- 1Key Laboratory of Aquatic Botany and Watershed Ecology, Wuhan Botanical Garden, Chinese Academy of Sciences, Wuhan, China
- 2Hubei Key Laboratory of Wetland Evolution and Ecological Restoration, Wuhan Botanical Garden, Chinese Academy of Sciences, Wuhan, China
- 3University of Chinese Academy of Sciences, Beijing, China
- 4Sino-African Joint Research Center, Chinese Academy of Sciences, Wuhan, China
Submerged macrophytes play a structuring role in the shallow freshwater ecosystem by increasing the heterogeneous state in freshwaters. The macrophytes in genus Ottelia were featured for their broad leaves, which might consequently produce specialized functions that differed from other submerged species. To explore the potential ecological role of Ottelia, a field investigation was conducted on leaf traits in eight populations of Ottelia ranging from the southwestern Yunnan–Guizhou plateau to the southern Hainan island in China covering a distance of >1,700 km. The eight populations included all the extant Ottelia species and varieties in China except the well-documented O. alismoides. Carbon-related traits [bicarbonate usage, photosynthetic characteristics, capability of Crassulacean acid metabolism (CAM)], pigment content and parameters of chlorophyll fluorescence, morphology and mass of the leaves were determined. The different populations showed distinct functional traits of mature leaves; O. acuminata var. songmingensis had the thickest and longest leaf with CaCO3 precipitation on the both sides of the leaf, and O. cordata showed putative CAM activity with the highest diel acidity changes 12.5 μequiv g-1 FW. Our results indicated an important role of Ottelia populations in carbon cycling as the dominant species in karst freshwaters in China.
Introduction
Submerged macrophytes are considered as one of the most important primary producers in shallow oligotrophic freshwaters and strongly affect the nutrient turnover for freshwater ecosystem (Wetzel, 1964; Epstein et al., 2012; Olsen et al., 2017). In addition, submerged macrophytes can interact with other organisms, e.g., protecting the zooplankton from fish grazing or providing substrate for periphyton growth (Jeppesen et al., 1998; Cao et al., 2014, 2017). Plant functional traits, as a series of core properties describing the growth, survival and reproduction of plants, are useful tools to explore the ecological function of submerged macrophytes in freshwater systems (Grime, 1974). Most studies related with plant functional traits are focusing on terrestrial forest or grass (Kraft et al., 2008; Klimešová et al., 2016). For example, Kraft et al. (2015) stated that using the combination of functional traits (e.g., the growth form), but not simplistic usage of single functional trait, was important to infer community assembly processes in grassland. For submerged macrophytes, there are only few recent studies related with functional traits, which have investigated the functional traits at the community level along water depth gradients in natural lakes (Fu et al., 2014, 2018; Liu and Wang, 2018).
Leaf is the most important photosynthetic organ, and leaf traits are one of central plant functional traits (Petter et al., 2016; Damián et al., 2018). Especially the concept of ‘leaf economic spectrum’ (LES) has revealed the importance of leaf traits; a typical trade-off between leaf functional traits of >2,000 species has been found, which shows that leaves with the higher photosynthesis rate are featured with shorter life span and lower leaf mass per area (MPA; Wright et al., 2004). In addition, Cornwell et al. (2010) linked the decomposition rate of terrestrial plant litter with the position of the species in the LES, indicating a close relationship between leaf traits and ecosystem function. The submerged macrophytes also have contrasting decomposition rates (Potamogeton crispus vs. P. macckianus), which can significantly affect the carbon cycling in shallow lakes (Wang et al., 2016). However, none of submerged species has been included in LES. Compared with other submerged species, leaves of Ottelia are usually much broader with long petiole, and meantime these leaves can play an extra role in ecosystem carbon cycling through CaCO3 precipitation on the leaf surface compared with those of terrestrial plants (Prins et al., 1982; Yin et al., 2017).
The genus Ottelia widely distributes from tropical to temperate areas and consists of ca. 21 species in the globe1. Among these species, O. alismoides has been under extensive investigation. The species used to widely spread in China and presently under threat due to habitat fragmentation (Chen et al., 2008). As an annual species, the seed germination was featured with density dependence (Yin et al., 2009, 2013). In addition, O. alismoides was found with three carbon concentrating mechanisms, i.e., bicarbonate usage, Crassulacean acid metabolism (CAM) and C4 (Zhang et al., 2014; Shao et al., 2017), which showed potentially strong influence on carbon cycling in freshwater ecosystems dominated by the species (Maberly and Gontero, 2017; Shao et al., 2017). While other species in genus Ottelia were less investigated, and the main focus was about the phylogenetic relationship among these species based on the characteristic of isozyme, flower, seed and qualitative description of the species (Kaul, 1969; Cook et al., 1984; He, 1991). For instance, Chen et al. (2017) has studied five recorded varieties of O. acuminata, an endemic species in China, based on molecular proofs, and the authors stated that most of collected varieties reflected genetically differentiated group, and the genetic divergences could be linked with the past tectonic movements. Other than O. alismoides, the Ottelia species or varieties grew in a localized and relatively stable karst freshwater (Chen et al., 2017). Consistently, Li et al. (2018) assumed a fast speciation process of O. acuminata due to geographic features in these areas. As the dominant submerged species in pristine karst freshwaters, populations of O. acuminata are potentially important factors of carbon source/sink in the ecosystem (Wang et al., 2017). Therefore, an in-situ investigation of leaf traits could give the indispensable information of ecological functions of the Ottelia populations in the freshwater ecosystem.
In this study, we sampled eight Ottelia populations across the distance of 1,700 km in karst freshwaters in China, and we hypothesized that leaf traits of the Ottelia populations can correlate with phylogenetic relationship, and a detailed analysis of leaf traits facilitates to reveal the role of Ottelia populations in the carbon cycling in karst freshwaters.
Materials and Methods
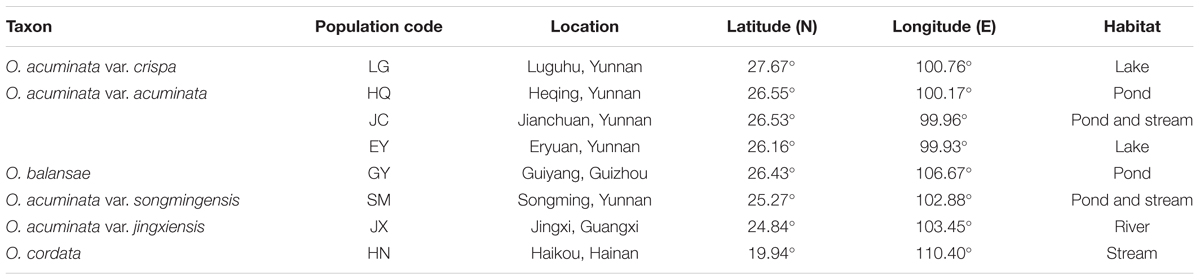
Eight populations in Luguhu (LG), Heqing (HQ), Jianchuan (JC), Eryuan (EY), Guiyang (GY), Songming (SM), Jingxi (JX), and Haikou (HN) were distributed in the provinces of Yunnan, Guizhou, Guangxi and Hainan, covering a distance of >1,700 km. Most of the collected species grew in localized karst freshwaters, and the geographic information of the sampling sites was listed in Table 1.
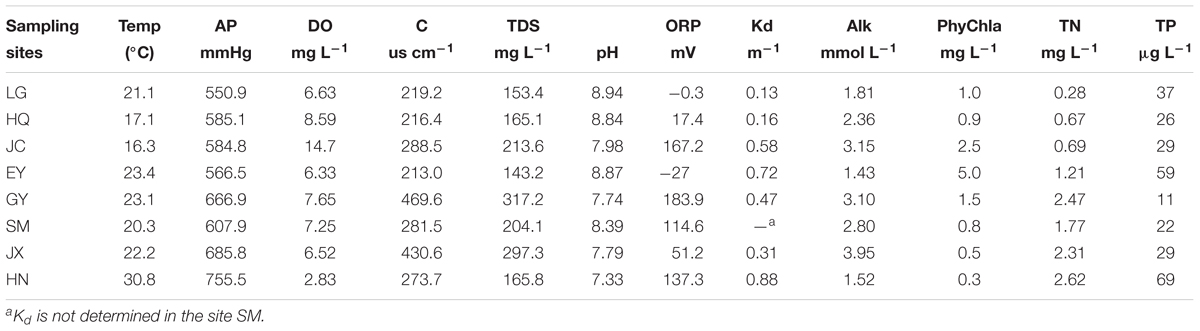
The physico-chemical variables in each site including atmospheric pressure (AP), dissolved oxygen (DO), conductivity (C), total dissolved solid (TDS), pH, and oxidation–reduction potential (ORP) were measured in situ by a YSI Pro Plus multiparameter meter (Xylem, United States). Photosynthetically active radiation (PAR) was determined at the water depth of 0 cm and 40 cm by a LI-1400 Data Logger and a LI-192 underwater quantum sensor (LI-COR, United States), and the light attenuation was calculated assuming an exponential decay of PAR in the water column (Kirk, 1977). Two or more liters of water samples were collected by a plastic tube sampler and separated into aliquot for the determination of total nitrogen (TN), total phosphorus (TP), alkalinity (Alk), and phytoplankton chlorophyll a (PhyChla). Samples for TN and TP were frozen at -20°C, transferred into lab and determined by the K2S2O8 digestion (Huang et al., 1999). Alkalinity (Alk) was determined using the Gran titration of 0.1 M HCl. At least 1 l of water was filtered through the GF/C filter for the determination of PhyChla, and the filter was extracted by 95% ethanol and determined by a spectrophotometer (Jespersen and Christoffersen, 1987).
