- 1Division of Cardiovascular Sciences, University of Manchester, Manchester, United Kingdom
- 2Institute of Systems, Molecular and Integrative Biology, University of Liverpool, Liverpool, United Kingdom
- 3Faculty of Life Sciences, University of Bradford, Bradford, United Kingdom
- 4Department of Anatomy, Jagiellonian University Medical College, Kraków, Poland
Heart failure is associated with atrioventricular (AV) node dysfunction, and AV node dysfunction in the setting of heart failure is associated with an increased risk of mortality and heart failure hospitalisation. This study aims to understand the causes of AV node dysfunction in heart failure by studying changes in the whole nodal transcriptome. The mouse transverse aortic constriction model of pressure overload-induced heart failure was studied; functional changes were assessed using electrocardiography and echocardiography and the transcriptome of the AV node was quantified using RNAseq. Heart failure was associated with a significant increase in the PR interval, indicating a slowing of AV node conduction and AV node dysfunction, and significant changes in 3,077 transcripts (5.6% of the transcriptome). Many systems were affected: transcripts supporting AV node conduction were downregulated and there were changes in transcripts identified by GWAS as determinants of the PR interval. In addition, there was evidence of remodelling of the sarcomere, a shift from fatty acid to glucose metabolism, remodelling of the extracellular matrix, and remodelling of the transcription and translation machinery. There was evidence of the causes of this widespread remodelling of the AV node: evidence of dysregulation of multiple intracellular signalling pathways, dysregulation of 109 protein kinases and 148 transcription factors, and an immune response with a proliferation of neutrophils, monocytes, macrophages and B lymphocytes and a dysregulation of 40 cytokines. In conclusion, inflammation and a widespread transcriptional remodelling of the AV node underlies AV node dysfunction in heart failure.
Introduction
Heart failure (HF) is associated with atrioventricular (AV) node dysfunction. First-degree AV block is present in 15%–51% of HF patients as compared to a prevalence of 2.1%–4% in the general population (Aro et al., 2014; Nikolaidou et al., 2016). First-degree AV block in HF is associated with an increased risk of mortality and HF hospitalisation, and optimisation of AV delay in patients by means of cardiac resynchronisation therapy is an important treatment (Nikolaidou et al., 2016). AV node dysfunction has also been widely observed in animal models of HF (e.g., Nikolaidou et al., 2015). The aim of this study was to investigate the causes of the AV node dysfunction by studying all changes occurring in the AV node in HF. For this, we used RNAseq to study the transcriptome of the AV node. The aetiology of HF in patients is likely to be mixed and complicated (Roger, 2021). Nevertheless, hypertension is the commonest cause of HF (Bui et al., 2011) and, although it is not a model of hypertension per se (Riehle and Bauersachs, 2019), we studied a mouse model of pressure overload-induced HF: the transverse aortic constriction model (Richards et al., 2019). Widespread changes were observed (in 3,077 transcripts), showing that AV node dysfunction is the result of the failure of multiple cellular systems.
Materials and methods
Male eight-week-old C57Bl/6N mice underwent transverse aortic constriction (TAC) or sham operations. Mice were terminated 8 weeks post-surgery or when HF symptoms developed. In the conscious mouse, the heart rate was measured weekly following surgery using an ECGenie. Prior to termination, in anaesthetised mice, echocardiography was carried out to determine heart function, and the electrocardiogram (ECG) was recorded to determine the PR interval, etc. AV node biopsies were collected from the triangle of Koch bordered by the coronary sinus, tendon of Todaro and tricuspid valve annulus (Li et al., 2008); the base, height and area of the triangle was ∼0.5 mm, ∼0.75 mm and 0.19 mm2, respectively. AV node biospies taken from three mice were pooled and three such replicates were obtained from HF (TAC) and control (sham-operated) mice. RNA was extracted and sequenced (RNAseq) to yield the expression level of 55,385 transcripts. Statistical analysis was carried out to determine significant differences in transcript expression, and gene ontology and pathway analysis was carried out to understand the functional significance of the data. All experimental procedures were approved by the University of Manchester and were in accordance with the United Kingdom Animals (Scientific Procedures) Act 1986. Further details of the methods used are given in the Supplementary Materials and Methods.
Results
TAC mouse model of pressure overload-induced HF and AV node dysfunction
Figure 1A shows that from day 36 following TAC surgery, some mice reached prescribed endpoints and had to be culled, whereas none of the control sham-operated mice had to be culled. Figures 1B–D shows that by the end of the experiment, in the case of the mice subjected to TAC surgery, the body weight was significantly reduced, and the heart weight and heart weight:body weight ratio were significantly increased, indicative of HF. The relative loss of body weight (as compared to the body weight of the control mice) occurred progressively after the TAC surgery (Supplementary Figure S1A). Following TAC surgery, the mice developed congestive HF—the lung weight:body weight ratio was significantly increased in the mice subjected to TAC surgery by the end of the experiment (Figure 1E). Echocardiography at the end of the experiment confirmed HF: fractional shortening and ejection fraction were significantly reduced and left ventricular mass and end diastolic and systolic diameters were significantly increased (Supplementary Figure S1B–F). HF was accompanied by electrophysiological changes: the ECG was recorded in the anaesthetised mouse at the end of the experiment, and in the HF mice there was a significant increase in the PR interval indicative of AV node dysfunction (a slowing of AV node conduction) (Figure 1G). There was also a significant decrease in the heart rate and significant increases in the QRS duration and uncorrected and corrected QT intervals in the HF mice as expected (Figures 1F,H–J). See the Supplementary Material for further discussion of the data as well as justification of the mouse TAC model as a model of HF. From this point onwards, pressure overload-induced HF will be simply referred to as HF.
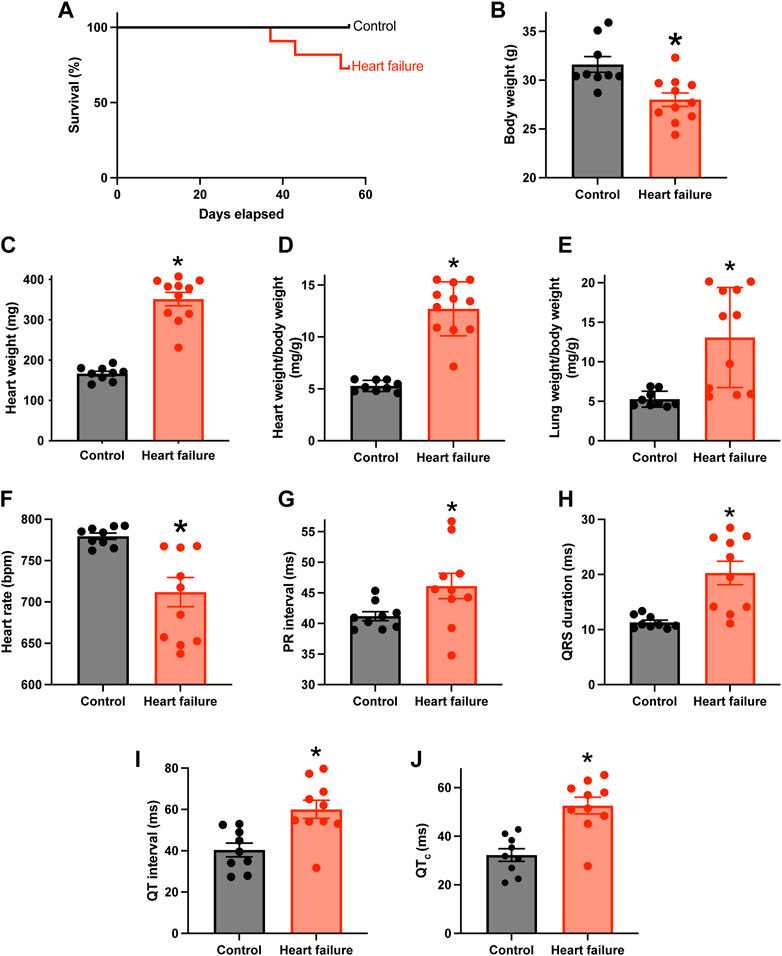
FIGURE 1. HF model with AV node dysfunction. (A), percentage survival of control (n = 9; subject to sham surgery) and HF (n = 11; subject to TAC surgery) mice in the days following surgery. (B–F), body weight (B), heart weight (C), heart weight:body weight ratio (D), lung weight:body weight ratio (E) and in vivo heart rate ((F); measured using the ECGenie) of the two cohorts of mice (n = 9 and 11) at the end of the experiment. (G–J), PR interval (G), QRS duration (H), QT interval (I) and corrected QT interval (J) of the two cohorts of mice (n = 9 and 10) measured in the anaesthetised animals at the end of the experiment. In B-J, means ± SEM as well as individual data points shown. *p < 0.05.
Transcriptomic remodelling of the AV node
The transcriptome of the AV node was determined using RNAseq, and to have sufficient material, AV node tissue isolated from three mice was pooled. Three biological replicates (each a pool from three mice) were isolated from each cohort of mice (the study therefore involved nine HF and nine control mice). 55,385 transcripts were detected and their abundance quantified. Principal component analysis (PCA) for each of the three technical replicates in the transcriptomics dataset showed clear distinction of the samples into two groups corresponding to the HF and control cohorts of mice (Figure 2A). 64% of the variation between samples (PC1) was attributed to HF, whereas 19% of the variation (PC2) was attributed to variation between technical replicates (Figure 2A). Figure 2B shows a volcano plot of the Benjamini-Hochberg-adjusted p-value against the fold change for all transcripts. The dashed line corresponds to p = 0.05, the threshold for significance. There were significant changes in the abundance of 3,077 transcripts (5.6% of the total). Unsupervised hierarchical clustering of the transcripts changing significantly in HF resulted in a grouping of the HF and control cohorts of mice into two distinct clusters (Figure 2C). 1,337 transcripts were significantly downregulated and 1,740 transcripts including well-known HF markers were significantly upregulated in HF (Figures 2B,C; Supplmentary Figure S2). A gene ontology analysis was carried out of the transcripts significantly downregulated or upregulated in HF using topGO and this showed that HF had widespread effects on the cellular systems of the AV node. In Figures 2D,E the most significant of these pathways is shown (p < 0.01). The set of transcripts downregulated in HF is enriched for transcripts related to “excitation and ion transport” for example, (Figure 2D), whereas the set of transcripts upregulated in HF is enriched for transcripts related to “intracellular Ca2+,” “contraction,” “metabolism,” “apoptosis,” “signalling,” “extracellular matrix” and the “immune system” for example, (Figure 2E). The effects of HF on some of these systems is now considered in detail.
