- 1Department of Environmental Science, University of Calcutta, Kolkata, India
- 2Centre for Mathematical Biology and Ecology, Department of Mathematics, Jadavpur University, Kolkata, India
- 3Systems Ecology and Ecological Modelling Laboratory, Department of Zoology, Visva-Bharati University, Santiniketan, India
- 4Department of Environmental Science and Engineering, Indian Institute of Technology (Indian School of Mines), Dhanbad, India
- 5Department of Biological Sciences, Indian Institute of Science Education and Research Kolkata, Nadia, India
Urbanization affects concurrent human-animal interactions as a result of altered resource availability and land use pattern, which leads to considerable ecological consequences. While some animals have lost their habitat due to urban encroachment, few of them managed to survive within the urban ecosystem by altering their natural behavioral patterns. The feeding repertoire of folivorous colobines, such as gray langur, largely consists of plant parts. However, these free-ranging langurs tend to be attuned to the processed high-calorie food sources to attain maximum benefits within the concrete jungle having insignificant greenery. Therefore, besides understanding their population dynamics, the effective management of these urbanized, free-ranging, non-human primate populations also depends on their altered feeding habits. Here, we have used a field-based experimental setup that allows gray langurs to choose between processed and unprocessed food options, being independent of any inter-specific conflicts over resources due to food scarcity. The multinomial logit model reveals the choice-based decision-making of these free-ranging gray langurs in an urban settlement of West Bengal, India, where they have not only learned to recognize the human-provisioned processed food items as an alternative food source but also shown a keen interest in it. However, such a mismatch between the generalized feeding behavior of folivorous colobines and their specialized gut physiology reminds us of Liem's paradox and demands considerable scientific attention. While urbanization imposes tremendous survival challenges to these animals, it also opens up for various alternative options for surviving in close proximity to humans which is reflected in this study, and could guide us for the establishment of a sustainable urban ecosystem in the future.
Introduction
The global urban human population is set to reach the 5 billion mark by 2028 (ONU, 2018), facilitating urban sprawling and subsequently contributing to natural habitat loss worldwide at an unprecedented rate. This is expected to affect a large number of animals whose habitat ranges overlap with urban areas (Mcdonald et al., 2008; He et al., 2014; Martinuzzi et al., 2015; Murray and St. Clair, 2015). Habitat fragmentation and encroachment due to such urban expansion, which is often irreversible, has forced many homeless animals to live in close proximity to humans (Bateman and Fleming, 2012), giving rise to frequent human-animal conflict (Messmer, 2000; Omondi, 2004; Woodroffe et al., 2005; Devi and Saikia, 2008). At the same time, some of these animals have also shown considerable behavioral adaptations like altered nesting or denning habits, vocalization, migratory activities, mating and breeding patterns, feeding behavior (Kettlewell, 1961; Able and Belthoff, 1998; Estes and Mannan, 2003; Slabbekoorn and Peet, 2003; Swedell et al., 2011; Lowry et al., 2013) together with life history modification to survive amidst anthropogenic stress. Such anthropogenic stress often creates unpredictable selection pressure on these urban animals, leading to a sharp decline in species richness and composition within an urban ecosystem (Vitousek et al., 1997; Kumara and Singh, 2004; Singh and Raghunatha Rao, 2004; Fuentes, 2012; Kale et al., 2012; Paul et al., 2016; Erinjery et al., 2017). However, despite significant loss of biodiversity, urban expansion offers various high-calorie resource options to the generalist species who have higher dietary as well as foraging plasticity, and therefore, can adjust more readily to the altered habitat in contrast to the specialists (Vázquez and Simberloff, 2002; Fisher and Owens, 2004). Moreover, such anthropogenic food sources remain available throughout the year, thus providing a risky yet reliable and easily accessible resource option which is thought to be one of the major driving forces behind human-animal co-existence within urban settlements (Bateman and Fleming, 2012; Lowry et al., 2013; Widdows et al., 2015; Thabethe and Downs, 2018). In some cases, urban-dwelling free-ranging animals have been shown to acquire a preference toward anthropogenic food items to minimize their foraging activities, so that could invest more energy and time in nurturing social relationships which is essential to attain better fitness benefits (Saj et al., 1999; Hoffman and O'Riain, 2012; Sha and Hanya, 2013; Bryson-Morrison et al., 2016, 2017; Thatcher et al., 2019).
India has more than 400 mammalian species, of which 17 are non-human primates with different conservation status (Molur et al., 2003; Karanth et al., 2010; Kumara et al., 2010) who have ecological as well as socio-cultural importance. Three of these non-human primate species i.e., Rhesus macaques (Macaca mulatta), bonnet macaques (Macaca radiata) and gray langurs (Semnopithecus entellus) are frequently found in Indian cities, market places, temples, roadside settlements, etc., where they are often provisioned with human offered food items and space, worshiped and protected by Hindus (Sharma et al., 2011). Their wide distribution range and various feeding habits reflect their generalistic nature where they use a handful of novel strategies such as “coo-calls,” begging gestures, car raiding, etc. to acquire the available food items directly from humans (Sinha, 2005; Arbib et al., 2008; Sha et al., 2009; Deshpande et al., 2018). However, such close human-animal interaction is often lethal, affecting their chances of survival within urban ecosystems (Grinder and Krausman, 2001; Vijayan and Pati, 2002; Gosselink et al., 2007; Paul et al., 2016). Furthermore, these high-calorie processed food items could have a substantial effect on the physiology of these animals underlying their behavioral patterns, thereby reshaping intra and inter-specific group dynamics in contrast to their natural counterparts (Orams, 2002; Higginbottom and Scott, 2008; Trave et al., 2017). In this scenario, it is imperative to understand how the oppression of urban expansion has thinned down the natural resources and influenced the lives of these animals, leading to urban-adaptation in these species.
While several studies have been carried out on the naturally omnivorous macaques (Oppenheimer, 1977; Goldstein and Richard, 1989; Laska, 2001; Ganguly and Chauhan, 2018), to understand their opportunistic feeding behavior to co-exist with human settlements, there has been no study yet to quantify and compare the dietary preference of folivorous gray langurs in urban areas. Due to their deity value, this species is endowed with ample human provisioning. However, their specialized tripartite stomach structure largely aids in the digestion of a leafy diet (Bauchop and Martucci, 1968; Caton, 1999). Moreover, unlike the terrestrial macaques, the arboreal nature of gray langurs (Khanal et al., 2018) is also barring them from availing enough human provisioning which could supplement their cellulose-based diet. Therefore, it seems to be all the more difficult for these large-bodied colobines to obtain sufficient resources that could be invested in maintaining reproductive fitness within an ecosystem where their natural food options are either unavailable or scarce to support their energy demand.
Gray langurs (Semnopithecus entellus), commonly called Hanuman langurs have colonized various parts of the Indian subcontinent, ranging from the desert to forest fringes, and have lived with a diversified resource structure and human interference (Oppenheimer, 1977; Ashalakshmi et al., 2014; Chetan et al., 2014). In comparison to the other species of langur, social organization of the gray langur is highly flexible (Newton, 1988; Caton, 1999; Sterck, 1999; Rajpurohit et al., 2006) and is often modified by the male-male competition followed by infanticides (Hrdy, 1974; Broom et al., 2004; Sharma et al., 2010). Besides unimale-bisexual troops, all-male bands are also common in these gray langurs (Rajpurohit et al., 2003). Even though they exploit a wide range of plant species including various plant parts, only a fraction of these so-called “Least Concern species” (IUCN 2003) can reach their reproductive age, which is again expected to reduce due to the adverse effect of urban encroachment (Kumara et al., 2020). On the contrary, such urban settlements provide easy access to various anthropogenic low-fiber food sources which are mostly processed yet offer high-calorie to these ruminant folivores (Sayers, 2013). Few articles have reported human-langur cooperation through the food provisioning in Indian cities and towns, portraying them as ecological generalists in terms of habitat and diet. This mismatch in their expected and observed diet has made them one of the prime examples of Liem's paradox which refers to the odd pairing of specialized anatomical features with a generalistic diet (Liem, 1980; Binning et al., 2009). However, it was later argued that such “asymmetry allows optimally foraging consumers to evolve phenotypic specializations on non-preferred resources without compromising their ability to use preferred resources” (Robinson and Wilson, 1998). Hence, the development of alternate feeding patterns in these urbanized free-ranging gray langurs demands considerable scientific attention which could provide important insights into their eco-ethological adaptation for better management and policy-making to develop a sustainable urban ecosystem. Several studies have manifested “food-resources” as one of the major contributing factors that limit group size and composition of primates (Chapman, 1990). The Van Schaik model posits that in folivorous non-human primates, the intra-group scramble feeding-competition leads to differential reproductive success which has an immense role in establishing the hierarchical relationship within the group (Van Schaik, 1989; Borries, 1993). Therefore, the feeding behavior of the gray langurs can provide interesting insights into the social dynamics, as well as the urban adaptation of the species. In this article, we have focused on the feeding preference of group-living, free-ranging gray langurs in the urban areas of West Bengal, India, which unraveled their behavioral plasticity to utilize anthropogenic habitats.
Methods
Study Area and Study Animals
Free-ranging gray langur groups were identified through regular census between September 2018 and December 2018 in various parts of West Bengal, India, of which three distinct langur groups [one in Dakshineswar (22.6573°N, 88.3624°E), one in Nangi (22.4973°N, 88.2214°E), and one in Sarenga, Nalpur (22.5307°N, 88.1840°E)] were selected for long-term observations, considering the various level of human interferences received by the gray langurs (Supplementary Data D1, D2).
