Does Chronic Kidney Disease Really Affect the Complications and Prognosis After Liver Resection for Hepatocellular Carcinoma? A Meta-Analysis
- 1Department of Gastrointestinal Surgery, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 2Department of General Surgery, Qijiang Hospital of the First Affiliated Hospital of Chongqing Medical University, Chongqing, China
Purpose: The purpose of this meta-analysis was to analyze whether chronic kidney disease (CKD) affected the complications and prognosis after liver resection for hepatocellular carcinoma.
Methods: The PubMed, Embase, and Cochrane Library databases were searched from inception to 22 February 2022 to find eligible studies. Complications, overall survival (OS), and disease-free survival (DFS) were collected, and this meta-analysis was performed with RevMan 5.3.
Results: A total of nine studies including 6,541 patients were included in this meta-analysis. After pooling all baseline information, the CKD group had a higher rate of Child-Pugh grade B than the Non-CKD group (OR = 1.58, 95% CI = 1.3 to 1.93, P < 0.00001). As for surgery-related information, the CKD group had larger blood loss (MD = −404.79, 95% CI = −509.70 to −299.88, P < 0.00001), and higher rate of blood transfusion (OR = 2.47, 95% CI = 1.85 to 3.3, P < 0.00001). In terms of complications, the CKD group had a higher rate of overall complications (OR = 2.1, 95% CI = 1.57 to 2.81, P < 0.00001) and a higher rate of ≥ grade III complications (OR = 2.04, 95% CI = 1.57 to 2.81, P = 0.0002). The CKD group had poor OS compared with the non-CKD group (HR = 1.28, 95% CI = 1.1 to 1.49, P = 0.001). However, in terms of DFS, no significant difference was found (HR = 1.11, 95% CI = 0.96 to 1.28, P = 0.16).
Conclusion: Preexisting CKD was associated with higher ratio of complications and poor OS.
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common malignant tumor and the fourth most common cause of cancer-related deaths in the world (1, 2). It is estimated that 840,000 newly diagnosed HCC cases are reported each year worldwide (3). Liver resection is commonly radical treatment (4, 5).
Chronic kidney disease (CKD) is a serious public health problem in the 21st century (6) and leads to premature death, reduced quality of life, and heavy economic burden on patients (7). The morbidity and mortality of CKD are increasing rapidly worldwide (8), and compared with the non-CKD population, the morbidity and mortality usually come from cardiovascular disease (9, 10).
Previous studies have reported the relationship between CKD and cancer surgeries, and CKD increased complications and mortality (11, 12). Multiple systems are impaired with progression of CKD (11), which might account for higher complications and poorer overall survival.
As for HCC, CKD might have a potential effect on outcomes. However, it remains controversial whether preexisting CKD has an impact on complications and prognosis after liver resection for hepatocellular carcinoma. Some studies reported that CKD had no effect on complications or prognosis; (13, 14) however, other studies reported that CKD was associated with increased complications and poor prognosis (15, 16). Therefore, the purpose of this meta-analysis was to analyze whether CKD affected the complications and prognosis after liver resection for hepatocellular carcinoma.
Methods
Literature Search Strategy
The PubMed, Embase, and Cochrane Library databases were systematically searched by two authors, and searching date was 22 February 2022. The search strategy mainly focused on the following two items, HCC and CKD. For HCC, the search strategy was as follows: (liver cancer) OR (hepatocellular carcinoma) OR (hepatocarcinoma) OR (HCC); for CKD, the search strategy was as follows: (chronic kidney disease) OR (hemodialysis) OR (estimated glomerular filtration rate) OR (dialysis) OR (cystatin c). Then, we used “and” to combine two key words. The scope of the search strategy was limited to the Title and Abstract, and the language was limited to articles published in English.
Inclusion and Exclusion Criteria
The inclusion criteria of this meta-study were as follows: (1) Patients diagnosed with HCC and received primary liver resection; (2) The preexisting disease included CKD group and Non-CKD group; and (3) Postoperative short-term complications or long-term prognosis were reported. The exclusion criteria were as follows: (1) data that could not be extracted in the meta-analysis; and (2) research type was case report, letter to editor, comments, reviews, or and conference. Two reviewers conducted the inclusion and exclusion separately, and a disagreement was resolved by another reviewer.
