- 1Institute for Brain Disorders, Beijing University of Chinese Medicine, Beijing, China
- 2Department of Neurology, Dongzhimen Hospital, Beijing University of Chinese Medicine, Beijing, China
- 3Beijing University of Chinese Medicine, Beijing, China
- 4Dongfang Hospital, Beijing University of Chinese Medicine, Beijing, China
- 5Department of Cardiology, Dongzhimen Hospital, Beijing University of Chinese Medicine, Beijing, China
- 6Department of Rheumatology, Guang’anmen Hospital, China Academy of Chinese Medical Sciences, Beijing, China
- 7Department of Acupuncture and Moxibustion, Beijing Hospital of Traditional Chinese Medicine, Capital Medical University, Beijing, China
- 8Institute of Basic Research in Clinical Medicine, China Academy of Chinese Medical Sciences, Beijing, China
- 9Dongzhimen Hospital, Beijing University of Chinese Medicine, Beijing, China
- 10Chinese Medicine Key Research Room of Brain Disorders Syndrome and Treatment of the National Administration of Traditional Chinese Medicine, Beijing, China
Background: Edaravone alleviates neurological deficits among patients with intracerebral hemorrhage; however, its effects on mortality and long-term functional outcomes remain unknown.
Objective: To assess clinical outcomes associated with edaravone initiated within 7 days of symptoms onset in intracerebral hemorrhage.
Methods: We systematically searched PubMed, Embase, Cochrane Library, CiNii, China National Knowledge Infrastructure, Chinese VIP information, Wanfang Data, and SinoMed for relevant randomized controlled trials from their inception to 1 May 2021 and conducted a comprehensive systematic review and meta-analysis (PROSPERO registration number: CRD42019147801). All-cause mortality and long-term functional outcomes were taken as the primary outcomes.
Results: A total of 38 randomized controlled trials including 3,454 participants with acute intracerebral hemorrhage were included. The selected articles were of poor quality. Meta-analysis revealed that edaravone could not reduce all-cause mortality [relative risk (RR) = 0.51; 95% confidence interval (CI) (0.11–2.32); p = 0.38]. No studies reported on long-term functional outcomes in those trials. In addition, edaravone alleviated neurological deficits [mean difference (MD) = −5.44; 95% CI (−6.44 to −4.44); p<0.00001], improved the activities of daily living [MD = 8.44; 95% CI (7.65–9.23); p<0.00001], reduced the hematoma volume [MD = −4.71; 95% CI (−5.86 to −3.56); p<0.00001], and increased treatment response [RR = 1.26; 95% CI (1.22–1.31); p<0.00001]. In terms of safety outcome, there was no significant difference between the edaravone group and the control groups [RR = 1.67; 95% CI (0.92 to 3.06); p = 0.09].
Conclusion: Till date, edaravone does not associate with mortality reduction when initiated within 7 days of intracerebral hemorrhage onset. The effect of edaravone on long-term functional outcomes remains unknown due to lack of data. Although edaravone alleviated neurological deficits, improved activities of daily living, and reduced hematoma volume, we cautiously interpreted the results owing to the overall poor quality and high heterogeneity of the included trials. Presently, the results are insufficient to support edaravone as a routine treatment option for acute intracerebral hemorrhage.
1 Introduction
Acute spontaneous, non-traumatic intracerebral hemorrhage (ICH) is a life-threatening disease associated with a mean of 9.46 disability-adjusted life-years, which is defined as the combination of years of life lost and years lived with disability, and bring about enormous health care and economic burden (Steiner et al., 2014; Cordonnier et al., 2018; GBD 2019 Diseases and Injuries Collaborators, 2020; Cao et al., 2020; Haupenthal et al., 2021). Currently, neuroprotection of the peripheral injured brain tissue, which is widely used by clinical researchers and doctors, is a rational but unproven approach to improve clinical outcomes of patients with acute ICH (Hemphill et al., 2015).
The pathological mechanisms of ICH can generally be divided into primary injuries caused by the physical damage of hematoma and secondary injuries triggered by the extravasated blood components and the disruption of mitochondria and intrinsic antioxidant systems (Keep et al., 2012; Shang et al., 2015). Oxidative stress is prominently involved in secondary injuries due to the massive oxidative damage to proteins, nucleic acids, carbohydrates, and lipids (Xi et al., 2006; Aronowski and Zhao, 2011; Shang et al., 2015). The effect of antioxidants to reduce ICH-induced brain injury has also been confirmed in animal models (Peeling et al., 1998; Peeling et al., 2001).
Edaravone (MCI-186, 3-methyl-1-phenyl-2-pyrazolin-5-one) is a free radical scavenger which can not only reduce oxidative DNA damage by reducing apurinic/apyrimidinic sites and 8-OHdG levels in rat models with acute ICH but also attenuate brain edema and ameliorate neurologic deficits by reducing iron- and thrombin-induced injury as well as suppressing NLRP3 in microglia (Nakamura et al., 2008; Shang et al., 2015; Zhang et al., 2016a; Miao et al., 2020). Edaravone is widely used in China and India and has been adopted by the Chinese Guidelines for Diagnosis and Treatment of Acute Intracerebral Hemorrhage 2019 with a weak recommendation (Class Ⅱ, level of evidence C) (Yang et al., 2011; Chinese Society of Neurology, and Chinese Stroke Society, 2019).
Nevertheless, the aforementioned recommendation was drawn on the unrobust results of previous systematic reviews, suggesting that edaravone could exert slightly promising effects on the neurological deficit improvement of ICH (Yang et al., 2011; Yang et al., 2015). The conclusive effects of edaravone treatment on survival or long-term functional outcomes in patients with ICH remain unclear. Recent reviews reveal that several randomized controlled trials (RCTs) have demonstrated that edaravone could not only improve the activities of daily living but also reduce the hematoma volume and the edema zone without increasing mortality and adverse events. Furthermore, several RCTs have reported long-term fatality and functional status, as well as adverse reactions associated with edaravone use (Abe et al., 2007).
Taken together, we conducted this updated systematic review and meta-analysis to evaluate the efficacy and safety of edaravone in the treatment of acute ICH to obtain conclusive evidence and provide clinicians and patients with the latest evidence-based options for edaravone use in ICH.
2 Materials and methods
The systematic review and meta-analysis were reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guidelines (Page et al., 2021). We also used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system to evaluate the certainty of the evidence derived from the meta-analysis results (Guyatt et al., 2011a; Guyatt et al., 2011b; Guyatt et al., 2011c; Guyatt et al., 2011d; Guyatt et al., 2011e). This study was conducted according to our previously published protocol (CRD42019147801) (Feng et al., 2020).
2.1 Search strategy and study screening
We conducted a comprehensive search on PubMed, Embase, Cochrane Library, CiNii, China National Knowledge Infrastructure (CNKI), Chinese VIP information (VIP), Wanfang Data, and SinoMed for relevant randomized controlled trials from their respective inception dates to 1 May 2021. All searches were conducted by combining free-text and MESH terms, and the following terms were used: “cerebral hemorrhage,” “edaravone,” and “randomized controlled trial” (Supplementary Table S1). The registered clinical trials, ongoing or unpublished trials, dissertations, and gray literature were also searched, regardless of language limitations. A secondary manual search was also performed based on the references of the included studies. After removing duplicate studies, two reviewers (Feng L. and Jiang P.) screened the articles together through abstracts and full texts successively, to assess eligibility.
