- State Key Laboratory of Southwestern Chinese Medicine Resources, School of Pharmacy, Chengdu University of Traditional Chinese Medicine, Chengdu, China
In recent years, the incidence and mortality of cardio-cerebrovascular diseases have been increasing year by year, which has become global burden and challenge. Based on the holistic thinking of “brain disease affects the heart” and “heart disease affects the brain,” as well as the characteristics of multi-target and multi-path effects of Chinese medicine, Chinese medicine is more advantageous in the treatment of cardio-cerebrovascular diseases. As a botanical medicine, storax is known for its resuscitation, filth avoidance and pain-relieving effects in the treatment of cardio-cerebrovascular diseases. By reviewing and collating the relevant domestic and international literature in the past 10 years, we have sorted out an overview of the medicinal parts, traditional uses and chemical composition of storax. For the first time, based on the idea of “cerebral and cardiac simultaneous treatment,” the pharmacological activities and mechanisms of heart and brain protection of storax for treating cardio-cerebrovascular diseases were summarized and analyzed, showing that storax has the pharmacological effects of anti-cerebral ischemia, regulation of blood-brain barrier, bidirectional regulation of the central nervous system, anti-myocardial ischemia, anti-arrhythmia, anti-thrombosis and anti-platelet aggregation. It mainly exerts its protective effects on the brain and heart through mechanisms such as inhibition of inflammatory immune factors, anti-oxidative stress, anti-apoptosis, pro-neovascularization and regulation of NO release. On the basis of the current findings and limitations, the future research strategies and perspectives of storax are proposed, with a view to providing a reference for further application and development of this medicine, as well as contributing new thoughts and visions for the clinical application of “treating brain-heart synchronously”.
1 Introduction
Storax (ST), which is also named Styrax or Su-Hexiang, is a kind of processed and refined aromatic resin exuded from the trunk of the Liquidambar orientalis Mill in the family Hamamelidaceae (State Pharmacopoeia Committee, 2020). It has been not only widely used as a folk medicine in East Asia, India, Africa and Turkey for many years (U.S. Pharmacopeial Convention, 2017; Duru et al., 2010), but also being a popular spice for food and wine (Zhang et al., 2020). During the Qin and Han dynasties, ST was introduced to China by the Silk Road and was widely used in healthcare and social life. Due to its high safety profile and beneficial effects on various diseases, ST is used as a crucial ingredient in the preparation of various traditional Chinese prescriptions, for example, Guanxin Suhe Pill, Shexiang Baoxin Pill, Su Bing Dripping Pill and ST Pill. Nowadays, Chinese medicine compounds containing ST are widely used in Asian countries for the prevention and treatment of cardio-cerebrovascular diseases (CCVDs) (Xu, 2014; Li et al., 2016; Zhou et al., 2016; Lu et al., 2018; Zou et al., 2020).
CCVDs are ischaemic or hemorrhagic diseases of the heart and brain caused by thickened blood, atherosclerosis, hypertension and hyperlipidemia, including cardiovascular and cerebrovascular lesions, which pose a serious threat to human health. According to Global Burden of disease 2016, there were approximately 80.1 million people with cerebrovascular disease worldwide, of which 67.6 million had ischemic stroke and 2.7 million died (Benjamin et al., 2019). Similar to ischemic stroke, the incidence of coronary heart disease was high, too. In 2016, it was estimated that 17.9 million people died from cardiovascular disease, accounting for 31% of deaths around the world (Benjamin et al., 2019). Therefore, in order to prevent and treat these diseases, it is urgent to identify the pathogenesis of CCVDs and clarify the relationship between cardiovascular diseases and cerebrovascular diseases, so as to find medications with better efficacy and fewer side effects.
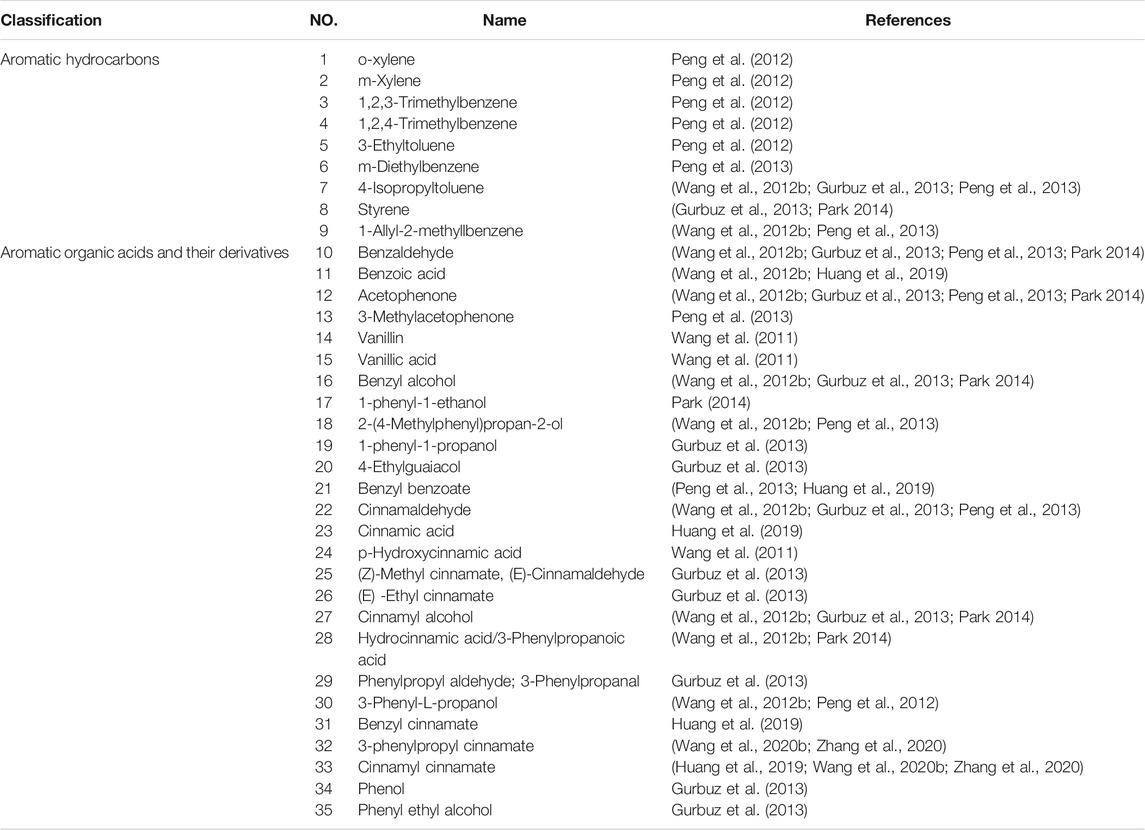
According to traditional Chinese medicine, human beings are an organic whole. Although the “brain” and “heart” are two different organs, they are pathologically related to each other, and CCVDs often exist at the same time. The incidence of stroke has been reported to be more than double in patients with coronary heart disease, more than triple in those with hypertension, quadruple in those with heart failure, and quintuple in those with atrial fibrillation (Chen et al., 2017). Likewise, stroke (cerebral ischemia, cerebral hemorrhage, or subarachnoid hemorrhage) can lead to cardiac insufficiency, arrhythmias, and even heart failure ultimately. The interaction of the brain and heart after stroke is described in Figure 1. Cardiac dysfunction can be induced after stroke by modulating the hypothalamic-pituitary-adrenal axis, activating sympathetic and parasympathetic nerves to release catecholamine (Vaseghi et al., 2017; Veltkamp et al., 2019), disrupting the gut microbiota (Nam 2019), inducing immune response (Zhao et al., 2019) and inflammation (Kelly et al., 2021) as well as releasing microvesicles (Huo et al., 2021). Hence, it is reasonable and inevitable to adopt the methodology of “regulating the brain and heart simultaneously,” and the flexible application of this principle can help improve the curative effect of CCVDs. At present, 84 chemical components have been isolated from ST, mainly including terpenoids, aromatic organic acids, and their derivatives, which have various pharmacological activities (e.g., anticoagulant, anti-inflammatory and antibacterial).
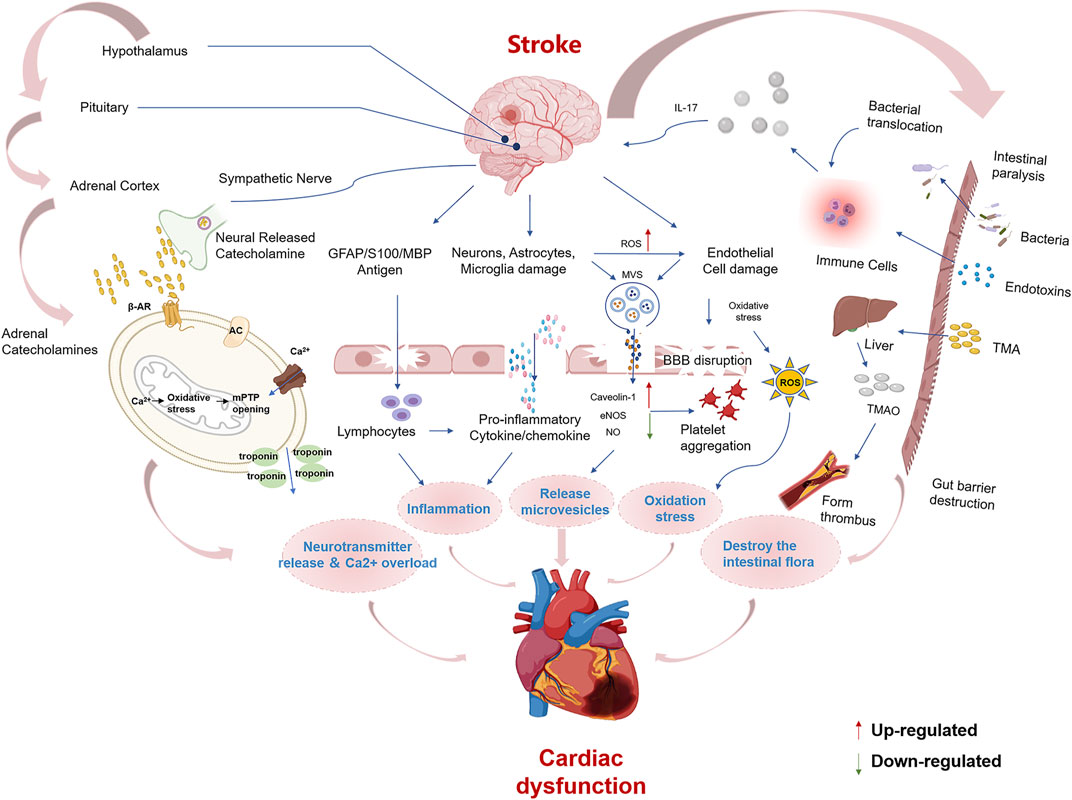
FIGURE 1. Summary of mechanisms for brain-heart association after stroke Cardiac dysfunction after stroke may be caused by the following mechanisms, including ① Activation of the hypothalamic-pituitary-adrenal axis and sympathetic modulation cause a surge of catecholamines, which act on β-receptors. β-receptor coupling stimulates G proteins to activate adenylate cyclase (AC), causing mitochondrial Ca2+ overload and failure of ATP synthesis leading to cardiomyocyte death. ② Brain cells that die after stroke release damaging antigens that can enter the body circulation through the ruptured blood-brain barrier, and the antigen-specific autoimmune response leads to the secretion of pro-inflammatory cytokines. Damaged neurons, microglia, endothelial and astrocytes, and the spleen can stimulate the release of pro-inflammatory cytokines and chemokines. ③ Microvesicles (MVs) released from damaged astrocytes, neurons and microglia after stroke inhibit eNOS function and increase Caveolin-1 while decreasing NO synthesis, leading to endothelial dysfunction and platelet aggregation. ④ Elevated reactive oxygen species and endothelial cell damage lead to oxidative stress. ⑤ Stroke increases the permeability of the intestinal barrier, causing dysbiosis of the intestinal flora and translocation of bacteria and endotoxin into the bloodstream. TMAO is a hepatic oxidation product of the microbial metabolite TMA. TMAO induces thrombosis and atherosclerosis formation.
Until recently, researchers at home and abroad have achieved great findings on CCVDs. However, there has been no published systematic and comprehensive review that focuses on various important aspects of ST in the treatment of CCVDs. In this paper, with “Storax,” “Styrax” and “Su-Hexiang” as keywords, the medicinal parts, traditional usages, phytochemistry, and pharmacological activities of ST in the past 10 years are summarized by searching the relevant documents in Wan Fang, China National Knowledge Infrastructure, Web of Science, PubMed and other databases. The limitations of current works are also debated, coupled with prospects on the future research directions of ST, which are paramount for deeper exploration, development and utilization of ST, and will offer a new outlook for the clinical diagnosis and treatment of CCVDs, besides supplying the basis for the application of the theory of “simultaneous regulation of the brain and heart.”
2 Medicinal Parts of ST
Liquidambar orientalis Mill. is a tree whose branches and leaves are shown in Figure 2A. The plant is native to southern Asia Minor, in particular, Turkey and northern Syria. Nowadays there are several introductions in Guangxi Province, China. The bark is usually struck or broken in early summer to reach the wood so that the resin is secreted and seeped into the bark. In autumn, the injured bark is peeled off and used to extract the resin. The remaining residue is boiled in water and pressed again, then filtering out the impurities and extracting the balsam as ordinary ST. The balsam is dissolved in ethanol, filtered and steamed out of the ethanol. Then it becomes refined balsam, also known as ST oil or flowing ST, characterized as semi-fluid, brown or dark brown, translucent, viscous, aromatic, shown in Figure 2B (Editorial Board of the State Administration of TCM, 1998).