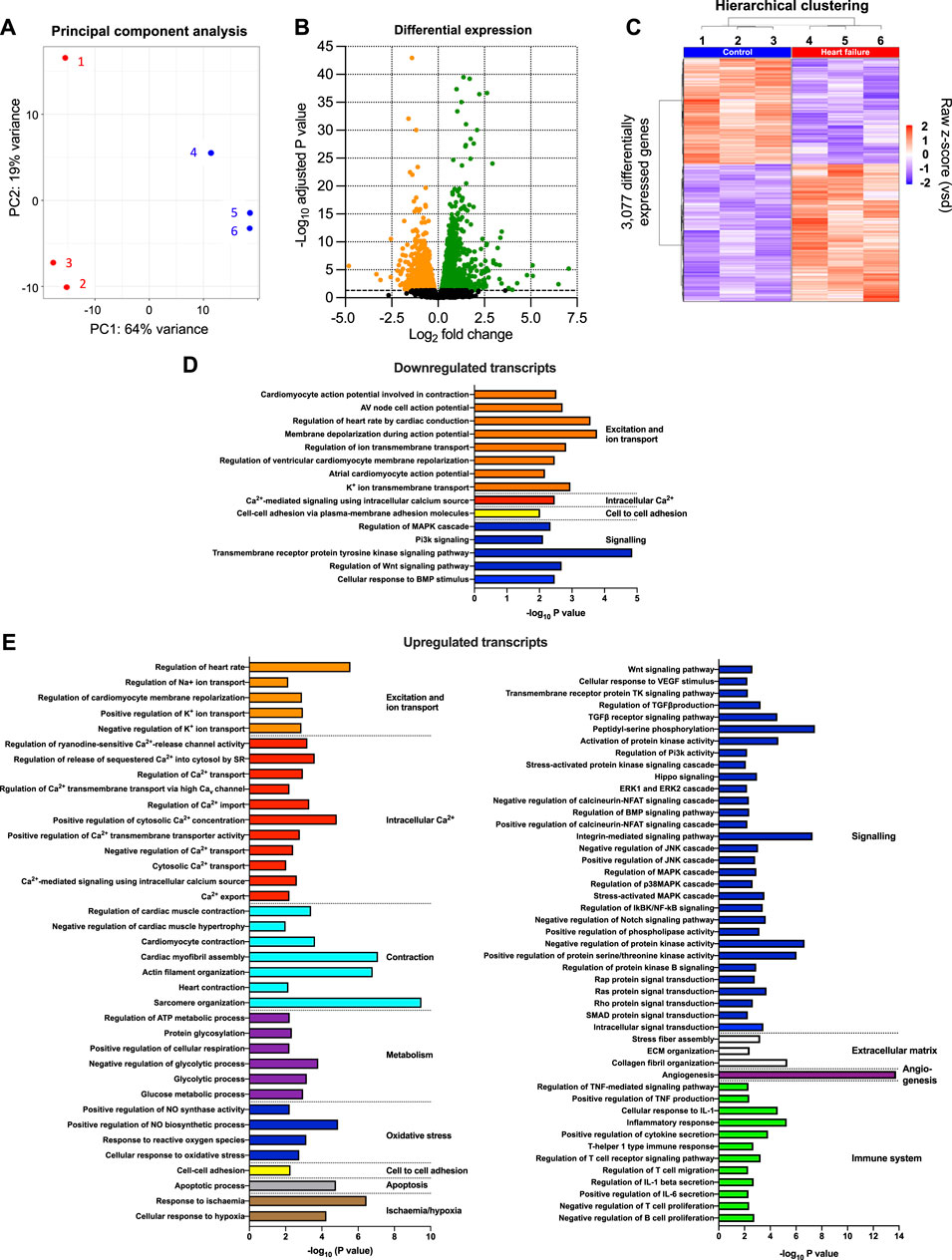
FIGURE 2. Transcriptomic remodelling of the AV node in HF. (A), principal component analysis (PCA) of all six replicates based on the 500 most highly expressed transcripts. The x-axis shows the PCA 1 score, which represents the maximum variance direction in the data set. This is principal component 1 (PC1) and it accounts for 64% of the variance in the samples and can be attributed to the variance between the control and HF cohorts. The y-axis shows the PCA 2 score, which represents the second highest variance direction in the data set. This is principal component 2 (PC2) and it accounts for 19% of the variance in the samples and can be attributed to the variance among all samples. The red symbols (1, 2 and 3) represent the three HF technical replicates and the blue symbols (4, 5 and 6) represent the three control technical replicates. Each technical replicate contained three pooled AV node biopsies. (B), volcano plot showing -log10 adjusted p-value plotted against log2 fold change for each transcript measured. Dashed line corresponds to adjusted p = 0.05. The red and blue symbols show the 3,077 transcripts significantly upregulated (red symbols; 1,740 transcripts) or downregulated (blue symbols; 1,337 transcripts). (C), hierarchical clustering of the 3,077 differentially expressed transcripts (adjusted p < 0.05) performed using Pearson’s correlation distance and ward. D2 agglomeration method. Data for the six samples shown. Expression (raw z-score) goes from high (red) to low (blue). (D and E), functional enrichment analysis performed using the topGO R package on downregulated (D) and upregulated (E) differentially expressed genes in the HF samples compared to the control samples. Significantly enriched biological process GO terms related to cardiac function are presented.
Downregulation of transcripts in the AV node expected to impact conduction
There was a remodelling of ion channel transcripts in the AV node in HF, some of which are expected to impact AV node conduction. Whereas Scn5a responsible for the principal Na+ channel in the heart, Nav1.5, was unaffected (Figure 3A), other less abundant Na+ channel transcripts were significantly downregulated (Figure 3B). Scn10a (responsible for Nav1.8) was scarce compared to Scn5a, but it was significantly downregulated (Figure 3B) and this may be important: GWAS studies have implicated Scn10a as a modulator of cardiac conduction (Park and Fishman, 2014) including the PR interval (Chambers et al., 2010; Holm et al., 2010; Pfeufer et al., 2010), and mice treated with a selective inhibitor of Nav1.8 channels show a marked prolongation of PR and QRS intervals (Park and Fishman, 2014). The mechanism of action of Scn10a is unclear (Park and Fishman, 2014). Three key Ca2+ channel transcripts were significantly downregulated: Cacna1d, Cacna1g and Cacna1h, responsible for the L- and T-type Ca2+ channels, Cav1.3, Cav3.1 and Cav3.2 (Figures 3A,B). This is likely to be important, because in mice, loss-of-function of Cav1.3 and Cav3.1 impairs AV conduction and in humans AV block has been attributed to loss-of-function of Cav1.3 and Cav3.1 (Mesirca et al., 2015). The Ca2+ channel accessory subunit, Cacna2d3, was significantly downregulated (Figure 3B) and it too has been linked to cardiac conduction (Auxerre-Plantié et al., 2019). However, Cacna2d1 was significantly upregulated (Figure 3B) and this is expected to increase the L-type Ca2+ current (Templin et al., 2011). Three key abundant K+ channel transcripts were significantly downregulated, Kcnh2, Kcnj3 and Kcnj5 (Figure 3C); Kcnh2 is responsible for the ERG (Kv11.1) channel, and Kcnj3 and Kcnj5 are responsible for Kir3.1 and Kir3.4, which together make up the ACh-activated K+ channel. Regan et al. (Regan et al., 2005) observed that selective pharmacological block of ERG (responsible for the K+ current, IK,r) and KvLQT1 (responsible for the K+ current, IK,s) both cause a prolonged AH interval and AV node effective refractory period in rats. Although Kcnh2 responsible for ERG was downregulated in HF, Kcnq1 responsible for KvLQT1 was not (Figure 3C). Regardless, this demonstrates that K+ channels are able to affect AV node conduction and refractoriness. Mesirca et al. (Mesirca et al., 2016) reported that ablation of the ACh-activated K+ current (carried by Kir3.1 and Kir3.4) relieves the AV block in Cav1.3 knockout mice. The downregulation of Kcnj3 and Kcnj5 responsible for Kir3.1 and Kir3.4 in HF (Figure 3C) could, therefore, be a compensatory mechanism, although it is also expected to reduce the response of the AV node to vagal stimulation. There were many significant changes of the less abundant K+ channel transcripts (Figure 3D). Kcna1 and Kcna2 (responsible for Kv1.1 and Kv1.2) were both significantly downregulated in HF (Figure 3D). In the mouse, systemic knockout of Kcna1 (Kv1.1) results in AV block–although this was attributed to the autonomic nervous system, it could be a direct action on the AV node (Glasscock et al., 2010; Glasscock, 2019). Transgenic mice expressing high levels of activated RhoA (a GTPase implicated in cardiac hypertrophy) develop lethal ventricular failure (Sah et al., 1999). The mice develop AV block and Sah et al, (1999) hypothesised that this was the result of RhoA physically associating with and suppressing the activity of Kv1.2 (Cachero et al., 1998). Interestingly, RhoA was significantly upregulated in the AV node in HF in the present study (data not shown). Transcripts for four Ca2+-activated K+ channels were present and Kcnn2 (responsible for KCa2.2 or SK2) was the most abundant (Figure 3D). Kcnn2 and Kcnn1 (responsible for KCa2.1 or SK1) were significantly downregulated in HF (Figure 3D) and this is likely to be functionally important. In the mouse, deletion of one copy of the Kcnn2 gene results in AV node dysfunction–a prolongation of the AV node action potential, a slowing of AV node spontaneous activity and an increase in the PR interval–and deletion of both copies results in complete AV block (Zhang et al., 2008).
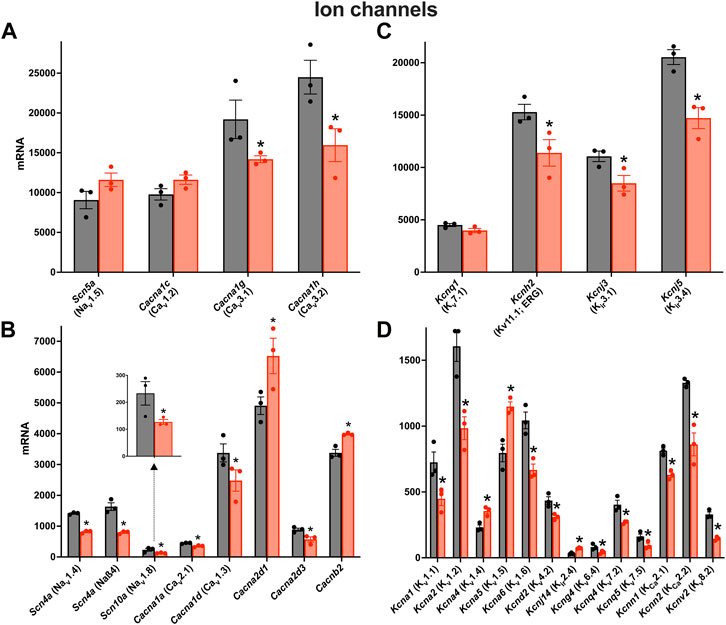
FIGURE 3. Downregulation of many key ion channel transcripts in the AV node in HF. (A–D), expression of transcripts for Na+ and Ca2+ channels (A and B) and K+ channels (C and D) in control (black bars) and HF (red bars). Highly expressed ion channel transcripts are shown in the upper panels (A and C) and more poorly expressed ion channel transcripts are shown in the lower panels (B and D). The data for Scn10a are also shown in a zoomed panel. Gene names and more common names are shown. Means ± SEM as well as individual data points shown *p < 0.05.
