- 1School of Medicine, Sungkyunkwan University, Seoul, South Korea
- 2Department of Psychiatry, Hanyang University Medical Center, Seoul, South Korea
- 3Department of Psychiatry, Depression Center, Samsung Medical Center, Seoul, South Korea
- 4Department of Health Sciences and Technology, Department of Medical Device Management and Research, Department of Clinical Research Design and Evaluation, Samsung Advanced Institute for Health Sciences and Technology, Sungkyunkwan University, Seoul, South Korea
In this study, a literature survey was conducted of research into the development and use of wearable devices and sensors in patients with depression. We collected 18 studies that had investigated wearable devices for assessment, monitoring, or prediction of depression. In this report, we examine the sensors of the various types of wearable devices (e.g., actigraphy units, wristbands, fitness trackers, and smartwatches) and parameters measured through sensors in people with depression. In addition, we discuss future trends, referring to research in other areas employing wearable devices, and suggest the challenges of using wearable devices in the field of depression. Real-time objective monitoring of symptoms and novel approaches for diagnosis and treatment using wearable devices will lead to changes in management of patients with depression. During the process, it is necessary to overcome several issues, including limited types of collected data, reliability, user adherence, and privacy concerns.
Introduction
The rapid development of wearable devices has led to their active use in research on depression. Sensors in wearable devices can collect physiological data related to mental health. These devices enable monitoring and assessment of patients in real time and in an unobtrusive way. In addition, the data collected through wearable devices can be monitored by the patient, and health care providers can receive and use the patient-generated health data to personalize healthcare. It is expected that the big data obtained by wearable technologies will facilitate the delivery of personalized, interactive, noncontact healthcare in a cost-effective manner.