Based on Kraft et al. (2015), we used the combination of functional traits (not a single trait) to evaluate the ecological functions of the leaves of Ottelia. We classified the leaf traits into three categories: leaf morphology and mass, pigment content and parameters of chlorophyll fluorescence and carbon-related traits (bicarbonate usage, capability of CAM and photosynthetic characteristics).
The individuals of Ottelia were harvested gently from the ponds or rivers. Twenty intact leaves were randomly chosen for the determination of leaf morphology and fresh weight. The maximum width and the maximum length were measured by a ruler, and the thickness was determined by a vernier caliper. The leaf was then placed into a standard plate and photographed for the determination of the leaf area (Shao et al., 2017). Afterward, the leaf was blotted by an absorbent paper and submerged into a half-filled measuring cylinder, and the volume changes of the water in the cylinder was estimated as the leaf volume.
Leaf pigment content including chlorophyll a (Chla), chlorophyll b (Chlb), and carotenoids (Car) was measured by the extraction of 95% ethanol (Huang et al., 1999). The parameters of leaf chlorophyll fluorescence were determined by chlorophyll fluorometer MONITORING-PAM (Walz, Germany) following the methods in Kramer et al. (2004) and Jiang et al. (2017). ΦPSII is derived from a pure ‘lake’ or ‘puddle’ model referring to the fraction of the energy absorbed by photosystem (PS) II that is used in photochemistry. ΦNPQ and ΦNO are used to estimate the flux of excitation energy into the non-photochemical pathways, and ΦNPQ refers to the yield induced by downregulatory processes, ΦNO for the yield of other energy losses. ΦPSII, ΦNPQ, and ΦNO were obtained by an induction curve by setting the PAR of the active light at 127 μmol E-1 s-2. rETRmax and Ik were acquired by a rapid light curve using the 12 steps of PAR gradients from ca. 2 to 1500 μmol E-1 s-2.
The end-point pH in 1 mM Na/KHCO3 solution for the leaves of each population was determined by a pH-drift method (Zhang et al., 2014). CAM capability was determined according to the diel change of acidity in leaves using the titration of 0.01M NaOH to pH 8.3 (Shao et al., 2017). Photosynthesis rates were determined by measuring the changes of DO in the 50 ml glass bottles prior to and 30 min after adding the leaves into the bottle at the concentrations of 0, 1, 2, 4, 6, 8, and 10 mM dissolved inorganic carbon (DIC, represented as Na/KHCO3), respectively. As a commonly used method, the DIC in the water was stable during the determination (Zhang et al., 2014; Clement et al., 2016). The light was provided by LED light bulb (ca. 100 μmol E-1 s-2), and the dark respiration rates were determined in the brown bottles prior to and 30 min after adding the leaves into the bottle at the concentrations of 0 and 10 mM DIC. The net photosynthesis rates at different concentrations of DIC was fitted to a slightly modified Michaelis–Menten equation that considered the compensation point for DIC (Clement et al., 2016). The equation is:
where (rate as mg O2 g-1 FW h-1 and concentration as mM) Vmax is the maximum rate of net photosynthesis; CP is the DIC compensation concentration; Khalf is the concentration of DIC producing half-maximal rates of net photosynthesis.
Five replicates were measured for the CAM capability, and three replicates were measured for other indicators of macrophyte leaves.
Statistical Analysis
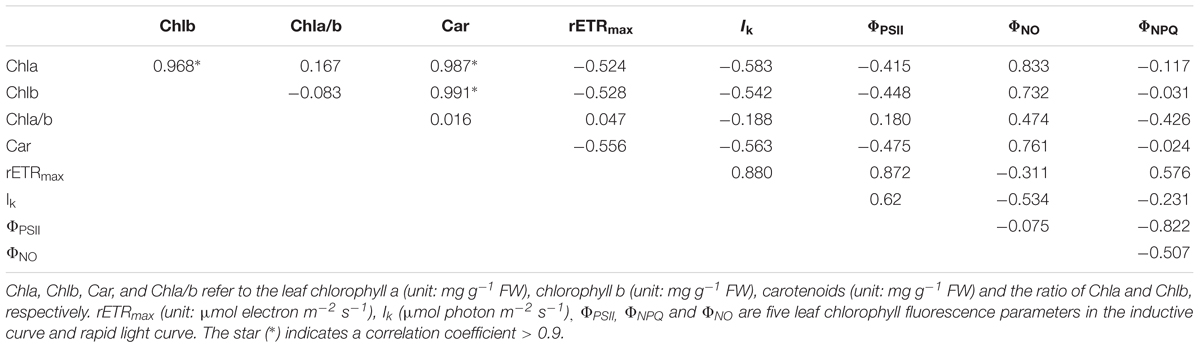
For most indicators of leaf traits, one-way ANOVA was used to analyze the difference among the eight populations. Post hoc test was conducted using Tukey method at the significance level of 0.05. The data was log-transformed to achieve variance homogeneity prior to the statistical analyses, if needed. Because the diel change of leaf acidity was determined by the difference of mean leaf acidity at dusk and at dawn and thus had no replicates, it was not quantitatively analyzed by ANOVA. Vmax and Khalf were estimated by fitting the Michaelis–Menten equation with standard errors (as stated above). The traits were classified into three categories: leaf morphology and mass, pigment content and parameters of chlorophyll fluorescence and carbon-related traits (bicarbonate usage, capability of CAM and photosynthetic characteristics). Pearson correlation and the cluster analysis were analyzed within each aspect. In this study the correlation coefficient that is >0.9 was defined as a strong relationship between the leaf traits, and only one trait was included in the further clustering analysis to reduce the statistical bias. The clustering analysis was conducted by package ‘mclust’ using the ‘euclidean distance’ after ‘scale’ the data. All the statistical analyses were determined in R 3.4.0. Data is presented in mean ± SD if not explicitly stated.
Results
Physic-Chemical Conditions in the Eight Sampling Sites
As shown in Table 2, most sites had low nutrient levels, low phytoplankton biomass, low light attenuation, and slight alkaline with relative high alkalinity, indicating a pristine status with clear water.
