- 1Institute for Systems Genomics, University of Connecticut, Storrs, CT, United States
- 2School of Nursing, University of Connecticut, Storrs, CT, United States
- 3Department of Statistics, University of Connecticut, Storrs, CT, United States
- 4Division of Neonatology, Connecticut Children’s Medical Center, Hartford, CT, United States
- 5Department of Pediatrics, School of Medicine, University of Connecticut, Farmington, CT, United States
- 6Yale University School of Nursing, Orange, CT, United States
Introduction: Black African American (B/AA) women have a 2-fold to 3-fold elevated risk compared with non-Hispanic White (W) women for preterm birth. Further, preterm birth is the leading cause of mortality among B/AA infants, and among survivors, preterm infant adverse health outcomes occur disproportionately in B/AA infants. Racial inequities in maternal and infant health continue to pose a public health crisis despite the discovery >100 years ago. The purpose of this study was to expand on reported preterm infant outcome disparities. A life-course approach, accumulation of lifelong stress, including discrimination, may explain social factors causing preterm birth rate and outcome inequities in B/AA mothers.
Methods: Anthropometric measures and clinical treatment information for 197 consented participants were milled from electronic health records across 4 years. The Neonatal Infant Stressor Scale was used to tally acute and chronic painful/stressful procedures. Neurobehavioral differences were investigated using the Neonatal Intensive Care Unit (NICU) Network Neurobehavioral Scale.
Results: B/AA mothers gave birth to preterm infants earlier than W mothers. NICU hospitalization stays were extended more than 2 weeks for the significantly smaller B/AA preterm infants in comparison to the age-matched W preterm infants. A higher number of chronic lifesaving procedures with demonstrated altered stress response patterns were recorded for B/AA preterm infants.
Discussion: This cross-sectional analysis of preterm birth rates and preterm infant developmental and neurodevelopmental outcomes are presented in the context of NICU stress and pain, with attendant implications for infant mortality and future health disparities. Preterm birth rate and outcome inequities further support the need to develop interventions and policies that will reduce the impact of discrimination and improve social determinants of health for Black, Indigenous, and other People of Color.
Introduction
Preterm births are a significant healthcare concern worldwide, with an estimated 15 million infants born preterm (<37 weeks’ gestational age [GA]) globally each year.1 Preterm births also represent the second most common cause of infant mortality in the US population overall (1). Unfortunately, national statistical systems reports show an increased incidence of preterm births among live births from 9.8 to 10.5% between 2011 to 2021.2 There is further evidence among data for stark racial disparities in preterm birth risks and infant health outcomes.3 The preterm birth rate for Black/African Americans (B/AA) in the United States is 52% higher than that of all other races.4 Preterm birth is the leading cause of infant mortality among B/AA (2) and for the preterm infants surviving infant mortality, reaching their first birthday, there is a high risk for infections, developmental difficulties, breathing problems, and lifelong health complications. Social determinants of health, including neighborhood environmental exposures and built environment, experiences of discrimination and limited access to quality health care can explain many pregnancy disparities (3). However, although the first report of racial differences in pregnancy outcomes was made 125 years ago (4) the outreach and funding priorities of health organizations remain strategies to reduce preterm births as a public health goal.5 At the same time, researchers continue to seek answers to explain disparity determinants.
Being born preterm is a perturbation in development; any additional disturbance is deemed a stress event. Preterm infants are hospitalized in neonatal intensive care units (NICU) for many reasons and often extensive periods, undergoing a mean of 17.3 painful procedures in the first 2 weeks of life (5). Pain and stress are inflicted on infants simply as part of routine, lifesaving care (6). Infants in the NICU, especially the extremely preterm (<28 weeks’ GA), the very pre-term (28–32 weeks’ GA), are likely to receive invasive interventions with higher pain intensity (e.g., gastric tube insertion and blood collection), however repositioning, diaper changes and bathing, while less invasive, are still stressful (7). Preterm infants have a heightened sensitivity to tactile and nociceptive (sensory) neuronal input making it challenging to distinguish between noxious and non-noxious stimuli (8). The number and duration of repeated stress/pain-inducing events harm the preterm infants’ rapidly developing central nervous and neuroendocrine systems, (9, 10) with associated long-term pathological outcomes recognized as poor neurodevelopment, autonomic nervous system dysfunction, and psychiatric or behavioral disorders (11).
Communication with infants about perceived pain is not realizable given crying may also indicate other conditions (hunger, tiredness), and facial expression interpretation is highly subjective. Behavioral, physiological, and biochemical markers are thus adopted as indicators of pain. Biochemically, stressful/painful procedures are accompanied by increased cortisol levels. An infant’s cortisol levels are higher during the early days in the NICU (12) when an infant is still adapting to acute stressors, a period called allostasis (13). Tracking physiological exposures to pain and stress is collected on the Neonatal Infant Stressor Scale (14). Acute pain procedures resolve relatively quickly, including, for example, peripheral venous catheter placement, blood collection via heel stick, or endotracheal tube placement. Longer-term procedures such as mechanical ventilation, supplemental oxygen administration, or central catheter placement are tallied as chronic pain procedures. Early detection of neurobehavioral delays may be identified by the use of standardized assessments such as the NICU Network Neurobehavioral Scale (NNNS) (15).
