A Review of Prebiotics Against Salmonella in Poultry: Current and Future Potential for Microbiome Research Applications
- 1Department of Food Science, Center for Food Safety, University of Arkansas, Fayetteville, AR, United States
- 2Division of Microbiology, National Center for Toxicological Research, U.S. Food and Drug Administration, Jefferson, AR, United States
- 3Diamond V, Cedar Rapids, IA, United States
Prebiotics are typically fermentable feed additives that can directly or indirectly support a healthy intestinal microbiota. Prebiotics have gained increasing attention in the poultry industry as wariness toward antibiotic use has grown in the face of foodborne pathogen drug resistance. Their potential as feed additives to improve growth, promote beneficial gastrointestinal microbiota, and reduce human-associated pathogens, has been well documented. However, their mechanisms remain relatively unknown. Prebiotics increasing short chain fatty acid (SCFA) production in the cecum have long since been considered a potential source for pathogen reduction. It has been previously concluded that prebiotics can improve the safety of poultry products by promoting the overall health and well-being of the bird as well as provide for an intestinal environment that is unfavorable for foodborne pathogens such as Salmonella. To better understand the precise benefit conferred by several prebiotics, “omic” technologies have been suggested and utilized. The data acquired from emerging technologies of microbiomics and metabolomics may be able to generate a more comprehensive detailed understanding of the microbiota and metabolome in the poultry gastrointestinal tract. This understanding, in turn, may allow for improved administration and optimization of prebiotics to prevent foodborne illness as well as elucidate unknown mechanisms of prebiotic actions. This review explores the use of prebiotics in poultry, their impact on gut Salmonella populations, and how utilization of next-generation technologies can elucidate the underlying mechanisms of prebiotics as feed additives.
Introduction
Salmonella can be spread through the fecal-oral route (1, 2), and is a concern for pathogenic contamination of poultry meats and eggs used for human consumption. Previously this concern had been mitigated through the use of antibiotics, which also promoted animal growth (3). However, with the rise of multidrug-resistant bacteria (4–6), the food industry has been pursuing alternative control measures for pathogenic Salmonella contamination. These approaches include but are not limited to chemical-based interventions, such as organic acids and essential oils, or biological-based treatments, such as bacteriophage, probiotic, and prebiotic therapies.
The recent use of prebiotics has been well documented. The term “prebiotic” was first coined by Gibson and Roberfroid in 1995 and defined as “a nondigestible food ingredient that beneficially affects the host by selectively stimulating the growth and/or activity of one or a limited number of bacteria in the colon, and thus improves host health” (7). Gibson and Roberfroid (8) demonstrated that the intake of prebiotics could regulate specific gastrointestinal tract (GIT) microorganisms to alter the microbiome. Over the years, further findings have led to several suggested modifications of the definition such as the addition of the term “selectively fermentable” (9) or the term “nonviable” (10, 11). More recently, an expert consensus from the International Scientific Association for Probiotics and Prebiotics (ISAPP) defined prebiotics as “a substrate that is selectively utilized by host microorganisms conferring a health benefit” (12).
Prebiotics have been used to influence the growth of reported beneficial bacteria in the GIT, such as Bacteroides and Bifidobacterium (13–16). Van Loo et al. (17) detailed several natural sources of prebiotics including garlic, onions, and asparagus. Typically including fiber and oligosaccharides (18), prebiotics in chickens increase amylase production in the GIT and therefore improve the overall growth rate of broilers (16). They reduce colonization of Salmonella during hen molting (19). Some prebiotics have also influenced protection against Salmonella by providing binding sites for bacteria to be flushed out of the digestive tract (18). Numerous studies have also seen the reduction of Salmonella populations by increasing short chain fatty acids (SCFAs) concentrations (20–22) which can be accomplished through prebiotic administration (23, 24).
Furthermore, several studies (25–29) investigated prebiotic effects on the GIT microbiota through 16S microbiome sequencing. By also noting changes in metabolite concentrations or metabolomics, this approach may be able to correlate changes in the microbiome to changes in the metabolite concentration such as SCFAs and other, possibly unknown, metabolites that can stymie Salmonella growth. The scope of this paper to provide an overview of the literature linking the use of prebiotics to the overall reduction in the number of foodborne Salmonella and the repression of virulence factors. The scope of this paper will not detail the other benefits of prebiotics in poultry such as impact on growth performance or antioxidant capacity, as they are covered extensively in Dhama et al. (30, 31), Yadav et al. (32), and other literature reviews. By investigating SCFA production, microbiomic, and metabolomic technologies, and currently utilized prebiotics, notably oligosaccharides, this review attempts to elucidate novel avenues of research into the reduction of virulent pathogens via prebiotics, which may improve the safety of the poultry industry and improve the overall public health by reducing the incidence and or severity of poultry-acquired salmonellosis.
The Poultry Gastrointestinal Tract
The gastrointestinal tract of chickens is complex due to the bird's large energy requirements (33). The chicken GIT includes the crop, gizzard, duodenum, ileum, and cecum, which are microbiologically abundant with over 900 documented bacterial species (34). Included in the upper segment of the GIT, is the crop, which is used for fermentation, hydrolysis of starch to sugar, food storage, and as an acid barrier with a pH of ~4.5. The gizzard grinds food particles in a highly acidic environment (pH 2.6) (35–38). While the mean retention time throughout the GIT is ~6 h, feed can remain in the crop and gizzard for as little as 8 and 50 min, respectively (39). The crop contains numerous anaerobic bacteria attached to the epithelium, including Lactobacillus, and they produce SCFA's and lactic acid (40, 41). The continuous layer of Lactobacillus, enterococci, coliforms, and yeast promote digestion of most carbohydrates, with the remainder digested in the ceca after passage through the lower GIT (37, 42).
Lower in the GIT is the duodenum, ileum, and cecum. Digestive enzymes and bile from the pancreas and gallbladder are added to the duodenum to break down food further, allowing for better absorption into the bloodstream through the villi (43). This process is continued through the ileum in the lower small intestine (43). The small intestine is dominated by anaerobic bacteria (44), and contains Lactobacillus and Bifidobacterium species in high concentrations as well as Enterococcus faecium and Pediococcus spp. (35, 45, 46). However, despite the presence of these bacteria in the small intestine, the concentrations of bacteria in the ceca are reported to be the highest in the chicken GIT, at ~1011 bacteria/g (35, 47, 48).
The ceca are located where the small and large intestines meet, and while they serve no identifiable purpose for digestion in mammals, it is important in chickens for fermentation and overall animal health (33, 35, 43). Due to culturing poultry cecal microbiota on arabinoxylan, it has been suggested the cecum may be involved in the breakdown of grains (42). The cecum plays additional roles in water adsorption and urea recycling, although the full nutritional significance remains unclear (49, 50). However, despite its importance, in an experiment involving ligation of the cecum, it was shown that while nitrogen availability was disturbed by a cecectomy, it was not necessary for survival (51, 52). The ceca, from a food safety standpoint, is also of major significance because it is one of the leading sites for Salmonella colonization along with the crop (53–55).
Salmonella can be found in varying concentrations in all regions of the poultry GIT of challenged chickens (56, 57). In Fanelli et al. (56), 1 day after the birds were challenged with Salmonella, the duodenum and the small intestines were examined, and 5–45% of the samples tested positive depending on the region viewed. However, cecal samples in this study were nearly 100% positive for Salmonella colonization (56). This trend continued throughout the 13-day trial. Additional studies found that, when challenged with a lower concentration, Salmonella was not recoverable from the duodenum and small intestine despite being isolated from the crop, because bacteria were often destroyed in passaging through the acid lumen of the proventriculus and gizzard (58). While other studies have focused on the crop and even the gizzard as colonization sites of Salmonella, the ceca remain the most commonly investigated section of GIT for Salmonella (39, 55, 58, 59). This is likely because of the relatively high bacterial counts of up to 1011 cells/g of digesta by the day three post-hatch (35, 60). Other reasons may include the ceca being the environment in the GIT most advantageous for Salmonella to colonize (56), and because the ceca can be ruptured during processing. However, it should be noted, Hargis et al (55) found that crops was 86 fold more likely to rupture than ceca during processing. Despite this focus on the ceca, with the potential for each organ's microbial composition to influence the next downstream, it is vital to understand the microbiota of each region of the avian GIT.
