **P < 0.01.
Discussion
The results from the current study showed no significant effect of dietary vitamin B12 levels on the production performance of laying hens including egg production, feed intake, and egg weight. Although the concentration of vitamin B12 in B0 is below the detectable limit, no negative impact on the production performance due to deficiency of vitamin B12 was noticed. This is probably because the layers might get enough reservation of vitamin B12 in the tissues from pretrial diets to support the need of production. The similar results were reported by other researchers (46–48). Kominato reported that 2–5 months may be needed to completely deplete hens of vitamin B12 stored in tissues (49). The high dietary levels of vitamin B12 (100 or 400 μg/kg) used in this trial did not affect the production performance of layers, but significantly increased the thickness and the percentage of eggshell. Leeson and Caston reported that a high dietary vitamin B12 had no effect on the egg production of laying hens (15). The improvement of eggshell quality is probably due to the change in microbiota and their metabolites in the gut. A positive correlation between the abundance of butyrate-producing bacterium and Lactobacillus in the cecal content and dietary vitamin B12 level was found in this trail. Many studies indicated the beneficial effect of dietary inclusion of butyrate or probiotic including Lactobacillus on the eggshell quality (50–53).
Vitamin B12 is a critical nutrient for human beings, animals, as well as microbes. Studies indicated that dietary inclusion of vitamin B12 increased the level of vitamin B12 in the hind gut and modulated the structure and function of microbial communities in humans and mice (22, 54, 55). In the current study, although different dietary levels of vitamin B12 did not alter the diversity of cecal microbiota of layer hens as evident by the similar α-diversity index among different treatments, the community structure and abundance of the microbiota in the cecum were significantly changed. A positive correlation between dietary level of vitamin B12 and cecal abundance of Firmicutes was detected. Studies found that Firmicutes is one of the dominant microorganisms in the cecum of chicken, and the abundance of Firmicutes is beneficial to the health of the bird because of its anti-inflammatory effects (56–58). The negative correlation between the dietary level of vitamin B12 and the richness of Bacteroidetes in the cecal content observed in this trial did not support the results reported by Kelly et al. and Wang (55, 59). The abundance changes in microbiota due to dietary vitamin B12 level could impact the energy metabolism of laying hens. This is based on some evidence showing the close relationship between the abundance of Firmicutes or Bacteroidetes and body fat accumulation in humans and some obesity-related parameters in mice (60, 61). However, further investigation is needed.
It is well-documented that butyrate can positively impact intestinal health through the synergistic effect of providing energy to the intestinal cell and anti-inflammatory effect (62–64). Major butyrate-producing bacteria in the gastral intestinal tract included F. prasusnitzii in Faecalibacterium genus and Butyricoccus (65, 66). They all played a very important role in host intestinal health (58, 66–68). Results from the current study indicated that Faecalibacterium was the core genus in the cecum of laying hens and was positively correlated with dietary supplemental levels of vitamin B12, and dietary supplementation of high-level vitamin B12 (100 or 400 μg/kg) increased the abundance of Butyricoccus. The increased abundance of Lactobacillus, Paraprevotella, Succinatimonas, Paludibacter, and Sphaerochaeta in the cecal content due to high dietary vitamin B12 (100 or 400 μg/kg) observed in this trial could also positively impact the gut health of laying hens. This is because bacteria in the gut, including Lactobacillus and Paraprevotella, have been proven to play an active and protective role in intestinal health of the host through producing antimicrobial molecules, competitive exclusion, and changing bile acid metabolism in the gut (69–71). Succinatimonas, Paludibacter, and Sphaerochaeta were found to produce volatile fatty acids including acetic acid, propionic acid, and succinic acid that could positively impact energy metabolism of the host and regulate hindgut pH to inhibit the growth of harmful bacteria (72–77). Dietary supplementation of high-level vitamin B12 depressed the richness of Acinetobacter and Bilophila that were inflammation-associated genera (78). The composition changes in cecal microbiota of laying hens found in this trial due to high dietary vitamin B12 was also significantly correlated to the predicted metabolism function of microbial communities in the cecum including carbohydrate, lipid, and many other metabolisms. Similar results were reported by other researchers (79).