B/AA preterm infants are born at an earlier gestational age than preterm infants of other races; as such, B/AA preterm infants are likely exposed to more painful/invasive procedures in the NICU (16, 17). We have also reported B/AA preterm infant outcome disparities in gestational age at birth (p < 0.005) and in birth weight, length, and head circumference (p < 0.0001). Growing evidence suggests that life-course epidemiology, including racism-related stress over time, may contribute to health inequities in preterm birth rate and infant mortality (18–22). To expand the data and knowledge base for preterm infant studies, we conducted a cross-sectional study of the developmental and neurodevelopmental outcomes of B/AA preterm infants resulting from stressful and painful procedures in the NICU. Stressful/painful procedures are quantified using the Neonatal Infant Stressor Scale, categorizing procedures as “acute” or “chronic” (14). Examples of highly stressful/painful acute procedures include surgery, insertion of umbilical arterial or venous central catheters, multiple intravenous catheter placement and eye exams for retinopathy of prematurity. Examples of highly stressful/painful chronic procedures include chest tube in situ, conventional ventilation in situ, nasopharyngeal suctioning, replogle irrigation and in situ and systemic infections such as sepsis, meningitis or pneumonia (14). We proposed that given earlier gestational ages, and lower birthweights of B/AA preterm infants, chronic painful procedures would outnumber acute pain/stress events. Significantly higher incidences of diabetes, hypertension, coronary artery disease, and stroke have been explained by race in B/AA adults (23). However, improvement in neonatal trauma-informed care necessitates steps toward understanding the impact of disparities to reduce subsequent prolonged adverse health outcomes for preterm infants.
Methods
Participants
This human participant research study was reviewed and approved by Connecticut Children’s Medical Center Institutional Review Board (IRB #16–001). The inclusion criteria were infants with GA at ≤32 weeks or birth weight less than 1,500 grams, and delivery during 2016–2020. Premature infants were cared for at either the urban (Hartford) or suburban (Farmington) locations of Connecticut Children’s Medical Center NICU departments. Exclusion criteria included major brain lesions (intraventricular hemorrhage >Grade 2, periventricular leukomalacia), neurosensorial deficits (retinopathy of prematurity >Stage 2), and a clinical history of genetic syndromes and/or major malformations. Informed consent was provided verbally in English or Spanish. Parents/legal guardians provided written informed consent on behalf of the infant to participate in the study. Deidentified electronic health record data, including demographic and clinical course of treatment, were entered into a password-secured REDCap database.
Measurements and assessments
The Neonatal Infant Stressor Scale (NISS) is the standardized instrument (14, 16) for tabulating the number and types of distress events an infant is exposed to during a NICU stay. The number of marked acute stressor columns out of 44 possible acute stressors is added to the marked columns out of 24 chronic stressors (Supplementary Table 1) for a cumulative stress score daily.
Before the creation of the NICU Network Neurobehavioral Scale (NNNS) (24), Pretchel and Beintema (25) posited that for valid interpretation of the standardized NISS measure, observational and clinical data must be collected during specific physical and behavioral infant conditions or “states”. The NISS (25) and, later, a neonatal intensive care unit-specific measure, the NICU Network Neurobehavioral Scale (24), defined infants attending and orienting themselves and producing organized motor acts as neurodevelopmentally proficient (24). As neurological scales evolved, additional criteria were added, e.g., orientation, habituation, tone, and reflexes (26). Practitioners and nurses could evaluate the items on the neurological scales to gauge an infant’s neurological status, guided by the fact that an intact brain can organize states. In contrast, an impaired brain cannot organize these states (25). Consolidation of neurodevelopment criteria was the impetus for developing the NICU Network Neurobehavioral Scale (NNNS) (27) The NNNS is a standardized neurobehavioral assessment with good psychometric properties (internal and concurrent validity) used in the NICU for early detection of neurobehavioral challenges in at-risk populations (15, 28). The assessment records 13 states: habituation, attention, arousal, regulation, handling procedures, quality of movement, excitability, lethargy, nonoptimal reflexes, asymmetric reflexes, hypertonicity, hypotonicity, and stress/abstinence (28). Measurements for the NNNS are plotted against the theoretical scale to identify tendency and percentile values (29). The NNNS was utilized on inpatient preterm infants observed by a trained assessor at 36–37 weeks postmenstrual age. Postmenstrual age considers the baby’s gestational age at birth plus the postnatal age (days of life) (30).
Statistical analyses
Descriptive statistics were calculated for quantitative variables, including mean, median, and interquartile range. Qualitative variables used to describe characteristics were compared using frequency calculations. A Wilcoxon test/t-test for numerical variables and a Pearson chi-square test/Fisher exact test for categorical variables were performed to assess differences between groups. Data was cleaned, processed, and analyzed using R (version 4.2.0) (31). Statistical calculations were considered significant at p = 0.05.
Results
A limited number of consented patients were of varying races, as identified by parents or legal guardians and abstracted from the electronic health record. The number of B/AA infants in each of the GA categories (<28 weeks, 28–32 weeks, >32 weeks) was statistically significantly different than the numbers of W infants in the same respective GA categories (p = 0.016). A Fisher’s exact test for count data indicted that 31 B/AA infants were born extremely preterm, 19 were born very preterm and 1 was born in the preterm GA category. The numbers for W infants were 55, 86, and 5, respectively. Black/African American non-Hispanic (hereto referred to as B/AA) or White non-Hispanic (now referred to as W) preterm infants. B/AA mothers gave birth to preterm infants earlier than W mothers, with a median GA at birth of 27.2 weeks and 28.6 weeks, respectively, (p < 0.001) (Figure 1). B/AA preterm infants were born smaller with lower birthweight, birth length and head circumference (Table 1). The demographic data revealed a disparity in the length of hospital stay by race (p = 0.0289) (Table 1).
