- 1School of Sports Medicine and Health, Chegdu Sport University, Chengdu, China
- 2Guizhou University of Traditional Chinese Medicine, Guiyang, China
- 3Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 4Central of Reproductive Medicine, Department of Obstetrics and Gynecology, School of Medicine, Sichuan Academy of Medical Sciences and Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China, Chengdu, China
- 5Personalized Drug Therapy Key Laboratory of Sichuan Province, Sichuan Academy of Medical Science and Sichuan Provincial People’s Hospital, School of Medicine of University of Electronic Science and Technology of China, Chengdu, China
Pulmonary fibrosis is a fatal chronic progressive respiratory disease, characterized by continuous scarring of the lung parenchyma, leading to respiratory failure and death. The incidence of PF has increased over time. There are drugs, yet, there are some limitations. Hence, it is of importance to find new therapies and new drugs to replace the treatment of pulmonary fibrosis. In recent years, there have been a great number of research reports on the treatment of traditional Chinese medicine polysaccharides in various system fields. Among them, the treatment of PF has also gained extensive attention. This review summarized the source of polysaccharides, the drug activity of traditional Chinese medicine, and the protective effects on targets of Pulmonary fibrosis. We hope it can inspire researchers to design and develop polysaccharides, serving as a reference for potential clinical therapeutic drugs.
Introduction
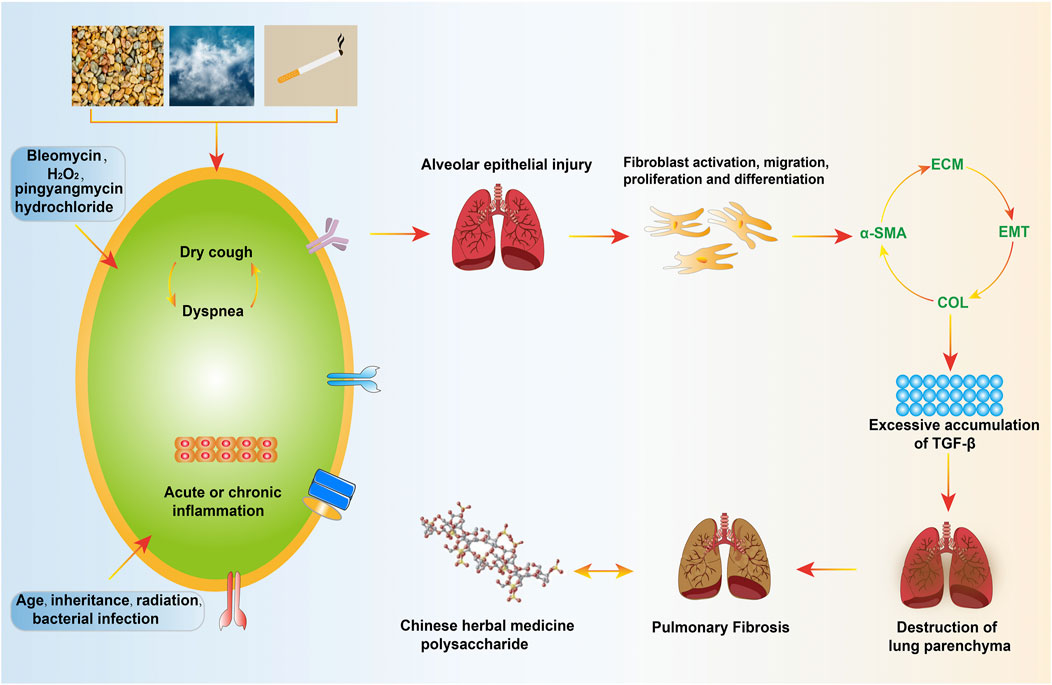
Pulmonary fibrosis (PF) is a large category of pulmonary diseases refers to the proliferation of fibroblasts and the accumulation of a large amount of extracellular matrix (ECM), accompanied by inflammatory damage, and tissue structure destruction, which can cause breathing difficulties, cough, hypoxemia, and hinder gas exchange, eventually leading to respiratory failure (Phan et al., 2021). PF’s pathogenesis has undergone fibroblast activation, migration, proliferation and differentiation into myofibroblasts, inducing ECM aggregation, destroying the pulmonary parenchyma, changing the expansion of the pulmonary and the diffusion of O2/CO2, resulting in respiratory abnormalities caused by insufficient gas exchange (limitation reduced pulmonary volume and diffusing capacity) (Ruigrok et al., 2021). The triggering factors for fibrosis include genetic susceptibility and other risk factors, such as persistent viruses, bacterial infections, cigarettes, drug damage, and other medical diseases. Hereditary factors account for the largest proportion among them, one that the cause is unknown and the most common type of the disease is called idiopathic PF. (Richeldi et al., 2017; Majewski and Piotrowski, 2020). Radiology of chest high-resolution CT showed that about 90% of patients had interstitial changes, the histology of pulmonary biopsy showed that scar tissue replaced the normal pulmonary parenchyma, which was also used as a standard for the diagnosis of PF (Solomon et al., 2013). The occurrence and development of PF and the decline of pulmonary function are closely related to the survival rate. According to reports, the incidence of PF is increasing year by year and is positively correlated with age, with males higher than females (Hutchinson et al., 2015). With ageing, patients will inevitably experience the gradual loss of physiological integrity, the decrease of steady-state controlling ability and the increase of vulnerability to death. If not taking corresponding treatment measures after diagnosis, most patients’ pulmonary function, if not all, will be progressively and irreversibly worsening, and their survival time is within 3–5 years (Lederer and Martinez, 2018) (Figure 1).
Currently, only two anti-fibrosis drugs, named pirfenidone and nintedanib, have been approved by the US Food and Drug Administration to treat PF, but, due to their side effects (headache, nausea, diarrhea, skin rash, and impaired liver function) and high cost, they cannot meet the medical needs (Galli et al., 2017). Lung transplantation can improve the survival rate, but the limited organ supply and the complexity of the surgery and medical treatment make it affordable for only a small number of patients (Ryu et al., 2014). Colchicine, prednisone, and cyclophosphamide were also used to reduce the incidence and mortality of PF, studies on PF reported (Miniati and Matucci Cerinic, 2007; Fiorucci et al., 2008). In the case of acute exacerbations, international guidelines recommend high-dose glucocorticoids, but there are no data to prove its safety and effectiveness (Song et al., 2011). It has also been suggested that the therapeutic effects of prednisolone, cyclophosphamide, interferon-1b, and N-acetylcysteine have no obvious advantages, but various adverse reactions and increase the economic burden of patients (Datta et al., 2011; Canestaro et al., 2016; Raimundo et al., 2016). Therefore, it is critical to developing new therapies and new drugs for PF. Many traditional Chinese medicines (TCM) have played a certain role in the treatment of various systems of diseases (Huang et al., 2018). Chinese herbal extracts also have many effective pharmacological ingredients to treat PF (Ji et al., 2016; Huo et al., 2020). In the past 30 years, the pharmacological activity of polysaccharides has attracted increasing attention (Layek and Mandal, 2020). Japanese scholar, Chihara Hiroro, firstly discovered that lentinan has an anti-tumor effect in 1968, thus striking the research boom of natural active polysaccharides (Chihara et al., 1969). Polysaccharides are natural macromolecular compounds composed of monosaccharides. They, with abundant biological activities and obviously low toxicity, are one of the main active components of Chinese medicine. Polysaccharides have received wide attention thanks to their effective biological activities and multiple molecular targets (Muhamad et al., 2019; Cao et al., 2020; Mohammed et al., 2021). Studies have shown that polysaccharides have a variety of biological activities, such as anti-tumor, anti-oxidation, anti-radiation, anti-virus, and hypoglycemic effects (Mu et al., 2021; Niu et al., 2021). The development and application of polysaccharides in Chinese herbal medicine have enriched the treatment methods of modern medicine. Nowadays, TCM polysaccharides have been widely used clinically to treat related diseases, such as astragalus polysaccharide injection, ganoderma polysaccharide injection, ginseng polysaccharide injection, etc. (Li et al., 2019b). As expected, TCM polysaccharides have protective effects on PF. For example, astragalus polysaccharides (APS) can reduce the degree of PF by inhibiting transforming growth factor-β1 (TGF-β1) in vitro and in vivo (Zhang, Xu, 2020). Ganoderma lucidum polysaccharides (GIP) inhibit bleomycin (BLM)-induced adult male SD rats by improving lung antioxidant capacity (Chen et al., 2016). Therefore, Chinese herbal polysaccharides provide a new way to discover and develop anti-PF.
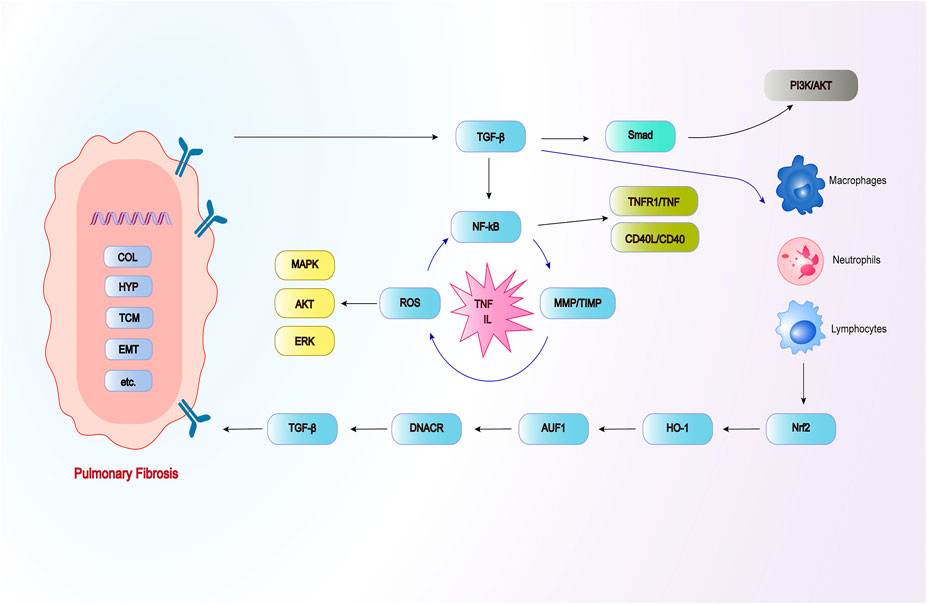
Repeated local micro-injuries play a fatal role in the aging alveolar epithelium, these micro-injuries start the connection of abnormal epithelial cell with fibroblast, induce myofibroblasts that produce matrix, and remodel a large amount of ECM accumulation and lung interstitial. There are many sources of myofibroblasts, including resident mesenchymal cell proliferatsion, pulmonary interstitial cells, circulating fibroblasts, epithelial-mesenchymal transition (EMT) and endothelial-mesenchymal transition (Todd et al., 2012). The histological characteristics of PF are excessive deposition of matrix collagen, increased fibrosis area and increased hydroxyproline (HYP) content, which can damage the normal structure and function of the lung. Activated alveolar epithelial cells secrete large amounts of fibrogenic growth factors and cytokines, including TGF-β1 and platelet-derived growth factors. TGF-β1 is an important regulator of fibrogenesis, which can activate fibroblasts and transform them into myofibroblasts (Organ et al., 2015), the initiation and regression of myofibroblasts herald disease progression. TGF-β1 not only promotes ECM deposition, but also concentrates it in the accumulated matrix, thereby accelerating it the pro-fibrotic reaction. The current pathogenesis of PF presumably includes abnormal accumulation of TGF-β, cell recruitment, apoptosis, inflammatory factors, oxidative stress, and the imbalance of matrix metalloproteinase/tissue inhibitor of metalloproteinase (MMP/TIMP) (Ryu et al., 2014; Spagnolo et al., 2021) (Figure 2). Therefore, the use of various activities of Chinese herbal polysaccharides brings hope to the treatment of PF through these targets. Chinese herbal polysaccharides can participate in reversing the down-regulation of TGF-β and protecting PF, and have broad prospects in the field of anti-PF research.
