- 1Department of Pharmacy, Women’s Hospital, School of Medicine, Zhejiang University, Hangzhou, China
- 2Department of Pharmacy, Hangzhou First People’s Hospital, Hangzhou, China
- 3Department of Pharmacy, Hangzhou Women’s Hospital, Hangzhou, China
Rheumatoid arthritis (RA) is an autoimmune disease characterized by synovial inflammation and bone destruction. Microbial infection is considered to be the most important inducement of RA. The pregnancy planning of women in childbearing age is seriously affected by the disease activity of RA. Gut microbiome, related to immunity and inflammatory response of the host. At present, emerging evidence suggested there are significant differences in the diversity and abundance of gut microbiome during pregnancy and lactation, which may be associated with the fluctuation of RA disease activity. Based on these research foundations, we pioneer the idea of regulating gut microbiome for the treatment of RA during pregnancy and lactation. In this review, we mainly introduce the potential treatment strategies for controlling the disease activity of RA based on gut microbiome during pregnancy and lactation. Besides, we also briefly generalize the effects of conventional anti-rheumatic drugs on gut microbiome, the effects of metabolic changes during pregnancy on gut microbiome, alteration of gut microbiome during pregnancy and lactation, and the effects of anti-rheumatic drugs commonly used during pregnancy and lactation on gut microbiome. These will provide a clear knowledge framework for researchers in immune-related diseases during pregnancy. Regulating gut microbiome may be a potential and effective treatment to control the disease activity of RA during pregnancy and lactation.
Introduction
Rheumatoid arthritis (RA) is an inflammatory autoimmune disease characterized by synovitis and bone destruction (Cai et al., 2020). The pathogenesis of RA is not clear, but it is generally considered to be related to the interactions between genetic and environmental factors (Scherer et al., 2020). And at present, microbial infection is considered to be the most important inducement of RA (Li et al., 2013). RA is prevalent among women, the ratio of males to females is about 1:3, and it mostly occurs between 20 and 55 years old (Croia et al., 2019). In the past decades, the exploration between RA and women indicated that the disease activity of RA fluctuate apparently during pregnancy and lactation. The disease activity of some patients with RA decreased during pregnancy, and some increased during lactation (de Man et al., 2008; Jethwa et al., 2019; Chen et al., 2020). These conditions seriously affect family planning and the health management of women in childbearing age.
Pregnancy is a complicated and delicate process, in which the maternal hormone levels, immunity and metabolism change conspicuously to ensure the normal growth and development of the fetus (Mu et al., 2019). As early as 1931, scholars began to study the relationship between RA disease activity and pregnancy or lactation. Here, a retrospective analysis based on objective markers of RA disease activity and related scores in 2019 showed that the disease activity of 60.3% of pregnant women with RA is improved during pregnancy and that of 46.7% is increased during lactation (Jethwa et al., 2019). What causes this phenomenon? Currently, it is generally believed that this is mainly associated with hormone levels of mother. Briefly, the level of estrogen with the anti-inflammatory effect increased significantly during pregnancy and decreased rapidly during lactation (Ince-Askan et al., 2017), which might be one of the important factors that cause the fluctuation in RA disease activity during pregnancy and lactation (de Jong and Dolhain, 2017). But this cannot explain the fact that many women are still having active disease activity during pregnancy and the proportion of improvement during pregnancy has gradually decreased for decades. The disease activity of RA during pregnancy and lactation severely affects the outcome of mother and fetus and postpartum health management (Bermas, 2017). However, apart from hormonal effects, the current understanding of the relationship between pregnancy or lactation and RA is very limited. Therefore, a clear demonstration of the impact of pregnancy and lactation on RA disease activity and its mechanisms is particularly important for managing the health of pregnant women with RA.
In recent years, the role of gut microbiome in the host health during pregnancy and lactation has received widespread attention. The maternal gut microbiome undergoes tremendous changes throughout pregnancy and lactation (Gomez-Arango et al., 2016b; Mutic et al., 2017; Stanislawski et al., 2017). This provides a novel idea for exploring the relationship between pregnancy or lactation and RA disease activity. During pregnancy and lactation, there are significant changes in the diversity, species richness, and composition of the maternal gut microbiome (Nuriel-Ohayon et al., 2016; Mu et al., 2019). The human gut microbiome is complex and composed of more than 500–1,500 different bacteria, archaea, fungi and viruses (de Brito Alves et al., 2019; DeMartino and Cockburn, 2020; Weersma et al., 2020). They are not only participating in the digestion and absorption of food but also play an important role in regulating immunity and metabolism of the host (Guerreiro et al., 2018; Peterson et al., 2020; Singh, 2020). Gut microbiome disorder leads to changes in microbial metabolites and increased permeability of the intestinal mucosa. These usually disrupt the balance between non-self antigen tolerance and immunity, which may promote antigen absorption and increase the persistence or worsening of immune-mediated diseases like RA (Morrison and Preston, 2016; Guerreiro et al., 2018; Picchianti-Diamanti et al., 2018).
More and more evidence showed that gut microbiome is involved in the formation of adaptive immunity and autoimmunity (Van de Wiele et al., 2016; Agorastos and Bozikas, 2019). The balance between helper T cell subsets and regulatory T cells is to be influenced by specific gut microbiome (Wu et al., 2010). For example, the colonization of segmental filamentous bacteria in mice can induce the production of Th17 cells. In contrast, Clostridia can induce the production of regulatory T cells, thereby suppressing the autoimmune response (Lee and Kim, 2017). Additionally, gut microbiome can directly or indirectly bind NOD-like receptors (Nod-like receptors) and/or Toll-like receptors (TLRs) to activate the immune system and produce metabolites of short-chain fatty acids (Short-chain fatty acids) (Hasegawa et al., 2006; Reichardt et al., 2014). Moreover, based on the study of gut microbiome at present, probiotics and specific gut microbiome metabolites are applicable in the treatment of various autoimmune diseases (Weis, 2018; Marietta et al., 2019).
In this review, we outline the role of gut microbiome in RA, specific changes in gut microbiome during pregnancy or lactation in recent years and the possibility of improving RA disease activity during pregnancy or lactation by regulating gut microbiome. Besides, we also briefly generalize the impacts of conventional anti-rheumatic drugs on gut microbiome, the effects of metabolic changes during pregnancy on gut microbiome, the outcome of mothers and fetuses in pregnant women with RA (Preterm delivery, Cesarean section, small for gestational age), and the anti-rheumatic drugs commonly used during pregnancy and lactation. This review will provide a clear knowledge framework for researchers in the field of pregnancy-related immune diseases. Regulating gut microbiome might be a potential and effective treatment to control the disease activity of RA during pregnancy and lactation.
The Role of Gut Microbiome in Rheumatoid Arthritis
Gut Microbiome in the Etiology of Rheumatoid Arthritis
RA is an inflammatory autoimmune disease characterized by synovial inflammation and bone destruction (Yao et al., 2018; Yao et al., 2019). The pathogenesis of RA is complicated, including the interaction between innate immune response and acquired immune response, which involves the formation of self-reactive T cells, antigen presentation and the formation of autoantibodies. These autoantibodies may come from various parts of the body, such as the gastrointestinal tract, and eventually enter the blood circulation, causing systemic joint inflammation (Malmstrom et al., 2017; Favalli et al., 2019).
The studies showed that the development of RA is based on heredity and epigenetics, but environmental factors also play an important role, such as cigarette smoke and dust contact, especially the microbiome representing the “internal” environment (Figure 1) (Amariuta et al., 2020; Scherer et al., 2020). And the susceptibility of RA is related to environmental factors, such as smoking and infection, which may be mediated by gut microbiome (Klareskog et al., 2006; Luckey et al., 2013). In particular, genotype and sex are strong determinants of gut microbiome, although gut microbiome changes dynamically with age (Gomez et al., 2012; Morgan et al., 2012). In humans, age-related alterations of gut microbial composition are associated with enrichment of pathobionts (Yatsunenko et al., 2012; Rampelli et al., 2013). These changes may cause an increase or loss of intestinal metabolic function, leading to disorders of gut microbiome. Additionally, smoking has been shown to affect the diversity of gut microbiome and quitting smoking will increase the diversity of gut microbiome (Biedermann et al., 2013; Savin et al., 2018). These findings indicate that the decrease in the diversity of gut microbiome is one of the reasons for the development of RA.
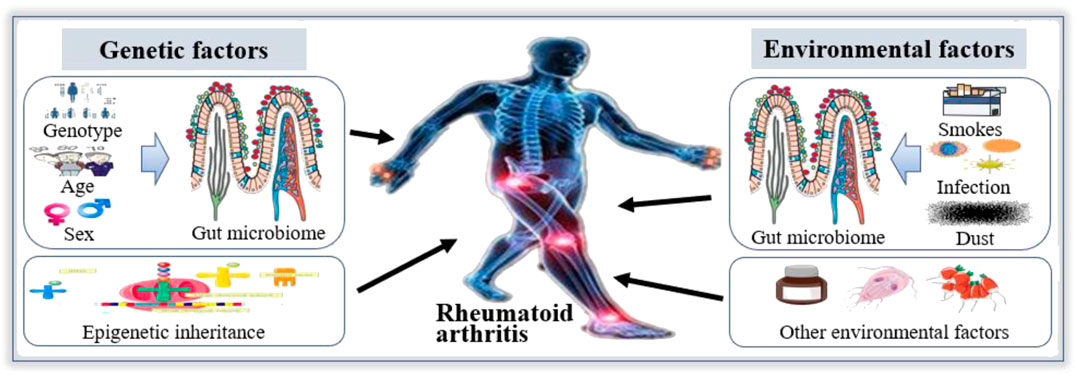
FIGURE 1. Gut microbiome in the etiology of Rheumatoid arthritis (RA). The etiology of RA is mainly related to environmental factors and genetic factors. Among the environmental factors, smoking, infection and dust can affect the structure of gut microbiome to participate in the occurrence and development of RA. Among genetic factors, the composition of gut microbiome is associated with genotype and sex, which is linked to the occurrence and development of RA. Therefore, gut microbiome play an important role in the etiology of RA.