Stanley et al. (35) compiled data from several papers detailing the most prevalent microbial groups in each of the GIT regions. They found that while Lactobacillus was prominent, if not dominant in all systems, a myriad of differences was reported, including Clostridiaceae and Enterococcus in the crop and gizzard, and that a majority of cecal bacteria were not culturable or described. However, these profiles can vary greatly, as it has been suggested that host genotype, sex, and age play an important role in determining microbial composition (61). Furthermore, a majority of the collected papers reported information using community-fingerprinting techniques such as temporal temperature gradient electrophoresis (TTGE) and terminal-restriction fragment length polymorphism (T-RFLP), as well as culture-based methods. These techniques provide useful information, such as the application of T-RFLP in Torok et al. (25), which helped identify the presence of over 600 bacteria species and 100 distinct genera in the GIT of chickens. However, each of these techniques exhibits significant issues. Community fingerprinting techniques in general, are considered only semi-quantitative and are only capable of detecting taxa in abundance of >1% (61, 62). Additionally, culture-dependent methods are particularly limited. For example, in the cecum, only 10–60% of bacterial strains have been cultured (63, 64). Therefore, while these techniques have generated valuable information, to accurately detail the complex and minute changes to the microbiota under the effect of prebiotics, further investigation with more sensitive methodologies is needed. The changes, however, often depend on the type of prebiotic utilized.
Commonly Used Prebiotics
Prebiotic studies have focused largely on oligosaccharides such as mannanoligosaccharides (MOS), galactooligosaccharides (GOS), and fructooligosaccharides (FOS) including inulin (12, 24, 65–67). Oligosaccharides are polymer chains with 3 to 10 of simple sugars (Figure 1) (68). Oligosaccharides and fiber have been combined and amended with feed products to create commercially viable sources of prebiotics in the poultry industry with a range of results. Illustrations of the modes of action of prebiotics within poultry can be found in Yadav et al. (32) and Pourabedin and Zhao (67).
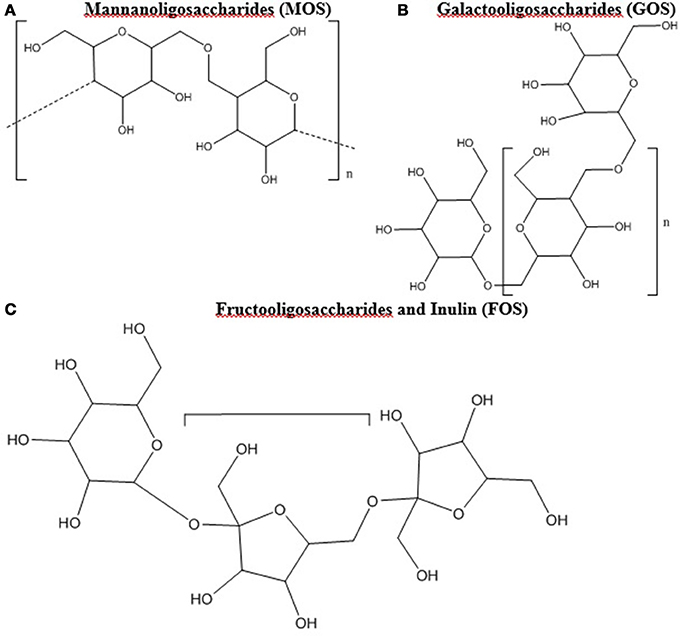
Figure 1. (A–C) Chemical Structure of oligosaccharides. All chemical structures were drawn in ChemBioDraw Ultra (PerkenElmer, Waltham, MA).
Several commercial prebiotics have been studied and utilized, such as Biolex® MB40 and Leiber® ExCel (Leiber, Hafenstraße 24, Germany), which are brewer's yeast cell walls composed of MOS (27–29, 69). These products were found to reduce Campylobacter concentrations and alter the microbiome, and there is an expectation of MOS-based products to reduce pathogens that utilize mannose-specific type 1 fimbriae such as Salmonella (28, 70). Furthermore, Lee et al. (71) did evaluate the effect of these products against Salmonella in commercially raised broilers, and while a lower prevalence was noted, only 10 samples were utilized, and a challenge study was not performed. As another example, the commercialized yeast-fermentate product XPC (Diamond V, Cedar Rapids, IA), has reduced Salmonella in chickens and increase butyrate in the GIT (27, 29, 72–74). Furthermore, during a Salmonella challenge experiment, the addition of XPC, which is comprised of 25% fiber, to chicken feed decreased the expression of virulence factor hilA, which is a regulator and promoter within a pathogenicity island (SPI-1) (72, 74). These findings imply that XPC may reduce Salmonella virulence and invasion.
While these effects are detectable, synergistic effects can also be created by combining probiotics and prebiotics to create synbiotics. Probiotic products such as All-Lac® have been used in conjunction with Bio-MOS® to alter the microbiome, whereas Fasttrack® (Fasttrack, Conklin, Kansas City MO) and PoultryStar® (PoultryStar, BIOMIN GmbH, Herzogenburg, Austria), contain FOS and have been shown to reduce Salmonella and improve feed conversion efficiency (65, 75–77). These products, along with numerous others, have been found to improve poultry GIT health, increase animal weight, and inhibit Salmonella and Campylobacter. As a consequence, because of the range of available prebiotic products, methodologies of application, and the yield of numerous and sometimes inconsistent results (24, 78, 79), it is vital to understand these prebiotics better. Moreover, it is essential to detail their currently elucidated or suggested mechanisms to refine further ways to improve poultry health and production practices. To capture the effects of the breadth of prebiotics available, several types of prebiotics and their impact on Salmonella in poultry will be discussed in this section.
Mannanoligosaccharides (Figure 1A) are found in the cell wall of numerous fungal species including brewer's yeast (Saccharomyces cerevisiae) and Saccharomyces boulardii, as well as certain plants (67, 66). Comprised of mannose oligomers linked via β-1,4 glycosidic bonds, MOS have been demonstrated to suppress enteric pathogens and enhance the poultry immune system (80). Broiler chickens do not possess enzymes to break down MOS, as such it is suggested that bacteria in the lower GIT, such as the ceca, are responsible for their digestion (67). One particular advantage of MOS as a prebiotic is its stability as a pellet during steaming, which allows it to be easily added to feed (66). Studies have shown that Salmonella possessing type 1 fimbriae can be sensitive to the presence of MOS, which can disrupt attachment and adhesion from the intestinal lining by encouraging attachment to the mannose in the lumen (69, 81). The disruption of attachment and adhesion was reported for 53% of tested Salmonella strains (81, 82). However not every S. Typhimurium strain possesses type 1 fimbriae, as out of 13 tested strains by Mirelmann et al. (83), only 4 expressed type 1 fimbriae.
Mannanoligosaccharides have also been reported to improve overall gut health through increasing villi length and providing an adjuvant-like effect by acting as a microbial antigen (66, 84, 85). One study in particular exhibited a reduction in Salmonella ceca population by day 10 in challenged chicks fed a diet consisting of (0.40%) MOS (86). Stanley et al. (87) also demonstrated a one to three log reduction of cecal Salmonella counts in 21-day old chicks when supplemented with 0.05% MOS and MgSO4. A meta-analysis, which was designed to increase power by combining results from multiple studies, was performed by Hooge (66), which indicated MOS addition to feed generated improved body weight, feed conversion ratios, and survivability. This meta-analysis listed seven selection criteria including date of publication and age of bird and consisted of 29 pen trials from separate studies that were analyzed using a paired T-test. However, some discrepancies were noted in MOS ability to improve beneficial microorganisms (80), and there was no set standardization among studies involving the administration of the amount of the prebiotic.
Fructooligosaccharides (Figure 1C) are naturally occurring, typically of plant origin, contain β-(2,1) linkages, and can be food ingredients, functional foods, and prebiotics (8, 88). Due to the β-(2,1)-linkages, enzymatic degradation is difficult in the upper GIT, leading to primary breakdown occurring in the ceca (8, 24, 89). Fructooligosaccharides support the growth of Lactobacillus and Bifidobacterium, resulting in an increase in SCFAs and lactate, an enhancement of the immune system, and the reduction of Salmonella colonization (23, 24, 90, 91). The elucidated mechanism of action for many of these benefits is that FOS is fermented by Lactobacillus and Bifidobacterium which increases SCFAs and lactate in the cecum resulting in lower Salmonella colonization (23, 24). The ability to ferment FOS is present in most strains of Lactobacillus and Bifidobacterium (24, 92, 93). However, only 8 of 55 strains tested by Rossi et al. (94) were capable of using inulin, which is a long chain FOS derivative, as the sole carbon source.
Furthermore, it was suggested that adverse consequences might exist with the implementation of FOS in poultry feed. Ten Bruggencate et al. (95) demonstrated, in rats, a decrease in Salmonella resistance occurred due to an increase in intestinal permeability. Additionally, SCFAs may lead to an enhanced expression of Salmonella virulence genes despite reductions in colonization (20, 96). However, inulin amended diets have yielded middling results with Rehman et al. (93) demonstrating that inulin supplementation did not significantly impact the microbial community of the chicken cecum and Ramnani et al. (97) showed no impact on SCFA production in human diets supplemented with inulin. The effectiveness of FOS and inulin is dependent on a number of factors including the composition of the basal diet, degree of FOS polymerization, the presence of Bifidobacteria strains, host animal characteristics, and even host stress factors (91, 98). The FOS amended diets in poultry studies have appeared to yield inconclusive results; however, it has been demonstrated that FOS, when supplemented with probiotics, can produce consistently significant reductions in Salmonella (24, 79). This potential synergism has led to its implementation in products such as PoultryStar™ that directly impact aspects of the GIT (99, 76).
