- 1Center for Reproductive Medicine, Department of Obstetrics and Gynecology, Peking University Third Hospital, Beijing, China
- 2First Affiliated Hospital, School of Medicine, Shihezi University, Beijing, China
Polycystic ovary syndrome (PCOS) is a common endocrine and metabolic disorder in women of childbearing age. Individual heterogeneity is evident, and the prevalence rate ranges between 6 and 15% globally. The prevalence rate of PCOS in Chinese women of childbearing age is 5.6%. The main manifestations are infertility, sparse menstruation, irregular vaginal bleeding, long-term endometrial hyperplasia, and endometrial cancer. PCOS is often associated with hyperandrogenemia, insulin resistance, hyperinsulinemia, obesity, metabolic syndrome, and intestinal flora disorder. Although there have been many studies in the past, the underlying pathophysiological mechanism of the disease is still unclear. Studies have shown that PCOS diseases and related complications are closely related to local oxidative stress imbalance in the endometrium, leading to poor endometrial receptivity and effects on pregnancy. Previous reviews have mainly focused on the abnormal mechanism of ovarian oxidative stress in women with PCOS, while reviews on endometrial receptivity and oxidative stress are relatively insufficient. This study reviews the abnormal cellular and molecular mechanisms of oxidative stress due to comorbidities in women with PCOS, leading to a downregulation of endometrial receptivity.
Introduction
Polycystic ovary syndrome (PCOS) is a common endocrine and metabolic disorder in women of childbearing age with evident individual heterogeneity and a prevalence rate of between 6 and 15% (Fauser et al., 2012). For Chinese women of childbearing age, the prevalence rate is 5.6% (Li et al., 2013). The main manifestations of PCOS are infertility, oligomenorrhea, irregular vaginal bleeding, long-term endometrial hyperplasia, and endometrial cancer, which are associated with hyperandrogenemia, insulin resistance (IR), hyperinsulinemia, obesity, and metabolic syndrome (MetS). PCOS was first reported by Stein and Leventhal in 1935 and is also known as Stein–Leventhal syndrome. Due to its clinical heterogeneity, the diagnosis of PCOS has always been controversial. The NIH, Rotterdam, AES, and other diagnostic criteria have been developed internationally, but the Rotterdam standard is the most widely used (Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group, 2004). The Rotterdam criteria are as follows: 1. anovulation or sparse ovulation; 2. polycystic ovarian changes, revealed by an ultrasound showing that one or both ovaries have ≥12 follicles with a 2–9-mm diameter and/or an ovarian volume ≥10 ml; and 3. if two of the three are present, the patient can be diagnosed with clinical hyperandrogenemia and/or biochemical hyperandrogenemia manifestations. PCOS can be diagnosed if two of the three items are met, but other diseases causing hyperandrogenism, hyperprolactinemia, and abnormal thyroid function should be excluded.
The underlying cause of PCOS has not been identified; however, PCOS has been related to genetic and environmental factors. Women of childbearing age mainly present with ovulation disorders. After lifestyle adjustments are made, and metabolic diseases are corrected; clomiphene, letrozole, tamoxifen, gonadotropins, and other drugs are given to induce ovulation. The ovulation rate is approximately 60–80%, although the clinical pregnancy rate is only approximately 35–40% (Bansal et al., 2021; Yland et al., 2022). Therefore, the proportions of failed embryo implantations and spontaneous abortions in women with PCOS are still relatively high. During assisted reproductive technology (ART), oocytes donated by women with PCOS do not reduce the overall pregnancy success rate compared with those donated by healthy women (Ashkenazi et al., 1995), which strongly suggests that the decreased fertility of women with PCOS is related to a disturbance of the body’s environment, decreased oocyte quality, and decreased endometrial receptivity (ER). ER refers to the ability of the endometrium to allow the embryo to adhere and invade, inducing a series of corresponding changes in the endometrium that enable embryo implantation. This period is also called the “implantation window period” and occurs 5–7 days after ovulation. To cope with these changes, precise mechanisms involving numerous molecules and pathways are needed. Two-thirds of clinical repeated implantation failures are caused by insufficient ER (Craciunas et al., 2019), so poor ER has become the primary reason for implantation failure.
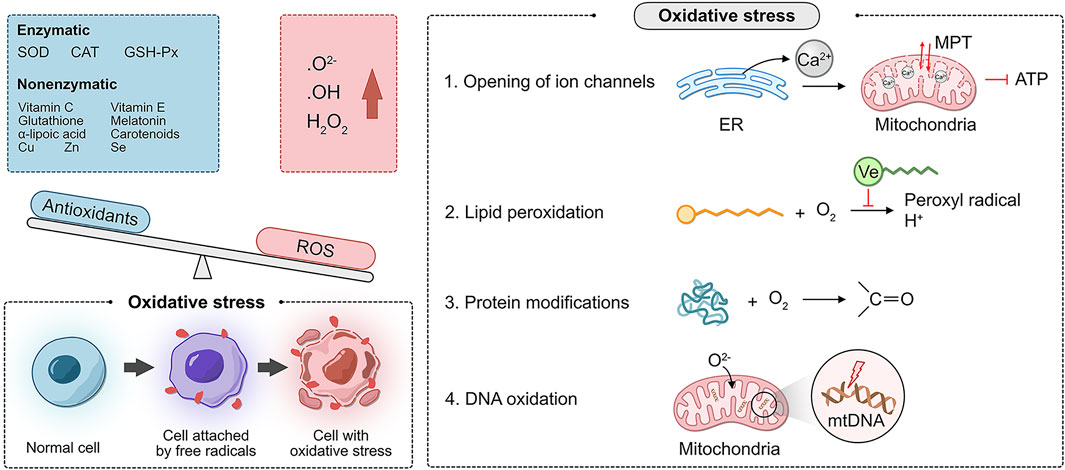
Oxidative stress (OS) refers to the excessive production of reactive oxygen species (ROS), an oxidation degree exceeding the scavenging ability of oxides, and an imbalance between the oxidation and antioxidant defense systems. ROS include superoxide anion (O₂-), hydroxyl radical (·OH), and hydrogen peroxide (H₂O₂). There are two types of antioxidant systems in the body: the enzyme antioxidant system and the non-enzymatic antioxidant system. The enzyme antioxidant system includes superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px). The non-enzymatic antioxidant system includes vitamin C, vitamin E, glutathione, melatonin, α-lipoic acid, carotenoids, and the trace elements copper, zinc, and selenium.
