- Jiangsu Provincial Hospital of Chinese Medicine, Affiliated Hospital of Nanjing University of Chinese Medicine, Nanjing, China
Background and Objectives: Ulcerative Colitis (UC) subtypes defined by disease extent and shared pathophysiology are important. Analyzing the clinical characteristics of UC with different disease extent and optimizing clinical typing are conducive to the pathogenesis research, disease monitoring and precise treatment.
Methods: 188 patients with active UC were divided into distal and extensive colitis. The clinical characteristics of the two groups were analyzed by propensity score. Spearman is used for correlation analysis, and receiver operating characteristic (ROC) curve was used to evaluate the ability of clinical indicators to predict Mayo endoscopic subscore (MES).
Results: Compared with distal colitis, extensive colitis had more severe disease activity, younger age, higher utilization rate of corticosteroids and incidence of extra intestinal manifestations (EIMs), and clinical indicators were differentially expressed in the two groups. After using propensity score, the incidence of EIMs in the extensive colitis was still higher than that in distal colitis. Inflammation, coagulation and immune indicators like CRP, FC, IL-10, D-D and α1-MG are higher in extensive colitis, and metabolic indicators like LDL-C, HDL-C, TC, GSP and albumin are higher in distal colitis. The correlation between clinical indicators and MES is affected by disease extent. The area under curve (AUC) of CRP + D-D + α2-MG for predicting distal colitis MES3 was 0.85, and the AUC of IL-6+ GSP+ α1-MG predicted extensive colitis MES3 can reach 0.82.
Conclusion: Differential clinical indicators can become potential markers for predicting disease progression and prognosis, and have significance for UC mechanism research and drug development. We can select biomarkers according to lesion site.
1 Introduction
Ulcerative colitis is a chronic intestinal inflammatory disease. Different from Crohn’s disease (CD), which is another type of inflammatory bowel disease (IBD), UC is confined to the colon, and mucosal inflammation may extend continuously from the rectum to the proximal end as the disease progresses (Lamb et al., 2019). Severe ulcerative colitis can cause serious complications such as gastrointestinal hemorrhage, perforation and toxic megacolon, and the incidence of UC related colon cancer is significantly higher than that of the general population. Studies have found that the incidence of colorectal cancer in UC patients was 1.7 times higher than that in the control group (Damas and Abreu, 2020). Regrettably, the pathogenesis of UC is still not clear, although the clinical application of 5-aminosalicylic acid (5-ASA), hormone and biological agents have achieved good clinical results (Raine et al., 2022).
A gene association analysis study in The Lancet proposed a new classification of IBD based on different pathogenesis, providing a new idea for the mechanism research and clinical precision treatment of IBD (Cleynen et al., 2016).This study tested the phenotype-genotype correlation of 156,154 genetic variants in IBD population and showed that there were significant differences in the genetics between UC, colonic CD and ileal CD. Atreya and Siegmund (2021) summarized from clinical behavior, epidemiology, genetics, and intestinal microbial groups, and pointed out that there are differences between colonic and ileal CD, and colonic CD overlapped with UC in disease behavior. In addition, a great number of studies have shown that different lesion sites have different therapeutic responses to biological agents. The transverse colon presented the highest mucosa healing rate, while the right colon stenosis showed the worst improvement (Wu et al., 2020). When adalimumab was used, the ulcer changes in the rectum, sigmoid colon/left colon and transverse colon were more obvious than those in the right colon and ileum (Reinisch et al., 2017). In addition, similar conclusions were obtained in the clinical trial of vedolizumab, and he patient response to anti-integrin drugs may depend on the distribution of α4β7+T cells in the colon (Lobatón et al., 2014; Zundler et al., 2017; Atreya and Siegmund, 2021). This means that IBD has some heterogeneity. The simple classification of CD and UC cannot fully describe the complex IBD phenotype. Subtypes defined by lesion site and shared pathophysiology are also important and will affect treatment decisions.
Compared with CD, there are fewer studies on the site phenotype of UC. The lesion site of UC is limited to the colon and the intestinal inflammation is continuous, which is different from the jumping distribution of CD in the whole digestive tract. According to Montreal classification, UC can be divided into ulcerative proctitis (E1), left-sided colitis (E2) and extensive colitis (E3). A systematic review showed that 69.5% of UC patients had distal colitis, and patients with extensive colitis accounted for 30.5%, and the 10 year colectomy rate is 19% for those with extensive colitis, 8% with left-sided colitis and 5% with proctitis (Fumery et al., 2018). The distal colitis is not a static disease state and may continue to extend over time. The 5-year incidence of progression to extensive colitis in patients with limited UC was 17.8%, and the 10-year incidence was 31% (Roda et al., 2017). Some studies show that patients with limited lesion sites have higher risk of colectomy if the disease range is extended (Gallo et al., 2018). Although it is generally believed that patients with extensive colitis experience more severe disease activity than localized colitis, studies found no significant difference in terms of quality of life, disability index and overall cost.
Currently, some studies have focused on the potential value of clinical site phenotype in the selection of treatment regimen and pathogenesis of UC, and actively explored the predictive markers related to disease site progression (Qiu et al., 2019; Argmann et al., 2021). However, no study summarized and analyzed the clinical characteristics of distal colitis and extensive colitis. This study retrospectively analyzed the expression of clinical indicators in 188 patients with active UC from inflammation, coagulation, immunity and metabolism, and used propensity score matching the difference factors between two groups, in order to obtain the clinical characteristics of patients with distal or extensive UC, and further guide the individualized treatment and efficacy evaluation of UC, effectively slow the progress of UC.
2 Materials and methods
2.1 Study population
In this retrospective study, we collected 188 UC patients who visited the Department of Gastroenterology, Jiangsu Provincial Hospital of traditional Chinese medicine from 1 January 2019, to 30 April 2022.
This study was approved by the ethics committee of Jiangsu Provincial Hospital of Chinese Medicine from January.
