- Jilin Provincial Key Laboratory on Molecular and Chemical Genetic, The Second Hospital of Jilin University, Changchun, China
Herbal medicines, as an important part of traditional Chinese medicine (TCM), have been used to treat digestive system malignancies (DSM) for many years, and have gradually gained recognition worldwide. The role of herbal medicines in the comprehensive treatment of DSM is being improved from adjuvant treatment of the autologous immune function in cancer patients, to the treatment of both the symptoms and disease, direct inhibition of tumor cell growth and proliferation, and induction of tumor cell autophagy and apoptosis. Their specific mechanisms in these treatments are also being explored. The paper reviews the current anti-tumor mechanisms of TCM, including single herbal medicines, Chinese herbal formulations, Chinese medicine preparations and TCM extract, and their application in the comprehensive treatment of digestive system tumors, providing a reference for clinical application of TCM.
Introduction
Digestive system malignancies (DSM) are a common cancer worldwide. They include esophageal, liver, pancreatic, gallbladder, gastric, and colorectal cancers. Due to their high incidence, rapid disease progression, and poor prognosis, they are a leading cause of death, and a public health burden around the world (Siegel et al., 2018). Of the available treatments for DSM, including surgery, radiotherapy, chemotherapy, targeted therapy, and immunotherapy, no current treatment can completely prevent tumor recurrence and metabolism (Tomasello et al., 2017; Finn et al., 2018; Iñarrairaegui et al., 2018; Toesca et al., 2018). Therefore, the development of new methods and drugs is particularly urgent for patients with DSM. Herbal medicine, as represented by traditional Chinese medicine (TCM), is an important component of complementary and alternative medicine, and has been developed in Asian countries, especially China.
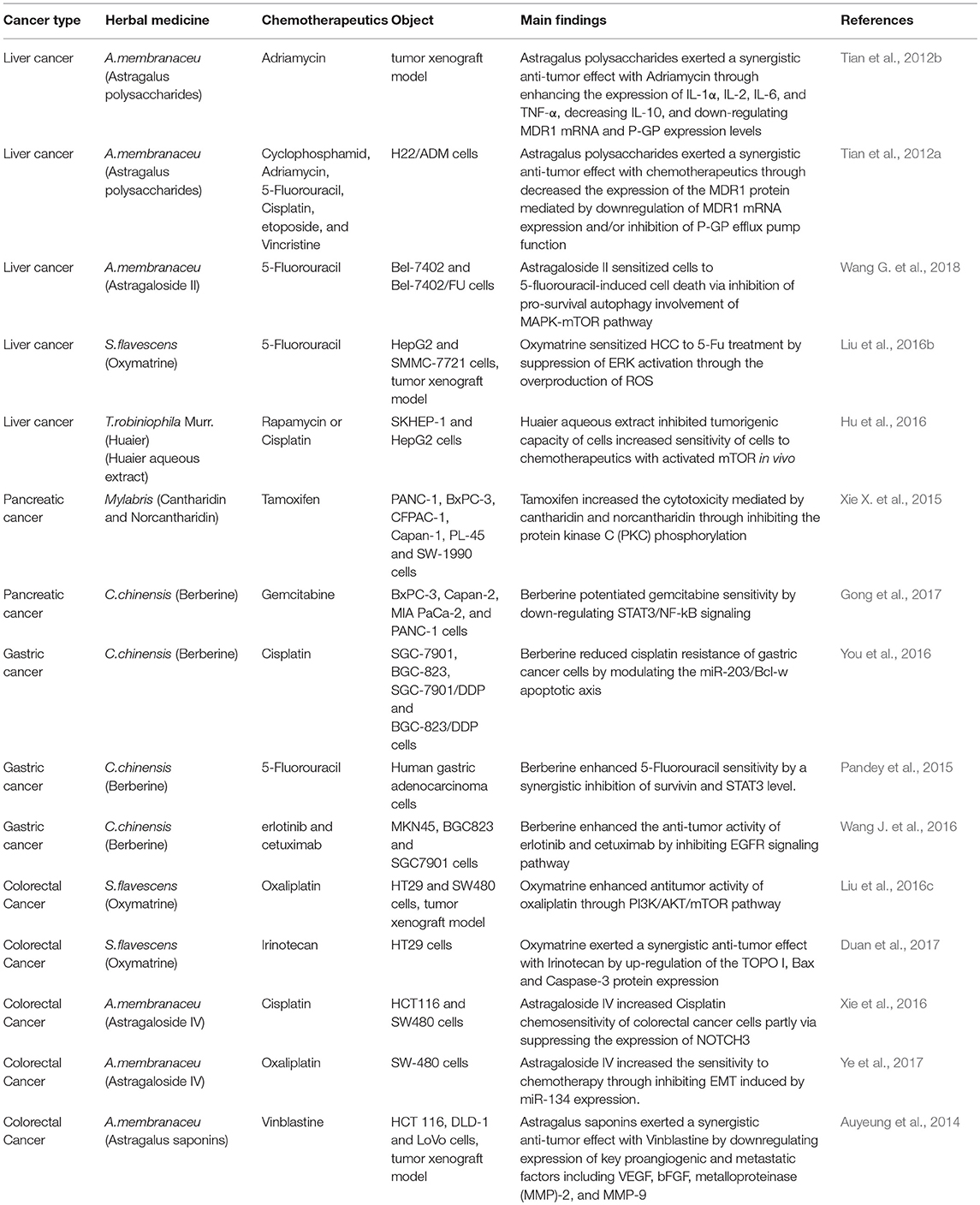
Because of its potential for preventing and treating cancer, TCM has received wide attention from around the world, including Western countries. Many studies found that TCM combined with radiotherapy or chemotherapy for malignant tumors could significantly reduce the incidence of adverse reactions to radiotherapy or chemotherapy and improve cellular immunity and quality of life for patients (Qi et al., 2010; Ge et al., 2016; Liu et al., 2016a,b). Even some TCM preparations have a synergistic effect on chemotherapy, which can both prolong patient survival and reduce tumor recurrence and metastasis (Tian et al., 2012a; Pandey et al., 2015). Many studies have also shown that TCM could directly inhibit tumor cell growth and proliferation and directly or indirectly mediate their autophagy and apoptosis (Tang et al., 2009; Wang X. et al., 2012; Deng et al., 2013). Therefore, TCM, as an anti-tumor therapy, could play an important role in the treatment of DSM. In this paper, we mainly review the clinical application and possible mechanisms of herbal medicine in treating DSM, and consider their possible applications, to provide a reference for treatment of DSM.
Survey Methodology
PubMed was mainly used to search for related articles published using the keyword “herbal medicine,” “liver cancer,” “cholangiocarcinoma,” “hepatocellular carcinoma,” “gastrointestinal tumors,” “pancreatic cancer,” “gastric cancer,” “colon cancer,” “colorectal cancer,” “pancreatic cancer,” and “gallbladder cancer.” Then, screened articles were used as references for this review.
Single Herbal Medicines for DSM
Astragalus Membranaceus
Astragalus membranaceus is a kind of tonifying TCM. It is often used in various advanced cancers to strengthen and consolidate body resistance (Li et al., 2017; Qi et al., 2017). Traditional Chinese medicine indications for A. membranaceus focus on “qi deficiency,” and patients often feel anorexia, a lack of strength, spontaneous sweating. Chemical extraction analysis shows that A. membranaceus contain many active components, such as astragalosides I–VIII, flavonoids, polysaccharides, amino acids and trace elements (Liu et al., 2013; Lu et al., 2016). Several clinical studies and animal experiments have confirmed that the effective components of A. membranaceus can enhance immune function and have anti-tumor effect (Cho and Leung, 2007; Huang et al., 2013; He et al., 2016; Li J. et al., 2016).
Astragalus polysaccharides are among the main active components of A. membranaceus, which are extracted with water from Astragalus roots (Xu et al., 2008). They have many biological functions such as immunomodulation, anti-inflammatory, antioxidants, anti-HBV, etc. (Dang et al., 2009; Huang et al., 2013; Yang et al., 2013; Zhang W. et al., 2017). Some studies have found that Astragalus polysaccharides can inhibit or kill tumor cells of digestive system (Tian et al., 2012b; Huang et al., 2016). Lai X et al. found that astragalus polysaccharides (100 mg·kg−1) could inhibit hepatoma tumor growth in H22 cell tumor-bearing mice. They also increase serum interleukin (IL) 2, IL-6, and tumor necrosis factor (TNF)-α concentrations (Lai et al., 2017), which suggests that astragalus polysaccharides play an anti-tumor role by regulating the immune system. The study also found that Astragalus polysaccharides could affect tumors by regulating the expression of Bcl-2 family proteins (up-regulated Bax, down-regulated Bcl-2; Lai et al., 2017). Notch receptors (Notch 1–4) are a kind of transmembrane protein receptor composed of extracellular domain, transmembrane domain and intracellular region. The main function of the extracellular domain is to bind specific ligands, and the intracellular region is mainly used for transcriptional regulation (Yoshida et al., 2013). Huang WH et al. found that, compared with normal tissues, mRNA expression and protein expression of Notch1 in HCC tissues increased significantly, and knockout of Notch1 significantly inhibited the expression of apoptosis suppressor gene Bcl-2, and enhanced the expression of pro-apoptotic gene Bax, which indicates that astragalus polysaccharides may mediate apoptosis of human HCC cells through regulating Bcl-2 family proteins by inhibiting Notch1 (Huang et al., 2016). Some studies have found that astragalus polysaccharides can reduce adverse effects of chemotherapeutics while enhancing tumor cell sensitivity to them, thus functioning as an adjuvant therapy and improving therapeutic effects in DSM (Cao et al., 2014; Zhang D. et al., 2017).
Multidrug resistance (MDR) is a difficult problem in treating cancer; one cause of multidrug resistance is overexpression of P-glycoprotein (P- GP) in cells, which is encoded by the Multidrug Resistance 1 (MDR1) gene and acts as an efflux pump for various chemotherapeutics (Syed and Coumar, 2016). Tian et al. found that astragalus polysaccharides increased sensitivity of H22 HCC cell lines resistant to Adriamycin (H22/ADM cell lines) to chemotherapeutic agents, which may be related to downregulation of P-glycoprotein and MDR1 mRNA expression (Tian et al., 2012a).
Astragalus saponins are active ingredients extracted from Astragalus roots, and mainly include astragalosides (I–VIII) and isomer iso-astragalosides (I, II) (Auyeung et al., 2010; Liu et al., 2010). Many recent studies suggest their potential value in the treatment of DSM (Li et al., 2017; Qi et al., 2017; Zhang S. et al., 2017). In a study of patients with advanced gastric cancer, Auyeung et al. found that astragalus saponins may mediate apoptosis in gastric cancer cells by activating caspase 3 and subsequently degradation of poly (ADP-ribose) polymerase. In addition, Astragalus saponins reportedly can induce stagnation of cell cycle of G2/M phase in gastric cancer cells and promote the down-regulation of the angiogenic protein vascular endothelial growth factor (VEGF) and metastatic proteins metalloproteinase (MMP), thus inhibiting tumor cell growth (Auyeung et al., 2012). This suggests that astragalus saponins may inhibit angiogenesis and metastasis of digestive system tumor cells. Wang et al. have found that astragalosides can combine with calpain inhibitor to induce endoplasmic reticulum stress-mediated apoptosis in colon cancer cells (Wang Y. et al., 2014). Wang T et al. found that astragalus saponins can inhibit the growth of human gastric cancer cell line BGC-823 cells in vitro and vivo, decrease its invasive ability (Wang T. et al., 2013). In addition, some studies have found that astragalosides can enhance the sensitivity of tumor cells to chemotherapeutic drugs (Auyeung et al., 2014; Xie et al., 2016; Ye et al., 2017). Wang et al. found Astragalus Saponin II could enhance the sensitivity of human HCC cells to 5- fluorouracil by suppressing autophagy via MAPK-mTOR pathway, thus improving the antitumor effects of chemotherapeutic drugs (Wang M. et al., 2017). These findings suggest that Astragalus saponins could be developed into an effective chemotherapeutic agent or adjuvant drug for the comprehensive treatment of DSM.
