- 1Department of Psychiatry and Behavioral Sciences, Northwestern University Feinberg School of Medicine, Chicago, IL, United States
- 2Department of Psychiatry and Behavioral Sciences, University of Washington School of Medicine, Seattle, WA, United States
- 3Department of Neuroscience, Northwestern University Feinberg School of Medicine, Chicago, IL, United States
Antipsychotic drugs are still widely prescribed to control various severe neuropsychiatric symptoms in the elderly and dementia patients although they are off-label use in the United States. However, clinical practice shows greater side effects and lower efficacy of antipsychotics for this vulnerable population and the mechanisms surrounding this aged-related sensitivity are not well understood. Our previous studies have shown that aging-induced epigenetic alterations may be involved in the increasing severity of typical antipsychotic haloperidol induced side effects in aged mice. Still, it is unknown if similar epigenetic mechanisms extend to atypical antipsychotics, which are most often prescribed to dementia patients combined with severe neuropsychiatric symptoms. In this study, we report that atypical antipsychotic risperidone also causes increased motor side effect behaviors in aged mice and 5xFAD mice. Histone deacetylase (HDAC) inhibitor Valproic Acid and Entinostat can mitigate the risperidone induced motor side effects. We further showed besides D2R, reduced expression of 5-HT2A, one of the primary atypical antipsychotic targets in the striatum of aged mice that are also mitigated by HDAC inhibitors. Finally, we demonstrate that specific histone acetylation mark H3K27 is hypoacetylated at the 5htr2a and Drd2 promoters in aged mice and can be reversed with HDAC inhibitors. Our work here establishes evidence for a mechanism where aging reduces expression of 5-HT2A and D2R, the key atypical antipsychotic drug targets through epigenetic alteration. HDAC inhibitors can restore 5-HT2A and D2R expression in aged mice and decrease the motor side effects in aged and 5xFAD mice.
Introduction
Though antipsychotics are primarily selected for treatment of psychoses, these pharmaceuticals are often being applied to control the severe neuropsychiatric symptoms in dementia patients with an off-label use in the United States (1–3). Alzheimer’s Disease (AD), the most common type of dementia, encompasses 60–80% of all dementias (4, 5). Over 90% of neuropsychiatric symptoms occur in AD patients, making these symptoms an integral part of this disease (6). As the US population is progressing toward a record number of individuals reaching advanced age, the number of AD cases are likely to rise precipitously, reflecting potential increased use of antipsychotics to treat these symptoms. Despite the frequent off-label use of antipsychotics in the elderly, these drugs have been shown to have decreasing efficacy and increased side-effects specifically in elderly populations, most notably with the emergence of higher risk of developing extrapyramidal side effects, stroke, and death (7, 8). Recent concern regarding the higher mortality rate observed in elderly patients prescribed antipsychotic drugs has prompted the FDA to require a “black box” warning on marketing materials (9, 10). The mechanism of these increased side-effects and lower efficacy is likely multifactorial, with pharmacokinetic and pharmacodynamic changes indicated (11, 12). Of these mechanisms, epigenetic influences at gene promoters for antipsychotic targets seems to be a key driver of the narrowing therapeutic window with age (13–15). In particular, our group has demonstrated that reduced dopamine receptor 2 (D2R; gene name Drd2) expression leads to increased extrapyramidal side effects with the typical antipsychotic haloperidol (14, 15). This reduced expression of D2R is driven by hypoacetylation of histones at the gene promoter for Drd2 in the striatum and reversal of the hypoacetylation with histone deacetylase (HDAC) inhibitors leads to decreased extrapyramidal side effects, specifically in aged but not in young mice (14, 16). Combined treatment of antipsychotic with HDAC inhibitor to widen the therapeutic window represents a novel strategy to increase functionality and reduce side-effects with these drugs in the elderly population (11, 15).
Despite this initial promising work with typical antipsychotics, the vast majority of elderly patients are treated with atypical antipsychotics, such as risperidone, olanzapine, and quetiapine (17). Although the receptor profiles for every antipsychotic are complex, typical antipsychotics favor blockade of D2R while atypical antipsychotics display greater antagonism of the serotonin receptor 2A (5-HT2A, gene name 5htr2a) (18, 19), much less antagonism of the D2R. While in general atypical antipsychotics display fewer extrapyramidal side effects and greater safety profiles overall in the elderly (20, 21), the motor side effects of these drugs are still a significant concern in clinical practice (2). In this study, we determined the severity of risperidone induced motor side effects with dose response in aged mice and 5xFAD mice, a common mouse model of AD (22). Additionally, we investigated whether adjunct HDAC inhibitor treatment with risperidone could mitigate such side effects. Risperidone was selected as it is the only federally approved drug in Europe to treat neuropsychiatric symptoms in AD (23) and has the higher rate of extrapyramidal side effects compared to other atypical antipsychotics (24, 25). We further investigated how 5-HT2A and D2R expression changes during aging, and after HDAC inhibitor administration. Finally, we characterized histone acetylation mark H3K27ac at the 5htr2a and Drd2 promoter to determine whether these epigenetic changes could be implicated in the differences in atypical antipsychotic efficacy and tolerability in the elderly.
Materials and methods
Animals
Young (2–3 months old, n = 6–8 per group) and aged (20–24 months, n = 5–6 per group) C57BL/6 J mice and 5xFAD mice and wildtype (WT) littermates at 11 months of age (n = 8–19) of both sexes from JAX laboratory [B6SJL-Tg(APPSwFlLon,PSEN1*M146L*L286V)6799Vas/Mmjax] were used for this study (RRID:MMRRC_034840-JAX). Animals were group housed on a 12-hour light/dark cycle and given food and water ad libitum. All procedures were performed according to NIH guidelines for the treatment of animal subjects and the Current Guide for the Care and Use of Laboratory Animals (2011, eighth edition) under a protocol approved by the Northwestern University Animal Care and Use Committee.
Drugs
All drugs were purchased from Sigma (St. Louis, MO, USA). Risperidone (RIS; 0.01, 0.5, and 0.1 mg/kg) was prepared in 0.9% saline with pH adjusted to 5–6, titrated with 0.1 M NaOH. Valproic Acid (VPA, 400 mg/kg) was dissolved in 0.9% saline with a pH of 7.3 and Entinostat (MS-275, 10 mg/kg) was dissolved in DMSO and 0.9% saline with pH adjusted to 5–6 (13, 14). Vehicle (Veh) was used as control. All treatments were administered by intra-peritoneal injection (IP) at a constant volume of 10 ul/g of body weight once a day for 14 consecutive days. VPA or MS-275 was injected 30 min before RIS administration.
Behavioral tests
Prior to behavioral procedures, animals were acclimated for at least 30 min to a soundproof behavioral testing room. Assays were performed during the light portion of the 24-hour light/dark cycle. Arenas and behavioral testing equipment were cleaned with 70% ethanol between each trial.
Catalepsy
Catalepsy is widely used for testing extrapyramidal side effects induced by antipsychotics in preclinical studies (26–29) including our group (14). We performed the catalepsy assay as described in our previous publications (14, 16). Briefly, a 1-cm diameter plastic rod was suspended 3.5 cm above a laboratory bench. Thirty minutes after all drug administration, the front paws of the mouse were placed on the rod while the hind paws rested on the bench. The duration of a cataleptic episode was defined and recorded as the latency to remove the forepaws from the rod during a 300-s (5 min) trial.
Open field
The open field test was applied for evaluation of the locomotor activity (14). Briefly, each mouse was placed in a Plexiglass box (25 cm × 25 cm × 25 cm) that was evenly illuminated in a soundproof testing room. Any-Maze Automated tracking system was used to track open field locomotor activity. Locomotion was measured as distance traveled in centimeters during a 10-minute period.