pH-Drift
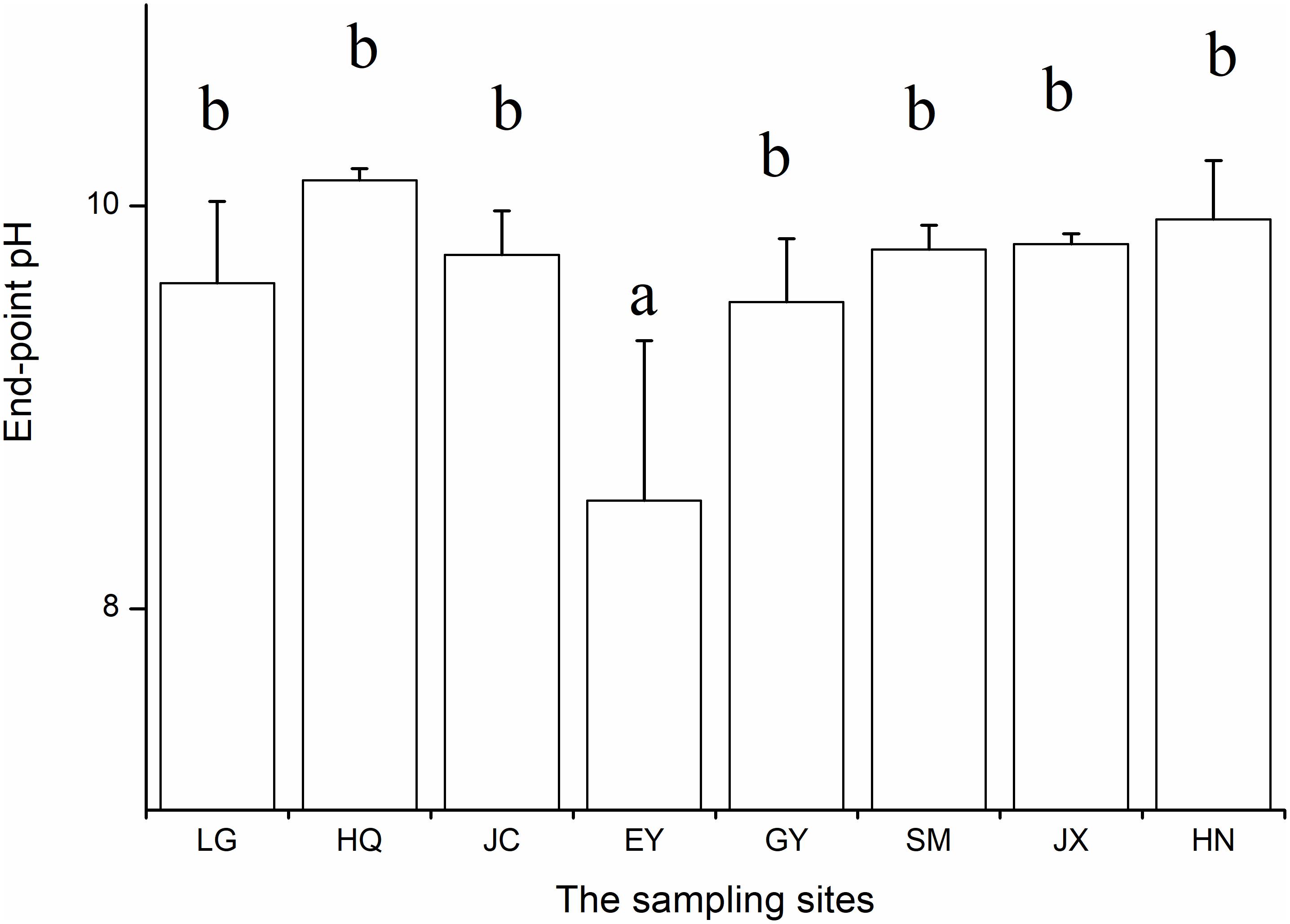
At the end of the pH-drift experiment pH was lowest in the EY population (Figure 1). After excluding the EY population, the end-point pH did not differ among the rest seven populations (ANOVA, F = 1.98, p > 0.05). Only the end-point pH in the HQ population is >10 (10.13 ± 0.06). Consistently, we observed that CaCO3 precipitation on the surface of the mature leaves in all eight populations, especially on two sides of leaves of O. acuminata var. songmingensis.
Carbon-Related Leaf Traits
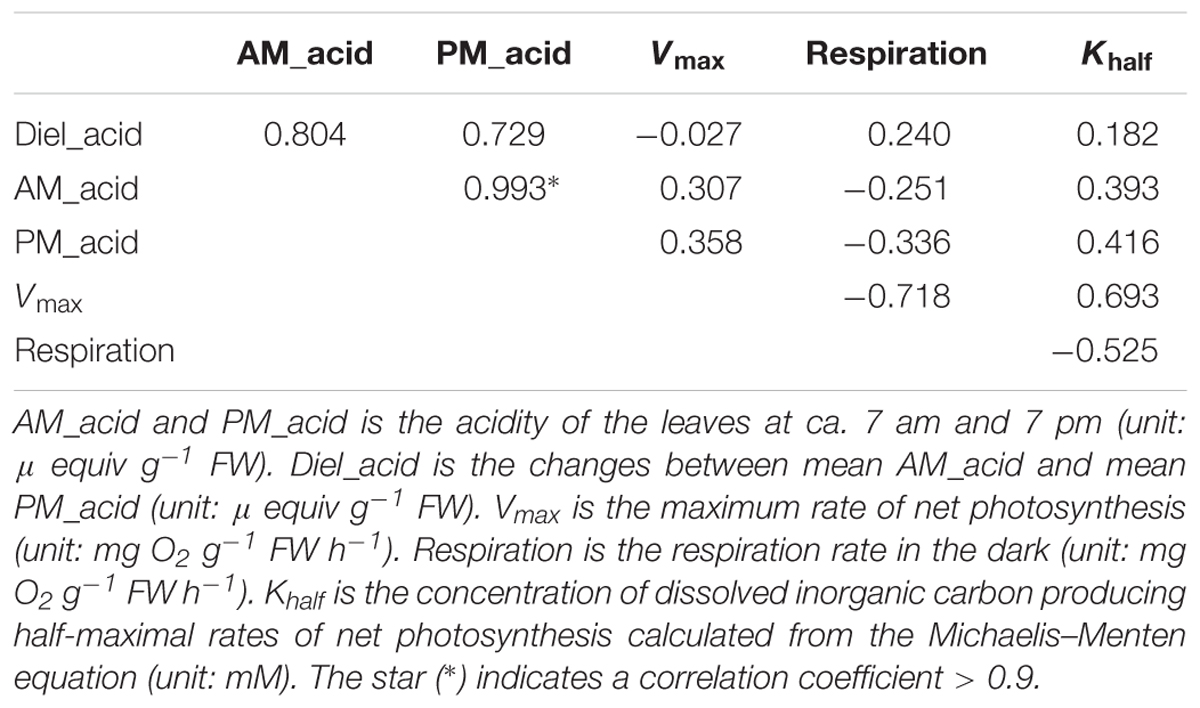
The leaf acidity at dawn and dusk was highest in the HN population (Figure 2 and Supplementary Table S1). The largest diel acidity change was also detected in the HN population, arriving at 12.5 μequiv g-1 FW. Vmax and Khalf were both high in the SM population but with large variation among the replicates. The respiration rate in the dark was highest in the JC population, intermediate in the EY population, and lowest in the HN population. Since there was strong correlation between the leaf acidity at dusk and at dawn (Table 3), the further cluster analysis only included the leaf acidity at dusk. The hierarchical clustering revealed three clusters in the eight populations (Figure 3). The HQ, GY, EY, and JC populations were grouped into one cluster, and the HN population were one cluster, with the rest three as one cluster.
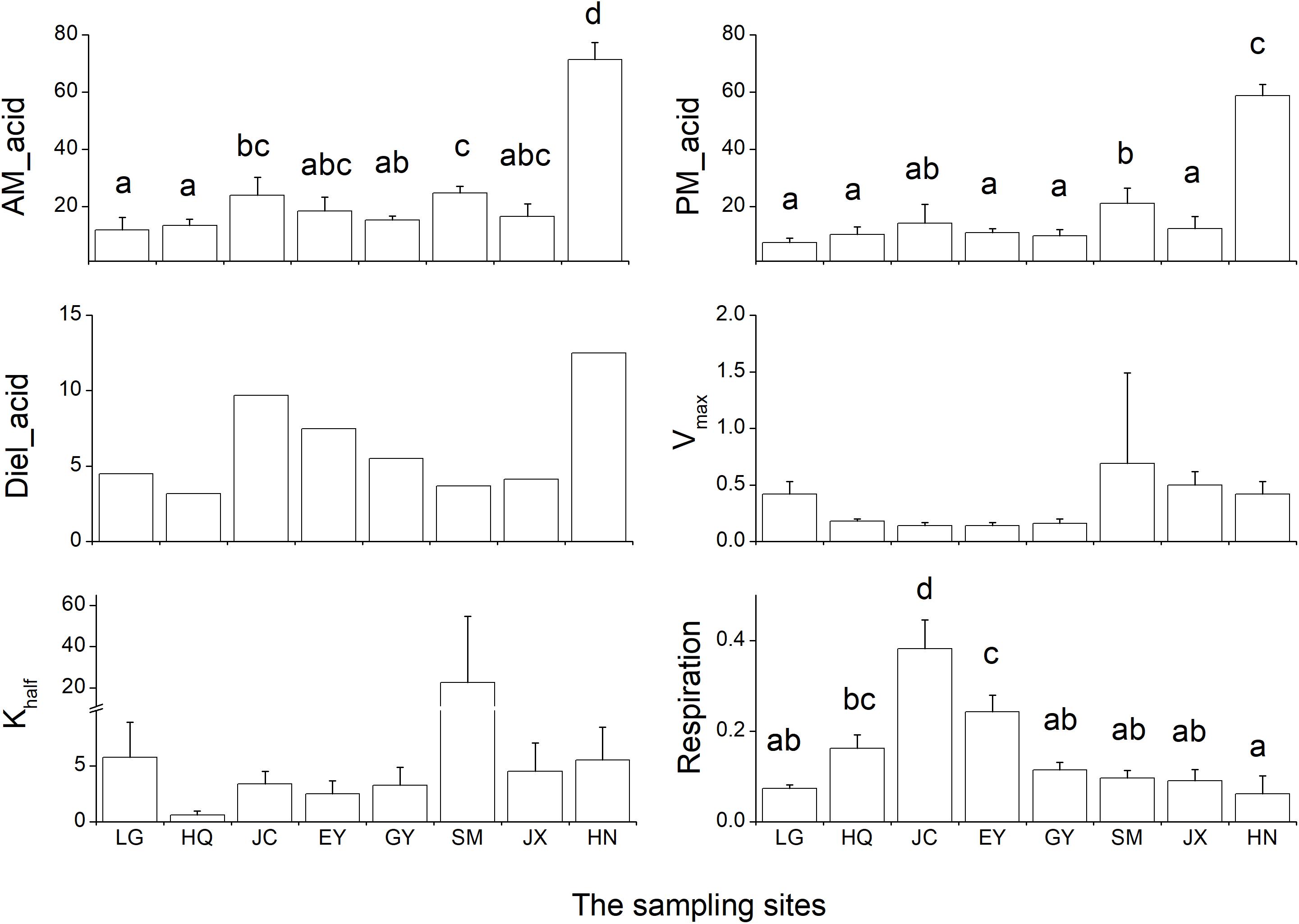
Figure 2. The six carbon-related leaf traits in the eight Ottelia populations. AM_acid and PM_acid is the acidity of the leaves at ca. 7 am and 7 pm (unit: μ equiv g-1 FW). Diel_acid is the changes between mean AM_acid and mean PM_acid (unit: μ equiv g-1 FW). Vmax is the maximum rate of net photosynthesis (unit: mg O2 g-1 FW h-1), and Khalf is the concentration of dissolved inorganic carbon producing half-maximal rates of net photosynthesis calculated from the Michaelis–Menten equation (unit: mM). Respiration is the respiration rate in the dark (unit: mg O2 g-1 FW h-1). Due to the method of calculation, Diel_acid, Vmax and Khalf are not analyzed by ANOVA and therefore no statistical results for post hoc test. The different letters in the figure indicate the significant difference among the eight population.