Galactooligosaccharides (Figure 1B) can be naturally found in human and cow milk, and consist of β-(1,6) and β-(1,4) linkages that avoid digestion in the upper GIT (100–103). Commercially, GOS can be prepared through hydrolyzing lactose from cow's milk and often commercial products contain lactose and a myriad of GOS oligomers (104, 105, 106). For instance, Bimuno (Clasado Ltd) is composed of varying concentrations of lactose and di-, tri-, tetra-, and pentose oligomers of GOS (104, 107, 106). Bimuno, in vitro and in mice ileal gut loops, caused reduction of S. Typhimurium adhesion and invasion, and but not when GOS was removed from the Bimuno mixture (107). Despite these positive effects, no significant differences in Salmonella concentrations was found when poultry was provided feed amended with 1% GOS, although significant alterations to the cecal microbiome were observed (108).
Despite this contrast, while GOS has not been as well studied in poultry compared to FOS and MOS (67), several publications have suggested some potential for GOS as a prebiotic in poultry. A bifidogenic effect has been observed by showing increased counts of Bifidobacterium in feces of birds fed 3 g of GOS per 25 kg of feed for 40 days (100). The addition of GOS to feed has also been shown to increase the Lactobacillus population in cecal contents (109), and when compared to xylooligosaccharides (XOS), FOS, and MOS, GOS significantly improved L. reuteri growth on minimal media (110). Besides promoting the growth of Bifidobacterium and Lactobacillus, GOS has demonstrated other potentially beneficial effects such as reducing heat stress in the jejunum, but not the ileum (111). GOS has been demonstrated to significantly alter the poultry transcriptome when injected in ovo compared to the addition of inulin and Lactococcus lactis (112), and also improve cell-mediated immunity when in low concentrations (0.1%) (109).
Additionally, GOS has been utilized as part of a synbiotic in some studies. Synbiotics are defined as a combination of probiotics and prebiotics (113). When Bifidobacterium was added to poultry feed along with GOS, this synbiotic affected total anaerobic microbial populations in feces, increasing them from 9.71 to 10.26 log colony forming units per gram (CFU/g) (100). This addition also increased Lactobacillus and Bifidobacterium fecal counts by 0.53 log and 1.32 log units, respectively (100). When injected in ovo, commercialized GOS and Lactococcus lactis elevated the body weight of broilers at the end of the rearing period (102, 113). This data differed from Biggs et al. (114) which used only the prebiotic, and by Jung et al. (100) and Abiuso et al. (115), which found no change in body weight when GOS was administered in feed. A cursory examination suggests this variation may be due to the differences in the basal diet and genetic variation of the chickens but more in-depth studies must be performed to ascertain the reason.
Other prebiotics have also been investigated to varying degrees. The implementation of 2 g/kg of XOS increased Lactobacillus and acetate in the cecum and after a 5-week treatment, significantly reduced cecal colonization and spleen translocation of S. Enteritidis (92, 116). Approximately a one log reduction of S. Enteritidis in the cecum was found by Pourabedin et al. (117) when XOS was implemented, but this was lower than the reduction observed by MOS (1.6 log reduction). Additionally, it was found that isomaltooligosaccharides (IMO) improved growth of Lactobacillus in vitro, exhibited a bifidogenic effect, and inhibited Salmonella in vitro (110, 118, 119). Thitaram et al. (120) found that diets supplemented with 1% IMO could reduce Salmonella by a two-log reduction and enhance growth during the first 3 weeks of growth, as well as increasing butyrate concentrations in the jejunum (121).
The effects of dietary fiber has also been investigated and suggested to possess prebiotic properties in poultry (10, 122). Fiber, depending on the derivative, source, and concentration, can accelerate feed passage and can alter the weight of the organs of the poultry GIT in a way that is indicative of improved functioning of the GIT (122–125). Organic acids, such as SCFAs, are a by-product of anaerobic fermentation of dietary fiber, and this suggests the possibility of inhibiting Salmonella growth in the GIT (126). As a consequence, there is some discussion if fiber should be considered a prebiotic (10). In Japan, while the term prebiotic is not defined, fiber, along with oligosaccharides are considered “foods to modify the gastrointestinal conditions” and can be considered “foods with specific health uses” (10, 127). Dietary fiber does meet the definition of a prebiotic purported in Gibson et al. (12). However, Roberfroid 128 suggests the need for several additional criteria such as resistance to gastrointestinal absorption, fermentation by intestinal microbiota, and selective stimulation of growth or activity of beneficial bacteria. Under this definition fiber, as well as inulin does not match the criteria for being a prebiotic, despite having some prebiotic effects (46, 128). As such, regulatory agencies such as the FDA and the European Food Safety Authority (EFSA) do not currently consider fiber to be a prebiotic (10, 129).
Regardless of their defined role from a regulatory consideration, there is an apparent variance in the effects these molecules have on the chicken GIT. Due to the complexity of some of these molecules such as fiber, and their effects, to elucidate their mechanisms on Salmonella reduction, the changes in the gut microbiota must be observed. To capture these alterations, microbiomic technologies can be employed.
Microbiomics
With the advent of whole genome and 16S rRNA genomic sequencing, researchers have been able to more accurately quantify microbial population shifts and host responses to the addition of prebiotics (25). By sequencing portions of the highly conserved 16S rRNA gene, such as the V1-V3 or the V4 region, and comparing it to databases, such as the Greengenes database, accurate identification of the microbiome can be determined efficiently and at a relatively lower cost (130, 131).
It should be noted that the rapid advancement in DNA sequencing technologies is continuously allowing for higher throughput at a lower cost (132, 133), and this section will attempt to provide as recent information as possible. Currently, Illumina-based microbiome sequencing can provide Operational Taxonomic Unit (OTU) detection at a very low abundance due to sequencing short DNA strands up to 300 bp. With the Illumina MiSeq Benchtop sequencer (Illumina, San Diego, CA, USA), a three-day sequencing run can return 7.5 Gb from 15 million 300-base paired-end reads to yield bulk data for small-scale projects (132). This efficiency is only increasing as technology allows for faster returns of more substantial data. Large-scale projects to study numerous samples can also use the Illumina HiSeq which allows for parallel sequencing at a comparably lower cost (132). The Illumina HiSeq returns 1,500 Gb from 5 billion 150 base paired-end reads but is typically only considered for production scale laboratory studies (132). Additionally, the Ion Torrent PGM system operates by detecting hydrogen ions that are released during DNA synthesis to sequence the genome israpid and easily scalable (Thermo Fisher Scientific, Waltham, MA, USA) (134–136). To analyze this ever-expanding capacity for bulk genomic data, bioinformatics programs are be employed such as Quantitative Insights Into Microbial Ecology (QIIME) and mothur (131, 137). Despite several differences, such as the programing language utilized, both programs have been shown to compile genomic data and evaluate species richness and equality with little statistical variation (131, 138–141). Using these bioinformatic programs, data can be efficently processed and changes in the GIT microbiome can be elucidated.
Investigative research into prebiotics greatly benefits from the sensitive high throughput technology that can quantitatively measure the differences between testing conditions. Park et al. (26) utilized Illumina based technology and the QIIME pipeline program to assess the changes in the cecal microbiota when subjected to the yeast-based prebiotics, Biolex® MB40, and Leiber® ExCel. They found significant changes in concentrations of Campylobacteraceae, Faecalibacterium, and, on the whole, in the phyla Firmicutes and Proteobacteria (26). This data was supported by Rastall and Gibson (142), and Park et al. (28), which also found an increase in Faecalibacterium OTU's during prebiotic treatment and suggested this increase helped facilitate a healthy microbiome, as an increase in Faecalibacterium has been linked to health benefits in poultry. Additional investigations into prebiotics found that MOS implementation can significantly alter the bacterial community phylogenetically (143, 144). Park et al. (28) also reported that FOS increased species diversity in pasture flock chickens demonstrated the prominence of Firmicutes across all trials, and showed that Bacteroidetes decreased in birds fed with diets amended with FOS and GOS. This study also investigated the use of fiber and found it increased the presence of the butyrate-producing Fusobacterium (28).
However, these changes only represent broad stroke differences in previously identified major taxa of importance. The aforementioned studies, as well as studies such as Pan (145), have generated not only general information about major taxa shifts but also seemingly negligible differences in the abundance and presence or absence of previously undetailed bacterial strains. While it is important to report changes in previously identified taxa of importance, Illumina sequencing allows for investigation into more nuanced changes or differences found in previously undescribed taxa. For instance, in Park et al. (26), several bacteria that could only be classified to the order Bacteroidales were present in chickens fed Biolex® MB40, but were not noted in the control group or birds fed with Leiber® ExCel. These unspecified species may play a potential role in the overall health of the GIT and may have previously gone undetected by culture and community fingerprinting techniques. Some of these nuanced differences can be attributed to variation in individual chicken microbiomes, but, when taken in composite, these data may yield vast and potentially vital information for understanding changes in the avian GIT incurred by prebiotics.
