- 1Department of Psychiatry, University of Pittsburgh, Pittsburgh, PA, United States
- 2Department of Bioengineering, University of Pittsburgh, Pittsburgh, PA, United States
- 3Department of Statistics, University of Pittsburgh, Pittsburgh, PA, United States
- 4Department of Neuroscience, University of Pittsburgh, Pittsburgh, PA, United States
- 5Department of Psychology, University of Pittsburgh, Pittsburgh, PA, United States
Childhood adversity is associated with altered or dysregulated stress reactivity; these altered patterns of physiological functioning persist into adulthood. Evidence from both preclinical animal models and human neuroimaging studies indicates that early life experience differentially influences stressor-evoked activity within central visceral neural circuits proximally involved in the control of stress responses, including the subgenual anterior cingulate cortex (sgACC), paraventricular nucleus of the hypothalamus (PVN), bed nucleus of the stria terminalis (BNST) and amygdala. However, the relationship between childhood adversity and the resting-state connectivity of this central visceral network remains unclear. To this end, we examined relationships between childhood threat and childhood socioeconomic deprivation, the resting-state connectivity between our regions of interest (ROIs), and affective symptom severity and diagnoses. We recruited a transdiagnostic sample of young adult males and females (n = 100; mean age = 27.28, SD = 3.99; 59 females) with a full distribution of maltreatment history and symptom severity across multiple affective disorders. Resting-state data were acquired using a 7.2-min functional magnetic resonance imaging (fMRI) sequence; noted ROIs were applied as masks to determine ROI-to-ROI connectivity. Threat was determined by measures of childhood traumatic events and abuse. Socioeconomic deprivation (SED) was determined by a measure of childhood socioeconomic status (parental education level). Covarying for age, race and sex, greater childhood threat was significantly associated with lower BNST-PVN, amygdala-sgACC and PVN-sgACC connectivity. No significant relationships were found between SED and resting-state connectivity. BNST-PVN connectivity was associated with the number of lifetime affective diagnoses. Exposure to threat during early development may entrain altered patterns of resting-state connectivity between these stress-related ROIs in ways that contribute to dysregulated neural and physiological responses to stress and subsequent affective psychopathology.
Introduction
Due to its high prevalence (Hillis et al., 2016; Merrick et al., 2018; Cuartas et al., 2019) and importance as a predictor of affective risk, childhood adversity is at the forefront of psychiatry’s public health burden (Sara and Lappin, 2017). One sensitivity analysis of global past year violence against children found that a minimum of 1.4 out of 2 billion children aged 2–17 experienced physical, sexual or emotional violence (Hillis et al., 2016). Further, the COVID-19 pandemic has exacerbated systemic challenges, increasing children’s risk of violence exposure (M’jid, 2020; Pereda and Díaz-Faes, 2020). Childhood adversity is a risk factor for and prospective predictor of greater affective symptoms and disorders (Danese et al., 2009; Nanni et al., 2012; Baldwin et al., 2021; Mayer et al., 2021; Russotti et al., 2021). Thus, greater mechanistic understanding of childhood adversity-related neural and physiological differences is necessary to mitigate these risks and guide treatment efforts.
Childhood adversity is associated with dysregulated (heightened or diminished) stress reactivity in childhood and later in life (Al’Absi et al., 2021), with alterations in both neuroendocrine and autonomic physiology and stress reactivity (Heim et al., 2000, 2001; Chen et al., 2004; Koopman et al., 2004; Carpenter et al., 2007, 2011; Gunnar et al., 2009; Lovallo et al., 2011; Hackman et al., 2012). There is evidence to suggest that there may be differential influences of childhood adversity dimensions, threat (e.g., abuse, traumatic events) and deprivation (e.g., neglect, socioeconomic deprivation, institutional rearing) (McLaughlin et al., 2014; Sheridan and McLaughlin, 2014) on stress reactivity, with threat blunting (Carpenter et al., 2007, 2011; Doom et al., 2014; Peckins et al., 2015; Bernard et al., 2017) and deprivation (i.e., low socioeconomic status, SES) heightening reactivity (Lupien et al., 2001; Cohen et al., 2006; Chen et al., 2009; Lê-Scherban et al., 2018). Despite evidence linking childhood threat and deprivation to altered physiological stress systems, how childhood adversity shapes specific, proximally stress-responsive neural circuits remains unclear.
The subgenual anterior cingulate cortex (sgACC), paraventricular nucleus of the hypothalamus (PVN), bed nucleus of the stria terminalis (BNST) and amygdala form a stress-responsive, central visceral network. These limbic forebrain and hypothalamic regions are central to reciprocal descending preautonomic/visceromotor and ascending viscerosensory (i.e., central visceral) pathways (see Figure 1) that control and modulate autonomic outflow and neuroendocrine function (Banihashemi and Rinaman, 2006; Card and Sved, 2011; Rinaman et al., 2011). Further, connections between these regions are important for stress regulation; the PVN, a gateway of hypothalamic-pituitary-adrenal (HPA)/neuroendocrine and autonomic regulation (Luiten et al., 1985; Herman et al., 2002), is directly innervated and influenced by the BNST (Dong et al., 2001b; Povysheva et al., 2021). With little to no innervation of the PVN (Freedman et al., 2000), the sgACC/Brodmann area 25 may access the PVN via direct connections to the BNST (Freedman et al., 2000; Vertes, 2004). The amygdala also has reciprocal connections to the sgACC (Heilbronner and Haber, 2014; Oler and Fudge, 2019; Sharma et al., 2019) and BNST (Dong et al., 2001a; Bienkowski et al., 2013; deCampo and Fudge, 2013; Oler et al., 2017; Figure 1). Further, the intrinsic functional connectivity of the extended amygdala (BNST/amygdala) appears to align with known anatomical connectivity; Tillman et al. (2018) showed that compared to the central nucleus of the amygdala, the BNST displayed stronger coupling with anterior cortical areas, including ventromedial prefrontal cortex/sgACC and brainstem/dorsal periaqueductal gray. This network (along with its central visceral connections) is involved in affective processes (e.g., emotional memory, threat responses, fear and anxiety) (Fendt et al., 2005; Schweimer et al., 2005; Somerville et al., 2010; Avery et al., 2014, 2016; Herrmann et al., 2016), implicated in psychopathology (e.g., depression, anxiety, trauma-related disorders) (Thayer and Lane, 2000; Gotlib et al., 2005; Drevets et al., 2008b; Lebow and Chen, 2016; Clauss, 2019; Clauss et al., 2019), and targeted for affective disorder treatments (e.g., deep brain stimulation for depression and obsessive compulsive disorder) (Johansen-Berg et al., 2008; Gutman et al., 2009; Drobisz and Damborská, 2018; Fitzgerald et al., 2018; Mosley et al., 2021).
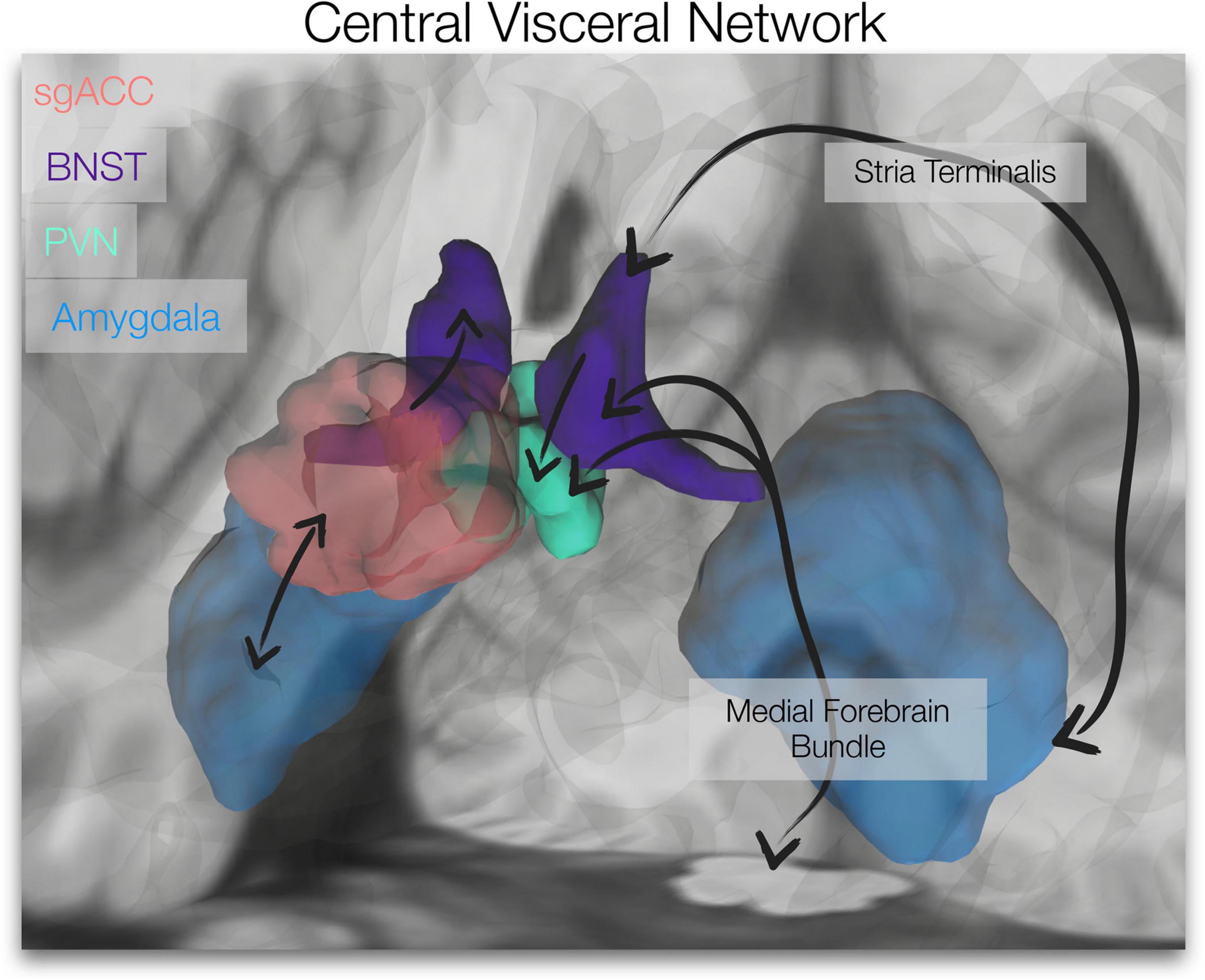
Figure 1. Central Visceral Network. The brain image depicts the regions of interest utilized in our resting-state analyses, the subgenual anterior cingulate cortex (sgACC, red), bed nucleus of the stria terminalis (BNST, violet), paraventricular nucleus of the hypothalamus (PVN, green) and amygdala (blue). Simplified connections are depicted (black arrows; not specific with respect to subnuclei). Ascending viscerosensory and descending preautonomic/visceromotor pathways course through the medial forebrain bundle (BNST and PVN connections depicted). Amygdala, BNST and PVN are connected via the stria terminalis. BNST projections to PVN and sgACC innervation of the BNST are displayed, as well as reciprocal connections between the sgACC and amygdala. The amygdalofugal pathway is also integral to this network (not depicted here). (Unilateral representations are shown).
Previous research in preclinical animal models (Banihashemi and Rinaman, 2010; Banihashemi et al., 2011) and human neuroimaging (Banihashemi et al., 2015) indicates that early life experience differentially influences stressor-evoked activity within this visceral, stress-responsive network. In physically and mentally healthy adults, childhood threat (i.e., physical abuse) is significantly associated with greater stressor-evoked activity across this central visceral, limbic forebrain-hypothalamic network (Banihashemi et al., 2015). Yet, how childhood adversity may influence resting-state connectivity of this central visceral network is virtually unknown.
Studies focused on childhood adversity and resting-state connectivity have spanned development and have primarily focused on amygdala-related or large-scale network connectivity (Teicher et al., 2020). In youth, findings tend to indicate that greater childhood maltreatment or trauma exposure is associated with greater amygdala-related connectivity (amygdala-sgACC cortex, amygdala-hippocampus, amygdala-salience network) (Marusak et al., 2015; Thomason et al., 2015; Rakesh et al., 2021b), while late adolescents/adults tend to display an opposing trend with greater maltreatment associated with less amygdala-related connectivity (amygdala-sgACC, amygdala-ventromedial prefrontal cortex, amygdala-orbitofrontal cortex/insula/hippocampus, amygdala-cuneus/precuneus) (Werff et al., 2012; Herringa et al., 2013; Souza-Queiroz et al., 2016). Rabellino et al. (2018) investigated BNST resting-state connectivity in PTSD and its dissociative subtype; however, they found no significant relationship between childhood maltreatment and BNST resting-state connectivity in subsequent analyses. To our knowledge, no studies of childhood adversity have focused exclusively on the resting-state connectivity of the central visceral network described. Thus, the current study is unique in examining this network in a transdiagnostic sample recruited specifically to represent a spectrum of severity across self-reported childhood physical abuse.
Our recent work discovered opposing relationships of childhood threat (both abuse and traumatic events) and socioeconomic deprivation with white matter structural integrity (i.e., generalized fractional anisotropy, gFA) of the stria terminalis (Banihashemi et al., 2021), which connects several of our regions of interest (ROIs) (caudomedial amygdala, BNST and hypothalamus) (Rafal et al., 2015; Oler et al., 2017; Weiss et al., 2021). Greater threat was associated with less, while greater deprivation was associated with greater, stria terminalis gFA. Thus, we hypothesized that threat and deprivation would have congruent, opposing effects on resting-state connectivity within this central visceral network (i.e., threat would be associated with lower, while deprivation would be associated with greater, resting-state connectivity). To this end, our goal was to examine differential relationships of threat and deprivation with resting-state connectivity between our central visceral ROIs, as well as to examine how their functional connectivity may contribute to affective symptoms or disorders. These findings may contribute to a greater understanding of how childhood-adversity shapes this central visceral network at rest, how these resting state dynamics may prime functional activity in response to emotionally salient stimuli and how this network may contribute to childhood adversity-related differences in affective symptoms and disorders.