FIGURE 2. Branches of storax (A); storax oil (B) (https://image.baidu.com/).
3 Traditional Usages
ST is widely used in both Asia and Africa, not only as the main element in the formulation of numerous Chinese medicine prescriptions but also as a prevalent spice in the manufacture of food and wine (Zhang et al., 2020). In Turkey, in addition to using ST to preparing medicines, it is also applied in the production of perfume, which is one of Turkey’s export commodities (Kültür, 2007). In Turkish folk medicine, ST is also utilized to treat varied ailments, for instance, cleft lips, burns, peptic ulcers, nocturnal enuresis, parasitic infections, and as an antiseptic or phlegmatic agent (Gurbuz et al., 2013). For the treatment of peptic ulcers, the mixture of ST and honey (1:5 ratio) is favored by the population (Honda et al., 1996). It was also used in ancient Egypt for mummification.
The usage of ST in medicine has been recorded since the Southern and Northern Dynasties in China. “Long-term intake is good for the spirit and makes the body lighter and healthier so that people live longer” was recorded in “Mingyi Bielu” compiled by Hongjing Tao (456–536 CE) during the Wei and Jin Dynasties, describing the medicinal value of ST for the first time. “Compendium of Materia Medica” authored by Shizhen Li (1518-1593 CE), stated that “ST, the aroma of which is able to move and flow though all the orifices and organs, so it can ward off all pathogenic qi.” According to another Chinese ancient classical book “Bencao Biandu” in the Qing Dynasty, the fragrance of ST is stronger than all kinds of incense, warm in nature and non-toxic, as soon as attributing to heart and spleen meridian, can be inducing resuscitation and expelling the pathogens as well. As a result, all pathological conditions with the inner invasion of pathogens, let’s say stroke, phlegm stroke, and qi stroke can use it to treat. Currently, ST is considered to have aromatic resuscitation, warming the heart, dispersing cold and relieving pain effects. It is frequently used for stroke, heatstroke, phlegm coma, heart and stomach pains, suggesting a therapeutic effect on chest paralysis and cerebral stroke. Presently, the fat-soluble components of ST oil are also extracted to obtain a water-insoluble perfume, which is incorporated into lavender recipes to provide a lingering effect, similar to “fragrance fixative.”
In traditional Chinese medicine applications, ST is often combined with musk and borneol, such as ST Pill from “Taiping Huimin Heji Ju Fang” in Song Dynasty. It is a common formula for the clinical treatment of angina pectoris in coronary heart disease and cerebral ischemia. Modern scientists have simplified and created new preparations based on this formula, such as Guanxin Suhe Pill (Capsule) and Su Bing Dripping Pill, which are commonly used for coronary heart disease and angina pectoris. Fangfei Zhang used ST Pill to treat abdominal pain, hypochondriac pain, and pinnacle headaches that were caused by qi stagnation, blood stasis, and cold clotting, with remarkable grades (Zhao et al., 2006). ST mixed with olive oil and applied externally to treat scabies (Editorial Board of the State Administration of TCM, 1998). Topical application of ST is effective in the reduction of eczema and itchiness by relieving inflammation and promoting the healing of ulcers, suggesting its efficacy in the treatment of skin conditions (Editorial Board of the State Administration of TCM, 1998). Simplified and improved from ST Pill, Shexiang Baoxin Pill and Guanxin Suhe Pill are commonly used in clinical practice for the treatment of CCVDs. Guanxin Suhe Capsule has the effect of regulating qi, broadening the chest and reducing soreness, mainly treating chest congestion and breath-holding. It is commonly used clinically for the prevention and treatment of angina pectoris with precise efficacy, increasing coronary flow and improving microcirculation, also lowering blood lipids and raising high-density lipoprotein, and has no adverse effects on liver and kidney. Wei Wan et al. compared the efficacy of Guanxin Suhe Pill with that of Suxiao Jiuxin Pill and found that Guanxin Suhe Pill was slightly more effective than Suxiao Jiuxin Pill, and there was no significant difference in the onset of action between the two, but the duration of Guanxin Suhe Pill was significantly longer than that of Suxiao Jiuxin Pill (Wan and Hang, 2005). Ma et al. used ST Pill in combination with conventional western medical measures to treat acute heavy craniocerebral injury identified as qi blockage of the brain, and the efficacy was significantly better than conventional western medical treatment, effectively reducing the disability rate and the incidence of complications (Ma and Xie, 2008).
4 Phytochemical Constituents
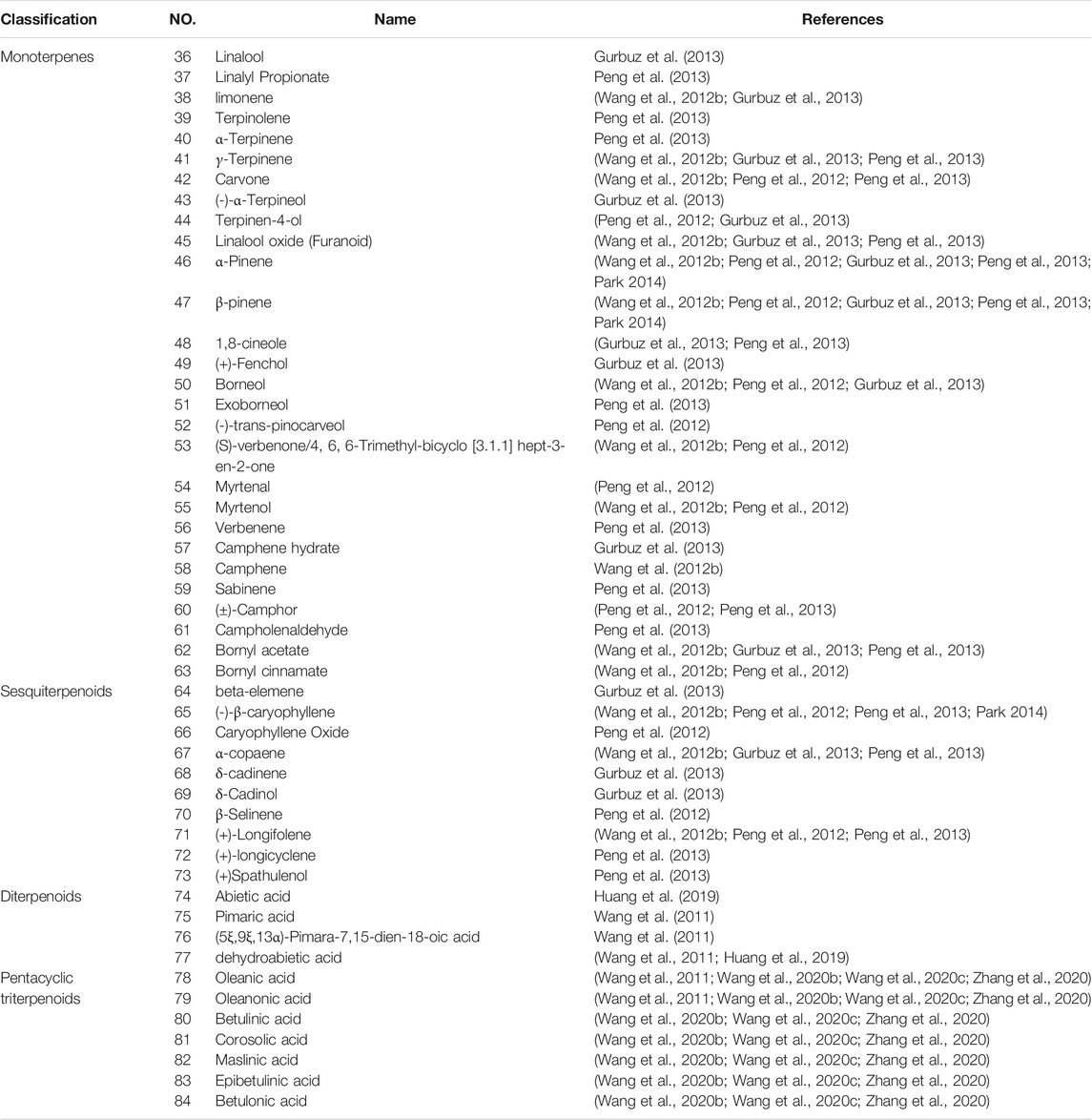
Despite its long history of use as a botanical, the systematic study of the chemical composition of ST has started relatively late. ST consists mainly of resin (about 36%), water (about 14–21%), and oily liquid. To date, 84 compounds have been isolated and identified from ST, and the composition obtained by different extraction methods is not entirely consistent. Among them, aromatic compounds and terpenoids are the most important active components of ST, which are considered to be important for future evaluation of medicinal efficacy.
4.1 Aromatic Compounds
At present, 35 aromatic compounds (1–35) have been isolated from ST as shown in Table 1, with physiological activities such as anti-myocardial ischemia, anti-cerebral ischemia and anti-thrombotic. These include nine aromatic hydrocarbon compounds (1–9) as shown in Figure 3A, and a total of 26 aromatic organic acids and their derivatives (10–35) have been identified, which have benzoic acid (Figure 3B) and cinnamic acid (Figure 3C) skeletons as their parent structures, respectively. Among them, cinnamic acid is an index component for the determination of the content of ST in the 2020 edition of the Chinese Pharmacopoeia and may also be one of the main components for its pharmacological action (State Pharmacopoeia Committee, 2020). Cinnamic acid has been shown to have a cardioprotective effect on isoproterenol-induced acute myocardial ischemia models (Song et al., 2013). Furthermore, cinnamic acid activates PPARα to stimulate lysosomal biogenesis to reduce amyloid plaque pathology in mice models of alzheimer’s disease, thus demonstrating its cerebral protective effect (Chandra et al., 2019).

FIGURE 3. Chemical structures of aromatic compounds from storax. Aromatic hydrocarbons (A); aromatic acids and their derivatives with the skeleton of benzoic acid (B) or cinnamic acid (C) as the core structure.
4.2 Terpenoids
ST is rich in terpenoids (Table 2), which have pharmacological effects such as anti-inflammatory, hypolipidemic and hypotensive, and are classified by structure into monoterpenes (36–63) (Figure 4A), sesquiterpenes (64–73) (Figure 4B), diterpenes (74–77) (Figure 4C) and pentacyclic triterpenes (78–84) (Figure 4D). These seven pentacyclic triterpenoids including oleanolic acid (78), oleanolone acid (79), betulinic acid (80), corosolic acid (81), maslinic acid (82), epibetulinic acid (83) and betulonic acid (84) are not only widely used as food additives, but also efficiently inhibit Human carboxylesterase 1A1 (hCES1A) and Pancreatic lipase (PL) activities (Wang et al., 2020b; Wang et al., 2020c; Zhang et al., 2020). Thereby ST could be a potential drug for the treatment of obesity and obesity-related metabolic disorders such as stoke and coronary heart disease, which is a hot research topic at the moment. Furthermore, oleanolic acid has been shown to have neuroprotective effects in rats with subarachnoid hemorrhage via SIRT1-mediated deacetylation of HMGB1 (Han et al., 2021). At the same time, oleanolic acid pretreatment also reduce ischemia-reperfusion injury in mice, reflecting its cerebroprotective effect (Shi et al., 2021). Betulinic acid has also been shown to reduce brain injury not only by reducing oxidative stress but also by activating the SIRT1/FoxO1 pathway to inhibit autophagy and ameliorate brain injury in middle cerebral artery occlusion (MCAO) rats (Zhao et al., 2021). Corosolic acid plays a cardioprotective role against dox-induced cardiotoxicity by restoring autophagic flux and improving mitochondrial function (Che et al., 2021). Maslinic acid ameliorates isoprenaline-induced myocardial necrosis by inhibiting the free radical generating enzyme XO (Shaik et al., 2021). All these findings suggest that terpenoids may exert protective effects in the heart and brain and may be the main medicinal substances of ST.
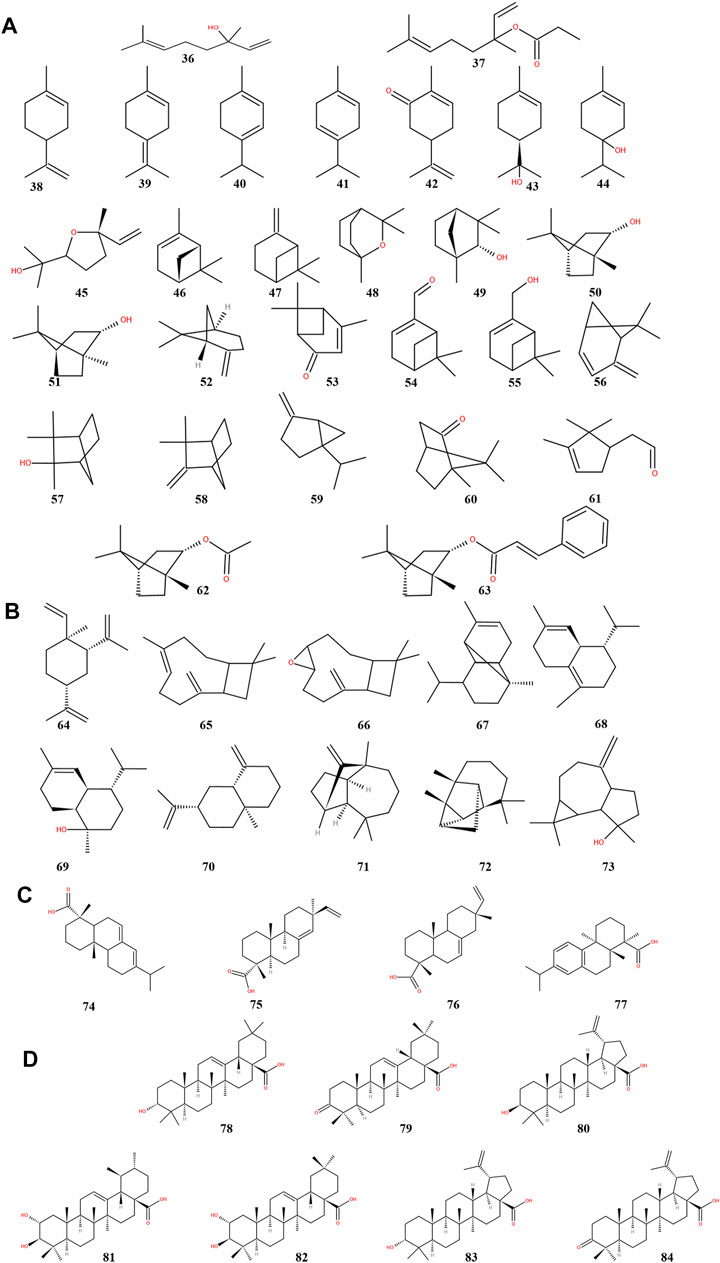
FIGURE 4. Chemical structures of terpenoids from storax. Monoterpenoids (A), sesquiterpenoids (B), diterpenoids (C) and pentacyclic triterpenoids (D).