2.2 Clinical data
All patients were diagnosed according to the combination of UC clinical, endoscopic, and histopathological criteria. Montreal classification was used to assess the lesion site, modified Truelove and Witt were used to classify the disease severity.
Inclusion criteria: 1) meet the diagnostic criteria of ulcerative colitis; 2) In the activity period of ulcerative colitis. Exclusion Criteria: 1) diseases such as shigellosis, intestinal tuberculosis, amoebiasis, Ischemic colitis and Crohn’s disease were excluded; 2) complicated with immune system diseases such as Rheumatoid Arthritis, Sjögren’s syndrome and systemic lupus erythematosus; 3) complicated with heart, brain, kidney, hematopoietic system and other important organs damage or serious infection.
Clinical information on patients with UC, including age, sex, symptoms, and colonoscopy and histopathology results, was collected from the electronic medical record system.
2.3 Statistical analysis
R (version 4.2.1) was used for data analysis, and the normality test was carried out for the measurement data. The measurement data conforming to normal distribution or approximate normal distribution is expressed as ‾x ± s, and two independent sample T-tests were used for analysis; nonparametric rank sum test was used for data not conforming to normal distribution. The counting data are expressed as the number of cases or the rate (%), the χ2 test is used for comparison. Spearman was used for multivariate correlation analysis. The difference is statistically significant if p < 0.05, and the difference is statistically significant if p < 0.01.