Ad libitum Study
Observers visited the areas at random times during the day and walked on all roads, by-lanes, and fields covered with vegetation of the above three selected urban settlements. Whenever a group of gray langurs was sighted, it was observed by using ad libitum for a minimum of 15 min to a maximum of 1 h, during which the observer recorded its location, group size, and behaviors (later categorized as either solitary or paired interactions; paired interactions were further subdivided into intra- and interspecific interactions). These behavioral interactions were used to prepare an ethogram which was used for long-term observations. We used physical and behavioral characteristics such as fur color, body size, locomotion, feeding, and sexual behavior to discriminate various life stages (Infant-dark fur color and fully dependent on adults for their movement and feeding; Juvenile-light fur color similar to adults but smaller in size and partially dependent on adults for their movement and feeding; sub adult-fully independent of adults but yet to attain sexual maturity, body size is typically in between that of juveniles and adults; adult- fully grown, independent individual who is sexually mature) of free-ranging gray langurs. Their ranges were accurately recorded during observation by using GPS- eTrex30, Garmin.
Food-Census
We recorded the food habits of these three gray langur groups between September 2018 and December 2018 through regular census. Whenever any feeding behavior was observed, the observer recorded the details of food types (food-census), and source of the food item (whether foraged from roadside tree patches, raided or stolen from the agricultural field, household garden, or vegetable market, being provisioned by humans, etc.). We categorized these feeding behaviors into “solitary” (when a gray langur independently fed on some food item) and “interactive” (which involved interactions with other group members or humans). For interactive feeding behaviors, we recorded the identity of the “initiator” and “recipient.” “Initiator” represents the individual (gray langur or human) who initiates a behavior (stealing or provisioning) that facilitates gray langurs to acquire a food item, whereas “recipient” is the individual who received the behavior (human and gray langur are recipients for food stealing and food provisioning behaviors, respectively). The feeding repertoire of these gray langurs consisted of either unprocessed (various plant parts such as raw vegetables, fruits, tree leaves, flowers, etc.) or processed food items (factory-made such as bread, biscuit, peanut, chips, puffed rice, cake, etc.), which are associated with human presence and involve some degree of human interference within an urban ecosystem (Supplementary Data D3a,b).
Food-Choice Test
We carried out a choice-based experiment between January and March for two consecutive years 2019 and 2020 to find the feeding preferences of free-ranging gray langurs at Dakshineswar where they received human interference, including food offerings (Unpublished data) and depended mostly on “processed foods,” unlike the gray langurs of Nalpur and Nangi where they relied on plant parts such as vegetables, fruits, leaves, flowers (Figure 1). We recorded maximum human provisioning at Dakhineswar between January and March when gray langurs receive both processed and unprocessed food items from the pilgrims (Unpublished data). Therefore, we used this context to explore the feeding preference of free-ranging gray langurs in presence of various human interferences (Bhattacharjee et al., 2020). Here, we conduct the choice-based experiment where we offered a food tray of cardboard, with four types of food items, each of them having a comparable quantity and size (Supplementary Data D4), between 0600 and 1800 h. We used cauliflower and brinjal as “unprocessed food” items, whereas bread and peanuts as “processed food” items. All of these offered food items were fresh and suitable for human consumption. These were presented on the food tray in random order to avoid any “side-bias.” Since peanuts were seen to be one of the most frequently eaten processed food items, we used it for the choice-based experiment and offered it in a small paper bag (which is usually used by people to offer peanuts to gray langurs during provisioning), making its quantity visually similar to the other food items.
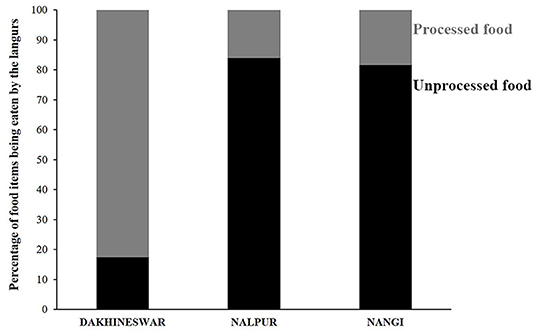
Figure 1. Stacked bar diagram showing the feeding habit of free-ranging langurs at three locations, Dakshineswar, Nalpur, and Nangi. Black and gray bars represent the percentage of “unprocessed” and “processed” food items being eaten by the free-ranging langurs.
Conducting Experiment at Various Zones of Dakshineswar
Based on the food-census data, we sub-divided the study area, Dakshineswar, into three distinct zones representing various feeding options available to gray langurs (Supplementary Data D5). The experimenter conducted experiments at various zones of Dakshineswar and presented the food tray at a spot where most of the group members could have equal access to the food tray. Since multiple gray langurs visited the food tray during each trial, we considered food items to be our focal object for the analysis instead of gray langurs. The experimenter either waited until the food tray was empty or waited for 10 min if the food tray remained unattended or partially attended by the gray langurs, before closing the session. Once started, the experiment remained undisturbed i.e., no human interference was allowed and the entire experiment was video-recorded. In order to avoid any bias, which could influence subsequent trials, the videos were decoded only in April 2020, after the completion of all experiments. We conducted a total of 83 trials in the field set-up of which 74 trials (where the food trays were attended by the gray langurs without having any human interference) were considered for the final analysis (Supplementary Data D6). Since the movement of free-ranging gray langurs is independent of human supervision, they forage in a group and every group member has easy access to the human-provisioned food items, we did not interfere if more than one gray langur explored the food tray and interact between themselves during food acquisition.
Scoring Method
For each experiment, we recorded the time points (in seconds) when a food item was attempted to be received by the gray langurs for the first time during a trial i.e., first attempt received by a food item (FA), chosen to be eaten (FC), latency between FA and FC (in seconds) (delay), number of rejection received by a food item (RJ), and the presence or absence of aggression shown by the gray langurs to possess a food item (AG) (Supplementary Data D7). A food item was considered to be rejected if it was not chosen to be eaten followed by FA. One food item could receive multiple RJ between FA and FC (Supplementary Data D7). Then we scored each of the four food items for FA, FC, RJ, AG, and “delay” to check the sequence of food items being picked up and consumed by the free-ranging gray langurs separately for each trial and used these data to reflect the feeding preference of gray langurs (Supplementary Data D7). Since the food tray had four food items, each of them had five scoring options for FA and FC. The food that was attempted to be taken first received a score of five and the last (fourth) one was given a score of two. If a food item remained unattempted, it received a score of one. Similar scoring was done for FC, where the food scored “one” if it was not chosen to be eaten and “five” for being eaten first. RJ was scored on a scale of a maximum of eight to a minimum of “zero,” where food items scored “zero” if they were not rejected at all, and scored “eight” when rejected for FA. Foods were scored “one” if they received aggression and “zero” if not, considering AG as an indication of the possessiveness for the most preferred food item which gray langurs did not want to share with. For “delay” we scored them between “zero” to “five” where “zero” represents no delay, “four” for the maximum delay between FA and FC, and “five” for the foods which were approached but not chosen to be eaten until the end of the experimental session (Supplementary Data D8).
Statistical Analysis
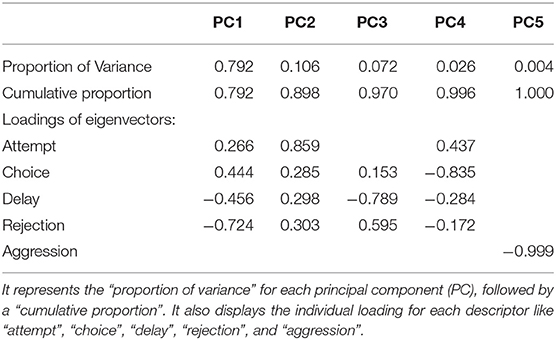
We used the scores for FA, FC, RJ, AG, and delay for all statistical analyses which were carried out using StatistiXL (version 2.0), and R (version 4.0.2) (Team, 2020). We ran a correlation analysis to check the inter-relation between various factors including “attempt” (FA), “choice” (FC), “delay,” “rejection” (RJ), and “aggression” (AG) which were affecting the final food selection by the gray langurs. To verify the results of the correlation we used a generalized linear model (GLM) and checked which parameter was finally affecting the final selection of the food items. We used the FC as the response variable, whereas FA, AG, RJ, and “delay” were incorporated into the model as the predictor variables. We used a “Poisson” distribution for the response variable to run the model. The distribution of the residuals was evaluated to check how well the model fits the data (Supplementary Data D9). A “principal component analysis” (PCA) was conducted for descriptors including FA, FC, AG, RJ, and delay to check their effect on the food selection separately for three zones (Figure 2).
