- The Affiliated Brain Hospital of Guangzhou Medical University, School of Mental Health, Guangzhou Medical University, Guangzhou, China
Objective: We previously found that chronic ketamine usages were associated with various psychotic and cognitive symptoms mimicking schizophrenia. The blockade of the NMDA receptor and subsequent nitric oxide synthase 1 (NOS1) dysfunction were found to be closely correlated with schizophrenia including NOS1 gene polymorphisms. We examined the allelic variants of the gene coding neuronal nitric oxide synthase 1 (NOS1) in chronic ketamine users in the Chinese population and analyzed the association between NOS1 gene polymorphism and psychopathological symptoms in chronic ketamine users. The association between the NOS1 polymorphism and ketamine use characteristics was also examined.
Methods: One hundred ninety seven male chronic ketamine users and 82 controls were recruited. Four common SNPs of the NOS1 gene, rs6490121, rs41279104, rs3782206, and rs3782219, were examined by real-time PCR with the TaqMan® assay system. Psychopathological symptoms were assessed using the Positive and Negative Syndrome Scale (PANSS), Beck Depression Inventory (BDI), and the Beck Anxiety Inventory (BAI).
Results: The genotype distribution of rs6490121 and rs41279104 in chronic ketamine users was significantly different from that in the control (p = 0.0001 and p = 0.002). The G allele frequency of rs6490121 in ketamine users was higher than that in the control (p = 2.23 * 10−6, OR = 3.07, 95% CI = 1.93–4.90). The T allele frequency of rs41279104 in chronic ketamine users was higher than that in the control (p = 0.01, OR = 1.76, 95% CI = 1.14–2.72). The BAI score was significantly different among the three genotypic groups of rs6490121 (F = 6.21, p = 0.002) in ketamine users; subjects of genotype AG and GG had a lower score than subjects of genotype AA. The score of the negative symptom subscale of PANSS was significantly different among the three genotypic groups of rs41279104 (F = 5.39, p = 0.005); in ketamine users, subjects of genotype CT and TT had a higher score than subjects of genotype CC. There was no difference in drug use characteristics in different genotypes of the four NOS1 gene polymorphisms tested in ketamine users (p > 0.05).
Introduction
Ketamine, a derivative of phencyclidine (PCP), is an N-methyl-D-aspartic acid receptor (NMDAR) antagonist; NMDAR was widely associated with many critical physiological processes like neurodevelopment, neuroplasticity, neurocognition, sensory, and mood function (1, 2). Ketamine has been widely abused as a psychoactive substance since the late 1990s (3), and it is the third most commonly abused substance after morphine and methamphetamine in China (4).
Acute administration of ketamine in healthy volunteers induces schizophrenic-like symptoms including positive and negative symptoms and cognitive impairment (5–8). Our study showed that chronic ketamine users exhibited greater similarity than the acute ketamine injection in the normal control group to the symptom domains of schizophrenia (9). Ketamine administration was considered as a pharmacological model in studying schizophrenia, and chronic ketamine users could potentially be a natural human model for schizophrenic research especially in exploring the various physiological consequences after chronic NMDAR blockade in the human brain (10–13).
Alteration of the nitric oxide (NO) level via NMDAR blockade and subsequent nitric oxide synthase 1 (NOS1) dysfunction was found to be closely correlated with schizophrenia (14). NMDAR, NOS1, and postsynaptic density protein 95 (PSD95) form a tertiary protein complex (15). Phencyclidine (PCP) was another NMDAR blocker and was widely used in schizophrenia animal models. Blockade of NOS1 was found to enhance PCP-induced schizophrenia-like symptoms and to increase c-fos gene expression in the cortical region (16). Moreover, PCP cannot induce psychotic symptoms in NOS1 knockout mice (17). On the contrary, the NO donor can block these changes and effectively eliminate the psychotic symptoms induced by NMDAR blockers and improve cognitive function (16, 18, 19). Clinical studies found that levels of NO metabolites were lower in plasma and cerebrospinal fluid of patients with schizophrenia than that in normal individuals and increased after 8 weeks of risperidone treatment and the NO metabolite concentration was negatively correlated with the severity of negative symptoms in patients (14, 20). The genetic polymorphisms of NOS1 of the NMDAR-NO-cGMP pathway were also found to be closely related to schizophrenia (21, 22). NOS1 is located in 12q24.2 and contains a total of 29 exons with a total length of ~149.4 kb (https://www.genecards.org/). NOS1 is a relevant functional and positional candidate gene for schizophrenia (23).
Clinically, it has been observed that only some ketamine users developed ketamine-related psychosis while others might not develop psychotic symptoms even if they used higher doses and longer durations (24). Moreover, some psychotic symptoms developed after ketamine use could last for weeks to months or even longer after stopping ketamine usage (25). Since the ketamine model is one of the most common schizophrenic models, there is no study to test whether the genetic variation of NOS1 is involved in the occurrence of psychosis in some chronic ketamine users. Based on previous evidences, we hypothesized that SNPs of NOS1 may play a role in ketamine-induced psychosis.