Materials and Methods
Participants
Participants were recruited from Allegheny County, Pittsburgh, PA, United States, using various methods (e.g., referrals from other research studies, and online and bus advertisements). Of the 1,020 contacts made, 111 (18.5%) underwent informed consent and were enrolled in the study. Of those consented, 100 (90%) participants completed study procedures. Participants were 59 female and 41 male young adults (n = 100, mean age = 27.28, SD = 3.99). Of the 100 participants that completed the study, 45% self-reported their race as White, 36% as Black or African American, 13% as Asian, 4% as multiracial, and 2% as biracial. Within this sample, 7 individuals reported their ethnicity as Hispanic or Latin American. All participants provided informed consent after receiving an explanation of study protocols and were examined with the approval of the University of Pittsburgh Institutional Review Board.
Exclusion criteria were: Magnetic resonance imaging (MRI) contraindications (e.g., claustrophobia, metal in the body, severe visual or auditory impairment), pregnancy, left-handedness, cardiovascular disease and diabetes, neurological disorders (including seizure disorders, migraine disorder, traumatic brain injury, or neurodegenerative disorders), psychotropic medications or any medications affecting cardiovascular or neural function, suicidality or marked functional impairment, and current psychiatric disorders (bipolar, psychotic disorders, substance abuse or dependence) except for depression, anxiety or trauma-related disorders.
Individuals were also screened using the five childhood physical abuse items from the Childhood Trauma Questionnaire (CTQ) with the goal of achieving an even distribution of participants across four physical abuse severity classifications as defined by CTQ guidelines (Bernstein et al., 1994). The following distribution of physical abuse severity was achieved in the final sample (n = 100): 29% None-Minimal, 23% Low-Moderate, 21% Moderate-Severe, 27% Severe-Extreme. A balanced distribution was also achieved across childhood SES (as assessed by maximum parental education level), 31% Low (GED – some college, no degree), 34% Middle (Associate or Bachelor’s), and 35% High (Master’s or Doctorate). All participants had at least one parent with a GED or higher education level.
Study Protocol and Measures
The study comprised two visits completed within one month (mean number of days between visits: 14.39 ± 10.96), an intake visit followed by an MRI scan visit at the University of Pittsburgh Magnetic Resonance Research Center. At the first visit, eligibility was determined using medical history, two-week medication history, current substance use and traumatic brain injury inventories. Participants were excluded if deemed ineligible by these assessments.
Childhood Threat
Childhood Threat was assessed with the Childhood Trauma Questionnaire (CTQ) and the Trauma History Questionnaire (THQ). The CTQ is a 28-item Likert-type scale that examines five subscales of maltreatment: physical, emotional and sexual abuse, and physical and emotional neglect (Bernstein et al., 1994). Each subscale contains five items with scores ranging from 1-Never to 5-Very Often True. A sum of the three abuse subscales represented our CTQ Threat variable (with 15 indicating no abuse and 75 indicating extreme abuse). (A sum of the two neglect subscales represented our CTQ Deprivation variable, which was used in secondary analyses. See section “Variable Selection”).
The THQ is a 24-item questionnaire that assesses the occurrence of traumatic events throughout the life course (Stamm, 1996). An adapted version of the THQ was used in which participants responded yes or no to indicate whether a particular event occurred and then selected the relevant age range(s): age 0–11, age 12–17, and age > 18 (Insana et al., 2012). Traumatic events included experiences with crime, environmental disasters, injury or death, as well as physical or sexual abuse. THQ 0-11 was used as a primary measure of childhood threat and THQ > 18 was used as a covariate (see sections “Variable Selection” and “Data Analysis”).
Childhood Socioeconomic Deprivation
A sociodemographic inventory was used to assess childhood and adulthood SES. Maximum parental education level was used to determine childhood SES; the participants’ own educational level determined adulthood SES. Both were presented as a 9-point education level scale (0 - No high school diploma, 1 – GED, 2 – High school diploma, 3 – Technical training, 4 – Some college, no degree, 5 – Associate degree, 6 – Bachelor’s degree, 7 – Master’s degree, 8 – MD/PhD/JD/PharmD). The Childhood Deprivation construct encompasses low SES, socioeconomic disadvantage or neighborhood deprivation (McLaughlin et al., 2014; Webb et al., 2017; Berti and Pivetti, 2019; Morris et al., 2019). Further, education level is often used as a measure of SES and is associated with mental health inequalities (Reiss, 2013), physiological stress (Ursache et al., 2017) and physical health, especially cardiovascular disease risk (Winkleby et al., 1992). Thus, we used maximum parental education level (reverse coded) as our primary measure of childhood socioeconomic deprivation (SED). Adulthood SES was used as a covariate.
Negative Life Events
The 24-item Life Events List assesses significant life events experienced by the participant within the past 12 months (e.g., unemployment, separation or divorce, serious illness, death of someone close) (Cohen et al., 1991). Participants indicate whether or not they have experienced an event in the past year with follow up questions assessing valence and/or details if endorsed. This inventory was used to assess the total number of negative life events, which was used as a covariate.
Affective Symptom Severity
Depression and post-traumatic stress symptom severity were assessed using the Beck’s Depression Inventory (BDI-II) and the PTSD Checklist - Civilian Version (PCL-C), respectively. The BDI-II is a 21-item questionnaire that assesses the presence and severity of depression within the past two weeks; it probes whether participants have experienced a thought or behavior related to depressive symptoms on a scale of 0 to 3, with scores > 20 considered moderate-to-severe (Beck et al., 1996). The PCL-C is a 20-item measure that reliably assesses post-traumatic stress symptom severity in the last month on a 5-point Likert scale ranging from not at all (Cuartas et al., 2019) to extremely (M’jid, 2020), with scores > 30 considered moderate-to-severe. It includes assessment of re-experiencing, avoidance and arousal symptoms, as well as negative cognitions (Wilkins et al., 2011).
Diagnostic Assessment
Psychiatric diagnoses of mood, anxiety or trauma-related disorders were evaluated and confirmed via in-person interview using the Structured Clinical Interview for DSM-IV Axis I Disorders by a trained interviewer. Of the 100 participants who completed the study, 29% were healthy (had no history of the affective disorders evaluated), whereas 71% had a history of affective diagnosis (47 participants had one or more current affective diagnoses). Of those with a diagnostic history, 30 had a trauma-related disorder, 24 had a depressive disorder and 17 had an anxiety disorder, as their primary lifetime diagnosis. Posttraumatic stress disorder was the most frequent diagnosis (30% of the sample) followed by major depressive disorder (15% of the sample). Further, 37% had comorbid lifetime mood and anxiety/trauma-related disorders.
Sample Characterization
Participants also completed questionnaires to characterize the sample, including the Perceived Stress Scale (PSS, 10-item version) to assess frequency of stress-related feelings (Cohen et al., 1983), the State Trait Anxiety Inventory (STAI-Y2) to assess presence and severity of trait anxiety (Spielberger et al., 1983) and the NEO Five-Factor Inventory-3 (NEO-FFI-3, 60 items) to assess personality (McCrae and Costa, 2007). See Supplementary Table S1 for Participant Characteristics.
Magnetic Resonance Imaging Protocol and Data Acquisition
Magnetic resonance imaging data were collected on a 3-Tesla Trio TIM whole-body MRI scanner (Siemens, Erlangen, Germany), equipped with a 32-channel head coil. Prior to the resting-state sequence, participants were instructed to “gaze at the fixation cross and rest” and reminded to remain as still as possible. A custom, localized shimming procedure was implemented that extended from the bottom-most slice to the ventral aspect of the corpus callosum. Resting-state functional MRI data were acquired using a 7.2-min, T2*-weighted gradient-echo echoplanar imaging (EPI) sequence (TR = 2000 ms, TE = 29 ms, flip angle = 65°, slices = 22, Multiband Factor = 3, FoV = 220 × 220 mm2, voxel size = 2 × 2 × 2.0 mm3). The FOV was angled 15–20° to ensure visualization of our ROIs. For registration purposes, anatomical images were acquired using a 4.8-min T1-weighted sagittal MPRAGE sequence (TR = 1500 ms, TE = 3.19 ms, flip angle = 8°, 176 slices, FoV = 256 × 256 mm2, voxel size = 1 × 1 × 1.0 mm3). The resting-state sequence followed the MPRAGE acquisition, which was the first sequence in the protocol. Additional sequences were collected during the MR (not reported here) with a total duration of approximately 50–55 min.
Resting-State Functional Magnetic Resonance Imaging Preprocessing and Analysis
Resting state fMRI data were preprocessed using Statistical Parametric Mapping software (SPM121). Motion correction was applied through realignment of each blood-oxygen-level dependent (BOLD) image to the mean reference image. The structural image was then co-registered to the mean functional image. Segmentation was performed on the structural image using probability maps for six tissue classes, generating a deformation field that was then applied to the functional images during normalization of all images to standard Montreal Neurological Institute (MNI) space (2 mm isotropic resolution). Smoothing was applied to functional images using a 4 mm full-width-at-half-maximum Gaussian kernel.
Resting-state connectivity analyses were performed using standard SPM-based functions (in-house MATLAB code was used to wrap these functions). Translation (mm) and rotation (deg) was assessed for each participant; motion was low across the sample of 100 participants (Translation: mean = 1.13 mm, SD = 0.37; Rotation: mean = 0.86 degrees, SD = 0.73). Our threshold for maximum translation was 3 mm of motion and none exceeded this. Motion artifact reduction was applied to smoothed functional images using the SPM BrainWavelet Toolbox wavelet despiking methods to identify and filter spike artifacts. A principal component analysis was performed by extracting five eigenvariates of the BOLD signal principal time series from the white matter and cerebrospinal fluid simultaneously using singular value decomposition. Using multiple linear regression, the time series at each voxel was adjusted by applying these tissue components and the raw values of the six motion parameters (not their derivatives) from preprocessing as covariates. The residual time series was extracted from each voxel and we used a series of cosines to model the band pass Butterworth filter (0.008–0.15 Hz), which was applied on the residuals.
Region of Interest-to-Region of Interest Analyses
The sgACC, BNST and PVN ROI masks were created using manual segmentation with MRIcron on the ch2better template. The BNST and PVN ROIs were based on the Atlas of the Human Brain (Mai et al., 2008) [BNST: plates 18 (Talairach reference systems, y = −2.7 mm) through 24 (y = + 2.7 mm); PVN: plates 20 (y = −1.3 mm) through 28 (y = + 8.0 mm)]. The sgACC ROI was based on its depiction in Cingulate Neurobiology and Disease (Vogt, 2009). These ROIs were described initially (Banihashemi et al., 2015) and utilized/reported elsewhere (Andreescu et al., 2015a,b; Price et al., 2018; Wu et al., 2019). The amygdala ROI was created from the SPM Anatomy toolbox using the 50% probabilistic map (Amunts et al., 2005; Eickhoff et al., 2005). Each ROI was applied as a mask on the covariate-processed functional data. Using principal component analysis, we extracted the first eigenvariate within each ROI for each subject. The correlation (Pearson) between the eigenvariates for each ROI-to-ROI pair was calculated to determine connectivity between the two regions.
Variable Selection
Our rationale for childhood threat and deprivation variable selection has been described previously (Banihashemi et al., 2021). Briefly, preliminary data analyses from the current sample demonstrated that our childhood threat and deprivation variables were correlated (Table 1), however, among deprivation measures, socioeconomic deprivation [SED, maximum parental education level (reverse coded, such that higher values reflected greater deprivation)] was the least correlated with the threat measures (Pearson r = 0.175 to 0.411, Table 1). CTQ Threat (abuse) and CTQ Deprivation (neglect) were strongly correlated (r = 0.769). As such, CTQ Deprivation was considered only in exploratory analyses (data not shown) and SED was used as the primary measure of childhood deprivation. As early childhood experiences are critical for brain development (Tottenham and Sheridan, 2010), our primary analyses of trauma utilized early traumatic events (THQ 0-11); exploratory analyses examining later traumatic events (THQ 12-17) are included in the Supplementary Material. Because CTQ Threat and THQ 0-11 are highly correlated (r = 0.535), these threat measures were considered in separate models, allowing separate examination of broader traumatic events (THQ 0-11) and abuse (CTQ Threat). Because threat co-occurs with SED (and these constructs are not independent from one another), we examined the additive effects (Fahrmeir et al., 2013) of threat and deprivation similar to Lawson et al. (2017).
Data Analysis
Childhood Adversity and Resting-State Connectivity
We examined whether childhood threat and SED variables were associated with resting-state connectivity between our ROIs; six resting-state ROI-to-ROI connections were examined (Amygdala-BNST, Amygdala-PVN, Amygdala-sgACC, BNST-PVN, BNST-sgACC and PVN-sgACC). All hierarchical linear regression models covaried for age, race and sex in Step 1 and examined the additive effects of childhood threat and SED together in Step 2 (i.e., Model 1: early traumatic events and SED, and Model 2: abuse and SED). We also evaluated whether our findings remained after multiple comparison correction (FDR < 0.05, for six tests, one for each ROI-to-ROI connection) (Benjamini and Hochberg, 1995) and after adjusting for adulthood trauma (all traumatic events occurring after age 18, THQ > 18), adulthood SES (education level) and negative life events within the past year (Life Events List); these variables were entered together in Step 3. Where a significant relationship was found between abuse (CTQ Threat) and resting-state connectivity, post hoc analyses were performed substituting each abuse subscale in the model to examine which type of abuse may have been driving the relationship.
Resting-State Connectivity and Affective Symptoms/Disorders
Where a significant relationship was found between childhood threat and resting-state connectivity between our ROIs, we also examined whether ROI-to-ROI connectivity was associated with depressive or post-traumatic stress symptom severity or the number of lifetime diagnoses. Hierarchical regression models covaried for age, race and sex in Step 1 and examined the effect of ROI-to-ROI connectivity in Step 2 in separate models. We also evaluated whether our findings remained after multiple comparison correction (FDR < 0.05, for three tests, one for each measure of affect) and after adjusting for adulthood trauma (THQ > 18), adulthood SES (education level) and negative life events within the past year (Life Events List); these variables were entered together in Step 3.
