- 1Department of Nephropathy, Hubei Provincial Hospital of TCM, Wuhan, China
- 2Clinical College of Chinese Medicine, Hubei University of Chinese Medicine, Wuhan, China
- 3Department of Oncology, Hubei Provincial Hospital of TCM, Wuhan, China
Objectives: To evaluate the efficacy of Traditional Chinese Medicine, specifically Jianpi Bushen (JPBS) therapy, for treatment of patients with chronic kidney disease (CKD) anemia.
Methods: Randomized controlled trials of JPBS therapy for CKD anemia were searched and selected from seven electronic databases. The Cochrane collaboration tool was used to conduct methodological quality assessment. RevMan v5.3 software was utilized to perform data analysis.
Results: In total, 12 randomized controlled trials with 799 patients met the meta-analysis criteria. The aggregated results indicated that JPBS therapy is beneficial for CKD anemia by improving the clinical efficacy rate [risk ratio (RR) = 1.23, 95% confidence interval (CI): (1.14, 1.33), P < 0.00001] and hemoglobin (Hb) [weighted mean difference (WMD) = 9.55, 95% CI: (7.97, 11.14), P < 0.00001], serum ferritin (SF) [WMD = 6.22, 95% CI: (2.65, 9.79), P = 0.0006], red blood cell (RBC) [WMD = 0.31, 95% CI: (0.24, 0.38), P < 0.00001], hematocrit (HCT) [WMD = 2.95, 95% CI: (2.36, 3.54), P < 0.00001], serum creatinine (SCr) [WMD = 64.57, 95% CI: (33.51, 95.64), P < 0.0001], and blood urea nitrogen (BUN) levels [WMD = 3.76, 95% CI: (2.21, 5.31), P <0.00001]. Furthermore, evidence suggests that JPBS therapy is safe and does not increase adverse reactions compared with western medicine (WM) alone.
Conclusion: This study found that JPBS therapy has a positive effect on the treatment of CKD anemia. However, more well-designed, double-blind, large-scale randomized controlled trials are needed to assess the efficacy of JPBS therapy in the treatment of CKD anemic patients.
Introduction
Anemia is one of the most common complications in patients with chronic kidney disease (CKD), and its prevalence progressively increases as the glomerular filtration rate declines (Hsu et al., 2002; Li et al., 2016). Chronic kidney disease anemia is associated with increased risk of reduced quality of life, cardiovascular disease, cognitive impairment, and mortality (Hörl, 2013). One of the main causes of CKD anemia is a decrease in the renal production of erythropoietin (EPO). In addition, iron, which is an important raw material for hemoglobin production, is often deficient in CKD patients due to impaired dietary iron absorption and increased iron loss (Babitt and Lin, 2012; Gafter-Gvili et al., 2019). Thus, erythropoiesis-stimulating agent (ESA) administration and iron supplementation are the primary Western medicine (WM) treatments for CKD patients with anemia. Although they are effective drugs that clearly avoid the need of blood transfusions, the controversy and safety issues have been raised for both drugs in recent years, especially when they are given at high doses. Evidence has shown that intravenous iron contributes to cardiovascular morbidity and mortality in patients with end-stage renal disease (ESRD) (Kuragano et al., 2014; Bailie et al., 2015). Treatment with ESAs has also been linked to stroke, thrombotic events, and hypertension in CKD patients (Koulouridis et al., 2013). Moreover, 5–10% of patients exhibit an inadequate response to ESAs (Costa et al., 2008), resulting in an increased risk of faster progression to ESRD and all-cause mortality (Solomon et al., 2010; Minutolo et al., 2012). Current WM treatment is also only designed to increase hemoglobin production, not necessarily to improve renal function. The production of EPO will decline with the decrease in glomerular filtration rate and progress of CKD, thereby worsening patient anemia (Prasad-Reddy et al., 2017). Hence, faced with the limitations of current treatment, complementary and/or alternative medicines that improve renal function and alleviate anemia are urgently needed.
Chinese herbal medicine (CHM) has been widely used to treat anemia (Bradley et al., 1999; Chen et al., 2018). According to Traditional Chinese Medicine (TCM) theory, blood deficiency is the main feature of anemia and spleen and kidney deficiencies are the basic pathological features. Based on the relationship between the spleen and kidney and blood generation, classic TCM therapy for the treatment of anemia involves invigorating the spleen and reinforcing the kidney (known as Jianpi Bushen, JPBS). For instance, Wu et al. (2016) found that treatments based on kidney support had a positive effect on treating chronic aplastic anemia. Lin et al. (1990) also found the therapy of warming and tonifying the spleen and kidney improve hemopoietic function of chronic aplastic anemia patients. In recent decades, several clinical trials have evaluated the efficacy and renoprotection of JPBS therapy for the treatment of anemia in CKD (Wang, 2013; Li, 2016; Zhang, 2017). Therefore, in the current study, we performed a meta-analysis to assess JPBS therapy for patients with CKD anemia. The results reported here will help lay a foundation for the treatment of CKD anemia with JPBS therapy.
Materials and Methods
Database Searching
Seven different databases (i.e., PubMed, Cochrane Library, EMBASE, Wanfang Database, China National Knowledge Infrastructure, Chinese Science and Technique Journals Database, and China Biological Medicine Database) were independently searched from inception to April 2019 (by authors YZ and QX). The search terms used were: (jianpi OR bushen OR invigorating spleen OR reinforcing kidney) AND (renal anemia OR anemia of chronic kidney disease OR anemia of renal disease). These terms were translated into Chinese when searching the Chinese databases. Additional studies missed by the electronic search were also searched manually.
Inclusion Criteria
The inclusion criteria were as follows: (1) randomized controlled trial (RCT); (2) patients diagnosed with CKD anemia according to the KDIGO Clinical Practice Guideline for Anemia in Chronic Kidney Disease (McDonald, 2012) and the Diagnosis and Treatment of Renal Anemia with Chinese Expert Consensus (Revision 2018) (Renal Physicians Association Branch of China Consensus Group of Experts on the Diagnosis and Treatment of Renal Anemia, 2018), with no limitations related to age, ethnicity, gender, or primary disease; (3) adoption of JPBS therapy combined with WM (iron supplements, ESAs) as the treatment group, with WM alone applied as the control group. Treatment needed to last at least eight weeks and the sample size needed to be ≥15; (4) primary outcomes to include clinical effective rate and hemoglobin (Hb) level, secondary outcomes to include serum ferritin (SF), red blood cell (RBC), hematocrit (HCT), serum creatinine (SCr), blood urea nitrogen (BUN) levels and number of adverse events; and (5) results available in either English or Chinese.
Exclusion Criteria
The exclusion criteria were as follows: (1) non-randomized controlled trial; (2) study subjects did not meet the diagnostic criteria of renal anemia; (3) both treatment and control groups were treated with CHM only; and (4) studies were duplicated publications or articles with unavailable data.
Quality Assessment and Data Extraction
The first author, year of publication, region, sample size, age, intervention, follow-up time, and main outcomes of each eligible study were collected by two authors (YZ and QX). Based on bias risk assessment of the Cochrane collaboration tool, the methodological quality of the selected studies was evaluated by YZ and QX. The assessed items included: (1) method of random allocation and allocation concealment; (2) blinding method of participants and personnel; (3) blinding method of outcome assessment; (4) integrity of data; (5) selectivity of result reporting; and (6) other biases. For any disagreement, the issue was resolved by discussion with a third author (CL).
Data Analysis
RevMan v5.3 from Cochrane was employed to analyze data. For dichotomous outcomes, risk ratio (RR) with a 95% confidence interval (CI) was used. For continuous outcomes, mean difference (MD) with a 95% confidence interval (CI) was adopted. Data heterogeneity was assessed by χ2 and I2 tests. If heterogeneity existed in pooled studies (I2 ≥ 50%), a random model was applied; if not, a fixed model was applied. Statistically significant differences were considered at P < 0.05. Publication bias was evaluated by funnel plots and calculated using Begg’s/Egger’s tests through STATA v15.0. Sensitivity analysis was conducted by removing individual studies to assess the stability of the results.