Studies have shown that some types of data obtained from various wearable devices concern depression. Vallance et al. (1) reported that greater physical activity as measured by an accelerometer was correlated with lower rates of depression. Other research found that skin conductance as measured by wearable patches can be a sensitive biomarker for depression (2–4). A meta-analysis reported that heart rate variability (HRV), which is defined as spontaneous fluctuations in heart rate mainly reflecting the activity of the autonomic system, is reduced in patients with depression, even without concomitant cardiovascular disease (5, 6). HRV often is measured by photoplethysmography (PPG) to determine changes in microvascular perfusion by illuminating the skin with light and measuring the transmitted or reflected amount (7).
Although the market of wearable devices is growing rapidly, the use of wearable technology for diagnosis and treatment of depression remains limited. Therefore, the purpose of this study was to provide a current overview of the developments and utility of wearable devices in research on depression. Here, we collected original studies examining wearable devices for diagnosis and treatment of depression and highlight the developments and trends in this field.
Methods and Results
To identify studies on the use of wearable devices in depression research, a literature search was performed in PubMed and the Web of Science databases, focusing on articles published prior to December 31, 2020. The specific search string was as follows: (wearable* OR actigraph* OR actigraphy OR actiwatch OR actimetry OR smartwatch OR wrist-worn OR “fitness tracker” OR “inertial sensor” OR “digital outcome measure”) AND (depress* OR MDD OR “major depressive disorder” OR bipolar OR unipolar OR “affective disorder” OR “mood disorder”). This search string was applied only to article titles.
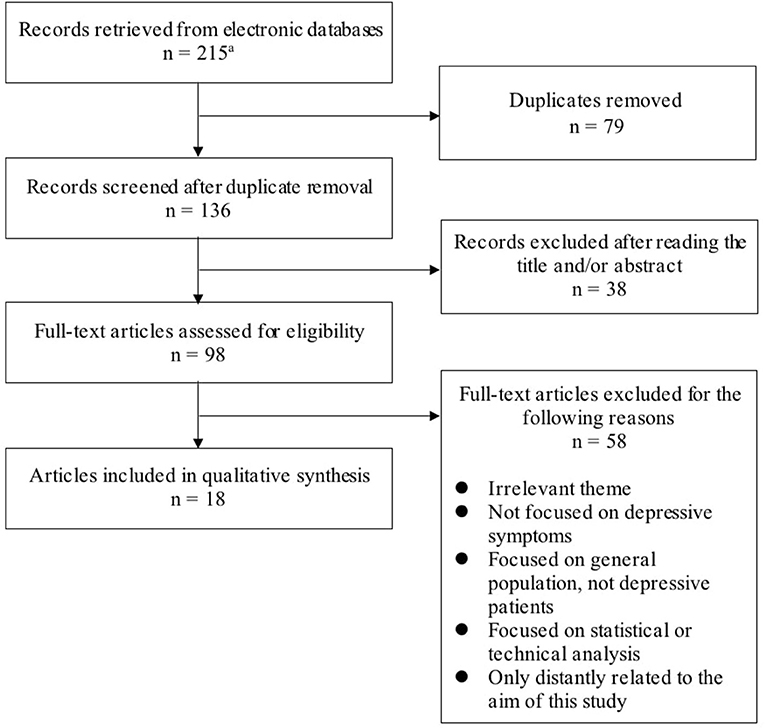
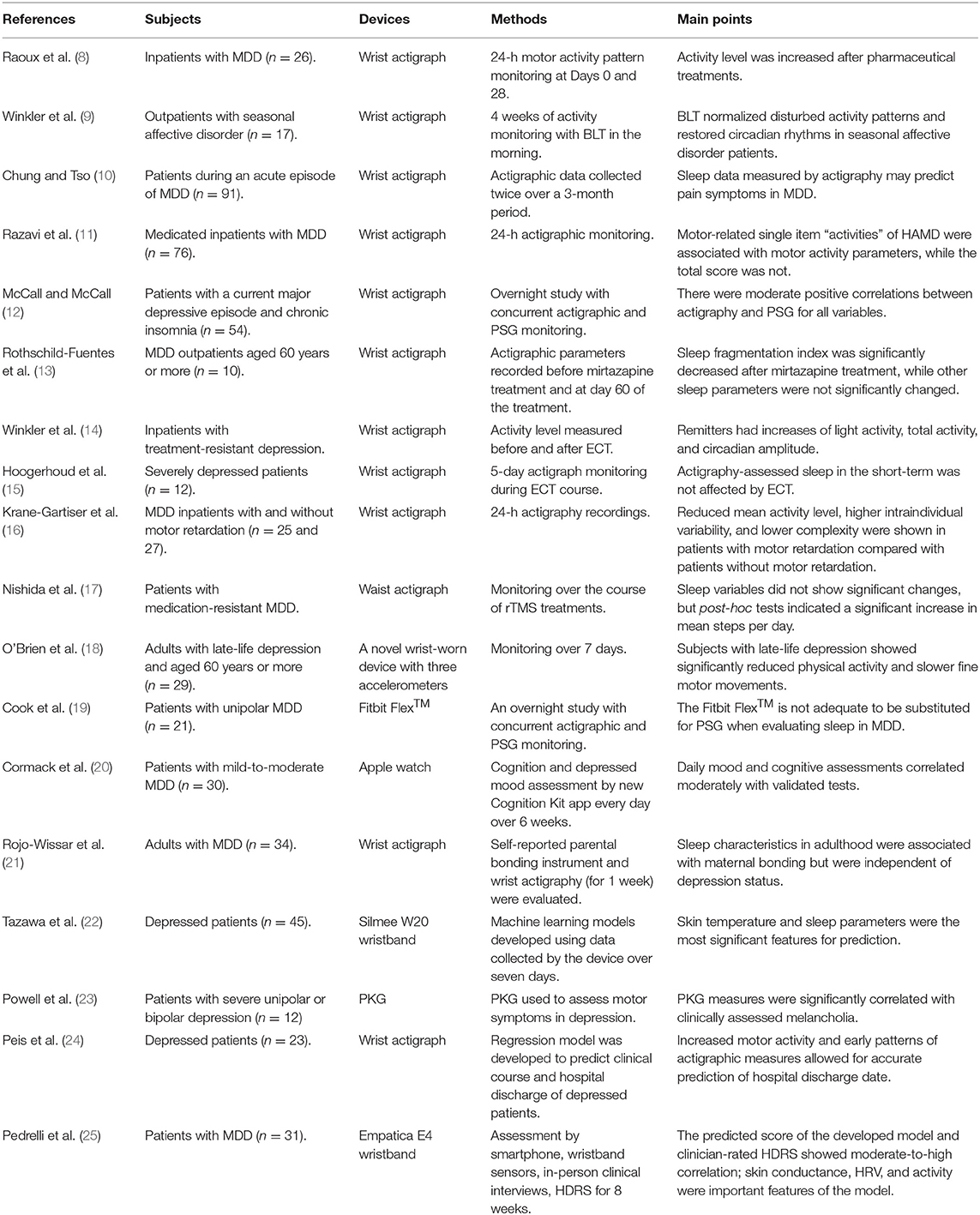
For this study, we excluded papers not written in English and the following types of manuscripts: conference papers, meeting abstracts, research notes, brief/short reports, letters, corrections, protocols, reviews, systematic reviews, meta-analyses, and editorial materials. We also excluded papers with an irrelevant theme (e.g., wearable bipolar batteries) or that were only distantly related to the aim of this study. In addition, we excluded papers that were not focused on depressive symptoms or focused on the general population or statistical and technical analysis. The search process is presented in Figure 1. We collected a total of 18 studies that made use of wearable devices to assess or monitor depressive symptoms or to predict major depressive disorder (MDD) (Table 1).
