- 1Department of Toxicology, College of Public Health, Zhengzhou University, Zhengzhou, China
- 2Department of Chemical Engineering, Quaid-e-Awam University of Engineering, Science & Technology (QUEST), Nawabshah, Pakistan
- 3Department of Occupational and Environmental Health, College of Public Health, Zhengzhou University, Zhengzhou, China
Objective: The primary aim of this systematic review was to examine the relationship of polycyclic aromatic hydrocarbon (PAH) exposure with cardiovascular diseases (CVDs) and elaborate the current knowledge and recent advances in the area of PAH and its effects on CVDs and discuss the growing epidemiological evidence linking PAH to CVDs on the health of human populations. In this systematic review, the increased risk of cardiovascular diseases and their relationship with PAHs were discussed in detail.
Methods: On 05th April 2021, a systematic literature search was conducted using PubMed/Medline and Web of Science search engines in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria. The search was limited to articles that were written in English and dealt with human issues. All original peer-review publications were considered for inclusion. Comments, case reports, reviews, duplicated papers, and conference reports were excluded. Data was collected from included papers by two independent reviewers.
Results: Conclusively, 20 research articles published between 2005 and 2021 were chosen for the final analysis. The systemic review included 20 studies with a variety of geographical studies. The most common research category among the nominated studies were time-series studies followed by retrospective cohort, cross-sectional, quasi-experimental, panel, and case-control studies. Most of the studies were conducted in the United States, whereas others were showed in various geographical countries around the world, such as Denmark, Germany, Finland, Netherlands, France, China, Norway, Korea, Sweden, Saudi Arabia, and Belgium. Eight studies assessed the association between PAH exposure and CVDs, four articles observed this relationship with blood pressure (BP), two observed association between atherosclerotic CVD and PAH, one congenital heart disease, cardiovascular events, and two with obesity. Furthermore, in some investigations, a favorable association between PAH exposure and hypertension as well as PAH exposure and obesity was found.
Conclusion: In conclusion, this systematic review examined the relationship of PAH exposure with CVDs and CVD-related risk factors by searching several digital databases. After a comprehensive literature searches and summarizing findings from 20 articles, the authors concluded that a positive relationship was observed between PAH exposure and CVD risks.
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are made up of two or more fused benzene rings. These ubiquitous contaminants are formed naturally or by incomplete combustion or through organic matter (1). Furthermore, to the well-documented oncogenic impacts of PAH (2), PAH exposure in the surrounding environment and at work can lead to the growth of CVDs, such as peripheral arterial disease (PAD), coronary heart disease (CAD), myocardial infarction (MI), and stroke (3–7). In the U.S. general population, for example, urinary PAH biomarkers, such as 2-OH-PH are associated with raised CVD events (median = 0.061 μg/L for the 2001–2002 and 2003-2004 surveys, correspondingly) (5). In addition, raised prevalence of fatal ischemic heart disease in relation to work-related exposure to benzo(a)pyrene B[a]P (n = 12,367 males asphalt pavers from 7 nations) was identified in earlier research of asphalt pavers (3). The underlying mechanisms are not known, but it has been advised that oxidative stress and systemic inflammation perform a role in CVD caused by PAH (8, 9). Moreover, it is predicted that particle matter in the ambient air population caused around 3 million fatalities and 85 million disability-adjusted live years (DALYs) in 2012 (10). In most parts of the world, ambient air pollution has been on the rise rapidly due to industrialization, urbanization, and motorization (10). Ambient air contaminants in the atmosphere consist of particulate matter (PM), organic compounds, gases, and toxic metals (11). Liquid droplets (aerosols) and solid particles such as dirt, dust, smoke, and soot are absorbed by the PM. The PM is a kind of particle which is detected in smoke, exhaust fumes, and smog that is produced by combustion or as a consequence of a reaction to gases, sunlight, or air (12). As demonstrated in the literature, various factors such as poor diet, stress, and environmental contaminant exposure (13), might CVDs caused by atherosclerosis, myocardial infarction, and angina pectoris (14). Epidemiological studies showed that exposure to definite substances in the air can cause increased CVDs risk in human individuals (15). A major component of air is PAHs, which are positively correlated with cardio-metabolic risk factors and atherosclerosis (8, 9, 16).
The body's natural response to damage is inflammation, which includes vascular hyper-permeability, white cell proliferation, and vascular remodeling. Exposure to PAHs was found to be favorably linked with an inflammatory response in both in vivo and in vitro investigations (15, 17). Furthermore, intrinsic and extrinsic factors (including angiotensin-II and air pollution) can cause oxidative stress and activation of inflammatory cytokines (like IL-1β and TNF-α), resulting in endothelial dysfunction, smooth muscle cell relation reduction, and carotid artery intima-media thickening. Though endothelial dysfunction may have a role in the onset of blood vessels inflammation and the formation of vessels remodeling, it has also been shown to exacerbate plaque formation and instability in atherosclerosis (18). As a result, inflammation is regarded as an initial stage in the development of atherosclerosis.
PAH exposure is known to be associated with a decrease in cardiac autonomic function (19). In addition, PAH exposures have been documented to be positively associated in certain occupational circumstances with CVD-caused mortality (3, 20). In addition, exposure to PAH has been shown to aggravate atherosclerosis via inflammation (21). The primary aim of this systematic review was to examine the relationship of polycyclic aromatic hydrocarbon (PAH) exposure with cardiovascular diseases (CVDs) and elaborate the current knowledge and recent advances in the area of PAH and its effects on CVDs and discuss the growing epidemiological evidence linking PAH to CVDs on the health of human populations. In this systematic review, the increased risk of cardiovascular diseases and their relationship with PAHs were discussed in detail.