It is possible that the changes in ion channel expression are dependent on the severity of HF. To explore this, for each of the six pooled samples measured, the expression of transcripts for key ion channel subunits was plotted against Myh7, a well-known HF marker, and in all cases with one exception there was a significant correlation (Supplementary Figure S3). However, they were not correlated with Nppa and Nppb, two other HF markers.
Changes of further ion channels and transporters are considered in the (Supplementary Figures S4–S7).
Evidence of a shift from fatty acid to glucose metabolism
It is well known that HF is associated with metabolic remodelling and energy insufficiency (Doenst et al., 2013; Wende et al., 2017). This insufficiency is thought to play an important role in the development of HF. Under normal conditions, the heart derives 50%–70% of the ATP needed from fatty acids with glucose contributing less (Wende et al., 2017). However, under stress conditions, there can be a shift from fatty acid to glucose utilisation (Tran Diem and Wang Zhao, 2019). Glucose generates more ATP than fatty acid for each mole of oxygen (Shiojima and Walsh, 2006). For the first time, we show that a qualitatively similar metabolic remodelling occurs in the AV node. Transcripts which change significantly in HF and are associated with glycolysis, fatty acid beta-oxidation, the tricarboxylic cycle, and the electron transport chain are shown in Figure 4. Glucose entry into cardiac myocytes is facilitated by GLUT1-13 transporters (Kewalramani and Rodrigues, 2009), but there were no changes in the corresponding transcripts (Slc23a1-13; Supplementary Figure S8A). Transcripts for six key enzymes in glycolysis were significantly upregulated in HF (Figure 4B) and are highlighted red in the metabolic pathway (Figure 4A). In two cases there appeared to be a partial isoform switch: whereas Aldoa and Eno1 were significantly upregulated in HF, Aldob and Eno2 were significantly downregulated (Figures 4A,B). Fatty acid is transported into cardiac myocytes by FAT/CD36 (Cd36), FABPpm (Got2) and FATP1-6 (Slc27a1-6) transporters (Chabowski et al., 2008); Cd36 was significantly upregulated and Slc27a1 was significantly downregulated (Supplementary Figure S8B). Transcripts for two key enyzmes in the fatty acid beta-oxidation pathway (Hadh and Acaa2) were significantly downregulated in HF (Figure 4C) and are highlighted blue in the metabolic pathway shown in Figure 4A. Although in the same set of transcripts shown in Figure 4C, Irs2 and Akt1 were upregulated in HF, AKT enhances glycolysis by promoting glucose uptake and may inhibit fatty acid oxidation (Shiojima and Walsh, 2006), and IRS similarly enhances glycolysis and inhibits fatty acid oxidation by acting via AKT (as well as AMP-activated protein kinase) (Long et al., 2011). Ppargc1a (PGC-1α) is a master regulator of mitochondrial functions (Austin et al., 2011) and Ppargc1a and also Ppara are involved in transcriptional regulation of fatty acid oxidation enzymes (Wende et al., 2017); both were upregulated (Supplementary Figure S9A). AMP-activated protein kinase is a central regulator of metabolism and it promotes both glycolysis and fatty acid oxidation (Kewalramani and Rodrigues, 2009), and there was an upregulation of three key AMP-activated protein kinase subunits (Supplementary Figure S9B). Transcripts for three key enzymes in the tricarboxylic acid cycle were significantly upregulated in HF (Figure 4D) and are again highlighted red in the metabolic pathway (Figure 4A). Finally, transcripts for three key enzymes in the electron transport chain were significantly upregulated in HF (Figure 4E) and are highlighted red in the metabolic pathway (Figure 4A); mt-Nd5 is responsible for NADH dehydrogenase 5, part of complex I, and mt-Co1 and Cox5a are responsible for cytochrome c oxidase subunits I and 5a of complex IV. In addition, Ppargc1a (the master regulator of mitochondrial functions) was significantly upregulated (Figure 4E) as already discussed and this has been shown to affect the electron transport chain (Austin et al., 2011).
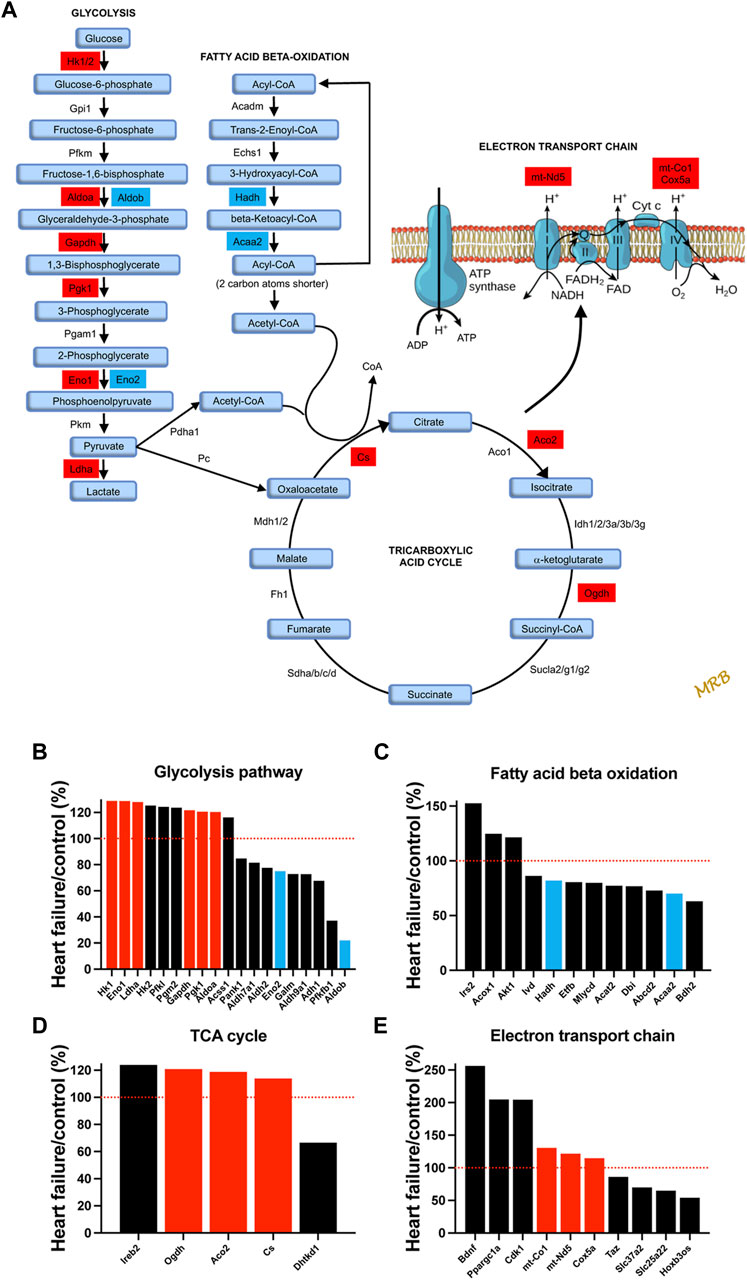
FIGURE 4. Evidence of a shift from fatty acid to glucose metabolism in the AV node in HF. (A), schematic diagram of glycolysis, fatty acid beta-oxidation, the tricarboxylic acid cycle, and the electron transport chain. For glycolysis, fatty acid beta-oxidation, and the tricarboxylic acid cycle, key enzymes and metabolic intermediaries (in light blue) are shown. Transcripts of enzymes highlighted in red were significantly upregulated in HF and transcripts of enzymes highlighted in dark blue were significantly downregulated. For the electron transport chain, ATP synthase and complexes I to IV are shown. Transcripts associated with complexes I and IV were significantly upregulated and are highlighted in red. (B–E), expression of transcripts associated with glycolysis (B), fatty acid beta-oxidation (C), the tricarboxylic acid cycle (D) and the electron transport chain (E) in HF mice as a percentage of that in control mice. All transcripts changed significantly in HF. Significantly upregulated transcripts highlighted in A are shown in red and significantly downregulated transcripts highlighted in A are shown in blue. Red dotted lines correspond to 100%.
Evidence of activation of intracellular signalling pathways and transcription factors
There was a significant upregulation of key transcripts in various intracellular signalling pathways and, as an example, the mitogen-activated protein (MAP) kinase pathway will be considered here. MAP kinases play an important signalling role in the heart (Rose et al., 2010). Various members of the family were significantly affected in the AV node in HF (Figure 5A). In particular, various members of the p38 signalling pathway (one arm of the MAP kinase signalling network) were upregulated (Figure 5B). MAP kinase signalling is prototypically activated by three tiers of phosphorylation: a MAP kinase kinase phosphorylates and activates a MAP kinase, which in turn phosphorylates and activates a MAP kinase (mediator of biological responses) (Rose et al., 2010). In the p38 pathway, Map3k6 was upregulated (Figure 5); Map3k6 is responsible for ASK2, which together with ASK1, acts as a MAP kinase kinase (Takeda et al., 2007). Map3k6 has previously been reported to be upregulated in the heart in the mouse model of pressure overload-induced HF (Matkovich et al., 2012). In the p38 pathway, Map2k3 was upregulated (Figure 5); Map2k3 is responsible for MKK3 and acts as a MAP kinase. Finally, in the p38 pathway, Mapk11 was upregulated (Figure 5); Mapk11 is responsible for p38β (one of four p38 isoforms present). p38α/β phosphorylates the downstream kinase, MAPKAPK2, which phosphorylates the heat shock protein HSP25/27 and the transcripts for both (Mapkapk2 and Hspb1) were significantly upregulated (Figure 5B). p21-activated kinases (PAKs) are a group of p38 activators and Pak2 and Pak3 were upregulated in HF (although Pak6 was downregulated; data not shown) (Zarubin and Han, 2005). The p38 pathway is activated in HF and the resultant pathological remodelling can lead to cardiac arrhythmias (Romero-Becerra et al., 2020). Interestingly, p38β regulates Hippo signalling (Huang et al., 2016). p38 inhibition improves heart function in pressure-overload right ventricular hypertrophy (Kojonazarov et al., 2017). The activity of MAP kinases are tightly controlled by a family of dual-specificity MAP kinase phosphatases (DUSPs) and they have been shown to have a variety of physiological and pathological roles (Liu and Molkentin, 2016). 10 DUSP transcripts were significantly up or downregulated in HF (data not shown).