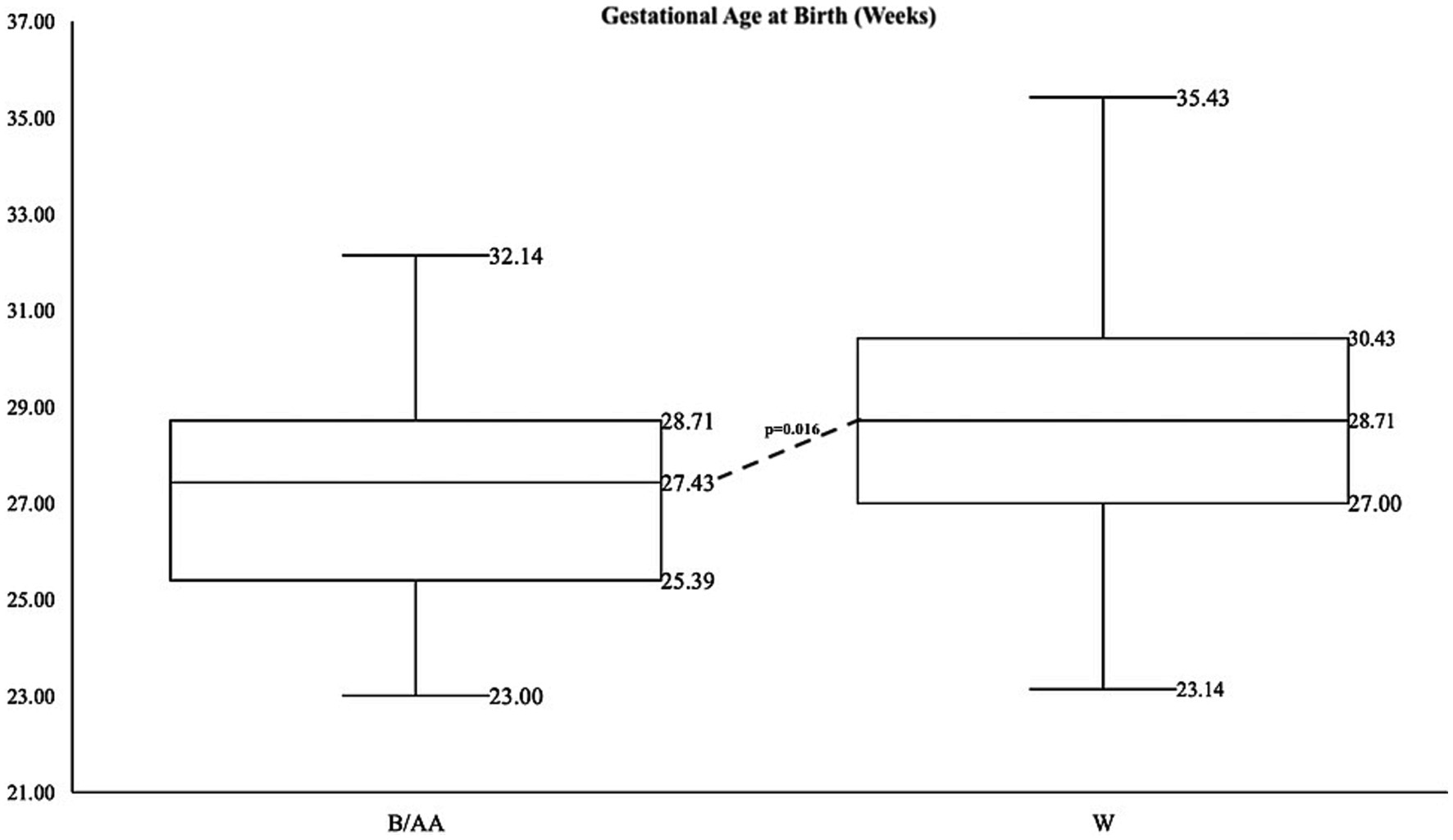
Figure 1. Summary statistics for gestational age by race with data points on box whisker plots from lowest value (minimum), first quartile, median, third quartile, and maximum value for B/AA infants on the left and W infants on the right.
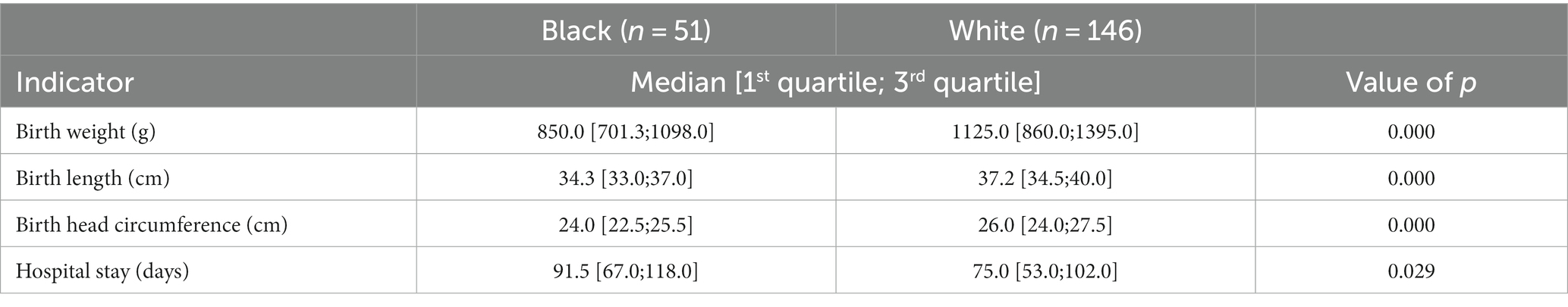
Table 1. B/AA preterm infants are smaller and have extended hospital stays versus W preterm infants.
Postnatal ages and numbers and types of acute and chronic painful/stressful procedures (Supplementary Table 1) were retrieved from patient medical records for significance calculations between B/AA and W infants. The number of acute painful/stressful procedures recorded on the Neonatal Infant Stressor Scale (NISS) over post-natal age (PNA) by day during preterm infant stays in the NICU produces a trendline evident in Figure 2A, that although not statistically significant, shows B/AA preterm infants experienced more acute pain experiences over time. A higher number of chronic painful/stressful procedures over PNA by day, with statistical significance, was identified for B/AA infants (Figure 2B). There was no statistically significant variation for 12 of the 13 NNNS scales evaluated across the B/AA and W infant populations. A statistically significant difference was identified for the NNNS scale measurement for handling between the B/AA and W infant populations (p = 0.036) (Supplementary Figure 1).