Rotarod
The TSE Rotarod System (Bad, Homburg, Germany) was used to assess motor coordination after drug administration. Mice were placed on an accelerating rod (4–40 rpm during the first 5 min) for 10 min, and the latency to fall from the rod was recorded. A total of three trials were conducted with a 10-min inter-trial interval. The average latency to fall from the rod across the three trials was calculated and used for comparison (14, 30).
Western blot
After behavioral test, the brain tissues were collected through a cardiac perfusion with 0.1 M PBS solution for 1 min, to wash-out the blood from blood vessels in the mouse brain. The brains were then removed and quickly dissected under ice with a dissecting scope. Tissues were frozen at −80°C until ready for processing for molecular analysis. For immunoblotting measuring the 5-HT2A and D2R expression levels, striatum tissue samples were collected and homogenized in an ice-cold RIPA buffer (Sigma, R0278) and centrifuged at 12,000 rpm for 20 min at 4°C. Protein quantification was measured using Pierce™ BCA Protein Assay Kit (ThermoFisher Scientific, 23225) according to protocol provided by the manufacturer. Polyvinylidene difluoride (PVDF) membranes (Biorad, 1620177) were used to transfer protein samples. Blots were blocked for 1-hour with 5% non-fat dry milk (Biorad, 1706404) and incubated using 1:1,000 5HT2A (Boster Biological, PA1373), 1:500 D2R (Millipore, AB5084P), or 1:1000 B-actin (Santa Cruz, sc-47778) overnight at 4°C. Blots were then incubated for 2 h at room temperature using secondary 1:3,000 goat anti-rabbit (Biorad, 170-6515) or 1:3,000 goat anti-mouse (Biorad, 172-1011). Blotted protein bands were detected using ChemiDoc imaging system (Biorad) and quantified using Image J Software. The levels of 5HT2A and D2R protein expression were normalized to β-actin (14, 16).
Chromatin immunoprecipitation (ChIP) assay
For measuring histone acetylation marks, the Magna ChIP G Tissue Kit (Millipore, 17-20000) assay was used and performed according to the kit protocol. Fragmented chromatin lysate was precipitated with 2–3 ul of antibody for H3K27ac (Abcam, ab4729). The DNA-histone complex was incubated with salmon sperm DNA/protein A-agarose beads overnight to estimate non-specific binding. The DNA-histone complex was eluted from the beads and dissociated at 65° for 4 h in a water bath under high-salt conditions. Proteins were digested using proteinase-K treatment, and the associated DNA was precipitated with 100% ethanol and re-suspended in 50 ul of PCR-grade water (14, 16).
Quantitative real-time PCR (qRT-PCR)
Levels of interaction between modified histones and the 5htr2a gene promoter were determined by measuring the amount of histone-associated DNA, isolated via chromatin immunoprecipitation (ChIP) and quantified using quantitative real-time PCR (qRT-PCR). We used custom primers for the 5-HT2A promoter (forward: 5′CTGGACCGCTACGTGGCTAT-3, reverse: 5′TATGGTCCACACCGCAATGA-3′), Drd2 promoter (forward: 5′-CTGGAGCCAAAAGCAGTCTG-3′, reverse: 5′-TCCTTCAGGTTTCCGACGCC-3′), and the b-actin promoter (forward: 5′-GCGTCCACCCGCGAGTACAA-3′, reverse: 5′-TCCATGGCGAACTGGTGGCG-3′) used as a control. Input and immunoprecipitated DNA amplification reactions were run in triplicate in the presence of SYBR Green (Applied Biosystems, 4472908) using an ABI Prism 7,700 thermocycler. Ct values from each sample were obtained using the Sequence Detector 1.1 software. Ct values were normalized to the endogenous gene, b-actin, to obtain a percent input. Fold differences (drug-treated vs. control) were then determined using the △△Ct method (14, 16).
Statistical analysis
All statistical analyses were conducted using Graph Prism Pad (San Diego, CA, USA). Data are expressed as mean ± standard error of the mean (S.E.M.). Catalepsy, locomotor activity, and rotarod data were analyzed using two-way analysis of variance (ANOVA) to detect age, treatment, and interaction effects followed by a multiple comparison analysis using Bonferroni’s post-hoc method. Significance was set at p < 0.05.
Results
Risperidone induces greater motor side effects in aged mice with dose response manner
In our study, we found that chronic RIS administration in aged mice displayed more severe motor side effects similar to the typical antipsychotic drug haloperidol measured through the behavioral tests we have applied before (14, 16).
Catalepsy
Two-way ANOVA showed significant effects of age (F1,47 = 32.88, p < 0.0001), drug (F3,47 = 24.25, p < 0.0001), and age and drug interaction (F3,47 = 7.06, p = 0.0005) on the duration of cataleptic episodes. In aged groups, post-hoc analysis revealed significant increases in the duration of cataleptic episodes at all doses selected in RIS as compared to their age-matched control mice (all p < 0.0001). There was no difference of cataleptic episodes between RIS treated groups (0.1–1.0 mg/kg) (Figure 1A). In addition, aged mice showed a greater degree of catalepsy than young mice at each RIS dose administered. In young groups, RIS induced cataleptic episodes are increased with dose response displaying a significant increase in the duration of cataleptic episodes at dose of 1.0 mg/kg (p = 0.0005). These data are comparable to the results we observed with haloperidol in aged mice (13, 14).