Conclusions
To our knowledge, this is the first trial to explore the relationship between high dietary levels of vitamin B12 and the cecal microbiota in laying hens. The results indicated that dietary supplementation of 100 μg/kg of vitamin B12 had equivalent effects with the supplemental level of 400 μg/kg. Both levels caused a significant change in composition and metabolic functions of cecal microorganisms, which could positively impact eggshell quality, metabolism, and gut health of laying hens during peak production period. These findings provided the valuable information for the application of high supplemental levels of vitamin B12 in the diet of laying hens for production or health purposes.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: BioProject ID PRJNA732929.
Ethics Statement
All experimental procedures were carried out in accordance with the Guidelines of the Shanxi Agricultural University Animal Experiment Ethics Committee, and the license number was SXAU-EAW-2017-002Chi.001.
Author Contributions
YY: conceptualized the study, supervised the study, was in charge of the project administration, and acquired the funding. RW: developed the methodology, performed the formal analysis and data curation, obtained the resources, and wrote and prepared the original draft. RRL and RW: conducted the investigation. RW, XTW, and YB wrote, reviewed, and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the 1331 Key Discipline Construction of Engineering Animal Husbandry of Shanxi Province (J202011315), Key Research and Development Project of Science and Technology (Agriculture) of Jinzhong City (Y182011), and Scientific and Technological Innovation Programs of Higher Education Institutions in Shanxi (2019L0959).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank relative personnel of Xingmin Animal Husbandry Cooperative (Taigu, China) for guidance in the feeding process. We would also like to thank Editage (www.editage.cn) for English language editing.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2021.712183/full#supplementary-material
References
1. Sahasrabudhe MR, Lakshminarayan Rao MV. Effect of Vitamin B12 on the synthesis of protein and nucleic acids in the liver. Nature. (1951) 168:605–6. doi: 10.1038/168605b0
2. Guest JR, Friedman S, Woods DD, Smith EL. A Methyl analogue of cobamide coenzyme in relation to methionine synthesis by bacteria. Nature. (1962) 195:340–2. doi: 10.1038/195340a0
3. Banerjee RV, Harder SR, Ragsdale SW, Matthews RG. Mechanism of reductive activation of cobalamin-dependent methionine synthase: an electron paramagnetic resonance spectroelectrochemical study. Biochemistry. (1990) 29:1129–35. doi: 10.1021/bi00457a005
4. Funada B, Tetsunori K, Tanaka N, Maekawa A, Mano H, Wada M. Change of the immune function in the rat vitamin B12 condition of depletion. Vitamins. (2002) 76:180.
5. Reynolds E. Vitamin B12, folic acid, and the nervous system. Lancet Neurol. (2006) 5:949–60. doi: 10.1016/S1474-4422(06)70598-1
6. Green R, Allen LH, Bjørke-Monsen AL, Brito A, Guéant JL, Miller JW. Vitamin B12 deficiency. Nat Rev Dis Primers. (2017) 3:17040. doi: 10.1038/nrdp.2017.40
7. Savage JE, Turner CW, Kempster HL, Hogan AG. The effects of vitamin B12 and thyroprotein on egg production, egg weight, shell quality and hatchability. Poult Sci. (1952) 31:22–30. doi: 10.3382/ps.0310022
8. Weberw GM. Improvement of flock productivity through supply of vitamins for higher laying performance and better egg quality. Worlds Poult Sci J. (2009) 65:443–58. doi: 10.1017/S0043933909000312
9. Kato RK, Bertechini AG, Fassani EJ, Santos CD, Dionizio MA, Fialho ET. Cobalt and vitamin B12 in diets for commercial laying hens on the second cycle of production. Braz J Poult Sci. (2003) 5:45–50. doi: 10.1590/S1516-635X2003000100006
10. Patel MB. Mcginnis J. The effect of levels of protein and vitamin B12 in hen diets on egg production and hatchability of eggs and on livability and growth of chicks. Poult Sci. (1977) 56:45–53. doi: 10.3382/ps.0560045
11. El-Husseiny OM, Soliman AZM, Elsherif HMR. Influence of dietary methionine, folic acid and cyanocobalamin and their interactions on the performance of broiler breeder. Int J Poult Sci. (2018) 17:189–96. doi: 10.3923/ijps.2018.189.196
12. Scott ML. Effect of processing on the availability and nutritional value of vitamins. In: Scott ML, editor. Effect of Processing on the Nutritional Value of Feeds. Washington, DC: National Academy of Sciences (1972) p. 119–130.