Sophora Flavescens
The dried root of Sophora flavescens Ait is used in TCM. Its active constituents include matrine, oxymatrine, sophocarpine, and oxysophocarpine (He et al., 2015; Ni et al., 2017). Basic studies found that alkaloids like matrine and oxymatrine could exert anti-tumor effects on various DSM, which has also been verified by many clinical studies and animal experiments (Zhou et al., 2014; Yong et al., 2015; Zhang and Yu, 2016; Wang X. et al., 2017).
Receptor-interacting protein-3(RIP3) is a serine–threonine kinase in the RIP kinase family, which can mediate the cell-death switch from apoptosis to necrosis in TNFα-induced necroptosis (Koo et al., 2015). Xu et al. found that matrine might promote expression of RIP3, thus promoting an anti-tumor effect in cholangiocarcinoma cells (Xu B. et al., 2017). The WNT signaling pathway is crucial for the generation and progression of both normal tissues and tumors (Pećina-Slaus, 2010). Ma Y et al. found that matrine (50 μg/ml) could inhibit migration and invasiveness of HPAC cells in pancreatic cancer. Moreover, expression of MT1-MMP, was reduced in matrine-treated HPAC cells, suggesting that matrine could be a pancreatic cancer treatment, through WNT-mediated down-regulation of MT1-MMP (Ma et al., 2015). Caspase-dependent apoptosis is a kind of programmed cell death. Wu et al. found that oxymatrine could mediate apoptosis in gallbladder cancer cells by activating caspase-3 and Bax and inhibiting Bcl-2 and NF-κB (Wu et al., 2014). Liang et al. found that oxymatrine regulated expression of epithelial-mesenchymal transition (EMT) markers such as E-cadherin, Snail and N-cadherin in colon cancer; expression of p65, a key protein in the NF-κB pathway was lowered at the same time (Liang and Huang, 2016). The results suggested that oxymatrine exerts an anti-tumor effect by down-regulating NF-κB, thus preventing EMT in colon cancer cells.
Moreover, studies found that matrine and oxymatrine could alleviate the toxicity of chemotherapeutic drugs, providing an adjuvant therapeutic approach for comprehensive treatment of digestive system malignancies (Liu et al., 2016b). The anti-tumor effect of gemcitabine on gallbladder carcinomas can be enhanced by suppressing the NF-κB pathway (Yang et al., 2012), which suggests that oxymatrine may increase efficacy of chemotherapeutic drugs by down-regulating the NF-κB pathway. Duan et al. found that the percentage of colon cancer cells suppressed with a combination of matrine and irinotecan (CPT-11), a first-line drug for colon cancer, was higher than that with either matrine or irinotecan alone, which might be associated with up-regulation of Topoisomerase (TOPO) I, Bax and Caspase-3 (Duan et al., 2017). The results suggested that matrine has a synergistic effect with irinotecan in the treatment of colon cancer. An experiment by Li et al. tested combined treatment with oxymatrine and 5-FU on human HCC both in vitro and in vivo, and found that tumor mass and volume in rats were more suppressed in the combined treatment group than in the single-drug groups (Liu et al., 2016a).
Chinese Herbal Formulations for DSM
Huangqin Tang/PHY906
Huangqin Tang, whose main components are Scutellaria baicalensis, Paeonia lactiflora Pall, Glycyrrhiza uralensis Fisch, and Ziziphus jujuba Mill, is a classical TCM preparation, first recorded in the book “Shang Han Lun” by Zhongjing Zhang during the Han Dynasty about 1800 years ago. It has been widely used to treating digestive system diseases that are accompanied with symptoms such as diarrhea, nausea, emesis and abdominal colic (Lam et al., 2010). PHY906, which is the improved version of Huangqin Tang, contains the same four herbs as Huangqin Tang but with a different weight ratio of 3:2:2:2 (Zhang et al., 2010). The application of PHY906 as an adjuvant for radiotherapy, chemotherapy, and targeted therapy in cancer has been explored by dozens of studies (Kummar et al., 2011; Liu and Cheng, 2012; Rockwell et al., 2013). Some studies confirmed the adjuvant role of PHY906 in treating DSMs, such as colon and pancreatic carcinoma (Farrell and Kummar, 2003; Yen et al., 2009; Kummar et al., 2011; Liu and Cheng, 2012; Saif et al., 2014). Lam et al. investigated PHY906 combined with irinotecan in a mouse colon cancer model (Lam et al., 2010). Overall survival in the PHY906 group was significantly better than in the control group; and intestinal mucosa in the control group was more severely damaged than in the PHY906 group, indicating that PHY906 decreases gastrointestinal damage caused by irinotecan. Yen Y et al. evaluated combined PHY906 and capecitabine for 42 patients with late-stage HCC who had lost the opportunity for resection (Yen et al., 2009). After two courses of treatment, 60% of patients showed stable or improved conditions; their median overall survival was 9.2 months with no significant quality of life reduction, implying that PHY906 could be used with capecitabine in treating HCC and providing support for a larger-cohort study. Saif et al. found that PHY906 significantly reduced nausea and emesis caused by capecitabine in 25 patients including 15 men and 10 women with pancreatic cancer in a Phase I clinical trial (Saif et al., 2014), which suggests that PHY906 combined with capecitabine is a safe substitute for gemcitabine for treating advanced pancreatic cancer.
Meanwhile, some studies investigated the mechanism of PHY906 in increasing the anti-tumor effect (Lam et al., 2015; Su et al., 2017). Sorafenib is the only drug approved by FDA for treating HCC, which inhibits the RAF/MEK/ERK pathways and tyrosine kinase receptors such as PDGF, VEGF and Kit (Wilhelm et al., 2004). Lam et al. discovered Huangqin and Shaoyao, which are the two main components of PHY906, might change the inflammatory state of tumor microenvironment and enhance the anti-tumor effect of sorefenib by inhibiting ERK1/2 phosphatase and thus increasing ERK1/2-P in HepG2 cells (Lam et al., 2015). Another study found that PHY906 could mediate apoptosis of colon cancer cells by regulating IFN-γ and activating responses toward steroid hormones, thus exerting an anti-tumor effect by protecting the epithelial barrier from being invaded by tumor cells (Su et al., 2017). The role of PHY906 in the comprehensive treatment of DSM warrants further research.
Bu-Zhong-Yi-Qi-Tang
Bu-zhong-yi-qi-tang (also known as TJ-41) is a traditional medicine widely used in China, Japan and Korea. It contains 7 herbs, including Scutellaria baicalensis, Pinellia tuber, Zizyphi fructus, Zingiberis rhizoma, Glycyrrhiza radix, Coptidis rhizoma, and Panax ginseng (Qi et al., 2010). Reportedly, TJ-41 can decrease side effects and increase curative effects of radiotherapy and chemotherapy in treating DSM (Qi et al., 2010). Jeong et al. randomly divided 40 patients with liver-cancer into an experimental group who received two weeks' treatment with TJ-41, and a control group. They found that the experimental group had significantly less fatigue than did the control group (Jeong et al., 2010). Interleukin-6 (IL6) affects development of cachexia, which is a major cause of cancer-related death. Yae et al. found that macrophages and serum IL-6 expression were reduced, and cachexia alleviated, in mice with colon cancer treated by TJ-41(Yae et al., 2012). Kuo et al. found that TJ-41 enhanced toxicity of mitomycin C for human gastric adenocarcinoma MKN-74 cells, probably through a non-apoptotic mechanism (Kuo et al., 2014). Kao et al. found that TJ-41 mediated apoptosis by inducing stagnation of G0/G1 cell-cycle phases and inhibiting DNA synthesis, thus preventing proliferation of human Hep3B and HepG2 liver cancer cell (Kao et al., 2001).
Shi-Quan-Da-Bu-Tang
Shi-quan-da-bu-tang (also known as TJ-48) is a TCM based on ten herbs: Paeonia lactiflora, Poria cocos, A. membranaceus, Cinnamomum cassia, Glycyrrhiza inflata, Liqusticum wallichii, Angelica sinensis, Atractylodes macrocephala, and Rehmannia glutinosa (Qi et al., 2010). Recently, some studies revealed TJ-48 might play a role in immune regulation and tumor treatment (Ikemoto et al., 2014). This medication can alleviate side effects caused by chemotherapy and radiotherapy during the treatment of DSM, and also prevent metastasis (Nishiuchi et al., 2013; Amitani et al., 2015). Ikemoto discovered TJ-48 could increase activity of T cells, based on decreased Foxp3+ Tregs in patients with advanced pancreatic cancer (Ikemoto et al., 2014). Another study indicated that TJ-48 might slow liver cancer progression and lengthen recurrence-free survival by suppressing Kupffer cell-mediated oxidative stress (Nishiuchi et al., 2013).
Daikenchuto
Daikenchuto (TJ-100) is a traditional herbal medicine, also called Kampo, composed of Capsicum Annuum, dried ginger, ginseng and cerealose (Endo et al., 2017) and can be used for gastrointestinal diseases including intestinal obstruction and Crohn's disease (Kominato et al., 2016; Okada et al., 2016). Some studies found that TJ-100 can suppress tumor development and alleviate side effects of surgery (Yoshikawa et al., 2015; Nagata et al., 2016; Hasebe and Matsukawa, 2017). Hasebe et al. discovered TJ-100 can inhibit the downstream pathway activated by EGFR and effectively suppress tumor growth in a mouse model of colon cancer (Hasebe and Matsukawa, 2017). Yoshikawa et al. postoperatively treated 245 patients with gastric cancer who underwent total gastrectomy, with either TJ-100 or placebo (Yoshikawa et al., 2015). The result showed theTJ-100 group had a shorter median time to first bowel movement than the placebo group (94.7 vs. 113.9 h), and a lower incidence of gastrointestinal dysfunction at 12 days after surgery than did the placebo group.
Traditional Chinese Medicine Extract for DSM
Cantharidin(C10H12O4) and Norcantharidin(C8H8O4)
Mylabris has been used in cancer treatment for more than 2000 years (Lao et al., 2013; Zeng et al., 2016). Cantharidin (CTD) (Supplementary Figure 1), a terpenoid, is the main active ingredient of Mylabris, and it has a significantly cytotoxic effect on tumor cells (Kadioglu et al., 2014; Hsia et al., 2016). However, its clinical application is limited by strong irritation of the urinary system and digestive system when taken orally or intravenously (Wang G. et al., 2018). Norcantharidin (NTCD), a demethylated derivative of CTD, has relatively low toxicity and similarly antitumor activity to cantharidin (Puerto Galvis et al., 2013). Studies have shown that CTD and NCTD might mediate apoptosis to inhibit tumor development and have a metabolic impact on tumor cells (Rauh et al., 2007). CTD and NCTD were also shown to inhibit serine/threonine protein phosphatase 1 and serine/threonine protein phosphatase 2A (Bian et al., 2014). These terpenoids affect intracellular signal transduction and cell-cycle progression (Yeh et al., 2010).
Reportedly, CTD and NCTD inhibit DSM proliferation and metabolism, including liver cancer (Shen et al., 2015; Su et al., 2015). Le et al. found that CTD (5 μM) had an antitumor effect on HCC stem cells in a dose- and time-dependent manner, which may be associated with down-regulation of β-catenin and cyclin D1, and inhibition of cell self-renewal ability (Le et al., 2016). Shen et al. found that CTD inhibited invasiveness of pancreatic cancer cells by downregulating matrix metalloproteinase 2, which has a major role in remodeling extracellular matrix (Shen et al., 2015). Another study suggested that CTD might induce apoptosis of SGC-7901 and BGC-823 cells and G2/M phase arrest by activating Bcl-2 proteins in gastric cancer (Wang T. et al., 2015). Overexpression of family-with-sequence-similarity-46C (FAM46C) reportedly inhibits invasiveness of liver cancer cells by suppressing transforming growth factor-β-Smad signaling and EMT; FAM46C-knockout can change the anti-metastatic effect of NCTD on tumor cells, suggesting the anti-hepatoma impact of NCTD is affected by up-regulating FAM46C (Wan et al., 2017). MiR-214 is significantly down-regulated in HCC tissues, which is associated with low clinical progression and poor prognosis in HCC (Shih et al., 2012). Overexpression of miR-214 could inhibit growth and invasiveness in HCC (Wang J. et al., 2012, 2013). Lu S et al. found that NCTD could significantly inhibit tumor growth in liver cancer-bearing mice, and the inhibitory effect may be associated with enhanced anti-tumor activity of tumor-associated macrophages (Lu et al., 2014). In addition, NCTD significantly inhibited β-catenin expression, which could be reversed by miR-214 inhibitor (Lu et al., 2014).
