Seroprevalence of Brucellosis in Buffalo Worldwide and Associated Risk Factors: A Systematic Review and Meta-Analysis
- 1College of Chinese Medicine Materials, Jilin Agricultural University, Changchun, China
- 2College of Animal Science and Technology, Jilin Agricultural University, Changchun, China
- 3Laboratory of Production and Product Application of Sika Deer of Jilin, Jilin Agricultural University, Changchun, China
- 4Key Lab of Animal Production, Product Quality and Security, Ministry of Education, Jilin Agricultural University, Changchun, China
Background: Brucellosis is an important zoonotic disease caused by Brucella spp. Brucellosis is widely distributed in more than 160 or 170 countries around the world, where it poses a huge threat to animal husbandry and human health. About 150 million head of water buffalo, distributed across more than 40 countries worldwide, are kept for the purposes of service, milk, and meat. High incidence of Brucella spp. in buffalo has negatively affected dairy products and meat products.
Results: We searched all research related to seroprevalence of brucellosis in water buffalo anywhere in the world in PubMed, Science Direct, SpringerLink, China National Knowledge Infrastructure, Wanfang Database, and VIP Chinese Journal Databases. A total of 26 articles published from 1985 to 2020 met the final selection criteria. The overall seroprevalence of buffalo brucellosis worldwide was 9.7%. The seroprevalence before 2010 (20.8%) (95% CI: 5.6–42.2) was much higher than the seroprevalence rate from 2010 to 2020 (4.2%) (95% CI: 1.8–7.5). Subgroup analysis by feeding mode found that the point estimate of seroprevalence in stock buffalo (11.5%) (95% CI: 3.6–23.0) was higher than that in captive buffalo (10.6%) (95% CI: 4.9–18.1). Subgroup analysis by farming mode found that the seroprevalence was higher in captive-bred buffalo (10.7%) (95% CI: 6.6–15.7) than in intensively farmed buffalo (8.5) (95% CI: 0.9–22.2). The seroprevalence in buffalo living in dry lands (6.4%) (95% CI: 2.0–12.9) is greater than that in buffalo living in wetlands (5.1%) (95% CI: 1.8–10.4) (P < 0.05). The seroprevalence in female buffalo (10.1%) (95% CI: 3.4–19.7) was higher than that in male buffalo (4.4%) (95% CI: 2.0–7.4). The seroprevalence in lactating buffalo was higher than that in buffalo of other ages (26.9%) (95% CI: 1.8–66.5). Subgroup analysis by detection method found that the seroprevalence detected by the complement fixation test (27.3%) (95% CI: 0.7–70.8) was much higher than that detected by other methods.
Conclusion: The results of this meta-analysis showed that buffalo brucellosis infection is very common in buffalo herds around the world. Although the seroprevalence of brucellosis in buffalo and humans is relatively low, serious effects upon animal husbandry and public health make it necessary to take effective control and preventive measures to control the spread of this disease.
Introduction
Brucellosis, also known as Mediterranean fever or Malta fever (1), is one of the most common zoonotic diseases worldwide, endemic in more than 170 countries and regions (2–4). Classical taxonomists recognize six species of the causative bacterium, Brucella, based on subtle phenotypic and antigenic differences and host specificity: B. abortus (bovine), B. melitensis (caprine and ovine), B. ovis (ovine), B. canis (canine), and B. neotomae (desert wood rat) (5); some of these Brucella spp. are classically divided into biovars. Brucellosis can affect phagocytes, mainly infecting the reproductive system and lymph nodes. Its typical clinical presentation in female animals includes abortion of the calf, and in male animals, orchitis, epididymitis, and arthritis (6, 7). The highest infection rate among wild animals is found in American bison and bison (39.9%), followed by alpine bison and goats (33%). Water buffalo, a common domestic animal, is also a major locus of brucellosis infection (8).
Although water buffalo are particularly well-suited to rivers and marshy areas in humid tropical regions, they are kept as livestock on all inhabited continents. Not only are they used to reclaim farmland, but their yield of milk, leather, and meat is of economic importance (9–13). Animals infected with Brucella can spread it to humans through unpasteurized milk, meat, and animal by-products (14–17). Human brucellosis may result in fever, severe arthritis, and infertility; unfortunately, through misdiagnosis and mistreatment, it often becomes chronic. Repeated episodes may cause a serious economic burden to the family and nation through the loss of working days (18, 19). By developing effective vaccination plans and control strategies, some high-income countries have effectively controlled or even eliminated bovine brucellosis (20, 21); however, each year, some 500,000 persons continue to be infected, mostly in developing countries such as the Middle East and Southeast Asia (22–29). Despite the fact that Office International Des Epizooties (OIE) has proposed control measures for Brucella farms since 2010 (30), brucellosis infection has improved and the incidence of brucellosis in animals and humans has shown a significant upward trend in recent years.
As far as we know, no research has previously been done on the overall seroprevalence of buffalo brucellosis worldwide. Therefore, we conducted a systematic review and meta-analysis to analyze the total seroprevalence of buffalo brucellosis and assess the risk factors for the disease.
Materials and Methods
Search Strategy
We searched six databases for all publications on buffalo brucellosis: PubMed, Science Direct, Springer, China National Knowledge Infrastructure (CNKI), Wanfang Database (Wan Fang), and VIP Chinese Journal Databases (VIP). We searched for all studies between 1985 and May 16, 2020. In this study, titles and articles were searched using corresponding nouns in English or Chinese.
We searched using the Medical Subject Headings (MeSH) term “brucellosis” and related terms “Brucelloses,” “Malta Fever,” “Fever, Malta,” “Gibraltar Fever,” “Fever, Gibraltar,” “Rock Fever,” “Fever, Rock,” “Cyprus Fever,” “Fever,” “Cyprus,” “Brucella Infection,” “Brucella Infections,” “Infection, Brucella,” “Undulant Fever,” “Fever,” “Undulant,” “Brucellosis,” “Pulmonary,” “Brucelloses,” “Pulmonary,” and “Pulmonary Brucelloses.” We thus built Search Formula A: (((((((((((((((((“Brucellosis” [Mesh]) OR (Brucelloses)) OR (Malta Fever)) OR (Fever, Malta)) OR (Gibraltar Fever)) OR (Fever, Gibraltar)) OR (Rock Fever)) OR (Fever, Rock)) OR (Cyprus Fever)) OR (Fever, Cyprus)) OR (Brucella Infection)) OR (Brucella Infections)) OR (Infection, Brucella)) OR (Undulant Fever)) OR (Fever, Undulant)) OR (Brucellosis, Pulmonary)) OR (Brucelloses, Pulmonary)) OR (Pulmonary Brucelloses).
We then used the MeSH term “Buffalo” and its entries “Buffaloes,” “Syncerus,” “Water Buffaloes,” “Water Buffalo,” “Buffalo, Water,” and “Bubalus” to construct the search Formula B: ((((((“Buffaloes”[Mesh]) OR (Buffalo)) OR (Syncerus)) OR (Water Buffaloes)) OR (Water Buffalo)) OR (Buffalo, Water)) OR (Bubalus).
Finally, we used the logical “AND” of the two formulas to identify items satisfying both Search Formula A AND Search Formula B: ((((((((((((((((((“Brucellosis”[Mesh]) OR (Brucelloses)) OR (Malta Fever)) OR (Fever, Malta)) OR (Gibraltar Fever)) OR (Fever, Gibraltar)) OR (Rock Fever)) OR (Fever, Rock)) OR (Cyprus Fever)) OR (Fever, Cyprus)) OR (Brucella Infection)) OR (Brucella Infections)) OR (Infection, Brucella)) OR (Undulant Fever)) OR (Fever, Undulant)) OR (Brucellosis, Pulmonary)) OR (Brucelloses, Pulmonary)) OR (Pulmonary Brucelloses)) AND (((((((“Buffaloes”[Mesh]) OR (Buffalo)) OR (Syncerus)) OR (Water Buffaloes)) OR (Water Buffalo)) OR (Buffalo, Water)) OR (Bubalus)).