With the increased understanding of gut microbiome, there has been a wide area of related researches, especially in autoimmune diseases. The evidence supporting the association of gut microbiome with RA is as follows: 1) Compared with the control group, the composition of gut microbiome changed in early RA patients, the abundance of certain bacteria in the genus Bifidobacterium and Bacteroides decreased, and genus Prevotella increased significantly (Vaahtovuo et al., 2008; Bernard, 2014; Jeong et al., 2019). 2) In the germ-free model, parenteral injection of cell wall fragments of exogenous intestinal bacteria can induce arthritis (Scher et al., 2013; Bernard, 2014). 3) Partial gut microbiome of clinical RA patients restored to normality after antirheumatic treatment (Zhang et al., 2015; Bodkhe et al., 2019). 4) Existing reports have shown that diet could affect the activity of inflammation, and high-fat diet has a negative effect on intestinal permeability (Skoldstam et al., 2003; Rohr et al., 2020). 5) Many drugs for the treatment of RA have antibacterial properties, such as minocycline, chloroquine, roxithromycin and sulfadiazine (Ogrendik, 2009; Ogrendik and Karagoz, 2011). 6) The alpha diversity index of gut microbiome in the feces of RA patients is significantly lower than that of healthy people. The bacterial genera Bacteroides and Escherichia-Shigella were more abundant in RA patients. In contrast, Lactobacillus, Odoribacter, Enterobacter, and Alloprevotella were less abundant in RA patients than that in healthy people (Drago, 2019; Sun et al., 2019). Based on the above facts, we conclude that gut microbiome may be closely related to RA, and the imbalance of gut microbiome may be one of the causes of RA.
The Mechanism of Gut Microbiome in Rheumatoid Arthritis
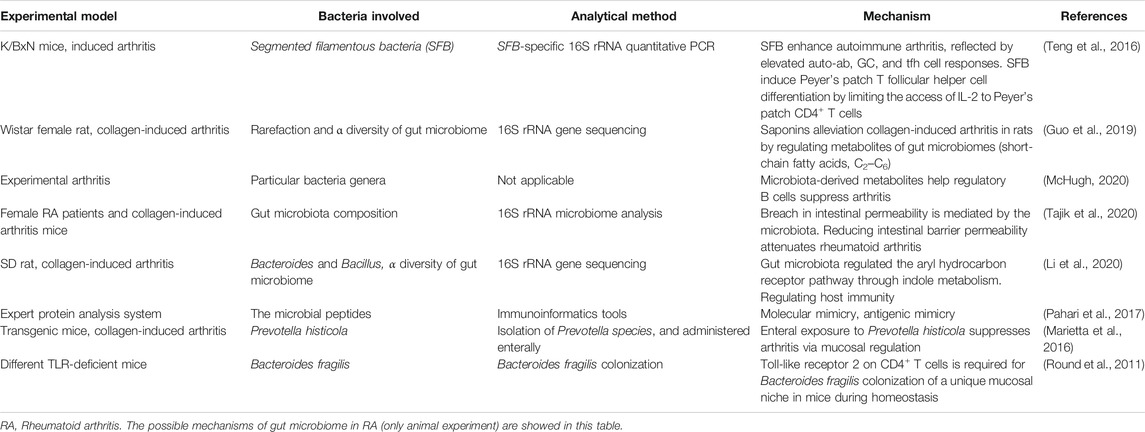
The studies of gut microbiome in animal models, the effects of diet and probiotics on the extent of inflammatory activity support the potential role of gut microbiome in the pathogenesis of RA (Badsha, 2018; Jubair et al., 2018). Researches in RA patients or animal models indicated that the abundance of many genera of gut microbiome increased or decreased significantly compared with the control group (healthy people or animal). The influence mechanism of gut microbiome on the pathophysiology of RA may be multifactorial, and it mainly includes the following aspects: the ability to produce citrullinization of peptides by enzymatic action, activation of antigen-presenting cells through an effect on TLRs or NLRs, antigenic mimicry, control of host immune system (triggering T cell differentiation), alterations in permeability of intestinal mucosal, and increase of T helper type 17-mediated mucosal inflammation [Table 1 (Round et al., 2011; Marietta et al., 2016; Teng et al., 2016; Pahari et al., 2017; Guo et al., 2019; Li et al., 2020; McHugh, 2020; Tajik et al., 2020)].
The evidence related to gut microbiome participating in the pathogenesis of RA all came from animal experiments based on the current research. In humans, it is only proven that there is a difference in gut microbiome between RA patients and healthy people, which cannot clarify the causal relationship between RA and gut microbiome. To predict the role of gut microbiome in the pathogenesis of RA, an improved understanding related to gut microbiome of the ecology and interactions with other microorganisms and hosts is still needed. Furthermore, the interaction between gut microbiome and the immune system might be the most likely influencing factor for gut microbiome to participate in the pathogenesis of RA, especially the influence of gut microbiome on Th17 cells. And the in-depth study of animal experiments will lay a solid foundation for clinical research.
Rheumatoid Arthritis Treatment Based on Gut Microbiome
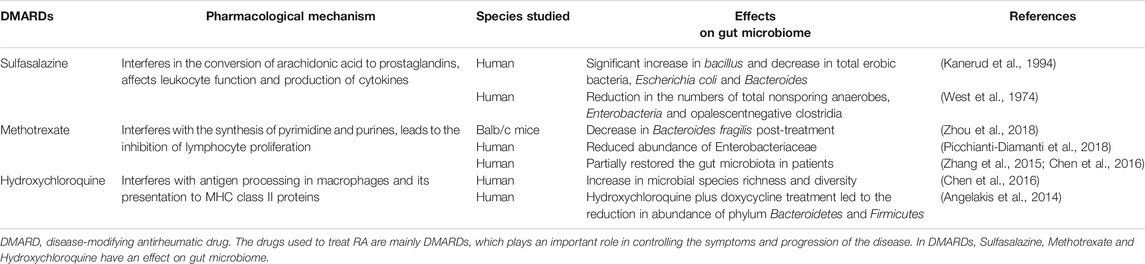
The pathogenesis of RA is considered to be the result of the interaction between genetics and the environment. The drugs used to treat RA are mainly disease-modifying antirheumatic drugs (DMARDs), which play an important role in controlling the symptoms and progression of the disease (Burmester and Pope, 2017). Recently, more and more evidence showed that gut microbiome regulates the host immune system directly or indirectly, for example, some gut microbiome in RA patients returned to normal after treatment with DMARDs (Bodkhe et al., 2019). Microbiome refers to a collection of genomes within an ecological community of microorganisms (Boon et al., 2014). It is difficult to clarify the role and mechanism of gut microbiome in human diseases due to only about 1% of microbes are culturable (Prakash et al., 2013). Fortunately, the development of DNA sequencing technology has made it possible to study unculturable microbes (The Human Microbiome Project Consortium, 2012; Ranjan et al., 2016). Gut microbiome has a strong metabolic potential and an important influence on the stability and activity of drugs that enter the intestine (Vande Voorde et al., 2014; Lehouritis et al., 2015). For example, the action of sulfasalazine (SSZ, a drug used to treat RA) must depend on anaerobic bacteria in the colon (Sousa et al., 2014). Moreover, drug treatment also has a significant effect on the composition of gut microbiome in the host (Sousa et al., 2008; Maier et al., 2018). Therefore, alterations of gut microbiome caused by drug affect the therapeutic effect and the host’s immune response [Table 2 (West et al., 1974; Kanerud et al., 1994; Angelakis et al., 2014; Zhang et al., 2015; Chen et al., 2016; Picchianti-Diamanti et al., 2018; Zhou et al., 2018)].
The drugs currently used to treat RA mainly include traditional DMARDs, biologic DMARDs, biologics, and alternative medicines such as herbs and probiotics (Demoruelle and Deane, 2012). Traditional DMARDs such as leflunomide, methotrexate (MTX), SSZ and hydroxychloroquine are essential in the treatment of RA (Scher et al., 2013). Compared with the control group, the gut microbiome diversity of RA patients increased and the disease activity of RA decreased after receiving these drugs (Zhang et al., 2015; Chen et al., 2016). Table 2 summarizes the effects of some DMARDs on gut microbiome (West et al., 1974; Kanerud et al., 1994; Angelakis et al., 2014; Zhang et al., 2015; Chen et al., 2016; Picchianti-Diamanti et al., 2018; Zhou et al., 2018). In general, biologic DMARDs (bDMARDs) will be considered when RA patients are not effective in the treatment of traditional DMARDs. As with traditional DMARDs, treatment with bDMARDs also causes changes in the abundance of gut microbiome (Picchianti-Diamanti et al., 2018). For example, with the treatment of etanercept (belongs to bDMARDs), the abundance of Cyanobacteria in the intestine increased significantly compared with untreated patients (Bodkhe et al., 2019). Chinese herbal medicine has been used to treat various incurable diseases since ancient times (Rigau-Perez, 2016). In recent years, a relationship between the therapeutic effect of Chinese herbal medicine and gut microbiome has been proved by researchers. Xiao M et al. found that the diversity of gut microbiome increased in collagen-induced arthritis mice treated with Paederia scandens (Chinese herbal medicine) (Xiao et al., 2018). In addition, the therapeutic effect of another Chinese herbal medicine, Tripterygium wilfordii (Chinese herbal medicine), used to treat RA has also been shown to be related to gut microbiome (Miao et al., 2015). After receiving the treatment of Tripterygium wilfordii, the abundance of Prevotella intermedia in the intestine of RA patients was increased (Zhang et al., 2015). What’s more, the direct application of probiotics proves the importance of regulating gut microbiome to RA (Pineda Mde et al., 2011; Vaghef-Mehrabany et al., 2014). Therefore, the treatment of RA based on gut microbiome may be a potential and effective strategy.
Gut Microbiome in Pregnancy and Lactation
Alterations of Gut Microbiome During Pregnancy
Pregnancy is a complex and delicate process with remarkable changes in the hormone level, immunity and metabolism of the mother to ensure the successful growth and development of the fetus (Pazos et al., 2012; Fejzo et al., 2019). The immune system and some complications during pregnancy are related to gut microbiome (Nyangahu et al., 2018; de Brito Alves et al., 2019). Several studies have proved that microbial infections such as bacteria, fungi or viruses during pregnancy are important risk factors for adverse pregnancy outcomes, including eclampsia, premature rupture of membranes, recurrent miscarriage, intrauterine growth retardation and premature delivery (Lamont, 2015; Young et al., 2015). Notably, there is a conspicuous change in the diversity of gut microbiome during the process of pregnancy (Chen et al., 2017). The whole microbial community undergoes an amazing rearrangement related to the host’s physiology and immunity (Koren et al., 2012; Paysour et al., 2019). Recently, some scholars suggested that gut microbiome remodeling during pregnancy is a positive reaction of the mother and necessary for a successful pregnancy, which may contribute to the state of the immunity and metabolism (Konstantinov et al., 2013). However, more researchers believe that changes in gut microbiome during pregnancy are harmful to pregnant women and will cause related complications (Morffy Smith et al., 2019).
The physiological changes of pregnant women are accompanied by the alteration of microbes, especially gut microbiome (Koren et al., 2012; Edwards et al., 2017). The gut microbiome of first trimester is similar in many aspects to that of healthy nonpregnant male and female controls. However, by the third trimester, the structure and composition of the community resemble a disease-associated dysbiosis that differs among women (Koren et al., 2012). Generally, pregnancy is characterized by increased bacterial load and profound changes in the composition of intestinal microorganisms (Nuriel-Ohayon et al., 2016; Smid et al., 2018). During pregnancy, the changes of gut microbiome mainly include the decrease of individual richness (α diversity), the change of specific species richness and the increase of between-subject diversity (β diversity) (Koren et al., 2012; Chen et al., 2017). Table 3 summarizes alterations of gut microbiome during pregnancy (Koren et al., 2012; Gohir et al., 2015; Smid et al., 2018; Chen Y. et al., 2019; Ji et al., 2019; Liu et al., 2019).
