- 1Faculty of Social Welfare and Health Sciences, Department of Occupational Therapy, University of Haifa, Haifa, Israel
- 2E. J. Safra Brain Research Center for the Study of Learning Disabilities, Haifa, Israel
- 3Laboratory for Functional Brain Imaging and Learning Research, Sagol Department of Neurobiology, University of Haifa, Haifa, Israel
In young adults without attention-deficit/hyperactivity disorder (ADHD) training on a novel movement sequence results not only in large within-session (online) gains in task performance but also in additional (delayed, off-line) gains in the performance, expressed after an interval of sleep. In contrast, young people with ADHD, given an identical practice, were shown to improve online but expressed much smaller delayed gains overnight. As delayed gains in performance are taken to reflect procedural (“how to”) memory consolidation processes, this may explain skill learning deficits in persons with ADHD. However, motor training is usually provided in morning sessions, and, given that persons with ADHD are often evening types, chronobiological constraints may constitute a hidden factor. Here, we tested the hypothesis that evening training, compared to morning training, would result in larger overnight consolidation gains following practice on a novel motor task in young women with ADHD. Participants with (N = 25) and without (N = 24) ADHD were given training on a finger opposition sequence tapping task, either in the morning or at evening. Performance was assessed before and immediately after training, overnight, and at 2 weeks post-training. Individuals with ADHD reported a general preference for evening hours. Evening training was equally effective in participants with and without ADHD, both groups showing robust consolidation gains in task performance overnight. However, the ability to express delayed gains overnight was significantly reduced in participants with ADHD if trained in the morning. Typical peers were as effective in expressing overnight consolidation phase gains irrespective of the time-of-day wherein the training session was afforded. Nevertheless, even after morning training, participants with ADHD fully retained the gains acquired within the first 24 h over an interval of about 2 weeks. Our results suggest that procedural memory consolidation processes are extant and effective in ADHD, but require that specific biobehavioral conditions be met. The affordance of training in the evening hours can relax some of the constraints on these processes in ADHD. The current results are in line with the notion that the control of what is to be retained in procedural memory is atypical or more stringent in ADHD.
Introduction
Motor Learning in Healthy Adults
Procedural memory processes (1–4) subserve the mastering and retention of motor skills and have been characterized in typical adults by a distinct time course (5–8). Large improvements in speed of motor performance occur early on during training on a novel motor task, with no costs of accuracy (i.e., within-session gains, “fast” learning phase). Performance within-session reaches an asymptote as practice accumulates [e.g., Ref. (5)]. However, within several hours after the end of the training session, additional robust gains in performance can be expressed (as delayed, “off-line” gains), reflected in improved speed and accuracy of task performance, as well as in a reduction in performance variance (9–11). The off-line gains are considered to constitute a behavioral marker for the successful accomplishment of procedural memory consolidation processes, which are initiated by the training experience but requiring hours to evolve; processes whereby the neuronal substrates engaged during practice are changed according to the accrued experience (3, 12–15). The level of motor performance attained at the consolidation phase is typically retained over a period of weeks and even months in young adults (3, 6, 7, 16). In the FOS task, the task used in the current study, these gains were correlated with experience dependent motor cortex activity pattern changes (17).
Training-related factors such as the number of task repetitions and instruction are critical in determining the effectiveness and course of skill learning (10, 18–20). However, after the termination of the training experience, conflicting experience, or the availability of a post-training sleep interval can also critically affect the course of learning a new skill, specifically, by interacting with the consolidation processes (8, 14, 21). Sleep has been identified as a state wherein the consolidation of newly acquired information in memory is promoted, depending on the specific conditions of training, instruction, and proximity to sleep episode (22, 23). Sleep supports both quantitative and qualitative changes of memory representations (5, 8, 15, 24–27), and age- or health-related changes in sleep architecture were shown to disrupt normal consolidation processes (23). In typical young adults training in the FOS task, memory consolidation processes (as reflected in the expression of delayed, off-line, gains in performance, but also in a decreased susceptibility to interference by subsequent conflicting experiences) were shown to be accelerated and successfully completed not only by a night’s sleep but also by relatively shorter daytime naps (8). Thus, in the FOS task [with training in the standard protocol (5, 7, 8)], the evidence clearly indicates that rather than time per se, time in sleep is the critical factor gating the successful completion of consolidation processes, in both young adults and the elderly (28) [but not in preadolescent children (29)].
Attention-Deficit/Hyperactivity Disorder (ADHD) Condition and Motor Learning Deficits in ADHD
Attention-deficit disorder (ADD)/ADHD is a neurological condition characterized by inattention and/or hyperactivity–impulsivity that interferes with everyday functioning. While attention problems are recognized as a core deficit (30), deficits in executive functioning (e.g., fluency, planning, inhibition, and set-shifting) (31) and motor functioning (32–34) are recognized as key characteristics.
Some theoretical accounts implicate deficits in procedural memory (skill acquisition) as a central deficit in ADHD (35). Findings from structural and functional neuroimaging studies of brains of individuals with ADHD have revealed differences compared to typical peers in multiple brain systems including circuits implicated in repeated task performance and skill learning (36–39). It is not clear, however, whether these differences relate to a less effective acquisition, or deficient consolidation or retention processes in ADHD. A simple view of ADHD as a procedural memory deficit may need to be qualified. The evidence from studies of implicit (SRT task) and explicit learning of movement sequences in adults and children with ADHD is equivocal; some studies report deficits vis-à-vis typical controls (32, 33, 40) while in other task conditions, participants with ADHD were as effective learners as their typical peers (40, 41). Deficits in the sustained engagement of attention resources and reduced inhibition of incorrect responses (42) were proposed as important factors leading to ineffective learning in ADHD.
A number of studies wherein the FOS task was used as the to-be-learned task suggest that young adults with ADHD may exhibit an atypical procedural memory consolidation process rather than a critical deficit (32, 43, 44). For example, in a study that compared the time course of learning following the FOS training in young females with and without ADHD, the ADHD group exhibited normal within-session gains in performance speed, but the delayed gains measured at the 24 h post-training retest, were reduced (32). Given the pivotal role of procedural memory for everyday functioning (e.g., the skill of driving) (3, 4), it would be reasonable to expect that short-term motor deficiencies occurring in the individuals with ADHD would have to be at least partially compensated on the long run. Indeed, the study by Adi-Japha and her colleagues (32) demonstrated that the relative initial deficits in the expression of overnight delayed gains turned out to be temporary; the performance gap vis-à-vis typical peers training in an identical protocol diminished within a few days. The real potential of the individuals with ADHD to acquire novel skills may, therefore, be uncovered by manipulating factors that gate brain plasticity; for example, more stringent demands may be set on factors such as attention and arousal levels or post-training sleep quality and timing in ADHD. Indeed, some of the relative learning deficits, in persons with ADHD, could be corrected when training was shortened (32, 33, 44, 45) perhaps decreasing the burden of long repetitive practice on mechanisms of sustained attention (46). The upregulation of arousal levels, which are typically low in ADHD (47), for example, by the means of whole body vibration (48) and white noise (49) stimulation, has been shown to enhance both attention and motor performance.
In typically developing adults, sleep after practicing a new motor skill supports memory consolidation processes, contributing to the generation of stable, enhanced and long lasting procedural memory representations (5, 7, 50–52). A comorbidity of ADHD and sleep disorders is recognized; over 65% of individuals with ADHD may present with one or more sleep disorders (53–56). A recent review suggests that sleep problems and ADHD interact in a complex bidirectional manner with sleep disturbances exacerbating or exacerbated by ADHD symptoms (53).
Chronotype, Sleep, and Learning in ADHD
Chronotype is an individual characteristic reflecting the time of day at which a person is “at his best” (57); “eveningness” (delayed sleep period, alertness reaches the maximum values at 11 p.m.) and “morningness” (advanced sleep period, alertness reaches the maximum at 8 a.m.) are the two extremes with most individuals in the general healthy population preferring the period between these extremes (58, 59).
Adult ADHD is associated with the evening chronotype (60–62). More than 40% of adults with ADHD display an evening preference; in age-matched healthy peers in general population, only 10.8% exhibit evening preference (63). Morning preference is switched, respectively, 40.2% in the typical population and 18.5% in ADHD. Greater eveningness correlates with the core symptoms of inattention and increased impulsivity; as eveningness is associated with shorter night sleep period, sleep debt may play a causal role in these symptoms (63). Additional evidence for a link between ADHD symptoms and circadian disruption comes from findings that seasonal affective disorder, a depression disorder directly linked to circadian disruption shows high comorbidity with ADHD (55, 64, 65). It was also suggested that the hyperactivity of people with ADHD may lead to sleep deprivation (66). The core symptoms of ADHD, such as inattention, impulsivity, and impatience, are typical outcomes of sleep deprivation in typical adults (67).