The present study tested four SNPs of NOS1 (rs6490121, rs41279104, rs3782206, rs3782219) commonly found in schizophrenic patients to determine if there were differences in genotypes and allele distributions of NOS1 gene polymorphism between ketamine users and healthy controls. We also analyzed the association between NOS1 gene polymorphism and the severity of psychopathological symptoms and the relationship between NOS1 gene polymorphism and ketamine use characteristics in ketamine users.
Materials and Methods
Subject Recruitment
A total of 197 chronic ketamine users and 82 controls were recruited. All ketamine users and controls were male. Chronic ketamine users were recruited voluntarily at Guangzhou Baiyun Voluntary Medical Detoxification Hospital. Healthy controls were recruited through advertisement. All chronic ketamine users underwent a 2-h semistructured interview which was conducted by clinicians with 3 or more years of clinical experience. Inclusion criteria required (1) chronic ketamine users be admitted for detoxification or treatment of ketamine-related symptoms; (2) no other substance dependence other than ketamine and tobacco according to DSM-IV-TR; and (3) subjects use ketamine as a drug of choice for longer than 6 months. Ketamine subjects with a history of psychiatric and neurological diseases prior to ketamine use disorder were excluded. The control subjects were free from a history of drug abuse or psychiatric disorder. Written informed consent for this study was provided by all subjects, and the study was approved by the Institutional Ethics Committee.
Assessment of Psychotic Symptoms
Clinical symptoms among ketamine users were evaluated with the Positive and Negative Syndrome Scale (PANSS) (26), administered by two trained psychiatrists with 3 or more years of clinical experience. The intraclass correlation coefficient (ICC) between raters on the PANSS was >0.8. In addition, ketamine users were asked to complete the Beck Depression Inventory (BDI) (27) and the Beck Anxiety Inventory (BAI) (28), which assessed their depressive and anxiety symptoms during the 2 weeks before they were hospitalized.
Sample Collection and Genotyping
Briefly, peripheral blood samples were collected from subjects in 6-ml EDTA vacuum tubes. Genomic DNA was extracted from peripheral blood using the standard phenol–chloroform extraction method (29). The final concentrations of all DNA samples were 20 ng/μl with good purity (OD260/OD280 = 1.8–2.0) and stored at −80°C until genotyping. The TaqMan real-time quantitative PCR (q-PCR) method was used to test DNA samples using the TaqMan® Genotyping Assay (cat#25800731, cat#27481263, cat#86363451, cat#29122242, Applied Biosystems, Shanghai, China) on an ABI ViiA™ 7 sequence detection system instrument (Applied Biosystems). PCR was performed in 25-μL reactions containing Genotyping Master Mix (Applied Biosystems, Shanghai, China), TaqMan® Genotyping Assay, and DNA template. Each 96-well-plate was quality-controlled with two no-template controls. The PCR program for the amplification was as follows: 95°C for 10 min and then 40 cycles of denaturation (95°C for 15 s) and annealing (60°C for 1 min). We further randomly selected 10% samples for each of the selected SNPs for re-genotyping, and the results were 100% concordant.
Statistical Analysis
Demographics and clinical data of ketamine users were statistically analyzed with μ ± SD. The Hardy–Weinberg equilibrium of all pairs of SNPs was calculated using SHEsis software (http://analysis.bio-x.cn) (30). Chi-square tests were used to compare the allelic and genotypic frequencies between ketamine users and healthy controls. Odds ratios (ORs) were used to measure the association of genotypes of the four SNPs between ketamine users and control subjects. Logistic regression analysis was used to obtain ORs and their 95% confidence intervals (95% CI). Age and years of education were evaluated as confounding factors. Bonferroni corrections were applied to adjust for multiple comparisons of different genotypes of SNPs in ketamine users and control subjects. To adjust for multiple comparisons, the significance level was set to p < 0.017 (α/3 = 0.05/3, two-tailed) for all multiple comparisons. Between-group differences grouped by genotypes were evaluated using one-way analysis of variance (ANOVA), followed by Fisher's least significant difference (LSD) multiple-range test for between-group comparison. Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) version 22.0. The level of significance was set at α = 0.05 (except multiple comparisons).
Results
Participant Characteristics
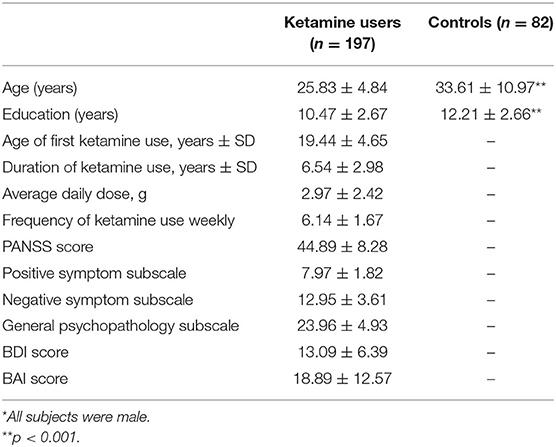
Socio-demographic characteristics, drug use conditions, and PANSS, BDI, and BAI scores are presented in Table 1. Age and years of education were significantly different between the two groups (p < 0.001).