Oxidative phosphorylation (OXPHOS) is fundamental for life. Mitochondria maintain OXPHOS by generating a membrane potential gradient generated by the electron transport chain (ETC) to drive ATP synthesis. The ETC is the main source of ROS generation (Chakrabarty and Chandel, 2021). Moderate levels of ROS stimulate cell growth and proliferation and are necessary for maintaining physiological functions (Mittler, 2017). Chronic ROS accumulation can occur in various cell types, such as vascular endothelial cells, oocytes, endometrial epithelial cells, and stromal cells. Internal signaling pathways directly or indirectly induce cell and tissue damage (i.e., damage to DNA, lipid membranes, and proteins), leading to various female reproductive system disorders (Zhang et al., 2019). These deleterious attacks are mediated by the following more specialized mechanisms (Agarwal et al., 2012):
1. Opening of ion channels: excess ROS leads to Ca2+ release from the endoplasmic reticulum, resulting in increased mitochondrial permeability. As a result, the mitochondrial membrane potential is altered, affecting the production of ATP.
2. Lipid peroxidation: this alteration is common in regions, where unsaturated fatty acid side chains are present. These side chains react with O2 to produce peroxyl radicals, which can acquire H+ from another fatty acid, forming a continuous reaction. Due to the lipid solubility and hydrophobic tail of vitamin E, it can break this chain reaction.
3. Modification of proteins: amino acids are targets of oxidative damage, and direct oxidation of side chains can lead to the formation of carbonyl groups.
4. DNA oxidation: mitochondrial DNA is vulnerable to ROS attack because of the lack of histone protection and repair mechanisms of O2- in the ETC (Figure 1).
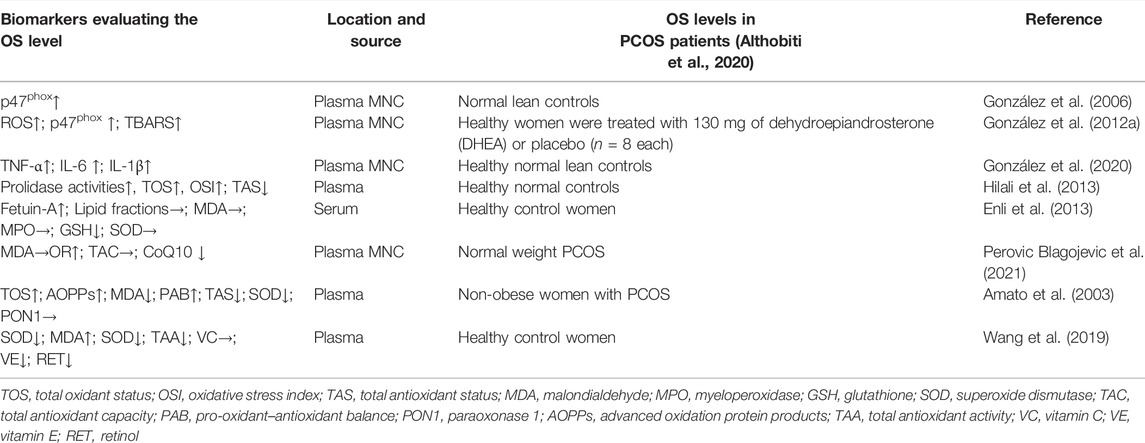
OS is closely related to the PCOS onset. Compared with the level in a healthy control group, the OS level in women with PCOS was significantly increased (Table 1). The aforementioned literature suggests that the imbalance of OS in the body is associated with adverse pregnancy outcomes in patients with PCOS. Further studies have shown that ER in women with PCOS is significantly reduced, which is closely related to IR, hyperandrogenemia, metabolic disorders, and intestinal flora imbalance, resulting in an imbalance of endometrial OS (Luo et al., 2017). However, previous reviews have mainly focused on the imbalance of OS and oocyte development, the follicular developmental microenvironment, and embryonic development in PCOS patients, and little attention has been given to research on ER. Therefore, the next section will review the decrease in ER and the cellular and molecular mechanisms of OS in PCOS women with distinct metabolic profiles.
Insulin Resistance and Oxidative Stress
Insulin is a protein hormone secreted by pancreatic β cells. Insulin binds to the insulin receptor (INSR) and is transferred from intracellular vesicles to the plasma membrane through glucose transporter-4 (GLUT-4) to achieve glucose uptake and utilization. Inhibiting glycogen degradation and gluconeogenesis play a vital role in glucose homeostasis. The anti-OS effect of insulin has been confirmed in vitro, in vivo, and by physiological and pharmacological studies (Lin et al., 2015; Ruegsegger et al., 2018). Approximately 75% of lean women and 95% of obese women with PCOS have IR (Stepto et al., 2013), and IR and hyperinsulinemia are the core mechanisms of PCOS. In addition, women with PCOS exhibit localized IR, and endometrial IR is mainly due to impaired key molecules in the endometrial insulin pathway and disturbed signaling, leading to decreased glucose uptake (Palomba et al., 2021). These key molecules and pathways include endometrial GLUT-4 (Cabrera-Cruz et al., 2020), INSR substrate phosphorylation downregulation, and PI3K/Akt pathway abnormalities. Local endometrial IR is associated with hyperandrogenemia, obesity, and chronic low-grade inflammation and is particularly closely related to OS imbalance, leading to an upregulation of the OS induced by ROS and pregnancy impairment. The infusion of physiological insulin into obese women with PCOS inhibits ROS generation and the activation of NF-κB (Dandona et al., 2001). Animal experiments (Hu et al., 2019) have shown that compared with that of a control group, IR in mice was related to changes in uterine morphology and the abnormal expression of genes related to endometrial decidualization, placenta formation, angiogenesis, and insulin signaling. Moreover, murine IR is associated with endometrial mitochondrial function and homeostasis (i.e., mitochondrial DNA copy number and the expression of genes responsible for mitochondrial fusion, division, biogenesis, and phagocytosis) changes and the inhibition of oxidative and antioxidant defenses (i.e., reactive oxygen species, nuclear factor erythroid-2 related factor 2 (Nrf2) signaling, and antioxidation). However, most research has been performed on rats, and it is unclear whether these insights can be transferred to long-lived mammals such as humans. The interactive network of the OS response to hyperandrogenemia and IR suggests that both induce mitochondrial-mediated damage in the uterus during pregnancy and lead to the unbalanced relationship between oxidative and antioxidative stress.