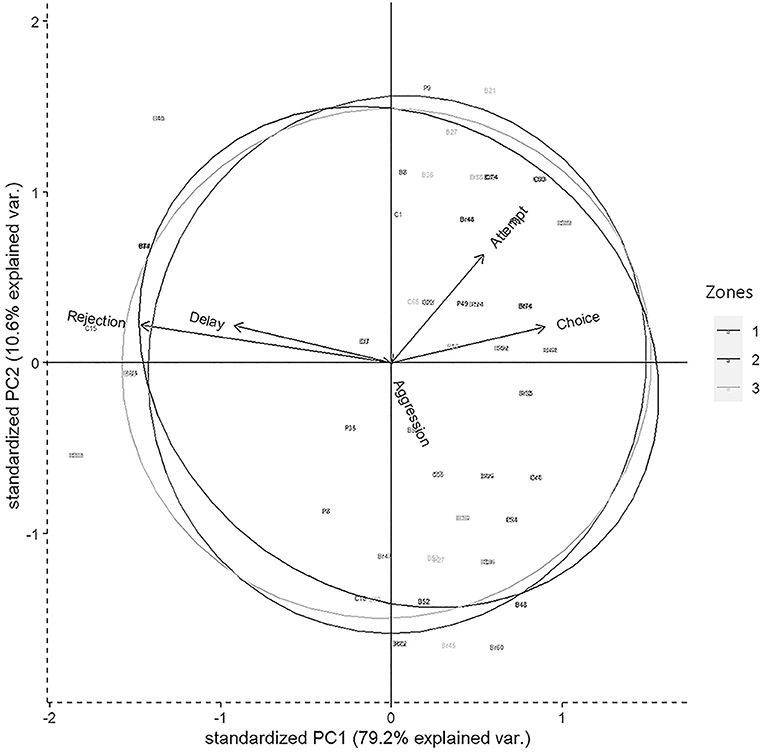
Figure 2. Biplot representing the distribution of variables in 2D space for the Principal component analysis (PCA) having descriptors like attempt, choice, delay, rejection, and aggression. Circles represent three different zones of Dakshineswar.
Multinomial Logit Model
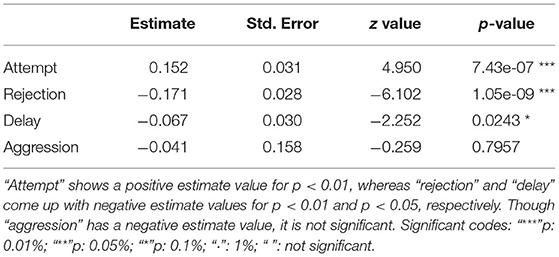
To explain the preference of one food over another, i.e., food choice, we ran varied combination of multinomial logit models (MLM) (Croissant, 2020). Since we were interested in checking the predictive values of different independent variables like aggression, rejection, etc. on the outcome of food choice, we ran two different sets of MLMs – separately for the “attempt” and “choice” probabilities (Table 1). These two sets had four sub-models each where we employed a “leave one component out” (LOCO) approach to meet our goal. The LOCO approach leaves one food component out at each sub-model step to check the order of selection of the subsequent food item. Besides, the models also evaluate the importance of the independent variables or descriptors in the outcome (Supplementary Data D10).
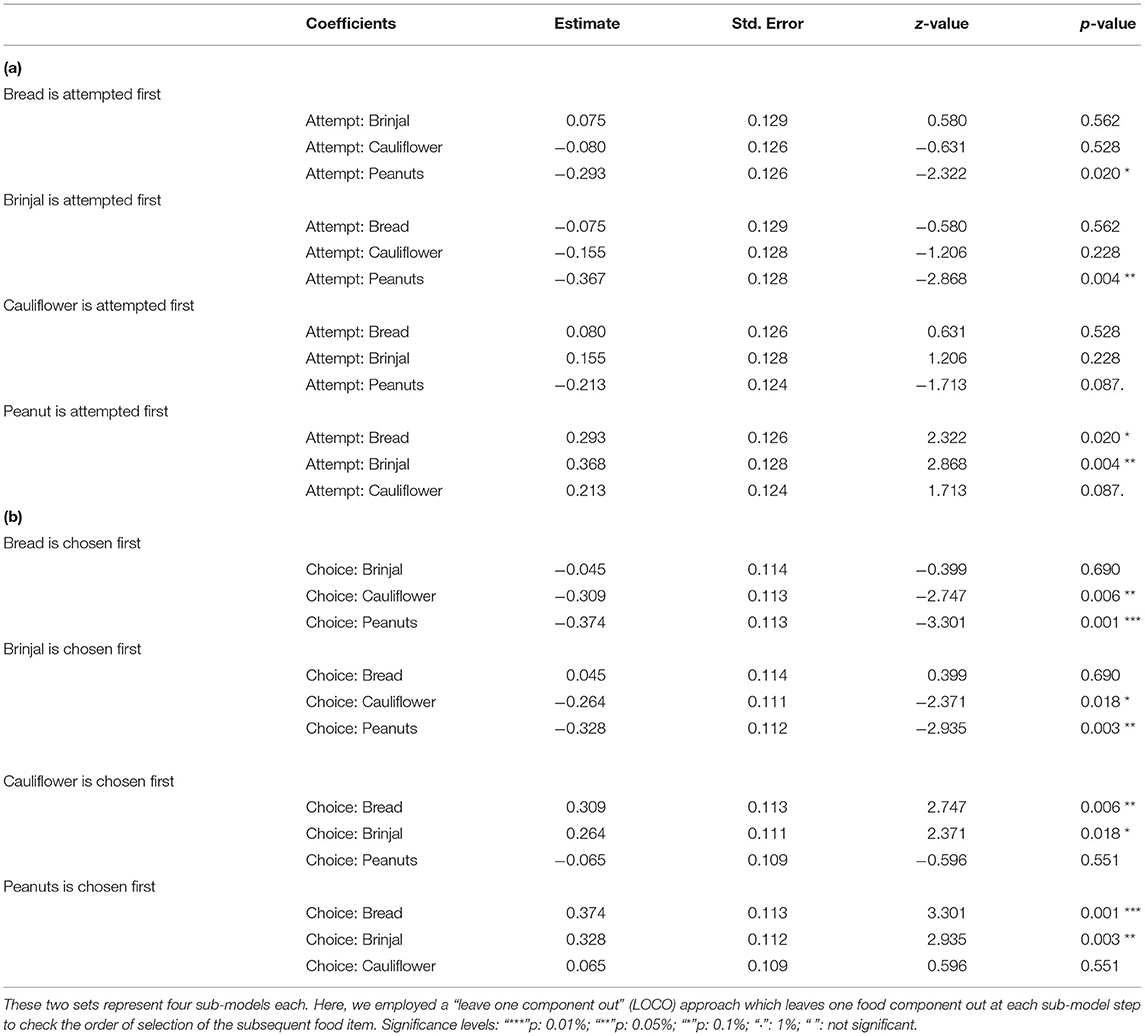
Table 1. Table representing the estimates, and p-values of the multinomial logit models, set 1 and 2, respectively for (a) attempt, and (b) choice probabilities.
The first set used the food attempt as the outcome and the second set used the goal function of final food choice. When one food was attempted to be received by gray langurs, the probabilities of attempting for the next food items can be determined subsequently by using set 1 MLMs. We ran four sub-set MLMs to check what would be the next approached food items separately while considering either brinjal, bread, cauliflower, or peanuts as the “first approached” food item (Table 1a). Higher scores of the odds ratio confirm the results of MLM estimates thereafter (Table 2a) and subsequently rank the different food attempt preferences according to the LOCO tactic.
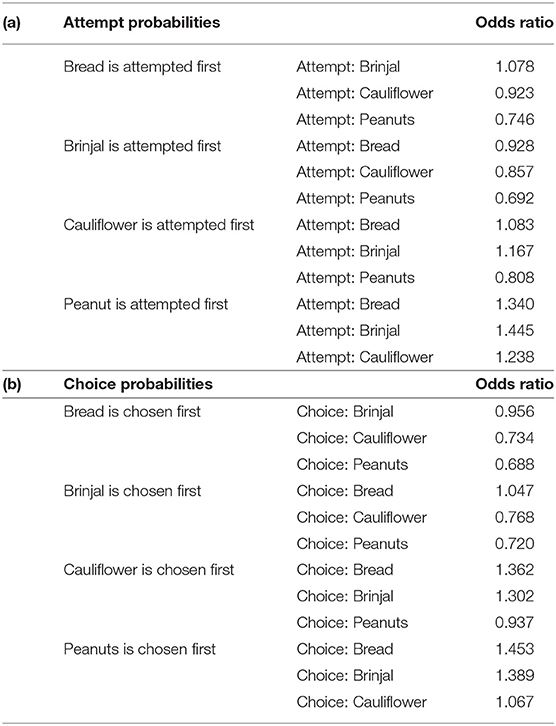
Table 2. Table representing the odds ratio separately for (a) attempt, and (b) choice probabilities.
Simultaneously, the set 2 MLMs were processed to establish and validate the preferred order of food items being chosen (final food choice) by the gray langurs during the experiment (Tables 1b, 2b). The LOCO here assumes that one food has been consumed (and thus exhausted) and subsequently calculates the probabilities of choosing the next item. Since all food items were provided equally (i.e., equal probability of choice at the beginning), the model considered the frequencies of alternatives equal to 0.25. We used the Newton-Ralphson method from the package “mlogit” in R to run the MLM (Croissant, 2020). The estimates of the MLM were plotted by using “tidyverse” (Wickham et al., 2019) after normalizing to 1.0 (to avoid the negative values) for the visual representation (Figure 3).
