- 1Department of Environmental Health Engineering, School of Public Health and Environmental Technologies Research Center, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
- 2Air Pollution and Respiratory Diseases Research Center, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
- 3Department of Nursing, Nursing Care Research Center in Chronic Diseases, School of Nursing and Midwifery, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
- 4Department of Health Promotion and Education, School of Health, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
- 5Department of Biostatistics and Epidemiology, School of Health, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
Toxic air pollutants are one of the main factors that have the effect of synergism to increase the incidence of multiple sclerosis (MS). This review aims to investigate the effects of toxic air pollutants on the occurrence of multiple sclerosis (MS). A narrative review of the literature was done from 2000 to 2022 based on various databases such as Google Scholar, Web of Science, Springer, PubMed, and Science Direct. In this study, according to the databases, three hundred and sixty articles were retrieved. Of these, 28 studies were screened after review and 14 full-text articles entered into the analysis process. Finally, 9 articles were selected in this study. According to the finding of this study, toxic air pollutants including polycyclic aromatic hydrocarbons (PAHs), heavy metals (HM), volatile organic compounds (VOCs), particulate matter (PM), and gases are the main agents that cause the development and spread of chronic diseases such as respiratory and cardiovascular diseases, chronic obstructive pulmonary disease (COPD), and multiple sclerosis. The result of this study showed that the main sources of emission of toxic air pollutants include industries, cars, power plants, and the excessive consumption of fossil fuels. In general, the inhalation of high concentration of toxic air pollutants can increase the risk of chronic diseases and multiple sclerosis.
Introduction
Health and environment are related to each other and have interaction effects (1). Both humans and other creatures in some way affect their environment (2, 3). With regards to the advancement of technology and its incompatibility with the environment and the change of human civilization during different eras, ignoring the correct relationship between human needs and the environment has made the life of creatures on earth more complex (4, 5). The main sources of emission and production of toxic air pollutants are human's activities such as industrial processes, agriculture, activities of power plants (man-made origin), road traffic, combustion of fuel (petroleum, oil, gasoline, diesel, wood, coal), pollutants enter the oceans through evaporation, volcanic eruptions (natural), and forest fires (6–8).
Toxic air pollutants can cause dysfunction and destroy the functions of the cardiovascular, respiratory, circulatory, reproductive, and nervous systems (9–11). Also, these pollutants can cause dangerous diseases such as cancers of the lungs, heart, liver, blood, kidney, bladder, and brain (9–11). This pollution has unpleasant effects on the health of humans and other organisms (12, 13). One of the most important effects of dangerous air pollutants is the effect on multiple sclerosis (MS) (14). Multiple sclerosis is known as a disease of the modern and industrial world, but this does not mean that no one has had MS before. Even in previous years there were patients, but with the advent of the modern world, the disease has become more severe (15, 16).
This means that in the last 20 years, we have been facing an abundance of diseases that are due to the industrialization or modernization of society, and the incidence of MS is increasing (17, 18). The cause of MS is still unknown (19). Multiple sclerosis is exacerbated by factors such as air pollution, vitamin D deficiency, environmental pollution, severe stress, and traffic (20, 21). The risk of developing MS in metropolitan areas due to air pollution is higher than in other areas (22, 23). There are several factors involved in this upward trend, including the industrialization of society (24). Smoking or hookah, vitamin D deficiency, and air pollution are other factors that increase the incidence of multiple sclerosis (25, 26).
In families with a history of MS, the risk of other family members being infected is about 10%, which indicates that environmental factors are involved in the development of multiple sclerosis (27). Multiple sclerosis is not curable like many diseases, but if treated, it can be controlled and the patient can lead a relatively normal life (28, 29).
In the world that has between 2.5 and 3 million people with MS (22), the latest statistics provided in the country show that about 70,000 Iranians also suffer from multiple sclerosis (30). In urban areas, the risk of developing multiple sclerosis is higher than in rural areas because of the high concentration of air pollutants and long-term exposure to toxic pollutants (31). The prevalence of MS in areas with low-level of air pollutants is much lower, which indicates that air pollution can be one of the high-risk factors for MS (32).
Considering the importance of neurological systems' dysfunction caused by exposure to air pollutants, the aim of this narrative review was the evaluation of toxic air pollutants and their effect on multiple sclerosis.
Methods
Eligibility Criteria and Search Strategy
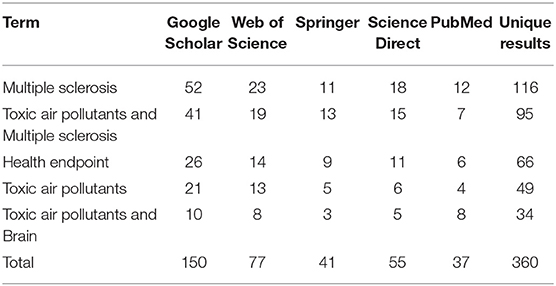
This narrative review study was conducted on references available in various databases: Science Direct, PubMed, Web of Science, Springer, and Google Scholar (Table 1). Years of publication 2000–2022 and English language were the main criteria for search limitation. Three hundred and sixty articles according to databases were retrieved.
Collection Data
“Multiple sclerosis,” “toxic air pollutants and multiple sclerosis,” “health endpoint,” “toxic air pollutants” and “toxic air pollutants and brain” were terms that were used in the first stage search.
Studies were prepared based on searches of 150 articles in the Google Scholar, Web of Sciences received 77 articles, Springer database 41 articles, Science Direct 55 articles, and PubMed database 37 articles. Review time efficiency of studies was limited to the range of 2000–2022.
