- 1Department of Pharmacy, The Affiliated Brain Hospital of Guangzhou Medical University (Guangzhou Huiai Hospital), Guangzhou, China
- 2Guangdong Engineering Technology Research Center for Translational Medicine of Mental Disorders, Guangzhou, China
Background: There is a crucial link between the gut microbiota and the host central nervous system, and the communication between them occurs via a bidirectional pathway termed the “microbiota-gut-brain axis.” The gut microbiome in the modern environment has markedly changed in response to environmental factors. These changes may affect a broad range of host psychiatric disorders, such as depression, by interacting with the host through metabolic, immune, neural, and endocrine pathways. Nevertheless, the general aspects of the links between the gut microbiota and depression have not been systematically investigated through bibliometric analysis.
Aim: This study aimed to analyze the current status and developing trends in gut microbiota research in the depression field through bibliometric and visual analysis.
Methods: A total of 1,962 publications published between 1999 and 2019 were retrieved from the Web of Science Core Collection. CiteSpace (5.6 R5) was used to perform collaboration network analysis, co-citation analysis, co-occurrence analysis, and citation burst detection.
Results: The number of publications has been rapidly growing since 2010. The collaboration network analysis revealed that the USA, University College Cork, and John F. Cryan were the most influential country, institute, and scholar, respectively. The most productive and co-cited journals were Brain Behavior and Immunity and Proceedings of the National Academy of Sciences of the United States of America, respectively. The co-citation analysis of references revealed that the most recent research focus was in the largest theme cluster, “cytokines,” thus reflecting the important research foundation in this field. The co-occurrence analysis of keywords revealed that “fecal microbiota” and “microbiome” have become the top two research hotspots since 2013. The citation burst detection for keywords identified several keywords, including “Parkinson's disease,” “microbiota-gut-brain axis,” “microbiome,” “dysbiosis,” “bipolar disorder,” “impact,” “C reactive protein,” and “immune system,” as new research frontiers, which have currently ongoing bursts.
Conclusions: These results provide an instructive perspective on the current research and future directions in the study of the links between the gut microbiota and depression, which may help researchers choose suitable cooperators or journals, and promote their research illustrating the underlying molecular mechanisms of depression, including its etiology, prevention, and treatment.
Introduction
Depression is a common mental illness that can affect both mental and physical health (1). It affects ~350 million of the world's population, as reflected in limited functioning and diminished quality of life (2). According to the Global Burden of Disease Study, in 2017, depression disorders ranked as the third leading cause of global years lived with disability in females, and the fifth leading cause in males (3). However, the pathophysiology of depression disorders has not been fully elucidated, and no single mechanism can adequately explain all aspects of this disease (4). The key molecular mechanisms related to depression disorders include “the monoamine hypothesis,” “hypothalamic-pituitary-adrenal (HPA) axis changes,” “inflammation,” “neuroplasticity and neurogenesis,” “structural and functional brain changes,” “genes,” “environmental milieu,” and “epigenetics (gene-environment interactions)” (4). Therefore, multiple biological and psychosocial determinants are known to be important factors influencing depression (4). Among the factors associated with depression pathology, the gut microbiota can affect a broad range of host psychiatric disorders, such as depression, by interacting with the host through metabolic, immune, neural, and endocrine pathways (5, 6). The communication between the host central nervous system (CNS) and the human gut microbiota occurs via a bidirectional pathway, termed the “microbiota-gut-brain axis,” in which the microbiota and its metabolism are major components (7, 8). The communication pathways linking the gut microbiota with the CNS are incompletely understood but appear to have four main routes (6) (Figure 1). Microbial metabolism affects mediators of gut-brain-communication, including neurotransmitters [e.g., serotonin (5-HT) and γ-aminobutyric acid (GABA)], short-chain fatty acids (SCFAs) (e.g., butyrate), hormones (e.g., cortisol), and immune system modulators (e.g., quinolinic acid) (5). The metabolites modulated by the gut microbiota can influence mood, possibly through the activation of peripheral receptors on immune, neural, or endocrine pathways (8). In addition to the peripheral stimulation of these mediators, other direct and indirect mechanisms have been proposed to explain how metabolites affect depressive behavior, such as through direct stimulation of central receptors and epigenetic regulation of histone acetylation or DNA methylation (8).
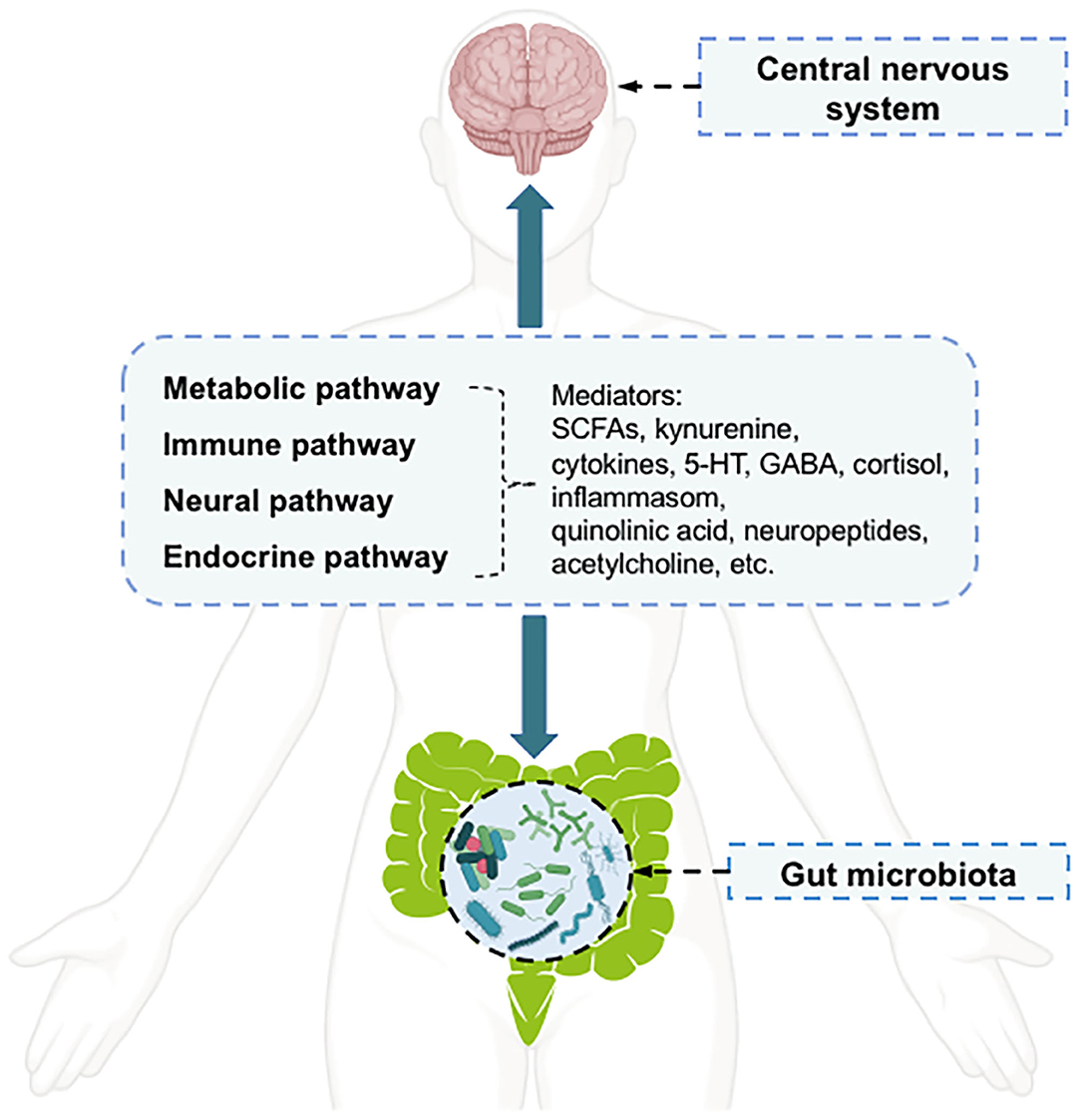
Figure 1. The pathways and mediators of gut microbiota-central nervous system bidirectional communication.
Compared with our ancestral microbiome, the gut microbiome in the modern environment has been markedly altered because of some environmental factors, such as diet, antibiotic exposure, and proton pump inhibitors. These differences may have important effects on host brain health (9). Fortunately, the rapid development of culture-independent molecular methods (e.g., 16S ribosomal RNA and metagenomic sequencing analysis) (10), metabolomics (11), available animal models of depression (12), and modern neuroimaging and computational biology (13, 14), has enabled quantitative analysis of the components of the gut microbiome and microbial metabolism, and exploration of the pathophysiological roles of the gut microbiota in depression and microbiome-gut-brain interactions. All aspects of these techniques are contributing to gut microbiota research, thus making the gut microbiota a hotspot in depression research. In recent decades, the relationships between the gut microbiota and depression in animals and humans have been widely studied (6). For instance, many probiotic strains known as “psychobiotics,” such as Lactobacillus paracasei PS23 and Lactobacillus helveticus NS8 (15, 16), as well as multi-species probiotics (17), have been shown to have antidepressant-like effects in rodent studies. Multiple studies in animal models have presented evidence that depression leads to microbiome changes or microbiota changes lead to depression, and indicated a causal relationship between them (6). Many human studies have also shown that probiotics are associated with a significant reduction in depression (18), possibly through an anti-inflammatory mechanism (19). Additionally, some clinical trials have been conducted to explore the feasibility of probiotics as adjuvants for traditional antidepressant therapies (20, 21). Although research on the connection between the gut microbiota and depression is widely available worldwide, the general aspects of this Research Topic, to our knowledge, have not been systematically analyzed through bibliometric analysis. Therefore, our study aimed to fill this gap in the literature.
Bibliometrics involves the use of a series of defined metrics and enables researchers to assess the published research output, impact, and developing trends by using statistical and quantitative methods (22). It can allow researchers to extract quantitative information on distribution by country/region, institution, author, and journal, and can aid in identifying research hotspots and frontiers in a particular field in a short time. Over the past 10 years, bibliometrics has been widely used to analyze scientific research in medical research worldwide (23). Previous bibliometric studies have focused on the gastrointestinal microbiome (24), the intestinal microbiota in obesity (25), or the microbiome-gut-brain axis (26), but have not addressed the gut microbiota in depression. Our bibliometric analysis involves this new and important field. The results should be helpful for researchers examining the gut microbiota in the depression field, aiding them in identifying journals to publish in and collaborators. The timely review and analysis of the hotspots and research trends may also promote the development of this field and advance research illustrating the molecular mechanisms underlying depression, such as its etiology, prevention, and treatment.
Therefore, this study aimed to comprehensively analyze the current status and developing trends in publications between 1999 and 2019 in gut microbiota research in the depression field through a bibliometric and visual analysis. The Web of Science™ Core Collection (WoSCC) database and CiteSpace software were used to conduct the bibliometric and visual analysis. Our focus was to: (i) investigate the outputs and growth trends in publications; (ii) construct international scientific collaboration networks among countries/regions, institutions, and authors; (iii) determine the core countries/regions, institutions, researchers, and journals; and (iv) explore the key topics, hotspots, and research trends, to guide future research and applications.
Materials and Methods
Introduction to CiteSpace
Science mapping, one of the main approaches used to explore a field of research in bibliometrics, is a general process of domain analysis and visualization. Its research scope includes a scientific discipline, and a research field or topics of particular research questions (27). CiteSpace, the Java application chosen to perform the bibliometric analysis, was developed in 2004 by Professor Chaomei Chen (College of Computing and Informatics, Drexel University, Philadelphia, PA, USA), an international expert in the information visualization field (28). It is an interactive analytic tool enabling visualization tasks in science mapping through a combination of bibliometrics, visual analytic methods, and data mining algorithms. CiteSpace supports several types of bibliometric studies, including collaboration network analysis, co-citation analysis, and co-occurrence analysis, and can generate visual maps such as geospatial visualizations (27). Collaboration network analysis can identify the core countries/regions, institutions, and authors in a particular field as well as their cooperative relationships. Additionally, co-citation analysis and co-occurrence analysis can reflect the research foundation and hotspots of the field, respectively. To date, it has been continually developed and widely used in the field of medical research (29, 30).
Data Acquisition and Search Terms
Bibliometric analysis relies on literature databases. One of the best-known databases is the WoSCC database, a curated collection of high-quality scholarly content on the Web of Science™ platform (WoS; previously known as Web of Knowledge), an online subscription-based scientific citation indexing service maintained by Thomson Reuters (31). To avoid the bias due to the daily database updates, because the database remains open, we performed the literature retrieval from WoSCC on a single day, May 15, 2020. The search index used in WoSCC was standardized to include all the relevant publications to ensure the comprehensiveness of the bibliographic data.