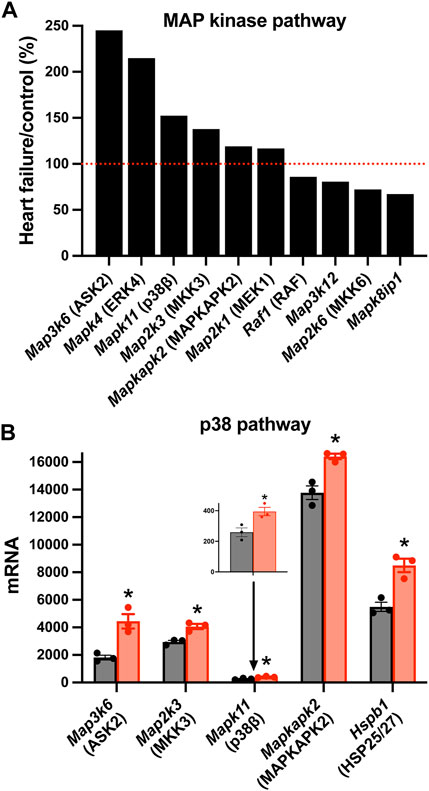
FIGURE 5. Changes in MAP kinase pathway transcripts in the AV node in HF. (A), significant changes in the expression of MAP kinase pathway transcripts. Expression in HF mice is shown as a percentage of that in control mice. Red dotted line corresponds to 100%. (B), mean (+SEM) expression (as well as individual data points) of p38 pathway transcripts in control (black bars) and HF (red bars) mice. The data for Mapk11 are also shown in a zoomed in panel. *p < 0.05.
There was also a significant upregulation of key transcripts in the protein kinase A, Ca2+-calmodulin-dependent protein kinase II (CaMKII), Hippo, WNT, JAK-STAT, and NOTCH signalling pathways (Supplementary Figures S10–S13). Protein phosphorylation is an important signalling mechanism, and it is determined by a balance of protein kinases and phosphatases and there was a remodelling of both protein kinases and phosphatases at the transcript level (Supplementary Figures S14, S15). Transcript expression is primarily governed by transcription factors, and in the mouse Zhou et al. (2017) have identified 941 transcription factors. Of these, transcripts for 927 were identified in the AV node and 148 (16%) changed significantly in HF (Supplementary Figure S16). 81 (55%) were upregulated and 67 (45%) downregulated (Supplementary Figure S16).
Remodelling of extracellular matrix transcripts
Nodal dysfunction has frequently been attributed to fibrosis, i.e., a proliferation of the fibrillar collagens (Suarez-Mier et al., 1995; Bailey et al., 2007; Swaminathan et al., 2011; Csepe et al., 2015), an important component of the extracellular matrix. The “Matrisome” from Naba et al, (2016) lists 1,110 transcripts in the mouse known to be associated with the extracellular matrix divided into four different categories. In the AV node in HF, many significant changes in Matrisome transcripts were observed (221 transcripts, i.e. 20% of the total) showing that there was likely a remodelling of the extracellular matrix. Collagens are the most abundant proteins in the extracellular matrix. Transcripts for two fibrillar collagens were present, types 1 and 3; they were the most abundant of the collagens, but they did not change in HF (Figure 6). Basement membranes are dense extracellular matrix structures, and previously we have shown that basement membrane surrounds each sinus node myocyte (Linscheid et al., 2019). Collagen type 4 is a major component of basement membranes (Steffensen and Rasmussen, 2018) and Col4a1 and Col4a2, the two most abundant collagen type 4 α-chain transcripts, were significantly upregulated in the AV node in HF (Figure 6). Collagen type 4 expression is known to be altered in various pathophysiological conditions (Shamhart and Meszaros, 2010). An increase in collagen type 4 may thicken the basement membrane of myocytes and impede extracellular communication with other cells (de Castro Brás et al., 2014). Collagen type 6 α-chain transcripts were significantly downregulated in the AV node in HF (Figure 6). Collagen type 6 carries out cytoprotective functions as well as mechanical roles (Cescon et al., 2015). In part by binding to collagen type 4 in the basement membrane, collagen type 6 links the myocyte to the extracellular matrix. In dilated cardiomyopathy in the human, Gil-Cayuela et al, (2016) reported there is an increase in the transcript (Col8a1) and protein for the non-fibrillar collagen eight α1 subunit, and there is a significant relationship between left ventricular mass and Col8a1 expression. There was also a significant upregulation of Col8a1 in the AV node in HF (Figure 6). Gil-Cayuela et al, (2016) hypothesise that overexpression of a number of non-fibrillar collagen genes may promote pathological remodelling by inducing apoptosis and hypertrophy of cardiomyocytes. The sum of all collagen transcripts was not significantly altered in HF (data not shown). Changes in other components of the Matrisome are considered in the Supplementary Results (Supplementary Figures S17–S19).
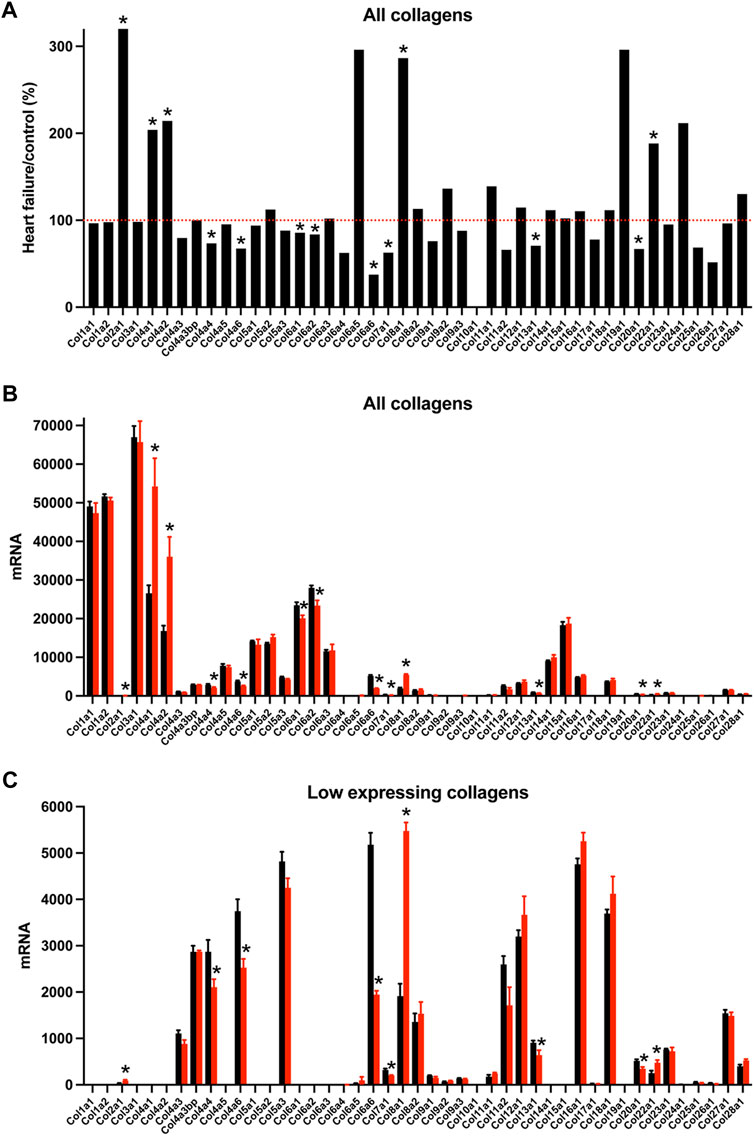
FIGURE 6. Transcriptomic remodelling of the extracellular matrix of the AV node in HF. (A), expression of all collagen transcripts in HF mice as a percentage of that in control mice. Red dotted line corresponds to 100%. (B and C), expression of highly expressed collagen transcripts (B) and more poorly expressed collagen transcripts (C) in control (black bars) and HF (red bars) mice. Only means + SEM shown (individual data points not shown for clarity). *p < 0.05.
Evidence of an immune response
In HF, although inflammation plays a key role in the development of ventricular dysfunction (Martini et al., 2019; Strassheim et al., 2019), it is not known whether it plays a similar role in AV node dysfunction. Using single cell RNAseq to measure the expression of transcript markers, Martini et al, (2019) showed that the myocardium is infiltrated by a variety of immune cell types in the mouse TAC model of HF. Figures 7A,B shows the expression of various immune cell marker transcripts in the AV node which changed significantly in HF. S100a8 and S100a9, expressed in neutrophils and monocytes, were upregulated (Figures 7A,B). During inflammation, S100A8/A9 is released and modulates the inflammatory response by stimulating leukocyte recruitment and inducing cytokine secretion (Wang et al., 2018). Cd14 is expressed in monocytes, macrophages, dendritic cells and neutrophils (Marcos et al., 2010) and was upregulated in the AV node in HF (Figures 7A,B). Ncf1 forms a subunit of NADPH oxidase, which is primarily active in phagocytes (including monocytes and macrophages) and plays an essential role in the immune system; Ncf1 was upregulated in the AV node in HF (Figures 7A,B). These data suggest that neutrophils, monocytes and macrophages are infiltrating the AV node in HF. However, this may not be true of all macrophages, which exist as M1 cells (pro-inflammatory) or M2 cells (involved with resolution of inflammation and repair of damaged tissues). The data suggest a decline in the M2 cell type: the M2-like marker, Cd163 (Martini et al., 2019), was downregulated and also the repair associated transcript, Mmp9 (Martini et al., 2019), was downregulated (Figures 7A,B). Cd1d1 is involved in natural killer T cell development (Sundararaj et al., 2018) and this too was downregulated (Figures 7A,B). However, phagocytes may not be the only immune cell type to be infiltrating the AV node in HF, because Ccr7, a marker of B lymphocytes (Mcheik et al., 2019), was also upregulated (Figures 7A,B). Cytokines are signalling molecules, largely secreted by cells of the immune system (predominantly macrophages and T helper cells), which mediate communication among immune and non-immune cells and are the means by which immune cells may impact the heart. Figure 7C shows that a large number of cytokine transcripts were significantly altered in the AV node in HF, some with known connections to HF; see SupplementaryFigure S20 for further information.