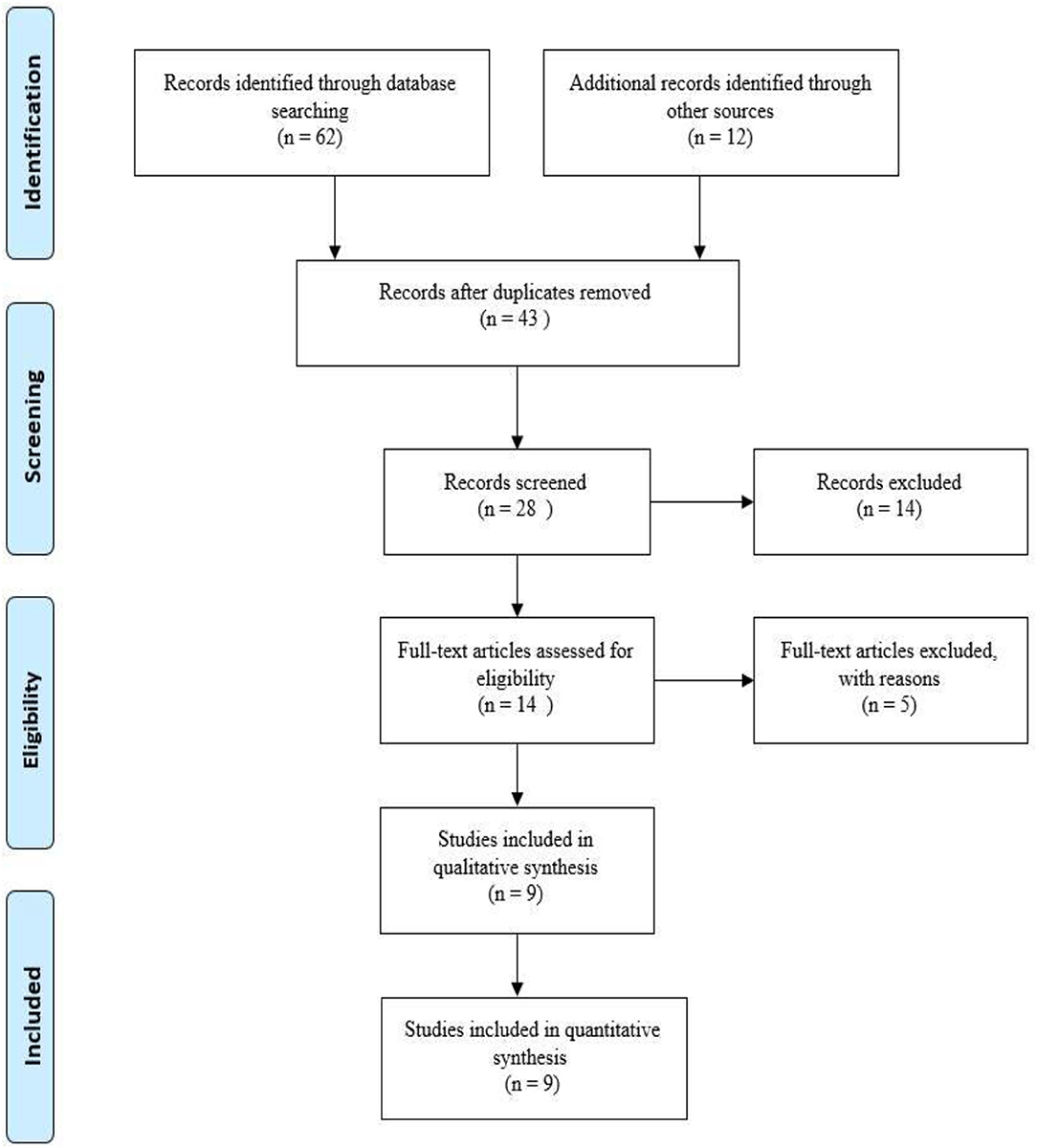
A total of 62 and 14 articles were found and selected based on records identified through database searching, and additional records were identified through other sources. In the next stage, 28 studies were screened after review and 14 full-text articles were entered into the analysis process. Finally, 9 articles were selected for this study. How to prepare studies and the selection process of articles based on the PRISMA flow diagram is shown in Figure 1.
Ethical Approval
Ethical issues (including plagiarism, misconduct, data fabrication, falsification, submission, double publication, and redundancy) have been completely observed by the authors. The Ethics License of the present study was acquired from the Ethics Committee of Ahvaz Jundishapur University of Medical Sciences (Code of ethics: IR.AJUMS.REC.1400.708).
Results And Discussion
Multiple Sclerosis (MS)
Nowadays, the main autoimmune inflammatory and degenerative disorder of the central nervous system that has clinical outcomes which are extremely variable is multiple sclerosis (16). Multiple sclerosis is a chronic autoimmune disease in which the immune system attacks the nerve tissue and destroys fat around the nerves (myelin) (33). In multiple sclerosis, the myelin of the central nerves (brain and spinal cord) becomes inflamed and damaged in the form of individual or large plaques of small or large size (34). Myelin can be compared to the insulation coating on an electrical wire (35). When myelin is damaged and nerve fiber is exposed, the messages that travel along that nerve may slow down or stop, failing to transmit the message properly (36). In this case, the brain loses control of its organs. In the central nervous system, symptoms are very different depending on where there are plaques (37). The age range for multiple sclerosis is between 20 and 50 years, and in recent years the incidence of the disease in children and people over 50 years has increased sharply (38, 39). MS is more prevalent in women, with a ratio of 3 to 4 women to one man (40).
Sex hormones are involved in the disease process since the sex hormones cause the disease to spread in women. Most people with multiple sclerosis have a relapsing–remitting illness (41). Patients with multiple sclerosis experience periods of new symptoms or recurrences that occur over days or weeks and usually improve partially or completely. Following these recurrences, there are periods of recovery that can last for months or even years (42, 43).
Low body temperature can temporarily worsen the signs and symptoms of MS, but is not considered a recurrence (44). About 60 to 70% of patients with relapsing–remitting MS eventually develop symptoms that progress with or without periods of recovery that is known as secondary progressive MS (45, 46). Worsening symptoms usually include movement and walking problems (47). The rate of disease progression varies among patients with progressive secondary MS (46). Some people with MS experience a gradual onset and continuous progression of symptoms without any recurrence (42). These patients are known to have primary progressive MS (48).
The diagnosis of multiple sclerosis is often based on the rejection of other diagnoses that may cause similar signs and symptoms, which are called differential diagnoses (49, 50). A person with inflammation has all the neurological signs or symptoms (51). The most common of these symptoms are autonomic, visual, motor, and sensory problems (52).
The main specific symptoms among people with multiple sclerosis include lethargy or drowsiness such as tingling, spasm, muscle weakness, involuntary reactions, muscle cramps or inability to move, inability to coordinate and balance muscle coordination, difficulty urinating and defecating, tiredness, difficulty speaking or dysphasia, visual problems (eyeball movement, blurred vision, or diplopia), severe or chronic pain, difficulty to think, and depression and emotional distress (53–56). Figure 2 shows the route of the central nerves system after the immune system attacks the nerve tissue.