Synonyms for gut microbiota and depression were included in the search strategy, as follows: TS = (((gut OR intestin* OR gastrointestin* OR gastro-intestin*) AND (microbiot* OR microbiome* OR flora OR microflora OR bacteria)) OR prebiotic OR probiotic OR antibiotic OR dysbiosis) AND TS = (depression OR depressed OR depressions OR depressive OR despondent OR gloomy). In the present study, the inclusive and exclusion criteria were as follows: (i) the timespan ranged from 1999 to 2019, encompassing 20 years in total, (ii) only articles and reviews were included, whereas other document types (e.g., letters, meeting abstracts, retracted publications, and book chapters) were excluded, (ii) no species limitations were set, (iv) the publication language was restricted to English, and (v) duplicate publications were excluded. The search results were directly analyzed with the WoSCC literature analysis wire and exported for further analysis in CiteSpace. The present study used published data from secondary sources and did not involve any interactions with human subjects; hence, the requirement for institutional review board approval was waived (32).
Analysis Tools
Features such as the publication outputs, subject categories of WoS, h-index, and impact factor (IF) were analyzed with the WoSCC literature analysis wire (33). The h-index is a measure of productivity and the impact of a researcher who has published h articles cited at least h times each; that is, if the h-index of a scientist is 10, the scientist published 10 articles with at least 10 citations per article (34). The IF from Thomson Reuters indicates the impact of journals, referring to the number of citations to a given journal in a specific year (35).
CiteSpace (5.6 R5) (http://cluster.cis.drexel.edu/~cchen/citespace/) was used to perform collaboration network analysis (authors, countries/regions, and institutions), co-citation analysis (journals, authors, and references), analysis of keyword co-occurrence, and citation burst detection for keywords and references. The specific parameters used in CiteSpace were set as follows: time slicing (from 1999 to 2019, years per slice = 1), term source (title, abstract, author, keyword, and keywords plus), node type (one option chosen at a time from author, institution, country, keyword, cited reference, cited author, and cited journal), link strength (cosine), link scope (within slices), selection criteria (top 50 per slice), and pruning (none).
The visualizations generated by CiteSpace consisted of nodes and link lines. The nodes in the network maps represented the type of study being analyzed, such as the authors, countries/regions, institutions, cited references, and keywords; link lines between the nodes indicated cooperative, co-cited, or co-occurring relationships (36). The size of a node indicated the number of citation or occurrence, and the width of a line indicated the strength of the relationship (36, 37). The color of a node, represented by a series of annual rings, indicated the distribution time (36, 38). The importance of the publication was identified and we measured its betweenness centrality. A key node or pivot point in the scientific collaboration or reference co-citation network was marked with a purple ring if its centrality was ≥ 0.1 (39, 40). The calculated Q-value and silhouette-value were indicators representing the modularity and homogeneity of the cluster network, respectively. The larger the Q-value, the better the modularity. The closer the silhouette-value to zero, the higher the homogeneity. A Q-value > 0.3 identified the cluster structure as significant, and a mean silhouette > 0.5 or > 0.7 indicated that the clustering result was reasonable or highly credible, respectively (40). A citation burst had two attributes: the intensity and the duration of the burst. The burst detection revealed abrupt changes in terms or citations over a specified period, thereby identifying emerging research trends (41).
IBM SPSS Statistics, version 25.0 (SPSS Inc., Chicago, IL, USA) was used to depict and visualize the growth trend in annual publication outputs by using a polynomial regression model. We tested for possible correlation relationships between the total number of publication outputs and the betweenness centrality for each country/region, institution, and author that reached a centrality threshold above zero. Pearson's correlation coefficient (r) was calculated in SPSS. A p-value < 0.05 was considered to indicate statistical significance.
Results
Analysis of Publication Outputs and Growth Trend Prediction
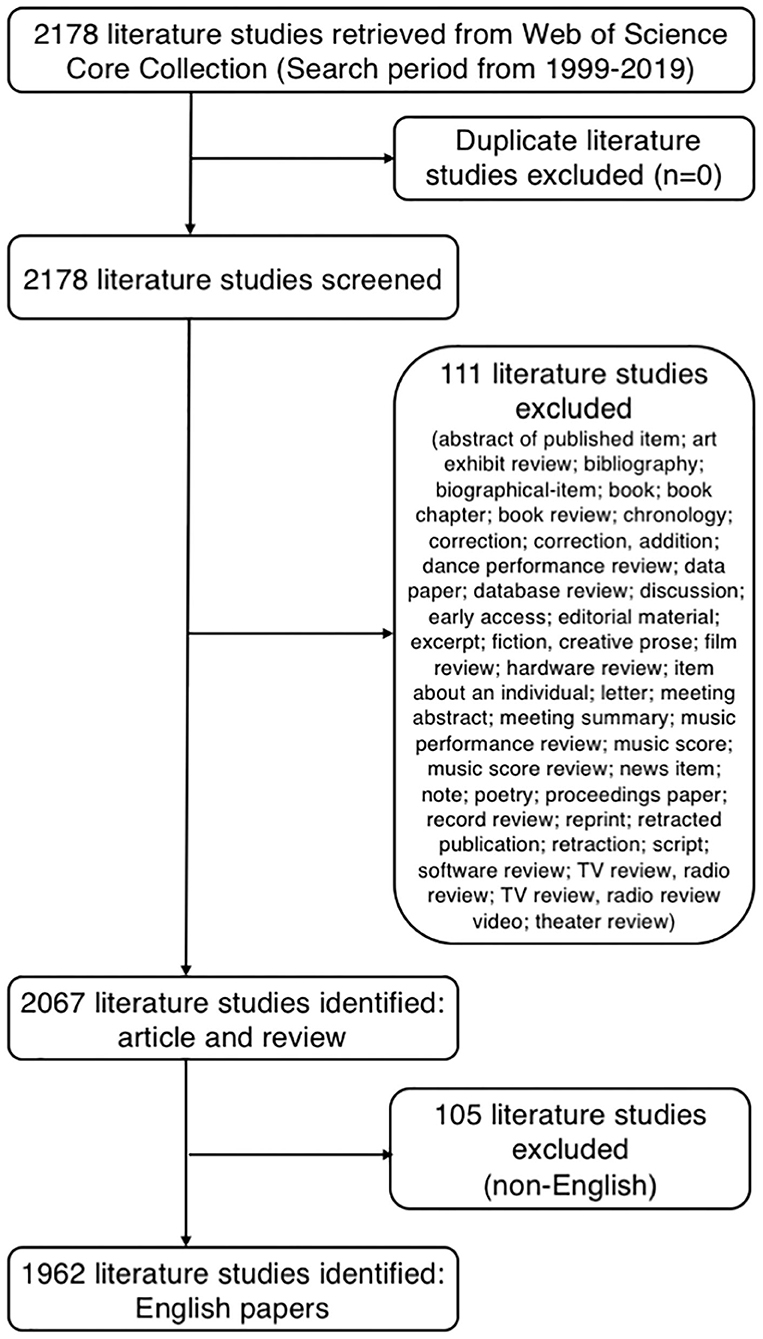
The search retrieved 1,962 publications that met the inclusion and exclusion criteria (Figure 2), and 1,520 and 442 articles and reviews, respectively, were identified. Over the past 20-year period, the development track showed two stages: one was the initial period (1999–2009), which had a very slow development speed, and the other was a rapid development period (2010–2019). The number of publication outputs increased from 31 publications in 1999 to 407 publications in 2019, with an average of 98 publications per year (Figure 3A). Compared with those in the year 1999, the cumulative growth rate reached 1212.903, 920.690, and 5450.000% in 2019 for total publications, articles, and reviews, respectively, and the compound annual growth rate (CAGR) was 13.740, 12.317, and 22.240%, respectively (Figure 3B). The CAGR was the annualized average rate of growth between the years 1999 and 2019, calculated as follows: CAGR = [(value in year 2019/value in year 1999) (1/20)-1]. As presented in Figure 3C, the publication trend remained relatively limited and stable before the 2010s, whereas continual growth of research on gut microbiota in the depression field has occurred since then. The model fitting curves of growth in document number showed a strongly positive correlation with the year of publication (R2 = 0.976, 0.964, and 0.980 for total publications, articles, and reviews, respectively). We conservatively estimated that the total publication, article, and review outputs will exceed 450, 325, and 125, respectively, in 2020. The top five WoS categories of the analyzed publications were neurosciences (n = 322, 16.412%), psychiatry (n = 240, 12.232%), pharmacology/pharmacy (n = 194, 9.888%), clinical neurology (n = 123, 6.269%), and gastroenterology/hepatology (n = 119, 6.065%).

Figure 3. Trends in the number of publications from 1999 to 2019 on gut microbiota research in the depression field. (A) The annual number of publications. (B) The cumulative growth rate for publications. (C) The polynomial fitting curves of growth trends.
Analysis of Scientific Collaboration Network
Fifty-two countries/regions contributed to the publications on gut microbiota research in the depression field. The top 10 countries/regions according to publications and centrality are listed in Table 1. The top five countries/regions, in order of the number of publications, were the USA, People's Republic of China, Canada, Australia, and England. The top five countries/regions by centrality were the USA, Germany, Australia, England, and Italy. Pearson's correlation analysis revealed a significant correlation between publications and centrality at the country/region level (r = 0.861, p < 0.001). Comprehensive analyses of publications and centrality indicated that the USA (publications: 580, centrality: 0.35), Australia (publications: 120, centrality: 0.16), and England (publications: 118, centrality: 0.16) were the most influential in the field. The collaboration network map among countries/regions is presented in Figure 4A, in which there were 52 nodes and 319 link lines. The nodes and the link lines between them represent the countries/regions and their cooperative relationships, respectively. The larger the node, the more publications. The wider the line, the stronger the relationships. Changes in node color represent the distribution time of publications. The node marked with a purple ring represents centrality ≥ 0.1. Overall, the USA (collaborators: 38), England (collaborators: 29), Germany (collaborators: 29), Canada (collaborators: 28), and Australia (collaborators: 28) had the largest number of national partners.
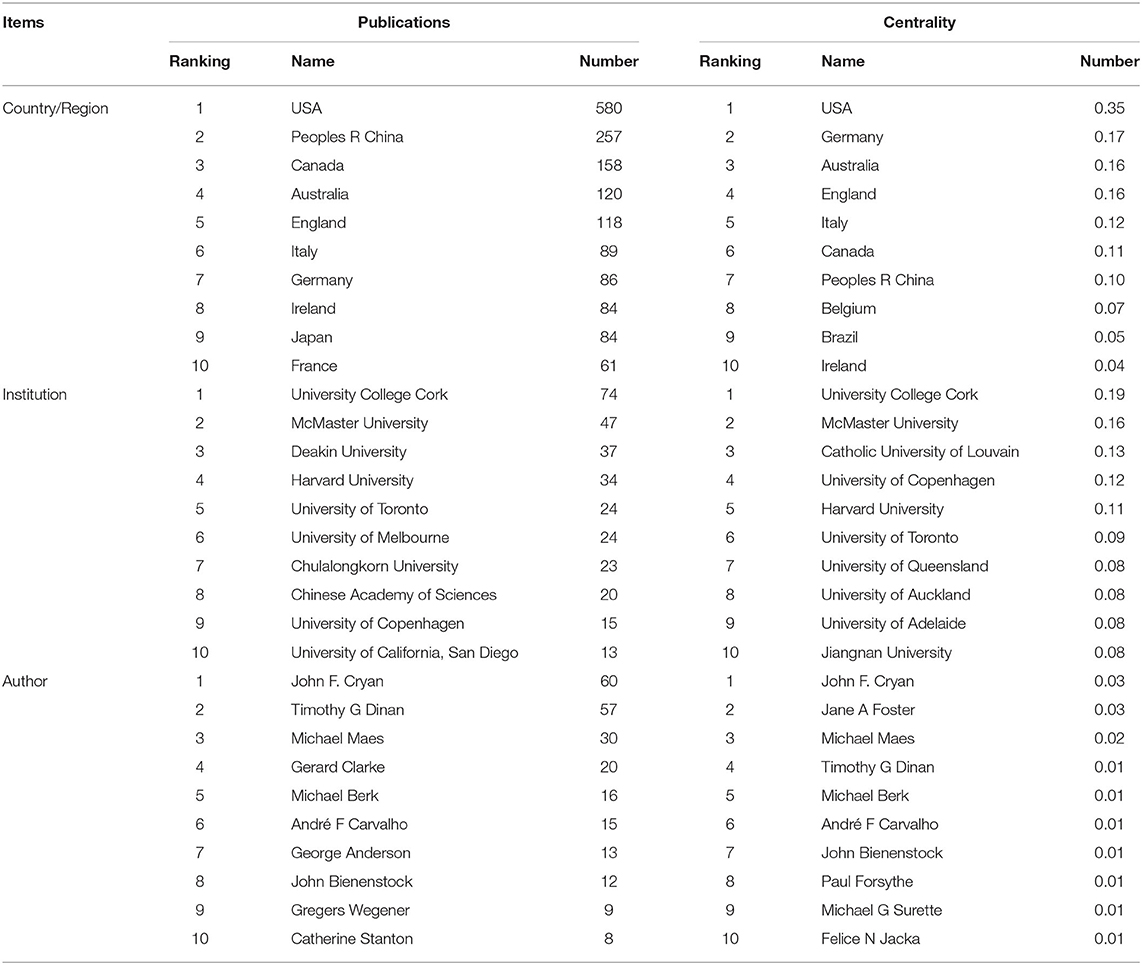
Table 1. The top 10 countries/regions, institutions, and authors in terms of publications and centrality.