Data Extraction
The extracted data were as follows: (1) first author, year of publication, country, study design, study date, definition of CKD; (2) baseline information including sex, age, tumor size, vascular invasion, intrahepatic metastases, Child-Pugh grade, and liver cirrhosis; (3) surgery-related information including blood loss, blood transfusion, and postoperative hospital stay; (4) postoperative information including overall complications, surgical site infection, bile leakage, liver failure, pleural effusion, ascites, and postoperative bleeding; and (5) overall survival (OS) and disease-free survival (DFS).
Quality Assessment
To assess the methodological quality of included retrospective studies, we adopted the Newcastle-Ottawa Scale (NOS) (17)If the NOS score was equal to nine points, we considered the methodological quality of a study to be high; if the NOS score was less than seven points, we considered the methodological quality of a study to be low; if the NOS score was between seven and eight, we considered the methodological quality of a research study was moderate.
Statistical Analysis
The meta-analysis was performed using RevMan 5.3 (The Cochrane Collaboration, London, United Kingdom). In this meta-analysis, pooled hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated for the OS and DFS of patients with hepatocellular carcinoma, and HRs were extracted from multivariate analyses and/or univariate analyses, or estimated from Kaplane-Meier survival curves (18, 19). Continuous variables were presented as the mean and standard deviation (SD), and categorical variables were presented as proportions. For dichotomous and continuous variables, odds ratios (ORs) and mean differences (MDs), and 95% CIs were calculated. The I2 value and the results of the chi-squared test were used to assess statistical heterogeneity (20, 21). High heterogeneity was considered when I2 > 50%; in such a case, a random effects model was used, and p < 0.1 was considered statistically significant. A fixed effects model was used when I2 ≤ 50%, and p < 0.05 was considered statistically significant.
Results
Study Selection
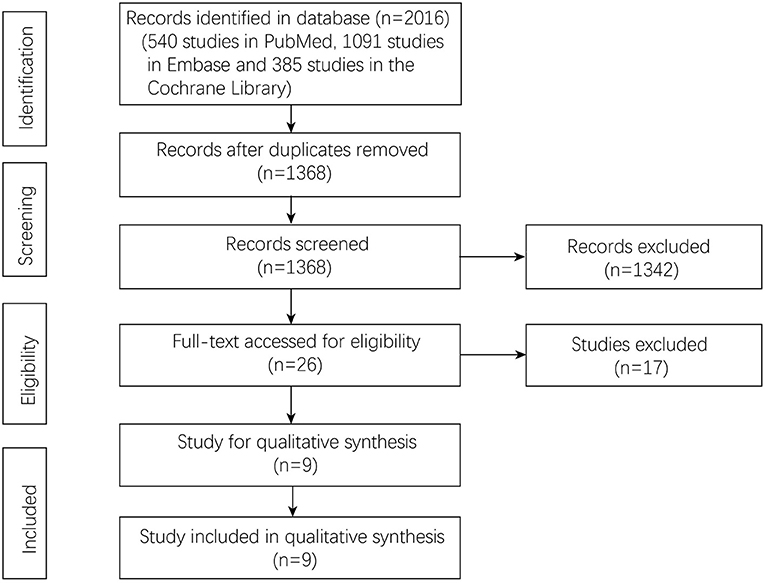
A total of 2,016 studies were identified in the three databases, 540 studies in Pubmed, 1,091 studies in Embase, and 385 studies in the Cochrane Library. After removing duplicate studies, 1,368 studies were left for initial screening. After that, 26 studies were accessed with full text for potential eligibility. In total, nine studies were included in this meta-analysis, and the flow chart is shown in Figure 1.
Patient Characteristics
Nine studies (13–16, 22–26) with a total of 6,541 patients were included in this meta-analysis. Five studies were from Japan, and four studies were from China. The year of publication was from 2001 to 2021, and all the studies were retrospective. The definition of CKD and non-CKD was according to eGFR, dialysis, and creatinine. Newcastle-Ottawa Scale (NOS) scores were accessed in each study and are shown in Table 1.