5 Pharmacological Activities
As ST is mainly used clinically for the treatment of CCVDs, there is a wealth of research data on its pharmacological action in recent years, which is also the main focus of this review. In accordance with the general requirements of phytopharmacology research (Heinrich et al., 2020), we observed type of extract used, the model used, the dose range tested, duration, the minimal active dose, if and what controls were used as shown specifically in Tables 3, 4. In addition, it also has assorted pharmacological effects, e.g., antibacterial (Wang et al., 2010; Wang et al., 2011), insecticidal (Imam, 2013), antidepressant (Min et al., 2018), inhibiting abnormal excitation of uterine smooth muscle (Wang et al., 2014), anti-prostate cancer cells (Atmaca et al., 2020), promoting wound healing (Ocsel et al., 2012) and hepatoprotective (Suzek et al., 2016), which will not be discussed in this article.
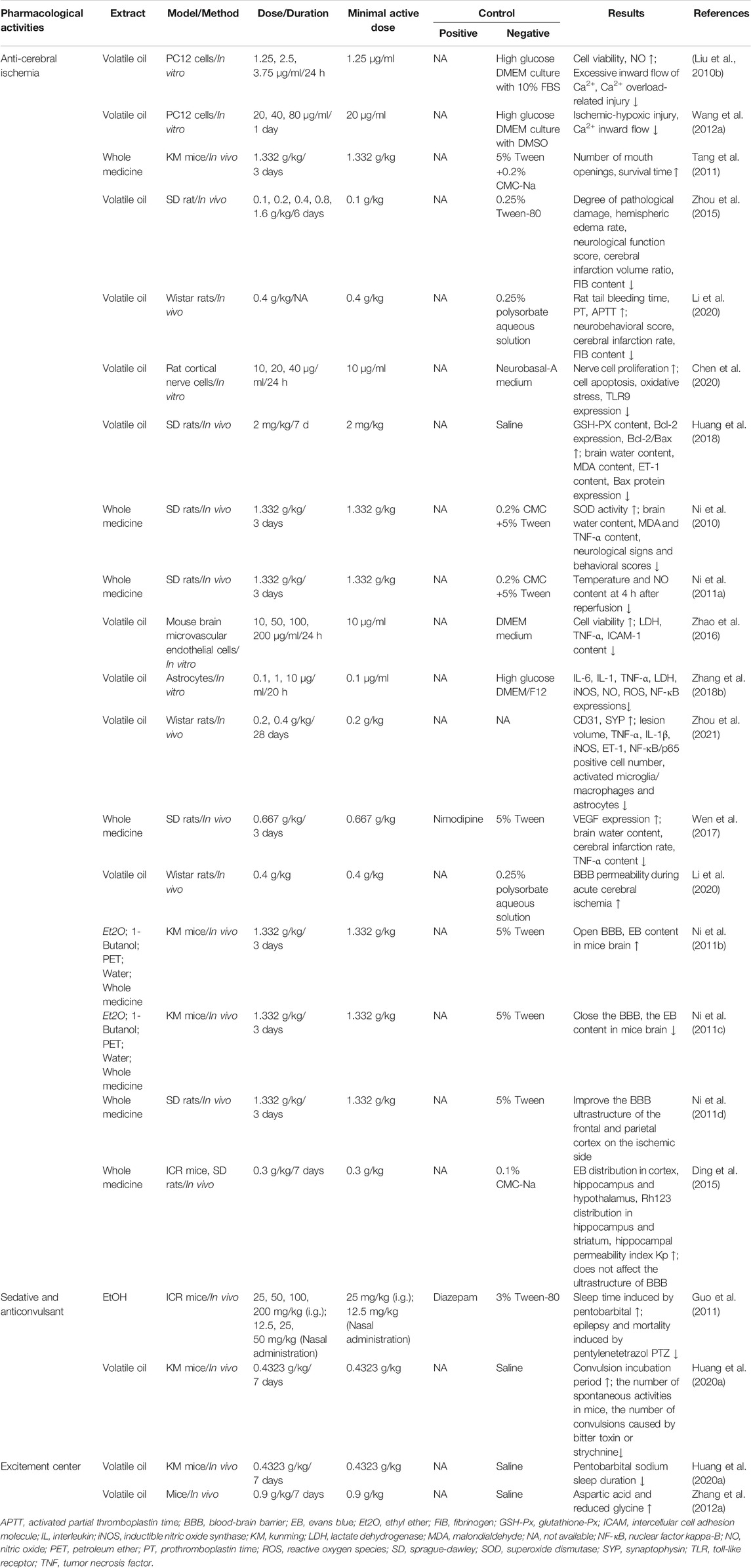
TABLE 3. Pharmacological effects of storax on cerebrovascular diseases (“↓,” decrease; “↑,” increase).
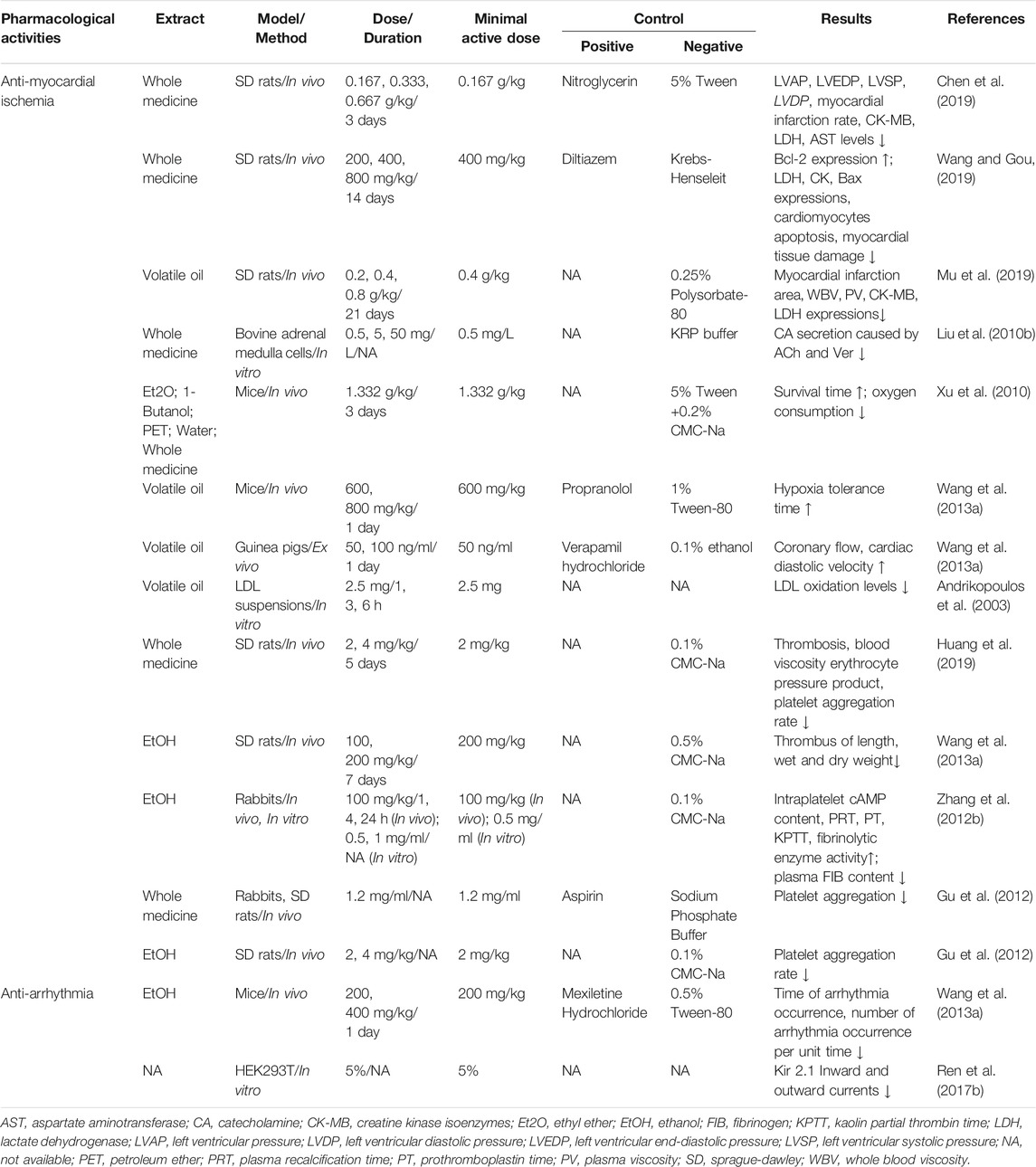
TABLE 4. Pharmacological effects of storax on cardiovascular diseases (”↓,” decrease; “↑,” increase).
5.1 The Effects on Cerebrovascular Diseases
ST has a variety of cerebral protective effects, for instance, anti-cerebral ischemia, anti-convulsion and central excitement, as demonstrated in Table 3.
5.1.1 Anti-cerebral Ischemia
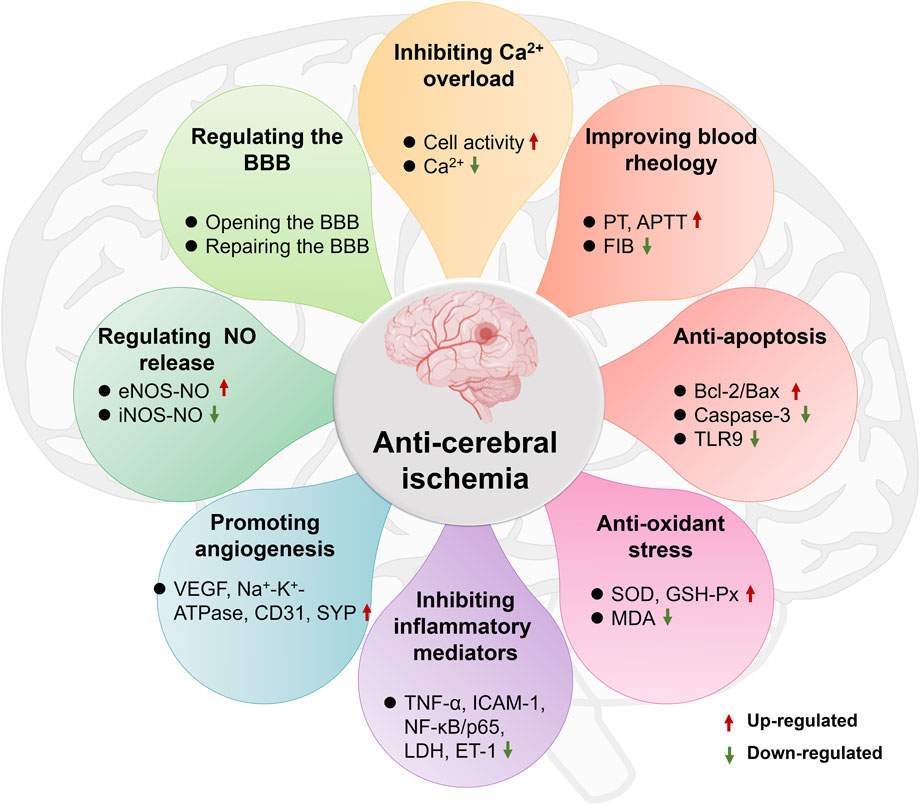
Ischemic brain injury is a complex pathophysiological process involving multiple factors such as NO neurotoxicity, inflammatory mediator stimulation and release, calcium overload and apoptosis. However, ST has shown the characteristics of multi-link and multi-pathway effects in the intervention of cerebral ischemic pathological damage. It was showed that ST significantly inhibited Na2S2O4-induced ischemic-hypoxic injury in PC12 cells as well as OGD/R-induced injury in cerebral microvascular endothelial cells, astrocytes and cortical neuronal cells, and enhanced cell viability (Liu et al., 2010a; Wang D. et al., 2012). It was also found that ST significantly increased the number of mouth openings in mice after decapitation and also prolonged the duration of mouth opening in mice injected with saturated magnesium chloride solution causing hypoxia (Tang et al., 2011), as well as reducing the degree of histopathological damage to the brain and the volume of cerebral infarction in MCAO rats. These results have indicated that ST has a noticeable improvement on ischemic brain tissue, and its primary mechanisms of action are shown in Figure 5, including inhibition of Ca2+ overload, improvement of blood rheology, anti-apoptosis of brain cells, anti-oxidative stress, inhibition of inflammatory mediators, promotion of angiogenesis, regulation of NO production and the blood-brain barrier (BBB). The main mechanisms as follows:
① Inhibiting Ca2+ overload
Under normal conditions, cells are in a dynamic equilibrium of calcium homeostasis, which is important for the maintenance of cellular physiological functions. Ca2+ is the most important “second messenger”, which plays a crucial role in the physiological activities of cells and the structural and functional integrity of neurons. Once the homeostasis of Ca2+ concentration in neurons is maladjusted, calcium overload occurs, which will lead to degeneration and death of nerve cells (He et al., 2020). While ST can reduce the excessive influx of Ca2+ in PC12 cells damaged by ischemia and hypoxia to improve cell viability (Liu et al., 2010a; Wang D. et al., 2012).