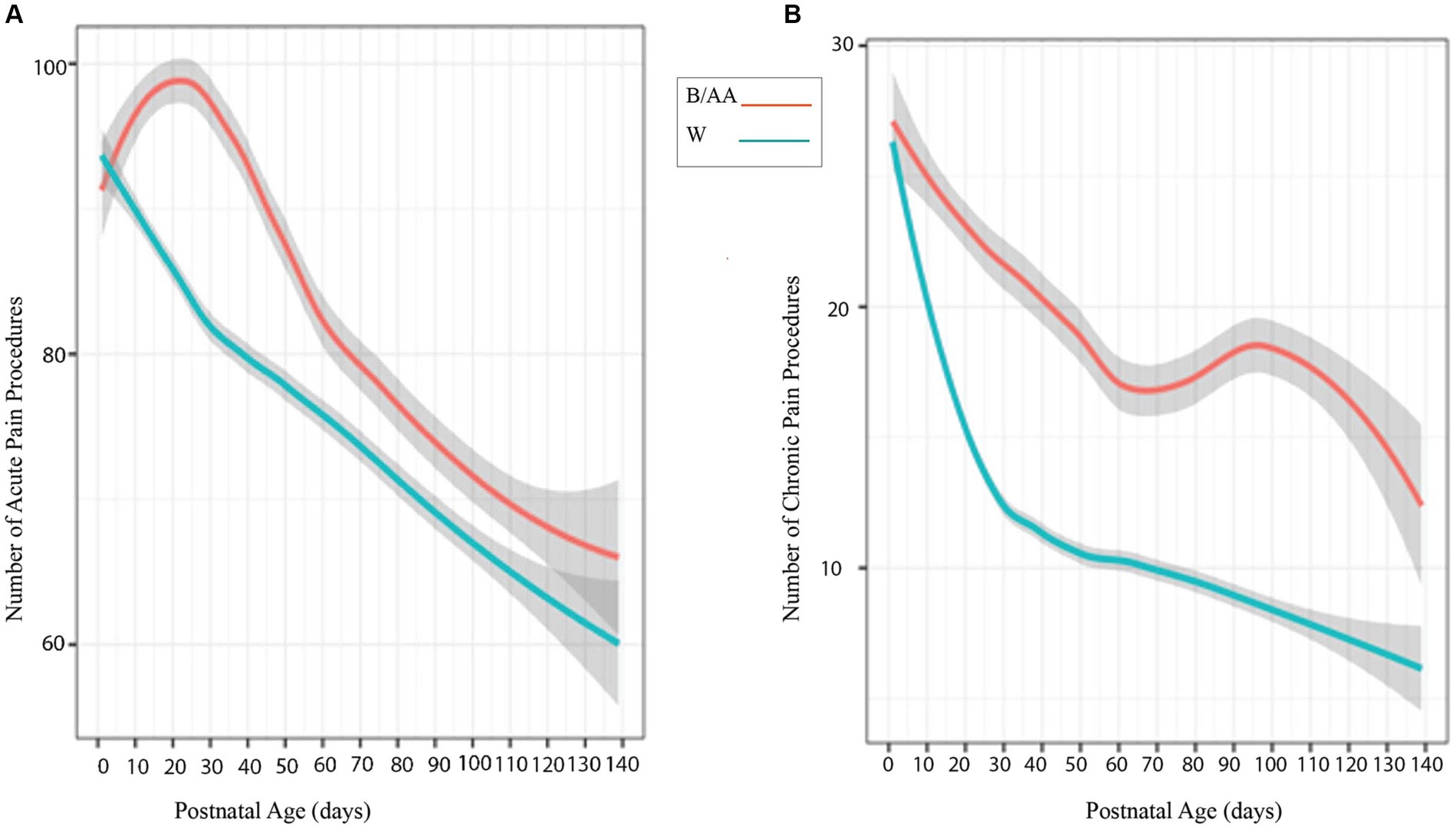
Figure 2. (A) Looking at mean over time, the number of Neonatal Infant Stressors for B/AA preterm infants (pink/top line) and W preterm infants (blue/bottom line) over postnatal age by day during the NICU stay. B/AA preterm infants experienced more acute pain procedures (n.s.) and chronic painful/stressful procedures (p = 0.01) than the W preterm infants (B).
Discussion
Preterm birth rates and infant mortality statistics document evidence of continued racial disparities, making preterm birth a significant global health concern. Evidence of inequities resulting from preterm birth include lower gestational age at birth and lower anthropometric measures in B/AA non-Hispanic infants compared to W non-Hispanic. Infants with a lower gestational age at birth will lack maturity in crucial organ systems, including the lungs, brain, and gastrointestinal tract. Very early GA births will thus have a higher likelihood of accompanying medical complications needing interventions for the survivability of the preterm infant. Mean event trend lines of the Neonatal Infant Stressor Scale (NISS) data provide visual confirmation of the proposal that longer NICU stays coincide with a higher number of pain procedures (Figure 2). Separation of the acute stressor trend line (n.s.) and the significant separation (p = 0.01) between the chronic pain trend lines for B/AA preterm infants and W preterm infants (Figure 2) supports the racial disparity in gestational age at birth (Table 1) and correlates with the statistically significant difference in length of hospital stay between the B/AA and W preterm infants.