Factors Affecting Gut Microbiome During Pregnancy
The initial changes in the mother during pregnancy are hormone levels. Progesterone and estrogen levels rise sharply, which cause many physiological effects (Di Renzo et al., 2016; Hudon Thibeault et al., 2019). On the one hand, these hormone levels are likely to affect the structure of gut microbiome because it has been proven that the composition of microorganisms is involved in responding and regulating host hormone levels, and host hormone levels affect bacterial growth (Gomez-Arango et al., 2016a). On the other hand, microorganisms can also secrete or produce hormones. Therefore, the interaction between hormones and microorganisms is bidirectional, and the two interact with each other (Neuman et al., 2015). However, there is a lack of direct evidence for the effect of gut microbiome on these hormones and the effect of estrogen or progesterone on gut microbiome. Further research is necessary. Besides, the immune system of mothers changes during pregnancy to support the successful growth of the fetus. Many studies have proven that gut microbiome participates in the regulation of the immune system, and the possible mechanism is antigen simulation or change in intestinal permeability (Rooks and Garrett, 2016; Neuman and Koren, 2017).
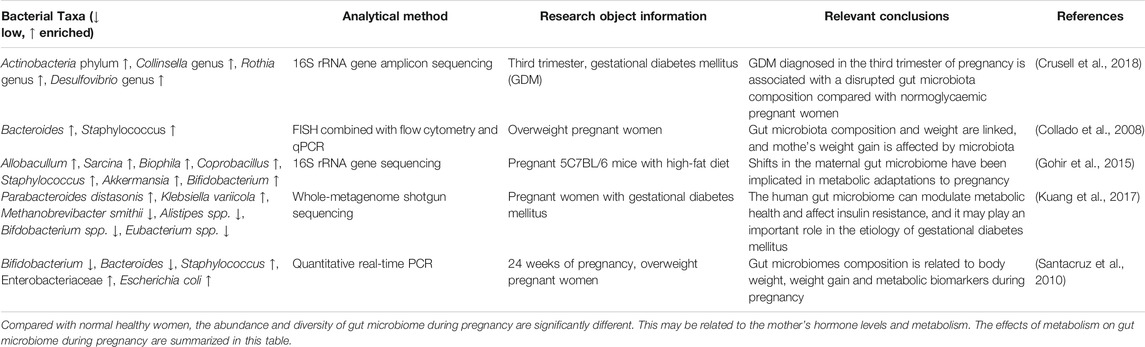
Apart from hormones, the relationship between metabolic changes during pregnancy and gut microbiome has received widespread attention at present. Metabolic changes during pregnancy are very similar to those in metabolic syndrome, including changes in metabolic hormone levels, weight gain, glucose intolerance, elevated fasting blood glucose levels, low-grade inflammation, and insulin resistance (Meo and Hassain, 2016; Nuriel-Ohayon et al., 2016; Zeng et al., 2017). These changes are closely connected with the composition of gut microbiome. Table 4 summarizes the effects of different metabolic changes during pregnancy on gut microbiome (Collado et al., 2008; Santacruz et al., 2010; Gohir et al., 2015; Kuang et al., 2017; Crusell et al., 2018).
Alterations of Gut Microbiome During Lactation
Lactation refers to the period from a mother secretes milk from her mammary glands to the stop of breastfeeding, which generally lasts for 10–12 months. The level of prolactin in postpartum mothers increased significantly, while the level of progesterone decreased rapidly (Vieira Borba and Shoenfeld, 2019). As mentioned above, changes in hormones affect the structure of gut microbiome. At present, the study of gut microbiome in lactation is less than that in pregnancy. Unlike the fluctuations observed during gestation, the bacterial composition appeared to be relatively stable over different stages of lactation (Liu et al., 2019). Liu et al. found that Christensenellaceae, Lachnospiraceae, and Escherichia coli are enriched during lactation in sows (Liu et al., 2019). Besides, another analysis of gut microbiome of the sow from pregnancy to weaning showed that the abundance of Tenericutes during lactation is significantly increased while Bacteroidetes and Fibrobacteres are decreased (Ji et al., 2019). These studies indicate that the gut microbiome during lactation is also in a state of disorder and continues the characteristics of pregnancy. The diversity of gut microbiome during lactation is similar to that in late pregnancy, and there was no significant change over time (Jost et al., 2014). However, we need more evidence to demonstrate the characteristic of gut microbiome in lactation. The difference between gut microbiome in lactation and normal women needs to be clarified to explore the specific genus of gut microbiome during lactation. This may help us to further study the role and mechanism of gut microbiome in disease states and to manage the health of lactating women.
In addition to lactation, there are also significant differences in the composition of gut microbiome in postpartum women. Operational taxonomic units richness and Shannon index decreased from late pregnancy to postpartum regardless of metabolic status (Crusell et al., 2018; Hasan et al., 2018). However, it lack of evidence to demonstrate the difference between breastfeeding and non-breastfeeding women, and further researches are needed.
The Treatment of Rheumatoid Arthritis During Pregnancy and Lactation Based on Gut Microbiome
Fluctuation of Disease Activity During Pregnancy and Lactation, and Feasibility of the Treatment Based on Gut Microbiome
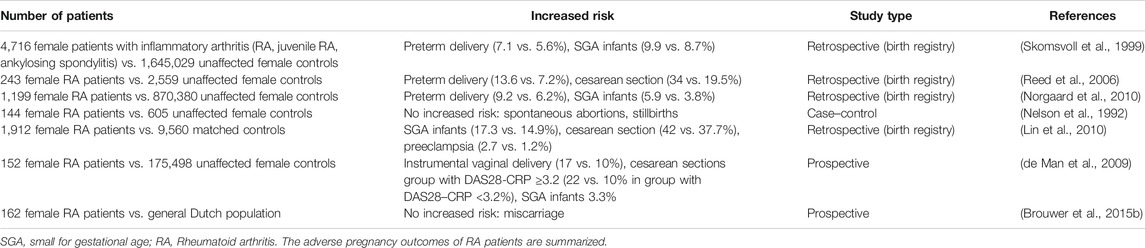
RA is common among women, and the ratio of men to women is close to 1:3. In women with RA, it seems to be more difficult to conceive, and the disease activity affects the mother and fetal outcome (Wallenius et al., 2011; Wallenius et al., 2012; Yao et al., 2020). For example, RA patients have a longer time to pregnancy (TTP). Table 5 summarizes some studies on pregnancy outcomes of RA patients (Nelson et al., 1992; Skomsvoll et al., 1999; Reed et al., 2006; de Man et al., 2009; Lin et al., 2010; Norgaard et al., 2010; Brouwer et al., 2015b). The disease activity of some patients usually improves during pregnancy. However, a large number of RA patients (>50%) still suffer from active diseases during pregnancy. Thus, clinical treatment is necessary, especially considering the negative correlation between active diseases and pregnancy outcomes (de Jong and Dolhain, 2017). At present, during pregnancy and lactation, antirheumatic drugs are mainly used to control disease activities. The main side effect of the approved antirheumatic drugs could increase TTP, including nonsteroidal anti-inflammatory drugs (NSAIDs) and prednisone (in a dose >7.5 mg daily) (Brouwer et al., 2015a). In addition, the use of MTX may damage fertility (Provost et al., 2014). Therefore, a treat-to-target strategy aiming for low disease activity is recommended for RA patients with the plan of conceive.
RA improves during pregnancy and relapses during lactation, but the improvement effect is not as previously thought (Gerosa et al., 2016). This results in a substantial number of RA patients with moderate to high disease activity during pregnancy (Ince-Askan and Dolhain, 2015). Therefore, how to take a scientific and reasonable plan to improve the maternal and infant outcomes and improve or control the condition of these patients has become the focus of clinical research in the field of rheumatism. The causes of the disease activity in pregnancy and lactation are still unclear at present. Through the study of pregnancy and RA and reviewing the relevant reports at home and abroad in recent years, we summarized the possible mechanisms as follows: 1) After pregnancy, the increase in progesterone and estrogen causes the level of glucocorticoid to rise, and estrogen itself also has anti-inflammatory effects. 2) The conversion of hormone secretion after pregnancy, the balance of Thl/Th2 type immune response and the activity of T cells can be indirectly regulated and suppressed by higher levels of estrogen. 3) Changes in regulatory T cell subsets can reduce the immune response to inflammation, which not only makes the mother immune tolerance to the fetus but also reduces the immune response to RA. 4) The conversion of postpartum hormone secretion makes the level of hormones with anti-inflammatory effect drop rapidly so that the pro-inflammatory effect of Th1-related cytokines is dominant. 5) Prolactin influences the negative selection of autoreactive B cells, promoting their proliferation, survival, and antibody production.
However, mechanisms of the fluctuation of RA disease activity during pregnancy and lactation are mainly based on the level of hormones. These cannot explain the decrease in improvement rate of RA during pregnancy in recent years. The improvement of RA during pregnancy can be attributed to not just a single mechanism but most likely several different pathogenic mechanisms (Ince-Askan and Dolhain, 2015; Murakawa, 2016). Controlling RA disease activity during pregnancy and lactation is challenging, especially because several antirheumatic drugs are contraindicated in pregnancy (Forger and Villiger, 2016; Ngian et al., 2016). Extensive studies on gut microbiome in RA and pregnancy revealed that gut microbiome might play an important role in pregnancy with RA. Changes in the diversity of gut microbiome during pregnancy and lactation may be one of the key factors in the fluctuation of RA disease activity. Moreover, compared with anti-rheumatic drugs, regulating the gut microbiome to control the disease activity of RA may be safer for pregnant women and fetuses. Based on the recent studies of gut microbiome between RA and pregnancy or lactation, we propose a new treatment strategy: regulating gut microbiome. Here, we summarize several methods for the treatment of RA during pregnancy and lactation based on gut microbiome.
Therapeutic Method Based on Gut Microbiome
Fecal Microbiota Transplantation
Fecal microbiota transplantation (FMT) therapy refers to the transfer of fecal microbial extracts from healthy donors to diseased recipients through colonoscopy, oral, nasogastric tube, nasal duodenum, or enema, to reconstruct the complete intestinal microbial community and reverse the imbalance of microecology (Vindigni and Surawicz, 2017; Wang et al., 2019). The research on FMT could be traced back to 1958. Four patients with pseudomembranous colitis survived through FMT (Eiseman et al., 1958). At present, FMT is mainly used to combat Clostridium difficile infectious colitis, and the cure rate can reach 90% (Vindigni and Surawicz, 2017). Additionally, FMT is also used in other diseases, such as Parkinson’s disease, obesity and metabolic syndrome, cancer, and multiple sclerosis (Choi and Cho, 2016; Chen D. et al., 2019; Zhou et al., 2019). FMT has not been used in patients with RA. However, when discussing the potential application of this method in inflammatory arthritis, some scholars suggested that the research results of FMT in inflammatory bowel disease are related to inflammatory arthritis. Especially compared with ulcerative colitis, there is a stronger association between spinal arthritis and Crohn’s disease (Abdollahi-Roodsaz et al., 2016). Therefore, FMT may be a therapeutic method for improving RA disease activity during pregnancy after the mechanism of gut microbiome in pregnancy with RA has been elucidated.