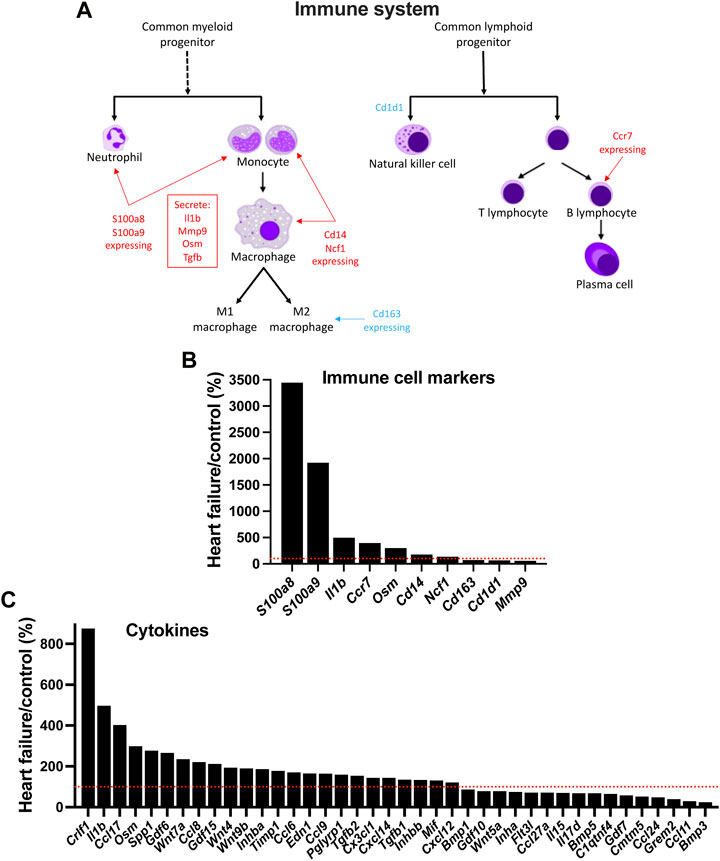
FIGURE 7. Evidence of infiltration of the AV node by immune cells in HF. (A), schematic diagram of the cells of the immune system. (B), expression of immune cell markers (transcripts) in HF mice as a percentage of that in control mice. (C), expression of cytokine transcripts in HF mice as a percentage of that in control mice. Red dotted lines correspond to 100%.
HF-dependent changes in transcripts identified by GWAS as determinants of the PR interval or associated with hereditary AV node dysfunction
GWAS studies have identified 44 genes associated with an abnormal PR interval (van Setten et al., 2018). Of these, the transcripts associated with 11 genes changed significantly in the AV node in HF (Table 1) and therefore could be involved in the AV node dysfunction in HF. The function of some of the transcripts (Fat1, Fermt2, Fign, Lrch1, Tmem182) is either unknown or has an unknown relevance for the AV node; some transcripts are involved in heart development (Meis1, Id2, Tbx3); two transcripts are known to be involved in HF (Camk2d, Myocd); and one transcript (Scn10a) is known to modulate AV node conduction (Table 1). Three transcripts known to be associated with hereditary AV node dysfunction (Lmna, Prkag2, Trpm4; Table 1) changed significantly in the AV node in HF.
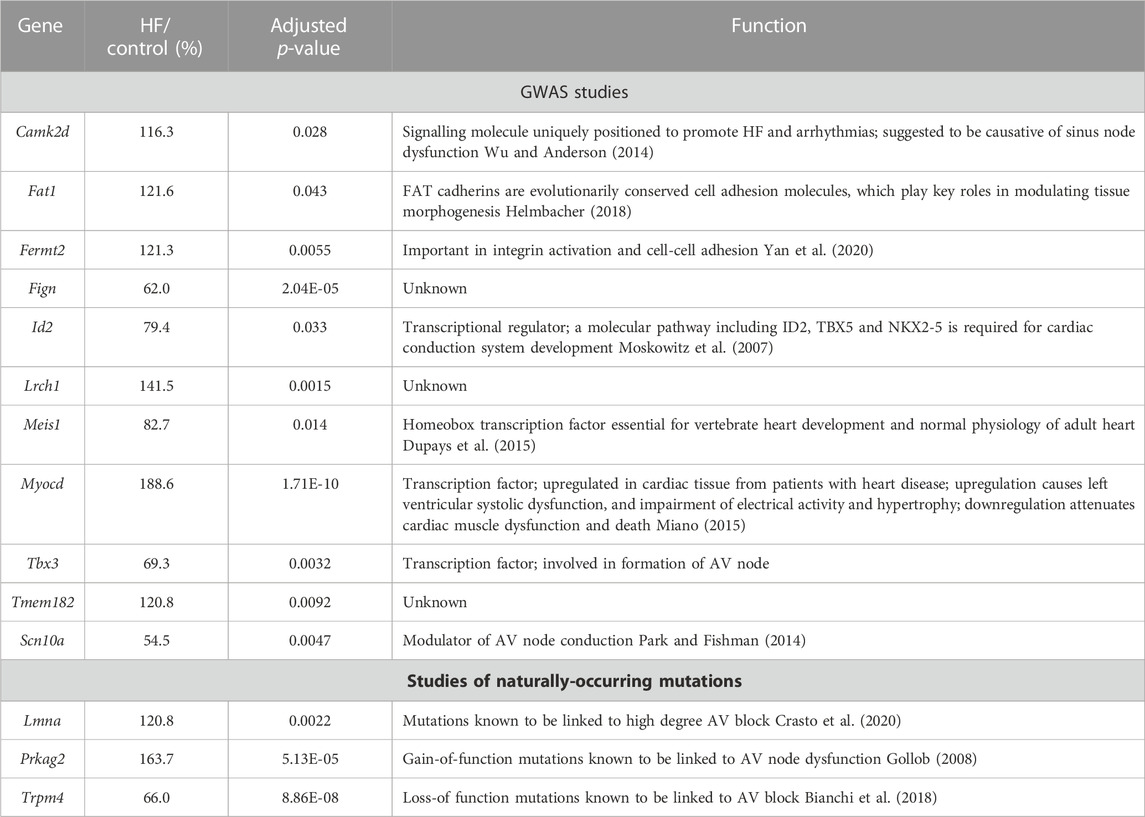
TABLE 1. Transcripts which changed significantly in the AV node in HF and the corresponding genes have been identified by GWAS studies as being associated with the PR interval or naturally-occurring mutations in the corresponding genes are associated with AV block. Gene/transcript name, transcript abundance in HF as a percentage of that in control animals, adjusted p-value of the change in HF, and notes on the functions of the genes shown.
Other HF-dependent changes
There were many other HF-dependent changes and some of these are considered in the Supplementary Figures S22–S25.
Discussion
This study has shown that in a model of pressure overload (hypertension)-induced cardiac hypertrophy and HF there is a significant prolongation of the PR interval, evidence of AV node dysfunction as observed in HF patients (Nikolaidou et al., 2016). This was accompanied by significant changes in 5.6% of the transcriptome of the AV node: for example, in transcripts responsible for the PR interval, the sarcomere, fatty acid and glucose metabolism, the extracellular matrix, and the transcription and translation machinery. Importantly, there were also significant changes in transcripts involved in multiple signalling molecules and pathways, all of which have the potential to be involved in cardiac remodelling. Finally, there was evidence of an activation of the immune system and a proliferation or infiltration of macrophages and other immune cells in the AV node. The complexity and multiplicity of effects of HF on the AV node is a novel finding. There are likely to be many more effects on the AV node; the reader can peruse the list of all transcripts in the Supplementary Material (All transcripts. xlsx).
Potential immediate cause of AV dysfunction
In the AV node in HF there was a downregulation of key ion channel transcripts involved in AV node conduction: Scn10a responsible for Nav1.8; Cacna1d (Cav1.3) in part responsible for the L-type Ca2+ current, ICa,L; Cacna1g (Cav3.1) and Cacna1h (Cav3.2) responsible for the T-type Ca2+ current, ICa,T; and Kcnh2 (ERG) responsible for the rapid delayed rectifier K+ current, IK,r. These changes could be responsible for the slowing of AV node conduction and AV node dysfunction (Regan et al., 2005; Chambers et al., 2010; Holm et al., 2010; Pfeufer et al., 2010; Park and Fishman, 2014; Mesirca et al., 2015; Auxerre-Plantié et al., 2019).
Widespread transcriptomic remodelling of the AV node
Transcriptomic remodelling of the AV node in HF was not restricted to ion channels–there was a remodelling of other systems as well. There was a transcriptomic remodelling of receptors and G proteins, which potentially could impact AV node conduction (Supplementary Figure S22). Some of the transcriptomic remodelling in the AV node in HF is similar to that reported for the rest of the heart, for example, the changes in natriuretic peptide and myosin heavy chain transcripts (Supplementary Figure S2) (Dirkx et al., 2013; Man et al., 2018). The observed changes in metabolic transcripts are qualitatively similar to changes observed in whole heart studies: in a rat TAC model of HF, Bugger et al, (2009) reported that six proteins involved in fatty acid beta-oxidation were downregulated including HADH (downregulation of Hadh was observed in this study–Figure 4C), three involved in glycolysis were upregulated, two involved in the tricarboxylic acid cycle were upregulated including aconitase 2 (upregulation of Aco2 was observed in this study–Figure 4D), and some proteins involved in the electron transport chain were upregulated and some downregulated including subunits of NADH dehydrogenase and cytochrome c oxidase. In the mouse TAC model of HF, a decreased abundance of proteins involved in fatty acid metabolism and increased abundance of proteins involved in glycolysis has been reported in the ventricles (Dai et al., 2012). HF is reported to result in fibrosis and this can be arrhythmogenic by interrupting the conduction of the action potential (Nguyen et al., 2017). However, there was no evidence of upregulation of transcripts for the fibrillar collagens, Col1 and Col3, in the AV node in HF, although there was a widespread transcriptional remodelling of the extracellular matrix (Figure 6 and Supplementary S17-S19).
Potential causes of the adverse remodelling of the AV node
This study has shown a transcriptomic remodelling of the protein kinase A, CaMKII, p38-MAP kinase, Hippo, WNT, JAK-STAT and Notch signalling pathways as well as the protein phosphatase interactome in the AV node in HF; it has also shown evidence of an immune response; each of these has been shown in other studies to be involved in cardiac pathological remodelling in disease. This study has also shown significant changes in signalling molecules: 40 cytokines, 109 protein kinases and 148 transcription factors (Figure 7C, Supplementary S14 and S16). Ingenuity pathway analysis was used to identify the canonical pathways significantly associated with the cytokines, protein kinases and transcription factors and the results are given in a Supplementary file (Canonical pathways. xlsx) and summarised in Figures 8A–C; in each case, many molecules are associated with the immune system especially the interleukin pathway, many are associated with intracellular signalling pathways (both signalling pathways highlighted in this study and many other pathways), and some are associated with Ca2+ signalling, metabolism and fibrosis. Figure 8D shows a large amount of overlap between the three sets of associated canonical pathways and this increases confidence in the predictions. The highlighted signalling pathways identified by the canonical pathway analysis include protein kinase A signalling, p38 MAPK signalling, PAK signalling, HIPPO signalling, WNT/β-catenin signalling, JAK/Stat signalling, STAT3 pathway and Notch signalling–this increases the likelihood that these pathways are involved. The involvement of multiple signalling pathways in the AV node in HF is not surprising because there were widespread changes (involving >3,000 transcripts) in the AV node in HF. This raises reservations concerning therapeutic strategies targeting only one pathway.