Results
Childhood Threat, Deprivation and Central Visceral Network Resting-State Connectivity
Early Traumatic Events (Trauma History Questionnaire, Age 0–11)
Of the six resting-state ROI-to-ROI connections examined, THQ 0-11 had a significant, negative association with BNST-PVN (ß = −0.224; p = 0.033, Figure 2A), Amygdala-sgACC (ß = −0.312; p = 0.003, Figure 2B) and PVN-sgACC connectivity (ß = −0.264; p = 0.008, Figure 2C) (standardized ß values reported throughout; Table 2). Among these, relationships between THQ 0-11 and Amygdala-sgACC (adjusted p = 0.018) and PVN-sgACC (adjusted p = 0.024) survived multiple comparison correction, and both relationships remained significant when adulthood trauma (age > 18), adulthood SES and negative life events were added to the model [Amygdala-sgACC (ß = −0.328; p = 0.005); PVN-sgACC (ß = −0.241; p = 0.030) (Table 2)]. Socioeconomic deprivation (SED, maximum parental education level reverse coded) did not have a significant association with any ROI-to-ROI connection examined.
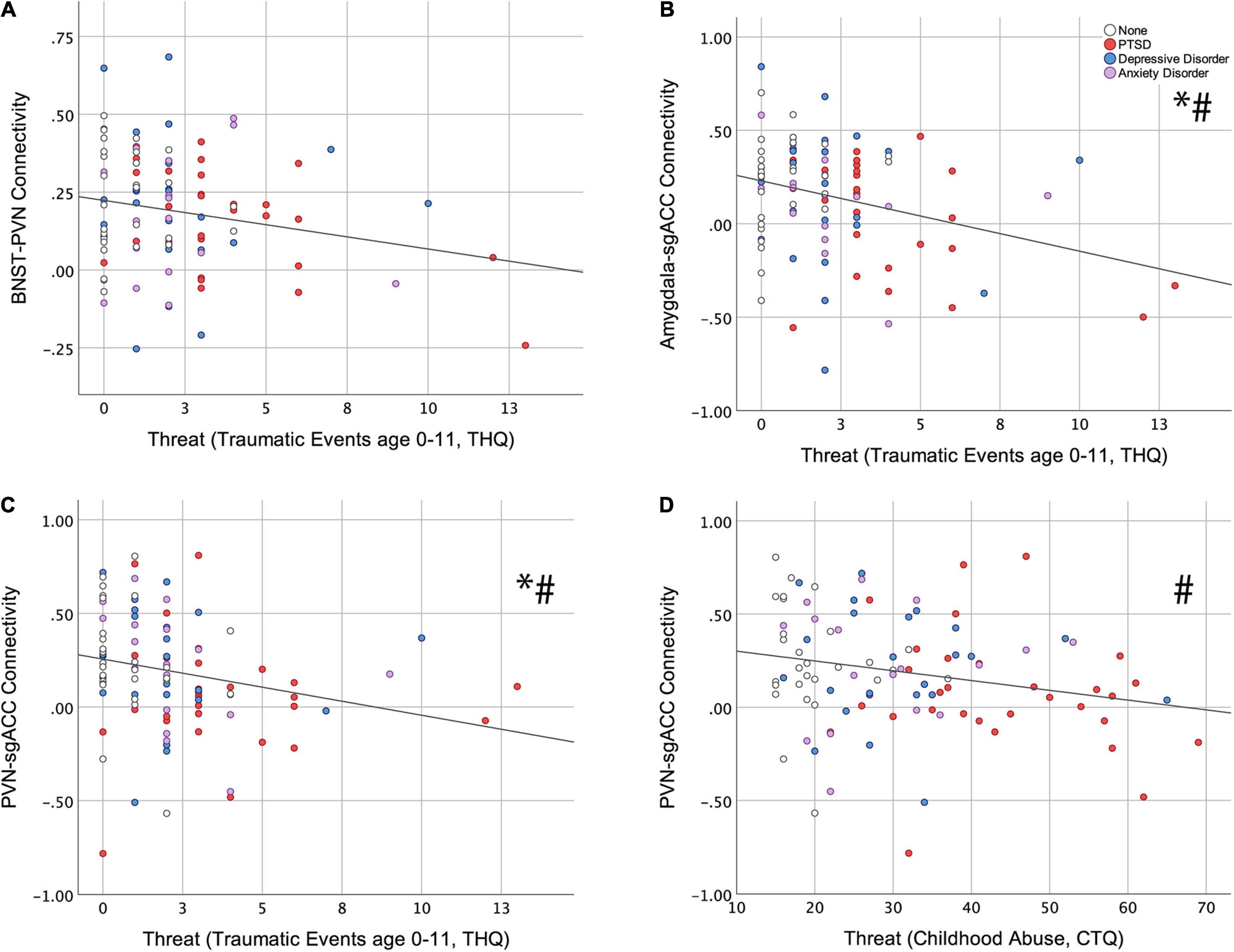
Figure 2. Relationships between childhood threat and central visceral network resting-state connectivity. Hierarchical linear regression was used to examine associations between early traumatic events (Trauma History Questionnaire, THQ, age 0–11) and (A) BNST-PVN (ß = −0.224; p = 0.033), (B) Amygdala-sgACC (ß = −0.312; p = 0.003), and (C) PVN-sgACC (ß = −0.264; p = 0.008) connectivity. (D) The association between childhood abuse (Childhood Trauma Questionnaire, CTQ) and PVN-sgACC (ß = −0.258; p = 0.018) connectivity. * Indicates survival of FDR correction (0.05) for six tests; # indicates that the childhood threat variable remained significant with the addition of adulthood covariates (adulthood trauma, socioeconomic status and negative life events) to the model. (BNST, bed nucleus of the stria terminalis; PVN, paraventricular nucleus of the hypothalamus; sgACC, subgenual anterior cingulate cortex).
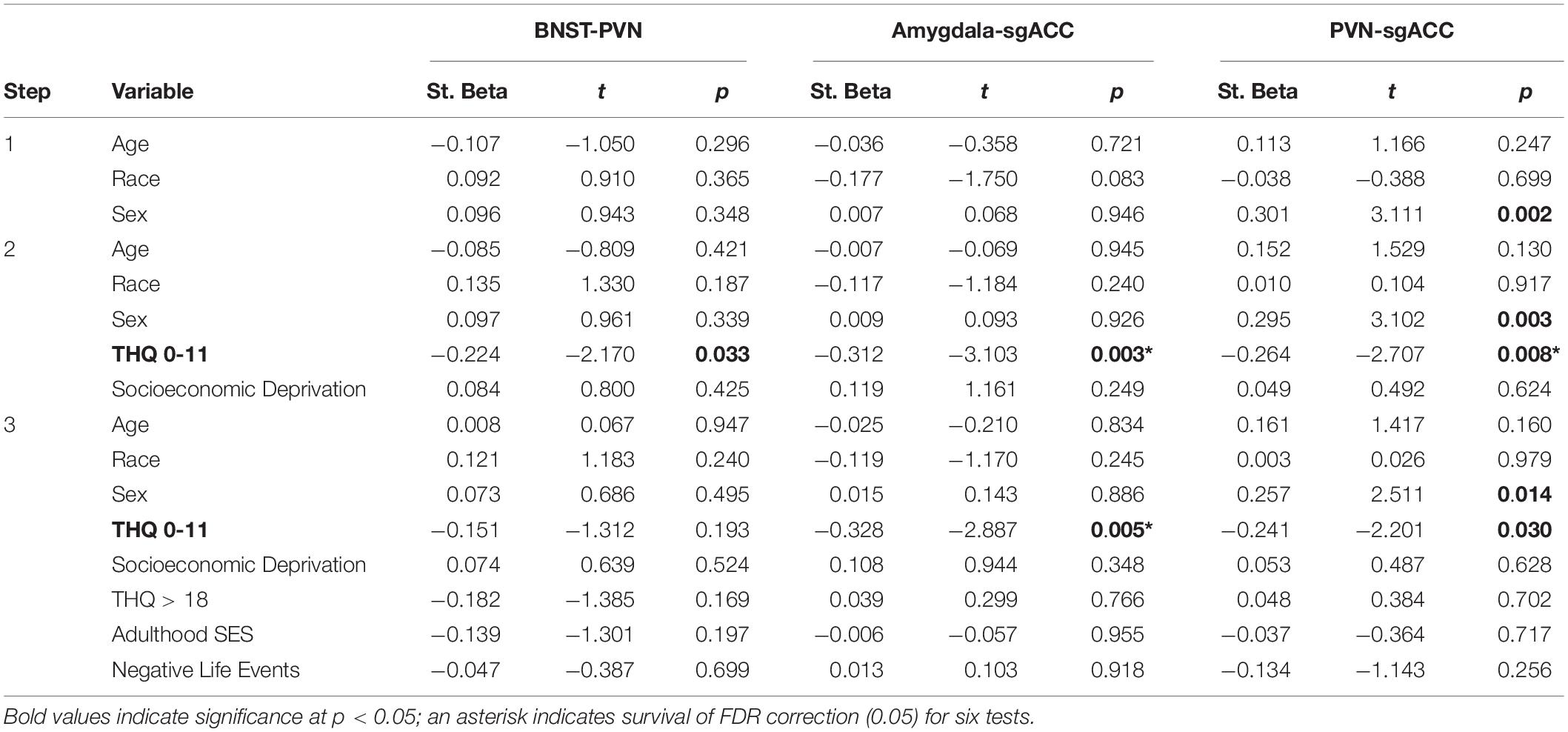
Table 2. Hierarchical linear regression results: childhood threat (early traumatic events, age 0–11) and central visceral network resting-state connectivity.
An examination of potential outliers is reported in the Supplementary Material [Results, Early Traumatic Events (THQ, age 0–11, Supplementary Tables S2–S4)]. When removing the largest THQ 0-11 value, the amygdala-sgACC and PVN-sgACC results remain significant, continue to survive multiple comparison correction and remain significant with the additional adulthood covariates.
Abuse (Childhood Trauma Questionnaire Threat)
CTQ Threat had a significant, negative association with only one resting-state ROI-to-ROI connection, PVN-sgACC connectivity (ß = −0.258; p = 0.018, Figure 2D and Table 3). This finding did not survive multiple comparison correction (adjusted p = 0.108); however, it did remain significant (ß = −0.228; p = 0.043) with the additional adulthood covariates (trauma, SES and negative life events) (Table 3). SED did not have a significant association with any ROI-to-ROI connection examined.
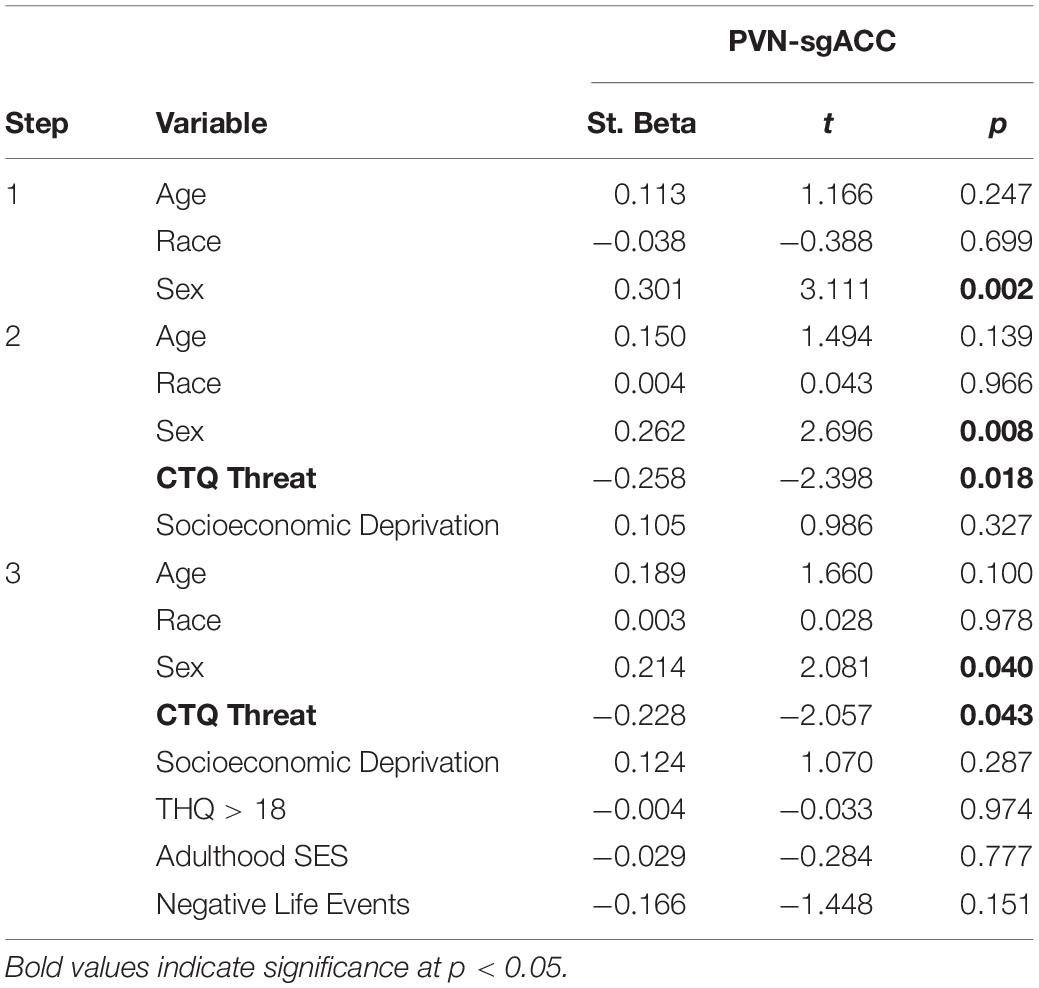
Table 3. Hierarchical linear regression results: childhood threat (abuse) and central visceral network resting-state connectivity.
Post hoc analyses examining CTQ Threat subscales (physical, emotional and sexual abuse) revealed that physical (ß = −0.255; p = 0.023, Supplementary Table S5) and sexual (ß = −0.250; p = 0.014, Supplementary Table S6) abuse were each negatively associated with PVN-sgACC connectivity. Both findings survived multiple comparison correction for 3 tests (one for each abuse type) [physical abuse (adjusted p = 0.035); sexual abuse (adjusted p = 0.042)]; however, only sexual abuse remained significant (ß = −0.248; p = 0.015) with the additional adulthood covariates (Supplementary Tables S5, S6).
Exploratory Analyses
Exploratory analyses examining later traumatic events (THQ 12-17) are included in Supplementary Table S7.
When CTQ Deprivation (Neglect) was substituted for SED in the THQ 0-11 or CTQ Threat models, no significant associations of CTQ Deprivation with any ROI-to-ROI connection were observed (data not shown).