2.2 Inclusion and exclusion criteria
We included RCTs that initiated edaravone within 7 days of symptom onset and determined its efficacy and safety compared with no treatment or placebo. In addition, trials wherein conventional treatments, surgeries, and other co-interventions with edaravone were administered equally to all groups were also included. Non-RCTs, animal studies, reviews, commentaries, and meta-analyses were excluded from this study. In addition, studies on patients with traumatic hemorrhage, primary intraventricular hemorrhage, or subarachnoid hemorrhage were also excluded.
2.3 Data extraction
Information from the eligible RCTs was independently extracted by reviewers, in pairs (Qin M., Guan J., Zhang X., and Shi X.), using a preformulated data collection form which included authors, publication year, number of participating sites, sample size, patient characteristics, intervention details, and outcomes. The primary outcomes were all-cause mortality and unfavorable functional outcomes, defined as modified Rankin Scale (mRS) grades 3–6, Glasgow Outcome Scale (GOS) grades 1–3, or Barthel Index (BI) less than or equal to 60 at the end of the follow-up. The secondary outcomes included neurological impairments, which were assessed using clinical scales including the National Institutes of Health Stroke Scale (NIHSS), Canadian Neurological Scale (CNS), European Stroke Scale (ESS), Scandinavian Stroke Scale (SSS), and Modified Edinburgh-Scandinavian Stroke Scale (MESSS) as well as other related scales, and the activities of daily living measured with BI, hematoma volume, and the total efficiency rate calculated by the ratio of effective number to total number after treatment. Edaravone-induced adverse events included liver impairment, kidney impairment, skin irritation, and nausea.
2.4 Risk of bias assessment and grading of evidence
Two reviewers (Yang C. and Wei D.) independently assessed the risk of bias of all included studies using the Cochrane Risk of Bias Tool. The GRADE system was used for each meta-analysis to ascertain the overall strength of evidence across the trials, and two reviewers (Qin M. and Li T.) downgraded evidence based on the risk of bias, inconsistency, indirectness, imprecision, and publication bias. Disagreements were resolved by discussion or by involving another review author (Gao Y.) to arbitrate.
2.5 Data synthesis and analysis
Statistical analyses were performed using STATA 16.0 and Revman 5.4 software. We calculated the relative risk (RR) from the number of events and participants and mean difference (MD) from the number of participants as well as the mean and standard deviation (SD) after treatment in each group. The effect estimates of continuous data (neurological deficits, activities of daily living, and hematoma volume) were measured by MD with a 95% confidence interval (CI), whereas RR with 95% CI was adopted for dichotomous data (mortality, total efficiency rate, and adverse events). Heterogeneity between studies was calculated using I2 statistics. We calculated the pooled effect size using the fixed effect model when I2 was less than 25% and the random effect or qualitative analysis model when I2 was 25% or greater. A two-tailed p < 0.05 was considered statistically significant. A sensitivity analysis was performed by omitting each study one at a time to calculate the pooled effect size. A Subgroup analysis was performed according to the severity, co-intervention, duration of treatment, and dose range of edaravone per day. The potential publication bias was assessed by visual inspection of funnel plot symmetry and validated by Egger’s test.
3 Results
3.1 Search results
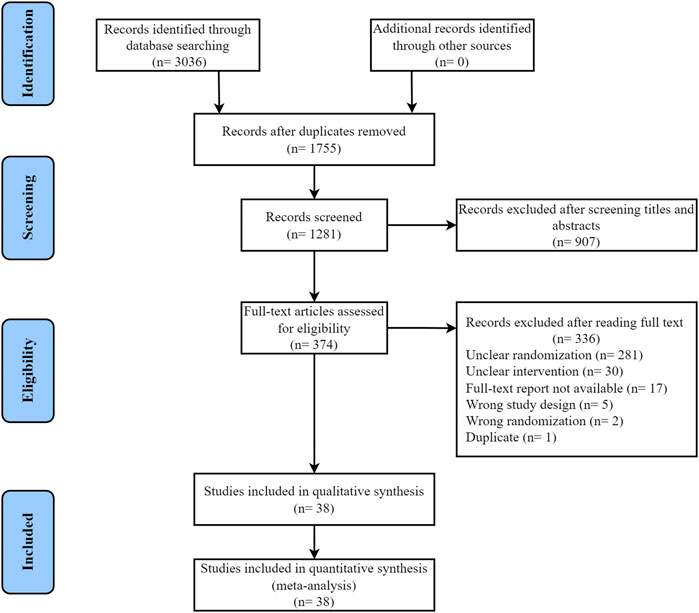
A total of 3,036 articles were obtained by our systematic search, 1,755 of which were excluded owing to duplications and 907 were excluded for inappropriate research type and content after reviewing the title or abstract. A total of 374 articles were subjected to full-text review. Of these, 336 studies were excluded for inappropriate study design, including unclear randomization (281 articles), unclear intervention (30 articles), unavailable full-text report (17 articles), wrong study design (5 articles), wrong randomization (2 articles), and duplicate (1 article). A total of 38 relevant studies were included in the final dataset, and the literature search and article selection are depicted in Figure 1 (Geng et al., 2004; Li and Shan, 2005; Wang et al., 2007; He et al., 2009a; He et al., 2009b; Hao, 2009; Liu, 2009; Zhao et al., 2009; Wang et al., 2010a; Wang et al., 2010b; Ma and Qiao, 2010; Sang and Gong, 2010; Wu, 2011; Zhao et al., 2011; Zhou, 2011; Liang and Zhou, 2013; Zhan et al., 2013; He, 2014; Qu and Wei, 2015; Ran, 2015; Zhang et al., 2016b; Duan and Cao, 2016; Jiang, 2016; Li et al., 2016; Sun et al., 2016; Tian and Pan, 2016; Cheng, 2017; Guo et al., 2017; Zhang et al., 2017; Li et al., 2018; Wan, 2018; Wang et al., 2018; Chen et al., 2019; Xu et al., 2019; Chi, 2020; Li et al., 2020; Wang, 2020; Ye et al., 2020).