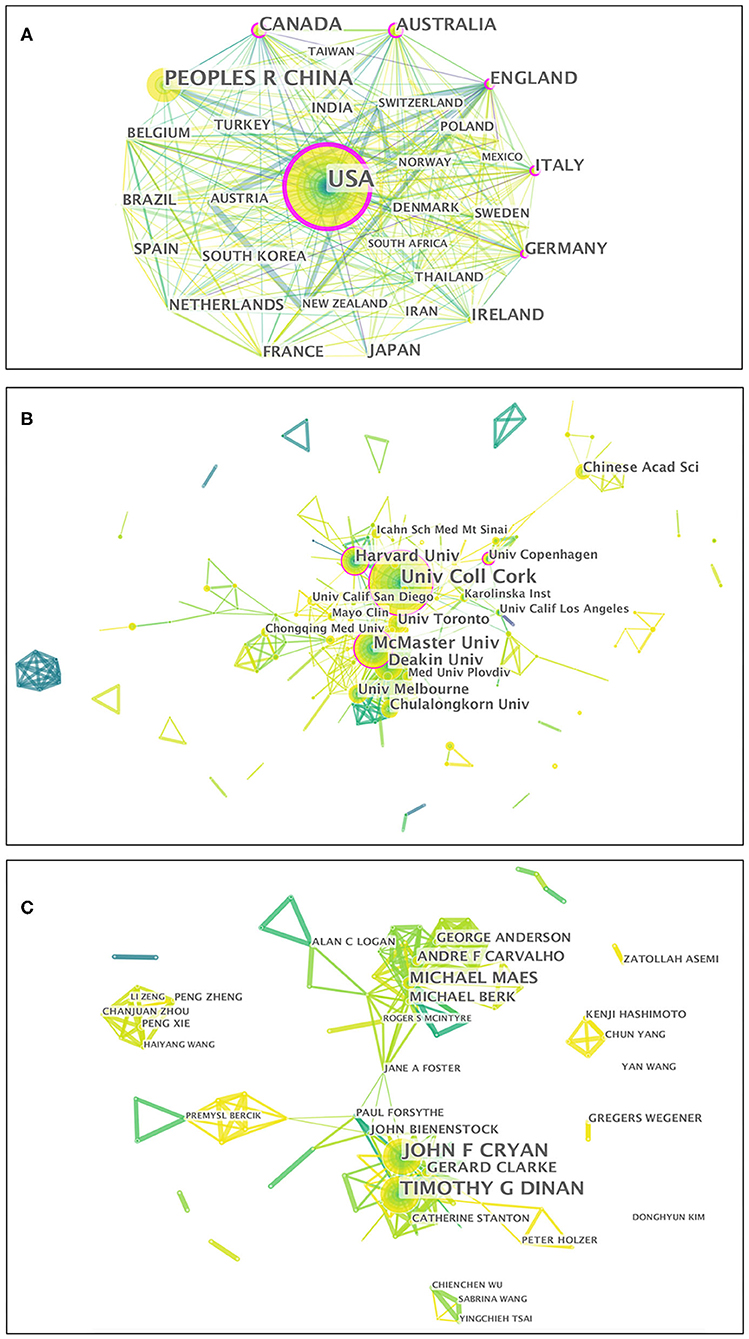
Figure 4. Visualization map of the scientific collaboration network analysis of gut microbiota research in the depression field from 1999 to 2019. Collaboration among countries/regions (A), institutions (B), and authors (C). The nodes in the map denote elements such as an author, country/region, or institute, and link lines between nodes denote collaborative relationships. The larger the circle, the more articles published. The wider the line, the stronger the relationship. The outermost purple ring denotes the centrality level, and highly central nodes are considered pivotal points in the research field.
From 1999 to 2019, a total of 266 institutions published in this field. Table 1 illustrates the top ten institutions according to publications and centrality. The top five institutions with the greatest contribution to this field were University College Cork, McMaster University, Deakin University, Harvard University, and University of Toronto. In terms of centrality, the top five institutions were University College Cork, McMaster University, Catholic University of Louvain, University of Copenhagen, and Harvard University. A significant correlation between publications and centrality was also observed at the institution level (Pearson r = 0.734, p < 0.001). On the basis of the analyses of publications and centrality, University College Cork (publications: 74, centrality: 0.19), McMaster University (publications: 47, centrality: 0.16), and Harvard University (publications: 34, centrality: 0.11) were the major research institutions. The generated institution network map identified 266 nodes and 581 link lines, which represented the institutions and their cooperative relationships, respectively; thus extensive collaborations between institutions were found (Figure 4B). For instance, University College Cork, represented by the largest node marked with a purple ring, had the most publications and the most extensive cooperation, with more than 25 institutions, such as TEAGASC, McMaster University, Harvard University, Deakin University, University of Auckland, University of Gothenburg, University of California-Los Angeles, and Catholic University of Louvain. Although Catholic University of Louvain did not have as many publications, it had many research partners, such as Medical University of Graz, University of Reading, University College Cork, McMaster University, University of Copenhagen, and University of Auckland.
In total, 368 authors published in this field in the past two decades. Table 1 presents the top 10 authors according to publications and centrality. Eight authors published more than 10 publications in this field. Among these active authors, John F. Cryan was ranked first, with 60 publications, followed by Timothy G. Dinan and Michael Maes. The top three authors ranked by centrality were John F. Cryan, Jane A. Foster, and Michael Maes, whereas none had a centrality ≥ 0.1, thus indicating that international cooperation among top scientific researchers was insufficient. Furthermore, there was no significant correlation between publications and centrality at the author level (Pearson r = 0.380, p = 0.249). The co-authorship network map is shown in Figure 4C, containing 368 nodes and 924 collaboration lines. The nodes and the link lines between them represent the authors and their cooperative relationships, respectively. None of the nodes are marked with a purple ring, owing to their very low centrality (<0.1). As presented in the map, Michael Maes, represented by the third largest node, had restricted relationships with John F. Cryan or Timothy G. Dinan. Notably, Jane A. Foster played a central role in international collaborations but did not have considerable publication productivity.
Analysis of Journals and Co-cited Journals
The articles included were published in 1,038 different journals, many of which were specialized journals. As one of the most important indicators, co-citation analysis has been widely used in bibliometrics. Table 2 presents the top 10 journals and co-cited journals for gut microbiota research in the depression field. The most prolific journal in this field was Brain Behavior and Immunity, followed by PLoS One, Poultry Science, and Scientific Reports. All the highly productive journals had an IF above 2.0. Co-cited journals were those cited together by other researchers. The top three journals according to co-citation count were Proceedings of the National Academy of Sciences of the United States of America (PNAS), PLoS One, and Nature. The journals with betweenness centrality ≥ 0.1 included PNAS (centrality: 0.20), British Journal of Nutrition (citations: 432, centrality: 0.16), Science (centrality: 0.12), and Nature (centrality: 0.11). The published references of those top journals reflect the foundation of this field.
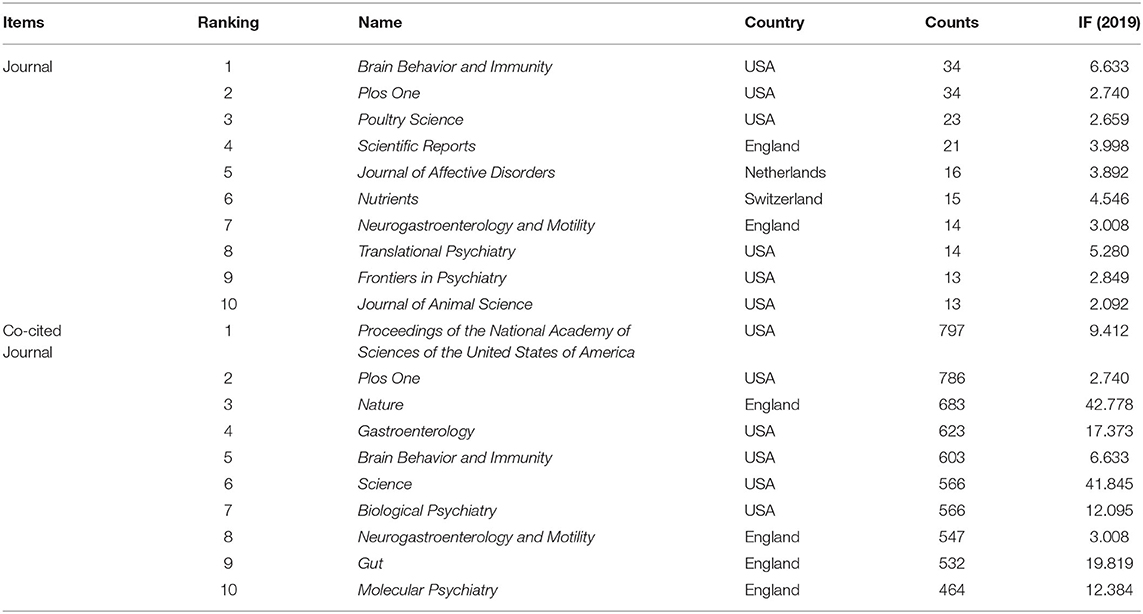
Table 2. The top 10 journals and co-cited journals for gut microbiota research in the depression field.
Analysis of Co-cited Authors
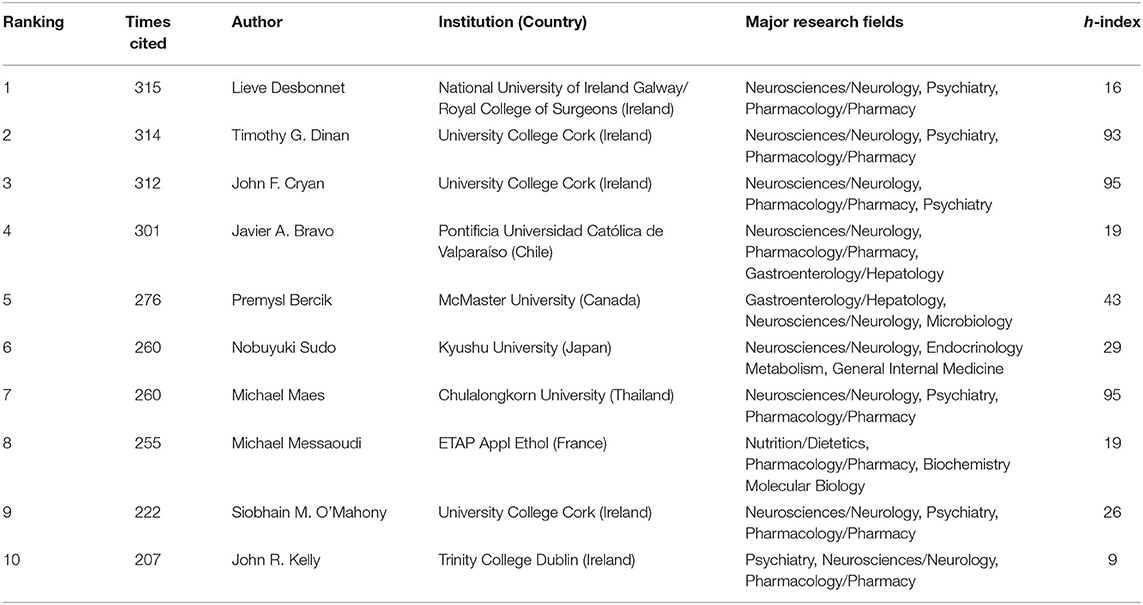
Co-cited authors are those cited together by other researchers. The co-citation analysis by author included 678 cited authors, 14 of whom were cited at least 200 times. The top 10 co-cited authors and their affiliates, major research fields, and h-indexes are listed in Table 3. The top three co-cited authors all came from Ireland. Lieve Desbonnet ranked first, with 315 citations, followed by Timothy G. Dinan, John F. Cryan, and Javier A. Bravo, whereas the remaining authors had fewer than 300 citations. The uppermost h-index value was for Michael Maes, followed by John F. Cryan and Timothy G. Dinan. Their research areas were mainly neurosciences, psychiatry, and pharmacology. These authors contributed to the work in this field, which provided the research basis.