Polysaccharides
Ginseng Polysaccharide (GPS)
GPS is a polysaccharide isolated from ginseng roots, with an average molecular weight (Mw) of 1.5 × 106 Da, mainly composed of Glc and Gal (over 90%, w/w) and 5–8% Man and Ara (Chen et al., 2020; Ahn et al., 2011; Ahn et al., 2011; Chen et al., 2020). It makes a variety of immunomodulatory functions, such as anti-oxidation, anti-tumor, anti-cancer and anti-adhesion (Akhter et al., 2018; Xiong et al., 2019). The Smad pathway is essential for TGF-β mediated signal transduction (Wilkes et al., 2005). Studies have shown that GPS inhibits the phosphorylation of Smad2 and Smad3 in fibroblasts through TGF-β, but has no response to the levels of Smad6 and Smad7, weakens the phosphorylation level of extracellular signal-regulated kinase/protein kinase B (ERK/AKT) and reverses the synthesis of collagen (COL)-1 and fibronectin (FN) also significantly reduces the protein expression of TGF-β1 receptor (TβRI) and TβRII in NIH/3T3 cells, preventing TβRII from its known that the protein expression of receptor TβRIII decreases, and it also inhibits the expression of a-smooth muscle actin (a-SMA) in IMR-90 and WI-38 cells (Ahn et al., 2011). Later, studies reported that ginsenosides can reduce EMT of lung tissue by inhibiting TGF-β1/Smad pathway (Guan et al., 2017). There are further reports that ginsenosides can prevent renal fibrosis (Li et al, 2018b). Recent observers have shown that ginseng can prevent liver, lung, kidney and myocardial fibrosis through TGF-β (Liu et al., 2020a). Therefore, the possible mechanism of GPS anti-PF is that the downstream Smad2 and Smad3 signals of TGF-β1 and its TβRI and TβRII can achieve the treatment purpose, and reduce the phosphorylation of ERK and AKT, and reduce the level of MMPs, which is beneficial to lung tissue damage (Figure 3 and Table 1).
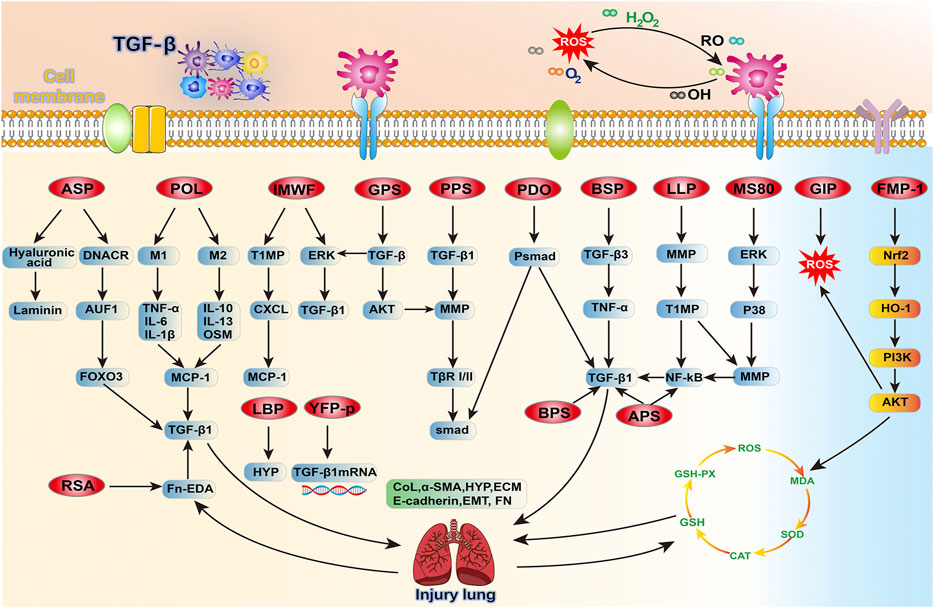
FIGURE 3. An overview of the target signaling pathways of 16 Chinese herbal polysaccharides that antagonize PF, mainly by reversing TGF-β and improving ROS to treat pulmonary fibrosis, and further linking the relationship with these related pathways.
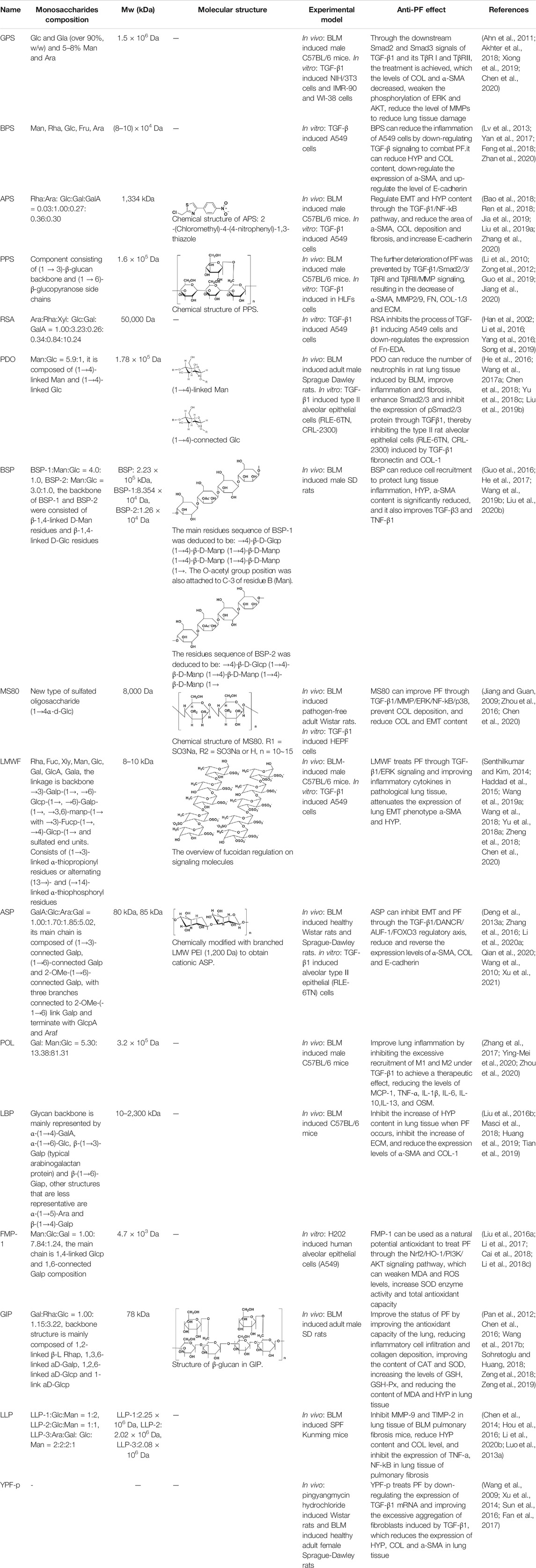
TABLE 1. Overview of the research progress of Chinese herbal medicine polysaccharides in the treatment of PF.
Basil Polysaccharide (BPS)
BPS is extracted from the Chinese herbal medicine basil, basil is a daily dish prepared in summer. BPS is a compound polysaccharide composed of fructose. It is mainly composed of Man, Rha, Glc, Fru, and Ara, the Mw is (8–10)×104 Da (Zhan et al., 2020). It has anti-tumor, anti-oxidation, anti-inflammatory, hypolipidemic, anti-diabetic and anti-liver cancer effects (Lv et al., 2013; Feng et al., 2018). Proves have shown that BPS has a potential inhibitory effect on the PF of human A549 cells induced by TGF-β, and down-regulates the expression of a-SMA and COL-1, up-regulates E-cadherin levels, and reduces HYP content (Yan et al., 2017), and proposed that BPS has an inhibitory effect on EMT (Feng et al., 2019). Therefore, BPS can reduce the inflammation of A549 cells by down-regulating TGF-β signaling to combat PF.
Astragalus polysaccharide (APS)
Astragalus as a medicinal and tonic food can keep fit and improve health. APS is a water-soluble polysaccharide isolated and purified from astragalus, the Mw is 1,334 kDa, composed of Rha, Ara, Glc, Gal, and GalA, the molar ratio is 0.03:1.00:0.27:0.36:0.30. The backbone of APS consisted of 1,2,4-linked Rhap, α-1, 4-linked Glcp, α-1, 4-linked GalAp6Me, β-1, 3, 6-linked Galp, with it branched at O-4 of the 1,2,4-linked Rhap and O-3 or O-4 of β-1, 3, 6-linked Galp. The side chains mainly consisted of α-T-Araf and α-1, 5-linked Araf with O-3 as branching points, having trace Glc and Gal, the terminal residues were T-linked Araf, T-linked Glcp, and T-linked Galp (Bao et al., 2018), its chemical structure is 2-(chloromethyl)-4-(4-nitrophenyl)-1,3-thiazole (Liu et al., 2019a). APS has been reported that have a variety of biological activities, including anti-inflammatory, anti-tumor, anti-diabetic and anti-oxidant (Ren et al., 2018; Jia et al., 2019). Previous reports suggested that astragalus injection and astragaloside IV have therapeutic effects on fibrosis (Qian et al., 2018). APS can protect renal fibrosis (Ren et al., 2019). Astragalus can improve EMT through β-catenin (Yu et al., 2018b). The overproduction of TGF-β1 is always closely related to PF, and TGF-β1 plays a key role in inducing EMT (Walton et al., 2017), nuclear factor-kB (NF-kB) regulates the expression of a variety of cytokines and inflammatory mediators, by inhibiting the expression of TGF-β1/NF-kB to block related signal pathways and prevent inflammation and reduce liver fibrosis (Li et al., 2018a). A recent observation proposed that APS can reduce PF through the TGF-β1/NF-kB pathway, which can effectively improve the deposition of COL-1, COL-3, and FN in vivo, APS reduce the area of fibrosis and HYP content in the matrix, and reduce the expression of a-SMA neutralizes E-cadherin, it significantly reduces the activation of the EMT and NF-kB pathways of TGF-β1 in vitro (Zhang et al., 2020). Therefore, the mechanism of APS protecting PF is the regulation of TGF-β1/NF-kB pathway.
Polyporus Polysaccharide (PPS)
The pharmaceutical ingredients of polyporus umbellatus include polysaccharides, ergosterol, biotin, protein and other molecules, among which PPS is one of the main biologically active substances of polyporus umbellatus, the main components of PPS are (1–3)-β-Glc main chain and (1–6)-β-Glc side chain β-glucan, the Mw is about 1.6 × 105 Da (Zong et al., 2012). Previous studies have reported various pharmacological activities of PPS, such as anti-tumor, anti-cancer, anti-oxidation and anti-inflammatory (Li et al., 2010; Guo et al., 2019). In renal fibrosis, PPS can improve the accumulation of TGF-β1, restore the balance of MMP/TIMP factors and improve renal fibrosis (Li et al., 2019a). PPS can significantly inhibit the ECM components in human lung fibrosis (HLFs) cells treated with TGF-β1, and inhibit Smad2/3 phosphorylation even reduce TβRI and TβRII, but it has no effect on TβRIV and can also inhibit medium MMP-2/MMP-9 expression (Jiang et al., 2020). Therefore, PPS prevented the further deterioration of PF through TGF-β1/Smad2/3/TβRI, and TβR II/MMP level signals.