Painful/stressful procedures trigger the hypothalamic–pituitary–adrenal (HPA) axis (12) to release cortisol with each procedure. However, the concept of allostasis and allostatic load must also be introduced (32). McEwen identified four stages that give rise to allostatic load, (the tipping point where the body is not responding appropriately) and their potential pathological impact. Those stages are (1) Normal allostatic response to a stressor (in this case, release of glucocorticoids) where the response is maintained for an appropriate amount of time and then inactivates; (2) Undergoing repeated “hits” from multiple stressors; (3) Lack of adaption leading to a delay in inactivation; and, (4) Inability to mount an adequate response which leads to compensatory hyperactivity of other mediators (i.e., inadequate secretion of glucocorticoids leading to increased concentration of cytokines typically regulated by glucocorticoids) (6). Therefore, once the body reaches stage four, we expect to see an absence of glucocorticoids. The “nhandle” value of the NNNS measurement may be used to reflect allostatic load, the scenario of an inadequate HPA axis response. Neurotypical infants require ~6 maneuvers to be soothed either by swaddling, rocking, walking, pacifier, or other methods (27). In our study, a median handling value of 0.25 (Supplementary Figure 1) shows that the B/AA infants require very few maneuvers to be soothed.
Although limited by lower participation numbers of B/AA women, our findings support disparities among B/AA preterm infant births and outcomes (3, 17, 33–38). We continue to expand recruiting efforts for a more diverse representation of mothers and infants as our research moves toward clarifying variables that may lead to B/AA pregnancy disparities. Longer NICU stays exacerbate the number and types of painful/stressful procedures for the infant but may also stress the mother-preterm infant relationship. A more extended stay has the potential to strain the mother-preterm infant relationship due to separation and the delicate yet dynamic interplay of oxytocin (the bonding hormone) between mother and infant (39). Maternal mental health may also be harmed when the bonding relationship is disrupted (40). Extended hospital stays with concomitant higher preterm infant care bills impart additional maternal stress. Of the live births in the United States in 2021, 10.5% (383,979) were preterm, with an annual price tag of $25 billion or $656,286 per preterm infant.6 Hospital expenditures for preterm infant care differ depending on gestational age and weight at birth. The average costs from 2008 to 2016 for late preterm births were $76,153; for low birthweight preterm infants, $114,437; and extremely preterm infants at 24 weeks GA were $603,778 (41).
Provision of colostrum and breastmilk is associated with long-term positive health outcomes, including risk reduction for infectious diseases, cancer, autoimmune disorders, high blood pressure, and more. B/AA communities face heightened risks for diabetes, infant obesity, high blood pressure, and ovarian and breast cancer. Maternal racial disparity, in part, also influences breastmilk provision and milk nutrient composition (42, 43). Support for mothers providing breast milk significantly affects the decision to start and maintain breastfeeding in B/AA women (44, 45). Insufficient milk delivery accounts for some sluggish weight gain and differences in anthropometric measures in B/AA preterm infants compared to W preterm infants during hospitalization and hospital discharge (17, 46). Breastmilk support is an area of investigation that may be important in reducing B/AA preterm infant health disparities. Most very preterm or extremely preterm infants cannot produce the sequential processes of swallowing and breathing simultaneously to eat by mouth until closer to term GA. Therefore, nutrition is delivered either via central line catheterization or placement of a naso- or oro-gastric tube, both painful and stressful.