Currently, through analysis of clustered data, it appears the predominant driver of the poultry microbiota composition is host age (28). This deterministic variable was independent of treatments with feeds amended with 1 kg of FOS or plum fibers per ton and 2 kg of GOS per ton (28). While Original XPC™ was able to reduce Salmonella cecal populations in Park et al. (27), the microbiota was impacted more by the age of the bird even when in the presence of a coccidiosis vaccine (27, 29). These findings agree with previous assertions regarding the age of the poultry GIT, as it is reported that at birth the GIT is colonized by aerobic organisms followed by anaerobic microbial domination (146). Despite the strong influence of age and other uncontrollable variables such as gender (61), data still indicate that the microbiome can be shifted due to feed amendments. Therefore, because prebiotics can still be utilized to shift the microbial composition of poultry GIT, it is possible to generate environments that are unfavorable for Salmonella colonization. This can be accomplished by increasing populations of “healthy” bacteria, preventing space for Salmonella colonization as well as increasing SCFA production (67). To understand how these environments can be chemically altered, microbiome technologies can be employed in conjunction with investigative metabolomics technologies.
Metabolomics
Metabolomics is the qualitative and quantitative identification of all metabolites in a biological system such as the GIT. Metabolites are the final products of cellular processes and can be quantified through a number of instruments such as nuclear magnetic resonance (NMR) and mass spectrometry (MS) (147, 148). Due to its high selectivity, NMR is widely accepted as the primary choice for metabolite elucidation. However, MS is more sensitive comparatively, allowing for detection down to femtomolar (10−15) concentrations. Because of this sensitivity, for mixed samples, such as cecal and fecal contents, MS analysis is more readily utilized (147, 149, 150). Mass Spectrometry can also be coupled with chromatography to elucidate the macro-contents of complex mixtures (151). Gas Chromatography (GC) coupled with MS has allowed for the analyses of both volatile and nonvolatile compounds (152). Using GC-MS, Rubinelli et al. (153) investigated the effects of rice bran on Salmonella in cecal cultures in vitro and detected 578 metabolites. Of these, 367 were unknown, and the change in metabolite concentration was causally linked to the reduction of Salmonella. Liquid chromatography has also been used to identify thermolabile molecules in the form of high-pressure liquid chromatography (HPLC) which demonstrated FOS when fed to layers, could reduce cholesterol in eggs (154).
Metagenomic outputs in Sergeant et al. (155) indicated over 200 enzymes that can degrade non-starch polysaccharides in cecal contents, some of which are involved in pathways that produce SCFAs and are vital to the mechanistic understanding of modifying the environment. Unfortunately, one significant drawback to this methodology is the current inability to incorporate genomic information by providing definitive linkages between genotypes and the metabolome (147). Furthermore, the dynamic range of current MS technologies resolving power is ~106, which is far below the estimated concentration of cellular metabolites (147). However, with advances in both high throughput microbiome sequencing and mass spectrometry, it may be possible to derive causal relationships between the presence of phylogenetically related species and concentrations of metabolites.
Conclusions
The potential for prebiotics to alter the GIT of broiler chickens has been demonstrated with previous generation technologies such as DDGE, T-RFLP, and conventional plating techniques (35). However, despite the success of altering the microbiome, the precise mechanisms, and changes, such as the exact impact of SCFAs on the cecal microbiota, were historically undetermined due to the incomplete analysis offered by the technologies available at the time (156). Furthermore, with a range of variables such as age, type of bird, and genotype, the underlying mechanisms affecting the GIT seemed unlikely to be elucidated. However, with the rising use and affordability of “omic” technologies such as metagenomics and metabolomics, new investigative strategies can be employed. Through the use of bioinformatics pipeline applications on the bulk deep-sequencing data produced by these technologies, there is potential to produce a complete image of the GIT affected by prebiotics. This image may provide predictive power and allow for the understanding and creation, through prebiotics, of an environment that controls for and inhibits Salmonella colonization and growth. Moreover, while Salmonella is not the only pathogen of concern in the poultry industry, with the potential for virulence gene repression, it is likely prebiotics will continue to play a role in the control of this pathogen. With the ability to utilize next-generation technologies and more fully understand the complexity of the microbiome of poultry GIT, impacts of prebiotics on pathogen control will continue to be elucidated, investigated, and utilized in food safety.
Author Contributions
AM, SF, and SR have made substantial, direct and intellectual contribution to the work, and approved it for publication. HP and DM have been involved in the editing process and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
AM is supported by a Distinguished Doctoral Fellowship and support from the Department of Food Science at the University of Arkansas. Diamond V is acknowledged for its support and assistance.
References
2. Meltzer E, Stienlauf S, Leshem E, Sidi Y, Schwartz E. A large outbreak of Salmonella Paratyphi A infection among Israeli travelers to Nepal. Clin Infect Dis. (2013) 58:359–64. doi: 10.1093/cid/cit723
3. Nosanchuk JD, Lin J, Hunter RP, Aminov RI. Low-dose antibiotics: current status and outlook for the future. Front Microbiol. (2014) 5:478–9. doi: 10.3389/fmicb.2014.00478
4. Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States (2013). Available online at: http://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf (Accessed June 20, 2017).
5. Medalla F, Gu W, Mahon BE, Judd M, Folster J, Griffin PM, et al. Estimated incidence of antimicrobial drug–resistant nontyphoidal Salmonella infections, United States. 2004–2012 Emerg Infect Dis. (2017) 23:29–37. doi: 10.3201/eid2301.160771
6. World Health Organization. Antimicrobial Resistance: Global Report on Surveillance. Geneva: World Health Organization (2014).
7. Butel MJ, Waligora-Dupriet AJ. Probiotics and prebiotics: what are they and what can they do for us? In: Henderson B, Nibali L editors The Human Microbiota and Chronic Disease: Dysbiosis as a Cause of Human Pathology. Hoboken, NJ: John Wiley & Sons (2016). p. 467–478.
8. Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. (1995) 125:1401.
9. Langen LV, Dieleman LA. Prebiotics in chronic intestinal inflammation. Inflamm Bowel Dis. (2009) 15:454–62. doi: 10.1002/ibd.20737
10. Hutkins RW, Krumbeck JA, Bindels LB, Cani PD, Fahey G, Goh YJ, et al. Prebiotics: why definitions matter. Curr Opin Biotechnol. (2016) 37:1–7. doi: 10.1016/j.copbio.2015.09.001
11. Pineiro M, Asp NG, Reid G, Macfarlane S, Morelli L, Brunser O, et al. FAO Technical meeting on prebiotics. J Clin Gastroenterol. (2008) 42:S156–9. doi: 10.1097/MCG.0b013e31817f184e
12. Gibson GR, Hutkins RW, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, et al. The international scientific association for probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. (2017) 14:491. doi: 10.1038/nrgastro.2017.75
13. Johnson LP, Walton GE, Psichas A, Frost GS, Gibson GR, Barraclough TG. Prebiotics modulate the effects of antibiotics on gut microbial diversity and functioning in vitro. Nutrients (2015) 7:4480–97. doi: 10.3390/nu7064480
14. Lee HW, Park YS, Jung JS, Shin WS. Chitosan oligosaccharides, dp 2–8, have prebiotic effect on the Bifidobacterium bifidium and Lactobacillus sp. Anaerobe (2002) 8:319–24. doi: 10.1016/S1075-9964(03)00030-1
15. Kaplan H, Hutkins RW. Fermentation of fructooligosaccharides by lactic acid bacteria and bifidobacteria. Appl Environ Microbiol. (2000) 66:2682–4. doi: 10.1128/AEM.66.6.2682-2684.2000
16. Xu ZR, Hu CH, Xia MS, Zhan XA, Wang MQ. Effects of dietary fructooligosaccharide on digestive enzyme activities, intestinal microflora and morphology of male broilers. Poultry Sci. (2003) 82:1030–6. doi: 10.1093/ps/82.6.1030
17. Van Loo J, Coussement P, de Leenheer L, Hoebregs H, Smits G. On the presence of inulin and oligofructose as natural ingredients in the western diet. Crit Rev Food Sci Nutr. (1995) 35:525–52. doi: 10.1080/10408399509527714
18. Charalampopoulos D, Rastall RA. editors. Prebiotics and Probiotics Science and Technology Vol. 1. New York, NY: Springer (2009).