Propensity score was used to minimize bias and adjust for confounding factors. We used a propensity score to match the gender, age, disease duration and disease activity of the two groups. Using a greedy nearest neighbor matching approach, in which each patient in the distal colon group was matched to an extensive colon group, ultimately producing the smallest within-pair difference among all available pairs with treated patients. Patients were matched only if the Logit difference in paired propensity scores between the two groups was less than or equal to 0.5 times the pooled estimate of standard deviation.
3 Results
3.1 Clinical characteristics of included participants
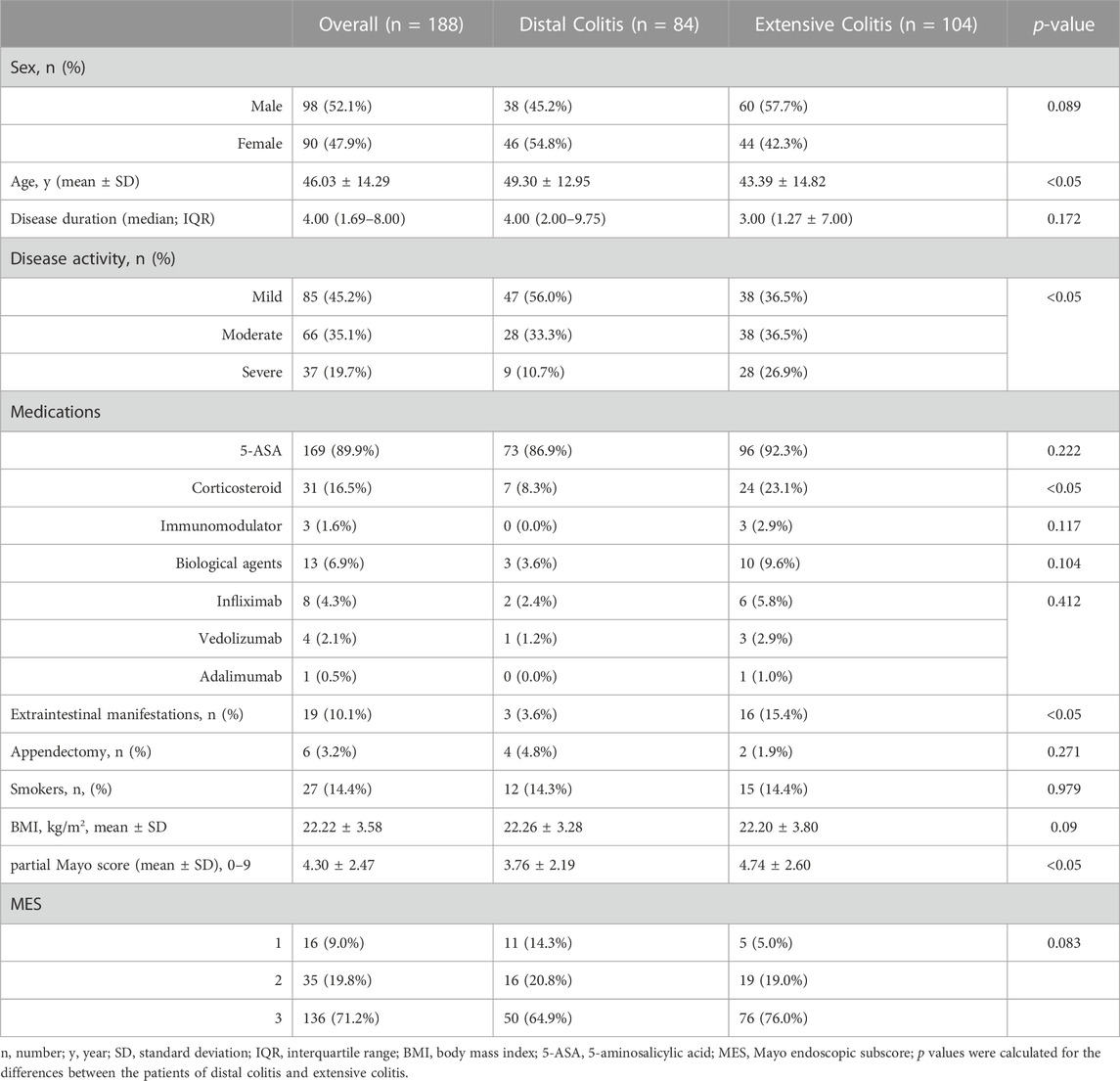
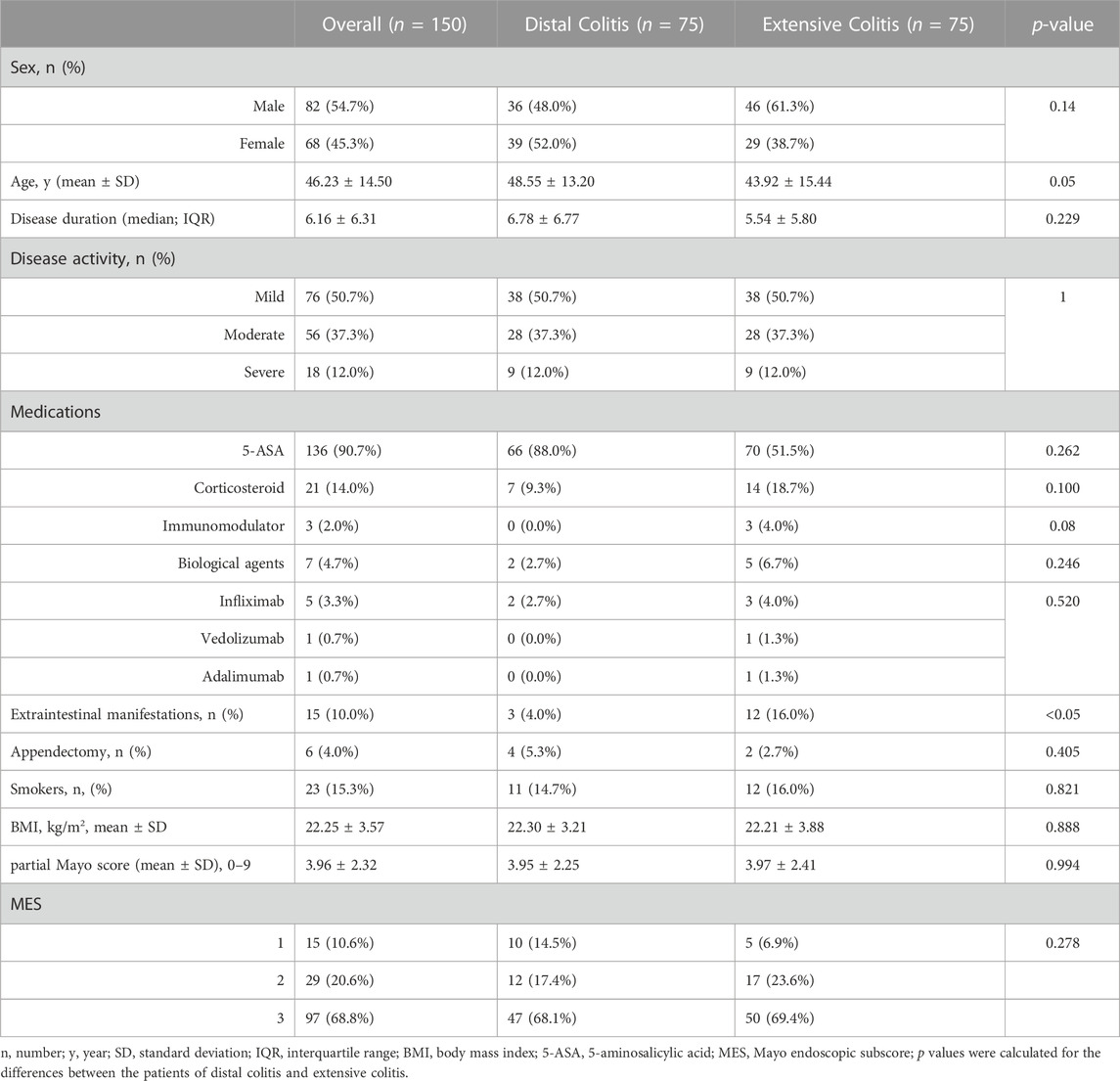
A total of 188 patients with active UC were included in this study. There were significant differences in age, disease activity, incidence of EIMs and utilization rate of corticosteroids between the two groups. Patients with distal colitis were mainly mild to moderate, and patients with extensive colitis were mostly moderate to severe. Partial Mayo scores were also statistically different between the two groups, but there was no difference in MES. (Table 1).
3.2 Clinical indicators of patients with distal and extensive ulcerative colitis
3.2.1 Expression of serum inflammatory markers in distal and extensive ulcerative colitis
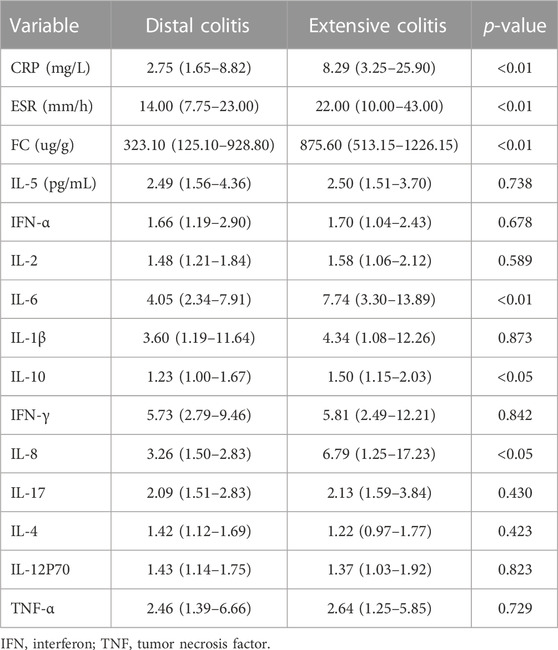
Table 2 summarizes the expression levels of C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), fecal calprotectin (FC) and serum cytokines in patients with distal and extensive ulcerative colitis. The expression of CRP, ESR, FC, interleukin-6(IL-6), IL-8 and IL-10 were different between the two groups, and except for IL-4 and IL-12P70, the expression of inflammatory markers was higher in extensive colitis.