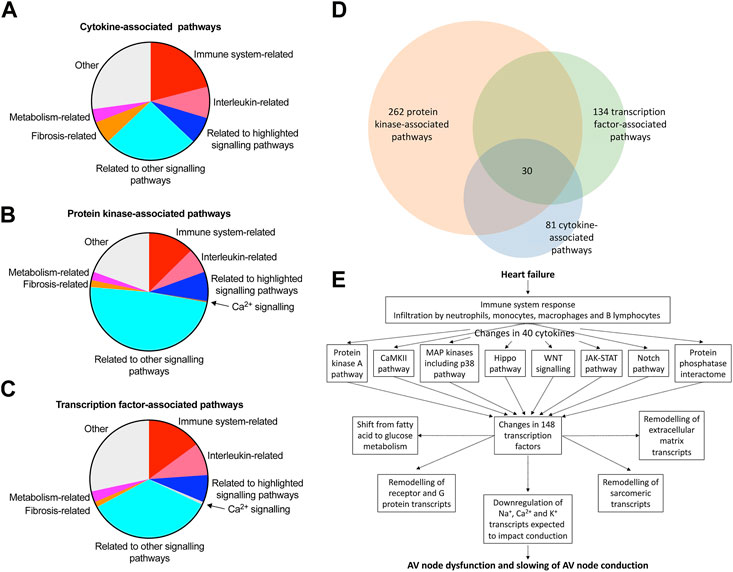
FIGURE 8. Pathways involved in AV node dysfunction in HF. (A–C), summary of canonical pathways (identified by Ingenuity Pathway Analysis) associated with the cytokines (A), protein kinases (B) and transcription factors (C) changing significantly in the AV node in HF. Details of the pathways are given in the Supplementary File, Canonical pathways. xlsx. 81, 262 and 134 pathways associated with the cytokines, protein kinases and transcription factors, respectively, were identified in total and the area of the pie chart for each group of pathways reflects the number of pathways in each group. (D), Venn diagram of the pathways associated with the cytokines, protein kinases and transcription factors; there is substantial overlap of the pathways and 30 pathways were common to the cytokines, protein kinases and transcription factors. (E), schematic diagram showing a potential chain of events in the AV node during HF leading to AV node dysfunction. Although the schema is based on the results of this study, it remains hypothetical. Although unidirectional arrows are drawn, they could be bidirectional.
The activation of the immune system has the potential to be the driver of all the other changes observed in the AV node. The function of the immune system is not only to protect the animal from invasion by foreign organisms. In the case of the heart, it is also concerned with repair of the heart after myocardial infarction (Kologrivova et al., 2021), the adverse remodelling of the working myocardium in heart disease (Strassheim et al., 2019; Murphy et al., 2020), and even ageing (Appel et al., 2021). For the first time, we show here evidence that in HF there is a proliferation of immune cells (neutrophils, monocytes, macrophages and B lymphocytes) in the AV node (Figure 7). One of the principal lines of evidence for a proliferation of neutrophils and monocytes were increases in S100a8 and S100a9 to 3,447% and 1,923% of control (Figure 7B). During inflammation, S100A8/A9 is actively released and exerts a critical role in modulating the inflammatory response and serves as a candidate biomarker for disease (Wang et al., 2018). S100A8/A9 plasma levels are significantly higher in chronic HF patients (Wang et al., 2018).
In the AV node in HF there was an increase in transcripts for proinflammatory cytokines (and proinflammatory cytokine-related molecules): Il1b (IL-1b), Osm (oncostatin M; cytokine of interleukin-6 family), Tnfrsf1a (TNF receptor 1), Tnfrsf1b (TNF receptor 2), Tgfb1 (TGFβ-1) and Tgfb2 (TGFβ-2) (Figure 7C). Lgals3, responsible for galectin-3, which can have cytokine-like regulatory actions in immune cells (Jeon et al., 2010), was also significantly upregulated in the AV node in HF (Supplmentary Figure S20C). These upregulated cytokines and cytokine-related molecules have a known role in HF: Il1b is upregulated in HF and associated with worse prognosis and trials have been undertaken to evaluate the role of interleukine-1 blockade to reduce inflammation and ventricular remodelling in HF (Van Tassell et al., 2015; Szekely and Arbel, 2018). There is evidence that IL-1b downregulates ICa,L and K+ current and Ca2+-handling proteins (Szekely and Arbel, 2018). Patients suffering chronic dilated cardiomyopathy show upregulation of oncostatin M and its receptor, Oβ (oncostatin M receptor; Osmr) (Kubin et al., 2011). Cardiac oncostatin M (Osm) induces dedifferentiation of cardiomyocytes and promotes progression of HF, and an antibody targeting Oβ or genetic inactivation of a single allele of Osmr reduces cardiomyocyte remodelling and dedifferentiation and improves cardiac performance and survival (Kubin et al., 2011; Pöling et al., 2013). TNF-α and its receptors have both pathogenic and cardioprotective roles in heart disease (Rolski and Błyszczuk, 2020). In the mouse TAC model of HF, there is a correlation between progressive hypertrophy and increasing myocardial levels of TNF-α and TNF receptor 1 (Rolski and Błyszczuk, 2020). Increased concentrations of circulating TNF receptors 1 and 2 are associated with increased risks of cardiovascular events and mortality in patients with stable coronary heart disease (Carlsson et al., 2018). TGF-β is another cytokine. Myocardial TGF-β expression is upregulated in animal models of myocardial infarction and cardiac hypertrophy and in patients with dilated or hypertrophic cardiomyopathy, and it plays an important role in cardiac remodelling and fibrosis (Dobaczewski et al., 2011). Galectin-3 overexpression and secretion is associated with several diseases including HF, in which galectin-3 has been shown to be a useful biomarker (Gehlken et al., 2018; Suthahar et al., 2018). Galectin-3 induces pathologic remodelling of the heart, and is a culprit in the development of cardiac fibrosis in HF and electrical remodelling in atrial fibrillation (Takemoto et al., 2016; Gehlken et al., 2018). Proof that inflammation is the cause of AV node remodelling and dysfunction in HF is beyond the scope of this study. However, if it is the cause there may be a correlation between the two and with one exception there are significant correlations between the two well-known heart failure markers, Nppa and Nppb (responsible for ANP and BNP) and the macrophage marker, Cd14, the cytokine transcript, Crlf1, and the transcript for the cytokine-like galectin-3, Lgals3 (Supplementary Figure S21).
In conclusion, the ultimate reason for the dysfunction of the AV node in HF is likely to be the downregulation of Na+, Ca2+ and K+ channel transcripts, as a result of transcription factors—as well as micro-RNAs (Yanni et al., 2020). It is feasible that these changes are driven by the protein kinase A, CaMKII, p38-MAP kinase, Hippo, WNT, JAK-STAT and Notch signalling pathways as well as the proliferation of immune cells and upregulation of cytokine transcripts in the AV node in HF. Figure 8E presents a hypothetical scheme of the possible hierarchy of changes. In addition to this, the changes in transcripts known to be related to AV block listed in Table 1 have also to be considered.
Data availability statement
The raw data from this study are available in a repository:https://www.ebi.ac.uk/biostudies/arrayexpress/arrayexpress/studies/E-MTAB-12384?accession=E-MTAB-12384. Further data are available in the Supplementary Material.
Ethics statement
The animal study was reviewed and approved by Experiments carried out in the UK were in accordance with the UK Animals (Scientific Procedures) Act (1986) and UK Home Office project licence PPL PBA6A87CA.
Author contributions
MRB and HD obtained funding for the study and supervised the experiments. CW and MZ carried out the experiments. MRB and CW with assistance and direction from AS and MS analysed the data. MRB and CW wrote the manuscript with contributions from others. CW, MZ, MS, MH, AS, HD, and MRB read and approved the manuscript.
Funding
This work was supported by a British Heart Foundation programme grant to MRB and HD(PG/15/16/31330), a Fondation Leducq grant to MRB, HD and others (TNE FANTASY 19CV03), a British Heart Foundation Intermediate Fellowship to AS (FS/19/1/34035) and a British Heart Foundation Ph.D. Studentship to MS
Acknowledgments
We thank Andy Hayes and I-Hsuan Lin of the Genomic Technologies and Bioinformatics Facilities at the University of Manchester for providing support with regard to RNAseq.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1083910/full#supplementary-material
Abbreviations
AV, atrioventricular; ECG, electrocardiogram; HF, heart failure; RNAseq, RNA-sequencing; TAC, transverse aortic constriction.
References
Appel, M., Frantz, S., and Campos Ramos, G. (2021). Myocardial inflammation comes of age. Curr. Opin. Physiol. 19, 47–54. doi:10.1016/j.cophys.2020.09.006
Aro, A. L., Anttonen, O., Kerola, T., Junttila, M. J., Tikkanen, J. T., Rissanen, H. A., et al. (2014). Prognostic significance of prolonged PR interval in the general population. Eur. Heart J. 35, 123–129. doi:10.1093/eurheartj/eht176
Austin, S., Klimcakova, E., and St-Pierre, J. (2011). Impact of PGC-1α on the topology and rate of superoxide production by the mitochondrial electron transport chain. Free Radic. Biol. Med. 51, 2243–2248. doi:10.1016/j.freeradbiomed.2011.08.036
Auxerre-Plantié, E., Nakamori, M., Renaud, Y., Huguet, A., Choquet, C., Dondi, C., et al. (2019). Straightjacket/α2δ3 deregulation is associated with cardiac conduction defects in myotonic dystrophy type 1. eLife 8, e51114. doi:10.7554/eLife.51114
Bailey, S., Burkhardt, J. D., and Bhargava, M. (2007). “Bradycardia,” in Handbook of cardiac electrophysiology. Editors A. Natale, and F. Marchlinski (CRC Press), 51–60.