Discussion
Overview
Among the collected studies on wearable devices for patients with depression, two-thirds used actigraph units, while the rest used other devices such as a novel wearable device with three accelerometers (18); medically used wearable devices, such as a Parkinson's KinetiGraph (PKG) (23) and the E4 wristband (Empatica, Boston, MA, USA) (25); and commercial wearable devices not originally intended for medical use, such as the Fitbit Flex™ (Fitbit, Inc., San Francisco, CA, USA) (19), Apple watch (Apple Inc., Cupertino, CA, USA) (20), and Silmee™ W20 wristband (TDK Corporation, Tokyo, Japan) (22). Among the reviewed studies, no wearable devices were used for treatment. In all studies, specific physiological, activity/sleep, or subjective parameters of individuals were collected through wearable devices, and the relationship between these parameters and depression was investigated. Except for one study that employed a waist actigraph unit (17), all included studies used wrist-worn devices to assess their study populations. Because wearing accessories on the wrist is nondistracting and familiar to most people, wrist-worn wearable devices have been used actively for research.
The use of actigraphy for monitoring depressive symptoms has been investigated since the 1990s and involves using wristwatch-like devices to collect activity or sleep data generated by movements (26). An actigraph unit is typically equipped with linear or a three-axis accelerometer to detect movements. Actigraph units are produced by various companies, with most studies (9, 11, 14, 15) using actigraph units manufactured by Cambridge Neurotechnology Ltd (Cambridgeshire, England). However, new devices and algorithms are being developed constantly, and there is no consensus regarding which device or algorithm is most appropriate for assessing patients with depression (26).
Some relevant medical devices include PKG and the Empatica E4 wristband. The PKG is a wristwatch-like device with a three-axis accelerometer originally designed to assess motor symptoms such as bradykinesia and tremor in patients with Parkinson's disease (27). However, Powell et al. (23) recently used it to assess motor symptoms in patients with depression. On the other hand, the Empatica E4 is a wrist-worn device that contains a PPG sensor capable of detecting heart rate, an electrodermal activity (EDA) sensor, an optical thermometer for measuring peripheral skin temperature, and the three-axis accelerometer for estimating motion and sleep characteristics. This device was used by Pedrelli et al. (25) to monitor changes in depressive symptom severity of patients.
Some consumer devices not originally indicated for medical use are the Fitbit Flex™, Apple watch, and Silmee™ W20 wristband. The Fitbit Flex™ is a commercially available fitness tracker equipped with a three-axis accelerometer that measures motion patterns of users. The utility of the Fitbit Flex™ in evaluating sleep patterns in depressive patients was compared against polysomnography (PSG) and actigraphy (19). On the other hand, the Apple watch is a so-called “smartwatch” featuring various apps as well as capabilities for phone calls and text messaging. It contains a small touchscreen, which was used by Cormack et al. (20) to offer tasks like cognitive and mood assessments to study participants. Finally, the Silmee™ W20 wristband was used previously (22) for unintended medical use and is equipped with a three-axis acceleration sensor, pulse sensor, ultraviolet sensor, and temperature sensor.
Measured Parameters
Various parameters related to depression have been measured by each wearable device and can be classified as activity/sleep, physiological, and subjective parameters.
Activity characteristics, sleep patterns, and circadian rhythm can be estimated by accelerometers in wearable devices. Previous studies have consistently revealed the association between physical activity and MDD. People who do not regularly engage in physical activity are more likely to show depressive symptoms (28), and depression can be a significant risk factor for a sedentary lifestyle (29). Most studies employing wearable devices have reported results consistent with these statements. When inpatients with MDD were treated with antidepressants, their activity level as measured by actigraphy was significantly increased upon discharge (8). Elsewhere, Winkler et al. (9) observed that activity was increased even by means of electroconvulsive therapy (ECT). Finally, Peis et al. (24) reported that increased motor activity and early patterns of actigraphic measures allowed for accurate prediction of hospital discharge date using a Hierarchical Generalized Linear Regression Model.
In a previous study, MDD patients with motor retardation had decreased level of activity compared with those without motor retardation (16). Meanwhile, Powell et al. (23) showed the potential for PKG to be used to evaluate movement patterns such as bradykinesia in patients with MDD.