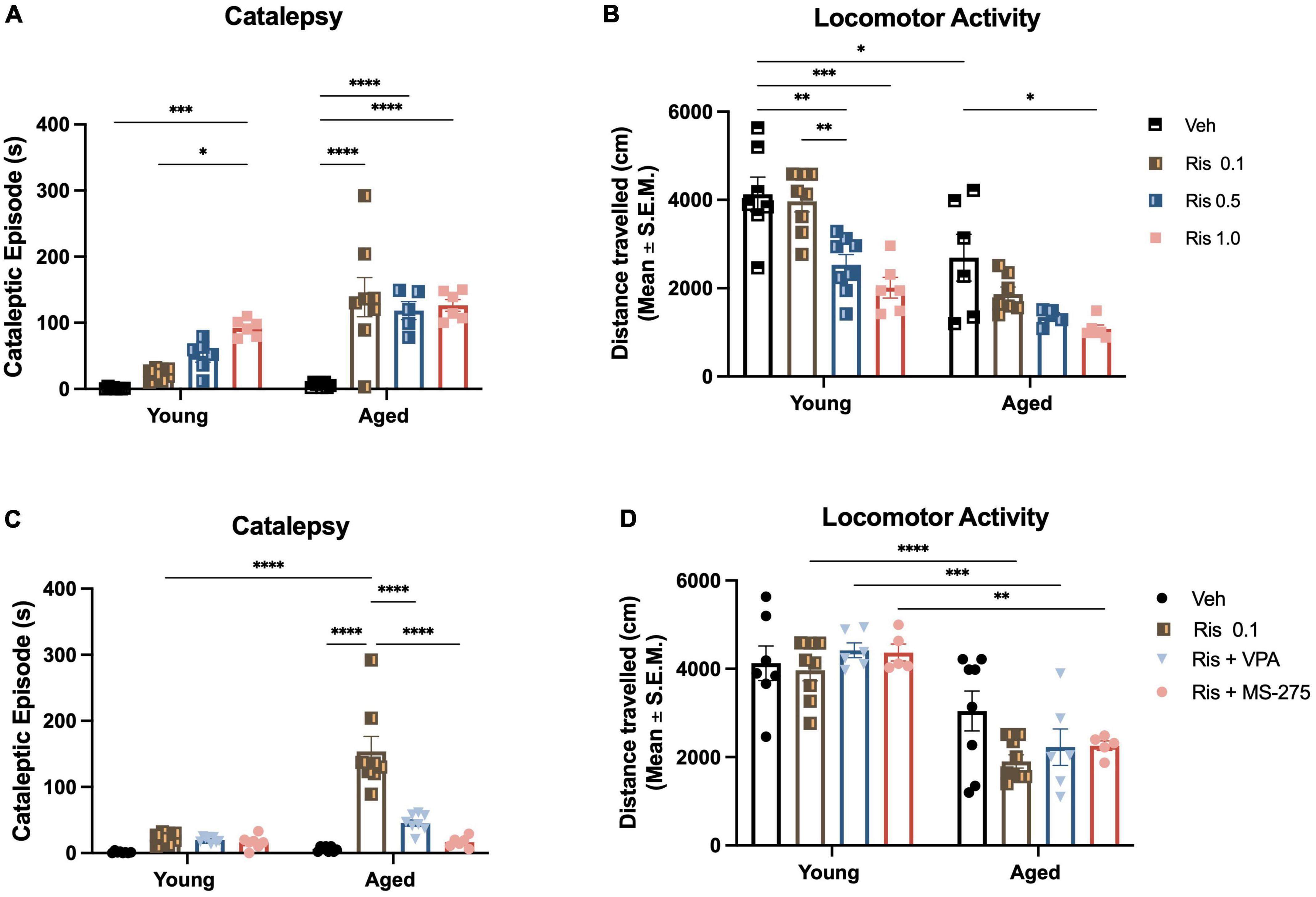
Figure 1. Risperidone induces a dose response of motor side effects in aged mice and histone deacetylase (HDAC) inhibitors could prevent the side effects. (A) Motor side effects measured by cataleptic episode in 5 min. Lower dose of RIS at 0.1 mg/kg already showed a significant increase in cataleptic behavior in aged mice but not young mice. However, the higher doses of RIS could cataleptic behavior in both young and aged mice, although more severe in aged mice. (B) General locomotor activity measured by open field. Again, the lower dose of RIS at 0.1 mg/kg displayed a decrease in the distance traveled in aged mice but not in young group. However, higher doses of RIS at 0.5 and 1.0 mg/kg induced the decreased in the distance traveled with a dose respond manner. (C) Co-treatment of lower dose of RIS (0.1 mg/kg) with VPA (400 mg/kg) or MS-275 (10 mg/kg) reduced the duration of catalepsy in aged mice. (D) Lower dose of RIS (0.1 mg/kg) with VPA (400 mg/kg) or MS-275 (10 mg/kg) did mitigate the RIS-induced decrease in locomotor activity in the young but not old mice. Data represent mean ± SEM (n = 6–8/group). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Locomotor activity
Two-way ANOVA showed significant effects of age (F1,44 = 45.00, p < 0.001) and drug (F3,44 = 16.64, p < 0.001) but no interaction between age and drug (F3,44 = 1.55, p = 0.2155) on the distance traveled in the open field. In the aged groups, RIS- induced decrease in the distance traveled displayed a dose response with higher doses being more significant (0.5 mg/kg, p < 0.05 and 1.0 mg/kg, p < 0.01) (Figure 1B). In young groups, the decrease in the distance traveled also displayed a dose response (0.5 mg/kg, p < 0.01; 1.0 mg/kg p < 0.001). However, there were no significance in the distance traveled between young and aged group.
HDAC inhibitor mitigate the risperidone induced motor side effects
After behavior tests for dosing response of RIS in young and aged mice, we determined whether HDAC inhibitor could reverse such effect. We selected the dose of RIS at 0.1 mg/kg as this dose is comparable to the dose that use for elderly patients and showed the motor side effects in aged mice but not young mice.
Catalepsy
Two-way ANOVA showed significant effects of age (F1,53 = 41.11, p < 0.0001), drug (F3,53 = 36.35, p < 0.0001), and age and drug interaction (F3,53 = 24.28, p < 0.0001) on the duration of cataleptic episodes. Specifically, post-hoc analysis demonstrated that either VPA or MS-275 can reduce the duration of catalepsy for the aged mice given a 0.1 mg/kg dose of RIS (both p < 0.0001) (Figure 1C). There were no differences detected between VPA or MS-275 on RIS-induced catalepsy duration. Similar to our results for haloperidol, these data demonstrate that atypical antipsychotic-induced catalepsy can be mitigated with adjunct HDAC inhibitor treatment.
Locomotor activity
Interestingly, Two-way ANOVA did not show any significant effects of age (F1,47 = 69.31, p < 0.0001), drug (F3,47 = 1.76, p = 0.1673), or interaction of drug and age (F3,47 = 1.50, p = 0.2263) in the distance traveled. Post-hoc analysis showed there were no differences in the distance traveled in the Veh treated groups between young and aged groups. RIS administration significantly decreased the distance traveled in the aged mice as compared to young mice (p < 0.001) (Figure 1D). However, adjunct treatment RIS with HDAC inhibitor VPA and MS275 did not mitigate the RIS-induced decrease in locomotor activity in aged mice (Figure 1D). Similar to our previous work with haloperidol, the atypical antipsychotic RIS reduced locomotion but this was not reversed with either HDAC inhibitor.
Risperidone induces greater motor side effects in 5xFAD mice that can be reversed with HDAC inhibitors
Antipsychotics are often prescribed to dementia patients to control some severe behavioral and psychological symptoms, such as agitation, aggression, and psychosis Yurusa (31). In this study, we evaluated whether the typical antipsychotic drug RIS could induce more severe side effects on 5xFAD mice at 12 months of age, when the memory deficits and neuropathology manifest, and whether HDAC inhibitors could reverse such side effects.
Catalepsy
Two-way ANOVA revealed significant effects of genotype (F3,111 = 25.90, p < 0.0001) and drug (F3,111 = 14.21, p < 0.0001), and genotype and drug interaction (F3,111 = 56.13, p < 0.0001) on cataleptic episodes (Figure 2A). Post-hoc analysis revealed 5xFAD mice but not WT mice administered RIS displayed severe cataleptic behavior compared to 5xFAD Veh treated mice, and WT RIS treated mice (both p < 0.0010). However, 5xFAD mice co-treated with MS-275 showed a significant decrease in cataleptic behavior, when compared to 5xFAD mice treated with RIS alone (p < 0.0001). These results suggest selective vulnerability of atypical antipsychotic drug induced cataleptic behavior in 5xFAD mice compared to WT controls, and co-treatment with MS-275 can reduce RIS-induced catalepsy in 5xFAD mice.