13. Killeit V. The Stability of Vitamins-A Selection of Current Literature. Basel: F. Hoffmann-LaRoche Co. Ltd. (1988).
14. Coelho M. Fate of vitamins in premixes and feeds: vitamin stability. Feed Mgmt. (1991) 42:24–35.
15. Leeson S, Caston LJ. Vitamin enrichment of eggs. J Appl Poult Res. (2003) 12:24–6. doi: 10.1093/japr/12.1.24
16. Sahlin A, House JD. Enhancing the vitamin content of meat and eggs: implications for the human diet. Can J Anim Sci. (2006) 86:181–95. doi: 10.4141/a05-060
17. Halick JV, Reid BL, Brown CL, Couch JR. The vitamin B12 content of egg yolks as influenced by oral and parenteral administration of the vitamin. J Nutr. (1953) 50:331–40. doi: 10.1093/jn/50.3.331
18. Denton CA, Kellogg WL, Sizemore JR, Lillie RJ. Effect of injecting and feeding vitamin B12 to hens on content of the vitamin in the egg and blood. J Nutr. (1954) 54:571–7. doi: 10.1093/jn/54.4.57
19. Squires MW, Naber EC. Vitamin profiles of eggs as indicators of nutritional status in the laying hen: vitamin B12 study. Poult Sci. (1992) 71:2075–82. doi: 10.3382/ps.0712075
20. Naber EC, Squires MW. Vitamin profiles of eggs as indicators of nutritional status in the laying hen: diet to egg transfer and commercial flock survey. Poult Sci. (1993) 72:1046–53. doi: 10.3382/ps.0721046
21. Giannella RA, Broitman SA, Zamcheck N. Cobalamin uptake by intestinal microorganisms: mechanism and relevance to syndromes of intestinal bacterial overgrowth. J Clin Inves. (1971) 50:1100–7. doi: 10.1172/JCI106581
22. Degnan PH, Taga ME, Goodman AL. Vitamin B12 as a modulator of gut microbial ecology. Cell Metab. (2014) 20:769–78. doi: 10.1016/j.cmet.2014.10.002
23. Miki T, Goto R, Fujimoto M, Okada N, Hardt W. The bactericidal lectin regiii beta prolongs gut colonization and enteropathy in the streptomycin mouse model for Salmonella diarrhea. Cell Host Microbe. (2017) 21:195–207. doi: 10.1016/j.chom.2016.12.008
24. Sperandio V. Take your pick: vitamins and microbiota facilitate pathogen clearance. Cell Host Microbe. (2017) 21:130–1. doi: 10.1016/j.chom.2017.01.013
25. Li YX, Wang JK, Sun H, PengYJ Mao HL, Wu YM, et al. In vitro rumen fermentation and microbial enzyme activities influenced by vitamin B12 supplementation in different concentrate to forage ratios. Chin J Anim Nutr. (2012) 24:1888–96. doi: 10.3969/j.issn.1006-267x.2012.10.007
26. Anderson CJ, Clark DE, Mazhar A, Kendall MM, Baumler AJ. Ethanolamine signaling promotes Salmonella niche recognition and adaptation during infection. PLoS Pathog. (2015) 11:e1005278. doi: 10.1371/journal.ppat.1005278
27. Thiennimitr P, Winter SE, Winter MG, Xavier MN, Tolstikov V, Huseby DL, et al. Intestinal inflammation allows Salmonella to use ethanolamine to compete with the microbiota. Proc Natl Acad Sci USA. (2011) 108:17480–5. doi: 10.1073/pnas.1107857108
28. Boran P, Baris HE, Kepenekli E, Erzik C, Dinh DM. The impact of vitamin B12 deficiency on infant gut microbiota. Eur J Pediatr. (2019) 179:385–93. doi: 10.1007/s00431-019-03517-2
29. Zhu X, Xiang S, Feng X, Wang H, Tian S, Xu Y, et al. Impact of cyanocobalamin and methylcobalamin on inflammatory bowel disease and the intestinal microbiota composition. J Agr Food Chem. (2018) 67:916–26. doi: 10.1021/acs.jafc.8b05730
30. Xu Y, Xiang S, Ye K, Zheng Y, Feng X, Zhu X, et al. Cobalamin (Vitamin B12) induced a shift in microbial composition and metabolic activity in an in vitro colon simulation. Front Microbiol. (2018) 9:2780. doi: 10.3389/fmicb.2018.02780
31. Hy-Line International. Hy-Line W-36 Performance Standards Manual. (2012). Available online at: http://www.hyline.com/aspx/productsandservices/managementmanuals.aspx