The data presented within adds to the growing field of study about racial health inequities, specifically disparities in preterm birth rates and outcomes (34). Research has moved toward efforts to identify the causative factors of recognized differences. Discerning the equation to explain disparities in preterm birth rates and outcomes for B/AA women requires, at minimum, the inclusion of NICU standards, trauma-informed chronic care practices, and social determinants of health. We propose life-course stress as the overarching theme for disparities in social health determinants. B/AA women disproportionately experience stress with measurable clinical outcomes of increased cardiometabolic syndromes (Figure 3A) and an increased incidence of preterm births (Figure 3B). Preterm infants of B/AA women are born earlier and have accompanying findings of lower birth weight, smaller length, and more extended hospital stays (Figure 3B).
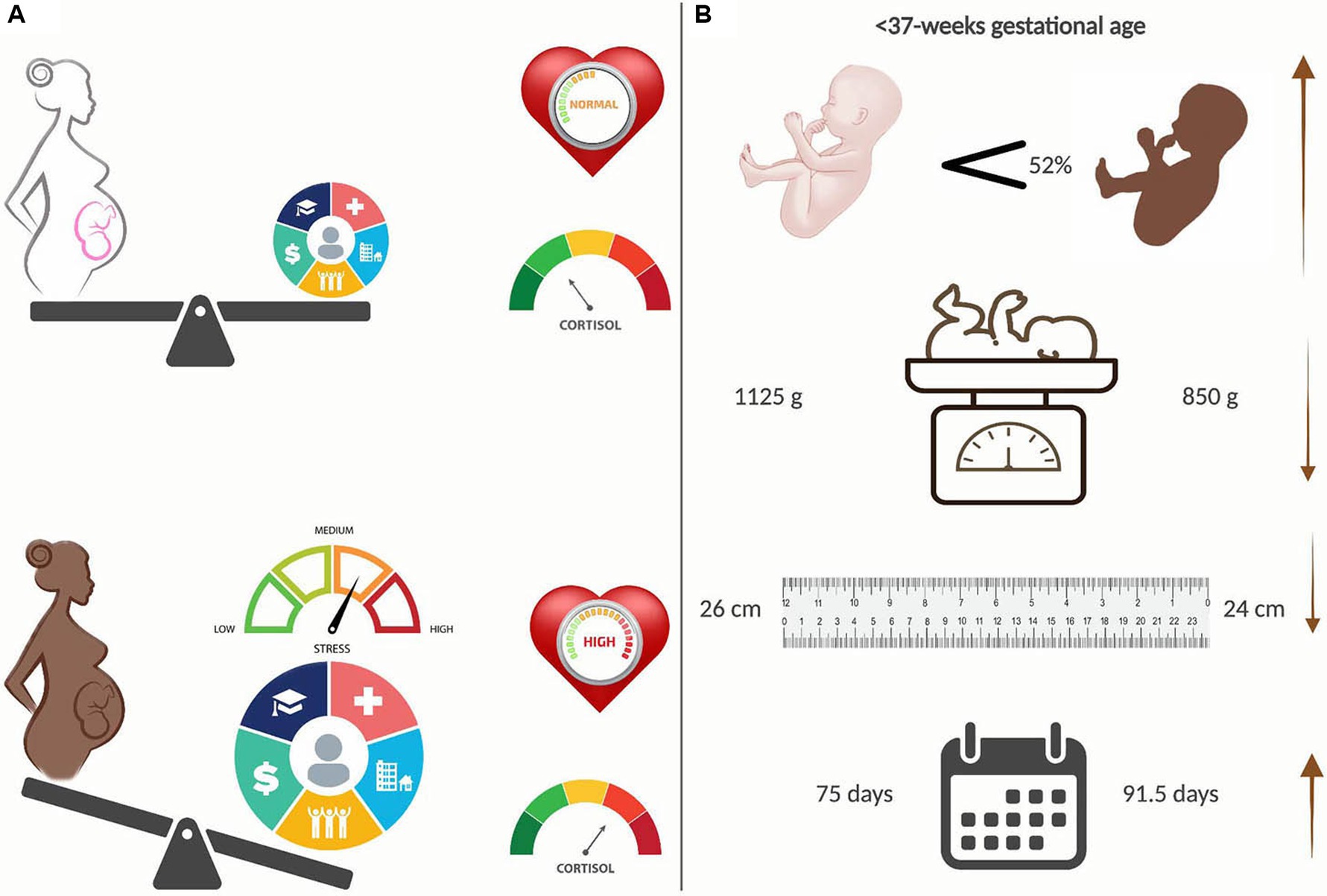
Figure 3. Racial disparities in preterm births. (A) Black/African American (B/AA) women experience disproportionately heavier stress than their White (W) counterparts, primarily because of Social Determinants of Health (SDOH), leading to increased measures of cardiometabolic syndromes, e.g. blood pressure and cortisol levels. (B) B/AA preterm babies are born 52 percent more often, are significantly smaller and have longer hospital stays than W preterm babies. Created with BioRender.com.
Furthermore, preterm infants with associated delayed development will have increased exposure to more frequent and chronic pain procedures. Improving health equity is crucial to reducing the number of preterm births and their challenging courses of treatment, often resulting in adverse developmental outcomes. While this study highlights significant health inequities in preterm birth rates and outcomes, further research is needed to explore potential interventions to address social determinants of health that contribute to these inequities and to offer recommendations on how healthcare providers and policymakers might improve outcomes for preterm infants.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Connecticut Children’s Medical Center Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
JB: Conceptualization, Writing – original draft, Writing – review & editing, Visualization. XCh: Writing – review & editing, Formal analysis. AM: Data curation, Writing – review & editing. SL: Writing – review & editing, Data curation. M-HC: Supervision, Writing – review & editing, Methodology, Resources, Software. XCo: Data curation, Supervision, Writing – review & editing. SC: Conceptualization, Investigation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Research reported in this publication was supported by the National Institute of Nursing Research of the National Institutes of Health (NIH) under Award No. 1F32NR018591 (SC) and Award No. 5R01NR016928 (XC). The content is solely the authors’ responsibility and does not necessarily represent the official views of the NIH. The University of Connecticut Institute for Collaboration on Health, Intervention and Policy also provided support (SC).
Acknowledgments
Thank you to Dr. Chen and his graduate statistics students Hongfei Li, Md. Tuhan Sheikh and Joochul Lee for data analysis support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2023.1275776/full#supplementary-material
Supplementary Figure 1 | NNNS handling scores. Summary statistics for NNNS handling score with data points on box whisker plots from lowest value (minimum), first quartile, median, mean (x), third quartile, and maximum value for B/AA infants on the left and W infants on the right. Dashed line between median scores represents significant difference at p = 0.036.
Footnotes
1. ^WHO. 2022. “Fact Sheet: Preterm Birth.” accessed January 10. https://www.who.int/news-room/fact-sheets/detail/preterm-birth.
2. ^Dimes Mo. 2022 Report Card [July 1, 2023]. Available from: https://www.marchofdimes.org/sites/default/files/2022-11/2022-MarchofDimes-ReportCard-UnitedStates.pdf.
3. ^National Vital Statistics System - Natality (NVSS-N) 2021. Available from: https://health.gov/healthypeople/objectives-and-data/data-sources-and-methods/data-sources/national-vital-statistics-system-natality-nvss-n.
4. ^Dimes Mo. 2022 Report Card [July 1, 2023]. Available from: https://www.marchofdimes.org/sites/default/files/2022-11/2022-MarchofDimes-ReportCard-UnitedStates.pdf.
5. ^National Vital Statistics System - Natality (NVSS-N) 2021. Available from: https://health.gov/healthypeople/objectives-and-data/data-sources-and-methods/data-sources/national-vital-statistics-system-natality-nvss.
6. ^https://www.marchofdimes.org/peristats/reports/united-states/prematurity-profile
References
2. Ely, D, and Driscoll, A. Infant mortality in the United States, 2017: Data from the period linked birth/infant death file Natl Vital Stat Rep. (2019); 68:1–20.