Methods
Search Strategy and Selection Criteria
On 05th April 2021, a systematic literature search was conducted using PubMed/Medline, and Web of Science search engines in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria (22). The study enrollment method was described using a Preferred Reporting Items for Systemic Review and Meta-Analysis (PRISMA) 2009 flow diagram (Figure 1). In our search approach, we utilized MeSH keywords and Emtree terms, as well as other relevant free-text terms. The keywords used for search were “PAHs,” “polycyclic aromatic hydrocarbons,” “polycyclic aromatic compounds,” “particulate matter,” combined with “cardiovascular diseases,” “cardio-metabolic disorders,” “hypertension,” and “cardiovascular disease risk.” Search strategy is detailed in Supplementary Table 1.
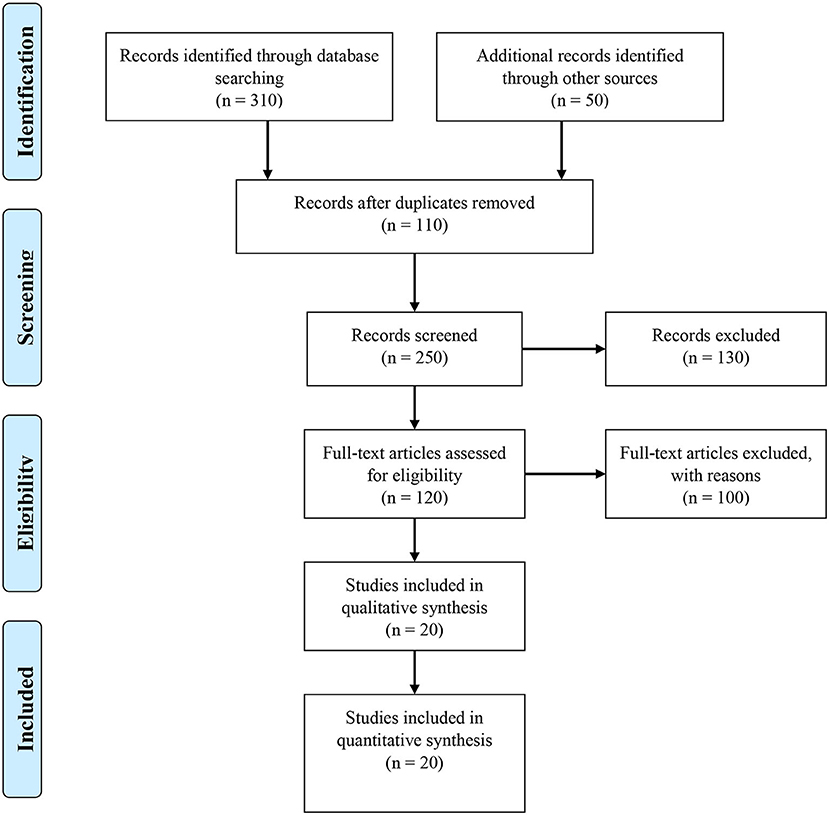
Figure 1. Identification, screening, eligibility and inclusion of studies for the systematic review.
The search was limited to articles that were written in English and dealt with human issues. The inclusion criteria were followed based on: papers that focused on PAHs and cardiovascular diseases, an association of urinary PAHs and hypertension, exposure of PAHs and cardiovascular diseases, and association of cardiovascular risk factors and PAHs. We carefully examined the bibliographies of the papers that were included in order to find any relevant studies that were missed by the first search. All original peer-review publications were considered for inclusion. When more than one report has almost the same material, the most recent research publications were chosen. Comments, case reports, reviews, duplicated papers, and conference reports were excluded.
Data Extraction and Quality Assessment
Data was collected from included papers by two independent reviewers (SB and MS). The author's name, publication year, study design, study participants, geographical locations, importance, and key findings were all extracted using Microsoft Excel. Newcastle-Ottawa scale for cohort studies and the modified Newcastle-Ottawa scale for cross-sectional studies were used to assess the quality of the included research, both of which have been validated and widely used in prior studies (23, 24). These studies were assessed using these scales by two independent reviewers (SB & MS), and any disagreements were addressed through discussion and the average score was utilized. A consensus has been made if there were any disagreements between the two reviewers concerning the methodological quality of an article. We used a practical technique to choose the majority of possible literature when data from the same study was provided in two or more publications.
Results
Study Selection and General Characteristics
Figure 1 shows a flowchart of the publication selection technique. In the first stage, a total 360 studies were collected from various internet sources. The remaining 250 studies were reviewed based on title and abstract, with 110 being duplicated articles that were eliminated. A total of 130 reports were eliminated, including 100 irrelevant studies and 30 duplicates. 120 studies were evaluated for eligibility and 100 articles were eliminated based on inadequate data (n = 80), irrelevant studies (n = 15), and duplicate (n = 5). Conclusively, 20 research articles published between 2005 and 2021 were chosen for the final analysis. All of the studies that were chosen were written in English. The characteristics of the studies are detailed in Table 1.
Characteristics of Included Studies
The systemic review included 20 studies with a variety of geographical studies. The most common research category among the nominated studies were time-series studies (5, 17, 21, 36, 40), followed by retrospective cohort (3, 28, 33, 38, 41, 42), cross-sectional (19, 26, 27, 30, 35, 37, 43), quasi-experimental (34), panel (39) and case-control (29) studies. The maximum number of individuals was 13,156 individuals, as described in one time-series study (5), whereas the lowest number of participants was described as 88 individuals in a panel study (39). Most of the studies (5, 17, 21, 33, 34, 36–38, 40, 41, 44) were conducted in the United States, while others were presented in various geographical countries around the world, such as Denmark, Germany, Finland, Netherlands, France, Norway (3), China (19, 25, 27–30, 45), Korea (26), Sweden (31), Saudi Arabia (35), and Belgium (39). In most of the published studies, the result (outcome) was reported as CVDs (5, 17, 25, 33), including as fatal ischemic heart disease (3), heart rate variability (19, 27, 34), atherosclerotic cardiovascular disease (28, 46), congenital heart disease (29), cardiovascular events (47), inflammation and atherosclerosis (21), and cardio-metabolic heart rate (38). Though the outcome was described as blood pressure (BP) with the titles of hypertension (26, 36, 37) and systolic and diastolic BP (35, 39) in three of the studies, and as obesity (38, 41) in one of them.
