- 1Compassionate Mind Research Group, School of Psychology, The University of Queensland, Brisbane, QLD, Australia
- 2The Queensland Brain Institute, The University of Queensland, Brisbane, QLD, Australia
- 3The Centre for Advanced Imaging, The University of Queensland, Brisbane, QLD, Australia
- 4Stanford University School of Medicine, California, CA, United States
Whilst research has shown how self-criticism may increase both neural and self-report markers of negative emotion, less well-known is how self-reassurance—a compassionately-motivated cognitive self-relating style—may regulate negative emotion. Using fMRI, we invited participants to engage in self-criticism and self-reassurance toward written descriptions of negative life events (mistakes, setbacks, failures). Our results identify that neural markers of negative emotion and self-report markers of trial intensity during fMRI are down-regulated under conditions of self-reassurance, relative to self-criticism. Future work to control for autobiographical memory during this fMRI task is needed, as are controls for how well participants can engage in both thinking styles, to explore how memory/task engagement can contribute to self-reassurance and self-criticism. Engagement in self-reassurance can reduce the “sting” of negative life-events, both neural and self-report, which holds important implications for therapy.
Introduction
Adverse life events are inescapable, be it a disruption in a career, dissolution of a relationship, or even a world-wide pandemic. These factors are known to take a toll on both physical and mental health outcomes (Solís et al., 2015) which can increase the likelihood of mortality (Puterman et al., 2020). These disappointments (e.g., making mistakes), losses (e.g., of hoped love) and fears (e.g., of rejection) are all triggers to self-criticism (Halamová et al., 2018; Kim et al., 2020a,b,c). Indeed, self-criticism is a common self-relating style people use to cope, often resulting in an individual taking the frustration and anger out on themselves, which compounds the experience of pain psychologically and neurophysiologically (Kim et al., 2020a,b,c). Whilst research has shown how self-criticism may increase both self-report (Cox et al., 2000; Gilbert et al., 2004) and neural (Longe et al., 2010; Lutz et al., 2016) markers of negative emotion, less well-known is how self-reassurance—a compassionately-motivated cognitive self-relating style—may regulate how the brain responds toward negative life events.
Here we conducted an fMRI experiment which examined two distinct self-relating styles, self-criticism and self-reassurance (Petrocchi et al., 2019; Kim et al., 2020a,b,c), when participants imagined themselves responding to mistakes, setbacks or failures. Importantly, we designed our experiment to deliberately tease apart neural markers of negative emotion, first by manipulating an emotional—neutral contrast at the first level of fMRI analysis, and explored how this activation may differ across self-criticism and self-reassurance. Accordingly, we set out to investigate how the brain encodes negative emotions under differing (thinking) conditions of self-criticism vs. self-reassurance; specifically exploring how self-reassurance may regulate neural markers of negative emotion.
Methods
Whilst the program of research within the present paper has been reported on previously, this examined the (neuro)physiological correlates of a brief, two-week compassion training paradigm (Kim et al., 2020a,b,c). Here we focus on the novel whole brain markers of criticism and reassurance which have not been reported. As our fMRI method as reported in the previous paper is also the same imaging method used for the present paper, we have reproduced this section for clarity under a CC BY open access license.
Participants
Forty participants (Mean age = 22 years, SD = 0.49, 27 female) took part in the present study. A University's Ethics Sub-Committee approved the experimental protocol, and this project complies with the provisions contained in the National Statement of Ethical Conduct in Human Research and complies with the regulations governing experimentation on humans. Participants provided informed and voluntary, written and/or electronic consent.