Genotype and Allele Frequencies of SNPs in Ketamine Users and Control Subjects
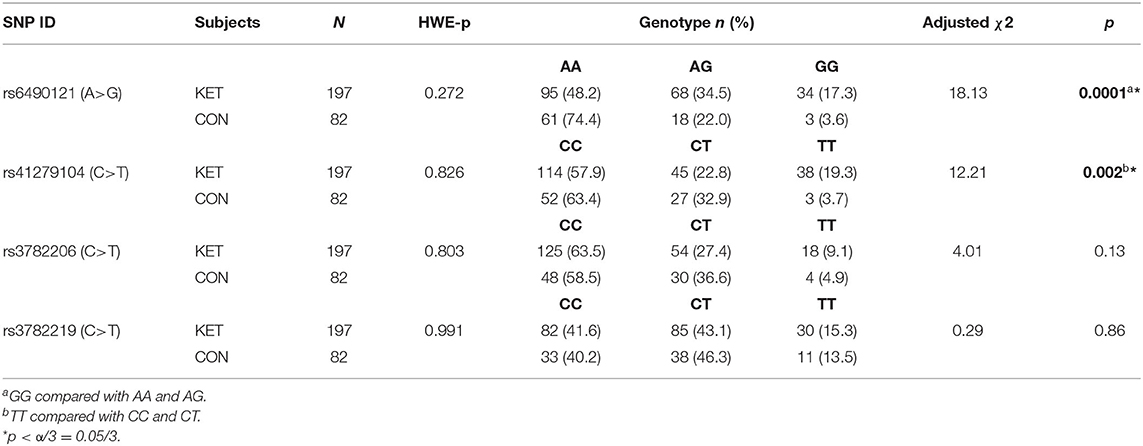
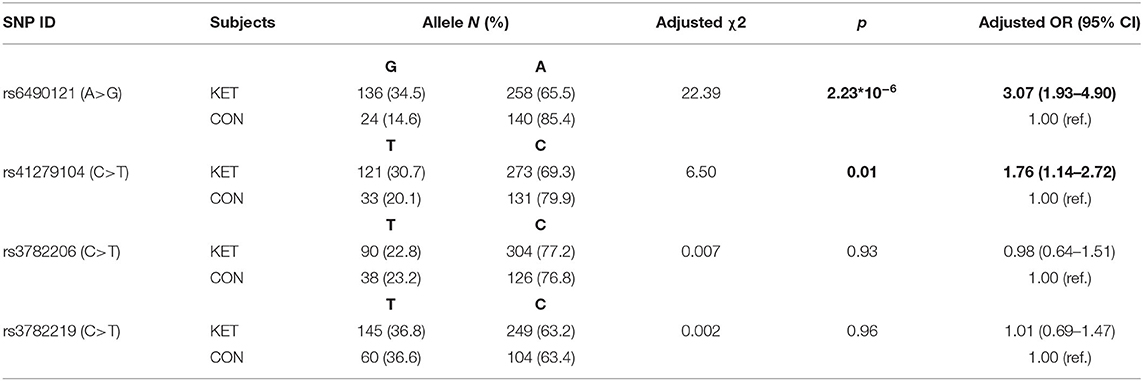
Genotypes and allele frequencies of the four SNPs in gene NOS1 are shown in Tables 2, 3. Distributions of each SNP genotype were consistent with Hardy–Weinberg equilibrium (HWE) in healthy controls (p > 0.05). The genotype distribution of rs6490121 and rs41279104 had a significant difference between ketamine users and control subjects (p = 0.0001 and p = 0.002), respectively. Genotypes GG and TT in ketamine users were higher than those in normal control. The G allele frequency of rs6490121 in ketamine users was higher than that in the controls (p = 2.23 * 10−6, OR = 3.07, 95% CI = 1.93–4.90). The T allele frequency of rs41279104 in ketamine users was higher than that in the controls (p = 0.01, OR = 1.76, 95% CI = 1.14–2.72). For the other two SNPs (rs3782206 and rs3782219), neither the genotype distributions nor allele frequencies were significantly different between ketamine users and control subjects (p > 0.05).
Relationship Between Psychotic Symptom Measures and Genotypes of Four NOS1 SNPs
Associations between psychotic symptom measures and genotypes of four SNPs in ketamine users are shown in Table 4. The BAI score was significantly different among the three genotypic groups of rs6490121 (F = 6.21, p = 0.002) in ketamine users; subjects of genotype AG and GG had a lower score than subjects of genotype AA (respectively, p = 0.043, p = 0.037). The score of the negative symptom subscale was significantly different among the three genotypic groups of rs41279104 (F = 5.39, p = 0.005); subjects of genotype CT and TT had a higher score than subjects of genotype CC (respectively, p = 0.048, p = 0.045). There were no significant genotype effects on rs3782206 and rs3782219 (all p > 0.05).