3.2.2 Expression of coagulation indicators in distal and extensive ulcerative colitis
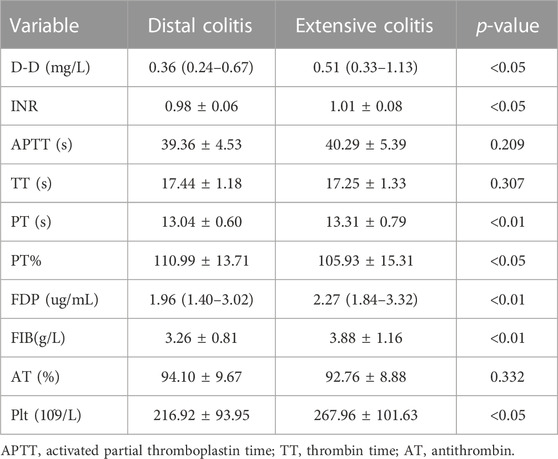
The coagulation function of UC patients is closely related to the degree of disease activity and the state of inflammation. The results in Table 3 show that the expression of D-Dimer (D-D), international normalized ratio (INR), platelet (Plt), prothrombin time (PT), fibrin degradation product (FDP) and fibrinogen (FIB) in patients with extensive colitis are significantly higher than that in distal colitis.
3.2.3 Expression of serum immune indicators in distal and extensive ulcerative colitis
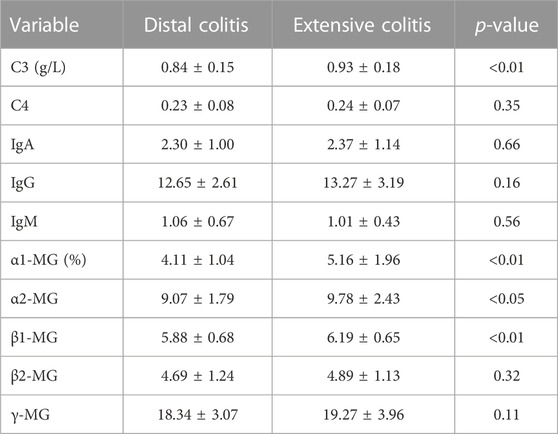
We next carried out a comparative analysis of the patient’s immunological indicators, mainly including complement(C), immunoglobulin (Ig) and immunoprotein electrophoresis. The results showed no significant difference in immunoglobulins between the two groups, and the serum level of C3 in distal ulcerative colitis was lower than that in extensive colitis, but there was no significant difference in C4. The results of immunoprotein electrophoresis showed that the ratio of α1- Micro globulin (MG), α2-MG, β1-MG in patients with distal colitis was also significantly lower than that in patients with extensive colitis (Table 4).
3.2.4 Expression of biochemical indicators in distal and extensive ulcerative colitis
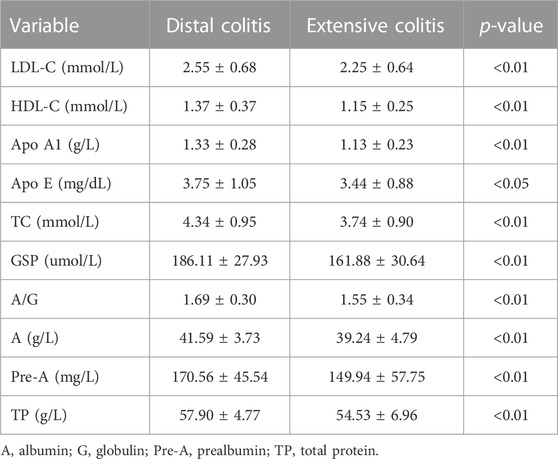
We classified and compared some metabolic related indicators in the biochemical indicators, including lipid metabolism, glucose metabolism and protein metabolism (Table 5). The comparison results showed that the levels of low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), apolipoprotein A1 (ApoA1), Apo E and total cholesterol (TC) in distal colitis were significantly higher than extensive colitis. In the glucose metabolism index, there was no significant difference in fasting blood glucose and HbA1c levels between the two groups, but the Glycosylated serum protein (GSP) level of distal colitis was significantly higher than that of extensive colitis.
3.3 Comparative study of distal colitis and extensive colitis based on propensity score
From the above comparison results, it can be seen that the disease activity level of extensive colitis is higher than distal ulcerative colitis. In order to further clarify whether there are differences in clinical indicators between distal and extensive colitis, we use propensity score to balance the degree of disease activity of the two groups of patients (Table 6).
The results showed that after excluding the influence of disease activity factors, there was no difference in age, utilization rate of corticosteroids and pMayo between the two groups, but the incidence of EIMs in extensive colitis was still higher than that in distal colitis. Although some clinical objective indicators have become similar after balanced treatment with propensity score (ESR, IL-6, IL-8, Pt, FDP, etc.), there are still many other indicators that are differentially expressed between the two groups (Table 7).
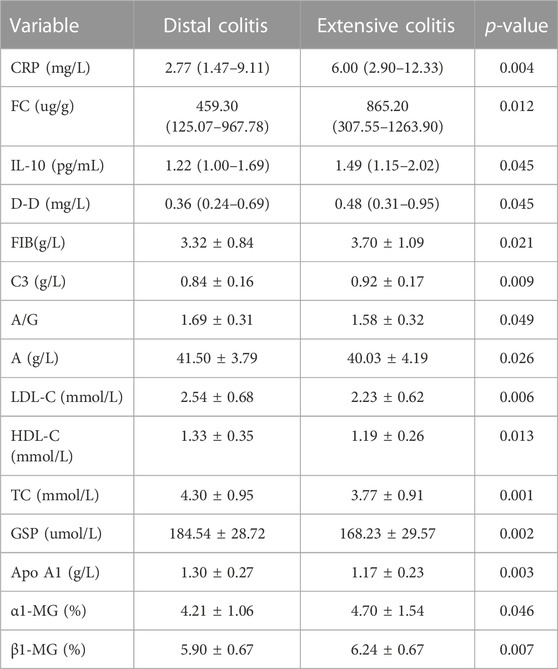
TABLE 7. Difference of clinical indexes between distal and extensive ulcerative colitis after propensity score.