Rhodiolasachalinensis Polysaccharide A (RSA)
RSA is an acidic heteropolysaccharide isolated from rhodiola alpina, its mainly composed of Ara, Rha, Xyl, Glc, Gal, and GalA, the molar ratio is 1.00:3.23:0.26:0.34:0.84:10.24, and the relative Mw is about 50,000 Da (Han et al., 2002), has anti-oxidant, anti-viral, anti-oxidant and anti-tumor effects (Yang et al., 2016; Song et al., 2019). Previous evaluations have reported that salidroside protects mice from acute lung injury through the NF-kB pathway by controlling the production of inflammatory cytokines, and is very effective in preventing oxidative stress after exercise in mice (Guan et al., 2012). Studies have suggested that RSA can significantly inhibit the death rate of A549 cells induced by TGF-β1 and the expression of the mesenchymal marker Fibronection-EDA (Fn-EDA) (Li, et al., 2016). Rhodiola rosea significantly reduced the expression levels of MMP-9 and α-SMA in PF in a dose-dependent manner (Zhang et al., 2016). Therefore, RSA can improve the abnormality of Fn-EDA through TGF-β1 to achieve the purpose of treating PF.
Polysaccharides From Dendrobium Officinale (PDO)
Dendrobium officinale is an epiphytic herb of the orchid family with mainly grows on semi-shaded moist rocks at an altitude of about 1,600 m in the Nanshan mountains of china. PDO is a neutral heteropolysaccharide isolated from the Chinese medicinal material dendrobium candidum, with composed of (1→4)-linked Man and (1→4)-linked Glc, the molar ratio is 5.9:1 and the Mw is 1.78 × 105 Da (He et al., 2016; Yu W. et al., 2018). It has the effects of anti-oxidation, anti-tumor, anti-viral, anti-aging, lowering blood sugar and promoting hair growth (Wang J.-H. et al., 2017; Liu et al., 2019b), and is often valued in the field of dermatology. Dendrobium nobile polysaccharides can improve the antioxidant capacity of rats and reduce liver inflammation, so it has an antagonistic effect on liver fibrosis (Sibinska et al., 2017). Early studies on dendrobium candidum can treat myocardial fibrosis (Zhang et al., 2016b; Zeng et al., 2020). PDO can reduce the number of neutrophils in lung tissue induced by BLM, improve inflammation and fibrosis, enhance Smad2/3 and inhibit the expression of pSmad2/3 protein through TGFβ1, thereby inhibiting the type II rat alveolar epithelial cells (RLE-6TN, CRL-2300) induced by TGF-β1 fibronectin and COL-1 (Chen et al., 2018). Therefore, PDO treatment of PF lies in the regulation of TGF-β1/Smad2, Smad3/pSmad2/3.
Bletilla Striata Polysaccharide (BSP)
Two water-soluble polysaccharides, BSP-1 and BSP-2, were extracted and purified from bacillus striata tuber, and their Mw are 8.354 × 104 and 1.26 × 104 Da, both BSP-1 and BSP-2 are composed of Man and Glc, and the molar ratio are 4.0:1.0 and 3.0:1.0. The backbone is mainly composed of repeated β-1,4-linked d-Man residues and β-1, 4-linked d-Glc residue composition. It has anti-ulcer, anti-oxidation, anti-inflammatory, anti-tumor and immunomodulatory activities (He et al., 2017; Liu et al., 2020). BSP can protect renal fibrosis through the down-regulation of TβRI, TβRII and a-SMA mediated by TGF-β (Wang et al., 2014). Some researchers have confirmed that a Mw of 2.23 × 105 kDa, BSP can reduce cell recruitment to protect lung tissue inflammation, the content of HYP is significantly reduced, TGF-β3 and TNF-β1 are also improved, they also proved that bletilla striata extract also has a certain degree of resistance the role of fibrosis (Guo et al., 2016). Therefore, BSP improves lung inflammation and abnormal cell recruitment through TGF-β/TNF to improve lung tissue fibrosis.
Seaweed Sulfated Oligosaccharide (MS80)
Marine macroalgae are mainly divided into four parts, green algae, cyanobacteria, brown algae and red algae. MS80 is a new type of sulfated oligosaccharide (1→4α-d-Glc) extracted from seaweed, with the Mw is 8,000 Da, MS80 can anti-cancer, anti-inflammatory and anti-tumor (Chen et al., 2020). MS80 inhibits BLM-induced PF in vivo, antagonizes TGF-β1-induced human embryo pulmonary fibroblast (HEPF) cells proliferation in vitro, prevents COL deposition and MMP activity, and inactivates ERK and p38 signaling pathways (Jiang and Guan, 2009). In addition, the MS80 targeting protein is receptor interacting protein 2 (a key component of CD40 signal transduction). MS80 inhibits the activation of NF-kB induced by CD40 linkage, thereby inhibiting the secretion of inflammatory cytokines, COL synthesis and fibrogenesis the excessive proliferatsion of cells confirms that MS80 has anti-fibrosis effects both in vivo and in vitro (Du et al., 2010). MS80 can also effectively inhibit standardized TGF-β1/Smad signaling, thereby improving the changes in EMT marker levels (Zhou et al., 2016). Therefore, MS80 can improve PF through TGF-β1/MMP/ERK/NF-kB/p38.
Low Molecular Weight Fucoidan (LMWF)
LMWF is a sulfated polysaccharide extracted from brown algae, the Mw is 8–10 kDa, containing Rha, Fuc, Xly, Man, Glc, Gal, GlcA and Gala, the linkage is backbone →3)-Galp-(1→, →6)-Glcp-(1→, →6)-Galp-(1→, →3,6)-manp-(1→ with →3)-Fucp-(1→, →4)-Glcp-(1→ and sulfated end units (Chen et al., 2020), consists of (1→3)-linked α-thiopropionyl residues or alternating (13→)- and (→14)-linked α-thiophosphoryl residues. Depending on previous studies, LMWF can lower blood sugar, anti-inflammatory, and promote angiogenesis (Haddad et al., 2015; Wang et al., 2018; Zheng et al., 2018). LMWF has an antagonistic effect on liver fibrosis through the TGF-β1/Smad3 pathway (Hayashi et al., 2008). Its also lnhibit the proliferatsion of breast cancer cells and the expression of EMT biomarkers (Hsu et al., 2013). In addition, LMWF can reduce inflammation of the inflammatory cytokines TIMP-1, chemokine ligand 1 (CXCL1), monocyte chemotactic protein-1 (MCP-1), and macrophage inflammation protein-2 (MIP-2) of lung tissue, TNF content to improve radiation-induced pneumonia in mice and further PF (Yu H.-H. et al., 2018). LMWF inhibits the morphological changes and proliferatsion of A549 cells induced by TGF-β1. In the male C57BL/6 mice model, LMWF attenuates the lung EMT phenotype. LMWF down-regulates TGF-β1/ERK signals in vivo and in vitro to regulate PF (Wang et al., 2019a). Therefore, LMWF treats PF through TGF-β1/ERK signal and improves inflammatory cytokines in pathological lung tissue.
Angelica Sinensis Polysaccharide (ASP)
ASP is an acidic heteropolysaccharide extracted from the root of angelica sinensis, its composed of GalA, Glc, Ara and Gal with the molar ratio of 1.00:1.70:1.85:5.02, the Mw is 80 kDa. Its main chain is composed of (1→3)-connected Galp, (1→6)-connected Galp and 2-OMe-(1→6)-connected Galp, with three branches connected to 2-OMe-(- 1→6) link Galp and terminate with GlcpA and Araf (Zhang et al., 2016a). ASP has immune regulation, anti-tumor, anti-oxidation and anti-proliferatsion functions (Li M. M. et al., 2020; Xu et al., 2021). There is also the ability of the compound Danggui Buxue Decoction total glycosides (containing astragalus and angelica) to inhibit abnormal lung tissue ECM and reverse the expression of MMP/TIMP (Gao et al., 2012). ASP can improve the content of hyaluronic acid and laminin in the lung tissue of rats with PF induced by BLM (Wang et al., 2010). The latest report points out that ASP inhibits PF by down-regulating the expression of differentiation-antagonizing non-protein coding RNA (DANCR), which inactivates FOXO3 translation after transcription in an AU binding factor 1 (AUF1)-dependent manner (Qian et al., 2020). Therefore, ASP can inhibit EMT and PF through the TGF-β1/DANCR/AUF-1/FOXO3 regulatory axis.
Total Polysaccharide From O, Lanpingensis (POL)
POL is an insect fungal polysaccharide isolated from cordyceps Lanping. It is mainly distributed in northwestern Yunnan, china as a new species reported in recent years, which is closely a relative species of cordyceps sinensis. POL Mw is 3.2 × 105 Da, composed of Gal, Man and Glc, and the molar ratio is 5.30:13.38:81.31 (Zhou et al., 2020). It was reported earlier that POL has an anti-inflammatory effect, which can improve liver fibrosis by alleviating the body’s oxidative stress, reduce inflammation and anti-apoptosis of liver cells (Zhang et al., 2017). POL can Treat renal insufficiency by enhancing antioxidant capacity and improving immune regulation ability (Ying-Mei et al., 2020). Ophiocordyceps lanpingensis can treat respiratory diseases. POL can significantly reduce the content of collagen in lung tissue and inflammation of lung tissue by inhibiting classically activated macrophages 1 (M1) and alternately activated M2 in lung tissue The recruitment of PF inhibits the occurrence of PF (Zhou et al., 2020). Therefore, POL can improve lung inflammation by inhibiting the excessive recruitment of M1 and M2 under TGF-β1 to achieve a therapeutic effect.
Lycium Barbarum Polysaccharide (LBP)
In the wolfberry extract, LBP isolated from the fruit of lycium barbarum caused the biological activity of the wolfberry. LBP is a group of water-soluble sugar conjugates with a Mw is 10–2,300 kDa, accounting for 5–8% of dried fruit. LBP glycan backbone is mainly represented by α-(1→4)-galA, α-(1→6)-glc, β-(1→3)-galp (typical arabinogalactan protein) and β-(1→6)-giap, other structures that are less representative are α-(1→5)-ara and β-(1→4)-galp, which have different branching and terminal sites (Tian et al., 2019). LBP has the effects of lowering blood sugar, lowering blood lipid, anti-oxidation and anti-tumor effect (Masci et al., 2018; Huang et al., 2019). LBP possibly inhibit the expression of genes such as COL-1 and α-SMA in inflamed lung tissues, weaken the excessive proliferatsion of fibroblasts and prevent their differentiation into myofibroblasts, thereby reducing the content of HYP in lung tissues to inhibit the development of PF in C57BL/6 mice (Liu et al., 2016b). Therefore, LBP inhibits PF by improving the degree of fibers in the lung tissue.