In ScienceDirect, we used the themes “Brucellosis” and “Buffaloes” and “seroprevalence” to search. In SpringerLink, we used the themes “Brucellosis” and “Buffaloes” and “seroprevalence” and “Buffaloes” to search. In Wanfang, we used the themes “Buffalo” and “Brucella” or “Buffalo” and “Brucella” to search. We used the themes “Buffalo” (in Chinese) and “Brucellosis” (in Chinese) in the CNKI database. In VIP, we used the themes or keywords “Buffalo” (in Chinese) and “Brucella” (in Chinese) or “Buffalo” (in Chinese) and “Brucella” (in Chinese); article types were limited to papers, either in journals or elsewhere, and collections.
In the three Chinese databases, i.e., CNKI, Wanfang, and VIP, the same search strategy for document retrieval was also used.
Studies were eligible if they satisfied all of the following inclusion criteria:
• The subjects of the research must be buffalo.
• The study's aim must be to investigate the seroprevalence of Buffalo brucellosis.
• Data must include the number of buffalo examined and the number of brucellosis-positive buffalo.
• The study must be published in Chinese or English.
Articles meeting any of the following exclusion criteria were excluded:
• host other than buffalo;
• review article;
• reviews or no data in studies;
• DOI does not match the article; and
• test method other than serology.
Data Extraction and Quality Assessment
We collected data using standardized forms (31). The data records were as follows: country, region, environment, sampling year, method, breeding mode, gender, age group, national per capita income level, sample collection, health status, and whether the herd had contact with wild animals. We classified buffalo aged <1 year as suckling, buffalo aged 1 and 3 years as juvenile, and buffalo aged ≥3 years as adult. Articles were sorted according to region, sample classification (serum or milk), detection method, income level, gender, living environment, and age group of buffalo.
We assessed the quality of the included studies on a scale of 0–5 points, where 1 point was awarded for each of the following items: sampling time, clear detection method, detailed sampling method, random sampling, and analysis of four or more subgroups. According to this standard, we categorized studies by their total score as high (4–5 points), medium (2, 3), or low (0–1) in quality.
Statistical Analysis
The “meta” package in R software (version 3.5.2) was used to obtain pooled prevalence rates (32). The degree of heterogeneity between the included studies, assessed using the χ2-based Q-test and I2, was used as the basis for selecting the effect model. Heterogeneity was considered insignificant when P > 0.1 and I2 < 50%. A fixed-effects model was used when I2 < 50%, P < 0.05, and a random-effects model was chosen when I2 > 50%, P < 0.05. Forest plots were used to visualize the statistical results of the meta-analysis (33, 34).
We used funnel plots, Egger's test, sensitivity analysis, and trim-and-fill analysis to evaluate the bias and stability of the selected articles (35). Publication bias was considered significant when Egger's test yielded P < 0.05 (36–38). A symmetric funnel plot exhibits no evidence of publication bias or heterogeneity (39). To estimate the impact of a single article on the results, we carried out a sensitivity analysis, repeating the meta-analysis with each article in turn deleted.
We used subgroup analysis to examine heterogeneity. The factors investigated included region (Europe vs. other continents), year of publication (publication year ≥ 2010 vs. < 2010), detection methods (serological vs. molecular biology methods), source of collected samples (serum vs. milk vs. other methods), farming mode (captive vs. stocking), living environment (wetlands vs. dry land), gender (female vs. male), age group (suckling vs. juvenile vs. adult), national income (high-income countries vs. middle- and low-income countries), whether the herd had contact with wild animals (with contact vs. without contact), and quality score (4–5 vs. 2–3 vs. 0–1).
Results
Search Results and Quality of the Eligible Studies
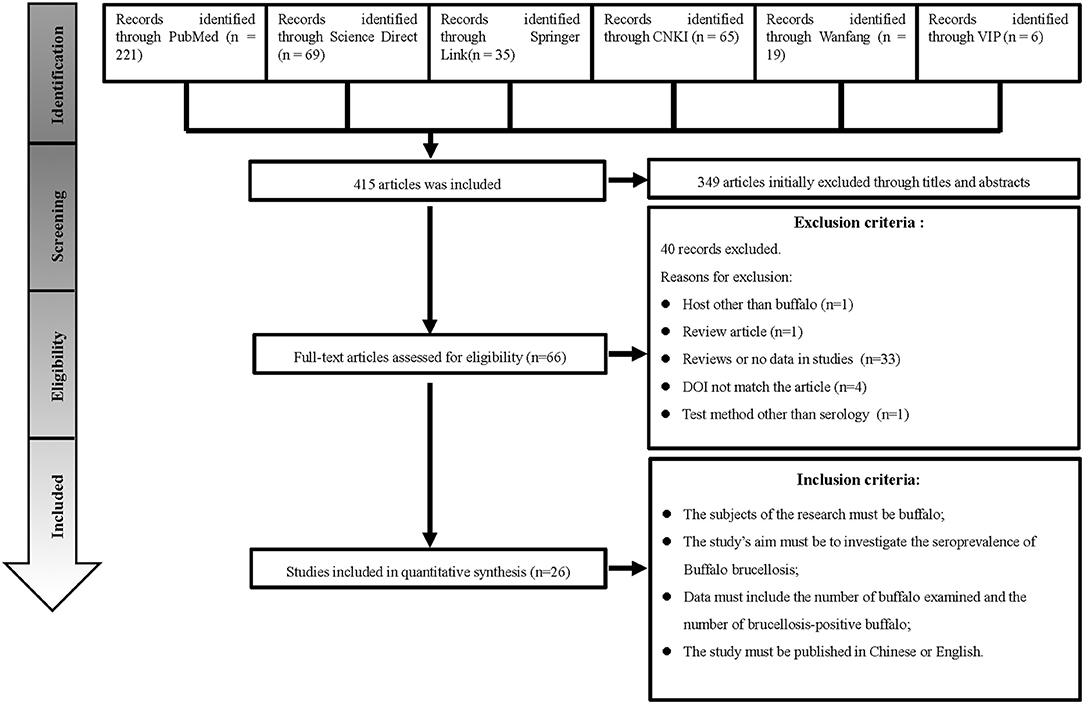
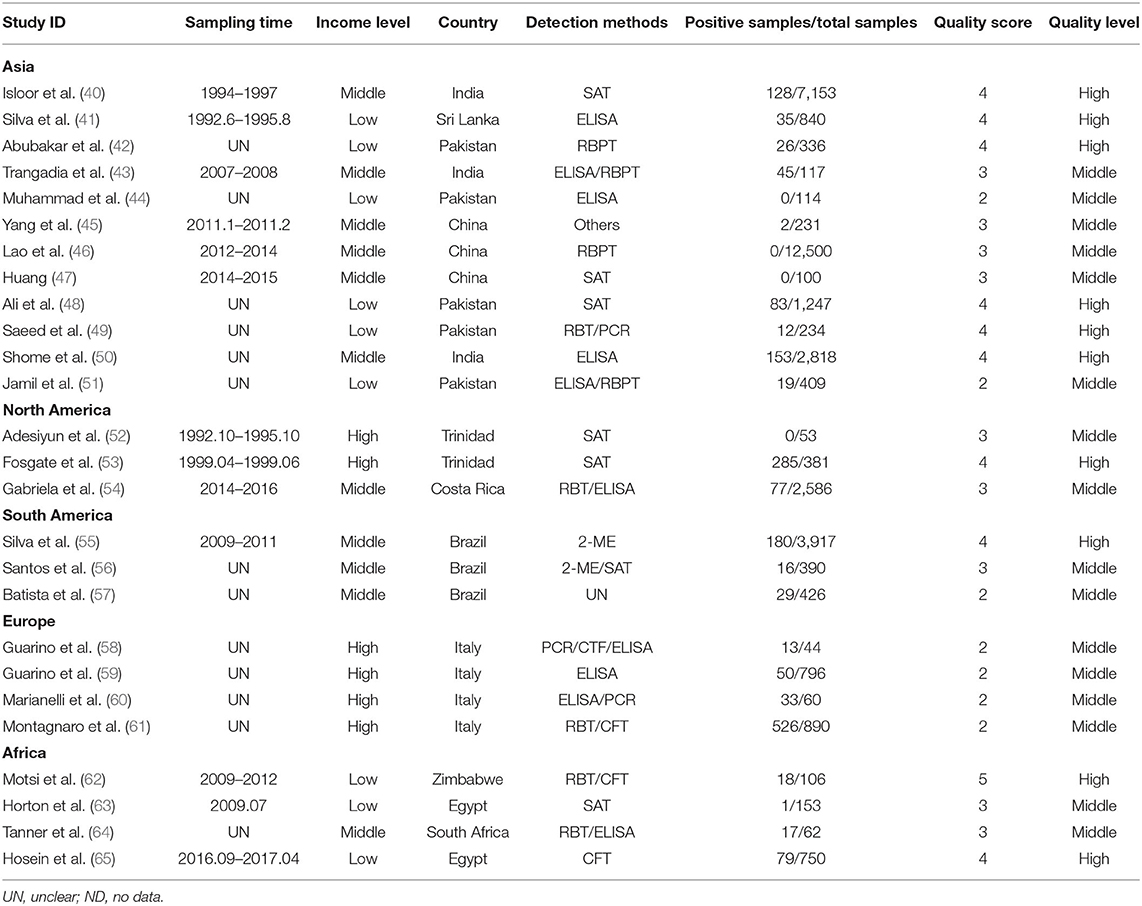
We searched 415 studies from six databases. Based on the inclusion and exclusion rules, we analyzed 26 articles published between 1985 and 2020 (Figure 1). Ten papers were rated as high quality (4–5 points), and 16 papers were rated as medium quality (0–3 points); none of the included papers were rated as low quality (0–1).