Baseline Information
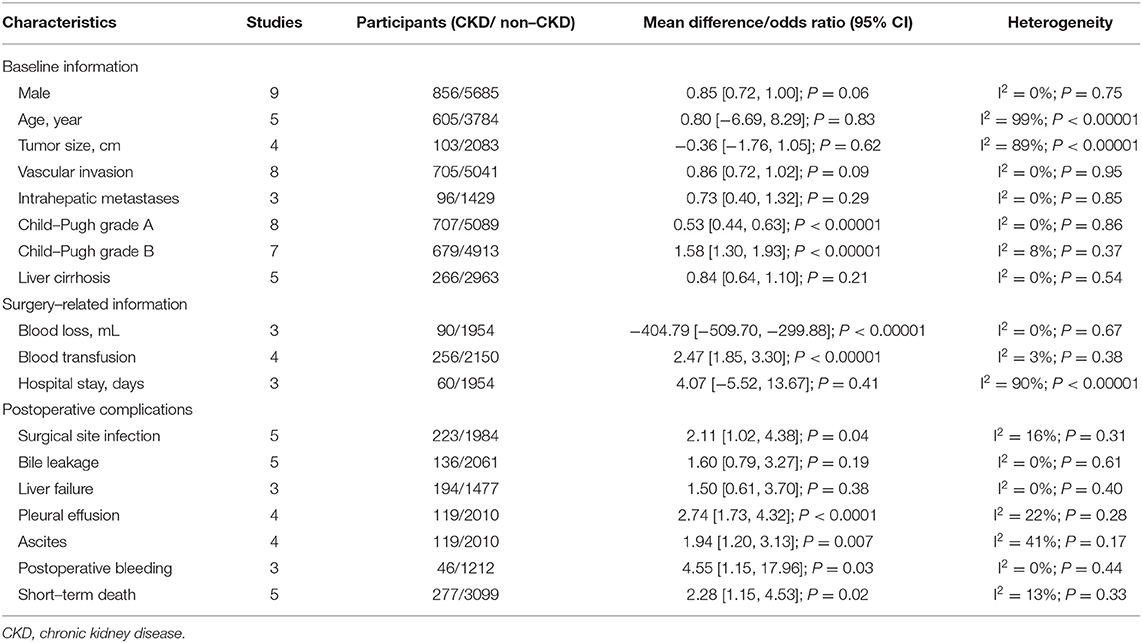
The baseline information included sex, age, tumor size, vascular invasion, intrahepatic metastases, liver cirrhosis and Child-Pugh classification, and was compared between the CKD group and the non-CKD group. After pooling all the data, the CKD group had a higher rate of Child-Pugh grade B than the non-CKD group (OR = 1.58, 95% CI = 1.3 to 1.93, P < 0.00001) (Table 2).
Surgery-Related Information
The surgery-related information included blood loss, blood transfusion, and hospital stay. After pooling all the data, the CKD group had larger blood loss (MD = −404.79, 95% CI = −509.7 to −299.88, P < 0.00001) and a higher rate of blood transfusion (OR = 2.47, 95% CI = 1.85 to 3.3, P < 0.00001). However, no significant difference was found between the CKD group and the non-CKD group (MD = 4.07, 95% CI = −5.52 to 13.67, P = 0.41).
Postoperative Complications
Seven studies reported postoperative complications, and after pooling the data, the CKD group had a higher rate of overall complications (OR = 2.1, 95% CI = 1.57 to 2.81, P < 0.00001) (Figure 2A). In terms of ≥ grade III complications, the CKD group had a higher rate of ≥ grade III complications as well (OR = 2.04, 95% CI = 1.57 to 2.81, P = 0.0002) (Figure 2B).