Morel Polysaccharide-1 (FMP-1)
Morels belong to the category of mushrooms, with high nutritional value and delicious taste, both the flavor and the pharmacological effects have been highly regarded (Tietel and Masaphy, 2018). FMP-1 is a heteropolysaccharide in morchella fruiting body, the Mw is 4.7 × 103 Da, composed of Man, Glc, and Gal with the molar ratio of 1.00:7.84:1.24, the main chain is 1,4-linked Glcp and 1,6-connected Galp composition (Cai et al., 2018). Previous studies have shown that morel contains many biologically active ingredients, such as polysaccharides, protein, dietary fiber and vitamins. FMP-1 has immunomodulatory, anti-oxidant, anti-value-added and anti-tumor effects (C. Liu et al., 2016a; Li et al., 2017). Controlling oxidative stress after lung injury has been shown to be effective in inhibiting fibrosis, it is proposed that polysaccharides can activate antioxidant defenses and improve oxidative stress damage (Hao et al., 2016; Chen et al., 2017). The phosphatidylinositol 3-kinase (PI3K)/AKT pathway is one of the effective ways to fight oxidative stress (Mozaffari et al., 2010), and it has a protective effect on lung epithelial cell death induced by oxidative stress (Deng et al., 2013b). Many examines have confirmed that the up-regulation of heme oxidase-1 (HO-1) is involved in the cells defense mechanism against the results of oxidation (Lee et al., 2012; Jang et al., 2016). Related1 research reports that FMP-1 can promote nuclear factor erythroid 2-related factor 2 (Nrf2) phosphorylation and nuclear translocation, and up-regulate downstream protein HO-1 through the PI3K/AKT-Nrf2 signaling pathway, thereby protecting human alveolar epithelial cells (A549) from hydrogen peroxide oxidative stress (Li et al., 2018c). Therefore, FMP-1 can be used as a natural potential antioxidant to treat PF through the Nrf2/HO-1/PI3K/AKT signaling pathway.
Ganoderma Lucidum Polysaccharide (GIP)
Ganoderma is an edible medicinal mushroom, which has been praised for 2000 years. It can enhance human vitality. GIP is a neutral heteropolysaccharide isolated from ganoderma lucidum. It is composed of Gal, Rha and Glc with the molar ratio of 1.00:1.15:3.22, the Mw is 78 kDa, and the backbone structure is mainly composed of 1,2-linked bL- Rhap, 1,3,6-linked aD-Galp, 1,2,6-linked aD-Glcp, and 1-link aD-Glcpconsisted mainly of 1,2-linked-β-L-Rhap, 1,3,6-linked-a-D-Galp, 1,2,6-linked-a-D-Glcp, and1-linked a-D-Glcp. (Pan et al., 2012). It is reported that a pure Glc polymer named β-glucan is considered to be one of the active ingredients of GIP (Zeng et al., 2019). GIP has many therapeutic effects on human diseases, including anti-oxidation, anti-inflammatory, anti-tumor, lowering blood sugar, lowering blood lipids, and anti-aging (Wang et al., 2017b; Sohretoglu and Huang, 2018; Zeng et al., 2018). In addition, it has been reported in the literatsure that GIP 100–300 mg/kg can reverse PF after 28 days. The main mechanism is related to the increase of lung antioxidant capacity. GIP can increase pathological lung tissue CAT (catalase), SOD (superoxide dismutation), GSH (glutathione), and GSH-Px (glutathione peroxidase) levels, simultaneously, reduce the content of MDA (malondialdehyde) and HYP in lung tissue (Chen et al., 2016). Later reports confirmed that ganoderma lucidum has obvious protective effect on oxidative damage caused by oxidants (Laçin et al., 2019; Lin and Deng, 2019). Ganoderic acid can treat renal fibrosis through TGF-β/Smad and MAPK signaling (Geng et al., 2020). Therefore, GIP improves the status of PF by improving the lung’s antioxidant capacity.
Lily Polysaccharide (LLP)
LLP-1, LLP-2 and LLP-3 are the three new polysaccharide components in lily, their Mw are estimated to be 2.25 × 106, 2.02 × 106, and 2.08 × 106 Da, LLP-1 and LLP-2 are mainly composed of Glc and Man, with the molar ratio of 1:2 and 1:1, respectively, LLP-3 is mainly composed of Ara, Gal, Glc, and Man, with the molar ratio is 2:2:2:1 (Chen et al., 2014; Hou et al., 2016). LLP has a variety of activities, such as hypoglycemic, anti-oxidant and anti-cancer effects (Li et al., 2020b). LLP can improve the PF alveolar compartment and reduce the infiltratsion of inflammatory cells. The mechanism is to inhibit the protein expression of MMP-9 and TIMP-2 in the fibrosis model (Luo, et al., 2013a). LLP combined with bone marrow mesenchymal stem cell transplantation inhibits the expression of TNF-α and NF-kB in the lung tissue of PF mice, improves the recruitment of COL and reduces the content of HYP. LLP can reduce the pathological damage of PF (Luo, et al., 2013b). Therefore, LLP treats PF by adjusting the balance of MMP/TIMP in rats, improving the NF-kB signaling pathway and inflammatory TNF-a abnormalities in lung tissue.
Yupingfeng–Polysaccharide (YPF–p)
Yupingfeng powder is a well-known TCM compound consisting of Astragalus, Atractylodes and Fangfeng. There are many compounds in Yupingfeng powder, including total polysaccharides, total saponins and volatile oil. Among them, YPF-p is one of the important components extracted from Yupingfeng powder, it has anti-inflammatory, anti-allergic, immune-regulating and alleviating effects of lung qi deficiency (Sun et al., 2016; Fan et al., 2017). Anti-fibrosis studies have found that YPF-p can improve the level of HYP in Wistar rats induced by pingyangmycin hydrochloride and the content of COL-3, COL-4, laminin, and hyaluronic acid in serum, and can down-regulate Wistar rats lungs. TGF-β1 mRNA expression in tissues (Wang et al., 2009). The mechanism presumably is that inhibit the increase of TGF-β1 mediated fibroblast activation and thus reduce synthesis (Xu et al., 2014). In addition, the total glycosides of Yupingfeng reduced the protein expression of box1 in the high mobility group, and reversed TGF-β1 to improve PF (Cui et al., 2015; Li et al., 2015). Therefore, YPF-p treats PF by down-regulating the expression of TGF-β1 mRNA and improving the excessive aggregation of fibroblasts induced by TGF-β1.
Conclusion and Future Prospects
These polysaccharides can significantly improve the abnormal recruitment and apoptosis of various cells in the lung tissue induced by the TGF-β signaling pathway, regulate the imbalance of the body caused by lung inflammation, and can control lung tissue damage through oxidative stress, thus confirming these polysaccharides can stabilize PF lung function and prevent further damage.
Our understanding of this evolving deadly disease is constantly improving, but effective treatments are still elusive. PF is also one of the main complications of COVID-19. Studying the properties of polysaccharides and combining polysaccharides with other drugs may provide medical help for PF patients with COVID-19. The epidemiological risk factors and biological process of PF and COVID-19 are similar. After severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) induced pneumonia may cause idiopathic PF related to its physical damage. Hence, modulating the mechanism of fibrosis in SARS-CoV-2 infection to exert a therapeutic effect may be acceptable. The chemical structure of Chinese herbal medicine polysaccharides is the basis of biological activity. Using the hydroxyl, carboxyl, amino, and other groups of sugar residues to modify the structure of polysaccharide molecules on the surface of polysaccharide molecules, For example, the GIP isolated from Fudan-Yueyang-G. lucidu has undergone methylation analysis, periodate oxidation, Smith degradation, and NMR characterization analysis to obtain a neutral polysaccharide with four residues A, B, C, and D which is significantly enhanced improve the lung’s antioxidant capacity to achieve the purpose of treating PF, The structure determines the activity, can makes the multiple structure of the plant clearer, and seeks the regularity between the nanostructure and the biological activity to improve the immune activity of polysaccharides and reduce toxic effects. Chemical polysaccharides with different structures have great differences in biological activity. Further explore the relationship between the biological effects and efficacy of Chinese herbal medicine polysaccharides to explain the therapeutic mechanism of polysaccharides in PF. It is very important to provide a certain reference for the in-depth study and exploration of the structure-activity relationship of Chinese herbal medicine polysaccharides and the development and application of carbohydrate products. This provides important medical theory and economic value for the development of Chinese herbal polysaccharides to treat fibrotic diseases, and has a very important society. Polysaccharides can be a new type of anti-fibrosis treatment drugs. While, the structure of traditional Chinese medicine polysaccharides is complex and diverse, and its special active mechanism needs to be further studied. The establishment of specific, efficient and practical methods for the stability of polysaccharides is of great significance to the research and development of modern medicine.
Author Contributions
JS guided the experiment. XW and JH collected supporting evidence and wrote the paper. All authors read and approved the final article.