Results of Publication Bias
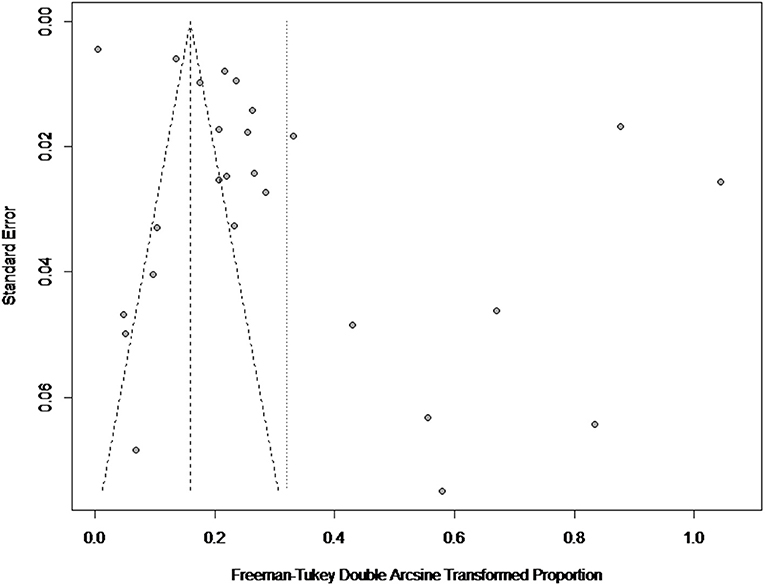
There was a high degree of heterogeneity between studies (I2 = 99.0%, P < 0.001) (Figure 2). The funnel plot shows evidence of publication bias in the selected articles (Figure 3), a result confirmed by Egger's test (P = 0.004) (Supplementary Figure 1). However, the test found that trimming did not add any research. The trim-and-fill analysis suggests that our own study is not publication bias (Supplementary Figure 2). Sensitivity analysis shows that after excluding a study, the results obtained are consistent with excluding the results obtained from other studies, which also suggests that our meta-analysis is trustworthy (Table 1 and Figure 4).
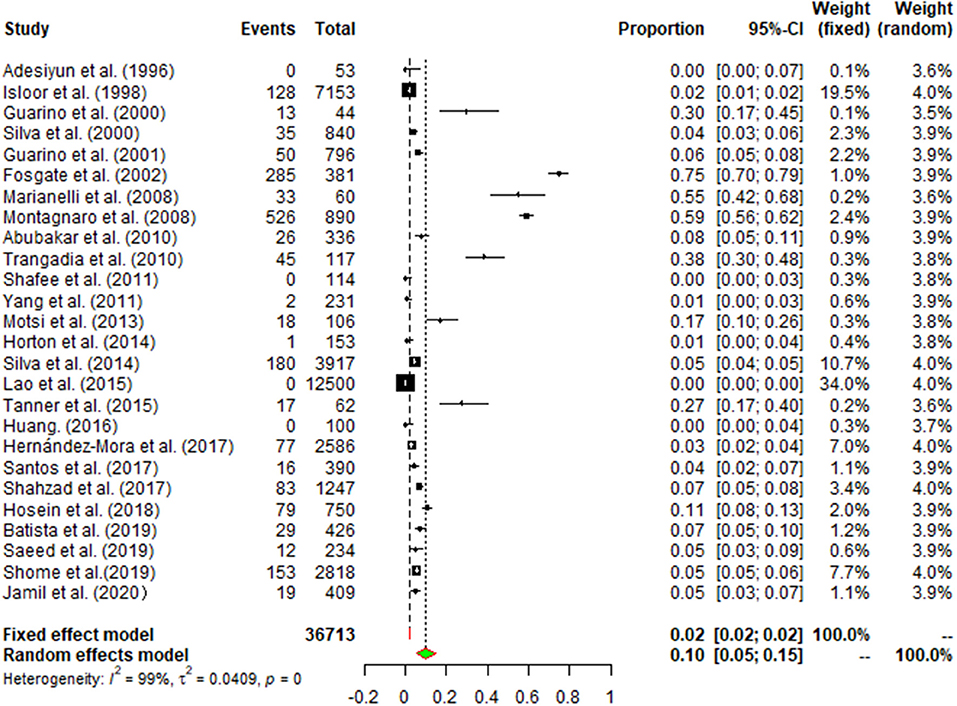
Figure 2. Forest plot of buffalo brucellosis among studies conducted anywhere in the world. The horizontal line represents the 95% CI; the diamond represents the pooled effect.
Table 1. Normal distribution test of the original rates and the different transformations of the original rates.
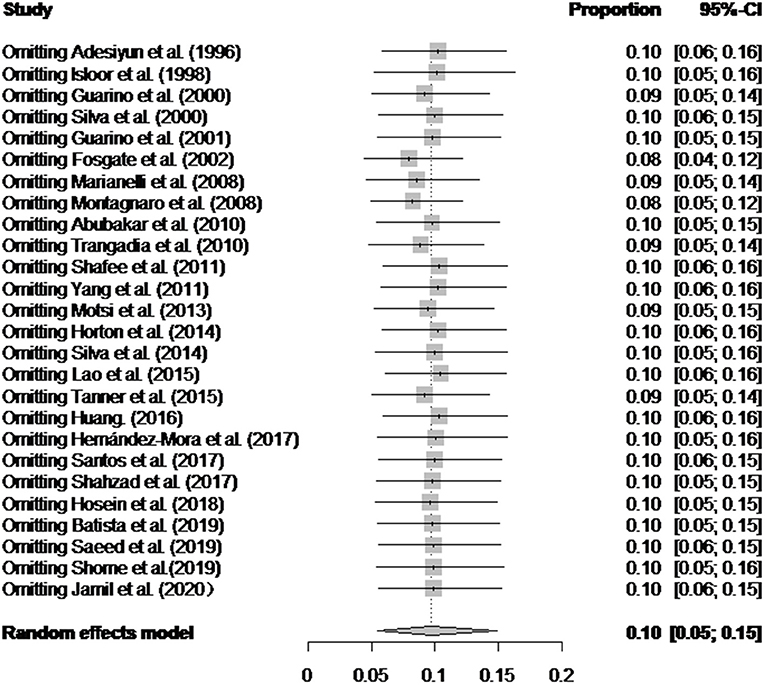
Figure 4. Sensitivity analysis. After one study at a time was removed, the remaining studies were recombined using a random-effects model to verify the impact of a single study on the overall results.