Central Visceral Network Resting-State Connectivity and Affective Symptoms or Diagnoses
Of the three ROI-to-ROI connections that showed an association with childhood threat (early traumatic events and/or childhood abuse) (BNST-PVN, Amygdala-sgACC, PVN-sgACC), only BNST-PVN connectivity was associated with affective (depressive or post-traumatic stress) symptoms or diagnoses. BNST-PVN connectivity was negatively associated with depressive symptoms (ß = −0.138; p = 0.169, non-significant), post-traumatic stress symptoms (ß = −0.202; p = 0.045) and the number of lifetime affective diagnoses (ß = −0.236; p = 0.011, Table 4). The relationship between BNST-PVN connectivity and the number of lifetime diagnoses survived multiple comparison correction (adjusted p = 0.033) and remained significant when adulthood trauma, adulthood SES and negative life events were added to the model (ß = −0.191; p = 0.032, Table 4). Relationships between childhood adversity and affective symptoms/diagnoses are in Supplementary Table S8.
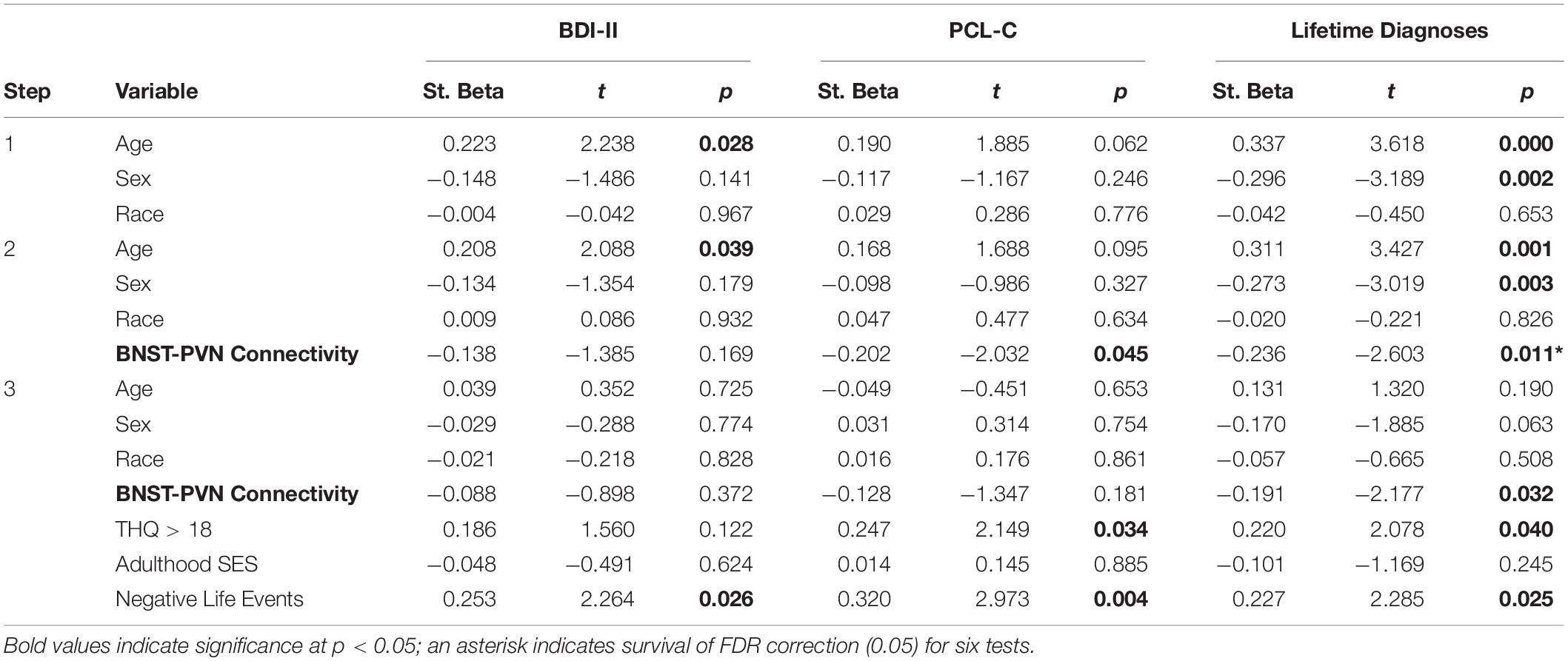
Table 4. Hierarchical linear regression results: central visceral network resting-state connectivity and affective symptoms or diagnoses.
Discussion
An expanding literature has linked childhood adversity to neural measures of brain structure, (e.g., gray matter volume, van Harmelen et al., 2010; Walsh et al., 2014) and white matter structural integrity (Choi et al., 2012; Hanson et al., 2013, 2015) and function [e.g., emotion or threat processing (Fani et al., 2010; McCrory et al., 2011, 2013; van Harmelen et al., 2012) and resting-state connectivity] (Teicher et al., 2016; Ho and King, 2021). Further, threat and deprivation dimensions of childhood adversity may have different neural correlates (McLaughlin et al., 2014; Sheridan and McLaughlin, 2014) and/or influence the same neural circuits in different ways (Banihashemi et al., 2021). How threat and deprivation may differentially influence a proximally stress-responsive, central visceral network (PVN, BNST, amygdala and sgACC) is unclear; previous work demonstrated that early experience may shape the sensitivity of these regions to stress (Banihashemi et al., 2011, 2015), however, childhood adversity-related differences in resting-state connectivity specific to this network have not been examined. To this end, the present study examined effects of childhood threat (traumatic events and childhood abuse) and socioeconomic deprivation on the resting-state connectivity of this neural circuit. We hypothesized that threat and deprivation would have differential, potentially opposing effects on resting-state connectivity within this central visceral network.
Overall, we found that childhood threat (namely, early traumatic events, age 0–11) was associated with lower resting-state-connectivity among our central visceral, limbic forebrain-hypothalamic ROIs (BNST-PVN, Amygdala-sgACC and PVN-sgACC). Of these, our most robust findings were that greater exposure to early traumatic events was associated with less PVN-sgACC and amygdala-sgACC connectivity, both of which withstood multiple comparison correction, as well as the addition of adulthood covariates to the model. Contrary to our hypothesis of differential effects of threat and deprivation, we only identified childhood threat as being related to resting-state connectivity within our network, with no significant associations of socioeconomic deprivation (SED) on any ROI-to-ROI connections. Lastly, despite the well-known clinical significance of amygdala-sgACC connectivity, only BNST-PVN connectivity was associated with affective symptoms and disorders, implicating this connection as a potential mediator between childhood threat and affective vulnerability.
Relationships Between Childhood Threat and Subgenual Anterior Cingulate Cortex-Related Resting-State Connectivity
Two of the three identified ROI-to-ROI relationships with childhood threat involved the sgACC (PVN-sgACC and amygdala-sgACC), a central visceral/visceromotor limbic forebrain region (Vertes, 2004; Alexander et al., 2020) involved in negative affect (Shackman et al., 2011) and emotion regulation, that is also dysregulated in affective disorders (Gotlib et al., 2005; Drevets et al., 2008a,b; Matthews et al., 2009; Alexander et al., 2019). The present study demonstrated a relationship between childhood threat and PVN-sgACC connectivity; this was shown with both early traumatic events (THQ 0-11) and childhood abuse (CTQ Threat), where greater threat was associated with lower PVN-sgACC connectivity. Interestingly, the sgACC has little to no direct projection to the PVN (Öngür et al., 1998; Freedman et al., 2000; Floyd et al., 2001). The sgACC may influence PVN activity via its direct innervation of the BNST (Freedman et al., 2000; Dong et al., 2001a), which sends dense projections to the PVN from its anterolateral and fusiform subnuclei (Figure 1; Dong et al., 2001b; Dong and Swanson, 2004; Maita et al., 2021). Indeed, our findings did indicate that greater exposure to early traumatic events was associated with less BNST-PVN connectivity (discussed further below, Relationship between Childhood Threat and BNST-PVN Resting-State Connectivity).
Our findings also revealed a negative relationship between early traumatic events and amygdala-sgACC resting-state connectivity. The amygdala and sgACC are directly and reciprocally connected (Figure 1); from amygdala to sgACC, projections primarily stem from basal, accessory basal and lateral nuclei (Kim et al., 2018; Sharma et al., 2019). Projections from sgACC to amygdala innervate various subnuclei, including basal, accessory basal, medial and intercalated nuclei (Freedman et al., 2000). Blunt dissection and tractography techniques have also identified putative connections between them (Johansen-Berg et al., 2008; Vergani et al., 2016). To further elaborate the white matter these connections traverse, elegant work by Folloni et al. (2019) demonstrated that in both macaques and humans the amygdalofugal pathway and the uncinate fasciculus extend between the amygdala and sgACC. Our present finding that greater exposure to early traumatic events was associated with less amygdala-sgACC connectivity may indicate microstructural differences in these white matter pathways. Indeed, childhood adversity has been associated with less uncinate fasciculus fractional anisotropy (Eluvathingal et al., 2006; Kumar et al., 2013; Hanson et al., 2015; McCarthy-Jones et al., 2018); however, only a medial bundle from the uncinate extends along the sgACC, while a major section of the amygdalofugal pathway extends along the sgACC (Folloni et al., 2019). Thus, the amygdalofugal pathway may be a promising candidate neural mechanism underlying the relationship between childhood threat and amygdala-sgACC connectivity found here. Further, a recent study showed greater neurite density within the ventral amygdalofugal pathway with age, perhaps indicating greater fiber packing density and/or myelination of the tract (Azad et al., 2021). Future work will be needed to examine childhood adversity-related microstructural differences within this pathway across development and to determine its multimodal relationship to functional connectivity among these ROIs.
Our finding that greater exposure to early traumatic events is associated with less amygdala-sgACC connectivity in a sample of transdiagnostic young adults converges with that of Herringa et al. (2013) who found that greater childhood maltreatment was associated with less amygdala-sgACC connectivity in a late adolescent sample. In younger individuals, however, this relationship may be reversed. Thomason et al. (2015) found that trauma-exposed youth displayed greater centromedial amygdala-sgACC connectivity compared to controls; this work highlights the need for future work examining the connectivity of this central visceral network across development.
The amygdala-sgACC connection has long been thought to be clinically important (Drevets et al., 2008b). Herringa et al. (2013) found that amygdala-sgACC connectivity contributed substantially in mediating the relationship between maltreatment and internalizing symptoms. Depressed adolescents display elevated sgACC-amygdala connectivity (Connolly et al., 2013; Ho et al., 2014) or weaker bottom-up amygdala-sgACC connectivity (Musgrove et al., 2015). Amygdala-sgACC connectivity may also predict treatment response (Taylor et al., 2018; Nakamura et al., 2021). Recent studies highlight the role of amygdala-sgACC connectivity in fear-related encoding, emotional processing/regulation and anxiety (Hakamata et al., 2020; Scharnowski et al., 2020). Hakamata et al. (2020) demonstrated that greater fear encoding strength is associated with greater basolateral amygdala-sgACC connectivity, and that this connectivity was also elevated in anxious participants. Scharnowski et al. (2020) have examined the role of amygdala-sgACC connectivity during automated and effortful emotion regulation; during more automated/less effortful emotion regulation, they found greater amygdala-to-sgACC connectivity. Additionally, they found greater amygdala-to-sgACC modulation among anxious participants during effortful emotion upregulation (Scharnowski et al., 2020). In this context, our amygdala-sgACC findings may suggest less adaptive emotional processing or regulation related to fear-inducing or emotionally salient stimuli, however, such childhood threat-related differences could be indicative of functional impairments that yield vulnerability to affective disorders and/or neuronal adaptations to the early environment that yield resilience (Champagne et al., 2003; Teicher and Samson, 2016; Teicher et al., 2016; Ioannidis et al., 2020).
Relationship Between Childhood Threat and Bed Nucleus of the Stria Terminalis-Paraventricular Nucleus of the Hypothalamus Resting-State Connectivity
The present study also revealed an association between early traumatic events and BNST-PVN resting-state connectivity; however, this effect was less robust (i.e., did not withstand multiple comparison correction or the addition of adulthood covariates). This relationship did, however, converge with our previous finding that greater childhood threat (both traumatic events and childhood abuse) was associated with less stria terminalis white matter structural integrity (Banihashemi et al., 2021), a white matter bundle that connects these regions (Figure 1; De Olmos and Ingram, 1972; Nieuwenhuys et al., 2008). Taken together, these findings may indicate a reciprocal relationship between BNST-PVN structural and functional connectivity. Further, previous work in rodents shows that ascending noradrenergic/viscerosensory pathways from caudal brainstem collateralize to both BNST and PVN, thus, enabling coordinated modulation of both structures’ response to stress (Banihashemi and Rinaman, 2006). These viscerosensory pathways course through the medial forebrain bundle (Figure 1), which our previous study also revealed may be diminished by childhood threat (Banihashemi et al., 2021). Thus, the medial forebrain bundle may be an indirect pathway underlying the resting-state relationship between BNST and PVN, as well as a neural mechanism underlying the current findings.
The BNST regulates physiological responses to stress not only via its own preautonomic projections but also through its direct connections to the PVN (Maita et al., 2021). Various BNST subnuclei differentially regulate physiological responses to stress (Choi et al., 2007; Crestani et al., 2013). PVN-projecting BNST neurons are primarily GABAergic and recent work has shown that the anteroventral BNST exerts inhibitory influence over HPA responses to stress (Radley et al., 2009; Johnson et al., 2016; Radley and Johnson, 2018) via potential peptidergic mechanisms (Zheng et al., 2019; Povysheva et al., 2021). Considered together, our finding that childhood threat (early traumatic events) was associated with lower BNST-PVN connectivity may indicate the BNST’s diminished capacity to constrain the PVN and stressor-evoked HPA responses, perhaps yielding greater stress reactivity.
It has been proposed that anteroventral BNST-related circuitry is recruited by stress-inducing stimuli, but is uninvolved in tonic HPA regulation (Johnson et al., 2016). Our findings suggest that childhood threat may shape basal BNST-PVN connectivity, however, effects of childhood threat on sgACC-related connectivity (PVN-sgACC and amygdala-sgACC) were more robust, despite BNST-PVN connectivity having closer proximity to the control of stress responses. It is possible that childhood threat may shape the resting-state connectivity of this central visceral network in ways that prime the network to engage differently during stress, with indirect connections (sgACC-related connectivity) more active at rest and direct connections (BNST-PVN) more active during stress.