Funding
The authors of this review were supported by the National Natural Science Foundation of China (82073311), the Key Research and Development Projects in Chengdu (2020-YF05-00058-SN), the Key Research and Development Projects in Sichuan Province (2020YFS0399), the Clinical Research and Transformation Foundation of Sichuan Provincial People’s Hospital (2021LZ03), the National Key Research and Development Program of China (2020YFC2005500), and the Key Research and Development Program of Science and Technology Department of Sichuan Province (2019YFS0514).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahn, J.-Y., Kim, M.-H., Lim, M.-J., Park, S., Lee, S.-l. -o., Yun, Y.-S., et al. (2011). The Inhibitory Effect of Ginsan on TGF-β Mediated Fibrotic Process. J. Cel. Physiol. 226 (5), 1241–1247. doi:10.1002/jcp.22452
Akhter, K. F., Mumin, M. A., Lui, E. M. K., and Charpentier, P. A. (2018). Fabrication of Fluorescent Labeled Ginseng Polysaccharide Nanoparticles for Bioimaging and Their Immunomodulatory Activity on Macrophage Cell Lines. Int. J. Biol. Macromolecules 109, 254–262. doi:10.1016/j.ijbiomac.2017.12.050
Bao, W.-R., Li, Z.-P., Zhang, Q.-W., Li, L.-F., Liu, H.-B., Ma, D.-L., et al. (2018). Astragalus Polysaccharide RAP Selectively Attenuates Paclitaxel-Induced Cytotoxicity toward RAW 264.7 Cells by Reversing Cell Cycle Arrest and Apoptosis. Front. Pharmacol. 9, 1580. doi:10.3389/fphar.2018.01580
Cai, Z.-N., Li, W., Mehmood, S., Pan, W.-J., Wang, Y., Meng, F.-J., et al. (2018). Structural Characterization, In Vitro and In Vivo Antioxidant Activities of a Heteropolysaccharide from the Fruiting Bodies of Morchella Esculenta. Carbohydr. Polym. 195, 29–38. doi:10.1016/j.carbpol.2018.04.069
Canestaro, W. J., Forrester, S. H., Raghu, G., Ho, L., and Devine, B. E. (2016). Drug Treatment of Idiopathic Pulmonary Fibrosis. Chest 149 (3), 756–766. doi:10.1016/j.chest.2015.11.013
Cao, P., Wu, S., Wu, T., Deng, Y., Zhang, Q., Wang, K., et al. (2020). The Important Role of Polysaccharides from a Traditional Chinese Medicine-Lung Cleansing and Detoxifying Decoction against the COVID-19 Pandemic. Carbohydr. Polym. 240, 116346. doi:10.1016/j.carbpol.2020.116346
Chen, J., Lu, J., Wang, B., Zhang, X., Huang, Q., Yuan, J., et al. (2018). Polysaccharides from Dendrobium Officinale Inhibit Bleomycin-Induced Pulmonary Fibrosis via the TGFβ1-Smad2/3 axis. Int. J. Biol. Macromol. 118 (Pt B), 2163–2175. doi:10.1016/j.ijbiomac.2018.07.056
Chen, J., Shi, Y., He, L., Hao, H., Wang, B., Zheng, Y., et al. (2016). Protective Roles of Polysaccharides from Ganoderma Lucidum on Bleomycin-Induced Pulmonary Fibrosis in Rats. Int. J. Biol. Macromolecules 92, 278–281. doi:10.1016/j.ijbiomac.2016.07.005
Chen, L., Liu, L., Li, C., Hu, C., Su, F., Liu, R., et al. (2017). A Mix of Apple Pomace Polysaccharide Improves Mitochondrial Function and Reduces Oxidative Stress in the Liver of High-Fat Diet-Induced Obese Mice. Mol. Nutr. Food Res. 61 (3), 1600433. doi:10.1002/mnfr.201600433
Chen, R.-r., Li, Y.-j., Chen, J.-j., and Lu, C.-l. (2020). A Review for Natural Polysaccharides with Anti-pulmonary Fibrosis Properties, Which May Benefit to Patients Infected by 2019-nCoV. Carbohydr. Polym. 247, 116740. doi:10.1016/j.carbpol.2020.116740
Chen, Z.-G., Zhang, D.-N., Zhu, Q., Yang, Q.-H., and Han, Y.-B. (2014). Purification, Preliminary Characterization and In Vitro Immunomodulatory Activity of Tiger Lily Polysaccharide. Carbohydr. Polym. 106, 217–222. doi:10.1016/j.carbpol.2014.02.004
Chihara, G., Maeda, Y., Hamuro, J., Sasaki, T., and Fukuoka, F. (1969). Inhibition of Mouse Sarcoma 180 by Polysaccharides from Lentinus Edodes (Berk.) Sing. Nature 222 (5194), 687–688. doi:10.1038/222687a0
Cui, W., Li, L., Li, D., Mo, X., Zhou, W., Zhang, Z., et al. (2015). Total Glycosides of Yupingfeng Protects against Bleomycin-Induced Pulmonary Fibrosis in Rats Associated with Reduced High Mobility Group Box 1 Activation and Epithelial-Mesenchymal Transition. Inflamm. Res. 64 (12), 953–961. doi:10.1007/s00011-015-0878-x
Datta, A., Scotton, C. J., and Chambers, R. C. (2011). Novel Therapeutic Approaches for Pulmonary Fibrosis. Br. J. Pharmacol. 163 (1), 141–172. doi:10.1111/j.1476-5381.2011.01247.x
Deng, W., Fu, M., Cao, Y., Cao, X., Wang, M., Yang, Y., et al. (2013a). Angelica Sinensis Polysaccharide Nanoparticles as Novel Non-viral Carriers for Gene Delivery to Mesenchymal Stem Cells. Nanomedicine: Nanotechnology, Biol. Med. 9 (8), 1181–1191. doi:10.1016/j.nano.2013.05.008
Deng, X., Rui, W., Zhang, F., and Ding, W. (2013b). PM2.5 Induces Nrf2-Mediated Defense Mechanisms against Oxidative Stress by Activating PIK3/AKT Signaling Pathway in Human Lung Alveolar Epithelial A549 Cells. Cell. Biol. Toxicol. 29 (3), 143–157. doi:10.1007/s10565-013-9242-5
Du, X., Jiang, S., Liu, H., Xin, X., Li, J., Geng, M., et al. (2010). MS80, a Novel Sulfated Polysaccharide, Inhibits CD40-NF-kappaB Pathway via Targeting RIP2. Mol. Cel. Biochem. 337 (1-2), 277–285. doi:10.1007/s11010-009-0309-9
Fan, W., Zheng, P., Wang, Y., Hao, P., Liu, J., and Zhao, X. (2017). Analysis of Immunostimulatory Activity of Polysaccharide Extracted from Yu-Ping-Feng In Vitro and In Vivo. Biomed. Pharmacother. 93, 146–155. doi:10.1016/j.biopha.2017.05.138
Feng, B., Zhu, Y., Su, Z., Tang, L., Sun, C., Li, C., et al. (2018). Basil Polysaccharide Attenuates Hepatocellular Carcinoma Metastasis in Rat by Suppressing H3K9me2 Histone Methylation under Hepatic Artery Ligation-Induced Hypoxia. Int. J. Biol. Macromolecules 107, 2171–2179. doi:10.1016/j.ijbiomac.2017.10.088
Feng, B., Zhu, Y., Sun, C., Su, Z., Tang, L., Li, C., et al. (2019). Basil Polysaccharide Inhibits Hypoxia-Induced Hepatocellular Carcinoma Metastasis and Progression through Suppression of HIF-1α-Mediated Epithelial-Mesenchymal Transition. Int. J. Biol. Macromolecules 137, 32–44. doi:10.1016/j.ijbiomac.2019.06.189
Fiorucci, E., Lucantoni, G., Paone, G., Zotti, M., Li, B. E., Serpilli, M., et al. (2008). Colchicine, Cyclophosphamide and Prednisone in the Treatment of Mild-Moderate Idiopathic Pulmonary Fibrosis: Comparison of Three Currently Available Therapeutic Regimens. Eur. Rev. Med. Pharmacol. Sci. 12 (2), 105–111. doi:10.1016/S0924-977X(08)70017-8
Galli, J. A., Pandya, A., Vega-Olivo, M., Dass, C., Zhao, H., and Criner, G. J. (2017). Pirfenidone and Nintedanib for Pulmonary Fibrosis in Clinical Practice: Tolerability and Adverse Drug Reactions. Respirology 22 (6), 1171–1178. doi:10.1111/resp.13024
Gao, J., Feng, L.-j., Huang, Y., Li, P., Xu, D.-j., Li, J., et al. (2012). Total Glucosides of Danggui Buxue Tang Attenuates Bleomycin-Induced Pulmonary Fibrosis via Inhibition of Extracellular Matrix Remodelling. J. Pharm. Pharmacol. 64 (6), 811–820. doi:10.1111/j.2042-7158.2012.01490.x
Geng, X.-q., Ma, A., He, J.-z., Wang, L., Jia, Y.-l., Shao, G.-y., et al. (2020). Ganoderic Acid Hinders Renal Fibrosis via Suppressing the TGF-β/Smad and MAPK Signaling Pathways. Acta Pharmacol. Sin. 41 (5), 670–677. doi:10.1038/s41401-019-0324-7
Guan, S., Liu, Q., Han, F., Gu, W., Song, L., Zhang, Y., et al. (2017). Ginsenoside Rg1 Ameliorates Cigarette Smoke-Induced Airway Fibrosis by Suppressing the TGF-β1/Smad Pathway In Vivo and In Vitro. Biomed. Res. Int. 2017, 1–12. doi:10.1155/2017/6510198
Guan, S., Xiong, Y., Song, B., Song, Y., Wang, D., Chu, X., et al. (2012). Protective Effects of Salidroside fromRhodiola Roseaon LPS-Induced Acute Lung Injury in Mice. Immunopharmacology and Immunotoxicology 34 (4), 667–672. doi:10.3109/08923973.2011.650175
Guo, N., Bai, Z., Jia, W., Sun, J., Wang, W., Chen, S., et al. (2019). Quantitative Analysis of Polysaccharide Composition in Polyporus Umbellatus by HPLC-ESI-TOF-MS. Molecules 24 (14), 2526. doi:10.3390/molecules24142526
Guo, Q., Meng, Y., Zhao, Y., Cheng, X. C., Lu, Y. X., and Zhang, Q. L. (2016). Therapeutic Effect of Baiji Extract on Pulmonary Fibrosis Induced by Bleomycin in Rats. J. Int. Pharm. Res. 43 (3), 518–523. doi:10.13220/j.cnki.jipr.2016.03.022
Haddad, O., Guyot, E., Marinval, N., Chevalier, F., Maillard, L., Gadi, L., et al. (2015). Heparanase and Syndecan-4 Are Involved in Low Molecular Weight Fucoidan-Induced Angiogenesis. Mar. Drugs 13 (11), 6588–6608. doi:10.3390/md13116588
Han, L. P., Liang, Z. Y., Han, L. M., Gong, R. C., and Ma, X. H. (2002). Isolation, Purification and Composition Analysis of the Polysaccharide RSA of Rhodiola Alpina. Chi. Pharm. J. 06, 19–22. doi:10.3321/j.issn:1001-2494.2002.06.00610.1142/s0219030302001933
Hao, L., Sheng, Z., Lu, J., Tao, R., and Jia, S. (2016). Characterization and Antioxidant Activities of Extracellular and Intracellular Polysaccharides from Fomitopsis Pinicola. Carbohydr. Polym. 141, 54–59. doi:10.1016/j.carbpol.2015.11.048
Hayashi, S., Itoh, A., Isoda, K., Kondoh, M., Kawase, M., and Yagi, K. (2008). Fucoidan Partly Prevents CCl4-Induced Liver Fibrosis. Eur. J. Pharmacol. 580 (3), 380–384. doi:10.1016/j.ejphar.2007.11.015
He, T.-B., Huang, Y.-P., Yang, L., Liu, T.-T., Gong, W.-Y., Wang, X.-J., et al. (2016). Structural Characterization and Immunomodulating Activity of Polysaccharide from Dendrobium Officinale. Int. J. Biol. Macromolecules 83, 34–41. doi:10.1016/j.ijbiomac.2015.11.038
He, X., Wang, X., Fang, J., Zhao, Z., Huang, L., Guo, H., et al. (2017). Bletilla Striata: Medicinal Uses, Phytochemistry and Pharmacological Activities. J. Ethnopharmacology 195, 20–38. doi:10.1016/j.jep.2016.11.026
Hou, R., Chen, J., Yue, C., Li, X., Liu, J., Gao, Z., et al. (2016). Modification of Lily Polysaccharide by Selenylation and the Immune-Enhancing Activity. Carbohydr. Polym. 142, 73–81. doi:10.1016/j.carbpol.2016.01.032