Consistent failure to meet basic sleep needs is currently viewed as a significant contributor to the cognitive and behavioral deficits in individuals with ADHD (53). As many as 70% of children and up to 83% of adults with ADHD have been reported as having sleep problems (68, 69) with sleep onset insomnia (SOI), the most common problem (54). A study that compared ADHD with and without SOI reported that 78% of adults with ADHD reported SOI, but when tested objectively by actigraphy, no difference in basic sleep parameters (duration and efficiency of sleep, as well as sleep onset latency) were found between those reporting or not reporting SOI (55). However, compared to typical controls, the participants with ADHD showed extended sleep onset latencies and lower sleep efficiency. Adults with ADHD also report reduced sleep quality, difficulty in getting to sleep, and difficulty in waking up (70). Individuals with ADHD were found to sleep on average an hour less than controls on nights prior to work days (but not prior to free days) and showed larger variability in bedtimes and sleep latencies (64). More than 60% of adults with ADHD reported increased sleepiness during day time (54, 55, 70). Delayed timing of melatonin secretion is systematically found in children and adults with ADHD (54, 55, 71). Rybak et al. suggested that a substantial circadian phase delay considerably impacts the core pathology of the ADHD (72).
Brain plasticity, the basis for skill and knowledge, is a slow and highly controlled (selective) process, wherein synaptic and cellular modifications occur at brain circuits in which the memory was initially encoded during salient experiences. Multiple lines of evidence suggest that these processes proceed “off-line,” during both wakefulness and sleep, and culminate in the consolidation of new information and it’s integration into previously existed knowledge (3, 5, 8, 15). Whether these off-line processes will be allowed to proceed to a successful completion is under strict control (“gating”) (73). Optimal arousal level during encoding is considered a prerequisite gating factor mediating the long-term memory formation (47). Memory systems (74) and cognitive processes such as attention and executive functions (75, 76), as well as reward processes (77–79), are sensitive to disruptions of sleep and circadian rhythms. Indeed, the circadian clock, the reward system, and memory processes directly or indirectly affect neurogenesis and neural growth and shaping processes (80). Light acts on all three systems through common basic signaling pathways (80) and all three are affected by the hypothalamic–pituitary–adrenal axis via cortisol (81). Moreover, the evidence that most of the genes that shape the biological clock are expressed in brain areas that are associated with learning, memory, and reward, such as the amygdala, the hippocampus, and the ventral tegmental areas, is in line with the notion that the endogenous ~24 h time-generator (suprachiasmatic nucleus) has a role in gating neuronal plasticity following daily experiences (81).
The Current Study
The majority of training protocols used in memory research afford training sessions during morning or early afternoon, a time of day that may be suboptimal for individuals with evening chronotype and/or with higher susceptibility to interference [like the persons with ADHD (55) or the elderly (28)]. Recently, it was proposed that post-training sleep and its timing relative to the training experience is a critical factor in the control (gating) of motor skill memory selectivity in young adolescents (82) and in the elderly (28). Similar constrains may be imposed on mnemonic processes in individuals with ADHD during the morning hours so as to limit the generation of long-term memory from experiences gained in less than optimal practice-learning conditions; i.e., when alertness and cognitive abilities are at the diurnal minimum (83). Thus, memory deficits may be, at least in part, a result of the timing of the training experience rather than a general deficit in motor skill consolidation. In the current study, we tested the hypothesis that practice in the evening hours compared to morning hours may provide better conditions for the engagement of, the presumably atypical, consolidation processes in young women with ADHD. Operationally, we expected that training in the evening hours will result in higher delayed, overnight, gains in performance than training in the morning in the ADHD groups. In contrast, we expected that in the control groups (typical adults), delayed gains in performance will evolve regardless of the timing of training session.
We chose to address in the current study only young women with ADHD because (i) the performance of skilled movements in ADHD were suggested to be gender dependent (84, 85), (ii) between individual variances in the symptomatology of ADHD is smaller in females compared to males (86) and thus a smaller number of participants can be used in an exploratory study, and (iii) to enable a direct comparison to the results of previous studies (32, 44) wherein consolidation processes were systematically explored in young women with ADHD, using the FOS task (the task used in the current study).
Materials and Methods
The study was approved by the Human Experimentation Ethics committee of the University of Haifa and the participants signed an informed consent form in accordance with the Declaration of Helsinki before beginning the experiments. Subjects were paid 150 shekels (approximately $37) for their participation.
Participants
Forty-nine right-handed (87) young (age between 20 and 35 years) females, University of Haifa students, enrolled in the study. Participants were recruited through advertisement boards at the University of Haifa and the University center for students with disabilities, for a “motor learning and memory study.” 24 participants met the criteria for a DSM-IV diagnosis of ADHD, and 25 typically developing adults matched by age and education, served as a control group. Inclusion criteria for the ADHD groups were as follows: (1) a formal psycho-didactic diagnosis of an attention-deficit disorder (either ADD or ADHD) from an authorized clinician, psychiatrist, or neurologist, approved by the University center for students with disabilities within 5 years of the current study; (2) a positive screening on the adult ADHD self-report scale (ASRS) (88, 89); and (3) no stimulant treatment for ADHD (methylphenidate or other stimulant drugs) during the recent period (> month). The participants of the ADHD group had on average 11 out of 18 items (10.9 ± 2.7, mean ± SD) positive responses on the ASRS. The control participants met less than 3 out of 6 criteria of the ASRS screening questionnaire (first 6 items). All control participants affirmed that they were not suggested (by family members or teachers) to have or were never diagnosed as having ADHD/ADD during their childhood or adulthood.
All participants underwent a semi-structured interview to exclude persons with diagnosed sleep, neurological or psychiatric disorders, motor-skeletal diseases, and use of chronic medications or drugs. Four of the participants with ADHD, but none of the participants in the control group, reported that they were previously diagnosed as having dyslexia. All participants underwent chronotype assessment using the Horne–Östberg Morningness–Eveningness Questionnaire (MEQ) (90). MEQ assesses whether a person’s peak alertness is in the morning, in the evening, or in between. Higher sum scores are associated with morningness, while lower scores point to eveningness. The MEQ is a widely used and reliable scale to measure circadian type (91, 92).
Participants reporting skilled “blind” typing or professional string instrument playing and those reporting sleeping less than 6 h per night routinely, were excluded (5, 32, 43). The participants were instructed not to practice the study task that they were trained on between the scheduled meetings and not to drink caffeine containing drinks during the experiment.
Task and Procedure
The participants were trained and tested in performing an explicitly instructed five-element finger-to-thumb opposition sequence (Figure 1A) (5, 32). All tests were performed with the participants sited in the arm-chair with their left (task performing) arm positioned, comfortably extended, with the palm facing up to allow video recording of all finger movements. Visual feedback was not allowed; the participants were instructed to avert the gaze away from the fingers of the performing hand.
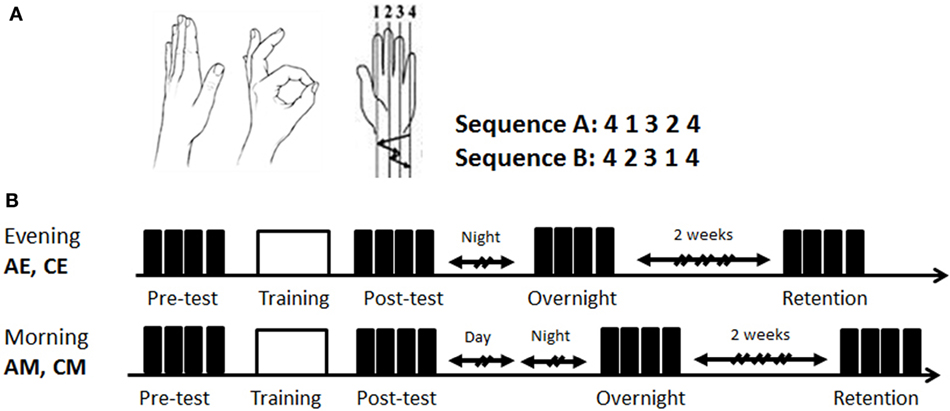
Figure 1. Task and study design. (A) The finger-to-thumb opposition task. The two 5-element finger-to-thumb opposition sequences were mirror reversed order of movements to each other. Each participant was randomly assigned one of the sequences (A or B); (B) the time line of the two experimental conditions. All participants were trained in an identical single session (160 cued repetitions of the sequence) (white box) afforded either at 7:00 p.m. to 9 p.m. (evening) or at 8:00 a.m. to 10:30 a.m. (morning). Both the evening and the morning groups were retested in the morning hours of the next day. Performance was tested at four time-points: pretest, posttest, overnight, and 2 weeks retention post-training (black boxes—test blocks; h—hours). EA, ADHD training at evening; EC, typical controls trained at evening; MA, ADHD trained at morning; MC, typical controls trained at morning.