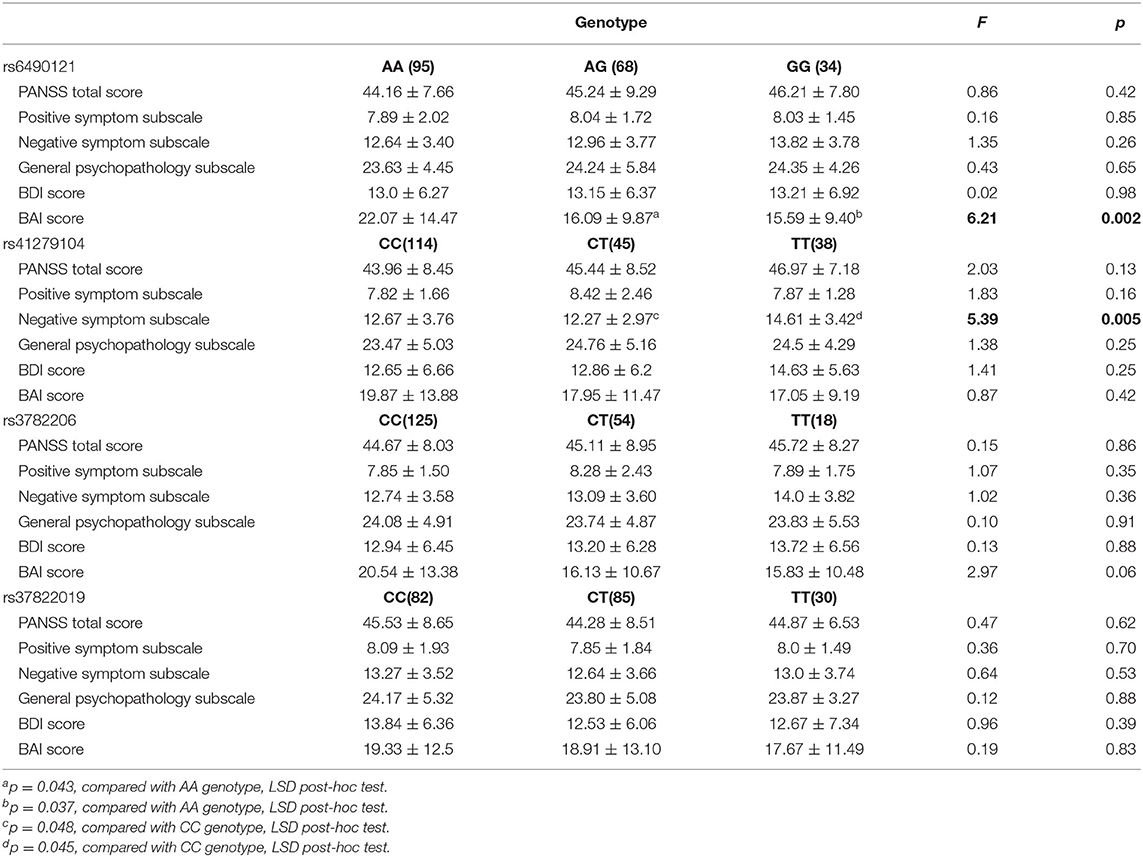
Table 4. Association between psychotic symptom measures and genotypes of four NOS1 SNPs in ketamine users.
Relationship Between Drug Use Characteristics and Genotypes of Four NOS1 SNPs
The association between drug use characteristics and genotypes of the four NOS1 SNPs in ketamine users was analyzed (Table 5). No significant differences in drug use characteristics among genotype groups of rs6490121, rs41279104, rs3782206, and rs3782219 were observed (p > 0.05).