fMRI Stimuli
We created 60 written stimuli in total, consisting of a personal mistake, setback or failure. Thirty statements were of emotional valence whereas 30 were neutral (i.e., “I fail to keep up with my commitments in life,” and “I keep up with my commitments in life,” respectively). Our neutral stimuli were created to describe a non-emotive, non-intense control to counterbalance the emotional stimuli set. For both emotional and neutral sets we assessed two metrics, valence (1–5, where 1 = Very Unpleasant and 5 = Very Pleasant) and intensity (1–5, where 1 = Not Intense and 5 = Very Intense). Our emotional statements (n = 30) were rated with a mean of 1.89 for unpleasantness (SD = 0.25) and a mean of 3.54 for intensity (SD = 0.41), with neutral statements (n = 30) exhibiting a mean of 3.80 for unpleasantness (SD = 0.33) and a mean of 2.34 for intensity (SD = 0.44).
fMRI Design
Within the scanner we examined participants' neural responses to the validated (emotional and neutral) written stimuli when engaged in self-criticism and self-reassurance (Figure 1). Participants were given one practice block with 16 trials (eight emotional and eight neutral) repeated for self-criticism and self-reassurance, and made button press ratings on how intense their self-critical or self-reassuring thoughts were. This same task instruction was used during the fMRI experiment. Within the scanner, participants made button-press ratings on an MR-compatible button box (that ranged from 1–4, where 1 = Not Intense, and 4 = Very Intense). A typical trial consisted of stimuli presented for a 6 s duration, followed by a rating of intensity for a 3 s duration, and an inter-trial-interval of 0.5 s. The first order of instruction for a particular block, that is, self-reassurance verses self-criticism, was counterbalanced for a total of 8 blocks. As our focal contrast, we manipulated the emotionality of the statements within scan runs (“emotional” vs. “neutral”), in a counterbalanced order across participants. Thirty statements were quasi-randomized across participants and presented for a total of 30 trials per fMRI run (~6.5 min total duration) over a total of 8 repeated fMRI runs.
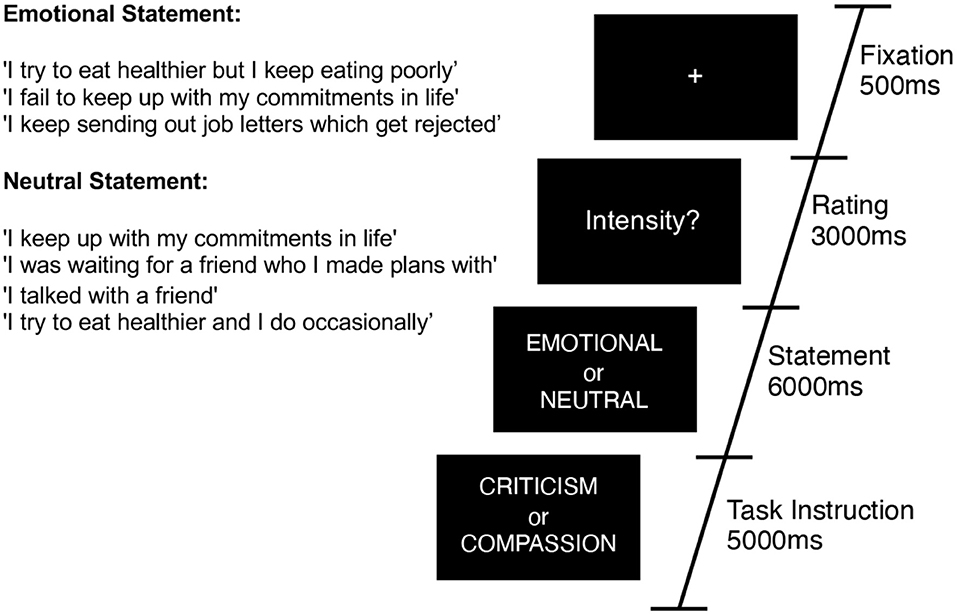
Figure 1. Task diagram for a typical trial. Participants were presented with 30 alternating trials of emotional or neutral statements which describe a mistake, setback or failure. Across scan runs of 6 min each, participants were asked to engage with these statements from two different perspectives—four blocks of self-criticism, and four blocks of self-reassurance (order counterbalanced across participants). Example statements are presented inset.