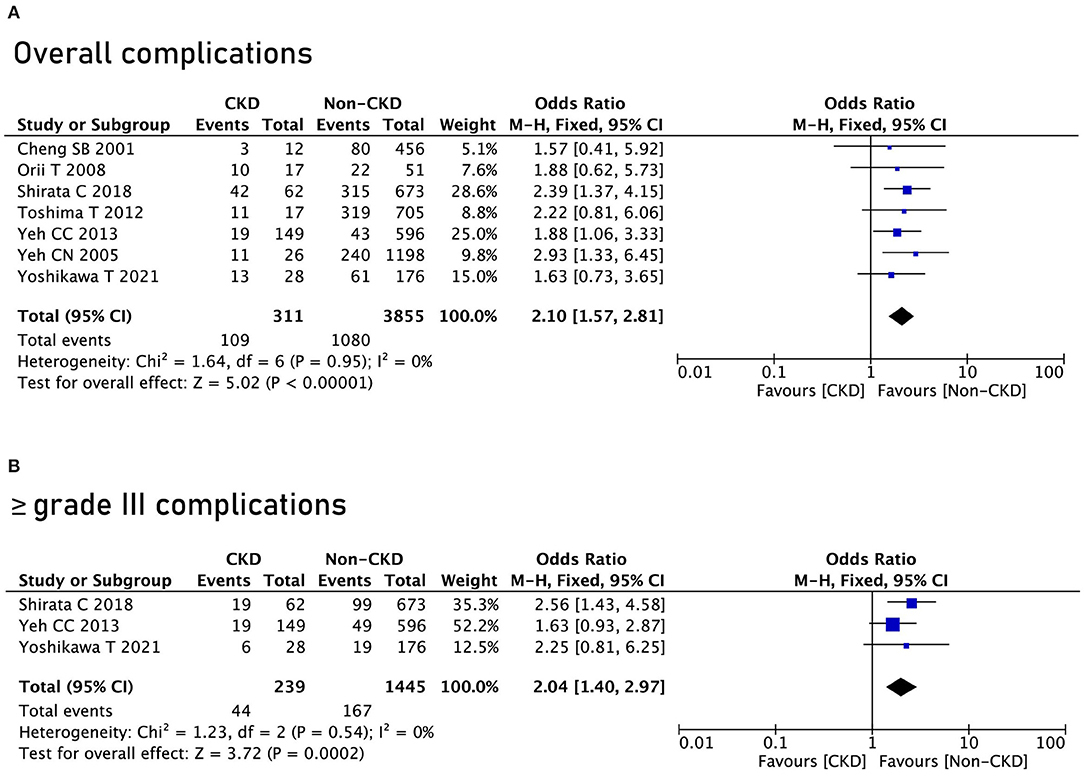
Figure 2. Complications between the chronic kidney disease (CKD) group and the non-CKD group. (A) Overall complications; (B) ≥ grade III complications.
As for specific complications, the CKD group had a higher rate of surgical site infection (OR = 2.04, 95% CI = 1.57 to 2.81, P = 0.0002), pleural effusion (OR = 2.04, 95% CI = 1.57 to 2.81, P = 0.0002), ascites (OR = 2.04, 95% CI = 1.57 to 2.81, P = 0.0002), postoperative bleeding (OR = 2.04, 95% CI = 1.57 to 2.81, P = 0.0002), and short-term death (OR = 2.04, 95% CI = 1.57 to 2.81, P = 0.0002) (Table 2).
Overall Survival
All the nine studies reported OS between the CKD group and the non-CKD group, and the CKD group had poor OS compared with the non-CKD group (HR = 1.28, 95% CI = 1.1 to 1.49, P = 0.001) (Figure 3A). However, in terms of DFS, no significant difference was found between the CKD group and the non-CKD group (HR = 1.11, 95% CI = 0.96 to 1.28, P = 0.16) (Figure 3B).