Insomnia is a risk factor for depression, and sleep-related parameters can be used for prediction of depression (30). In addition, depressive patients tend to report more unsatisfactory sleep quality than healthy controls (31). However, this finding was not supported by most studies using actigraphy for sleep assessment of depressive patients, even though McCall and McCall (12) showed that actigraphic measurement of sleep is closely approximated those of PSG. Specifically, with mirtazapine medication for MDD patients, no significant improvement was found for actigraphic sleep measures (13). Also, both Winkler et al. (14) and Hoogerhoud et al. (15) reported that ECT did not affect actigraphy-assessed sleep. Rojo-Wissar et al. (21) suggested that maternal bonding characteristics were associated with sleep characteristics in adulthood but were not dependent on depression status. However, unlike other studies, Chung and Tso (10) showed that insomnia symptoms measured by actigraphy can be used to predict pain symptoms in MDD patients by Pearson correlation analysis, so further research is needed.
Several studies have shown that circadian rhythm–related parameters are correlated with depressive symptoms. Winkler et al. (9) reported that circadian rhythms in patients with seasonal affective disorder can be restored with bright light therapy (BLT). In another study, remitters had increases in circadian amplitude (the gap between the peak and the mean of a wave), but no significant changes were observed in circadian acrophase (the time at which the peak of a rhythm occurs) (14).
Skin temperature (ST), skin conductance (SC), and HRV as measured by an EDA sensor, optical thermometer, and PPG sensor, respectively, are physiological parameters collected by wearable devices. Tazawa et al. (22) reported that ST can be an indicator of depression using a machine learning model with parameters detected from the Silmee™ W20 wristband. This corresponds with the report that depressive patients have a higher body temperature than that of healthy controls (32).
The study by Pedrelli et al. (25) corresponds with previous research suggesting that SC is significantly correlated with depression (33), and that HRV reflects autonomic dysregulation affected by mental health status (34). They have proposed a machine learning model that predicts clinical scores of MDD from various data collected through a smartphone and wristband. According to them, the 10 most predictive features for MDD include parameters related to SC and HRV. However, further research is necessary since machine learning models usually remain black boxes and do not clearly conclude to what degree and why the underlying parameters are related to the prediction (35).
Future Trends
The advantage of incorporating wearable devices into research in patients with depression is that wearable devices enable continuous and objective monitoring of patients. With this technology, real-time changes between patient hospital visits can be tracked objectively and treatment effects can be monitored more accurately. Assessments of MDD often are conducted by mood questionnaires such as the Hamilton Depressing Rate Scale (HDRS). However, these types of mood assessments are easily disturbed by various biases, such as recall bias (36). This can lead to inaccurate results depending upon the degree of inconsistency among patients or clinicians (37). In addition, errors that can occur during manual data entry by physicians or researchers can be prevented when using wearable devices.
Wearable devices also enable patients to monitor their symptoms. For example, a patient can check their current level of stress or depression using wearable devices or a smartphone connected via Bluetooth. In a study using Psymate (PsyMate BV, Maastricht, the Netherlands), a “personal digital assistant”–based system for mood assessment, personalized feedback interventions appeared to help patients improve their depressive symptoms and prevent maladaptive behaviors that can worsen their moods, suggesting that the provision of such feedback through wearable devices will have similar effects (38). It has shown that, if patients are notified about their mood frequently, it may help them to manage their own depression.
Wearable devices can potentially be used to develop novel diagnostic methods or treatments. Sensors can measure various parameters other than those introduced above; for example, speech pattern and voice analyses through sensors can be used to assess depression severity and treatment response (39). In addition, using sensors like inertial sensors, bending sensors, and electromyography signals, it is possible to capture human motion through wearable devices (40). Considering studies showing that depressive patients can exhibit gait variability (41) and motor abnormalities (42), future research is expected to use novel wearable devices to investigate how movement changes with depression.
Recently, wearable devices that can treat depression at home have been developed. One study (43) showed that daily morning light therapy for 60 min using home light-therapy glasses can trigger an improvement in depressive symptoms. If this technology can be applied to normal glasses with light sensors tracking the daily light exposure of patients, daily use can help to monitor and treat depression.
Wearable devices can be used as a part of personalized exercise-treatment programs for MDD (44). In addition, wearable devices can increase patient physical activity and medication adherence according to an ongoing trial enrolling patients with heart failure and diabetes mellitus (45); based on step count data from wearable devices in this study, individualized feedback is provided to patients. We can expect a study in the future that attempts this type of intervention for patients with depression.
An aripiprazole tablet with an ingestible sensor (Abilify MyCite; Otsuka America Pharmaceutical, Inc., Rockville, MD, USA) was approved recently by the United States Food and Drug Administration. The sensor on the pill sends a signal to a cutaneous wearable so that clinicians can track the medication adherence of patients. This model is expected to be used in depression studies considering that aripiprazole can be administrated as an adjunctive treatment for depression.