Air Pollutants
Air pollution occurs when large volumes of harmful particles such as gases, particles, and biomolecules enter the Earth's atmosphere (57). Air pollution is a mixture of suspended particles and gases whose concentration has reached a range harmful to humans.
Growth population, emissions of greenhouse gases, destroying vegetation and forests and cutting down trees indiscriminately, excessive consumerism of the world, excessive production of carbon dioxide and global warming, increasing industrialization, and excessive use of fossil fuels are the most important causes for the release of toxic air pollutants, their entry into the food cycle (plants and animals), and consumption by people (58, 59).
Air pollution results in several major causes of death in the world including stroke, heart disease, lung cancer, and respiratory diseases (COPD, bronchitis and asthma) (60, 61). Air pollution in various ways can have long-term and short-term harmful effects on human health (62). The impact of air pollution on people is different because some people are much more vulnerable to air pollution (63). Younger children and the elderly are more likely to be affected by air pollution (64). The extent of damage usually depends on the time exposed to toxic air pollutants and the concentration of toxic pollutants (62, 64).
Toxic air pollutants through the lungs, skin, mucous membranes, mucous membranes of the eyes, ears, nose, and gastrointestinal tract enter the body and destroy the body's cells (65). Air pollution is associated with behavioral changes because because it causes people to spend less time in the open and lead a more sedentary lifestyle, which can cause psychological distress and social isolation (66, 67). The main negative effects of air pollution on the nervous system are more irritability, aggression, anger, and intolerance (68, 69). Figure 3 shows sources of emission of toxic air pollutants in the environment.
Toxic Air Pollutants and Effects on Multiple Sclerosis
Toxic air pollutants (polycyclic aromatic hydrocarbons (PAHs), BTEX (benzene, toluene, ethylbenzene, and xylene), heavy metals, VOCs, and particulate matter) through the bloodstream reach the brain and can directly damage the neurons (58, 70–72).
Toxic air pollutants get into the body mainly through breathing. They can also be ingested (e. g., children eating soil contaminated with lead) or absorbed through the skin (73, 74).
Inhalation of toxic air pollutants (breathing), swallowing, or ingestion of foods infected with toxic air pollutants (eating foods) and dermal contact with heavy metal and polycyclic aromatic hydrocarbons (skin) are the main routes of entire toxic air pollutants and destruction on human (75, 76). Air pollutants can cause extensive damage by disrupting the regulation of microglia and immune cells in the brain. Microglia may confuse intruders with pathogens and release chemicals to kill them (77, 78). These chemicals can accumulate and cause inflammation, and chronic inflammation of the brain is involved in the destruction of nerve cells (79, 80).
According to the reports of different studies, toxic air pollutants can slip through the plasma membrane of the alveoli and small air sacs in the lungs, collect through the capillaries, and then the pollutants are distributed in the blood throughout the body (77). Although some of these pollutants may eventually break down the blood–brain barrier, there is no need for a contaminant to enter the brain to cause a problem even though the immune system can also react to particles in the lungs or bloodstream, causing widespread inflammation that affects the brain (81).
A swallowed particle can have indirect neurological effects through the intestine (82). Researchers are now recognizing strong links between the gut microbiome and the brain, and studies show that delivering small particles to the gut can cause systemic inflammation (83, 84). Exposure to toxic air pollutants is known to be an environmental risk factor that causes brain aging (85, 86). The risk of dementia and Alzheimer's disease increases with exposure to toxic air pollutants (87).
Nitrogen dioxide (NO2) and hydrocarbons (HC) are produced by the combustion of vehicle fuels (88, 89). NO2 and HC increase the risk of developing autism (90, 91). Short- or long-term exposure to air pollution is also associated with an increased risk of stroke and acute coronary syndrome, such as heart attack (92–94). Exposure to toxic air pollutants is directly related to the underlying clinical diseases of stroke (systemic inflammation, oxidative stress, atherosclerosis, thrombosis, and arrhythmia) (95–97).
Multiple sclerosis has a variety of causes and some environmental factors such as air pollution can trigger an abnormal immune response (98, 99). Air pollution can also be directly linked to affect neurological diseases such as Alzheimer's and Parkinson's (100, 101). Inhalable toxic air pollutants can increase the risk of developing MS (102, 103). Since MS is an inflammatory disease, air pollution can affect it and increase MS attacks. Smoking is one of the main agents that as an environmental factorplays a main role in increasing the risk of prevalence multiple sclerosis (104–106). Based on the results of different studies, the risk of the incidence multiple sclerosis has a direct relationship with cigarette smoking because of the presence of high amounts of cadmium that affect the susceptibility to MS (104, 107, 108). Seasonal changes in the condition of patients with MS show that climate change and air pollution can be effective in relapsing MS. The toxic air pollutants are absorbed into the lungs through the respiratory tract, causing systemic pulmonary inflammation and activation of cells that then secrete proinflammatory cytokines in the brain. Second, toxic air pollutants are absorbed directly through the olfactory bulb and may cause inflammatory reactions in the brain, and third, genetic mutations caused by toxic air pollutants affect its onset (109–113). Figure 4 shows sources of toxic air pollutants and its effects on multiple sclerosis.
Controlling the level of vitamin D in the body using cod liver oil, cheese, yogurt, butter, milk; regular use of prescription drugs; avoiding negative thoughts; reducing daily stress; exercises such as yoga, swimming, and walking; avoiding smoking; consumption of fish; coffee consumption; maintaining a healthy body mass index (BMI); and living in places with very low levels of air pollution are among the most important measures that can help prevent multiple sclerosis.
Conclusion
In this study, we investigated toxic air pollutants and their effect on the increased prevalence of multiple sclerosis. The results showed that the inhalation and exposure to air pollution such as polycyclic aromatic hydrocarbons, heavy metals, volatile organic compounds, and particulate matter were related to the actual risks of different systems and organs of the body such as the brain, lungs, and heart.
The finding this study showed is that toxic air pollutants can have synergistic and aggravating properties in increasing cases of multiple sclerosis. It should be noted that according to health organizations, such as World Health Organization (WHO), the trend incidence and severity of recurrent MS attacks have been increasing. Increase acknowledge of the relationship between toxic air pollutants on multiple sclerosis are the main applications of the results in this study.
Also, based on reports to better understand and more knowledge of the characteristics of toxic air pollutants can be a great help to health managers and policymakers in taking preventive and protective measures. An increase in the number of air monitoring stations, preparation and approval of government regulations related to reducing air pollutant emissions, and reducing the use of these fossil fuels are the most important activities that can causes reduces emissions and have a significant effect on decreasing the health endpoint of toxic air pollutants.