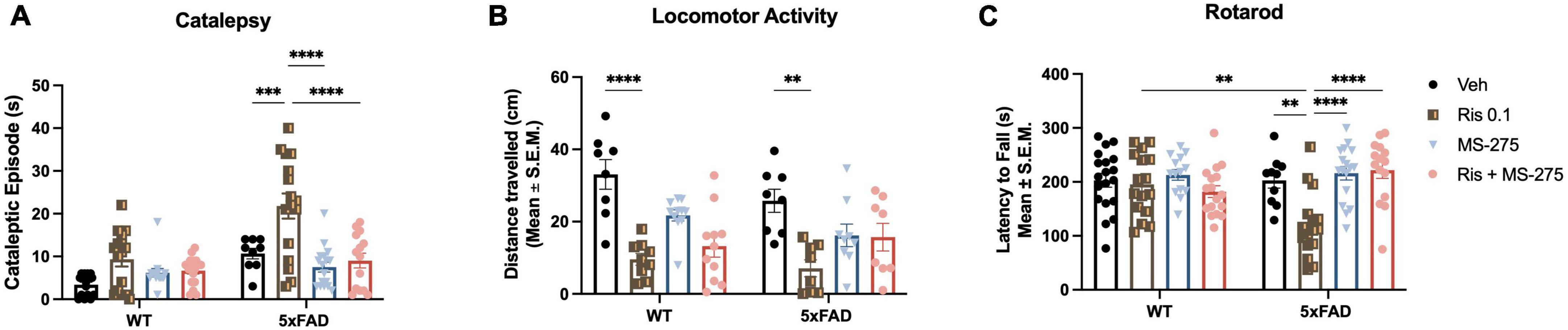
Figure 2. Risperidone induces motor side effects in 5xFAD mice and histone deacetylase (HDAC) inhibitor could prevent the side effects. (A) Lower dose of RIS (0.1 mg/kg) induced the cataleptic episode in 5xFAD but not wild type (WT) mice at 12 months of age. Co-treatment of RIS with MS-275 significantly reduced cataleptic episodes of 5xFAD mice. (B) RIS induced reduced general locomotor function in both WT and 5xFAD mice measured by the distance traveled in the open field. However, co-treatment of RIS with MS-275 did not improve general locomotor activity. (C) RIS also effected on motor coordination in 5xFAD mice but not WT controls by decreasing the latency to fall measured the rotarod. Co-treatment of RIS with MS-275 significantly improved motor coordination. Data represent mean ± SEM (n = 8–19/group). **p < 0.01, ***p < 0.001, ****p < 0.0001.
Locomotor activity
Two-way ANOVA only revealed a significant effect of drug (F3,64 = 17.36, p < 0.0001) but not genotype and drug and genotype interaction in the total distance traveled (Figure 2B). Post-hoc analysis showed a significant decreased in the total distance traveled in WT RIS treated mice compared to WT Veh treated mice (p < 0.0001). Similarly, 5xFAD RIS treated mice showed a significant decrease in the total distance traveled compared to 5xFAD Veh treated mice (p = 0.0014). No significant difference was found between Veh and RIS treated groups between WT and 5xFAD mice. Co-administration of RIS and MS-275 did not significantly improve general locomotor activity. These results suggest that general locomotor activity declines with chronic RIS administration, regardless of genotype, however, chronic administration of MS-275 is not sufficient to improve general locomotion in both RIS treated WT and 5xFAD mice.
Rotarod
Two-way ANOVA revealed a significant effect of drug but not genotype (F3,116 = 7.27, p < 0.0001), and a genotype and drug interaction (F3,116 = 6.90, p < 0.0001) on the latency to fall from the rod (Figure 2C). Post-hoc analysis showed a significant decline of motor coordination in 5xFAD mice treated with RIS compared to Veh (p < 0.01), however, no significant difference in motor coordination was found in WT RIS treated mice compared to WT Veh treated mice. Additionally, RIS treated 5xFAD mice showed a significant decrease in motor coordination when compared to RIS treated WT mice (p < 0.01). Furthermore, co-treatment of RIS and MS-275 showed a significant increase of motor coordination in 5xFAD mice compared to RIS treated 5xFAD mice (p < 0.0001). These results suggest atypical antipsychotic drug induced motor coordination dysfunction specifically in 5xFAD mice and co-treatment with MS-275 can improve motor coordination in RIS treated 5xFAD mice.
Aging decreased 5-HT2A expression in the striatum in aged mice and HDAC inhibitors reversed this expression
Atypical antipsychotics primarily target 5-HT2A, therefore, we examined whether this receptor is decreased in aged mice and whether RIS administration differentially affects expression of this receptor with aging. We analyzed 5-HT2A expression in the striatum via western blot in young and aged mice treated with Veh, RIS, and HDAC inhibitors VPA or MS-275. Two-way ANOVAs showed significant effects of age (F1,32 = 15.90, p = 0.0004), drug (F3,32 = 5.68, p = 0.0031) and age and drug interaction (F1,32 = 4.59, p = 0.0088) on 5HT2A protein levels in the striatum. Post-hoc testing indicated a significantly decreased 5-HT2A expression in aged mice compared to young mice for Veh treated groups (p = 0.0112). Moreover, RIS induced a significant decrease in 5-HT2A protein levels in the striatum in aged mice (p < 0.05) but not young mice although young mice displayed a tendency of decreased 5-HT2A expression but not significant (Figures 3A, C). However, in aged but not young mice, significant increases in 5-HT2A protein levels were found in co-treated RIS and MS-275 (p = 0.0124) mice and a trend of increased 5-HT2A protein levels in co-treated RIS and VPA mice as compared to those treated with RIS alone. Overall, the atypical antipsychotic target 5-HT2A is reduced with aging, and chronic treatment of RIS further reduces 5-HT2A expression, which can be restored with HDAC inhibitor in old but not young mice in the striatum as compared to RIS group (Figures 3A, C).
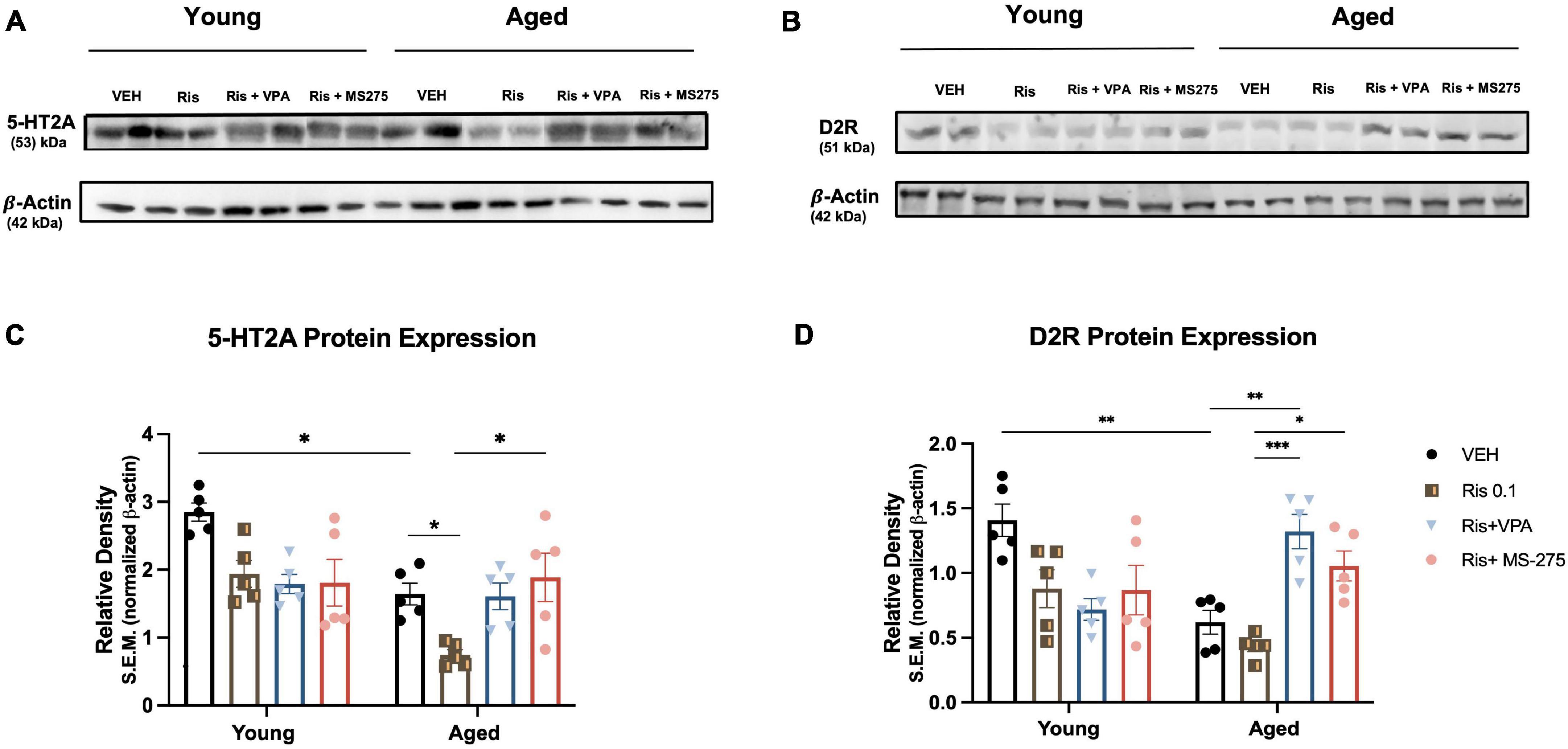
Figure 3. Effects of histone deacetylase (HDAC) inhibitors on 5-HT2A and D2R protein expression in the striatum of young and aged mice. (A, C) 5-HT2A expression was significantly decreased in the striatum in aged mice but not young mice, RIS further induced decreased expression in of 5-HT2A expression in aged mice. Co-treatment of RIS with VPA and MS-275 could restore 5-HT2A expression in aged mice. (B, D) D2R expression was decreased in the striatum in aged mice as compared to young mice administration of both VPA and MS-275 with RIS significantly increased D2R expression in the striatum as compared to Veh and RIS treated aged mice. Data represent mean ± SEM (n = 5). *p < 0.05, **p < 0.01, ***p < 0.001.