3.4 The value of clinical indicators in distal and extensive ulcerative colitis is different
3.4.1 Correlation between clinical indexes and MES in distal and extensive ulcerative colitis
Patients with distal and extensive ulcerative colitis have different disease activity, and this difference significantly affects the expression of clinical markers. We performed Spearman correlation analysis for the above clinical indicators. (Figure 1A). There is no doubt that MES and pMayo, as the scores reflecting the activity of UC, have a good correlation with CRP, ESR, FC and IL-6. The correlation between α1-MG and pMayo was the highest (r = 0.698), and the correlation between α1-MG and CRP was as high as 0.867. This suggests that α1-MG may serve as a potential marker for predicting ulcerative colitis disease activity.
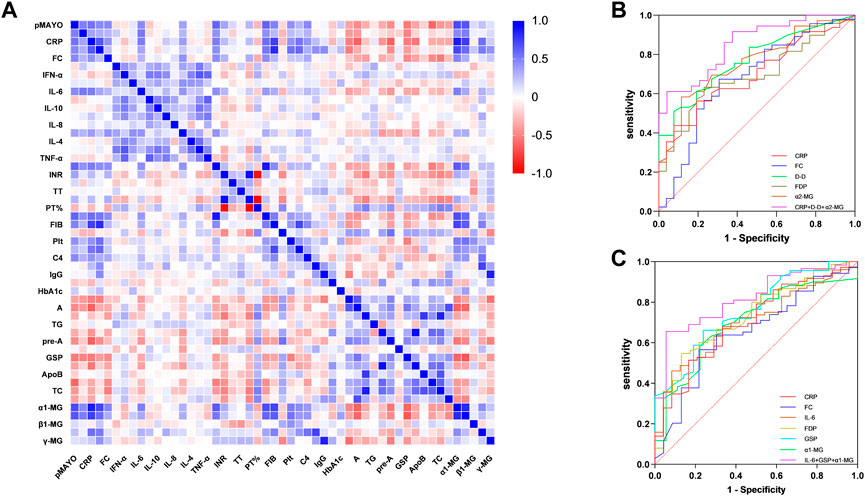
FIGURE 1. (A) Spearman correlation analysis between Mayo score and clinical objective indexes; (B) ROC curve of clinical biomarkers predicting MES 3 in active distal ulcerative colitis; (C) Figure 3 ROC curve of clinical biomarkers predicting MES 3 in active extensive ulcerative colitis.
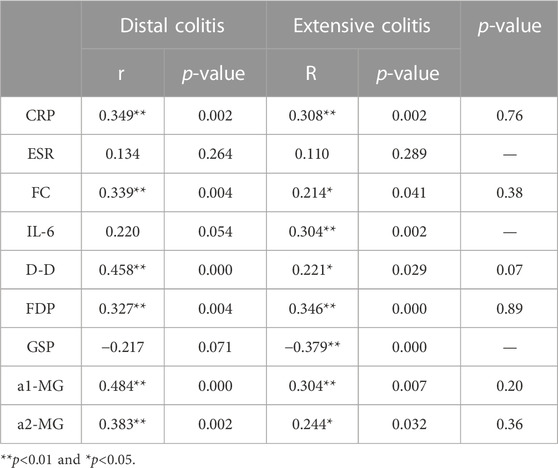
We selected the indicators with strong correlation with MES for group analysis. The results showed that there was no correlation between ESR and MES in both groups of patients, while IL-6 and GSP were only correlated with MES in extensive colitis and not in distal colitis. CRP, FC, D-D, FDP, a1-MG and a2-MG were correlated with MES in the two groups. Although the correlation coefficients were different, there was no statistical difference between the two groups (Table 8).
3.4.2 The ability of clinical indicators to predict MES 3 in patients with distal and extensive colitis
We further analyzed the ability of clinical indicators to predict MES 3 in both groups of patients, and the research results are presented as ROC curves in Figures 1A, B. In patients with distal ulcerative colitis, the AUC of CRP, FC, D-D, FDP, and α2-MG independently predicted MES 3 is 0.70, 0.68, 0.77, 0.69, and 0.76, respectively, and the AUC of CRP + D-D + α2-MG for predicting distal colitis MES 3 was 0.85, with a sensitivity and specificity of 61% and 96%, respectively.
In patients with extensive colitis, the AUC of CRP, FC, IL-6, FDP, GSP and α1-MG independently predicted MES 3 is 0.70, 0.65, 0.70, 0.73, 0.76, and 0.70, respectively, and the AUC of IL-6 + GSP+ α1-MG predicted MES3 can reach 0.82, with sensitivity and specificity of 66% and 94%, respectively (Figures 1B, C).
4 Discussion
The site phenotype of ulcerative colitis is dynamic and closely related to the disease activity, which suggests that we cannot judge the disease condition based on the initial lesion site, so dynamic follow-up is required during treatment. In this study, we divided the patients into two groups: distal and extensive ulcerative colitis, and compared their clinical characteristics and objective indicators.
The results showed that the age, disease activity and the incidence of EIMs were in great differences between the two groups. These three are interrelated, and were listed as high-risk characteristics of ulcerative colitis in the 2019 ACG and AGA adult UC guidelines, emphasizing more active treatment in the early stage (Ko et al., 2019; Rubin et al., 2019). In the second part, we homogenized the disease activity of the two groups and found that when the disease activity was the same, the incidence of EIMs in extensive colitis was still higher than those with distal colitis. Therefore, EIMS are often considered to be inflammation located outside the gut of IBD patients, and their pathogenesis depends on the extension of intestinal immune response and has a common environmental or genetic predisposition with IBD (Hedin et al., 2019).