3.2 Characteristics of the included studies
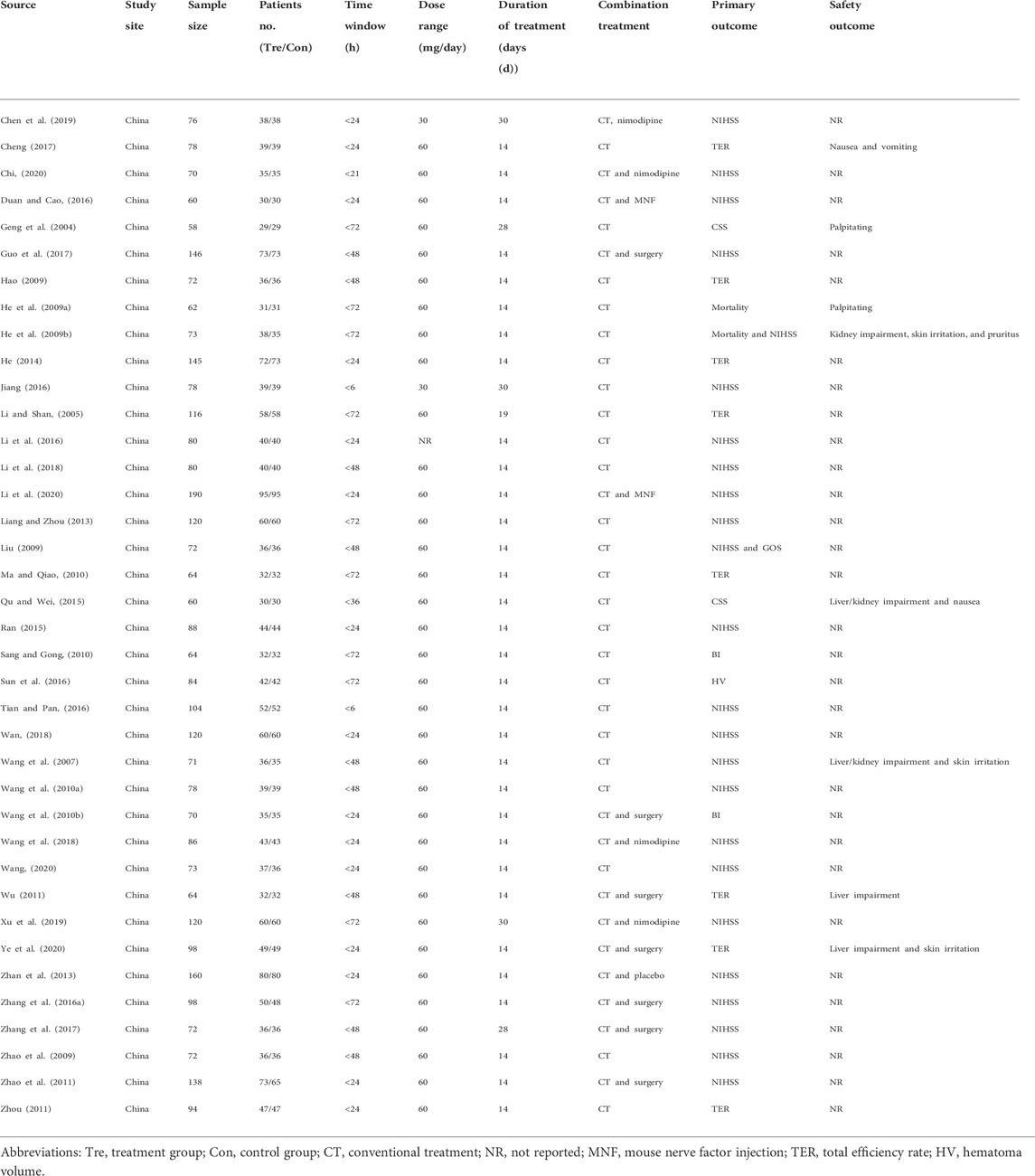
Overall, 38 studies involving 3,454 participants were included, all of which were single-center studies conducted in mainland China. The characteristics of these 38 studies are listed in Table 1. The sample size ranged from 58 to 190, and the mean age ranged from 45.8 to 71.03 years. The patients were admitted to the hospital within 72 h of ICH onset, and the average bleeding volume was 9–66 ml, according to the image findings. The dose range of edaravone was 30 mg or 60 mg per day, and the duration of treatment was 14–30 days. The co-interventions included conventional treatment, surgery, or neuroprotective agents. Only one study conducted placebo control, whereas the others added on co-interventions.
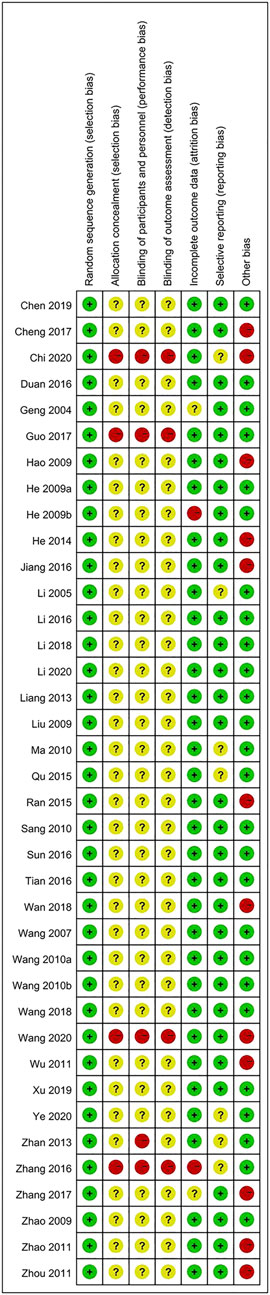
Evaluation of bias risk for all articles is presented in Figure 2. The risk of bias in the RCTs was examined based on seven items. Most of the included articles did not meet these standards, indicating that the selected articles were of poor quality.
3.3 Primary outcomes
3.3.1 All-cause mortality
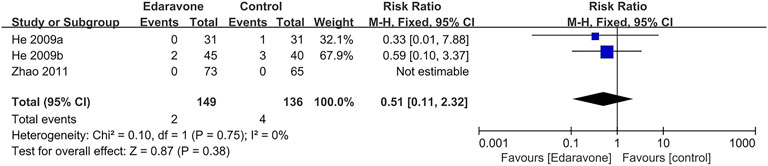
Three articles (He et al., 2009a; He et al., 2009b; Zhao et al., 2011) provided no evidence proving that edaravone could reduce the all-cause mortality of acute ICH (RR = 0.51; 95% CI [0.11 to 2.32]; p = 0.38; I2 = 0%) (Figure 3).
3.3.2 Long-term functional outcomes
One of the studies reported the long-term functional outcome with BI at 60 days; however, its random sequence generation and onset time were unknown. Therefore, we excluded this study (Ze, 2015).
3.4 Secondary outcomes
3.4.1 Improvement of neurological impairment
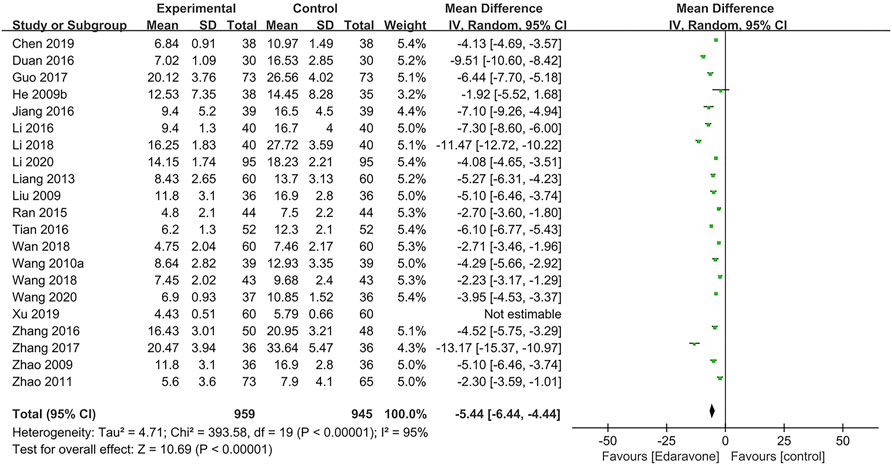
A total of 21 articles (He et al., 2009b; Liu, 2009; Zhao et al., 2009; Wang et al., 2010a; Zhao et al., 2011; Liang and Zhou, 2013; Ran, 2015; Zhang et al., 2016a; Duan and Cao, 2016; Jiang, 2016; Li et al., 2016; Tian and Pan, 2016; Guo et al., 2017; Zhang et al., 2017; Li et al., 2018; Wan, 2018; Wang et al., 2018; Chen et al., 2019; Xu et al., 2019; Li et al., 2020; Wang, 2020) used the NIHSS, and the pooled data illustrated that edaravone could reduce the NIHSS score [MD = −5.44; 95% CI (−6.44 to −4.44); p < 0.00001] (Figure 4). Considering the high heterogeneity in the meta-analysis of the NIHSS score (I2 = 95%, p < 0.00001), we further performed a sensitivity analysis, and one study was excluded (Xu et al., 2019). Subgroup analyses were also conducted, respectively, by severity [moderate-to-severe was defined as the NIHSS score 5–25 before treatment, MD = −4.32; 95% CI (−5.12 to −3.52); p < 0.00001; I2 = 89%; severe was defined as the NIHSS score above 25 before treatment, MD = −8.13; 95% CI (−11.00 to −5.26); p < 0.00001; I2 = 98%] (Supplementary Figure S1), by co-intervention [conventional treatment, MD = −4.95; 95% CI (−6.38 to −3.53); p < 0.00001; I2 = 95%; conventional treatment plus surgery, MD = −4.42; 95% CI (−6.74 to −2.11); p = 0.0002; I2 = 90%; other co-interventions, MD = −6.64; 95% CI (−8.63 to −4.64); p < 0.00001; I2 = 97%] (Supplementary Figure S2), by duration of treatment (14 days, MD = −5.05; 95% CI (−6.13 to −3.97); p < 0.00001; I2 = 95%; 28 days, MD = −13.17; 95% CI (−15.37 to −10.97); p < 0.00001; I2 = not applicable; 30 days, MD = −5.42; 95% CI (−8.31 to −2.54); p = 0.0002; I2 = 85%] (Supplementary Figure S3), and by dose range of edaravone per day [30 mg, MD = −5.42; 95% CI (−8.31 to −2.54); p = 0.0002; I2 = 85%; 60 mg, MD = −5.33; 95% CI (−6.49 to −4.18); p < 0.00001; I2 = 96%] (Supplementary Figure S4).