The experiment included three sessions. In the first session, lasting approximately 30 min, the experimenter showed the thumb-to-finger opposition movements, without demonstration of the sequence. Participants received verbal instructions, informed which sequence they were assigned to (randomly chosen A or B, Figure 1A) and performed self-paced warm-up sequences three times. Following the correct performance of the three warm-up trials, a pre-training performance test, a training, and a post-training performance test were afforded. A performance test consisted of four self-paced blocks, each 30 s long. An explicit instruction was provided before each block to perform the assigned sequence of finger movements “as fast and as accurate as possible” between the start and the stop sounds, given by the computer. Occasional errors should not be corrected. A 30 s rest interval was afforded between the test blocks. Following completion of the four test blocks (pretest), the participants performed 20, 30-s, cued training blocks (training), with a 30-s rest between blocks, altogether 160 repetitions of the sequence. Rest periods could be prolonged if requested by the participant.
Start of each sequence was signaled by a beep at a rate 2.5 s per sequence. No feedback was provided on correctness and speed of performance. Following the training, participants again performed four test blocks (posttest), with identical instructions to the initial tests.
The participants were randomly assigned to four groups (Figure 1B): two groups, ADHD-morning (AM, n = 12) and control morning (CM, n = 13) were trained in the morning (8:00 a.m. to 10:30 a.m.); and two additional groups, ADHD-evening (AE, n = 12) and control evening (CE, n = 12), received an identical training session in the evening (7:00 a.m. to 9:00 p.m.) on the first day of the experiment. The first session (first day, evening, or morning) included the baseline performance test on the assigned sequence (pretest), the training session and the post-test. All participants were retested during second session in the morning of the next day (overnight retest, 5 min long). The third, retention test (third day session, 15 min long), took place on average 14 days (±2) after the second session and was performed again during morning hours (8:00 a.m. to 10:30 a.m.).
Participants were asked to wear an actiwatch (Actigraph Co.) starting from the end of the immediate post-training test to the next 5–7 days, so as to record sleep times and quality. Actigraphy was optional; a consent to wear an actiwatch did not constitute an inclusion criterion. The data were analyzed using ActiLife 6 software.
Statistical Analysis
Performance data were analyzed off-line in terms of speed (number of correct sequences) and accuracy (number of errors) performed per test block from video recordings. Average speed and accuracy of the four test-blocks at each of the four time points (pre-training; post-training; 12–24 h post-training; retention) was calculated. Speed and accuracy of performance were analyzed separately using: (a) a repeated measures analysis of variance with the four time points as within-subject factors × 4 groups [ADHD morning (AM); ADHD evening (AE); CM; CE] as a between subjects factor; and (b) a repeated measures analysis of variance with two consecutive time points to test performance changes across different stages of learning: acquisition phase—fast learning (pre-training vs. post-training), consolidation phase—slow learning (post-training vs. 24 h post-training) and retention phase (24 h post-training vs. retention). Two-tailed t-tests corrected for multiple comparisons were used in the analysis of the normalized performance gains with level of significance of p < 0.05.
Results
Chronotype and Sleep Data
Mean group MEQ scores differed between persons with ADHD and healthy controls (two-sample t-test, t = −4.127, p < 0.001); lower scores, corresponding to larger eveningness were found for the ADHD group (Table 1). The proportion of participants expressing a certain chronotype was significantly different between the groups (χ = 9.17, p = 0.043; Fisher’s exact test, p = 0.048). More eveningness types were found in the ADHD group than in the control group (45.8 vs. 12%). Also, there was a significant difference between the ADHD and the control groups when the MEQ score (continuous measure) were compared (two-sample t-test, t = 4.290, p < 0.001). There was, however, no significant difference in the MEQ scores of the participants with ADHD who were trained in the morning as compared to those receiving evening training (two-sample t-test, t = 0.660, p = 0.516; EA group—5/12, MA group—6/13). Similarly, no significant difference in the MEQ scores of the control participants who were trained in the morning as compared to those trained in the evening was found (two-sample t-test, t = −0.510, p = 0.615; EC group—1/12, MC group—2/12).
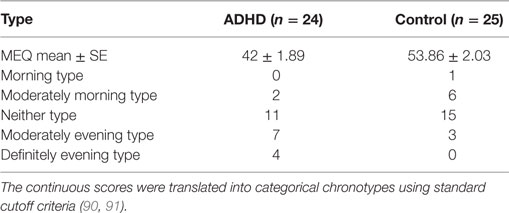
Table 1. Morningness–eveningness questionnaire (MEQ) continuous and categorical scores for the ADHD and control participants.
As the participation in actigraphy was voluntary, the actigraphy data sample is limited and contains selected participants in each condition [ADHD n = 16, control n = 12; AM n = 7, AE n = 9, CM n = 6, CE n = 6]. Average time-in-bed, sleep latency (time to fall asleep), total sleep time (minutes), and sleep efficiency parameters, averaged across 5–7 nights starting from the first night following the training session, were analyzed using two-tailed independent sample t-tests. Results showed significant main effect of group (ADHD, control) for total sleep time (t = −2.722, p = 0.011), reflecting shorter night sleep in ADHD participants (ADHD: 400.18 ± 74 min, control: 498.46 ± 115 min). No significant differences were found with regard to sleep efficiency (mean 92.3 ± 8.6%), sleep latency (mean 4.3 ± 2.9 min) and time-in-bed. All participants reported a high subjective sleep quality during the experimental period. No significant correlations between chronotypes and sleep parameters and the observed gains in performance speed at the posttest, overnight, and retention test points were found.
Behavioral Data
First, we excluded the possibility of a confounding effect of pre-training differences in performance between the experimental groups. Independent samples, two-tailed t-tests showed that there were no significant difference between the pretest performance of the two control groups (CM, CE) (p = 0.12) as well as between the two ADHD groups (AM, AE) (p = 0.558). There were also no significant differences between the participants with ADHD and their corresponding control groups when tested in the morning (MA, MC; p = 0.22) or in the evening (EA, EC; p = 0.33). Thus, the baseline performance of all participants was not significantly affected by the time of test (morning or evening) or ADHD status (Figure 2).
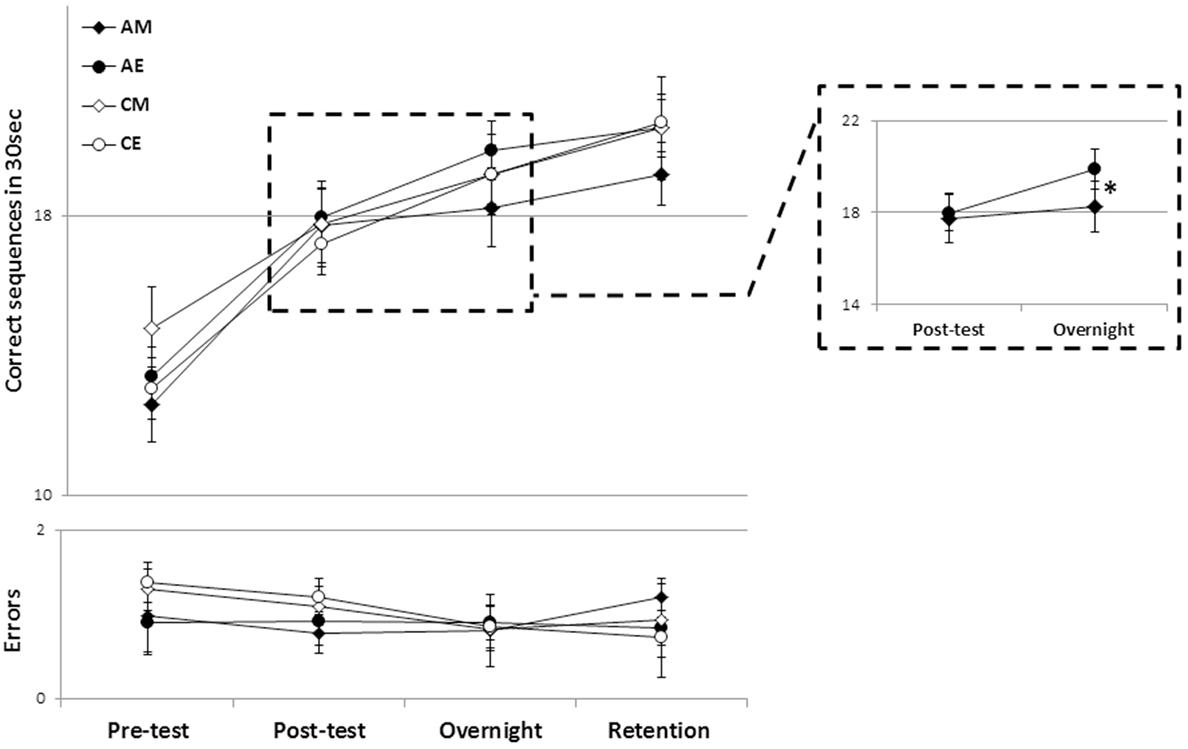
Figure 2. The time course of performance improvement in the four groups. There were clear within-session gains as well as delayed (post-training) “off-line” gains in speed that were well maintained across a 2-week retention interval (upper panel) with no costs in accuracy (lower panel). Each data point depicts the mean group performance for four time-points. Note that the overnight time-point denotes performance on the morning of the post-training day (~12 or 24 h post-training for the evening and morning training groups, respectively). AM, ADHD morning; AE, ADHD evening; CM, control morning; CE, control evening (bars, SEM). Inset: a magnified view of the AM and AE groups’ performance across the first overnight interval (consolidation phase). *Significant interaction effect.