fMRI Acquisition and Pre-processing
We collected our fMRI data on a Siemens Magnetom Prisma Fit 3 Tesla MRI scanner utilizing a 64-channel head-coil. A gradient-echo, echo-planar imaging sequence was used to acquire functional images, with the following sequence parameters: 60 horizontal slices (2 x 2-mm in-plane voxel resolution and 2-mm slice thickness plus 10% gap), repetition time (TR) 1,000 ms; echo time (TE) 30 ms. Eight identical fMRI runs of 292 images (6 min each) were acquired. A 3D high-resolution, unified and denoised T1-weighted MP2RAGE image across the entire brain was also acquired and used as anatomical reference for subsequent pre-processing in SPM12 (TR = 4,000 ms, TE = 2.93 ms, FA = 6°, 176 cube matrix, voxel size = 1-mm). Functional imaging data were pre-processed and analyzed using SPM12, implemented in MATLAB. Structural T1-scans were co-registered to the average of the spatially realigned functional slices. Next, an inbuilt segmentation routine was applied to register each structural T1-image to the standard MNI template in MNI space. These transform parameters elicited from segmentation were subsequently applied to all realigned images, resliced to a 2 x 2 x 2-mm resolution and smoothed with 6-mm full-width-at-half-maximum (FWHM) isotropic Gaussian kernel.
fMRI First and Second-Level Analyses
For first-level data analysis, block-related neural responses to stimuli were modeled as two separate conditions (all combinations of emotional/neutral, self-criticism/self-reassurance) and convolved with the canonical hemodynamic response function (HRF). For group level analysis, whole-brain contrasts of self-criticism (emotional-neutral) stimuli were reported at a cluster-level threshold of p < 0.05, corrected for family-wise error, with clusters formed with a voxel-level height threshold at p < 0.001, uncorrected, with a cluster extent threshold of K = 144. Whole-brain contrasts of self-reassurance (emotional-neutral) stimuli were reported at a cluster-level threshold of p < 0.05, corrected for family-wise error, with clusters formed with a voxel-level height threshold at p < 0.001, uncorrected, with a cluster extent threshold of K = 144. Whole-brain repeated-measures contrasts of self-criticism (emotional-neutral)—self-reassurance (emotional-neutral) stimuli were reported at a cluster-level threshold of p < 0.05, corrected for family-wise error, with clusters formed with a voxel-level height threshold at p < 0.001, uncorrected, with a cluster extent threshold of K = 110. A whole-brain repeated-measures contrast of self-reassurance (emotional-neutral)—self-criticism (emotional-neutral) was also conducted, yet no brain regions were shown to survive an initial cluster-forming height threshold of p < 0.001 (uncorrected). Brain regions shown to be significant had their anatomical labels identified with the Automated Anatomical Labelling (AAL) toolbox implemented in SPM12.
Results
First, group-level one-sample t-tests of the whole brain contrasts of emotional—neutral stimuli were conducted overall. We then examined how this contrast differed across self-criticism and self-reassurance. For neural markers of negative emotion during self-criticism, we observed activation in the “salience” (midcingulo-insular), “default-mode” (medial frontoparietal), and the occipital network (Uddin et al., 2019). Whilst neural markers of negative emotion during self-reassurance recruited activation in regions such as the medial-prefrontal cortex (MPFC) and visual cortex, we observed no activation of the salience network as shown under self-criticism. Across both these contrasts, clusters were formed at a cluster-level threshold of p < 0.05, corrected for family-wise error, with clusters formed with a voxel-level height threshold at p < 0.001, uncorrected (cluster extent threshold K = 144).