Aging alters D2R expression in the striatum in aged mice and HDAC inhibitors reversed this expression
In addition to 5-HT2A, atypical antipsychotics also target D2R, therefore, in this study we also measured D2R expression in the striatum. Two-way ANOVA revealed significant effect of drug (F3,32 = 3.93, p = 0.0017) but not age (F1,32 = 1.62, p = 0.2119), and an age and drug interaction (F3,32 = 12.79, p < 0.0001) in D2R expression in the striatum (Figures 3B, D). Post-hoc analysis showed a significant decrease in D2R expression in aged mice as compared to young mice in Veh treated groups (p = 0.0018). Administration of RIS showed a trend of decreased D2R expression, however, it was not significant in both ages. Administration of either VPA or MS-275 with RIS significantly increased D2R expression in the striatum of aged mice as compared to Veh (p = 0.001) and RIS treated (p < 0.0001) aged groups. No significant differences were found in young mice between treatment groups.
HDAC inhibitor increases histone acetylation at the 5htr2a gene promoter in young and aged mice
We previously found that histone hypoacetylation at the Drd2 gene promoter in the striatum led to the reduced protein expression and lower tolerability of typical antipsychotic drug haloperidol (14, 16). To determine whether similar changes of histone modification can be observed on the 5htr2a gene with aging, we used ChIP to measure histone acetylation marker H3K27ac, at the 5htr2a gene promoter in the striatum of young and aged mice treated with combinations of Veh, RIS, and HDAC inhibitors. Two-way ANOVA showed significant effect of drug (F3,32 = 28.52, p < 0.0001) and an interaction of drug and age (F3,32 = 4.86, p = 0.0066), but not age (F1,32 = 0.00015, p = 0.9902), in the binding of H3K27ac to the 5htr2a promoter. Post-hoc analysis found no significant differences of H3K27ac level binding to the 5htr2a promoter in Veh treated groups between young and aged mice, but RIS did show a trend of decreasing H3K27ac levels in both groups. Interestingly, a significant increase in H3K27ac level binding to the 5htr2a promoter was found in young and aged mice co-treated with RIS and VPA (p < 0.001) and RIS and MS-275 (p < 0.001) compared to those treated with Veh or RIS alone (Figure 4A).
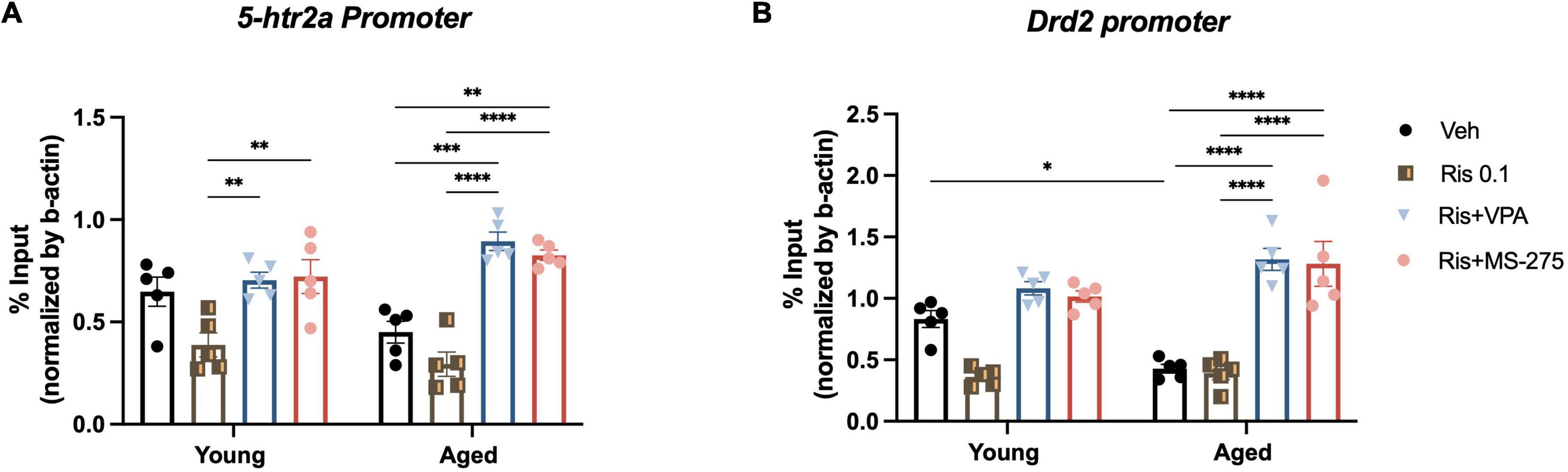
Figure 4. Effects of histone deacetylase (HDAC) inhibitors on histone acetylation at the 5htr2a and Drd2 gene promoters in the striatum in young and aged mice. (A) No significant differences of H3K27ac binding at the 5htr2a promoter between young and aged group was found, but a significant increase in H3K27ac binding at the 5ht2a promoter in the striatum was found in aged and young mice co-treated with RIS and VPA or MS-275 compared to those treated with RIS alone. (B) H3K27ac binding at the Drd2 promoter was significantly decreased in aged mice as compared to young mice in Veh treated group. However, H3K27ac binding at the Drd2 promoter in the striatum was observed to be significantly increased in aged mice co-treated with RIS and HDAC inhibitors VPA or MS-275 when compared to those treated with Veh alone. Data are presented as mean ± SEM, (n = 5). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
HDAC inhibitor decreases RIS induced-motor side effect through restoring histone acetylation at the Drd2 gene promoter in aged mice
Given Drd2 plays a significant role in antipsychotic induced side effects, we then investigated if the severe motor side effects observed in RIS treated mice are due to hypoacetylation at Drd2 promoter resulting in the decreased D2R expression and function. ChIP was used to measure histone acetylation marker H3K27ac levels at the Drd2 gene promoter in the striatum in aged and young mice treated with Veh, RIS, and HDAC inhibitors. Two-way ANOVA showed significant effect of drug (F3,32 = 46.13, p < 0.0001), and a drug and age interaction (F3,32 = 6.84, p = 0.0011), but not age (F1,32 = 0.30, p = 0.5907) in the binding of H3K27ac to the Drd2 gene promoter in the striatum. Post-hoc analysis found a significant decrease in H3K27ac level binding at the Drd2 promoter in aged mice as compared to young mice treated with Veh. Moreover, significant increase in H3K27ac level binding at the Drd2 promoter was found in aged but not young mice co-treated with RIS and VPA (p < 0.0001) and RIS and MS-275 (p < 0.0001) compared to those treated with Veh (Figure 4B).