3.4.2 Improvement of activities of daily living
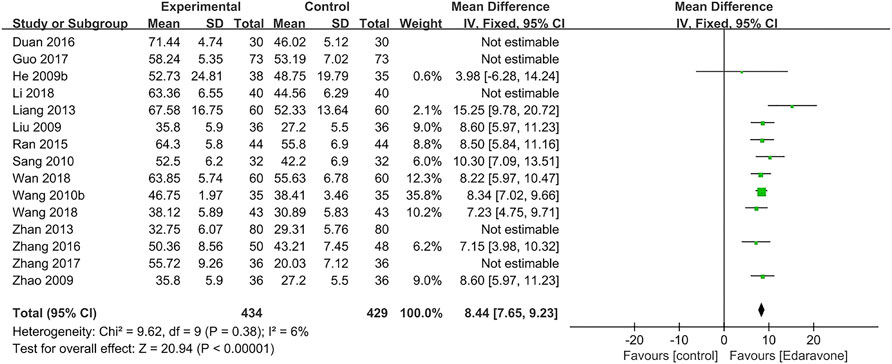
As for the activities of daily living, 15 articles (He et al., 2009b; Liu, 2009; Zhao et al., 2009; Wang Z. et al., 2010; Sang and Gong, 2010; Liang and Zhou, 2013; Zhan, et al., 2013; Ran, 2015; Zhang et al., 2016b; Duan and Cao, 2016; Guo et al., 2017; Zhang et al., 2017; Li et al., 2018; Wan, 2018; Wang et al., 2018) reported the grading according to BI. Considering the high heterogeneity in BI (I2 = 95%, p < 0.00001), we further conducted a sensitivity analysis, and five studies were removed (Zhan, et al., 2013; Duan and Cao, 2016; Guo et al., 2017; Zhang et al., 2017; Li et al., 2018). The outcome indicated that edaravone could improve the BI [MD = 8.44; 95% CI (7.65–9.23); p < 0.00001; I2 = 6%] (Figure 5).
3.4.3 Reduction of hematoma volume
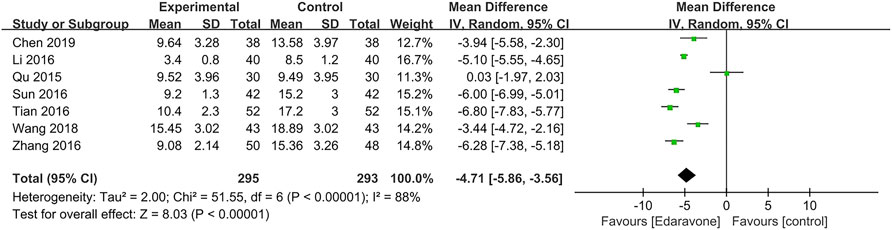
A total of seven articles (Qu and Wei, 2015; Zhang et al., 2016a; Li et al., 2016; Sun et al., 2016; Tian and Pan, 2016; Wang et al., 2018; Chen et al., 2019) reported the hematoma volume, and the pooled data showed that edaravone could reduce the hematoma volume [MD = −4.71; 95% CI (−5.86 to −3.56); p < 0.00001] (Figure 6). Since high heterogeneity existed (I2 = 88%, p < 0.00001), we conducted a sensitivity analysis, and no study was removed. Subgroup analyses were performed as well, and we divided it into four subgroups, respectively, by severity (moderate-to-severe, MD = −4.91; 95% CI (−6.17 to −3.65); p < 0.00001; I2 = 84%; severe, MD = −6.28; 95% CI [−7.38 to −5.18]; p < 0.00001; I2 = not applicable] (Supplementary Figure S5), by co-intervention [by conventional treatment, MD = −4.40; 95% CI (−7.46 to −1.35); p = 0.005; I2 = 94%; by convention treatment plus surgery, MD = −6.28; 95% CI (−7.38 to −5.18); p < 0.00001; I2 = not applicable; by other co-interventions, MD = −4.30; 95% CI (−5.49 to −3.12); p < 0.00001; I2 = 72%] (Supplementary Figure S6), by duration of treatment [14 days, MD = −4.81, 95% CI (−6.07 to −3.55); p < 0.00001; I2 = 90%; 30 days, MD = −3.94; 95% CI (−5.58 to −2.30); p < 0.00001; I2 = not applicable] (Supplementary Figure S7), and by dose range of edaravone per day [30 mg, MD = −3.94; 95% CI (−5.58 to −2.30); p < 0.00001; I2 = not applicable; 60 mg, MD = −4.64; 95% CI (−6.51 to −2.78); p < 0.00001; I2 = 92%] (Supplementary Figure S8).