Training on the assigned sequence of movements resulted in early (within-session) and delayed (post-training, time-dependent) gains in performance triggered by a single training session in all groups (Figure 2). An analysis of variance with repeated measures (rm-ANOVA) with four groups × 4 time points, showed that, overall, there was a significant improvement in speed [F(3, 43) = 78.46, p < 0.001] (Figure 2, upper panel) across the study period in all groups. There was no significant group effect (p = 0.705). There was, however, a trend toward a significant interaction of time-point × group [F(9, 135) = 1.78, p = 0.079] suggesting that the performance changes were dissimilar across the four groups.
On average, the participants in all four groups tended to commit, if any, very few errors (Figure 2, lower panel). Absolute accuracy did not change significantly across the period tested [F(3, 43) = 1.396, p = 0.247], suggesting that in all groups the improvements in speed were not at the cost of increased errors.
To explore which of the time intervals contributed to the trend toward an interaction of time-point and group, in performance speed, post hoc rm-ANOVA comparing pairs of consecutive time-points were conducted across the four groups. A significant interaction of time-point × group was found only for the post-session consolidation interval, i.e., in comparing between the posttest and the overnight post-training retest [F(3, 45) = 3.31, p = 0.028]; indicating a significant difference in the rate of performance improvement overnight in the different groups. As can be seen in Figure 2 (inset), the ADHD morning group lagged behind their peers who received the identical training protocol but in the evening, as well as behind the participants in the two control groups. To directly test the contribution of the time of training to the expression of overnight, delayed, gains in performance, in participants with ADHD, an rm-ANOVA was performed comparing the two time-points (posttest, overnight) in the two ADHD groups (AM, AE). Although there was no significant group effect (p = 0.49), there was a significant time-point effect [F(1, 22) = 22.24, p < 0.001] indicating overall gains, but also a significant interaction of time-point × group [F(1, 22) = 7.55, p = 0.012] reflecting the smaller gains in the ADHD morning group (Figure 2, inset). A similar analysis comparing the overnight, delayed gains in performance speed in the two Control groups showed a significant overall improvement in both groups (CM, CE) [F(1, 23) = 39.9, p < 0.001] but no significant group (i.e., time of day) effect (p = 0.58) as well as, importantly, no significant time-point × group interaction (p = 0.29) suggesting that both groups improved at a similar rate.
The time of day in which training was afforded had, however, no significant effect on the ability to retain the gains in speed across the 2 weeks interval (Figure 2). An rm-ANOVA comparing performance in the last two time-points (overnight, retention) in the four groups showed that, rather than forgetting, there was a significant improvement in speed across the retention interval [F(1, 45) = 12.26, p = 0.001], but no significant group effect (p = 0.788), reflecting the finding that the gap that opened between the AM groups performance and that of their peers (irrespective of ADHD status) did not close at 2 weeks post training. The relatively smaller speed gains of the AM group were as well retained as those of the other participant groups.
Although there were no significant differences between the four groups’ average performance, there were large individual differences in pre-training performance, irrespective of ADHD status. To ensure that these large differences between individuals’ task performance levels did not bias the analyses based on absolute performance measures, we also assessed the differences in the expression of delayed gains in performance, with respect to the time of day training was afforded, using normalized data (Figure 3).
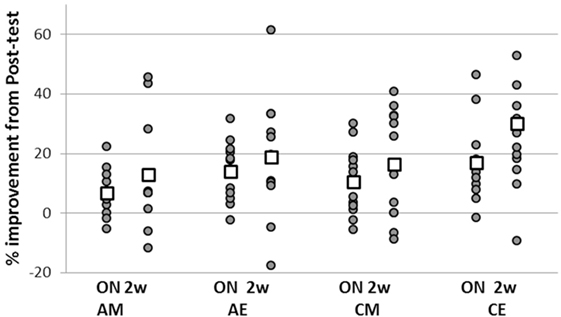
Figure 3. Individual normalized gains in task performance speed expressed at overnight and retention. Overnight gains (ON—the difference between overnight and posttest) were normalized to performance in the posttest; total gains (2 w—difference between retention and the immediate posttest) were normalized to pretest performance for each individual participant. Positive values indicate delayed gains in task performance; negative values correspond to a slowing down of performance speed relative to immediate post training levels. Squares—group averages.
To this end, each participant’s gains in the overnight post-training interval (i.e., the difference between overnight and posttest) were normalized to pre-training performance. In addition, normalization to pre-training performance was done for the total post-training gains expressed in the retention test (i.e., for the difference between retention and the immediate posttest) (Figure 3). There was a significant difference between the two ADHD groups (AM, AE) in the overnight interval (two-sample t-test, t = −0.81, p = 0.042) reflecting an advantage for the evening group. In addition, the overnight performance gains of the ADHD morning group were significantly smaller compared to the control participants trained in the evening (two-sample t-test, t = −2.085, p = 0.05) though not significantly smaller than the gains of the CM group (p = 0.36). However, the overnight gains of the ADHD evening group (AE) were not significantly different from the gains attained by their typical peers trained either at morning (CM) or evening (CE) (p = 0.43, p = 0.54, respectively). There were no significant differences in the normalized performance gains expressed over the 2 weeks retention period in the four groups.
Discussion
The present findings suggest that procedural memory consolidation processes are extant and effective in ADHD, but necessitate specific circadian conditions in order to be fully expressed. The current results, therefore, suggest a new effective learning strategy for ADHD. In line with previous studies (32), persons with ADHD showed the expected gains within the training session but less-than-expected performance gains, evolving overnight, during the procedural memory consolidation phase, if the training session took place in the morning hours. The same training experience afforded in the evening was equally effective in participants with and without ADHD, with both groups improving within-session as well as expressing additional, robust gains in task performance overnight. Nevertheless, morning training afforded to individuals with ADHD was as effective as evening training in terms of the ability to retain the gains acquired within the first 24 h post-training over an interval of about 2 weeks. Moreover, the retention of the training induced gains in performance was as effective in individuals with ADHD as in their typical peers with no ADHD.
Importantly, the current results show that the disadvantage of morning training for ADHD was not related to their ability to improve within session, regardless of the time of training. This result is in line with previous studies (32). However, the relative disadvantage of the morning trained individuals with ADHD was in their ability to express delayed, consolidation phase gains following their quite effective within-session learning. This relative performance lag was maintained over the retention interval.
Chronotype and Sleep
There is good evidence supporting the notion that the affordance of an interval of sleep after a training experience constitutes an important factor in the expression of practice-dependent delayed (“off-line”) gains in the performance of the FOS task in young adults (5, 14, 93) and perhaps more so in elderly individuals (28). There are ample data suggesting that sleep structure may be atypical in persons with ADHD (54, 69, 94). In line with these notions, in the current study, individuals with ADHD tended to be evening chronotypes and to have on average shorter sleep durations. However, the robust overnight expression of delayed gains in the performance of the FOS, in persons with and without ADHD, after evening training, suggests that the post-training sleep intervals were equally sufficient in both groups in supporting the consolidation process.