The mechanistic target of rapamycin (mTOR) is a member of the phosphatidylinositol 3-kinase-related kinase superfamily. As an essential molecule for signal transduction, cell proliferation, growth, differentiation, and apoptosis, mTOR plays a vital role in mammalian growth control (Somers and Paul, 2015; Wataya-Kaneda, 2015; Vahidnezhad et al., 2016). The expression of molecules related to the mTOR signaling pathway is closely related to ER (Wollenhaupt et al., 2013; Mahdi et al., 2015). Previous studies have shown that an injection of the mTOR inhibitor rapamycin reduces ER (Li et al., 2017). In maternal hyperinsulinemic mice, phosphorylated mTOR (p-mTOR) and p-p70S6K protein expression are reduced, and the expression of genes related to uterine receptivity, namely, Esr1, Pgr, Hoxa10, and Esr2, is deregulated. After insulin treatment, the impaired ER is reversed (Li et al., 2017). Likewise, in women with PCOS and IR, the use of the insulin sensitizer metformin may directly act on the endometrium, reducing IR by increasing the GLUT4 expression and thereby indirectly restoring endometrial function (Supplementary Figure S1). However, these studies either have a small sample size, or the mechanism research is not in-depth enough. More large-sample prospective randomized studies or more in-depth direct mechanism studies are urgently needed. The aforementioned studies show that ER dysfunction in women with PCOS and IR is closely related to the local OS imbalance in the endometrium.
Hyperandrogenemia and Oxidative Stress
Hyperandrogenemia (HA) is characterized by elevated testosterone levels in women, leading to clinical manifestations such as acne, hirsutism, and alopecia. Androgens are primarily produced by the ovaries, adrenal glands, and peripheral organs (skin and liver). Among them, the ovaries are the main source of androgens (Rosas et al., 2016). The mechanism of the ovarian origin of HA is as follows (Supplementary Figure S2):
1 Abnormal neurotransmitter-related synthase and receptors in the central nervous system lead to abnormal secretion of the neurotransmitter kisspeptin (Varikasuvu et al., 2019; Skorupskaite et al., 2020) and an increase in the frequency of hypothalamic GnRH release and consequently the production of luteinizing hormone (LH). As the frequency and quantity of kisspeptin release are increased, follicular membrane cells are stimulated, and excessive androgen is secreted.
2 Women with PCOS have a variety of androgen synthase abnormalities, such as the increased activity of 3β-hydroxysteroid dehydrogenase (HSD3β), 17α-hydroxylase (CYP17), cytochrome P450 side-chain lyase (CYPscc), and steroidogenic acute regulatory protein (StAR) overexpression, as well as reduced 21-hydroxylase activity. The impairment of the 21-hydroxylase activity blocks the conversion of 17α-hydroxyprogesterone, which is a precursor hormone and, thus, its entry into the androgen synthesis pathway, leading to increased androgen synthesis.
HA is a typical feature of PCOS. 80% of women with PCOS have elevated serum androgen levels (Li et al., 2013). Clinically, total serum testosterone, androstenedione, dehydroepiandrosterone, and dehydroepiandrosterone sulfate are tested. Most studies find that serum-free testosterone may be a more sensitive indicator for detecting hyperandrogenic diseases (Azziz et al., 2009). In addition, the OS imbalance and inflammatory activation in women with PCOS contribute to the continuous occurrence of HA in PCOS, which may change the expression of proteins related to endometrial development and embryo implantation (Rahman et al., 2018; Mokhtar et al., 2020), thereby impairing the receptivity of the endometrium. Furthermore, women with PCOS and HA have high-density lipoprotein (HDL) antioxidant/anti-inflammatory damage, and the decrease in plasma sex hormone-binding globulin (SHBG) levels is an important cause of HA in PCOS (Sun et al., 2021). Nuclear factor-κB (NF-κB) is a potential crucial mediator of hyperandrogenemia-induced inflammation (González et al., 2012b). WNT5a acts as a pro-inflammatory factor in the ovarian granulosa cells of patients with PCOS. Upregulated expression of WNT5a in PCOS primarily increases inflammation and OS through the PI3K/AKT/NF-κB signaling pathway. Moreover, inducible pro-inflammatory cytokines may further enhance the NF-κB-dependent regulation of the WNT5a expression (Zhao et al., 2015). In addition, a cross-sectional study from China showed that OS inhibits the SHBG expression and secretion by downregulating hepatic nuclear factor-4a (HNF-4a) in vitro, which may be an important factor in promoting HA in PCOS (Sun et al., 2021). But some of these studies only compared weight-matched controls, ignoring the fact that a large proportion of polycystic ovary syndrome is overweight. These prospective studies provide us with strong evidence that HA is closely related to OS and is an important reason for decreasing endometrial receptivity in women with PCOS.
Metabolic Disorders and Oxidative Stress
MetS is a complex group of metabolic disorders in which the metabolism of proteins, fats, carbohydrates, and other substances in the human body is disturbed. The central aspects of MetS are obesity and IR, with the main manifestation being obesity, especially central obesity. MetS is a global public health problem. According to statistics, the prevalence of MetS is approximately 20–30% (LeRoith, 2008). The diagnosis of MetS is made when three of the following five criteria are met (Supplementary Figure S3) (Alberti et al., 2009):
1. Central obesity or abdominal obesity: men with MetS have a waist circumference of 90 cm or more, and women with MetS have a waist circumference of 85 cm or more.
2. Elevated fasting blood triglycerides (>150 mg/dl or drug therapy for elevated triglycerides).
3. Decreased high-density lipoprotein (HDL) cholesterol (<40 mg/dl or pharmacological treatment for decreased HDL cholesterol).
4. Elevated blood pressure (systolic pressure >130 mmHg and/or diastolic pressure >85 mmHg, or hypertension has been diagnosed and treatment has been started).
5. Elevated fasting blood glucose (>100 mg/dl or pharmacological treatment for elevated blood glucose).
MetS is characterized by an abnormal increase in multiple body indicators, especially plasma-free fatty acids (FFAs) (Saklayen, 2018). Elevated plasma FFAs cause a progressive decline in insulin secretion by promoting pancreatic β-cell death through increased production of ROS, which activates the generation of ROS (Inoguchi et al., 2000). Compared with women in a control group, women with or without MetS combined with PCOS had a lower antioxidant capacity, and the OS level in the combined MetS group was higher. Similarly, the pathogenetic analysis showed that compared with the level in a control group, the average MDA level in women with PCOS was increased (Pei et al., 2021). In contrast, the exposure of adipose tissues to OS under MetS circumstances, leading to reduced secretion of adiponectin, which exerts anti-inflammatory effects and increased secretion of inflammatory cytokines (Soares et al., 2005; Otani, 2011), led to a compromised insulin signaling pathway through the induction of insulin receptor phosphorylation and the exacerbation of GLUT4 translocation and gene transcription (Bloch-Damti and Bashan, 2005).