32. DSM Vitamin Supplementation Guidelines 2016 for Animal Nutrition. Available online at: http://www.dsm.com
33. National Research Council Nutrient Requirements of Poultry. 9th ed. Washington, DC: National Academy Press (1994).
34. Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. Qiime allows analysis of high-throughput community sequencing data. Nat Methods. (2010) 7:335–6. doi: 10.1038/nmeth.f.303
35. Gill SR, Pop M, DeBoy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, et al. Metagenomic analysis of the human distal gut microbiome. Science. (2006) 12:1355–9. doi: 10.1371/journal.pone.0231197
36. Chen H, Jiang W. Application of high-throughput sequencing in understanding human oral microbiome related with health and disease. Front Microbiol. (2014) 5:508–508. doi: 10.3389/fmicb.2014.00508
37. Magoc T, Salzberg SL FLASH fast length adjustment of short reads to improve genome assemblies. Bioinformatics. (2011) 27:2957–63. doi: 10.1093/bioinformatics/btr507
38. Edgar RC. search and clustering orders of magnitude faster than BLAST. Bioinformatics. (2010) 26:2460–1. doi: 10.1093/bioinformatics/btq461
39. DeSantis TZ, Rojas M, Dalevi D, Keller K, Huber T, Andersen GL, et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microb. (2006) 72:5069–72. doi: 10.1128/AEM.03006-05
40. Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. (1997) 25:3389–402. doi: 10.1093/nar/25.17.3389
41. Huson DH, Mitra S, Ruscheweyh HJ, Weber N, Schuster SC. Integrative analysis of environmental sequences using MEGAN4. Genome Res. (2011) 21:1552–60. doi: 10.1101/gr.120618.111
42. Asnicar F, Weingart G, Tickle TL, Huttenhower C, Segata N. Compact graphical representation of phylogenetic data and metadata with GraPhlAn. PeerJ. (2015) 3:e1029. doi: 10.7717/peerj.1029
43. Zaura E, Keijser BJ, Huse SM, Crielaard W. Defining the Healthy “core microbiome” of oral microbial communities. BMC Microbiol. (2009) 9:259–259. doi: 10.1186/1471-2180-9-259
44. White JR, Nagarajan N, Pop M. Statistical methods for detecting differentially abundant features in clinical metagenomic samples. PLoS Comput Biol. (2009) 5:e1000352. doi: 10.1371/journal.pcbi.1000352
45. Langille MGI, Zaneveld J, Caporaso JG, McDonald D, Knights DJ, Reyes A, et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol. (2013) 31:814–21. doi: 10.1038/nbt.2676
46. Kachana S, Banchasak C, Harnbanchong A. Effect of dietary folate and vitamin B12 on egg composition and liver triglyceride of laying hens at 64 weeks of age. Songklanak J Sci Technol. (2005) 27:789–97.
47. Bunchasak C, Kachana S. Dietary folate and vitamin B12 supplementation and consequent vitamin deposition in chicken eggs. Trop Anim Health Pro. (2009) 41:1583–9. doi: 10.1007/s11250-009-9350-7
48. Janist N, Srichana P, Asawakarn T, Kijparkorn S. Effect of supplementing the laying hen diets with choline, folic acid, and vitamin B12 on production performance, egg quality, and yolk phospholipid. Livest Sci. (2019) 223:24–31. doi: 10.1016/j.livsci.2019.02.019