Results
Search Results and Study Characteristics
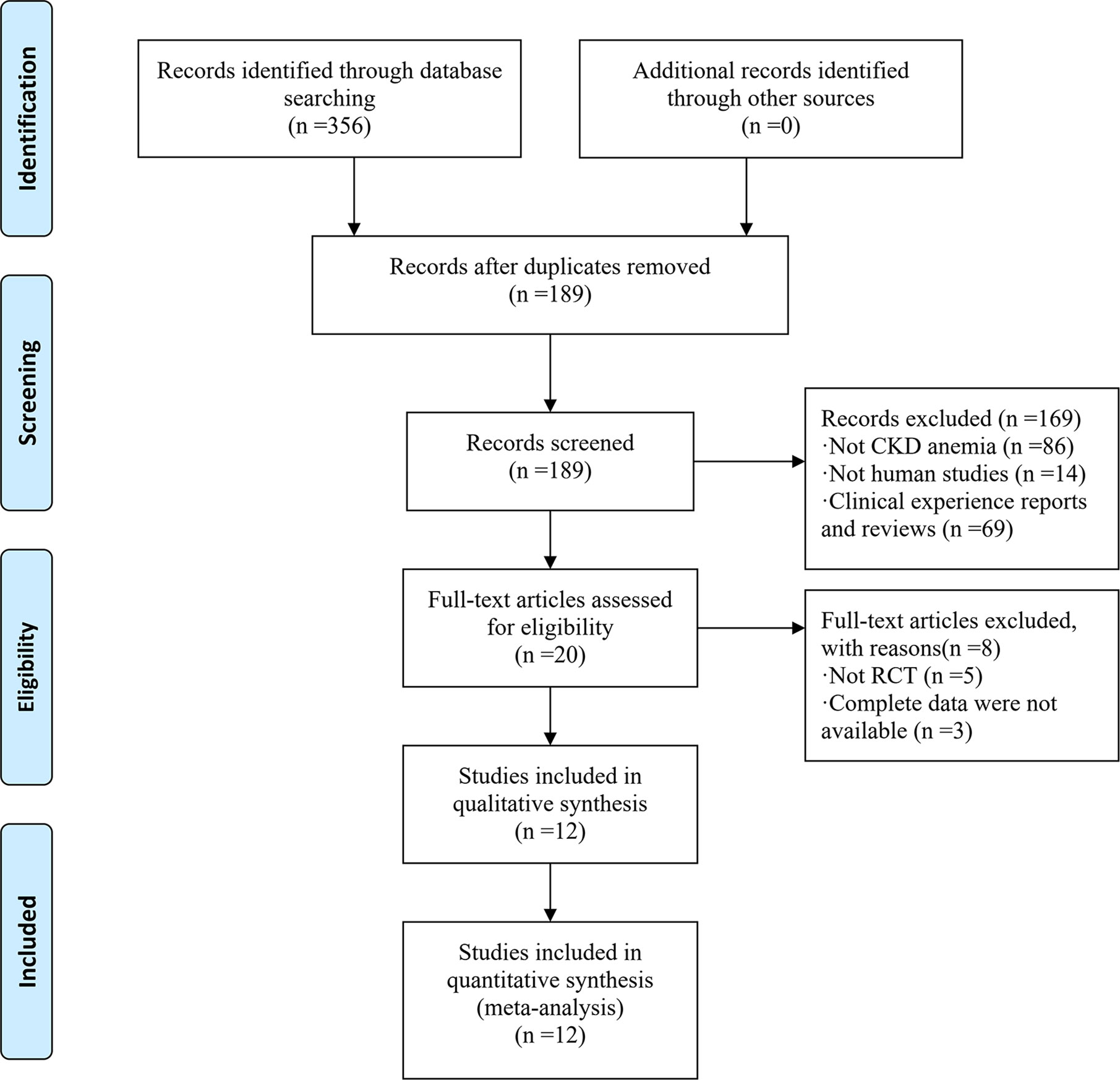
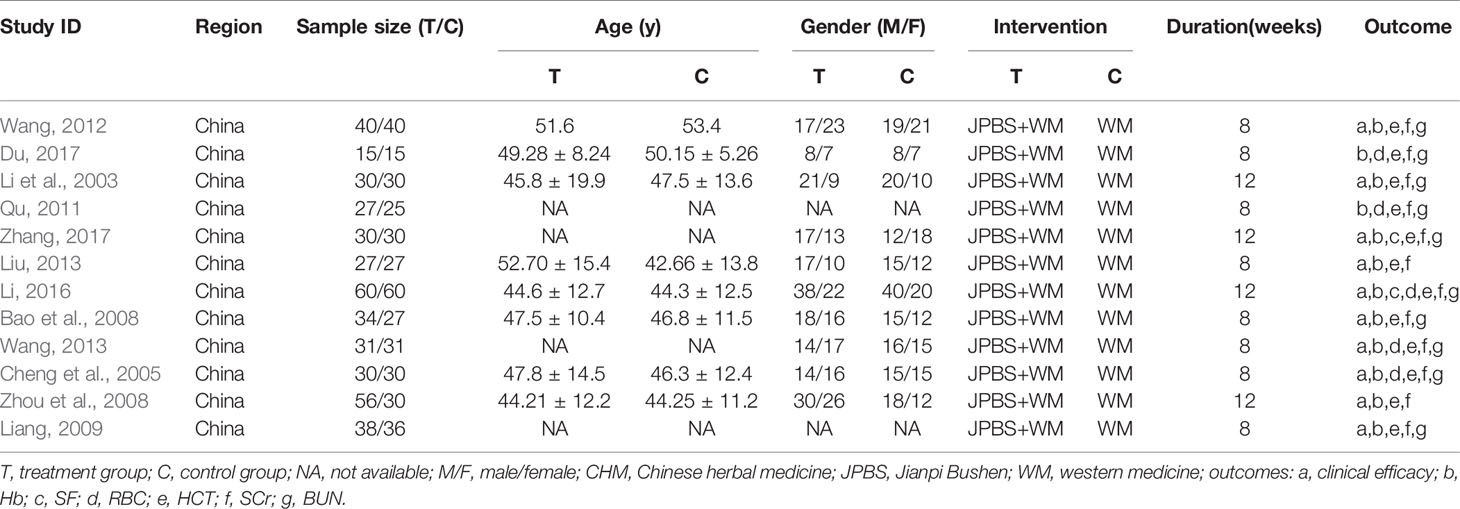
Figure 1 shows a flow diagram of the study. Based on our database search, 356 articles reporting on CKD anemia treatment using JPBS therapy were identified. Of these articles, 167 duplicated publications were excluded. After going through the titles and abstracts, another 169 articles were excluded due to non-CKD anemia, non-human studies, and clinical experience reports and reviews. The 20 remaining full-text articles were further screened, with another eight eliminated because of non-RCT or incomplete data. Finally, a total of 12 articles with 799 patients were enrolled in this meta-analysis (Li et al., 2003; Cheng et al., 2005; Bao et al., 2008; Zhou et al., 2008; Liang, 2009; Qu, 2011; Wang, 2012; Liu, 2013; Wang, 2013; Li, 2016; Du, 2017; Zhang, 2017). Among them, 418 patients received JPBS therapy and 381 patients received WM treatment. The main characteristics of the enrolled articles are presented in Table 1.