Moreover, some studies have also found that combining radiotherapy or chemotherapy with CTD and NCTD could reduce the side effects of radiotherapy and chemoradiotherapy, and increase sensitivity of tumor cells to chemotherapeutic drugs, thus enhancing their efficacy (Sun et al., 2016; Zhang Y. et al., 2017). Wang et al. have found that CTD and NCTD might enhance the toxicity of gemcitabine and erlotinib for human pancreatic cancer cells by inhibiting the beta-catenin pathway, thus augmenting treatment of pancreatic cancer (Wang W. J. et al., 2015).
Berberine(C20H18CLNO4)
Berberine (Supplementary Figure 2) is an isoquinoline alkaloid, extracted as a quaternary ammonium compound from Coptis chinensis (Huanglian in Chinese), Hydrastis canadensis, Berberis aristata, Berberis vulgaris, and Berberis aquifolium (Tang et al., 2009). Berberine is used for bringing down fevers and fighting intestinal bacterial infections due to its antibiotic effect. Berberine usually utilized in TCM to treat infectious diseases, including bacterial diarrhea, intestinal parasitic infection and ocular Chlamydia trachomatis infection, based on its robust resistance to bacteria, viruses, fungi, protozoa, worms and chlamydia (Tang et al., 2009). In the recent years, several studies have found that berberine might suppress tumor invasiveness and metastasis through multiple mechanisms (Wang et al., 2010; Liu et al., 2015). Yu et al. found that berberine could mediate apoptosis and autophagic death in HepG-2 cells through activation of AMPK, which is a kind of metabolic-sensing protein kinase (Yu et al., 2014). Wang et al. found that berberine (>100 μM) may inhibit HCC cell invasiveness and metastasis through up-regulation of plasminogen activator inhibitor-1 and down-regulation of urokinase-type plasminogen activator (Wang X. et al., 2016). Yi et al. found that berberine can significantly inhibit activation of the Akt pathway and inhibit the growth of tumors (Yi et al., 2015).
Other studies found that berberine could be combined with chemotherapeutics to neutralize their toxicity, thereby enhancing their anti-tumor effects (Pandey et al., 2015; You et al., 2016; Gong et al., 2017). Epidermal growth factor receptor (EGFR), a receptor for the ErbB family, is usually overexpressed in gastric cancer cells, which is associated with poor prognosis (Kim et al., 2008). Wang J et al. found that berberine can mediate apoptosis by inhibiting the EGFR pathway and enhance the efficacy of cetuximab or erlotinib in gastric cancer cell lines, which provide strong support for the potential of berberine in the treatment of DSM (Wang J. et al., 2016).
Chinese Medicine Preparations for DSM
Huaier Granules
Huaier granules are a new anti-tumor drug extracted from Trametes robiniophila Murr (Huaier) (Zhao et al., 2017). Its main active ingredient is a proteoglycan composed of 41.53% polyose, 12.93% amino acid, and 8.72% H2O (Zou et al., 2015). The drug has been approved by (CFDA) the Chinese Food and Drug Administration for patients with malignancies, especially those with primary liver cancer who have lost the opportunity for surgery and chemotherapy (CFDA approval number, Z20000109; Bao et al., 2016). Various studies confirm that Huaier granules can inhibit tumor growth, promote tumor cell apoptosis, induce secretion of various cytokines, and increase immunity, while enhancing sensitivity to chemotherapeutics and reversing drug resistance. Because of the wide potential applications of Huaier granules, many studies have focused on its use and mechanisms for the treatment of DSM (Wang X. et al., 2012; Song et al., 2015).
Pathways for hypoxia-inducible factor (HIF)-1α, VEGF, RNA-binding factor 1(AUF-1), and astrocyte elevated gene-1 (AEG-1) play roles in the progression of liver cancer (Yang et al., 2014; Liu et al., 2017). Cong et al. did a series of in-vivo and in-vitro experiments with human HCC SMMC-7721 cells (Li C. et al., 2015). The results suggested that Huaier polysaccharide (TP-1) inhibits the above pathways by partially down-regulating HIF-1α, VEGF, AUF-1, and AEG-1 proteins, thereby suppressing revascularization, growth and metabolism of liver cancer cells. Mitogen-activated protein kinase (MAPK), which mediates cell proliferation, differentiation, stress reaction and apoptosis, mainly via p38 MAPK pathway, can induce apoptosis by adjusting the expression of several relevant proteins including p53 and Bcl-2 (Chang and Karin, 2001; Taylor et al., 2013; Hui et al., 2014; Zhang C. et al., 2014). Activity of the p38–MAPK pathway is significantly reduced in liver cancer cells and activation of p38 may induce apoptosis (Lamy et al., 2013). Bao HD et al. tested the anti-tumor effect of TP-1 in HepG2 and Huh7 HCC cells. Expression of Bax, Bcl-2 and survivin in the cells treated with TP-1 was obviously enhanced, leading to apoptosis of liver cancer cells due to activation of p38–MAPK pathway (Bao et al., 2016). A study by Xie et al. found that Huaier could mediate apoptosis of gastric cancer cells through the PI3K–AKT signaling pathway and prevent their proliferation by suppressing cyclin B1 expression and promoting G2/M-phase arrest (Xie H. X. et al., 2015).
Huaier granules can also increase the sensitivity of human hepatoma cell lines SKHEP-1 and HepG2 to rapamycin and cisplatin and thus enhance the anti-tumor effect of chemotherapeutics, probably by activating thr mTOR pathway (Hu et al., 2016). Huaier granules combined with DC-CIK showed increased efficacy in treating mice with HT-29 colon carcinoma cell line than either single method (Sun et al., 2017). Even so, the mechanisms of Huaier granules require further study.
Shenqi Fuzheng Injection
Shenqi fuzheng injection (SFI) was approved by Chinese Food and Drug Administration in 1999 for clinical application (CFDA approval number, Z19990065). It mainly includes Codonopsis pilosula and A. membranaceus (ratio of 1:1) (Wang J. et al., 2014). Studies showed that SFI significantly suppressed tumor growth, neutralized toxicity of chemotherapeutics and increased immunity (Qi et al., 2015). Yang et al. conducted an animal experiment to treat nude mice induced by cyclophosphamide with SFI of low, medial and high doses (Wang J. et al., 2012). The results showed that SFI could dose-dependently increase the spleen index of mice, promote recovery of peripheral white blood cells and marrow cells, stimulate the proliferation of T cells and B cells, increase activity of splenic natural killer cells and peritoneal macrophages, and renew the level of serous IL-2. Many studies have indicated that SFI combined with systemic chemotherapeutics can generate a synergetic anti-tumor effect that improves the objective response rate (ORR), increases Karnofsky performance score (KPS) and immunity, and decreases the adverse event rate (Li J. et al., 2015; Zhang D. et al., 2017). In a meta-analysis of 15 random clinical trials (RCTs) among patients with late-stage gastric cancer, Yao et al. showed the ORR for patients treated with SFI and chemotherapy was significantly higher than for patients treated with chemotherapy alone (OR = 1.66, 95% confidence interval [CI]: 1.20–2.29, P < 0.05; Yao et al., 2014). Furthermore, KPS was significantly increased by the combinatorial treatment (OR = 3.74, 95% CI: 2.66–5.27, P < 0.05). Another meta-analysis that included a combined cohort of 722 patients with colon cancer found that the group treated with SFI combined with chemotherapeutics had a higher ORR and lower gastrointestinal toxicity than did the group treated with chemotherapy only. This result suggests that SFI can enhance the efficacy of chemotherapeutics while decreasing their side effects (Xu R. et al., 2017).
Aidi Injection
Aidi injection (CFDA approval number, Z52020236) is an anti-tumor Chinese medicine from the spotted jellyfish, A. membranaceus, Acanthopanax, and ginseng (Xiao et al., 2017). Aidi injections can reportedly alleviate side effects of chemotherapeutics and exert an adjuvant effect on the comprehensive treatment of DSM (Wang T. et al., 2014; Ge et al., 2016). In a meta-analysis of 32 RCTs by Wang et al., Aidi injections combined with chemotherapeutics improved the effective rate of chemotherapeutics for gastric cancer and the patient's quality of life, and decreased incidences of side effects such as nausea and vomiting, diarrhea, leukopenia III–IV, and thrombocytopenia III–IV (Jiancheng et al., 2015). Wang et al. treated patients with advanced colon cancer using Aidi injections combined with FOLFOX4 and found that the experimental group (n = 63) had a much lower rate of grade-II nausea, vomiting, and diarrhea than did the control group (n = 58) for 7 days (Wang J. et al., 2014).
Kanglaite Injection
Kanglaite injection is an anti-tumor medication mainly extracted from Chinese herb-coix seed (Semen coicis yokuinin), and has been approved by CFDA to treat gastric cancer, liver cancer, etc. (Qi et al., 2015). Kanglaite injection can reduce side effects of chemotherapy and radiotherapy, improve patients' quality of life, and reverse drug resistance to some extent. In a meta-analysis of Kanglaite injection combined with hepatic arterial intervention for the treatment of non-resectable HCC, the ORR and KPS of patients who received the combined treatments were both higher than for patients treated with a single method (Fu et al., 2014).
Conclusions
In summary, we have reviewed the current anti-tumor mechanisms of TCM, including single herbal medicines (Table 1), Chinese herbal formulations, Chinese medicine preparations (Table 2), and TCM extract (Table 3), and their application in the comprehensive treatment of digestive system tumors (Table 4). TCM has a long history, and its application in many diseases, especially malignant tumors, has been widely reported (Wong et al., 2015; Xu et al., 2015; Chen X. et al., 2016; Zhang et al., 2016). However, due to the variety of medicinal plants (estimated over 12,000 species) and their complex components, TCM has not been widely recognized in Western countries (Chen et al., 2014). In recent years, with more extensive TCM investigations, continuous progress has been made in extraction technology, and standardization of TCM. The value of TCM in modern medical and health services is becoming more widely recognized. However, there are still some difficulties in the study of TCM for DSM, such as the determination of active substances and the accuracy of chemical composition determination (Zhang et al., 2018).