Bianchi, B., Ozhathil, L. C., Medeiros-Domingo, A., Gollob, M. H., and Abriel, H. (2018). Four TRPM4 cation channel mutations found in cardiac conduction diseases lead to altered protein stability. Front. Physiol. 9, 177. doi:10.3389/fphys.2018.00177
Bugger, H., Schwarzer, M., Chen, D., Schrepper, A., Amorim, P. A., Schoepe, M., et al. (2009). Proteomic remodelling of mitochondrial oxidative pathways in pressure overload-induced heart failure. Cardiovasc. Res. 85, 376–384. doi:10.1093/cvr/cvp344
Bui, A. L., Horwich, T. B., and Fonarow, G. C. (2011). Epidemiology and risk profile of heart failure. Nat. Rev. Cardiol. 8, 30–41. doi:10.1038/nrcardio.2010.165
Cachero, T. G., Morielli, A. D., and Peralta, E. G. (1998). The small GTP-binding protein RhoA regulates a delayed rectifier potassium channel. Cell 93, 1077–1085. doi:10.1016/S0092-8674(00)81212-X
Carlsson, A. C., Ruge, T., Kjøller, E., Hilden, J., Kolmos, H. J., Sajadieh, A., et al. (2018). 10-year associations between tumor necrosis factor receptors 1 and 2 and cardiovascular events in patients with stable coronary heart disease: A CLARICOR (effect of clarithromycin on mortality and morbidity in patients with ischemic heart disease) trial substudy. J. Am. Heart Assoc. 7, e008299. doi:10.1161/JAHA.117.008299
Cescon, M., Gattazzo, F., Chen, P., and Bonaldo, P. (2015). Collagen VI at a glance. J. Cell Sci. 128, 3525–3531. doi:10.1242/jcs.169748
Chabowski, A., Górski, J., Glatz, J. F. C., P Luiken, J. J. F., and Bonen, A. (2008). Protein-mediated fatty acid uptake in the heart. Curr. Cardiol. Rev. 4, 12–21. doi:10.2174/157340308783565429
Chambers, J. C., Zhao, J., Terracciano, C. M., Bezzina, C. R., Zhang, W., Kaba, R., et al. (2010). Genetic variation in SCN10A influences cardiac conduction. Nat. Genet. 42, 149–152. doi:10.1038/ng.516
Crasto, S., My, I., and Di Pasquale, E. (2020). The broad spectrum of LMNA cardiac diseases: From molecular mechanisms to clinical phenotype. Front. Physiology 11, 761. doi:10.3389/fphys.2020.00761
Csepe, T. A., Kalyanasundaram, A., Hansen, B. J., Zhao, J., and Fedorov, V. V. (2015). Fibrosis: A structural modulator of sinoatrial node physiology and dysfunction. Front. Physiol. 6, 37. doi:10.3389/fphys.2015.00037
Dai, D. F., Hsieh, E. J., Liu, Y., Chen, T., Beyer, R. P., Chin, M. T., et al. (2012). Mitochondrial proteome remodelling in pressure overload-induced heart failure: The role of mitochondrial oxidative stress. Cardiovasc. Res. 93, 79–88. doi:10.1093/cvr/cvr274
de Castro Brás, L. E., Toba, H., Baicu, C. F., Zile, M. R., Weintraub, S. T., Lindsey, M. L., et al. (2014). Age and SPARC change the extracellular matrix composition of the left ventricle. BioMed Res. Int. 2014, 810562. doi:10.1155/2014/810562
Dirkx, E., da Costa Martins, P. A., and De Windt, L. J. (2013). Regulation of fetal gene expression in heart failure. Biochim. Biophys. Acta 1832, 2414–2424. doi:10.1016/j.bbadis.2013.07.023
Dobaczewski, M., Chen, W., and Frangogiannis, N. G. (2011). Transforming growth factor (TGF)-β signaling in cardiac remodeling. J. Mol. Cell. Cardiol. 51, 600–606. doi:10.1016/j.yjmcc.2010.10.033
Doenst, T., Nguyen Tien, D., and Abel, E. D. (2013). Cardiac metabolism in heart failure: Implications beyond ATP production. Circulation Res. 113, 709–724. doi:10.1161/CIRCRESAHA.113.300376
Dupays, L., Shang, C., Wilson, R., Kotecha, S., Wood, S., Towers, N., et al. (2015). Sequential binding of MEIS1 and NKX2-5 on the Popdc2 gene: A mechanism for spatiotemporal regulation of enhancers during cardiogenesis. Cell Rep. 13, 183–195. doi:10.1016/j.celrep.2015.08.065
Gehlken, C., Suthahar, N., Meijers, W. C., and de Boer, R. A. (2018). Galectin-3 in heart failure: An update of the last 3 years. Heart Fail. Clin. 14, 75–92. doi:10.1016/j.hfc.2017.08.009
Gil-Cayuela, C., Roselló-Lletí, E., Ortega, A., Tarazón, E., Triviño, J. C., Martínez-Dolz, L., et al. (2016). New altered non-fibrillar collagens in human dilated cardiomyopathy: Role in the remodeling process. PloS One 11, e0168130. doi:10.1371/journal.pone.0168130
Glasscock, E., Yoo, J. W., Chen, T. T., Klassen, T. L., and Noebels, J. L. (2010). Kv1.1 potassium channel deficiency reveals brain-driven cardiac dysfunction as a candidate mechanism for sudden unexplained death in epilepsy. J. Neurosci. 30, 5167–5175. doi:10.1523/JNEUROSCI.5591-09.2010
Glasscock, E. (2019). Kv1.1 channel subunits in the control of neurocardiac function. Channels 13, 299–307. doi:10.1080/19336950.2019.1635864
Gollob, M. H. (2008). Modulating phenotypic expression of the PRKAG2 cardiac syndrome. Circulation 117, 134–135. doi:10.1161/CIRCULATIONAHA.107.747345
Helmbacher, F. (2018). Tissue-specific activities of the Fat1 cadherin cooperate to control neuromuscular morphogenesis. PLoS Biol. 16, e2004734. doi:10.1371/journal.pbio.2004734
Holm, H., Gudbjartsson, D. F., Arnar, D. O., Thorleifsson, G., Thorgeirsson, G., Stefansdottir, H., et al. (2010). Several common variants modulate heart rate, PR interval and QRS duration. Nat. Genet. 42, 117–122. doi:10.1038/ng.511
Huang, D., Li, X., Sun, L., Huang, P., Ying, H., Wang, H., et al. (2016). Regulation of Hippo signalling by p38 signalling. J. Mol. Cell Biol. 8, 328–337. doi:10.1093/jmcb/mjw036
Jeon, S. B., Yoon, H. J., Chang, C. Y., Koh, H. S., Jeon, S. H., and Park, E. J. (2010). Galectin-3 exerts cytokine-like regulatory actions through the JAK-STAT pathway. J. Immunol. 185, 7037–7046. doi:10.4049/jimmunol.1000154
Kewalramani, G., and Rodrigues, B. (2009). AMP-Activated protein kinase in the heart: Role in cardiac glucose and fatty acid metabolism. Clin. Lipidol. 4, 643–661. doi:10.2217/clp.09.43
Kojonazarov, B., Novoyatleva, T., Boehm, M., Happe, C., Sibinska, Z., Tian, X., et al. (2017). p38 MAPK inhibition improves heart function in pressure-loaded right ventricular hypertrophy. Am. J. Respir. Cell Mol. Biol. 57, 603–614. doi:10.1165/rcmb.2016-0374OC
Kologrivova, I., Shtatolkina, M., Suslova, T., and Ryabov, V. (2021). Cells of the immune system in cardiac remodeling: Main players in resolution of inflammation and repair after myocardial infarction. Front. Immunol. 12, 664457. doi:10.3389/fimmu.2021.664457
Kubin, T., Pöling, J., Kostin, S., Gajawada, P., Hein, S., Rees, W., et al. (2011). Oncostatin M is a major mediator of cardiomyocyte dedifferentiation and remodeling. Cell Stem Cell 9, 420–432. doi:10.1016/j.stem.2011.08.013
Li, J., Greener, I. D., Inada, S., Nikolski, V. P., Yamamoto, M., Hancox, J. C., et al. (2008). Computer three-dimensional reconstruction of the atrioventricular node. Circ. Res. 102, 975–985. doi:10.1161/CIRCRESAHA.108.172403
Linscheid, N., Logantha, S., Poulsen, P. C., Zhang, S., Schrolkamp, M., Egerod, K. L., et al. (2019). Quantitative proteomics and single-nucleus transcriptomics of the sinus node elucidates the foundation of cardiac pacemaking. Nat. Commun. 10, 2889. doi:10.1038/s41467-019-10709-9
Liu, R., and Molkentin, J. D. (2016). Regulation of cardiac hypertrophy and remodeling through the dual-specificity MAPK phosphatases (DUSPs). J. Mol. Cell. Cardiol. 101, 44–49. doi:10.1016/j.yjmcc.2016.08.018
Long, Y. C., Cheng, Z., Copps, K. D., and White, M. F. (2011). Insulin receptor substrates Irs1 and Irs2 coordinate skeletal muscle growth and metabolism via the Akt and AMPK pathways. Mol. Cell. Biol. 31, 430–441. doi:10.1128/MCB.00983-10
Man, J., Barnett, P., and Christoffels, V. M. (2018). Structure and function of the Nppa-Nppb cluster locus during heart development and disease. Cell. Mol. Life Sci. 75, 1435–1444. doi:10.1007/s00018-017-2737-0
Marcos, V., Latzin, P., Hector, A., Sonanini, S., Hoffmann, F., Lacher, M., et al. (2010). Expression, regulation and clinical significance of soluble and membrane CD14 receptors in pediatric inflammatory lung diseases. Respir. Res. 11, 32. doi:10.1186/1465-9921-11-32
Martini, E., Kunderfranco, P., Peano, C., Carullo, P., Cremonesi, M., Schorn, T., et al. (2019). Single-cell sequencing of mouse heart immune infiltrate in pressure overload-driven heart failure reveals extent of immune activation. Circulation 140, 2089–2107. doi:10.1161/CIRCULATIONAHA.119.041694
Matkovich, S. J., Hu, Y., Eschenbacher, W. H., Dorn, L. E., Dorn, G. W., and 2nd, (2012). Direct and indirect involvement of microRNA-499 in clinical and experimental cardiomyopathy. Circ. Res. 111, 521–531. doi:10.1161/circresaha.112.265736
Mcheik, S., Van Eeckhout, N., De Poorter, C., Galés, C., Parmentier, M., and Springael, J.-Y. (2019). Coexpression of CCR7 and CXCR4 during B cell development controls CXCR4 responsiveness and bone marrow homing. Front. Immunol. 10, 2970. doi:10.3389/fimmu.2019.02970
Mesirca, P., Torrente, A. G., and Mangoni, M. E. (2015). Functional role of voltage gated Ca2+ channels in heart automaticity. Front. Physiology 6, 19. doi:10.3389/fphys.2015.00019
Mesirca, P., Bidaud, I., Briec, F., Evain, S., Torrente, A. G., Le Quang, K., et al. (2016). G protein-gated IKACh channels as therapeutic targets for treatment of sick sinus syndrome and heart block. Proc. Natl. Acad. Sci. U. S. A. 113, E932–E941. doi:10.1073/pnas.1517181113
Miano, J. M. (2015). Myocardin in biology and disease. J. Biomed. Res. 29, 3–19. doi:10.7555/JBR.29.20140151