Challenges
There are challenges in the application of wearable devices in the field of depression. First, it is difficult to detect various symptoms of depression using wearable devices. In particular, evaluation of subjective mood symptoms is difficult; for example, wearable devices mostly detect physiological data and have limitations in evaluating subjective symptoms. However, a recent study using the Apple watch showed the possibility of high-frequency cognitive and mood assessments using wearable devices (20). Second, physiological data that can be measured by wearable devices are lacking. For example, although a change in appetite is a common symptom in patients with depression, it is difficult to measure it with wearable devices. Vu et al. (46) previously tried to estimate eating behaviors by detecting wrist movements or the sound of eating, but the accuracy was low.
There are various issues with adherence and compliance of patients. Wearable devices have variations in the degree of convenience of use, and there is a difference in user comfort when wearing the devices. The effectiveness of mobile health interventions varies greatly according to design of the intervention (47), and acceptance of wearable devices can vary depending on user age (48). Therefore, further research is required about how to increase adherence and compliance rates so that users can continuously wear wearable devices for 24 h.
Unreliability and inaccuracy are problems in use of wearable devices. Various wearable devices for fitness and wellness are available on the market. However, since these devices are heterogeneous, they are not simple to use for those wishing to monitor clinical symptoms. The reliability of the sensor system and data-processing algorithm of wearable devices also makes it difficult to introduce these devices into the medical field. Furthermore, data can be disrupted by various kinds of noises generated by the surrounding environment and the physical condition of the person wearing the device. Although some studies have been carried out to validate the accuracy of wrist actigraphy in comparison with PSG (49, 50), accelerometers on the wrist are not effective in detecting sleep patterns not involving limb motion, so other instruments like a pressure sensor sheet or chest-worn sensor are needed to obtain a higher degree of accuracy comparable to that of PSG (51, 52).
Another issue involves feature extraction with regard to unreliability issues. According to a layered hierarchical framework presented by Mohr et al. (53), raw sensor data (e.g., location, movement) are transformed into low-level features (e.g., activity, total sleep time). The low-level features are combined and constitute high-level behavioral markers (e.g., psychomotor activity, sleep disruption). The clinical state (e.g., depression) is inferred through a combination of high-level behavioral markers. Although it is a logically valid framework, whether the data, such as the movements measured with an accelerometer, can accurately represent psychomotor agitation or retardation is unclear. For example, sleep is estimated based on patient motion and pulse not by measuring their brainwaves (54).
There also is a problem of privacy and ethics. Since data obtained through wearable devices are stored on an external server, there is a possibility that this data can be leaked. Because of this problem, legal regulations for use in the medical field will be essential.
Conclusion
There are rapid ongoing developments of wearable devices for clinical use. Depressive symptoms can be estimated by many parameters collected objectively in real-time by wearable devices. The possibility of diagnosis and prediction remotely using these devices has been supported in this review. Future trends are expected with the emergence of new wearable devices that will bring novel diagnostic and therapeutic approaches like motion capture, speech analysis, and portable light therapy. This suggests the potential for fundamental changes in diagnosis and treatment of depression in the future by developments of wearable devices. These developments can lead to early and accurate diagnosis of depression, the capability to provide more personalized treatment to patients with depression, and to develop preventive measures for groups at risk of depression. Wearable devices will have a critical role in medicine with the advent of personalized telemedicine.
Author Contributions