Biomarkers for disease activity monitoring and treatment response have been a notable topic for research of UC in recent years. In this study, we incorporated the clinical objective indicators including cytokines as much as possible. In the first part of the study, we found that a large number of clinical indicators were differentially expressed in the two groups. Most of these clinical indicators have been confirmed to be related to the disease activity of ulcerative colitis in previous studies (Kato et al., 2016; Langhorst et al., 2016; Sollelis et al., 2019; Ge et al., 2022). In order to exclude the influence of clinical markers of disease activity, the analyses were adjusted by the propensity score. CRP, ESR and FC are the most commonly used indicators to reflect the disease activity of UC, so why do CRP and FC levels have significant differences when the disease activity is the same? Compared with distal colitis, patients with extensive colitis have a wider range of intestinal lesions, and the above results suggest that CRP and FC are more sensitive than ESR in responding to intestinal inflammation. Thus, elevated levels of CRP and FC in patients with distal colitis may serve as potential markers to predict progression of the lesion. Similarly, the expression of D-D, FIB, C3, α1-MG, β1-MG also has the potential to become biomarkers.
The abnormal immune response of intestinal mucosa is an important internal factor causing inflammation and tissue damage in UC (Leppkes and Neurath, 2020). Cytokines play an important role in mediating the abnormal immune response. Compared with non -specific inflammatory indicators such as CRP, cytokines can be used as biomarkers to respond to intestinal inflammation, and also closely related to IBD mechanism research and drug research and development. As of now, the clinical treatment of IBD has entered the era of biological agents, but the research status of new biological agents are not satisfactory. Our results suggest that IL-10 expression in patients with extensive colitis is significantly higher than that in patients with distal colitis when disease activity is similar between the two groups. IL-10 is generated by CD4+TH2 sub-groups, and is also known as cytokine synthesis inhibitory factor (CSIF). It can inhibit macrophages to secrete cytokines and have extensive immune regulation activity. Studies have shown that IL-10 knockout mice have spontaneous enteritis, suggesting that the occurrence of UC is related to the reduction of IL-10. However, when inflammation occurs, the expression of IL-10 in the inflammatory and non-inflammatory areas of intestinal mucosa is significantly increased to play an anti-inflammatory role (Melgar et al., 2003). In addition, studies show IL-10 and IL-10R1 levels were increased in transverse colon biopsies of patients with extensive/pancolitis, compared with control subjects and patients with limited distal disease (Wittmann Dayagi et al., 2021). On 6 July 2022, Applied Molecular Transport (AMT) announced the results of the 2-stage MARKET test IL-10 inhibitor in patients with moderate to severe ulcerative colitis. The combination of IL-10 inhibitor and adalimumab did not show better clinical efficacy compared to that of adalimumab monotherapy. But, for patients with UC less than 5 years, the clinical remission rate of patients receiving combination therapy was 43.8%, while that of patients receiving adalimumab alone was 15.4%, suggesting that combination therapy may be beneficial in the early stage of the disease (Applied Molecular Transport, 2022). These results all indicate that IL-10 has great potential in the clinical study of UC.
TC, LDL-C, HDL-C, Albumin, GSP and other metabolic markers were also differentially expressed in the two groups. The metabolic process of the body is regulated by many physiological and pathological links. The dysregulation of intestinal flora is related to a variety of human metabolic diseases. Studies have found that the amount and types of beneficial bacteria which has important and unique significance for the integrity of intestinal mucosal barrier are significantly reduced in the gut of IBD patients. (Turnbaugh et al., 2009; Henao-Mejia et al., 2012; Leung et al., 2016; Vatanen et al., 2018). Intestinal flora can produce a great number of metabolites in the colon that can affect the physiological process of the body. Gut microbiota-derived metabolites were also recognized as key actors in IBD (Lavelle and Sokol, 2020). The results showed that the metabolic indexes of patients with extensive colitis were lower than those with distal colitis, among which lipid metabolism were the most obvious. This difference is closely related to the proximal colonic inflammation. Take lipid metabolism as an example, bile acids are the most widely studied gut microbiota-derived metabolites. Studies have shown that bile acid is the main way to decompose metabolism. In the state of inflammation, bile acid metabolism rises to promote inflammation. The intestinal flora can also play an important role in regulating dietary fat absorption and lipid metabolism by affecting bile acid metabolism, generating short -chain fatty acids, and regulating intestinal endocrine systems (Yu et al., 2019). When the expression of bile acid in the inflammation area is increased, the synthesis of cholesterol will be reduced accordingly, which can reflect intestinal inflammation. Dongke Xu’s study indicated that the Cholesterol Sulfate (CS) can promote the biological synthesis of cholesterol, thereby alleviating ulcerative colitis (Xu et al., 2022). Total cholesterol levels can be used as a short-term therapeutic target. Maiko et al. demonstrated that during induction therapy in patients with acute ulcerative colitis, patients with mucosal healing had higher white blood cell counts and total cholesterol than those without (Motobayashi et al., 2019).
A large number of studies have focused on clinical markers used to predict disease activity, but few have examined whether the predictive value of different biomarkers is influenced differently by lesion site. To this end, we preliminarily explored the correlation between clinical biomarkers and MES in two groups. ESR was associated with MES overall, but after grouping ESR was not associated with MES in either distal colitis or extensive colitis. Furthermore, IL-6 and GSP were associated with MES in extensive colitis but not in distal colitis. CRP, FC, D-D, FDP, a1-MG and a2-MG were correlated with MES in the two groups. Although the correlation coefficients were different, there was no statistical difference between the two groups. In addition, the ability of biomarkers to predict MES 3 in the two groups are different. The lesion site of ulcerative colitis may affect the predictive ability of biomarkers.