49. Kominato T. Speed of vitamin B12 turnover and its relation to the intestine in the rat. Vitamins. (1971) 44:76–83.
50. Fathi M, Al-Homidan I, Al-Dokhail A, Ebeid T, Abou-Emera O, Alsagan A. Effects of dietary probiotic (Bacillus subtilis) supplementation on productive performance, immune response and egg quality characteristics in laying hens under high ambient temperature. Ital J Anim Sci. (2018) 17:804–14. doi: 10.1080/1828051X.2018.1425104
51. Soltan MA. Effect of dietary organic acid supplementation on egg production, egg quality and some blood serum parameters in laying hens. Int J Poult Sci. (2008) 7:613–21. doi: 10.3923/ijps.2008.613.621
52. Khong C, Sen S, Lee S, Choi Y, Kim KY, Ingale S, et al. Effect of sodium butyrate supplementation on performance, egg quality and bacterial load in the excreta of laying hens. J Anim Res. (2014) 4:141–53. doi: 10.5958/2277-940X.2014.00499.9
53. Pires MF, Leandro NSM, Jacob DV, Carvalho FB, Oliveira HF, Stringhini JH, et al. Performance and egg quality of commercial laying hens fed with various levels of protected sodium butyrate. S Afr J Anim Sci. (2020) 50:758–65. doi: 10.4314/sajas.v50i5.14
54. Wang HH, Shou Y, Zhu X, Xu Y, Shi L, Xiang S, et al. Stability of vitamin B12 with the protection of whey proteins and their effects on the gut microbiome. Food Chem. (2018) 276:298–306. doi: 10.1016/j.foodchem.2018.10.033
55. Kelly CJ, Alexeev EE, Farb L, Vickery TW, Zheng L, Campbell EL, et al. Oral vitamin B12 supplement is delivered to the distal gut, altering the corrinoid profile and selectively depleting Bacteroides in C57BL/6 mice. Gut Microbes. (2019) 10:1–9. doi: 10.1080/19490976.2019.1597667
56. Dong XY, Azzam MMM, Zou XT. Effects of dietary threonine supplementation on intestinal barrier function and gut microbiota of laying hens. Poult Sci. (2017) 96:3654–63. doi: 10.3382/ps/pex185
57. Kumar S, Adhikari P, Oakley BB, Kim WK. Changes in cecum microbial community in response to total sulfur amino acid (TSAA: DL-methionine) in antibiotic-free and supplemented poultry birds. Poult Sci. (2019) 98:5809–19. doi: 10.3382/ps/pez380
58. Eeckhaut V, Machiels K, Perrier C, Romero C, Maes S, Flahou B, et al. Butyricicoccus pullicaecorum in inflammatory bowel disease. Gut. (2013) 62:1745–52. doi: 10.1136/gutjnl-2012-303611
59. Wang JX. The gut and hemolymph microbiotas of crustacean, composition, functions and homeostatic regulation. Chin Acta Microbiol Sin. (2018) 58:760–72. doi: 10.13343/j.cnki.wsxb.20170407
60. Stephens RW, Arhire LI, Covasa M. Gut microbiota: from microorganisms to metabolic organ influencing obesity. Obesity. (2018) 26:801–9. doi: 10.1002/oby.22179
61. Zhang J, Zhao Y, Ren D, Yang X. Effect of okra fruit powder supplementation on metabolic syndrome and gut microbiota diversity in high fat diet-induced obese mice. Food Res Int. (2020) 130:108929. doi: 10.1016/j.foodres.2019.108929
62. Guilloteau P, Martin L, Eeckhaut V, Ducatelle R, Zabielski R, Van Immerseel F. From the gut to the peripheral tissues: the multiple effects of butyrate. Nutr Res Rev. (2010) 23:366–84. doi: 10.1017/S0954422410000247
63. Bedford A, Gong J. Implications of butyrate and its derivatives for gut health and animal production. Anim Nutr. (2018) 4:151–9. doi: 10.1016/j.aninu.2017.08.010
64. Antongiovanni M, Buccioni A, Petacchi F, Leeson S, Minieri S, Martini A. Butyric acid glycerides in the diet of broiler chickens: effects on gut histology and carcass composition. Ital J Anim Sci. (2007) 6:19–26. doi: 10.4081/ijas.2007.19
65. Leylabadlo HE, Ghotaslou R, Feizabadi MM, Farajnia S, Moaddab SY, Ganbarov K, et al. The critical role of Faecalibacterium prausnitzii in human health: an overview. Microb Pathogenesis. (2020) 149:104344. doi: 10.1016/j.micpath.2020.104344