In general, further studies are vital and required to determine the effects of toxic air pollutants on all body systems (respiratory, cardiac, vascular, gastrointestinal, nervous, circulatory, urinary, and reproductive) and body organs (brain, heart, kidney, lung and liver).
Author Contributions
MM, KZ, NH, ASa, and ASh were principal investigators and advisors of the study, drafted the manuscript, and performed the statistical analysis. All authors contributed to the design, data analysis, assisted in the preparation of the final version of the manuscript, read, and approved the final version of the manuscript.
Funding
This study was funded by the Ahvaz Jundishapur University of Medical Sciences.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors are grateful to the Ahvaz Jundishapur University of Medical Sciences (with code IR.AJUMS.REC.1400.708) for providing the necessary facilities to perform this research.
References
1. Shahriyari HA, Nikmanesh Y, Jalali S, Tahery N, Zhiani Fard A, Hatamzadeh N, et al. Air pollution and human health risks: mechanisms and clinical manifestations of cardiovascular and respiratory diseases. Toxin Rev. (2022) 41:606–17. doi: 10.1080/15569543.2018.1463266
2. Doherty TS, Hays GC, Driscoll DA. Human disturbance causes widespread disruption of animal movement. Nat Ecol Evol. (2021) 5:513–9. doi: 10.1038/s41559-020-01380-1
3. Javanmardi P, Morovati P, Farhadi M, Geravandi S, Khaniabadi YO, Angali KA, et al. Monitoring the impact of ambient ozone on human health using time series analysis and air quality model approaches. Fresenius Environ Bull. (2018) 27:533–44. Available online at: https://www.researchgate.net/publication/320894033
4. Overstreet RM. Parasitic Diseases of Fishes and their Relationship with Toxicants and Other Environmental Factors. Pathobiology of Marine and Estuarine Organisms. https://www.google.com/search?biw=1408&bih=652&q=Boca+Raton&stick=H4sIAAAAAAAAAOPgE-LUz9U3ME7LK09S4gAxk03LjLS0spOt9POL0hPzMqsSSzLz81A4VhmpiSmFpYlFJalFxYtYuZzykxMVghJL8vN2sDLuYmfiYAAAm_ncwFgAAAA&sa=X&ved=2ahUKEwiTten57qX4AhXEnuAKHRQwC2EQmxMoAHoECEkQAg Boca Raton, FL: CRC press (2021). p. 111–56.
5. Davar H, Taghavirad S, Mohammadi M. The investigation of effects of silica on the environment and prevention of release of the silica particles with simulation of gas-solid flow in a gas cyclone. Res J Chem Environ. (2014) 18:28–30. Available online at: https://www.researchgate.net/publication/287270383
6. Nazaroff WW, Weschler CJ. Cleaning products and air fresheners: exposure to primary and secondary air pollutants. Atmos Environ. (2004) 38:2841–65. doi: 10.1016/j.atmosenv.2004.02.040
7. Borsi SH, Goudarzi G, Sarizadeh G, Dastoorpoor M, Geravandi S, Shahriyari HA, et al. Health endpoint of exposure to criteria air pollutants in ambient air of on a populated in Ahvaz City, Iran. Front Public Health. (2022) 10:869656. doi: 10.3389/fpubh.2022.869656
8. Zhang X, Zhang M, Cui Y, He Y. Estimation of Daily Ground-Received Global Solar Radiation Using Air Pollutant Data. Front Public Health. (2022) 10:860107. doi: 10.3389/fpubh.2022.860107
9. Hajir S, Al Aaraj L, Zgheib N, Badr K, Ismaeel H, Abchee A, et al. The association of urinary metabolites of polycyclic aromatic hydrocarbons with obstructive coronary artery disease: a red alert for action. Environ Poll. (2021) 272:115967. doi: 10.1016/j.envpol.2020.115967
10. Turner MC, Andersen ZJ, Baccarelli A, Diver WR, Gapstur SM, Pope III CA, et al. Outdoor air pollution and cancer: An overview of the current evidence and public health recommendations. CA Cancer J Clin. (2020) 70:460–79. doi: 10.3322/caac.21632
11. Hashim D, Boffetta P. Occupational and environmental exposures and cancers in developing countries. Ann Global Health. (2014) 80:393–411. doi: 10.1016/j.aogh.2014.10.002
12. Abdollahbeigi M. Non-climatic factors causing climate change. J Chem Rev. (2020) 2:292–308. doi: 10.22034/JCR.2020.249615.1087
13. Mazumdar M, Goswami H, Debnath A. Brick industry as a source of pollution-its causes and impacts on human rights: a case study of brick kilns of palasbari revenue circle. Int J Human Soc Sci. (2018) 6:220–40.
14. Gregory II AC, Shendell DG, Okosun IS, Gieseker KE. Multiple Sclerosis disease distribution and potential impact of environmental air pollutants in Georgia. Sci Total Environ. (2008) 396:42–51. doi: 10.1016/j.scitotenv.2008.01.065
15. Moghadam VK, Dickerson AS, Shahedi F, Bazrafshan E, Seyedhasani SN, Sarmadi M. Association of the global distribution of multiple sclerosis with ultraviolet radiation and air pollution: an ecological study based on GBD data. Environ Sci Poll Res. (2021) 28:17802–11. doi: 10.1007/s11356-020-11761-5
16. Bevens W, Weiland TJ, Gray K, Neate SL, Nag N, Simpson-Yap S, et al. The feasibility of a web-based educational lifestyle program for people with multiple sclerosis: a randomized controlled trial. Front Public Health. (2022) 10:852214. doi: 10.3389/fpubh.2022.852214
17. Sahraian MA, Sahebkar M, Dehghani R, Derakhshan-Jazari M, Kazami-Moghaddam V, Kouchaki E. Multiple sclerosis-A disease on a dramatically rising trend in Iran: Review of possible reasons. Iran J Neurol. (2017) 16:34.