Discussion
In this study we aimed to investigate whether atypical antipsychotics could induce more severe motor side effects in aged mice and 5xFAD mice, an animal model of AD. We also explored whether the changes of histone modification at antipsychotic target genes play a significant role in response to such side effects in aging. We found that atypical antipsychotic RIS leads to greater motor side-effects in aged mice as well as 5xFAD mice, and that these side-effects can be reversed with adjunct HDAC inhibitor VPA, or MS-275. RIS induced age-related motor side effects are similar to that of typical antipsychotic drug haloperidol (14, 16), confirming the increased sensitivity of atypical antipsychotic drugs in aged mice. We also demonstrated that similar epigenetic regulation at the Drd2 and 5htr2a gene promoters is hypoacetylated at a key residue H3K27ac and can be reversed with adjunct HDAC inhibitor in aged mice.
Due to the higher risk of extrapyramidal side effects, such as tardive dyskinesia and death, typical antipsychotics have been replaced by atypical antipsychotics for the treatment of psychiatric disorders as well as to control severe psychiatric symptoms of AD in the elderly (32–34). In the US, there are currently no FDA-approved drugs to treat behavioral psychiatric symptoms in AD and antipsychotics have a black box warning due to the increase in mortality in elderly patients treated with these drugs (9, 10). Despite this, RIS, one of the most common antipsychotics prescribed to elderly patients, is approved to treat psychiatric symptoms in dementia in Europe, Canada, New Zealand, and Australia (23). In general, atypical antipsychotics, including RIS, have decreased the extrapyramidal side effects as compared to the typical antipsychotic haloperidol (35). However, in elderly patients, the higher risk of motor side effects still exists. For this reason, we chose to study this medication in our models of aging and AD to reveal if an epigenetic mechanism we found in our previous work with typical antipsychotic drug, haloperidol, is similar in our study with atypical antipsychotic drug RIS.
As expected, RIS induced very similar motor side effects to haloperidol in aged mice. Both haloperidol and risperidone caused an increase in catalepsy in aged compared to young mice but do not cause greater age-dependent hypolocomotion in the open field test. In addition, adjunct treatment with a broad HDAC inhibitor, VPA, and a more selective Class 1 HDAC inhibitor, MS-275, reversed the age-exaggerated increase in catalepsy without altering the cataleptic responses of young mice to RIS. These results suggest that transcriptional restriction of antipsychotic drug targets by histone hypoacetylation due to aging can be reversed with HDAC inhibitors to increase the therapeutic window of these drugs in elderly patients, expanding the pre-clinical evidence for reducing side-effects with this adjunct treatment to atypical antipsychotics. The mechanism of extrapyramidal side effects such as catalepsy are largely tied to the degree of antagonism, receptor occupancy, and receptor kinetics of D2R or imbalance of D2R and 5-HT2A (23, 36–38). Additionally, out of the atypical antipsychotics, RIS displays the highest antagonism at these two receptors, suggesting that the age-related increases in catalepsy seen with typical antipsychotics and RIS depend on D2R and 5-HT2A expression functioning. In fact, evidence suggests that concomitant 5-HT2A antagonism expands the therapeutic window of antipsychotics through indirect or direct cross-talk with D2R (39, 40). Thus, to investigate the age-related 5-HT2A and D2R expression as well as histone modification on the gene promoters will help to better understand the age-related increase in catalepsy by RIS. In our study, we showed significant reduction of both 5-HT2A and D2R expression in the striatum of aged mice as compared to young mice (Veh groups), and 0.1 mg/kg RIS selectively further reduced 5-HT2A expression in aged mice but not in young mice, although young mice showed a trend to reduce the expression, it was not significant. These results support that decreased 5-HT2A and D2R expression at the base line during aging, which may contribute to the increased sensitivity to antipsychotic drug side effects. Additionally, these results suggest that young and aged mice in response to antipsychotic drugs are differently expressed at the cellular level. After HDAC inhibitor treatment, MS-275 increased both 5-HT2A and D2R expression and VPA selectively increased D2R expression in the striatum in aged mice but not young mice, suggesting HDAC inhibitors could restore antipsychotic drug target receptor expression in aged mice. Our results determined the effects of age and RIS treatment on 5-HT2A and D2R expression and histone acetylation at the 5htr2a and Drd2 promoters. Similar to our findings with D2R, aging also leads to a reduction in 5-HT2A expression that is associated with histone hypoacetylation at the 5htr2a promoter, with alterations in individual acetylated histone marks depending on age, RIS treatment status, and HDAC inhibitor. The same pattern of age-related hypoacetylation and transcriptional repression we found on the D2R in this study and previous report (14, 16), which suggests that epigenetic changes during aging may broadly affect numerous antipsychotic drug targets.
Our current and previous work make a compelling case for adjunct HDAC inhibitors to improve the therapeutic efficacy for antipsychotics in both aged mice as well as the mouse model of AD. However, we have mentioned that 12-month-old 5xFAD mice, which genetically overexpressed APP/PS1, two mutation caused familiar AD but are not naturally aged. Currently, we did not find significant changes D2R and 5-HT2A expression in our AD model, but a trend of decreased D2R and 5-HT2A in the striatum (data not shown) and these results need further confirmation. Nevertheless, we propose that AD neuropathogenesis could influence drug targeted genes that may cause motor side effects. Indeed, antipsychotics are among some of the least selective medications in terms of receptor antagonism, and it is still unknown how aging and/or AD pathogenesis influence D2R, 5-HT2A and other important target receptors, including histaminergic, adrenergic, muscarinic, sigma, and serotonergic and dopaminergic receptors in other brain areas (41). In addition, although the imbalance of D2R and 5-HT2A are one potential reason, 5-HT2A-related side effects have yet to be described conclusively in the literature (42, 43). Future work will be needed to determine if aging-related epigenetic influences at histaminergic, adrenergic, and muscarinic gene promoters lead to greater metabolic and cardiovascular side-effects that may occur with age and are closely associated with these signaling systems (41). Additionally, adding 18-month-old 5xFAD mice as a comparison will provide important information for age and neuropathology interaction effect on antipsychotic actions.