Prebiotics or Probiotics Supplementation
Prebiotics is an indigestible food ingredient that selectively stimulates the growth and activity of probiotics in the intestine, thereby improving host health (Naseer et al., 2020; Ricke et al., 2020). It is currently defined as “a substrate that is selectively utilized by host microorganisms endowed with health benefits,” and its “selectivity” mainly refers to Lactobacillus and Bifidobacterium (da Silva et al., 2020). Oral prebiotics supplements, especially those added with plant polysaccharides, are effective to improve gut microbiome (Roberfroid et al., 2010). Probiotics are beneficial to human health, such as Bifidobacterium, Lactobacillus and Yeast (Morovic and Budinoff, 2020). Probiotics/prebiotics is widely used in the medical field to prevent or treat some diseases, and their therapeutic effect on RA has been confirmed in animals and humans (Mohammed et al., 2017). Probiotics/prebiotics intervention may be a safe and effective method to regulate gut microbiome.
Reasonable Use of Antibiotics
The treatment of gut microbiome also includes the use and management of antibiotics (Iizumi et al., 2017). Different kinds of antibiotics have different effects on gut microbiome (Lange et al., 2016). A study showed that the delayed onset of RA flares after the use of specific antibiotics might be mediated through the gut microbiome (Nagra et al., 2019). However, the use of antibiotics during pregnancy could affect the health of infant and the exposure of the breastfed infant to antibiotics is related to bacterial resistance and the development of gut microbiome (van Wattum et al., 2019; Zimmermann and Curtis, 2020). Therefore, the use of antibiotics to regulate gut microbiome during pregnancy should be carefully considered. And reasonable use of antibiotics can effectively control the disease activity of RA during pregnancy or lactation.
Gut Pharmacomicrobiomics
The ability of gut microbiome to metabolize drugs is comparable to that of the liver (Enright et al., 2016). Gut pharmacomicrobiomics reflects the influence of changes in gut microbiome on drug pharmacokinetics and pharmacodynamics (Saad et al., 2012; Doestzada et al., 2018). Most drugs have little contact with gut microbiome because they are rapidly and completely absorbed by the upper gastrointestinal tract (Kim, 2015). However, some drugs are converted into active, inactive or toxic metabolites by gut microbiome located in the ileum and colon (Wilson and Nicholson, 2017). For example, the antirheumatic drug sulfasalazine is broken down into sulfapyridine and 5-aminosalicylic acid by the bacterial azoreductase in the colon, which is not metabolized when antibiotics are given (Peppercorn and Goldman, 1972). Pharmacomicrobiomics based on gut microbiome may be a potential treatment for RA during pregnancy or lactation. Current research needs to further clarify the mechanism of interaction between gut microbiome and drugs. The intervention of enzymes produced by specific bacteria may be of great significance for the treatment of RA during pregnancy or lactation.
Diet Regulation
Gut microbiome could be involved in energy balance by affecting the efficiency of energy harvesting from the diet and the expression of host genes related to the regulation of energy storage and expenditure (Guo et al., 2018). It is well known that diet is closely related to obesity and hyperlipidemia. Obesity and hyperlipidemia have a great influence on gut microbiome during pregnancy. The abundance of the phylum Firmicutes was lower and Bacteroidetes was higher in strict vegetarians, with the genus Prevotella being increased among other changes (Franco-de-Moraes et al., 2017). A randomized controlled trial showed that differences exited in the relative abundance of several genera in pregnancy women on a vegetarian diet, specifically a reduction in Collinsella, Holdemania, and increases in the relative abundances of Roseburia and Lachnospiraceae (Barrett et al., 2018). Therefore, diet control may be an effective treatment method for RA during pregnancy or lactation.
Discussion
Presently, there is limited knowledge about the fluctuation of RA activity during pregnancy or lactation in addition to hormonal effects. We speculate that the changes of gut microbiome during pregnancy are harmful to the disease activity of RA based on current studies. The improvement of the disease activity of RA during pregnancy may be the result of neutralization between the harmful effects of gut microbiome and the beneficial effects of estrogen and progesterone. From late pregnancy to lactation, the levels of estrogen and progesterone decreased rapidly, and the leading role of gut microbiome appeared, showing the recurrence of RA at this time. Therefore, regulation of gut microbiome may be a potential strategy for the treatment of RA during pregnancy and lactation. Combined with domestic and foreign literature reports, the treatment of RA based on gut microbiome mainly includes fecal microbiota transplantation, prebiotics or probiotics supplementation, reasonable use of antibiotics, gut pharmacomicrobiomics and diet regulation.
However, current knowledge in this field is limited. Most studies related to gut microbiome during pregnancy or lactation are animal experiments. Therefore, there is a long journey before conclusion, the first step is to clarify the difference of gut microbiome between pregnant women with RA and healthy pregnant women. Then the specific bacteria associated with pregnancy with RA and its mechanism should be demonstrated. Finally, the method based on regulating gut microbiome to improve the disease activity of RA during pregnancy or lactation should be explored. The mechanisms of gut microbiome in RA during pregnancy and lactation may be (Figure 2): 1) The ability to produce citrullinization of peptides by enzymatic action. 2) Activation of antigenpresenting cells through an effect on TLRs or NLRs. 3) Antigenic mimicry 4) Control of the host immune system (triggering T cell differentiation). 5) Alterations in the permeability of intestinal mucosal. 6) Increase of T helper type 17-mediated mucosal inflammation.
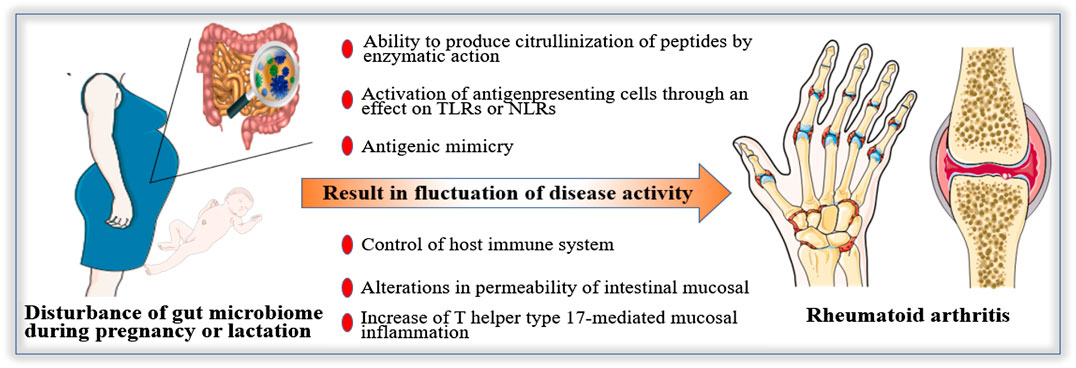
FIGURE 2. Possible mechanisms of gut microbiome in Rheumatoid arthritis (RA) during pregnancy and lactation. The fluctuation of disease activity of RA during pregnancy and lactation may be affected by gut microbiome. The possible mechanisms are as follows: 1) The ability to produce citrullinization of peptides by enzymatic action. 2) Activation of antigenpresenting cells through an effect on Toll-like receptors or Nod-like receptors. 3) Antigenic mimicry 4) Control of host immune system (triggering T cell differentiation). 5) Alterations in permeability of intestinal mucosal. 6) Increase of T helper type 17-mediated mucosal inflammation.
In general, gut microbiome is a double-edged sword. Understanding the mechanism of gut microbiome in RA during pregnancy and lactation will provide potential and effective ideas for controlling the disease activity of RA. Mastering it will be beneficial to the treatment of RA during pregnancy or lactation.
Conclusion
In summary, the conclusions of this paper are as follows: 1) Gut microbiome participates in the pathogenesis of RA and plays an important role in the development of RA. 2) There are significant differences in the diversity and abundance of gut microbiome during pregnancy and lactation compared with healthy women. 3) The disease activity of RA fluctuates abnormally during pregnancy or lactation, and the overall manifestation is aggravated, which affects the pregnancy plan. 4) Regulation of gut microbiome during pregnancy or lactation may be an effective treatment for RA. 5) Mechanisms of gut microbiome affecting the disease activity of RA during pregnancy or lactation may be related to the immune system, citrullinization of peptides and permeability of intestinal mucosal.
Author Contributions
YY and XC drafted the manuscript. Conceptualization was carried out by YY and CZ. WF assisted in reviewing literature. FR and FW modified the manuscript. FC and XL helped in reviewing the first draft of the manuscript. YY and CZ reviewed and edited the final manuscript. All authors approved the final manuscript and agreed to be accountable for all aspects of the work.
Funding
This study was supported by The National Natural Science Foundation of China (Grant No. 81873838), and Zhejiang Provincial Natural Science Foundation of China (Grant No. LYY18H310001).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
CIA, collagen-induced arthritis; DMARDs, disease-modifying antirheumatic drugs; FMT, fecal microbiota transplantation; GDM, gestational diabetes mellitus; MTX, methotrexate; NLRs, Nod-like receptors; RA, rheumatoid arthritis; SCFAs, short-chain fatty acids; SFB, segmented filamentous bacteria; SGA, small for gestational age; SSZ, sulfasalazine; Th1/Th2/Th17, T helper cell 1/2/17; TLRs, Toll-like receptors; TTP, time to pregnancy.