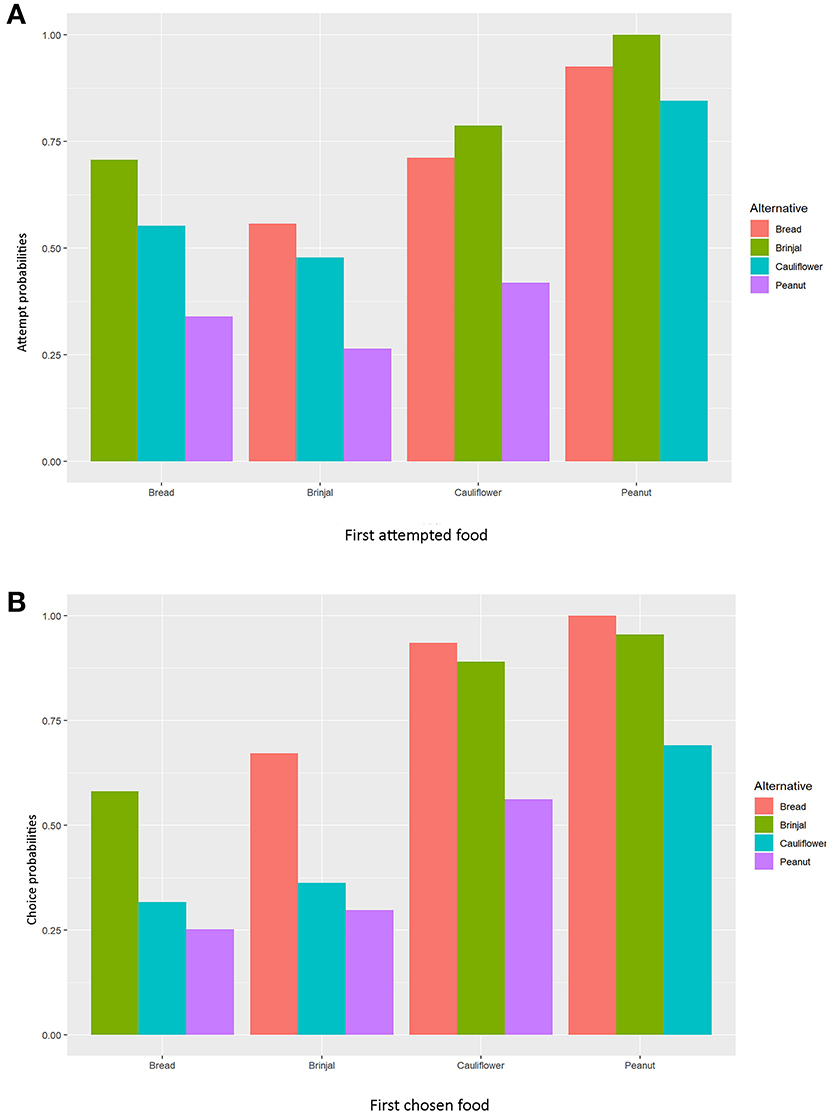
Figure 3. Bar diagrams representing the normalized values of MLM estimates separately for (A) “attempt,” and (B) “choice” probabilities. The X-axis represents the (A) first approached and (B) first chosen foods.
Food Sharing
We recorded the incidents of food sharing between gray langurs, if any, out of the total 221 successful cases (where the food items were attended by gray langurs) from a total of 296 cases (four food options for 74 experiments). We used the term “primary recipient” for the gray langurs who received food items directly from the food tray. Food sharing was recorded either when “primary recipient” provided a piece of their food to another group member (secondary recipient), or when the “secondary recipient” attempted to take the food from “primary recipient” and was successful. Therefore, we recorded the details of initiator (who initiated the act), and recipient for each “food sharing” behavior, along with the proportion of food being shared. We used social network analysis (SNA) by using Cytoscape (Shannon et al., 2003) where we used various life stages (adult, subadult, juvenile, and infant) as a “node” and an incident of food sharing between them as a “link,” separately for each food type (Figure 4). Here, we calculated the “indegree” and “outdegree” for each node representing the number of food sharing behavior initiated and received by them respectively.
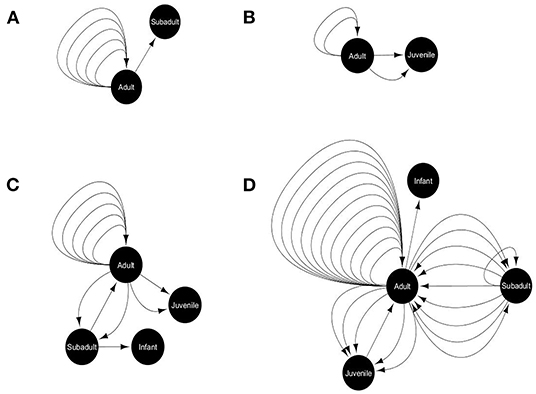
Figure 4. Food-sharing networks of free-ranging hanuman langurs for various food items like (A) bread, (B) brinjal, (C) cauliflower, and (D) peanuts. The solid black circle represents a node, depicting a particular life stage of langurs. The black arrow represents one food-sharing behavior between two nodes, which originated from the initiator and directed toward the recipient.
Ethical Note
No gray langurs were harmed during this work. All work reported here was purely observation-based and did not involve direct handling of gray langurs in any manner, therefore, was in accordance with approved guidelines of animal rights regulations of the Government of India. The research reported in this paper was sanctioned by DST-INSPIRE, Government of India (approval number: DST/INSPIRE/04/2018/001287, dated 24th July 2018), and was also notified to the Principal Chief Conservator of Forests (PCCF), West Bengal, India.
Results
Feeding Habit
Feeding habit of free-ranging gray langurs greatly varied between locations (Contingency chi square: χ2 = 122.15, df = 2, p > 0.0001). Gray langurs of Dakshineswar largely depended on processed food items (83%) which were mostly human offered (Unpublished data), whereas in Nalpur and Nangi they mostly relied on the plant-based, unprocessed food items (84 and 82%, respectively) (Figure 1).
Effects of Attempt, Delay, and Rejection to the Final Food Selection
We carried out a total of 83 experimental trials in Dakshineshwar, of which 74 were successful. The experimental outcomes were perused by “Correlation analysis” and “Generalized linear model (GLM).” Correlation analysis- Rejection (RJ) was seen to be highly correlated to attempt (FA), choice (FC), and “delay.” A significant positive correlation (r = 0.755, p < 0.01) was found between RJ and “delay.” On the other hand, high negative correlations with FA and FC (r = FA: −0.504, FC: −0.814; p < 0.01) represented inverse relations of the same with these factors. FC was highly and positively correlated to FA (r = 0.685, p < 0.01), especially toward a few food items like bread and brinjal (bread = 0.786, brinjal = 0.726, cauliflower = 0.594, peanuts = 0.606). On the contrary, “delay” had significant negative effects on FC (r = −0.76, p < 0.01) (Figure 5).
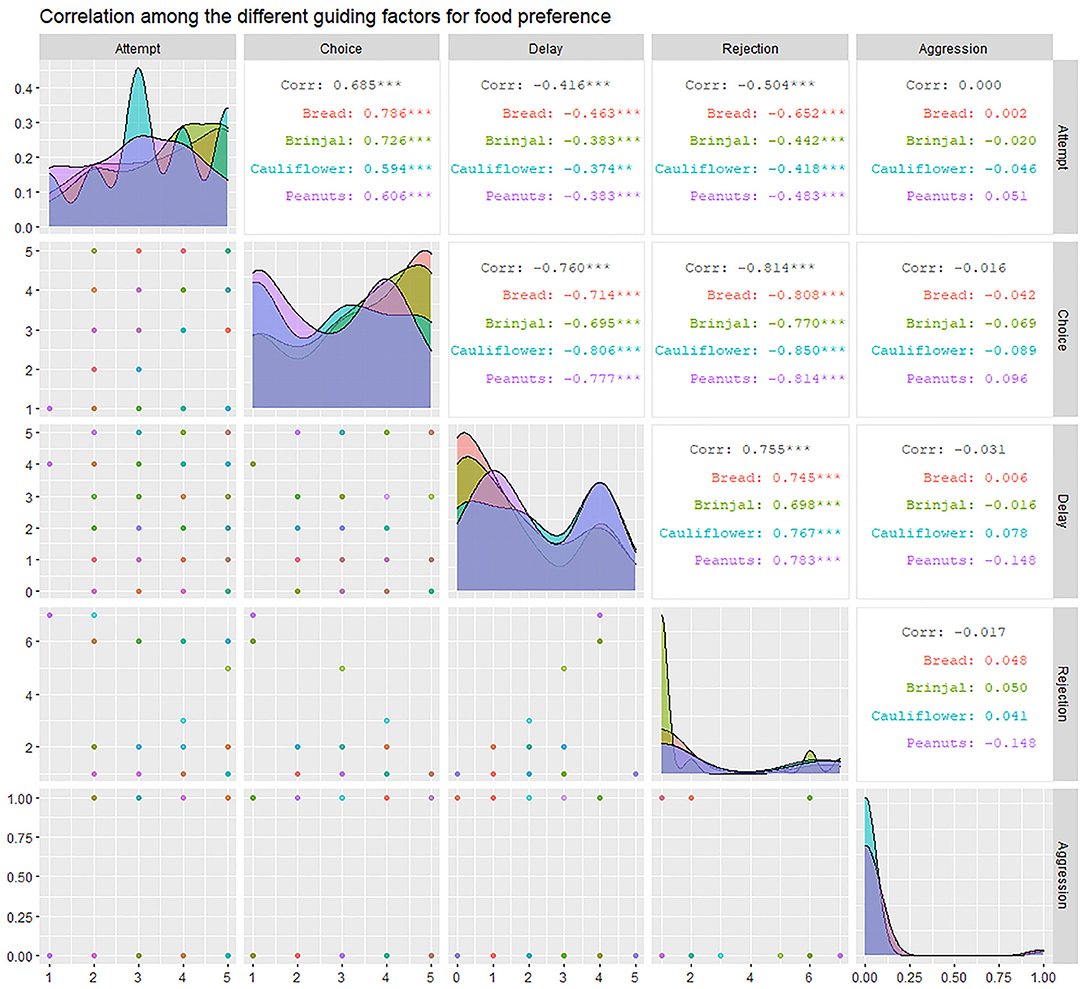
Figure 5. The correlogram representing the inter-relation between factors like “attempt” (FA), “choice” (FC), “rejection” (RJ), “aggression” (AG), and “delay.” It provides the correlation coefficient values (r) for each combination of factors and separately for each of the four food items along with their level of significance.