HK, MP, and HJ contributed to conception and design of the study. HK and MP performed the bibliographical search. SL wrote the first draft of the manuscript. SL, HK, MP, and HJ wrote sections of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by the Bio & Medical Technology Development Program of the NRF funded by the Korean government, MSIP (No. NRF-2017M3A9F1027323; PI HJ); the Healthcare AI Convergence Research & Development Program through the National IT Industry Promotion Agency of Korea (NIPA) funded by the Ministry of Science and ICT (No. S1601-20-1041); and a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (Grant No. HI21C0885).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Vallance JK, Winkler EA, Gardiner PA, Healy GN, Lynch BM, Owen N. Associations of objectively-assessed physical activity and sedentary time with depression: NHANES (2005–2006). Prev Med. (2011) 53:284–8. doi: 10.1016/j.ypmed.2011.07.013
2. Ward NG, Doerr HO, Storrie MC. Skin conductance: a potentially sensitive test for depression. Psychiatry Res. (1983) 10:295–302. doi: 10.1016/0165-1781(83)90076-8
3. Noble P, Lader M. The symptomatic correlates of the skin conductance changes in depression. J Psychiatry Res. (1971) 9:61–9. doi: 10.1016/0022-3956(71)90008-2
4. Ward NG, Doerr HO. Skin conductance: a potentially sensitive and specific marker for depression. J Nerv Ment Dis. (1986) 174:553–9. doi: 10.1097/00005053-198609000-00008
5. Kemp AH, Quintana DS, Gray MA, Felmingham KL, Brown K, Gatt JM. Impact of depression and antidepressant treatment on heart rate variability: a review and meta-analysis. Biol Psychiatry. (2010) 67:1067–74. doi: 10.1016/j.biopsych.2009.12.012
6. Carney RM, Freedland KE. Depression and heart rate variability in patients with coronary heart disease. Cleve Clin J Med. (2009) 76:S13. doi: 10.3949/ccjm.76.s2.03
7. Allen J. Photoplethysmography and its application in clinical physiological measurement. Physiol Meas. (2007) 28:R1. doi: 10.1088/0967-3334/28/3/R01
8. Raoux N, Benoit O, Dantchev N, Denise P, Franc B, Allilaire JF, et al. Circadian pattern of motor activity in major depressed patients undergoing antidepressant therapy: relationship between actigraphic measures and clinical course. Psychiatry Res. (1994) 52:85–98. doi: 10.1016/0165-1781(94)90122-8
9. Winkler D, Pjrek E, Praschak-Rieder N, Willeit M, Pezawas L, Konstantinidis A, et al. Actigraphy in patients with seasonal affective disorder and healthy control subjects treated with light therapy. Biol Psychiatry. (2005) 58:331–6. doi: 10.1016/j.biopsych.2005.01.031
10. Chung KF, Tso KC. Relationship between insomnia and pain in major depressive disorder: a sleep diary and actigraphy study. Sleep Med. (2010) 11:752–8. doi: 10.1016/j.sleep.2009.09.005
11. Razavi N, Horn H, Koschorke P, Hügli S, Höfle O, Müller T, et al. Measuring motor activity in major depression: the association between the hamilton depression rating scale and actigraphy. Psychiatry Res. (2011) 190:212–6. doi: 10.1016/j.psychres.2011.05.028
12. McCall C, McCall WV. Comparison of actigraphy with polysomnography and sleep logs in depressed insomniacs. J Sleep Res. (2012) 21:122–7. doi: 10.1111/j.1365-2869.2011.00917.x
13. Rothschild-Fuentes B, Roche A, Jiménez-Genchi A, Sánchez-Ferrer J, Fresan A, Muñoz-Delgado J. Effects of mirtazapine on the sleep wake rhythm of geriatric patients with major depression: an exploratory study with actigraphy. Pharmacopsychiatry. (2013) 46:59–62. doi: 10.1055/s-0032-1323655
14. Winkler D, Pjrek E, Lanzenberger R, Baldinger P, Eitel D, Kasper S, et al. Actigraphy in patients with treatment-resistant depression undergoing electroconvulsive therapy. J Psychiatr Res. (2014) 57:96–100. doi: 10.1016/j.jpsychires.2014.06.006
15. Hoogerhoud A, Hazewinkel AW, Reijntjens RH, van Vliet IM, van Noorden MS, Lammers GJ, et al. Short-term effects of electroconvulsive therapy on subjective and actigraphy-assessed sleep parameters in severely depressed inpatients. Depress Res Treat. (2015) 2015:764649. doi: 10.1155/2015/764649
16. Krane-Gartiser K, Henriksen TE, Vaaler AE, Fasmer OB, Morken G. Actigraphically assessed activity in unipolar depression: a comparison of inpatients with and without motor retardation. J Clin Psychiatry. (2015) 76:1181–7. doi: 10.4088/JCP.14m09106
17. Nishida M, Kikuchi S, Nisijima K, Suda S. Actigraphy in patients with major depressive disorder undergoing repetitive transcranial magnetic stimulation: an open label pilot study. J ECT. (2017) 33:36–42. doi: 10.1097/YCT.0000000000000352