Risk of Bias
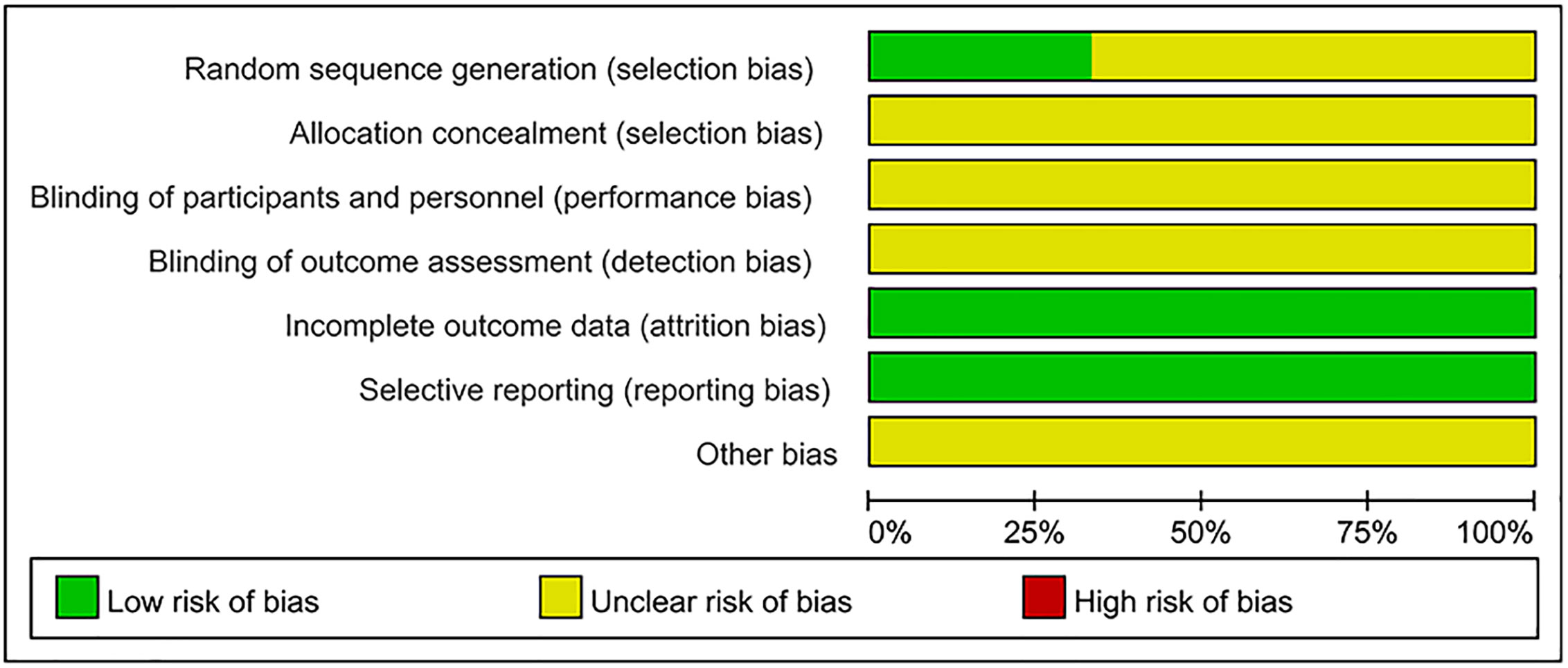
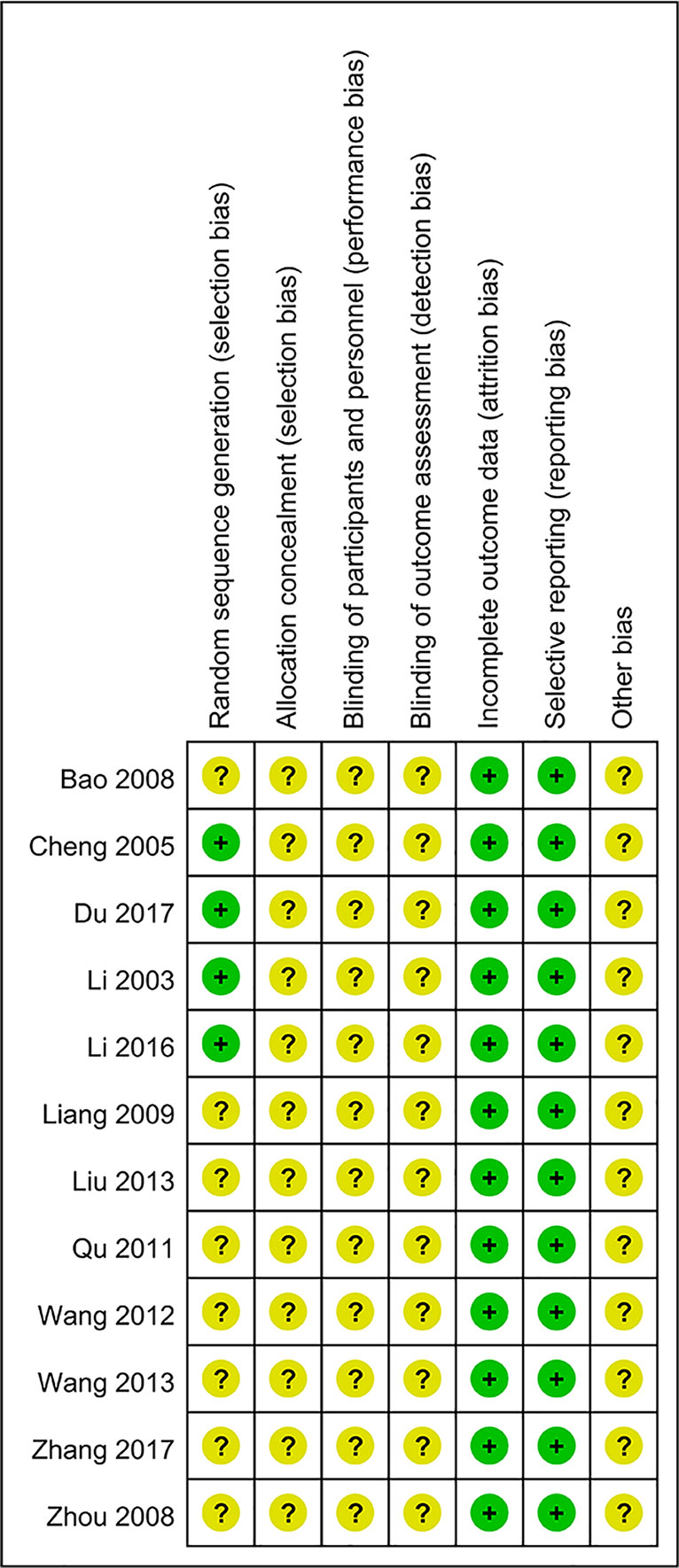
As shown in Figures 2 and 3, the methodological quality of the included articles was relatively low. All enrolled articles claimed to be randomized, but only four described their random methods (Li et al., 2003; Cheng et al., 2005; Li, 2016; Du, 2017). No article mentioned allocation concealment or blinding methods. All articles had complete data, and there was no selective reporting or other biases.
Effects of Intervention
Clinical Efficacy Rate
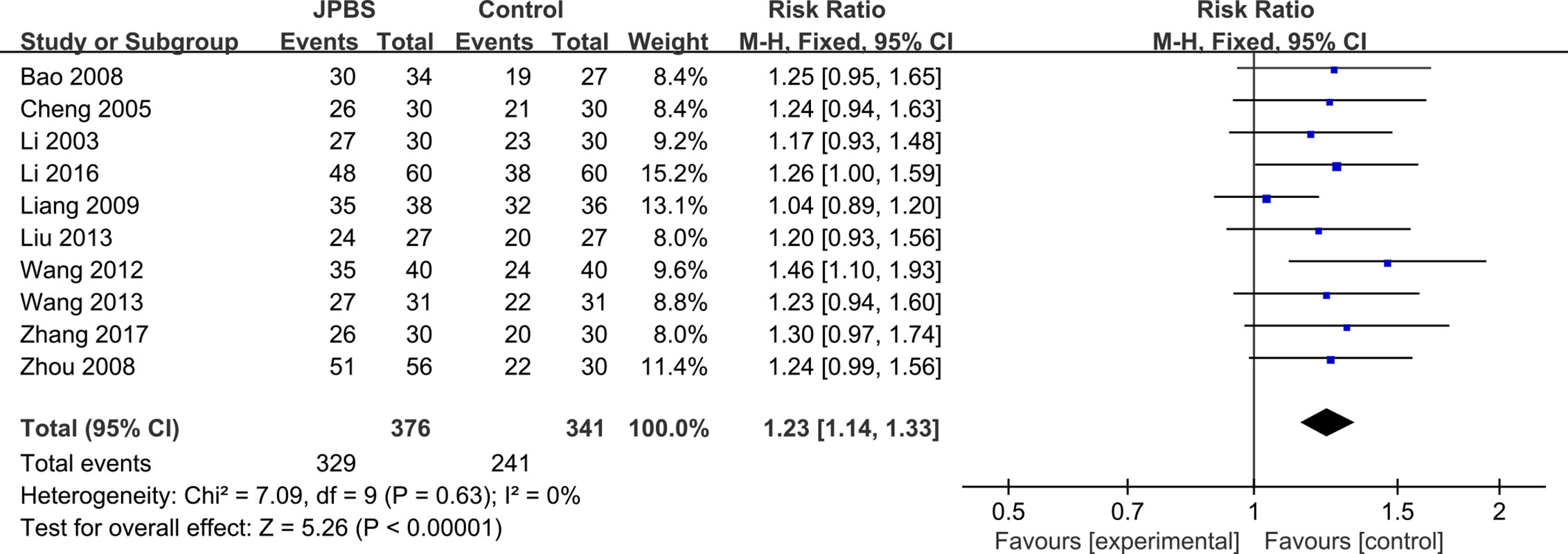
Clinical efficacy rates were reported in 10 studies (Li et al., 2003; Cheng et al., 2005; Bao et al., 2008; Zhou et al., 2008; Liang, 2009; Wang, 2012; Liu, 2013; Wang, 2013; Li, 2016; Zhang, 2017), totaling 717 patients (376 cases in the JPBS therapy group and 341 cases in the WM treatment group). Results indicated that the clinical efficacy rates of the JPBS therapy group were higher than those of the WM treatment group [RR = 1.23, 95% CI: (1.14, 1.33), P < 0.00001, Figure 4].