The GLM confirmed the significant effects of predictor variables like attempt, rejection, and delay on the final food choice. Considering the estimates and p-values, while FA (positive) and RJ (negative) showed more significant effects on the FC (p < 0.01), “delay” had a lesser impact (negative) (p < 0.05) (Table 3). An even distribution of residuals on either side of the “0.0 line” indicated that the model had a good valid fit (Supplementary Data D9).
Influence of Aggression on Food Selection
Aggression (AG) had a slight negative influence on both FC and RJ (−0.29 ≤ r ≤ 0 i.e., weak negative) (Figure 5, Table 3). However, the linear model (LM) plot revealed that when AG was not present (left panel, aggression = 0) and “delay” was minimum (red color bands), FC was highest for lower RJ and vice-versa. Right panel showed that the presence of AG increased the “delay” in FC (width of the color bands represents the “increase”) (Figure 6A). Furthermore, a detailed LM plot revealed that with an increase in the FA, probabilities for FC increased, but both RJ and “delay” lowered the FC (Figure 6B).
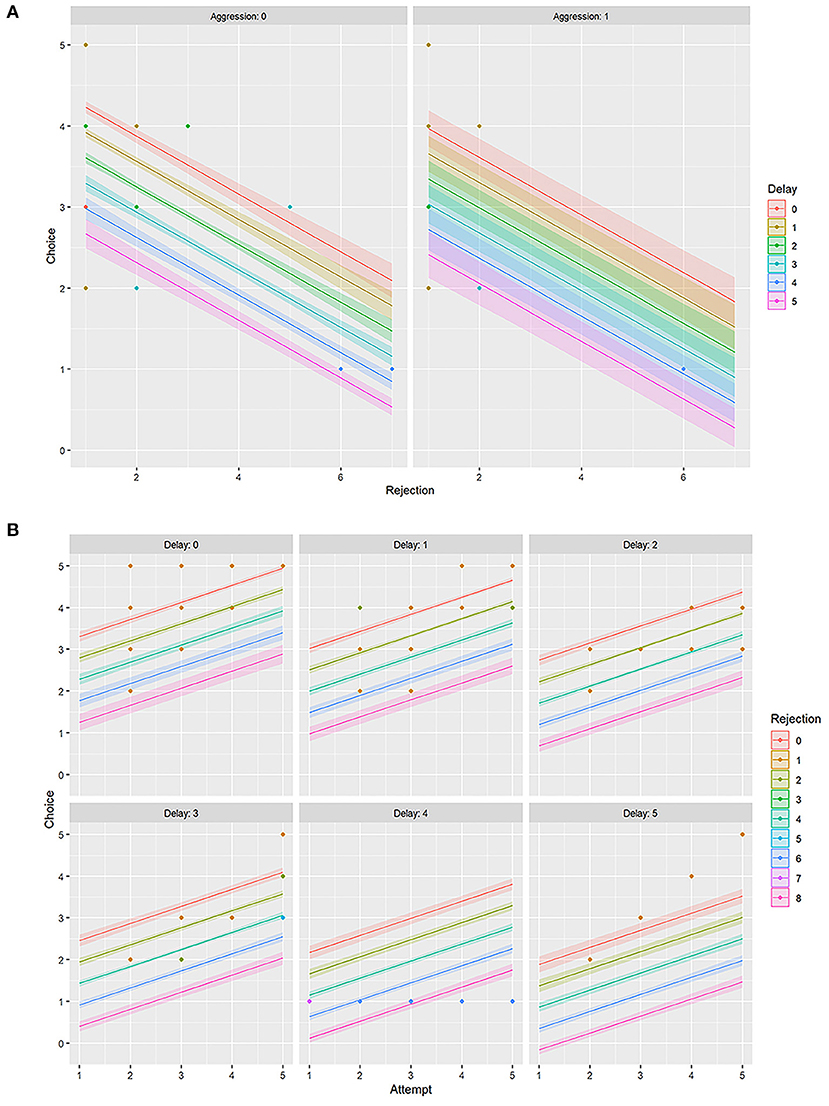
Figure 6. Linear model (LM) plot representing the variations in the choice of food for “rejection” and “delay.” The width of the color bands increases with the delay. (A) LM plots showing different levels of “aggression” has different effects on food choice. The left panel represents data for “zero aggression,” whereas the right panel shows that the “presence of aggression” increases the delay in food choice. (B) LM plots showing the effects of “attempt” on food choice, together with “delay” and “rejection.” Each panel represents a particular “delay” score. For example, the top left panel is for “no delay” or “zero” delay score, and the bottom right is for the “maximum delay” i.e., score five.
Impact of Descriptors on the Final Food Selection
Results of PCA showed that most of the variability in the experimental observations could be explained through PC1 (79.20%), and subsequently another 10.60% by PC2 (Table 4). The PCA biplot revealed that the “zones” had no impact on food selection by gray langurs. The arrows associated with descriptors “attempt” and “choice” remained close to each other, and pointed in the direction of the increasing values of both PC1 and PC2 (the signs of the eigenvectors are also positive for both PC1 and 2, Table 4, Figure 2), thereby confirming their positive effects on the food selection. However, “delay” almost overlapped with the “rejection” and pointed in the direction of the low value of PC1 but high value of PC2 (the signs of the eigenvectors for PC1 is negative and positive for PC2, Table 4, Figure 2), revealing their negative impact on the food selection. The individual loading of “aggression” was only −0.99 on PC5, therefore considered to have a minimal effect (Table 4).
Preference for Food Items
The multinomial logit model (MLM) provided a higher score for bread (estimate value: −0.075) among others, revealing the probability of approaching bread as the second alternative, followed by cauliflower (estimate value: −0.155), and peanuts (estimate value: −0.368) when brinjal was attempted first. Similarly, the MLM picked up bread, cauliflower, and peanuts, one by one, as the first attempted food item, and checked the probability of attempt for the rest. Together with MLM estimate values, odds ratio confirmed the highest approach probabilities for brinjal, followed by bread, cauliflower, and peanuts (Tables 1a, 2a, Figure 3A). However, for the choice probabilities, bread scored highest for both the MLM estimates and odds ratio, followed by brinjal, cauliflower, and peanuts (Tables 1b, 2b, Figure 3B).
First Attempted vs. Eaten Food
Bread and brinjal were chosen as the first attempted food item (scored “five” for FA) for 31 and 32% cases respectively, followed by cauliflower (23%) and peanuts (14%). However, not always the first attempted foods were chosen to be eaten first. Gray langurs switched their preference between the first attempt to first choice for 29.7% cases, and mostly for bread (Goodness of fit: χ2 = 31.08, df = 3, p < 0.0001) (Supplementary Data D6).
Food Sharing
Only 18% of the total successful cases were recorded where gray langurs shared the received food items with their troop members during the experimental trials. However, the shared food items mostly consisted of the least preferred peanuts (53%), and cauliflower (22%) (Goodness of fit: χ2 = 44.72, df = 3, p < 0.0001) (Supplementary Data D11). Social network analysis revealed that food sharing mostly occurs between adults (Goodness of fit: Outdegree: χ2 = 75.35, df = 3, p < 0.0001; Indegree: χ2 = 40.39, df = 3, p < 0.0001) and largely for peanuts, and cauliflower (Figure 4).
Discussion
Folivorous colobines have received considerable research attention because of their unique ability to ingest large quantities of foliage (Struhsaker and Oates, 1975; Oates, 1988; Newton, 1992). Their multipartite stomachs are lined with mucus-secreting glands which facilitate the fermentation of leafy diet in the presence of cellulolytic bacteria (Caton, 1999). However, the dietary composition of free-ranging gray langurs (Semnopithecus entellus) seems to be relatively complex. They often use a diverse array of plant parts including leaves, stalks, shoots, buds, flowers, and fruit to utilize the available resources at their best (Yoshiba, 1967; Vandercone et al., 2012). Besides, Srivastava and Winkler added insectivory and human-provisioning to the feeding repertoire of Hanuman langurs (Winkler, 1988; Srivastava, 1989, 1991). However, these feeding habits were mostly seasonal and plant parts still accounted for a significant portion of their regular diet (Koenig and Borries, 2001) similar to the gray langur group of Nungi, and Nalpur.
Surprisingly, the gray langur group of Dakhineswar, West Bengal, India, was spotted to thrive largely (83% of the total diet) on the processed food items for their sustenance within human settlements, throughout the year. Similar to other free-ranging scavengers like dogs, jackals, monkeys (Butler and du Toit, 2002; Sanyal et al., 2010; Paul et al., 2016), this gray langur group was observed to rely upon human generosity and food provisioning, seeking easy access to the anthropogenic food sources (Unpublished data). However, unlike these carnivorous, and omnivorous scavengers, the stomach physiology of gray langur looks alike to that of herbivores such as Macropodidae (Caton, 1999). Therefore, human-provisioned processed foods could have an inevitable health impact, followed by potential behavioral alteration in these urban-adapted free-ranging gray langurs.