Meta-Analysis of Buffalo Brucella Seroprevalence in Buffalo Worldwide
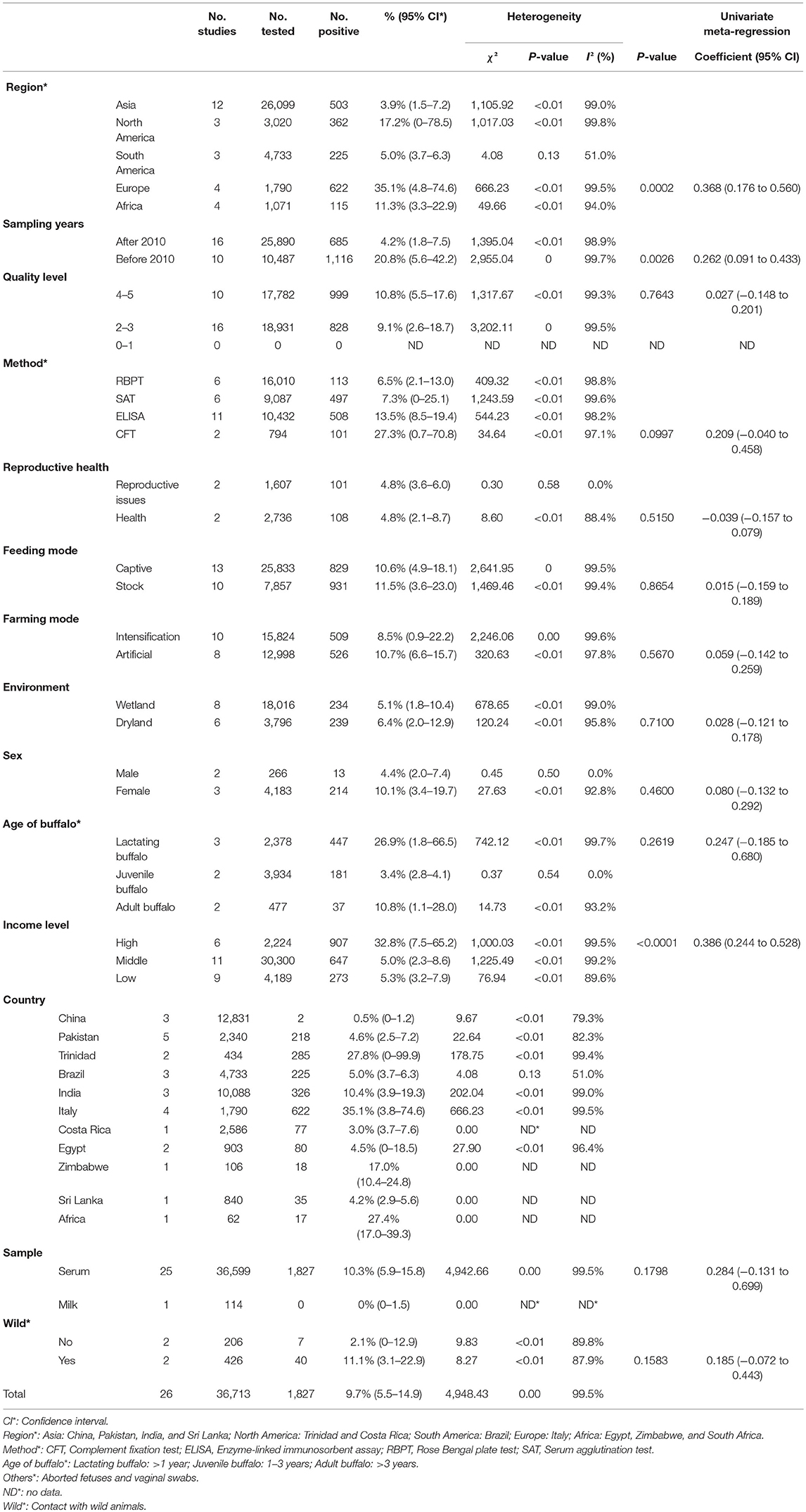
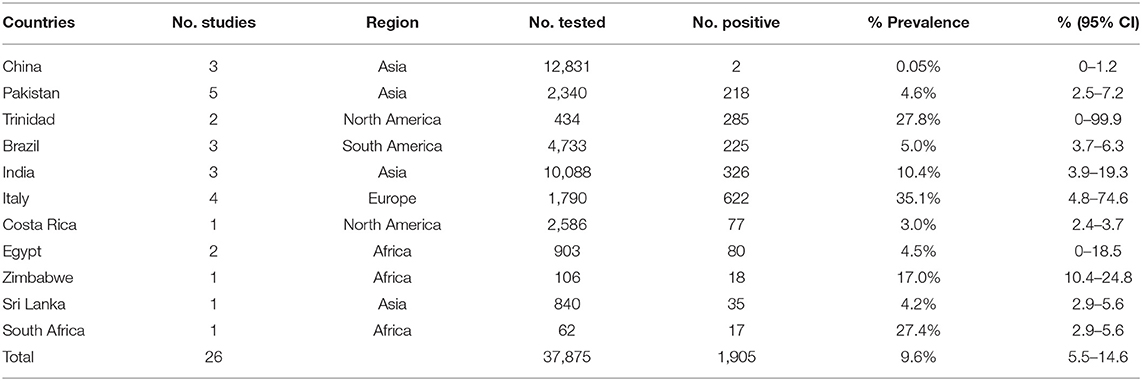
The pooled seroprevalence of buffalo brucellosis was (9.7%) (95% CI: 5.5–14.9), based on data from 36,713 buffalo in selected articles (Table 2). In general, the data on seroprevalence mostly show a skewed distribution. The total seroprevalence of brucellosis in buffalo varies across different geographic regions (Tables 2, 3). Among regional groupings, Europe had the highest estimate of the midpoint, namely, 35.1% (95% CI: 4.8–74.6), and there was a statistically significant difference (P < 0.05) (Tables 2, 3). In the subgroup analysis by sampling year, the point estimates before 2010 (20.8%) (95% CI: 5.6–42.2) were higher than those after 2010 (4.2%) (95% CI: 1.8–7.5) (Table 2). The subgroup analysis by feeding mode found that the point estimate in stocked buffalo (11.5%) (95% CI: 3.6–23.0), was higher than that in captive buffalo (10.6%) (95% CI: 4.9–18.1); however, the difference was not statistically significant (P > 0.05). The subgroup analysis by farming mode found that the seroprevalence in artificially bred buffalo (10.7%) (95% CI: 6.6–15.7) was higher than that in intensively farmed buffalo (8.5%) (95% CI: 0.9–22.2). The subgroup analysis by farming environment found that the seroprevalence in buffalo living on dry land (6.4%) (95% CI: 2.0–12.9) was greater than that in buffalo living in wetlands (5.1%) (95% CI: 1.8–10.4) (P < 0.05). The point estimate in female buffalo (10.1%) (95% CI: 3.4–19.7) was higher than that in male buffalo (4.4%) (95% CI: 2.0–7.4); however, the difference was not statistically significant (P > 0.05) (Table 2). The point estimate of seroprevalence in high-income countries (32.8%) (95% CI: 7.5–65.2) was higher than that in middle-and low-income countries (Table 4). The age group with the highest estimated value was the lactating buffalo (26.9%) (95% CI: 1.8–66.5), but the difference between age groups was not significant (P > 0.05). The point estimate of Brucella in serum (10.3%) (95% CI: 5.9–15.8) was higher than that in milk (0%) (95% CI: 0–1.5). The subgroup analysis by study quality found the highest seroprevalence (10.8%) (95% CI: 5.5–17.6) for studies with a score of 4–5. The subgroup analysis by detection method found that the seroprevalence (27.3%) (95% CI: 0.7–70.8) detected by the complement fixation test (CFT) method was much higher than the rates detected by other methods.
Results of Sensitivity Analysis
Italy's point estimate (35.1%) (95% CI: 3.8–74.6) was the highest for any country; however, according to Table 2, the sampling time was unclear for all four of the studies from Italy, all of which were published before 2010. Guarino found a seropositive rate of 10% in the detection of Brucella in Italy (59); Montagnaro tested for Brucella in Campania in southern Italy with a seropositive rate of 59.1% (60). Therefore, it is unclear whether the very high value reported for the country is still representative.
Results of univariate multiple regression are shown for variables including region, country, sampling year (1985–2020), presence or absence of problems with reproduction, reproduction method, living environment, income, and contact with wild animals. These groups may be the main source of heterogeneity in the meta-analysis (Table 2).
Discussion
Brucellosis is a major epidemic disease in cattle-raising areas around the world, which can cause buffalo abortion and cause economic losses (66). In addition, due to the close relationship between buffalo and humans, brucellosis can be transmitted to humans (67). This study is the world's first meta-analysis of the seroprevalence of buffalo brucellosis. In this study, we searched six databases and found a total of 26 related articles, which contained qualifying data.