Association of PAH Exposure With Cardiovascular Diseases
A total of twenty studies comprised in this review, eight studies evaluated the association between PAH exposure and CVDs (9, 11, 13, 14, 16, 18, 48), four articles observed this relationship with BP (33, 49–51), two observed association between atherosclerotic cardiovascular disease and PAH (28, 46), one Congenital heart disease (29), Cardiovascular events (47) and two with obesity (13, 17). Table 1 presents a list of key findings of these research articles. The majority of evidence has shown a substantial positive relationship between exposures to PAH and the risk of CVDs (5, 19, 33, 34, 38). However, the findings of Clark et al. (17) indicated the contrary, claiming that there was no substantial association between exposures of PAH and CVDs risk. Furthermore, a positive relationship was testified between PAH exposure and hypertension (21, 35–37, 39), as well as between PAH exposure and obesity (38, 41) in some of the studies.
Discussion
In this systematic review, we evaluated the association between exposure to PAH and cardiovascular diseases. The results indicated that exposure of PAH and risk of CVDs were significantly positively associated. PAH-rich sources are recognized risk factors affecting the human cardiovascular system, including cigarette smoke (3), cooking smoke, and exhaust smokes (52–54). According to studies conducted in this area, in PHA-contaminated settings, people with cardiometabolic risk factors are more vulnerable; the elderly (55), as well as people with diabetes (56), obesity (57), heart disease (58), and high systemic inflammation (59), are more affected. A cross-sectional study (5) found that the prevalence of self-reported CVDs is positively associated with PAH exposure. Nevertheless, another study (17) reported no significant correlation between PAH exposure and inflammatory CVDs; however, this study did not answer the potential underlying explanations for their results being adequately supported. In addition, another study showed that PAH biomarkers were linked with elevated diastolic blood pressure, indicating that PAH exposure is a contributing element in the development of CVD (31). Wuhan-Zhuhi (WHZH) cohort study, which included 2,715 individuals aged 30–74 years, observed a strong positive association between urinary OH-PAHs levels with the 10-year ASCVD risk (28). A U.S. population-based study, merged 2001–02, 2003–04, 2005–06, 2007–08, and 2009–10 data cycles, included 7,301 total participants, found a positive association between PAH exposure and CVD (33).
Furthermore, studies demonstrated that urinary low molecular weight OH-PAHs were linked with raised prevalence of 10-years ASCVD in a Chinese population (25). Urinary 8-oxodG was significantly linked with PAH exposure and 10-year risk of ASCVD (25, 28). In addition, an association was observed between PAH exposures and hypertension in people (26). In the United States, there was a positive dose-response association was noticed for urinary 2-naphthol and 2-hydroxyphenanthrene and hypertension (26, 38). A study evaluated those greater maternal levels of PAHs exposure during pregnancy may be linked to an elevated prevalence of fetal CHDs and CHDs subtypes (29). Earlier studies in laboratory model systems have indicated that prenatal PAH exposure is linked with CHDs (60, 61).
Moreover, the findings indicated that sensitivity to PAH be associated with raised BP significantly. Accordingly, systolic and diastolic BP has been reported to be greater in school students near the oil factories and in those who are exposed to significant amounts of this substance than in schools outside that area (35). Another research found that with rising age, living in high-traffic areas, and body mass index, the prevalence of hypertension increases (37). Similarly, reports conducted on people with raised cholesterol, myocardial infarction history, or diabetes, and those with physical disabilities, presented an increased prevalence of hypertension due to exposure to PAH. A positive association is also documented between exposure to PAH and the level of BP (39). Experimental studies have shown that exposure to organic compounds containing PAH may lead to elevated arterial BP (50).
The pathways that underlying the substantial link between PAH exposure and CVD are yet unclear. Detoxification occurs in response to PAH exposure, resulting in the production of extremely reactive metabolites that can interact with DNA (62). PAH exposure was reported to have dose-dependent effects on plaque development in animals (63). Pre-clinical investigations have also shown that PAHs may cause atherosclerosis by inducing an inflammatory response that results in an enhanced infiltration of pro-inflammatory cells into plaques (9). Inflammation has been recognized as a risk factor for the development of CVD (64, 65). There was an association observed between PAH and inflammation, according to population studies (15, 17). Recent research also found an association between PAHs and a variety of obesity-related cardio-metabolic risk factors (38). PAH exposure is positively linked with systemic inflammation and oxidative stress in the pathogenesis of atherosclerosis, according to accumulating evidence (15, 66). The expression of pro-inflammatory cytokines in carotid plaques was triggered by DNA adducts in animal arteries (9). Furthermore, exposure to PAHs exacerbated atherosclerosis in HepG2 cells via the activation of p53 and causing down-regulation of the liver X receptor-mediated genes (67).