The prevalence of late chronotypes among young adults in general population is less frequent than in those with ADHD but still significant, reaching 10–15% (95, 96); the low number of participants with evening chronotype in the control groups of the current study is in line with these reported frequencies. However, little is known about the contribution of chronotype to memory in healthy typical individuals. Future studies should address whether evening persons in the general population, those with no ADHD symptoms, may benefit from scheduling of learning session to evening hours, in analogy to the effects found for persons with ADHD. This is especially pertinent in adolescence, a phase of development wherein the circadian profiles are skewed toward eveningness (97).
Procedural Memory Processes in ADHD
The current results provide support for several notions pertaining to skill memory processes in adults with ADHD. First, there is evidence suggesting that the acquisition and consolidation of a recently acquired memory trace, pertaining to a trained movement sequence, interact, but nevertheless constitute independent processes; each of these processes may require a different set of specific conditions to be effectively completed (15, 98, 99). Our results support this notion—young women with ADHD were as effective learners in the morning and evening hours as their typical peers, but they did differ in terms of their ability to subsequently (overnight) express consolidation phase gains. Thus, learning (acquisition, potentially reversible) and memory (dependent on consolidation) may differ from each other with regard to critically important control processes and gating factors. Proximity of evening training to sleep interval may be critical for successful engagement of consolidation processes for persons with ADHD. Not mutually exclusive is the possibility that, in the evening type persons, circadian factors affecting consolidation processes, for example, more effective synaptic tagging (100), are (also) at work.
A second notion is that while procedural memory mechanisms in young adults with ADHD may differ from those subserving skill consolidation in typical individuals, individuals with ADHD nevertheless can generate and effectively retain procedural memory. Atypical procedural memory consolidation processes in young adults with ADHD were indicated in previous studies of motor learning using the FOS task (32, 101). Nevertheless, in both studies, as well as in a study addressing FOS task learning and motor memory consolidation in adolescents with ADHD receiving methylphenidate treatment (43), there was clear evidence, despite atypical learning patterns, for effective long-term retention of skill in the individuals with ADHD. The current results, however, support the notion that young women with ADHD practicing the FOS task may differ from their typical peers in the conditions under which the engagement of consolidation processes occurs. Thus, young women with ADHD may atypically engage consolidation processes when trained in the morning, but not when trained in the evening.
A third notion concerns the training conditions. Conditions that are well suited for typical young adults may be less than optimal for individuals with ADHD. Thus, the apparent consolidation phase deficits in individuals with ADHD may reflect an interaction of the specific learning (and test) conditions with the individuals’ predispositions and chronotype, rather than the latter’s specific deficits per se. For example, Fox and colleagues (101) showed that halving (shortening) the training session may be beneficial for the training of persons with ADHD; perhaps because individuals with ADHD tend to commit more errors in tasks and tests that require multiple repetitions (33, 42, 45, 102). We extend this notion to account for time of training as an important condition, given that in people with ADHD show predominant chronotypes that are skewed toward eveningness. Optimal arousal level during encoding is considered to be a prerequisite, gating factor, mediating the process of long-term memory formation (47). Thus, the endogenous biological clock should be considered as gating factor to neuronal plasticity induced by daily experiences (81).
Altogether, we propose that consolidation processes are under stricter control in individuals with ADHD compared to their typically developing peers. A similar notion of extant procedural memory consolidation mechanisms that may be under stricter constraints compared to that of typical young adults has been recently suggested in explaining the findings in elderly individuals (28). Korman and her colleagues have shown that motor skill acquisition is well preserved in healthy elderly individuals, however, unless a post-training nap was afforded, overnight (consolidation phase, “off-line”) gains were under-expressed. The current findings indicate a similar pattern, with evening training critical for the expression of the full potential for overnight gains, in young women with ADHD. Thus, in analogy to the case of the healthy elderly, we propose that the apparent deficits observed after morning training in individuals with ADHD may reflect suboptimal engagement of procedural, “how to” memory consolidation processes rather than a core deficit in procedural memory consolidation abilities per se [as suggested for example by Nicolson and Fawcett (35)]. We do not suggest that the processes underlying the hypothesized under-engagement (or stricter control) of procedural memory processes in healthy elderly and in young adults with ADHD are identical. The proposal rather is that, in both populations, some added constraints are imposed on the selection of what is to be maintained in long-term memory after a given learning experience, compared to the constraints imposed on consolidation processes in typical young adults. Different constraints on consolidation processes (rather than differences in the capacity to learn or generate long-term procedural memory per se) have also been indicated by recent studies addressing developmental effects in FOS consolidation, i.e., before and after puberty in typically developing individuals (20, 29, 103).
Limitations
Several considerations may limit the interpretation of our findings, given the different first retest periods across study groups trained in the morning and evening hours. Unequal time periods from training to subsequent testing may have contributed to processes of interference or enhancement (104), independently of circadian optimal time-windows for skill acquisition.
An increased susceptibility to interference (e.g., by everyday activities, following training session for which the new movement sequence is irrelevant) was suggested as a mechanism for applying a stricter consistency criterion on what is to be incorporated into long-term procedural memory (28, 105). One could suppose that, in adults with ADHD, there is an increased susceptibility to interference experiences during the waking hours after the training session, leading to smaller consolidation gains in performance. This possibility should be further investigated. However, a recent study suggested that overall susceptibility to interference by a subsequent conflicting experience is not enhanced, but rather is reduced in young women with ADHD (44), compared to typical peers.
The protocol using different delay periods across study groups trained in the morning and evening hours (all groups were retested the next morning) was implemented in to neutralize the possible differences in performance resulting from the time of post-training testing. Thus, the morning groups had more time (~24 h) to consolidate the newly acquired knowledge compared to the evening groups (~13 h). If time per se would be the critical factor to determine the amount of the delayed gains in performance, one would expect to find different levels of overnight performance in the control (evening and morning) groups. However, our findings clearly indicate that this is not the case. As well, in order to control for the confounding effect of the different time-periods following training, we have tested for skill retention at 2-week post-training, allowing ample time to complete the memory consolidation process. The results clearly indicate that: (1) all groups show robust retention (thus no forgetting) and, in fact, additional gains compared to the performance at the first retest; (2) the morning trained ADHD group still lags behind. Thus, our data show no forgetting in time intervals as long as 12–14 days post-training, and support the main interpretation of our results, of the disadvantage of morning training in young adults with ADHD.
Further, we note that current results are limited to a population of highly functional young females with and without ADHD (university students). Additional studies should be conducted in males or mixed experimental groups and in different age groups to afford more general conclusions.
Conclusion
The current study provides evidence to suggest that in individuals with ADHD (frequently exhibiting evening chronotypes), training session afforded during morning hours negatively affect procedural memory consolidation (off-line, delayed) processes. Thus, individuals with ADHD may benefit from training protocols that have been optimized for their own advantage rather than from protocols optimized for their typical peers. Just as the length of the training session (101) or the spacing (rest periods) within and between practice sessions (106, 107) need to be taken into consideration when adapting training protocols for the benefit of persons with ADHD, an adjustment of the diurnal scheduling of the training protocol may be necessary for the full expression of the potential for skill acquisition and its consolidation in persons with ADHD.
Ethics Statement
The study was approved by the Human Experimentation Ethics committee of the University of Haifa and the participants signed an informed consent form in accordance with the Declaration of Helsinki before beginning the experiments. Subjects were paid 150 shekels (approximately $37) for their participation.
Author Contributions
MK, IL, and AK conceived and designed the experiments. IL collected the data. MK and IL analyzed the raw data. MK made the statistical analysis and interpretation of the data. MK, IL, and AK wrote the article.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer, HT, and handling editor declared their shared affiliation, and the handling editor states that the process nevertheless met the standards of a fair and objective review.
Funding
The E. J. Safra Brain Research Center for the Study of Learning Disabilities is gratefully acknowledged for partially funding this project.