We next conducted a repeated-measures contrast between self-criticism (emotional—neutral) minus self-reassurance (emotional—neutral), as a marker of neural markers of negative emotion which differs between these two self-relating styles. Here, we identified brain activation across bilateral hippocampus (with a cluster which also included left putamen and left insula), thalamus, ACC, and occipital lobe, revealing neural markers of negative emotion are driven by self-criticism but not self-reassurance (cluster-level threshold of p < 0.05, corrected for family-wise error, with clusters formed with a voxel-level height threshold at p < 0.001, uncorrected, with a cluster extent threshold of K = 110). A repeated-measures contrast between self-reassurance (emotional—neutral) minus self-criticism (emotional—neutral) returned non-significant. Our experimental design is shown in Figures 1, 2 depicts the whole-brain results. Tables which present the thresholded output for each contrast are available online (https://osf.io/9wzu4/?view_only=91484c009daa4b03b676cbfa70940a6f).
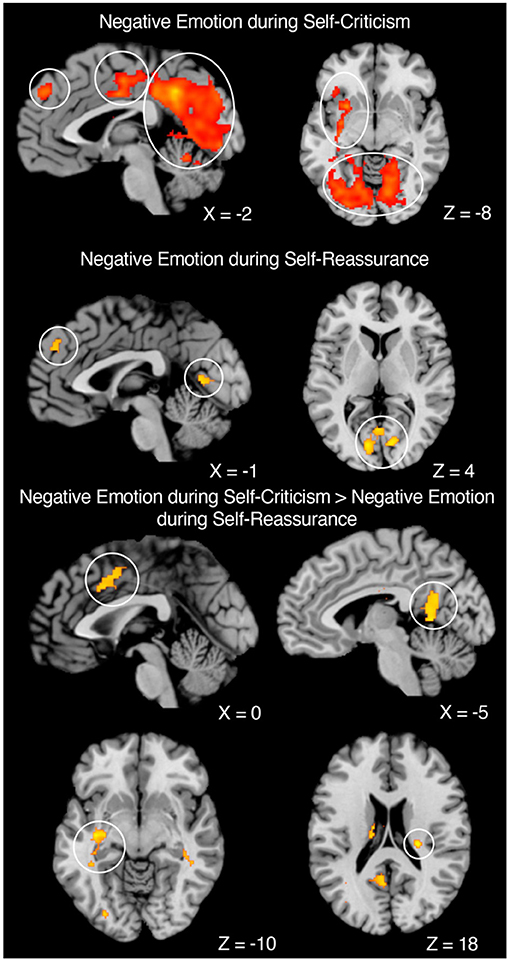
Figure 2. Neural markers of negative emotion across self-criticism and self-reassurance. Negative Emotion during Self-Criticism: Left. Sagittal image of MPFC (Left Circle), ACC (Middle Circle), and Left Lingual Gyrus and Cerebellum (Right Circle). Right. Axial image of Subcortical Regions (Top Circle) and Bilateral Visual Cortex (Bottom Circle). Negative Emotion during Self-Reassurance: Left. Sagittal image of MPFC (Left Circle) and Visual Cortex (Right Circle). Right. Axial image of Visual Cortex. Negative Emotion during Self-Criticism—Negative Emotion during Self-Reassurance: Top Left. Sagittal image of ACC. Top Right. Sagittal image of posterior cingulate. Bottom Left. Axial image of left putamen. Bottom Right. Axial image of Right Hippocampus. Coordinates reported in MNI-space. N = 40.
Self-Report Markers of Intensity During fMRI
Analysis of participants' mean level of intensity ratings for reassuring trials (emotional stimuli: M = 2.45, SD = 0.48, neutral statements: M = 2.63, SD = 0.64) and critical trials (emotional stimuli: M = 2.92, SD = 0.45, neutral stimuli: M = 2.07, SD = 0.52) revealed intensity ratings were significantly higher for critical (emotional—neutral) but not for reassuring (emotional—neutral) trials [t(38) = 7.300, p < 0.001, and t(38) = −1.372, p = 0.178, ns, respectively], as depicted in Figure 3.