3.4.4 Total efficiency rate
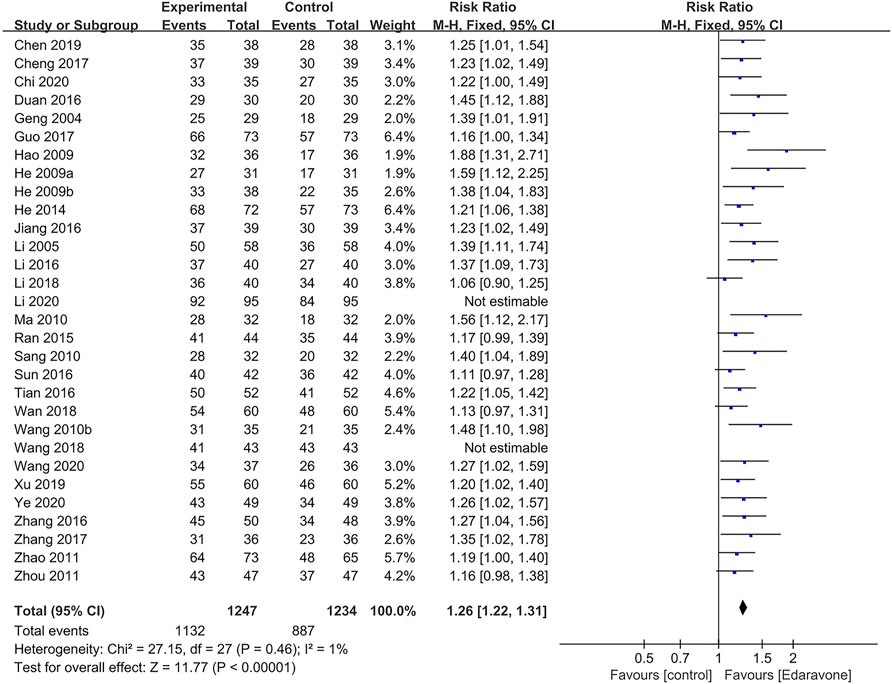
A total of 30 articles (Geng et al., 2004; Li and Shan, 2005; He et al., 2009a; He et al., 2009b; Hao, 2009; Wang et al., 2010b; Ma and Qiao, 2010; Sang and Gong, 2010; Zhao et al., 2011; Zhou, 2011; He, 2014; Ran, 2015; Zhang et al., 2016b; Duan and Cao, 2016; Jiang, 2016; Li et al., 2016; Sun et al., 2016; Tian and Pan, 2016; Cheng, 2017; Guo et al., 2017; Zhang et al., 2017; Li et al., 2018; Wan, 2018; Wang et al., 2018; Chen et al., 2019; Xu et al., 2019; Chi, 2020; Li et al., 2020; Wang, 2020; Ye et al., 2020) reported the total efficiency rate at the end of follow-up, while two of them were excluded in the sensitivity analysis as meta-analyses could not be performed (Wang et al., 2018; Li et al., 2020). The outcomes showed that edaravone was more likely to improve neurological impairment [RR = 1.26; 95% CI (1.22–1.31); p < 0.00001; I2 = 1%] (Figure 7).
3.4.5 Adverse events
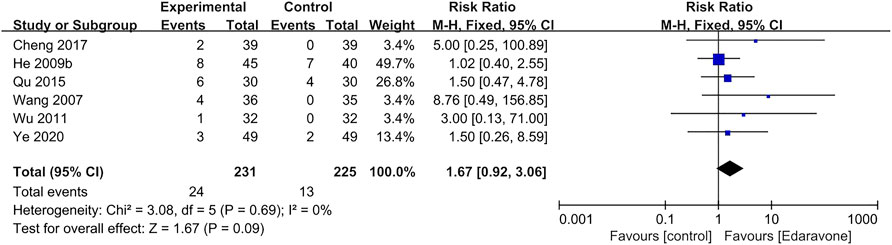
In all studies, the data on adverse events were too disparate and inconsistently reported to allow for further formal analysis. A total of six articles (Wang et al., 2007; He et al., 2009b; Wu, 2011; Qu and Wei, 2015; Cheng, 2017; Ye et al., 2020) reported adverse events, and there was no significant difference between the experimental group and the control group [RR = 1.67; 95% CI (0.92–3.06); p = 0.09; I2 = 0%] (Figure 8). A total of 24 participants developed adverse events during or after edaravone treatment, and the most frequently reported adverse events were kidney impairment, liver impairment, and skin irritation.
3.5 Bias of publication
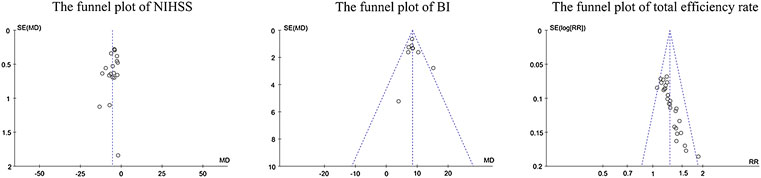
Visual inspection of funnel plots did not reveal marked asymmetry, suggesting no significant publication bias for the effect of edaravone on the NIHSS score and BI. The distribution of scatter points regarding the total efficiency rate was asymmetric, suggesting publication bias might be present (Figure 9). Egger’s test showed no obvious publication bias risk in the NIHSS score (p > 0.05) and BI (p > 0.05), while it showed high bias risk in the total efficiency rate (p = 0.012).
3.6 Risk of bias and grading of recommendations assessment, development and evaluation assessment
No study was rated as having a low risk of bias, while some showed a high risk of bias or near high risk of bias. The certainty of evidence was rated as low for all-cause mortality, activities of daily living, hematoma volume, total efficiency rate, and safety outcomes, while it was rated very low for neurologic deficits, which meant that confidence in the effect size estimates was very limited. The main reasons for downgrading the evidence were risk of bias, inconsistency, and imprecision (Supplementary Figure S9).
4 Discussion
This study did not favor edaravone for reducing all-cause mortality compared with placebo or added on co-interventions. Simultaneously, we could not draw a conclusion regarding the effects of edaravone on long-term functional outcomes owing to the lack of corresponding data. We are supposed to attach great importance to the aforementioned two outcomes, as acute ICH is a medical emergency that endangers patients’ lives. Moreover, edaravone is a relatively expensive medicine, costing approximately 600–860 US dollars for one standard course of treatment per stroke patient in China (Yang et al., 2011). Irrational use of edaravone may bring an enormous economic burden to patients and society.
As for secondary outcomes, very low-certainty evidence supported that edaravone could alleviate neurological deficits. Low-certainty evidence revealed that edaravone could improve the activities of daily living and reduce hematoma volume. Heterogeneity was substantial for the NIHSS score and hematoma volume. Although we attempted to perform subgroup analyses by severity, co-intervention, duration of treatment, and dose range of edaravone per day to explore the heterogeneity source, the heterogeneity did not decrease. In addition, low-certainty evidence suggested that no difference existed between the two groups regarding safety outcomes. The reported adverse reactions (including kidney impairment, liver impairment, skin irritation, nausea, vomiting, pruritus, and palpitations) may be related to edaravone or other therapeutic agents or procedures. Compared with previous systematic reviews and meta-analyses, our study attempted to explore whether edaravone could reduce mortality or not (Yang et al., 2011; Yang et al., 2015). Consistent with previous studies revealing that edaravone could improve short-term neurological deficits, we confirmed this finding numerically. However, caution should be paid in interpreting these results. Among the 14 studies included in the systematic review and meta-analysis published in 2015, only 10 studies overlapped with the 38 studies included in this study. Although we attempted to contact the authors, the random methods were still unclear in the four studies; therefore, we did not include them (Pang and Li, 2007; Cen, 2008; Sang et al., 2008; Shi et al., 2008). In addition, the heterogeneities of the original studies were too high to be reduced by sensitivity and subgroup analyses. After reading the full text, we used the random effects model to pool data in view of clinical similarity. We suggest being cautious in understanding and interpreting the results.
Some articles reported the total efficiency rate, which could be improved by edaravone via pooling the corresponding data. Compared with other outcomes, the total efficiency rate remains controversial since the essence of surrogate composite outcome has not been rigorously validated for treatment effect assessment. Objective bias was unavoidable when selecting and setting standards. Concurrently, pooling data may increase the probability of error (Armstrong and Westerhout, 2017; McCoy, 2018).