Moreover, the evidence suggested that PAH exposure was linked with obesity and cardio-metabolic risk factors (38, 68). In children and non-diabetic adults, urinary PAHs metabolites were positively associated with biomarkers of cardio-metabolic risk (such as BMI and WC), IR, and improved prevalence of metabolic syndrome (68, 69). PAHs may be found in all internal organs of humans, particularly in adipose tissue, due to their high lipophilicity. Parent PAHs and their metabolites have been linked to estrogen receptor activation and thyroid receptor inhibition (70). They modify fat cell metabolism, resulting in a rise in weight and fat mass, which is linked to the alternation of metabolic homeostasis and IR during the development of adipose tissue via elevated gene expression, adiponectin, and reduced DNA methylation of peroxisome proliferator-activated receptor γ (71). Toxicological research found a substantial link between low-dose PAH exposure and adiposity (72). According to the research, adipose tissue can act as a reservoir for inflammatory cytokines and numerous chemicals, including PAHs, and can play a crucial role in the advancement of atherosclerosis and carcinogenesis in both acute and chronic states (73). PAH exposure has been linked to metabolic outcomes of amino acids, purine, lipid, and glucuronic acid along with human oxidative stress state (74). One of the most powerful PAH carcinogens, benzo[a]pyrene, affects phase I and phase II enzyme activity via increasing AhR-dependent gene expression, raises oxidative stress, and hence causes cellular dysfunction, including muscle protein breakdown and adipocyte differentiation (75). Furthermore, PAH exposure causes an inflammatory response, the production of PAH-DNA adducts, and the suppression of DNA repair in the heart tissue, with larger PAH-DNA adducts in smokers' hearts and other tissues than non-smokers' (76). Moreover, oxidative stress-related mitochondrial DNA damage has been linked to the development of several atherogenic pathways (73). Adiposity has been linked to a greater risk of CVDs as an independent health risk factor (77, 78).
PAHs are prevalent environmental contaminants across the world, and chronic CVDs, including congestive heart failure, coronary artery disease, angina, heart failure, and stroke are the main globally public health concerns, the findings of our study have significant global health consequences. The positive exposure-response relationship between PAH exposure and the increased prevalence of CVDs from our study indicates that regulating atmospheric PAHs may be an efficient strategy to lower the increased risk of CVDs and prevent cardiovascular disease.
Limitations
Our study has some noteworthy limitations. First, the most notable was the absence of sufficient evidence to address a number of the review questions. Our ability to synthesize the available evidence was also limited by the variation in design, methodology, samples, analysis, and presentation of results of the included studies. However, given the limited number of studies available in the area, and the aim of our study to provide an overview of the literature, it was important to include all available evidence regardless of design. Second, our study is an only systematic review, our study is limited only to the selected database source and English-language publications. Third, although this analysis provides evidence for the harmful effects of PAH, we are unable to comment on the potential of interventions, such as PAH pathways and their effects on a cellular and molecular level. Fourth, we did not do a meta-analysis and have an adequate number of studies to pool to assess the association.
Prospects
This systematic review may be useful for future investigation and development of therapeutic strategies for the removal of PAHs from the environment. Most studies are from developed countries and it is important to highlights the impacts of PAH pollution in developing countries and identify the existing gap in human health and environmental exposures. In this area, longitudinal research types with long-term follow-up are important. However, our study provides important data from which future practice-changing prospective trials can be designed.
Conclusions
This systematic review examined the relationship of PAH exposure with CVDs and CVD-related risk factors by searching several digital databases. After a comprehensive literature searches and summarizing findings from 20 articles, the authors concluded that a positive relationship was observed between PAH exposure and CVD risks. Overall, epidemiological results in both occupational and the general population recommend potential relationships between environmental PAH exposure and CVDs, and other well CVDs risk factors.
Recommendations for Health and Environmental Protection
• Due to the widespread presence of PAHs in the environment and their toxicological significance, the assessment of exposure to PAHs is significant.
• The biological impact should be precisely monitored in terms of the total PAHs intake into the body via respiratory, dermal, and gastrointestinal routes.
• PAH exposure in occupational settings should be eliminated, or reduced due to its toxic effects