② Improving blood rheology
ST has been found to alter blood viscosity after cerebral ischemia. ST can significantly prolong plasma prothromboplastin time (PT), activated partial thromboplastin time (APTT), and reduce fibrinogen (FIB) content after cerebral ischemia. These explained that the anti-ischemic effect of ST may be related to improved cerebral blood flow by improving post-ischemic blood hypercoagulation (Zhou et al., 2015; Li et al., 2020).
③ Anti-apoptosis of brain cells
ST is able to inhibit the apoptosis of cortical nerve cells injured by CI/RI and reduce the expression of Caspase-3 protein significantly, whose regulatory mechanism is related to TLR9 (Chen, 2020). It may up-regulate the Bcl-2/Bax ratio in ischemic brain tissues of CI/RI rats as well (Huang et al., 2018). What’s more, it can ameliorate the damage of capillary endothelial cells, astrocytes and neurons in the frontal-parietal cortex of the ischemic hemisphere in rats to varying degrees (Ni et al., 2010; Ni et al., 2011a). These outcomes have evidenced that ST has an anti-apoptosis impact on brain cells.
④ Anti-oxidant stress
During cerebral ischemia, the imbalance between free radical generation and removal causes free radicals to accumulate, destroys the protein components in the membrane structure, causes membrane lipid peroxidation and leads to membrane damage, mitochondrial dysfunction, lysosome rupture, cell lysis, and tissue edema such a series of damage effects. These further promote the formation of oxidative stress, which in turn aggravates brain tissue damage (Liu et al., 2015). Malondialdehyde (MDA) is a breakdown product of lipid peroxidation and is one of the indicators reflecting the level of tissue oxidation. Superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px), which are the main antioxidant enzymes in the body, are free radical scavengers. Insufficient activity of SOD and GSH-Px means free radical scavenging ability decline. ST can increase the SOD activity of cortical nerve cells injured by CI/RI, reduce the level of MDA, and increase the level of GSH-Px in the serum of CI/RI rats (Huang et al., 2018; Chen, 2020). These results have testified that ST has an antioxidant effect.
⑤ Inhibiting the release of inflammatory mediators
After cerebral ischemia, the expression of a large number of inflammatory factors is upregulated, which promotes neutrophils to adhere to the vascular wall and enter the central nervous tissue. Subsequently, monocytes and macrophages infiltrate to destroy the endothelial cells and basement membrane of cerebral microvessels, further leading to vasogenic edema and bleeding. ST has been found to ameliorate OGD/R-induced damage to brain microvascular endothelial cells, astrocytes and cortical neuronal cells, increase cell viability, decrease LDH, TNF-α and ICAM-1 release, and reduce NF-κB expression (Zhao et al., 2016; Zhang M. et al., 2018). It was also found that ST significantly suppressed TNF-α and IL-1β expression in brain tissue and ET-1 levels in serum, reduced the number of NF-κB/p65-positive cells, and decreased the number of activated microglia/macrophages and astrocytes while reversing their morphological change 24 h after cerebral ischemia in rats (Zhou et al., 2021), and also significantly reduced hemispheric edema rates, neurological function scores and brain infarct volume ratios after cerebral ischemia in rats. These results have confirmed that ST may exert neuroprotective effects by inhibiting the NF-κB signaling pathway and thus reducing the expression of related inflammatory factors.
⑥ Promoting angiogenesis
Angiogenesis after cerebral ischemia is essential to promote the recovery of nerve function. VEGF is a multifunctional factor that acts on vascular endothelial cells and is a specific mitogenic source of endothelial cells. It can promote endothelial cell division, proliferation and metastasis. VEGF can induce the proliferation of endothelial cells by activating the expression of its receptors, thereby promoting the formation of new blood vessels and the establishment of collateral circulation, which is currently recognized as the most critical factor in angiogenesis (Wang H. et al., 2020). ST can increase VEGF content in serum and Na+-K+-ATPase activity in ischemic brain tissues of CI/RI rats (Wen et al., 2017). Long-term neurological studies have found that ST significantly alleviates neurological impairment starting 7 days after cerebral ischemia and continuing up to 28 days, with a significant reduction in lesion volume 28 days after stroke, as well as increasing the density of CD31 and SYP around the infarcted area (Zhou et al., 2021).
⑦ Regulating the production of NO
NO is believed to perform at least four actions in the central nervous system (CNS): 1) As a vasodilator, it plays an important role in controlling cerebral blood flow. 2) As a neuroprotective agent, it can reduce excessive calcium influx. 3) As a neurotransmitter, it can increase cAMP level by combining with guanylate cyclase and inactivate toxic oxygen-free radicals. But at high concentrations, it can directly damage DNA and interfere with the respiratory chain. 4) As a cytotoxin, it may contribute to neuronal cell death (Gao et al., 2020; Lv et al., 2020). Whereas NOS is a key determinant of the dual role of NO, different subtypes of NOS have different roles in cerebral ischemic injury. NO production by nNOS and iNOS is associated with nerve injury. nNOS is expressed in the early stages of ischemic neuronal injury, with activity rising 10 min after ischemia and peaking at 3 h, whereas iNOS is expressed in the late stages of ischemic neuronal injury, with activity rising 12 h after ischemia and peaking at 48 h (Liu et al., 2020). In contrast, NO produced by eNOS has a neuroprotective effect, dilating cerebral blood vessels and increasing cerebral blood flow in ischemic areas, with activity rising at 1 h and peaking at 24 h (Yagita et al., 2013). Studies have found that ST can not only increase the expression of eNOS and stimulate the production of NO to play a neuroprotective effect (Liu et al., 2010a), but also inhibit the production of iNOS and eliminate the neurotoxicity of NO to play a dual brain protection effect (Ni et al., 2011a; Zhang M. et al., 2018).
⑧ Regulating the BBB
The BBB is a protective structure that exists between blood and brain tissue. It is responsible for regulating the exchange of substances between blood and brain tissue and allowing the nutrients needed by brain tissue in blood circulation (Huang Y. et al., 2020). The passage of substances can also effectively prevent the intrusion of harmful substances, thereby maintaining the stability of the internal environment of the CNS (Jiang et al., 2018). In pathological conditions, let’s say ischemic stroke could make the BBB disrupted, with subsequent extravasation of blood components into the brain, impairing normal neuronal function. So how to enhance BBB repair is essential to promote long-term functional recovery after ischemic stroke. Under physiological conditions, ST significantly increased the level of Evans Blue (EB) in mice brain tissue and had an opening effect on the BBB in normal mice, which increased with time and was significantly different from the control group at 72 h, and the results of all studies are consistent (Ni et al., 2011b; Li et al., 2020).
In the state of cerebral ischemia, Caixia Ni et al. considered that ST tends to reduce the content of EB in brain tissue (Ni et al., 2011c), which seems to be able to repair BBB and prevent harmful substances from entering the brain parenchyma. In contrast, Dongna Li et al. believed that ST could increase the openness of the BBB in the acute phase of cerebral ischemia, that is, 2 h of ischemia. By 48 h, there was a significant increase in Evans Blue transmission compared to the model group (Li et al., 2020). Similar to the results of Li et al., Liu et al. observed that ST could significantly increase the concentration of combined sulpiride in the brain and blood of cerebral ischemia rats by using microdialysis technology (MD) combined with high-performance liquid chromatography (HPLC), indicating that ST could increase the permeability of BBB and promote the penetration of sulpiride through BBB (Liu et al., 2012). These two findings seem to be somewhat contradictory, but at the same time they are consistent with the characteristics of aromatic resuscitation Chinese medicines, which on the one hand can exert neuroprotective effects, and on the other hand can open the BBB so that the drug itself, as well as the concomitant drugs, can enter the brain parenchyma and exert neuroprotective effects more directly, while the exact time window when the BBB is opened still needs to be further examined.
The addition of benzaldehyde and vanillin, the active ingredients of ST, to caco-2 cells with high expression of P-glycoprotein (P-gp), was found to significantly promote the uptake of alkaline rhodopsin-123 (Rho-123) by caco-2 cells, suggesting that the inhibition of P-gp efflux function may be one of the mechanisms by which ST improves the permeability of the BBB (Yang et al., 2012). Further research found that ST significantly increased the distribution of EB in the cortex, hippocampus and hypothalamus of normal rats, as well as the distribution of Rho-123 in the hippocampus and striatum, and increased the permeability index Kp of the hippocampus, suggesting that ST could induce the opening of the BBB in the cortex, hippocampus and hypothalamus. It is speculated that the opening effect of ST on the hippocampus is related to the inhibition of P-gp function, while the increase of EB distribution in other brain regions may have nothing to do with P-gp function and may be related to other ATP-binding box proteins (such as Mrps and Bcrp) (Ding et al., 2015). In addition, ST has been found not to affect the ultrastructure of the BBB in normal rats (Ding et al., 2015), but to improve the cortical BBB ultrastructure in the frontoparietal region of the ischemic side of the hemisphere in CI/RI rats (Ni et al., 2011d). In summary, ST can not only open the BBB non-destructively, but also directly repair the BBB. Still, its time window and mechanism of action need to be further clarified.
5.1.2 Sedation and Anticonvulsant
Convulsion is a symptom of central nervous overexcitation caused by various causes. Pentatetrazole induces convulsion by blocking the activity of γ -aminobutyric acid (GABA) in the CNS. Studies have found that ST can significantly prolong the incubation period of pentatetrazole-induced convulsion and thus reduce the mortality rate of convulsion. These results suggest that ST may play an anticonvulsant effect through the GABA neuroregulatory pathway (Guo et al., 2011). It also has significant effects on convulsion induced by strychnine and picrotoxin (Huang et al., 2020a). It was also found that long-term inhalation of volatile oils of ST could prolong pentobarbital induced sleep, and nasal inhalation had a sedative and anticonvulsant effect faster than gavage, and significantly reduced the content of MDA and glutamate in brain tissue, suggesting that volatile oil of ST had a certain sedative and antioxidation effect. As the excitatory amino acids are released in the state of oxidative stress, it is believed that the anticonvulsant effect of ST is also related to its antioxidant effect, which provides a theoretical basis for its clinical use in the treatment of convulsions (Guo et al., 2011). Nasal administration itself can improve drug dosage because there are two ways for drugs to be absorbed into the brain through the nose: one is directly absorbed into the brain through the olfactory mucosa; the other is absorbed into the systemic circulation through the mucosa of the respiratory area, and then transported into the brain through BBB, which is effective due to rapid absorption (Zhao et al., 2012; Pardeshi and Belgamwar 2013; Long et al., 2020). Therefore, nasal administration is very important for the treatment of acute central system diseases, and ST naturally adjusts the advantages of BBB combined with the optimization of drug delivery mode is of great significance for the development of new and efficient central targeted drugs.
5.1.3 Excitatory Centers
Aromatic resuscitation drugs have a two-way regulating effect on the excitability of the CNS, which is both “tranquilizing the mind” and “inducing-resuscitation.” Studies have shown that oral administration of ST can increase the content of aspartic acid in the striatum of rats and decrease the content of glycine, which plays an excitatory role in neurotransmitters. Different from the previous results of prolonging the sleep duration of pentobarbital sodium, ST can also shorten the sleep duration of pentobarbital sodium, showing the effect of brain awakening and excitation (Zhang N. et al., 2012; Huang et al., 2020a). The reason for the two opposite results may be that prolonged inhalation of the volatile oil of ST is equivalent to an increased dose of the drug, which has a sedative effect similar to that of “gaseous anesthesia,” while oral administration has an “inducing-resuscitation” effect, which plays a role of excitement center. However, this hypothesis has not been confirmed in the same overall experiment, so it should be verified by means of multiple models and multiple indicators.
5.2 The Effects on Cardiovascular Diseases
ST mainly treats cardiovascular diseases in terms of anti-myocardial ischemia and anti-arrhythmia, as described in Table 4.