Before 2010, the global infection rate was much higher than after 2010. We speculate that the global reduction in infection rate after 2010 is attributable to on-site control measures for Brucella that the World Organization for Animal Health (OIE) proposed in the chapter on brucellosis in its 2010–2011 terrestrial animal health regulations. Greater attention to brucellosis has led to improved buffalo breeding technology in countries around the world. The control of diseases in developing countries has gradually matured, and certain diseases have been well-controlled. However, countries whose preventive measures are not yet adequate need to be vigilant and, when formulating and applying animal food safety standards, should prioritize pathogens that affect human health. A research focus on developing countries and countries with transitional economies would support the aim of reducing global poverty (58).
Our research shows that the seroprevalence of buffalo brucellosis is different for different regions and different periods. We believe that many factors contribute to different epidemics in different regions. First of all, there are significant regional differences in breeding scale, breeding technology, and on-site sanitation conditions. In some areas, especially underdeveloped areas, the proportion of free-range buffalo is relatively large (68). Secondly, buffalo often inhabit muddy or flooded areas, where Brucella can survive longer (69); therefore, the point estimate is higher in areas with tropical climates. Finally, we should note that our systematic search found few studies on buffalo brucellosis seroprevalence relative to the number of countries where buffalo are raised, and in some countries, only one or two studies have been analyzed. Therefore, our conclusions are necessarily limited and should be applied with caution in consideration of local conditions that may affect seroprevalence. Regional diversity in the infection of buffalo by Brucella needs further study.
The seroprevalence of buffalo brucellosis may be related to the age of the buffalo. Studies have pointed out that Brucella exposure may occur in the first year after weaning. For animals older than 1 year, the average probability of seropositivity decreases with age (70, 71). We attribute this trend to incomplete development of immune function during the suckling period, with the result that resistance to diseases becomes more sufficient only as the animal continues to mature. From the results of gender subgroups, the seroprevalence of females is higher than that of males, but the difference is not significant. As the number of male diseased buffalo in our included articles was only 266, this is not sufficient to prove that female buffalo are more prevalent than males. Female animals can transmit infection to other livestock through skin and mucous membranes (72). At the same time, most farms have far more female animals than male animals, the young animals can be infected by contact with the secretions and excrement of the mother animals; male animals may be infected through sexual contact with the mother animals. Our limited data on fetal miscarriage are treated separately, and since our search found only one article on fetal miscarriage, a pooled analysis was not possible. We recommend that future epidemiological research into brucellosis examine the positive rate of aborted fetuses.
Our results found that from the perspective of national income and farming methods, in countries with a high per-capita income and middle-income countries, such as Italy and China, respectively, buffalo breeding adopts intensive breeding strategies; the infection rate of buffalo brucellosis is high in countries with low per-capita income such as Pakistan and India [see, e.g., (57)]. Where artificial breeding is the main strategy for buffalo, the infection rate of buffalo brucellosis is higher. The potential explanation for this model may be that low- and middle-income levels are represented in this analysis by Pakistan, South Africa, and China, which are characterized by developing agriculture, so the trend may be generalized to other countries with similar per-capita income. The results of a previous study showed that the burden of buffalo brucellosis in low-income countries was lighter than that in higher-income countries, which also supports our results (73). As human settlements have expanded, they have encroached upon the habitat of wild animals, thereby increasing the possibility of contact between people and wild animals (74, 75). This may contribute to increased chances of brucellosis infection, first in livestock and consequently in humans consuming infected food. Therefore, it is necessary to conduct regular inspections and to enhance breeders' awareness because their perceptions of brucellosis may otherwise interfere with taking effective preventive measures (68). Once an inspection finds a positive result, the sick cattle must be dealt with promptly by processing methods that include culling and burying (76). Treatment should focus on incineration and landfill as much as possible (30). At the same time, most buffalo live in humid environments, tropical climates or tropical rain forest climates are mostly wetlands, and humid environments are more susceptible to buffalo infections than dry environments (30). However, data show that the seropositive rate of brucellosis in buffalo living on dry land was higher than that in those living in wetlands. This may be due to the fact that most of the dry land in the extracted data is in low- and middle-income countries and to the herders' lack of awareness of brucellosis prevention.
Based on the classification of the samples collected from buffalo, Brucella is more likely to be detected in serum than in other sources, possibly because most tests use blood as a sample. Milk with mastitis and other breast diseases is not suitable for testing and is prone to false positives (77), which affects the detection result. The Rose Bengal plate test (RBPT) mainly detects IgG antibodies (77, 78). This simple method of qualitative analysis can complete preliminary screening quickly (79). However, due to the interference of non-specific antibodies, non-specific agglutination sometimes occurs. The serum agglutination test (SAT) is a serological test that detects the agglutination of an antigen when it binds to the corresponding antibody, so that results can be directly observed in the test tube by the naked eye. However, not only does the test take 18–24 h to complete, but it also requires a large amount of Brucella agglutination antigen and diseased livestock serum, and a standard turbidimetric tube needs to be made during the test, and the results must be compared one by one, which is time-consuming and laborious (80). The enzyme-linked immunosorbent assay (ELISA) is divided into I-ELISA and C-ELISA (81). ELISA refers to a qualitative and quantitative detection method in which soluble antigens or antibodies are bound to solid-phase carriers such as polystyrene, and the specific binding of antigen and antibody is used to carry out the immune response (82). The CFT method works mainly through the combination of the Brucella complement binding test antigen and the antibody in the serum to be tested, thereby forming a complex (83), and the complex is combined with the complement, no longer causing hemolysis of red blood cells in the indicator system, and the result is positive; otherwise, it is negative. From the point of view of detection methods, the CFT method for detection of brucellosis detected the highest positive rate. As a commonly used detection technique in serology, CFT has the characteristics of strong specificity, high sensitivity, and easy promotion compared with other serological detection methods such as the SAT, so the OIE has approved it for serological diagnosis of brucellosis.
Our research has some limitations. First, we tried to identify buffalo brucellosis infection by using several different MeSH terms in all studies in the selected database. Second, we found few studies on buffalo brucellosis infection in countries around the world, sampling locations are concentrated, and the sample size included in the study in some countries is very small. Therefore, it is difficult to objectively determine the true incidence of buffalo brucellosis in any specific locality. Third, limitation of the language of publication to Chinese or English may have led to the omission of relevant research in other languages, so the included studies may not be fully representative of certain regions. Fourth, some of the included studies did not indicate whether sampling was random, which may lead to heterogeneity. Finally, because some included papers have not reported the sample area and number of sample years, we did not conduct a joint test of the sampling area and year for each risk factor, which may lead to instability.
Despite notable improvements since 2010, buffalo brucellosis has remained a high-risk factor for human infection even in recent years. To improve public health, it is necessary to take appropriate management and monitoring measures for brucellosis infection, so it is strongly recommended to enhance herders' awareness of this disease and reduce errors in the feeding process. Better monitoring and control of the disease in animals can keep meat and dairy products from causing brucellosis in humans.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
J-ML and RD: idea contributions. RD, XL, and YZ: funding. Z-YC, Y-HS, YY, and B-YM: data extraction. BZ: database establishment. QW and XL: data analysis. J-FS: writing—original draft. Q-LG and YZ: writing—review and editing. All authors contributed to manuscript editing and approved the final manuscript.
Funding
This study was supported by the National Key R&D Program of China (2018YFD0500900), the Science and Technology Development Program of Jilin Province (20190304004YY), and the Scientific Research Planning Project of the Jilin Provincial Department of Education (JJKH20200364KJ).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the scientists and personnel of the College of Animal Science and Technology, Jilin Agricultural University, and the College of Chinese Medicine Materials, Jilin Agricultural University, for their collaboration.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2021.649252/full#supplementary-material
Supplementary Figure 1. Egger's test for publication bias.
Supplementary Figure 2. Funnel plot with trim and fill analysis for the publication bias test.
Abbreviations
OIE, World Organization for Animal Health; CFT, Complement fixation test; ELISA, Enzyme-Linked Immunosorbent Assay; RBPT, Rose Bengal plate test; SAT, Serum agglutination test.