Analysis of Co-cited References
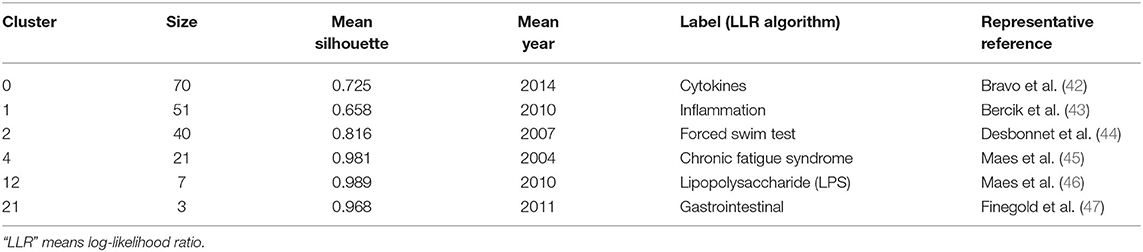
References cited are often considered a core component of bibliometric research. Co-cited references are those co-cited in the reference lists of other articles. Figure 5A shows a cluster visualization of the reference co-citation network generated by CiteSpace software, which was divided into 90 clusters, of which only the largest six extracted from the references, on the basis of indexing terms and identified by a log-likelihood ratio algorithm. They are shown with different convex hulls in the figure, including cytokines (cluster #0), inflammation (cluster #1), forced swim test (cluster #2), chronic fatigue syndrome (cluster #4), lipopolysaccharide (LPS) (cluster #12), and gastrointestinal (cluster #21). The different nodes in the map represent cited references, and their authors are labeled in black. The representative authors in the largest six clusters are shown in Figure 5A. The details of the largest six clusters of references in the co-citation network are shown in Table 4. The total Q-value was 0.6901, and each cluster had a mean silhouette above 0.5, indicating that the cluster quality was reasonable. Figure 5B shows the top six clusters in a timeline view, which depicts clusters along with horizontal timelines and has a vertical arrangement according to descending size, thus indicating the scientific relevance of the published articles. Figure 5B and Table 4 show that the most recent research focus was in cluster #0, “cytokines” (mean year 2014). The cluster and timeline visualization of the reference co-citation map contained 410 nodes and 1,796 links, representing the cited references and their co-cited relationships, respectively.
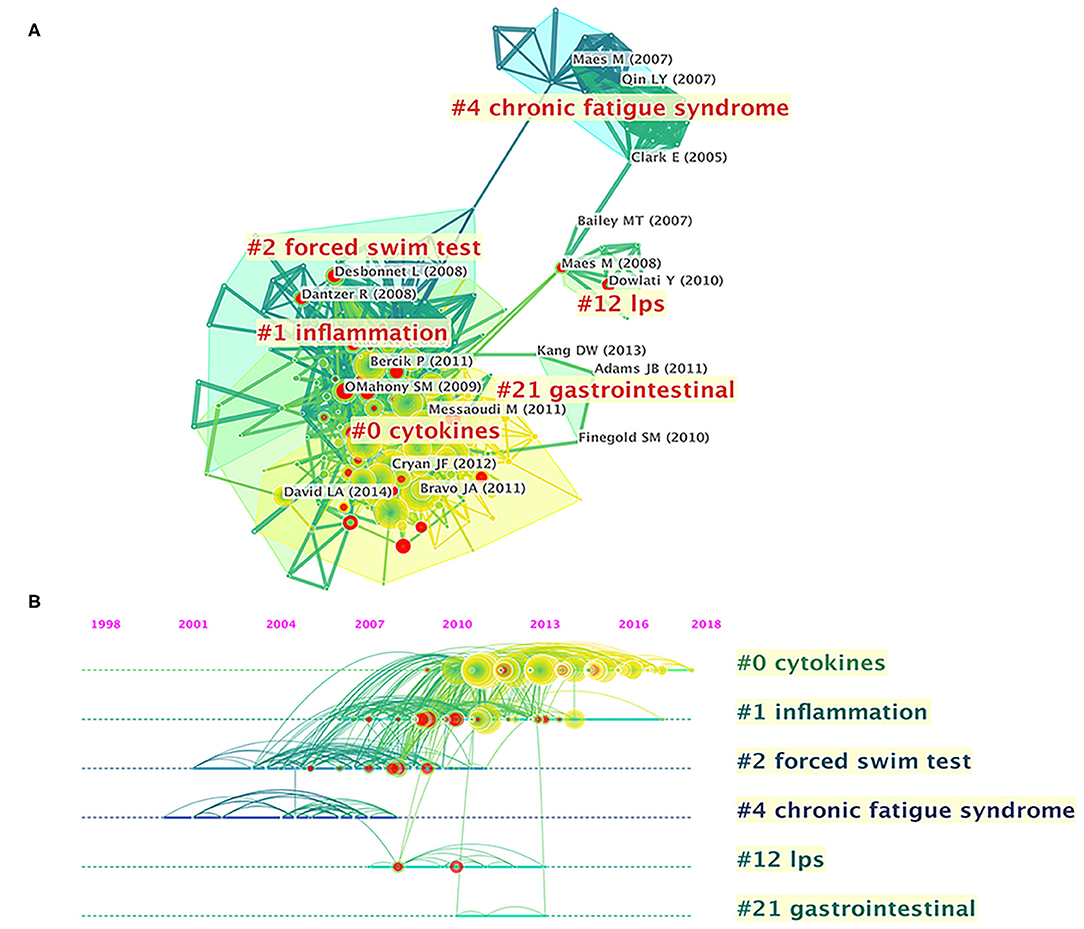
Figure 5. Reference co-citation network analysis of publications on gut microbiota research in the depression field between 1999 and 2019. (A) Cluster visualization of the reference co-citation map. (B) Timeline visualization of the reference co-citation map. Top clusters and respective landmark references are labeled. The nodes in the map denote co-cited references, and links between nodes denote co-citation relationships. The citation rings denote the citation history of a reference. Large nodes or nodes with red tree-rings are either highly cited or have citation bursts in a given time slice.
The characteristics of the top 10 highly co-cited references concerning gut microbiota research in the depression field are summarized in Table 5. All were found in cluster #0, and the top-ranked reference was published by Javier A Bravo (42), with a co-citation count of 283. The articles with the maximum co-citations are generally important foundational studies in this field.
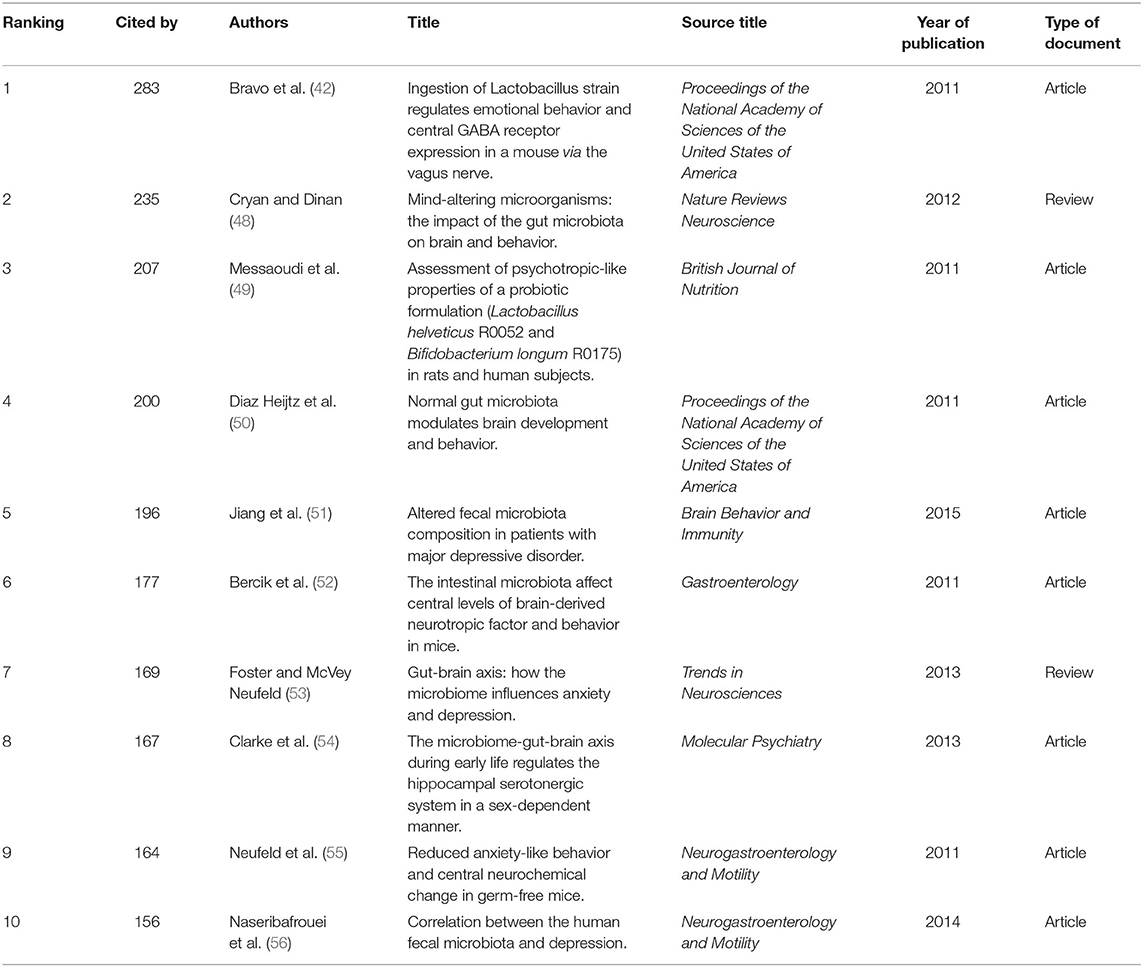
Table 5. The top10 co-cited references related to gut microbiota research in the depression field between 1999 and 2019.
Analysis of Co-occurring Keywords
A map of keyword co-occurrence reflects research hotspots. Figure 6 presents a time-zone view of the keyword co-occurrence network to map the knowledge structure of research, resulting in 350 nodes and 2,168 links, representing the keywords and their co-occurring relationships, respectively. The larger the node, the greater the occurrence the keyword. In terms of co-occurrence frequency, the top 10 keywords were “depression,” “gut microbiota,” “anxiety,” “inflammation,” “microbiota,” “stress,” “irritable bowel syndrome,” “gut-brain axis,” “intestinal microbiota,” and “brain” (Table 6). However, the keywords “fecal microbiota” and “microbiome” have become the top two research hotspots since 2013 (Figure 6), appearing in 98 and 94 citing studies, respectively (Table 6).
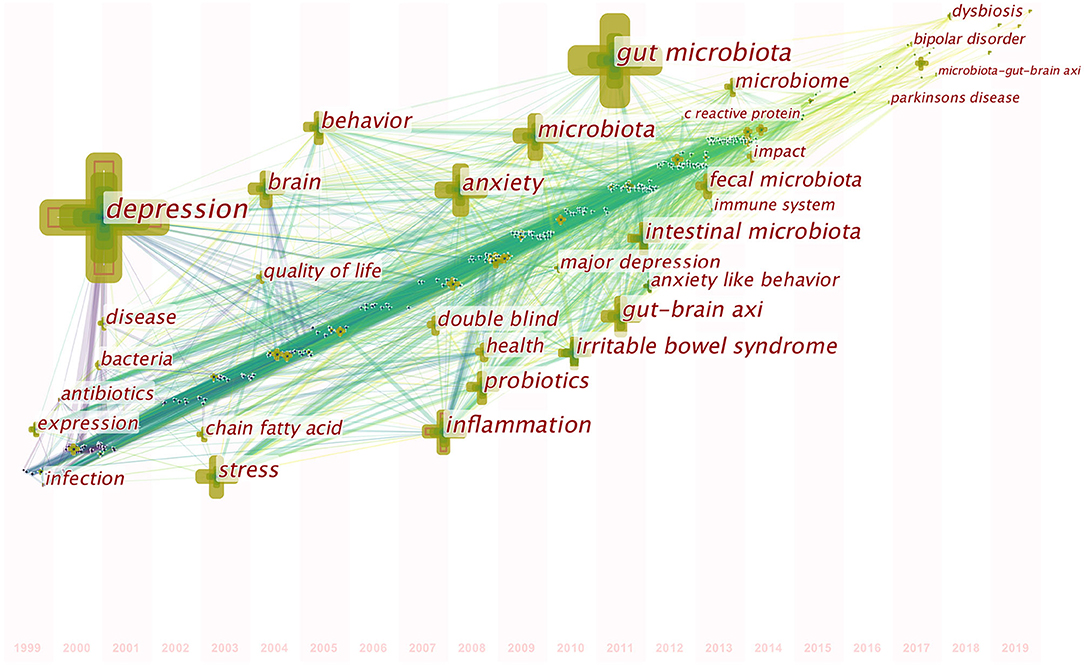
Figure 6. Time-zone view of keyword co-occurrence in publications from 1999 to 2019 on gut microbiota research in the depression field. The nodes represent keywords, and the chronological order in which keywords appear can be found on the map. The size of a node is proportional to the frequency of keyword occurrence.