5.2.1 Anti-myocardial Ischemia
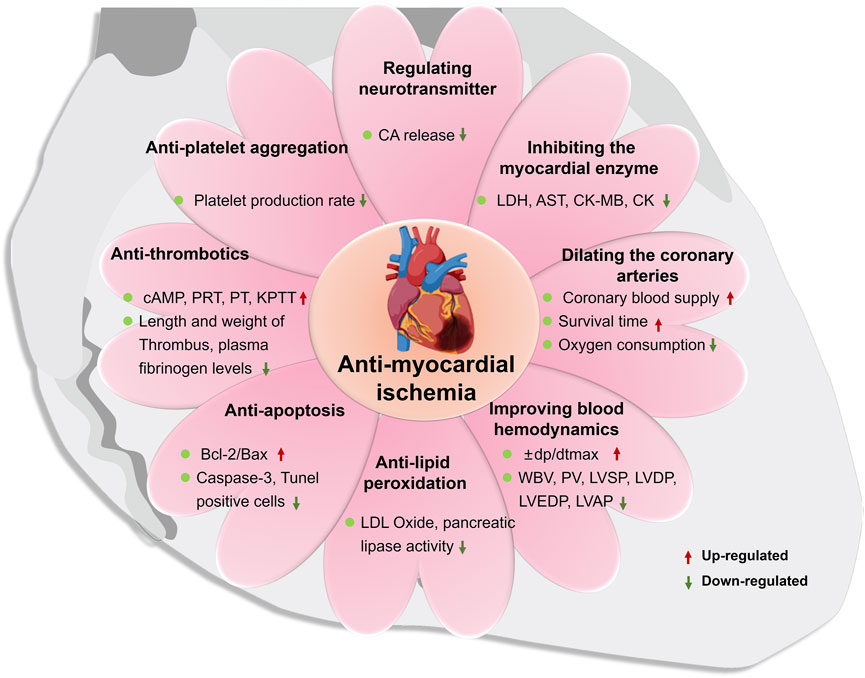
Myocardial ischemia is a pathological condition in which the blood supply and perfusion of the heart are reduced and the oxygen supply is reduced, which leads to abnormal energy metabolism and the heart cannot work normally. It is the main pathogenesis of a variety of cardiovascular diseases, such as myocardial infarction and atherosclerosis. A number of studies have confirmed that ST has an inhibitory effect on the myocardial infarction area and myocardial infarction rate in rats with myocardial ischemia. Pathological examination shows that it can improve myocardial cell necrosis, degeneration, bleeding and edema caused by ischemia, indicating that it has a protective effect on the ischemic myocardium (Chen et al., 2019; Mu et al., 2019; Wang and Gou, 2019). Due to the complex and diverse pathogenesis of myocardial ischemia, the main mechanisms of ST in anti-myocardial ischemia are shown in Figure 6, including regulation of neurotransmitters, regulation of myocardial enzyme spectrum, dilation coronary arteries, improvement of hemorheology and hemodynamics, anti-lipid quality peroxidation, anti-cardiomyocyte apoptosis, anti-thrombotics and anti-platelet aggregation. The main mechanisms as follows:
① Regulating the release of neurotransmitters
About 3/4 of ischemic heart diseases are caused by autonomic nerve dysfunction, which is typically characterized by a sharp decrease in vagal tone accompanied by an increase in sympathetic nerve activity (Zhang D. et al., 2018). Increased sympathetic excitability leads to increased catecholamine (CA) neurotransmitter secretion. CA is an important physiological substance that regulates cardiovascular activities. Under normal conditions, it acts on adrenergic receptors and dopamine receptors to produce contraction of blood vessels, enhancing myocardial contractility and improving the excitability of cardiomyocytes. It is an important neurohumoral hormone that maintains the physiological functions of the body, and its increase has a certain rhythm. However, in the pathological state, this rhythm is disrupted, which is manifested by the continuous or sudden increase of catecholamine, and the excessive increase of catecholamine has direct damage to the myocardium. The release of large amounts of catecholamines can cause an increase in myocardial oxygen consumption, resulting in coronary artery spasm, leading to myocardial ischemia and hypoxia, and even necrosis. However, ST can inhibit the secretion of CA caused by acetylcholine stimulation of bovine adrenal medulla cells in a dose-dependent manner. The mechanism is mainly related to the nAChR pathway on the cell membrane and the voltage-dependent sodium ion channel (Liu et al., 2010b), suggesting that ST can achieve the effect of “brain regulates the heart” by regulating the CNS in the treatment of cardiovascular diseases.
② Inhibiting the myocardial enzyme profile
Cardiac enzymes are enzymes within cardiac muscle cells that catalyze cardiomyocyte metabolism and regulate cardiac electrical activity in cardiac muscle cells. In clinical practice, serum glutamic-oxalacetic transaminase (AST), lactate dehydrogenase (LDH), creatine kinase (CK) and creatine kinase isoenzyme (CK-MB) are commonly used as reference indexes for myocardial ischemia and hypoxia injury. Once the cardiomyocytes become necrotic and ruptured, these enzymes will be released into the serum, causing the enzyme concentration to increase. Several studies have demonstrated that both pre-administration and combined prophylactic and therapeutic administration of ST inhibited the rise in serum cardiac enzymes induced by myocardial ischemia (Chen et al., 2019; Mu et al., 2019; Wang and Gou, 2019), suggesting that ST has a role in modulating the cardiac enzyme profile in rats with myocardial ischemia.
③ Dilating the coronary arteries
The imbalance between myocardial oxygen demand and supply can lead to myocardial ischemia and hypoxia, causing metabolites such as lactic acid to accumulate in the heart tissue, resulting in an attack of angina. Dilating the coronary arteries reduces myocardial oxygen consumption and improves coronary blood supply. Placing mice pre-administered with ST in a hypoxic environment prolongs survival time by reducing oxygen consumption (Xu et al., 2010; Wang Y. et al., 2013). It has also been shown that ST increases coronary blood flow as well as cardiac diastolic velocity in guinea pigs with ligated lateral left ventricular vessels (Wang Y. et al., 2013). The results of these studies all tentatively suggest that ST has a coronary vasodilating effect, but its mechanism still needs to be studied in depth.
④ Improving blood rheology and hemodynamics
Blood rheology and hemodynamics focus on the viscosity of blood and its movement through the circulatory system. High blood viscosity, poor fluidity, the risk of myocardial infarction is higher, on the contrary, low blood viscosity, good fluidity, the risk of myocardial infarction is small. It was found that ST significantly reduced whole blood viscosity (WBV) and plasma viscosity (PV) in rats with myocardial infarction. In addition, ST inhibited increases in left ventricular systolic pressure (LVSP), left ventricular diastolic pressure (LVDP), left ventricular end-diastolic pressure (LVEDP) and mean left ventricular intraventricular pressure (LVAP) in rats with myocardial ischemia (Chen et al., 2019), and also increased the reduction of ±dp/dtmax induced by ischemia, and acted earlier than diltiazem (Wang and Gou, 2019). The above research results indicate that ST can reduce blood viscosity and improve its fluidity, thereby improving left ventricular function.
⑤ Anti-lipid peroxidation
Low-density lipoprotein (LDL) is the main carrier of cholesterol for transport to peripheral tissue cells and is associated with the deposition of cholesterol into endothelial tissue, which can lead to the formation of atheromatous plaques, while the rupture of vulnerable atherosclerotic plaque is a major cause of myocardial infarction (Gustafsen et al., 2017). Moreover, LDL is highly susceptible to oxidation in an atherogenic environment. Oxidized low-density lipoprotein has been shown to enhance the formation of foam cells in atherosclerotic plaques, thereby increasing the risk of coronary heart disease, such a vicious circle (Stein et al., 2010). ST can protect low-density lipoprotein from oxidation and prevent coronary heart disease, and its active components may be pentacyclic triterpenoids (Andrikopoulos et al., 2003). Inhibition of pancreatic lipase activity is one of the most effective methods for the treatment of obesity and obesity-related metabolic disorders, such as atherosclerosis and hyperlipidemia. ST contains five pentacyclic triterpenoid acids, which have a strong inhibitory effect on pancreatic lipase, with IC50 values ranging from 0.49 to 6.35 μm. Among them, oleanonic acid and betulonic acid are the most effective two pancreatic lipase inhibitors, and they have a strong inhibitory effect on pancreatic lipase in a reversible and mixed manner, revealing the chemical composition of ST for the treatment of lipid metabolic diseases such as coronary heart disease (Wang et al., 2020b).
⑥ Anti-apoptosis of cardiomyocytes
Apoptosis, also known as programmed cell death, is an active and orderly cell death mode controlled by genes (Zhai et al., 2017). Myocardial apoptosis occurs during ischemia and hypoxia. Therefore, inhibition of myocardial apoptosis has become a research direction in the treatment of myocardial ischemia. The molecular mechanisms of apoptosis are complex. The Bcl-2 family, calpain and caspases are all involved in the initiation, occurrence, and development of apoptosis. Apoptosis-promoting protein Bax and apoptosis-inhibiting protein Bcl-2 restrict each other, and their ratio reflects the state of apoptosis. Studies have found that ST can significantly reduce the ratio of Bax to Bcl-2, suggesting that it can inhibit cardiomyocyte apoptosis. Further Tunel staining analysis revealed that ST significantly reduced the number of positive cardiomyocytes and its regulatory mechanism may be related to the PI3K/Akt signaling pathway, suggesting that it may improve myocardial ischemia and myocardial ischemia/reperfusion (MI/RI) injury in rats by inhibiting myocardial apoptosis (Chen et al., 2019; Wang and Gou, 2019).
⑦ Anti-thrombotics
The occurrence and development of thrombotic diseases are not only related to vascular wall lesions and blood flow disorders but also related to platelet function, coagulation function and fibrinolytic activity. In vitro experiments on rat thrombosis showed that ST could significantly inhibit thrombus length, wet weight and dry weight of thrombus (Wang Y. et al., 2013), and in vivo experiments showed that ST could inhibit thrombus formation, reduce blood viscosity and hematocrit in rats (Huang et al., 2019). In vivo and in vitro experiments in rabbits have also shown that ST can significantly increase the cAMP content in platelets, prolong plasma recalcification time (PRT), prothrombin time (PT) and kaolin partial thrombin time (KPTT), reduce plasma FIB content and plasmin activity (Zhang N. et al., 2012). These results all tentatively suggested that ST could alleviate blood coagulation and act as an antithrombotic agent. Neverthless, there are few relevant studies and animal models such as rabbits and rats are costly, difficult to manipulate and prone to thrombolysis. Future studies can use ferric chloride to induce thrombosis in zebrafish models, which is much more microdosing and efficient than other traditional animal thrombosis models and can screen antithrombotic active ingredients in high throughput.
⑧ Anti-platelet aggregation
Platelet plays an important role in thrombosis and inflammatory response, and its role runs through the whole process of cardiovascular diseases. Its activation and aggregation are related to unstable atherosclerotic lesions and are serious risk factors for patients with coronary heart disease (Moraes, et al., 2013). ST and its component cinnamic acid have obvious anti-aggregation effects on rabbit and rat platelets. In vitro experiments showed that ST and cinnamic acid used collagen as the inducer, the inhibition rates for rabbits were 33 and 52%, and the inhibition rates for rats were 24 and 42%. With ADP as an inducer, the inhibitory rates for rabbits were 32 and 72%, and for rats were 35 and 77%. The effect of cinnamic acid was equivalent to that of aspirin and ferulic acid (Gu et al., 2012). These results indicated that ST had an inhibitory effect on platelet aggregation. Unfortunately, only the cinnamic acid contained in it has been tested, and it is impossible to judge whether other ingredients have a more significant inhibitory effect, which should be discussed in the future.
Although the above studies reveal the pharmacological mechanism of ST on myocardial ischemia and hypoxia from lots of aspects, it is still necessary to supplement the longitudinal and in-depth molecular mechanism.
5.2.2 Anti-arrhythmia
Arrhythmia refers to the abnormality of cardiac rhythm, frequency, or activation sequence caused by cardiac pacing and conduction dysfunction, mainly manifested as tachycardia, bradycardia, arrhythmia, conduction block and arrest, which can lead to syncope, sudden death, and other critical situations (Bernardi et al., 2020). Modern antiarrhythmic drugs for arrhythmia are effective but have a large number of adverse effects, in addition to liver and kidney damage, and even some antiarrhythmic drugs have the same arrhythmogenic adverse effects (Wang X. et al., 2013). Traditional Chinese medicine has good clinical effects and safety in anti-arrhythmia. It can improve the quality of life of patients while improving clinical symptoms. Studies have shown that ST can significantly reduce the incidence of chloroform-induced arrhythmias in mice, with a tendency to enhance with increasing dose (Wang Y. et al., 2013). Ren et al. screened 25 kinds of traditional Chinese medicines that were thought to alleviate arrhythmia by the TI+flux screening method. It was found that ST could block the inward and outward currents of Kir2.1, and it was the first traditional Chinese medicine to block Kir2.1 and its mutants, which might become a targeted drug for type 3 short QT syndrome (sqt3). Further studies found that the effective component of its role was hydrocinnamic acid (Ren et al., 2017a; Ren et al., 2017b), but its mechanism still needs to be clarified.