A study strongly suggested that impaired lipid patterns may lead to OS activation and weakened antioxidant capacity in women with PCOS combined with MetS (Wang et al., 2019). In addition, in the circulation, the level of HDL, which is an antioxidant, is reduced, and the level of LDL that induces OS is increased. The two work together to further activate OS (Holvoet, 2008; Zelzer et al., 2011). These results suggest that women with PCOS with MetS have higher OS levels and lower antioxidant capacity than women with PCOS without MetS. Studies in rodent livers and endothelial cells have shown that fructose can drive OS by increasing triglyceride synthesis and uric acid production (Johnson et al., 2013). Fructose-induced hyperglycemia and fatty liver mouse models suggest that impaired antioxidant defenses contribute to the pro-oxidant environment in the uterus. In C57BL/6 mice fed with a high-fat diet, proliferating cell nuclear antigen (PCNA) expression decreased, and activated B cells (NF-κB) and the nuclear factor κ-light chain enhancer of the peroxisome proliferator-activated receptor gamma (PPAR gamma) signaling pathway increased (Cheng et al., 2018). With the increase in OS, the high-fat diet led to increased endometrial cell apoptosis by increasing the expression of 8-hydroxydeoxyguanosine (8-OHdG) (Heard et al., 2016); compared with the control group, the high-fat diet in the hypercholesterolemia rat model promoted oxidative and inflammatory stress and significantly increased tumor necrosis factor-α (TNF-α) and F4/80 macrophage infiltration (El-Mansi et al., 2019). The aforementioned studies all suggest that the impaired lipid pattern in PCOS patients with MetS can activate ROS through a variety of molecular adjustment mechanisms, resulting in an imbalance in OS in the body, especially in the endometrial region, which in turn affects ER and leads to adverse pregnancy outcomes.
Disturbance of the Gut Microbiota and Oxidative Stress
The gut microbiota is the most plentiful and functionally critical microflora, encompassing approximately 1014 resident microorganisms and commensals within the human intestinal tract (Sun et al., 2012). The intestinal microbiota plays a vital role in metabolism and the immune system (Chung et al., 2012; Krajmalnik-Brown et al., 2012). The composition of the intestinal microbiota of women with PCOS is significantly altered (Liang et al., 2020), which may be related to IR, metabolic abnormalities, and sex hormone abnormalities in women with PCOS. Whole-genome shotgun sequencing showed no significant difference in bacterial alpha diversity between women with PCOS and healthy controls; however, the beta diversity of the PCOS microbiome was significantly reduced, and the community structure among women with PCOS was more even, especially in the obese PCOS group. In addition, compared with that in healthy controls, the abundance of common Bacteroides vulgatus in women with PCOS was significantly increased (Lindheim et al., 2017; Liu et al., 2017; Torres et al., 2018; Qi et al., 2019; Zeng et al., 2019). Tremellen et al. proposed the “gut barrier-endotoxemia-inflammation mechanism” hypothesis, which reflects PCOS pathogenesis (Tremellen and Pearce, 2012), where changes in serum markers such as zonulin, intestinal fatty acid-binding protein 2 (FABP2), and bacterial lipopolysaccharide (LPS) are a result of intestinal barrier damage and inflammation (Sturgeon and Fasano, 2016; Stevens et al., 2018). Endotoxemia could play a role in PCOS pathogenesis by initiating the inflammatory activity (Tremellen and Pearce, 2012). LPS produced by intestinal Gram-negative bacteria is a key molecule in the early development of inflammation and metabolic diseases and has an endotoxin effect, inducing macrophage activation (Wellen and Hotamisligil, 2005), which leads to increases in serum TNF-α and interleukin 6 (IL-6) and triggers IR, which in turn leads to excessive ROS production in the gastrointestinal system (Gyuraszova et al., 2017). Moreover, chronic low-grade inflammation promotes HA and obesity in women with PCOS (Franks and Hardy, 2010; Glintborg et al., 2016; Belani et al., 2018), forming a vicious cycle. In short, gut dysbiosis may mediate systemic low-grade inflammation and IR. Changes affecting sex hormones, the gut-brain axis, and other pathological mechanisms are involved in PCOS development. As discussed earlier, IR, HA, and gut microbiota disturbances can form a vicious cycle that mediates OS imbalances, downregulating ER through various mechanisms and resulting in adverse pregnancy outcomes.
In summary, PCOS is a common multifactorial endocrine and metabolic disorder in women of childbearing age with marked individual heterogeneity, and it mainly manifests as infertility, oligomenorrhea, irregular vaginal bleeding, and long-term complications such as endometrial hyperplasia or endometrial cancer. Genetic susceptibility is one of the risk factors for PCOS, and poor lifestyle habits can induce the disease phenotype. Chronic inflammation in PCOS is systemic. In women with PCOS, higher levels of serum inflammation markers, namely, IL-6, IL-16, IL-18, TNF-α, and CRP, are found (Samy et al., 2009; Ebejer and Calleja-Agius, 2013; Long et al., 2017; Blumenfeld, 2019), and the expressions of intercellular adhesion molecule 1 (ICAM-1), TNF-α, and MCP are upregulated (Peng et al., 2016), indicating an OS imbalance (Long et al., 2017). In addition, it should be noted that PCOS patients suffer from inflammation, regardless of body weight. Obesity is only an aggravating factor. IR, HA, metabolic disorders, and gut microbiota imbalance can lead to endometrial OS imbalance through different signaling pathways and downregulation of ER. The changes in the implantation environment caused by inflammatory mediators may cause ER damage in women with PCOS and embryo implantation failure (Ashary et al., 2018). The crosstalk between embryo implantation and the maternal–fetal interface has not been fully elucidated and still needs to be explored in more in-depth, multicenter studies.
Author Contributions
HS, RL, and XG wrote the manuscript. ZY and TP sorted out ideas. FL, ZY, and RL revised the manuscript. All the authors read and approved the final manuscript.