Our field-based observational data reflected the highest degree of human interference in Dakhineswar where gray langurs frequently approached humans to acquire processed high-calorie food sources, in contrast to the gray langurs of Nangi, and Nalpur where they opted for foraging and scavenging and depended mostly on plant-based food sources. Therefore, high human-langur interactions could be considered as an intriguing driving force behind altered feeding habits of gray langurs in Dakhineswar. Moreover, the scarcity of plants and crop fields within the urban settlement at Dakhineswar might be another reason behind their consistent dependence on human-provisioned processed food items. In this context, the choice-based field experiment allowed us to understand whether it was the scarcity of plant-based, unprocessed food sources or the easy accessibility of processed food sources that lured them to get accustomed to the urban ecosystem.
The experimental setup allowed gray langurs to choose between processed and unprocessed food items, keeping aside the factors like scantiness, and human influences. Gray langurs chose brinjal, and bread consistently either as the first or second food options, in all the three zones of Dakhineswar, reflecting their feeding preferences within urban settlements. The outcomes of the experiments manifested the “attempt” to be a significant precursor to food selection. Moreover, its close association to the descriptor, “food choice,” for the increasing values of both principal components 1 and 2 in the PCA confirmed the significance of attempt shown by the gray langur toward a particular food item. Therefore, it can be interpreted that the food had to be attempted first prior to the final selection, allowing gray langurs for choice-based decision-making. However, the effects of “rejection” and “delay” were also substantial, and the final food selection by the gray langurs seemed to be non-random but a consequence of all the above three factors. A significant positive correlation between “rejection” and “delay” revealed that more delays in food selection might lead to the ultimate rejection of that particular food item. A greater rate of rejection negatively facilitated the final choice, whereas swifter attempts toward food led to less rejection. Therefore, when an increased “attempt” escalated the probabilities for the final food selection, “delay” gave rise to a dilemma between “food choice” and “rejection” which finally lowered the chances of selection for a given food item. “Aggression” also had some negative effects on both “choice” and “rejection.” Although it increased the “delay” in final food selection, gray langurs used aggression to possess their chosen food items without being forced to share them with other group members. Hence, our experiments revealed that these gray langurs have developed a fondness for both processed and unprocessed food items within an urban settlement at Dakhineswar, which is driven neither by human interference, nor the scantiness of natural food options but by a keen interest in specific food items. The multinomial logit model contemplated all of these factors for the final food selection by the gray langurs and revealed brinjal and bread to be the most attempted food items, followed by cauliflower and peanuts. However, bread outperformed brinjal as the most chosen food item for which gray langurs often switched their first attempted food to the final selection, indicating their inclination to the processed food option.
In the case of food-provisioning where humans provide a food item of their choice to the animals, the animals have no scope to choose but to receive the offered food items. In our experimental setup, gray langurs had the liberty to choose from a platter of offered food items, without any human interference, and the underlying assumption was that the outcome of the experiment would be influenced only by their preference if any. Our findings suggested that even in the presence of plant-based unprocessed vegetables, which is more akin to natural food items of folivorous colobines, these free-ranging gray langurs chose processed food items that were offered in the food-tray. Moreover, the social network analysis revealed that these gray langurs rarely shared bread and brinjal with groupmates, thereby reflecting their comparable fondness for both processed and unprocessed food items. Although the impact of such processed food items on the physiology of gray langurs is still debatable (Maréchal et al., 2016; Geffroy et al., 2017), it can be interpreted that these free-ranging gray langurs of Dakhineswar not only learned to recognize the human-provisioned food items as an alternative to the natural food sources but they displayed behavioral plasticity to take the advantages of anthropogenic habitats which could facilitate their successful survival within an urban ecosystem. However, resource provisioning is often being correlated to the intentions of people to get in touch with the wildlife, imposing a considerable threat to the survival chances of free-ranging animals (Orams, 2002; Trave et al., 2017). Yet, such man-animal interaction opens up possibilities for alternative easy access to resources like food and shelter for these animals who have lost their home due to urban encroachment (Theobald et al., 1997; Lowry et al., 2013; Cox and Gaston, 2018). Moreover, it has been shown that the ability to digest carbohydrates provided ancestral dog populations an advantage over wolves, facilitating the process of domestication, as the dogs could now utilize human-generated resources (Axelsson et al., 2013). Undoubtedly, our experimental results are an example of such urban adaptation where folivorous arboreal gray langurs find their interest in terrestrial processed food items. Therefore, besides their deity value, the survival of free-ranging gray langurs within human settlements and their wide distribution throughout the Indian subcontinent could be well-explained by their altered yet opportunistic feeding pattern.
Data Availability Statement
Raw data is available at the Dryad data repository. https://doi.org/10.5061/dryad.8cz8w9gpz.
Ethics Statement
The animal study was reviewed and approved by DST-INSPIRE, Government of India (approval number: DST/INSPIRE/04/2018/001287, dated 24th July 2018).
Author Contributions
DD, RK, DB, SM, SK, ABh, and SG carried out the field work. MP and DD coded the entire data. ABa and MP carried out all the statistical analyses. ABa prepared the correlation matrix, ran PCA, GLM, and MLM to interpret the data. MP conceptualized the study, got grants to support the work, designed the fieldwork, and supervised the work. MP, DD, and PB drafted the manuscript. PS prepared the GIS map. PB provided the laboratory support to carry out the analysis. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by a project from the Department of Science and Technology, India (DST/INSPIRE/04/2018/001287) and was supported by the Department of Environmental Science, University of Calcutta, Kolkata, India. ABa acknowledges the DSKPD Fellowship of University grants commission (no. BL/17-18/0490).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
All the authors acknowledge Dr. Anindita Bhadra, Associate Professor, Indian Institute of Science Education and Research- Kolkata, India, for her valuable inputs to the study and gave final approval for publication.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyg.2021.649027/full#supplementary-material
References
Able, K. P., and Belthoff, J. R. (1998). Rapid “evolution” of migratory behaviour in the introduced house finch of eastern North America. Proc. R. Soc. B Biol. Sci. 265, 2063–2071. doi: 10.1098/rspb.1998.0541
Arbib, M. A., Liebal, K., and Pika, S. (2008). Primate vocalization, gesture, and the evolution of human language. Curr. Anthropol. 49, 1053–1076. doi: 10.1086/593015
Ashalakshmi, N. C., Nag, K. S. C., and Karanth, K. P. (2014). Molecules support morphology: species status of South Indian populations of the widely distributed Hanuman langur. Conserv. Genet. 16, 43–58. doi: 10.1007/s10592-014-0638-4
Axelsson, E., Ratnakumar, A., Arendt, M. L., Maqbool, K., Webster, M. T., Perloski, M., et al. (2013). The genomic signature of dog domestication reveals adaptation to a starch-rich diet. Nature 495, 360–364. doi: 10.1038/nature11837
Bateman, P. W., and Fleming, P. A. (2012). Big city life: carnivores in urban environments. J. Zool. 287, 1–23. doi: 10.1111/j.1469-7998.2011.00887.x
Bauchop, T., and Martucci, R. W. (1968). Ruminant-like digestion of the langur monkey. Science 161, 698–699. doi: 10.1126/science.161.3842.698
Bhattacharjee, D., Sau, S., and Bhadra, A. (2020). ‘Bolder’ together — response to human social cues in groups of free-ranging dogs. Behaviour 157, 363–384. doi: 10.1163/1568539X-bja10005
Binning, S. A., Chapman, L. J., and Cosandey-Godin, A. (2009). Specialized morphology for a generalist diet: evidence for Liem's Paradox in a cichlid fish. J. Fish Biol. 75, 1683–1699. doi: 10.1111/j.1095-8649.2009.02421.x
Borries, C. (1993). Ecology of female social relationships: Hanuman langurs (Presbytis entellus) and the van Schaik model. Folia Primatologica 61, 21–30. doi: 10.1159/000156723
Broom, M., Borries, C., and Koenig, A. (2004). Infanticide and infant defence by males - modelling the conditions in primate multi-male groups. J. Theor. Biol. 231, 261–270. doi: 10.1016/j.jtbi.2004.07.001
Bryson-Morrison, N., Matsuzawa, T., and Humle, T. (2016). Chimpanzees in an anthropogenic landscape: examining food resources across habitat types at Bossou, Guinea, West Africa. Am. J. Primatol. 78, 1237–1249. doi: 10.1002/ajp.22578
Bryson-Morrison, N., Tzanopoulos, J., Matsuzawa, T., and Humle, T. (2017). Activity and habitat use of Chimpanzees (Pan troglodytes verus) in the anthropogenic landscape of Bossou, Guinea, West Africa. Int. J. Primatol. 38, 282–302. doi: 10.1007/s10764-016-9947-4
Butler, J. R. A., and du Toit, J. T. (2002). Diet of free-ranging domestic dogs (Canis familiaris) in rural Zimbabwe: implications for wild scavengers on the periphery of wildlife reserves. Anim. Conserv. 5, 29–37. doi: 10.1017/S136794300200104X
Caton, J. M. (1999). Digestive strategy of the Asian colobine genus Trachypithecus. Primates 40, 311–325. doi: 10.1007/BF02557555
Chapman, C. A. (1990). Ecological constraints on group size in three species of neotropical primates. Folia Primatol. 55, 1–9. doi: 10.1159/000156492
Chetan, N., Praveen, K. K., and Vasudeva, G. K. (2014). Delineating ecological boundaries of hanuman langur species complex in peninsular India using MaxEnt modeling approach. PLoS ONE 9:e0087804. doi: 10.1371/journal.pone.0087804
Cox, D. T. C., and Gaston, K. J. (2018). Human–nature interactions and the consequences and drivers of provisioning wildlife. Philos. Trans. R. Soc. B Biol. Sci. 373:62. doi: 10.1098/rstb.2017.0092
Croissant, Y. (2020). mlogit: Multinomial Logit Models. R package version 1.1-0. Available online at: https://CRAN.R-project.org/package=mlogit
Deshpande, A., Gupta, S., and Sinha, A. (2018). Intentional communication between wild bonnet macaques and humans. Sci. Rep. 8, 1–12. doi: 10.1038/s41598-018-22928-z
Devi, O. S., and Saikia, P. K. (2008). Human-monkey conflict: a case study at Gauhati University Campus, Jalukbari, Kamrup, Assam. Zoos' Print J. 23, 15–18.