6 Conclusion and Future Perspectives
As one of the most popular folk medicines, ST has a history of widespread use in Asia and Africa, with remarkable benefits in the prevention and treatment of cardiovascular and cerebrovascular diseases (e.g., angina pectoris, myocardial infarction, stroke and cerebral atherosclerosis). In conclusion, ST can play a brain-heart protective role through the common pharmacological mechanisms of anti-inflammation, anti-oxidative stress, anti-apoptosis and pro-angiogenesis, the specific regulation schematic is shown in Figure 7. However, the present domestic and international literature does not go far enough into the investigation of ST. Some reports are outdated, with single doses, lack of controls or in vitro studies only. The pharmacological research of herbal medicine should be carefully designed, with detailed records of dose, route and frequency of administration, appropriate models and accurate measurement methods. Neither in vitro nor in silico can replicate the complex environment of the organism, so it is not enough to accurately evaluate the effects of drugs. Therefore, we recommend the use of a combination of in vivo and in vitro studies, with multiple doses being investigated simultaneously for pharmacological studies, following the requirements of the consensus document (Heinrich et al., 2020). This review explores future research directions and priorities for ST as indicated below:
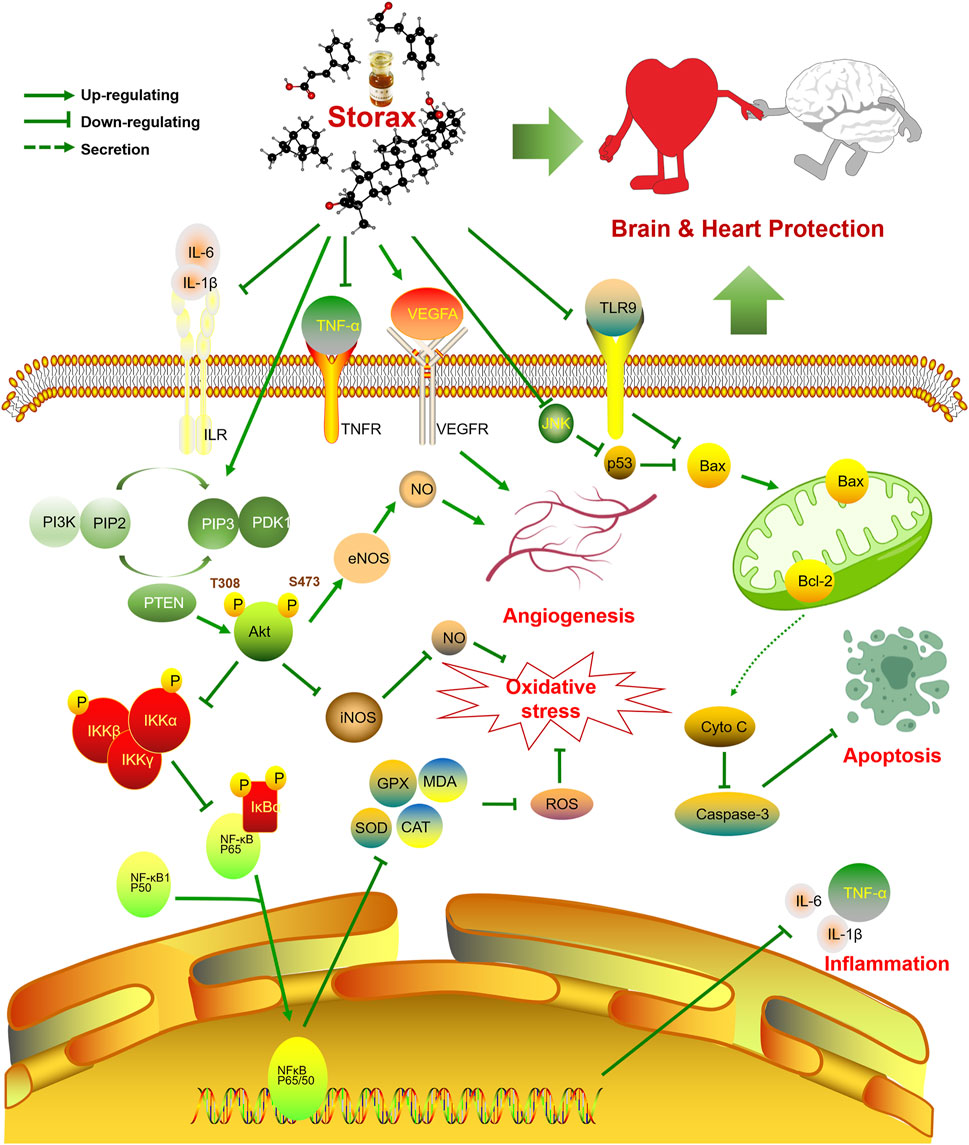
FIGURE 7. Schematic diagram of the regulatory mechanism of the brain-heart protective effect of storax. Storax can activate PI3K/Akt signaling pathway and inhibit NF-κB signaling pathway, which in turn can play a role in regulating NO production, anti-inflammatory, anti-oxidative stress and pro-angiogenic pharmacological effects, as well as anti-apoptosis by elevating Bcl-2 and inhibiting the expression of Caspase-3 and Bax, thus playing a co-protective role in the brain and heart.
① Digging deeper into the chemical composition of ST on the protection for the brain and heart. Although a large number of compounds have been isolated and identified from ST, only a few have been evaluated pharmacologically. Therefore, an in-depth study of the chemical composition of ST and its pharmacological action, especially the mechanism of action of its bioactive components, in order to illustrate the correlation between ethnomedical use and biological activity, will undoubtedly be the focus of further research. As the study of the chemical composition of its potency is still at the stage of crude extraction, its specific active ingredients have not yet been isolated and identified, making it difficult to establish the relationship between the two, thus affecting further research in all aspects. So that the current version of the Pharmacopoeia of the People’s Republic of China (2020 edition) has clear quality standards for ST, but it is still not possible to effectively differentiate between forgeries due to its weak specificity and low content limits. There has been a great deal of progress in scientific research on the identification and fingerprinting of indicator components (Liu et al., 2019; Sun et al., 2021). Yet further efforts are needed to incorporate these results into legal quality standards. To establish a more reasonable and effective evaluation system to improve the quality of ST, as well as to find specific ranges of administration to ensure safe and valid clinical medication, thus providing a reference basis for the development and utilization of ST resources.
② Building “brain-heart” disease models are the future development direction. The therapeutic effect of ST on CCVDs fully reflects the TCM principle of “treating the brain and the heart simultaneously”, but it still needs to be verified by constructing a model for both cardio-cerebral vascular diseases, so as to treat both and reduce the pathological damage of cardiac and cerebral tissues at the same time. In addition, the specific potency and strength of ST in compound formulas are unknown, and there are fewer studies on the formulation of effective components. Extensive cross-sectional studies have been conducted on the interventional effects of multiple pathological aspects after cardiovascular and cerebrovascular injury, but there are fewer in-depth longitudinal studies on individual pathological aspects, as well as few studies at the molecular biological level. Therefore, there is a necessity to further explore the biological activity and potential pharmacological mechanism through systems pharmacology approaches, combined with multi-omics tools to screen and predict the targets of active substances and to elucidate their specific pharmacological mechanisms.
③ The homeostatic mechanism of BBB regulation by ST remains to be studied in depth. The special function of the BBB makes the treatment of cerebrovascular diseases difficult because the majority of drugs are difficult to pass through the BBB, making it impossible for drugs to be delivered to the CNS and failing to accumulate in the brain to reach a sufficient dose to affect the efficacy. In recent years, how to promote the efficient opening of the BBB to improve the curative effect in CNS diseases has gradually become a hot research topic. On the one hand, ST can perform neuroprotective effects, and on the other hand, it can open the BBB so that the drug itself and the accompanying drugs can enter the brain parenchyma and directly perform neuroprotective effects. However, it is worrying whether any harmful matter may enter the brain parenchyma along with the drug and aggravate the neurological damage while the BBB is opened by ST. Further investigations into the mechanism and time window for BBB opening are awaited.
④The pharmacokinetic processes of the pharmacodynamic substances in ST need to be explored. So far, there have been relatively few reports on the pharmacokinetics of ST. This sparse report still used HPLC to establish a pharmacokinetic study using cinnamic acid as an indicator for determination. Owing to its multi-component, volatile nature, however, the determination of pharmacokinetic parameters of a single component may not be representative of the actual processes of other components in vivo. There are very few toxicological studies on ST. According to the literature (Zhang et al., 2020), ST inhibits human cytochrome P450 enzymes (CYPS), which is a double-edged sword. On the one hand, the combination of ST with some CYP-based drugs with a narrow therapeutic window (e.g. warfarin, digoxin, etc.) may trigger herb-drug interactions, which may inhibit drug metabolism and induce clinical adverse effects or complications. On the other hand, ST may prolong the duration of CYP-based drugs by blocking their metabolic clearance, which is beneficial for CYP-based drugs with relatively rapid clearance rates and thus improves therapeutic efficacy. The active ingredients for these effects are the pentacyclic triterpenes. In summary, the in vivo processes of ST need to be systematically understood and studied in depth. Studies on the blood and metabolic components of ST itself and in combination with other drugs will allow for better regulation of the indications and contraindications for clinical use.
In view of the above, the therapeutic effect of ST on CCVDs adequately reflects the TCM treatment principle of “treating both heart and brain”, which is in line with the treatment strategy of holistic medicine and has great potential for development and research value. In the future, further in-depth examinations should be planned on the basis of existed studies to avoid low-level repetition in order to obtain new advancements and breakthroughs.
Author Contributions
ZX obtained the literatures, designed the figures, and finalized the paper. DL, JY, and MR arranged the tables and checked the accuracy of the manuscript. RM, QX, YL, and JL discussed the manuscript layout. JW revised this article. All authors contributed to the article and approved the submitted version.
Funding
The present study was supported by the National Natural Science Foundation of China (grant nos. 81873023 and 81473371), the Innovation Team in Chengdu University of Traditional Chinese Medicine (grant no. CXTD2018004) and the Open Research Fund of the Key Laboratory of Southwestern Characteristic Chinese Medicine Resources, Chengdu University of Traditional Chinese Medicine (grant no. 2020XSGG025).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Andrikopoulos, N. K., Kaliora, A. C., Assimopoulou, A. N., and Papapeorgiou, V. P. (2003). Biological Activity of Some Naturally Occurring Resins, Gums and Pigments against In Vitro LDL Oxidation. Phytother Res. 17, 501–507. doi:10.1002/ptr.1185
Atmaca, H., Camli Pulat, C., and Cittan, M. (2020). Liquidambar Orientalis Mill. Gum Extract Induces Autophagy via PI3K/Akt/mTOR Signaling Pathway in Prostate Cancer Cells. Int. J. Environ. Health Res. 1-9, 1–9. doi:10.1080/09603123.2020.1818187
Benjamin, E. J., Muntner, P., Alonso, A., Bittencourt, M. S., Callaway, C. W., Carson, A. P., et al. (2019). Heart Disease and Stroke Statistics-2019 Update: a Report from the American Heart Association. Circulation 139, e56–e528. doi:10.1161/cir.0000000000000659
Bernardi, J., Aromolaran, K. A., and Aromolaran, A. S. (2020). Neurological Disorders and Risk of Arrhythmia. Ijms 22, 188–0067. doi:10.3390/ijms22010188
Chandra, S., Roy, A., Jana, M., and Pahan, K. (2019). Cinnamic Acid Activates PPARα to Stimulate Lysosomal Biogenesis and Lower Amyloid Plaque Pathology in an Alzheimer's Disease Mouse Model. Neurobiol. Dis. 124, 379–395. doi:10.1016/j.nbd.2018.12.007
Che, Y., Wang, Z., Yuan, Y., Zhou, H., Wu, H., Wang, S., et al. (2021). By Restoring Autophagic Flux and Improving Mitochondrial Function, Corosolic Acid Protects against Dox-Induced Cardiotoxicity. Cell Biol Toxicol. doi:10.1007/s10565-021-09619-8
Chen, N., Wang, J., Fan, Y., Ma, X., Guo, X., Dong, T., et al. (2019). Effects of Styrax on Myocardial Ischemia in Rats. Chin. Traditional Patent Med. 41, 533–538. CNKI:SUN:ZCYA.0.2019-03-008.
Chen, Y., Lin, G. C., and Bai, L. (2020). Effects of Suhexiang Volatile Oil on Neuronal Injury Induced by Cerebral Ischemia-Reperfusion. Chin. Traditional Patent Med. 42, 3298–3302. doi:10.3969/j.issn.1001-1528.2020.12.036
Chen, Z., Venkat, P., Seyfried, D., Chopp, M., Yan, T., and Chen, J. (2017). Brain-Heart Interaction: Cardiac Complications after Stroke. Circ. Res. 121, 451–468. doi:10.1161/circresaha.117.311170
Ding, J., Zhang, Y., Wu, Y., Xia, T., Li, B., and Yu, B. (2015). Study on Specific Opening Effects and Mechanism of Musk, Benzoin and Styrax on Blood-Brain Barrier Brain. Chin. J. Hosp. Pharm. 35, 279–282. doi:10.13286/j.cnki.chinhosppharmacyj.2015.04.01
Duru, M. E., Cakir, A., and Harmandar, M. (2002). Composition of the Volatile Oils Isolated from the Leaves ofLiquidambar Orientalis Mill. var.Orientalis andL. Orientalis var.Integriloba from Turkey. Flavour Fragr. J. 17, 95–98. doi:10.1002/ffj.1050
Editorial Board of the State Administration of TCM (1998). Chinese Materia Medica. ShangHai: Science and Technology Publishing House.