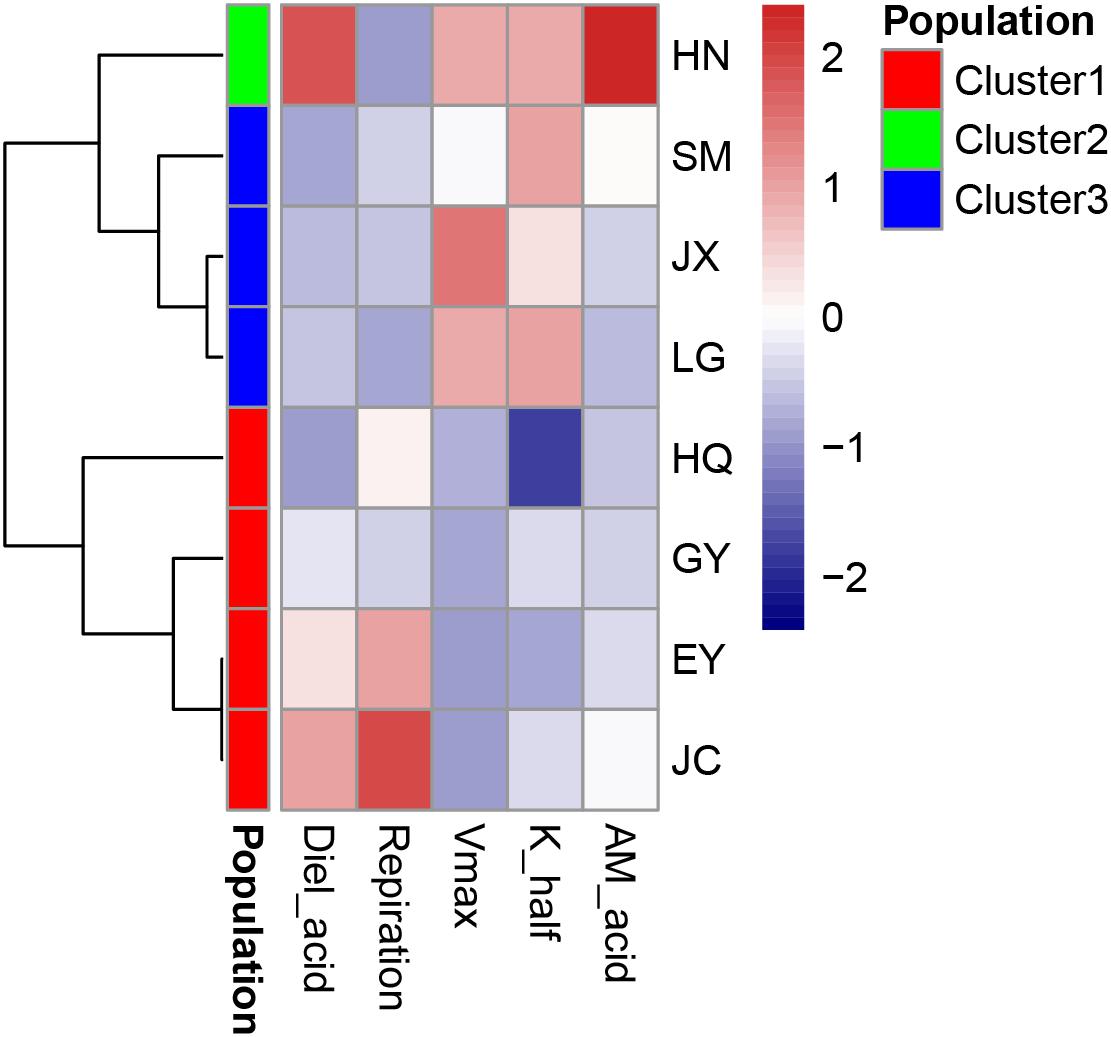
Figure 3. The cluster analysis of carbon-related leaf traits in the eight Ottelia populations. AM_acid is the acidity of the leaves at ca. 7 am (unit: μ equiv g-1 FW). Diel_acid is the changes of leaf acidity between ca. 7 am (dusk) and 7 pm (dawn) (unit: μ equiv g-1 FW). Vmax is the maximum rate of net photosynthesis (unit: mg O2 g-1 FW h-1), and Khalf is the concentration of dissolved inorganic carbon producing half-maximal rates of net photosynthesis calculated from the Michaelis–Menten equation (unit: mM). Respiration is the respiration rate in the dark (unit: mg O2 g-1 FW h-1).
Leaf Pigment Content and Chlorophyll Fluorescence
The leaf chlorophyll a (Chla), chlorophyll b (Chlb) and carotenoids were highest in the JC population, intermediate in the JX population and lowest in the HN population (Figure 4). In contrast, the ratio of Chla and Chlb (Chla/b) was lowest in the GY population.