Erinjery, J. J., Kavana, T. S., and Singh, M. (2017). Behavioural variability in macaques and langurs of the Western Ghats, India. Folia Primatol. 88, 293–306. doi: 10.1159/000480010
Estes, W. A., and Mannan, R. W. (2003). Feeding behavior of Cooper's Hawks at urban and rural nests in southeastern Arizona. Condor 105, 107–116. doi: 10.1093/condor/105.1.107
Fisher, D. O., and Owens, I. P. F. (2004). The comparative method in conservation biology. Trends Ecol. Evol. 19, 391–398. doi: 10.1016/j.tree.2004.05.004
Fuentes, A. (2012). Ethnoprimatology and the anthropology of the human-primate interface. Annu. Rev. Anthropol. 41, 101–117. doi: 10.1146/annurev-anthro-092611-145808
Ganguly, I., and Chauhan, N. S. (2018). Dietary preference and feeding patterns of the urban Rhesus Macaque Macaca mulatta (Mammalia: Primates: Cercopithecidae) in Asola-Bhatti Wildlife Sanctuary in India. J. Threat. Taxa 10, 12907–12915. doi: 10.11609/jott.4347.10.15.12907-12915
Geffroy, B., Sadoul, B., and Ellenberg, U. (2017). “Physiological and behavioral consequences of human visitation,” in Ecotourism's Promise and Peril, eds D. Blumstein, B. Geffroy, D. S. M. Samia, and E. Bessa (Cham: Springer International Publishing), 9–27. doi: 10.1007/978-3-319-58331-0_2
Goldstein, S. J., and Richard, A. F. (1989). Ecology of rhesus macaques (Macaca mulatta) in northwest Pakistan. Int. J. Primatol. 10, 531–567. doi: 10.1007/BF02739364
Gosselink, T. E., Van Deelen, T. R., Warner, R. E., and Mankin, P. C. (2007). Survival and cause-specific mortality of red foxes in agricultural and urban areas of Illinois. J. Wildl. Manage. 71, 1862–1873. doi: 10.2193/2006-020
Grinder, M., and Krausman, P. R. (2001). Morbidity - mortality factors and survival of an urban coyote population in Arizona. J. Wildl. Dis. 37, 312–317. doi: 10.7589/0090-3558-37.2.312
He, C., Liu, Z., Tian, J., and Ma, Q. (2014). Urban expansion dynamics and natural habitat loss in China: a multiscale landscape perspective. Glob. Chang. Biol. 20, 2886–2902. doi: 10.1111/gcb.12553
Higginbottom, K., and Scott, N. (2008). Strategic planning of wildlife tourism in Australia. J. Ecotour. 7, 102–115. doi: 10.1080/14724040802140485
Hoffman, T. S., and O'Riain, M. J. (2012). Landscape requirements of a primate population in a human-dominated environment. Front. Zool. 9, 1–17. doi: 10.1186/1742-9994-9-1
Hrdy, S. B. (1974). Male-male competition and infanticide among the langurs (Presbytis entellus) of Abu, Rajasthan. Folia Primatol. 22, 19–58. doi: 10.1159/000155616
Kale, M., Dudhe, N., Kasambe, R., Chakane, S., and Bhattacharya, P. (2012). Impact of urbanization on avian population and its status in Maharashtra state, India. Int. J. Appl. Environ. Sci. 7, 59–76.
Karanth, K. K., Nichols, J. D., and Hines, J. E. (2010). Occurrence and distribution of Indian primates. Biol. Conserv. 143, 2891–2899. doi: 10.1016/j.biocon.2010.02.011
Kettlewell, H. B. D. (1961). The phenomenon of industrial melanism in Lepidoptera. Annu. Rev. Entomol. 6, 245–262. doi: 10.1146/annurev.en.06.010161.001333
Khanal, L., Chalise, M. K., Wan, T., and Jiang, X. (2018). Riverine barrier effects on population genetic structure of the Hanuman langur (Semnopithecus entellus) in the Nepal Himalaya. BMC Evol. Biol. 18, 1–16. doi: 10.1186/s12862-018-1280-4
Koenig, A., and Borries, C. (2001). Socioecology of Hanuman langurs: the story of their success. Evol. Anthropol. 10, 122–137. doi: 10.1002/evan.1026
Kumara, H. N., Kumar, A., and Singh, M. (2020). Semnopithecus entellus. New Delhi, NY: The IUCN Red List of Threatened Species 2020: e.T39832A17942050.
Kumara, H. N., Kumar, S., and Singh, M. (2010). Of how much concern are the “least concern” species? Distribution and conservation status of bonnet macaques, rhesus macaques and Hanuman langurs in Karnataka, India. Primates 51, 37–42. doi: 10.1007/s10329-009-0168-8
Kumara, H. N., and Singh, M. (2004). Distribution and abundance of primates in rain forests of the Western Ghats, Karnataka, India and the conservation of Macaca silenus. Int. J. Primatol. 25, 1001–1018. doi: 10.1023/B:IJOP.0000043348.06255.7f
Laska, M. (2001). A comparison of food preferences and nutrient composition in captive squirrel monkeys, Saimiri sciureus, and pigtail macaques, Macaca nemestrina. Physiol. Behav. 73, 111–120. doi: 10.1016/S0031-9384(01)00439-5
Liem, K. F. (1980). Adaptive significance of intra- and interspecific differences in the feeding repertoires of cichlid fishes. Integr. Comp. Biol. 20, 295–314. doi: 10.1093/icb/20.1.295
Lowry, H., Lill, A., and Wong, B. B. M. (2013). Behavioural responses of wildlife to urban environments. Biol. Rev. 88, 537–549. doi: 10.1111/brv.12012
Maréchal, L., Semple, S., Majolo, B., and MacLarnon, A. (2016). Assessing the effects of tourist provisioning on the health of wild Barbary macaques in Morocco. PLoS ONE 11:e0155920. doi: 10.1371/journal.pone.0155920
Martinuzzi, S., Withey, J. C., Pidgeon, A. M., Plantinga, A. J., McKerrow, A. J., Williams, S. G., et al. (2015). Future land-use scenarios and the loss of wildlife habitats in the southeastern United States. Ecol. Appl. 25, 160–171. doi: 10.1890/13-2078.1
Mcdonald, R. I., Kareiva, P., and Forman, R. T. T. (2008). The implications of current and future urbanization for global protected areas and biodiversity conservation. Biol. Conserv. 141, 1695–1703. doi: 10.1016/j.biocon.2008.04.025
Messmer, T. A. (2000). The emergence of human–wildlife conflict management: turning challenges into opportunities. Int. Biodeteriorat. Biodegr. 45, 97–102. doi: 10.1016/S0964-8305(00)00045-7
Molur, S., Brandon-Jones, D., Dittus, W., Eudey, A. A., Kumar, A., Singh, M., et al. (2003). Status of South Asian Primates. Apple Valley, MN: CBSG, South Asia. IUCN/SSC Primate Specialist Group.
Murray, M. H., and St. Clair, C. C. (2015). Individual flexibility in nocturnal activity reduces risk of road mortality for an urban carnivore. Behav. Ecol. 26, 1520–1527. doi: 10.1093/beheco/arv102
Newton, P. (1992). Feeding and ranging patterns of forest hanuman langurs (Presbytis entellus). Int. J. Primatol. 13, 245–285. doi: 10.1007/BF02547816
Newton, P. N. (1988). The variable social organization of hanuman langurs (Presbytis entellus), infanticide, and the monopolization of females. Int. J. Primatol. 9, 59–77. doi: 10.1007/BF02740198
Oates, J. F. (1988). The diet of the olive colobus monkey, Procolobus verus, in Sierra Leone. Int. J. Primatol. 9, 457–478. doi: 10.1007/BF02736220
Omondi, S. A. (2004). The role of civil society in conflict management: a case study of the Catholic Church in the 1994 Rwanda genocide. Doctoral dissertation, University of Nairobi, Nairobi, Kenya.
ONU (2018). United Nations, Department of Economic and Social Affairs, Population Division (2019). World Urbanization Prospects: The 2018 Revision (ST/ESA/SER.A/420). New York, NY: United Nations, 10.
Oppenheimer, J. R. (1977). Presbytis entellus, the Hanuman Langur. Primate Conservation, United Kingdom edition. London, UK: Academic Press, INC. 469–512.