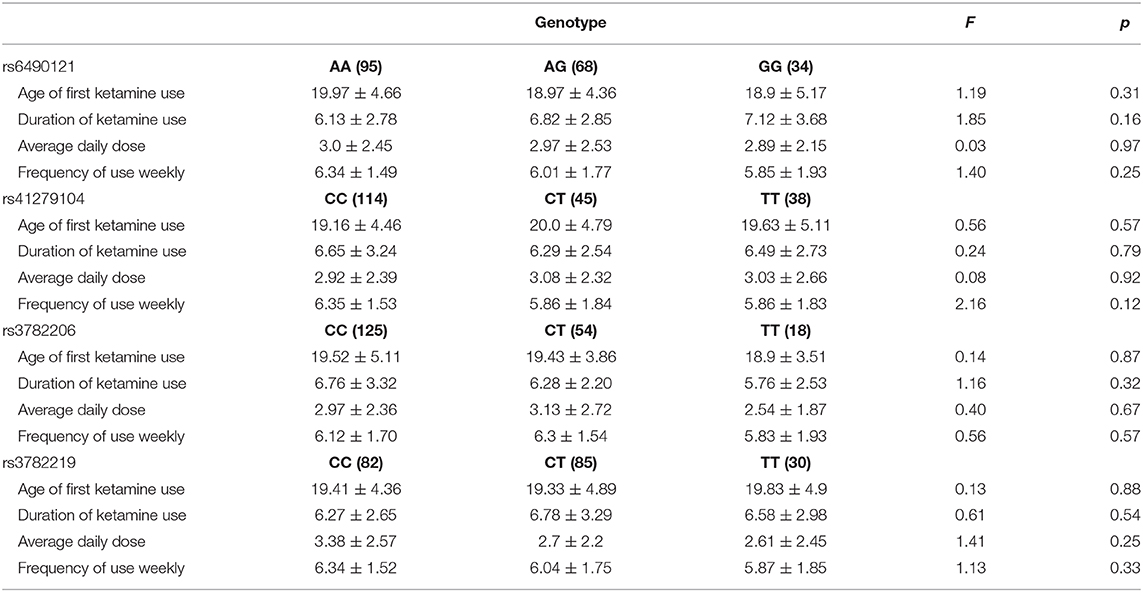
Table 5. Association between drug use characteristics and genotypes of four NOS1 SNPs in ketamine users.
Discussion
To our knowledge, this is the first study to investigate the gene polymorphism of NOS1 in chronic ketamine users. We found more genotypic variations of rs6490121 and rs41279104 in ketamine users than those in normal control. Among ketamine users, the BAI score was significantly lower with the genotypic variation of rs6490121 and the score of the PANSS negative symptom subscale was significantly higher in the genotypic TT group of rs41279104. There is no significant correlation between tested gene polymorphism with ketamine use characteristics.
In previous schizophrenic studies, it has been found that variations in the locus of the functional promoter region of SNP rs41279104 leads to a decrease in NOS1 gene expression and the variation of rs41279104 results in decreased prefrontal lobe expression of NOS1, which consequently reduced brain executive function, increased error in continuous operation testing, and increased P300 latency (31). In the high-density EEG study, the rs41279104 G allele carrier showed a decrease in P1 response compared to non-carriers (32). Another SNP rs6490121 of NOS1 was also found to be associated with working memory impairment in schizophrenic patients, and the working memory impairment of risk allele carriers was declining (33).
Our results were in line with these findings in schizophrenia studies. The rs41279104 and rs6490121 variations were identified in chronic ketamine users similar to that found in schizophrenia. Moreover, such variations were found closely correlated with lower anxiety symptoms and higher negative symptoms not associated with positive symptoms. Although the cognitive assessment was not included in our current analysis, the PANSS negative symptoms also include part of cognition test items and the score of negative symptoms was found to be highly related with the cognitive functions (34, 35). Our previous study analyzed the short-term and long-term symptom features in 187 chronic ketamine users and found that the short-term symptoms were closer to the positive symptoms, while long-term symptoms were more similar to the schizophrenic negative symptoms including more social withdrawal symptoms like personality change, being unsociable and eccentric, reduced social activities, reduced number of friends, and cognitive symptoms including memory loss, slow reaction time, and decreased work efficiency (36). It suggested that such variations were potentially more related with cognitive impairment and negative symptoms instead of positive psychotic symptoms as shown in schizophrenia. The presence of critical genetic variations in the NOS1 gene in ketamine users could increase the susceptibility of schizophrenic negative symptoms and potentially with more cognitive impairment while being challenged by chronic ketamine usage which could further reduce the functioning of NOS1 by upstream NMDAR blockade. Further studies are needed to investigate the role of NOS1 and NO in ketamine-related psychosis.
The present study did not detect significant differences of the other two NOS1 SNPs (rs3782206 and rs3782219). However, a study reported that there is a link at rs3782206 and cognitive functions, which underpinned at the inferior frontal gyrus (23). Another study showed that a link between NOS1 gene polymorphism at rs3782206 and cognitive functions and their neural underpinnings at the IFG (22). The absence of a positive finding in our study suggests that any effect of these gene polymorphisms on ketamine users might be weak in the Chinese population.
Although we did not found relationship between NOS1 polymorphisms and ketamine use characteristics, it has been found that mutation of the NOS1 gene substantially increased vulnerability to alcohol-induced cell loss in a brain region where the gene is expressed (37). Targeted disruption of the NOS1 gene worsens the behavioral impact of developmental alcohol exposure and allows alcohol-induced learning problems to emerge (38). Findings of roles for NO and NOS1 in alcohol-related mental disease indicate that further studies are needed to explore the role of NO and NOS1 in ketamine users.