• Public awareness and education should be strengthened about the causes and health effects of PAH exposure.
• Air pollution should be documented throughout the year and not only just seasonally.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: Pubmed, Scopus and Web of Science.
Author Contributions
All authors contributed to conception and design, interpretation of the data, critical revision of the manuscript, and approval of the final version to be published.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors greatly acknowledge QZ for her guidance and insightful comments on this systematic review. We are very grateful to Dr. Saifullah Bhullo, Begum Nusarat Bhutto University, Sukkur and Dr. Muhammad Sohail, Nanjing Normal University, China for their quality assessment of this systematic review.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2021.763706/full#supplementary-material
References
1. Samanta SK, Singh OV, Jain KR. Polycyclic aromatic hydrocarbons: environmental pollution and bioremediation. Trends Biotechnol. (2002) 20:243–8. doi: 10.1016/S0167-7799(02)01943-1
2. Diggs DL, Huderson AC, Harris KL, Myers JN, Banks LD, Rekhadevi PV, et al. Polycyclic aromatic hydrocarbons and digestive tract cancers: a perspective. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. (2011) 29:324–57. doi: 10.1080/10590501.2011.629974
3. Burstyn I, Kromhout H, Partanen T, Svane O, Langard S, Ahrens W, et al. Polycyclic aromatic hydrocarbons and fatal ischemic heart disease. Epidemiology. (2005) 16:744–50. doi: 10.1097/01.ede.0000181310.65043.2f
4. Xu X, Hu H, Kearney GD, Kan H, Sheps SD. Studying the effects of polycyclic aromatic hydrocarbons on peripheral arterial disease in the United States. Sci Total Environ. (2013) 461:341–7. doi: 10.1016/j.scitotenv.2013.04.089
5. Xu X, Cook RL, Ilacqua VA, Kan H, Talbott EO, Kearney G. Studying associations between urinary metabolites of polycyclic aromatic hydrocarbons (PAHs) and cardiovascular diseases in the United States. Science of the total Environment. (2010) 408:4943–8. doi: 10.1016/j.scitotenv.2010.07.034
6. Gustavsson P, Plato N, Hallqvist J, Hogstedt C, Lewne M, Reuterwall C, et al. A population-based case-referent study of myocardial infarction and occupational exposure to motor exhaust, other combustion products, organic solvents, lead, and dynamite. Stockholm Heart Epidemiology Program (SHEEP) study group. Epidemiology. (2001) 12:222–8. doi: 10.1097/00001648-200103000-00015
7. Friesen MC, Demers PA, Spinelli JJ, Eisen EA, Lorenzi MF, Le DN. Chronic and acute effects of coal tar pitch exposure and cardiopulmonary mortality among aluminum smelter workers. Am J Epidemiol. (2010) 172:790–9. doi: 10.1093/aje/kwq208
8. Jeng HA, Pan CH, Diawara N, Chang-Chien GP, Lin WY, Huang CT, et al. Polycyclic aromatic hydrocarbon-induced oxidative stress and lipid peroxidation in relation to immunological alteration. Occup Environ Med. (2011) 68:653–8. doi: 10.1136/oem.2010.055020
9. Curfs DM, Knaapen AM, Pachen DM, Gijbels MJ, Lutgens E, Smook ML, et al. Polycyclic aromatic hydrocarbons induce an inflammatory atherosclerotic plaque phenotype irrespective of their DNA binding properties. FASEB J. (2005) 19:1290–2. doi: 10.1096/fj.04-2269fje
10. WHO Organization. Ambient air pollution: A global assessment of exposure and burden of disease (2016). Available online at: https://www.who.int/publications/i/item/9789241511353
11. Ravindra K, Sokhi R, Van Grieken R. Atmospheric polycyclic aromatic hydrocarbons: source attribution, emission factors and regulation. Atmosph Environ. (2008) 42:2895–921. doi: 10.1016/j.atmosenv.2007.12.010
12. Yan D, Wu S, Zhou S, Tong G, Li F, Wang Y, et al. Characteristics, sources and health risk assessment of airborne particulate PAHs in Chinese cities: A review. Environ Pollut. (2019) 248:804–14. doi: 10.1016/j.envpol.2019.02.068
13. Cohen AJ, Brauer M, Burnett R, Anderson HR, Frostad J, Estep K, et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. The Lancet. (2017) 389:1907–18. doi: 10.1016/S0140-6736(17)30505-6
14. Rabadán-Diehl C, Alam D, Baumgartner J. household air pollution in the early origins of cvd in developing countries. Glob Heart. (2012) 7:235–42. doi: 10.1016/j.gheart.2012.06.014
15. Alshaarawy O, Zhu M, Ducatman A, Conway B, Andrew EM. Polycyclic aromatic hydrocarbon biomarkers and serum markers of inflammation: a positive association that is more evident in men. Environ Res. (2013) 126:98–104. doi: 10.1016/j.envres.2013.07.006
16. Bhatnagar A. Environmental cardiology: studying mechanistic links between pollution and heart disease. Circ Res. (2006) 99:692–705. doi: 10.1161/01.RES.0000243586.99701.cf
17. Clark JD, Serdar B, Lee DJ, Arheart K, Wilkinson JD, Fleming EL. Exposure to polycyclic aromatic hydrocarbons and serum inflammatory markers of cardiovascular disease. Environ Res. (2012) 117:132–7. doi: 10.1016/j.envres.2012.04.012
18. Ellulu MS, Patimah I, Khaza'ai H, Rahmat A, Abed Y, Ali F. Atherosclerotic cardiovascular disease: a review of initiators and protective factors. Inflammopharmacology. (2016) 24:1–10. doi: 10.1007/s10787-015-0255-y
19. Feng Y, Sun H, Song Y, Bao J, Huang X, Ye J, et al. A community study of the effect of polycyclic aromatic hydrocarbon metabolites on heart rate variability based on the Framingham risk score. Occup Environ Med. (2014) 71:338–45. doi: 10.1136/oemed-2013-101884