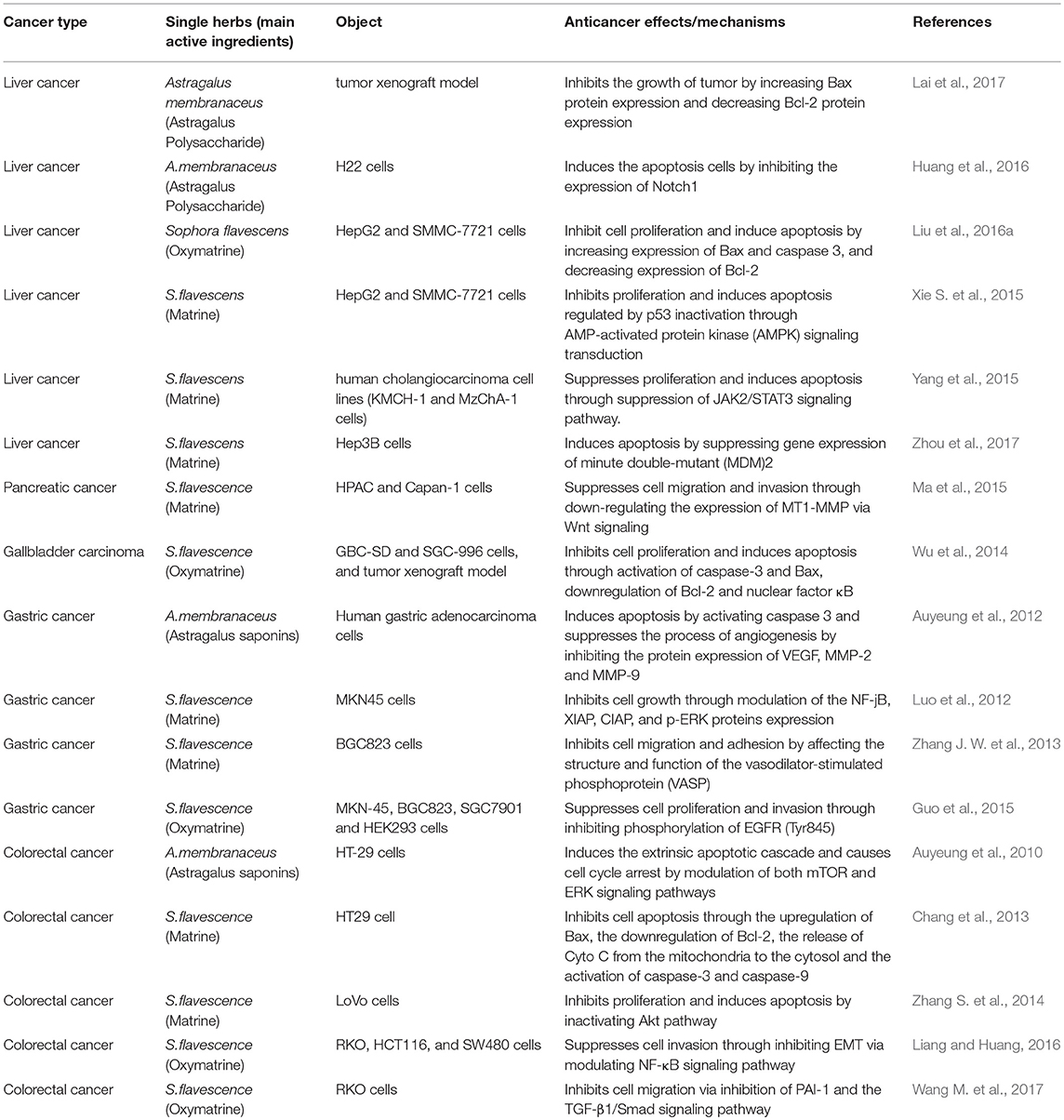
Table 1. Recent advances in anti-tumor mechanisms of single herbal medicines for digestive system malignancies.
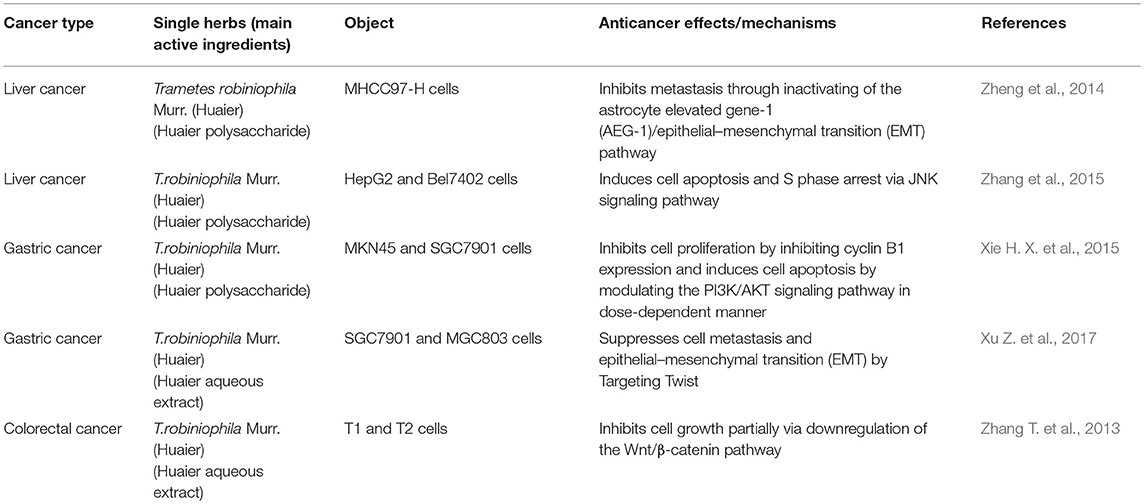
Table 2. Recent advances in anti-tumor mechanisms of Chinese medicine preparations for digestive system malignancies.
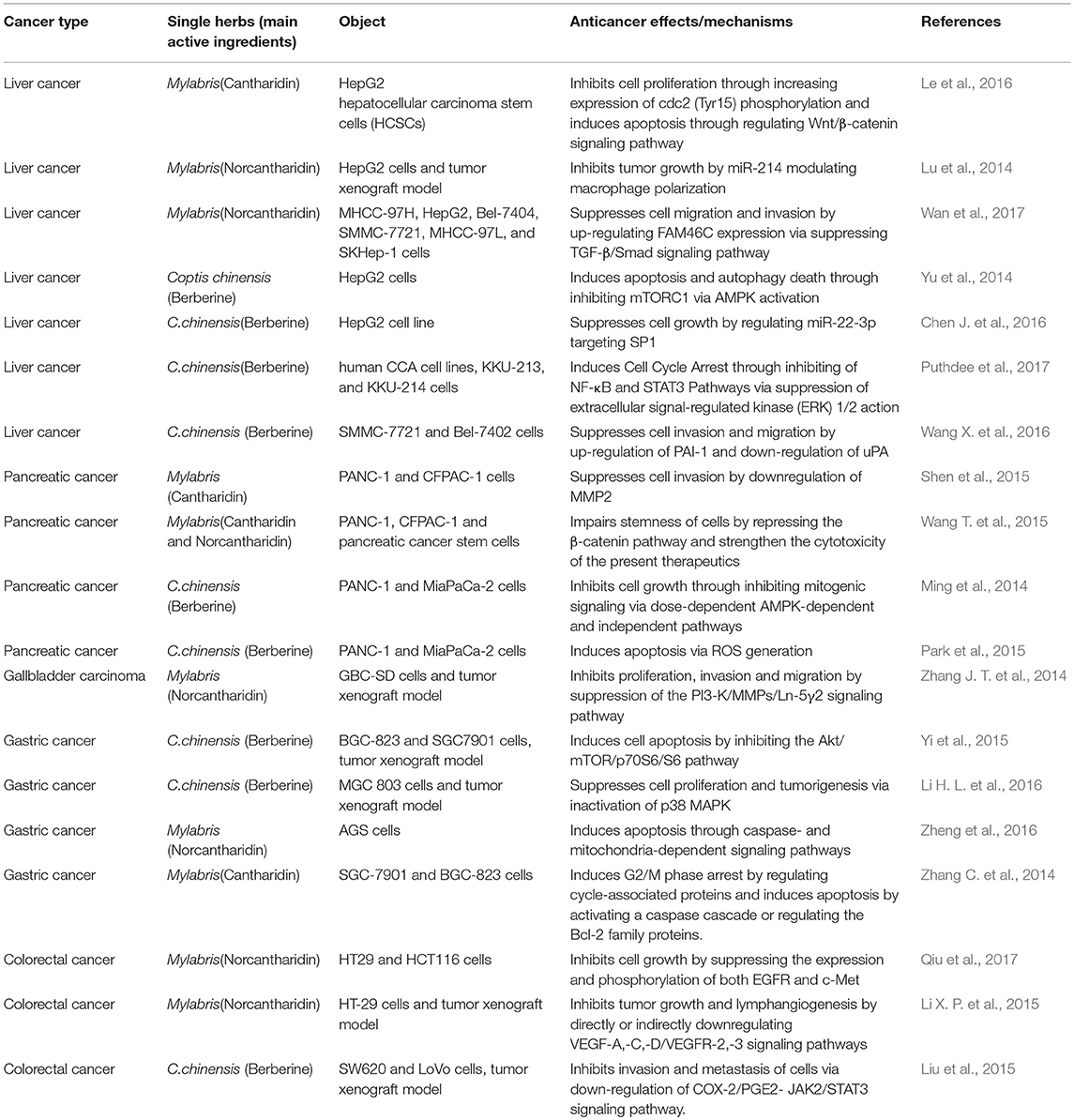
Table 3. Recent advances in anti-tumor mechanisms of traditional Chinese medicine extract for digestive system malignancies.
Unlike Western medicine, the anti-metastatic effects of TCM reflect comprehensive treatment of multiple targets and components, which can inhibit the various links of metastasis from many aspects, with less toxicity. Although some progress has been made in basic research and clinical application of TCM in the treatment of DSM, many components and mechanisms still need to be clarified. Modern scientific and technological means are being used to study anti-tumor mechanisms of TCM at the cellular, molecular and genome levels, to make full use of TCM in the management of digestive system malignancies.
Author Contributions
JS, ZC, XZ and YX wrote the manuscript. JS, WY, YL and RC provided the critical revisions. All authors approved the final version of the manuscript for submission.
Funding
This work was supported by grants from Natural Science Foundation of China (NSFC: 81802613), Jilin Science and Technology Agency funding (20180101114JC), and Finance Department of Jilin Province (2018).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2018.01249/full#supplementary-material
Supplementary Figure 1. Chemical structure of cantharidin and norcantharidin.
Supplementary Figure 2. Chemical structure of berberine.
References
Amitani, M., Amitani, H., Sloan, R. A., Suzuki, H., Sameshima, N., Asakawa, A., et al. (2015). The translational aspect of complementary and alternative medicine for cancer with particular emphasis on Kampo. Front. Pharmacol. 6:150. doi: 10.3389/fphar.2015.00150
Auyeung, K. K., Law, P. C., and Ko, J. K. (2014). Combined therapeutic effects of vinblastine and Astragalus saponins in human colon cancer cells and tumor xenograft via inhibition of tumor growth and proangiogenic factors. Nutr. Cancer 66, 662–674. doi: 10.1080/01635581.2014.894093
Auyeung, K. K., Mok, N. L., Wong, C. M., Cho, C. H., and Ko, J. K. (2010). Astragalus saponins modulate mTOR and ERK signaling to promote apoptosis through the extrinsic pathway in HT-29 colon cancer cells. Int. J. Mol. Med. 26, 341–349. doi: 10.3892/ijmm-00000471
Auyeung, K. K., Woo, P. K., Law, P. C., and Ko, J. K. (2012). Astragalus saponins modulate cell invasiveness and angiogenesis in human gastric adenocarcinoma cells. J. Ethnopharmacol. 141, 635–641. doi: 10.1016/j.jep.2011.08.010
Bao, H., Liu, P., Jiang, K., Zhang, X., Xie, L., Wang, Z., et al. (2016). Huaier polysaccharide induces apoptosis in hepatocellular carcinoma cells through p38 MAPK. Oncol. Lett. 12, 1058–1066. doi: 10.3892/ol.2016.4686
Bian, Y., Kitagawa, R., Bansal, P. K., Fujii, Y., Stepanov, A., and Kitagawa, K. (2014). Synthetic genetic array screen identifies PP2A as a therapeutic target in Mad2-overexpressing tumors. Proc. Natl. Acad. Sci. U.S.A. 111, 1628–1633. doi: 10.1073/pnas.1315588111
Cao, Y., Ruan, Y., Shen, T., Huang, X., Li, M., Yu, W., et al. (2014). Astragalus polysaccharide suppresses doxorubicin-induced cardiotoxicity by regulating the PI3k/Akt and p38MAPK pathways. Oxid. Med. Cell Longev. 2014:674219. doi: 10.1155/2014/674219
Chang, C., Liu, S. P., Fang, C. H., He, R. S., Wang, Z., Zhu, Y. Q., et al. (2013). Effects of matrine on the proliferation of HT29 human colon cancer cells and its antitumor mechanism. Oncol. Lett. 6, 699–704. doi: 10.3892/ol.2013.1449
Chang, L., and Karin, M. (2001). Mammalian MAP kinase signalling cascades. Nature 410, 37–40. doi: 10.1038/35065000
Chen, J., Wu, F. X., Luo, H. L., Liu, J. J., Luo, T., Bai, T., et al. (2016). Berberine upregulates miR-22-3p to suppress hepatocellular carcinoma cell proliferation by targeting Sp1. Am.J. Transl. Res. 8, 4932–4941.