Gao, J. Q., Wang, P., Yan, J. W., Ba, L. N., Shi, P. L., Wu, H. M., et al. (2020). Shear Stress Rescued the Neuronal Impairment Induced by Global Cerebral Ischemia Reperfusion via Activating PECAM-1-eNOS-NO Pathway. Front Cel Dev Biol 8, 631286. doi:10.3389/fcell.2020.631286
Gu, N., Chen, Z., Zhao, Q., Qu, Y., and Huang, C. (2012). Study on Mechanism of Heart-protection Musk Pill on Ventricular Remodeling in Rats with Acute Myocardial Infarction. Liaoning J. Traditional Chin. Med. 39, 965–968. doi:10.13192/j.ljtcm.2012.06.12.gun.081
Guo, J., Duan, J. A., Tang, Y., and Li, Y. (2011). Sedative and Anticonvulsant Activities of Styrax after Oral and Intranasal Administration in Mice. Pharm. Biol. 49, 1034–1038. doi:10.3109/13880209.2011.561438
Gurbuz, I., Yesilada, E., Demirci, B., Sezik, E., Demirci, F., and Baser, K. H. (2013). Characterization of Volatiles and Anti-ulcerogenic Effect of Turkish Sweetgum Balsam (Styrax Liquidus). J. Ethnopharmacol 148, 332–336. doi:10.1016/j.jep.2013.03.071
Gustafsen, C., Olsen, D., Vilstrup, J., Lund, S., Reinhardt, A., Wellner, N., et al. (2017). Heparan Sulfate Proteoglycans Present PCSK9 to the LDL Receptor. Nat. Commun. 8, 503. doi:10.1038/s41467-017-00568-7
Han, Y., Tong, Z., Wang, C., Li, X., and Liang, G. (2021). Oleanolic Acid Exerts Neuroprotective Effects in Subarachnoid Hemorrhage Rats through SIRT1-Mediated HMGB1 Deacetylation. Eur. J. Pharmacol. 893, 173811. doi:10.1016/j.ejphar.2020.173811
He, J., Liu, J., Huang, Y., Zhuo, Y., Chen, W., Duan, D., et al. (2020). Olfactory Mucosa Mesenchymal Stem Cells Alleviate Cerebral Ischemia/Reperfusion Injury via Golgi Apparatus Secretory Pathway Ca2+ -ATPase Isoform1. Front. Cel Dev Biol 8, 586541. doi:10.3389/fcell.2020.586541
Heinrich, M., Appendino, G., Efferth, T., Fürst, R., Izzo, A. A., Kayser, O., et al. (2020). Best Practice in Research - Overcoming Common Challenges in Phytopharmacological Research. J. Ethnopharmacol. 246, 112230. doi:10.1016/j.jep.2019.112230
Honda, G., Yeşilada, E., Tabata, M., Sezik, E., Fujita, T., Takeda, Y., et al. (1996). Traditional Medicine in Turkey. VI. Folk Medicine in West Anatolia: Afyon, Kütahya, Denizli, Muğla, Aydin Provinces. J. Ethnopharmacol 53, 75–87. doi:10.1016/s0378-8741(96)01426-2
Huang, L., Zhu, C., Lin, L., Wang, J., and Li, L. (2020a). Effects of Different Resuscitation-Inducing Herbs on Excitation-Sedation Condition of Nervous System. Acta Chin. Med. 35, 1501–1504. doi:10.16368/j.issn.1674-8999.2020.07.335
Huang, L., Zhu, C., Zhang, Q., Fang, Y., and Li, L. (2018). Protective Effects of Resuscitation-Inducing Herbs on Acute Incomplete Cerebral Ischemia in Rats. Chin. J. Comp. Med. 28, 77–82. CNKI:SUN:ZGDX.0.2018-08-014.
Huang, X. J., Li, T. T., Xiao, C. X., Xu, L., Zhang, D. K., Wen, Y. S., et al. (2019). Quality Evaluation of Styrax Based on Quantitative Determination of 7 Chemical Components Using HPLC Wavelength Switching Method and Chemometrics. Zhongguo Zhong Yao Za Zhi 44, 3070–3077. doi:10.19540/j.cnki.cjcmm.20190423.201
Huang, Y., Chen, S., Luo, Y., and Han, Z. (2020b). Crosstalk between Inflammation and the BBB in Stroke. Curr. Neuropharmacol 18, 1227–1236. doi:10.2174/1570159x18666200620230321
Huo, S., Kränkel, N., Nave, A. H., Sperber, P. S., Rohmann, J. L., Piper, S. K., et al. (2021). Endothelial and Leukocyte-Derived Microvesicles and Cardiovascular Risk after Stroke: PROSCIS-B. Neurology 96, e937–e946. doi:10.1212/wnl.0000000000011223
Imam, H., Riaz, L., and Sofi, G. (2013). Mosquito Larvicidal Efficacy of Storax ( Liquidambar Orientalis ) against aedes Aegypti L. Larvae. J. Nat. Remedies 13, 104–108. doi:10.18311/jnr/2013/103
Jiang, X., Andjelkovic, A. V., Zhu, L., Yang, T., Bennett, M. V. L., Chen, J., et al. (2018). Blood-brain Barrier Dysfunction and Recovery after Ischemic Stroke. Prog. Neurobiol. 163-164, 144–171. doi:10.1016/j.pneurobio.2017.10.001
Kelly, P. J., Lemmens, R., and Tsivgoulis, G. (2021). Inflammation and Stroke Risk: a New Target for Prevention. Stroke 52, 2697–2706. doi:10.1161/strokeaha.121.034388
Kültür, S. (2007). Medicinal Plants Used in Kirklareli Province (Turkey). J. Ethnopharmacol 111, 341–364. doi:10.1016/j.jep.2006.11.035
Li, C., Zhang, F., and Huang, C. (2016). Clinical Trial of Xue Saitong Injection Combined with Su Hexiang Pill in the Treatment of Acute Cerebral Infarction. Chin. J. Clin. Pharmacol. 32, 1350–1352+1366. doi:10.13699/j.cnki.1001-6821.2016.15.002
Li, D., Ma, J., Li, L., Zhuang, P., Sun, L., Guo, H., et al. (2020). Experimental Study of Storax on Brain protection and Blood-Brain Barrier Permeability of Rats with Cerebral Ischemia Reperfusion Injury. Drug Eval. Res. 43, 1977–1982. CNKI:SUN:YWPJ.0.2020-10-009.
Liu, L., Huang, X., Wang, X., Zeng, Z., Kong, W., and Li, T. (2019). Simultaneous Determination of Four Components in Styrax by GC. West China Med. J. 34, 498–501. doi:10.13375/j.cnki.wcjps.2019.05.014
Liu, P., Yu, S. S., and He, X. R. (2012). Effect of Storax on Concentration of Sulpiride in Brain and Blood by MD-HPLC. Zhongguo Zhong Yao Za Zhi 37, 3307–3311. CNKI:SUN:ZGZY.0.2012-21-034.
Liu, P., Zhao, H., Wang, R., Wang, P., Tao, Z., Gao, L., et al. (2015). MicroRNA-424 Protects against Focal Cerebral Ischemia and Reperfusion Injury in Mice by Suppressing Oxidative Stress. Stroke 46, 513–519. doi:10.1161/strokeaha.114.007482
Liu, Y., Wang, J., Yao, H., Wang, D., Zeng, N., and Xia, H. (2010a). Effect of Resuscitation Medicine on PC12 Cells Damaged by Ischemia and Hypoxia and its Mechanism. Phamacology Clin. Chin. Materia Med. 26, 35–38. doi:10.13412/j.cnki.zyyl.2010.04.017
Liu, P., Yu, s., and He, X. (2010b). Progress on Styrax. Chin. J. Drug Appl. Monit. 07, 315–317. doi:10.3969/j.issn.1672-8157.2010.05.018
Liu, Y., Deng, S., Zhang, Z., Gu, Y., Xia, S., Bao, X., et al. (2020). 6-Gingerol Attenuates Microglia-Mediated Neuroinflammation and Ischemic Brain Injuries through Akt-mTOR-STAT3 Signaling Pathway. Eur. J. Pharmacol. 883, 173294. doi:10.1016/j.ejphar.2020.173294
Long, Y., Yang, Q., Xiang, Y., Zhang, Y., Wan, J., Liu, S., et al. (2020). Nose to Brain Drug Delivery - A Promising Strategy for Active Components from Herbal Medicine for Treating Cerebral Ischemia Reperfusion. Pharmacol. Res. 159, 104795. doi:10.1016/j.phrs.2020.104795
Lu, L., Sun, X., Chen, C., Qin, Y., and Guo, X. (2018). Shexiang Baoxin Pill, Derived from the Traditional Chinese Medicine, Provides Protective Roles against Cardiovascular Diseases. Front. Pharmacol. 9, 1161. doi:10.3389/fphar.2018.01161
Lv, M. H., Li, S., Jiang, Y. J., and Zhang, W. (2020). The Sphkl/SlP Pathway Regulates Angiogenesis via NOS/NO Synthesis Following Cerebral Ischemia-Reperfusion. CNS Neurosci. Ther. 26, 538–548. doi:10.1111/cns.13275
Ma, Z., and Xie, Y. (2008). Clinical Observation on Syndrome Differentiation Treatment of Acute Severe Craniocerebral Injury by Integrated Traditional Chinese and Western Medicine. J. Guangzhou Univ. Traditional Chin. Med., 503–506. doi:10.13359/j.cnki.gzxbtcm.2008.06.026
Min, L., Du, Y., Li, W., Xi, Y., and Shi, H. (2018). SuHeXiang Essential Oil Inhalation Produces Antidepressant- and Anxiolytic-like Effects in Adult Mice. Biol. Pharm. Bull. 41, 1040–1048. doi:10.1248/bpb.b18-00082
Moraes, L. A., Vaiyapuri, S., Sasikumar, P., Ali, M. S., Kriek, N., Sage, T., et al. (2013). Antithrombotic Actions of Statins Involve PECAM-1 Signaling. Blood 122 (18), 3188–3196. doi:10.1182/blood-2013-04-491845
Mu, F., Li, R., Zhang, H., Wang, F., Li, F., Zhao, M., et al. (2019). Metabonomics Study on the Effects of Traditional Chinese Medicine Storax for Treating Heart Blood Stasis Syndrome of Coronary Heart Disease in Rats. Chin. J. New Drugs 28, 620–627. CNKI:SUN:ZXYZ.0.2019-05-020.
Nam, H. S. (2019). Gut Microbiota and Ischemic Stroke: The Role of Trimethylamine N-Oxide. J. Stroke 21, 151–159. doi:10.5853/jos.2019.00472
Ni, C., Zeng, N., Xu, F., Gou, L., Liu, J., Wang, J., et al. (2011d). Effects of Aromatic Resuscitation Drugs on Blood Brain Barrier in Cerebral Ischemia-Reperfusion Injury Model Rats. Zhongguo Zhong Yao Za Zhi 36, 2562–2566. CNKI:SUN:ZGZY.0.2011-18-026.
Ni, C., Zeng, N., Gou, L., Liu, J., He, L., Huang, X., et al. (2011a). Protective Mechanism of Aromatic Resuscitation Drugs in Cerebral Ischemia-Reperfusion Injury Model Rats. Phamacology Clin. Chin. Materia Med. 27, 65–68. doi:10.13412/j.cnki.zyyl.2011.05.023
Ni, C., Zeng, N., Tang, Q., Gou, L., Xu, F., He, L., et al. (2010). The Protective Effect of Aromatic Resuscitation Drugs on Cerebral Ischemia-Reperfusion Injury in Rats and its Mechanism. Phamacology Clin. Chin. Materia Med. 26, 64–66. doi:10.13412/j.cnki.zyyl.2010.05.037
Ni, C., Zeng, N., Tang, Q., Tang, Y., Wang, J., and Xia, H. (2011b). Effect of Aromatic Resuscitation Drugs on the Permeability of Blood-Brain Barrier in normal Mice. Jiangsu J. Traditional Chin. Med. 43, 88–89. CNKI:SUN:JSZY.0.2011-02-061.
Ni, C., Zeng, N., Tang, Q., Xu, F., Gou, L., Wang, J., et al. (2011c). Effects of Aromatic Resuscitation Drugs on the Permeability of Blood-Brain Barrier in Mice with Cerebral Ischemia-Reperfusion Injury. Lishizhen Med. Materia Med. Res. 22, 2639–2640. CNKI:SUN:SZGY.0.2011-11-031.
Ocsel, H., Teke, Z., Sacar, M., Kabay, B., Duzcan, S. E., and Kara, I. G. (2012). Effects of oriental Sweet Gum Storax on Porcine Wound Healing. J. Invest. Surg. 25, 262–270. doi:10.3109/08941939.2011.639847
Pardeshi, C. V., and Belgamwar, V. S. (2013). Direct Nose to Brain Drug Delivery via Integrated Nerve Pathways Bypassing the Blood-Brain Barrier: an Excellent Platform for Brain Targeting. Expert Opin. Drug Deliv. 10, 957–972. doi:10.1517/17425247.2013.790887
Park, I. K. (2014). Fumigant Toxicity of Oriental Sweetgum (Liquidambar Orientalis) and Valerian (Valeriana Wallichii) Essential Oils and Their Components, Including Their Acetylcholinesterase Inhibitory Activity, against Japanese Termites (Reticulitermes Speratus). Molecules 19, 12547–12558. doi:10.3390/molecules190812547
Peng, Y., Xia, H., Wang, J., Zhou, Y., and Huang, L. (2012). Analysis of Active Fractions of Styrax and Benzoinum by GC-MS. Pharm. Clin. Chin. Materia Med. 3, 15–17+24.
Peng, Y., Xia, H., Zhou, Y., Wang, J., Wu, Q., Guo, S., et al. (2013). Comparative Analysis of Volatile Oil Components in Liquidambar Orientalis and Styrax Tonkinensis. China Pharm. 24, 241–243. doi:10.6039/j.issn.1001-0408.2013.03.17
Ren, S., Pang, C., Huang, Y., Xing, C., Zhan, Y., and An, H. (2017a). Hydrocinnamic Acid Inhibits the Currents of WT and SQT3 Syndrome-Related Mutants of Kir2.1 Channel. J. Membr. Biol. 250, 425–432. doi:10.1007/s00232-017-9964-z
Ren, S., Pang, C., Li, J., Huang, Y., Zhang, S., Zhan, Y., et al. (2017b). Styrax Blocks Inward and Outward Current of Kir2.1 Channel. Channels (Austin) 11, 46–54. doi:10.1080/19336950.2016.1207022
Shaik, A. H., Shaik, S. R., Shaik, A. S., Daoud, A., Salim, M., and Kodidhela, L. D. (2021). Analysis of Maslinic Acid and Gallic Acid Compounds as Xanthine Oxidase Inhibitors in Isoprenaline Administered Myocardial Necrotic Rats. Saudi J. Biol. Sci. 28 (4), 2575–2580. doi:10.1016/j.sjbs.2021.01.062
Shi, Y. J., Sun, L. L., Ji, X., Shi, R., Xu, F., and Gu, J. H. (2021). Neuroprotective Effects of Oleanolic Acid against Cerebral Ischemia-Reperfusion Injury in Mice. Exp. Neurol. 343, 113785. doi:10.1016/j.expneurol.2021.113785
Song, F., Li, H., Sun, J., and Wang, S. (2013). Protective Effects of Cinnamic Acid and Cinnamic Aldehyde on Isoproterenol-Induced Acute Myocardial Ischemia in Rats. J. Ethnopharmacol 150, 125–130. doi:10.1016/j.jep.2013.08.019
State Pharmacopoeia Committee (2020). Pharmacopoeia of the People's Republic of China. Part Ⅰ. Beijing: China Pharmaceutical Science and Technology Press.