Hsu, H.-Y., Lin, T.-Y., Hwang, P.-A., Tseng, L.-M., Chen, R.-H., Tsao, S.-M., et al. (2013). Fucoidan Induces Changes in the Epithelial to Mesenchymal Transition and Decreases Metastasis by Enhancing Ubiquitin-dependent TGF Receptor Degradation in Breast Cancer. Carcinogenesis 34 (4), 874–884. doi:10.1093/carcin/bgs396
Huang, C., Yao, R., Zhu, Z., Pang, D., Cao, X., Feng, B., et al. (2019). A Pectic Polysaccharide from Water Decoction of Xinjiang Lycium Barbarum Fruit Protects against Intestinal Endoplasmic Reticulum Stress. Int. J. Biol. Macromolecules 130, 508–514. doi:10.1016/j.ijbiomac.2019.02.157
Huang, K.-C., Su, Y.-C., Sun, M.-F., and Huang, S.-T. (2018). Chinese Herbal Medicine Improves the Long-Term Survival Rate of Patients with Chronic Kidney Disease in Taiwan: A Nationwide Retrospective Population-Based Cohort Study. Front. Pharmacol. 9, 1117. doi:10.3389/fphar.2018.01117
Huo, J., Lu, Y., Xia, L., and Chen, D. (2020). Structural Characterization and Anticomplement Activities of Three Acidic Homogeneous Polysaccharides from Artemisia Annua. J. Ethnopharmacology 247, 112281. doi:10.1016/j.jep.2019.112281
Hutchinson, J., Fogarty, A., Hubbard, R., and McKeever, T. (2015). Global Incidence and Mortality of Idiopathic Pulmonary Fibrosis: a Systematic Review. Eur. Respir. J. 46 (3), 795–806. doi:10.1183/09031936.00185114
Jang, H. J., Hong, E. M., Kim, M., Kim, J. H., Jang, J., Park, S. W., et al. (2016). Simvastatin Induces Heme Oxygenase-1 via NF-E2-Related Factor 2 (Nrf2) Activation through ERK and PI3K/Akt Pathway in colon Cancer. Oncotarget 7 (29), 46219–46229. doi:10.18632/oncotarget.10078
Ji, S., Li, Z., Song, W., Wang, Y., Liang, W., Li, K., et al. (2016). Bioactive Constituents ofGlycyrrhiza uralensis(Licorice): Discovery of the Effective Components of a Traditional Herbal Medicine. J. Nat. Prod. 79 (2), 281–292. doi:10.1021/acs.jnatprod.5b00877
Jia, N., Qiao, H., Zhu, W., Zhu, M., Meng, Q., Lu, Q., et al. (2019). Antioxidant, Immunomodulatory, Oxidative Stress Inhibitory and Iron Supplementation Effect of Astragalus Membranaceus Polysaccharide-Iron (III) Complex on Iron-Deficiency Anemia Mouse Model. Int. J. Biol. Macromolecules 132, 213–221. doi:10.1016/j.ijbiomac.2019.03.196
Jiang, H.-d., and Guan, H.-s. (2009). MS80, a Novel Sulfated Oligosaccharide, Inhibits Pulmonary Fibrosis by Targeting TGF-Β1 Both In Vitro and In Vivo. Acta Pharmacol. Sin. 30 (7), 973–979. doi:10.1038/aps.2009.86
Jiang, J., Wang, F., Luo, A., Lin, S., Feng, X., Yan, W., et al. (2020). Polyporus Polysaccharide Ameliorates Bleomycin-Induced Pulmonary Fibrosis by Suppressing Myofibroblast Differentiation via TGF-β/Smad2/3 Pathway. Front. Pharmacol. 11, 767. doi:10.3389/fphar.2020.00767
Layek, B., and Mandal, S. (2020). Natural Polysaccharides for Controlled Delivery of Oral Therapeutics: a Recent Update. Carbohydr. Polym. 230, 115617. doi:10.1016/j.carbpol.2019.115617
Laçin, N., İzol, S. B., İpek, F., and Tuncer, M. C. (2019). Ganoderma Lucidum, a Promising Agent Possessing Antioxidant and Anti-inflammatory Effects for Treating Calvarial Defects with Graft Application in Rats. Acta Cir. Bras. 34 (9), e201900904. doi:10.1590/s0102-865020190090000004
Lederer, D. J., and Martinez, F. J. (2018). Idiopathic Pulmonary Fibrosis. N. Engl. J. Med. 378 (19), 1811–1823. doi:10.1056/NEJMra1705751
Lee, Y.-J., Jeong, H.-Y., Kim, Y.-B., Lee, Y.-J., Won, S. Y., Shim, J.-H., et al. (2012). Reactive Oxygen Species and PI3K/Akt Signaling Play Key Roles in the Induction of Nrf2-Driven Heme Oxygenase-1 Expression in Sulforaphane-Treated Human Mesothelioma MSTO-211H Cells. Food Chem. Toxicol. 50 (2), 116–123. doi:10.1016/j.fct.2011.10.035
Li, D., Li, W., Chen, Y., Liu, L., Ma, D., Wang, H., et al. (2018a). Anti-fibrotic Role and Mechanism of Periplaneta americana Extracts in CCl4-Induced Hepatic Fibrosis in Rats. Acta Biochim. Biophys. Sin. 50 (5), 491–498. doi:10.1093/abbs/gmy024
Li, H., Yan, Z., Xiong, Q., Chen, X., Lin, Y., Xu, Y., et al. (2019a). Renoprotective Effect and Mechanism of Polysaccharide from Polyporus Umbellatus Sclerotia on Renal Fibrosis. Carbohydr. Polym. 212, 1–10. doi:10.1016/j.carbpol.2019.02.026
Li, L., Li, D., Xu, L., Zhao, P., Deng, Z., Mo, X., et al. (2015). Total Extract of Yupingfeng Attenuates Bleomycin-Induced Pulmonary Fibrosis in Rats. Phytomedicine 22 (1), 111–119. doi:10.1016/j.phymed.2014.10.011
Li, M. M., Zhang, Y., Wu, J., and Wang, K. P. (2020a). Polysaccharide from Angelica Sinensis Suppresses Inflammation and Reverses Anemia in Complete Freund’s Adjuvant-Induced Rats. Curr. Med. Sci. 40 (2), 265–274. doi:10.1007/s11596-020-2183-3
Li, N., Yu, X., Yu, Q. H., and Wang, M. (2019b). Research Progress on Stability of Polysaccharides in Traditional Chinese Medicine. Zhongguo Zhong Yao Za Zhi 44 (22), 4793–4799. doi:10.19540/j.cnki.cjcmm.20190916.309
Li, S.-s., He, A.-l., Deng, Z.-y., and Liu, Q.-f. (2018b). Ginsenoside-Rg1 Protects against Renal Fibrosis by Regulating the Klotho/TGF-β1/Smad Signaling Pathway in Rats with Obstructive Nephropathy. Biol. Pharm. Bull. 41 (4), 585–591. doi:10.1248/bpb.b17-00934
Li, W., Cai, Z.-N., Mehmood, S., Wang, Y., Pan, W.-J., Zhang, W.-N., et al. (2018c). Polysaccharide FMP-1 from Morchella Esculenta Attenuates Cellular Oxidative Damage in Human Alveolar Epithelial A549 Cells through PI3K/AKT/Nrf2/HO-1 Pathway. Int. J. Biol. Macromolecules 120 (Pt A), 865–875. doi:10.1016/j.ijbiomac.2018.08.148
Li, W., Wang, Y., Wei, H., Zhang, Y., Guo, Z., Qiu, Y., et al. (2020b). Structural Characterization of Lanzhou Lily ( Lilium Davidii Var. Unicolor ) Polysaccharides and Determination of Their Associated Antioxidant Activity. J. Sci. Food Agric. 100 (15), 5603–5616. doi:10.1002/jsfa.10613
Li, X., Xu, W., and Chen, J. (2010). Polysaccharide Purified from Polyporus Umbellatus (Per) Fr Induces the Activation and Maturation of Murine Bone-Derived Dendritic Cells via Toll-like Receptor 4. Cell Immunol. 265 (1), 50–56. doi:10.1016/j.cellimm.2010.07.002
Li, Y., Yuan, Y., Lei, L., Li, F., Zhang, Y., Chen, J., et al. (2017). Carboxymethylation of Polysaccharide from Morchella Angusticepes Peck Enhances its Cholesterol-Lowering Activity in Rats. Carbohydr. Polym. 172, 85–92. doi:10.1016/j.carbpol.2017.05.033
Li, Z. L., Gao, Y., Zhao, L., and Hong, B. (2016). Study on Rhodiola Polysaccharide against Pulmonary Fibrosis. J. Mol. Sci. 32 (01), 34–39. doi:10.13563/j.cnki.jmolsci.2016.01.004
Lin, Z., and Deng, A. (2019). Antioxidative and Free Radical Scavenging Activity of Ganoderma (Lingzhi). Adv. Exp. Med. 1182, 271–297. doi:10.1007/978-981-32-9421-9_12
Liu, C., Li, H., Wang, K., Zhuang, J., Chu, F., Gao, C., et al. (2019a). Identifying the Antiproliferative Effect of Astragalus Polysaccharides on Breast Cancer: Coupling Network Pharmacology with Targetable Screening from the Cancer Genome Atlas. Front. Oncol. 9, 368. doi:10.3389/fonc.2019.00368
Liu, C., Sun, Y., Mao, Q., Guo, X., Li, P., Liu, Y., et al. (2016a). Characteristics and Antitumor Activity of Morchella Esculenta Polysaccharide Extracted by Pulsed Electric Field. Ijms 17 (6), 986. doi:10.3390/ijms17060986
Liu, D., Dong, L. J., Lei, T., and Ming, L. G. (2016b). The Interventional Effect and Mechanism ofLycium Barbarum Polysaccharides on Bleomycin-Induced Pulmonary Fibrosis in Mice. J. Med. Postgraduates 29 (09), 918–922. doi:10.16571/j.cnki.1008-8199.2016.09.006
Liu, H., Lv, C., and Lu, J. (2020a). Panax Ginseng C. A. Meyer as a Potential Therapeutic Agent for Organ Fibrosis Disease. Chin. Med. 15 (1), 124. doi:10.1186/s13020-020-00400-3
Liu, J., Li, Y., Liu, W., Qi, Q., Hu, X., Li, S., et al. (2019b). Extraction of Polysaccharide from Dendrobium Nobile Lindl. By Subcritical Water Extraction. ACS. Omega. 4 (24), 20586–20594. doi:10.1021/acsomega.9b02550
Liu, Y., Sun, C., Zhang, G., Wu, J., Huang, L., Qiao, J., et al. (2020b). Bio-responsive Bletilla Striata Polysaccharide-Based Micelles for Enhancing Intracellular Docetaxel Delivery. Int. J. Biol. Macromolecules 142, 277–287. doi:10.1016/j.ijbiomac.2019.09.099
Luo, Y. L., Cheng, X. L., Wang, Y. L., Li, N. L., She, Y. L., and Li, Y. (2013a). Lily Polysaccharide Inhibits the Expression of MMP-9 and TIMP-2 in Lung Tissue of Mice with Bleomycin-Induced Pulmonary Fibrosis. Basic Clin. Med. 3, 363–364. doi:10.16352/j.issn.1001-6325.2013.03.002
Luo, Y. L., Wang, Y. L., Pan, Z., Li, N. L., Li, Y., Yan, X., et al. (2013b). Effect of Lily Polysaccharides Combined with BMSCs Transplantation on Expression of TNF-Alpha and NF-kappaB in Bleomycin-Induced Pulmonary Fibrosis Mice. J. Third Mil. Med. Univ. 35 (5), 431–434. doi:10.16016/j.1000-5404.2013.05.004
Lv, J., Shao, Q., Wang, H., Shi, H., Wang, T., Gao, W., et al. (2013). Effects and Mechanisms of Curcumin and Basil Polysaccharide on the Invasion of SKOV3 Cells and Dendritic Cells. Mol. Med. Rep. 8 (5), 1580–1586. doi:10.3892/mmr.2013.1695
Majewski, S., and Piotrowski, W. J. (2020). Air Pollution-An Overlooked Risk Factor for Idiopathic Pulmonary Fibrosis. Jcm 10 (1), 77. doi:10.3390/jcm10010077
Masci, A., Carradori, S., Casadei, M. A., Paolicelli, P., Petralito, S., Ragno, R., et al. (2018). Lycium Barbarum Polysaccharides: Extraction, Purification, Structural Characterisation and Evidence about Hypoglycaemic and Hypolipidaemic Effects. A Review. Food Chem. 254, 377–389. doi:10.1016/j.foodchem.2018.01.176
Miniati, I., and Matucci Cerinic, M. (2007). Pulmonary Fibrosis in Systemic Sclerosis: Is Treatment with Cyclophosphamide More Effective Than Placebo?. Nat. Rev. Rheumatol. 3 (7), 372–373. doi:10.1038/ncprheum0507
Mohammed, A. S. A., Naveed, M., and Jost, N. (2021). Polysaccharides; Classification, Chemical Properties, and Future Perspective Applications in Fields of Pharmacology and Biological Medicine (A Review of Current Applications and Upcoming Potentialities). J. Polym. Environ. 29, 2359–2371. doi:10.1007/s10924-021-02052-2