Chen, S., Pang, X., Song, J., Shi, L., Yao, H., Han, J., et al. (2014). A renaissance in herbal medicine identification: from morphology to DNA. Biotechnol. Adv. 32, 1237–1244. doi: 10.1016/j.biotechadv.2014.07.004
Chen, X., Deng, L., Jiang, X., and Wu, T. (2016). Chinese herbal medicine for oesophageal cancer. Cochr. Database Syst. Rev. Cd004520. doi: 10.1002/14651858.CD004520.pub7
Cho, W. C., and Leung, K. N. (2007). In vitro and in vivo anti-tumor effects of Astragalus membranaceus. Cancer Lett. 252, 43–54. doi: 10.1016/j.canlet.2006.12.001
Dang, S. S., Jia, X. L., Song, P., Cheng, Y. A., Zhang, X., Sun, M. Z., et al. (2009). Inhibitory effect of emodin and Astragalus polysaccharide on the replication of HBV. World J. Gastroenterol. 15, 5669–5673. doi: 10.3748/wjg.15.5669
Deng, L. P., Dong, J., Cai, H., and Wang, W. (2013). Cantharidin as an antitumor agent: a retrospective review. Curr. Med. Chem. 20, 159–166. doi: 10.2174/0929867311320020001
Duan, L., Deng, L., Wang, D., Ma, S., Li, C., and Zhao, D. (2017). Treatment mechanism of matrine in combination with irinotecan for colon cancer. Oncol. Lett. 14, 2300–2304. doi: 10.3892/ol.2017.6407
Endo, M., Hori, M., Mihara, T., Ozaki, H., Oikawa, T., Odaguchi, H., et al. (2017). Zingiberis siccatum rhizoma, the active component of the Kampo formula Daikenchuto, induces anti-inflammatory actions through alpha7 nicotinic acetylcholine receptor activation. Neurogastroenterol. Motil. 29. doi: 10.1111/nmo.13139
Farrell, M. P., and Kummar, S. (2003). Phase I/IIA randomized study of PHY906, a novel herbal agent, as a modulator of chemotherapy in patients with advanced colorectal cancer. Clin. Colorectal. Cancer 2, 253–256. doi: 10.3816/CCC.2003.n.007
Finn, R. S., Zhu, A. X., Farah, W., Almasri, J., Zaiem, F., Prokop, L. J., et al. (2018). Therapies for advanced stage hepatocellular carcinoma with macrovascular invasion or metastatic disease: a systematic review and meta-analysis. Hepatology 67, 422–435. doi: 10.1002/hep.29486
Fu, F., Wan, Y., and Wu, T. (2014). Kanglaite injection combined with hepatic arterial intervention for unresectable hepatocellular carcinoma: a meta-analysis. J. Cancer Res. Ther. 10(Suppl. 1), 38–41. doi: 10.4103/0973-1482.139753
Ge, L., Wang, Y. F., Tian, J. H., Mao, L., Zhang, J., Zhang, J. H., et al. (2016). Network meta-analysis of Chinese herb injections combined with FOLFOX chemotherapy in the treatment of advanced colorectal cancer. J. Clin. Pharm. Ther. 41, 383–391. doi: 10.1111/jcpt.12410
Gong, J., Munoz, A. R., Pingali, S., Payton-Stewart, F., Chan, D. E., Freeman, J. W., et al. (2017). Downregulation of STAT3/NF-kappaB potentiates gemcitabine activity in pancreatic cancer cells. Mol. Carcinog. 56, 402–411. doi: 10.1002/mc.22503
Guo, B., Zhang, T., Su, J., Wang, K., and Li, X. (2015). Oxymatrine targets EGFR(p-Tyr845) and inhibits EGFR-related signaling pathways to suppress the proliferation and invasion of gastric cancer cells. Cancer Chemother. Pharmacol. 75, 353–363. doi: 10.1007/s00280-014-2651-1
Hasebe, T., and Matsukawa, J. (2017). Daikenchuto (TU-100) suppresses tumor development in the azoxymethane and APCmin/+ mouse models of experimental colon cancer. 31, 90–99. doi: 10.1002/ptr.5735
He, C. S., Liu, Y. C., Xu, Z. P., Dai, P. C., Chen, X. W., and Jin, D. H. (2016). Astragaloside IV enhances cisplatin chemosensitivity in non-small cell lung cancer cells through inhibition of B7-H3. Cell Physiol. Biochem. 40, 1221–1229. doi: 10.1159/000453175
He, X., Fang, J., Huang, L., Wang, J., and Huang, X. (2015). Sophora flavescens Ait.: traditional usage, phytochemistry and pharmacology of an important traditional Chinese medicine. J. Ethnopharmacol. 172, 10–29. doi: 10.1016/j.jep.2015.06.010
Hsia, T. C., Yu, C. C., Hsiao, Y. T., Wu, S. H., Bau, D. T., Lu, H. F., et al. (2016). Cantharidin impairs cell migration and invasion of human lung cancer NCI-H460 Cells via UPA and MAPK signaling pathways. Anticancer Res. 36, 5989–5997. doi: 10.21873/anticanres.11187
Hu, Z., Yang, A., Fan, H., Wang, Y., Zhao, Y., Zha, X., et al. (2016). Huaier aqueous extract sensitizes cells to rapamycin and cisplatin through activating mTOR signaling. J. Ethnopharmacol. 186, 143–150. doi: 10.1016/j.jep.2016.03.069
Huang, W. H., Liao, W. R., and Sun, R. X. (2016). Astragalus polysaccharide induces the apoptosis of human hepatocellular carcinoma cells by decreasing the expression of Notch1. Int. J. Mol. Med. 38, 551–557. doi: 10.3892/ijmm.2016.2632
Huang, W. M., Liang, Y. Q., Tang, L. J., Ding, Y., and Wang, X. H. (2013). Antioxidant and anti-inflammatory effects of Astragalus polysaccharide on EA.hy926 cells. Exp. Ther. Med. 6, 199–203. doi: 10.3892/etm.2013.1074
Hui, K., Yang, Y., Shi, K., Luo, H., Duan, J., An, J., et al. (2014). The p38 MAPK-regulated PKD1/CREB/Bcl-2 pathway contributes to selenite-induced colorectal cancer cell apoptosis in vitro and in vivo. Cancer Lett. 354, 189–199. doi: 10.1016/j.canlet.2014.08.009
Ikemoto, T., Shimada, M., Iwahashi, S., Saito, Y., Kanamoto, M., Mori, H., et al. (2014). Changes of immunological parameters with administration of Japanese Kampo medicine (Juzen-Taihoto/TJ-48) in patients with advanced pancreatic cancer. Int. J. Clin. Oncol. 19, 81–86. doi: 10.1007/s10147-013-0529-6
Iñarrairaegui, M., Melero, I., and Sangro, B. (2018). Immunotherapy of hepatocellular carcinoma: facts and hopes. Clin. Cancer Res. 24, 1518–1524. doi: 10.1158/1078-0432.ccr-17-0289
Jeong, J. S., Ryu, B. H., Kim, J. S., Park, J. W., Choi, W. C., and Yoon, S. W. (2010). Bojungikki-tang for cancer-related fatigue: a pilot randomized clinical trial. Integr. Cancer Ther. 9, 331–338. doi: 10.1177/1534735410383170
Jiancheng, W., Long, G., Ye, Z., Jinlong, L., Pan, Z., Lei, M., et al. (2015). Effect of Aidi injection plus chemotherapy on gastric carcinoma: a meta-analysis of randomized controlled trials. J Tradit Chin Med 35, 361–374. doi: 10.1016/S0254-6272(15)30111-4
Kadioglu, O., Kermani, N. S., Kelter, G., Schumacher, U., Fiebig, H. H., Greten, H. J., et al. (2014). Pharmacogenomics of cantharidin in tumor cells. Biochem. Pharmacol. 87, 399–409. doi: 10.1016/j.bcp.2013.10.025
Kao, S. T., Yeh, C. C., Hsieh, C. C., Yang, M. D., Lee, M. R., Liu, H. S., et al. (2001). The Chinese medicine Bu-Zhong-Yi-Qi-Tang inhibited proliferation of hepatoma cell lines by inducing apoptosis via G0/G1 arrest. Life. Sci. 69, 1485–1496. doi: 10.1016/S0024-3205(01)01226-7
Kim, M. A., Lee, H. S., Lee, H. E., Jeon, Y. K., Yang, H. K., and Kim, W. H. (2008). EGFR in gastric carcinomas: prognostic significance of protein overexpression and high gene copy number. Histopathology 52, 738–746. doi: 10.1111/j.1365-2559.2008.03021.x
Kominato, K., Yamasaki, H., Mitsuyama, K., Takedatsu, H., Yoshioka, S., Kuwaki, K., et al. (2016). Increased levels of circulating adrenomedullin following treatment with TU100 in patients with Crohn's disease. Mol. Med. Rep. 14, 2264–2268. doi: 10.3892/mmr.2016.5488
Koo, G. B., Morgan, M. J., Lee, D. G., Kim, W. J., Yoon, J. H., Koo, J. S., et al. (2015). Methylation-dependent loss of RIP3 expression in cancer represses programmed necrosis in response to chemotherapeutics. Cell. Res. 25, 707–725. doi: 10.1038/cr.2015.56
Kummar, S., Copur, M. S., Rose, M., Wadler, S., Stephenson, J., O'Rourke, M., et al. (2011). A phase I study of the chinese herbal medicine PHY906 as a modulator of irinotecan-based chemotherapy in patients with advanced colorectal cancer. Clin. Colorectal. Cancer 10, 85–96. doi: 10.1016/j.clcc.2011.03.003
Kuo, C. C., Chen, J. J., Tsai, J. Y., and Hsueh, C. T. (2014). Effects of Chinese herbal medicine in combination with mitomycin C on gastric cancer cells. Biomark. Res. 2:26. doi: 10.1186/s40364-014-0026-8
Lai, X., Xia, W., Wei, J., and Ding, X. (2017). Therapeutic effect of astragalus polysaccharides on hepatocellular carcinoma H22-bearing mice. Dose Response 15:1559325816685182. doi: 10.1177/1559325816685182
Lam, W., Bussom, S., Guan, F., Jiang, Z., Zhang, W., Gullen, E. A., et al. (2010). The four-herb Chinese medicine PHY906 reduces chemotherapy-induced gastrointestinal toxicity. Sci. Transl. Med. 2:45ra59. doi: 10.1126/scitranslmed.3001270.