Relationship Between Bed Nucleus of the Stria Terminalis-Paraventricular Nucleus of the Hypothalamus Resting-State Connectivity and Affective Disorders
The BNST’s involvement in mediating responses to more distant, less predictable threats implicate it in future-oriented anxiety states, as well as addiction and other psychiatric disorders (Avery et al., 2016; Lebow and Chen, 2016; Clauss, 2019; Clauss et al., 2019; Limbachia et al., 2020). Recent work also indicates stronger BNST-hypothalamus structural connectivity in women, which may underlie sex differences in symptoms related to abstinence from alcohol and risk for relapse (Flook et al., 2021). As the BNST’s anatomical connection to the PVN contributes in part to its ability to respond to threat, our findings may indicate childhood threat-related differences in vulnerability to affective disorders. Indeed, the present study found that greater BNST-PVN resting-state connectivity was associated with less affective symptoms and disorders (i.e., fewer lifetime diagnoses), implicating this connection as a potential mediator between childhood threat and affective vulnerability, although future, larger studies will be necessary to test formal mediation models (Fritz and MacKinnon, 2007).
Convergence With Large-Scale Networks
The sgACC is considered to be part of the default mode network (DMN), which is involved in self-related mental activity; the DMN is most active when individuals are not engaged in goal-oriented tasks and is deactivated when engaged in cognitive processing (Menon, 2013; Seitzman et al., 2019). The sublenticular extended amygdala and hypothalamus are considered to be part of the salience network (Menon, 2013), although the amygdala may also be considered part of the affective or limbic network (Seitzman et al., 2019). Nevertheless, our findings involved childhood threat-related differences in functional connectivity in cortico-amygdalar-hypothalamic regions that overlap with DMN and salience networks. Expanding literatures indicate that these networks, their nodes and connections between them are altered by childhood adversity (Werff et al., 2012; Marusak et al., 2015; Hoffmann et al., 2018; Cheng et al., 2021; Huang et al., 2021; Rakesh et al., 2021a; Merrick et al., 2018; Silveira et al., 2021), and that these networks are dysregulated in affective disorders (Greicius et al., 2007; Seeley et al., 2007; Jacobs et al., 2014, 2016; Iadipaolo et al., 2018). Thus, our findings may also reflect alterations within these large-scale networks that impact emotion regulation processes (DMN) and orientation to salient internal and external stimuli (salience) (Menon, 2013). Additionally, Kleckner et al. (2017) provided evidence of a large-scale, intrinsic allostatic-interoceptive system and demonstrated that stronger connectivity between hubs within this system supported greater interoceptive ability. This allostatic-interoceptive system is comprised of DMN and salience network regions and includes limbic cortices and subcortical and brainstem visceromotor regions (Kleckner et al., 2017; Ruiz-Rizzo et al., 2020; Sennesh et al., 2022) that converge with our central visceral network of interest (Myers et al., 2005; Banihashemi and Rinaman, 2006; Rinaman et al., 2011; Banihashemi et al., 2015, 2021). Further, Schaan et al. (2019) have shown that childhood maltreatment was associated with less stress-related interoceptive accuracy during a heartbeat perception task. Taken together, our findings indicating lower childhood threat-related central visceral network connectivity may have implications for diminished interoceptive ability and/or accuracy. Future work will be necessary to explicitly examine the neural mechanisms underlying links between childhood adversity and interoceptive ability/capacity.
Differential Relationships of Childhood Threat and Deprivation on Resting-State Connectivity
Several studies have examined differential relationships between resting-state connectivity and aspects of threat and deprivation dimensions (Cheng et al., 2021; Fadel et al., 2021; Park et al., 2021; Rakesh et al., 2021a). A study examining mesocorticolimbic circuitry in young children found opposing influences of threat and socioeconomic deprivation on ventral tegmental area (VTA)-related resting-state connectivity (Park et al., 2021), with greater threat associated with less VTA-somatomotor connectivity and greater deprivation associated with greater VTA-intraparietal sulcus connectivity. In a large adolescent sample, Rakesh et al. (2021a) found differential effects of threat and deprivation across development: at age 16, greater abuse was associated with less within salience network connectivity, while at age 19, greater neglect was associated with greater within-salience network connectivity, potentially indicating different trajectories for adversity dimensions. Fadel et al. (2021) also found differential relationships of threat and deprivation on salience network connectivity; in a sample of healthy and depressed adults they found opposing relationships of abuse and neglect on within salience network connectivity (i.e., prefrontal cortex-insula), in which greater abuse was associated with greater resting-state connectivity and greater neglect was associated with less resting-state connectivity. The present study did not find effects of socioeconomic deprivation, as defined by maximum parental education level (reverse coded), on any ROI-to-ROI connection. Effects of CTQ Deprivation (neglect) on central visceral network connectivity were also explored and no significant relationships were found (data not shown). It is possible that socioeconomic deprivation will have a greater impact on stressor-evoked activity and connectivity within this central visceral network than on its resting-state connectivity. Future work on this network will be needed to investigate different aspects of the deprivation construct (e.g., neighborhood and cognitive deprivation).
Limitations and Future Directions
A limitation of this study is its cross-sectional design examining young adults; however, participants were specifically recruited across a continuum of physical abuse severity, with individuals across a spectrum of affective symptom severity including those with depression, anxiety and trauma-related disorders. This recruitment strategy achieved a relatively even distribution across childhood socioeconomic status, as well. Nevertheless, future prospective work will be needed to examine distinct dimensions of childhood adversity and how they differentially impact this central visceral network across development. Additionally, future studies designed to be statistically powered for detecting realistic effect sizes for mediation are necessary to further examine central visceral network components as mediators of the relationship between childhood adversity and affective symptoms/disorders.
Regions of interest in the present study were defined using a template for manual segmentation [sgACC, BNST and PVN (Banihashemi et al., 2015; Wu et al., 2019)]. Greater accuracy and precision are necessary to define specific subnuclei within these ROIs and to examine additional components of the network (e.g., brainstem nuclei), which may benefit from high-field acquisitions. Improvements in manual segmentation approaches for these regions and continued advancements in automated segmentations would also benefit the examination of these brain regions, particularly in humans. Future work will be needed to capitalize on current advances (Avery et al., 2014; Saygin et al., 2017; Wolff et al., 2018).
Summary and Conclusion
This study provides novel evidence that childhood threat may influence a central visceral network. Analyses revealed that childhood threat is associated with lower connectivity among our ROIs (PVN-sgACC, amygdala-sgACC and BNST-PVN). These findings have functional and clinical implications that suggest potential alterations in emotion regulation and processing, orienting responses to salient stimuli, and stress and threat reactivity. Further, our results demonstrate that BNST-PVN connectivity may provide a novel link between childhood threat and affective symptoms and disorders. In conclusion, exposure to threat during early development may entrain altered patterns of resting-state connectivity between these stress-related regions in ways that contribute to dysregulated neural and physiological responses to stress and subsequent affective psychopathology. Investigating how this network links childhood adversity and affective symptoms may elucidate underlying neural mechanisms of affective disorders, as well as guide interventions targeting these brain structures.
Data Availability Statement
The datasets presented in this article are not readily available because they are still undergoing primary analyses. The data that support the findings of this study will be made available from the corresponding author upon request in the future. Requests to access the datasets should be directed to LB, layla.banihashemi@pitt.edu.
Ethics Statement
The studies involving human participants were reviewed and approved by the University of Pittsburgh Institutional Review Board. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
LB conceptualized the research, acquired funding, conducted the research, performed formal analysis, wrote the original draft, and reviewed and edited the manuscript. CP conducted the research, managed and coordinated the research, performed formal analysis, and wrote portions of the Methods. AR performed formal analysis and implemented code and algorithms. HK provided programming, implemented code and algorithms, and reviewed and edited the manuscript. MW contributed to creation of models and reviewed and edited the manuscript. BS wrote portions of the Discussion and reviewed and edited the manuscript. JS wrote portions of the Introduction. MS provided data curation. AG provided mentorship, oversight, and resources. HA provided mentorship, oversight, and resources and reviewed and edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by the National Institute of Mental Health Grants K01 MH102406 and R01 MH120065 to LB.
Conflict of Interest
MW is a statistical consultant for Noctem, unrelated to this work. AG serves as CEO and holds equity in Rehat, LLC and has also served as a consultant for Jazz Pharmaceuticals, Inc., unrelated to this work.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Many thanks to Noelle Rode for providing database construction, Mark Jones for providing clinical interview supervision and Dr. Tae Kim of the Magnetic Resonance Research Center (MRRC) for providing sequence optimization. The authors would like to thank the reviewers of this manuscript for their time and effort in contributing their feedback.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyg.2022.805049/full#supplementary-material
Footnotes
References
Al’Absi, M., Ginty, A. T., and Lovallo, W. R. (2021). Neurobiological mechanisms of early life adversity, blunted stress reactivity and risk for addiction. Neuropharmacology 188:108519. doi: 10.1016/j.neuropharm.2021.108519
Alexander, L., Clarke, H. F., and Roberts, A. C. A. (2019). Focus on the functions of area 25. Brain Sci. 9:129. doi: 10.3390/brainsci9060129
Alexander, L., Wood, C. M., Gaskin, P. L. R., Sawiak, S. J., Fryer, T. D., Hong, Y. T., et al. (2020). Over-activation of primate subgenual cingulate cortex enhances the cardiovascular, behavioral and neural responses to threat. Nat Commun. 11:5386. doi: 10.1038/s41467-020-19167-0
Amunts, K., Kedo, O., Kindler, M., Pieperhoff, P., Mohlberg, H., Shah, N., et al. (2005). Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat. Embryol. 210, 343–352. doi: 10.1007/s00429-005-0025-5
Andreescu, C., Mennin, D., Tudorascu, D., Sheu, L. K., Walker, S., Banihashemi, L., et al. (2015a). The many faces of anxiety-neurobiological correlates of anxiety phenotypes. Psychiatry Res. 234, 96–105. doi: 10.1016/j.pscychresns.2015.08.013
Andreescu, C., Sheu, L. K., Tudorascu, D., Gross, J. J., Walker, S., Banihashemi, L., et al. (2015b). Emotion reactivity and regulation in late-life generalized anxiety disorder: functional connectivity at baseline and post-treatment. Am. J. Geriatr. Psychiatry 23, 200–214. doi: 10.1016/j.jagp.2014.05.003
Avery, S. N., Clauss, J. A., and Blackford, J. U. (2016). The human BNST: functional role in anxiety and addiction. Neuropsychopharmacology 41, 126–141. doi: 10.1038/npp.2015.185
Avery, S. N., Clauss, J. A., Winder, D. G., Woodward, N., Heckers, S., and Blackford, J. U. (2014). BNST neurocircuitry. NeuroImage 91, 311–323.