18. O'Brien JT, Gallagher P, Stow D, Hammerla N, Ploetz T, Firbank M, et al. A study of wrist-worn activity measurement as a potential real-world biomarker for late-life depression. Psychol Med. (2017) 47:93–102. doi: 10.1017/S0033291716002166
19. Cook JD, Prairie ML, Plante DT. Utility of the fitbit flex to evaluate sleep in major depressive disorder: a comparison against polysomnography and wrist-worn actigraphy. J Affect Disord. (2017) 217:299–305. doi: 10.1016/j.jad.2017.04.030
20. Cormack F, McCue M, Taptiklis N, Skirrow C, Glazer E, Panagopoulos E, et al. Wearable technology for high-frequency cognitive and mood assessment in major depressive disorder: longitudinal observational study. JMIR Ment Health. (2019) 6:e12814. doi: 10.2196/12814
21. Rojo-Wissar DM, McQuaid JR, Ancoli-Israel S, Gengler DN, Haynes PL. Maternal bonding predicts actigraphy-measured sleep parameters in depressed and nondepressed adults. J Nerv Ment Dis. (2020) 208:33–7. doi: 10.1097/NMD.0000000000001071
22. Tazawa Y, Liang KC, Yoshimura M, Kitazawa M, Kaise Y, Takamiya A, et al. Evaluating depression with multimodal wristband-type wearable device: screening and assessing patient severity utilizing machine-learning. Heliyon. (2020) 6:e03274. doi: 10.1016/j.heliyon.2020.e03274
23. Powell A, Graham D, Portley R, Snowdon J, Hayes MW. Wearable technology to assess bradykinesia and immobility in patients with severe depression undergoing electroconvulsive therapy: a pilot study. J Psychiatr Res. (2020) 130:75–81. doi: 10.1016/j.jpsychires.2020.07.017
24. Peis I, López-Moríñigo JD, Pérez-Rodríguez MM, Barrigón ML, Ruiz-Gómez M, Artés-Rodríguez A, et al. Actigraphic recording of motor activity in depressed inpatients: a novel computational approach to prediction of clinical course and hospital discharge. Sci Rep. (2020) 10:17286. doi: 10.1038/s41598-020-74425-x
25. Pedrelli P, Fedor S, Ghandeharioun A, Howe E, Ionescu DF, Bhathena D, et al. Monitoring changes in depression severity using wearable and mobile sensors. Front Psychiatry. (2020) 11:584711. doi: 10.3389/fpsyt.2020.584711
26. Sadeh A, Acebo C. The role of actigraphy in sleep medicine. Sleep Med Rev. (2002) 6:113–24. doi: 10.1053/smrv.2001.0182
27. Griffiths RI, Kotschet K, Arfon S, Xu ZM, Johnson W, Drago J, et al. Automated assessment of bradykinesia and dyskinesia in Parkinson's disease. J Parkinsons Dis. (2012) 2:47–55. doi: 10.3233/JPD-2012-11071
28. De Mello MT, de Aquino Lemos V, Antunes HKM, Bittencourt L, Santos-Silva R, Tufik S. Relationship between physical activity and depression and anxiety symptoms: a population study. J Affect Disord. (2013) 149:241–6. doi: 10.1016/j.jad.2013.01.035
29. Roshanaei-Moghaddam B, Katon WJ, Russo J. The longitudinal effects of depression on physical activity. Gen Hosp Psychiatry. (2009) 31:306–15. doi: 10.1016/j.genhosppsych.2009.04.002
30. Fang H, Tu S, Sheng J, Shao A. Depression in sleep disturbance: a review on a bidirectional relationship, mechanisms and treatment. J Cell Mol Med. (2019) 23:2324–32. doi: 10.1111/jcmm.14170
31. Mayers AG, Baldwin DS. The relationship between sleep disturbance and depression. Intern J Psychiatry Clin Pract. (2006) 10:2–16. doi: 10.1080/13651500500328087
32. Rausch JL, Johnson M, Corley K, Hobby H, Shendarkar N, Fei Y, et al. Depressed patients have higher body temperature: 5-HT transporter long promoter region effects. Neuropsychobiology. (2003) 47:120–7. doi: 10.1159/000070579
33. Lin HP, Lin HY, Lin WL, Huang ACW. Effects of stress, depression, and their interaction on heart rate, skin conductance, finger temperature, and respiratory rate: sympathetic-parasympathetic hypothesis of stress and depression. J Clin Psychol. (2011) 67:1080–91. doi: 10.1002/jclp.20833
34. Kemp AH, Quintana DS. The relationship between mental and physical health: insights from the study of heart rate variability. Intern J Psychophysiol. (2013) 89:288–96. doi: 10.1016/j.ijpsycho.2013.06.018
35. Watson DS, Krutzinna J, Bruce IN, Griffiths CE, McInnes IB, Barnes MR, et al. Clinical applications of machine learning algorithms: beyond the black box. BMJ. (2019) 364:l886. doi: 10.2139/ssrn.3352454
36. Kruijshaar ME, Barendregt J, Vos T, De Graaf R, Spijker J, Andrews G. Lifetime prevalence estimates of major depression: an indirect estimation method and a quantification of recall bias. Eur J Epidemiol. (2005) 20:103–11. doi: 10.1007/s10654-004-1009-0
37. Aboraya A, Rankin E, France C, El-Missiry A, John C. The reliability of psychiatric diagnosis revisited: the clinician's guide to improve the reliability of psychiatric diagnosis. Psychiatry. (2006) 3:41.