Orams, M. B. (2002). Feeding wildlife as a tourism attraction: a review of issues and impacts. Tour. Manage. 23, 281–293. doi: 10.1016/S0261-5177(01)00080-2
Paul, M., Sen Majumder, S., Sau, S., Nandi, A. K., and Bhadra, A. (2016). High early life mortality in free-ranging dogs is largely influenced by humans. Sci. Rep. 6, 1–8. doi: 10.1038/srep19641
Rajpurohit, L., Chhangani, A., and Mohnot, S. (2006). Population dynamics of Hanuman langur, Semnopithecus entellus around Jodhpur (India) during 1995-2000. Proc. Nat. Sci. India 76, 141–147.
Rajpurohit, L. S., Chhangani, A. K., Rajpurohit, R. S., and Mohnot, S. M. (2003). Observation of a sudden resident-male replacement in a unimale bisexual troop of Hanuman langurs, Semnopithecus entellus, around Jodhpur (India). Folia Primatol. 74, 85–87. doi: 10.1159/000070002
Robinson, B. W., and Wilson, D. S. (1998). Optimal foraging, specialization, and a solution to Liem's paradox. Am. Nat. 151, 223–235. doi: 10.1086/286113
Saj, T., Sicotte, P., and Paterson, J. D. (1999). Influence of human food consumption on the time budget of vervets. Int. J. Primatol. 20, 977–994. doi: 10.1023/A:1020886820759
Sanyal, A. K., Dey, J. K., and Kankane, P. L. (2010). The jackals of Tollygunge Club, Kolkata. Rec. Zool. Surv. India 111, 37–45.
Sayers, K. (2013). On folivory, competition, and intelligence: generalisms, overgeneralizations, and models of primate evolution. Primates 54, 111–124. doi: 10.1007/s10329-012-0335-1
Sha, J. C. M., Gumert, M. D., Lee, B. P. Y. H., Jones-Engel, L., Chan, S., and Fuentes, A. (2009). Macaque-Human interactions and the societal perceptions of macaques in Singapore. Am. J. Primatol. 71, 825–839. doi: 10.1002/ajp.20710
Sha, J. C. M., and Hanya, G. (2013). Diet, activity, habitat use, and ranging of two neighboring groups of food-enhanced long-tailed Macaques (Macaca fascicularis). Am. J. Primatol. 75, 581–592. doi: 10.1002/ajp.22137
Shannon, P., Markiel, A., Ozier, O., Baliga, N. S., Wang, J. T., Ramage, D., et al. (2003). Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504. doi: 10.1101/gr.1239303
Sharma, G., Ram, C., and Rajpurohit, L. S. (2010). A case study of infanticide after resident male replacement in Semnopithecus entellus around Jodhpur (India). Proc. Zoo. Soc. 63, 93–98. doi: 10.1007/s12595-010-0013-5
Sharma, G., Ram, C., and Rajpurohit, L. S. (2011). Study of man-monkey conflict and its management in Jodhpur, Rajasthan (India). J. Evol. Biol. Res. 3, 1–3. Available online at: http://www.academicjournals.org/jebr
Singh, M., and Raghunatha Rao, N. (2004). Population dynamics and conservation of commensal bonnet macaques. Int. J. Primatol. 25, 847–859. doi: 10.1023/B:IJOP.0000029125.54747.ee
Sinha, A. (2005). Not in their genes: phenotypic flexibility, behavioural traditions and cultural evolution in wild bonnet macaques. J. Biosci. 30, 51–64. doi: 10.1007/BF02705150
Slabbekoorn, H., and Peet, M. (2003). Birds sing at a higher pitch in urban noise. Nature 424:267. doi: 10.1038/424267a
Srivastava, A. (1989). Feeding Ecology and Behaviour of Hanuman langur, Presbytis entellus. PhD Thesis, University of Jodhpur, Jodhpur, India.
Srivastava, A. (1991). Insectivory and its significance to langur diets. Primates 32, 237–241. doi: 10.1007/BF02381181
Sterck, E. H. M. (1999). Variation in langur social organization in relation to the socioecological model, human habitat alteration, and phylogenetic constraints. Primates 40, 199–213. doi: 10.1007/BF02557711
Struhsaker, T. T., and Oates, J. F. (1975). “Comparison of the behavior and ecology of red colobus and black-and-white colobus monkeys in Uganda: a summary,” in Socio-Ecology and Psychology of Primates, ed R. H.Tuttle (The Hagu: Mouton), 103–123. doi: 10.1515/9783110803839.103
Swedell, L., Saunders, J., Schreier, A., Davis, B., Tesfaye, T., and Pines, M. (2011). Female “dispersal” in hamadryas baboons: transfer among social units in a multilevel society. Am. J. Phys. Anthropol. 145, 360–370. doi: 10.1002/ajpa.21504
Team, R. Core (2020). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. Available online at: https://www.R-project.org/
Thabethe, V., and Downs, C. T. (2018). Citizen science reveals widespread supplementary feeding of African woolly-necked storks in suburban areas of KwaZulu-Natal, South Africa. Urban Ecosyst. 21, 965–973. doi: 10.1007/s11252-018-0774-6
Thatcher, H. R., Downs, C. T., and Koyama, N. F. (2019). Anthropogenic influences on the time budgets of urban vervet monkeys. Landscape Urban Plann. 181, 38–44. doi: 10.1016/j.landurbplan.2018.09.014
Theobald, D. M., Miller, J. R., and Hobbs, N. T. (1997). Estimating the cumulative effects of development on wildlife habitat. Landsc. Urban Plan. 39, 25–36. doi: 10.1016/S0169-2046(97)00041-8
Trave, C., Brunnschweiler, J., Sheaves, M., Diedrich, A., and Barnett, A. (2017). Are we killing them with kindness? Evaluation of sustainable marine wildlife tourism. Biol. Conserv. 209, 211–222. doi: 10.1016/j.biocon.2017.02.020
Van Schaik, C. P. (1989). “The ecology of social relationships amongst female primates,” in Comparative Socioecology: The Behavioural Ecology of Humans And Other Mammals, eds V. Standen and R. A. Foley (London: Blackwell Scientific Publications), 195–218.
Vandercone, R. P., Dinadh, C., Wijethunga, G., Ranawana, K., and Rasmussen, D. T. (2012). Dietary diversity and food selection in Hanuman Langurs (Semnopithecus entellus) and Purple-Faced Langurs (Trachypithecus vetulus) in the Kaludiyapokuna Forest Reserve in the Dry Zone of Sri Lanka. Int. J. Primatol. 33, 1382–1405. doi: 10.1007/s10764-012-9629-9
Vázquez, D. P., and Simberloff, D. (2002). Ecological specialization and susceptibility to disturbance: conjectures and refutations. Am. Nat. 159, 606–623. doi: 10.1086/339991
Vijayan, S., and Pati, B. P. (2002). Impact of changing cropping patterns on man-animal conflicts around Gir protected area with specific reference to Talala sub-district, Gujarat, India. Popul. Environ. 23, 541–559. doi: 10.1023/A:1016317819552
Vitousek, P. M., Mooney, H. A., Lubchenco, J., and Melillo, J. M. (1997). Human domination of Earth's ecosystems. Science 277, 494–499. doi: 10.1126/science.277.5325.494
Wickham, H., Averick, M., Bryan, J., Chang, W., McGowan, L. D., François, R., et al. (2019). Welcome to the tidyverse. J. Open Source Softw. 4:1686. doi: 10.21105/joss.01686
Widdows, C. D., Ramesh, T., and Downs, C. T. (2015). Factors affecting the distribution of large spotted genets (Genetta tigrina) in an urban environment in South Africa. Urban Ecosyst. 18, 1401–1413. doi: 10.1007/s11252-015-0449-5
Winkler, P. (1988). “Feeding behavior of a food-enhanced troop of Hanuman langurs (Presbytis entellus) in Jodhpur, India,” in Ecology and Behavior of Food Enhanced Primate Groups, eds J. E. Fa and C. H. Southwick (New York, NY: Alan R. Liss, Inc.), 3–24.
Woodroffe, R., Thirgood, S., and Rabinowitz, A., (eds.). (2005). People and Wildlife, Conflict or Co-existence? (No. 9). Cambridge: Cambridge University Press. doi: 10.1017/CBO9780511614774
Keywords: gray langur, free-ranging, urbanization, co-existence, feeding preference
Citation: Dasgupta D, Banerjee A, Karar R, Banerjee D, Mitra S, Sardar P, Karmakar S, Bhattacharya A, Ghosh S, Bhattacharjee P and Paul M (2021) Altered Food Habits? Understanding the Feeding Preference of Free-Ranging Gray Langurs Within an Urban Settlement. Front. Psychol. 12:649027. doi: 10.3389/fpsyg.2021.649027
Received: 03 January 2021; Accepted: 15 March 2021;
Published: 26 April 2021.
Edited by:
Nicholas T. Bello, Rutgers, The State University of New Jersey, United StatesReviewed by:
Mewa Singh, University of Mysore, IndiaAnindya Sinha, National Institute of Advanced Studies, India
Ashni Kumar Dhawale contributed to the review of Anindya Sinha
Copyright © 2021 Dasgupta, Banerjee, Karar, Banerjee, Mitra, Sardar, Karmakar, Bhattacharya, Ghosh, Bhattacharjee and Paul. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Manabi Paul, manabii.paul@gmail.com
†These authors have contributed equally to this work and share first authorship
 Dishari Dasgupta1†
Dishari Dasgupta1† Arnab Banerjee
Arnab Banerjee Srijita Karmakar
Srijita Karmakar Pritha Bhattacharjee
Pritha Bhattacharjee Manabi Paul
Manabi Paul