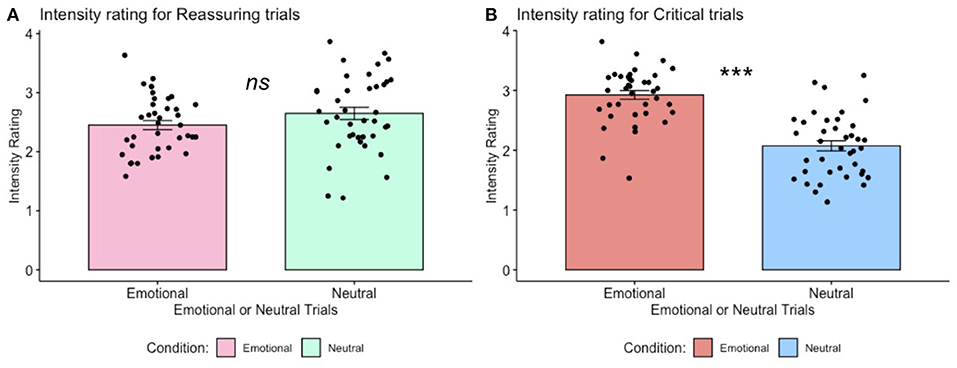
Figure 3. Trial-by-trial ratings of intensity for self-critical and reassuring trials. (A). Intensity ratings for self-reassuring trials, across emotional vs. neutral stimuli. One-sample paired t-test returned non-significant, p > 0.05, ns. (B). Intensity ratings for self-critical trials, across emotional vs. neutral stimuli. For these self-critical trials, one-sample paired t-tests revealed self-report ratings of intensity for emotional stimuli were greater than intensity ratings for neutral stimuli, p < 0.001. Error bars indicate standard error.
Discussion
Here we investigated neural markers of negative emotion when participants engaged in self-criticism and self-reassurance toward negative life events (i.e., mistakes, setbacks or failures). Across both self-criticism and self-reassurance, our fMRI study revealed common activation across diverse regions such as the visual cortex (associated with mental imagery), salience network (associated with processing pain and threat), and default-mode network (associated with self-referential thought) (Uddin et al., 2019). Brain activation overall was more extensive for self-critical than self-reassuring trials, even though both contrasts did activate similar regions such as the MPFC and visual cortex. Furthermore, self-reassurance did not activate regions such as the insula, anterior cingulate cortex and amygdala. In addition, self-report ratings of intensity for emotional stimuli were significantly lower for self-reassuring vs. self-critical trials. Importantly, a contrast of negative emotion between self-criticism minus self-reassurance revealed brain activation in regions such as the anterior cingulate cortex, insula and hippocampus. Taken together, our data show that neural and self-report markers of negative emotion are down-regulated during self-reassurance compared with self-criticism, providing evidence for how cultivating a reassuring self-relating style may regulate neural markers of negative emotion.
Whilst recruitment of the insula and anterior cingulate cortex have previously been shown for self-criticism (Hooley et al., 2012; Lutz et al., 2016), it is important to remark on bilateral hippocampus activation within the current experiment, which may be an indicator of autobiographical memory recall (Aly, 2020; McCormick et al., 2020). Given our paradigm instructions were for participants to engage in self-critical thoughts from the stimuli presented, it is entirely possible that for reference participants engaged in their own first-person accounts from situations in their own lives (Holland and Kensinger, 2010). Indeed, previous work has highlighted an important relationship between mood and memory recall (Parrott and Sabini, 1990), that suggests the need to control for how these processes may contribute to the neural markers of self-criticism or self-reassurance observed within this experiment.
Indeed, one of the considerations for future research would be to optimize this experiment against potential participant fatigue, and to examine potential movement artifacts or differences in affective (i.e., neural, self-report) ratings that might differ across the first and last blocks of the task, given the scanning sessions were fairly long (i.e., 48 min). To do so would increase sensitivity and specificity of the observed neural responses, and provide greater confidence in the results. Furthermore, future work will need to establish how well participants can engage in self-critical vs. self-reassuring thoughts, as this ability may differ across individuals and influence the regulatory function that self-reassurance may provide toward negative emotion.