Hb Levels
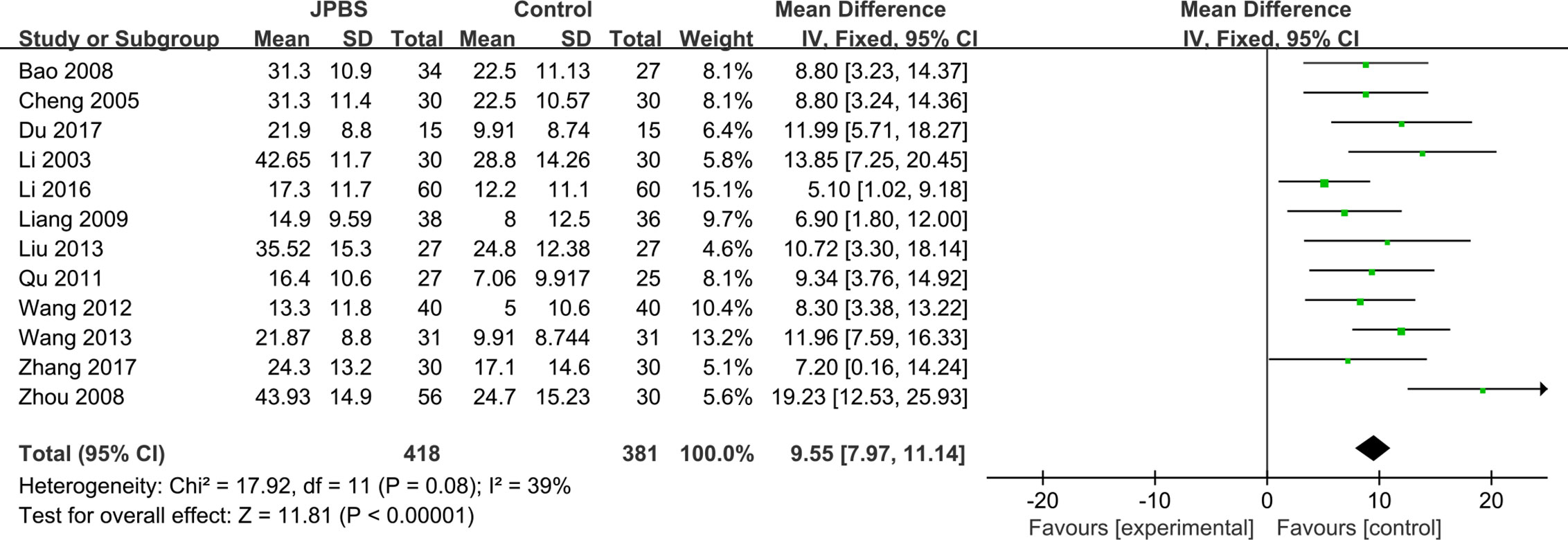
Hb data were provided in all studies (Li et al., 2003; Cheng et al., 2005; Bao et al., 2008; Zhou et al., 2008; Liang, 2009; Qu, 2011; Wang, 2012; Liu, 2013; Wang, 2013; Li, 2016; Du, 2017; Zhang, 2017), totaling 799 patients (418 cases in the JPBS therapy group and 381 cases in the WM treatment group). As data heterogeneity was not found (I2 = 39%, P = 0.08), the fixed effect model was applied (Figure 5). Results indicated that the increase in serum Hb levels was greater in the JPBS therapy group than in the WM treatment group [WMD = 9.55, 95% CI: (7.97, 11.14), P < 0.00001].
SF Levels
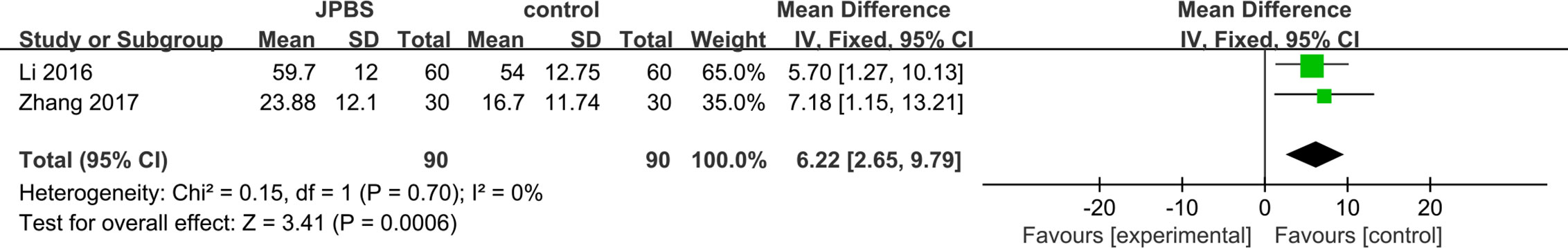
SF data were provided in two studies (Li, 2016; Zhang, 2017), totaling 180 patients (90 cases in the JPBS therapy group and 90 cases in the WM treatment group). As data heterogeneity was not found (I2 = 0%, P = 0.70), the fixed effect model was applied (Figure 6). Results indicated that the increase in serum SF levels was greater in the JPBS therapy group than in the WM treatment group [WMD = 6.22, 95% CI: (2.65, 9.79), P = 0.0006].
RBC Levels
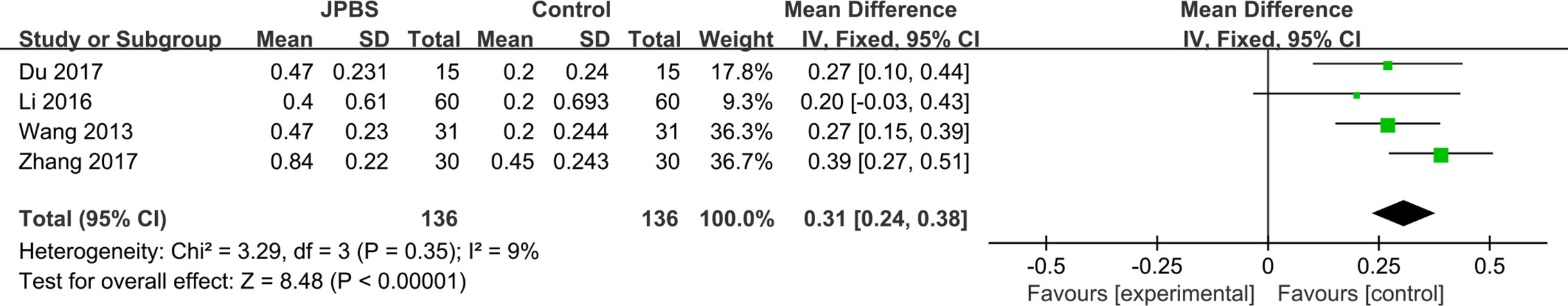
RBC data were provided in four studies, totaling 272 patients (136 cases in the JPBS therapy group and 136 cases in the WM treatment group) (Wang, 2013; Li, 2016; Du, 2017; Zhang, 2017). No significant heterogeneity was found across the studies (I2 = 9%, P = 0.35), and thus the fixed effect model was used (Figure 7). Results indicated that the increase in serum RBC levels was greater in the JPBS therapy group than in the WM treatment group [WMD = 0.31, 95% CI: (0.24, 0.38), P < 0.00001].