19. Donalson LM, McReynolds JL, Kim WK, Chalova VI, Woodward CL, Kubena LF, et al. The influence of a fructooligosaccharide prebiotic combined with alfalfa molt diets on the gastrointestinal tract fermentation, Salmonella Enteritidis infection, and intestinal shedding in laying hens. Poult Sci. (2008) 87:1253–62. doi: 10.3382/ps.2007-00166
20. Durant JA, Corrier DE, Ricke SC. Short-chain volatile fatty acids modulate the expression of the hilA and invF genes of Salmonella Typhimurium. J Food Protect. (2000) 63:573–8. doi: 10.4315/0362-028X-63.5.573
21. Durant JA, Lowry VK, Nisbet DJ, Stanker LH, Corrier DE, Ricke SC. Short chain fatty acids alter HEp-2 cell association and invasion by stationary growth phase Salmonella Typhimurium. J Food Sci. (2000) 65:1206–9. doi: 10.1111/j.1365-2621.2000.tb10266.x
22. Durant JA, Lowry VK, Nisbet DJ, Stanker LH, Corrier DE, Ricke SC. Late logarithmic Salmonella Typhimurium Hep-2 cell association and invasion response to short-chain fatty acid addition. J Food Saf. (2000) 20:1–11. doi: 10.1111/j.1745-4565.2000.tb00284.x
23. Cummings JH, Macfarlane GT. Gastrointestinal effects of prebiotics. Br J Nutr. (2002) 87:S145–51. doi: 10.1079/BJNBJN/2002530
24. Ricke SC. Potential of fructooligosaccharide prebiotics in alternative and nonconventional poultry production systems. Poultry Sci. (2015) 94:1411–8. doi: 10.3382/ps/pev049
25. Torok VA, Hughes RJ, Mikkelsen LL, Perez-Maldonado R, Balding K, MacAlpine R, et al. Identification and characterization of potential performance-related gut microbiotas in broiler chickens across various feeding trials. Appl Environ Microbiol. (2011) 77:5868–78. doi: 10.1128/AEM.00165-11
26. Park SH, Lee SI, Ricke SC. Microbial populations in naked neck chicken ceca raised on pasture flock fed with commercial yeast cell wall prebiotics via an Illumina MiSeq platform. PLoS ONE (2016) 11:e0151944. doi: 10.1371/journal.pone.0151944
27. Park SH, Kim S, Lee SI, Rubinelli PM, Roto SM, Pavlidis HO, et al. Original XPC™ effect on Salmonella Typhimurium and cecal microbiota from three different ages of broiler chickens when incubated in an anaerobic in vitro culture system. Front Microbiol. (2017) 8:1070. doi: 10.3389/fmicb.2017.01070
28. Park SH, Perrotta A, Hanning I, Diaz-Sanchez S, Pendleton S, Alm E, et al. Pasture flock chicken cecal microbiome responses to prebiotics and plum fiber feed amendments. Poultry Sci. (2017) 96:1820–30. doi: 10.3382/ps/pew441
29. Park SH, Roto S, Pavlidis H, McIntyre D, Striplin K, Brammer L, et al. Effects of feeding Original XPC™ to broilers with a live coccidiosis vaccine under industrial conditions: Part 2. Cecal microbiota analysis. Poult Sci. (2017) 96:2400–11. doi: 10.3382/ps/pex014
30. Dhama K, Tiwari R, Khan RU, Chakraborty S, Gopi M, Karthik K, et al. Growth promoters and novel feed additives improving poultry production and health, bioactive principles and beneficial applications: the trends and advances- a review. Int J Pharmacol. (2014) 10:129–59. doi: 10.3923/ijp.2014.129.159
31. Dhama K, Mahendran M, Tomar S, Chauhan RS. Beneficial effects of probiotics and prebiotics in livestock and poultry: the current perspectives. Intas Polivet. (2008) 9:1–12.
32. Yadav AS, Kolluri G, Gopi M, Karthik K, Singh Y. Exploring alternatives to antibiotics as health promoting agents in poultry-A review. J Exp Biol. (2016) 4:3S. doi: 10.18006/2016.4(3S).368.383
34. Wei S, Morrison M, Yu Z. Bacterial census of poultry intestinal microbiome. Poult Sci. (2013) 92:671–83. doi: 10.3382/ps.2012-02822
35. Stanley D, Hughes RJ, Moore RJ. Microbiota of the chicken gastrointestinal tract: influence on health, productivity and disease. Appl Microbiol Biotechnol. (2014) 98:4301–10. doi: 10.1007/s00253-014-5646-2
36. Chang MH, Chen TC. Reduction of Campylobacter jejuni in a simulated chicken digestive tract by lactobacilli cultures. J Food Protect. (2000) 63:1594–7. doi: 10.4315/0362-028X-63.11.1594
37. Gabriel I, Lessire M, Mallet S, Guillot JF. Microflora of the digestive tract: critical factors and consequences for poultry. World's Poult Sci J. (2006) 62:499–511. doi: 10.1017/S0043933906001115
39. Heres L, Wagenaar JA, van Knapen F, Urlings BA. Passage of Salmonella through the crop and gizzard of broiler chickens fed with fermented liquid feed. Avian Pathol. (2003) 32:173–81. doi: 10.1080/0307945021000071597
40. Bolton W. Digestion in the crop of the fowl. Br Poultry Sci. (1965) 6:97–102. doi: 10.1080/00071666508415561
41. Fuller R, Brooker BE. Lactobacilli which attach to the crop epithelium of the fowl. Am J Clin Nutr. (1974) 27:1305–12. doi: 10.1093/ajcn/27.11.1305
42. Mead GC. Microbes of the avian cecum: types present and substrates utilized. J Exp Zool Ecol Integrat Physiol. (1989) 3:48–54. doi: 10.1002/jez.1402520508
43. Turk DE. The anatomy of the avian digestive tract as related to feed utilization. Poult Sci. (1982) 61:1225–44.
44. Sun Y, O'Riordan MX. Regulation of bacterial pathogenesis by intestinal short-chain fatty acids. Adv Appl Microbiol. (2013) 85:93–118. doi: 10.1016/B978-0-12-407672-3.00003-4
45. Shin MS, Han SK, Ji AR, Kim KS, Lee WK. Isolation and characterization of bacteriocin-producing bacteria from the gastrointestinal tract of broiler chickens for probiotic use. J Appl Microbiol. (2008) 105:2203–12. doi: 10.1111/j.1365-2672.2008.03935.x
46. Roto SM, Rubinelli PM, Ricke SC. An introduction to the avian gut microbiota and the effects of yeast-based prebiotic-type compounds as potential feed additives. Front Vet Sci. (2015) 2:28. doi: 10.3389/fvets.2015.00028
47. Rehman HU, Vahjen W, Awad WA, Zentek J. Indigenous bacteria and bacterial metabolic products in the gastrointestinal tract of broiler chickens. Arch Anim Nutr. (2007) 61:319–35. doi: 10.1080/17450390701556817
48. Barnes EM, Mead GC, Barnuml DA, Harry EG. The intestinal flora of the chicken in the period 2 to 6 weeks of age, with particular reference to the anaerobic bacteria. Br Poult Sci. (1972) 13:311–26. doi: 10.1080/00071667208415953
49. Svihus B, Choct M, Classen HL. Function and nutritional roles of the avian caeca: a review. World's Poult Sci J. (2013) 69:249–64. doi: 10.1017/S0043933913000287
50. Deusch S, Tilocca B, Camarinha-Silva A, Seifert J. News in livestock research—use of Omics-technologies to study the microbiota in the gastrointestinal tract of farm animals. Comput Struct Biotechnol J. (2015) 13:55–63. doi: 10.1016/j.csbj.2014.12.005
51. Son JH, Karasawa Y. Effect of removal of caecal contents on nitrogen utilisation and nitrogen excretion in caecally ligated chickens fed on a low protein diet supplemented with urea. Br Poult Sci. (2000) 41:69–71. doi: 10.1080/00071660086420
52. Parsons CM. Influence of caecectomy and source of dietary fibre or starch on excretion of endogenous amino acids by laying hens. Br J Nutr. (1984) 51:541–8.