Funding
This study was supported by the “National Science Foundation for Distinguished Young Scholars, grant number 81925013” and the “China Postdoctoral Science Foundation grant number 2021M690259”.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.904942/full#supplementary-material
References
Agarwal, A., Aponte-Mellado, A., Premkumar, B. J., Shaman, A., and Gupta, S. (2012). The Effects of Oxidative Stress on Female Reproduction: a Review. Reprod. Biol. Endocrinol. 10, 49. doi:10.1186/1477-7827-10-49
Alberti, K. G. M. M., Eckel, R. H., Grundy, S. M., Zimmet, P. Z., Cleeman, J. I., Donato, K. A., et al. (2009). Harmonizing the Metabolic Syndrome. Circulation 120 (16), 1640–1645. doi:10.1161/CIRCULATIONAHA.109.192644
Althobiti, M., Muftah, A. A., Aleskandarany, M. A., Joseph, C., Toss, M. S., Green, A., et al. (2020). The Prognostic Significance of BMI1 Expression in Invasive Breast Cancer Is Dependent on its Molecular Subtypes. Breast Cancer Res. Treat. 182 (3), 581–589. doi:10.1007/s10549-020-05719-x
Amato, G., Conte, M., Mazziotti, G., Lalli, E., Vitolo, G., Tucker, A. T., et al. (2003). Serum and Follicular Fluid Cytokines in Polycystic Ovary Syndrome during Stimulated Cycles. Obstet. Gynecol. 101 (6), 1177–1182. doi:10.1016/s0029-7844(03)00233-3
Ashary, N., Tiwari, A., and Modi, D. (2018). Embryo Implantation: War in Times of Love. Endocrinology 159 (2), 1188–1198. doi:10.1210/en.2017-03082
Ashkenazi, J., Farhi, J., Orvieto, R., Homburg, R., Dekel, A., Feldberg, D., et al. (1995). Polycystic Ovary Syndrome Patients as Oocyte Donors: the Effect of Ovarian Stimulation Protocol on the Implantation Rate of the Recipient. Fertil. Steril. 64 (3), 564–567. doi:10.1016/s0015-0282(16)57793-0
Azziz, R., Carmina, E., Dewailly, D., Diamanti-Kandarakis, E., Escobar-Morreale, H. F., Futterweit, W., et al. (2009). The Androgen Excess and PCOS Society Criteria for the Polycystic Ovary Syndrome: the Complete Task Force Report. Fertil. Steril. 91 (2), 456–488. doi:10.1016/j.fertnstert.2008.06.035
Bansal, S., Goyal, M., Sharma, C., and Shekhar, S. (2021). Letrozole versus Clomiphene Citrate for Ovulation Induction in Anovulatory Women with Polycystic Ovarian Syndrome: A Randomized Controlled Trial. Int. J. Gynecol. Obstet. 152 (3), 345–350. doi:10.1002/ijgo.13375
Belani, M., Deo, A., Shah, P., Banker, M., Singal, P., and Gupta, S. (2018). Differential Insulin and Steroidogenic Signaling in Insulin Resistant and Non-insulin Resistant Human Luteinized Granulosa Cells-A Study in PCOS Patients. J. Steroid Biochem. Mol. Biol. 178, 283–292. doi:10.1016/j.jsbmb.2018.01.008
Bloch-Damti, A., and Bashan, N. (2005). Proposed Mechanisms for the Induction of Insulin Resistance by Oxidative Stress. Antioxid. Redox Signal 7 (11-12), 1553–1567. doi:10.1089/ars.2005.7.1553
Blumenfeld, Z. (2019). The Possible Practical Implication of High CRP Levels in PCOS. Clin. Med. Insights Reprod. Health 13, 1179558119861936. doi:10.1177/1179558119861936
Cabrera-Cruz, H., Oróstica, L., Plaza-Parrochia, F., Torres-Pinto, I., Romero, C., and Vega, M. (2020). The Insulin-Sensitizing Mechanism of Myo-Inositol Is Associated with AMPK Activation and GLUT-4 Expression in Human Endometrial Cells Exposed to a PCOS Environment. Am. J. Physiol. Endocrinol. Metab. 318 (2), E237–E248. doi:10.1152/ajpendo.00162.2019
Chakrabarty, R. P., and Chandel, N. S. (2021). Mitochondria as Signaling Organelles Control Mammalian Stem Cell Fate. Cell. Stem Cell. 28 (3), 394–408. doi:10.1016/j.stem.2021.02.011
Cheng, Y., Lv, Q., Xie, B., Yang, B., Shan, W., Ning, C., et al. (2018). Estrogen and High-Fat Diet Induced Alterations in C57BL/6 Mice Endometrial Transcriptome Profile. Endocr. Connect. 7 (1), 36–46. doi:10.1530/EC-17-0315
Chung, H., Pamp, S. J., Hill, J. A., Surana, N. K., Edelman, S. M., Troy, E. B., et al. (2012). Gut Immune Maturation Depends on Colonization with a Host-specific Microbiota. Cell. 149 (7), 1578–1593. doi:10.1016/j.cell.2012.04.037
Craciunas, L., Gallos, I., Chu, J., Bourne, T., Quenby, S., Brosens, J. J., et al. (2019). Conventional and Modern Markers of Endometrial Receptivity: a Systematic Review and Meta-Analysis. Hum. Reprod. Update 25 (2), 202–223. doi:10.1093/humupd/dmy044
Dandona, P., Aljada, A., Mohanty, P., Ghanim, H., Hamouda, W., Assian, E., et al. (2001). Insulin Inhibits Intranuclear Nuclear Factor kappaB and Stimulates IkappaB in Mononuclear Cells in Obese Subjects: Evidence for an Anti-inflammatory Effect? J. Clin. Endocrinol. Metab. 86 (7), 3257–3265. doi:10.1210/jcem.86.7.7623
Ebejer, K., and Calleja-Agius, J. (2013). The Role of Cytokines in Polycystic Ovarian Syndrome. Gynecol. Endocrinol. 29 (6), 536–540. doi:10.3109/09513590.2012.760195
El-Mansi, A. A., ElSayyad, H. I., Elshershaby, E. M., and Al-Ashry, N. E. (2019). Dietary Supplementation of Barley And/or Dates Attenuate Hypercholesterolemic-Induced Endometrial Dysfunction in Wistar Albino Rats via Alleviation of Apoptotic Pathways and Enhancing Oxidative Capacity. J. Food Biochem. 43 (11), e13001. doi:10.1111/jfbc.13001