In conclusion, the current systematic review and meta-analysis revealed that no association was found between the edaravone intervention and all-cause mortality in patients with ICH when initiated within 7 days of symptom onset. Although the outcomes showed that edaravone was effective in alleviating neurological impairment, improving the activities of daily living, and reducing hematoma volume, they were summarized with significant statistical heterogeneity and acted as surrogate outcomes in terms of assessment of specific treatment for acute ICH.
Our review has some potential limitations. The included trials varied in several aspects, such as patient characteristics, co-interventions, and follow-up period. Substantial heterogeneity was observed, which lowered the evidence grade. Despite the significant statistical heterogeneity, we still pooled the data of the NIHSS score and hematoma volume because we found acceptable clinical heterogeneity in terms of age, sex, onset time, amount of bleeding, and lesion sites. Furthermore, only one study reported long-term functional outcomes measured with the BI at 60 days. However, we had to exclude this study owing to the undescribed sequence generation and ICH onset time even though we contacted the authors via email and telephone. Moreover, nearly half of the included trials were at high risk of bias, which mainly resulted from unreported allocation concealment, blinding of participants and personnel, and blinding of outcome assessment. The faulty methodology brought about selection bias, performance bias, and detection bias. Future studies should overcome these shortcomings. To improve research quality and reduce associated bias, future studies should focus on the implementation of allocation concealment, including central randomization, and sequentially numbered, opaque, sealed envelopes. Furthermore, the research is supposed to report rigorously whether the blinding method is used. If done, it had better provide specific information on the implementation of the blinding method and characteristics of drug consistency. Most studies were conducted based on co-interventions rather than the placebo control. The efficacy of a single therapy may be better assessed using the placebo control. In addition, future research should focus on exploring the association between edaravone and other clinical measures that have been proven to affect patient mortality and quality of life. Given that no study has been performed outside the Chinese mainland, the aforementioned results of limited generalizability should be interpreted cautiously when used for reference to other countries in the future.
5 Conclusion
In this review, edaravone was not associated with mortality reduction when initiated within 7 days of ICH onset. The effect of edaravone on long-term functional outcomes remained unknown. Although the pooled data showed that edaravone could alleviate neurological deficits, improve activities of daily living, and reduce hematoma volume, the interpretation of these results still required particular caution. Presently, the results are insufficient to support edaravone as a routine treatment option for acute ICH. The conclusive efficacy and safety of edaravone for the treatment of acute ICH need to be further validated by rigorous studies to update the evidence.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.
Author contributions
Study conception: YG; study design: YG, CZ, and LF; systematic literature search: LF and PJ; study screening: LF and PJ; data extraction: MQ, JG, XZ, and XS; risk of bias assessment: CY and DW; grading of evidence: MQ and TL; statistical analysis: MQ; drafting the manuscript: LF and MQ; critical revision of the manuscript for important intellectual content: all authors; and supervision: YG, CZ, NL, XL, and LZ.
Funding
This work was supported by the National Key Research and Development Project (grant number 2018YFC1705000), Chinese Medicine Inheritance and Innovation Talent Project-National Leading Talent Support Program for Traditional Chinese Medicine 2018 (No. 12), and the Dongzhimen Hospital Project (grant number 2020TSRC-002).
Acknowledgments
The authors acknowledge contributions from all the included studies.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.935198/full#supplementary-material
Abbreviations
AP, apurinic/apyrimidinic; BI, Barthel index; CI, confidence interval; CNKI, China National Knowledge Infrastructure; CNS, Canadian Neurological Scale; ESS, European Stroke Scale; GOS, Glasgow Outcome Scale; ICH, intracerebral hemorrhage; MD, mean difference; MESSS, Modified Edinburgh-Scandinavian Stroke Scale; mRS, Modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; RCTs, randomized controlled trials; RR, relative risk; SSS, Scandinavian Stroke Scale; and VIP, Chinese VIP information
References
Abe, M., Kaizu, K., and Matsumoto, K. (2007). A case report of acute renal failure and fulminant hepatitis associated with edaravone administration in a cerebral infarction patient. Ther. Apher. Dial. 11, 235–240. doi:10.1111/j.1744-9987.2007.00480.x
Armstrong, P. W., and Westerhout, C. M. (2017). Composite end points in clinical research: A time for reappraisal. Circulation 135, 2299–2307. doi:10.1161/CIRCULATIONAHA.117.026229
Aronowski, J., and Zhao, X. (2011). Molecular pathophysiology of cerebral hemorrhage secondary brain injury. Stroke 42, 1781–1786. doi:10.1161/STROKEAHA.110.596718
Cao, Y., Yu, S., Zhang, Q., Yu, T., Liu, Y., Sun, Z., et al. (2020). Chinese Stroke Association guidelines for clinical management of cerebrovascular disorders: Executive summary and 2019 update of clinical management of intracerebral haemorrhage. Stroke Vasc. Neurol. 5, 396–402. doi:10.1136/svn-2020-000433
Chinese Society of Neurology, and Chinese Stroke Society (2019). Chinese guidelines for diagnosis and treatment of acute intracerebral hemorrhage. Chin. J. Neurology 52, 994–1005. doi:10.3760/cma.j.issn.1006‐7876.2019.12.003
Cen, Y. (2008). Study on the clinical efficacy of edaravone in the treatment of hypertensive cerebral hemorrhage. Hainan Med. J. 19, 77–78.
Chen, X., Zhang, H., and Kou, T. (2019). Effect of edaravone combined with nimodipine sequential therapy on neurological function and expression of serum NSE, OPN and BDNF in patients with cerebral hemorrhage. Mod. Chin. Dr. 57, 23–26.
Cheng, Q. (2017). Clinical efficacy and safety of edaravone on acute cerebral hemorrhage. Clin. Med. 37, 38.
Chi, H. (2020). Effects of edaravone combined with nimodipine on serum S100β and NSE levels in patients with hypertensive intracerebral hemorrhage. Contemp. Med. 26, 44–46.
Cordonnier, C., Demchuk, A., Ziai, W., and Anderson, C. S. (2018). Intracerebral haemorrhage: Current approaches to acute management. Lancet 392, 1257–1268. doi:10.1016/S0140-6736(18)31878-6
Duan, Z., and Cao, Y. (2016). Clinical effect of rat nerve growth factor combined with edaravone in the treatment of intracerebral hemorrhage and its effect on neurological function. Chin. J. Clin. 44, 44–47.
Feng, L., Liang, N., Li, T., Yang, Q., Jiang, P., Guo, S., et al. (2020). Efficacy and safety of edaravone for acute intracerebral haemorrhage: Protocol for a systematic review and meta-analysis. BMJ Open 10, e039366. doi:10.1136/bmjopen-2020-039366
GBD 2019 Diseases and Injuries Collaborators (2020). Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: A systematic analysis for the global burden of disease study 2019. Lancet 396, 1204–1222. doi:10.1016/S0140-6736(20)30925-9
Geng, Z., Sun, X., Shen, J., Chen, J., and Du, Z. (2004). Clinical efficacy of edaravone on acute cerebral hemorrhage. Shanghai Med. J. 27, 917–919.
Guo, Q., Li, L., Yang, Y., Ma, T., and Xie, M. (2017). Clinical effects of minimally invasive catheterized suction drainage combined with edaravone on cerebral hemorrhage. Prog. Mod. Biomed. 17, 5850–5852.