3. Thoma, ME, Drew, LB, Hirai, AH, Kim, TY, Fenelon, A, and Shenassa, ED. Black-white disparities in preterm birth: geographic, social, and health determinants. Am J Prev Med. (2019) 57:675–86. doi: 10.1016/j.amepre.2019.07.007
4. Hoffman, FL. The race traits and tendenties of the American negro. Publications of the American Economic Association. (1896); 11: 1–329. New York
5. Schmidt, AR, and Ramamoorthy, C. Bronchopulmonary dysplasia. Paediatr Anaesth. (2022) 32:174–80. doi: 10.1111/pan.14365
6. Casavant, S, Cong, X, Fitch, RH, Moore, J, Rosenkrantz, T, and Starkweather, A. Allostatic load and biomarkers of stress in the preterm infant: an integrative review. Biol Res Nurs. (2019) 21:210–23. doi: 10.1177/1099800418824415
7. Gardner, FC, Adkins, CS, Hart, SE, Travagli, RA, and Doheny, KK. Preterm stress behaviors, autonomic indices, and maternal perceptions of infant colic. Adv Neonatal Care. (2018) 18:49–57. doi: 10.1097/ANC.0000000000000451
8. Grunau, RE, Holsti, L, Haley, DW, Oberlander, T, Weinberg, J, Solimano, A, et al. Neonatal procedural pain exposure predicts lower cortisol and behavioral reactivity in preterm infants in the NICU. Pain. (2005) 113:293–300. doi: 10.1016/j.pain.2004.10.020
9. Duerden, EG, Grunau, RE, Guo, T, Foong, J, Pearson, A, Au-Young, S, et al. Early procedural pain is associated with regionally-specific alterations in thalamic development in preterm neonates. J Neurosci. (2018) 38:878–86. doi: 10.1523/JNEUROSCI.0867-17.2017
10. Epel, ES, and Prather, AA. Stress, telomeres, and psychopathology: toward a deeper understanding of a triad of early aging. Annu Rev Clin Psychol. (2018) 14:371–97. doi: 10.1146/annurev-clinpsy-032816-045054
11. Montirosso, R, Provenzi, L, Fumagalli, M, Sirgiovanni, I, Giorda, R, Pozzoli, U, et al. Serotonin transporter gene (SLC6A4) methylation associates with neonatal intensive care unit stay and 3-month-old termperment in preterm infants. Child Dev. (2016) 87:38–48. doi: 10.1111/cdev.12492
12. Brummelte, S, Chau, CMY, Cepeda, IL, Degenhardt, A, Weinberg, J, Synnes, AR, et al. Cortisol levels in former preterm children at school age are predicted by neonatal procedural pain-related stress. Psychoneuroendocrinology. (2015) 51:151–63. doi: 10.1016/j.psyneuen.2014.09.018
13. McEwen, BS. Allostasis and the epigenetics of brain and body health over the life course: the brain on stress. JAMA Psychiatry. (2017) 74:551–2. doi: 10.1001/jamapsychiatry.2017.0270
14. Newnham, C, Inder, T, and Milgrom, J. Measuring preterm cumulative stressors within the NICU: the neonatal infant stressor scale. Early Hum Dev. (2009) 85:549–55. doi: 10.1016/j.earlhumdev.2009.05.002
15. Lester, BM, Andreozzi-Fontaine, L, Tronick, E, and Bigsby, R. Assessment and evaluation of the high risk neonate: the NICU network neurobehavioral scale. JoVE. (2014) 90:e3368. doi: 10.3791/3368
16. Pourkaviani, S, Zhang, X, Spear, EA, D'Agostino, M, Satty, RE, Liu, SH, et al. Clinical validation of the neonatal infant stressor scale with preterm infant salivary cortisol. Pediatr Res. (2020) 87:1237–43. doi: 10.1038/s41390-019-0713-0
17. Bridgeman-Bunyoli, AM, Cheyney, M, Monroe, SM, Wiggins, N, and Vedam, S. Preterm and low birthweight birth in the United States: black midwives speak of causality, prevention, and healing. Birth. (2022) 49:526–39. doi: 10.1111/birt.12624
18. Braveman, P, Dominguez, TP, Burke, W, Dolan, SM, Stevenson, DK, Jackson, FM, et al. Explaining the black-white disparity in preterm birth: a consensus statement from a multi-disciplinary scientific work group convened by the March of Dimes. Front Reprod Health. (2021) 3:684207. doi: 10.3389/frph.2021.684207
19. Alhusen, JL, Bower, KM, Epstein, E, and Sharps, P. Racial discrimination and adverse birth outcomes: an integrative review. J Midwifery Womens Health. (2016) 61:707–20. doi: 10.1111/jmwh.12490
20. Burris, HH, Lorch, SA, Kirpalani, H, Pursley, DM, Elovitz, MA, and Clougherty, JE. Racial disparities in preterm birth in USA: a biosensor of physical and social environmental exposures. Arch Dis Child. (2019) 104:931–5. doi: 10.1136/archdischild-2018-316486
21. Lu, MC, and Chen, B. Racial and ethnic disparities in preterm birth: the role of stressful life events. Am J Obstet Gynecol. (2004) 191:691–9. doi: 10.1016/j.ajog.2004.04.018
22. Nurius, PS, Green, S, Logan-Greene, P, Longhi, D, and Song, C. Stress pathways to health inequalities: embedding ACEs within social and behavioral contexts. Int Public Health J. (2016) 8:241–56.
23. Odlum, M, Moise, N, Kronish, IM, Broadwell, P, Alcántara, C, Davis, NJ, et al. Trends in poor health indicators among black and Hispanic middle-aged and older adults in the United States, 1999-2018. JAMA Netw Open. (2020) 3:e2025134-e. doi: 10.1001/jamanetworkopen.2020.25134
24. Lester, B, and Tronick, E. History and description of the neonatal intensive care unit network neurobehavioral scale. Pediatrics. (2004) 113:634–40. doi: 10.1542/peds.113.S2.634
25. Prechtl, H, and Beintema, D. The neurological examination of the full-term newborn infants. London, United Kingdom: Heinemann (1964).