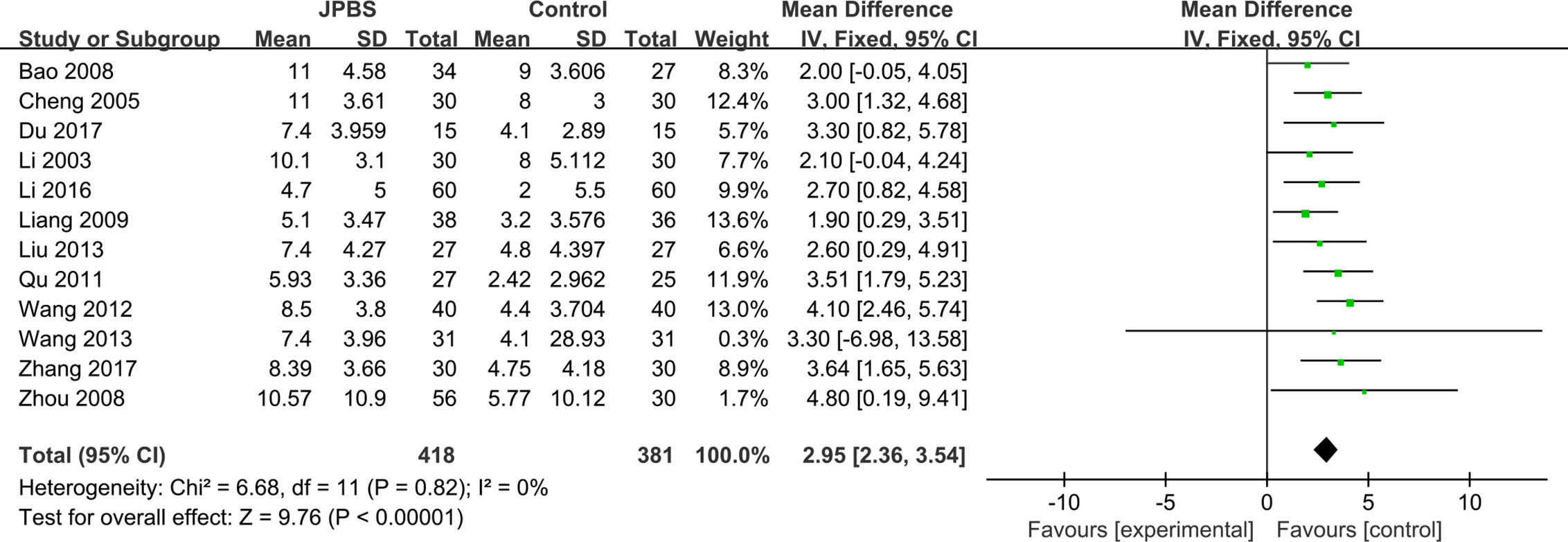
HCT Levels
HCT data were provided in all included studies, totaling 799 patients (418 cases in the JPBS therapy group and 381 cases in the WM treatment group) (Li et al., 2003; Cheng et al., 2005; Bao et al., 2008; Zhou et al., 2008; Liang, 2009; Qu, 2011; Wang, 2012; Liu, 2013; Wang, 2013; Li, 2016; Du, 2017; Zhang, 2017). The fixed effect model was applied as no data heterogeneity was found among the studies (I2 = 0%, P= 0.82) (Figure 8). Compared with that in the WM treatment group, the level of serum HCT showed greater improvement in the JPBS therapy group (WMD = 2.95, 95% CI: (2.36, 3.54), P < 0.00001).
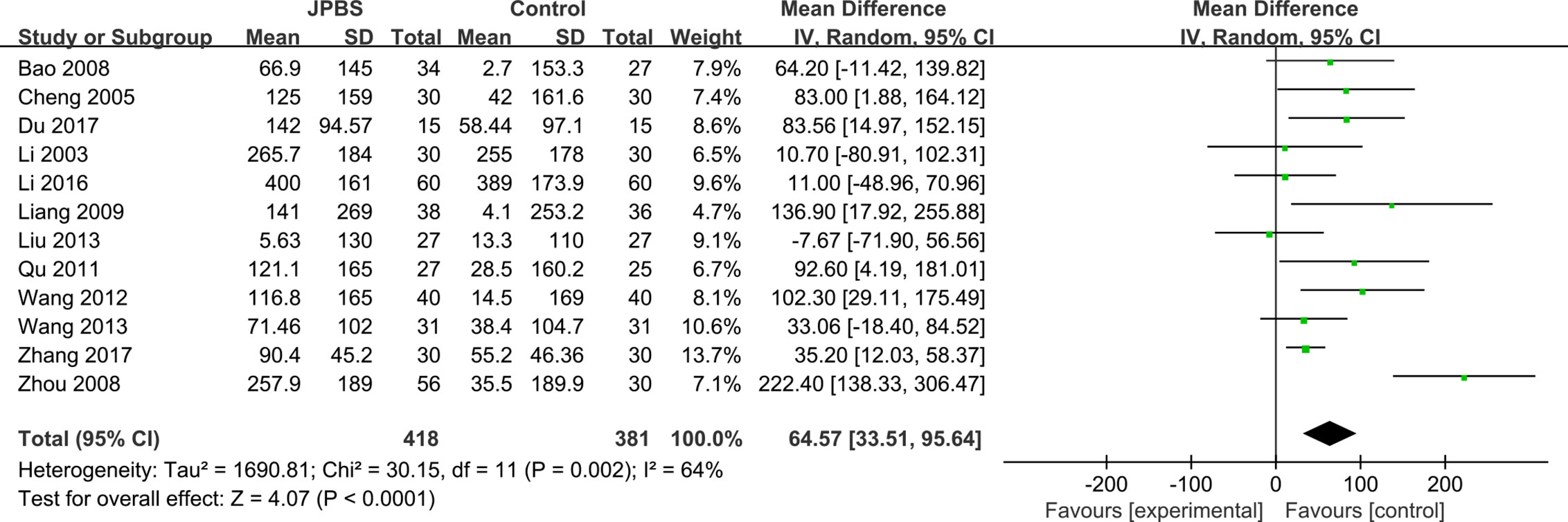
SCr Levels
SCr data were reported in all studies, totaling 799 patients (418 cases in the JPBS therapy group and 381 cases in the WM treatment group) (Li et al., 2003; Cheng et al., 2005; Bao et al., 2008; Zhou et al., 2008; Liang, 2009; Qu, 2011; Wang, 2012; Liu, 2013; Wang, 2013; Li, 2016; Du, 2017; Zhang, 2017). Data heterogeneity was found among the studies (I2 = 64%, P = 0.002), and thus we applied the random effect model (Figure 9). Compared with that in the WM treatment group, the level of serum SCr showed greater improvement in the JPBS therapy group (WMD = 64.57, 95% CI: (33.51, 95.64), P < 0.0001).
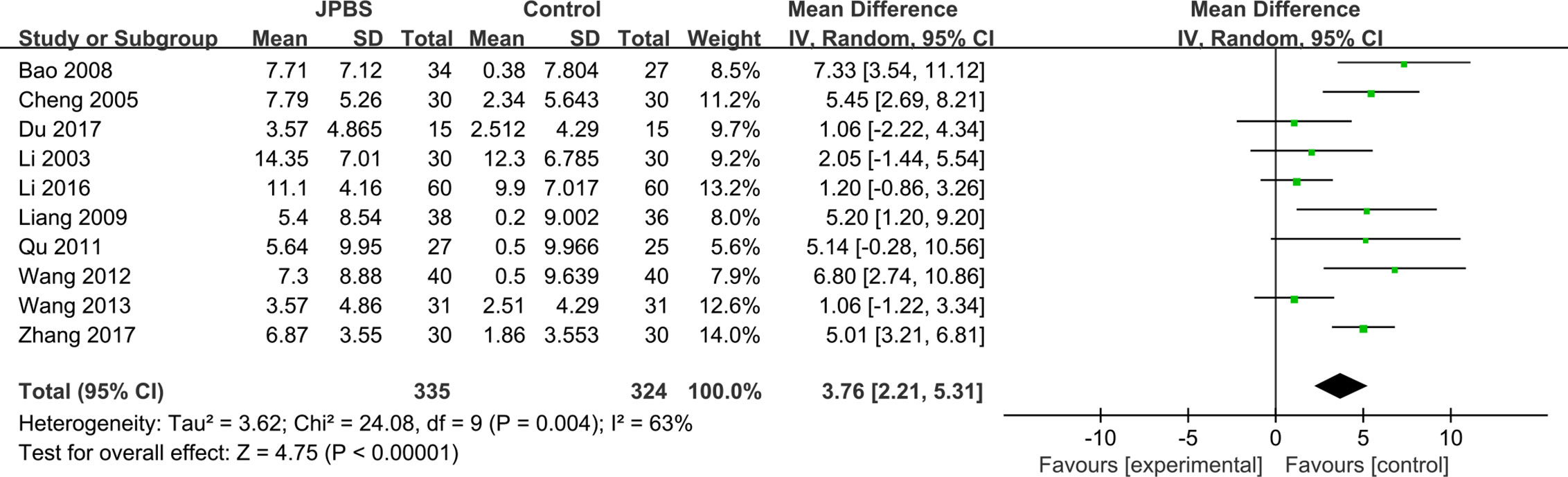
BUN Levels
BUN data were reported in 10 studies, totaling 659 patients (335 cases in the JPBS therapy group and 324 cases in the WM treatment group) (Li et al., 2003; Cheng et al., 2005; Bao et al., 2008; Liang, 2009; Qu, 2011; Wang, 2012; Wang, 2013; Li, 2016; Du, 2017; Zhang, 2017). Based on data heterogeneity analysis (I2 = 63%, P = 0.004), the random effect model was adopted (Figure 10). Results showed that the level of serum BUN decreased in the JPBS therapy group compared with that in the WM treatment group [WMD = 3.76, 95% CI: (2.21, 5.31), P < 0.00001].