Enli, Y., Fenkci, S. M., Fenkci, V., and Oztekin, O. (2013). Serum Fetuin-A Levels, Insulin Resistance and Oxidative Stress in Women with Polycystic Ovary Syndrome. Gynecol. Endocrinol. 29 (12), 1036–1039. doi:10.3109/09513590.2013.829442
Fauser, B. C., Tarlatzis, B. C., Rebar, R. W., Legro, R. S., Balen, A. H., Lobo, R., et al. (2012). Consensus on Women's Health Aspects of Polycystic Ovary Syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil. Steril. 97 (1), 28–e25. doi:10.1016/j.fertnstert.2011.09.024
Franks, S., and Hardy, K. (2010). Aberrant Follicle Development and Anovulation in Polycystic Ovary Syndrome. Ann. Endocrinol. Paris. 71 (3), 228–230. doi:10.1016/j.ando.2010.02.007
Glintborg, D., Petersen, M. H., Ravn, P., Hermann, A. P., and Andersen, M. (2016). Comparison of Regional Fat Mass Measurement by Whole Body DXA Scans and Anthropometric Measures to Predict Insulin Resistance in Women with Polycystic Ovary Syndrome and Controls. Acta Obstet. Gynecol. Scand. 95 (11), 1235–1243. doi:10.1111/aogs.12964
González, F., Considine, R. V., Abdelhadi, O. A., and Acton, A. J. (2020). Lipid-induced Mononuclear Cell Cytokine Secretion in the Development of Metabolic Aberration and Androgen Excess in Polycystic Ovary Syndrome. Hum. Reprod. 35 (5), 1168–1177. doi:10.1093/humrep/deaa056
González, F., Nair, K. S., Daniels, J. K., Basal, E., Schimke, J. M., and Blair, H. E. (2012). Hyperandrogenism Sensitizes Leukocytes to Hyperglycemia to Promote Oxidative Stress in Lean Reproductive-Age Women. J. Clin. Endocrinol. Metab. 97 (8), 2836–2843. doi:10.1210/jc.2012-1259
González, F., Nair, K. S., Daniels, J. K., Basal, E., and Schimke, J. M. (2012). Hyperandrogenism Sensitizes Mononuclear Cells to Promote Glucose-Induced Inflammation in Lean Reproductive-Age Women. Am. J. Physiol. Endocrinol. Metab. 302 (3), E297–E306. doi:10.1152/ajpendo.00416.2011
González, F., Rote, N. S., Minium, J., and Kirwan, J. P. (2006). Reactive Oxygen Species-Induced Oxidative Stress in the Development of Insulin Resistance and Hyperandrogenism in Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 91 (1), 336–340. doi:10.1210/jc.2005-1696
Gyuraszova, M., Kovalcikova, A., and Gardlik, R. (2017). Association between Oxidative Status and the Composition of Intestinal Microbiota along the Gastrointestinal Tract. Med. Hypotheses 103, 81–85. doi:10.1016/j.mehy.2017.04.011
Heard, M. E., Melnyk, S. B., Simmen, F. A., Yang, Y., Pabona, J. M., and Simmen, R. C. (2016). High-Fat Diet Promotion of Endometriosis in an Immunocompetent Mouse Model Is Associated With Altered Peripheral and Ectopic Lesion Redox and Inflammatory Status. Endocrinology 157 (7), 2870–2882. doi:10.1210/en.2016-1092
Hilali, N., Vural, M., Camuzcuoglu, H., Camuzcuoglu, A., and Aksoy, N. (2013). Increased Prolidase Activity and Oxidative Stress in PCOS. Clin. Endocrinol. (Oxf) 79 (1), 105–110. doi:10.1111/cen.12110
Holvoet, P. (2008). Relations between Metabolic Syndrome, Oxidative Stress and Inflammation and Cardiovascular Disease. Verh. K. Acad. Geneeskd. Belg 70 (3), 193–219.
Hu, M., Zhang, Y., Guo, X., Jia, W., Liu, G., Zhang, J., et al. (2019). Perturbed Ovarian and Uterine Glucocorticoid Receptor Signaling Accompanies the Balanced Regulation of Mitochondrial Function and NFκB-Mediated Inflammation under Conditions of Hyperandrogenism and Insulin Resistance. Life Sci. 232, 116681. doi:10.1016/j.lfs.2019.116681
Inoguchi, T., Li, P., Umeda, F., Yu, H. Y., Kakimoto, M., Imamura, M., et al. (2000). High Glucose Level and Free Fatty Acid Stimulate Reactive Oxygen Species Production through Protein Kinase C--dependent Activation of NAD(P)H Oxidase in Cultured Vascular Cells. Diabetes 49 (11), 1939–1945. doi:10.2337/diabetes.49.11.1939
Johnson, R. J., Nakagawa, T., Sanchez-Lozada, L. G., Shafiu, M., Sundaram, S., Le, M., et al. (2013). Sugar, Uric Acid, and the Etiology of Diabetes and Obesity. Diabetes 62 (10), 3307–3315. doi:10.2337/db12-1814
Krajmalnik-Brown, R., Ilhan, Z. E., Kang, D. W., and DiBaise, J. K. (2012). Effects of Gut Microbes on Nutrient Absorption and Energy Regulation. Nutr. Clin. Pract. 27 (2), 201–214. doi:10.1177/0884533611436116
LeRoith, D. (2008). Endocrinology and Metabolism Clinics of North America. Foreword. Endocrinol. Metab. Clin. North Am. 37 (3), xiii. doi:10.1016/j.ecl.2008.07.004
Li, R., Wu, J., He, J., Wang, Y., Liu, X., Chen, X., et al. (2017). Mice Endometrium Receptivity in Early Pregnancy Is Impaired by Maternal Hyperinsulinemia. Mol. Med. Rep. 15 (5), 2503–2510. doi:10.3892/mmr.2017.6322
Li, R., Zhang, Q., Yang, D., Li, S., Lu, S., Wu, X., et al. (2013). Prevalence of Polycystic Ovary Syndrome in Women in China: a Large Community-Based Study. Hum. Reprod. 28 (9), 2562–2569. doi:10.1093/humrep/det262
Liang, Y., Ming, Q., Liang, J., Zhang, Y., Zhang, H., and Shen, T. (2020). Gut Microbiota Dysbiosis in Polycystic Ovary Syndrome: Association with Obesity - a Preliminary Report. Can. J. Physiol. Pharmacol. 98 (11), 803–809. doi:10.1139/cjpp-2019-0413
Lin, X. H., Liu, M. E., Xu, H. Y., Chen, X. J., Wang, H., Tian, S., et al. (2015). Leptin Down-Regulates γ-ENaC Expression: a Novel Mechanism Involved in Low Endometrial Receptivity. Fertil. Steril. 103 (1), 228–e3. doi:10.1016/j.fertnstert.2014.10.002