38. Snippe E, Simons CJ, Hartmann JA, Menne-Lothmann C, Kramer I, Booij SH, et al. Change in daily life behaviors and depression: within-person and between-person associations. Health Psychol. (2016) 35:433. doi: 10.1037/hea0000312
39. Mundt JC, Snyder PJ, Cannizzaro MS, Chappie K, Geralts DS. Voice acoustic measures of depression severity and treatment response collected via interactive voice response (IVR) technology. J Neuroling. (2007) 20:50–64. doi: 10.1016/j.jneuroling.2006.04.001
40. Dong M, Fang B, Li J, Sun F, Liu H. Wearable sensing devices for upper limbs: a systematic review. Proc Inst Mech Eng H. (2021) 235:117–30. doi: 10.1177/0954411920953031
41. Radovanović S, Jovičić M, Marić NP, Kostić V. Gait characteristics in patients with major depression performing cognitive and motor tasks while walking. Psychiatry Res. (2014) 217:39–46. doi: 10.1016/j.psychres.2014.02.001
42. Lohr JB, May T, Caligiuri MP. Quantitative assessment of motor abnormalities in untreated patients with major depressive disorder. J Affect Disord. (2013) 146:84–90. doi: 10.1016/j.jad.2012.08.043
43. Swanson LM, Burgess HJ, Zollars J, Arnedt JT. An open-label pilot study of a home wearable light therapy device for postpartum depression. Arch Women Ment Health. (2018) 21:583–6. doi: 10.1007/s00737-018-0836-z
44. Burns MN, Begale M, Duffecy J, Gergle D, Karr CJ, Giangrande E, et al. Harnessing context sensing to develop a mobile intervention for depression. J Med Int Res. (2011) 13:e55. doi: 10.2196/jmir.1838
45. Sharma A, Mentz RJ, Granger BB, Heitner JF, Cooper LB, Banerjee D, et al. Utilizing mobile technologies to improve physical activity and medication adherence in patients with heart failure and diabetes mellitus: rationale and design of the TARGET-HF-DM trial. Am Heart J. (2019) 211:22–33. doi: 10.1016/j.ahj.2019.01.007
46. Vu T, Lin F, Alshurafa N, Xu W. Wearable food intake monitoring technologies: a comprehensive review. Computers. (2017) 6:4. doi: 10.3390/computers6010004
47. Morrison LG, Yardley L, Powell J, Michie S. What design features are used in effective e-health interventions? A review using techniques from critical interpretive synthesis. Telemed J E Health. (2012) 18:137–44. doi: 10.1089/tmj.2011.0062
48. Steele R, Lo A, Secombe C, Wong YK. Elderly persons' perception and acceptance of using wireless sensor networks to assist healthcare. Int J Med Inform. (2009) 78:788–801. doi: 10.1016/j.ijmedinf.2009.08.001
49. Marino M, Li Y, Rueschman MN, Winkelman JW, Ellenbogen J, Solet JM, et al. Measuring sleep: accuracy, sensitivity, and specificity of wrist actigraphy compared to polysomnography. Sleep. (2013) 36:1747–55. doi: 10.5665/sleep.3142
50. Sadeh A. The role and validity of actigraphy in sleep medicine: an update. Sleep Med Rev. (2011) 15:259–67. doi: 10.1016/j.smrv.2010.10.001
51. Nam Y, Kim Y, Lee J. Sleep monitoring based on a tri-axial accelerometer and a pressure sensor. Sensors. (2016) 16:750. doi: 10.3390/s16050750
52. Razjouyan J, Lee H, Parthasarathy S, Mohler J, Sharafkhaneh A, Najafi B. Improving sleep quality assessment using wearable sensors by including information from postural/sleep position changes and body acceleration: a comparison of chest-worn sensors, wrist actigraphy, and polysomnography. J Clin Sleep Med. (2017) 13:1301–10. doi: 10.5664/jcsm.6802
53. Mohr DC, Zhang M, Schueller SM. Personal sensing: understanding mental health using ubiquitous sensors and machine learning. Ann Rev Clin Psychol. (2017) 13:23–47. doi: 10.1146/annurev-clinpsy-032816-044949
Keywords: wearable devices, sensors, major depression, biomarkers in psychiatry, mood monitoring
Citation: Lee S, Kim H, Park MJ and Jeon HJ (2021) Current Advances in Wearable Devices and Their Sensors in Patients With Depression. Front. Psychiatry 12:672347. doi: 10.3389/fpsyt.2021.672347
Received: 25 February 2021; Accepted: 21 May 2021;
Published: 17 June 2021.
Edited by:
Andrea De Bartolomeis, University of Naples Federico II, ItalyReviewed by:
Fay Horak, Oregon Health and Science University, United StatesBin Fang, Tsinghua University, China
Copyright © 2021 Lee, Kim, Park and Jeon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Jin Jeon, jeonhj@skku.edu
 Seunggyu Lee
Seunggyu Lee Hyewon Kim
Hyewon Kim Mi Jin Park3
Mi Jin Park3 Hong Jin Jeon
Hong Jin Jeon