Besides NOS1, other gene variations were also found to be related with the function of ketamine. A study found that brain-derived neurotrophic factor (BDNF) Val66Met polymorphism was a susceptible gene for ketamine abuse. BDNF Val66Met polymorphism leads to the decrease of BDNF concentration in brain tissue, which resulted in the change of addiction behavior (39). The resting-state functional connectivity (rs-fc) reduction was more pronounced in norepinephrine transporter (NET) rs28386840 [AA] homozygous subjects than in [T] carriers after ketamine administration, which may contribute to understanding the antidepressant effect of ketamine (40). It implicated that more genetic variations besides the NMDAR-NOS1-NO pathway were correlated with ketamine's effects and more exploratory studies were needed to indentify the critical factors contributing to ketamine addiction and to ketamine-induced psychotic symptoms.
A few limitations of this study have to be mentioned. Firstly, only four SNPs of NOS1 were examined. Further genetic and mechanism studies with more variants of the NOS1 gene and other genes would be necessary. Secondly, all ketamine users were male and in-patients, and the sample size was relatively small for such genetic studies. In a further study, subjects from the community with larger samples size should be considered. Thirdly, the control group was not matched in age and education, since ketamine users were mainly male with a relatively low education level and it was hard to recruit a control group with a perfect match. We set age and education as the covariables during group comparisons to minimize its possible confounding effects. Lastly, some ketamine users had used other psychoactive drugs other than ketamine. It may be a confounding factor for the study. However, all the subjects recruited used ketamine as a drug of choice for longer than 6 months and they had no other substance dependence other than ketamine and tobacco. So confounding is likely to be limited.
Conclusion
Our study showed that chronic ketamine users carried more genotypic variations of rs6490121 and rs41279104 in the NOS1 gene than the control group and such variations were associated with more negative psychotic symptoms and fewer anxiety symptoms. It implicated that patients with genetic variations in the NOS1 gene potentially were more vulnerable to chronic ketamine usages, and it warranted further studies to use chronic ketamine users as models to explore the role of NOS1 dysfunction in ketamine-induced psychological symptoms.
Data Availability Statement
According to national legislation/guidelines, specifically the Administrative Regulations of the People's Republic of China on Human Genetic Resources (http://www.gov.cn/zhengce/content/2019-06/10/content_5398829.htm, http://english.www.gov.cn/policies/latest_releases/2019/06/10/content_281476708945462.htm), no additional raw data is available at this time. Data of this project can be accessed after an approval application to the China National Genebank (CNGB, https://db.cngb.org/cnsa/). Please refer to https://db.cngb.org/, or email: CNGBdb@cngb.org for detailed application guidance. The accession code CNP0001402 should be included in the application.
Ethics Statement
The studies involving human participants were reviewed and approved by Institutional Ethics Committee of affiliated Brain Hospital of Guangzhou Medical University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
HH and NF designed the study, wrote the protocol, and helped on the manuscript revision. YD and CZ recruited the subjects and assessed the psychotic symptoms. MZ collected the blood samples. JC undertook the genotyping and statistical analysis and wrote the manuscript. All authors contributed to and have approved the final manuscript.
Funding
This work was supported by grants to HH from Science and Technology Program of Guangzhou, China (grant number 201804010056) and by grants to NF from the National Natural Science Foundation of China (grant number 81571304), Natural Science Foundation of Guangdong Province (grant number 2017A030313671).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. psychotomimetic, perceptual, cognitive, neuroendocrine responses. Arch Gen Psychiatry. (1994) 51:199–214. doi: 10.1001/archpsyc.1994.03950030035004
2. Jansen KLR. Ketamine: dreams and realities. In: Jon Hanna, editor. Multidisciplinary Association for Psychedelic Studies (MAPS). Sarasota, FL: Multidisciplinary Association for Psychedelic Studies (MAPS) (2004). p. 5–7.
3. Mion G. History of anaesthesia: the ketamine story - past,present and future. Eur J Anaesthesiol. (2017) 34:571–75. doi: 10.1097/EJA.0000000000000638
4. National Drug Control Commission 2015 China Drug Report [R]. Beijing: National Narcotics Control Commission of Ministry of Public Security (2018).