66. Lopezsiles M, Duncan SH, Garciagil LJ, Martinezmedina M. Faecalibacterium prausnitzii: from microbiology to diagnostics and prognostics. ISME J. (2017) 11:841–52. doi: 10.1038/ismej.2016.176
67. Ferreira-Halder CV, Faria AVS, Andrade SS. Action and function of Faecalibacterium prausnitzii in health and disease. Best Pract Res Clin Gastroenterol. (2017) 31:243–8. doi: 10.1016/j.bpg.2017.09.011
68. Trachsel J, Humphrey S, Allen HK. Butyricicoccus porcorum sp. nov., a butyrate-producing bacterium from swine intestinal tract. Int J Syst Evol Microbiol. (2018) 68:1737–42. doi: 10.1099/ijsem.0.002738
69. Mu Q, Tavella VJ, Luo XM. Role of Lactobacillus reuteri in human health and diseases. Front Microbiol. (2018) 9:757. doi: 10.3389/fmicb.2018.00757
70. Koenen ME, Heres L, Claassen E, Boers WJA. Lactobacilli as probiotics in chicken feeds. Biosci. Microflora. (2002) 21:209–16. doi: 10.12938/bifidus1996.21.209
71. Zhang S, Zhong G, Shao D, Wang Q, Hu Y, Wu TX, et al. Dietary supplementation with Bacillus subtilis promotes growth performance of broilers by altering the dominant microbial community. Poult Sci. (2021) 100:3. doi: 10.1016/j.psj.2020.12.032
72. Stackebrandt E, Hespell RB. The family Succinivibrionaceae. In: Rosenberg E, DeLong EF, Lory S, Stackebrandt E, Thompson F, editors. The Prokaryotes. Berlin; Heidelberg: Springer (2006). p. 639–48. doi: 10.1007/0-387-30743-5_20
73. Hu Y, Wang LD, Shao D, Wang Q, Wu YY, Han YM, et al. Selective and reshaped early dominant microbial community in the cecum with similar proportions and better homogenization and species diversity due to organic acids as AGP alternatives mediate their effects on broilers growth. Front Microbiol. (2020) 10:2948. doi: 10.3389/fmicb.2019.02948
74. Qiu YL, Kuang XZ, Shi XS, Yuan XZ, Guo RB. Paludibacter jiangxiensis sp. nov a strictly anaerobic, propionate-producing bacterium isolated from rice paddy. Field Arch Microbiol. (2014) 196:149–55. doi: 10.1007/s00203-013-0951-1
75. Koh A, De Vadder F, Kovatcheva-Datchary P, Bäckhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. (2016) 165:1332–45. doi: 10.1016/j.cell.2016.05.041
76. Liu T, Tang J, Feng F. Medium-chain α-monoglycerides improves productive performance and egg quality in aged hens associated with gut microbiota modulation. Poult Sci. (2020) 99:7122–32. doi: 10.1016/j.psj.2020.07.049
77. Li Z, Lin Z, Lu Z, Feng Z, Ying Z. Coix seed improves growth performance and productivity in post-weaning pigs by reducing gut pH and modulating gut microbiota. AMB Express. (2019) 9:115–29. doi: 10.1186/s13568-019-0828-z
78. Htay H, Cho Y, Ascoe PM, Hawley CM, Johnson DW. Outcomes of Acinetobacter peritonitis in peritoneal dialysis patients: a multicenter registry analysis. Peritoneal Dial Int Perit Dial Int. (2018) 38:257–65. doi: 10.3747/pdi.2017.00199
Keywords: vitamin B12, laying hens, production performance, eggshell quality, cecal microbiota
Citation: Wang R, Bai Y, Yang Y, Wu XT and Li RR (2021) A Comparison of Production Performance, Egg Quality, and Cecal Microbiota in Laying Hens Receiving Graded Levels of Vitamin B12. Front. Vet. Sci. 8:712183. doi: 10.3389/fvets.2021.712183
Received: 20 May 2021; Accepted: 16 September 2021;
Published: 21 October 2021.
Edited by:
Shourong Shi, Poultry Institute, Chinese Academy of Agricultural Sciences (CAAS), ChinaReviewed by:
Siaka Seriba Diarra, University of the South Pacific, FijiYueping Chen, Nanjing Agricultural University, China
Copyright © 2021 Wang, Bai, Yang, Wu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu Yang, 345605203@qq.com
 Rui Wang
Rui Wang Yan Bai1
Yan Bai1  Yu Yang
Yu Yang