18. Zarghami A, Li Y, Claflin SB, van der Mei I, Taylor BV. Role of environmental factors in multiple sclerosis. Exp Rev Neurotherap. (2021) 21:1389–1408. doi: 10.1080/14737175.2021.1978843
19. Ji H, Xu Y, Lu H, Zhang Z. Deep MS/MS-aided structural-similarity scoring for unknown metabolite identification. Anal Chem. (2019) 91:5629–37. doi: 10.1021/acs.analchem.8b05405
20. Scartezzini A, Tateo F, Perini P, Benacchio L, Ermani M, Ferro A., et al. Association of Multiple Sclerosis with PM 25 levels Further evidence from the highly polluted area of Padua Province, Italy. Multiple Sclerosis Relat Disord. (2021) 48:102677. doi: 10.1016/j.msard.2020.102677
21. Türk Börü Ü, Bölük C, Taşdemir M, Gezer T, Serim VA. Air pollution, a possible risk factor for multiple sclerosis. Acta Neurol Scand. (2020) 141:431–7. doi: 10.1111/ane.13223
22. Bai L, Burnett RT, Kwong JC, Hystad P, van Donkelaar A, Brook JR, et al. Long-term exposure to air pollution and the incidence of multiple sclerosis: a population-based cohort study. Environ Res. (2018) 166:437–43. doi: 10.1016/j.envres.2018.06.003
23. Tang C, Li Q-R, Mao Y-M, Xia Y-R, Guo H-S, Wang J-P, et al. Association between ambient air pollution and multiple sclerosis: a systemic review and meta-analysis. Environ Sci Poll Res. (2021) 28:58142–8153. doi: 10.1007/s11356-021-14577-z
24. Kaiser R, Romieu I, Medina S, Schwartz J, Krzyzanowski M, Künzli N. Air pollution attributable postneonatal infant mortality in US metropolitan areas: a risk assessment study. Environ Health. (2004) 3:1–6. doi: 10.1186/1476-069X-3-4
25. Ashtari F, Esmaeil N, Mansourian M, Poursafa P, Mirmosayyeb O, Barzegar M, et al. An 8-year study of people with multiple sclerosis in Isfahan, Iran: association between environmental air pollutants and severity of disease. J Neuroimmunol. (2018) 319:106–11. doi: 10.1016/j.jneuroim.2018.02.019
26. Eskandari G, Ghajarzadeh M, Yekaninejad MS, Sahraian MA, Gorji R, Rajaei F, et al. Comparison of serum vitamin D level in multiple sclerosis patients, their siblings, and healthy controls. Iran J Neurol. (2015) 14:81.
27. Sadovnick D. The place of environmental factors in multiple sclerosis: genes, environment and the interactions thereof in the etiology of multiple sclerosis. Rev Neurol. (2019) 175:593–6. doi: 10.1016/j.neurol.2019.08.003
28. Crayton H, Heyman RA, Rossman HS. A multimodal approach to managing the symptoms of multiple sclerosis. Neurology. (2004) 63:S12–S8. doi: 10.1212/WNL.63.11_suppl_5.S12
29. Dodd KJ, Taylor N, Shields N, Prasad D, McDonald E, Gillon A. Progressive resistance training did not improve walking but can improve muscle performance, quality of life and fatigue in adults with multiple sclerosis: a randomized controlled trial. Multiple Sclerosis J. (2011) 17:1362–74. doi: 10.1177/1352458511409084
30. Mohammad Mousaei F, Zendehtalb HR, Zare M, Behnam Vashani HR. Effect of family-centered empowerment model on self-care behaviors of patients with multiple sclerosis. Evidence Based Care. (2021) 11:35–43. doi: 10.22038/EBCJ.2021.58299.2521
31. Tateo F, Grassivaro F, Ermani M, Puthenparampil M, Gallo P. PM2 5 levels strongly associate with multiple sclerosis prevalence in the Province of Padua, Veneto Region, North-East Italy. Multiple Sclerosis J. (2019) 25:1719–27. doi: 10.1177/1352458518803273
32. Chen H, Kwong JC, Copes R, Tu K, Villeneuve PJ, Van Donkelaar A, et al. Living near major roads and the incidence of dementia, Parkinson's disease, and multiple sclerosis: a population-based cohort study. Lancet. (2017) 389:718–26. doi: 10.1016/S0140-6736(16)32399-6
33. Podbielska M, Banik NL, Kurowska E, Hogan EL. Myelin recovery in multiple sclerosis: the challenge of remyelination. Brain Sci. (2013) 3:1282–324. doi: 10.3390/brainsci3031282
34. Lassmann H. The pathologic substrate of magnetic resonance alterations in multiple sclerosis. Neuroimaging Clin N Am. (2008) 18:563–76. doi: 10.1016/j.nic.2008.06.005
35. Tian D-S, Jing J-H, Qian J, Chen L, Zhu B. Effect of oscillating electrical field stimulation on motor function recovery and myelin regeneration after spinal cord injury in rats. J Phys Ther Sci. (2016) 28:1465–71. doi: 10.1589/jpts.28.1465
36. Delalande B, Verpilliere L. Another Train Paradox: May the Myelin Be with You! Open Access Libr J. (2021) 8:1. doi: 10.4236/oalib.1107379
37. Shechter R, Schwartz M. Harnessing monocyte-derived macrophages to control central nervous system pathologies: no longer ‘if'but ‘how'. J Pathol. (2013) 229:332–46. doi: 10.1002/path.4106
38. Talebi M, Sadigh-Eteghad S, Sahraian MA, Fahidi A. Age and sex adjusted prevalence and annual incidence of multiple sclerosis in East-Azerbaijan, Iran. Mult Scler Relat Disord. (2021) 50:102839. doi: 10.1016/j.msard.2021.102839
39. Harris ML, Egan N, Forder PM, Loxton D. Increased chronic disease prevalence among the younger generation: Findings from a population-based data linkage study to inform chronic disease ascertainment among reproductive-aged Australian women. PLoS ONE. (2021) 16:e0254668. doi: 10.1371/journal.pone.0254668
40. Koutsouraki E, Costa V, Baloyannis S. Epidemiology of multiple sclerosis in Europe: a review. Int Rev Psychiatry. (2010) 22:2–13. doi: 10.3109/09540261003589216
41. Sparaco M, Bonavita S. The role of sex hormones in women with multiple sclerosis: From puberty to assisted reproductive techniques. Front Neuroendocrinol. (2021) 60:100889. doi: 10.1016/j.yfrne.2020.100889
42. Zeydan B, Kantarci OH. Progressive forms of multiple sclerosis: distinct entity or age-dependent phenomena. Neurol Clin. (2018) 36:163–71. doi: 10.1016/j.ncl.2017.08.006
43. Tsang BK, Macdonell R. Multiple sclerosis: diagnosis, management and prognosis. Aust Fam Physician. (2011) 40:948–55.