Regarding acetylation at 5ht2a and Drd2 promoters, we found that H3K27 levels were significantly decreased in Drd2 promoter as compared to young mice but have a trend to decrease of H3K27 levels at 5-htr2a promoter in both young and aged mice. As expected, we found chronic HDAC inhibitor treatment significantly increased Drd2 and 5-htr2a gene promoters in aged mice compared to Veh treated aged mice. Although we found a trend of increased H3K27 levels at both Drd2 and 5-htr2a gene promoters in young mice when compared to the Veh treated group, that trend did not reach significance. These results suggest that the changes of H3K27 acetylation at Drd2 and 5-htr2a gene promoters differently in the antipsychotic targeted receptor between young and aged mice. Our current study is focused on histone acetylation specifically H3K27, other histone modification marks may also modulate at 5ht2a and Drd2 gene promoters during aging. Therefore, in future work we will include more histone acetylation marks to identify age-related changes of histone acetylation contributing to 5-HT2A and D2R expression. Additionally, Histone Acetyltransferases (HATs), another important regulator system of histone acetylation (44) might be driving the hypoacetylation seen at the Drd2 or 5htr2a promoters. It is unclear how the individual balance of specific HDACs and HATs leads to aging-or disease related narrowing of the antipsychotic therapeutic window. As HDAC inhibitors are powerful drugs, which itself could potentially induce the side-effects (45), understanding which individual HDACs or HATs involved in the aging-induced hypoacetylation of key histones would greatly improve the potential for adjunct HDAC inhibitors, as more selective HDAC inhibitors may lead to better tolerability of this adjunct treatment approach in elderly and AD patients.
In summary, our current study demonstrated that typical antipsychotic drug RIS could reduce more severe motor side effects in aged mice and 5xFAD mice. This work also expands our previous findings from typical antipsychotics to atypical antipsychotics in terms of the aging-related mechanism of hypoacetylation of key drug targets and subsequent increase in side-effect severity in aged mice. Future work will determine the mechanisms of epigenetic regulation in AD involved in antipsychotics along with expanding the investigation of side-effects beyond motor side effects that may provide greater evidence for the adjunct HDAC inhibitor treatment as a viable option for AD patients treated with antipsychotics to reduce side-effects and increase efficacy of these important medications.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was reviewed and approved by Institutional Animal Care and Use Committee (IACUC) Office at Northwestern University.
Author contributions
GR and HD designed the experiments and supervised the project. GR, BM, and JM-O were responsible for mice handling, drug preparations, and IP injections. GR, BM, and QC conducted the experiments and data collection. HD, DF, JM-O, CC, and GR revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Institute of Mental Health grant (1 R01 MH109466) for HD and the National Institute of Neurological Disorders and Stroke (R01NS069777) for CC.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Tampi RR, Tampi DJ, Rogers K, Alagarsamy S. Antipsychotics in the management of behavioral and psychological symptoms of dementia: maximizing gain and minimizing harm. Neurodegener Dis Manag. (2020) 10:5–8. doi: 10.2217/nmt-2019-0036
2. Yunusa I, Rashid N, Demos GN, Mahadik BS, Abler VC, Rajagopalan K. Comparative outcomes of commonly used off-label atypical antipsychotics in the treatment of dementia-related psychosis: a network meta-analysis. Adv Ther. (2022) 39:1993–2008. doi: 10.1007/s12325-022-02075-8
3. Jenraumjit R, Somboon J, Chainan S, Chuenchom P, Wongpakaran N, Wongpakaran T. Drug-related problems of antipsychotics in treating delirium among elderly patients: a real-world observational study. J Clin Pharm Ther. (2021) 46:1274–80. doi: 10.1111/jcpt.13423
4. Breijyeh Z, Karaman R. Comprehensive review on Alzheimer’s disease: causes and treatment. Molecules. (2020) 25:5789. doi: 10.3390/molecules25245789
5. Knopman DS, Amieva H, Petersen RC, Chételat G, Holtzman DM, Hyman BT, et al. Alzheimer disease. Nat Rev Dis Primers. (2021) 7:33. doi: 10.1038/s41572-021-00269-y
6. Aupperle P. Management of aggression, agitation, and psychosis in dementia: focus on atypical antipsychotics. Am J Alzheimers Dis Other Demen. (2006) 21:101–8. doi: 10.1177/153331750602100209
7. Volkow ND, Gur RC, Wang GJ, Fowler JS, Moberg PJ, Ding YS, et al. Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. Am J Psychiatry. (1998) 155:344–9.
8. Kaasinen V, Vilkman H, Hietala J, Någren K, Helenius H, Olsson H, et al. Age-related dopamine D2/D3 receptor loss in extrastriatal regions of the human brain. Neurobiol Aging. (2000) 21:683–8. doi: 10.1016/S0197-4580(00)00149-4
9. Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA. (2005) 294:1934–43. doi: 10.1001/jama.294.15.1934
10. Steinberg M, Lyketsos CG. Atypical antipsychotic use in patients with dementia: managing safety concerns. Am J Psychiatry. (2012) 169:900–6. doi: 10.1176/appi.ajp.2012.12030342
11. McClarty BM, Fisher DW, Dong H. Epigenetic alterations impact on antipsychotic treatment in elderly patients. Curr Treat Options Psychiatry. (2018) 5:17–29. doi: 10.1007/s40501-018-0134-4
12. Sykes DA, Moore H, Stott L, Holliday N, Javitch JA, Lane JR, et al. Extrapyramidal side effects of antipsychotics are linked to their association kinetics at dopamine D2 receptors. Nat Commun. (2017) 8:763. doi: 10.1038/s41467-017-00716-z
13. Montalvo-Ortiz JL, Keegan J, Gallardo C, Gerst N, Tetsuka K, Tucker C, et al. HDAC inhibitors restore the capacity of aged mice to respond to haloperidol through modulation of histone acetylation. Neuropsychopharmacology. (2014) 39:1469–78. doi: 10.1038/npp.2013.346
14. Montalvo-Ortiz JL, Fisher DW, Rodríguez G, Fang D, Csernansky JG, Dong H. Histone deacetylase inhibitors reverse age-related increases in side effects of haloperidol in mice. Psychopharmacology. (2017) 234:2385–98. doi: 10.1007/s00213-017-4629-2
15. Keszycki RM, Fisher DW, Dong H. The hyperactivity-impulsivity-irritiability-disinhibition-aggression-agitation domain in Alzheimer’s disease: current management and future directions. Front Pharmacol. (2019) 10:1109. doi: 10.3389/fphar.2019.01109
16. McClarty B, Rodriguez G, Dong H. Dose effects of histone deacetylase inhibitor tacedinaline (CI-994) on antipsychotic haloperidol-induced motor and memory side effects in aged mice. Front Neurosci. (2021) 15:674745. doi: 10.3389/fnins.2021.674745
17. Jeste D, Savla G, Thompson W, Vahia I, Glorioso D, Martin A, et al. Association between older age and more successful aging: critical role of resilience and depression. Am J Psychiatry. (2013) 170:188–96. doi: 10.1176/appi.ajp.2012.12030386
18. Li P, Snyder GL, Vanover KE. Dopamine targeting drugs for the treatment of schizophrenia: past, present and future. Curr Top Med Chem. (2016) 16:3385–403. doi: 10.2174/1568026616666160608084834
19. Seeman P. Atypical antipsychotics: mechanism of action. Can J Psychiatry. (2002) 47:27–38. doi: 10.1177/070674370204700106
20. Gareri P, Fazio PD, Manfredi VG, Sarro GD. Use and safety of antipsychotics in behavioral disorders in elderly people with dementia. J Clin Psychopharmacol. (2014) 34:109–23. doi: 10.1097/JCP.0b013e3182a6096e
21. Yen YC, Lung FW, Chong MY. Adverse effects of risperidone and haloperidol treatment in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. (2004) 28:285–90. doi: 10.1016/j.pnpbp.2003.10.006
22. Oblak A, Lin P, Kotredes K, Pandey R, Garceau D, Williams H, et al. Comprehensive evaluation of the 5XFAD mouse model for preclinical testing applications: a MODEL-AD study. Front Aging Neurosci. (2021) 13:713726. doi: 10.3389/fnagi.2021.713726
23. Yunusa I, Alsumali A, Garba AE, Regestein QR, Eguale T. Assessment of reported comparative effectiveness and safety of atypical antipsychotics in the treatment of behavioral and psychological symptoms of dementia: a network meta-analysis. JAMA Netw Open. (2019) 2:e190828. doi: 10.1001/jamanetworkopen.2019.0828
24. Weiden PJ. EPS profiles: the atypical antipsychotics are not all the same. J Psychiatr Pract. (2007) 13:13–24. doi: 10.1097/00131746-200701000-00003
25. Ho MY, Mobini S, Chiang TJ, Bradshaw CM, Szabadi E. Theory and method in the quantitative analysis of “impulsive choice” behaviour: implications for psychopharmacology. Psychopharmacology. (1999) 146:362–72. doi: 10.1007/PL00005482
26. Bardin L, Kleven MS, Barret-Grévoz C, Depoortère R, Newman-Tancredi A. Antipsychotic-like vs cataleptogenic actions in mice of novel antipsychotics having D2 antagonist and 5-HT1A agonist properties. Neuropsychopharmacology. (2006) 31:1869–79. doi: 10.1038/sj.npp.1300940
27. Fink-Jensen A, Schmidt LS, Dencker D, Schülein C, Wess J, Wörtwein G, et al. Antipsychotic-induced catalepsy is attenuated in mice lacking the M4 muscarinic acetylcholine receptor. Eur J Pharmacol. (2011) 656:39–44. doi: 10.1016/j.ejphar.2011.01.018
28. Nishchal BS, Rai S, Prabhu MN, Ullal SD, Rajeswari S, Gopalakrishna HN. Effect of tribulus terrestris on haloperidol-induced catalepsy in mice. Indian J Pharm Sci. (2014) 76:564–7.