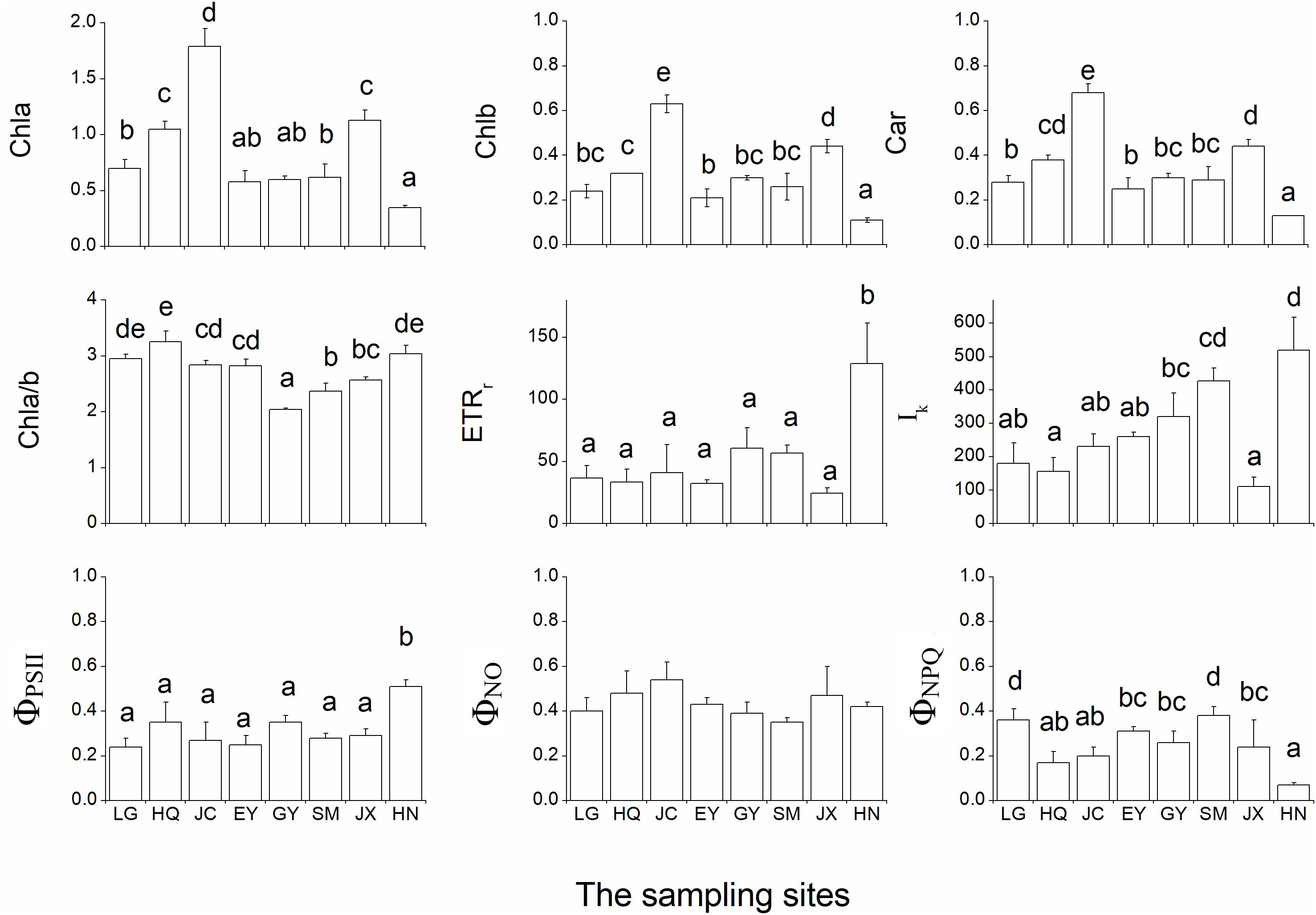
Figure 4. The nine pigment-related leaf traits in the eight populations. Chla, Chlb, Car, and Chla/b refer to the leaf chlorophyll a (unit: mg g-1 FW), chlorophyll b (unit: mg g-1 FW), carotenoids (unit: mg g-1 FW) and the ratio of Chla and Chlb, respectively. rETRmax (unit: μmol electron m-2 s-1), Ik (μmol photon m-2 s-1), ΦPSII, ΦNPQ and ΦNO are five leaf chlorophyll fluorescence parameters in the inductive curve and rapid light curve. The different letters in the figure indicate the significant difference among the eight population.
Both rETRmax and ΦPSII were significantly higher in the HN than in the other populations (Figure 4). Ik was low in the HQ and JX populations and high in the SM and HN populations. ΦNO did not differ among the eight populations while ΦNPQ was highest in LG and SM populations.
The strong correlation was found among Chla, Chlb, and carotenoids (Table 4). The cluster analysis discovers only one cluster for the eight populations (Figure 5).
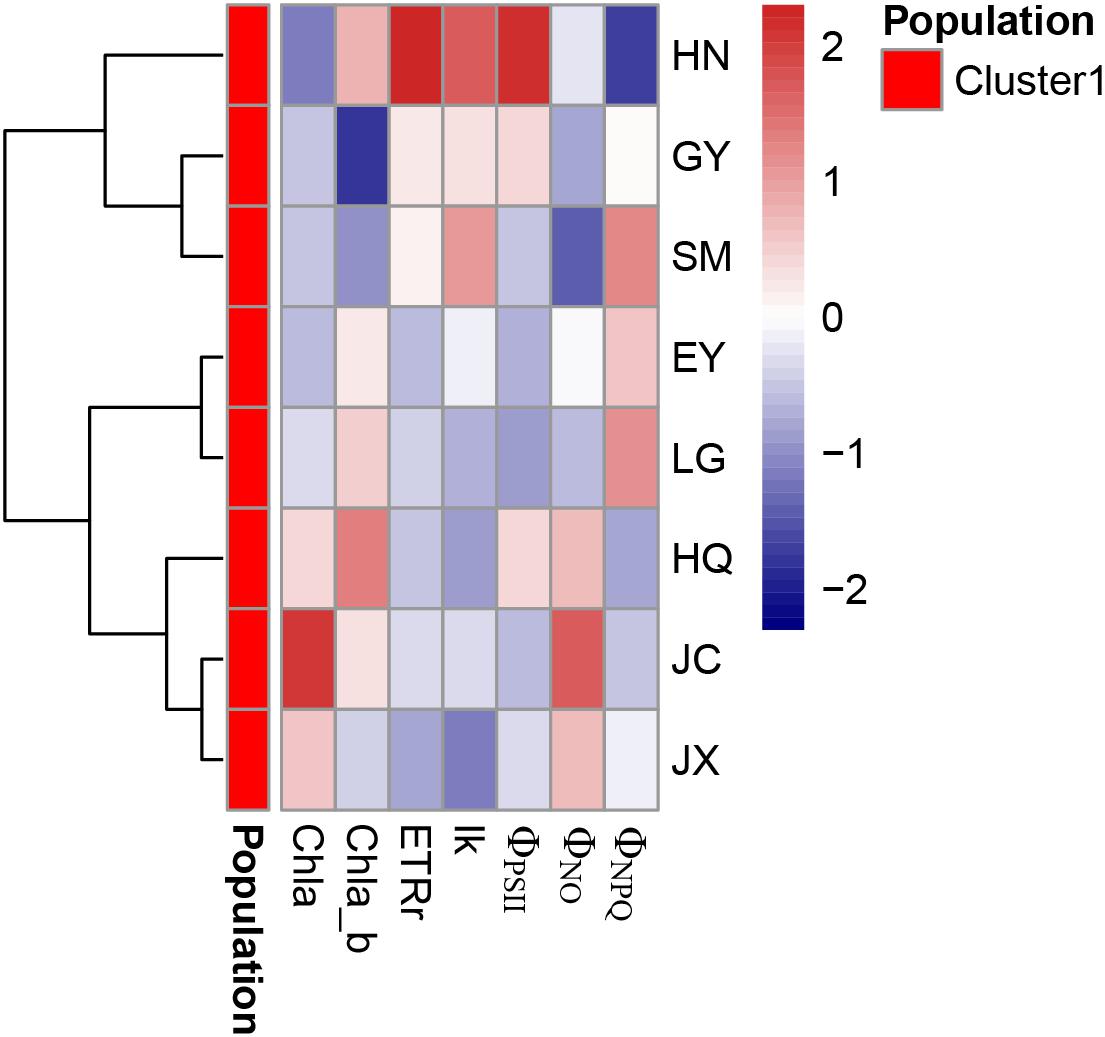
Figure 5. The cluster analysis of pigment-related leaf traits in the eight populations. Chla, Chlb, Car, and Chla/b refer to the leaf chlorophyll a (unit: mg g-1 FW), chlorophyll b (unit: mg g-1 FW), carotenoids (unit: mg g-1 FW) and the ratio of Chla and Chlb, respectively. rETRmax (unit: μmol electron m-2 s-1), Ik (μmol photon m-2 s-1), ΦPSII, ΦNPQ, and ΦNO are five leaf chlorophyll fluorescence parameters in the inductive curve and rapid light curve.
Leaf Traits of Morphology and Mass
The SM population had the largest leaf length (reaching ca. 100 cm), length/width ratio (Len/width), thickness, area, volume, fresh weight, MPA compared with the rest seven populations (Figure 6). While for leaf width, the EY population had the highest values, the LG population the lowest.