44. Davis SL, Wilson TE, White AT, Frohman EM. Thermoregulation in multiple sclerosis. J Appl Physiol. (2010) 109:1531–7. doi: 10.1152/japplphysiol.00460.2010
45. Scalfari A, Neuhaus A, Daumer M, Muraro PA, Ebers GC. Onset of secondary progressive phase and long-term evolution of multiple sclerosis. J Neurol Neurosurg Psychiatry. (2014) 85:67–75. doi: 10.1136/jnnp-2012-304333
46. Inojosa H, Proschmann U, Akgün K, Ziemssen T, A. focus on secondary progressive multiple sclerosis (SPMS): challenges in diagnosis and definition. J Neurol. (2021) 268:1210–21. doi: 10.1007/s00415-019-09489-5
47. Weikert M, Motl RW, Suh Y, McAuley E, Wynn D. Accelerometry in persons with multiple sclerosis: measurement of physical activity or walking mobility? J Neurol Sci. (2010) 290:6–11. doi: 10.1016/j.jns.2009.12.021
48. Tsagkas C, Magon S, Gaetano L, Pezold S, Naegelin Y, Amann M, et al. Preferential spinal cord volume loss in primary progressive multiple sclerosis. Multiple Sclerosis J. (2019) 25:947–57. doi: 10.1177/1352458518775006
49. Freedman MS, Thompson EJ, Deisenhammer F, Giovannoni G, Grimsley G, Keir G, et al. Recommended standard of cerebrospinal fluid analysis in the diagnosis of multiple sclerosis: a consensus statement. Arch Neurol. (2005) 62:865–70. doi: 10.1001/archneur.62.6.865
51. Leppert D, Lindberg RL, Kappos L, Leib SL. Matrix metalloproteinases: multifunctional effectors of inflammation in multiple sclerosis and bacterial meningitis. Brain Res Rev. (2001) 36:249–57. doi: 10.1016/S0165-0173(01)00101-1
52. Christogianni A, Bibb R, Davis SL, Jay O, Barnett M, Evangelou N, et al. Temperature sensitivity in multiple sclerosis: an overview of its impact on sensory and cognitive symptoms. Temperature. (2018) 5:208–23. doi: 10.1080/23328940.2018.1475831
53. Aghaei N, Karbandi S, Gorji MAH, Golkhatmi MB, Alizadeh B. Social support in relation to fatigue symptoms among patients with multiple sclerosis. Indian J Palliat Care. (2016) 22:163. doi: 10.4103/0973-1075.179610
54. Motl RW, Sandroff BM, Kwakkel G, Dalgas U, Feinstein A, Heesen C, et al. Exercise in patients with multiple sclerosis. Lancet Neurol. (2017) 16:848–56. doi: 10.1016/S1474-4422(17)30281-8
55. Shema-Shiratzky S, Gazit E, Sun R, Regev K, Karni A, Sosnoff J, et al. Deterioration of specific aspects of gait during the instrumented 6-min walk test among people with multiple sclerosis. J Neurol. (2019) 266:3022–30. doi: 10.1007/s00415-019-09500-z
56. Palladino R, Marrie RA, Majeed A, Chataway J. Evaluating the risk of macrovascular events and mortality among people with multiple sclerosis in England. JAMA Neurol. (2020) 77:820–8. doi: 10.1001/jamaneurol.2020.0664
57. Steenhof M, Janssen NA, Strak M, Hoek G, Gosens I, Mudway IS, et al. Air pollution exposure affects circulating white blood cell counts in healthy subjects: the role of particle composition, oxidative potential and gaseous pollutants–the RAPTES project. Inhal Toxicol. (2014) 26:141–65. doi: 10.3109/08958378.2013.861884
58. Dastoorpoor M, Riahi A, Yazdaninejhad H, Borsi SH, Khanjani N, Khodadadi N, et al. Exposure to particulate matter and carbon monoxide and cause-specific Cardiovascular-Respiratory disease mortality in Ahvaz. Toxin Rev. (2020) 40:1362–372. doi: 10.1080/15569543.2020.1716256
59. Goudarzi G, Alavi N, Geravandi S, Idani E, Behrooz HRA, Babaei AA, et al. Health risk assessment on human exposed to heavy metals in the ambient air PM 10 in Ahvaz, southwest Iran. Int J Biometeorol. (2018) 62:1075–83. doi: 10.1007/s00484-018-1510-x
60. Schraufnagel DE, Balmes JR, Cowl CT, De Matteis S, Jung S-H, Mortimer K, et al. Air pollution and noncommunicable diseases: a review by the Forum of International Respiratory Societies' Environmental Committee, Part 2: Air pollution and organ systems. Chest. (2019) 155:417–26. doi: 10.1016/j.chest.2018.10.041
61. Tahery N, Zarea K, Cheraghi M, Hatamzadeh N, Farhadi M, Dobaradarn S, et al. Chronic Obstructive Pulmonary Disease (COPD) and air pollution: a review. Jundishapur J Chron Dis Care. (2021) 10. doi: 10.5812/jjcdc.110273
62. Le Tertre A, Medina S, Samoli E, Forsberg B, Michelozzi P, Boumghar A, et al. Short-term effects of particulate air pollution on cardiovascular diseases in eight European cities. J Epidemiol Commun Health. (2002) 56:773–9. doi: 10.1136/jech.56.10.773
63. Gouveia N, Fletcher T. Time series analysis of air pollution and mortality: effects by cause, age and socioeconomic status. J Epidemiol Commun Health. (2000) 54:750–5. doi: 10.1136/jech.54.10.750
64. Manisalidis I, Stavropoulou E, Stavropoulos A, Bezirtzoglou E. Environmental and health impacts of air pollution: a review. Front Public Health. (2020) 8:14. doi: 10.3389/fpubh.2020.00014
65. Sahoo G, Wani AM, Swamy SL, Rout S, Gupta S editors. Indoor pollution and human health. AIP Conference Proceedings. (2022). Melville, NY: AIP Publishing LLC.