To position our results in the broader literature on the neuroscience of empathy and compassion, we have shown that brain regions for processing negative emotion toward others (Zaki et al., 2016; Ashar et al., 2017; Kim et al., 2020a,b,c) were shown to not be recruited during compassion to the self. Specifically, we have shown that neural markers of negative emotion are down-regulated during attempts to be compassionate and reassuring to one's suffering, in contrast with previous work that has suggested dissociable neural regions for self-reassurance and self-criticism (Longe et al., 2010). While more work is needed to explore the potentially unique neural substrates of these processes, our data suggest that engagement in self-reassurance can reduce the “sting” of negative life-events, both neural and self-report, which is a timely finding in our current global environment.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: Data is available online (https://osf.io/9wzu4/?view_only=91484c009daa4b03b676cbfa70940a6f).
Ethics Statement
The studies involving human participants were reviewed and approved by The University of Queensland Health and Behavioural Sciences, Low & Negligible Risk Ethics Sub-Committee approved the experimental protocol, and this project complies with the provisions contained in the National Statement of Ethical Conduct in Human Research and complies with the regulations governing experimentation on humans. Participants provided informed and voluntary, written and/or electronic consent. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
JJK, RC, and JNK conceived and designed the experiment. JJK acquired, analyzed, and interpreted the data. JJK, JNK, and JD wrote the manuscript. All authors have given final approval for submission.
Funding
This research was supported by a University of Queensland Research and Teaching fellowship awarded to JNK. JJK was supported by an Australian Postgraduate Scholarship.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
JJK would like to thank ATH for the help and support throughout this manuscript process.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyg.2021.658118/full#supplementary-material
References
Aly, M. (2020). Spotlight brain dynamics underlying memory for lifetime experiences. Trends Cogn. Sci. 24, 1–2. doi: 10.1016/j.tics.2020.06.010
Ashar, Y. K., Andrews-Hanna, J. R., Dimidjian, S., and Wager, T. D. (2017). Empathic care and distress: predictive brain markers and dissociable brain systems. Neuron 94, 1263–1273.e4. doi: 10.1016/j.neuron.2017.05.014
Cox, B. J., Rector, N. A., Bagby, R. M., Swinson, R. P., Levitt, A. J., and Joffe, R. T. (2000). Is self-criticism unique for depression? A comparison with social phobia. J. Affect. Disord. 57, 223–228. doi: 10.1016/S0165-0327(99)00043-9
Gilbert, P., Clarke, M., Hempel, S., Miles, J. N. V., and Irons, C. (2004). Criticizing and reassuring oneself: an exploration of forms, styles and reasons in female students. Br. J. Clin. Psychol. 43, 31–50. doi: 10.1348/014466504772812959
Halamová, J., Kanovský, M., Gilbert, P., Troop, N. A., Zuroff, D. C., Hermanto, N., et al. (2018). The factor structure of the forms of self-criticising/attacking & self-reassuring scale in thirteen distinct populations. J. Psychopathol. Behav. Assess. 40, 736–751. doi: 10.1007/s10862-018-9686-2
Holland, A. C., and Kensinger, E. A. (2010). Emotion and autobiographical memory. Phys. Life Rev. 7, 88–131. doi: 10.1016/j.plrev.2010.01.006