References
1. Shravasti RK, Jadhav VB, Shah NM, Kulkarni RB, Shah AD. A case of PUO-Human Brucellosis. Int J Biomed Res. (2014) 5:533. doi: 10.7439/ijbr.v5i8.727
2. Abedi AS, Hashempour-Baltork F, Alizadeh AM, Beikzadeh S, Hosseini H, Bashiry M, et al. The prevalence of Brucella spp. in dairy products in the Middle East region: A systematic review and meta-analysis. Acta Trop. (2020) 202:105241. doi: 10.1016/j.actatropica.2019.105241
3. Ma S, Li JJ, Zhao M, Wang Y, Liu SQ, Li C. Research progress of brucellosis. China Anim Quarantine. (2020) 37:73–9. Available online at: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFDLAST2020&filename=ZGDW202007016&v=Bh8a8ZT44%25mmd2BWtXQCmc1KclAR0RXRvANylsYS5JejfeRhDWx73%25mmd2BO4NgckDJR0Vcxs6
4. Ran XH, Cheng JJ, Wang MM, Chen XH, Wang H, Ge Y, et al. Brucellosis seroprevalence in dairy cattle in China during 2008-2018: a systematic review and meta-analysis. Acta Trop. (2019) 189:117–23. doi: 10.1016/j.actatropica.2018.10.002
5. Zeng QK, Huang F, Lu YQ, Xie BK, Li SK, Sun N. Investigation report on the Italian milk buffalo industry. Guangxi Anim Husbandry Vet. (2010) 26:33–5.
6. Wernery U. Camelid brucellosis. A review. Rev Sci Tech. (2014) 33:839–57. doi: 10.20506/rst.33.3.2322
7. Narnaware SD, Dahiya SS, Kumar S, Tuteja FC, Nath K, Patil NV. Pathological and diagnostic investigations of abortions and neonatal mortality associated with natural infection of Brucella abortus in dromedary camels. Comp Clin Pathol. (2017) 26:79–85. doi: 10.1007/s00580-016-2348-4
8. Yan ZF. Prevention and control of brucellosis in cattle and sheep. Anim Breed Feed. (2021) 20:80–1. doi: 10.13300/j.cnki.cn42-1648/s.2021.04.040
10. Huang JX, Huang F, Tan GS, Shang JH, Yang CJ, Li L, et al. Overview of the development of China's dairy buffalo industry-national profile and technological innovation. Chin Dairy Cow. (2019) 37:1–8. Available online at: https://d.wanfangdata.com.cn/periodical/ChlQZXJpb2RpY2FsQ0hJTmV3UzIwMjEwNDE1Eg16Z25uMjAxOTExMDAxGghoeG5ocjhpYg%3D%3D
11. Konrad JL, Campero LM, Caspe GS, Brihuega B, Draghi G, Moore DP, et al. Detection of antibodies against Brucella abortus, Leptospira spp., and Apicomplexa protozoa in water buffaloes in the Northeast of Argentina. Trop Anim Health Pro. (2013) 45:1751–6. doi: 10.1007/s11250-013-0427-y
12. Reddy PRK, Kumar DS, Rao ER, Seshiah CV, Sateesh K, Rao KA, et al. Environmental sustainability assessment of tropical dairy buffalo farming vis-a-vis sustainable feed replacement strategy. Sci Rep. (2019) 9:16745. doi: 10.1038/s41598-019-53378-w
13. Dadar M, Shahali Y, Fakhri Y, Godfroid J. The global epidemiology of Brucella infections in terrestrial wildlife: a meta-analysis. Transbound Emerg Dis. (2021) 68:715–29. doi: 10.1111/tbed.13735
14. Yun WX. What happens if I eat milk infected with Brucella. Life Disaster. (2015) 23:28–9. Available online at: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFDLAST2015&filename=MFFF201502012&v=lcWCvZx9%25mmd2FkMl6s6Wg%25mmd2FaNiYjmQUQ9TqbvX15eGtwRook1dvYFLKDqJaGZ3U%25mmd2BSMHQ5
15. Garcell HG, Garcia EG, Pueyo PV, Martín IR, Arias AV, Serrano RNA. Outbreaks of brucellosis related to the consumption of unpasteurized camel milk. J Infect Public Health. (2016) 9:523–7. doi: 10.1016/j.jiph.2015.12.006
16. Musallam II, Abo-Shehada MN, Hegazy YM, Holt HR, Guitian FJ. Systematic review of brucellosis in the Middle East: disease frequency in ruminants and humans and risk factors for human infection. Epidemiol Infect. (2016) 144:671–85. doi: 10.1017/S0950268815002575
17. Dadar M, Fakhri Y, Shahali Y, Khaneghah AM. Contamination of milk and dairy products by Brucella species: a global systematic review and meta-analysis. Food Res Int. (2020) 128:108775. doi: 10.1016/j.foodres.2019.108775
18. Chen JD, Ke CW, Deng XL, Jiang S, Liang WJ, Ke BX, et al. Brucellosis in Guangdong Province, People's Republic of China, 2005–2010. Emerg Infect Dis. (2013) 19:817–8. doi: 10.3201/eid1905.120146
19. Chen Y. Risk analysis of human brucellosis. Breeding Feed. (2020) 9:128–9. doi: 10.13300/j.cnki.cn42-1648/s.2020.06.059
20. Almér LO. A case of brucellosis complicated by endocarditis and disseminated intravascular coagulation. Acta Med Scand. (1985) 217:139–40. doi: 10.1111/j.0954-6820.1985.tb01647.x
21. Aznar MN, Samartino LE, Humblet MF, Saegerman C. Bovine brucellosis in Argentina and bordering countries: update. Transbound Emerg Dis. (2014) 61:121–33. doi: 10.1111/tbed.12018
22. Moreno E. Brucellosis in Central America. Vet Microbiol. (2002) 90:31–8. doi: 10.1016/S0378-1135(02)00242-0
23. Lucero NE, Ayala SM, Escobar GI, Jacob NR. Brucella isolated in humans and animals in Latin America from 1968 to 2006. Epidemiol Infect. (2008) 136:496–503. doi: 10.1017/S0950268807008795
24. Sofian M, Aghakhani A, Velayati AA, Banifazl M, Eslamifar A, Ramezani A. Risk factors for human brucellosis in Iran: a case–control study. Int J Infect Dis. (2008) 12:157–61. doi: 10.1016/j.ijid.2007.04.019
25. Zhang WY, Guo WD, Sun SH, Jiang JF, Sun HL, Li SL, et al. Human brucellosis, Inner Mongolia, China. Emerg Infect Dis. (2010) 16:2001–3. doi: 10.3201/eid1612.091081
26. Maurice NA, Wungak SY, Gana BA, Nanven MB, Ngbede EO, Ibrahim A, et al. Seroprevalence of bovine brucellosis in northern Plateau State, North Central Nigeria. Asian Pac J Trop Dis. (2013) 3:337–40. doi: 10.1016/S2222-1808(13)60081-X
27. Scacchia M, Provvido AD, Ippoliti C, Kefle U, Sebhatu TT, D'Angelo A, et al. Prevalence of brucellosis in dairy cattle from the main dairy farming regions of Eritrea. Onderstepoort J Vet Res. (2013) 80:448. doi: 10.4102/ojvr.v80i1.448
28. Lindahl E, Sattorov N, Boqvist S, Sattori I, Magnusson U. Seropositivity and risk factors for Brucella in dairy cows in urban and peri-urban small-scale farming in Tajikistan. Trop Anim Health Pro. (2014) 46:563–9. doi: 10.1007/s11250-013-0534-9
29. Dadar M, Shahali Y, Whatmore AM. Human brucellosis caused by raw dairy products: a review on the occurrence, major risk factors and prevention. Int J Food Microbiol. (2019) 292:39–47. doi: 10.1016/j.ijfoodmicro.2018.12.009
30. Knight-Jones TJD, Mylrea GE, Kahn S. Animal production food safety: priority pathogens for standard setting by the World Organisation for animal health. Rev Sci Tech. (2010) 29:523–35. doi: 10.20506/rst.29.3.1994
31. Ni HB, Gong QL, Zhao Q, Li XY, Zhang XX. Prevalence of Haemophilus parasuis “Glaesserella parasuis” in pigs in China: a systematic review and meta-analysis. Prev Vet Med. (2020) 182:105083. doi: 10.1016/j.prevetmed.2020.105083
32. Gong QL, Li J, Li D, Tian T, Leng X, Li JM, et al. Seroprevalence of Toxoplasma gondii in cattle in China from 2010 to 2019: a systematic review and meta-analysis. Acta Trop. (2020) 211:105439. doi: 10.1016/j.actatropica.2020.105439
33. Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta-analysis of prevalence. J Epidemiol Community Health. (2013) 67:974–8. doi: 10.1136/jech-2013-203104
34. Li X, Ni HB, Ren WX, Jiang J, Gong QL, Zhan XX. Seroprevalence of Toxoplasma gondii in horses: a global systematic review and meta-analysis. Acta Trop. (2020) 201:105222. doi: 10.1016/j.actatropica.2019.105222
35. Gong QL, Li D, Diao NC, Liu Y, Li BY, Tian T, et al. Mink aleutian disease seroprevalence in China during 1981-2017: a systematic review and meta-analysis. Microb Pathog. (2020) 139:103908. doi: 10.1016/j.micpath.2019.103908