66. Holt-Lunstad J, Smith TB, Baker M, Harris T, Stephenson D. Loneliness and social isolation as risk factors for mortality: a meta-analytic review. Perspect Psychol Sci. (2015) 10:227–37. doi: 10.1177/1745691614568352
67. Steptoe A, Shankar A, Demakakos P, Wardle J. Social isolation, loneliness, and all-cause mortality in older men and women. Proc Nat Acad Sci. (2013) 110:5797–801. doi: 10.1073/pnas.1219686110
68. Hammond FM, Zafonte RD, Tang Q, Jang JH. Carbamazepine for irritability and aggression after traumatic brain injury: a randomized, placebo-controlled study. J Neurotrauma. (2021) 38:2238–46. doi: 10.1016/j.apmr.2021.07.401
69. Romero-Martínez Á, Bressanutti S, Moya-Albiol L, A. systematic review of the effectiveness of non-invasive brain stimulation techniques to reduce violence proneness by interfering in anger and irritability. J Clin Med. (2020) 9:882. doi: 10.3390/jcm9030882
70. Idani E, Geravandi S, Akhzari M, Goudarzi G, Alavi N, Yari AR, et al. Characteristics, sources, and health risks of atmospheric PM10-bound heavy metals in a populated middle eastern city. Toxin Rev. (2020) 39:266–74. doi: 10.1080/15569543.2018.1513034
71. Goudarzi G, Geravandi S, Alavi N, Idani E, Salmanzadeh S, Yari AR, et al. Association between cancer risk and polycyclic aromatic hydrocarbons' exposure in the ambient air of Ahvaz, southwest of Iran. Int J Biometeorol. (2018) 62:1461–70. doi: 10.1007/s00484-018-1543-1
72. Goudarzi G, Alavi N, Idani E, Babaei AA, Salmanzadeh S, Mohammadi MJ. Association of particulate maters attributed to outdoor air in Ahvaz, Iran during cold-warm season of 2017. Fresenius Environ Bull. (2017) 26:5428–33. Available online at: https://www.researchgate.net/publication/326849881
73. Suh HH, Bahadori T, Vallarino J, Spengler JD. Criteria air pollutants and toxic air pollutants. Environ Health Perspect. (2000) 108:625–33. doi: 10.1289/ehp.00108s4625
74. Anderson EL, Patrick DR. Introduction to Risk Assessment. Risk Assessment and Indoor Air Quality. Boca Raton, FL: CRC Press (2019). p. 1–33.
75. Crinnion WJ. The CDC Fourth National Report on Human Exposure to Environmental Chemicals: What it Tells Us About our Toxic Burden and How it Assists Environmental Medicine Physicians. Altern Med Rev. (2010) 15:101–9.
76. Qu C, Li B, Wu H, Wang S, Giesy JP. Multi-pathway assessment of human health risk posed by polycyclic aromatic hydrocarbons. Environ Geochem Health. (2015) 37:587–601. doi: 10.1007/s10653-014-9675-7
77. Peeples L. News Feature: How air pollution threatens brain health. Proc Nat Acad Sci. (2020) 117:13856–60. doi: 10.1073/pnas.2008940117
78. Hawkins R. Facing up to complexity: Implications for our social experiments. Sci Eng Ethics. (2016) 22:775–814. doi: 10.1007/s11948-015-9657-x
79. Khansari N, Shakiba Y, Mahmoudi M. Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Pat Inflamm Allergy Drug Discov. (2009) 3:73–80. doi: 10.2174/187221309787158371
80. Andersen HH, Johnsen KB, Moos T. Iron deposits in the chronically inflamed central nervous system and contributes to neurodegeneration. Cell Mol Life Sci. (2014) 71:1607–22. doi: 10.1007/s00018-013-1509-8
81. Genc S, Zadeoglulari Z, Fuss SH, Genc K. The adverse effects of air pollution on the nervous system. J Toxicol. (2012) 2012:782462. doi: 10.1155/2012/782462
82. Pedersen A, Bardow A, Jensen SB, Nauntofte B. Saliva and gastrointestinal functions of taste, mastication, swallowing and digestion. Oral Dis. (2002) 8:117–29. doi: 10.1034/j.1601-0825.2002.02851.x
83. Adamovsky O, Buerger AN, Wormington AM, Ector N, Griffitt RJ, Bisesi Jr JH, et al. The gut microbiome and aquatic toxicology: an emerging concept for environmental health. Environ Toxicol Chem. (2018) 37:2758–75. doi: 10.1002/etc.4249
84. Seifert A, Kashi Y, Livney YD. Delivery to the gut microbiota: a rapidly proliferating research field. Adv Colloid Interface Sci. (2019) 274:102038. doi: 10.1016/j.cis.2019.102038
85. von Ehrenstein OS, Aralis H, Cockburn M, Ritz B. In utero exposure to toxic air pollutants and risk of childhood autism. Epidemiology (Cambridge, Mass). (2014) 25:851. doi: 10.1097/EDE.0000000000000150
86. Moulton PV, Yang W. Air pollution, oxidative stress, and Alzheimer's disease. J Environ Public Health. (2012) 2012:472751. doi: 10.1155/2012/472751
87. Rhew SH, Kravchenko J, Lyerly HK. Exposure to low-dose ambient fine particulate matter PM2. 5 and Alzheimer's disease, non-Alzheimer's dementia, and Parkinson's disease in North Carolina. PloS ONE. (2021) 16:e0253253. doi: 10.1371/journal.pone.0253253
88. Saki H, Goudarzi G, Jalali S, Barzegar G, Farhadi M, Parseh I, et al. Study of relationship between nitrogen dioxide and chronic obstructive pulmonary disease in Bushehr, Iran. Clin Epidemiol Global Health. (2020) 8:446–9. doi: 10.1016/j.cegh.2019.10.006
89. Goudarzi G, Alavi N, Babaei AA, Geravandi S, Idani E, Salmanzadeh S, et al. Investigation of ambient polycyclic aromatic hydrocarbons in a populated middle eastern city. Polycyclic Aromatic Comp. (2021) 42:1978–993. doi: 10.1080/10406638.2020.1823857
90. Tripathi MK, Kartawy M, Amal H. The role of nitric oxide in brain disorders: autism spectrum disorder and other psychiatric, neurological, and neurodegenerative disorders. Redox Biol. (2020) 34:101567. doi: 10.1016/j.redox.2020.101567