5. Adler CM, Goldberg TE, Malhotra AK, Pickar D, Breier A. Effects of ketamine on thought disorder,working memory,and semantic memory in healthy volunteers. Biol Psychiatry. (1998) 43:811–6. doi: 10.1016/S0006-3223(97)00556-8
6. Malhotra AK, Pinals DA, Adler CM, Elman I, Clifton A, Pickar D, et al. Ketamine-induced exacerbation of psychotic symptoms and cognitive impairment in neuroleptic-free schizophrenics. Neuropsychopharmacology. (1997) 17:141–50. doi: 10.1016/S0893-133X(97)00036-5
7. Anticevic A, Cole MW, Repovs G, Savic A, Driesen NR, Yang G, et al. Connectivity, pharmacology, and computation: toward a mechanistic understanding of neural system dysfunction in schizophrenia. Front Psychiatry. (2013) 4:169. doi: 10.3389/fpsyt.2013.00169
8. Dawson N, McDonald M, Higham DJ, Morris BJ, Pratt JA. Subanesthetic ketamine treatment promotes abnormal interactions between neural subsystems and alters the properties of functional brain networks. Neuropsychopharmacology. (2014) 39:1786–98. doi: 10.1038/npp.2014.26
9. Xu K, Krystal JH, Ning Y, Chen DC, He H, Wang D, et al. Preliminary analysis of positive and negative syndrome scale in ketamine-associated psychosis in comparison with schizophrenia. J Psychiatr Res. (2015) 61:64–72. doi: 10.1016/j.jpsychires.2014.12.012
10. Abi-Saab WM, D'Souza DC, Moghaddam B, Krystal JH. (1998) The NMDA antagonist model for schizophrenia: promise and pitfalls. Pharmacopsychiatry. 31(Suppl 2):104–9. doi: 10.1055/s-2007-979354
11. Krystal JH, D'Souza DC, Mathalon D, Perry E, Belger A, Hoffman R. NMDA receptor antagonist effects,cortical glutamatergic function,and schizophrenia: toward a paradigm shift in medication development. Psychopharmacology. (2003) 169:215–33. doi: 10.1007/s00213-003-1582-z
12. Lahti AC, Weiler MA, Tamara Michaelidis BA, Parwani A, Tamminga CA. Effects of ketamine in normal and schizophrenic volunteers. Neuropsychopharmacology. (2001) 25:455–67. doi: 10.1016/S0893-133X(01)00243-3
13. Zugno AI, de Miranda IM, Budni J, Volpato AM, Luca RD, Deroza PF, et al. Effect of maternal deprivation on acetylcholinesterase activity and behavioral changes on the ketamine-induced animal model of schizophrenia. Neuroscience. (2013) 248:252–60. doi: 10.1016/j.neuroscience.2013.05.059
14. Ramirez J, Garnica R, Boll MC, Montes S, Rios C. Low concentration of nitrite and nitrate in the cerebrospinal fluid from schizophrenic patients: a pilot study. Schizophr Res. (2004) 68:357–61. doi: 10.1016/S0920-9964(03)00070-7
15. Jaffrey SR, Snowman AM, Eliasson MJ, Cohen NA, Snyder SH. CAPON: a protein associated with neuronal nitric oxide synthase that regulates its interactions with PSD95. Neuron. (1998) 20:115–24. doi: 10.1016/S0896-6273(00)80439-0
16. Bujas-Bobanovic M, Bird DC, Robertson HA, Dursun SM. Blockade of phencyclidine-induced effects by a nitric oxide donor. Br J Pharmacol. (2000) 130:1005–12. doi: 10.1038/sj.bjp.0703406
17. Bird DC, Bujas-Bobanovic M, Robertson HA, Dursun SM. Lack of phencyclidine-induced effects in mice with reduced neuronal nitric oxide synthase. Psychopharmacology. (2001) 155:299–309. doi: 10.1007/s002130100705
18. Huang L, Liu Y, Zhang P, Kang R, Liu Y, Li X, et al. In vitro dose-dependent inhibition of the intracellular spontaneous calcium oscillations in developing hippocampal neurons by ketamine. PLoS ONE. (2013) 8:e0059804. doi: 10.1371/journal.pone.0059804
19. Pitsikas N, Zisopoulou S, Sakellaridis N. Nitric oxide donor molsidomine attenuates psychotomimetic effects of the NMDA receptor antagonist MK-801. J Neurosci Res. (2006) 84:299–305. doi: 10.1002/jnr.20889
20. Lee BH, Kim YK. Reduced plasma nitric oxide metabolites before and after antipsychotic treatment in patients with schizophrenia compared to controls. Schizophr Res. (2008) 104:36–43. doi: 10.1016/j.schres.2008.06.005
21. Dordević VV, Jevtovićstoimenov T, Lazarević D. Association analysis for neuronal nitric oxide synthase gene polymorphism with plasma nitrite/nitrate concentration in schizophrenia. J Med Biochem. (2014) 33:364–70. doi: 10.2478/jomb-2014-0023
22. Zhang Z, Chen X, Yu P, Zhang Q, Sun X, Gu H, et al. Evidence for the contribution of NOS1 gene polymorphism (rs3782206) to prefrontal function in schizophrenia patients and healthy controls. Neuropsychopharmacology. (2015) 40:1383–94. doi: 10.1038/npp.2014.323
23. Shinkai T, Ohmori O, Hori H, Nakamura J. Allelic association of the neuronal nitric oxide synthase(NOS1) gene with schizophrenia. Mol Psychiatry. (2002) 7:560–3. doi: 10.1038/sj.mp.4001041