20. Brucker N, Charao MF, Moro AM, Ferrari P, Bubols G, Sauer E, et al. Atherosclerotic process in taxi drivers occupationally exposed to air pollution and co-morbidities. Environ Res. (2014) 131:31–8. doi: 10.1016/j.envres.2014.02.012
21. Everett CJ, King DE, Player MS, Matheson EM, Post RE, Mainous AG. Association of urinary polycyclic aromatic hydrocarbons and serum C-reactive protein. Environ Res. (2010) 110:79–82. doi: 10.1016/j.envres.2009.09.010
22. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. (2009) 6:e1000100. doi: 10.1371/journal.pmed.1000100
23. Modesti PA, Reboldi G, Cappuccio FP, Agyemang C, Remuzzi G, Rapi S, et al. Panethnic differences in blood pressure in europe: a systematic review and meta-analysis. PLoS ONE. (2016) 11:e0147601. doi: 10.1371/journal.pone.0147601
24. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The newcastle-ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Clinical Neurol Neurosurg. (2014) 197:106183. Available online at: https://www.semanticscholar.org/paper/The-Newcastle-Ottawa-Scale-%28NOS%29-for-Assessing-the-Wells-Wells/c293fb316b6176154c3fdbb8340a107d9c8c82bf
25. Cao L, Wang D, Zhu C, Wang B, Cen X, Chen A, et al. Polycyclic aromatic hydrocarbon exposure and atherosclerotic cardiovascular disease risk in urban adults: The mediating role of oxidatively damaged DNA. Environ Pollut. (2020) 265:114860. doi: 10.1016/j.envpol.2020.114860
26. Lee TW, Kim DH, Ryu YJ. Association between urinary polycyclic aromatic hydrocarbons and hypertension in the Korean population: data from the Second Korean National environmental health survey (2012-2014). Sci Rep. (2020) 10:17142. doi: 10.1038/s41598-020-74353-w
27. Yang L, Guo W, Zeng D, Ma L, Lai X, Fang Q, et al. Heart rate variability mediates the association between polycyclic aromatic hydrocarbons exposure and atherosclerotic cardiovascular disease risk in coke oven workers. Chemosphere. (2019) 228:166–173. doi: 10.1016/j.chemosphere.2019.04.101
28. Hu C, Hou J, Zhou Y, Sun H, Yin W, Zhang Y, et al. Association of polycyclic aromatic hydrocarbons exposure with atherosclerotic cardiovascular disease risk: A role of mean platelet volume or club cell secretory protein. Environ Pollut. (2018) 233:45–53. doi: 10.1016/j.envpol.2017.10.042
29. Li N, Mu Y, Liu Z, Deng Y, Guo Y, Zhang X, et al. Assessment of interaction between maternal polycyclic aromatic hydrocarbons exposure and genetic polymorphisms on the risk of congenital heart diseases. Sci Rep. (2018) 8:3075. doi: 10.1038/s41598-018-21380-3
30. Yin W, Hou J, Xu T, Cheng J, Li P, Wang L, et al. Obesity mediated the association of exposure to polycyclic aromatic hydrocarbon with risk of cardiovascular events. Sci Total Environ. (2018) 617:841–54. doi: 10.1016/j.scitotenv.2017.10.238
31. Alhamdow A, Lindh C, Albin M, Gustavsson P, Tinnerberg H, Broberg K. Early markers of cardiovascular disease are associated with occupational exposure to polycyclic aromatic hydrocarbons. Sci Rep. (2017) 7:9426. doi: 10.1038/s41598-017-09956-x
32. Yang K, Jiang X, Cheng S, Chen C, Cao X, Tu B. Effects of coke oven emissions and benzo[a]pyrene on blood pressure and electrocardiogram in coke oven workers. J Occup Health. (2017) 59:1–7. doi: 10.1539/joh.15-0264-OA
33. Alshaarawy O, Elbaz HA, Andrew EM. The association of urinary polycyclic aromatic hydrocarbon biomarkers and cardiovascular disease in the US population. Environ Int. (2016) 89:174–8. doi: 10.1016/j.envint.2016.02.006
34. Yang B, Deng Q, Zhang W, Feng Y, Dai X, Feng W, et al. Exposure to polycyclic aromatic hydrocarbons, plasma cytokines, and heart rate variability. Sci Rep. (2016) 6:19272. doi: 10.1038/srep19272
35. Trasande L, Urbina EM, Khoder M, Alghamdi M, Shabaj I, Alam MS, et al. Polycyclic aromatic hydrocarbons, brachial artery distensibility and blood pressure among children residing near an oil refinery. Environ Res. (2015) 136:133–40. doi: 10.1016/j.envres.2014.08.038
36. Shiue I. Are urinary polyaromatic hydrocarbons associated with adult hypertension, heart attack, and cancer? USA NHANES, 2011-2012. Environ Sci Pollut Res Int. (2015) 22:16962–8. doi: 10.1007/s11356-015-4922-8
37. Bangia KS, Symanski E, Strom SS, Bondy M. A cross-sectional analysis of polycyclic aromatic hydrocarbons and diesel particulate matter exposures and hypertension among individuals of Mexican origin. Environ Health. (2015) 14:51. doi: 10.1186/s12940-015-0039-2
38. Ranjbar M, Rotondi MA, Ardern CI, Kuk LJ. Urinary Biomarkers of Polycyclic Aromatic Hydrocarbons Are Associated with Cardiometabolic Health Risk. PLoS ONE. (2015) 10:e0137536. doi: 10.1371/journal.pone.0137536
39. Jacobs L, Buczynska A, Walgraeve C, Delcloo A, Potgieter-Vermaak S, Van Grieken R, et al. Acute changes in pulse pressure in relation to constituents of particulate air pollution in elderly persons. Environ Res. (2012) 117:60–67. doi: 10.1016/j.envres.2012.05.003
40. Liu H, Xu C, Jiang ZY, Gu A. Association of polycyclic aromatic hydrocarbons and asthma among children 6-19 years: NHANES 2001-2008 and NHANES 2011-2012. Respir Med. (2016) 110:20–7. doi: 10.1016/j.rmed.2015.11.003