In conclusion, this study conducted a comparative analysis of the clinical characteristics and objective indicators of distal colitis and extensive colitis, with the aim of finding biomarkers related to disease site extension and exploring the significance of different indicators in predicting disease activity, mechanism research and drug development. However, this study is only a preliminary exploration, subsequent large-scale retrospective studies and prospective clinical studies are needed to further verify the results.
Authors’ note
The manuscript, including related data, figures and tables has not been previously published and that the manuscript is not under consideration elsewhere.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
This study involving human participants were reviewed and approved by the ethics committee of Jiangsu Hospital of Chinese Medicine, and exemption from informed consent for retrospective studies was obtained (ethics number:2022NL-062-01).
Author contributions
CG and ZS drafted the manuscript. LZ and HS conceived and designed the study. YL, XL, YT, MZ and YL were responsible for data acquisition. CG and ZS were responsible for analysis. LZ critically reviewed the manuscript for important intellectual content. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Jiangsu Province Traditional Chinese Medicine Science and Technology Development Project in 2021 (No. ZT202103), Jiangsu Province Traditional Chinese Medicine Science and Technology Development Project in 2020 (No. YB2020008) and Research and Practice Innovation Program for graduate students in Jiangsu Province (SJCX22_0730).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Applied Molecular Transport. Applied molecular Transport announces top-line phase 2 results from MARKET combination trial of oral AMT-101 in patients with moderate-to-severe ulcerative colitis - applied molecular Transport inc. 2022. Available at: https://ir.appliedmt.com/news-releases/news-release-details/applied-molecular-transport-announces-top-line-phase-2-results/ (Accessed: August 25 2022).
Argmann, C., Tokuyama, M., Ungaro, R. C., Huang, R., Hou, R., Gurunathan, S., et al. (2021). Molecular characterization of limited ulcerative colitis reveals novel biology and predictors of disease extension. Gastroenterology 161, 1953–1968.e15. doi:10.1053/j.gastro.2021.08.053
Atreya, R., and Siegmund, B. (2021). Location is important: Differentiation between ileal and colonic crohn’s disease. Nat. Rev. Gastroenterol. Hepatol. 18, 544–558. doi:10.1038/s41575-021-00424-6
Cleynen, I., Boucher, G., Jostins, L., Schumm, L. P., Zeissig, S., Ahmad, T., et al. (2016). Inherited determinants of crohn’s disease and ulcerative colitis phenotypes: A genetic association study. Lancet 387, 156–167. doi:10.1016/S0140-6736(15)00465-1
Damas, O. M., and Abreu, M. T. (2020). Are patients with ulcerative colitis still at increased risk of colon cancer? Lancet 395, 92–94. doi:10.1016/S0140-6736(19)33225-8
Fumery, M., Singh, S., Dulai, P. S., Gower-Rousseau, C., Peyrin-Biroulet, L., and Sandborn, W. J. (2018). Natural history of adult ulcerative colitis in population-based cohorts: A systematic review. Clin. Gastroenterol. Hepatol. 16, 343–356. doi:10.1016/j.cgh.2017.06.016
Gallo, G., Kotze, P. G., and Spinelli, A. (2018). Surgery in ulcerative colitis: When? How? Best. Pract. Res. Clin. Gastroenterol. 32–33, 71–78. doi:10.1016/j.bpg.2018.05.017
Ge, C., Lu, Y., Shen, H., and Zhu, L. (2022). Monitoring of intestinal inflammation and prediction of recurrence in ulcerative colitis. Scand. J. Gastroenterol. 57, 513–524. doi:10.1080/00365521.2021.2022193
Hedin, C. R. H., Vavricka, S. R., Stagg, A. J., Schoepfer, A., Raine, T., Puig, L., et al. (2019). The pathogenesis of extraintestinal manifestations: Implications for IBD research, diagnosis, and therapy. J. Crohns Colitis 13, 541–554. doi:10.1093/ecco-jcc/jjy191
Henao-Mejia, J., Elinav, E., Jin, C., Hao, L., Mehal, W. Z., Strowig, T., et al. (2012). Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 482, 179–185. doi:10.1038/nature10809
Kato, J., Hiraoka, S., Nakarai, A., Takashima, S., Inokuchi, T., and Ichinose, M. (2016). Fecal immunochemical test as a biomarker for inflammatory bowel diseases: Can it rival fecal calprotectin? Intest. Res. 14, 5–14. doi:10.5217/ir.2016.14.1.5
Ko, C. W., Singh, S., Feuerstein, J. D., Falck-Ytter, C., Falck-Ytter, Y., Cross, R. K., et al. (2019). AGA clinical Practice guidelines on the management of mild-to-moderate ulcerative colitis. Gastroenterology 156, 748–764. doi:10.1053/j.gastro.2018.12.009
Lamb, C. A., Kennedy, N. A., Raine, T., Hendy, P. A., Smith, P. J., Limdi, J. K., et al. (2019). British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 68, s1–s106. doi:10.1136/gutjnl-2019-318484
Langhorst, J., Boone, J., Lauche, R., Rueffer, A., and Dobos, G. (2016). Faecal lactoferrin, calprotectin, PMN-elastase, CRP, and white blood cell count as indicators for mucosal healing and clinical course of disease in patients with mild to moderate ulcerative colitis: Post hoc analysis of a prospective clinical trial. J. Crohn’s Colitis 10, 786–794. doi:10.1093/ecco-jcc/jjw044