Guyatt, G. H., Oxman, A. D., Kunz, R., Brozek, J., Alonso-Coello, P., Rind, D., et al. (2011a). GRADE guidelines 6. Rating the quality of evidence—imprecision. J. Clin. Epidemiol. 64, 1283–1293. doi:10.1016/j.jclinepi.2011.01.012
Guyatt, G. H., Oxman, A. D., Kunz, R., Woodcock, J., Brozek, J., Helfand, M., et al. (2011b). GRADE guidelines: 7. Rating the quality of evidence—inconsistency. J. Clin. Epidemiol. 64, 1294–1302. doi:10.1016/j.jclinepi.2011.03.017
Guyatt, G. H., Oxman, A. D., Kunz, R., Woodcock, J., Brozek, J., Helfand, M., et al. (2011c). GRADE guidelines: 8. Rating the quality of evidence—indirectness. J. Clin. Epidemiol. 64, 1303–1310. doi:10.1016/j.jclinepi.2011.04.014
Guyatt, G. H., Oxman, A. D., Montori, V., Vist, G., Kunz, R., Brozek, J., et al. (2011d). GRADE guidelines: 5. Rating the quality of evidence—publication bias. J. Clin. Epidemiol. 64, 1277–1282. doi:10.1016/j.jclinepi.2011.01.011
Guyatt, G. H., Oxman, A. D., Vist, G., Kunz, R., Brozek, J., Alonso-Coello, P., et al. (2011e). GRADE guidelines: 4. Rating the quality of evidence—study limitations (risk of bias). J. Clin. Epidemiol. 64, 407–415. doi:10.1016/j.jclinepi.2010.07.017
Hao, W. (2009). Clinical observation of edaravone in the treatment of acute intracerebral hemorrhage. Clin. Med. Pract. 18, 626–627.
Haupenthal, D., Kuramatsu, J. B., Volbers, B., Sembill, J. A., Mrochen, A., Balk, S., et al. (2021). Disability-Adjusted Life-Years associated with intracerebral hemorrhage and secondary injury. JAMA Netw. Open 4, e2115859. doi:10.1001/jamanetworkopen.2021.15859
He, H. (2014). Clinical effect of edaravone on patients with intracerebral hemorrhage. Mod. Diagn. Treat. 25, 1566–1567.
He, J., Li, H., Zhang, H., Guo, Q., and Qu, J. (2009b). Clinical observation of edaravone in the treatment of acute intracerebral hemorrhage in stroke unit. J. Qiqihaer Med. Coll. 30, 2887–2888.
He, Z., Mei, H., Yan, J., and Hua, L. (2009a). Clinical application of edaravone in acute intracerebral hemorrhage. J. Chin. Physician 11, 974–975.
Hemphill, J. C., Greenberg, S. M., Anderson, C. S., Becker, K., Bendok, B. R., Cushman, M., et al. (2015). Guidelines for the management of spontaneous intracerebral hemorrhage: A guideline for healthcare professionals from the American heart association/American stroke association. Stroke 46, 2032–2060. doi:10.1161/STR.0000000000000069
Jiang, L. (2016). Edaravone combined with Xingnaojing injection in the treatment of acute intracerebral hemorrhage. Frontier Med. 6, 26–27.
Keep, R. F., Hua, Y., and Xi, G. (2012). Intracerebral haemorrhage: Mechanisms of injury and therapeutic targets. Lancet. Neurol. 11, 720–731. doi:10.1016/S1474-4422(12)70104-7
Li, C., Sun, K., Xu, Z., Feng, X., Huang, M., Lian, J., et al. (2016). Effect of Xingnaojing combined with edaravone on acute intracerebral hemorrhage. Chin. J. Pract. Nerv. Dis. 19, 62–64.
Li, D., and Shan, S. (2005). Clinical efficacy of edaravone on the treatment of acute cerebral hemorrhage. Ningxia Med. J. 27, 616–617.
Li, S., Wang, X., Zhang, D., Li, D., and Zhang, H. (2020). Clinical observation of edaravone combined with nerve growth factor in the treatment of hypertensive intracerebral hemorrhage. World Latest Med. Inf. (Electron. Version) 20, 118–119.
Li, X., Li, X., and An, G. (2018). Effect of edaravone on acute intracerebral hemorrhage. Med. J. Chin. People's Health 30, 20–21.
Liang, Y., and Zhou, Y. (2013). Effect of an oxygen free radical scavenger on serum inflammatory factors of patients with acute cerebral hemorrhage. J. Chin. Med. Pract. 17, 53–55.
Liu, X. (2009). The effect of edaravone on serum NSE and S-100β level and observation of therapeutic effect in the treatment of acute intracerebral hemorrhage. Chin. Pract. Med. 4, 48–50.
Ma, Q., and Qiao, Y. (2010). Effect of edaravone on acute intracerebral hemorrhage. J. Shanxi Med. Univ. 41, 642–643.
McCoy, C. (2018). Understanding the use of composite endpoints in clinical trials. West. J. Emerg. Med. 19, 631–634. doi:10.5811/westjem.2018.4.38383
Miao, H., Jiang, Y., Geng, J., Zhang, B., Zhu, G., Tang, J., et al. (2020). Edaravone administration confers neuroprotection after experimental intracerebral hemorrhage in rats via NLRP3 suppression. J. Stroke Cerebrovasc. Dis. 29, 104468. doi:10.1016/j.jstrokecerebrovasdis.2019.104468
Nakamura, T., Kuroda, Y., Yamashita, S., Zhang, X., Miyamoto, O., Tamiya, T., et al. (2008). Edaravone attenuates brain edema and neurologic deficits in a rat model of acute intracerebral hemorrhage. Stroke 39, 463–469. doi:10.1161/STROKEAHA.107.486654
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 372, n71. doi:10.1136/bmj.n71
Pang, H., and Li, X. (2007). Effect of edaravone on neurological rehabilitation after hypertensive intracerebral hemorrhage. Anhui Med. Pharm. J. 11, 1073–1074.
Peeling, J., Del Bigio, M. R., Corbett, D., Green, A. R., and Jackson, D. M. (2001). Efficacy of disodium 4-[(tert-butylimino)methyl]benzene-1, 3-disulfonate N-oxide (NXY-059), a free radical trapping agent, in a rat model of hemorrhagic stroke. Neuropharmacology 40, 433–439. doi:10.1016/S0028-3908(00)00170-2
Peeling, J., Yan, H., Chen, S., Campbell, M., and Del Bigio, M. R. (1998). Protective effects of free radical inhibitors in intracerebral hemorrhage in rat. Brain Res. 795, 63–70. doi:10.1016/S0006-8993(98)00253-4
Qu, D., and Wei, M. (2015). The clinical efficacy and safety evaluation for the treatment of edaravone in acute cerebral hemorrhage. J. Clin. Emerg. (China) 16, 520–522.
Ran, K. (2015). Impact of edaravone on serum levels of inflammatory cytokines and oxidative stress products in patients with acute cerebral hemorrhage. Pract. J. Cardiac Cereb. Pneumal Vasc. Dis. 23, 86–88.
Sang, D., Wei, D., Yin, L., Wang, W., Li, M., and Wu, G. (2008). Edaravone for treatment of cerebral hemorrhage: Report of 30 cases. J. Bengbu Med. Coll. 33, 533–534.