References
1. Brown RM, Robertson EM. Inducing motor skill improvements with a declarative task. Nat Neurosci (2007) 10(2):148–9. doi:10.1038/nn1836
2. Cohen N, Squire L. Preserved learning and retention of pattern-analyzing skill in amnesia: dissociation of knowing how and knowing that. Science (1980) 210(4466):207–10. doi:10.1126/science.7414331
3. Karni A. The acquisition of perceptual and motor skills: a memory system in the adult human cortex. Brain Res Cogn Brain Res (1996) 5(1–2):39–48. doi:10.1016/S0926-6410(96)00039-0
5. Korman M, Raz N, Flash T, Karni A. Multiple shifts in the representation of a motor sequence during the acquisition of skilled performance. Proc Natl Acad Sci U S A (2003) 100(21):12492–7. doi:10.1073/pnas.2035019100
6. Maquet P, Schwartz S, Passingham R, Frith C. Sleep-related consolidation of a visuomotor skill: brain mechanisms as assessed by functional magnetic resonance imaging. J Neurosci (2003) 23(4):1432–40.
7. Karni A, Meyer G, Rey-Hipolito C, Jezzard P, Adams MM, Turner R, et al. The acquisition of skilled motor performance: fast and slow experience-driven changes in primary motor cortex. Proc Natl Acad Sci U S A (1998) 95(3):861–8. doi:10.1073/pnas.95.3.861
8. Korman M, Doyon J, Doljansky J, Carrier J, Dagan Y, Karni A. Daytime sleep condenses the time course of motor memory consolidation. Nat Neurosci (2007) 10(9):1206–13. doi:10.1038/nn1959
9. Rozanov S, Keren O, Karni A. The specificity of memory for a highly trained finger movement sequence: change the ending, change all. Brain Res (2010) 1331(0):80–7. doi:10.1016/j.brainres.2010.03.019
10. Friedman J, Korman M. Kinematic strategies underlying improvement in the acquisition of a sequential finger task with self-generated vs. cued repetition training. PLoS One (2012) 7(12):e52063. doi:10.1371/journal.pone.0052063
11. Friedman J, Korman M. Offline optimization of the relative timing of movements in a sequence is blocked by retroactive behavioral interference. Front Hum Neurosci (2016) 10:623. doi:10.3389/fnhum.2016.00623
12. Diekelmann S, Wilhelm I, Born J. The whats and whens of sleep-dependent memory consolidation. Sleep Med Rev (2009) 13(5):309–21. doi:10.1016/j.smrv.2008.08.002
13. Krakauer JW, Shadmehr R. Consolidation of motor memory. Trends Neurosci (2006) 29(1):58–64. doi:10.1016/j.tins.2005.10.003
14. Walker MP, Stickgold R, Jolesz FA, Yoo SS. The functional anatomy of sleep-dependent visual skill learning. Cereb Cortex (2005) 15(11):1666–75. doi:10.1093/cercor/bhi043
15. Dudai Y, Karni A, Born J. The consolidation and transformation of memory. Neuron (2015) 88(1):20–32. doi:10.1016/j.neuron.2015.09.004
16. Dayan E, Cohen Leonardo G. Neuroplasticity subserving motor skill learning. Neuron (2011) 72(3):443–54. doi:10.1016/j.neuron.2011.10.008
17. Gabitov E, Manor D, Karni A. Patterns of modulation in the activity and connectivity of motor cortex during the repeated generation of movement sequences. J Cogn Neurosci (2015) 27(4):736–51. doi:10.1162/jocn_a_00751
18. Censor N, Karni A, Sagi D. A link between perceptual learning, adaptation and sleep. Vision Res (2006) 46(23):4071–4. doi:10.1016/j.visres.2006.07.022
19. Hauptmann B, Reinhart E, Brandt SA, Karni A. The predictive value of the leveling off of within session performance for procedural memory consolidation. Brain Res Cogn Brain Res (2005) 24(2):181–9. doi:10.1016/j.cogbrainres.2005.01.012
20. Wilhelm I, Metzkow-Mészàros M, Knapp S, Born J. Sleep-dependent consolidation of procedural motor memories in children and adults: the pre-sleep level of performance matters. Dev Sci (2012) 15(4):506–15. doi:10.1111/j.1467-7687.2012.01146.x
21. Brashers-Krug T, Shadmehr R, Bizzi E. Consolidation in human motor memory. Nature (1996) 382(6588):252–5. doi:10.1038/382252a0
22. Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci (2010) 11(2):114–26. doi:10.1038/nrn2762
23. Rasch B, Born J. About sleep’s role in memory. Physiol Rev (2013) 93(2):681–766. doi:10.1152/physrev.00032.2012
24. Born J, Wilhelm I. System consolidation of memory during sleep. Psychol Res (2012) 76(2):192–203. doi:10.1007/s00426-011-0335-6
25. Albouy G, King BR, Schmidt C, Desseilles M, Dang-Vu TT, Balteau E, et al. Cerebral activity associated with transient sleep-facilitated reduction in motor memory vulnerability to interference. Sci Rep (2016) 6:34948. doi:10.1038/srep34948
26. Lahl O, Wispel C, Willigens B, Pietrowsky R. An ultra short episode of sleep is sufficient to promote declarative memory performance. J Sleep Res (2008) 17(1):3–10. doi:10.1111/j.1365-2869.2008.00622.x
27. Nishida M, Walker MP. Daytime naps, motor memory consolidation and regionally specific sleep spindles. PLoS One (2007) 2(4):e341. doi:10.1371/journal.pone.0000341
28. Korman M, Dagan Y, Karni A. Nap it or leave it in the elderly: a nap after practice relaxes age-related limitations in procedural memory consolidation. Neurosci Lett (2015) 606:173–6. doi:10.1016/j.neulet.2015.08.051
29. Ashtamker L, Karni A. Motor memory in childhood: early expression of consolidation phase gains. Neurobiol Learn Mem (2013) 106:26–30. doi:10.1016/j.nlm.2013.07.003
30. Douglas VI. Cognitive control processes in attention deficit/hyperactivity disorder. In: Quay HC, Hogan AE, editors. Handbook of Disruptive Behavior Disorders. Boston, MA: Springer US (1999). p. 105–38.
31. Pennington BF, Ozonoff S. Executive functions and developmental psychopathology. J Child Psychol Psychiatry (1996) 37(1):51–87. doi:10.1111/j.1469-7610.1996.tb01380.x
32. Adi-Japha E, Fox O, Karni A. Atypical acquisition and atypical expression of memory consolidation gains in a motor skill in young female adults with ADHD. Res Dev Disabil (2011) 32(3):1011–20. doi:10.1016/j.ridd.2011.01.048
33. Mostofsky SH, Rimrodt SL, Schafer JGB, Boyce A, Goldberg MC, Pekar JJ, et al. Atypical motor and sensory cortex activation in attention-deficit/hyperactivity disorder: a functional magnetic resonance imaging study of simple sequential finger tapping. Biol Psychiatry (2006) 59(1):48–56. doi:10.1016/j.biopsych.2005.06.011
34. Goulardins JB, Marques JC, De Oliveira JA. Attention deficit hyperactivity disorder and motor impairment. Percept Mot Skills (2017) 124(2):425–40. doi:10.1177/0031512517690607
35. Nicolson RI, Fawcett AJ. Procedural learning difficulties: reuniting the developmental disorders? Trends Neurosci (2007) 30(4):135–41. doi:10.1016/j.tins.2007.02.003
36. Liston C, Malter Cohen M, Teslovich T, Levenson D, Casey BJ. Atypical prefrontal connectivity in attention-deficit/hyperactivity disorder: pathway to disease or pathological end point? Biol Psychiatry (2011) 69(12):1168–77. doi:10.1016/j.biopsych.2011.03.022
37. Konrad K, Eickhoff SB. Is the ADHD brain wired differently? A review on structural and functional connectivity in attention deficit hyperactivity disorder. Hum Brain Mapp (2010) 31(6):904–16. doi:10.1002/hbm.21058
38. Cubillo A, Halari R, Smith A, Taylor E, Rubia K. A review of fronto-striatal and fronto-cortical brain abnormalities in children and adults with attention deficit hyperactivity disorder (ADHD) and new evidence for dysfunction in adults with ADHD during motivation and attention. Cortex (2012) 48(2):194–215. doi:10.1016/j.cortex.2011.04.007
39. Nakao T, Radua J, Rubia K, Mataix-Cols D. Gray matter volume abnormalities in ADHD: voxel-based meta-analysis exploring the effects of age and stimulant medication. Am J Psychiatry (2011) 168(11):1154–63. doi:10.1176/appi.ajp.2011.11020281