36. Wang ZD, Wang SC, Liu HH, Ma HY, Li ZY, Wei F, et al. Prevalence and burden of Toxoplasma gondii infection in HIV-infected people: a systematic review and meta-analysis. Lancet Hiv. (2017) 4:177–88. doi: 10.1016/S2352-3018(17)30005-X
37. Wang ZH, Qin Z, Wei H, Wen XT, Cao SJ, Huang XB, et al. Prevalence and seroepidemiology of Haemophilus parasuis in Sichuan province, China. Peer J. (2017) 5:e3379. doi: 10.7717/peerj.3379
38. Assefa A, Bihon A. Bovine cysticercosis in Ethiopia: a systematic review and meta-analysis of prevalence from abattoir-based surveys. Prev Vet Med. (2019) 169:104707. doi: 10.1016/j.prevetmed.2019.104707
39. Sterne JAC, Sutton AJ, Ioannidis JPA, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. (2011) 343:d4002. doi: 10.1136/bmj.d4002
40. Isloor S, Renukaradhya GJ, Rajasekhar M. A serological survey of bovine brucellosis in India. Rev Sci Tech. (1998) 17:781–5. doi: 10.20506/rst.17.3.1131
41. Silva I, Dangolla A, Kulachelvy K. Seroepidemiology of Brucella abortus infection in bovids in Sri Lanka. Prev Vet Med. (2000) 46:51–9. doi: 10.1016/S0167-5877(00)00136-7
42. Abubakar M, Arshed MJ, Hussain M, Ehtisham-ul-Haq, Ali Q. Serological evidence of Brucella abortus prevalence in Punjab province, Pakistan–a cross-sectional study. Transbound Emerg Dis. (2010) 57:443–7. doi: 10.1111/j.1865-1682.2010.01171.x
43. Trangadia B, Rana SK, Mukherjee F, Srinivasan VA. Prevalence of brucellosis and infectious bovine rhinotracheitis in organized dairy farms in India. Trop Anim Health Pro. (2010) 42:203–7. doi: 10.1007/s11250-009-9407-7
44. Muhammad S, Masood R, Ahmad SA, Din AM, Abdul R. Prevalence of bovine brucellosis in organized dairy farms, using milk ELISA. in Quetta City, Balochistan, Pakistan. Vet Med Int. (2011) 2011:358950. doi: 10.4061/2011/358950
45. Yang SB, Xin AG, Zhao WH, Wang JP, Li FX, Hu Q, et al. Serological monitoring and prevention and control measures of foot-and-mouth disease and brucellosis in Yunnan milk and buffalo cattle. China Dairy Conf. (2011) 29:42–4. Available online at: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFD2011&filename=ZGNN201124013&v=4DOJc2Fi5xH6gV2YdLfEJcZUhIGfT%25mmd2Fhd%25mmd2FsVXL5bb8wBc4rBhchy4cRibLZsXbDN2
46. Lao YR, Huang M, Cao PY, Li YC. Epidemiological investigation of “two diseases” in some dairy buffalo farms in Qinzhou, Guangxi. Livest Ind. (2015) 26:65–6. Available online at: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFDLAST2015&filename=XQYI201508067&v=1147aFpOFSp4C1%25mmd2BivxmchMsai7mbp%25mmd2FWNfQUHVnLBMHbRRF%25mmd2F8kfqcL3URO%25mmd2FZe3pEw
47. Huang ZL. Sero-epidemiological survey of major diseases in cattle farms in selected areas of Guangxi (Master's thesis), University of Guangxi, Guangxi (2016).
48. Ali S, Akhter S, Neubauer H, Melzer F, Khan I, Abatih EN, et al. Seroprevalence and risk factors associated with bovine brucellosis in the Potohar Plateau, Pakistan. BMC Res Notes. (2017) 10:73. doi: 10.1186/s13104-017-2394-2
49. Saeed U, Ali S, Khan TM, El-Adawy H, Melzer F, Khan AU, et al. Seroepidemiology and the molecular detection of animal brucellosis in Punjab, Pakistan. Microorganisms. (2019) 7:449. doi: 10.3390/microorganisms7100449
50. Shome R, Triveni K, Swati S, Ranjitha S, Krithiga N, Shome BR, et al. Spatial seroprevalence of bovine brucellosis in India—A large random sampling survey. Comp Immunol Microbiol Infect Dis. (2019) 65:124–7. doi: 10.1016/j.cimid.2019.05.002
51. Jamil T, Melzer F, Saqib M, Shahzad A, Kasi KK, Hammad MH, et al. Serological and molecular detection of bovine brucellosis at institutional livestock farms in Punjab, Pakistan. Int J Env Res Pub He. (2020) 17:1412. doi: 10.3390/ijerph17041412
52. Adesiyun AA, Cazabon EP. Seroprevalences of brucellosis, Q-fever and toxoplasmosis in slaughter livestock in Trinidad. Rev Elev Med Vet Pays Trop. (1996) 49:28–30.
53. Fosgate GT, Adesiyun AA, Hird DW, Johnson WO, Hietala SK, Schurig GG, et al. Comparison of serologic tests for detection of Brucella infections in cattle and water buffalo (Bubalus bubalis). Am J Vet Res. (2002) 63:1598–605. doi: 10.2460/ajvr.2002.63.1598
54. Gabriela HM, Roberto BM, Osvaldo BG, Andrea ES, Danilo MC, Rocío GB, et al. Brucellosis in mammals of Costa Rica: an epidemiological survey. PLoS ONE. (2017) 12:e0182644. doi: 10.1371/journal.pone.0182644
55. Silva JB, Rangel CP, Fonseca AH, Morais E, Vinhote WMS, Lima DHS, et al. Serological survey and risk factors for brucellosis in water buffaloes in the state of Para, Brazil. Trop Anim Health Pro. (2014) 46:385–9. doi: 10.1007/s11250-013-0501-5
56. Santos LSD, Sá JC, Ribeiro DLDS, Chaves NP, Mol JPS, Santos RL, et al. Detection of Brucella sp. infection through serological, microbiological, and molecular methods applied to buffaloes in Maranhão State, Brazil. Trop Anim Health Pro. (2017) 49:675–9. doi: 10.1007/s11250-017-1238-3
57. Batista HR, Passos CTS, Neto OGN, Sarturi C, Coelho APL, Moreira TR, et al. Factors associated with the prevalence of antibodies against Brucella abortus in water buffaloes from Santarém, Lower Amazon region, Brazil. Transbound Emerg Dis. (2020) 67:44–8. doi: 10.1111/tbed.13192
58. Guarino A, Serpe L, Fusco G, Scaramuzzo A, Gallo P. Detection of Brucella species in buffalo whole blood by gene-specific PCR. Vet Rec. (2000) 147:634–6. doi: 10.1136/vr.147.22.634
59. Guarino A, Fusco G, Di Matteo A, Urbani G, Condoleo R, Serpe L, et al. Indirect ELISA for the diagnosis of brucellosis in water buffaloes (Bubalus bubalis) in Italy. Vet Rec. (2001) 149:88–90. doi: 10.1136/vr.149.3.88
60. Marianelli C, Martucciello A, Tarantino M, Vecchio R, Iovane G, Galiero G. Evaluation of molecular methods for the detection of Brucella species in water buffalo milk. J Dairy Sci. (2008) 91:3779–86. doi: 10.3168/jds.2008-1233
61. Montagnaro S, Longo M, Mallardo K, Pisanelli G, Martino LD, Fusco G, et al. Evaluation of a fluorescence polarization assay for the detection of serum antibodies to Brucella abortus in water buffalo (Bubalus bubalis). Vet Immunol Immunop. (2008) 125:135–42. doi: 10.1016/j.vetimm.2008.05.017
62. Motsi TR, Tichiwangana SC, Matope G, Mukarati NL. A serological survey of brucellosis in wild ungulate species from five game parks in Zimbabwe. Onderstepoort J Vet Res. (2013) 80:586. doi: 10.4102/ojvr.v80i1.586
63. Horton KC, Wasfy M, Samaha H, Abdel-Rahman B, Safwat S, Fadeel MA, et al. Serosurvey for zoonotic viral and bacterial pathogens among slaughtered livestock in Egypt. Vector Borne Zoonot Dis. (2014) 14:633–9. doi: 10.1089/vbz.2013.1525
64. Tanner M, Inlameia O, Michel A, Maxlhuza G, Pondja A, Fafetine J, et al. Bovine tuberculosis and brucellosis in cattle and African buffalo in the Limpopo National Park, Mozambique. Transbound Emerg Dis. (2015) 62:632–8. doi: 10.1111/tbed.12210
65. Hosein HI, Zaki HM, Safwat NM, Menshawy AMS, Rouby S, Mahrous A, et al. Evaluation of the General Organization of Veterinary Services control program of animal brucellosis in Egypt: an outbreak investigation of brucellosis in buffalo. Vet World. (2018) 11:748–57. doi: 10.14202/vetworld.2018.748-757
66. Mei LY. Serological investigation of cattle and sheep brucellosis and promotion of colloidal gold detection technology in Altay, Xinjiang (Master's thesis), Xinjiang Agricultural University, Altay (2015).