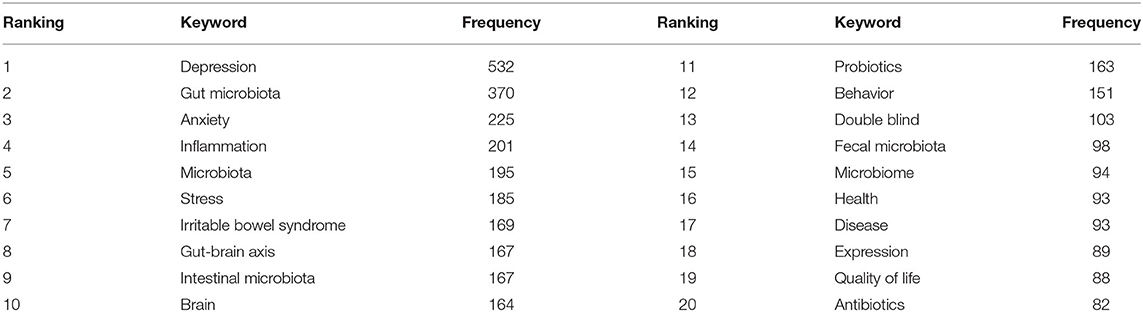
Table 6. The top 20 keywords in terms of frequency for gut microbiota research in the depression field.
Analysis of Burst Detection
References with citation bursts, defined as those that have frequent citations over time, can be used to indicate the evolution of a knowledge domain (57). A total of 59 references with strong citation bursts were detected (Figure 7). The duration of the burst is displayed by a red line segment. References with citation bursts first appeared in 2007 (58, 59), and the most recent references with citation bursts appeared in 2017 (60–65). The strongest burst (strength: 18.7714) appeared in 2010 for a 2008 article (44). Nine references had a burst that lasted until 2019 (60–68). The references with citation bursts between 2010 and 2017 accounted for 93.22%.
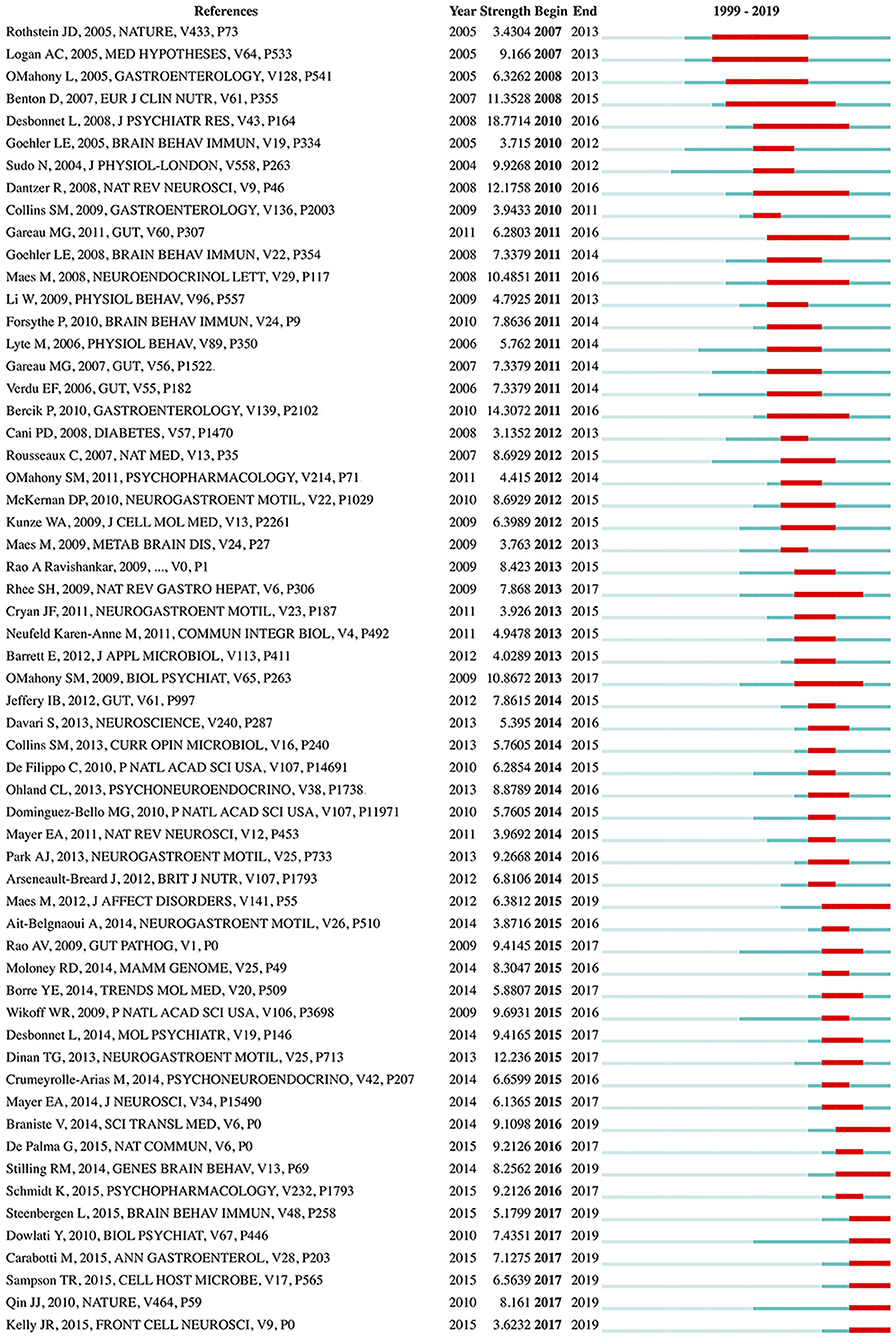
Figure 7. References with the strongest citation bursts in publications from 1999 to 2019 on gut microbiota research in the depression field. The strength values reflect the frequency of citation. The red bars indicate references cited frequently; the green bars indicate references cited infrequently.
Burst keywords can be used to predict new frontier topics in research in a particular field. Figure 8 shows the top 68 keywords with the strongest citation bursts. “Antibiotics” was the strongest burst keyword (strength: 16.4535) in this field from 1999 to 2012, and was followed by “infection (strength: 15.5516),” “anxiety-like behavior (strength: 13.0703),” and “performance (strength: 11.1118).” The keywords with a burst lasting until 2019 included “Parkinson's disease,” “microbiota-gut-brain axis,” “microbiome,” “dysbiosis,” “bipolar disorder,” “impact,” “C reactive protein (CRP),” and “immune system,” reflecting the most recent research trends. The bursts in certain keywords can also be used to analyze the evolution of research. For instance, Figure 8 reveals that rodents such as mice and rats have replaced non-rodent animals such as piglets, cattle, and horses, as the preferred animal models in gut microbiota-depression research. The results also suggest that studies in recent decades, have increasingly focused on the relationships between the gut microbiota and potential depression-related disorders (e.g., Alzheimer's disease, inflammatory bowel disease, irritable bowel syndrome, Parkinson's disease, and bipolar disorder), as well as the underlying mechanisms, involving the immune system, intestinal permeability, bacterial translocation, cytokines such as necrosis factor-alpha, CRP, and the microbiota-gut-brain axis.
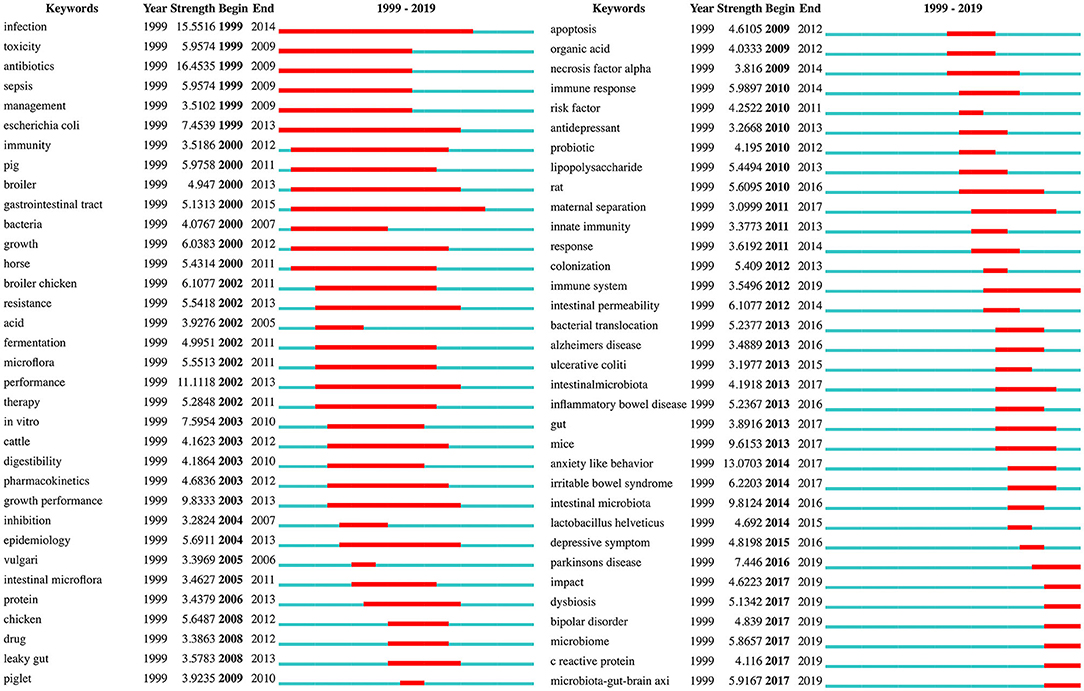
Figure 8. Keywords with the strongest citation bursts in publications from 1999 to 2019 on gut microbiota research in the depression field.
Discussion
This is the first application of bibliometric and visual analysis methods to gut microbiota research in the depression field. A total of 1,962 publications originating from WoSCC were analyzed, and we present a comprehensive overview of the worldwide hotspots and trends in gut microbiota research in the field of depression over the past two decades. Our analysis revealed the rapid growth in the number of publications since 2010 and the close international scientific cooperation in this field. The USA, University College Cork, and John F. Cryan were the most influential country, institute, and scholar, respectively. Brain Behavior and Immunity and PNAS were the most productive and co-cited journals, respectively. The most recent research focus was on the largest theme cluster “cytokines,” thus reflecting the important research foundation in this field. Since 2013, the top two research hotspots have been “fecal microbiota” and “microbiome.” Additionally, “Parkinson's disease,” “microbiota-gut-brain axis,” “microbiome,” “dysbiosis,” “bipolar disorder,” “impact,” “CRP,” and “immune system” reflected the most recent research trends in the field. Thus, our results provided insights and valuable information regarding gut microbiota research in depression; these findings may help researchers choose suitable cooperators or journals, and promote their research through our timely review and analysis of the hotspots and research trends.
Collaboration network analysis can provide detailed information for evaluating research collaborations and identifying the key cooperators. Among countries/regions, the USA had an absolute advantage in this field, possibly because of its better economics and expenditure in scientific research; for example, a special research project on the gut microbiota-brain axis was launched in 2013 (69), thus resulting in a positive influence on research productivity and quality (70). Remarkably, China, as a developing country, has shown vast progress in this field over the past two decades. Although China was the second most productive country, its influence in this field was inferior to that of the USA because of its insufficient international cooperation. Among institutions, University College Cork was a key player, possibly because the main researchers worldwide (including John F. Cryan, Timothy G. Dinan, Gerard Clarke, and Siobhain M. O'Mahony) in this area worked at this institute, whereas the institutions and researchers in the USA were quite scattered. Overall, close international scientific cooperation was found in this field. However, we discovered that the cooperation between top researchers appears to be insufficient, a finding that may be associated with their research being comparatively full-fledged (40). For example, we found that John F. Cryan and Timothy G. Dinan, who were academic leaders at the Alimentary Pharmabiotic Centre, University College Cork, Ireland, had the most productive publications and highest citations in this field. They were the first to define the term “psychobiotic,” which refers to a live organism that produces positive effects on the mental health of people with psychiatric illness when ingested in adequate amounts (71). In addition, their research team has conducted many studies in the field of psychobiotics, funded by Science Foundation Ireland, the Health Research Board of Ireland, and the European Community's Seventh Framework Programme, in close collaboration with several companies including GlaxoSmithKline, Pfizer, Cremo, Wyeth, and Mead Johnson (26, 72).