Mozaffari, M. S., Liu, J. Y., and Schaffer, S. W. (2010). Effect of Pressure Overload on Cardioprotection via PI3K-Akt: Comparison of Postconditioning, Insulin, and Pressure Unloading. Am. J. Hypertens. 23 (6), 668–674. doi:10.1038/ajh.2010.43
Mu, S., Yang, W., and Huang, G. (2021). Antioxidant Activities and Mechanisms of Polysaccharides. Chem. Biol. Drug Des. 97 (3), 628–632. doi:10.1111/cbdd.13798
Muhamad, , Zulkifli, N., Selvakumaran, S. a. p., and Lazim, N. A. M. (2019). Bioactive Algal-Derived Polysaccharides: Multi-Functionalization, Therapeutic Potential and Biomedical Applications. Cpd 25 (11), 1147–1162. doi:10.2174/1381612825666190618152133
Niu, W., Chen, X., Xu, R., Dong, H., Yang, F., Wang, Y., et al. (2021). Polysaccharides from Natural Resources Exhibit Great Potential in the Treatment of Ulcerative Colitis: A Review. Carbohydr. Polym. 254, 117189. doi:10.1016/j.carbpol.2020.117189
Organ, L., Bacci, B., Koumoundouros, E., Barcham, G., Kimpton, W., Nowell, C. J., et al. (2015). A Novel Segmental challenge Model for Bleomycin-Induced Pulmonary Fibrosis in Sheep. Exp. Lung Res. 41 (3), 115–134. doi:10.3109/01902148.2014.985806
Pan, D., Wang, L., Chen, C., Teng, B., Wang, C., Xu, Z., et al. (2012). Structure Characterization of a Novel Neutral Polysaccharide Isolated from Ganoderma Lucidum Fruiting Bodies. Food Chem. 135 (3), 1097–1103. doi:10.1016/j.foodchem.2012.05.071
Phan, T. H. G., Paliogiannis, P., Nasrallah, G. K., Giordo, R., Eid, A. H., Fois, A. G., et al. (2021). Emerging Cellular and Molecular Determinants of Idiopathic Pulmonary Fibrosis. Cell. Mol. Life Sci. 78 (5), 2031–2057. doi:10.1007/s00018-020-03693-7
Qian, W., Cai, X., Qian, Q., Wang, D., and Zhang, L. (2020). Angelica Sinensis Polysaccharide Suppresses Epithelial-Mesenchymal Transition and Pulmonary Fibrosis via a DANCR/AUF-1/FOXO3 Regulatory Axis. Aging Dis. 11 (1), 17–30. doi:10.14336/ad.2019.0512
Qian, W., Cai, X., Qian, Q., Zhang, W., and Wang, D. (2018). Astragaloside IV Modulates TGF ‐β1‐dependent Epithelial‐mesenchymal Transition in Bleomycin‐induced Pulmonary Fibrosis. J. Cel. Mol. Med. 22 (9), 4354–4365. doi:10.1111/jcmm.13725
Raimundo, K., Chang, E., Broder, M. S., Alexander, K., Zazzali, J., and Swigris, J. J. (2016). Clinical and Economic burden of Idiopathic Pulmonary Fibrosis: a Retrospective Cohort Study. BMC. Pulm. Med. 16, 2. doi:10.1186/s12890-015-0165-1
Ren, L., Guo, X.-Y., Gao, F., Jin, M.-L., and Song, X.-N. (2019). Identification of the Perturbed Metabolic Pathways Associating with Renal Fibrosis and Evaluating Metabolome Changes of Pretreatment with Astragalus Polysaccharide through Liquid Chromatography Quadrupole Time-Of-Flight Mass Spectrometry. Front. Pharmacol. 10, 1623. doi:10.3389/fphar.2019.01623
Ren, Q., Zhao, S., Ren, C., and Ma, Z. (2018). Astragalus Polysaccharide Alleviates LPS-Induced Inflammation Injury by Regulating miR-127 in H9c2 Cardiomyoblasts. Int. J. Immunopathol. Pharmacol. 31, 205873841875918. doi:10.1177/2058738418759180
Richeldi, L., Collard, H. R., and Jones, M. G. (2017). Idiopathic Pulmonary Fibrosis. The Lancet 389 (10082), 1941–1952. doi:10.1016/s0140-6736(17)30866-8
Ruigrok, M. J. R., Frijlink, H. W., Melgert, B. N., Olinga, P., and Hinrichs, W. L. J. (2021). Gene Therapy Strategies for Idiopathic Pulmonary Fibrosis: Recent Advances, Current Challenges, and Future Directions. Mol. Ther. - Methods Clin. Dev. 20, 483–496. doi:10.1016/j.omtm.2021.01.003
Ryu, J. H., Moua, T., Daniels, C. E., Hartman, T. E., Yi, E. S., Utz, J. P., et al. (2014). Idiopathic Pulmonary Fibrosis: Evolving Concepts. Mayo Clinic Proc. 89 (8), 1130–1142. doi:10.1016/j.mayocp.2014.03.016
Senthilkumar, K., and Kim, S.-K. (2014). Anticancer Effects of Fucoidan. Adv. Food Nutr. Res. 72, 195–213. doi:10.1016/b978-0-12-800269-8.00011-7
Sibinska, Z., Tian, X., Korfei, M., Kojonazarov, B., Kolb, J. S., Klepetko, W., et al. (2017). Amplified Canonical Transforming Growth Factor-β Signalling via Heat Shock Protein 90 in Pulmonary Fibrosis. Eur. Respir. J. 49 (2), 1501941. doi:10.1183/13993003.01941-2015
Sohretoglu, D., and Huang, S. (2018). Ganoderma Lucidum Polysaccharides as an Anti-cancer Agent. Acamc 18 (5), 667–674. doi:10.2174/1871520617666171113121246
Solomon, J. J., Olson, A. L., Fischer, A., Bull, T., Brown, K. K., and Raghu, G. (2013). Scleroderma Lung Disease. Eur. Respir. Rev. 22 (127), 6–19. doi:10.1183/09059180.00005512
Song, J. W., Hong, S.-B., Lim, C.-M., Koh, Y., and Kim, D. S. (2011). Acute Exacerbation of Idiopathic Pulmonary Fibrosis: Incidence, Risk Factors and Outcome. Eur. Respir. J. 37 (2), 356–363. doi:10.1183/09031936.00159709
Song, J., Wu, Y., Jiang, G., Feng, L., Wang, Z., Yuan, G., et al. (2019). Sulfated Polysaccharides from Rhodiola Sachalinensis Reduce D-Gal-Induced Oxidative Stress in NIH 3T3 Cells. Int. J. Biol. Macromolecules 140, 288–293. doi:10.1016/j.ijbiomac.2019.08.052
Spagnolo, P., Distler, O., Ryerson, C. J., Tzouvelekis, A., Lee, J. S., Bonella, F., et al. (2021). Mechanisms of Progressive Fibrosis in Connective Tissue Disease (CTD)-associated Interstitial Lung Diseases (ILDs). Ann. Rheum. Dis. 80 (2), 143–150. doi:10.1136/annrheumdis-2020-217230
Sun, H., Ni, X., Song, X., Wen, B., Zhou, Y., Zou, F., et al. (2016). Fermented Yupingfeng Polysaccharides Enhance Immunity by Improving the Foregut Microflora and Intestinal Barrier in Weaning rex Rabbits. Appl. Microbiol. Biotechnol. 100 (18), 8105–8120. doi:10.1007/s00253-016-7619-0
Tian, X., Liang, T., Liu, Y., Ding, G., Zhang, F., and Ma, Z. (2019). Extraction, Structural Characterization, and Biological Functions of Lycium Barbarum Polysaccharides: A Review. Biomolecules 9 (9), 389. doi:10.3390/biom9090389
Tietel, Z., and Masaphy, S. (2018). True Morels (Morchella)-Nutritional and Phytochemical Composition, Health Benefits and Flavor: A Review. Crit. Rev. Food Sci. Nutr. 58 (11), 1888–1901. doi:10.1080/10408398.2017.1285269
Todd, N. W., Luzina, I. G., and Atamas, S. P. (2012). Molecular and Cellular Mechanisms of Pulmonary Fibrosis. Fibrogenesis. Tissue Repair 5 (1), 11. doi:10.1186/1755-1536-5-11
Walton, K. L., Johnson, K. E., and Harrison, C. A. (2017). Targeting TGF-β Mediated SMAD Signaling for the Prevention of Fibrosis. Front. Pharmacol. 8, 461. doi:10.3389/fphar.2017.00461
Wang, J.-H., Zuo, S.-R., and Luo, J.-P. (2017a). Structural Analysis and Immuno-Stimulating Activity of an Acidic Polysaccharide from the Stems of Dendrobium Nobile Lindl. Molecules 22 (4), 611. doi:10.3390/molecules22040611
Wang, L., Zhang, P., Li, X., Zhang, Y., Zhan, Q., and Wang, C. (2019a). Low-molecular-weight Fucoidan Attenuates Bleomycin-Induced Pulmonary Fibrosis: Possible Role in Inhibiting TGF-Β1-Induced Epithelial-Mesenchymal Transition through ERK Pathway. Am. J. Transl. Res. 11 (4), 2590–2602.
Wang, S. X., Li, J., and Liu, Y. (2009). Interventional Effect of Yupingfeng Polysaccharide on Pulmonary Fibrosis in Rats and its Partial Mechanism. J. Anhui Med. Univ. 44 (05), 586–590. doi:10.3969/j.issn.1000-1492.2009.05.015
Wang, Y., Han, S., Li, R., Cui, B., Ma, X., Qi, X., et al. (2019b). Structural Characterization and Immunological Activity of Polysaccharides from the Tuber of Bletilla Striata. Int. J. Biol. Macromolecules 122, 628–635. doi:10.1016/j.ijbiomac.2018.10.201
Wang, Y., Liu, D., Chen, S., Wang, Y., Jiang, H., and Yin, H. (2014). A New Glucomannan from Bletilla Striata: Structural and Anti-fibrosis Effects. Fitoterapia 92, 72–78. doi:10.1016/j.fitote.2013.10.008
Wang, Y., Liu, Y., Yu, H., Zhou, S., Zhang, Z., Wu, D., et al. (2017b). Structural Characterization and Immuno-Enhancing Activity of a Highly Branched Water-Soluble β-glucan from the Spores of Ganoderma Lucidum. Carbohydr. Polym. 167, 337–344. doi:10.1016/j.carbpol.2017.03.016
Wang, Y. Q., Wang, X. Q., Zhang, X. M., Lin, X. Y., Wang, X. H., and An, Y. F. (2010). Effects Ofangelica Polysaccharides on Lung Function and Lung Coefficient in Rats with Pulmonary Fibrosis. Gansu Traditional Chin. Med. 23 (011), 28–31. doi:10.3969/j.issn.1004-6852.2010.11.013
Wang, Z., Liu, T., Chen, X., You, H., Zhang, Q., Xue, J., et al. (2018). Low Molecular Weight Fucoidan Ameliorates Hindlimb Ischemic Injury in Type 2 Diabetic Rats. J. Ethnopharmacology 210, 434–442. doi:10.1016/j.jep.2017.09.014
Wilkes, M. C., Mitchell, H., Penheiter, S. G., Doré, J. J., Suzuki, K., Edens, M., et al. (2005). Transforming Growth Factor-β Activation of Phosphatidylinositol 3-Kinase Is Independent of Smad2 and Smad3 and Regulates Fibroblast Responses via P21-Activated Kinase-2. Cancer Res. 65 (22), 10431–10440. doi:10.1158/0008-5472.Can-05-1522
Xiong, X., Huang, G., and Huang, H. (2019). The Antioxidant Activities of Phosphorylated Polysaccharide from Native Ginseng. Int. J. Biol. Macromolecules 126, 842–845. doi:10.1016/j.ijbiomac.2018.12.266
Xu, C., Ni, S., Zhuang, C., Li, C., Zhao, G., Jiang, S., et al. (2021). Polysaccharide from Angelica Sinensis Attenuates SNP-Induced Apoptosis in Osteoarthritis Chondrocytes by Inducing Autophagy via the ERK1/2 Pathway. Arthritis Res. Ther. 23 (1), 47. doi:10.1186/s13075-020-02409-3
Xu, L., Li, L.-c., Zhao, P., Qi, L.-w., Li, P., Gao, J., et al. (2014). Total Polysaccharide of Yupingfeng Protects against Bleomycin-Induced Pulmonary Fibrosis via Inhibiting Transforming Growth Factor-Β1-Mediated Type I Collagen Abnormal Deposition in Rats. J. Pharm. Pharmacol. 66 (12), 1786–1795. doi:10.1111/jphp.12308
Yan, D. D., Tian, J. Z., Zhang, D., Hou, L., Li, L., Zhang, C. H., et al. (2017). Inhibitory Effect of Basil Polysaccharide on TGF-Beta-Induced Epithelial-Mesenchymal Transition of A549 Cells. Traditional Chin. Drug Res. Clin. Pharmacol. 28 (02), 154–159.