Lam, W., Jiang, Z., Guan, F., Huang, X., Hu, R., Wang, J., et al. (2015). PHY906(KD018), an adjuvant based on a 1800-year-old Chinese medicine, enhanced the anti-tumor activity of Sorafenib by changing the tumor microenvironment. Sci. Rep. 5:9384. doi: 10.1038/srep09384
Lamy, E., Herz, C., Lutz-Bonengel, S., Hertrampf, A., Marton, M. R., and Mersch-Sundermann, V. (2013). The MAPK pathway signals telomerase modulation in response to isothiocyanate-induced DNA damage of human liver cancer cells. PLoS ONE 8:e53240. doi: 10.1371/journal.pone.0053240
Lao, M., Weissler, A., and Siegfried, E. (2013). Safe and speedy cantharidin application. J. Am. Acad. Dermatol. 69:e47. doi: 10.1016/j.jaad.2013.04.023
Le, A. P., Zhang, L. L., Liu, W., and Shi, Y. F. (2016). Cantharidin inhibits cell proliferation and induces apoptosis through G2/M phase cell cycle arrest in hepatocellular carcinoma stem cells. Oncol. Rep. 35, 2970–2976. doi: 10.3892/or.2016.4684
Li, C., Wu, X., Zhang, H., Yang, G., Hao, M., Sheng, S., et al. (2015). A Huaier polysaccharide restrains hepatocellular carcinoma growth and metastasis by suppression angiogenesis. Int. J. Biol. Macromol. 75, 115–120. doi: 10.1016/j.ijbiomac.2015.01.016
Li, H. L., Wu, H., Zhang, B. B., Shi, H. L., and Wu, X. J. (2016). MAPK pathways are involved in the inhibitory effect of berberine hydrochloride on gastric cancer MGC 803 cell proliferation and IL-8 secretion in vitro and in vivo. Mol. Med. Rep. 14, 1430–1438. doi: 10.3892/mmr.2016.5361
Li, J., Huang, L., Wang, S., Yao, Y., and Zhang, Z. (2016). Astragaloside IV attenuates inflammatory reaction via activating immune function of regulatory T-cells inhibited by HMGB1 in mice. Pharm. Biol. 54, 3217–3225. doi: 10.1080/13880209.2016.1216133
Li, J., Wang, J. C., Ma, B., Gao, W., Chen, P., Sun, R., et al. (2015). Shenqi Fuzheng injection for advanced gastric cancer: a systematic review of randomized controlled trials. Chin. J. Integr. Med. 21, 71–79. doi: 10.1007/s11655-014-1768-8
Li, L., Hou, X., Xu, R., Liu, C., and Tu, M. (2017). Research review on the pharmacological effects of astragaloside IV. Fundam. Clin. Pharmacol. 31, 17–36. doi: 10.1111/fcp.12232
Li, X. P., Jing, W., Sun, J. J., Liu, Z. Y., Zhang, J. T., Sun, W., et al. (2015). A potential small-molecule synthetic antilymphangiogenic agent norcantharidin inhibits tumor growth and lymphangiogenesis of human colonic adenocarcinomas through blocking VEGF-A,-C,-D/VEGFR-2,-3 “multi-points priming” mechanisms in vitro and in vivo. BMC Cancer 15:527. doi: 10.1186/s12885-015-1521-5
Liang, L., and Huang, J. (2016). Oxymatrine inhibits epithelial-mesenchymal transition through regulation of NF-kappaB signaling in colorectal cancer cells. Oncol. Rep. 36, 1333–1338. doi: 10.3892/or.2016.4927
Liu, J., Hu, X., Yang, Q., Yu, Z., Zhao, Z., Yi, T., et al. (2010). Comparison of the immunoregulatory function of different constituents in radix astragali and radix hedysari. J. Biomed. Biotechnol. 2010:479426. doi: 10.1155/2010/479426
Liu, J., Zhang, J. F., Lu, J. Z., Zhang, D. L., Li, K., Su, K., et al. (2013). Astragalus polysaccharide stimulates glucose uptake in L6 myotubes through AMPK activation and AS160/TBC1D4 phosphorylation. Acta Pharmacol. Sin. 34, 137–145. doi: 10.1038/aps.2012.133
Liu, P., Atkinson, S. J., Akbareian, S. E., Zhou, Z., Munsterberg, A., Robinson, S. D., et al. (2017). Sulforaphane exerts anti-angiogenesis effects against hepatocellular carcinoma through inhibition of STAT3/HIF-1alpha/VEGF signalling. 7:12651. doi: 10.1038/s41598-017-12855-w
Liu, S. H., and Cheng, Y. C. (2012). Old formula, new Rx: the journey of PHY906 as cancer adjuvant therapy. J. Ethnopharmacol. 140, 614–623. doi: 10.1016/j.jep.2012.01.047
Liu, X., Ji, Q., Ye, N., Sui, H., Zhou, L., Zhu, H., et al. (2015). Berberine inhibits invasion and metastasis of colorectal cancer cells via COX-2/PGE2 mediated JAK2/STAT3 signaling pathway. PLoS ONE 10:e0123478. doi: 10.1371/journal.pone.0123478
Liu, Y., Bi, T., Dai, W., Wang, G., Qian, L., Gao, Q., et al. (2016a). Effects of oxymatrine on the proliferation and apoptosis of human hepatoma carcinoma cells. Technol. Cancer Res.Treat. 15, 487–497. doi: 10.1177/1533034615587616
Liu, Y., Bi, T., Dai, W., Wang, G., Qian, L., Gao, Q., et al. (2016b). Oxymatrine synergistically enhances the inhibitory effect of 5-fluorouracil on hepatocellular carcinoma in vitro and in vivo. Tumour. Biol. 37, 7589–7597. doi: 10.1007/s13277-015-4642-1
Liu, Y., Bi, T., Wang, Z., Wu, G., Qian, L., Gao, Q., et al. (2016c). Oxymatrine synergistically enhances antitumor activity of oxaliplatin in colon carcinoma through PI3K/AKT/mTOR pathway. Apoptosis 21, 1398–1407. doi: 10.1007/s10495-016-1297-3
Lu, Q., Xu, L., Meng, Y., Liu, Y., Li, J., Zu, Y., et al. (2016). Preparation and characterization of a novel Astragalus membranaceus polysaccharide-iron (III) complex. Int. J. Biol. Macromol. 93(Pt A), 208–216. doi: 10.1016/j.ijbiomac.2016.08.049
Lu, S., Gao, Y., Huang, X., and Wang, X. (2014). Cantharidin exerts anti-hepatocellular carcinoma by miR-214 modulating macrophage polarization. Int. J.Biol.Sci. 10, 415–425. doi: 10.7150/ijbs.8002
Luo, C., Zhong, H. J., Zhu, L. M., Wu, X. G., Ying, J. E., Wang, X. H., et al. (2012). Inhibition of matrine against gastric cancer cell line MNK45 growth and its anti-tumor mechanism. Mol. Biol. Rep. 39, 5459–5464. doi: 10.1007/s11033-011-1346-5
Ma, Y., Zou, F., Xiong, J., Wan, W., Yin, L., Li, X., et al. (2015). Effect of Matrine on HPAC cell migration by down-regulating the expression of MT1-MMP via Wnt signaling. Cancer Cell. Int. 15:59. doi: 10.1186/s12935-015-0210-4
Ming, M., Sinnett-Smith, J., Wang, J., Soares, H. P., Young, S. H., Eibl, G., et al. (2014). Dose-dependent AMPK-dependent and independent mechanisms of berberine and metformin inhibition of mTORC1, ERK, DNA synthesis and proliferation in pancreatic cancer cells. PLoS ONE 9:e114573. doi: 10.1371/journal.pone.0114573
Nagata, T., Toume, K., Long, L. X., Hirano, K., Watanabe, T., Sekine, S., et al. (2016). Anticancer effect of a Kampo preparation Daikenchuto. J. Nat.Med. 70, 627–633. doi: 10.1007/s11418-016-0989-x
Ni, W., Li, C., Liu, Y., Song, H., Wang, L., Song, H., et al. (2017). Various bioactivity and relationship of structure-activity of matrine analogues. J. Agric. Food Chem. 65, 2039–2047. doi: 10.1021/acs.jafc.6b05474
Nishiuchi, T., Okutani, Y., Yamagishi, Y., Fujita, T., Imataki, O., Ohnishi, H., et al. (2013). Synergistic effect between Juzen-taiho-to, a Japanese traditional herbal medicine, and gemcitabine single-agent chemotherapy for advanced biliary tract cancer. J. Altern. Complement. Med. 19, 593–597. doi: 10.1089/acm.2012.0177
Okada, K., Kawai, M., Hirono, S., Fujii, T., Kodera, Y., Sho, M., et al. (2016). Evaluation of the efficacy of daikenchuto (TJ−100) for the prevention of paralytic ileus after pancreaticoduodenectomy: a multicenter, double-blind, randomized, placebo-controlled trial. Surgery 159, 1333–1341. doi: 10.1016/j.surg.2015.11.019
Pandey, A., Vishnoi, K., Mahata, S., Tripathi, S. C., Misra, S. P., Misra, V., et al. (2015). Berberine and curcumin target survivin and STAT3 in gastric cancer cells and synergize actions of standard chemotherapeutic 5-fluorouracil. Nutr. Cancer 67, 1293–1304. doi: 10.1080/01635581.2015.1085581
Park, S. H., Sung, J. H., Kim, E. J., and Chung, N. (2015). Berberine induces apoptosis via ROS generation in PANC-1 and MIA-PaCa2 pancreatic cell lines. Braz. J. Med. Biol. Res. 48, 111–119. doi: 10.1590/1414-431x20144293
Pećina-Slaus, N. (2010). Wnt signal transduction pathway and apoptosis: a review. Cancer Cell. Int. 10:22. doi: 10.1186/1475-2867-10-22
Puerto Galvis, C. E., Vargas Mendez, L. Y., and Kouznetsov, V. V. (2013). Cantharidin-based small molecules as potential therapeutic agents. Chem. Biol. Drug Des. 82, 477–499. doi: 10.1111/cbdd.12180
Puthdee, N., Seubwai, W., Vaeteewoottacharn, K., Boonmars, T., Cha'on, U., Phoomak, C., et al. (2017). Berberine induces cell cycle arrest in cholangiocarcinoma cell lines via inhibition of NF-kappaB and STAT3 Pathways. Biol. Pharm Bull. 40, 751–757. doi: 10.1248/bpb.b16-00428
Qi, F., Li, A., Inagaki, Y., Gao, J., Li, J., Kokudo, N., et al. (2010). Chinese herbal medicines as adjuvant treatment during chemo- or radio-therapy for cancer. Biosci. Trends 4, 297–307.
Qi, F., Zhao, L., Zhou, A., Zhang, B., Li, A., Wang, Z., et al. (2015). The advantages of using traditional Chinese medicine as an adjunctive therapy in the whole course of cancer treatment instead of only terminal stage of cancer. Biosci. Trends 9, 16–34. doi: 10.5582/bst.2015.01019
Qi, Y., Gao, F., Hou, L., and Wan, C. (2017). Anti-inflammatory and immunostimulatory activities of astragalosides. Am. J. Chin. Med. 45, 1157–1167. doi: 10.1142/s0192415x1750063x
Qiu, P., Wang, S., Liu, M., Ma, H., Zeng, X., Zhang, M., et al. (2017). Norcantharidin Inhibits cell growth by suppressing the expression and phosphorylation of both EGFR and c-Met in human colon cancer cells. BMC Cancer 17:55. doi: 10.1186/s12885-016-3039-x.