Journal analysis and co-cited journal analysis can provide important information that can help researchers select appropriate journals for article submission. Through our research, we discovered that the top 10 most active journals published less than a quarter (10.04%) of the total publications on gut microbiota research in the depression field, thus indicating a clearly dispersed distribution of the literature distribution across journals, possibly because of the diversified research directions involving neurosciences, psychiatry, pharmacology/pharmacy, clinical neurology, and gastroenterology/hepatology. Thus, on the one hand, researchers may have many journal choices, and on the other hand, they may have difficulty in choosing the most appropriate journal because of a lack of knowledge or experience (38). In addition, there was only a 30% concordance rate between the top 10 most active journals and the top 10 co-cited journals, thus suggesting that the quality of research in this field still must be improved, and the international cooperation among researchers should be strengthened to produce high-quality research (38).
According to reference co-citation network analysis, all the top 10 highly co-cited references were located in the largest theme cluster #0, “cytokines,” which formed an important basis for studies in this field. Mounting evidence suggests that the levels of some pro-inflammatory cytokines [e.g., interleukin (IL)-1β, IL-6, IL-18, and tumor necrosis factor (TNF)-α] are increased in depressive disorder, whereas some anti-inflammatory cytokines [e.g., IL-10 and transforming growth factor (TGF)- β1] are decreased (73, 74). Some meta-analyses have provided evidence that the alterations in the levels of peripheral cytokine (e.g., IL-8, TNF-α, IL-6, and IL-10) are associated with the antidepressant treatment response in patients with major depressive disorder (MDD) (75, 76). IL-1β, IL-6, and TNF-α are the three main cytokines that play a key role in mediating signaling via the gut microbiome-to-brain communication pathways (77). Other cytokine signaling pathways have also been widely explored. For example, a previous study has demonstrated that live Lactobacillus rhamnosus (JB-1) can modulate the immune system by inhibiting the synthesis of TNF-induced IL-8 in the human epithelial cell lines T84 and HT-29 (78). Among the top ten co-cited references, the first was an article published by Bravo et al. in PNAS in 2011 (42), in which the authors further found that L. rhamnosus (JB-1) modulates the GABAergic system in mice via the vagus nerve, thereby exerting beneficial effects in the treatment of stress-related psychiatric disorders such as depression and anxiety. The second most co-cited reference was a review published by Cryan and Dinan in Nature Reviews Neuroscience in 2012 (48), in which the authors describe how the gut microbiota influences brain function and behavior through neural (vagus and enteric nervous system), endocrine (cortisol), and immune (cytokines) pathways involving bidirectional communication between the gut microbiota and the brain. Those publications have laid important research foundations in this field.
Furthermore, the dynamics of this field was partly characterized by references with citation bursts (57, 79). The reference with the strongest burst, occurring in 2010, was an article published by Desbonnet et al. in the Journal of Psychiatric Research in 2008 (44), in which the authors revealed that Bifidobacteria treatment attenuates pro-inflammatory immune responses and elevates serotonergic precursors and tryptophan, thus suggesting that depressed people, particularly those with accompanying gastrointestinal inflammation, may benefit from this probiotic, owing to its potential antidepressant properties. An article published by Maes et al. in the Journal of Affective Disorders in 2012 (66), had a citation burst starting in 2015 that is still ongoing. Their findings suggest that in mesenteric lymph nodes, “translocated” commensal bacteria activate immune cells and elicit IgA and IgM responses, and increased bacterial translocation may play a role in the pathophysiology of chronic depression by causing progressive amplification of inflammatory and cell-mediated immune pathways. The most recent citation bursts occurred in 2017, among which the strongest burst was due to an article published by Qin et al. in Nature in 2010 (64). This was the largest metagenomic survey study to date of human gut microbial genes established by metagenomic sequencing, thus setting the stage for comparing gut microbiome profiles between healthy and diseased individuals (80, 81).
According to the co-occurring keyword analysis, we identified some of the most important hotspots in this field over the past two decades, including depression, gut microbiota, anxiety, and inflammation. We found that the publication outputs have increased rapidly since 2010, partly because of the key hotspot, the second highest frequency keyword “gut microbiota,” occurring in 2011. Since 2013, “fecal microbiota” and “microbiome” have become the new top hotspots in this field. The fecal microbiota, as a proxy for the gut microbiota, have been shown to be significantly associated with depression (51, 56). Moreover, fecal microbiota transplants (FMT) technology, an ancient administration route traced back to fourth century China (82), is receiving increasing attention. In this method, stool is transferred from a healthy donor into a recipient, with the goal of replacing the recipient's “bad” bacteria with “good” bacteria to normalize the gut microbiota composition and gain therapeutic benefits (83). Accumulating clinical studies suggest that FMT has potential therapeutic effects on depression-related disorders, owing to the increase in microbiota diversity (84, 85). FMT is also called stool transplantation and is an emerging tool used in animal models in gut microbiota research. For example, Li et al. (86) have used an antibiotic cocktail to deplete the gut microbiota in mice, and then colonized the gut, via FMT, with the microbiota from mice exposed to chronic unpredictable mild stress. Subsequently, similar anxiety- and depression-like behavior was observed, possibly because of the significant elevation in pro-inflammatory cytokines modulated by the gut microbiota. Another hotspot is “microbiome.” The amalgam of microorganisms in a particular habitat (such as the skin, mouth, vagina, or gut) is defined as a microbiota (87). Although the term microbiome refers specifically to the collective genomes of all microorganisms in a microbiota, it is sometimes used as a synonym for microbiota (88). Microbiome-host interaction, although not a novel concept, has recently been revisited in a surge of studies, particularly those focusing on the role of the gut microbiome in regulating the maturation and functionality of the host immune system (89). Notably, the gut microbiome composition is also under the influence of multiple intrinsic and extrinsic factors, such as host genetics (90), prolonged physiological stress (91), alcohol (92), dietary habits (93), and other lifestyle factors. Additionally, an integrated analysis of gut microbial community profiling and the host metabolomic signatures can help to provide a comprehensive understanding of microbiome-host interaction, thus providing a basis for studying the pathogenesis of depression (94). Notably, the traditional concepts will probably need to be revised by considering the reciprocal interactions between the gut microbiome and xenobiotics, termed the “microbiome-xenobiotic interactions” (95). For example, a recent study has shown that administration of psychotropics (such as fluoxetine, lithium, valproate, and aripiprazole) significantly alters the gut microbiome composition (96). Despite the increasing recognition of these hotspots, as these research directions develop, further innovations and breakthroughs may be hindered. Therefore, there is a need to pay more attention to frontier Research Topics identified by the burst keyword analysis in the years ahead.
Of note, many strong burst keywords associated with emerging areas of gut microbiota-depression-related research have appeared in recent years, including anxiety-like behavior, intestinal microbiota, and mice. Moreover, some keywords, such as the abovementioned hotspot “microbiome,” had bursts that are currently ongoing, among which the top keyword was “Parkinson's disease.”
Parkinson's disease is the second most common neurodegenerative disorder after Alzheimer's disease, with a median incidence in developed countries of 14 per 100,000 people in the total population, and 160 per 100,000 people at least 65 years of age (97). Apart from classical motor symptoms such as tremors, bradykinesia, and rigidity, some non-motor symptoms, including depression, anxiety, apathy, and psychosis, also affect quality of life in people with Parkinson's disease (98). Depression occurs in approximately 35% of people with Parkinson's disease, and the alterations in neurotransmitter systems, as well as neurotrophic factors, neuropeptides, immunomodulatory mediators, and stress hormones, may be associated with the depression observed in these individuals (99). Previous evidence supports the antidepressant properties of minocycline (a broad-spectrum tetracycline antibiotic), owing to its suppression of microglial activation, and neuro-protective or anti-inflammatory actions (100, 101). A population-based cohort study in older people (HEllenic Longitudinal Investigation of Aging and Diet) has found that adherence to the Mediterranean diet is associated with a lower probability of non-motor markers of prodromal Parkinson's disease, mainly depression and constipation (102). This finding may be due to decreased matrix metalloproteinase 9 activity (103), thereby maintaining the intestinal microbiota diversity and decreasing the severity of depression (104, 105). All the above findings suggest that manipulation of the microbiota may be beneficial in alleviating mood disorders in Parkinson's disease (104). Thus, modifying the microbiota to modulate depression in Parkinson's disease may be a future research direction. Moreover, depression, as a prodromal biomarker, can be used to identify the risk of developing Parkinson's disease, and assessment of the gut microbiota may also be considered an early biomarker of this disease in the future (104).
Other frontier topics in this field should also be noted, such as “microbiota-gut-brain axis,” “dysbiosis,” “CRP,” and “immune system,” which have currently ongoing bursts. Depression is a form of mood disorders that probably results from immunological deregulation (89). The immune system (including both the innate and adaptive immune system) is an important part of the microbiota-gut-brain axis that mediates the communication between the gut microbiota and CNS (106). The communication between the brain and the immune system occurs through different pathways, including neural and humoral pathways (107). The gut microbiota can activate epithelial cell gene expression via molecules such as Toll-like receptor (TLR), an innate immune system protein (108), thus inducing the production and release of pro-inflammatory cytokines (107). These cytokines then cross the blood brain barrier (BBB), possibly via a volume diffusion mechanism (109). Increased gut dysbiosis can also increase mucosal barrier permeability, thereby allowing bacteria and their pro-inflammatory products into the circulatory system and contributing to a chronic pro-inflammatory state (110). In individuals with MDD compared with healthy controls, higher elevated peripheral levels of CRP, an immune-inflammatory marker, have been confirmed by meta-analysis (111). CRP has also been demonstrated to have a relationship with depressive symptoms in obese people (112, 113). The probable pathway linking inflammation to these symptoms is an obesity-depression-inflammation cycle, involving systemic low-grade inflammation, partly owing to the changes in the gut microbiota (113). Thus, future clinical studies should focus on the feasibility of using anti-inflammatory strategies for patients with obesity and MDD, who might possibly benefit from reducing systemic inflammatory biomarkers such as CRP.
Our study has several limitations. First, publications were retrieved from only the WoSCC database, which may have led to bias and incompleteness in the included studies. In addition, our retrieval strategy may not have identified all the relevant references, owing to the limited terms, types of literature, and language; thus, our findings may not be comprehensive. Second, we cannot ensure that each publication retrieved was completely relevant to the topic meeting the search criteria. Third, some authors have the same name, and some keywords have different expressions, for example, “gut-brain axis” and “brain-gut axis”; thus, bias may have still existed despite our normalization procedures. Future studies should consider more databases, such as Scopus, more accurate and comprehensive search terms, and the latest software versions, in which bugs are addressed. However, we believe that our findings reflect the overall state of, and general trends in, this field, because the number of publications in our analysis was sufficiently large. We also believe that these limitations may be noted in future similar studies and addressed wherever possible.
Conclusions
This study comprised a bibliometric and visual analysis of gut microbiota research in the field of depression over the past two decades. The number of publications has been rapidly growing since 2010. Overall, close international scientific cooperation was observed in this field, but more cooperation among researchers may be necessary. There were multiple journals for researchers to choose from because of the diversified research directions. The most recent research focus was on “cytokines,” thus reflecting the important research foundation in this field. “Fecal microbiota” and “microbiome” have become the top two research hotspots since 2013. However, “Parkinson's disease,” “microbiota-gut-brain axis,” “microbiome,” “dysbiosis,” “bipolar disorder,” “impact,” “CRP,” and “immune system” were identified as new frontiers of research, whose bursts are currently ongoing. Therefore, our timely review and analysis of the hotspots and research trends may promote the development of this field.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
DS and YW conceived and designed the study. XZ wrote the original draft preparation. HL assisted in literature searching. JH conducted analysis based on WoS. XN, LL, and MZ conducted the CiteSpace analysis. SH, HC, and TX provided the figures and tablets. CQ, ZW, SD, and YT reviewed and edited the manuscript for content and style. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Natural Science Foundation of Guangdong Province (grant number 2018A0303130074), Guangzhou Municipal Psychiatric Disease Clinical Transformation Laboratory, Science and Technology Program of Guangzhou (grant number 201805010009), Science and Technology Plan Project of Guangdong Province (grant number 2019B030316001), Guangzhou municipal key discipline in medicine (2017-2019), and Guangzhou Municipal Science and Technology Project for Medicine and Healthcare (grant numbers 20201A011047 and 20202A011016).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript. We also would like to express our appreciation to Professor C. M. Chen, who developed the CiteSpace software, which is free to use.