Moskowitz, I. P. G., Kim, J. B., Moore, M. L., Wolf, C. M., Peterson, M. A., Shendure, J., et al. (2007). A molecular pathway including Id2, Tbx5, and Nkx2-5 required for cardiac conduction system development. Cell 129, 1365–1376. doi:10.1016/j.cell.2007.04.036
Murphy, S. P., Kakkar, R., McCarthy, C. P., and Januzzi, J. L. (2020). Inflammation in Heart Failure: JACC state-of-the-art review. J. Am. Coll. Cardiol. 75, 1324–1340. doi:10.1016/j.jacc.2020.01.014
Naba, A., Clauser, K. R., Ding, H., Whittaker, C. A., Carr, S. A., and Hynes, R. O. (2016). The extracellular matrix: Tools and insights for the “omics” era. Matrix Biol. 49, 10–24. doi:10.1016/j.matbio.2015.06.003
Nguyen, M. N., Kiriazis, H., Gao, X. M., and Du, X. J. (2017). Cardiac fibrosis and arrhythmogenesis. Compr. Physiol. 7, 1009–1049. doi:10.1002/cphy.c160046
Nikolaidou, T., Cai, X., Stephenson, R. S., Yanni, J., Lowe, T., Atkinson, A. J., et al. (2015). Congestive heart failure leads to prolongation of the PR Interval and atrioventricular junction enlargement and ion channel remodelling in the rabbit. PLoS One 10, e0141452. doi:10.1371/journal.pone.0141452
Nikolaidou, T., Ghosh, J. M., and Clark, A. L. (2016). Outcomes related to first-degree atrioventricular block and therapeutic implications in patients with heart failure. J. Am. Coll. Cardiol. Clin. Electrophysiol. 2, 181–192. doi:10.1016/j.jacep.2016.02.012
Park, D. S., and Fishman, G. I. (2014). Navigating through a complex landscape: SCN10A and cardiac conduction. J. Clin. Investigation 124, 1460–1462. doi:10.1172/jci75240
Pfeufer, A., van Noord, C., Marciante, K. D., Arking, D. E., Larson, M. G., Smith, A. V., et al. (2010). Genome-wide association study of PR interval. Nat. Genet. 42, 153–159. doi:10.1038/ng.517
Pöling, J., Gajawada, P., Richter, M., Lörchner, H., Polyakova, V., Kostin, S., et al. (2013). Therapeutic targeting of the oncostatin M receptor-β prevents inflammatory heart failure. Basic Res. Cardiol. 109, 396. doi:10.1007/s00395-013-0396-3
Regan, C. P., Cresswell, H. K., Zhang, R., and Lynch, J. J. (2005). Novel method to assess cardiac electrophysiology in the rat: Characterization of standard ion channel blockers. J. Cardiovasc. Pharmacol. 46, 68–75. doi:10.1097/01.fjc.0000162774.86780.9d
Richards, D. A., Aronovitz, M. J., Calamaras, T. D., Tam, K., Martin, G. L., Liu, P., et al. (2019). Distinct phenotypes induced by three degrees of transverse aortic constriction in mice. Sci. Rep. 9, 5844. doi:10.1038/s41598-019-42209-7
Riehle, C., and Bauersachs, J. (2019). Small animal models of heart failure. Cardiovasc. Res. 115, 1838–1849. doi:10.1093/cvr/cvz161
Roger, V. L. (2021). Epidemiology of heart failure: A contemporary perspective. Circ. Res. 128, 1421–1434. doi:10.1161/circresaha.121.318172
Rolski, F., and Błyszczuk, P. (2020). Complexity of TNF-α signaling in heart disease. J. Clin. Med. 9, 3267. doi:10.3390/jcm9103267
Romero-Becerra, R., Santamans, A. M., Folgueira, C., and Sabio, G. (2020). p38 MAPK pathway in the heart: new insights in health and disease. Int. J. Mol. Sci. 21, 7412. doi:10.3390/ijms21197412
Rose, B. A., Force, T., and Wang, Y. (2010). Mitogen-activated protein kinase signaling in the heart: Angels versus demons in a heart-breaking tale. Physiol. Rev. 90, 1507–1546. doi:10.1152/physrev.00054.2009
Sah, V. P., Minamisawa, S., Tam, S. P., Wu, T. H., Dorn, G. W., Iiross, J., et al. (1999). Cardiac-specific overexpression of RhoA results in sinus and atrioventricular nodal dysfunction and contractile failure. J. Clin. Invest. 103, 1627–1634. doi:10.1172/JCI6842
Shamhart, P. E., and Meszaros, J. G. (2010). Non-fibrillar collagens: Key mediators of post-infarction cardiac remodeling? J. Mol. Cell. Cardiol. 48, 530–537. doi:10.1016/j.yjmcc.2009.06.017
Shiojima, I., and Walsh, K. (2006). Regulation of cardiac growth and coronary angiogenesis by the Akt/PKB signaling pathway. Genes and Dev. 20, 3347–3365. doi:10.1101/gad.1492806
Steffensen, L. B., and Rasmussen, L. M. (2018). A role for collagen type IV in cardiovascular disease? Am. J. Physiol.-Heart Circ. Physiol. 315, H610–H625. doi:10.1152/ajpheart.00070.2018
Strassheim, D., Dempsey, E. C., Gerasimovskaya, E., Stenmark, K., and Karoor, V. (2019). Role of inflammatory cell subtypes in heart failure. J. Immunol. Res. 2019, 2164017. doi:10.1155/2019/2164017
Suarez-Mier, M. P., Fernandez-Simón, L., and Gawallo, C. (1995). Pathologic changes of the cardiac conduction tissue in sudden cardiac death. Am. J. Forensic Med. Pathol. 16, 193–202. doi:10.1097/00000433-199509000-00002
Sundararaj, S., Zhang, J., Krovi, S. H., Bedel, R., Tuttle, K. D., Veerapen, N., et al. (2018). Differing roles of CD1d2 and CD1d1 proteins in type I natural killer T cell development and function. Proc. Natl. Acad. Sci. 115, E1204–E1213. doi:10.1073/pnas.1716669115
Suthahar, N., Meijers, W. C., Silljé, H. H. W., Ho, J. E., Liu, F.-T., and de Boer, R. A. (2018). Galectin-3 activation and inhibition in heart failure and cardiovascular disease: An update. Theranostics 8, 593–609. doi:10.7150/thno.22196
Swaminathan, P. D., Purohit, A., Soni, S., Voigt, N., Singh, M. V., Glukhov, A. V., et al. (2011). Oxidized CaMKII causes cardiac sinus node dysfunction in mice. J. Clin. Invest. 121, 3277–3288. doi:10.1172/JCI57833
Szekely, Y., and Arbel, Y. (2018). A review of interleukin-1 in heart disease: Where do we stand today? Cardiol. Ther. 7, 25–44. doi:10.1007/s40119-018-0104-3
Takeda, K., Shimozono, R., Noguchi, T., Umeda, T., Morimoto, Y., Naguro, I., et al. (2007). Apoptosis signal-regulating kinase (ASK) 2 functions as a mitogen-activated protein kinase kinase kinase in a heteromeric complex with ASK1. J. Biol. Chem. 282, 7522–7531. doi:10.1074/jbc.M607177200
Takemoto, Y., Ramirez, R. J., Yokokawa, M., Kaur, K., Ponce-Balbuena, D., Sinno, M. C., et al. (2016). Galectin-3 regulates atrial fibrillation remodeling and predicts catheter ablation outcomes. JACC Basic Transl. Sci. 1, 143–154. doi:10.1016/j.jacbts.2016.03.003
Templin, C., Ghadri, J.-R., Rougier, J.-S., Baumer, A., Kaplan, V., Albesa, M., et al. (2011). Identification of a novel loss-of-function calcium channel gene mutation in short QT syndrome (SQTS6). Eur. Heart J. 32, 1077–1088. doi:10.1093/eurheartj/ehr076
Tran Diem, H., and Wang Zhao, V. (2019). Glucose metabolism in cardiac hypertrophy and heart failure. J. Am. Heart Assoc. 8, e012673. doi:10.1161/JAHA.119.012673
van Setten, J., Brody, J. A., Jamshidi, Y., Swenson, B. R., Butler, A. M., Campbell, H., et al. (2018). PR interval genome-wide association meta-analysis identifies 50 loci associated with atrial and atrioventricular electrical activity. Nat. Commun. 9, 2904. doi:10.1038/s41467-018-04766-9
Van Tassell, B. W., Raleigh, J. M., and Abbate, A. (2015). Targeting interleukin-1 in heart failure and inflammatory heart disease. Curr. Heart Fail. Rep. 12, 33–41. doi:10.1007/s11897-014-0231-7
Wang, S., Song, R., Wang, Z., Jing, Z., Wang, S., and Ma, J. (2018). S100A8/A9 in inflammation. Front. Immunol. 9, 1298. doi:10.3389/fimmu.2018.01298
Wende, A. R., Brahma, M. K., McGinnis, G. R., and Young, M. E. (2017). Metabolic origins of heart failure. JACC Basic Transl. Sci. 2, 297–310. doi:10.1016/j.jacbts.2016.11.009
Wu, Y., and Anderson, M. E. (2014). CaMKII in sinoatrial node physiology and dysfunction. Front. Pharmacol. 5, 48. doi:10.3389/fphar.2014.00048
Yan, Y., Zhao, A., Qui, Y., Li, Y., Yan, R., Wang, Y., et al. (2020). Genetic association of FERMT2, HLA-DRB1, CD2AP, and PTK2B polymorphisms with Alzheimer’s disease risk in the southern Chinese population. Front. Aging Neurosci. 12, 16. doi:10.3389/fnagi.2020.00016
Yanni, J., D’Souza, A., Wang, Y., Li, N., Hansen, B. J., Zakharkin, S. O., et al. (2020). Silencing miR-370-3p rescues funny current and sinus node function in heart failure. Sci. Rep. 10, 11279. doi:10.1038/s41598-020-67790-0
Zarubin, T., and Han, J. (2005). Activation and signaling of the p38 MAP kinase pathway. Cell Res. 15, 11–18. doi:10.1038/sj.cr.7290257
Zhang, Q., Timofeyev, V., Lu, L., Li, N., Singapuri, A., Long Melissa, K., et al. (2008). Functional roles of a Ca2+-activated K+ channel in atrioventricular nodes. Circulation Res. 102, 465–471. doi:10.1161/CIRCRESAHA.107.161778
Keywords: heart failure, atrioventricular node, heart block, RNA sequencing, transcriptome, ion channels, inflammation
Citation: Wilson C, Zi M, Smith M, Hussain M, D’Souza A, Dobrzynski H and Boyett MR (2023) Atrioventricular node dysfunction in pressure overload-induced heart failure—Involvement of the immune system and transcriptomic remodelling. Front. Pharmacol. 14:1083910. doi: 10.3389/fphar.2023.1083910
Received: 29 October 2022; Accepted: 13 March 2023;
Published: 04 April 2023.
Edited by:
Ming Lei, University of Oxford, United KingdomReviewed by:
Tamer M. A. Mohamed, University of Louisville, United StatesMatthew Lancaster, University of Leeds, United Kingdom
Andrew F. James, University of Bristol, United Kingdom
Copyright © 2023 Wilson, Zi, Smith, Hussain, D’Souza, Dobrzynski and Boyett. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Halina Dobrzynski, halina.dobrzynski@manchester.ac.uk; Mark R. Boyett, m.r.boyett@bradford.ac.uk
†These authors share senior authorship
 Claire Wilson
Claire Wilson Min Zi
Min Zi Matthew Smith1
Matthew Smith1 Munir Hussain
Munir Hussain Alicia D’Souza
Alicia D’Souza Halina Dobrzynski
Halina Dobrzynski Mark R. Boyett
Mark R. Boyett