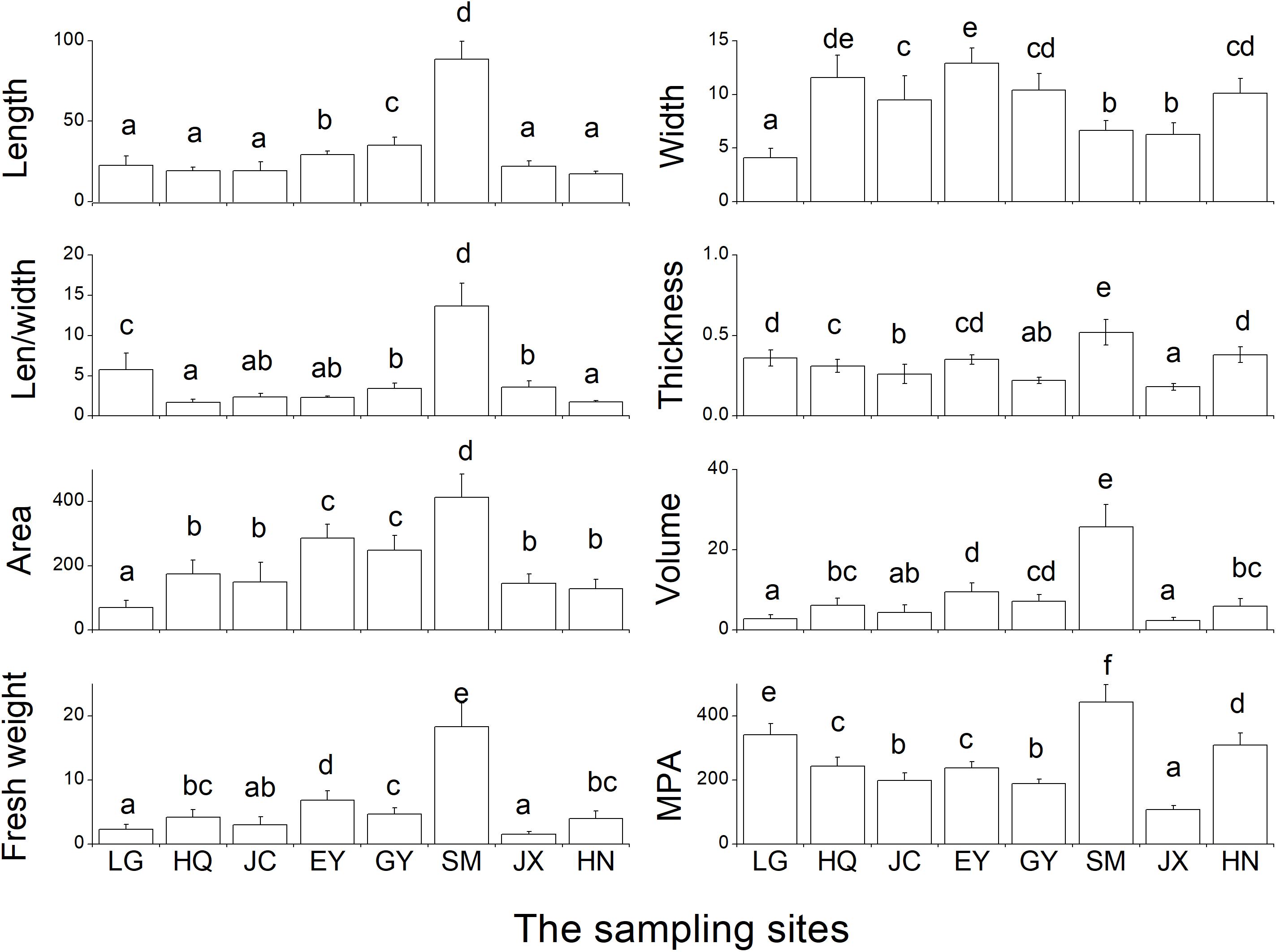
Figure 6. The eight leaf traits of morphology and biomass in the eight populations. Len/width refers to the ratio of leaf length and leaf width. MPA refers to the biomass per area of the determined leaf (unit: g m-2). Leaf length and width is with the unit of cm, thickness with the unit of mm, area with the unit of cm2, and volume with the unit of cm3, fresh weight with the unit of g. The different letters in the figure indicate the significant difference among the eight population.
There was strong correlation among several traits of leaf morphology and mass (Table 5), and only four traits (leaf length, width, area and thickness) were included in the further hierarchical clustering analysis (Figure 7). Seven clusters were identified with the HQ and JC populations as one cluster, and other populations are distinct from each other.
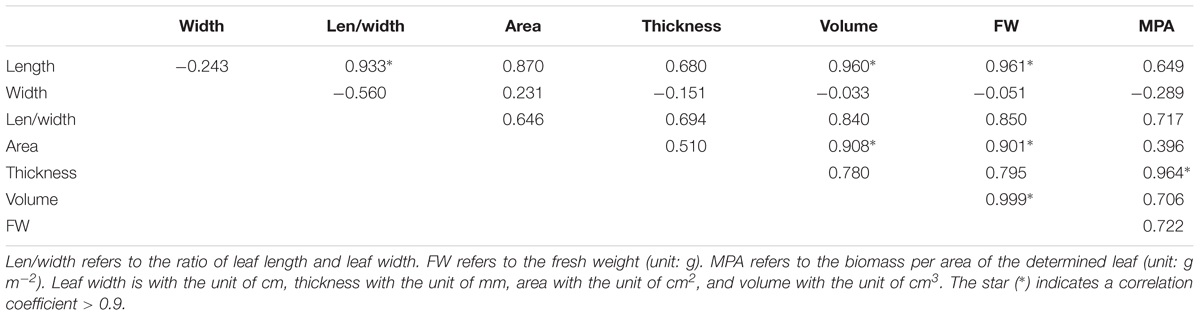
Table 5. The Pearson correlation between eight leaf traits of morphology and biomass in the eight populations.
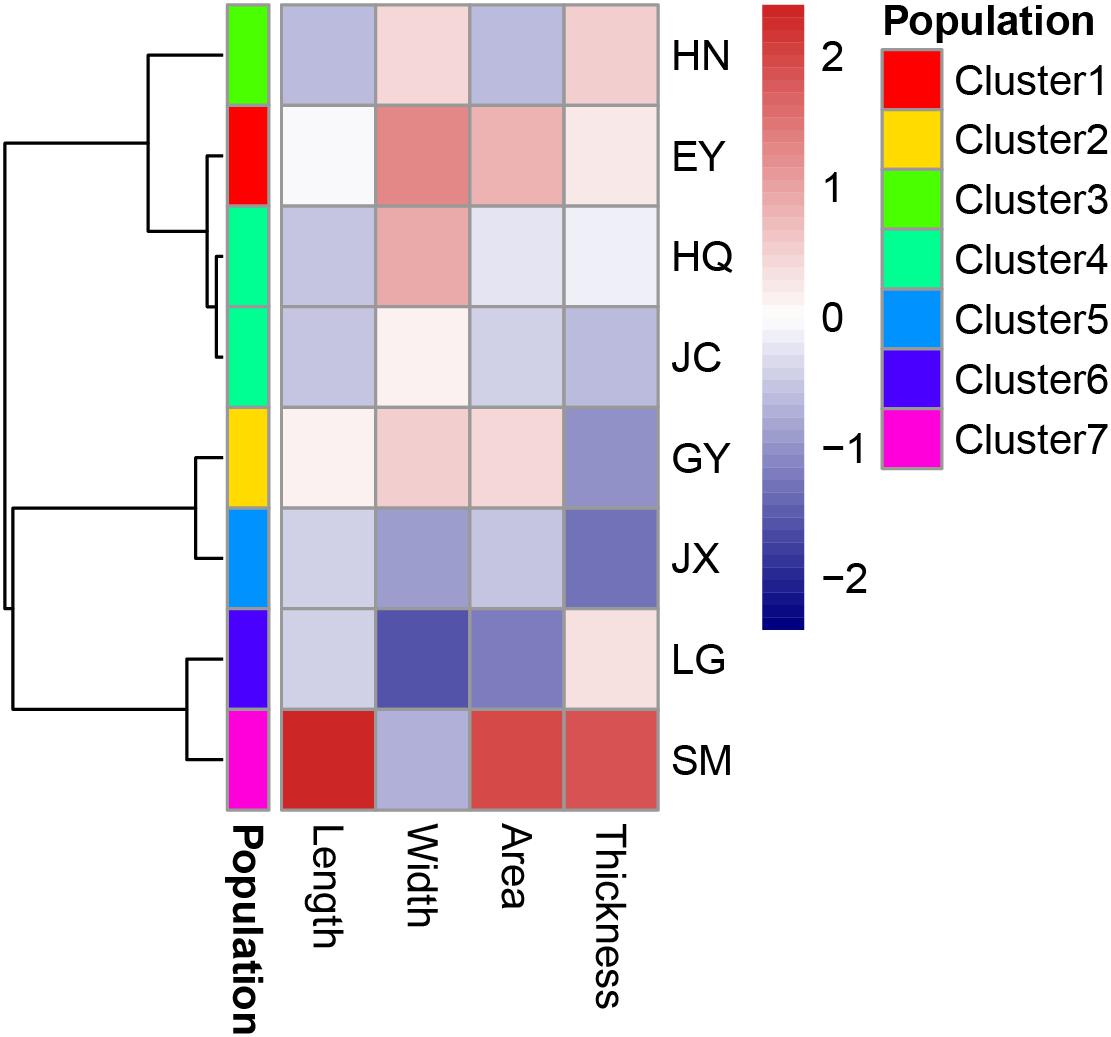
Figure 7. The cluster analysis of leaf traits of morphology and biomass in the eight populations. Leaf length and width is with the unit of cm, area with the unit of cm2 and thickness with the unit of mm.