References
Abdollahi-Roodsaz, S., Abramson, S. B., and Scher, J. U. (2016). The metabolic role of the gut microbiota in health and rheumatic disease: mechanisms and interventions. Nat. Rev. Rheumatol. 12 (8), 446–455. doi:10.1038/nrrheum.2016.68
Agorastos, A., and Bozikas, V. P. (2019). Gut microbiome and adaptive immunity in schizophrenia. Psychiatriki 30 (3), 189–192. doi:10.22365/jpsych.2019.303.189
Amariuta, T., Luo, Y., Knevel, R., Okada, Y., and Raychaudhuri, S. (2020). Advances in genetics toward identifying pathogenic cell states of rheumatoid arthritis. Immunol. Rev. 294 (1), 188–204. doi:10.1111/imr.12827
Angelakis, E., Million, M., Kankoe, S., Lagier, J. C., Armougom, F., Giorgi, R., et al. (2014). Abnormal weight gain and gut microbiota modifications are side effects of long-term doxycycline and hydroxychloroquine treatment. Antimicrob. Agents Chemother. 58 (6), 3342–3347. doi:10.1128/AAC.02437-14
Badsha, H. (2018). Role of diet in influencing rheumatoid arthritis disease activity. Open Rheumatol. J. 12, 19–28. doi:10.2174/1874312901812010019
Barrett, H. L., Gomez-Arango, L. F., Wilkinson, S. A., McIntyre, H. D., Callaway, L. K., Morrison, M., et al. (2018). A vegetarian diet is a major determinant of gut microbiota composition in early pregnancy. Nutrients 10 (7), 890. doi:10.3390/nu10070890
Bermas, B. L. (2017). Lactation and management of postpartum disease. Rheum. Dis. Clin. N. Am. 43 (2), 249–262. doi:10.1016/j.rdc.2016.12.002
Bernard, N. J. (2014). Rheumatoid arthritis: Prevotella copri associated with new-onset untreated RA. Nat. Rev. Rheumatol. 10 (1), 2. doi:10.1038/nrrheum.2013.187
Biedermann, L., Zeitz, J., Mwinyi, J., Sutter-Minder, E., Rehman, A., Ott, S. J., et al. (2013). Smoking cessation induces profound changes in the composition of the intestinal microbiota in humans. PLoS One 8 (3), e59260. doi:10.1371/journal.pone.0059260
Bodkhe, R., Balakrishnan, B., and Taneja, V. (2019). The role of microbiome in rheumatoid arthritis treatment. Ther. Adv. Musculoskelet. Dis. 11, 1759720X19844632. doi:10.1177/1759720X19844632
Boon, E., Meehan, C. J., Whidden, C., Wong, D. H., Langille, M. G., and Beiko, R. G. (2014). Interactions in the microbiome: communities of organisms and communities of genes. FEMS Microbiol. Rev. 38 (1), 90–118. doi:10.1111/1574-6976.12035
Brouwer, J., Hazes, J. M., Laven, J. S., and Dolhain, R. J. (2015a). Fertility in women with rheumatoid arthritis: influence of disease activity and medication. Ann. Rheum. Dis. 74 (10), 1836–1841. doi:10.1136/annrheumdis-2014-205383
Brouwer, J., Laven, J. S., Hazes, J. M., and Dolhain, R. J. (2015b). Brief report: miscarriages in female rheumatoid arthritis patients: associations with serologic findings, disease activity, and antirheumatic drug treatment. Arthritis Rheum. 67 (7), 1738–1743. doi:10.1002/art.39137
Burmester, G. R., and Pope, J. E. (2017). Novel treatment strategies in rheumatoid arthritis. Lancet 389 (10086), 2338–2348. doi:10.1016/S0140-6736(17)31491-5
Cai, X. Y., Zhu, Y., Wang, C., Tang, X. Y., Han, L., Shu, J. L., et al. (2020). Etanercept inhibits B cell differentiation by regulating TNFRII/TRAF2/NF-kappaB signaling pathway in rheumatoid arthritis. Front. Pharmacol. 11, 676. doi:10.3389/fphar.2020.00676 | Pubmed
Chen, D., Wu, J., Jin, D., Wang, B., and Cao, H. (2019). Fecal microbiota transplantation in cancer management: current status and perspectives. Int. J. Canc. 145 (8), 2021–2031. doi:10.1002/ijc.32003
Chen, J., Wright, K., Davis, J. M., Jeraldo, P., Marietta, E. V., Murray, J., et al. (2016). An expansion of rare lineage intestinal microbes characterizes rheumatoid arthritis. Genome Med. 8 (1), 43. doi:10.1186/s13073-016-0299-7
Chen, W. M. Y., Subesinghe, S., Muller, S., Hider, S. L., Mallen, C. D., and Scott, I. C. (2020). The association between gravidity, parity and the risk of developing rheumatoid arthritis: a systematic review and meta-analysis. Semin. Arthritis Rheum. 50 (2), 252–260. doi:10.1016/j.semarthrit.2019.09.003
Chen, X., Liu, S., Tan, Q., Shoenfeld, Y., and Zeng, Y. (2017). Microbiome, autoimmunity, allergy, and helminth infection: the importance of the pregnancy period. Am. J. Reprod. Immunol. 78 (2). doi:10.1111/aji.12654
Chen, Y., Li, Z., Tye, K. D., Luo, H., Tang, X., Liao, Y., et al. (2019) Probiotic supplementation during human pregnancy affects the gut microbiota and immune status. Front. Cell Infect. Microbiol. 9, 254. doi:10.3389/fcimb.2019.00254
Choi, H. H., and Cho, Y. S. (2016). Fecal microbiota transplantation: current applications, effectiveness, and future perspectives. Clin. Endosc. 49 (3), 257–265. doi:10.5946/ce.2015.117
Collado, M. C., Isolauri, E., Laitinen, K., and Salminen, S. (2008). Distinct composition of gut microbiota during pregnancy in overweight and normal-weight women. Am. J. Clin. Nutr. 88 (4), 894–899. doi:10.1093/ajcn/88.4.894
Croia, C., Bursi, R., Sutera, D., Petrelli, F., Alunno, A., and Puxeddu, I. (2019). One year in review 2019: pathogenesis of rheumatoid arthritis. Clin. Exp. Rheumatol. 37 (3), 347–357.
Crusell, M. K. W., Hansen, T. H., Nielsen, T., Allin, K. H., Ruhlemann, M. C., Damm, P., et al. (2018). Gestational diabetes is associated with change in the gut microbiota composition in third trimester of pregnancy and postpartum. Microbiome 6 (1), 89. doi:10.1186/s40168-018-0472-x
da Silva, T. F., Casarotti, S. N., de Oliveira, G. L. V., and Penna, A. L. B. (2020). The impact of probiotics, prebiotics, and synbiotics on the biochemical, clinical, and immunological markers, as well as on the gut microbiota of obese hosts. Crit. Rev. Food Sci. Nutr.: 1–19. doi:10.1080/10408398.2020.1733483
de Brito Alves, J. L., de Oliveira, Y., Carvalho, N. N. C., Cavalcante, R. G. S., Pereira Lira, M. M., Nascimento, L., et al. (2019). Gut microbiota and probiotic intervention as a promising therapeutic for pregnant women with cardiometabolic disorders: present and future directions. Pharmacol. Res. 145, 104252. doi:10.1016/j.phrs.2019.104252
de Jong, P. H., and Dolhain, R. J. (2017). Fertility, pregnancy, and lactation in rheumatoid arthritis. Rheum. Dis. Clin. N. Am. 43 (2), 227–237. doi:10.1016/j.rdc.2016.12.004
de Man, Y. A., Dolhain, R. J., van de Geijn, F. E., Willemsen, S. P., and Hazes, J. M. (2008). Disease activity of rheumatoid arthritis during pregnancy: results from a nationwide prospective study. Arthritis Rheum. 59 (9), 1241–1248. doi:10.1002/art.24003
de Man, Y. A., Hazes, J. M., van der Heide, H., Willemsen, S. P., de Groot, C. J., Steegers, E. A., et al. (2009). Association of higher rheumatoid arthritis disease activity during pregnancy with lower birth weight: results of a national prospective study. Arthritis Rheum. 60 (11), 3196–3206. doi:10.1002/art.24914
DeMartino, P., and Cockburn, D. W. (2020). Resistant starch: impact on the gut microbiome and health. Curr. Opin. Biotechnol. 61, 66–71. doi:10.1016/j.copbio.2019.10.008
Demoruelle, M. K., and Deane, K. D. (2012). Treatment strategies in early rheumatoid arthritis and prevention of rheumatoid arthritis. Curr. Rheumatol. Rep. 14 (5), 472–480. doi:10.1007/s11926-012-0275-1
Di Renzo, G. C., Giardina, I., Clerici, G., Brillo, E., and Gerli, S. (2016). Progesterone in normal and pathological pregnancy. Horm. Mol. Biol. Clin. Invest. 27 (1), 35–48. doi:10.1515/hmbci-2016-0038
Doestzada, M., Vila, A. V., Zhernakova, A., Koonen, D. P. Y., Weersma, R. K., Touw, D. J., et al. (2018). Pharmacomicrobiomics: a novel route towards personalized medicine? Protein Cell 9 (5), 432–445. doi:10.1007/s13238-018-0547-2
Drago, L. (2019). Prevotella copri and microbiota in rheumatoid arthritis: fully convincing evidence? J. Clin. Med. 8 (11), 1837. doi:10.3390/jcm8111837
Edwards, S. M., Cunningham, S. A., Dunlop, A. L., and Corwin, E. J. (2017). The maternal gut microbiome during pregnancy. MCN Am. J. Matern. Child Nurs. 42 (6), 310–317. doi:10.1097/NMC.0000000000000372
Eiseman, B., Silen, W., Bascom, G. S., and Kauvar, A. J. (1958). Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery 44 (5), 854–859.
Enright, E. F., Gahan, C. G., Joyce, S. A., and Griffin, B. T. (2016). The impact of the gut microbiota on drug metabolism and clinical outcome. Yale J. Biol. Med. 89 (3), 375–382.