53. Hudault S, Bewa H, Bridonneau C, Raibaud P. Efficiency of various bacterial suspensions derived from cecal floras of conventional chickens in reducing the population level of Salmonella Typhimurium in gnotobiotic mice and chicken intestines. Can J Microbiol. (1985) 31:832–8. doi: 10.1139/m85-155
54. Ricke SC. The gastrointestinal tract ecology of Salmonella Enteritidis colonization in molting hens. Poult Sci. (2003) 82:1003–7. doi: 10.1093/ps/82.6.1003
55. Hargis BM, Caldwell DJ, Brewer RL, Corrier DE, DeLoach JR. Evaluation of the chicken crop as a source of Salmonella contamination for broiler carcasses. Poult Sci. (1995) 74:1548–52. doi: 10.3382/ps.0741548
56. Fanelli MJ, Sadler WW, Franti CE, Brownell JR. Localization of Salmonellae within the intestinal tract of chickens. Avian Dis. (1971) 366–75. doi: 10.2307/1588708
57. Snoeyenbos GH, Soerjadi AS, Weinack OM. Gastrointestinal colonization by Salmonellae and pathogenic Escherichia coli in monoxenic and holoxenic chicks and poults. Avian Dis. (1982) 26:566–75. doi: 10.2307/1589903
58. Soerjadi AS, Stehman SM, Snoeyenbos GH, Weinack OM, Smyser CF. Some measurements of protection against paratyphoid Salmonella and Escherichia coli by competitive exclusion in chickens. Avian Dis. (1981) 25:706–12. doi: 10.2307/1590001
59. Huang DS, Li DF, Xing JJ, Ma YX, Li ZJ, Lv SQ. Effects of feed particle size and feed form on survival of Salmonella Typhimurium in the alimentary tract and cecal S. typhimurium reduction in growing broilers. Poult Sci. (2006) 85:831–6. doi: 10.1093/ps/85.5.831
60. Apajalahti J, Kettunen A, Graham H. Characteristics of the gastrointestinal microbial communities, with special reference to the chicken. World's Poult Sci J. (2004) 60:223–32. doi: 10.1079/WPS20040017
61. Zoetendal EG, Collier CT, Koike S, Mackie RI, Gaskins HR. Molecular ecological analysis of the gastrointestinal microbiota: a review. J Nutr. (2004) 134:465–72. doi: 10.1093/jn/134.2.465
62. Muyzer G, De Waal EC, Uitterlinden AG. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol. (1993) 59:695–700.
63. Salanitro JP, Fairchilds IG, Zgornicki YD. Isolation, culture characteristics, and identification of anaerobic bacteria from the chicken cecum. Appl Microbiol. (1974) 27:678–87.
64. Barnes EM. The intestinal microflora of poultry and game birds during life and after storage. Address of the president of the Society for Applied Bacteriology delivered at a meeting of the society on 10 January 1979. J Appl Bacteriol. (1979) 46:407–19 doi: 10.1111/j.1365-2672.1979.tb00838.x
65. Mundt E, Collett SR, Berghaus R, Pedroso AA, Lee MD, Maurer JJ. Can bacteriotherapy using commercially available probiotics, prebiotics, and organic acids ameliorate the symptoms associated with runting-stunting syndrome in broiler chickens?. Avian Dis. (2015) 59:201–6. doi: 10.1637/122013-Reg
66. Hooge DM. Meta-analysis of broiler chicken pen trials evaluating dietary mannan oligosaccharide 1993-2003. Int J Poult Sci. (2004) 3:163–74.
67. Pourabedin M, Zhao X. Prebiotics and gut microbiota in chickens. FEMS Microbiol Lett. (2015) 362:1–8. doi: 10.1093/femsle/fnv122
68. US National Library of Medicine. Oligosaccharides (1999). Available online at: https://meshb.nlm.nih.gov/record/ui?name=Oligosaccharides (Accessed June 20, 2017).
69. Park SH, Gibson KE, Almeida G, Ricke SC. Assessment of gastrointestinal microflora in pasture raised chickens fed two commercial prebiotics. J Prob Health (2014) 2:122. doi: 10.4172/2329-8901.1000122
70. Newman K. Mannan-oligosaccharides natural polymers with significant impact on the gastrointestinal microflora and the immune system. In: Lyons TP, Jacques KA editors. Biotechnology in the Feed Industry: Proceedings of Alltech's 10th Annual Symposium. Nottingham: Nottingham University Press (1994). p. 167–74.
71. Lee SI, Park SH, Ricke SC. Assessment of cecal microbiota, integron occurrence, fermentation responses, and Salmonella frequency in conventionally raised broilers fed a commercial yeast-based prebiotic compound. Poult Sci. (2016) 95:144–53. doi: 10.3382/ps/pev322
72. Feye KM, Anderson KL, Scott MF, McIntyre DR, Carlson SA. Inhibition of the virulence, antibiotic resistance, and fecal shedding of multiple antibiotic-resistant Salmonella Typhimurium in broilers fed Original XPC™. Poult Sci. (2016) 95:2902–10. doi: 10.3382/ps/pew254
73. Broomhead J, Severson D, Butler J, Frank J. Effects of a Saccharomyces cerevisiae fermentation product on volatile fatty acid production and growth of Salmonella in a complex fecal microbial population in vitro. Poult Sci. (2012) 91(Suppl. 1):132.
74. Nsereko VL, Broomhead JN, Butler J, Weigand T, Gingerich E, Diamond V. Effects of Original XPC on growth of Salmonella Arizonae and Heidelberg in a complex fecal microbial population. Poult Sci. (2013) 92:130.
75. Windschitl PM, Randall KM, Brainard DJ. Growth performance of Holstein dairy calves supplemented with a probiotic. In: Agricultural and Forestry Experiment Station School of Agriculture and Land Resources Management. University of Alaska Fairbanks (1991).
76. Sterzo EV, Paiva JB, Mesquita AL, Freitas Neto OC, Berchieri Jr A. Organic acids and/or compound with defined microorganisms to control Salmonella enterica serovar Enteritidis experimental infection in chickens. Revista Brasileira de Ciência Avícola (2007) 9:69–73. .doi: 10.1590/S1516-635X2007000100010
77. Pedroso AA, Hurley-Bacon AL, Zedek AS, Kwan TW, Jordan AP, Avellaneda G, et al. Can probiotics improve the environmental microbiome and resistome of commercial poultry production? Int J Environ Res Public Health (2013) 10:4534–59. doi: 10.3390/ijerph10104534
78. Torres-Rodriguez A, Higgins SE, Vicente JLS, Wolfenden AD, Gaona-Ramirez G, Barton JT, et al. Effect of lactose as a prebiotic on turkey body weight under commercial conditions. J Appl Poult Res. (2007) 16:635–41. doi: 10.3382/japr.2006-00127
79. Hajati H, Rezaei M. The application of prebiotics in poultry production. Int J Poult Sci. (2010) 9:298–304. doi: 10.3923/ijps.2010.298.304
80. Baurhoo B, Phillip L, Ruiz-Feria CA. Effects of purified lignin and mannan oligosaccharides on intestinal integrity and microbial populations in the ceca and litter of broiler chickens. Poult Sci. (2007) 86:1070–8. doi: 10.1093/ps/86.6.1070
81. Finucane M, Spring P, Newman KE. Incidence of mannose sensitive adhesions in enteric bacteria. Poult Sci. (1999) 78(Suppl.1):139.
82. Shane SM. Mannan oligosaccharides in poultry nutrition: mechanisms and benefits. In: Alltech‘s Annual Symposium Vol. 17. Nottingham, UK (2001). p. 65–77.
83. Mirelmann D Altman G, Eshdat Y. Screening of bacterial isolates for mannose-specific lectin activity by agglutination of yeast. J Clin Microbiol. (1980) 11:328–31.
84. Ferket PR, Parks CW, Grimes JL. Benefits of dietary antibiotic and mannanoligosaccharide supplementation for poultry. In: Multi-State Poultry Meeting Vol. 14 (2002).
85. Lourenço MC, Kuritza LN, Hayashi RM, Miglino LB, Durau JF, Pickler L, et al. Effect of a mannanoligosaccharide-supplemented diet on intestinal mucosa T lymphocyte populations in chickens challenged with Salmonella Enteritidis. J Appl Poult Res. (2015) 24:15–22. doi: 10.3382/japr/pfu002
86. Spring P, Wenk C, Dawson KA, Newman KE. Effect of mannan oligosaccharide on different cecal parameters and on cecal concentration on enteric bacteria in challenged broiler chicks. Poult Sci. (2000) 79:205–11. doi: 10.1093/ps/79.2.205
87. Stanley VG, Gray C, Kreuger WF, Hume ME. Combined effects of dietary carbohydrates and preslaughter flushing with magnesium sulfate on cecal coliform colonization in chicks. Texas J Agricult Nat Res. (2016) 14:47–52.