References
1. Cui R. Editorial: a systematic review of depression. Curr Neuropharmacol. (2015) 13:480. doi: 10.2174/1570159x1304150831123535
3. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. (2018) 392:1789–858. doi: 10.1016/S0140-6736(18)32279-7
5. Valles-Colomer M, Falony G, Darzi Y, Tigchelaar EF, Wang J, Tito RY, et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat Microbiol. (2019) 4:623–32. doi: 10.1038/s41564-018-0337-x
6. Yang Z, Li J, Gui X, Shi X, Bao Z, Han H, et al. Updated review of research on the gut microbiota and their relation to depression in animals and human beings. Mol Psychiatry. (2020) 25:2759–72. doi: 10.1038/s41380-020-0729-1
7. Hao WZ, Li XJ, Zhang PW, Chen JX. A review of antibiotics, depression, and the gut microbiome. Psychiatry Res. (2020) 284:112691. doi: 10.1016/j.psychres.2019.112691
8. Caspani G, Kennedy S, Foster JA, Swann J. Gut microbial metabolites in depression: understanding the biochemical mechanisms. Microb Cell. (2019) 6:454–81. doi: 10.15698/mic2019.10.693
9. Deans E. Microbiome and mental health in the modern environment. J Physiol Anthropol. (2016) 36:1. doi: 10.1186/s40101-016-0101-y
10. Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature. (2012) 486:207–14. doi: 10.1038/nature11234
11. Joshua CJ. Metabolomics: a microbial physiology and metabolism perspective. Methods Mol Biol. (2019) 1859:71–94. doi: 10.1007/978-1-4939-8757-3_3
12. Desbonnet L, Garrett L, Clarke G, Kiely B, Cryan JF, Dinan TG. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience. (2010) 170:1179–88. doi: 10.1016/j.neuroscience.2010.08.005
13. Liu P, Peng G, Zhang N, Wang B, Luo B. Crosstalk between the gut microbiota and the brain: an update on neuroimaging findings. Front Neurol. (2019) 10:883. doi: 10.3389/fneur.2019.00883
14. Dovrolis N, Kolios G, Spyrou GM, Maroulakou I. Computational profiling of the gut-brain axis: microflora dysbiosis insights to neurological disorders. Brief Bioinform. (2019) 20:825–41. doi: 10.1093/bib/bbx154
15. Wei CL, Wang S, Yen JT, Cheng YF, Liao CL, Hsu CC, et al. Antidepressant-like activities of live and heat-killed Lactobacillus paracasei PS23 in chronic corticosterone-treated mice and possible mechanisms. Brain Res. (2019) 1711:202–13. doi: 10.1016/j.brainres.2019.01.025
16. Liang S, Wang T, Hu X, Luo J, Li W, Wu X, et al. Administration of Lactobacillus helveticus NS8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience. (2015) 310:561–77. doi: 10.1016/j.neuroscience.2015.09.033
17. Abildgaard A, Kern T, Pedersen O, Hansen T, Wegener G, Lund S. The antidepressant-like effect of probiotics and their faecal abundance may be modulated by the cohabiting gut microbiota in rats. Eur Neuropsychopharmacol. (2019) 29:98–110. doi: 10.1016/j.euroneuro.2018.10.011
18. Huang R, Wang K, Hu J. Effect of probiotics on depression: a systematic review and meta-analysis of randomized controlled trials. Nutrients. (2016) 8:483. doi: 10.3390/nu8080483
19. Park C, Brietzke E, Rosenblat JD, Musial N, Zuckerman H, Ragguett RM, et al. Probiotics for the treatment of depressive symptoms: an anti-inflammatory mechanism? Brain Behav Immun. (2018) 73:115–24. doi: 10.1016/j.bbi.2018.07.006
20. Miyaoka T, Kanayama M, Wake R, Hashioka S, Hayashida M, Nagahama M, et al. Clostridium butyricum MIYAIRI 588 as adjunctive therapy for treatment-resistant major depressive disorder: a prospective open-label trial. Clin Neuropharmacol. (2018) 41:151–5. doi: 10.1097/WNF.0000000000000299
21. Bambling M, Edwards SC, Hall S, Vitetta L. A combination of probiotics and magnesium orotate attenuate depression in a small SSRI resistant cohort: an intestinal anti-inflammatory response is suggested. Inflammopharmacology. (2017) 25:271–4. doi: 10.1007/s10787-017-0311-x
22. Chen C, Dubin R, Kim MC. Emerging trends and new developments in regenerative medicine: a scientometric update (2000-2014). Expert Opin Biol Ther. (2014) 14:1295–317. doi: 10.1517/14712598.2014.920813
23. Thompson DF, Walker CK. A descriptive and historical review of bibliometrics with applications to medical sciences. Pharmacotherapy. (2015) 35:551–9. doi: 10.1002/phar.1586
24. Huang X, Fan X, Ying J, Chen S. Emerging trends and research foci in gastrointestinal microbiome. J Transl Med. (2019) 17:67. doi: 10.1186/s12967-019-1810-x
25. Yao H, Wan JY, Wang CZ, Li L, Wang J, Li Y, et al. Bibliometric analysis of research on the role of intestinal microbiota in obesity. PeerJ. (2018) 6:e5091. doi: 10.7717/peerj.5091
26. Zyoud SH, Smale S, Waring WS, Sweileh WM, Al-Jabi SW. Global research trends in microbiome-gut-brain axis during 2009-2018: a bibliometric and visualized study. BMC Gastroenterol. (2019) 19:158. doi: 10.1186/s12876-019-1076-z
27. Chen C. Science mapping: a systematic review of the literature. J Data Inf Sci. (2017) 2:1–40. doi: 10.1515/jdis-2017-0006
28. Chen C. Searching for intellectual turning points: progressive knowledge domain visualization. Proc Natl Acad Sci USA. (2004) 101 (Suppl. 1):5303–10. doi: 10.1073/pnas.0307513100
29. Zhang XM, Zhang X, Luo X, Guo HT, Zhang LQ, Guo JW. Knowledge mapping visualization analysis of the military health and medicine papers published in the web of science over the past 10 years. Mil Med Res. (2017) 4:23. doi: 10.1186/s40779-017-0131-8
30. Liang YD, Li Y, Zhao J, Wang XY, Zhu HZ, Chen XH. Study of acupuncture for low back pain in recent 20 years: a bibliometric analysis via CiteSpace. J Pain Res. (2017) 10:951-64. doi: 10.2147/JPR.S132808
31. Wáng YX, Arora R, Choi Y, Chung HW, Egorov VI, Frahm J, et al. Implications of Web of Science journal impact factor for scientific output evaluation in 16 institutions and investigators' opinion. Quant Imaging Med Surg. (2014) 4:453–61. doi: 10.3978/j.issn.2223-4292.2014.11.16
32. Xu X, Mishra GD, Jones M. Mapping the global research landscape and knowledge gaps on multimorbidity: a bibliometric study. J Glob Health. (2017) 7:10414. doi: 10.7189/jogh.07.010414
33. Hui J, Wang L, Liu R, Yang C, Zhang H, He S, et al. A bibliometric analysis of international publication trends in premature ejaculation research (2008-2018). Int J Impot Res. (2020). doi: 10.1038/s41443-019-0224-x. [Epub ahead of print].
34. Hirsch JE. An index to quantify an individual's scientific research output. Proc Natl Acad Sci USA. (2005) 102:16569–72. doi: 10.1073/pnas.0507655102
35. Garfield E. The history and meaning of the journal impact factor. JAMA. (2006) 295:90–3. doi: 10.1001/jama.295.1.90
36. Zheng K, Wang X. Publications on the association between cognitive function and pain from 2000 to 2018: a bibliometric analysis using CiteSpace. Med Sci Monit. (2019) 25:8940–51. doi: 10.12659/MSM.917742
37. Zheng KY, Dai GY, Lan Y, Wang XQ. Trends of repetitive transcranial magnetic stimulation from 2009 to 2018: a bibliometric analysis. Front Neurosci. (2020) 14:106. doi: 10.3389/fnins.2020.00106
38. Wang SQ, Gao YQ, Zhang C, Xie YJ, Wang JX, Xu FY. A bibliometric analysis using CiteSpace of publications from 1999 to 2018 on patient rehabilitation after total knee arthroplasty. Med Sci Monit. (2020) 26:e920795. doi: 10.12659/MSM.920795
39. Wang R, Weng LM, Peng MS, Wang XQ. Exercise for low back pain: a bibliometric analysis of global research from 1980 to 2018. J Rehabil Med. (2020) 52:jrm00052. doi: 10.2340/16501977-2674
40. Guo S, Wang L, Xie Y, Luo X, Zhang S, Xiong L, et al. Bibliometric and visualized analysis of stem cells therapy for spinal cord injury based on Web of Science and CiteSpace in the last 20 years. World Neurosurg. (2019) 132:e246–58. doi: 10.1016/j.wneu.2019.08.191
41. Li D, Dai FM, Xu JJ, Jiang MD. Characterizing hotspots and frontier landscapes of diabetes-specific distress from 2000 to 2018: a bibliometric study. Biomed Res Int. (2020) 2020:8691451. doi: 10.1155/2020/8691451
42. Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci USA. (2011) 108:16050–5. doi: 10.1073/pnas.1102999108
43. Bercik P, Park AJ, Sinclair D, Khoshdel A, Lu J, Huang X, et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol Motil. (2011) 23:1132–9. doi: 10.1111/j.1365-2982.2011.01796.x
44. Desbonnet L, Garrett L, Clarke G, Bienenstock J, Dinan TG. The probiotic Bifidobacteria infantis: an assessment of potential antidepressant properties in the rat. J Psychiatr Res. (2008) 43:164–74. doi: 10.1016/j.jpsychires.2008.03.009
45. Maes M, Mihaylova I, Leunis JC. Increased serum IgA and IgM against LPS of enterobacteria in chronic fatigue syndrome (CFS): indication for the involvement of gram-negative enterobacteria in the etiology of CFS and for the presence of an increased gut-intestinal permeability. J Affect Disord. (2007) 99:237–40. doi: 10.1016/j.jad.2006.08.021
46. Maes M, Kubera M, Leunis JC. The gut-brain barrier in major depression: intestinal mucosal dysfunction with an increased translocation of LPS from gram negative enterobacteria (leaky gut) plays a role in the inflammatory pathophysiology of depression. Neuro Endocrinol Lett. (2008) 29:117–24.
47. Finegold SM, Dowd SE, Gontcharova V, Liu C, Henley KE, Wolcott RD, et al. Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe. (2010) 16:444–53. doi: 10.1016/j.anaerobe.2010.06.008
48. Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. (2012) 13:701–12. doi: 10.1038/nrn3346
49. Messaoudi M, Lalonde R, Violle N, Javelot H, Desor D, Nejdi A, et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br J Nutr. (2011) 105:755–64. doi: 10.1017/S0007114510004319
50. Diaz Heijtz R, Wang S, Anuar F, Qian Y, Björkholm B, Samuelsson A, et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci USA. (2011) 108:3047–52. doi: 10.1073/pnas.1010529108
51. Jiang H, Ling Z, Zhang Y, Mao H, Ma Z, Yin Y, et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun. (2015) 48:186–94. doi: 10.1016/j.bbi.2015.03.016
52. Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. (2011) 141:599–609, 609.e1–3. doi: 10.1053/j.gastro.2011.04.052
53. Foster JA, McVey Neufeld KA. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. (2013) 36:305–12. doi: 10.1016/j.tins.2013.01.005
54. Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F, et al. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry. (2013) 18:666–73. doi: 10.1038/mp.2012.77
55. Neufeld KM, Kang N, Bienenstock J, Foster JA. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol Motil. (2011) 23:255–64 e119. doi: 10.1111/j.1365-2982.2010.01620.x
56. Naseribafrouei A, Hestad K, Avershina E, Sekelja M, Linløkken A, Wilson R, et al. Correlation between the human fecal microbiota and depression. Neurogastroenterol Motil. (2014) 26:1155–62. doi: 10.1111/nmo.12378
57. Synnestvedt MB, Chen C, Holmes JH. CiteSpace II: visualization and knowledge discovery in bibliographic databases. AMIA Annu Symp Proc. (2005) 2005:724–8.
58. Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, et al. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. (2005) 433:73–7. doi: 10.1038/nature03180
59. Logan AC, Katzman M. Major depressive disorder: probiotics may be an adjuvant therapy. Med Hypotheses. (2005) 64:533–8. doi: 10.1016/j.mehy.2004.08.019
60. Steenbergen L, Sellaro R, van Hemert S, Bosch JA, Colzato LS. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain Behav Immun. (2015) 48:258–64. doi: 10.1016/j.bbi.2015.04.003
61. Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. (2010) 67:446–57. doi: 10.1016/j.biopsych.2009.09.033
62. Carabotti M, Scirocco A, Maselli MA, Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol. (2015) 28:203–9.
63. Sampson TR, Mazmanian SK. Control of brain development, function, and behavior by the microbiome. Cell Host Microbe. (2015) 17:565–76. doi: 10.1016/j.chom.2015.04.011
64. Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. (2010) 464:59–65. doi: 10.1038/nature08821
65. Kelly JR, Kennedy PJ, Cryan JF, Dinan TG, Clarke G, Hyland NP. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci. (2015) 9:392. doi: 10.3389/fncel.2015.00392
66. Maes M, Kubera M, Leunis JC, Berk M. Increased IgA and IgM responses against gut commensals in chronic depression: further evidence for increased bacterial translocation or leaky gut. J Affect Disord. (2012) 141:55–62. doi: 10.1016/j.jad.2012.02.023
67. Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Tóth M, et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. (2014) 6:263ra158. doi: 10.1126/scitranslmed.3009759
68. Stilling RM, Dinan TG, Cryan JF. Microbial genes, brain & behaviour - epigenetic regulation of the gut-brain axis. Genes Brain Behav. (2014) 13:69–86. doi: 10.1111/gbb.12109
69. Wang HX, Wang YP. Gut Microbiota-brain Axis. Chin Med J. (2016) 129:2373–80. doi: 10.4103/0366-6999.190667
70. Rahman M, Fukui T. Biomedical research productivity: factors across the countries. Int J Technol Assess Health Care. (2003) 19:249–52. doi: 10.1017/s0266462303000229
71. Dinan TG, Stanton C, Cryan JF. Psychobiotics: a novel class of psychotropic. Biol Psychiatry. (2013) 74:720–6. doi: 10.1016/j.biopsych.2013.05.001
72. Dinan TG, Cryan JF. The microbiome-gut-brain axis in health and disease. Gastroenterol Clin North Am. (2017) 46:77–89. doi: 10.1016/j.gtc.2016.09.007
73. Zhao G, Liu X. Neuroimmune advance in depressive disorder. Adv Exp Med Biol. (2019) 1180:85–98. doi: 10.1007/978-981-32-9271-0_4
74. Pérez-Sánchez G, Becerril-Villanueva E, Arreola R, Martínez-Levy G, Hernández-Gutiérrez ME, Velasco-Velásquez MA, et al. Inflammatory profiles in depressed adolescents treated with fluoxetine: an 8-week follow-up open study. Mediators Inflamm. (2018) 2018:4074051. doi: 10.1155/2018/4074051
75. Liu JJ, Wei YB, Strawbridge R, Bao Y, Chang S, Shi L, et al. Peripheral cytokine levels and response to antidepressant treatment in depression: a systematic review and meta-analysis. Mol Psychiatry. (2020) 25:339–50. doi: 10.1038/s41380-019-0474-5
76. Köhler CA, Freitas TH, Stubbs B, Maes M, Solmi M, Veronese N, et al. Peripheral alterations in cytokine and chemokine levels after antidepressant drug treatment for major depressive disorder: systematic review and meta-analysis. Mol Neurobiol. (2018) 55:4195–206. doi: 10.1007/s12035-017-0632-1
77. D'Mello C, Swain MG. Immune-to-brain communication pathways in inflammation-associated sickness and depression. Curr Top Behav Neurosci. (2017) 31:73–94. doi: 10.1007/7854_2016_37
78. Ma D, Forsythe P, Bienenstock J. Live Lactobacillus rhamnosus [corrected] is essential for the inhibitory effect on tumor necrosis factor alpha-induced interleukin-8 expression. Infect Immun. (2004) 72:5308-14. doi: 10.1128/IAI.72.9.5308-5314.2004
79. Gao Y, Shi S, Ma W, Chen J, Cai Y, Ge L, et al. Bibliometric analysis of global research on PD-1 and PD-L1 in the field of cancer. Int Immunopharmacol. (2019) 72:374-84. doi: 10.1016/j.intimp.2019.03.045
81. Li H, Xu H, Li Y, Jiang Y, Hu Y, Liu T, et al. Alterations of gut microbiota contribute to the progression of unruptured intracranial aneurysms. Nat Commun. (2020) 11:3218. doi: 10.1038/s41467-020-16990-3
82. Zhang F, Luo W, Shi Y, Fan Z, Ji G. Should we standardize the 1,700-year-old fecal microbiota transplantation? Am J Gastroenterol. (2012) 107:1755; author reply 1755–6. doi: 10.1038/ajg.2012.251
83. Wang JW, Kuo CH, Kuo FC, Wang YK, Hsu WH, Yu FJ, et al. Fecal microbiota transplantation: review and update. J Formos Med Assoc. (2019) 118: S23–31. doi: 10.1016/j.jfma.2018.08.011
84. Kurokawa S, Kishimoto T, Mizuno S, Masaoka T, Naganuma M, Liang KC, et al. The effect of fecal microbiota transplantation on psychiatric symptoms among patients with irritable bowel syndrome, functional diarrhea and functional constipation: an open-label observational study. J Affect Disord. (2018) 235:506–12. doi: 10.1016/j.jad.2018.04.038
85. Mazzawi T, Lied GA, Sangnes DA, El-Salhy M, Hov JR, Gilja OH, et al. The kinetics of gut microbial community composition in patients with irritable bowel syndrome following fecal microbiota transplantation. PLoS ONE. (2018) 13:e0194904. doi: 10.1371/journal.pone.0194904
86. Li N, Wang Q, Wang Y, Sun A, Lin Y, Jin Y, et al. Fecal microbiota transplantation from chronic unpredictable mild stress mice donors affects anxiety-like and depression-like behavior in recipient mice via the gut microbiota-inflammation-brain axis. Stress. (2019) 22:592–602. doi: 10.1080/10253890.2019.1617267
87. Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. (2009) 9:313–23. doi: 10.1038/nri2515
88. Hall AB, Tolonen AC, Xavier RJ. Human genetic variation and the gut microbiome in disease. Nat Rev Genet. (2017) 18:690–9. doi: 10.1038/nrg.2017.63
89. Wang Y, Kasper LH. The role of microbiome in central nervous system disorders. Brain Behav Immun. (2014) 38:1–12. doi: 10.1016/j.bbi.2013.12.015
90. Kurilshikov A, Wijmenga C, Fu J, Zhernakova A. Host genetics and gut microbiome: challenges and perspectives. Trends Immunol. (2017) 38:633–47. doi: 10.1016/j.it.2017.06.003
91. Karl JP, Margolis LM, Madslien EH, Murphy NE, Castellani JW, Gundersen Y, et al. Changes in intestinal microbiota composition and metabolism coincide with increased intestinal permeability in young adults under prolonged physiological stress. Am J Physiol Gastrointest Liver Physiol. (2017) 312: G559–71. doi: 10.1152/ajpgi.00066.2017
92. Dubinkina VB, Tyakht AV, Odintsova VY, Yarygin KS, Kovarsky BA, Pavlenko AV, et al. Links of gut microbiota composition with alcohol dependence syndrome and alcoholic liver disease. Microbiome. (2017) 5:141. doi: 10.1186/s40168-017-0359-2
93. Rinninella E, Cintoni M, Raoul P, Lopetuso LR, Scaldaferri F, Pulcini G, et al. Food components and dietary habits: keys for a healthy gut microbiota composition. Nutrients. (2019) 11:2393. doi: 10.3390/nu11102393
94. Song J, Ma W, Gu X, Zhao L, Jiang J, Xu Y, et al. Metabolomic signatures and microbial community profiling of depressive rat model induced by adrenocorticotrophic hormone. J Transl Med. (2019) 17:224. doi: 10.1186/s12967-019-1970-8
95. Leprun PMB, Clarke G. The gut microbiome and pharmacology: a prescription for therapeutic targeting of the gut-brain axis. Curr Opin Pharmacol. (2019) 49:17–23. doi: 10.1016/j.coph.2019.04.007
96. Cussotto S, Strain CR, Fouhy F, Strain RG, Peterson VL, Clarke G, et al. Differential effects of psychotropic drugs on microbiome composition and gastrointestinal function. Psychopharmacology. (2019) 236:1671–85. doi: 10.1007/s00213-018-5006-5
97. Ascherio A, Schwarzschild MA. The epidemiology of Parkinson's disease: risk factors and prevention. Lancet Neurol. (2016) 15:1257–72. doi: 10.1016/S1474-4422(16)30230-7
98. Pfeiffer RF. Non-motor symptoms in Parkinson's disease. Parkinsonism Relat Disord. (2016) 22(Suppl. 1):S119–22. doi: 10.1016/j.parkreldis.2015.09.004
99. Aarsland D, Påhlhagen S, Ballard CG, Ehrt U, Svenningsson P. Depression in Parkinson disease–epidemiology, mechanisms and management. Nat Rev Neurol. (2011) 8:35–47. doi: 10.1038/nrneurol.2011.189
100. Miyaoka T, Wake R, Furuya M, Liaury K, Ieda M, Kawakami K, et al. Minocycline as adjunctive therapy for patients with unipolar psychotic depression: an open-label study. Prog Neuropsychopharmacol Biol Psychiatry. (2012) 37:222–6. doi: 10.1016/j.pnpbp.2012.02.002
101. Zemke D, Majid A. The potential of minocycline for neuroprotection in human neurologic disease. Clin Neuropharmacol. (2004) 27:293–8. doi: 10.1097/01.wnf.0000150867.98887.3e
102. Maraki MI, Yannakoulia M, Stamelou M, Stefanis L, Xiromerisiou G, Kosmidis MH, et al. Mediterranean diet adherence is related to reduced probability of prodromal Parkinson's disease. Mov Disord. (2019) 34:48–57. doi: 10.1002/mds.27489
103. Camargo A, Delgado-Lista J, Garcia-Rios A, Cruz-Teno C, Yubero-Serrano EM, Perez-Martinez P, et al. Expression of proinflammatory, proatherogenic genes is reduced by the Mediterranean diet in elderly people. Br J Nutr. (2012) 108:500–8. doi: 10.1017/S0007114511005812
104. Bobińska K, Szemraj J, Czarny P, Gałecki P. Expression and activity of metalloproteinases in depression. Med Sci Monit. (2016) 22:1334–41. doi: 10.12659/msm.895978
105. Felice VD, Quigley EM, Sullivan AM, O'Keeffe GW, O'Mahony SM. Microbiota-gut-brain signalling in Parkinson's disease: implications for non-motor symptoms. Parkinsonism Relat Disord. (2016) 27:1–8. doi: 10.1016/j.parkreldis.2016.03.012
106. Colpitts SL, Kasper LH. Influence of the gut microbiome on autoimmunity in the central nervous system. J Immunol. (2017) 198:596–604. doi: 10.4049/jimmunol.1601438
107. Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. (2008) 9:46–56. doi: 10.1038/nrn2297
108. Elson CO, Alexander KL. Host-microbiota interactions in the intestine. Dig Dis. (2015) 33:131–6. doi: 10.1159/000369534
109. Vitkovic L, Konsman JP, Bockaert J, Dantzer R, Homburger V, Jacque C. Cytokine signals propagate through the brain. Mol Psychiatry. (2000) 5:604–15. doi: 10.1038/sj.mp.4000813
110. Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. (2018) 15:505–22. doi: 10.1038/s41569-018-0064-2
111. Slyepchenko A, Maes M, Jacka FN, Köhler CA, Barichello T, McIntyre RS, et al. Gut microbiota, bacterial translocation, and interactions with diet: pathophysiological links between major depressive disorder and non-communicable medical comorbidities. Psychother Psychosom. (2017) 86:31–46. doi: 10.1159/000448957
112. Castanon N, Lasselin J, Capuron L. Neuropsychiatric comorbidity in obesity: role of inflammatory processes. Front Endocrinol. (2014) 5:74. doi: 10.3389/fendo.2014.00074
Keywords: gut microbiota, depression, bibliometric analysis, citespace, developing trends, microbiota-gut-brain axis, cytokines, microbiome
Citation: Zhu X, Hu J, Deng S, Tan Y, Qiu C, Zhang M, Ni X, Lu H, Wang Z, Li L, Chen H, Huang S, Xiao T, Shang D and Wen Y (2021) Bibliometric and Visual Analysis of Research on the Links Between the Gut Microbiota and Depression From 1999 to 2019. Front. Psychiatry 11:587670. doi: 10.3389/fpsyt.2020.587670
Received: 27 July 2020; Accepted: 04 December 2020;
Published: 08 January 2021.
Edited by:
Shaohua Hu, Zhejiang University, ChinaReviewed by:
Miao Qu, Xuanwu Hospital, Capital Medical University, ChinaPeng Zheng, Chongqing Medical University, China
Copyright © 2021 Zhu, Hu, Deng, Tan, Qiu, Zhang, Ni, Lu, Wang, Li, Chen, Huang, Xiao, Shang and Wen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dewei Shang, shang_dewei@163.com; Yuguan Wen, wenyuguandede@163.com
 Xiuqing Zhu
Xiuqing Zhu Jinqing Hu1,2
Jinqing Hu1,2 Lu Li
Lu Li Dewei Shang
Dewei Shang Yuguan Wen
Yuguan Wen