Azad, A., Cabeen, R. P., Sepehrband, F., Kim, R., Campbell, C. E., Lynch, K., et al. (2021). Microstructural properties within the amygdala and affiliated white matter tracts across adolescence. NeuroImage 243:118489. doi: 10.1016/j.neuroimage.2021.118489
Baldwin, J. R., Caspi, A., Meehan, A. J., Ambler, A., Arseneault, L., Fisher, H. L., et al. (2021). Population vs individual prediction of poor health from results of adverse childhood experiences screening. JAMA Pediatr. 175, 385–393. doi: 10.1001/jamapediatrics.2020.5602
Banihashemi, L., and Rinaman, L. (2006). Noradrenergic inputs to the bed nucleus of the stria terminalis and paraventricular nucleus of the hypothalamus underlie hypothalamic-pituitary-adrenal axis but not hypophagic or conditioned avoidance responses to systemic yohimbine. J. Neurosci. 26, 11442–11453. doi: 10.1523/JNEUROSCI.3561-06.2006
Banihashemi, L., and Rinaman, L. (2010). Repeated brief postnatal maternal separation enhances hypothalamic gastric autonomic circuits in juvenile rats. Neuroscience 165, 265–277. doi: 10.1016/j.neuroscience.2009.09.081
Banihashemi, L., O’Neill, E. J., and Rinaman, L. (2011). Central neural responses to restraint stress are altered in rats with an early life history of repeated brief maternal separation. Neuroscience 192, 413–428. doi: 10.1016/j.neuroscience.2011.06.052
Banihashemi, L., Peng, C. W., Verstynen, T., Wallace, M. L., Lamont, D. N., Alkhars, H. M., et al. (2021). Opposing relationships of childhood threat and deprivation with stria terminalis white matter. Hum. Brain Mapp. 42, 2445–2460. doi: 10.1002/hbm.25378
Banihashemi, L., Sheu, L. K., Midei, A. J., and Gianaros, P. J. (2015). Childhood physical abuse predicts stressor-evoked activity within central visceral control regions. Soc. Cogn. Affect. Neurosci. 10, 474–485. doi: 10.1093/scan/nsu073
Beck, A. T., Steer, R. A., Ball, R., and Ranieri, W. F. (1996). Comparison of beck depression inventories-IA and-II in psychiatric outpatients. J. Pers. Assess. 67, 588–597. doi: 10.1207/s15327752jpa6703_13
Benjamini, Y., and Hochberg, Y. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Series B 57, 289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x
Bernard, K., Frost, A., Bennett, C. B., and Lindhiem, O. (2017). Maltreatment and diurnal cortisol regulation: a meta-analysis. Psychoneuroendocrinology 78, 57–67. doi: 10.1016/j.psyneuen.2017.01.005
Bernstein, D. P., Fink, L., Handelsman, L., Foote, J., Lovejoy, M., Wenzel, K., et al. (1994). Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am. J. Psychiatry 151:1132. doi: 10.1176/ajp.151.8.1132
Berti, C., and Pivetti, M. (2019). Childhood economic disadvantage and antisocial behavior: intervening factors and pathways. Child. Youth Serv. Rev. 97, 120–126. doi: 10.1016/j.childyouth.2017.06.007
Bienkowski, M. S., Wendel, E. S., and Rinaman, L. (2013). Organization of multisynaptic circuits within and between the medial and the central extended amygdala. J. Comp. Neurol. 521, 3406–3431. doi: 10.1002/cne.23356
Card, J. P., and Sved, A. F. (2011). “Central autonomic pathways,” in Central Regulation of Autonomic Functions. 2nd Edn, eds I. J. Llewellyn-Smith and A. J. Verberne (New York, NY: Oxford University Press, Inc), 3–22. doi: 10.1093/acprof:oso/9780195306637.003.0001
Carpenter, L. L., Carvalho, J. P., Tyrka, A. R., Wier, L. M., Mello, A. F., Mello, M. F., et al. (2007). Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biol. Psychiatry 62, 1080–1087. doi: 10.1016/j.biopsych.2007.05.002
Carpenter, L., Shattuck, T., Tyrka, A., Geracioti, T., and Price, L. (2011). Effect of childhood physical abuse on cortisol stress response. Psychopharmacology 214, 367–375. doi: 10.1007/s00213-010-2007-4
Champagne, F. A., Francis, D. D., Mar, A., and Meaney, M. J. (2003). Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol. Behav. 79, 359–371. doi: 10.1016/s0031-9384(03)00149-5
Chen, E., Cohen, S., and Miller, G. E. (2009). How low socioeconomic status affects 2-year hormonal trajectories in children. Psychol. Sci. 21, 31–37. doi: 10.1177/0956797609355566
Chen, E., Langer, D. A., Raphaelson, Y. E., and Matthews, K. A. (2004). Socioeconomic status and health in adolescents: the role of stress interpretations. Child Dev. 75, 1039–1052. doi: 10.1111/j.1467-8624.2004.00724.x
Cheng, T. W., Mills, K. L., Dominguez, O. M., Zeithamova, D., Perrone, A., Sturgeon, D., et al. (2021). Characterizing the impact of adversity, abuse, and neglect on adolescent amygdala resting-state functional connectivity. Dev. Cogn. Neurosci. 47:100894. doi: 10.1016/j.dcn.2020.100894
Choi, D. C., Furay, A. R., Evanson, N. K., Ostrander, M. M., Ulrich-Lai, Y. M., and Herman, J. P. (2007). Bed nucleus of the stria terminalis subregions differentially regulate hypothalamic-pituitary-adrenal axis activity: implications for the integration of limbic inputs. J. Neurosci. 27, 2025–2034. doi: 10.1523/JNEUROSCI.4301-06.2007
Choi, J., Jeong, B., Polcari, A., Rohan, M. L., and Teicher, M. H. (2012). Reduced fractional anisotropy in the visual limbic pathway of young adults witnessing domestic violence in childhood. NeuroImage 59, 1071–1079. doi: 10.1016/j.neuroimage.2011.09.033
Clauss, J. (2019). Extending the neurocircuitry of behavioural inhibition: a role for the bed nucleus of the stria terminalis in risk for anxiety disorders. Gen. Psychiatry 32:e100137. doi: 10.1136/gpsych-2019-100137
Clauss, J. A., Avery, S. N., Benningfield, M. M., and Blackford, J. U. (2019). Social anxiety is associated with BNST response to unpredictability. Depress. Anxiety 36, 666–675. doi: 10.1002/da.22891
Cohen, S., Doyle, W. J., and Baum, A. (2006). Socioeconomic status is associated with stress hormones. Psychosom. Med. 68, 414–420. doi: 10.1097/01.psy.0000221236.37158.b9
Cohen, S., Kamarck, T., and Mermelstein, R. (1983). A global measure of perceived stress. J. Health Soc. Behav. 24, 385–396. doi: 10.2307/2136404
Cohen, S., Tyrrell, D. A. J., and Smith, A. P. (1991). Psychological stress and susceptibility to the common cold. N. Engl. J. Med. 325, 606–612. doi: 10.1056/NEJM199108293250903
Connolly, C. G., Wu, J., Ho, T. C., Hoeft, F., Wolkowitz, O., Eisendrath, S., et al. (2013). Resting-state functional connectivity of subgenual anterior cingulate cortex in depressed adolescents. Biol. Psychiatry 74, 898–907. doi: 10.1016/j.biopsych.2013.05.036
Crestani, C. C., Alves, F. H. F., Gomes, F. V., Resstel, L. B. M., Correa, F. M. A., and Herman, J. P. (2013). Mechanisms in the bed nucleus of the stria terminalis involved in control of autonomic and neuroendocrine functions: a review. Curr. Neuropharmacol. 11, 141–159. doi: 10.2174/1570159X11311020002
Cuartas, J., McCoy, D. C., Rey-Guerra, C., Britto, P. R., Beatriz, E., and Salhi, C. (2019). Early childhood exposure to non-violent discipline and physical and psychological aggression in low- and middle-income countries: national, regional, and global prevalence estimates. Child Abuse Negl. 92, 93–105. doi: 10.1016/j.chiabu.2019.03.021
Danese, A., Moffitt, T. E., Harrington, H. L., Milne, B. J., Polanczyk, G., Pariante, C. M., et al. (2009). Adverse childhood experiences and adult risk factors for age-related disease: depression, inflammation, and clustering of metabolic risk markers. Arch. Pediatr. Adolesc. Med. 163:1135. doi: 10.1001/archpediatrics.2009.214
De Olmos, J. S., and Ingram, W. (1972). The projection field of the stria terminalis in the rat brain. An experimental study. J. Comp. Neurol. 146, 303–333. doi: 10.1002/cne.901460303
deCampo, D. M., and Fudge, J. L. (2013). Amygdala projections to the lateral bed nucleus of the stria terminalis in the macaque: comparison with ventral striatal afferents. J. Comp. Neurol. 521, 3191–3216. doi: 10.1002/cne.23340
Dong, H.-W., and Swanson, L. W. (2004). Organization of axonal projections from the anterolateral area of the bed nuclei of the stria terminalis. J. Comp. Neurol. 468, 277–298. doi: 10.1002/cne.10949
Dong, H.-W., Petrovich, G. D., Watts, A. G., and Swanson, L. W. (2001b). Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. J. Comp. Neurol. 436, 430–455. doi: 10.1002/cne.1079
Dong, H.-W., Petrovich, G. D., and Swanson, L. W. (2001a). Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res. Rev. 38, 192–246. doi: 10.1016/s0165-0173(01)00079-0
Doom, J. R., Cicchetti, D., and Rogosch, F. A. (2014). Longitudinal patterns of cortisol regulation differ in maltreated and nonmaltreated children. J. Am. Acad. Child Adolesc. Psychiatry 53, 1206–1215. doi: 10.1016/j.jaac.2014.08.006
Drevets, W. C., Savitz, J., and Trimble, M. (2008b). The subgenual anterior cingulate cortex in mood disorders. CNS Spectr. 13:663. doi: 10.1017/s1092852900013754
Drevets, W. C., Price, J. L., and Furey, M. L. (2008a). Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct. Funct. 213, 93–118. doi: 10.1007/s00429-008-0189-x
Drobisz, D., and Damborská, A. (2018). Deep brain stimulation targets for treating depression. Behav Brain Res. 359, 266–273. doi: 10.1016/j.bbr.2018.11.004
Eickhoff, S. B., Stephan, K. E., Mohlberg, H., Grefkes, C., Fink, G. R., Amunts, K., et al. (2005). A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage 25, 1325–1335. doi: 10.1016/j.neuroimage.2004.12.034
Eluvathingal, T. J., Chugani, H. T., Behen, M. E., Juhász, C., Muzik, O., Maqbool, M., et al. (2006). Abnormal brain connectivity in children after early severe socioemotional deprivation: a diffusion tensor imaging study. Pediatrics 117, 2093–2100. doi: 10.1542/peds.2005-1727
Fadel, E., Boeker, H., Gaertner, M., Richter, A., Kleim, B., Seifritz, E., et al. (2021). Differential alterations in resting state functional connectivity associated with depressive symptoms and early life adversity. Brain Sci. 11:591. doi: 10.3390/brainsci11050591
Fahrmeir, L., Kneib, T., Lang, S., and Marx, B. (2013). “Regression models,” in Regression: Models, Methods and Applications, eds L. Fahrmeir, T. Kneib, S. Lang, and B. Marx (Berlin: Springer Berlin Heidelberg), 21–72.
Fani, N., Bradley-Davino, B., Ressler, K. J., and McClure-Tone, E. B. (2010). Attention bias in adult survivors of childhood maltreatment with and without posttraumatic stress disorder. Cogn. Ther. Res. 35, 57–67.
Fendt, M., Siegl, S., and Steiniger-Brach, B. (2005). Noradrenaline transmission within the ventral bed nucleus of the stria terminalis is critical for fear behavior induced by trimethylthiazoline, a component of fox odor. J. Neurosci. 25, 5998–6004. doi: 10.1523/JNEUROSCI.1028-05.2005
Fitzgerald, P. B., Segrave, R., Richardson, K. E., Knox, L. A., Herring, S., Daskalakis, Z. J., et al. (2018). A pilot study of bed nucleus of the stria terminalis deep brain stimulation in treatment-resistant depression. Brain Stimul. 11, 921–928. doi: 10.1016/j.brs.2018.04.013
Flook, E. A., Feola, B., Benningfield, M. M., Silveri, M. M., Winder, D. G., and Blackford, J. U. (2021). Alterations in connectivity of the bed nucleus of the stria terminalis during early abstinence in individuals with alcohol use disorder. Alcohol. Clin. Exp. Res. 45, 1028–1038. doi: 10.1111/acer.14596
Floyd, N. S., Price, J. L., Ferry, A. T., Keay, K. A., and Bandler, R. (2001). Orbitomedial prefrontal cortical projections to hypothalamus in the rat. J. Comp. Neurol. 432, 307–328. doi: 10.1002/cne.1105
Folloni, D., Sallet, J., Khrapitchev, A. A., Sibson, N., Verhagen, L., and Mars, R. B. (2019). Dichotomous organization of amygdala/temporal-prefrontal bundles in both humans and monkeys. eLife 8:e47175. doi: 10.7554/eLife.47175
Freedman, L. J., Insel, T. R., and Smith, Y. (2000). Subcortical projections of area 25 (subgenual cortex) of the macaque monkey. J. Comp. Neurol. 421, 172–188. doi: 10.1002/(sici)1096-9861(20000529)421:2<172::aid-cne4>3.0.co;2-8
Fritz, M. S., and MacKinnon, D. P. (2007). Required sample size to detect the mediated effect. Psychol. Sci. 18, 233–239. doi: 10.1111/j.1467-9280.2007.01882.x
Gotlib, I. H., Sivers, H., Gabrieli, J. D. E., Whitfield-Gabrieli, S., Goldin, P., Minor, K. L., et al. (2005). Subgenual anterior cingulate activation to valenced emotional stimuli in major depression. Neuroreport 16:1731. doi: 10.1097/01.wnr.0000183901.70030.82
Greicius, M. D., Flores, B. H., Menon, V., Glover, G. H., Solvason, H. B., Kenna, H., et al. (2007). Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol. Psychiatry 62, 429–437. doi: 10.1016/j.biopsych.2006.09.020
Gunnar, M. R., Frenn, K., Wewerka, S. S., and Van Ryzin, M. J. (2009). Moderate versus severe early life stress: associations with stress reactivity and regulation in 10–12-year-old children. Psychoneuroendocrinology 34:62. doi: 10.1016/j.psyneuen.2008.08.013
Gutman, D. A., Holtzheimer, P. E., Behrens, T. E. J., Johansen-Berg, H., and Mayberg, H. S. A. (2009). Tractography analysis of two deep brain stimulation white matter targets for depression. Biol Psychiatry 65, 276–282. doi: 10.1016/j.biopsych.2008.09.021
Hackman, D. A., Betancourt, L. M., Brodsky, N. L., Hurt, H., and Farah, M. J. (2012). Neighborhood disadvantage and adolescent stress reactivity. Front. Hum. Neurosci. 6:277. doi: 10.3389/fnhum.2012.00277
Hakamata, Y., Mizukami, S., Izawa, S., Moriguchi, Y., Hori, H., Kim, Y., et al. (2020). Basolateral amygdala connectivity with subgenual anterior cingulate cortex represents enhanced fear-related memory encoding in anxious humans. Biol. Psychiatry 5, 301–310. doi: 10.1016/j.bpsc.2019.11.008
Hanson, J. L., Adluru, N., Chung, M. K., Alexander, A. L., Davidson, R. J., and Pollak, S. D. (2013). Early neglect is associated with alterations in white matter integrity and cognitive functioning. Child Dev. 84, 1566–1578. doi: 10.1111/cdev.12069
Hanson, J. L., Knodt, A. R., Brigidi, B. D., and Hariri, A. R. (2015). Lower structural integrity of the uncinate fasciculus is associated with a history of child maltreatment and future psychological vulnerability to stress. Dev. Psychopathol. 27, 1611–1619. doi: 10.1017/S0954579415000978
Heilbronner, S. R., and Haber, S. N. (2014). Frontal cortical and subcortical projections provide a basis for segmenting the cingulum bundle: implications for neuroimaging and psychiatric disorders. J. Neurosci. 34, 10041–10054. doi: 10.1523/JNEUROSCI.5459-13.2014
Heim, C., Newport, D. J., Bonsall, R., Miller, A. H., and Nemeroff, C. B. (2001). Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. Am. J. Psychiatry 158, 575–581. doi: 10.1176/appi.ajp.158.4.575
Heim, C., Newport, D. J., Heit, S., Graham, Y. P., Wilcox, M., Bonsall, R., et al. (2000). Pituitary-adrenal and autonomic responses to stress in woman after sexual and physical abuse in childhood. JAMA 284, 592–597. doi: 10.1001/jama.284.5.592
Herman, J. P., Cullinan, W. E., Ziegler, D. R., and Tasker, J. G. (2002). Role of the paraventricular nucleus microenvironment in stress integration. Eur. J. Neurosci. 16, 381–385. doi: 10.1046/j.1460-9568.2002.02133.x
Herringa, R. J., Birn, R. M., and Ruttle, P. L. (2013). Childhood maltreatment is associated with altered fear circuitry and increased internalizing symptoms by late adolescence. Proc. Natl. Acad. Sci. U.S.A. 110, 19119–19124. doi: 10.1073/pnas.1310766110
Herrmann, M. J., Boehme, S., Becker, M. P. I., Tupak, S. V., Guhn, A., Schmidt, B., et al. (2016). Phasic and sustained brain responses in the amygdala and the bed nucleus of the stria terminalis during threat anticipation. Hum. Brain Mapp. 37, 1091–1102.