27. Tronick, E, and Lester, BM. Grandchild of the NBAS: the NICU network neurobehavioral scale (NNNS): a review of the research using the NNNS. J Child Adolesc Psychiatr Nurs. (2013) 26:193–203. doi: 10.1111/jcap.12042
28. Lester, B, Tronick, E, LaGasse, L, Seifer, R, Bauer, CR, Shankaran, S, et al. Summary statistics of neonatal intensive care unit network neurobehavioral scale scores from the maternal lifestyle study: a quasinormative sample. Pediatrics. (2004) 113:668–75. doi: 10.1542/peds.113.S2.668
29. Provenzi, L, Olson, K, Giusti, L, Montirosso, R, DeSantis, A, and Tronick, E. NICU network neurobehavioral scale: 1-month normative data and variation from birth to 1 month. Pediatr Res. (2018) 83:1104–9. doi: 10.1038/pr.2018.25
30. Engle, WA, American Academy of Pediatrics Committee on Fetus and Newborn. Age terminology during the perinatal period. Pediatrics. (2004) 114:1362–4. doi: 10.1542/peds.2004-1915
31. Team RC. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing (2019).
32. McEwen, BS. Protective and damaging effects of stress mediators. N Engl J Med. (1998) 338:171–9. doi: 10.1056/NEJM199801153380307
33. Mac Dorman, MF. Race and ethnic disparities in fetal mortality, preterm birth, and infant mortality in the United States: an overview. Semin Perinatol. (2011) 35:200–8. doi: 10.1053/j.semperi.2011.02.017
34. Manuck, TA. Racial and ethnic differences in preterm birth: a complex, multifactorial problem. Semin Perinatol. (2017) 41:511–8. doi: 10.1053/j.semperi.2017.08.010
35. McLemore, MR, Altman, MR, Cooper, N, Williams, S, Rand, L, and Franck, L. Health care experiences of pregnant, birthing and postnatal women of color at risk for preterm birth. Soc Sci Med. (2018) 201:127–35. doi: 10.1016/j.socscimed.2018.02.013
36. Giugescu, C, Misra, DP, Slaughter-Acey, JC, Gillespie, SL, Nowak, AL, Dove-Meadows, E, et al. Neighborhoods, racism, stress, and preterm birth among African American women: a review. West J Nurs Res. (2022) 44:101–10. doi: 10.1177/01939459211041165
37. Smith, GC, Gutovich, J, Smyser, C, Pineda, R, Newnham, C, Tjoeng, TH, et al. Neonatal intensive care unit stress is associated with brain development in preterm infants. Ann Neurol. (2011) 70:541–9. doi: 10.1002/ana.22545
38. Smith, SM, Nichols, TE, Vidaurre, D, Winkler, AM, Behrens, TE, Glasser, MF, et al. A positive-negative mode of population covariation links brain connectivity, demographics and behavior. Nat Neurosci. (2015) 18:1565–7. doi: 10.1038/nn.4125
39. Scatliffe, N, Casavant, S, Vittner, D, and Cong, X. Oxytocin and early parent-infant contact: a systematic review. Int J Nurs Sci. (2019) 6:445–53. doi: 10.1016/j.ijnss.2019.09.009
40. Hyman, SE. How adversity gets under the skin. Nat Neurosci. (2009) 12:241–3. doi: 10.1038/nn0309-241
41. Beam, AL, Fried, I, Palmer, N, Agniel, D, Brat, G, Fox, K, et al. Estimates of healthcare spending for preterm and low-birthweight infants in a commercially insured population: 2008–2016. J Perinatol. (2020) 40:1091–9. doi: 10.1038/s41372-020-0635-z
42. Gates, A, Marin, T, De Leo, G, Waller, JL, and Stansfield, BK. Nutrient composition of preterm mother's milk and factors that influence nutrient content. Am J Clin Nutr. (2021) 114:1719–28. doi: 10.1093/ajcn/nqab226
43. Merewood, A, Brooks, D, Bauchner, H, Mac Auley, L, and Mehta, SD. Maternal birthplace and breastfeeding initiation among term and preterm infants: a statewide assessment for Massachusetts. Pediatrics. (2006) 118:e1048–54. doi: 10.1542/peds.2005-2637
44. Gyamfi, A, Spatz, DL, Jefferson, U, Lucas, R, O’Neill, B, and Henderson, WA. Breastfeeding social support among African women in the United States: a meta-ethnography. Adv Neonatal Care. (2023) 23:72–80. doi: 10.1097/ANC.0000000000001021
45. Srisoap, P, and Lucas, R. Maternal perception of paternal breastfeeding support: a secondary qualitative analysis. Midwifery. (2021) 102:103067. doi: 10.1016/j.midw.2021.103067
Keywords: health inequities, preterm birth, neurodevelopment, pain, social determinants of health
Citation: Brown J, Chang X, Matson A, Lainwala S, Chen M-H, Cong X and Casavant SG (2023) Health disparities in preterm births. Front. Public Health. 11:1275776. doi: 10.3389/fpubh.2023.1275776
Edited by:
Kristen Rappazzo, United States Environmental Protection Agency (EPA), United StatesReviewed by:
David Hollar, Mercer University School of Medicine, United StatesOlufunmilayo Knutson, University of St. Thomas, United States
Adrien Wilkie, Oak Ridge Institute for Science and Education (ORISE), United States
Copyright © 2023 Brown, Chang, Matson, Lainwala, Chen, Cong and Casavant. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sharon G. Casavant, sharon.casavant@uconn.edu
 Judy Brown
Judy Brown Xiaolin Chang3
Xiaolin Chang3 Ming-Hui Chen
Ming-Hui Chen Sharon G. Casavant
Sharon G. Casavant