40. Pedersen A, Ohrmann P. Impaired behavioral inhibition in implicit sequence learning in adult ADHD. J Atten Disord (2012). doi:10.1177/1087054712464392
41. Karatekin C, White T, Bingham C. Incidental and intentional sequence learning in youth-onset psychosis and attention-deficit/hyperactivity disorder (ADHD). Neuropsychology (2009) 23(4):445–59. doi:10.1037/a0015562
42. Burden MJ, Mitchell DB. Implicit memory development in school-aged children with attention deficit hyperactivity disorder (ADHD): conceptual priming deficit? Dev Neuropsychol (2005) 28(3):779–807. doi:10.1207/s15326942dn2803_3
43. Fox O, Adi-Japha E, Karni A. The effect of a skipped dose (placebo) of methylphenidate on the learning and retention of a motor skill in adolescents with attention deficit hyperactivity disorder. Eur Neuropsychopharmacol (2014) 24(3):391–6. doi:10.1016/j.euroneuro.2013.11.005
44. Fox O, Adi-Japha E, Karni A. Motor memory consolidation processes in young female adults with ADHD may be less susceptible to interference. Neurosci Lett (2017) 637:91–5. doi:10.1016/j.neulet.2016.11.044
45. Barnes KA, Howard JH, Howard DV, Kenealy L, Vaidya CJ. Two forms of implicit learning in childhood ADHD. Dev Neuropsychol (2010) 35(5):494–505. doi:10.1080/87565641.2010.494750
46. Sergeant JA. Modeling attention-deficit/hyperactivity disorder: a critical appraisal of the cognitive-energetic model. Biol Psychiatry (2005) 57(11):1248–55. doi:10.1016/j.biopsych.2004.09.010
47. Zentall SS, Zentall TR. Optimal stimulation: a model of disordered activity and performance in normal and deviant children. Psychol Bull (1983) 94(3):446–71. doi:10.1037/0033-2909.94.3.446
48. Fuermaier AB, Tucha L, Koerts J, van Heuvelen MJ, van der Zee EA, Lange KW, et al. Good vibrations – effects of whole body vibration on attention in healthy individuals and individuals with ADHD. PLoS One (2014) 9(2):e90747. doi:10.1371/journal.pone.0090747
49. Baijot S, Slama H, Soderlund G, Dan B, Deltenre P, Colin C, et al. Neuropsychological and neurophysiological benefits from white noise in children with and without ADHD. Behav Brain Funct (2016) 12(1):016–0095. doi:10.1186/s12993-016-0095-y
50. Debas K, Carrier J, Orban P, Barakat M, Lungu O, Vandewalle G, et al. Brain plasticity related to the consolidation of motor sequence learning and motor adaptation. Proc Natl Acad Sci U S A (2010) 107(41):17839–44. doi:10.1073/pnas.1013176107
51. Gais S, Plihal W, Wagner U, Born J. Early sleep triggers memory for early visual discrimination skills. Nat Neurosci (2000) 3(12):1335–9. doi:10.1038/81881
52. Walker MP, Stickgold R. Overnight alchemy: sleep-dependent memory evolution. Nat Rev Neurosci (2010) 11(3):218. doi:10.1038/nrn2762-c1
53. Owens J, Gruber R, Brown T, Corkum P, Cortese S, O’Brien L, et al. Future research directions in sleep and ADHD: report of a consensus working group. J Atten Disord (2013) 17(7):550–64. doi:10.1177/1087054712457992
54. Van der Heijden KB, Smits MG, Van Someren EJ, Gunning WB. Idiopathic chronic sleep onset insomnia in attention-deficit/hyperactivity disorder: a circadian rhythm sleep disorder. Chronobiol Int (2005) 22(3):559–70. doi:10.1081/CBI-200062410
55. Van Veen MM, Kooij JJS, Boonstra AM, Gordijn MCM, Van Someren EJW.Delayed circadian rhythm in adults with attention-deficit/hyperactivity disorder and chronic sleep-onset insomnia. Biol Psychiatry (2010) 67(11):1091–6. doi:10.1016/j.biopsych.2009.12.032
56. Owens JA. A clinical overview of sleep and attention-deficit/hyperactivity disorder in children and adolescents. J Can Acad Child Adolesc Psychiatry (2009) 18(2):92–102.
57. Rhee MK, Lee H-J, Rex KM, Kripke DF. Evaluation of two circadian rhythm questionnaires for screening for the delayed sleep phase disorder. Psychiatry Investig (2012) 9(3):236–44. doi:10.4306/pi.2012.9.3.236
58. Natale V, Alzani A. Additional validity evidence for the composite scale of morningness. Pers Individ Dif (2001) 30(2):293–301. doi:10.1016/S0191-8869(00)00046-5
59. Smith ME, McEvoy LK, Gevins A. The impact of moderate sleep loss on neurophysiologic signals during working-memory task performance. Sleep (2002) 25(7):784–94. doi:10.1093/sleep/25.7.56
60. Baird AL, Coogan AN, Siddiqui A, Donev RM, Thome J. Adult attention-deficit hyperactivity disorder is associated with alterations in circadian rhythms at the behavioural, endocrine and molecular levels. Mol Psychiatry (2012) 17(10):988–95. doi:10.1038/mp.2011.149
61. Voinescu BI, Szentagotai A, David D. Sleep disturbance, circadian preference and symptoms of adult attention deficit hyperactivity disorder (ADHD). J Neural Transm (2012) 119(10):1195–204. doi:10.1007/s00702-012-0862-3
62. Coogan AN, McGowan NM. A systematic review of circadian function, chronotype and chronotherapy in attention deficit hyperactivity disorder. Atten Defic Hyperact Disord (2017). doi:10.1007/s12402-016-0214-5
63. Rybak YE, McNeely HE, Mackenzie BE, Jain UR, Levitan RD. Seasonality and circadian preference in adult attention-deficit/hyperactivity disorder: clinical and neuropsychological correlates. Compr Psychiatry (2007) 48(6):562–71. doi:10.1016/j.comppsych.2007.05.008
64. Bijlenga D, Van Someren EJ, Gruber R, Bron TI, Kruithof IF, Spanbroek EC, et al. Body temperature, activity and melatonin profiles in adults with attention-deficit/hyperactivity disorder and delayed sleep: a case-control study. J Sleep Res (2013) 22(6):607–16. doi:10.1111/jsr.12075
65. Amons PJ, Kooij JJ, Haffmans PM, Hoffman TO, Hoencamp E. Seasonality of mood disorders in adults with lifetime attention-deficit/hyperactivity disorder (ADHD). J Affect Disord (2006) 91(2–3):251–5. doi:10.1016/j.jad.2005.11.017
66. Philipsen A. Differential diagnosis and comorbidity of attention-deficit/hyperactivity disorder (ADHD) and borderline personality disorder (BPD) in adults. Eur Arch Psychiatry Clin Neurosci (2006) 256(1):i42–6. doi:10.1007/s00406-006-1006-2
67. Corkum P, Tannock R, Moldofsky H. Sleep disturbances in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry (1998) 37(6):637–46. doi:10.1097/00004583-199806000-00014
68. Bruni O, Alonso-Alconada D, Besag F, Biran V, Braam W, Cortese S, et al. Current role of melatonin in pediatric neurology: clinical recommendations. Eur J Paediatr Neurol (2015) 19(2):122–33. doi:10.1016/j.ejpn.2014.12.007
69. Philipsen A, Hornyak M, Riemann D. Sleep and sleep disorders in adults with attention deficit/hyperactivity disorder. Sleep Med Rev (2006) 10(6):399–405. doi:10.1016/j.smrv.2006.05.002
70. Kooij JJ, Bijlenga D. The circadian rhythm in adult attention-deficit/hyperactivity disorder: current state of affairs. Expert Rev Neurother (2013) 13(10):1107–16. doi:10.1586/14737175.2013.836301
71. Cubero-Millan I, Molina-Carballo A, Machado-Casas I, Fernandez-Lopez L, Martinez-Serrano S, Tortosa-Pinto P, et al. Methylphenidate ameliorates depressive comorbidity in ADHD children without any modification on differences in serum melatonin concentration between ADHD subtypes. Int J Mol Sci (2014) 15(9):17115–29. doi:10.3390/ijms150917115
72. Rybak YE, McNeely HE, Mackenzie BE, Jain UR, Levitan RD. An open trial of light therapy in adult attention-deficit/hyperactivity disorder. J Clin Psychiatry (2006) 67(10):1527–35. doi:10.4088/JCP.v67n1006
73. Korman M, Herling Z, Levy I, Egbarieh N, Engel-Yeger B, Karni A. Background matters: minor vibratory stimulation during motor skill acquisition selectively reduces off-line memory consolidation. Neurobiol Learn Mem (2017) 140:27–32. doi:10.1016/j.nlm.2017.02.002