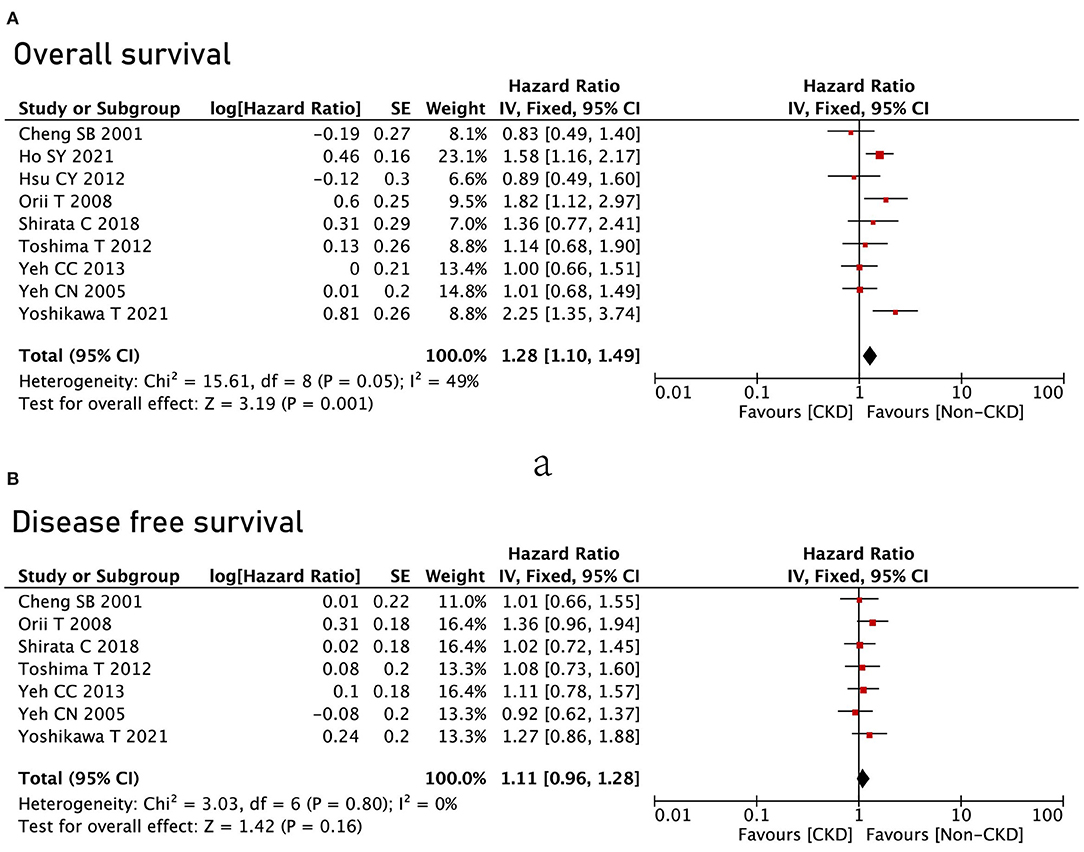
Figure 3. Survival analysis between the CKD group and the non-CKD group. (A) Overall survival; (B) disease-free survival.
Sensitivity Analysis and Publication Bias
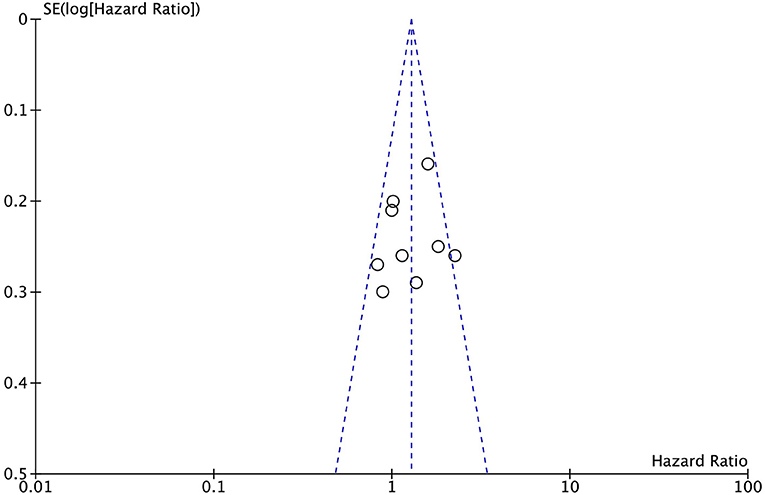
Sensitive analysis was conducted by excluding one study in turns, and the results remained the same after excluding one study in turns. Publication bias was determined according to a funnel plot; the funnel plot was symmetrical visually (Figure 4).
Discussion
A total of nine studies with 6,541 patients were included in this meta-analysis. After pooling all the baseline information, the CKD group had a higher rate of Child-Pugh grade B than the non-CKD group. As for the surgery-related information, the CKD group had larger blood loss and a higher rate of blood transfusion. In terms of complications, the CKD group had a higher rate of overall complications and a higher rate of ≥ grade III complications. The CKD group had poor OS compared with the non-CKD group; however, in terms of DFS, no significant difference was found.