Lavelle, A., and Sokol, H. (2020). Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 17, 223–237. doi:10.1038/s41575-019-0258-z
Leppkes, M., and Neurath, M. F. (2020). Cytokines in inflammatory bowel diseases – update 2020. Pharmacol. Res. 158, 104835. doi:10.1016/j.phrs.2020.104835
Leung, C., Rivera, L., Furness, J. B., and Angus, P. W. (2016). The role of the gut microbiota in NAFLD. Nat. Rev. Gastroenterol. Hepatol. 13, 412–425. doi:10.1038/nrgastro.2016.85
Lobatón, T., Vermeire, S., Van Assche, G., and Rutgeerts, P. (2014). Review article: Anti-adhesion therapies for inflammatory bowel disease. Aliment. Pharmacol. Ther. 39, 579–594. doi:10.1111/apt.12639
Melgar, S., Yeung, M. M-W., Bas, A., Forsberg, G., Suhr, O., Oberg, A., et al. (2003). Over-expression of interleukin 10 in mucosal T cells of patients with active ulcerative colitis. Clin. Exp. Immunol. 134, 127–137. doi:10.1046/j.1365-2249.2003.02268.x
Motobayashi, M., Matsuoka, K., Takenaka, K., Fujii, T., Nagahori, M., Ohtsuka, K., et al. (2019). Predictors of mucosal healing during induction therapy in patients with acute moderate-to-severe ulcerative colitis. J. Gastroenterol. Hepatol. 34, 1004–1010. doi:10.1111/jgh.14565
Qiu, Y., Chen, B., Li, Y., Xiong, S., Zhang, S., He, Y., et al. (2019). Risk factors and long-term outcome of disease extent progression in asian patients with ulcerative colitis: A retrospective cohort study. BMC Gastroenterol. 19, 7. doi:10.1186/s12876-018-0928-2
Raine, T., Bonovas, S., Burisch, J., Kucharzik, T., Adamina, M., Annese, V., et al. (2022). ECCO guidelines on therapeutics in ulcerative colitis: Medical treatment. J. Crohn’s Colitis 16, 2–17. doi:10.1093/ecco-jcc/jjab178
Reinisch, W., Colombel, J-F., D’Haens, G., Sandborn, W. J., Rutgeerts, P., Geboes, K., et al. (2017). Characterisation of mucosal healing with adalimumab treatment in patients with moderately to severely active crohn’s disease: Results from the EXTEND trial. J. Crohns Colitis 11, 425–434. doi:10.1093/ecco-jcc/jjw178
Roda, G., Narula, N., Pinotti, R., Skamnelos, A., Katsanos, K. H., Ungaro, R., et al. (2017). Systematic review with meta-analysis: Proximal disease extension in limited ulcerative colitis. Aliment. Pharmacol. Ther. 45, 1481–1492. doi:10.1111/apt.14063
Rubin, D. T., Ananthakrishnan, A. N., Siegel, C. A., Sauer, B. G., and Long, M. D. (2019). ACG clinical guideline: Ulcerative colitis in adults. Am. J. Gastroenterol. 114, 384–413. doi:10.14309/ajg.0000000000000152
Sollelis, E., Quinard, R. M., Bouguen, G., Goutte, M., Goutorbe, F., Bouvier, D., et al. (2019). Combined evaluation of biomarkers as predictor of maintained remission in Crohn’s disease. World J. Gastroenterol. 25, 2354–2364. doi:10.3748/wjg.v25.i19.2354
Turnbaugh, P. J., Hamady, M., Yatsunenko, T., Cantarel, B. L., Duncan, A., Ley, R. E., et al. (2009). A core gut microbiome in obese and lean twins. Nature 457, 480–484. doi:10.1038/nature07540
Vatanen, T., Franzosa, E. A., Schwager, R., Tripathi, S., Arthur, T. D., Vehik, K., et al. (2018). The human gut microbiome in early-onset type 1 diabetes from the TEDDY study. Nature 562, 589–594. doi:10.1038/s41586-018-0620-2
Wittmann Dayagi, T., Werner, L., Pinsker, M., Salamon, N., Barschak, I., Weiss, B., et al. (2021). Mucosal IL-10 and IL-10 receptor expression patterns in paediatric patients with ulcerative colitis. Int. J. Exp. Pathology 102, 4–10. doi:10.1111/iep.12382
Wu, Y., Zhang, L., Cao, J., Wang, H., Ye, C., Zhuoma, D., et al. (2020). Efficacy of infliximab treatment on the mucosal healing of different intestinal segments in patients with ileocolonic Crohn’s disease. Ther. Adv. Gastroenterol. 13, 1756284820976923. doi:10.1177/1756284820976923
Xu, D., Ma, R., Ju, Y., Song, X., Niu, B., Hong, W., et al. (2022). Cholesterol sulfate alleviates ulcerative colitis by promoting cholesterol biosynthesis in colonic epithelial cells. Nat. Commun. 13, 4428. doi:10.1038/s41467-022-32158-7
Yu, Y., Raka, F., and Adeli, K. (2019). The role of the gut microbiota in lipid and lipoprotein metabolism. J. Clin. Med. 8, E2227. doi:10.3390/jcm8122227
Keywords: ulcerative colitis, propensity score, clinical characteristics, disease extent, biomarkers
Citation: Ge C, Shen Z, Lu Y, Liu X, Tong Y, Zhang M, Liu Y, Shen H and Zhu L (2023) Propensity score analysis the clinical characteristics of active distal and extensive ulcerative colitis: a retrospective study. Front. Physiol. 14:1136659. doi: 10.3389/fphys.2023.1136659
Received: 03 January 2023; Accepted: 24 May 2023;
Published: 30 June 2023.
Edited by:
Stephen J. Pandol, Cedars Sinai Medical Center, United StatesReviewed by:
Satoshi Tamura, Hamamatsu University School of Medicine, JapanShin Hamada, Tohoku University, Japan
Copyright © 2023 Ge, Shen, Lu, Liu, Tong, Zhang, Liu, Shen and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Shen, shenhong999@njucm.edu.cn; Lei Zhu, zhulei5100@njucm.edu.cn
†These authors share first authorship
 Changchang Ge
Changchang Ge Zhaofeng Shen†
Zhaofeng Shen† Hong Shen
Hong Shen Lei Zhu
Lei Zhu