Rauh, R., Kahl, S., Boechzelt, H., Bauer, R., Kaina, B., and Efferth, T. (2007). Molecular biology of cantharidin in cancer cells. Chin. Med. 2:8. doi: 10.1186/1749-8546-2-8
Rockwell, S., Grove, T. A., Liu, Y., Cheng, Y. C., Higgins, S. A., and Booth, C. J. (2013). Preclinical studies of the Chinese Herbal Medicine formulation PHY906 (KD018) as a potential adjunct to radiation therapy. Int. J. Radiat. Biol. 89, 16–25. doi: 10.3109/09553002.2012.717733
Saif, M. W., Li, J., Lamb, L., Kaley, K., Elligers, K., Jiang, Z., et al. (2014). First-in-human phase II trial of the botanical formulation PHY906 with capecitabine as second-line therapy in patients with advanced pancreatic cancer. Cancer Chemother. Pharmacol. 73, 373–380. doi: 10.1007/s00280-013-2359-7
Shen, M., Wu, M. Y., Chen, L. P., Zhi, Q., Gong, F. R., Chen, K., et al. (2015). Cantharidin represses invasion of pancreatic cancer cells through accelerated degradation of MMP2 mRNA. Sci. Rep. 5:11836. doi: 10.1038/srep11836
Shih, T. C., Tien, Y. J., Wen, C. J., Yeh, T. S., Yu, M. C., Huang, C. H., et al. (2012). MicroRNA-214 downregulation contributes to tumor angiogenesis by inducing secretion of the hepatoma-derived growth factor in human hepatoma. J. Hepatol. 57, 584–591. doi: 10.1016/j.jhep.2012.04.031
Siegel, R. L., Miller, K. D., and Jemal, A. (2018). Cancer statistics, 2018. CA Cancer J. Clin. 2018. 68, 7–30. doi: 10.3322/caac.21442
Song, X., Li, Y., Zhang, H., and Yang, Q. (2015). The anticancer effect of Huaier (Review). Oncol. Rep. 34, 12–21. doi: 10.3892/or.2015.3950
Su, C. C., Liu, S. H., Lee, K. I., Huang, K. T., Lu, T. H., Fang, K. M., et al. (2015). Cantharidin induces apoptosis through the calcium/PKC-regulated endoplasmic reticulum stress pathway in human bladder cancer cells. Am. J. Chin. Med. 43, 581–600. doi: 10.1142/s0192415x15500366
Su, Z., Zhou, C., Qin, S., Jia, E., and Wu, Z. (2017). The significant pathways and genes underlying the colon cancer treatment by the traditional chinese medicine PHY906. Evid. Based Complement. Alternat. Med. 2017:8753815. doi: 10.1155/2017/8753815
Sun, W. W., Dou, J. X., Zhang, L., Qiao, L. K., Shen, N., Zhao, Q., et al. (2017). Killing effects of huaier granule combined with DC-CIK on nude mice transplanted with colon carcinoma cell line. Oncotarget 8, 46081–46089. doi: 10.18632/oncotarget.17687
Sun, X., Cai, X., Yang, J., Chen, J., Guo, C., and Cao, P. (2016). Cantharidin overcomes imatinib resistance by depleting BCR-ABL in chronic myeloid leukemia. Mol. Cells 39, 869–876. doi: 10.14348/molcells.2016.0023
Syed, S. B., and Coumar, M. S. (2016). P-Glycoprotein mediated multidrug resistance reversal by phytochemicals: a review of SAR & future perspective for drug design. Curr. Top. Med. Chem. 16, 2484–2508. doi: 10.2174/1568026616666160212123814
Tang, J., Feng, Y., Tsao, S., Wang, N., Curtain, R., and Wang, Y. (2009). Berberine and coptidis rhizoma as novel antineoplastic agents: a review of traditional use and biomedical investigations. J. Ethnopharmacol. 126, 5–17. doi: 10.1016/j.jep.2009.08.009
Taylor, C. A., Zheng, Q., Liu, Z., and Thompson, J. E. (2013). Role of p38 and JNK MAPK signaling pathways and tumor suppressor p53 on induction of apoptosis in response to Ad-eIF5A1 in A549 lung cancer cells. Mol. Cancer 12, 35. doi: 10.1186/1476-4598-12-35
Tian, Q. E., De Li, H., Yan, M., Cai, H. L., Tan, Q. Y., and Zhang, W. Y. (2012a). Effects of Astragalus polysaccharides on P-glycoprotein efflux pump function and protein expression in H22 hepatoma cells in vitro. BMC Compl. Altern. Med. 12:94. doi: 10.1186/1472-6882-12-94
Tian, Q. E., Li, H. D., Yan, M., Cai, H. L., Tan, Q. Y., and Zhang, W. Y. (2012b). Astragalus polysaccharides can regulate cytokine and P-glycoprotein expression in H22 tumor-bearing mice. World J. Gastroenterol. 18, 7079–7086. doi: 10.3748/wjg.v18.i47.7079
Toesca, D. A. S., Koong, A. J., Poultsides, G. A., Visser, B. C., Haraldsdottir, S., Koong, A. C., et al. (2018). Management of borderline resectable pancreatic cancer. Int. J. Radiat. Oncol. Biol. Phys. 100, 1155–1174. doi: 10.1016/j.ijrobp.2017.12.287
Tomasello, G., Petrelli, F., Ghidini, M., Russo, A., Passalacqua, R., and Barni, S. (2017). FOLFOXIRI plus bevacizumab as conversion therapy for patients with initially unresectable metastatic colorectal cancer: a systematic review and pooled analysis. JAMA Oncol. 3:e170278. doi: 10.1001/jamaoncol.2017.0278
Wan, X. Y., Zhai, X. F., Jiang, Y. P., Han, T., Zhang, Q. Y., and Xin, H. L. (2017). Antimetastatic effects of norcantharidin on hepatocellular carcinoma cells by up-regulating FAM46C expression. Am. J. Transl. Res. 9, 155–166.
Wang, G., Dong, J., and Deng, L. (2018). Overview of Cantharidin and Its Analogues. Curr Med Chem. 25, 2034–2044. doi: 10.2174/0929867324666170414165253
Wang, J., Li, J., Wang, X., Zheng, C., and Ma, W. (2013). Downregulation of microRNA-214 and overexpression of FGFR-1 contribute to hepatocellular carcinoma metastasis. Biochem. Biophys. Res. Commun. 439, 47–53. doi: 10.1016/j.bbrc.2013.08.032
Wang, J., Tong, X., Li, P., Cao, H., and Su, W. (2012). Immuno-enhancement effects of Shenqi Fuzheng Injection on cyclophosphamide-induced immunosuppression in Balb/c mice. J. Ethnopharmacol.139, 788–795. doi: 10.1016/j.jep.2011.12.019
Wang, J., Tong, X., Li, P., Liu, M., Peng, W., Cao, H., et al. (2014). Bioactive components on immuno-enhancement effects in the traditional Chinese medicine Shenqi Fuzheng Injection based on relevance analysis between chemical HPLC fingerprints and in vivo biological effects. J. Ethnopharmacol. 155, 405–415. doi: 10.1016/j.jep.2014.05.038
Wang, J., Yang, S., Cai, X., Dong, J., Chen, Z., Wang, R., et al. (2016). Berberine inhibits EGFR signaling and enhances the antitumor effects of EGFR inhibitors in gastric cancer. Oncotarget 7, 76076–76086. doi: 10.18632/oncotarget.12589
Wang, M., Huang, C., Su, Y., Yang, C., Xia, Q., and Xu, D. J. (2017). Astragaloside II sensitizes human hepatocellular carcinoma cells to 5-fluorouracil via suppression of autophagy. J. Pharm. Pharmacol. 69, 743–752. doi: 10.1111/jphp.12706
Wang, N., Feng, Y., Zhu, M., Tsang, C. M., Man, K., Tong, Y., et al. (2010). Berberine induces autophagic cell death and mitochondrial apoptosis in liver cancer cells: the cellular mechanism. J. Cell Biochem. 111, 1426–1436. doi: 10.1002/jcb.22869
Wang, T., Liu, J., and Xiao, X. Q. (2015). Cantharidin inhibits angiogenesis by suppressing VEGF-induced JAK1/STAT3, ERK and AKT signaling pathways. Arch. Pharm. Res. 38, 282–289. doi: 10.1007/s12272-014-0383-8
Wang, T., Nan, H., Zhang, C., Wang, Y., Zhang, X., and Li, Y. (2014). Aidi injection combined with FOLFOX4 chemotherapy regimen in the treatment of advanced colorectal carcinoma. J. Cancer Res. Ther. 10 (Suppl 1), 52–55. doi: 10.4103/0973-1482.139760
Wang, T., Xuan, X., Li, M., Gao, P., Zheng, Y., Zang, W., et al. (2013). Astragalus saponins affect proliferation, invasion and apoptosis of gastric cancer BGC-823 cells. Diagn. Pathol. 8:179. doi: 10.1186/1746-1596-8-179
Wang, W. J., Wu, M. Y., Shen, M., Zhi, Q., Liu, Z. Y., Gong, F. R., et al. (2015). Cantharidin and norcantharidin impair stemness of pancreatic cancer cells by repressing the beta-catenin pathway and strengthen the cytotoxicity of gemcitabine and erlotinib. Int. J. Oncol. 47, 1912–1922. doi: 10.3892/ijo.2015.3156
Wang, X., Chen, J., Li, F., Lin, Y., Zhang, X., Lv, Z., et al. (2012). MiR-214 inhibits cell growth in hepatocellular carcinoma through suppression of beta-catenin. Biochem. Biophys. Res. Commun. 428, 525–531. doi: 10.1016/j.bbrc.2012.10.039
Wang, X., Liu, C., Wang, J., Fan, Y., Wang, Z., and Wang, Y. (2017). Oxymatrine inhibits the migration of human colorectal carcinoma RKO cells via inhibition of PAI-1 and the TGF-beta1/Smad signaling pathway. Oncol. Rep. 37, 747–753. doi: 10.3892/or.2016.5292
Wang, X., Wang, N., Li, H., Liu, M., Cao, F., Yu, X., et al. (2016). Up-regulation of PAI-1 and down-regulation of upa are involved in suppression of invasiveness and motility of hepatocellular carcinoma cells by a natural compound berberine. Int. J. Mol. Sci. 17:577. doi: 10.3390/ijms17040577
Wang, X., Zhang, N., Huo, Q., and Yang, Q. (2012). Anti-angiogenic and antitumor activities of Huaier aqueous extract. Oncol. Rep. 28, 1167–1175. doi: 10.3892/or.2012.1961
Wang, Y., Auyeung, K. K., Zhang, X., and Ko, J. K. (2014). Astragalus saponins modulates colon cancer development by regulating calpain-mediated glucose-regulated protein expression. BMC Compl. Altern. Med. 14:401. doi: 10.1186/1472-6882-14-401
Wilhelm, S. M., Carter, C., Tang, L., Wilkie, D., McNabola, A., Rong, H., et al. (2004). BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 64, 7099–7109. doi: 10.1158/0008-5472.can-04-1443
Wong, A. S., Che, C. M., and Leung, K. W. (2015). Recent advances in ginseng as cancer therapeutics: a functional and mechanistic overview. Nat. Prod. Rep. 32, 256–272. doi: 10.1039/c4np00080c
Wu, X. S., Yang, T., Gu, J., Li, M. L., Wu, W. G., Weng, H., et al. (2014). Effects of oxymatrine on the apoptosis and proliferation of gallbladder cancer cells. Anticancer Drugs 25, 1007–1015. doi: 10.1097/cad.0000000000000124
Xiao, Z., Wang, C., Chen, L., Tang, X., Li, L., Li, N., et al. (2017). Has aidi injection the attenuation and synergistic efficacy to gemcitabine and cisplatin in non-small cell lung cancer? a meta-analysis of 36 randomized controlled trials. Oncotarget 8, 1329–1342. doi: 10.18632/oncotarget.13617
Xie, H. X., Xu, Z. Y., Tang, J. N., Du, Y. A., Huang, L., Yu, P. F., et al. (2015). Effect of Huaier on the proliferation and apoptosis of human gastric cancer cells through modulation of the PI3K/AKT signaling pathway. Exp. Ther. Med. 10, 1212–1218. doi: 10.3892/etm.2015.2600
Xie, S. B., He, X. X., and Yao, S. K. (2015). Matrine-induced autophagy regulated by p53 through AMP-activated protein kinase in human hepatoma cells. Int. J. Oncol. 47, 517–526. doi: 10.3892/ijo.2015.3023
Xie, T., Li, Y., Li, S. L., and Luo, H. F. (2016). Astragaloside IV enhances cisplatin chemosensitivity in human colorectal cancer via regulating NOTCH3. Oncol. Res. 24, 447–453. doi: 10.3727/096504016x14685034103590
Xie, X., Wu, M. Y., Shou, L. M., Chen, L. P., Gong, F. R., Chen, K., et al. (2015). Tamoxifen enhances the anticancer effect of cantharidin and norcantharidin in pancreatic cancer cell lines through inhibition of the protein kinase C signaling pathway. Oncol. Lett. 9, 837–844. doi: 10.3892/ol.2014.2711
Xu, B., Xu, M., Tian, Y., Yu, Q., Zhao, Y., Chen, X., et al. (2017). Matrine induces RIP3-dependent necroptosis in cholangiocarcinoma cells. Cell Death Discov. 3:16096. doi: 10.1038/cddiscovery.2016.96
Xu, D. J., Xia, Q., Wang, J. J., and Wang, P. P. (2008). Molecular weight and monosaccharide composition of Astragalus polysaccharides. Molecules 13, 2408–2415.