29. Luciani KR, Frie JA, Khokhar JY. An open source automated bar test for measuring catalepsy in rats. eNeuro. (2020) 7:ENEURO.488–419. doi: 10.1523/ENEURO.0488-19.2020
30. Kirschbaum KM, Hiemke C, Schmitt U. Rotarod impairment: catalepsy-like screening test for antipsychotic side effects. Int J Neurosci. (2009) 119:1509–22. doi: 10.1080/00207450902984002
31. Yunusa I, El Helou ML. The use of risperidone in behavioral and Psychological symptoms of dementia: a review of pharmacology, clinical evidence, regulatory approvals, and off-label use. Front Pharmacol. (2020) 11:596. doi: 10.3389/fphar.2020.00596
32. Gareri P, Fazio PD, Fazio SD, Marigliano N, Ibbadu GF, Sarro GD. Adverse effects of atypical antipsychotics in the elderly: a review. Drugs Aging. (2006) 23:937–56. doi: 10.2165/00002512-200623120-00002
33. Gareri P, Segura-García C, Manfredi VG, Bruni A, Ciambrone P, Cerminara G, et al. Use of atypical antipsychotics in the elderly: a clinical review. Clin Interv Aging. (2014) 9:1363–73. doi: 10.2147/CIA.S63942
34. Maust DT, Kim HM, Seyfried LS, Chiang C, Kavanagh J, Schneider LS, et al. Antipsychotics, other psychotropics, and the risk of death in patients with dementia: number needed to harm. JAMA Psychiatry. (2015) 72:438–45. doi: 10.1001/jamapsychiatry.2014.3018
35. Mühlbauer V, Möhler R, Dichter MN, Zuidema SU, Köpke S, Luijendijk HJ. Antipsychotics for agitation and psychosis in people with Alzheimer’s disease and vascular dementia. Cochrane Database Syst Rev. (2021) 12:CD013304. doi: 10.1002/14651858.CD013304.pub2
36. Wadenberg ML, Kapur S, Soliman A, Jones C, Vaccarino F. Dopamine D2 receptor occupancy predicts catalepsy and the suppression of conditioned avoidance response behavior in rats. Psychopharmacology. (2000) 150:422–9. doi: 10.1007/s002130000466
37. Hauber W, Neuscheler P, Nagel J, Müller CE. Catalepsy induced by a blockade of dopamine D1 or D2 receptors was reversed by a concomitant blockade of adenosine A(2A) receptors in the caudate-putamen of rats. Eur J Neurosci. (2001) 14:1287–93. doi: 10.1046/j.0953-816x.2001.01759.x
38. Wadenberg ML, Soliman A, VanderSpek SC, Kapur S. Dopamine D(2) receptor occupancy is a common mechanism underlying animal models of antipsychotics and their clinical effects. Neuropsychopharmacology. (2001) 25:633–41. doi: 10.1016/S0893-133X(01)00261-5
39. Wynchank D, Berk M. Efficacy of nefazodone in the treatment of neuroleptic induced extrapyramidal side effects: a double-blind randomised parallel group placebo-controlled trial. Hum Psychopharmacol. (2003) 18:271–5.
40. Meltzer HY, Huang M. In vivo actions of atypical antipsychotic drug on serotonergic and dopaminergic systems. Prog Brain Res. (2008) 172:177–97. doi: 10.1016/S0079-6123(08)00909-6
41. Kaar SJ, Gobjila C, Butler E, Henderson C, Howes OD. Making decisions about antipsychotics: a qualitative study of patient experience and the development of a decision aid. BMC Psychiatry. (2019) 19:309. doi: 10.1186/s12888-019-2304-3
42. Kusumi I, Boku S, Takahashi Y. Psychopharmacology of atypical antipsychotic drugs: from the receptor binding profile to neuroprotection and neurogenesis. Psychiatry Clin Neurosci. (2015) 69:243–58. doi: 10.1111/pcn.12242
43. Meltzer HY, Matsubara S, Lee JC. The ratios of serotonin2 and dopamine2 affinities differentiate atypical and typical antipsychotic drugs. Psychopharmacol Bull. (1989) 25:390–2.
44. Carrozza MJ, Utley RT, Workman JL, Côt JÉ. The diverse functions of histone acetyltransferase complexes. Trends Genet. (2003) 19:321–9. doi: 10.1016/S0168-9525(03)00115-X
Keywords: antipsychotics, HDAC inhibitors, risperidone, neuropsychiatric symptoms, epigenetic, motor side effects, aging, Alzheimer’s disease
Citation: Rodriguez G, Fisher DW, McClarty B, Montalvo-Ortiz J, Cui Q, Chan CS and Dong H (2023) Histone deacetylase inhibitors mitigate antipsychotic risperidone-induced motor side effects in aged mice and in a mouse model of Alzheimer’s disease. Front. Psychiatry 13:1020831. doi: 10.3389/fpsyt.2022.1020831
Received: 16 August 2022; Accepted: 12 December 2022;
Published: 06 January 2023.
Edited by:
Jian-hua Zhuang, Shanghai Changzheng Hospital, ChinaReviewed by:
Chuanjun Zhuo, Tianjin Anding Hospital, ChinaAnastasios Georgakopoulos, Icahn School of Medicine at Mount Sinai, United States
Antonella Borreca, National Research Council (CNR), Italy
Copyright © 2023 Rodriguez, Fisher, McClarty, Montalvo-Ortiz, Cui, Chan and Dong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongxin Dong,  h-dong@northwestern.edu
h-dong@northwestern.edu
 Guadalupe Rodriguez
Guadalupe Rodriguez Daniel W. Fisher1,2
Daniel W. Fisher1,2 Bryan McClarty
Bryan McClarty Janitza Montalvo-Ortiz
Janitza Montalvo-Ortiz Qiaoling Cui
Qiaoling Cui C. Savio Chan
C. Savio Chan Hongxin Dong
Hongxin Dong