Hillis, S., Mercy, J., Amobi, A., and Kress, H. (2016). Global prevalence of past-year violence against children: a systematic review and minimum estimates. Pediatrics 137:e20154079. doi: 10.1542/peds.2015-4079
Ho, T. C., and King, L. S. (2021). Mechanisms of neuroplasticity linking early adversity to depression: developmental considerations. Transl. Psychiatry 11:517. doi: 10.1038/s41398-021-01639-6
Ho, T. C., Yang, G., Wu, J., Cassey, P., Brown, S. D., Hoang, N., et al. (2014). Functional connectivity of negative emotional processing in adolescent depression. J. Affect. Disord. 155, 65–74. doi: 10.1016/j.jad.2013.10.025
Hoffmann, F., Viding, E., Puetz, V. B., Gerin, M. I., Sethi, A., Rankin, G., et al. (2018). Evidence for depressogenic spontaneous thoughts and altered resting-state connectivity in adolescents with a maltreatment history. J. Am. Acad. Child Adolesc. Psychiatry 57, 687.e4–695.e4. doi: 10.1016/j.jaac.2018.05.020
Huang, D., Liu, Z., Cao, H., Yang, J., Wu, Z., and Long, Y. (2021). Childhood trauma is linked to decreased temporal stability of functional brain networks in young adults. J. Affect. Disord. 290, 23–30. doi: 10.1016/j.jad.2021.04.061
Iadipaolo, A. S., Marusak, H. A., Paulisin, S. M., Sala-Hamrick, K., Crespo, L. M., Elrahal, F., et al. (2018). Distinct neural correlates of trait resilience within core neurocognitive networks in at-risk children and adolescents. NeuroImage 20, 24–34. doi: 10.1016/j.nicl.2018.06.026
Insana, S. P., Kolko, D. J., and Germain, A. (2012). Early-life trauma is associated with rapid eye movement sleep fragmentation among military veterans. Biol. Psychol. 89, 570–579. doi: 10.1016/j.biopsycho.2012.01.001
Ioannidis, K., Askelund, A. D., Kievit, R. A., and van Harmelen, A-L (2020). The complex neurobiology of resilient functioning after childhood maltreatment. BMC Med. 18:32.
Jacobs, R. H., Barba, A., Gowins, J. R., Klumpp, H., Jenkins, L. M., Mickey, B. J., et al. (2016). Decoupling of the amygdala to other salience network regions in adolescent-onset recurrent major depressive disorder. Psychol. Med. 46, 1055–1067. doi: 10.1017/S0033291715002615
Jacobs, R. H., Jenkins, L. M., Gabriel, L. B., Barba, A., Ryan, K. A., Weisenbach, S. L., et al. (2014). Increased coupling of intrinsic networks in remitted depressed youth predicts rumination and cognitive control. PLoS One 9:e104366. doi: 10.1371/journal.pone.0104366
Johansen-Berg, H., Gutman, D. A., Behrens, T. E. J., Matthews, P. M., Rushworth, M. F. S., Katz, E., et al. (2008). Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment-resistant depression. Cereb. Cortex 18, 1374–1383. doi: 10.1093/cercor/bhm167
Johnson, S. B., Emmons, E. B., Anderson, R. M., Glanz, R. M., Romig-Martin, S. A., Narayanan, N. S., et al. (2016). A basal forebrain site coordinates the modulation of endocrine and behavioral stress responses via divergent neural pathways. J. Neurosci. 36, 8687–8699. doi: 10.1523/JNEUROSCI.1185-16.2016
Kim, Y., Sakata, H., Nejime, M., Konoike, N., Miyachi, S., and Nakamura, K. (2018). Afferent connections of the dorsal, perigenual, and subgenual anterior cingulate cortices of the monkey: amygdalar inputs and intrinsic connections. Neurosci. Lett. 681, 93–99. doi: 10.1016/j.neulet.2018.05.028
Kleckner, I. R., Zhang, J., Touroutoglou, A., Chanes, L., Xia, C., Simmons, W. K., et al. (2017). Evidence for a large-scale brain system supporting allostasis and interoception in humans. Nat. Hum. Behav. 1:0069. doi: 10.1038/s41562-017-0069
Koopman, C., Carrion, V., Butler, L. D., Sudhakar, S., Palmer, L., and Steiner, H. (2004). Relationships of dissociation and childhood abuse and neglect with heart rate in delinquent adolescents. J. Trauma. Stress 17, 47–54. doi: 10.1023/B:JOTS.0000014676.83722.35
Kumar, A., Behen, M. E., Singsoonsud, P., Veenstra, A. L., Wolfe-Christensen, C., Helder, E., et al. (2013). Microstructural abnormalities in language and limbic pathways in orphanage-reared children: a diffusion tensor imaging study. J. Child Neurol. 29, 318–325. doi: 10.1177/0883073812474098
Lawson, G. M., Camins, J. S., Wisse, L., Wu, J., Duda, J. T., Cook, P. A., et al. (2017). Childhood socioeconomic status and childhood maltreatment: distinct associations with brain structure. PLoS One 12:e0175690. doi: 10.1371/journal.pone.0175690
Lebow, M. A., and Chen, A. (2016). Overshadowed by the amygdala: the bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Mol. Psychiatry 21, 450–463.
Lê-Scherban, F., Brenner, A. B., Hicken, M. T., Needham, B. L., Seeman, T., Sloan, R. P., et al. (2018). Child and adult socioeconomic status and the cortisol response to acute stress: evidence from the multi-ethnic study of atherosclerosis. Psychosom. Med. 80, 184–192. doi: 10.1097/PSY.0000000000000543
Limbachia, C., Morrow, K., Khibovska, A., Meyer, C., Padmala, S., and Pessoa, L. (2020). Controllability over stressor decreases responses in key threat-related brain areas. Commun. Biol. 4:42. doi: 10.1038/s42003-020-01537-5
Lovallo, W. R., Farag, N. H., Sorocco, K. H., Cohoon, A. J., and Vincent, A. S. (2011). Lifetime adversity leads to blunted stress axis reactivity: studies from the oklahoma family health patterns project. Biol. Psychiatry 71, 344–349. doi: 10.1016/j.biopsych.2011.10.018
Luiten, P. G. M., Ter Horst, G. J., Karst, H., and Steffens, A. B. (1985). The course of paraventricular hypothalamic efferents to autonomic structures in medulla and spinal cord. Brain Res. 329, 374–378. doi: 10.1016/0006-8993(85)90554-2
Lupien, S. J., King, S., Meaney, M. J., and McEwen, B. S. (2001). Can poverty get under your skin? Basal cortisol levels and cognitive function in children from low and high socioeconomic status. Dev. Psychopathol. 13, 653–676. doi: 10.1017/s0954579401003133
M’jid, N. M. (2020). Hidden scars: the impact of violence and the COVID-19 pandemic on children’s mental health. Child Adolesc. Psychiatry Ment. Health 14:33. doi: 10.1186/s13034-020-00340-8
Mai, J. K., Paxinos, G., and Voss, T. (2008). Atlas of the Human Brain, 3rd Edn. New York, NY: Academic Press.
Maita, I., Bazer, A., Blackford, J. U., and Samuels, B. A. (2021). Chapter 27 functional anatomy of the bed nucleus of the stria terminalis–hypothalamus neural circuitry: implications for valence surveillance, addiction, feeding, and social behaviors. Handb. Clin. Neurol. 179, 403–418. doi: 10.1016/B978-0-12-819975-6.00026-1
Marusak, H. A., Etkin, A., and Thomason, M. E. (2015). Disrupted insula-based neural circuit organization and conflict interference in trauma-exposed youth. Neuroimage Clin. 8, 516–525.
Matthews, S., Simmons, A., Strigo, I., Gianaros, P., Yang, T., and Paulus, M. (2009). Inhibition-related activity in subgenual cingulate is associated with symptom severity in major depression. Psychiatry Res. 172, 1–6. doi: 10.1016/j.pscychresns.2008.08.006
Mayer, S. E., Surachman, A., Prather, A. A., Puterman, E., Delucchi, K. L., Irwin, M. R., et al. (2021). The long shadow of childhood trauma for depression in midlife: examining daily psychological stress processes as a persistent risk pathway. Psychol. Med. Online ahead of print doi: 10.1017/S0033291721000921
McCarthy-Jones, S., Oestreich, L. K. L., Lyall, A. E., Kikinis, Z., Newell, D. T., Savadjiev, P., et al. (2018). Childhood adversity associated with white matter alteration in the corpus callosum, corona radiata, and uncinate fasciculus of psychiatrically healthy adults. Brain Imaging Behav. 12, 449–458. doi: 10.1007/s11682-017-9703-1
McCrae, R. R., and Costa, P. T. Jr. (2007). Brief versions of the NEO-PI-3. J. Individ. Differ. 28:116.
McCrory, E. J., De Brito, S. A., Kelly, P. A., Bird, G., Sebastian, C. L., Mechelli, A., et al. (2013). Amygdala activation in maltreated children during pre-attentive emotional processing. Br. J. Psychiatry 202, 269–276. doi: 10.1192/bjp.bp.112.116624
McCrory, E. J., De Brito, S. A., Sebastian, C. L., Mechelli, A., Bird, G., Kelly, P. A., et al. (2011). Heightened neural reactivity to threat in child victims of family violence. Curr. Biol. 21, R947–R948. doi: 10.1016/j.cub.2011.10.015
McLaughlin, K. A., Sheridan, M. A., and Lambert, H. K. (2014). Childhood adversity and neural development: deprivation and threat as distinct dimensions of early experience. Neurosci. Biobehav. Rev. 47, 578–591. doi: 10.1016/j.neubiorev.2014.10.012
Menon, V. (2013). Developmental pathways to functional brain networks: emerging principles. Trends Cogn. Sci. 17, 627–640. doi: 10.1016/j.tics.2013.09.015
Merrick, M. T., Ford, D. C., Ports, K. A., and Guinn, A. S. (2018). Prevalence of adverse childhood experiences from the 2011-2014 behavioral risk factor surveillance system in 23 statesprevalence of adverse childhood experiences from the behavioral risk factor surveillance systemprevalence of adverse childhood experiences from the behavioral risk factor surveillance system. JAMA Pediatr. 172, 1038–1044. doi: 10.1001/jamapediatrics.2018.2537
Morris, G., Berk, M., Maes, M., Carvalho, A. F., and Puri, B. K. (2019). Socioeconomic deprivation, adverse childhood experiences and medical disorders in adulthood: mechanisms and associations. Mol. Neurobiol. 56, 5866–5890. doi: 10.1007/s12035-019-1498-1
Mosley, P. E., Windels, F., Morris, J., Coyne, T., Marsh, R., Giorni, A., et al. (2021). A randomised, double-blind, sham-controlled trial of deep brain stimulation of the bed nucleus of the stria terminalis for treatment-resistant obsessive-compulsive disorder. Transl. Psychiatry 11:190. doi: 10.1038/s41398-021-01307-9
Musgrove, D. R., Eberly, L. E., Klimes-Dougan, B., Basgoze, Z., Thomas, K. M., Mueller, B. A., et al. (2015). Impaired bottom-up effective connectivity between amygdala and subgenual anterior cingulate cortex in unmedicated adolescents with major depression: results from a dynamic causal modeling analysis. Brain Connect. 5, 608–619. doi: 10.1089/brain.2014.0312
Myers, E. A., Banihashemi, L., and Rinaman, L. (2005). The anxiogenic drug yohimbine activates central viscerosensory circuits in rats. J. Comp. Neurol. 492, 426–441. doi: 10.1002/cne.20727
Nakamura, T., Tomita, M., Horikawa, N., Ishibashi, M., Uematsu, K., Hiraki, T., et al. (2021). Functional connectivity between the amygdala and subgenual cingulate gyrus predicts the antidepressant effects of ketamine in patients with treatment-resistant depression. Neuropsychopharmacol. Rep. 41, 168–178. doi: 10.1002/npr2.12165
Nanni, V., Uher, R., and Danese, A. (2012). Childhood maltreatment predicts unfavorable course of illness and treatment outcome in depression: a meta-analysis. Am. J. Psychiatry 169, 141–151. doi: 10.1176/appi.ajp.2011.11020335
Nieuwenhuys, R., Voogd, J., and van Huijzen, C. (2008). The Human Central Nervous System: A Synopsis and Atlas, 4th Edn. Berlin: Springer.
Oler, J. A., Tromp, D. P. M., Fox, A. S., Kovner, R., Davidson, R. J., Alexander, A. L., et al. (2017). Connectivity between the central nucleus of the amygdala and the bed nucleus of the stria terminalis in the non-human primate: neuronal tract tracing and developmental neuroimaging studies. Brain Struct. Funct. 222, 21–39. doi: 10.1007/s00429-016-1198-9
Öngür, D., An, X., and Price, J. L. (1998). Prefrontal cortical projections to the hypothalamus in macaque monkeys. J. Comp. Neurol. 401, 480–505.
Park, A. T., Tooley, U. A., Leonard, J. A., Boroshok, A. L., McDermott, C. L., Tisdall, M. D., et al. (2021). Early childhood stress is associated with blunted development of ventral tegmental area functional connectivity. Dev. Cogn. Neurosci. 47:100909. doi: 10.1016/j.dcn.2020.100909
Peckins, M. K., Susman, E. J., Negriff, S., Noll, J., and Trickett, P. K. (2015). Cortisol profiles: a test for adaptive calibration of the stress response system in maltreated and nonmaltreated youth. Dev. Psychopathol. 27, 1461–1470.