91. Tostes M, Teixeira H, Gattaz W, Brandão M, Raposo N. Altered neurotrophin, neuropeptide, cytokines and nitric oxide levels in autism. Pharmacopsychiatry. (2012) 45:241–3. doi: 10.1055/s-0032-1301914
92. Bourdrel T, Bind M-A, Béjot Y, Morel O, Argacha J-F. Cardiovascular effects of air pollution. Arch Cardiovasc Dis. (2017) 110:634–42. doi: 10.1016/j.acvd.2017.05.003
93. Pope III CA, Muhlestein JB, Anderson JL, Cannon JB, Hales NM, Meredith KG, et al. Short-term exposure to fine particulate matter air pollution is preferentially associated with the risk of ST-segment elevation acute coronary events. J Am Heart Assoc. (2015) 4:e002506. doi: 10.1161/JAHA.115.002506
94. Effatpanah M, Effatpanah H, Jalali S, Parseh I, Goudarzi G, Barzegar G, et al. Hospital admission of exposure to air pollution in Ahvaz megacity during 2010–2013. Clin Epidemiol Global Health. (2020) 8:550–6. doi: 10.1016/j.cegh.2019.12.001
95. Bevan GH, Al-Kindi SG, Brook RD, Münzel T, Rajagopalan S. Ambient air pollution and atherosclerosis: insights into dose, time, and mechanisms. Arterioscler Thromb Vasc Biol. (2021) 41:628–37. doi: 10.1161/ATVBAHA.120.315219
96. Yue C, Yang F, Li F, Chen Y. Association between air pollutants and atrial fibrillation in general population: a systematic review and meta-analysis. Ecotoxicol Environ Saf. (2021) 208:111508. doi: 10.1016/j.ecoenv.2020.111508
97. Guo J-m, Xing H-j, Cai J-z, Zhang H-f, Xu S-w. H2S exposure-induced oxidative stress promotes LPS-mediated hepatocyte autophagy through the PI3K/AKT/TOR pathway. Ecotoxicol Environ Safety. (2021) 209:111801. doi: 10.1016/j.ecoenv.2020.111801
98. Suzuki T, Hidaka T, Kumagai Y, Yamamoto M. Environmental pollutants and the immune response. Nat Immunol. (2020) 21:1486–495. doi: 10.1038/s41590-020-0802-6
99. Vodjgani M, Salehi Z, Izad M. The influence of reactive oxygen species in the immune system and pathogenesis of multiple sclerosis. Autoimmune Dis. (2020) 2020:5793817. doi: 10.1155/2020/5793817
100. Arias-Pérez RD, Taborda NA, Gómez DM, Narvaez JF, Porras J, Hernandez JC. Inflammatory effects of particulate matter air pollution. Environ Sci Poll Res. (2020) 27:42390–2404. doi: 10.1007/s11356-020-10574-w
101. Costa LG, Cole TB, Dao K, Chang Y-C, Garrick JM. Developmental impact of air pollution on brain function. Neurochem Int. (2019) 131:104580. doi: 10.1016/j.neuint.2019.104580
102. Rosso M, Chitnis T. Association between cigarette smoking and multiple sclerosis: a review. JAMA Neurol. (2020) 77:245–53. doi: 10.1001/jamaneurol.2019.4271
103. Wang Y, Xiong L, Tang M. Toxicity of inhaled particulate matter on the central nervous system: neuroinflammation, neuropsychological effects and neurodegenerative disease. J Appl Toxicol. (2017) 37:644–67. doi: 10.1002/jat.3451
104. Jafari N, Hintzen RQ. The association between cigarette smoking and multiple sclerosis. J Neurol Sci. (2011) 311:78–85. doi: 10.1016/j.jns.2011.09.008
105. Hawkes C. Smoking is a risk factor for multiple sclerosis: a metanalysis. Multiple Sclerosis J. (2007) 13:610–5. doi: 10.1177/1352458506073501
106. Ebers GC. Environmental factors and multiple sclerosis. Lancet Neurol. (2008) 7:268–77. doi: 10.1016/S1474-4422(08)70042-5
107. Aliomrani M, Sahraian MA, Shirkhanloo H, Sharifzadeh M, Khoshayand MR, Ghahremani MH. Blood concentrations of cadmium and lead in multiple sclerosis patients from Iran. Iran J Pharm Res. (2016) 15:825–833.
108. Wingerchuk DM. Smoking: effects on multiple sclerosis susceptibility and disease progression. Ther Adv Neurol Disord. (2012) 5:13–22. doi: 10.1177/1756285611425694
109. Martinelli V, Dalla Costa G, Colombo B, Dalla Libera D, Rubinacci A, Filippi M, et al. Vitamin D levels and risk of multiple sclerosis in patients with clinically isolated syndromes. Multiple Sclerosis J. (2014) 20:147–55. doi: 10.1177/1352458513494959
110. Di Rosanna P, Salvatore C. Reactive oxygen species, inflammation, and lung diseases. Curr Pharm Des. (2012) 18:3889–900. doi: 10.2174/138161212802083716
111. Block ML, Calderón-Garcidueñas L. Air pollution: mechanisms of neuroinflammation and CNS disease. Trends Neurosci. (2009) 32:506–16. doi: 10.1016/j.tins.2009.05.009
112. Calderón-Garcidueñas L, Solt AC, Henríquez-Roldán C, Torres-Jardón R, Nuse B, Herritt L, et al. Long-term air pollution exposure is associated with neuroinflammation, an altered innate immune response, disruption of the blood-brain barrier, ultrafine particulate deposition, and accumulation of amyloid β-42 and α-synuclein in children and young adults. Toxicol Pathol. (2008) 36:289–310. doi: 10.1177/0192623307313011
Keywords: multiple sclerosis, brain, toxic air pollutants, chronic diseases, health
Citation: Mohammadi MJ, Zarea K, Hatamzadeh N, Salahshouri A and Sharhani A (2022) Toxic Air Pollutants and Their Effect on Multiple Sclerosis: A Review Study. Front. Public Health 10:898043. doi: 10.3389/fpubh.2022.898043
Received: 16 March 2022; Accepted: 30 May 2022;
Published: 06 July 2022.
Edited by:
Yunquan Zhang, Wuhan University of Science and Technology, ChinaReviewed by:
Mehdi Vosoughi, Ardabil University of Medical Sciences, IranSabor Rahmati, University of Kurdistan Hewler, Iraq
Copyright © 2022 Mohammadi, Zarea, Hatamzadeh, Salahshouri and Sharhani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Asaad Sharhani, asaadsharhani3@gmail.com
 Mohammad Javad Mohammadi
Mohammad Javad Mohammadi Kourosh Zarea
Kourosh Zarea Nasser Hatamzadeh4
Nasser Hatamzadeh4 Asaad Sharhani
Asaad Sharhani