24. Ding Y, He N, Shoptaw S, Gao M, Detels R. Severity of club drug dependence and perceived need for treatment among a sample of adult club drug users in Shanghai, China. Soc Psychiatry Psychiatr Epidemiol. (2014) 49:395–404. doi: 10.1007/s00127-013-0713-z
25. Zhou C, Liu Y-p, Lan X-c, Duan L, Xu S-c, Ding Y, et al. Explore the influence factors of the addiction severity on the ketamine dependents. Chin J Drug Abuse Prev Treat. (2018) 24:138–40,153. doi: 10.15900/j.cnki.zylf1995.2018.03.003
26. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale(PANSS) for schizophrenia. Schizophr Bull. (1987) 13:261–76. doi: 10.1093/schbul/13.2.261
27. Beck AT, Beamesderfer A. Assessment of depression: the depression inventory. Mod Probl Pharmacopsychiatry. (1974) 7:151–69. doi: 10.1159/000395074
28. Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. (1988) 56:893–7. doi: 10.1037/0022-006X.56.6.893
29. Di Pietro F, Ortenzi F, Tilio M, Concetti F, Napolioni V. Genomic DNA extraction from whole blood stored from 15- to 30-years at−20 °C by rapid phenol-chloroform protocol: a useful tool for genetic epidemiology studies. Mol Cell Probes. (2011) 25:44–8. doi: 10.1016/j.mcp.2010.10.003
30. Shi YY, He L. SHEsis,a powerful software platform for analyses of linkage disequilibrium,haplotype construction,and genetic association at polymorphism loci. Cell Res. (2005) 15:97–8. doi: 10.1038/sj.cr.7290272
31. Reif A, Herterich S, Strobel A, Ehlis AC, Saur D, Jacob CP, et al. A neuronal nitric oxide synthase (NOS-I) haplotype associated with schizophrenia modifies prefrontal cortex function. Mol Psychiatry. (2006) 11:286–300. doi: 10.1038/sj.mp.4001779
32. O'Donoghue T, Morris DW, Fahey C, Da Costa A, Foxe JJ, Hoerold D, et al. A NOS1 variant implicated in cognitive performance influences evoked neural responses during a high density EEG study of early visual perception. Hum Brain Mapp. (2012) 33:1202–11. doi: 10.1002/hbm.21281
33. Callicott JH, Nicodemus KK, Higier RG. Allelic variation in NOS1AP(CAPON) is associated with altered prefrontal cortex function. In: Presented at the Annual Meeting of The Society for Neuroscience San Diego, CA (2007).
34. Leanza L, Egloff L, Studerus E, Andreou C, Heitz U, Ittig S, et al. The relationship between negative symptoms and cognitive functioning in patients at clinical high risk for psychosis. Psychiatry Res. (2018) 268:21–7. doi: 10.1016/j.psychres.2018.06.047
35. Ahmed AO, Richardson J, Buckner A, Romanoff S, Feder M, Oragunye N, et al. Do cognitive deficits predict negative emotionality and aggression in schizophrenia? Psychiatry Res. (2018) 259:350–7. doi: 10.1016/j.psychres.2017.11.003
36. Fan N, Xu K, Ning Y, Wang D, Ke X, Ding Y, et al. Development of a checklist of short-term and long-term psychological symptoms associated with ketamine use. Shanghai Arch Psychiatry. (2015) 27:186–94. doi: 10.11919/j.issn.1002-0829.214158
37. Todd D, Bonthius DJ Jr, Sabalo LM, Roghair J, Karacay B, et al. Regional patterns of alcohol-induced neuronal loss depend on genetics: implications for fetal alcohol spectrum disorder. Alcohol Clin Exp Res. (2018) 42:1627–39. doi: 10.1111/acer.13824
38. Karacay B, Bonthius NE, Plume J, Bonthius DJ. Genetic absence of nNOS worsens fetal alcohol effects in mice.I: behavioral deficits. Alcohol Clin Exp Res. (2015) 39:212–20. doi: 10.1111/acer.12616
39. Corominas M, Roncero C, Ribases M, Castells X, Casas M. Brain-derived neurotrophic factor and its intracellular signaling pathways in cocaine addiction. Neuropsychobiology. (2007) 55:2–13. doi: 10.1159/000103570
Keywords: nitric oxide synthase 1, single nucleotide polymorphisms, ketamine use, association analysis, psychopathological symptoms
Citation: Chen J, Zhang M, Zhou C, Ding Y, Fan N and He H (2020) Association Analysis of Neuronal Nitric Oxide Synthase 1 Gene Polymorphism With Psychopathological Symptoms in Chronic Ketamine Users. Front. Psychiatry 11:580771. doi: 10.3389/fpsyt.2020.580771
Received: 21 July 2020; Accepted: 23 November 2020;
Published: 23 December 2020.
Edited by:
Jennifer Evans, National Institutes of Health (NIH), United StatesReviewed by:
Thomas Liebe, Otto von Guericke University Magdeburg, GermanyWK Tang, The Chinese University of Hong Kong, China
Copyright © 2020 Chen, Zhang, Zhou, Ding, Fan and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongbo He, vulut2@126.com; Ni Fan, fanni2005@126.com
 Jiansong Chen
Jiansong Chen Minling Zhang
Minling Zhang Ni Fan
Ni Fan