Sang, J., and Gong, Q. (2010). Clinical observation of edaravone for acute cerebral hemorrhage. Clin. Focus 25, 2146–2148.
Shang, H., Cui, D., Yang, D., Liang, S., Zhang, W., Zhao, W., et al. (2015). The radical scavenger edaravone improves neurologic function and perihematomal glucose metabolism after acute intracerebral hemorrhage. J. Stroke Cerebrovasc. Dis. 24, 215–222. doi:10.1016/j.jstrokecerebrovasdis.2014.08.021
Shi, J., Li, Q., and Wang, G. (2008). Clinical efficacy of edaravone on the treatment of acute cerebral hemorrhage. Pharm. Clin. Res. 16, 134–136.
Steiner, T., Salman, R. A., Beer, R., Christensen, H., Cordonnier, C., Csiba, L., et al. (2014). European stroke organisation (ESO) guidelines for the management of spontaneous intracerebral hemorrhage. Int. J. Stroke 9, 840–855. doi:10.1111/ijs.12309
Sun, X., Wu, Y., Qin, M., and Li, J. (2016). Clinical efficacy and changes in brain natriuretic peptide of patients with acute cerebral hemorrhage in treatment with edaravone. J. Pharm. Res. 15, 1215–1218.
Tian, C., and Pan, Y. (2016). Clinical effect of edaravone on patients with acute intracerebral hemorrhage. Drugs Clin. 46, 74–75.
Wan, S. (2018). Influence of edaravone on inflammatory cytokines and oxidative stress products in patients with acute cerebral hemorrhage. Chin. J. Prim. Med. Pharm. 25, 1660–1663.
Wang, C., Xu, L., Chen, X., and Shi, Q. (2018). Effects of edaravone combined with nimodipine on oxidative stress and neurological function in patients with intracerebral hemorrhage. J. Guangxi Med. Univ. 35, 209–212.
Wang, G., Shen, X., and Xu, Z. (2007). Evaluation of edaravone in the treatment of acute intracerebral hemorrhage. Hainan Med. J. 18, 3–5.
Wang, X. (2020). Efficacy of edaravone in the treatment of acute cerebral hemorrhage in elderly patients and its effect on serum HMGB-1 and CRP expression. Chin. Med. Innov. 17, 57–59.
Wang, X., Zuo, X., Wang, W., Liu, Y., and Lu, Y. (2010a). Clinical observation of edaravone in the treatment of acute hypertensive intracerebral hemorrhage. Chin. J. Integr. Traditional West. Med. Intensive Crit. Care 17, 182.
Wang, Z., Zhao, L., Yang, Y., Xu, S., and Cui, C. (2010b). Clinical application of edaravone in neurological recovery after surgery of acute intracerebral hemorrhage Chinese. J. Mod. Drug Appl. 4, 126–128.
Wu, K. (2011). Clinical study of edaravone in the treatment of hypertensive intracerebral hemorrhage. Neural Inj. Funct. Reconstr. 6, 461–462.
Xi, G., Keep, R. F., and Hoff, J. T. (2006). Mechanisms of brain injury after intracerebral haemorrhage. Lancet. Neurol. 5, 53–63. doi:10.1016/S1474-4422(05)70283-0
Xu, B., Gong, X., and Liu, Q. (2019). Effects of edaravone combined with nimodipine on inflammatory factors and neurological function in patients with hypertensive intracerebral hemorrhage. Front. Pharmacol. 32, 36–39.
Yang, J., Cui, X., Li, J., Zhang, C., Zhang, J., Liu, M., et al. (2015). Edaravone for acute stroke: Meta-analyses of data from randomized controlled trials. Dev. Neurorehabil. 18, 330–335. doi:10.3109/17518423.2013.830153
Yang, J., Liu, M., Zhou, J., Zhang, S., Lin, S., Zhao, H., et al. (2011). Edaravone for acute intracerebral haemorrhage. Cochrane Database Syst. Rev. 16, CD007755. doi:10.1002/14651858.CD007755.pub2
Ye, F., Yang, K., Wang, Z., Ma, C., Chen, Z., Pan, Q., et al. (2020). Effect of edaravone on brain function, expression of serum S100β protein and NSE in patients with hypertensive cerebral hemorrhage after emergency treatment. World J. Emerg. Med. 27, 1583–1587.
Ze, Y. (2015). Clinical effect observation of edaravone in the treatment of 60 cases of acute cerebral hemorrhage. Community Tradit. Chin. Med. 31, 86–87.
Zhan, L., Ling, F., and Hua, M. (2013). Changes of serum HMGB1, ENA-78 and S100β protein levels in patients with acute cerebral hemorrhage and the intervention effect of edaravone. Stroke Nerv. Dis. 20, 306–310.
Zhang, J., Ren, X., Wen, S., Xu, D., Gao, X., Wang, J., et al. (2016a). Effect of edaravone on neurological function and quality of life in patients with intracerebral hemorrhage. Jiangxi Med. J. 51, 347–349.
Zhang, X., Liu, X., and Zhang, F. (2017). Efficacy observation of edaravone injection combined with Tongfuxingshen Decoction on the postoperative serum factors and neurological function for the patients with acute intracerebral hemorrhage. Chin. J. Pharmacovigil. 14, 78–81.
Zhang, Y., Yang, Y., Zhang, G., Gao, M., Ge, G., Wang, Q., et al. (2016b). Stereotactic administration of edaravone ameliorates collagenase‐induced intracerebral hemorrhage in rat. CNS Neurosci. Ther. 22, 824–835. doi:10.1111/cns.12584
Zhao, J., Qian, Z., Qiu, C., Wang, C., Zhu, J., and Qu, Z. (2011). Influence of micro-invasive evacuation hematoma and edaravone on neural functional recovery and inflammation factors in patients with acute cerebral hemorrhage. Chin. J. Pract. Nerv. Dis. 14, 6–8.
Zhao, R., Wang, C., Hu, C., He, J., Ji, Y., Fu, X., et al. (2009). Effects of edaravone on serum neuron specific enolase and S-100β in patients with acute cerebral hemorrhage. Clin. Focus 24, 1085–1086.
Keywords: intracerebral hemorrhage, edaravone, mortality, neurological deficits, systematic review, meta-analysis
Citation: Qin M, Feng L, Yang C, Wei D, Li T, Jiang P, Guan J, Zhang X, Shi X, Liang N, Lai X, Zhou L, Zhang C and Gao Y (2022) Edaravone use in acute intracerebral hemorrhage: A systematic review and meta-analysis of randomized controlled trials. Front. Pharmacol. 13:935198. doi: 10.3389/fphar.2022.935198
Received: 03 May 2022; Accepted: 14 July 2022;
Published: 12 August 2022.
Edited by:
Zhong Wang, Soochow University, ChinaReviewed by:
Daniela Oliveira De Melo, Federal University of São Paulo, BrazilShihong Zhang, Sichuan University, China
Copyright © 2022 Qin, Feng, Yang, Wei, Li, Jiang, Guan, Zhang, Shi, Liang, Lai, Zhou, Zhang and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Gao, gaoying973@163.com; Chi Zhang, saga618@126.com
†These authors have contributed equally to this work and share first authorship
 Mingzhen Qin
Mingzhen Qin Luda Feng
Luda Feng Chinyu Yang1,2,3
Chinyu Yang1,2,3 Ning Liang
Ning Liang Xinxing Lai
Xinxing Lai Chi Zhang
Chi Zhang Ying Gao
Ying Gao