Adverse Event Reporting
Two studies reported adverse events. Liang (2009) reported six cases of high blood pressure in the control group. Li (2016) reported seven cases of high blood pressure in the JPBS therapy group (adverse event frequency of 7/60), as well as one case of allergic reaction and eight cases of high blood pressure in the control group (adverse event frequency of 9/60). The Renal Association Clinical Practice Guideline on Anemia of Chronic Kidney Disease suggests there may be an increase in the risk of allergic reaction during intravenous iron injection and of hypertension with ESA administration (Mikhail et al., 2017). Thus, the adverse events reported above may be caused by WM treatment. Based on the adverse events reported in the selected studies, JPBS therapy appears to be safe and does not show an increase in adverse reactions compared with WM alone.
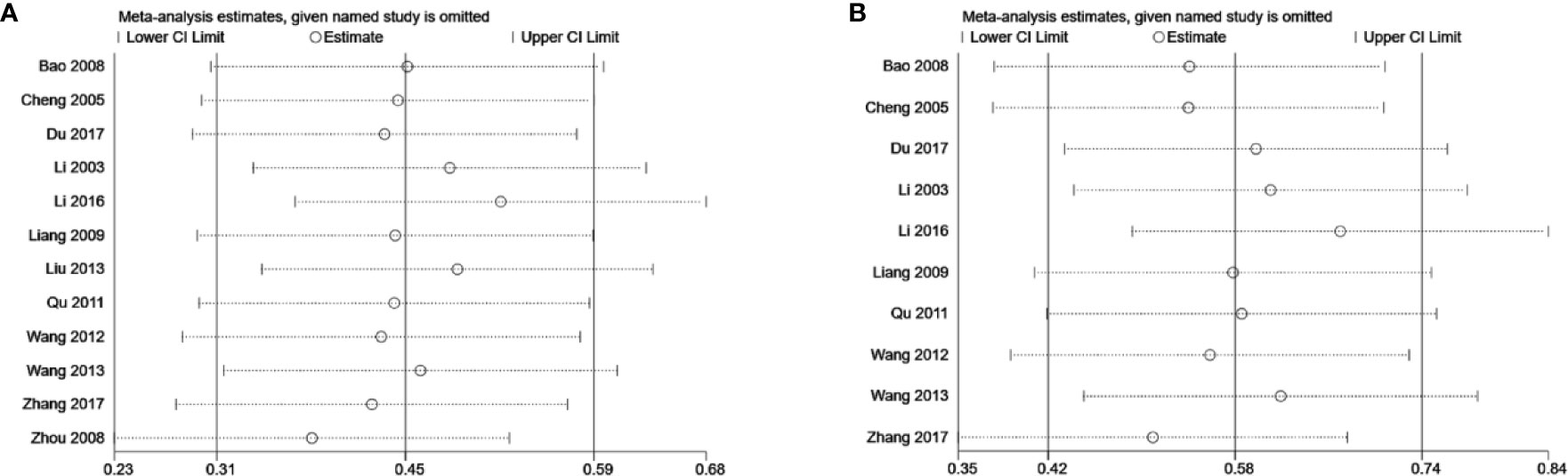
Sensitivity Analysis
Due to the considerable heterogeneity in the SCr and BUN results found among the studies, sensitivity analysis was carried out to investigate the source of this data heterogeneity. Sensitivity analysis suggested that exclusion of any one study for each outcome did not alter the overall results, indicating that the conclusions were robust (Figure 11).
Publication Bias
Publication bias was assessed based on the clinical efficacy and Hb, SCr, and BUN results. The clinical efficacy funnel plot revealed a slight asymmetry and Egger’s (t = 5.61, P = 0.001) and Begg’s tests (z = 2.15, P = 0.032) indicated possible publication bias (Figures 12A–C). Visual assessment of funnel plots and Egger’s (Hb: t = 1.86, P = 0.093; SCr: t = 1.77, P = 0.107; BUN: t = 0.89, P = 0.398) and Begg’s test results (Hb: z = 1.85, P = 0.064; SCr: z = 1.85, P = 0.064; BUN: z = 0.89, P = 0.371) showed no publication bias for Hb, SCr, and BUN (Figures 12D–L).
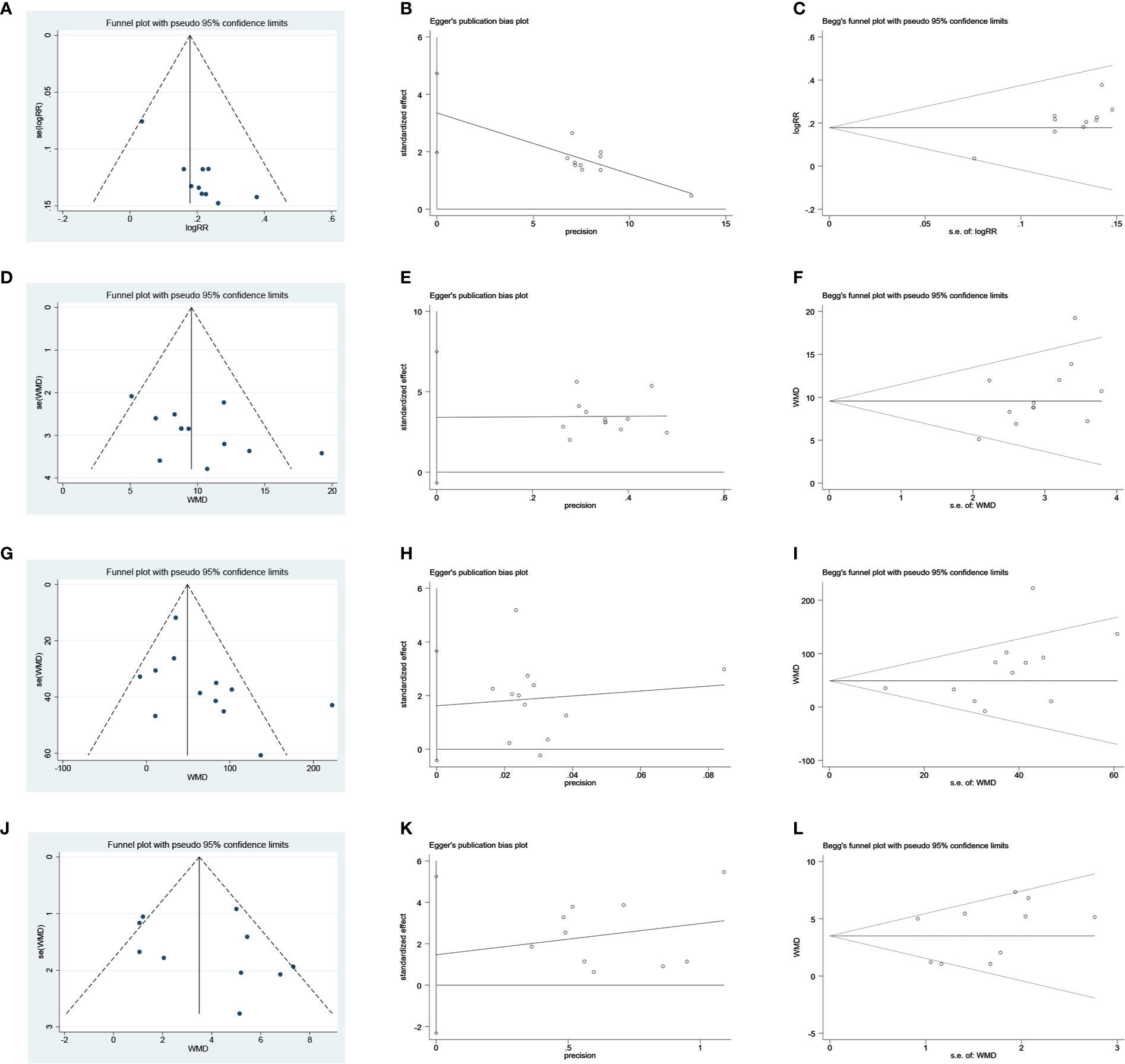
Figure 12 Publication bias plots. (A) Funnel plot of clinical efficacy rate; (B) Egger’s plot of clinical efficacy rate; (C) Begg’s plot of clinical efficacy rate; (D) Funnel plot of Hb; (E) Egger’s plot of Hb; (F) Begg’s plot of Hb; (G) Funnel plot of SCr; (H) Egger’s plot of SCr; (I) Begg’s plot of SCr; (J) Funnel plot of BUN; (K) Egger’s plot of BUN; (L) Begg’s plot of BUN.