Stein, S., Lohmann, C., Schäfer, N., Hofmann, J., Rohrer, L., Besler, C., et al. (2010). SIRT1 Decreases Lox-1-Mediated Foam Cell Formation in Atherogenesis. Eur. Heart J. 31, 2301–2309. doi:10.1093/eurheartj/ehq107
Sun, J., Zhu, Y. R., Hu, Q., Mao, X. H., and Ji, S. (2021). Quality Standard for Styrax. Chin. Traditional Patent Med. 43, 871–876. doi:10.3969/j.issn.1001-1528.2021.04.007
Suzek, H., Celik, I., Dogan, A., and Yildirim, S. (2016). Protective Effect and Antioxidant Role of Sweetgum (Liquidambar Orientalis) Oil against Carbon Tetrachloride-Induced Hepatotoxicity and Oxidative Stress in Rats. Pharm. Biol. 54, 451–457. doi:10.3109/13880209.2015.1045086
Tang, Y., Zeng, N., Ni, C., Wang, J., and Xia, H. (2011). Study on the Effect of Aromatic Resuscitation Drugs on Anti-cerebral Ischemia and Anoxia in Mice. Pharm. Clin. Chin. Materia Med. 2, 31–32+41. CNKI:SUN:LCZY.0.2011-01-013.
U.S. Pharmacopeial Convention (2017). United States Pharmacopoeia. Maryland: United States Pharmacopeial.
Vaseghi, M., Salavatian, S., Rajendran, P. S., Yagishita, D., Woodward, W. R., Hamon, D., et al. (2017). Parasympathetic Dysfunction and Antiarrhythmic Effect of Vagal Nerve Stimulation Following Myocardial Infarction. JCI Insight 2. doi:10.1172/jci.insight.86715
Veltkamp, R., Uhlmann, S., Marinescu, M., Sticht, C., Finke, D., Gretz, N., et al. (2019). Experimental Ischaemic Stroke Induces Transient Cardiac Atrophy and Dysfunction. J. Cachexia Sarcopenia Muscle 10, 54–62. doi:10.1002/jcsm.12335
Wan, W., and Hang, X. (2005). Comparison of Curative Effect between Guanxin Suhe Pill and Suxiao Jiuxin Pill. Med. Industry Inf. 61.
Wang, D., Wang, J., Peng, Y., Wang, Q., Ma, X., Tang, D., et al. (2012a). Effects of Volatile Constituents of Four Resuscitation Inducing Aromatic Herb on Oxygen Glucose Deprivation in PC12 Cells and Intracellular Ca2+. J. Xi'an Jiaotong Univ. (Medical Science) 33, 370–373. CNKI:SUN:XAYX.0.2012-03-027.
Wang, F., Cui, H., and Wang, S. (2011). Studies on Chemical Constituents of Storax. Chin. J. Exp. Traditional Med. Formulae 17, 89–91. doi:10.13422/j.cnki.syfjx.2011.08.058
Wang, H., Xu, X., Yin, Y., Yu, S., Ren, H., Xue, Q., et al. (2020a). Catalpol Protects Vascular Structure and Promotes Angiogenesis in Cerebral Ischemic Rats by Targeting HIF-1α/VEGF. Phytomedicine 78, 153300. doi:10.1016/j.phymed.2020.153300
Wang, J., Cheng, D., Zeng, N., Xia, H., Fu, Y., Yan, D., et al. (2011). Microcalorimetric Study of the Effect of Benzoinum and Styrax on the Growth of Escherichia coli. Nat. Prod. Res. 25, 457–463. doi:10.1080/14786419.2010.533670
Wang, J., Cheng, D., Zeng, N., Xia, H., Fu, Y., Yan, D., et al. (2010). Application of Microcalorimetry and Principal Component Analysis. J. Therm. Anal. Calorim. 102, 137–142. doi:10.1007/s10973-010-0929-z
Wang, L., Guan, X. Q., He, R. J., Qin, W. W., Xiong, Y., Zhang, F., et al. (2020c). Pentacyclic Triterpenoid Acids in Styrax as Potent and Highly Specific Inhibitors against Human Carboxylesterase 1A. Food Funct. 11, 8680–8693. doi:10.1039/d0fo01732a
Wang, L., Guan, X.-Q., He, R.-j., Huo, P.-C., Qin, W.-W., Cui, L.-T., et al. (2020b). Discovery and Characterization of Pentacyclic Triterpenoid Acids in Styrax as Potent and Reversible Pancreatic Lipase Inhibitors. J. Funct. Foods 74, 104159. doi:10.1016/j.jff.2020.104159
Wang, L., Wang, J., Zheng, X., Zhang, L., Xia, H., and Zeng, N. (2014). Reaserch on the Effect of Styrax on Uterus Smooth Muscle of Non-pregnant Rats In Vivo and In Vitro. Lishizhen Med. Materia Med. Res. (258), 1828–1830. CNKI:SUN:SZGY.0.2014-08-013.
Wang, S., Peng, Y., Xia, H., Zhou, Y., Wang, J., and Zeng, N. (2012b). GC-MS of Volatile Chemical Constituents of Liquidamobar Orientalis. China Pharm. 23, 1393–1394. doi:10.6039/j.issn.1001-0408.2012.15.19
Wang, X., Xie, X., Lu, H., and Shi, L. (2013b). Literature Analysis of the Effects of Antiarrhythmic Drugs on Arrhythmia. Zhongguo Yiyuan Yaoxue Zazhi 33, 1825–1826. doi:10.13286/j.cnki.chinhosppharmacyj.2013.21.029
Wang, Y., and Gou, D. (2019). Relationship between Dose and Effects of Suhexiang against Myocardial Ischemia of Model Rats. Acta Chin. Med. 34, 2157–2163. doi:10.16368/j.issn.1674-8999.2019.10.500
Wang, Y., Xu, Z., Wang, J., Zhou, Q., Zhang, X., and Liu, Y. (2013a). Summary of Storax. Pharm. Clin. Chin. Materia Med. 4, 49–52.
Wen, J., Wang, J., Chen, N., Xie, Q., and Ma, X. (2017). Study of Cerebral protection and Mechanism of Storax against Permanent Focal Cerebral Ischemia Injury in Rats. Lishizhen Med. Materia Med. Res. 28, 2305–2308. CNKI:SUN:SZGY.0.2017-10-001.
Xu, F., Zeng, N., Peng, X., Tang, Q., Ni, C., Wang, J., et al. (2010). Effect of Aromatic Resuscitation Medicine on Acute Hypoxic Injury in Mice. Pharmacol. Clin. Chin. Materia Med. 26, 72–74. doi:10.13412/j.cnki.zyyl.2010.05.040
Xu, X. (2014). Clinical Observation on the Treatment of 42 Cases of Cerebral Arteriosclerosis with Suhexiang Pills and Dihuang Yinzi. Hunan J. Traditional Chin. Med. 30, 56–57. doi:10.16808/j.cnki.issn1003-7705.2014.11.025
Yagita, Y., Kitagawa, K., Oyama, N., Yukami, T., Watanabe, A., Sasaki, T., et al. (2013). Functional Deterioration of Endothelial Nitric Oxide Synthase after Focal Cerebral Ischemia. J. Cereb. Blood Flow Metab. 33, 1532–1539. doi:10.1038/jcbfm.2013.112
Yang, Y., Wang, S., Fang, M., Yang, L., Meng, X., and Zheng, X. (2012). The Effect of Benzoin, Vanillin and β-asarone on the Function of P-Glycoprotein. Chin. Traditional Patent Med. 34, 1364–1366. CNKI:SUN:ZCYA.0.2012-07-040.
Zhai, M., Li, B., Duan, W., Jing, L., Zhang, B., Zhang, M., et al. (2017). Melatonin Ameliorates Myocardial Ischemia Reperfusion Injury through SIRT3-dependent Regulation of Oxidative Stress and Apoptosis. J. Pineal Res. 63 (2), e12419. doi:10.1111/jpi.12419
Zhang, B., Liu, X., Zhou, Y., and Hu, L. (2012b). Oral Pharmacokinetics of Different Dosage Forms of Storax in SD Rats. J. Shenyang Pharm. Univ. 29, 292–297. doi:10.14066/j.cnki.cn21-1349/r.2012.04.003
Zhang, D., Tu, H., Cao, L., Zheng, H., Muelleman, R. L., Wadman, M. C., et al. (2018a). Reduced N-type Ca2+ Channels in Atrioventricular Ganglion Neurons Are Involved in Ventricular Arrhythmogenesis. J. Am. Heart Assoc. 7. doi:10.1161/jaha.117.007457
Zhang, F., Huang, J., He, R. J., Wang, L., Huo, P. C., Guan, X. Q., et al. (2020). Herb-drug Interaction between Styrax and Warfarin: Molecular Basis and Mechanism. Phytomedicine 77, 153287. doi:10.1016/j.phymed.2020.153287
Zhang, M., Ma, Y., Chai, L., Mao, H., Zhang, J., and Fan, X. (2018b). Storax Protected Oxygen-Glucose Deprivation/Reoxygenation Induced Primary Astrocyte Injury by Inhibiting NF-Κb Activation In Vitro. Front. Pharmacol. 9, 1527. doi:10.3389/fphar.2018.01527
Zhang, N., Liu, P., and He, X. (2012a). Effect of Borneol, Moschus, Storax, and Acorus Tatarinowii on Expression Levels of Four Amino Acid Neurotransmitters in the Rat Corpus Striatum. Neural Regen. Res. 7, 440–444. doi:10.3969/j.issn.1673-5374.2012.06.006
Zhao, G., Dai, S., and Cheng, R. (2006). Dictionary of Traditional Chinese Medicine. ShangHai: Science and Technology Publishing House.
Zhao, J. Y., Lu, Y., Du, S. Y., Song, X., Bai, J., and Wang, Y. (2012). Comparative Pharmacokinetic Studies of Borneol in Mouse Plasma and Brain by Different Administrations. J. Zhejiang Univ. Sci. B 13, 990–996. doi:10.1631/jzus.B1200142
Zhao, P., Yue, S., Ma, Y., Zhu, J., Liang, J., Li, L., et al. (2016). Effects of Storax on the Injury of Brain Microvascular Endothelial Cells Induced by Oxygen - Glucose Deprivation/reoxygenation. Chin. J. Clin. Pharmacol. 32, 847–849. doi:10.13699/j.cnki.1001-6821.2016.09.025
Zhao, Q., Yan, T., Li, L., Chopp, M., Venkat, P., Qian, Y., et al. (2019). Immune Response Mediates Cardiac Dysfunction after Traumatic Brain Injury. J. Neurotrauma 36, 619–629. doi:10.1089/neu.2018.5766
Zhao, Y., Shi, X., Wang, J., Mang, J., and Xu, Z. (2021). Betulinic Acid Ameliorates Cerebral Injury in Middle Cerebral Artery Occlusion Rats through Regulating Autophagy. ACS Chem. Neurosci. 12 (15), 2829–2837. doi:10.1021/acschemneuro.1c00198
Zhou, M., Li, D., Li, L., Zhao, P., Yue, S., Li, X., et al. (2021). Post-stroke Treatment of Storax Improves Long-Term Outcomes of Stroke in Rats. J. Ethnopharmacol 280, 114467. doi:10.1016/j.jep.2021.114467
Zhao, Q., Yan, T., Li, L., Chopp, M., Venkat, P., Qian, Y., et al. (2019). Immune Response Mediates Cardiac Dysfunction after Traumatic Brain Injury. J. Neurotrauma. 36, 619–629. doi:10.1089/neu.2018.5766
Zhou, M., Zhao, P., Zhu, J., Wang, X., Zhang, M., and Zhang, B. (2015). Effects of Storax on Focal Cerebral Ischemia in Rats. Tianjin J. Traditional Chin. Med. 32, 496–500. CNKI:SUN:TJZY.0.2015-08-015.
Zhou, Z., Shen, W., Yu, L., Xu, C., and Wu, Q. (2016). A Chinese Patent Medicine, Shexiang Baoxin Pill, for Non-ST-elevation Acute Coronary Syndromes: A Systematic Review. J. Ethnopharmacol 194, 1130–1139. doi:10.1016/j.jep.2016.11.024
Keywords: storax, cardio-cerebrovascular diseases, chemical composition, pharmacological activities, cerebral and cardiac treatment synchronously
Citation: Xu Z, Lu D, Yuan J, Ren M, Ma R, Xie Q, Li Y, Li J and Wang J (2021) Storax, A Promising Botanical Medicine for Treating Cardio-Cerebrovascular Diseases: A Review. Front. Pharmacol. 12:785598. doi: 10.3389/fphar.2021.785598
Received: 29 September 2021; Accepted: 08 November 2021;
Published: 30 November 2021.
Edited by:
Lucian Hritcu, Alexandru Ioan Cuza University, RomaniaReviewed by:
Wang Ning, Anhui University of Chinese Medicine, ChinaMin Wu, China Academy of Chinese Medical Sciences, China
Copyright © 2021 Xu, Lu, Yuan, Ren, Ma, Xie, Li, Li and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Wang, lczyx712@163.com
 Zhuo Xu
Zhuo Xu Danni Lu
Danni Lu Rong Ma
Rong Ma Qian Xie
Qian Xie Jinxiu Li
Jinxiu Li Jian Wang
Jian Wang