Favalli, E. G., Biggioggero, M., Crotti, C., Becciolini, A., Raimondo, M. G., and Meroni, P. L. (2019). Sex and management of rheumatoid arthritis. Clin. Rev. Allergy Immunol. 56 (3), 333–345. doi:10.1007/s12016-018-8672-5
Fejzo, M. S., Trovik, J., Grooten, I. J., Sridharan, K., Roseboom, T. J., Vikanes, A., et al. (2019). Nausea and vomiting of pregnancy and hyperemesis gravidarum. Nat. Rev. Dis. Primers 5 (1), 62. doi:10.1038/s41572-019-0110-3
Forger, F., and Villiger, P. M. (2016). Treatment of rheumatoid arthritis during pregnancy: present and future. Expet Rev. Clin. Immunol. 12 (9), 937–944. doi:10.1080/1744666X.2016.1184973
Franco-de-Moraes, A. C., de Almeida-Pititto, B., da Rocha Fernandes, G., Gomes, E. P., da Costa Pereira, A., and Ferreira, S. R. G. (2017). Worse inflammatory profile in omnivores than in vegetarians associates with the gut microbiota composition. Diabetol. Metab. Syndrome 9, 62. doi:10.1186/s13098-017-0261-x
Gerosa, M., Schioppo, T., and Meroni, P. L. (2016). Challenges and treatment options for rheumatoid arthritis during pregnancy. Expet Opin. Pharmacother. 17 (11), 1539–1547. doi:10.1080/14656566.2016.1197204
Gohir, W., Whelan, F. J., Surette, M. G., Moore, C., Schertzer, J. D., and Sloboda, D. M. (2015). Pregnancy-related changes in the maternal gut microbiota are dependent upon the mother’s periconceptional diet. Gut Microb. 6 (5), 310–320. doi:10.1080/19490976.2015.1086056
Gomez, A., Luckey, D., Yeoman, C. J., Marietta, E. V., Berg Miller, M. E., Murray, J. A., et al. (2012). Loss of sex and age driven differences in the gut microbiome characterize arthritis-susceptible 0401 mice but not arthritis-resistant 0402 mice. PLoS One 7 (4), e36095. doi:10.1371/journal.pone.0036095
Gomez-Arango, L. F., Barrett, H. L., McIntyre, H. D., Callaway, L. K., Morrison, M., Dekker Nitert, M., et al. (2016a). Connections between the gut microbiome and metabolic hormones in early pregnancy in overweight and obese women. Diabetes 65 (8), 2214–2223. doi:10.2337/db16-0278
Gomez-Arango, L. F., Barrett, H. L., McIntyre, H. D., Callaway, L. K., Morrison, M., Dekker Nitert, M., et al. (2016b). Increased systolic and diastolic blood pressure is associated with altered gut microbiota composition and butyrate production in early pregnancy. Hypertension 68 (4), 974–981. doi:10.1161/HYPERTENSIONAHA.116.07910
Guerreiro, C. S., Calado, A., Sousa, J., and Fonseca, J. E. (2018). Diet, microbiota, and gut permeability-the unknown triad in rheumatoid arthritis. Front. Med. 5, (349. doi:10.3389/fmed.2018.00349
Guo, L. X., Wang, H. Y., Liu, X. D., Zheng, J. Y., Tang, Q., Wang, X. N., et al. (2019). Saponins from Clematis mandshurica Rupr. regulates gut microbiota and its metabolites during alleviation of collagen-induced arthritis in rats. Pharmacol. Res. 149, 104459. doi:10.1016/j.phrs.2019.104459
Guo, Y., Wang, Z., Chen, L., Tang, L., Wen, S., Liu, Y., et al. (2018). Diet induced maternal obesity affects offspring gut microbiota and persists into young adulthood. Food Funct. 9 (8), 4317–4327. doi:10.1039/c8fo00444g
Hasan, S., Aho, V., Pereira, P., Paulin, L., Koivusalo, S. B., Auvinen, P., et al. (2018). Gut microbiome in gestational diabetes: a cross-sectional study of mothers and offspring 5 years postpartum. Acta Obstet. Gynecol. Scand. 97 (1), 38–46. doi:10.1111/aogs.13252
Hasegawa, M., Yang, K., Hashimoto, M., Park, J. H., Kim, Y. G., Fujimoto, Y., et al. (2006). Differential release and distribution of Nod1 and Nod2 immunostimulatory molecules among bacterial species and environments. J. Biol. Chem. 281 (39), 29054–29063. doi:10.1074/jbc.M602638200
Hudon Thibeault, A. A., Sanderson, J. T., and Vaillancourt, C. (2019). Serotonin-estrogen interactions: what can we learn from pregnancy? Biochimie 161, 88–108. doi:10.1016/j.biochi.2019.03.023
Iizumi, T., Battaglia, T., Ruiz, V., and Perez Perez, G. I. (2017). Gut microbiome and antibiotics. Arch. Med. Res. 48 (8), 727–734. doi:10.1016/j.arcmed.2017.11.004
Ince-Askan, H., and Dolhain, R. J. (2015). Pregnancy and rheumatoid arthritis. Best Pract. Res. Clin. Rheumatol. 29 (4–5), 580–596. doi:10.1016/j.berh.2015.07.001
Ince-Askan, H., Hazes, J. M. W., and Dolhain, R. (2017). Identifying clinical factors associated with low disease activity and remission of rheumatoid arthritis during pregnancy. Arthritis Care Res. 69 (9), 1297–1303. doi:10.1002/acr.23143
Jeong, Y., Kim, J. W., You, H. J., Park, S. J., Lee, J., Ju, J. H., et al. (2019). Gut microbial composition and function are altered in patients with early rheumatoid arthritis. J. Clin. Med. 8 (5), 693. doi:10.3390/jcm8050693
Jethwa, H., Lam, S., Smith, C., and Giles, I. (2019). Does rheumatoid arthritis really improve during pregnancy? A systematic review and metaanalysis. J. Rheumatol. 46 (3), 245–250. doi:10.3899/jrheum.180226
Ji, Y. J., Li, H., Xie, P. F., Li, Z. H., Li, H. W., Yin, Y. L., et al. (2019). Stages of pregnancy and weaning influence the gut microbiota diversity and function in sows. J. Appl. Microbiol. 127 (3), 867–879. doi:10.1111/jam.14344
Jost, T., Lacroix, C., Braegger, C., and Chassard, C. (2014). Stability of the maternal gut microbiota during late pregnancy and early lactation. Curr. Microbiol. 68 (4), 419–427. doi:10.1007/s00284-013-0491-6
Jubair, W. K., Hendrickson, J. D., Severs, E. L., Schulz, H. M., Adhikari, S., Ir, D., et al. (2018). Modulation of inflammatory arthritis in mice by gut microbiota through mucosal inflammation and autoantibody generation. Arthritis Rheum. 70 (8), 1220–1233. doi:10.1002/art.40490
Kanerud, L., Scheynius, A., Nord, C. E., and Hafstrom, I. (1994). Effect of sulphasalazine on gastrointestinal microflora and on mucosal heat shock protein expression in patients with rheumatoid arthritis. Br. J. Rheumatol. 33 (11), 1039–1048. doi:10.1093/rheumatology/33.11.1039
Kim, D. H. (2015). Gut microbiota-mediated drug-antibiotic interactions. Drug Metab. Dispos. 43 (10), 1581–1589. doi:10.1124/dmd.115.063867
Klareskog, L., Padyukov, L., Ronnelid, J., and Alfredsson, L. (2006). Genes, environment and immunity in the development of rheumatoid arthritis. Curr. Opin. Immunol. 18 (6), 650–655. doi:10.1016/j.coi.2006.06.004
Konstantinov, S. R., van der Woude, C. J., and Peppelenbosch, M. P. (2013). Do pregnancy-related changes in the microbiome stimulate innate immunity? Trends Mol. Med. 19 (8), 454–459. doi:10.1016/j.molmed.2013.06.002
Koren, O., Goodrich, J. K., Cullender, T. C., Spor, A., Laitinen, K., Backhed, H. K., et al. (2012). Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 150 (3), 470–480. doi:10.1016/j.cell.2012.07.008
Kuang, Y. S., Lu, J. H., Li, S. H., Li, J. H., Yuan, M. Y., He, J. R., et al. (2017). Connections between the human gut microbiome and gestational diabetes mellitus. GigaScience 6 (8), 1–12. doi:10.1093/gigascience/gix058
Lamont, R. F. (2015). Advances in the prevention of infection-related preterm birth. Front. Immunol. 6, 566. doi:10.3389/fimmu.2015.00566
Lange, K., Buerger, M., Stallmach, A., and Bruns, T. (2016). Effects of antibiotics on gut microbiota. Dig. Dis. 34 (3), 260–268. doi:10.1159/000443360
Lee, N., and Kim, W. U. (2017). Microbiota in T-cell homeostasis and inflammatory diseases. Exp. Mol. Med. 49 (5), e340. doi:10.1038/emm.2017.36
Lehouritis, P., Cummins, J., Stanton, M., Murphy, C. T., McCarthy, F. O., Reid, G., et al. (2015). Local bacteria affect the efficacy of chemotherapeutic drugs. Sci. Rep. 5, 14554. doi:10.1038/srep14554
Li, S., Yu, Y., Yue, Y., Zhang, Z., and Su, K. (2013). Microbial infection and rheumatoid arthritis. J. Clin. Cell. Immunol. 4 (6), 174. doi:10.4172/2155-9899.1000174
Li, X., Lu, C., Fan, D., Lu, X., Xia, Y., Zhao, H., et al. (2020). Human umbilical mesenchymal stem cells display therapeutic potential in rheumatoid arthritis by regulating interactions between immunity and gut microbiota via the aryl hydrocarbon receptor. Front. Cell Dev. Biol. 8, 131. doi:10.3389/fcell.2020.00131
Lin, H. C., Chen, S. F., Lin, H. C., and Chen, Y. H. (2010). Increased risk of adverse pregnancy outcomes in women with rheumatoid arthritis: a nationwide population-based study. Ann. Rheum. Dis. 69 (4), 715–717. doi:10.1136/ard.2008.105262
Liu, H., Hou, C., Li, N., Zhang, X., Zhang, G., Yang, F., et al. (2019). Microbial and metabolic alterations in gut microbiota of sows during pregnancy and lactation. FASEB J. 33 (3), 4490–4501. doi:10.1096/fj.201801221RR
Luckey, D., Gomez, A., Murray, J., White, B., and Taneja, V. (2013). Bugs & us: the role of the gut in autoimmunity. Indian J. Med. Res. 138 (5), 732–743.
Maier, L., Pruteanu, M., Kuhn, M., Zeller, G., Telzerow, A., Anderson, E. E., et al. (2018). Extensive impact of non-antibiotic drugs on human gut bacteria. Nature 555 (7698), 623–628. doi:10.1038/nature25979
Malmstrom, V., Catrina, A. I., and Klareskog, L. (2017). The immunopathogenesis of seropositive rheumatoid arthritis: from triggering to targeting. Nat. Rev. Immunol. 17 (1), 60–75. doi:10.1038/nri.2016.124
Marietta, E., Horwath, I., Balakrishnan, B., and Taneja, V. (2019). Role of the intestinal microbiome in autoimmune diseases and its use in treatments. Cell. Immunol. 339, 50–58. doi:10.1016/j.cellimm.2018.10.005
Marietta, E. V., Murray, J. A., Luckey, D. H., Jeraldo, P. R., Lamba, A., Patel, R., et al. (2016). Suppression of inflammatory arthritis by human gut-derived Prevotella histicola in humanized mice. Arthritis Rheum. 68 (12), 2878–2888. doi:10.1002/art.39785
McHugh, J. (2020). Microbiota-derived metabolites help regulatory B cells suppress arthritis. Nat. Rev. Rheumatol. 16 (6), 297. doi:10.1038/s41584-020-0425-1
Meo, S. A., and Hassain, A. (2016). Metabolic physiology in pregnancy. J. Pakistan Med. Assoc. 66 (9 Suppl. 1), S8–S10.