67. McMichael AJ. The urban environment and health in a world of increasing globalization: issues for developing countries. B World Health Organ. (2000) 78:1117–26.
68. Aune K, Rhyan J, Russell R, Roffe T, Corso B. Environmental persistence of Brucella abortus in the Greater Yellowstone Area. J Wildlife Manage. (2012) 76:253–61. doi: 10.1002/jwmg.274
69. Nymo IH, Tryland M, Frie AK, Haug T, Foster G, Rødven R, et al. Age-dependent prevalence of anti-Brucella antibodies in hooded seals Cystophora cristata. Dis Aquat Organ. (2013) 106:187–96. doi: 10.3354/dao02659
70. Benavides JA, Caillaud D, Scurlock BM, Maichak EJ, Edwards WH, Cross PC. Estimating loss of Brucella abortus antibodies from age-specific serological data in Elk. Ecohealth. (2017) 14:234–43. doi: 10.1007/s10393-017-1235-z
71. Jin LZ, Wang F, Wan CY. Discussion on the prevention and control of brucellosis among livestock in Songyuan City. Friends Farmers Rich. (2017) 61:249–50. Available online at: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFDLAST2017&filename=NMZF201712243&v=VEhy0YowUs2%25mmd2BJdMom%25mmd2BFoUW%25mmd2FJDeP6ySJEUu2pQzn12Q%25mmd2FIx2ukEBiJATVWHc7S87Mp
72. Gazila T, Li XY, Lei CH, Ren RX, Mujapal A. Comparison of detection methods for brucellosis. Xinjiang Anim Husbandry. (2020) 35:44–6. Available online at: https://d.wanfangdata.com.cn/periodical/zgdwjy200606015
73. Rajala EL, Grahn C, Ljung I, Sattorov N, Boqvist S, Magnusson U. Prevalence and risk factors for Brucella seropositivity among sheep and goats in a peri-urban region of Tajikistan. Trop Anim Health Pro. (2016) 48:553–8. doi: 10.1007/s11250-015-0992-3
74. Lindahl-Rajala E, Hoffman T, Fretin D, Godfroid J, Sattorov N, Boqvist S, et al. Detection and characterization of Brucella spp. in bovine milk in small-scale urban and peri-urban farming in Tajikistan. PLoS Negl Trop Dis. (2017) 11:e0005367. doi: 10.1371/journal.pntd.0005367
75. Liu BC. Problems and countermeasures in prevention and treatment of cattle and sheep brucellosis. Livest Ind. (2020) 31:107. doi: 10.19567/j.cnki.1008-0414.2020.07.084
76. Zhang ZP, Zuo X. Epidemic characteristics, diagnosis, prevention and control measures of bovine brucellosis. Livest Environ. (2020) 36:73. Available online at: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFDLAST2020&filename=XMYH202001069&v=76JItinEKK5sy%25mmd2B9Cz7VCm0vnh6%25mmd2Fq3oPis0vIkYN%25mmd2FdVAFo2OPQJAfeDR%25mmd2BNomIhZLW
77. Wei RP, Wang MX, Song LT, Li XY, Guo W, Zhang WQ, et al. Comparative analysis of three methods for detecting brucellosis. Med Anim Control. (2019) 35:711–713. Available online at: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFDLAST2019&filename=YXDZ201907030&v=lxjZf8RnRMiipmojXRtQMbLUdjTDdQONIJCAQ%25mmd2BDLQvR4Qa1U7Qx6jzAyF4srh3XV
78. Gürtürk K, Boynukara B, Ilhan Z, Ekin IH, Gülhan T. Comparison of the dot-immunobinding assay with the serum agglutination test, the rose bengal plate test and the milk ring test for the detection of Brucella antibodies in bovine sera and milk. J Vet B. (1999) 46:279–85. doi: 10.1111/j.0931-1793.1999.0_273.x
79. Li JP, Guo HL, Xue XB, Xue J, Turhun N, Ma JJ, et al. Comparison of yak brucellosis detection by SAT RBPT and iELISA. Herbivorous Livest. (2014) 35:52–4. doi: 10.3969/j.issn.1003-6377.2014.03.013
80. Guo JY. Enzyme immunoassay in immunological testing. Chin J Lab Med. (2005) 28:98–101. doi: 10.3760/j:issn:1009-9158.2005.02.038
81. Al-Farwachi MI, Al-Iraqi OM, Al-Hankawe OK, Abdul-Majeed MO. Using of competitive ELISA in detection of Brucella antibodies in cattle sera. Iraqi J VET SCI. (2009) 23:97–103. doi: 10.33899/ijvs.2009.5739
82. Fan WX, Zhong Q, He QN, Wu DL, Cai YF, Zhao ZX. Comparative study on several methods of serological diagnosis of brucellosis. China Anim Quarantine. (2006) 23:31–3. doi: 10.3969/j.issn.1005-944X.2006.06.015
Keywords: brucellosis, Brucella, buffalo, meta-analysis, seroprevalence
Citation: Shi J-F, Gong Q-L, Zhao B, Ma B-Y, Chen Z-Y, Yang Y, Sun Y-H, Wang Q, Leng X, Zong Y, Li J-M and Du R (2021) Seroprevalence of Brucellosis in Buffalo Worldwide and Associated Risk Factors: A Systematic Review and Meta-Analysis. Front. Vet. Sci. 8:649252. doi: 10.3389/fvets.2021.649252
Received: 04 January 2021; Accepted: 03 May 2021;
Published: 04 June 2021.
Edited by:
Mujeeb Ur Rehman, Livestock and Dairy Development Department, PakistanReviewed by:
Maryam Dadar, Razi Vaccine and Serum Research Institute, IranMuhammad Saqib, University of Agriculture, Faisalabad, Pakistan
Amar Nasir, University of Veterinary and Animal Sciences, Pakistan
Copyright © 2021 Shi, Gong, Zhao, Ma, Chen, Yang, Sun, Wang, Leng, Zong, Li and Du. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian-Ming Li, 923202040@qq.com; Rui Du, durui197101@sina.com
†These authors have contributed equally to this work and share first authorship
 Jun-Feng Shi
Jun-Feng Shi Qing-Long Gong
Qing-Long Gong Bo Zhao
Bo Zhao Bao-Yi Ma
Bao-Yi Ma Zi-Yang Chen
Zi-Yang Chen Yang Yang
Yang Yang Yu-Han Sun
Yu-Han Sun Qi Wang
Qi Wang Xue Leng
Xue Leng Ying Zong
Ying Zong Jian-Ming Li
Jian-Ming Li Rui Du
Rui Du