74. Taki Y, Hashizume H, Thyreau B, Sassa Y, Takeuchi H, Wu K, et al. Sleep duration during weekdays affects hippocampal gray matter volume in healthy children. Neuroimage (2012) 60(1):471–5. doi:10.1016/j.neuroimage.2011.11.072
75. Nilsson JP, Soderstrom M, Karlsson AU, Lekander M, Akerstedt T, Lindroth NE, et al. Less effective executive functioning after one night’s sleep deprivation. J Sleep Res (2005) 14(1):1–6. doi:10.1111/j.1365-2869.2005.00442.x
76. Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol (2005) 25(1):117–29. doi:10.1055/s-2005-867080
77. Peigneux P, Laureys S, Fuchs S, Collette F, Perrin F, Reggers J, et al. Are spatial memories strengthened in the human hippocampus during slow wave sleep? Neuron (2004) 44(3):535–45. doi:10.1016/j.neuron.2004.10.007
78. Ellenbogen JM, Payne JD, Stickgold R. The role of sleep in declarative memory consolidation: passive, permissive, active or none? Curr Opin Neurobiol (2006) 16(6):716–22. doi:10.1016/j.conb.2006.10.006
79. Ruby NF, Hwang CE, Wessells C, Fernandez F, Zhang P, Sapolsky R, et al. Hippocampal-dependent learning requires a functional circadian system. Proc Natl Acad Sci U S A (2008) 105(40):15593–8. doi:10.1073/pnas.0808259105
80. Iyer R, Wang TA, Gillette MU. Circadian gating of neuronal functionality: a basis for iterative metaplasticity. Front Syst Neurosci (2014) 8:164. doi:10.3389/fnsys.2014.00164
81. Albrecht U. The circadian clock, reward, and memory. Front Mol Neurosci (2011) 4:41. doi:10.3389/fnmol.2011.00041
82. Holz J, Piosczyk H, Landmann N, Feige B, Spiegelhalder K, Riemann D, et al. The timing of learning before night-time sleep differentially affects declarative and procedural long-term memory consolidation in adolescents. PLoS One (2012) 7(7):e40963. doi:10.1371/journal.pone.0040963
83. Lange KW, Reichl S, Lange KM, Tucha L, Tucha O. The history of attention deficit hyperactivity disorder. Atten Defic Hyperact Disord (2010) 2(4):241–55. doi:10.1007/s12402-010-0045-8
84. Kadesjö B, Janols L-O, Korkman M, Mickelsson K, Strand G, Trillingsgaard A, et al. The FTF (five to fifteen): the development of a parent questionnaire for the assessment of ADHD and comorbid conditions. Eur Child Adolesc Psychiatry (2004) 13(3):3–13. doi:10.1007/s00787-004-3002-2
85. Cole WR, Mostofsky SH, Larson JC, Denckla MB, Mahone EM. Age-related changes in motor subtle signs among girls and boys with ADHD. Neurology (2008) 71(19):1514–20. doi:10.1212/01.wnl.0000334275.57734.5f
86. Arnett AB, Pennington BF, Willcutt EG, DeFries JC, Olson RK. Sex differences in ADHD symptom severity. J Child Psychol Psychiatry (2015) 56(6):632–9. doi:10.1111/jcpp.12337
87. Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia (1971) 9(1):97–113. doi:10.1016/0028-3932(71)90067-4
88. Adler LA, Kessler RC, Spencer T. Adult ADHD Self-Report Scale-v1. 1 (ASRS-v1. 1) Symptom Checklist. New York, NY: World Health Organization (2003).
89. Kessler RC, Adler L, Barkley R, Biederman J, Conners CK, Demler O, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry (2006) 163(4):716–23. doi:10.1176/ajp.2006.163.4.716
90. Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol (1976) 4(2):97–110.
91. Caci H, Deschaux O, Adan A, Natale V. Comparing three morningness scales: age and gender effects, structure and cut-off criteria. Sleep Med (2009) 10(2):240–5. doi:10.1016/j.sleep.2008.01.007
92. Di Milia L, Adan A, Natale V, Randler C. Reviewing the psychometric properties of contemporary circadian typology measures. Chronobiol Int (2013) 30(10):1261–71. doi:10.3109/07420528.2013.817415
93. Fischer S, Nitschke MF, Melchert UH, Erdmann C, Born J. Motor memory consolidation in sleep shapes more effective neuronal representations. J Neurosci (2005) 25(49):11248–55. doi:10.1523/JNEUROSCI.1743-05.2005
94. Oosterloo M, Lammers GJ, Overeem S, de Noord I, Kooij JJS. Possible confusion between primary hypersomnia and adult attention-deficit/hyperactivity disorder. Psychiatry Res (2006) 143(2):293–7. doi:10.1016/j.psychres.2006.02.009
95. Randler C, Freyth-Weber K, Rahafar A, Florez Jurado A, Kriegs JO. Morningness-eveningness in a large sample of German adolescents and adults. Heliyon (2016) 2(11):e00200. doi:10.1016/j.heliyon.2016.e00200
96. Cavallera GM, Giudici S. Morningness and eveningness personality: a survey in literature from 1995 up till 2006. Pers Individ Dif (2008) 44(1):3–21. doi:10.1016/j.paid.2007.07.009
97. Roenneberg T, Kuehnle T, Juda M, Kantermann T, Allebrandt K, Gordijn M, et al. Epidemiology of the human circadian clock. Sleep Med Rev (2007) 11(6):429–38. doi:10.1016/j.smrv.2007.07.005
98. Karni A, Korman M. When and where in skill memory consolidation: neuro-behavioral constraints on the acquisition and generation of procedural knowledge. BIO Web Conf (2011) 1:00047. doi:10.1051/bioconf/20110100047
99. Seitz AR, Dinse HR. A common framework for perceptual learning. Curr Opin Neurobiol (2007) 17(2):148–53. doi:10.1016/j.conb.2007.02.004
100. Redondo RL, Morris RGM. Making memories last: the synaptic tagging and capture hypothesis. Nat Rev Neurosci (2011) 12(1):17–30. doi:10.1038/nrn2963
101. Fox O, Karni A, Adi-Japha E. The consolidation of a motor skill in young adults with ADHD: shorter practice can be better. Res Dev Disabil (2016) 52:135–44. doi:10.1016/j.ridd.2016.01.014
102. Adi-Japha E, Karni A, Parnes A, Loewenschuss I, Vakil E. A shift in task routines during the learning of a motor skill: group-averaged data may mask critical phases in the individuals’ acquisition of skilled performance. J Exp Psychol Learn Mem Cogn (2008) 34(6):1544–51. doi:10.1037/a0013217
103. Julius MS, Adi-Japha E. Learning of a simple grapho-motor task by young children and adults: similar acquisition but age-dependent retention. Front Psychol (2015) 6:225. doi:10.3389/fpsyg.2015.00225
104. Walker MP, Brakefield T, Seidman J, Morgan A, Hobson JA, Stickgold R. Sleep and the time course of motor skill learning. Learn Mem (2003) 10(4):275–84. doi:10.1101/lm.58503
105. Dorfberger S, Adi-Japha E, Karni A. Reduced susceptibility to interference in the consolidation of motor memory before adolescence. PLoS One (2007) 2(2):e240. doi:10.1371/journal.pone.0000240
106. Kwon YH, Kwon JW, Lee MH. Effectiveness of motor sequential learning according to practice schedules in healthy adults; distributed practice versus massed practice. J Phys Ther Sci (2015) 27(3):769–72. doi:10.1589/jpts.27.769
Keywords: procedural learning, motor sequence, consolidation, attention-deficit/hyperactivity disorder, chronotype, evening training, young adults, training schedule
Citation: Korman M, Levy I and Karni A (2017) Procedural Memory Consolidation in Attention-Deficit/Hyperactivity Disorder Is Promoted by Scheduling of Practice to Evening Hours. Front. Psychiatry 8:140. doi: 10.3389/fpsyt.2017.00140
Received: 21 April 2017; Accepted: 19 July 2017;
Published: 03 August 2017
Edited by:
Tomiki Sumiyoshi, National Center of Neurology and Psychiatry, JapanReviewed by:
Hidetoshi Takahashi, National Center of Neurology and Psychiatry, JapanCaroline L. Horton, Bishop Grosseteste University, United Kingdom
Copyright: © 2017 Korman, Levy and Karni. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maria Korman, korman.maria@gmail.com
 Maria Korman
Maria Korman Ishay Levy
Ishay Levy Avi Karni2,3
Avi Karni2,3