Miao, G. P., Zhu, C. S., Feng, J. T., Han, L. R., and Zhang, X. (2015). Effects of plant stress signal molecules on the production of wilforgine in an endophytic actinomycete isolated from Tripterygium wilfordii Hook.f. Curr. Microbiol. 70 (4), 571–579. doi:10.1007/s00284-014-0758-6
Mohammed, A. T., Khattab, M., Ahmed, A. M., Turk, T., Sakr, N., , A, M. K., et al. (2017). The therapeutic effect of probiotics on rheumatoid arthritis: a systematic review and meta-analysis of randomized control trials. Clin. Rheumatol. 36 (12), 2697–2707. doi:10.1007/s10067-017-3814-3
Morffy Smith, C. D., Gong, M., Andrew, A. K., Russ, B. N., Ge, Y., Zadeh, M., et al. (2019). Composition of the gut microbiota transcends genetic determinants of malaria infection severity and influences pregnancy outcome. EBioMedicine 44. 639–655. doi:10.1016/j.ebiom.2019.05.052
Morgan, X. C., Tickle, T. L., Sokol, H., Gevers, D., Devaney, K. L., Ward, D. V., et al. (2012). Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 13 (9), R79. doi:10.1186/gb-2012-13-9-r79
Morovic, W., and Budinoff, C. R. (2020). Epigenetics: a new frontier in probiotic research. Trends Microbiol. doi:10.1016/j.tim.2020.04.008
Morrison, D. J., and Preston, T. (2016). Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microb. 7 (3), 189–200. doi:10.1080/19490976.2015.1134082
Mu, Q., Cabana-Puig, X., Mao, J., Swartwout, B., Abdelhamid, L., Cecere, T. E., et al. (2019). Pregnancy and lactation interfere with the response of autoimmunity to modulation of gut microbiota. Microbiome 7 (1), 105. doi:10.1186/s40168-019-0720-8
Murakawa, Y. (2016). Pregnancy and lactation in rheumatoid arthritis. Nihon Rinsho 74 (6), 1035–1041 [in Japanese].
Mutic, A. D., Jordan, S., Edwards, S. M., Ferranti, E. P., Thul, T. A., and Yang, I. (2017). The postpartum maternal and newborn microbiomes. MCN Am. J. Matern. Child Nurs. 42 (6), 326–331. doi:10.1097/NMC.0000000000000374
Nagra, N. S., Robinson, D. E., Douglas, I., Delmestri, A., Dakin, S. G., Snelling, S. J. B., et al. (2019). Antibiotic treatment and flares of rheumatoid arthritis: a self-controlled case series study analysis using CPRD GOLD. Sci. Rep. 9 (1), 8941. doi:10.1038/s41598-019-45435-1
Naseer, M., Poola, S., Uraz, S., and Tahan, V. (2020). Therapeutic effects of prebiotics in constipation: a review. Curr. Clin. Pharmacol. doi:10.2174/1574884715666200212125035
Nelson, J. L., Voigt, L. F., Koepsell, T. D., Dugowson, C. E., and Daling, J. R. (1992). Pregnancy outcome in women with rheumatoid arthritis before disease onset. J. Rheumatol. 19 (1), 18–21.
Neuman, H., Debelius, J. W., Knight, R., and Koren, O. (2015). Microbial endocrinology: the interplay between the microbiota and the endocrine system. FEMS Microbiol. Rev. 39 (4), 509–521. doi:10.1093/femsre/fuu010
Neuman, H., and Koren, O. (2017). The pregnancy microbiome. Nestle Nutr. Inst. Workshop Ser. 88, 1–9. doi:10.1159/000455207
Ngian, G. S., Briggs, A. M., Ackerman, I. N., and Van Doornum, S. (2016). Safety of anti-rheumatic drugs for rheumatoid arthritis in pregnancy and lactation. Int. J. Rheum. Dis. 19 (9), 834–843. doi:10.1111/1756-185X.12860
Norgaard, M., Larsson, H., Pedersen, L., Granath, F., Askling, J., Kieler, H., et al. (2010. Rheumatoid arthritis and birth outcomes: a Danish and Swedish nationwide prevalence study. J. Intern. Med. 268 (4), 329–337. doi:10.1111/j.1365-2796.2010.02239.x
Nuriel-Ohayon, M., Neuman, H., and Koren, O. (2016). Microbial changes during pregnancy, birth, and infancy. Front. Microbiol. 7, 1031. doi:10.3389/fmicb.2016.01031
Nyangahu, D. D., Lennard, K. S., Brown, B. P., Darby, M. G., Wendoh, J. M., Havyarimana, E., et al. (2018). Disruption of maternal gut microbiota during gestation alters offspring microbiota and immunity. Microbiome 6 (1), 124. doi:10.1186/s40168-018-0511-7
Ogrendik, M. (2009). Efficacy of roxithromycin in adult patients with rheumatoid arthritis who had not received disease-modifying antirheumatic drugs: a 3-month, randomized, double-blind, placebo-controlled trial. Clin. Therapeut. 31 (8), 1754–1764. doi:10.1016/j.clinthera.2009.08.014
Ogrendik, M., and Karagoz, N. (2011). Treatment of rheumatoid arthritis with roxithromycin: a randomized trial. Postgrad. Med. 123 (5), 220–227. doi:10.3810/pgm.2011.09.2478
Pahari, S., Chatterjee, D., Negi, S., Kaur, J., Singh, B., and Agrewala, J. N. (2017). Morbid sequences suggest molecular mimicry between microbial peptides and self-antigens: a possibility of inciting autoimmunity. Front. Microbiol. ,(1938. doi:10.3389/fmicb.2017.01938
Paysour, M. J., Bolte, A. C., and Lukens, J. R. (2019). Crosstalk between the microbiome and gestational immunity in autism-related disorders. DNA Cell Biol. 38 (5), 405–409. doi:10.1089/dna.2019.4653
Pazos, M., Sperling, R. S., Moran, T. M., and Kraus, T. A. (2012). The influence of pregnancy on systemic immunity. Immunol. Res. 54 (1–3), 254–261. doi:10.1007/s12026-012-8303-9
Peppercorn, M. A., and Goldman, P. (1972). The role of intestinal bacteria in the metabolism of salicylazosulfapyridine. J. Pharmacol. Exp. Therapeut. 181 (3), 555–562.
Peterson, S. N., Bradley, L. M., and Ronai, Z. A. (2020). The gut microbiome: an unexpected player in cancer immunity. Curr. Opin. Neurobiol. 62, 48–52. doi:10.1016/j.conb.2019.09.016
Picchianti-Diamanti, A., Panebianco, C., Salemi, S., Sorgi, M. L., Di Rosa, R., Tropea, A., et al. (2018). Analysis of gut microbiota in rheumatoid arthritis patients: disease-related dysbiosis and modifications induced by etanercept. Int. J. Mol. Sci. 19 (10), 2938. doi:10.3390/ijms19102938
Pineda Mde, L., Thompson, S. F., Summers, K., de Leon, F., Pope, J., and Reid, G. (2011). A randomized, double-blinded, placebo-controlled pilot study of probiotics in active rheumatoid arthritis. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 17 (6), CR347–CR354. doi:10.12659/msm.881808
Prakash, O., Shouche, Y., Jangid, K., and Kostka, J. E. (2013). Microbial cultivation and the role of microbial resource centers in the omics era. Appl. Microbiol. Biotechnol. 97 (1), 51–62. doi:10.1007/s00253-012-4533-y
Provost, M., Eaton, J. L., and Clowse, M. E. (2014). Fertility and infertility in rheumatoid arthritis. Curr. Opin. Rheumatol. 26 (3), 308–314. doi:10.1097/BOR.0000000000000058
Rampelli, S., Candela, M., Turroni, S., Biagi, E., Collino, S., Franceschi, C., et al. (2013). Functional metagenomic profiling of intestinal microbiome in extreme ageing. Aging (Albany NY) 5 (12), 902–912. doi:10.18632/aging.100623
Ranjan, R., Rani, A., Metwally, A., McGee, H. S., and Perkins, D. L. (2016). Analysis of the microbiome: advantages of whole genome shotgun versus 16S amplicon sequencing. Biochem. Biophys. Res. Commun. 469 (4), 967–977. doi:10.1016/j.bbrc.2015.12.083
Reed, S. D., Vollan, T. A., and Svec, M. A. (2006). Pregnancy outcomes in women with rheumatoid arthritis in Washington state. Matern. Child Health J. 10 (4), 361–366. doi:10.1007/s10995-006-0073-3
Reichardt, N., Duncan, S. H., Young, P., Belenguer, A., McWilliam Leitch, C., Scott, K. P., et al. (2014). Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. ISME J. 8 (6), 1323–1335. doi:10.1038/ismej.2014.14
Ricke, S. C., Lee, S. I., Kim, S. A., Park, S. H., and Shi, Z. (2020). Prebiotics and the poultry gastrointestinal tract microbiome. Poultry Sci. 99 (2), 670–677. doi:10.1016/j.psj.2019.12.018
Rigau-Perez, J. G. (2016). Unlicensed to prescribe herbs: a Chinese healer - medico chino - in Puerto Rico, 1851–1853. Puert. Rico Health Sci. J. 35 (2), 100–107.