Discussion
Anemia is a common and serious complication for millions of patients with CKD (Locatelli et al., 2013). However, debate continues about the best strategies for the management of CKD anemia; for example, Solomon et al. (2010) found that treatment of CKD anemia with ESAs is inferior to that using placebo. In this context, current treatment of CKD anemic patients only reduces the risk of blood transfusion, rather than managing anemia and its associated risks. Nowadays, CHM is frequently applied to improve the symptoms and signs associated with CKD anemia. In accordance with TCM theory, the pathogenesis of CKD anemia is related to deficiencies of the spleen and kidney. Therefore, invigorating the spleen and reinforcing the kidney (Jianpi Bushen) are the main principles of TCM treatment for CKD anemia. In recent years, an increasing number of randomized controlled trials have been conducted using JPBS therapy for the treatment of CKD anemia, providing opportunities for objective and comprehensive evaluation of this method. Here, we performed a meta-analysis to clarify the efficacy of this treatment strategy.
The main findings of our meta-analysis were as follows: (1) compared with WM alone, JPBS therapy combined with WM improved the clinical efficacy rate; (2) compared with WM alone, JPBS therapy combined with WM significantly improved serum Hb, SF, RBC, and HCT levels in patients suffering CKD anemia; (3) adjunctive treatment with JPBS therapy decreased serum SCr and BUN levels in CKD anemic patients; and (4) JPBS therapy did not increase adverse reactions compared with WM alone. These results suggest that JPBS therapy may have beneficial effects in the management of CKD anemic patients.
The significant improvements in clinical efficacy rate and Hb, SF, RBC, HCT, SCr, BUN levels may be attributed to the promotion of hemoglobin formation and the renoprotective effects of CHM. In the included studies, the most frequently used Chinese medicines with the JPBS therapy were Astragali Radix, Angelicae Sinensis Radix, Codonopsis Radix, and Lycium Barbarum (Supplementary Tables 1 and 2). The main components of Astragali Radix, i.e., flavonoids and polysaccharides, are reported to promote erythroid differentiation, increase fetal hemoglobin synthesis, and regulate EPO expression (Yang et al., 2010; Zheng et al., 2011; Zhang et al., 2017). Angelicae Sinensis Radix polysaccharides are thought to exhibit an anti-anemic effect, which can restore EPO production and improve iron availability via stabilizing HIF2α protein and suppressing inflammation (Wang et al., 2018). Codonopsis Radix polysaccharides significantly elevate peripheral blood Hb levels in mice (Gao et al., 2018). In respect of renoprotective effects, Zhao et al. (2016) reported that Lycium Barbarum polysaccharides improve renal function and alleviate kidney inflammation in diabetic rabbits. Therefore, a combination of these CHMs could theoretically alleviate anemia and improve renal function, with a clear therapeutic effect on CDK anemia.
The results of the present study are consistent with research from Li et al. (2004). However, our study varies from previous published meta-analysis related to CKD anemia. For example, Zhao et al. (2017) suggested that Danggui Buxue Decoction combined with WM may be more effective in the treatment of CKD anemia than WM alone. However, they did not specifically reveal the TCM pathogenesis of CKD anemia and JPBS therapy. Based on another meta-analysis, Elliott et al. (2017) reported that ESAs may not have robust or reproducible benefits on renal function in humans. In our meta-analysis, JPBS therapy combined with WM showed positive effects on renal function. However, there are several limitations to this study. First, all included studies were conducted in China, which may limit the wide application of the results. Second, the methodological quality of the enrolled articles was generally low, with none providing a description of allocation concealment or blinding method, which may affect the credibility of the results. Third, there was a potential publication bias in regard to the clinical efficacy rate, which may be due to the flawed research design of small studies or the lack of publication of small studies with negative results. Lastly, data heterogeneity among the studies, including for partial results, was also significant, although sensitivity analysis indicated that our results were stable and reliable. The heterogeneity among the studies may be due to differences in treatment dose, intervention duration, and primary disease of CKD.
Our study demonstrated that JPBS therapy shows good potential for the treatment of CKD anemia, with the possibility of alleviating anemia while improving renal function. However, further research is needed to explore the possible mechanisms of JPBS therapy in the treatment of CKD anemia.
Conclusion
The results of this study indicate that JPBS therapy combined with WM is more effective than WM alone in terms of inducing remission in CKD anemia, especially in regard to clinical efficacy rates and improvement in Hb, SF, RBC, HCT, SCr, and BUN levels. However, our conclusions should be interpreted with some caution due to the relatively poor quality of the included studies. Therefore, to determine the clinical value of JPBS therapy in the treatment of CKD anemia, further standard, double-blind, randomized studies are needed.
Author Contributions
LL and YB designed the study. YZ, QX, and CL contributed to the data collection and data analysis. Results were interpreted by ZW and XZ. LL drafted the original manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by Academic Experience Inheritance of the Sixth National Group of Old Chinese Medicine Experts of the State Administration of Traditional Chinese Medicine [No. 2017 (29)].
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the two reviewers for their helpful comments, which have significantly improved the quality of this manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2020.560920/full#supplementary-material
References
Babitt, J. L., Lin, H. Y. (2012). Mechanisms of anemia in CKD. J. Am. Soc Nephrol. 23, 1631–1634. doi: 10.1681/ASN.2011111078
Bailie, G. R., Larkina, M., Goodkin, D. A., Li, Y., Pisoni, R. L., Bieber, B., et al. (2015). Data from the Dialysis Outcomes and Practice Patterns Study validate an association between high intravenous iron doses and mortality. Kidney Int. 87, 162–168. doi: 10.1038/ki.2014.275
Bao, X. X., Jin, W. M., Zhang, L. (2008). Treatment of renal anemia 34 cases with Jianpi YishenXiezhuo recipe combined EPO. Shaanxi J. Tradit. Chin. Med. 29, 1596–1597. doi: 10.3969/j.issn.1000-7369.2008.12.020
Bradley, R. R., Cunniff, P. J., Pereira, B. J., Jaber, B. L. (1999). Hematopoietic effect of Radix angelicae sinensis in a hemodialysis patient. Am. J. Kidney Dis. 34, 349–354. doi: 10.1053/AJKD03400349
Chen, W. D., Huang, H. S., Su, Y. C., Chou, S. C., Ho, W. C., Kao, M. C., et al. (2018). The characteristics and prescription patterns of Chinese herbal medicine in clinical practice for the treatment of anemia. Taiwan J. ObstetGynecol. 57, 570–577. doi: 10.1016/j.tjog.2018.06.030
Cheng, W., Fu, W. R., Cao, E. Z., Wang, Y. P., Zhang, L., Lv, Y. (2005). Clinical observation on 30 cases of renal anemia treated by Jianpi Bushen Xiezhuo method. J. Anhui Tradit. Chin. Med. Coll. 24, 7–9. doi: 10.3969/j.issn.1000-2219.2005.04.003
Costa, E., Rocha, S., Rocha-Pereira, P., Nascimento, H., Castro, E., Miranda, V., et al. (2008). Neutrophil activation and resistance to recombinant human erythropoietin therapy in hemodialysis patients. Am. J. Nephrol. 28, 935–940. doi: 10.1159/000142147
Du, P. L. (2017). Curative Effect Observation of Self-made BupiQiangshenShengxue Decoction in Treating Anemia of Early Stage Chronic Renal Failure. Med. Innovation China 14, 130–133. doi: 10.3969/j.issn.1674-4985.2017.25.035
Elliott, S., Tomita, D., Endre, Z. (2017). Erythropoiesis stimulating agents and reno-protection: a meta-analysis. BMC Nephrol. 18, 14. doi: 10.1186/s12882-017-0438-4
Gafter-Gvili, A., Schechter, A., Rozen-Zvi, B. (2019). Iron Deficiency Anemia in Chronic Kidney Disease. Acta Haematol. 142, 44–50. doi: 10.1159/000496492
Gao, S. M., Liu, J. S., Wang, M., Cao, T. T., Qi, Y. D., Zhang, B. G., et al. (2018). Traditional uses, phytochemistry, pharmacology and toxicology of Codonopsis: A review. J. Ethnopharmacol. 219, 50–70. doi: 10.1016/j.jep.2018.02.039
Hörl, W. H. (2013). Anaemia management and mortality risk in chronic kidney disease. Nat. Rev. Nephrol. 9, 291–301. doi: 10.1038/nrneph.2013.21
Hsu, C. Y., McCulloch, C. E., Curhan, G. C. (2002). Epidemiology of anemia associated with chronic renal insufficiency among adults in the United States: results from the Third National Health and Nutrition Examination Survey. J. Am. Soc Nephrol. 13, 504–510.