Discussion
Based on 24 leaf traits, we have provided a quantitative profile of the eight Ottelia populations in field. After grouped into three aspects (traits of morphology and mass, carbon-related traits and leaf pigment and chlorophyll florescence parameters), the leaf traits showed different divergences in these populations. The physiological traits (such as diel acidity changes, leaf florescence parameters, and etc.) were usually measured in the studies of macrophytes exposed to toxicity or under other stress due to the sensitive responses (Zhang et al., 2014; Shao et al., 2017). While in this study, the physiological traits were less sensitively divergent than morphological ones (seven clusters) among the eight populations, probably reflecting the physiological adaptation to the clear water conditions benign for the growth of Ottelia. The quantitatively determined traits showed to some extent correlation with the phylogenetic relationship, implying that variation or speciation of Ottelia in these isolated water systems have produces various traits in each population. For example, the morphological traits were clustered into seven groups (the two populations from the same O. acuminata var. acuminata were merged into one cluster), and the HN population was singled out base on the carbon-related traits. This is consistent with the results of He (1991) using several qualitative or semi-quantitative traits such as flower, seed and other structures. We also found special features within two populations. The SM population (O. acuminata var. songmingensis) had thickest and longest leaves in all the population and interestingly with calcium precipitation on both sides of the leaves, an interesting phenomenon that requires further study. The HN population (O. cordata) had high diel acidity changes, only similar with O. alismoides but not with other species in the genus Ottelia in China, which provided a clue for future phylogenetic research.
The leaf functional traits have been well investigated in terrestrial plants, and the photosynthesis rate, leaf weight per area and leaf life span of >2,000 species of plants have been recorded (Wright et al., 2004). The authors proposed a LES and claimed that there was a typical trade-off between leaf functional traits that leaves with high photosynthesis rate are featured low life span and low leaf MPA (Wright et al., 2004). To our knowledge, this study is the first attempt to systematically analyze the leaf functional traits in submerged freshwater macrophytes. The low leaf MPA and high photosynthesis rate of the Ottelia leaves fit well with LES and placed in the spectrum of the quick return on nutrient and biomass investment. Accordingly, the leaf life span of Ottelia was expected to be short as other submerged species (Hemminga et al., 1999; Yamamoto et al., 1999; Kamermans et al., 2001). In addition, Cornwell et al. (2010) revealed the predominant influence of plant functional traits on decomposition rates at a global scale. Based on our findings on functional traits of Ottelia populations, the decomposition rate and carbon turnover of these populations should be very fast, similar to Potamogeton crispus (Wang et al., 2016), and thus a fast carbon cycling was expected for the Ottelia-dominated karst freshwaters. However, our results, together with Yin et al. (2017), indicated that all the species or varieties of the genus Ottelia in China could use bicarbonate as inorganic carbon supply though there was variation among the populations. Similar like Potamogeton lucens (Prins et al., 1982), the Ottelia leaves were expected with polarity of pH between the adaxial and abaxial side, but with much broader leaves probably stronger effects on the polarity. Therefore, the calcium precipitation (as CaCO3) on the surfaces of Ottelia leaves could bring abundant carbon burial when the macrophytes were dominant producers and thus strongly affect the carbon cycling. Thirdly, high diel acidity changes >10 μequiv g-1 FW were found even at high inorganic carbon supply, which is potentially inducible CAM feature similar with that O. alismoides showed in Shao et al. (2017). A dense macrophyte bed with strong CAM capacity could also affect the pH of water column at night (Keeley, 1983). Consequently, the changes of pH have a strong effect on the inorganic carbon species in the water column and other primary producers, e.g., periphyton on the leaves (Maberly, 1996; Hao et al., 2017). In summary, the role of Ottelia populations on the carbon cycles in the karst freshwaters warrants further studies.
There is also small variation of plant traits among the three populations (HQ, EY, and JC) belonging to the same variety O. acuminata var. acuminata. The lower end-point pH in EY population concurred with habitat fragmentation and destruction in Eryuan County. During our field survey, we found that the natural populations of O. acuminata var. lunanen and O. emersa disappeared due to habitat destruction. The extinct of the Ottelia populations should be highlighted in the future projects of macrophyte conservation.
Conclusion
Our trait analyses profiled the eight populations of genus Ottelia in details. With the unique growth form Otteliids and limited distribution in the localized area, the investigated species or varieties may form special effects on the growing freshwaters distinct from other submerged species. Our results indicated an important ecological role of submerged macrophyte Ottelia spp. in the karst freshwaters. More studies about whether direct uptake of bicarbonate or relying on extracellular carbonic anhydrase in Ottelia leaves would provide more accurate knowledge about the effects of the species on other primary producers and the carbon cycling in the karst freshwaters.
Author Contributions
YC, WL, and HJ designed the experiments. YC and LX determined the physic-chemical variables. YL determined the leave morphology. LN determined the photosynthesis rate. HJ determined the acidity of leaves. YC and HJ wrote the first edition of the manuscript, and other co-authors contributed to the modification of the manuscript.
Funding
This research was supported by the Strategic Priority Research Program of Chinese Academy of Sciences, Grant No. XDB31010000 and the National Natural Science Foundation of China (31870345 and 31670368).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to Zhizhong Li, Yu Cheng, Ying Zhang, Haisu Wang, and Quan Jin during the field sampling.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2018.01938/full#supplementary-material
Footnotes
- ^http://foc.eflora.cn/content.aspx?TaxonId=123432, accessed on 06-28-2018.
References
Cao, Y., Li, W., and Jeppesen, E. (2014). The response of two submerged macrophytes and periphyton to elevated temperatures in the presence and absence of snails: a microcosm approach. Hydrobiologia 738, 49–59. doi: 10.1007/s10750-014-1914-5
Cao, Y., Olsen, S., Gutierrez, M. F., Brucet, S., Davidson, T. A., Li, W., et al. (2017). Temperature effects on periphyton, epiphyton and epipelon under a nitrogen pulse in low-nutrient experimental freshwater lakes. Hydrobiologia 795, 267–279. doi: 10.1007/s10750-017-3140-4
Chen, J.-M., Du, Z.-Y., Long, Z.-C., Gichira, A. W., and Wang, Q.-F. (2017). Molecular divergence among varieties of Ottelia acuminata (Hydrocharitaceae) in the yunnan-guizhou plateau. Aquat. Bot. 140, 62–68. doi: 10.1016/j.aquabot.2017.03.001
Chen, Y.-Y., Li, X.-L., Yin, L.-Y., and Li, W. (2008). Genetic diversity of the threatened aquatic plant Ottelia alismoides in the yangtze river. Aquat. Bot. 88, 10–16. doi: 10.1016/j.aquabot.2007.08.002
Clement, R., Dimnet, L., Maberly, S. C., and Gontero, B. (2016). The nature of the CO2-concentrating mechanisms in a marine diatom, Thalassiosira pseudonana. New Phytol. 209, 1417–1427. doi: 10.1111/nph.13728
Cook, C. D. K., Symoens, J.-J., and Urmi-König, K. (1984). A revision of the genus Ottelia (hydrocharicaea) I. generic considerations. Aquat. Bot. 18, 263–274. doi: 10.1016/0304-3770(84)90068-8
Cornwell, W. K., Cornelissen, J. H., Amatangelo, K., Dorrepaal, E., Eviner, V. T., Godoy, O., et al. (2010). Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol. Lett. 11, 1065–1071. doi: 10.1111/j.1461-0248.2008.01219.x
Damián, X., Fornoni, J., Domínguez, C. A., and Boege, K. (2018). Ontogenetic changes in the phenotypic integration and modularity of leaf functional traits. Funct. Ecol. 32, 234–246. doi: 10.1111/1365-2435.12971
Epstein, D. M., Wurtsbaugh, W. A., and Baker, M. A. (2012). Nitrogen partitioning and transport through a subalpine lake measured with an isotope tracer. Limnol. Oceanogr. 57, 1503–1516. doi: 10.4319/lo.2012.57.5.1503
Fu, H., Yuan, G., Lou, Q., Taotao, D., Xu, J., Cao, T., et al. (2018). Functional traits mediated cascading effects of water depth and light availability on temporal stability of a macrophyte species. Ecol. Indic. 89, 168–174. doi: 10.1016/j.ecolind.2018.02.010
Fu, H., Zhong, J., Yuan, G., Xie, P., Guo, L., Zhang, X., et al. (2014). Trait-based community assembly of aquatic macrophytes along a water depth gradient in a freshwater lake. Freshw. Biol. 59, 2464–2471. doi: 10.1111/fwb.12443
Grime, J. P. (1974). Vegetation classification by reference to strategies. Nature 250, 26–31. doi: 10.1038/250026a0
Hao, B., Wu, H., Cao, Y., Xing, W., Jeppesen, E., and Li, W. (2017). Comparison of periphyton communities on natural and artificial macrophytes with contrasting morphological structures. Freshw. Biol. 62, 1783–1793. doi: 10.1111/fwb.12991
He, J. B. (1991). Systematic Botanical and Biosystematic Studies on Ottelia in China. Wuhan: Wuhan University Press.