Hooley, J. M., Siegle, G., and Gruber, S. A. (2012). Affective and neural reactivity to criticism in individuals high and low on perceived criticism. PLoS ONE 7:e44412. doi: 10.1371/journal.pone.0044412
Kim, J., Parker, S., Doty, J., Cunnington, R., Gilbert, P., and Kirby, J. (2020a). Neurophysiological and behavioural markers of compassion. Sci. Rep. 10:6789. doi: 10.1038/s41598-020-63846-3
Kim, J. J., Cunnington, R., and Kirby, J. N. (2020b). The neurophysiological basis of compassion: an fMRI meta-analysis of compassion and its related neural processes. Neurosci. Biobehav. Rev. 108, 112–123. doi: 10.1016/j.neubiorev.2019.10.023
Kim, J. J., Henderson, T., Best, T., Cunnington, R., and Kirby, J. N. (2020c). Neural and self-report markers of reassurance: a generalized additive modelling approach. Front. Psychiatry 11:566141. doi: 10.3389/fpsyt.2020.566141
Longe, O., Maratos, F. A., Gilbert, P., Evans, G., Volker, F., Rockliff, H., et al. (2010). Having a word with yourself: neural correlates of self-criticism and self-reassurance. NeuroImage 49, 1849–1856. doi: 10.1016/j.neuroimage.2009.09.019
Lutz, J., Bruhl, A. B., Doerig, N., Scheerer, H., Achermann, R., Weibel, A., et al. (2016). Altered processing of self-related emotional stimuli in mindfulness meditators. NeuroImage 124(Pt A), 958–967. doi: 10.1016/j.neuroimage.2015.09.057
McCormick, C., Barry, D. N., Jafarian, A., Barnes, G. R., and Maguire, E. A. (2020). vmPFC drives hippocampal processing during autobiographical memory recall regardless of remoteness. Cereb. Cortex 1–16. doi: 10.1093/cercor/bhaa172
Parrott, W. G., and Sabini, J. (1990). Mood and memory under natural conditions: evidence for mood incongruent recall. J. Pers. Soc. Psychol. 59, 321–336. doi: 10.1037/0022-3514.59.2.321
Petrocchi, N., Dentale, F., and Gilbert, P. (2019). Self-reassurance, not self-esteem, serves as a buffer between self-criticism and depressive symptoms. Psychol. Psychother. 92, 394–406. doi: 10.1111/papt.12186
Puterman, E., Weiss, J., Hives, B. A., Gemmill, A., Karasek, D., Mendes, W. B., et al. (2020). Predicting mortality from 57 economic, behavioral, social, and psychological factors. Proc. Natl. Acad. Sci. U. S. A. 117, 16273–16282. doi: 10.1073/pnas.1918455117
Solís, C. B., Kelly-Irving, M., Fantin, R., Darnaudéry, M., Torrisani, J., Lang, T., et al. (2015). Adverse childhood experiences and physiological wear-and-tear in midlife: findings from the 1958 British birth cohort. Proc. Natl. Acad. Sci. U. S. A.112, E738–E746. doi: 10.1073/pnas.1417325112
Uddin, L. Q., Yeo, B. T. T., and Spreng, R. N. (2019). Towards a universal taxonomy of macro - scale functional human brain networks how many functional brain networks are a fundamental construct in neuroscience is the definition. Brain Topogr. 32, 926–942. doi: 10.1007/s10548-019-00744-6
Keywords: compassion, self-criticism, fMRI, reassurance, emotion
Citation: Kim JJ, Doty JR, Cunnington R and Kirby JN (2021) Does Self-Reassurance Reduce Neural and Self-Report Reactivity to Negative Life Events? Front. Psychol. 12:658118. doi: 10.3389/fpsyg.2021.658118
Received: 25 January 2021; Accepted: 30 August 2021;
Published: 28 September 2021.
Edited by:
Christopher Chad Woodruff, Northern Arizona University, United StatesReviewed by:
David Sheffield, University of Derby, United KingdomFrances Anne Maratos, University of Derby, United Kingdom
Copyright © 2021 Kim, Doty, Cunnington and Kirby. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jeffrey J. Kim, jeffrey.kim@uqconnect.edu.au
 Jeffrey J. Kim
Jeffrey J. Kim James R. Doty
James R. Doty Ross Cunnington
Ross Cunnington James N. Kirby
James N. Kirby