As previously described, CKD has a certain impact on a variety of operations, including gastric cancer surgery (27), colorectal cancer surgery (28) and bladder cancer surgery (29). CKD could result in increased complications and reduced OS. However, in terms of liver resection for HCC, it remains controversial whether preexisting CKD has an impact on the complications and prognosis (13–16). Therefore, it is necessary for surgeons to explore whether there is a precise effect of CKD on liver resection for HCC.
In this meta-analysis, we found that postoperative complications were higher in the CKD group than in the non-CKD group. As for specific complications, we found that patients with CKD required more blood transfusions, and that the proportion of postoperative ascites was increased. The possible reason is that patients with CKD often suffer from other serious comorbidities, including diabetes and cardiovascular disease, which might inhibit wound repair and result in complications (15). In addition, because of nutritional deficiencies, loss of serum immune system components, and lymphocyte suppression, patients with CKD are in an immunosuppressive state; therefore, they might be more likely to suffer from postoperative infections and even death (30, 31).
There are many factors that could affect the prognosis after liver resection for HCC including presence of vascular penetration, satellite nodules, tumor differentiation, hepatitis, cirrhosis, and tumor staging (32, 33). In this meta-analysis, the CKD group had poor OS compared with the non-CKD group; however, in terms of DFS, no significant difference was found. The main reason was related to cardiovascular diseases. Patients with CKD had a higher risk of vascular disease and risk of events such as heart failure, myocardial infarction, stroke, atrial fibrillation, and peripheral arterial, after surgery (14, 15). Theses cardiovascular diseases might decrease OS. However, as for DFS, the main cause was the tumor itself; therefore, no significant difference was found.
Therefore, when patients with concurrent CKD and HCC are admitted to a hospital, examination of the progression of CKD is necessary. In addition, biological parameters including the immune system components and lymphocyte examination must be tested. For surgeons, it is important to reduce surgical time and give more attention to perioperative management.
There were some important data that were insufficient for meta-analysis and were presented in the included studies. Shirata et al. (24) reported that the CKD group had no effect on patients with Child-Pugh A liver function compared to the non-CKD group, and that poor OS was identified in patients with concurrent CKD and Child-Pugh B liver function. Hsu et al. (13) reported that CKD could result in a poor outcome of patients who underwent TACE. Another study did propensity score matching (PSM) and found that the CKD group had poor prognosis, (23) the baseline information had no significant difference after the PSM, and more studies with the PSM method were needed in the future.
There were some limitations that existed in this meta-analysis. First, only nine retrospective studies were included in this study, which was relatively small. Second, countries of the included studies were restricted to China and Japan; therefore, the results could only be applied to eastern Asians. Third, the definition of CKD group and non-CKD group was different, which might result in heterogeneity. Fourth, the included studies ranged from 20 years ago to now. Improvements in primary postoperative care of the patients during these two decades might have reduced mortality and morbidity; thus, bias might occur. Therefore, multicenter, high-quality randomized controlled trials should be performed in the future.
In conclusion, preexisting CKD was associated with higher ratio of complications and poor OS. However, no difference was found in terms of DFS.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
X-YL and Z-QZ: data extraction. C-YW: quality assessments. C-YW and X-YL: data analysis and writing (original draft). C-YW, X-YL, Z-QZ, Y-XC, WT, BZ, and CY: writing (review and editing). All authors read and approved the final version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We acknowledge all the authors whose publications are referenced in this article.