Xu, H., Zhao, X., Liu, X., Xu, P., Zhang, K., and Lin, X. (2015). Antitumor effects of traditional Chinese medicine targeting the cellular apoptotic pathway. Drug Des. Devel. Ther. 9, 2735–2744. doi: 10.2147/dddt.s80902
Xu, R., Lin, L., Li, Y., and Li, Y. (2017). ShenQi FuZheng injection combined with chemotherapy in the treatment of colorectal cancer: a meta-analysis. 12:e0185254. doi: 10.1371/journal.pone.0185254
Xu, Z., Zheng, G., Wang, Y., Zhang, C., Yu, J., Teng, F., et al. (2017). Aqueous huaier extract suppresses gastric cancer metastasis and epithelial to mesenchymal transition by targeting twist. J Cancer 8, 3876–3886. doi: 10.7150/jca.20380
Yae, S., Takahashi, F., Yae, T., Yamaguchi, T., Tsukada, R., Koike, K., et al. (2012). Hochuekkito (TJ-41), a Kampo formula, ameliorates cachexia induced by colon 26 adenocarcinoma in mice. Evid. Based Compl. Alternat. Med. 2012:976926. doi: 10.1155/2012/976926
Yang, B., Xiao, B., and Sun, T. (2013). Antitumor and immunomodulatory activity of Astragalus membranaceus polysaccharides in H22 tumor-bearing mice. Int. J. Biol. Macromol. 62, 287–290. doi: 10.1016/j.ijbiomac.2013.09.016
Yang, M. H., Lee, K. T., Yang, S., Lee, J. K., Lee, K. H., Moon, I. H., et al. (2012). Guggulsterone enhances antitumor activity of gemcitabine in gallbladder cancer cells through suppression of NF-kappaB. J. Cancer Res. Clin. Oncol. 138, 1743–1751. doi: 10.1007/s00432-012-1254-7
Yang, N., Han, F., Cui, H., Huang, J., Wang, T., Zhou, Y., et al. (2015). Matrine suppresses proliferation and induces apoptosis in human cholangiocarcinoma cells through suppression of JAK2/STAT3 signaling. Pharmacol. Rep. 67, 388–393. doi: 10.1016/j.pharep.2014.10.016
Yang, Y., Kang, P., Gao, J., Xu, C., Wang, S., Jin, H., et al. (2014). AU-binding factor 1 expression was correlated with metadherin expression and progression of hepatocellular carcinoma. Tumour. Biol. 35, 2747–2751. doi: 10.1007/s13277-013-1362-2
Yao, K., Ma, Y., Ma, W., Hu, J., Wang, C., Chen, J., et al. (2014). Shenqifuzheng injection combined with chemotherapy in the treatment of advanced gastric cancer: a systematic review and meta-analysis. J. Cancer Res. Ther. 10(Suppl. 1), 70–74. doi: 10.4103/0973-1482.139768
Ye, Q., Su, L., Chen, D., Zheng, W., and Liu, Y. (2017). Astragaloside IV Induced miR-134 expression reduces EMT and increases chemotherapeutic sensitivity by suppressing CREB1 signaling in colorectal cancer cell line SW-480. Cell Physiol. Biochem. 43, 1617–1626. doi: 10.1159/000482025
Yeh, C. B., Su, C. J., Hwang, J. M., and Chou, M. C. (2010). Therapeutic effects of cantharidin analogues without bridging ether oxygen on human hepatocellular carcinoma cells. Eur. J. Med. Chem. 45, 3981–3985. doi: 10.1016/j.ejmech.2010.05.053
Yen, Y., So, S., Rose, M., Saif, M. W., Chu, E., Liu, S. H., et al. (2009). Phase I/II study of PHY906/capecitabine in advanced hepatocellular carcinoma. Anticancer Res 29, 4083–4092.
Yi, T., Zhuang, L., Song, G., Zhang, B., Li, G., and Hu, T. (2015). Akt signaling is associated with the berberine-induced apoptosis of human gastric cancer cells. Nutr. Cancer 67, 523–531. doi: 10.1080/01635581.2015.1004733
Yong, J., Wu, X., and Lu, C. (2015). Anticancer advances of matrine and its derivatives. Curr. Pharm. Des. 21, 3673–3680.
Yoshida, R., Nagata, M., Nakayama, H., Niimori-Kita, K., Hassan, W., Tanaka, T., et al. (2013). The pathological significance of Notch1 in oral squamous cell carcinoma. Lab. Invest. 93, 1068–1081. doi: 10.1038/labinvest.2013.95
Yoshikawa, K., Shimada, M., Wakabayashi, G., Ishida, K., Kaiho, T., Kitagawa, Y., et al. (2015). Effect of daikenchuto, a traditional japanese herbal medicine, after total gastrectomy for gastric cancer: a multicenter, randomized, double-blind, placebo-controlled, phase II trial. J. Am. Coll. Surg. 221, 571–578. doi: 10.1016/j.jamcollsurg.2015.03.004
You, H. Y., Xie, X. M., Zhang, W. J., Zhu, H. L., and Jiang, F. Z. (2016). Berberine modulates cisplatin sensitivity of human gastric cancer cells by upregulation of miR-203. In Vitro Cell Dev. Biol. Anim. 52, 857–863. doi: 10.1007/s11626-016-0044-y
Yu, R., Zhang, Z. Q., Wang, B., Jiang, H. X., Cheng, L., and Shen, L. M. (2014). Berberine-induced apoptotic and autophagic death of HepG2 cells requires AMPK activation. Cancer Cell Int. 14:49. doi: 10.1186/1475-2867-14-49
Zeng, Y. B., Liu, X. L., Zhang, Y., Li, C. J., Zhang, D. M., Peng, Y. Z., et al. (2016). Cantharimide and its derivatives from the blister beetle mylabris phalerata palla. J. Nat. Prod. 79, 2032–2038. doi: 10.1021/acs.jnatprod.6b00332
Zhang, A., Sun, H., and Wang, X. (2018). Mass spectrometry-driven drug discovery for development of herbal medicine. Mass. Spectrom. Rev. 37, 307–320. doi: 10.1002/mas.21529
Zhang, C., Chen, Z., Zhou, X., Xu, W., Wang, G., Tang, X., et al. (2014). Cantharidin induces G2/M phase arrest and apoptosis in human gastric cancer SGC-7901 and BGC-823 cells. Oncol. Lett. 8, 2721–2726. doi: 10.3892/ol.2014.2611
Zhang, C., Zhang, J., Li, X., Sun, N., Yu, R., Zhao, B., et al. (2015). Huaier aqueous extract induces hepatocellular carcinoma cells arrest in S phase via JNK signaling pathway. Evid. Based Complement. Alternat. Med. 2015:171356. doi: 10.1155/2015/171356
Zhang, D., Zheng, J., Ni, M., Wu, J., Wang, K., Duan, X., et al. (2017). Comparative efficacy and safety of Chinese herbal injections combined with the FOLFOX regimen for treating gastric cancer in China: a network meta-analysis. Oncotarget 8, 68873–68889. doi: 10.18632/oncotarget.20320
Zhang, J. T., Sun, W., Zhang, W. Z., Ge, C. Y., Liu, Z. Y., Zhao, Z. M., et al. (2014). Norcantharidin inhibits tumor growth and vasculogenic mimicry of human gallbladder carcinomas by suppression of the PI3-K/MMPs/Ln-5gamma2 signaling pathway. BMC Cancer 14:193. doi: 10.1186/1471-2407-14-193
Zhang, J. W., Su, K., Shi, W. T., Wang, Y., Hu, P. C., Wang, Y., et al. (2013). Matrine inhibits the adhesion and migration of BCG823 gastric cancer cells by affecting the structure and function of the vasodilator-stimulated phosphoprotein (VASP). Acta Pharmacol. Sin. 34, 1084–1092. doi: 10.1038/aps.2013.15
Zhang, S., Cheng, B., Li, H., Xu, W., Zhai, B., Pan, S., et al. (2014). Matrine inhibits proliferation and induces apoptosis of human colon cancer LoVo cells by inactivating Akt pathway. Mol. Biol. Rep. 41, 2101–2108. doi: 10.1007/s11033-014-3059-z
Zhang, S., Tang, D., Zang, W., Yin, G., Dai, J., Sun, Y. U., et al. (2017). Synergistic inhibitory effect of traditional chinese medicine astragaloside iv and curcumin on tumor growth and angiogenesis in an orthotopic nude-mouse model of human hepatocellular carcinoma. Anticancer Res. 37, 465–473. doi: 10.21873/anticanres.11338
Zhang, T., Wang, K., Zhang, J., Wang, X., Chen, Z., Ni, C., et al. (2013). Huaier aqueous extract inhibits colorectal cancer stem cell growth partially via downregulation of the Wnt/beta-catenin pathway. Oncol. Lett. 5, 1171–1176. doi: 10.3892/ol.2013.1145
Zhang, W., Ma, W., Zhang, J., Song, X., Sun, W., and Fan, Y. (2017). The immunoregulatory activities of astragalus polysaccharide liposome on macrophages and dendritic cells. Int. J. Biol. Macromol. 105(Pt 1), 852–861. doi: 10.1016/j.ijbiomac.2017.07.108
Zhang, W., Saif, M. W., Dutschman, G. E., Li, X., Lam, W., Bussom, S., et al. (2010). Identification of chemicals and their metabolites from PHY906, a Chinese medicine formulation, in the plasma of a patient treated with irinotecan and PHY906 using liquid chromatography/tandem mass spectrometry (LC/MS/MS). J. Chromatogr. A. 1217, 5785–5793. doi: 10.1016/j.chroma.2010.07.045
Zhang, X., and Yu, H. (2016). Matrine inhibits diethylnitrosamine-induced HCC proliferation in rats through inducing apoptosis via p53, Bax-dependent caspase-3 activation pathway and down-regulating MLCK overexpression. Iran. J. Pharm. Res. 15, 491–499.
Zhang, Y., Yang, S. L., Zhang, H. R., Gao, L., Gao, X., Liu, P. J., et al. (2017). Combination radiotherapy and cantharidin inhibits lung cancer growth through altering tumor infiltrating lymphocytes. Future Oncol. 13, 1173–180. doi: 10.2217/fon-2016-0437
Zhang, Y. S., Shen, Q., and Li, J. (2016). Traditional Chinese medicine targeting apoptotic mechanisms for esophageal cancer therapy. Acta Pharmacol. Sin. 37, 295–302. doi: 10.1038/aps.2015.116
Zhao, G. S., Liu, Y., Zhang, Q., Li, C., Zhang, Y. W., Ren, Z. Z., et al. (2017). Transarterial chemoembolization combined with Huaier granule for the treatment of primary hepatic carcinoma: safety and efficacy. Medicine 96:e7589. doi: 10.1097/md.0000000000007589
Zheng, J., Li, C., Wu, X., Liu, M., Sun, X., Yang, Y., et al. (2014). Huaier polysaccharides suppresses hepatocarcinoma MHCC97-H cell metastasis via inactivation of EMT and AEG-1 pathway. Int. J. Biol. Macromol. 64, 106–110. doi: 10.1016/j.ijbiomac.2013.11.034
Zheng, L. C., Yang, M. D., Kuo, C. L., Lin, C. H., Fan, M. J., Chou, Y. C., et al. (2016). Norcantharidin-induced apoptosis of AGS human gastric cancer cells through reactive oxygen species production, and caspase- and mitochondria-dependent signaling pathways. Anticancer Res. 36, 6031–6042. doi: 10.21873/anticanres.11192
Zhou, H., Xu, M., Gao, Y., Deng, Z., Cao, H., Zhang, W., et al. (2014). Matrine induces caspase-independent program cell death in hepatocellular carcinoma through bid-mediated nuclear translocation of apoptosis inducing factor. Mol. Cancer 13:59. doi: 10.1186/1476-4598-13-59
Zhou, N., Li, J., Li, T., Chen, G., Zhang, Z., and Si, Z. (2017). Matrine-induced apoptosis in Hep3B cells via the inhibition of MDM2. Mol. Med. Rep. 15, 442–450. doi: 10.3892/mmr.2016.5999
Keywords: herbal medicine, digestive system malignancies, comprehensive treatment, chemotherapy, side effect
Citation: Sheng J, Zou X, Cheng Z, Xiang Y, Yang W, Lin Y and Cui R (2018) Recent Advances in Herbal Medicines for Digestive System Malignancies. Front. Pharmacol. 9:1249. doi: 10.3389/fphar.2018.01249
Received: 08 June 2018; Accepted: 15 October 2018;
Published: 20 November 2018.
Edited by:
Hongjie Zhang, Hong Kong Baptist University, Hong KongReviewed by:
Shuai Ji, Xuzhou Medical University, ChinaSubhalakshmi Ghosh, Jadavpur University, India
Copyright © 2018 Sheng, Zou, Cheng, Xiang, Yang, Lin and Cui. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yang Lin, linyang@jlu.edu.cn
 Jiyao Sheng
Jiyao Sheng Xiaohan Zou
Xiaohan Zou Ziqian Cheng
Ziqian Cheng Yien Xiang
Yien Xiang Ranji Cui
Ranji Cui