Lindheim, L., Bashir, M., Münzker, J., Trummer, C., Zachhuber, V., Leber, B., et al. (2017). Alterations in Gut Microbiome Composition and Barrier Function Are Associated with Reproductive and Metabolic Defects in Women with Polycystic Ovary Syndrome (PCOS): A Pilot Study. PLoS One 12 (1), e0168390. doi:10.1371/journal.pone.0168390
Liu, R., Zhang, C., Shi, Y., Zhang, F., Li, L., Wang, X., et al. (2017). Dysbiosis of Gut Microbiota Associated with Clinical Parameters in Polycystic Ovary Syndrome. Front. Microbiol. 8, 324. doi:10.3389/fmicb.2017.00324
Long, X., Li, R., Yang, Y., and Qiao, J. (2017). Overexpression of IL-18 in the Proliferative Phase Endometrium of Patients With Polycystic Ovary Syndrome. Reprod. Sci. 24 (2), 252–257. doi:10.1177/1933719116653681
Luo, L., Gu, F., Jie, H., Ding, C., Zhao, Q., Wang, Q., et al. (2017). Early Miscarriage Rate in Lean Polycystic Ovary Syndrome Women after Euploid Embryo Transfer - a Matched-Pair Study. Reprod. Biomed. Online 35 (5), 576–582. doi:10.1016/j.rbmo.2017.07.010
Mahdi, H., Xiu, J., Reddy, S. K., and DeBernardo, R. (2015). Alteration in PI3K/mTOR, MAPK Pathways and Her2 Expression/amplification Is More Frequent in Uterine Serous Carcinoma Than Ovarian Serous Carcinoma. J. Surg. Oncol. 112 (2), 188–194. doi:10.1002/jso.23993
Mittler, R. (2017). ROS Are Good. Trends Plant Sci. 22 (1), 11–19. doi:10.1016/j.tplants.2016.08.002
Mokhtar, M. H., Giribabu, N., and Salleh, N. (2020). Testosterone Reduces Tight Junction Complexity and Down-Regulates Expression of Claudin-4 and Occludin in the Endometrium in Ovariectomized, Sex-Steroid Replacement Rats. Vivo 34 (1), 225–231. doi:10.21873/invivo.11764
Otani, H. (2011). Oxidative Stress as Pathogenesis of Cardiovascular Risk Associated with Metabolic Syndrome. Antioxid. Redox Signal 15 (7), 1911–1926. doi:10.1089/ars.2010.3739
Palomba, S., Piltonen, T. T., and Giudice, L. C. (2021). Endometrial Function in Women with Polycystic Ovary Syndrome: a Comprehensive Review. Hum. Reprod. Update 27 (3), 584–618. doi:10.1093/humupd/dmaa051
Pei, C.-Z., Jin, L., and Baek, K.-H. (2021). Pathogenetic Analysis of Polycystic Ovary Syndrome from the Perspective of Omics. Biomed. Pharmacother. 142, 112031. doi:10.1016/j.biopha.2021.112031
Peng, Z., Sun, Y., Lv, X., Zhang, H., Liu, C., and Dai, S. (2016). Interleukin-6 Levels in Women with Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. PLoS One 11 (2), e0148531. doi:10.1371/journal.pone.0148531
Perovic Blagojevic, I. M., Vekic, J. Z., Macut, D. P., Ignjatovic, S. D., Miljkovic-Trailovic, M. M., Zeljkovic, A. R., et al. (2021). Overweight and Obesity in Polycystic Ovary Syndrome: Association with Inflammation, Oxidative Stress and Dyslipidaemia. Br. J. Nutr., 1–9. doi:10.1017/S0007114521003585
Qi, X., Yun, C., Sun, L., Xia, J., Wu, Q., Wang, Y., et al. (2019). Gut Microbiota-Bile Acid-Interleukin-22 axis Orchestrates Polycystic Ovary Syndrome. Nat. Med. 25 (8), 1225–1233. doi:10.1038/s41591-019-0509-0
Rahman, T. U., Ullah, K., Guo, M. X., Pan, H. T., Liu, J., Ren, J., et al. (2018). Androgen-induced Alterations in Endometrial Proteins Crucial in Recurrent Miscarriages. Oncotarget 9 (37), 24627–24641. doi:10.18632/oncotarget.24821
Rosas, C., Oróstica, L., Poblete, C., Carvajal, R., Gabler, F., Romero, C., et al. (2016). Hyperandrogenism Decreases GRP78 Protein Level and Glucose Uptake in Human Endometrial Stromal Cells. Reprod. Sci. 23 (6), 761–770. doi:10.1177/1933719115618283
Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group (2004). Revised 2003 Consensus on Diagnostic Criteria and Long-Term Health Risks Related to Polycystic Ovary Syndrome. Fertil. Steril. 81 (1), 19–25. doi:10.1016/j.fertnstert.2003.10.004
Ruegsegger, G. N., Creo, A. L., Cortes, T. M., Dasari, S., and Nair, K. S. (2018). Altered Mitochondrial Function in Insulin-Deficient and Insulin-Resistant States. J. Clin. Invest. 128 (9), 3671–3681. doi:10.1172/JCI120843
Saklayen, M. G. (2018). The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 20 (2), 12. doi:10.1007/s11906-018-0812-z
Samy, N., Hashim, M., Sayed, M., and Said, M. (2009). Clinical Significance of Inflammatory Markers in Polycystic Ovary Syndrome: Their Relationship to Insulin Resistance and Body Mass Index. Dis. Markers 26 (4), 163–170. doi:10.3233/DMA-2009-0627
Skorupskaite, K., George, J. T., Veldhuis, J. D., Millar, R. P., and Anderson, R. A. (2020). Kisspeptin and Neurokinin B Interactions in Modulating Gonadotropin Secretion in Women with Polycystic Ovary Syndrome. Hum. Reprod. 35 (6), 1421–1431. doi:10.1093/humrep/deaa104
Soares, A. F., Guichardant, M., Cozzone, D., Bernoud-Hubac, N., Bouzaïdi-Tiali, N., Lagarde, M., et al. (2005). Effects of Oxidative Stress on Adiponectin Secretion and Lactate Production in 3T3-L1 Adipocytes. Free Radic. Biol. Med. 38 (7), 882–889. doi:10.1016/j.freeradbiomed.2004.12.010
Somers, M. J., and Paul, E. (2015). Safety Considerations of Mammalian Target of Rapamycin Inhibitors in Tuberous Sclerosis Complex and Renal Transplantation. J. Clin. Pharmacol. 55 (4), 368–376. doi:10.1002/jcph.428