Roberfroid, M., Gibson, G. R., Hoyles, L., McCartney, A. L., Rastall, R., Rowland, I., et al. (2010). Prebiotic effects: metabolic and health benefits. Br. J. Nutr. 104 (Suppl. 2), S1–S63. doi:10.1017/S0007114510003363
Rohr, M. W., Narasimhulu, C. A., Rudeski-Rohr, T. A., and Parthasarathy, S. (2020). Negative effects of a high-fat diet on intestinal permeability: a review. Adv. Nutr. 11 (1), 77–91. doi:10.1093/advances/nmz061
Rooks, M. G., and Garrett, W. S. (2016). Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 16 (6), 341–352. doi:10.1038/nri.2016.42
Round, J. L., Lee, S. M., Li, J., Tran, G., Jabri, B., Chatila, T. A., et al. (2011). The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science 332 (6032), 974–977. doi:10.1126/science.1206095
Saad, R., Rizkallah, M. R., and Aziz, R. K. (2012). Gut Pharmacomicrobiomics: the tip of an iceberg of complex interactions between drugs and gut-associated microbes. Gut Pathog. 4 (1), 16. doi:10.1186/1757-4749-4-16
Santacruz, A., Collado, M. C., Garcia-Valdes, L., Segura, M. T., Martin-Lagos, J. A., Anjos, T., et al. (2010). Gut microbiota composition is associated with body weight, weight gain and biochemical parameters in pregnant women. Br. J. Nutr. 104 (1), 83–92. doi:10.1017/S0007114510000176
Savin, Z., Kivity, S., Yonath, H., and Yehuda, S. (2018). Smoking and the intestinal microbiome. Arch. Microbiol. 200 (5), 677–684. doi:10.1007/s00203-018-1506-2
Scher, J. U., Sczesnak, A., Longman, R. S., Segata, N., Ubeda, C., Bielski, C., et al. (2013). Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. elife 2, e01202. doi:10.7554/eLife.01202
Scherer, H. U., Haupl, T., and Burmester, G. R. (2020). The etiology of rheumatoid arthritis. J. Autoimmun. 110, 102400. doi:10.1016/j.jaut.2019.102400
Singh, A. (2020). Materials modulate immunity and gut microbiome. Nat. Mater. 19 (1), 3–4. doi:10.1038/s41563-019-0557-3
Skoldstam, L., Hagfors, L., and Johansson, G. (2003). An experimental study of a mediterranean diet intervention for patients with rheumatoid arthritis. Ann. Rheum. Dis. 62 (3), 208–214. doi:10.1136/ard.62.3.208
Skomsvoll, J. F., Ostensen, M., Irgens, L. M., and Baste, V. (1999). Perinatal outcome in pregnancies of women with connective tissue disease and inflammatory rheumatic disease in Norway. Scand. J. Rheumatol. 28 (6), 352–356. doi:10.1080/03009749950155337
Smid, M. C., Ricks, N. M., Panzer, A., McCoy, A. N., Azcarate-Peril, M. A., Keku, T. O., et al. (2018). Maternal gut microbiome biodiversity in pregnancy. Am. J. Perinatol. 35 (1), 24–30. doi:10.1055/s-0037-1604412
Sousa, T., Paterson, R., Moore, V., Carlsson, A., Abrahamsson, B., and Basit, A. W. (2008). The gastrointestinal microbiota as a site for the biotransformation of drugs. Int. J. Pharm. 363 (1–2), 1–25. doi:10.1016/j.ijpharm.2008.07.009
Sousa, T., Yadav, V., Zann, V., Borde, A., Abrahamsson, B., and Basit, A. W. (2014. On the colonic bacterial metabolism of azo-bonded prodrugsof 5-aminosalicylic acid. J. Pharm. Sci. 103 (10), 3171–3175. doi:10.1002/jps.24103
Stanislawski, M. A., Dabelea, D., Wagner, B. D., Sontag, M. K., Lozupone, C. A., and Eggesbo, M. (2017). Pre-pregnancy weight, gestational weight gain, and the gut microbiota of mothers and their infants. Microbiome 5 (1), 113. doi:10.1186/s40168-017-0332-0
Sun, Y., Chen, Q., Lin, P., Xu, R., He, D., Ji, W., et al. (2019). Characteristics of gut microbiota in patients with rheumatoid arthritis in shanghai, China. Front. Cell Infect. Microbiol. 9, 369. doi:10.3389/fcimb.2019.00369
Tajik, N., Frech, M., Schulz, O., Schalter, F., Lucas, S., Azizov, V., et al. (2020). Targeting zonulin and intestinal epithelial barrier function to prevent onset of arthritis. Nat. Commun. 11 (1), 1995. doi:10.1038/s41467-020-15831-7
Teng, F., Klinger, C. N., Felix, K. M., Bradley, C. P., Wu, E., Tran, N. L., et al. (2016). Gut microbiota drive autoimmune arthritis by promoting differentiation and migration of peyer's patch T follicular helper cells. Immunity 44 (4), 875–888. doi:10.1016/j.immuni.2016.03.013
The Human Microbiome Project Consortium (2012). Structure, function and diversity of the healthy human microbiome. Nature 486 (7402), 207–214. doi:10.1038/nature11234
Vaahtovuo, J., Munukka, E., Korkeamaki, M., Luukkainen, R., and Toivanen, P. (2008). Fecal microbiota in early rheumatoid arthritis. J. Rheumatol. 35 (8), 1500–1505.
Vaghef-Mehrabany, E., Alipour, B., Homayouni-Rad, A., Sharif, S. K., Asghari-Jafarabadi, M., and Zavvari, S. (2014). Probiotic supplementation improves inflammatory status in patients with rheumatoid arthritis. Nutrition 30 (4), 430–435. doi:10.1016/j.nut.2013.09.007
Van de Wiele, T., Van Praet, J. T., Marzorati, M., Drennan, M. B., and Elewaut, D. (2016). How the microbiota shapes rheumatic diseases. Nat. Rev. Rheumatol. 12 (7), 398–411. doi:10.1038/nrrheum.2016.85
van Wattum, J. J., Leferink, T. M., Wilffert, B., and Ter Horst, P. G. J. (2019). Antibiotics and lactation: an overview of relative infant doses and a systematic assessment of clinical studies. Basic Clin. Pharmacol. Toxicol. 124 (1), 5–17. doi:10.1111/bcpt.13098
Vande Voorde, J., Sabuncuoglu, S., Noppen, S., Hofer, A., Ranjbarian, F., Fieuws, S., et al. (2014). Nucleoside-catabolizing enzymes in mycoplasma-infected tumor cell cultures compromise the cytostatic activity of the anticancer drug gemcitabine. J. Biol. Chem. 289 (19), 13054–13065. doi:10.1074/jbc.M114.558924
Vieira Borba, V., and Shoenfeld, Y. (2019). Prolactin, autoimmunity, and motherhood: when should women avoid breastfeeding? Clin. Rheumatol. 38 (5), 1263–1270. doi:10.1007/s10067-018-04415-y
Vindigni, S. M., and Surawicz, C. M. (2017). Fecal microbiota transplantation. Gastroenterol. Clin. N. Am. 46 (1), 171–185. doi:10.1016/j.gtc.2016.09.012
Wallenius, M., Skomsvoll, J. F., Irgens, L. M., Salvesen, K. A., Nordvag, B. Y., Koldingsnes, W., et al. (2011). Fertility in women with chronic inflammatory arthritides. Rheumatology 50 (6), 1162–1167. doi:10.1093/rheumatology/keq458
Wallenius, M., Skomsvoll, J. F., Irgens, L. M., Salvesen, K. A., Nordvag, B. Y., Koldingsnes, W., et al. (2012). Parity in patients with chronic inflammatory arthritides childless at time of diagnosis. Scand. J. Rheumatol. 41 (3), 202–207. doi:10.3109/03009742.2011.641582
Wang, J. W., Kuo, C. H., Kuo, F. C., Wang, Y. K., Hsu, W. H., Yu, F. J., et al. (2019). Fecal microbiota transplantation: review and update. J. Formos. Med. Assoc. 118 (Suppl. 1), S23–S31. doi:10.1016/j.jfma.2018.08.011
Weersma, R. K., Zhernakova, A., and Fu, J. (2020). Interaction between drugs and the gut microbiome. Gut 69 (8), 1510–1519. doi:10.1136/gutjnl-2019-320204
Weis, M. (2018). Impact of the gut microbiome in cardiovascular and autoimmune diseases. Clin. Sci. (Lond.) 132 (22), 2387–2389. doi:10.1042/CS20180410
West, B., Lendrum, R., Hill, M. J., and Walker, G. (1974). Effects of sulphasalazine (Salazopyrin) on faecal flora in patients with inflammatory bowel disease. Gut 15 (12), 960–965. doi:10.1136/gut.15.12.960
Wilson, I. D., and Nicholson, J. K. (2017). Gut microbiome interactions with drug metabolism, efficacy, and toxicity. Transl. Res. 179, 204–222. doi:10.1016/j.trsl.2016.08.002
Wu, H. J., Ivanov, I. I., Darce, J., Hattori, K., Shima, T., Umesaki, Y., et al. (2010). Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity 32 (6), 815–827. doi:10.1016/j.immuni.2010.06.001
Xiao, M., Fu, X., Ni, Y., Chen, J., Jian, S., Wang, L., et al. (2018). Protective effects of Paederia scandens extract on rheumatoid arthritis mouse model by modulating gut microbiota. J. Ethnopharmacol. 226, 97–104. doi:10.1016/j.jep.2018.08.012
Yao, Y., Cai, X., Chen, C., Fang, H., Zhao, Y., Fei, W., et al. (2020). The role of microbiomes in pregnant women and offspring: research progress of recent years. Front. Pharmacol. 11, 643. doi:10.3389/fphar.2020.00643
Yao, Y., Cai, X., Yu, H., Xu, Q., Li, X., Yang, Y., et al. (2019). PSTPIP2 attenuates joint damage and suppresses inflammation in adjuvant-induced arthritis. Eur. J. Pharmacol. 859, 172558. doi:10.1016/j.ejphar.2019.172558
Yao, Y., Yu, H., Liu, Y., Xu, Q., Li, X., Meng, X., et al. (2018). PSTPIP2 inhibits the inflammatory response and proliferation of fibroblast-like synoviocytes in vitro. Front. Pharmacol. 9, 1432. doi:10.3389/fphar.2018.01432
Yatsunenko, T., Rey, F. E., Manary, M. J., Trehan, I., Dominguez-Bello, M. G., Contreras, M., et al. (2012). Human gut microbiome viewed across age and geography. Nature 486 (7402), 222–227. doi:10.1038/nature11053
Young, O. M., Tang, Z., Niven-Fairchild, T., Tadesse, S., Krikun, G., Norwitz, E. R., et al. (2015). Toll-like receptor-mediated responses by placental Hofbauer cells (HBCs): a potential pro-inflammatory role for fetal M2 macrophages. Am. J. Reprod. Immunol. 73 (1), 22–35. doi:10.1111/aji.12336
Zeng, Z., Liu, F., and Li, S. (2017). Metabolic adaptations in pregnancy: a review. Ann. Nutr. Metab. 70 (1), 59–65. doi:10.1159/000459633
Zhang, X., Zhang, D., Jia, H., Feng, Q., Wang, D., Liang, D., et al. (2015). The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat. Med. 21 (8), 895–905. doi:10.1038/nm.3914
Zhou, B., Xia, X., Wang, P., Chen, S., Yu, C., Huang, R., et al. (2018). Induction and amelioration of methotrexate-induced gastrointestinal toxicity are related to immune response and gut microbiota. EBioMedicine 33, 122–133. doi:10.1016/j.ebiom.2018.06.029
Zhou, Y., Xu, H., Huang, H., Li, Y., Chen, H., He, J., et al. (2019). Are there potential applications of fecal microbiota transplantation beyond intestinal disorders? BioMed Res. Int. 2019 (3469754. doi:10.1155/2019/3469754
Keywords: gut microbiome, rheumatoid arthritis, pregnancy, lactation, treatment
Citation: Yao Y, Cai X, Fei W, Ren F, Wang F, Luan X, Chen F and Zheng C (2020) Regulating Gut Microbiome: Therapeutic Strategy for Rheumatoid Arthritis During Pregnancy and Lactation. Front. Pharmacol. 11:594042. doi: 10.3389/fphar.2020.594042
Received: 12 August 2020; Accepted: 05 October 2020;
Published: 11 November 2020.
Edited by:
Maria Gerosa, University of Milan, ItalyReviewed by:
Caren Lee Hughes, Mayo Clinic Florida, United StatesSílvia M. Illamola, University of Minnesota Twin Cities, United States
Copyright © 2020 Yao, Cai, Fei, Ren, Wang, Luan, Chen and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yao Yao, yaoyaofb@zju.edu.cn Caihong Zheng, chzheng@zju.edu.cn
†These authors have contributed equally to this work and share first authorship.
 Yao Yao
Yao Yao Xiaoyu Cai2†
Xiaoyu Cai2† Weidong Fei
Weidong Fei