References
1. Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR, et al. Global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. (2019) 16:589–604. doi: 10.1038/s41575-019-0186-y
2. Mak LY, Cruz-Ramón V, Chinchilla-López P, Torres HA, LoConte NK, Rice JP, et al. Global epidemiology, prevention, and management of hepatocellular carcinoma. Am Soc Clin Oncol Educ Book. (2018) 38:262–79. doi: 10.1200/EDBK_200939
3. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
4. Gryziak M, Wozniak K, Kraj L, Stec R. Milestones in the treatment of hepatocellular carcinoma: A systematic review. Crit Rev Oncol Hematol. (2021) 157:103179. doi: 10.1016/j.critrevonc.2020.103179
5. Clark T, Maximin S, Meier J, Pokharel S, Bhargava P. Hepatocellular carcinoma: review of epidemiology, screening, imaging diagnosis, response assessment, and treatment. Curr Probl Diagn Radiol. (2015) 44:479–86. doi: 10.1067/j.cpradiol.2015.04.004
6. Dimitrijevic Z, Paunovic G, Tasic D, Mitic B, Basic D. Risk factors for urosepsis in chronic kidney disease patients with urinary tract infections. Sci Rep. (2021) 11:14414. doi: 10.1038/s41598-021-93912-3
7. Lv JC, Zhang LX. Prevalence and disease burden of chronic kidney disease. Adv Exp Med Biol. (2019) 1165:3–15. doi: 10.1007/978-981-13-8871-2_1
8. GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. (2020) 395:709–33. doi: 10.1016/S0140-6736(20)30045-3
9. Shajahan S, Amin J, Phillips JK, Hildreth CM. Relationship between sex and cardiovascular mortality in chronic kidney disease: A systematic review and meta-analysis. PLoS ONE. (2021) 16:e0254554. doi: 10.1371/journal.pone.0254554
10. Di Lullo L, House A, Gorini A, Santoboni A, Russo D, Ronco C. Chronic kidney disease and cardiovascular complications. Heart Fail Rev. (2015) 20:259–72. doi: 10.1007/s10741-014-9460-9
11. Cheng YX, Tao W, Liu XY, Zhang H, Yuan C, Zhang B, et al. Does chronic kidney disease affect the surgical outcome and prognosis of patients with gastric cancer? A meta-analysis. Nutr Cancer. (2021) 25:1–8. doi: 10.1080/01635581.2021.1993277
12. Hu WH, Cajas-Monson LC, Eisenstein S, Parry L, Ramamoorthy S. Association of dialysis with adverse postoperative outcomes in colorectal cancer-an analysis of ACS-NSQIP. Int J Colorectal Dis. (2015) 30:1557–62. doi: 10.1007/s00384-015-2347-y
13. Hsu CY, Hsia CY, Huang YH, Su CW, Lin HC, Chiou YY, et al. Differential prognostic impact of renal insufficiency on patients with hepatocellular carcinoma: a propensity score analysis and staging strategy. J Gastroenterol Hepatol. (2012) 27:690–9. doi: 10.1111/j.1440-1746.2011.06886.x
14. Toshima T, Shirabe K, Yoshiya S, Muto J, Ikegami T, Yoshizumi T, et al. Outcome of hepatectomy for hepatocellular carcinoma in patients with renal dysfunction. HPB (Oxford). (2012) 14:317–24. doi: 10.1111/j.1477-2574.2012.00452.x
15. Yoshikawa T, Nomi T, Hokuto D, Kamitani N, Matsuo Y, Sho M. Outcomes in patients with chronic kidney disease after liver resection for hepatocellular carcinoma. World J Surg. (2021) 45:598–606. doi: 10.1007/s00268-020-05829-z
16. Orii T, Takayama T, Haga I, Fukumori T, Amada N. Efficacy of a liver resection for hepatocellular carcinoma in patients with chronic renal failure. Surg Today. (2008) 38:329–34. doi: 10.1007/s00595-007-3634-1
17. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
18. Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. (1998) 17:2815–34. doi: 10.1002/(SICI)1097-0258(19981230)17:24<2815::AID-SIM110>3.0.CO;2-8
19. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. (2007) 8:16. doi: 10.1186/1745-6215-8-16
20. Ioannidis JP. Interpretation of tests of heterogeneity and bias in meta-analysis. J Eval Clin Pract. (2008) 14:951–7. 00986. x. doi: 10.1111/j.1365-2753.2008.00986.x
21. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 327:557–60. doi: 10.1136/bmj.327.7414.557
22. Cheng SB, Wu CC, Shu KH, Ho WL, Chen JT, Yeh DC, et al. Liver resection for hepatocellular carcinoma in patients with end-stage renal failure. J Surg Oncol. (2001) 78:241–6. doi: 10.1002/jso.1160
23. Ho SY, Liu PH, Hsu CY, Ko CC, Huang YH, Su CW, et al. ALBI grade in dialysis patients with hepatocellular carcinoma: prognostic impact and staging strategy. J Gastrointest Oncol. (2021) 12:722–34. doi: 10.21037/jgo-20-332
24. Shirata C, Hasegawa K, Kokudo T, Yamashita S, Yamamoto S, Arita J, et al. Liver resection for hepatocellular carcinoma in patients with renal dysfunction. World J Surg. (2018) 42:4054–62. doi: 10.1007/s00268-018-4698-3
25. Yeh CC, Lin JT, Jeng LB, Charalampos I, Chen TT, Lee TY, et al. Hepatic resection for hepatocellular carcinoma patients on hemodialysis for uremia: a nationwide cohort study. World J Surg. (2013) 37:2402–9. doi: 10.1007/s00268-013-2137-z
26. Yeh CN, Lee WC, Chen MF. Hepatic resection for hepatocellular carcinoma in end-stage renal disease patients: two decades of experience at Chang Gung Memorial Hospital. World J Gastroenterol. (2005) 11:2067–71. doi: 10.3748/wjg.v11.i14.2067
27. Oh HJ, Lee HA, Moon CM Ryu DR. Incidence risk of various types of digestive cancers in patients with pre-dialytic chronic kidney disease: a nationwide population-based cohort study. PLoS ONE. (2018) 13:e0207756. doi: 10.1371/journal.pone.0207756
28. Wu MY, Chang TC, Chao TY, Huang MT, Lin HW. Risk of colorectal cancer in chronic kidney disease: a matched cohort study based on administrative data. Ann Surg Oncol. (2013) 20:3885–91. doi: 10.1245/s10434-013-3065-8
29. Matsumoto A, Nakagawa T, Kanatani A, Ikeda M, Kawai T, Miyakawa J, et al. Preoperative chronic kidney disease is predictive of oncological outcome of radical cystectomy for bladder cancer. World J Urol. (2018) 36:249–56. doi: 10.1007/s00345-017-2141-2
30. James MT, Hemmelgarn BR, Wiebe N, Pannu N, Manns BJ, Klarenbach SW, et al. Glomerular filtration rate, proteinuria, and the incidence and consequences of acute kidney injury: a cohort study. Lancet. (2010) 376:2096–103. doi: 10.1016/S0140-6736(10)61271-8
31. Canedo J, Ricciardi K, DaSilva G, Rosen L, Weiss EG, Wexner SD. Are postoperative complications more common following colon and rectal surgery in patients with chronic kidney disease? Colorectal Dis. (2013) 15:85–90. 03099. x. doi: 10.1111/j.1463-1318.2012.03099.x
32. Wang XH, Liu QB, Xiang CL, Mao XH, Yang B, Li Q, et al. Multi-institutional validation of novel models for predicting the prognosis of patients with huge hepatocellular carcinoma. Int J Cancer. (2021) 149:127–38. doi: 10.1002/ijc.33516
Keywords: chronic kidney disease, hepatocellular carcinoma, overall survival, complications, meta-analysis
Citation: Liu X-Y, Zhao Z-Q, Cheng Y-X, Tao W, Yuan C, Zhang B and Wang C-Y (2022) Does Chronic Kidney Disease Really Affect the Complications and Prognosis After Liver Resection for Hepatocellular Carcinoma? A Meta-Analysis. Front. Surg. 9:870946. doi: 10.3389/fsurg.2022.870946
Received: 07 February 2022; Accepted: 10 March 2022;
Published: 06 April 2022.
Edited by:
Mattia Garancini, San Gerardo Hospital, ItalyReviewed by:
Farzad Kakaee, Tabriz University of Medical Sciences, IranChai Hong Rim, Korea University, South Korea
Copyright © 2022 Liu, Zhao, Cheng, Tao, Yuan, Zhang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chun-Yi Wang, 1962989853@qq.com
†These authors have contributed equally to this work
 Xiao-Yu Liu
Xiao-Yu Liu Zhi-Qiang Zhao2†
Zhi-Qiang Zhao2†  Yu-Xi Cheng
Yu-Xi Cheng Wei Tao
Wei Tao Chao Yuan
Chao Yuan Bin Zhang
Bin Zhang