Yang, S.-M., Wang, T., Wen, D.-G., Hou, J.-Q., and Li, H.-B. (2016). Protective Effect of Rhodiola Rosea Polysaccharides on Cryopreserved Boar Sperm. Carbohydr. Polym. 135, 44–47. doi:10.1016/j.carbpol.2015.08.081
Ying-Mei, K. E., Min, J., Shu-Bo, Z., Hong, Y. U., Juan, W., and Feng, G. E. (2020). Component Analysis of Ophiocordyceps Lanpingensis Polysaccharides and Study on Alleviation of Hepatic Fibrosis in Mice by Polysaccharides. Zhongguo Zhong Yao Za Zhi 45 (21), 5256–5264. doi:10.19540/j.cnki.cjcmm.20200628.401
Yu, H.-H., Chengchuan Ko, E., Chang, C.-L., Yuan, K., Wu, A., Shan, Y.-S., et al. (2018a). Fucoidan Inhibits Radiation-Induced Pneumonitis and Lung Fibrosis by Reducing Inflammatory Cytokine Expression in Lung Tissues. Mar. Drugs 16 (10), 392. doi:10.3390/md16100392
Yu, M., Shi, J., Sheng, M., Gao, K., Zhang, L., Liu, L., et al. (2018b). Astragalus Inhibits Epithelial-To-Mesenchymal Transition of Peritoneal Mesothelial Cells by Down-Regulating β-Catenin. Cell. Physiol. Biochem. 51 (6), 2794–2813. doi:10.1159/000495972
Yu, W., Ren, Z., Zhang, X., Xing, S., Tao, S., Liu, C., et al. (2018c). Structural Characterization of Polysaccharides from Dendrobium Officinale and Their Effects on Apoptosis of HeLa Cell Line. Molecules 23 (10), 2484. doi:10.3390/molecules23102484
Zeng, J., Li, D., Li, Z., Zhang, J., and Zhao, X. (2020). Dendrobium Officinale Attenuates Myocardial Fibrosis via Inhibiting EMT Signaling Pathway in HFD/STZ-Induced Diabetic Mice. Biol. Pharm. Bull. 43 (5), 864–872. doi:10.1248/bpb.b19-01073
Zeng, P., Chen, Y., Zhang, L., and Xing, M. (2019). Ganoderma Lucidum Polysaccharide Used for Treating Physical Frailty in China. Prog. Mol. Biol. Transl. Sci. 163, 179–219. doi:10.1016/bs.pmbts.2019.02.009
Zeng, P., Guo, Z., Zeng, X., Hao, C., Zhang, Y., Zhang, M., et al. (2018). Chemical, Biochemical, Preclinical and Clinical Studies of Ganoderma Lucidum Polysaccharide as an Approved Drug for Treating Myopathy and Other Diseases in China. J. Cel. Mol. Med. 22 (7), 3278–3297. doi:10.1111/jcmm.13613
Zhan, Y., An, X., Wang, S., Sun, M., and Zhou, H. (2020). Basil Polysaccharides: A Review on Extraction, Bioactivities and Pharmacological Applications. Bioorg. Med. Chem. 28 (1), 115179. doi:10.1016/j.bmc.2019.115179
Zhang, K., Si, X.-P., Huang, J., Han, J., Liang, X., Xu, X.-B., et al. (2016). Preventive Effects of Rhodiola Rosea L. On Bleomycin-Induced Pulmonary Fibrosis in Rats. Ijms 17 (6), 879. doi:10.3390/ijms17060879
Zhang, R., Xu, L., An, X., Sui, X., and Lin, S. (2020). Astragalus Polysaccharides Attenuate Pulmonary Fibrosis by Inhibiting the Epithelial-Mesenchymal transition and NF-Κb Pathway Activation. Int. J. Mol. Med. 46 (1), 331–339. doi:10.3892/ijmm.2020.4574
Zhang, Y., Du, Y., Yu, H., Zhou, Y., and Ge, F. (2017). Protective Effects of Ophiocordyceps Lanpingensis on Glycerol-Induced Acute Renal Failure in Mice. J. Immunol. Res. 2017, 1–8. doi:10.1155/2017/2012585
Zhang, Y., Zhou, T., Wang, H., Cui, Z., Cheng, F., and Wang, K.-p. (2016a). Structural Characterization and In Vitro Antitumor Activity of an Acidic Polysaccharide from Angelica Sinensis (Oliv.) Diels. Carbohydr. Polym. 147, 401–408. doi:10.1016/j.carbpol.2016.04.002
Zhang, Z., Zhang, D., Dou, M., Li, Z., Zhang, J., and Zhao, X. (2016b). Dendrobium officinale Kimura et Migo attenuates diabetic cardiomyopathy through inhibiting oxidative stress, inflammation and fibrosis in streptozotocin-induced mice. Biomed. Pharmacother. 84, 1350–1358. doi:10.1016/j.biopha.2016.10.074
Zheng, Y., Liu, T., Wang, Z., Xu, Y., Zhang, Q., and Luo, D. (2018). Low Molecular Weight Fucoidan Attenuates Liver Injury via SIRT1/AMPK/PGC1α axis in Db/db Mice. Int. J. Biol. Macromolecules 112, 929–936. doi:10.1016/j.ijbiomac.2018.02.072
Zhou, J., You, W., Sun, G., Li, Y., Chen, B., Ai, J., et al. (2016). The Marine-Derived Oligosaccharide Sulfate MS80, a Novel Transforming Growth Factor 1 Inhibitor, Reverses Epithelial Mesenchymal Transition Induced by Transforming Growth Factor- 1 and Suppresses Tumor Metastasis. J. Pharmacol. Exp. Ther. 359 (1), 54–61. doi:10.1124/jpet.116.234799
Zhou, S., Zhou, Y., Yu, J., Du, Y., Tan, Y., Ke, Y., et al. (2020). Ophiocordyceps Lanpingensis Polysaccharides Attenuate Pulmonary Fibrosis in Mice. Biomed. Pharmacother. 126, 110058. doi:10.1016/j.biopha.2020.110058
Zong, A., Cao, H., and Wang, F. (2012). Anticancer Polysaccharides from Natural Resources: a Review of Recent Research. Carbohydr. Polym. 90 (4), 1395–1410. doi:10.1016/j.carbpol.2012.07.026
Glossary
PF Pulmonary fibrosis
TCM Traditional Chinese medicine
HYP Hydroxyproline
ECM Extracellular matrix
TGF-β Transforming growth factor-β
BLM Bleomycin
COL Collagen
EMT Epithelial-mesenchymal transition
a-SMA a-smooth muscle actin
GPS Ginseng polysaccharide
BPS Basil polysaccharide
APS Astragalus polysaccharide
PPS Polyporus polysaccharide
RSA Rhodiolasachalinensis polysaccharide A
PDO Polysaccharides from dendrobium officinale
BSP Bletilla striata polysaccharide
MS80 Seaweed sulfated oligosaccharide
LMWF Low molecular weight fucoidan
ASP Angelica sinensis polysaccharide
POL Total polysaccharide from O, lanpingensis
LBP Lycium barbarum polysaccharide
FMP-1 Morel polysaccharide-1
GIP Ganoderma lucidum polysaccharide
LLP Lily polysaccharide
YPF-p Yupingfeng-polysaccharide
TβR1 Transforming growth factor-β1 receptor
HLFs Human lung fibrosis
HEPF Human embryo pulmonary fibroblast
DANCR Differentiation antagonistic non-protein coding RNA
Mw Molecular weight
Nrf2 Nuclear factor erythroid 2-related factor 2
AUF1 AU binding factor 1
MMP Matrix metalloproteinase
TIMP
Tissue inhibitor of metalloproteinase
NF-kB Nuclear factor-kB
IL Interleukin
MCP-1 Monocyte chemoattractant protein-1
MDA Malondialdehyde
ROS Reactive oxygen species
SOD Superoxide dismutase
TNF Tumor necrosis factor
Ho-1 Heme oxygenase-1
AKT Protein kinase B
ERK Extracellular signal-regulated kinase
FN Fibronectin
MIP-2 Macrophage inflammatory protein-2
M1 Macrophages 1
CXCL1 Chemokine ligand 1
PI3K Phosphatidylinositol 3-kinase
CAT Catalase
GSH Glutathione
GSH-Px Glutathione peroxidase
MAPK Mitogen -activated protein kinase
AP-1 Activator protein-1
Ara Arabinose
Glc Glucose
Rha Rhamnose
Man Mannose
Gal Galactose
Xyl Xylose
GalA Galacturonic acid
Fru Fructose
Keywords: pulmonary fibrosis, traditional Chinese medicine, polysaccharide, transforming growth factor-β, extracellular matrix, collagen-1, biological activity
Citation: Wu X, Huang J, Wang J, Xu Y, Yang X, Sun M and Shi J (2021) Multi-Pharmaceutical Activities of Chinese Herbal Polysaccharides in the Treatment of Pulmonary Fibrosis: Concept and Future Prospects. Front. Pharmacol. 12:707491. doi: 10.3389/fphar.2021.707491
Received: 10 May 2021; Accepted: 04 August 2021;
Published: 17 August 2021.
Edited by:
Huahao Shen, Zhejiang University, ChinaReviewed by:
Ganesh Prasad Mishra, Swami Vivekanand Subharti University, IndiaHamed Barabadi, Shahid Beheshti University of Medical Sciences, Iran
Copyright © 2021 Wu, Huang, Wang, Xu, Yang, Sun and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianyou Shi, shijianyoude@126.com; Minghan Sun, sunminghan26@gmail.com
†These authors have contributed equally to this work and share first authorship
 Xianbo Wu
Xianbo Wu Jianli Huang
Jianli Huang Jie Wang2
Jie Wang2 Jianyou Shi
Jianyou Shi