Pereda, N., and Díaz-Faes, D. A. (2020). Family violence against children in the wake of COVID-19 pandemic: a review of current perspectives and risk factors. Child Adolesc. Psychiatry Ment. Health 14:40. doi: 10.1186/s13034-020-00347-1
Povysheva, N., Zheng, H., and Rinaman, L. (2021). Glucagon-like peptide 1 receptor-mediated stimulation of a GABAergic projection from the bed nucleus of the stria terminalis to the hypothalamic paraventricular nucleus. Neurobiol. Stress 15:100363. doi: 10.1016/j.ynstr.2021.100363
Price, R. B., Cummings, L., Gilchrist, D., Graur, S., Banihashemi, L., Kuo, S. S., et al. (2018). Towards personalized, brain-based behavioral intervention for transdiagnostic anxiety: transient neural responses to negative images predict outcomes following a targeted computer-based intervention. J. Consult. Clin. Psychol. 86, 1031–1045. doi: 10.1037/ccp0000309
Rabellino, D., Densmore, M., Harricharan, S., Jean, T., McKinnon, M. C., and Lanius, R. A. (2018). Resting-state functional connectivity of the bed nucleus of the stria terminalis in post-traumatic stress disorder and its dissociative subtype. Hum. Brain Mapp. 39, 1367–1379. doi: 10.1002/hbm.23925
Radley, J. J., and Johnson, S. B. (2018). Anteroventral bed nuclei of the stria terminalis neurocircuitry: towards an integration of HPA axis modulation with coping behaviors - Curt Richter Award Paper 2017. Psychoneuroendocrinology 89, 239–249. doi: 10.1016/j.psyneuen.2017.12.005
Radley, J. J., Gosselink, K. L., and Sawchenko, P. E. A. (2009). Discrete GABAergic relay mediates medial prefrontal cortical inhibition of the neuroendocrine stress response. J. Neurosci. 29, 7330–7340. doi: 10.1523/JNEUROSCI.5924-08.2009
Rafal, R. D., Koller, K., Bultitude, J. H., Mullins, P., Ward, R., Mitchell, A. S., et al. (2015). Connectivity between the superior colliculus and the amygdala in humans and macaque monkeys: virtual dissection with probabilistic DTI tractography. J. Neurophysiol. 114, 1947–1962. doi: 10.1152/jn.01016.2014
Rakesh, D., Kelly, C., Vijayakumar, N., Zalesky, A., Allen, N. B., and Whittle, S. (2021b). Unraveling the consequences of childhood maltreatment: deviations from typical functional neurodevelopment mediate the relationship between maltreatment history and depressive symptoms. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 6, 329–342. doi: 10.1016/j.bpsc.2020.09.016
Rakesh, D., Allen, N. B., and Whittle, S. (2021a). Longitudinal changes in within-salience network functional connectivity mediate the relationship between childhood abuse and neglect, and mental health during adolescence. Psychol. Med. Online ahead of print doi: 10.1017/S0033291721003135
Reiss, F. (2013). Socioeconomic inequalities and mental health problems in children and adolescents: a systematic review. Soc. Sci. Med. 90, 24–31. doi: 10.1016/j.socscimed.2013.04.026
Rinaman, L., Banihashemi, L., and Koehnle, T. J. (2011). Early life experience shapes the functional organization of stress-responsive visceral circuits. Physiol. Behav. 104, 632–640. doi: 10.1016/j.physbeh.2011.04.008
Ruiz-Rizzo, A. L., Beissner, F., Finke, K., Müller, H. J., Zimmer, C., Pasquini, L., et al. (2020). Human subsystems of medial temporal lobes extend locally to amygdala nuclei and globally to an allostatic-interoceptive system. NeuroImage 207:116404. doi: 10.1016/j.neuroimage.2019.116404
Russotti, J., Warmingham, J. M., Duprey, E. B., Handley, E. D., Manly, J. T., Rogosch, F. A., et al. (2021). Child maltreatment and the development of psychopathology: the role of developmental timing and chronicity. Child Abuse Negl. 120:105215. doi: 10.1016/j.chiabu.2021.105215
Sara, G., and Lappin, J. (2017). Comment childhood trauma: psychiatry’s greatest public health challenge? Lancet Public Health 2, e300–e301. doi: 10.1016/S2468-2667(17)30104-4
Saygin, Z. M., Kliemann, D., Iglesias, J. E., Kouwe, A., Boyd, E., Reuter, M., et al. (2017). High-resolution magnetic resonance imaging reveals nuclei of the human amygdala: manual segmentation to automatic atlas. NeuroImage 155, 370–382. doi: 10.1016/j.neuroimage.2017.04.046
Schaan, V. K., Schulz, A., Rubel, J. A., Bernstein, M., Domes, G., Schächinger, H., et al. (2019). Childhood trauma affects stress-related interoceptive accuracy. Front. Psychiatry 10:750. doi: 10.3389/fpsyt.2019.00750
Scharnowski, F., Nicholson, A. A., Pichon, S., Rosa, M. J., Rey, G., Eickhoff, S. B., et al. (2020). The role of the subgenual anterior cingulate cortex in dorsomedial prefrontal–amygdala neural circuitry during positive-social emotion regulation. Hum. Brain Mapp. 41, 3100–3118. doi: 10.1002/hbm.25001
Schweimer, J., Fendt, M., and Schnitzler, H.-U. (2005). Effects of clonidine injections into the bed nucleus of the stria terminalis on fear and anxiety behavior in rats. Eur. J. Pharmacol. 507, 117–124. doi: 10.1016/j.ejphar.2004.11.044
Seeley, W. W., Menon, V., Schatzberg, A. F., Keller, J., Glover, G. H., Kenna, H., et al. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 27:2349. doi: 10.1523/JNEUROSCI.5587-06.2007
Seitzman, B. A., Snyder, A. Z., Leuthardt, E. C., and Shimony, J. S. (2019). The state of resting state networks. Top. Magn. Reson. Imaging 28, 189–196. doi: 10.1097/RMR.0000000000000214
Sennesh, E., Theriault, J., Brooks, D., Meent, J., Barrett, L. F., and Quigley, K. S. (2022). Interoception as modeling, allostasis as control. Biol. Psychol. 167:108242. doi: 10.1016/j.biopsycho.2021.108242
Shackman, A. J., Salomons, T. V., Slagter, H. A., Fox, A. S., Winter, J. J., and Davidson, R. J. (2011). The integration of negative affect. pain, and cognitive control in the cingulate cortex. Nat. Rev. Neurosci. 12, 154–167. doi: 10.1038/nrn2994
Sharma, K. K., Kelly, E. A., Pfeifer, C. W., and Fudge, J. L. (2019). Translating fear circuitry: amygdala projections to subgenual and perigenual anterior cingulate in the macaque. Cereb. Cortex 30, 550–562. doi: 10.1093/cercor/bhz106
Sheridan, M. A., and McLaughlin, K. A. (2014). Dimensions of early experience and neural development: deprivation and threat. Trends Cogn. Sci. 18, 580–585.
Silveira, S., Boney, S., Tapert, S. F., and Mishra, J. (2021). Developing functional network connectivity of the dorsal anterior cingulate cortex mediates externalizing psychopathology in adolescents with child neglect. Dev. Cogn. Neurosci. 49:100962. doi: 10.1016/j.dcn.2021.100962
Somerville, L. H., Whalen, P. J., and Kelley, W. M. (2010). Human bed nucleus of the stria terminalis indexes hypervigilant threat monitoring. Biol. Psychiatry 68, 416–424. doi: 10.1016/j.biopsych.2010.04.002
Souza-Queiroz, J., Boisgontier, J., Etain, B., Poupon, C., Duclap, D., d’Albis, M.-A., et al. (2016). Childhood trauma and the limbic network_ a multimodal MRI study in patients with bipolar disorder and controls. J. Affect. Disord. 200, 159–164. doi: 10.1016/j.jad.2016.04.038
Spielberger, C. D., Gorsuch, R. L., Lushene, R., Vagg, P. R., and Jacobs, G. A. (1983). Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press.
Stamm, B. H. (1996). Measurement of Stress, Trauma, and Adaptation. Baltimore, MD: The Sidran Press.
Taylor, S. F., Ho, S. S., Abagis, T., Angstadt, M., Maixner, D. F., Welsh, R. C., et al. (2018). Changes in brain connectivity during a sham-controlled, transcranial magnetic stimulation trial for depression. J. Affect. Disord. 232, 143–151. doi: 10.1016/j.jad.2018.02.019
Teicher, M. H., and Samson, J. A. (2016). Annual research review: enduring neurobiological effects of childhood abuse and neglect. J. Child Psychol. Psychiatry 57, 241–266. doi: 10.1111/jcpp.12507
Teicher, M. H., Ohashi, K., and Khan, A. (2020). Additional insights into the relationship between brain network architecture and susceptibility and resilience to the psychiatric sequelae of childhood maltreatment. Advers. Resil. Sci. 1, 49–64. doi: 10.1007/s42844-020-00002-w
Teicher, M. H., Samson, J. A., Anderson, C. M., and Ohashi, K. (2016). The effects of childhood maltreatment on brain structure, function and connectivity. Nat. Rev. Neurosci. 17, 652–666. doi: 10.1038/nrn.2016.111
Thomason, M. E., Marusak, H. A., Tocco, M. A., Vila, A. M., McGarragle, O., and Rosenberg, D. R. (2015). Altered amygdala connectivity in urban youth exposed to trauma. Soc. Cogn. Affect. Neurosci. 10, 1460–1468. doi: 10.1093/scan/nsv030
Tillman, R. M., Stockbridge, M. D., Nacewicz, B. M., Torrisi, S., Fox, A. S., Smith, J. F., et al. (2018). Intrinsic functional connectivity of the central extended amygdala. Hum. Brain Mapp. 39, 1291–1312. doi: 10.1002/hbm.23917
Tottenham, N., and Sheridan, M. (2010). A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Front. Hum. Neurosci. 3:68. doi: 10.3389/neuro.09.068.2009
Ursache, A., Merz, E. C., Melvin, S., Meyer, J., and Noble, K. G. (2017). Socioeconomic status, hair cortisol and internalizing symptoms in parents and children. Psychoneuroendocrinology 78, 142–150. doi: 10.1016/j.psyneuen.2017.01.020
van Harmelen, A. L., van Tol, M. J., Demenescu, L. R., van der Wee, N. J. A., Veltman, D. J., Aleman, A., et al. (2012). Enhanced amygdala reactivity to emotional faces in adults reporting childhood emotional maltreatment. Soc. Cogn. Affect. Neurosci. 8, 362–369. doi: 10.1093/scan/nss007
van Harmelen, A.-L., van Tol, M.-J., van der Wee, N. J. A., Veltman, D. J., Aleman, A., Spinhoven, P., et al. (2010). Reduced medial prefrontal cortex volume in adults reporting childhood emotional maltreatment. Biol. Psychiatry 68, 832–838. doi: 10.1016/j.biopsych.2010.06.011
Vergani, F., Martino, J., Morris, C., Attems, J., Ashkan, K., and Dell’Acqua, F. (2016). Anatomic connections of the subgenual cingulate region. Neurosurgery 79, 465–472. doi: 10.1227/NEU.0000000000001315
Vertes, R. P. (2004). Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse 51, 32–58. doi: 10.1002/syn.10279
Walsh, N. D., Dalgleish, T., Lombardo, M. V., Dunn, V. J., Van Harmelen, A.-L., Ban, M., et al. (2014). General and specific effects of early-life psychosocial adversities on adolescent grey matter volume. NeuroImage 4, 308–318. doi: 10.1016/j.nicl.2014.01.001
Webb, S., Janus, M., Duku, E., Raos, R., Brownell, M., Forer, B., et al. (2017). Neighbourhood socioeconomic status indices and early childhood development. SSM Popul. Health. 3, 48–56. doi: 10.1016/j.ssmph.2016.11.006
Weiss, A., Carlo, D. T. D., Russo, P. D., Weiss, F., Castagna, M., Cosottini, M., et al. (2021). Microsurgical anatomy of the amygdaloid body and its connections. Brain Struct. Funct. 226, 861–874. doi: 10.1007/s00429-020-02214-3
Werff, S. J., Pannekoek, J. N., Veer, I. M., van Tol, MJ, Aleman, A., Veltman, D. J., et al. (2012). Resting-state functional connectivity in adults with childhood emotional maltreatment. Psychol. Med. 43:1825. doi: 10.1017/S0033291712002942
Wilkins, K. C., Lang, A. J., and Norman, S. B. (2011). Synthesis of the psychometric properties of the PTSD checklist (PCL) military, civilian, and specific versions. Depress. Anxiety 28, 596–606. doi: 10.1002/da.20837
Winkleby, M. A., Jatulis, D. E., Frank, E., and Fortmann, S. P. (1992). Socioeconomic status and health: how education, income, and occupation contribute to risk factors for cardiovascular disease. Am. J. Public Health 82, 816–820. doi: 10.2105/ajph.82.6.816
Wolff, J., Schindler, S., Lucas, C., Binninger, A.-S., Weinrich, L., Schreiber, J., et al. (2018). A semi-automated algorithm for hypothalamus volumetry in 3 tesla magnetic resonance images. Psychiatry Res. 277, 45–51. doi: 10.1016/j.pscychresns.2018.04.007
Wu, M., Mennin, D. S., Ly, M., Karim, H. T., Banihashemi, L., Tudorascu, D. L., et al. (2019). When worry may be good for you: worry severity and limbic-prefrontal functional connectivity in late-life generalized anxiety disorder. J. Affect. Disord. 257, 650–657. doi: 10.1016/j.jad.2019.07.022
Zheng, H., Reiner, D. J., Hayes, M. R., and Rinaman, L. (2019). Chronic suppression of glucagon-like peptide-1 receptor (GLP1R) mRNA translation in the rat bed nucleus of the stria terminalis reduces anxiety-like behavior and stress-induced hypophagia. but prolongs stress-induced elevation of plasma corticosterone. J. Neurosci. 39:2649. doi: 10.1523/JNEUROSCI.2180-18.2019
Keywords: childhood trauma, subgenual anterior cingulate cortex, amygdala, bed nucleus of stria terminalis, extended amygdala, hypothalamus, affective disorders
Citation: Banihashemi L, Peng CW, Rangarajan A, Karim HT, Wallace ML, Sibbach BM, Singh J, Stinley MM, Germain A and Aizenstein HJ (2022) Childhood Threat Is Associated With Lower Resting-State Connectivity Within a Central Visceral Network. Front. Psychol. 13:805049. doi: 10.3389/fpsyg.2022.805049
Received: 29 October 2021; Accepted: 09 February 2022;
Published: 03 March 2022.
Edited by:
Annie Ginty, Baylor University, United StatesReviewed by:
Kaiping Burrows, Laureate Institute for Brain Research, United StatesYuta Katsumi, Massachusetts General Hospital and Harvard Medical School, United States
Copyright © 2022 Banihashemi, Peng, Rangarajan, Karim, Wallace, Sibbach, Singh, Stinley, Germain and Aizenstein. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Layla Banihashemi, layla.banihashemi@pitt.edu
 Layla Banihashemi
Layla Banihashemi Christine W. Peng1
Christine W. Peng1 Helmet T. Karim
Helmet T. Karim Brandon M. Sibbach
Brandon M. Sibbach Jaspreet Singh
Jaspreet Singh Mark M. Stinley
Mark M. Stinley Anne Germain
Anne Germain