41. Rundle A, Hoepner L, Hassoun A, Oberfield S, Freyer G, Holmes D, et al. Association of childhood obesity with maternal exposure to ambient air polycyclic aromatic hydrocarbons during pregnancy. Am J Epidemiol. (2012) 175:1163–1172. doi: 10.1093/aje/kwr455
42. Cao L, Wang D, Wen Y, He H, Chen A, Hu D, et al. Effects of environmental and lifestyle exposures on urinary levels of polycyclic aromatic hydrocarbon metabolites: A cross-sectional study of urban adults in China. Chemosphere. (2020) 240:124898. doi: 10.1016/j.chemosphere.2019.124898
43. Muangchinda C, Rungsihiranrut A, Prombutara P, Soonglerdsongpha S, Pinyakong O. 16S metagenomic analysis reveals adaptability of a mixed-PAH-degrading consortium isolated from crude oil-contaminated seawater to changing environmental conditions. J Hazard Mater. (2018) 357:119–127. doi: 10.1016/j.jhazmat.2018.05.062
44. Li Z, Romanoff LC, Lewin MD, Porter EN, Trinidad DA, Needham LL, et al. Variability of urinary concentrations of polycyclic aromatic hydrocarbon metabolite in general population and comparison of spot, first-morning, and 24-h void sampling. J Expo Sci Environ Epidemiol. (2010) 20:526–535. doi: 10.1038/jes.2009.41
45. Yang L, Yan K, Zeng D, Lai X, Chen X, Fang Q, et al. Association of polycyclic aromatic hydrocarbons metabolites and risk of diabetes in coke oven workers. Environ Pollut. (2017) 223:305–310. doi: 10.1016/j.envpol.2017.01.027
46. Han B, Liu Y, You Y, Xu J, Zhou J, Zhang J, et al. Assessing the inhalation cancer risk of particulate matter bound polycyclic aromatic hydrocarbons (PAHs) for the elderly in a retirement community of a mega city in North China. Environ Sci Pollut Res Int. (2016) 23:20194–20204. doi: 10.1007/s11356-016-7209-9
47. Yi T, Wang J, Zhu K, Tang Y, Huang S, Shui X, et al. Aryl hydrocarbon receptor: a new player of pathogenesis and therapy in cardiovascular diseases. Biomed Res Int. (2018) 2018:6058784. doi: 10.1155/2018/6058784
48. Taleb S. Inflammation in atherosclerosis. Arch Cardiovasc Dis. (2016) 109:708–715. doi: 10.1016/j.acvd.2016.04.002
49. Poursafa P, Amin MM, Hajizadeh Y, Mansourian M, Pourzamani H, Ebrahim K, et al. Association of atmospheric concentrations of polycyclic aromatic hydrocarbons with their urinary metabolites in children and adolescents. Environ Sci Pollut Res Int. (2017) 24:17136–44. doi: 10.1007/s11356-017-9315-8
50. Poursafa P, Moosazadeh M, Abedini E, Hajizadeh Y, Mansourian M, Pourzamani H, et al. A Systematic Review on the Effects of Polycyclic Aromatic Hydrocarbons on Cardiometabolic Impairment. Int J Prev Med. (2017) 8:19. doi: 10.4103/ijpvm.IJPVM_144_17
51. Freitas F, Brucker N, Durgante J, Bubols G, Bulcão R, Moro A, et al. Urinary 1-hydroxypyrene is associated with oxidative stress and inflammatory biomarkers in acute myocardial infarction. Int J Environ Res Public Health. (2014) 11:9024–9037. doi: 10.3390/ijerph110909024
52. Liu G, Niu Z, Van Niekerk D, Xue J, Zheng L. Polycyclic aromatic hydrocarbons (PAHs) from coal combustion: emissions, analysis, and toxicology. Rev Environ Contam Toxicol. (2008) 192:1–28. doi: 10.1007/978-0-387-71724-1_1
53. Ramesh A, Walker SA, Hood DB, Guillén MD, Schneider K, Weyand HE. Bioavailability and risk assessment of orally ingested polycyclic aromatic hydrocarbons. Int J Toxicol. (2004) 23:301–33. doi: 10.1080/10915810490517063
54. Simko P. Factors affecting elimination of polycyclic aromatic hydrocarbons from smoked meat foods and liquid smoke flavorings. Mol Nutr Food Res. (2005) 49:637–47. doi: 10.1002/mnfr.200400091
55. Jia X, Song X, Shima M, Tamura K, Deng F, Guo X. Effects of fine particulate on heart rate variability in Beijing: a panel study of healthy elderly subjects. Int Arch Occup Environ Health. (2012) 85:97–107. doi: 10.1007/s00420-011-0646-3
56. Whitsel EA, Quibrera PM, Christ SL, Liao D, Prineas RJ, Anderson GL, et al. Heart rate variability, ambient particulate matter air pollution, and glucose homeostasis: the environmental epidemiology of arrhythmogenesis in the women's health initiative. Am J Epidemiol. (2009) 169:693–703. doi: 10.1093/aje/kwn400
57. Chen JC, Cavallari JM, Stone PH, Christiani CD. Obesity is a modifier of autonomic cardiac responses to fine metal particulates. Environ Health Perspect. (2007) 115:1002–6. doi: 10.1289/ehp.9609
58. Wheeler A, Zanobetti A, Gold DR, Schwartz J, Stone P, Suh HH. The relationship between ambient air pollution and heart rate variability differs for individuals with heart and pulmonary disease. Environ Health Perspect. (2006) 114:560–6. doi: 10.1289/ehp.8337
59. Luttmann-Gibson H, Suh HH, Coull BA, Dockery DW, Sarnat SE, Schwartz J, et al. Systemic inflammation, heart rate variability and air pollution in a cohort of senior adults. Occup Environ Med. (2010) 67:625–30. doi: 10.1136/oem.2009.050625
60. Incardona JP, Collier TK, Scholz LN. Defects in cardiac function precede morphological abnormalities in fish embryos exposed to polycyclic aromatic hydrocarbons. Toxicol Appl Pharmacol. (2004) 196:191–205. doi: 10.1016/j.taap.2003.11.026
61. Farwell A, Nero V, Croft M, Bal P, Dixon GD. Modified Japanese medaka embryo-larval bioassay for rapid determination of developmental abnormalities. Arch Environ Contam Toxicol. (2006) 51:600–7. doi: 10.1007/s00244-005-0319-x