Koulouridis, I., Alfayez, M., Trikalinos, T. A., Balk, E. M., Jaber, B. L. (2013). Dose of erythropoiesis-stimulating agents and adverse outcomes in CKD: a metaregression analysis. Am. J. Kidney Dis. 61, 44–56. doi: 10.1053/j.ajkd.2012.07.014
Kuragano, T., Matsumura, O., Matsuda, A., Hara, T., Kiyomoto, H., Murata, T., et al. (2014). Association between hemoglobin variability, serum ferritin levels, and adverse events/mortality in maintenance hemodialysis patients. Kidney Int. 86, 845–854. doi: 10.1038/ki.2014.114
Li, Q. F., Zhu, C. F., Ma, Q. Y. (2003). Clinical observation of integrated Traditional Chinese Medicine and Western medicine in treatment of renal anemia. Hubei J. Tradit. Chin. Med. 25, 7–9. doi: 10.3969/j.issn.1000-0704.2003.12.003
Li, Q. F., Ma, Q. Y., Zhu, C. F. (2004). Effect of combination of bushenjianpi recipe and erythropoietin on serum tumor necrosis factor alpha in patients with anemia. Zhongguo Zhong Xi Yi Jie He Za Zhi 24, 106–108. doi: 10.1007/BF02836398
Li, Y., Shi, H., Wang, W. M., Peng, A., Jiang, G. R., Zhang, J. Y., et al. (2016). Prevalence, awareness, and treatment of anemia in Chinese patients with nondialysis chronic kidney disease: First multicenter, cross-sectional study. Med. (Baltimore) 95, e3872. doi: 10.1097/MD.0000000000003872
Li, W. (2016). Clinical observation of Warming Kidney and Spleen and Blood Paste in the treatment of hemodialysis patients with anemia .[D]. (Nanjing Univ. Chin. Med.).
Liang, X. P. (2009). Clinical study of BupiYishenShengxue recipe in treatment of renal anemia. Clinical Journal of Traditional Chinese Medicine. Clin. J. Tradit. Chin. Med. 21, 407–409. doi: 10.16448/j.cjtcm.2009.05.011
Lin, L., Wu, S., Tang, J. (1990). Clinical observation and experimental study of the treatment of aplastic anemia by warming and tonifying the spleen and kidney. Zhong Xi Yijie He Za Zhi Chin. J. Modern Develop. Tradit. Med. 10272-274, 259.
Liu, J. (2013). Clinical efficacy research on treating renal anemia with the methods of reinforcing kidney and invigorating spleen.[D]. Chengdu Univ. Chin. Med.
Locatelli, F., Bárány, P., Covic, A., De Francisco, A., Del Vecchio, L., Goldsmith, D., et al. (2013). Kidney Disease: Improving Global Outcomes guidelines on anaemia management in chronic kidney disease: a European Renal Best Practice position statement. Nephrol. Dial. Transplant. 28, 1346–1359. doi: 10.1093/ndt/gft033
McDonald, R. A. (2012). Kidney disease: Improving global outcomes (KDIGO) anemia work group. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int. Suppl. (2011) 2, 279–335. doi: 10.1038/kisup.2012.33
Mikhail, A., Brown, C., Williams, J. A., Mathrani, V., Shrivastava, R., Evans, J., et al. (2017). Renal association clinical practice guideline on Anaemia of Chronic Kidney Disease. BMC Nephrol. 18, 345. doi: 10.1186/s12882-017-0688-1
Minutolo, R., Conte, G., Cianciaruso, B., Bellizzi, V., Camocardi, A., De Paola, L., et al. (2012). Hyporesponsiveness to erythropoiesis-stimulating agents and renal survival in non-dialysis CKD patients. Nephrol. Dial. Transplant. 27, 2880–2886. doi: 10.1093/ndt/gfs007
Prasad-Reddy, L., Isaacs, D., Kantorovich, A. (2017). Considerations and controversies in managing chronic kidney disease: An update. Am. J. Health Syst. Pharm. 74, 795–810. doi: 10.2146/ajhp160559
Qu, X. S. (2011). Treatment of 27 cases of renal anemia with integrated Traditional Chinese Medicine and Western medicine. Chin. Med. Modern Distance Educ. China 9, 56. doi: 10.3969/j.issn.1672-2779.2011.14.037
Renal Physicians Association Branch of China Consensus Group of Experts on the Diagnosis and Treatment of Renal Anemia (2018). Diagnosis and Treatment of Renal Anemia with Chinese Expert Consensus (Revision 2018). Chin. J. Nephrol. 34, 860–866. doi: 10.3760/cma.j.issn.1001?7097.2018.11.012
Solomon, S. D., Uno, H., Lewis, E. F., Eckardt, K. U., Lin, J., Burdmann, E. A., et al. (2010). Erythropoietic response and outcomes in kidney disease and type 2 diabetes. N. Engl. J. Med. 363, 1146–1155. doi: 10.1056/NEJMoa1005109
Wang, K., Wu, J., Xu, J., Gu, S., Li, Q., Cao, P., et al. (2018). Correction of Anemia in Chronic Kidney Disease With Angelica sinensis Polysaccharide via Restoring EPO Production and Improving Iron Availability. Front. Pharmacol. 9, 803. doi: 10.3389/fphar.2018.00803
Wang, W. H. (2012). Treatment of 40 cases of renal anemia with self-made Jianpi YishenHuazhuo decoction. China’s Naturopathy 20, 22–23. doi: 10.3969/j.issn.1007-5798.2012.05.022
Wang, X. F. (2013). Clinical research on Jianpi YishenShengxue methods treat the renal anemia.[D]. Chengdu Univ. Chin. Med.
Wu, D., Shen, Y., Ye, B., Fang, B., Lin, S., Chen, Z., et al. (2016). Efficacy and advantages of modified Traditional Chinese Medicinetreatments based on “kidney reinforcing” for chronic aplastic anemia:a randomized controlled clinical trial. J. Tradit. Chin. Med. 36, 434–443. doi: 10.1016/s0254-6272(16)30059-0
Yang, M., Qian, X. H., Zhao, D. H., Fu, S. Z. (2010). Effects of Astragalus polysaccharide on the erythroid lineage and microarray analysis in K562 cells. J. Ethnopharmacol. 127, 242–250. doi: 10.1016/j.jep.2009.11.013
Zhang, L., Gong, A. G., Riaz, K., Deng, J. Y., Ho, C. M., Lin, H. Q., et al. (2017). A novel combination of four flavonoids derived from Astragali Radix relieves the symptoms of cyclophosphamide-induced anemic rats. FEBS Open Bio 7, 318–323. doi: 10.1002/2211-5463.12146
Zhang, J. (2017). Clinical observation on the treatment of renal anemia by the method of nourishing kidney and spleen and resolving phlegm .[D]. Heilongjiang Univ. Chin. Med.
Zhao, Q., Li, J., Yan, J., Liu, S., Guo, Y., Chen, D., et al. (2016). Lycium barbarum polysaccharides ameliorates renal injury and inflammatory reaction in alloxan-induced diabetic nephropathy rabbits. Life Sci. 157, 82–90. doi: 10.1016/j.lfs.2016.05.045
Zhao, M. M., Zhang, Y., Li, L. S., Yu, Z. K., Li, B. (2017). Efficacy and safety of DangguiBuxue Decoction in combination with western medicine treatment of anemia for renal anemia: a systematic review and meta-analysis. Ann. Transl. Med. 5, 136. doi: 10.21037/atm.2017.01.17
Zheng, K. Y., Choi, R. C., Cheung, A. W., Guo, A. J., Bi, C. W., Zhu, K. Y., et al. (2011). Flavonoids from Radix Astragali induce the expression of erythropoietin in cultured cells: a signaling mediated via the accumulation of hypoxia-inducible factor-1α. J. Agric. Food Chem. 59, 1697–1704. doi: 10.1021/jf104018u
Keywords: chronic kidney disease anemia, Jianpi Bushen therapy, meta-analysis, randomized controlled trials (RCT), Traditional Chinese Medicine
Citation: Li L, Li C, Zhou Y, Xu Q, Wang Z, Zhu X and Ba Y (2020) Effects of Jianpi Bushen Therapy for Treatment of CKD Anemia: A Meta-Analysis of Randomized Controlled Trials. Front. Pharmacol. 11:560920. doi: 10.3389/fphar.2020.560920
Received: 11 May 2020; Accepted: 25 August 2020;
Published: 15 September 2020.
Edited by:
Jianping Chen, Guangzhou University of Chinese Medicine, ChinaReviewed by:
Mingze Qin, Shenyang Pharmaceutical University, ChinaQian Zhou, Nanjing University of Chinese Medicine, China
Copyright © 2020 Li, Li, Zhou, Xu, Wang, Zhu and Ba. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuanming Ba, 1723426138@qq.com
 Liang Li
Liang Li Chengyin Li3
Chengyin Li3