Hemminga, M. A., Marba, N., and Stapel, J. (1999). Leaf nutrient resorption, leaf lifespan and the retention of nutrients in seagrass systems. Aquat. Bot. 65, 141–158. doi: 10.1016/S0304-3770(99)00037-6
Huang, X. F., Chen, W. M., and Cai, Q. M. (1999). “Survey, observation and analysis of lake ecology,” in Standard Methods for Observation and Analysis in Chinese Ecosystem Research Network, Series V, (Beijing: Standards Press of China).
Jeppesen, E., Sondergaard, M., Sondergaard, M., and Christofferson, K. (1998). The Structuring Role of Submerged Macrophytes in Lakes. New York, NY: Springer Science & Business Media. doi: 10.1007/978-1-4612-0695-8
Jespersen, A.-M., and Christoffersen, K. (1987). Measurements of chlorophyll—a from phytoplankton using ethanol as extraction solvent. Arch. Hydrobiol. 109, 445–454. doi: 10.3791/51441
Jiang, H. S., Yin, L. Y., Ren, N. N., Zhao, S. T., Li, Z., Zhi, Y., et al. (2017). Silver nanoparticles induced reactive oxygen species via photosynthetic energy transport imbalance in an aquatic plant. Nanotoxicology 11, 157–167. doi: 10.1080/17435390.2017.1278802
Kamermans, P., Hemminga, M. A., Marbà, N., Mateo, M. A., Mtolera, M., and Stapel, J. (2001). Leaf production, shoot demography, and flowering of Thalassodendron ciliatum along the east African coast. Aquat. Bot. 70, 243–258. doi: 10.1016/S0304-3770(01)00156-5
Kaul, R. B. (1969). Morphology and development of the flowers of Boottia cordata, Ottelia alismoides, and their synthetic hybrid (Hydrocharitaceae). Am. J. Bot. 56, 951–959. doi: 10.1002/j.1537-2197.1969.tb09746.x
Keeley, J. E. (1983). Crassulacean acid metabolism in the seasonally submerged aquatic Isoetes howellii. Oecologia 58, 57–62. doi: 10.1007/BF00384542
Kirk, J. T. O. (1977). Attenuation of light in natural waters. Mar. Freshw. Res. 28, 497–508. doi: 10.1071/MF9770497
Klimešová, J., Tackenberg, O., and Herben, T. (2016). Herbs are different: clonal and bud bank traits can matter more than leaf–height–seed traits. New Phytol. 210, 13–17. doi: 10.1111/nph.13788
Kraft, N. J. B., Godoy, O., and Levine, J. M. (2015). Plant functional traits and the multidimensional nature of species coexistence. Proc. Natl. Acad. Sci. U.S.A. 112, 797–802. doi: 10.1073/pnas.1413650112
Kraft, N. J. B., Valencia, R., and Ackerly, D. D. (2008). Functional traits and niche-based tree community assembly in an amazonian forest. Science 322, 580–582. doi: 10.1126/science.1160662
Kramer, D. M., Johnson, G., Kiirats, O., and Edwards, G. E. (2004). New fluorescence parameters for the determination of QA redox state and excitation energy fluxes. Photosynth. Res. 79, 209–218. doi: 10.1023/B:PRES.0000015391.99477.0d
Li, Z.-Z., Lu, M.-X., Gichira, A. W., Islam, M. R., Wang, Q.-F., and Chen, J.-M. (2018). Genetic diversity and population structure of Ottelia acuminata var. jingxiensis, an endangered endemic aquatic plant from southwest China. Aquat. Bot. 152, 20–26. doi: 10.1016/j.aquabot.2018.09.004
Liu, X., and Wang, H. (2018). Contrasting patterns and drivers in taxonomic versus functional diversity, and community assembly of aquatic plants in subtropical lakes. Biodivers. Conserv. 27, 3103–3118. doi: 10.1007/s10531-018-1590-2
Maberly, S. C. (1996). Diel, episodic and seasonal changes in pH and concentrations of inorganic carbon in a productive lake. Freshw. Biol. 35, 579–598. doi: 10.1111/j.1365-2427.1996.tb01770.x
Maberly, S. C., and Gontero, B. (2017). Ecological imperatives for aquatic carbon dioxide-concentrating mechanisms. J. Exp. Bot. 68, 3797–3814. doi: 10.1093/jxb/erx201
Olsen, S., Cao, Y., Florencia Gutierrez, M., Brucet, S., Landkildehus, F., Lauridsen, T. L., et al. (2017). Effect of a nitrogen pulse on ecosystem N processing at different temperatures: a mesocosm experiment with 15NO3- addition. Freshw. Biol. 62, 1232–1243. doi: 10.1111/fwb.12940
Petter, G., Wagner, K., Wanek, W., Sánchez Delgado, E. J., Zotz, G., Cabral, J. S., et al. (2016). Functional leaf traits of vascular epiphytes: vertical trends within the forest, intra- and interspecific trait variability, and taxonomic signals. Funct. Ecol. 30, 188–198. doi: 10.1111/1365-2435.12490
Prins, H. B. A., Snel, J. F. H., Zanstra, P. E., and Helder, R. J. (1982). The mechanism of bicarbonate assimilation by the polar leaves of potamogeton and elodea-CO2 concentrations at the leaf surface. Plant Cell Environ. 5, 207–214. doi: 10.1111/1365-3040.ep11571916
Shao, H., Gontero, B., Maberly, S. C., Jiang, H. S., Cao, Y., Li, W., et al. (2017). Responses of Ottelia alismoides, an aquatic plant with three CCMs, to variable CO2 and light. J. Exp. Bot. 68, 3985–3995. doi: 10.1093/jxb/erx064
Wang, H. J., Wang, H. Z., Liang, X. M., Pan, B. Z., and Kosten, S. (2016). Macrophyte species strongly affects changes in C, N, and P stocks in shallow lakes after a regime shift from macrophyte to phytoplankton dominance. Inl. Waters 6, 449–460. doi: 10.1080/IW-6.3.837
Wang, P., Hu, G., and Cao, J. (2017). Stable carbon isotopic composition of submerged plants living in karst water and its eco-environmental importance. Aquat. Bot. 140, 78–83. doi: 10.1016/j.aquabot.2017.03.002
Wetzel, R. G. (1964). A comparative study of the primary production of higher aquatic plants, periphyton, and phytoplankton in a large, shallow lake. Int. Rev. Hydrobiol. 49, 1–61. doi: 10.1002/iroh.19640490102
Wright, I. J., Reich, P. B., Mark, W., Ackerly, D. D., Zdravko, B., Frans, B., et al. (2004). The worldwide leaf economics spectrum. Nature 428, 821–827. doi: 10.1038/nature02403
Yamamoto, I., Tsuchiya, T., and Ikusima, I. (1999). Relationship between net photosynthetic rate and leaf life span of six submerged plants in experimental ponds. Jpn. J. Limnol. 60, 257–263. doi: 10.3739/rikusui.60.257
Yin, L., Li, W., Madsen, T. V., Maberly, S. C., and Bowes, G. (2017). Photosynthetic inorganic carbon acquisition in 30 freshwater macrophytes. Aquat. Bot. 140, 48–54. doi: 10.1016/j.aquabot.2016.05.002
Yin, L., Wang, C., Chen, Y., Cao, Y., Cheng, Y., and Li, W. (2009). Cold stratification, light and high seed density enhance the germination of Ottelia alismoides. Aquat. Bot. 90, 85–88. doi: 10.1016/j.aquabot.2008.05.002
Yin, L., Zhang, R., Xie, Z., Wang, C., and Li, W. (2013). The effect of temperature, substrate, light, oxygen availability and burial depth on Ottelia alismoides seed germination. Aquat. Bot. 111, 50–53. doi: 10.1016/j.aquabot.2013.09.001
Keywords: Ottelia, Crassulacean acid metabolism, bicarbonate usage, leaf traits, shallow freshwaters
Citation: Cao Y, Liu Y, Ndirangu L, Li W, Xian L and Jiang HS (2019) The Analysis of Leaf Traits of Eight Ottelia Populations and Their Potential Ecosystem Functions in Karst Freshwaters in China. Front. Plant Sci. 9:1938. doi: 10.3389/fpls.2018.01938
Received: 26 June 2018; Accepted: 12 December 2018;
Published: 07 January 2019.
Edited by:
ZhongQiang Li, Hubei University, ChinaReviewed by:
Lina Fusaro, Sapienza University of Rome, ItalyEnhua Li, Institute of Geodesy and Geophysics (CAS), China
Copyright © 2019 Cao, Liu, Ndirangu, Li, Xian and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Sheng Jiang, jhs@wbgcas.cn
 Yu Cao
Yu Cao Yang Liu1,2,3
Yang Liu1,2,3 Leah Ndirangu
Leah Ndirangu Ling Xian
Ling Xian Hong Sheng Jiang
Hong Sheng Jiang