62. Curfs DM, Lutgens E, Gijbels MJ, Kockx MM, Daemen MJ, van Schooten JF. Chronic exposure to the carcinogenic compound benzo[a]pyrene induces larger and phenotypically different atherosclerotic plaques in ApoE-knockout mice. Am J Pathol. (2004) 164:101–8. doi: 10.1016/S0002-9440(10)63101-X
63. Chen YY, Kao TW, Wang CC, Chen YJ, Wu CJ, Lai CH, et al. Exposure to polycyclic aromatic hydrocarbons and risk of disability among an elderly population. Environ Sci Pollut Res Int. (2019) 26:10719–26. doi: 10.1007/s11356-019-04498-3
64. Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO III, Criqui M, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the centers for disease control and prevention and the american heart association. Circulation. (2003) 107:499–511. doi: 10.1161/01.CIR.0000052939.59093.45
65. Goff DC, Lloyd-Jones DM, Bennett G, Coady S, D'Agostino RB, Gibbons R, et al. ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American college of cardiology/American heart association task force on practice guidelines. Circulation. (2014) 129:S49–73. doi: 10.1161/01.cir.0000437741.48606.98
66. Araujo JA, Barajas B, Kleinman M, Wang X, Bennett BJ, Gong KW, et al. Ambient particulate pollutants in the ultrafine range promote early atherosclerosis and systemic oxidative stress. Circulat Res. (2008) 102:589–96. doi: 10.1161/CIRCRESAHA.107.164970
67. Iwano S, Shibahara N, Saito T, Kamataki T. Activation of p53 as a causal step for atherosclerosis induced by polycyclic aromatic hydrocarbons. FEBS Lett. (2006) 580:890–3. doi: 10.1016/j.febslet.2006.01.009
68. Hu H, Kan H, Kearney GD, Xu X. Associations between exposure to polycyclic aromatic hydrocarbons and glucose homeostasis as well as metabolic syndrome in nondiabetic adults. Sci Total Environ. (2015) 505:56–64. doi: 10.1016/j.scitotenv.2014.09.085
69. Scinicariello F, Buser MC. Urinary polycyclic aromatic hydrocarbons and childhood obesity: nhanes (2001–2006). Environ Health Perspect. (2014) 122:299–303. doi: 10.1289/ehp.1307234
70. Sun Q, Yue P, Ying Z, Cardounel AJ, Brook RD, Devlin R, et al. Air pollution exposure potentiates hypertension through reactive oxygen species-mediated activation of Rho/ROCK. Arterioscler Thromb Vasc Biol. (2008) 28:1760–6. doi: 10.1161/ATVBAHA.108.166967
71. Yan Z, Zhang H, Maher C, Arteaga-Solis E, Champagne FA, Wu L, et al. Prenatal polycyclic aromatic hydrocarbon, adiposity, peroxisome proliferator-activated receptor (PPAR) γ methylation in offspring, grand-offspring mice. PLOS ONE. (2014) 9:e110706. doi: 10.1371/journal.pone.0110706
72. Irigaray P, Lacomme S, Mejean L, Belpomme D. Ex vivo study of incorporation into adipocytes and lipolysis-inhibition effect of polycyclic aromatic hydrocarbons. Toxicol Lett. (2009) 187:35–39. doi: 10.1016/j.toxlet.2009.01.021
73. Pulliero A, Godschalk R, Andreassi MG, Curfs D, Van Schooten FJ, Izzotti A. Environmental carcinogens and mutational pathways in atherosclerosis. Int J Hyg Environ Health. (2015) 218:293–312. doi: 10.1016/j.ijheh.2015.01.007
74. Wang ZJ, Zhou YJ, Galper BZ, Gao F, Yeh RW, Mauri L. Association of body mass index with mortality and cardiovascular events for patients with coronary artery disease: a systematic review and meta-analysis. Heart. (2015) 101:1631–8. doi: 10.1136/heartjnl-2014-307119
75. Penning TM, Drury JE. Human aldo–keto reductases: Function, gene regulation, and single nucleotide polymorphisms. Archiv Biochemistr Biophysic. (2007) 464:241–50. doi: 10.1016/j.abb.2007.04.024
76. Singh R, Sram RJ, Binkova B, Kalina I, Popov TA, Georgieva T, et al. The relationship between biomarkers of oxidative DNA damage, polycyclic aromatic hydrocarbon DNA adducts, antioxidant status and genetic susceptibility following exposure to environmental air pollution in humans. Mutat Res Fundament Mol Mechan Mutagen. (2007) 620:83–92. doi: 10.1016/j.mrfmmm.2007.02.025
77. Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, et al. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific statement on obesity and heart disease from the obesity committee of the council on nutrition, physical activity, and metabolism. Circulation. (2006) 113:898–918. doi: 10.1161/CIRCULATIONAHA.106.171016
78. Dale CE, Fatemifar G, Palmer TM, White J, Prieto-Merino D, Zabaneh D, et al. Causal associations of adiposity and body fat distribution with coronary heart disease, stroke subtypes, and type 2 diabetes mellitus: a mendelian randomization analysis. Circulation. (2017) 135:2373–2388. doi: 10.1161/CIRCULATIONAHA.116.026560
Keywords: cardiovascular diseases, hypertension, heart rate variability, polycyclic aromatic hydrocarbons, PAH exposure
Citation: Mallah MA, Mallah MA, Liu Y, Xi H, Wang W, Feng F and Zhang Q (2021) Relationship Between Polycyclic Aromatic Hydrocarbons and Cardiovascular Diseases: A Systematic Review. Front. Public Health 9:763706. doi: 10.3389/fpubh.2021.763706
Received: 24 August 2021; Accepted: 08 November 2021;
Published: 07 December 2021.
Edited by:
Guang Hao, Jinan University, ChinaReviewed by:
Fangchao Liu, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaNiti B. Jadeja, Ashoka Trust for Research in Ecology and the Environment (ATREE), India
Copyright © 2021 Mallah, Mallah, Liu, Xi, Wang, Feng and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiao Zhang, zhangqiao@zzu.edu.cn
 Manthar Ali Mallah
Manthar Ali Mallah Mukhtiar Ali Mallah2
Mukhtiar Ali Mallah2 Qiao Zhang
Qiao Zhang