Stepto, N. K., Cassar, S., Joham, A. E., Hutchison, S. K., Harrison, C. L., Goldstein, R. F., et al. (2013). Women with Polycystic Ovary Syndrome Have Intrinsic Insulin Resistance on Euglycaemic-Hyperinsulaemic Clamp. Hum. Reprod. 28 (3), 777–784. doi:10.1093/humrep/des463
Stevens, B. R., Goel, R., Seungbum, K., Richards, E. M., Holbert, R. C., Pepine, C. J., et al. (2018). Increased Human Intestinal Barrier Permeability Plasma Biomarkers Zonulin and FABP2 Correlated with Plasma LPS and Altered Gut Microbiome in Anxiety or Depression. Gut 67 (8), 1555–1557. doi:10.1136/gutjnl-2017-314759
Sturgeon, C., and Fasano, A. (2016). Zonulin, a Regulator of Epithelial and Endothelial Barrier Functions, and its Involvement in Chronic Inflammatory Diseases. Tissue Barriers 4 (4), e1251384. doi:10.1080/21688370.2016.1251384
Sun, L., Hu, W., Liu, Q., Hao, Q., Sun, B., Zhang, Q., et al. (2012). Metabonomics Reveals Plasma Metabolic Changes and Inflammatory Marker in Polycystic Ovary Syndrome Patients. J. Proteome Res. 11 (5), 2937–2946. doi:10.1021/pr3000317
Sun, Y., Li, S., Liu, H., Bai, H., Hu, K., Zhang, R., et al. (2021). Oxidative Stress Promotes Hyperandrogenism by Reducing Sex Hormone-Binding Globulin in Polycystic Ovary Syndrome. Fertil. Steril. 116 (6), 1641–1650. doi:10.1016/j.fertnstert.2021.07.1203
Torres, P. J., Siakowska, M., Banaszewska, B., Pawelczyk, L., Duleba, A. J., Kelley, S. T., et al. (2018). Gut Microbial Diversity in Women With Polycystic Ovary Syndrome Correlates With Hyperandrogenism. J. Clin. Endocrinol. Metab. 103 (4), 1502–1511. doi:10.1210/jc.2017-02153
Tremellen, K., and Pearce, K. (2012). Dysbiosis of Gut Microbiota (DOGMA)-a Novel Theory for the Development of Polycystic Ovarian Syndrome. Med. Hypotheses 79 (1), 104–112. doi:10.1016/j.mehy.2012.04.016
Vahidnezhad, H., Youssefian, L., and Uitto, J. (2016). Molecular Genetics of the PI3K-AKT-mTOR Pathway in Genodermatoses: Diagnostic Implications and Treatment Opportunities. J. Invest. Dermatol 136 (1), 15–23. doi:10.1038/JID.2015.331
Varikasuvu, S. R., Prasad, V. S., Vamshika, V. C., Satyanarayana, M. V., and Panga, J. R. (2019). Circulatory Metastin/kisspeptin-1 in Polycystic Ovary Syndrome: a Systematic Review and Meta-Analysis with Diagnostic Test Accuracy. Reprod. Biomed. Online 39 (4), 685–697. doi:10.1016/j.rbmo.2019.04.018
Wang, H., Ruan, X., Li, Y., Cheng, J., and Mueck, A. O. (2019). Oxidative Stress Indicators in Chinese Women with PCOS and Correlation with Features of Metabolic Syndrome and Dependency on Lipid Patterns. Arch. Gynecol. Obstet. 300 (5), 1413–1421. doi:10.1007/s00404-019-05305-7
Wataya-Kaneda, M. (2015). Mammalian Target of Rapamycin and Tuberous Sclerosis Complex. J. Dermatol Sci. 79 (2), 93–100. doi:10.1016/j.jdermsci.2015.04.005
Wellen, K. E., and Hotamisligil, G. S. (2005). Inflammation, Stress, and Diabetes. J. Clin. Invest. 115 (5), 1111–1119. doi:10.1172/jci20052510210.1172/JCI25102
Wollenhaupt, K., Brüssow, K. P., Albrecht, D., and Tomek, W. (2013). The Akt/mTor Signaling Cascade Is Modified during Placentation in the Porcine Uterine Tissue. Reprod. Biol. 13 (3), 184–194. doi:10.1016/j.repbio.2013.06.001
Yland, J. J., Chiu, Y.-H., Rinaudo, P., Hsu, J., Hernán, M. A., and Hernández-Díaz, S. (2022). Emulating a Target Trial of the Comparative Effectiveness of Clomiphene Citrate and Letrozole for Ovulation Induction. Hum. Reprod. Oxf. Engl. 37 (4), 793–805. doi:10.1093/humrep/deac005
Zelzer, S., Fuchs, N., Almer, G., Raggam, R. B., Prüller, F., Truschnig-Wilders, M., et al. (2011). High Density Lipoprotein Cholesterol Level Is a Robust Predictor of Lipid Peroxidation Irrespective of Gender, Age, Obesity, and Inflammatory or Metabolic Biomarkers. Clin. Chim. Acta 412 (15-16), 1345–1349. doi:10.1016/j.cca.2011.03.031
Zeng, B., Lai, Z., Sun, L., Zhang, Z., Yang, J., Li, Z., et al. (2019). Structural and Functional Profiles of the Gut Microbial Community in Polycystic Ovary Syndrome with Insulin Resistance (IR-PCOS): a Pilot Study. Res. Microbiol. 170 (1), 43–52. doi:10.1016/j.resmic.2018.09.002
Zhang, Y., Zhao, W., Xu, H., Hu, M., Guo, X., Jia, W., et al. (2019). Hyperandrogenism and Insulin Resistance-Induced Fetal Loss: Evidence for Placental Mitochondrial Abnormalities and Elevated Reactive Oxygen Species Production in Pregnant Rats that Mimic the Clinical Features of Polycystic Ovary Syndrome. J. Physiol. 597 (15), 3927–3950. doi:10.1113/JP277879
Keywords: polycystic ovary syndrome, endometrial receptivity, oxidative stress, molecular mechanism, hyperandrogenemia
Citation: Shan H, Luo R, Guo X, Li R, Ye Z, Peng T, Liu F and Yang Z (2022) Abnormal Endometrial Receptivity and Oxidative Stress in Polycystic Ovary Syndrome. Front. Pharmacol. 13:904942. doi: 10.3389/fphar.2022.904942
Received: 06 May 2022; Accepted: 20 June 2022;
Published: 25 July 2022.
Edited by:
Valentina Vellecco, University of Naples Federico II, ItalyReviewed by:
Helda Tutunchi, Tabriz University of Medical Sciences, IranCopyright © 2022 Shan, Luo, Guo, Li, Ye, Peng, Liu and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rong Li, roseli001@sina.com
 Hongying Shan
Hongying Shan Renxin Luo1
Renxin Luo1 Rong Li
Rong Li Fenting Liu
Fenting Liu Zi Yang
Zi Yang