88. Singh RS, Singh RP, Kennedy JF. Recent insights in enzymatic synthesis of fructooligosaccharides from inulin. Int J Biol Macromol. (2016) 85:565–72. doi: 10.1016/j.ijbiomac.2016.01.026
89. Roberfroid M, Gibson GR, Hoyles L, McCartney AL, Rastall R, Rowland I, et al. Prebiotic effects: metabolic and health benefits. Br J Nutr. (2010) 104:S1–63. doi: 10.1017/S0007114510003363
90. Emami NK, Samie A, Rahmani HR, Ruiz-Feria CA. The effect of peppermint essential oil and fructooligosaccharides, as alternatives to virginiamycin, on growth performance, digestibility, gut morphology and immune response of male broilers. Anim Feed Sci Technol. (2012) 175:57–64. doi: 10.1016/j.anifeedsci.2012.04.001
91. Bogusławska-Tryk M, Piotrowska A, Burlikowska K. Dietary fructans and their potential beneficial influence on health and performance parameters in broiler chickens. J Centr Eur Agricul. (2012) 13:272–91. doi: 10.5513/JCEA01/13.2.1045
92. Pourabedin M, Guan L, Zhao X. Xylo-oligosaccharides and virginiamycin differentially modulate gut microbial composition in chickens. Microbiome (2015) 3:3–15. doi: 10.1186/s40168-015-0079-4
93. Rehman H, Hellweg P, Taras D, Zentek J. Effects of dietary inulin on the intestinal short chain fatty acids and microbial ecology in broiler chickens as revealed by denaturing gradient gel electrophoresis. Poult Sci. (2008) 87:783–9. doi: 10.3382/ps.2007-00271
94. Rossi M, Corradini C, Amaretti A, Nicolini M, Pompei A, Zanoni S, et al. Fermentation of fructooligosaccharides and inulin by bifidobacteria: a comparative study of pure and fecal cultures. Appl Environ Microbiol. (2005) 71:6150–8. doi: 10.1128/AEM.71.10.6150-6158.2005
95. Ten Bruggencate SJ, Bovee-Oudenhoven IM, Lettink-Wissink ML, Van der Meer R. Dietary fructooligosaccharides increase intestinal permeability in rats. J Nutr. (2005) 135:837–42. doi: 10.1093/jn/135.4.837
96. Lawhon SD, Maurer R, Suyemoto M, Altier C. Intestinal short-chain fatty acids alter Salmonella Typhimurium invasion gene expression and virulence through BarA/SirA. Mol Microbiol. (2002) 46:1451–64. doi: 10.1046/j.1365-2958.2002.03268.x
97. Ramnani P, Gaudier E, Bingham M, van Bruggen P, Tuohy KM, Gibson GR. Prebiotic effect of fruit and vegetable shots containing Jerusalem artichoke inulin: a human intervention study. Br J Nutr. (2010) 104:233–40. doi: 10.1017/S000711451000036X
98. Lee JH, O'Sullivan DJ. Genomic insights into Bifidobacteria. Microbiol Mol Biol Rev. (2010) 74:378–416. doi: 10.1128/MMBR.00004-10
99. Bailey JS, Blankenship LC, Cox NA. Effect of fructooligosaccharide on Salmonella colonization of the chicken intestine. Poult Sci. (1991) 70:2433–8. doi: 10.3382/ps.0702433
100. Jung SJ, Houde R, Baurhoo B, Zhao X, Lee BH. Effects of galacto-oligosaccharides and a Bifidobacteria lactis-based probiotic strain on the growth performance and fecal microflora of broiler chickens. Poult Sci. (2008) 87:1694–9. doi: 10.3382/ps.2007-00489
101. Prenosil JE, Stuker E, Bourne JR. Formation of oligosaccharides during enzymatic lactose: Part I: State of art. Biotechnol Bioeng. (1987) 30:1019–25. doi: 10.1002/bit.260300904
102. Bednarczyk M, Stadnicka K, Kozłowska I, Abiuso C, Tavaniello S, Dankowiakowska A, et al. Influence of different prebiotics and mode of their administration on broiler chicken performance. Animal (2016) 10:1271–9. doi: 10.1017/S1751731116000173
103. Burvall A, Asp N, Dahlqvist A. Oligosaccharide formation during hydrolysis of lactose with “Saccharomyces lactis” lactase—part 3: digestibility by human intestinal enzymes in vitro. Food Chem. (1980) 5:189–94.
104. Tzortzis G, Goulas AK, Gee JM, Gibson GR. A novel galactooligosaccharide mixture increases the bifidobacterial population numbers in a continuous in vitro fermentation system and in the proximal colonic contents of pigs in vivo. J Nutr. (2005) 135:1726–31. doi: 10.1093/jn/135.7.1726
105. Al-Sheraji SH, Ismail A, Manap MY, Mustafa S, Yusof RM, Hassan FA. Prebiotics as functional foods: a review. J Funct Foods (2013) 5:1542–53. doi: 10.1016/j.jff.2013.08.009
106. Tzortzis G, Goulas AK, Gibson GR. Synthesis of prebiotic galactooligosaccharides using whole cells of a novel strain, Bifidobacterium bifidum NCIMB 41171. Appl Microbiol Biotechnol. (2005) 68:412–6. doi: 10.1007/s00253-005-1919-0
107. Searle LE, Best A, Nunez A, Salguero FJ, Johnson L, Weyer U, et al. A mixture containing galactooligosaccharide, produced by the enzymic activity of Bifidobacterium bifidum, reduces Salmonella enterica serovar Typhimurium infection in mice. J Med Microbiol. (2009) 58:37–48. doi: 10.1099/jmm.0.004390-0
108. Hughes RA, Ali R, Mendoz M, Hassan HM, Koci M. Impact of dietary galacto-oligosaccharide (GOS) on chicken's gut microbiota, mucosal gene expression, and Salmonella colonization. Front Vet Sci. (2017) 4:192. doi: 10.3389/fvets.2017.00192
109. Yousaf MS, Ahmad I, Ashraf K, Rashid MA, Hafeez A, Ahmad A, et al. Comparative effects of different dietary concentrations of β-galacto-oligosaccharides on serum biochemical metabolites, selected caecel microbiota and immune response in broilers. JAPS (2017) 27:98–105.
110. Saminathan M, Sieo CC, Kalavathy R, Abdullah N, Ho YW. Effect of prebiotic oligosaccharides on growth of Lactobacillus strains used as a probiotic for chickens. Afr J Microbiol Res. (2011) 5:57–64. doi: 10.5897/AJMR10.700
111. Varasteh S, Braber S, Akbari P, Garssen J, Fink-Gremmels J. Differences in susceptibility to heat stress along the chicken intestine and the protective effects of galacto-oligosaccharides. PloS ONE (2015) 10:e0138975. doi: 10.1371/journal.pone.0138975
112. Slawinska A, Plowiec A, Siwek M, Jaroszewski M, Bednarczyk M. Long-term transcriptomic effects of prebiotics and synbiotics delivered in ovo in broiler chickens. PloS ONE (2016) 11:e0168899. doi: 10.1371/journal.pone.0168899
113. Pruszynska-Oszmalek E, Kolodziejski PA, Stadnicka K, Sassek M, Chalupka D, Kuston B, et al. In ovo injection of prebiotics and synbiotics affects the digestive potency of the pancreas in growing chickens. Poult Sci. (2015) 94:1909–16. doi: 10.3382/ps/pev162
114. Biggs P, Parsons CM, Fahey GC. The effects of several oligosaccharides on growth performance, nutrient digestibilities, and cecal microbial populations in young chicks. Poult Sci. (2007) 86:2327–36. doi: 10.3382/ps.2007-00427
115. Abiuso C, Maiorano G, Stadnicka K, Bogucka J, Bednarczyk M. Influence of different prebiotics and their modality of administration on carcass traits and muscle fiber diameter in broiler chickens. In: XXVI International Poultry Symposium PB WPSA. Kazimierz Dolny: The Polish Branch of World's Poultry Science Association (2014). p. 24.
116. Eeckhaut V, Van Immerseel F, Dewulf J, Pasmans F, Haesebrouck F, Ducatelle R, et al. Arabinoxylooligosaccharides from wheat bran inhibit Salmonella colonization in broiler chickens. Poult Sci. (2008) 87:2329–34. doi: 10.3382/ps.2008-00193
117. Pourabedin M, Chen Q, Yang M, Zhao X. Mannan-and xylooligosaccharides modulate caecal microbiota and expression of inflammatory-related cytokines and reduce caecal Salmonella Enteritidis colonisation in young chickens. FEMS Microbiol Ecol. (2017) 93:fiw226. doi: 10.1093/femsec/fiw226
118. Chung CH, Day DF. Glucooligosaccharides from Leuconostoc mesenteroides B-742 (ATCC 13146): a potential prebiotic. J Indust Microbiol Biotechnol. (2002) 29:196–9. doi: 10.1038/sj.jim.7000269
119. Chung CH, Day DF. Efficacy of Leuconostoc mesenteroides (ATCC 13146) isomaltooligosaccharides as a poultry prebiotic. Poult Sci. (2004) 83:1302–6. doi: 10.1093/ps/83.8.1302
120. Thitaram SN, Chung CH, Day DF, Hinton Jr A, Bailey JS, Siragusa GR. Isomaltooligosaccharide increases cecal Bifidobacterium population in young broiler chickens. Poult Sci. (2005) 84:998–1003. doi: 10.1093/ps/84.7.998
121. Zhang WF, Li DF, Lu WQ, Yi GF. Effects of isomalto-oligosaccharides on broiler performance and intestinal microflora. Poult Sci. (2003) 82:657–63. doi: 10.1093/ps/82.4.657
122. Jiménez-Moreno E, González-Alvarado JM, González-Sánchez D, Lázaro R, Mateos GG. Effects of type and particle size of dietary fiber on growth performance and digestive traits of broilers from 1 to 21 days of age 1. Poult Sci. (2010) 89:2197–212. doi: 10.3382/ps.2010-00771
123. Amerah AM, Ravindran V, Lentle RG. Influence of insoluble fibre and whole wheat inclusion on the performance, digestive tract development and ileal microbiota profile of broiler chickens. Br Poult Sci. (2009) 50:366–75. doi: 10.1080/00071660902865901
124. Hetland H, Svihus B. Effect of oat hulls on performance, gut capacity and feed passage time in broiler chickens. Br Poult Sci. (2001) 42:354–61. doi: 10.1080/00071660120055331
125. Mateos GG, Jiménez-Moreno E, Serrano MP, Lázaro RP. Poultry response to high levels of dietary fiber sources varying in physical and chemical characteristics. J Appl Poult Res. (2012) 21:156–74. doi: 10.3382/japr.2011-00477
126. Jørgensen H, Zhao XQ, Knudsen KEB, Eggum BO. The influence of dietary fibre source and level on the development of the gastrointestinal tract, digestibility and energy metabolism in broiler chickens. Br J Nutr. (1996) 75:379–95. doi: 10.1079/BJN19960141
127. Japanese Ministry of Health. Labour and Welfare: Food for Specified Health Uses (FOSHU) (2010). Available online at: http://www.mhlw.go.jp/english/topics/foodsafety/fhc/02.html
128. Roberfroid M. Prebiotics: the concept revisited. J Nutr. (2007) 137:830S−7S. doi: 10.1093/jn/137.3.830S
129. Van Loveren H, Sanz Y, Salminen S. Health claims in Europe: probiotics and prebiotics as case examples. Ann Rev Food Sci Technol. (2012) 3:247–61. doi: 10.1146/annurev-food-022811-101206
130. DeSantis TZP, Hugenholz N, Larsen M, Rojas EL, Brodie K, et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol. (2006) 72:5069–72. doi: 10.1128/AEM.03006-05
131. Ricke SC, Hacker JC, Yearkey KL, Shi Z, Park SH, Rainwater CE. Unraveling food production microbiomes: concepts and future directions. In: Ricke SC, Park SH, Rainwater CE. editors. Food and Feed Safety Systems and Analysis. San Diego, CA: Elsevier Inc (2017). p. 347–74.
132. Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. (2012) 6:1621. doi: 10.1038/ismej.2012.8
133. Van Dijk EL, Auger H, Jaszczyszyn Y, Thermes C. Ten years of next-generation sequencing technology. Trends Genet. (2014) 30:418–26. doi: 10.1016/j.tig.2014.07.001
134. Ansorge WJ. Next-generation DNA sequencing techniques. N Biotechnol. (2009) 25:195–203. doi: 10.1016/j.nbt.2008.12.009
135. Glenn TC. Field guide to next-generation DNA sequencers. Mol Ecol Res. (2011) 11:759–69. doi: 10.1111/j.1755-0998.2011.03024.x
136. Milani C, Hevia A, Foroni E, Duranti S, Turroni F, Lugli GA, et al. Assessing the fecal microbiota: an optimized ion torrent 16S rRNA gene-based analysis protocol. PloS ONE (2013) 8:e68739. doi: 10.1371/journal.pone.0068739
137. Luscombe NM, Greenbaum D, Gerstein M. What is bioinformatics? A proposed definition and overview of the field. Methods Informat Med. (2001) 40:346–58. doi: 10.1055/s-0038-1634431
138. Plummer E, Twin J, Bulach DM, Garland SM, Tabrizi SN. A comparison of three bioinformatics pipelines for the analysis of preterm gut microbiota using 16S rRNA gene sequencing data. J Proteom Bioinformat. (2015) 8:283–91. doi: 10.4172/jpb.1000381
139. Robinson CK, Brotman RM, Ravel J. Intricacies of assessing the human microbiome in epidemiologic studies. Ann Epidemiol. (2016) 26:311–21. doi: 10.1016/j.annepidem.2016.04.005
140. Gensollen T, Blumberg RS. Correlation between early-life regulation of the immune system by microbiota and allergy development. J Allergy Clin Immunol. (2017) 139:1084–91. doi: 10.1016/j.jaci.2017.02.011
141. Nilakanta H, Drews KL, Firrell S, Foulkes MA, Jablonski KA. A review of software for analyzing molecular sequences. BMC Res Notes (2014) 7:830. doi: 10.1186/1756-0500-7-830
142. Rastall RA, Gibson GR. Recent developments in prebiotics to selectively impact beneficial microbes and promote intestinal health. Curr Opin Biotechnol. (2015) 32:42–6. doi: 10.1016/j.copbio.2014.11.002
143. Corrigan A, Horgan K, Clipson N, Murphy RA. Effect of dietary supplementation with a Saccharomyces cerevisiae mannan oligosaccharide on the bacterial community structure of broiler cecal contents. Appl Environ Microbiol. (2011) 77:6653–62. doi: 10.1128/AEM.05028-11
144. Corrigan A, de Leeuw M, Penaud-Frézet S, Dimova D, Murphy RA. Phylogenetic and functional alterations in bacterial community compositions in broiler ceca as a result of mannan oligosaccharide supplementation. Appl Environ Microbiol. (2015) 81:3460–70. doi: 10.1128/AEM.04194-14
145. Pan D, Yu Z. Intestinal microbiome of poultry and its interaction with host and diet. Gut Microb. (2014) 5:108–19. doi: 10.4161/gmic.26945
146. Hanning I, Diaz-Sanchez S. The functionality of the gastrointestinal microbiome in non-human animals. Microbiome (2015) 3:51. doi: 10.1186/s40168-015-0113-6
147. Park SH, Hanning I, Perrota A, Bench BJ, Alm E, Ricke SC. Modifying the gastrointestinal ecology in alternatively raised poultry and the potential for molecular and metabolomic assessment. Poult Sci. (2013) 92:546–61. doi: 10.3382/ps.2012-02734
148. Roberts T, Wilson J, Guthrie A, Cookson K, Vancraeynest D, Schaeffer J, et al. New issues and science in broiler chicken intestinal health: Emerging technology and alternative interventions. J Appl Poultry Res. (2015) 24:257–66. doi: 10.3382/japr/pfv023
149. Bedair M, Sumner LW. Current and emerging mass-spectrometry technologies for metabolomics. TrAC Trends Analyt Chem. (2008) 27:238–50. doi: 10.1016/j.trac.2008.01.006
150. Lei Z, Huhman DV, Sumner LW. Mass spectrometry strategies in metabolomics. J Biol Chem. (2011) 286:25435–42. doi: 10.1074/jbc.R111.238691
151. Lisec J, Schauer N, Kopka J, Willmitzer L, Fernie AR. Gas chromatography mass spectrometry-based metabolite profiling in plants. Nat Protocols (2006) 1:387. doi: 10.1038/nprot.2006.59
152. Babushok VI, Linstrom PJ, Reed JJ, Zenkevich IG, Brown RL, Mallard WG, et al. Development of a database of gas chromatographic retention properties of organic compounds. J Chromatogr A (2007) 1157:414–21. doi: 10.1016/j.chroma.2007.05.044
153. Rubinelli PM, Kim SA, Park SH, Roto SM, Nealon NJ, Ryan EP, et al. Differential effects of rice bran cultivars to limit Salmonella Typhimurium in chicken cecal in vitro incubations and impact on the cecal microbiome and metabolome. PloS ONE (2017) 12:e0185002. doi: 10.1371/journal.pone.0185002
154. Li X, Liu L, Li K, Hao K, Xu C. Effect of fructooligosaccharides and antibiotics on laying performance of chickens and cholesterol content of egg yolk. Br Poult Sci. (2007) 48:185–9. doi: 10.1080/00071660701261310
155. Sergeant MJ, Constantinidou C, Cogan TA, Bedford MR, Penn CW, Pallen MJ. Extensive microbial and functional diversity within the chicken cecal microbiome. PLoS ONE (2014) 9:e91941. doi: 10.1371/journal.pone.0091941
Keywords: prebiotics, Salmonella, poultry, microbiomics, metabolomics, fructooligosaccharides, mannanoligosaccharides, galactooligosaccharides
Citation: Micciche AC, Foley SL, Pavlidis HO, McIntyre DR and Ricke SC (2018) A Review of Prebiotics Against Salmonella in Poultry: Current and Future Potential for Microbiome Research Applications. Front. Vet. Sci. 5:191. doi: 10.3389/fvets.2018.00191
Received: 10 May 2018; Accepted: 25 July 2018;
Published: 15 August 2018.
Edited by:
Timothy J. Johnson, University of Minnesota Twin Cities, United StatesReviewed by:
Kuldeep Dhama, Indian Veterinary Research Institute (IVRI), IndiaChristi Swaggerty, United States Department of Agriculture, United States
Copyright © 2018 Micciche, Foley, Pavlidis, McIntyre and Ricke. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Steven C. Ricke, sricke@uark.edu
 Andrew C. Micciche
Andrew C. Micciche Steven L. Foley
Steven L. Foley Hilary O. Pavlidis
Hilary O. Pavlidis Donald R. McIntyre3
Donald R. McIntyre3  Steven C. Ricke
Steven C. Ricke