- 1Department of Anatomy, School of Traditional Chinese Medicine, Beijing University of Chinese Medicine, Beijing, China
- 2College of Special Education, Beijing Union University, Beijing, China
- 3Center for Scientific Research, School of Traditional Chinese Medicine, Beijing University of Chinese Medicine, Beijing, China
- 4Key Laboratory for Neurodegenerative Diseases of Ministry of Education, Capital Medical University, Beijing, China
- 5Beijing Key Lab for Immune-Mediated Inflammatory Diseases, Institute of Clinical Medical Science, China-Japan Friendship Hospital, Beijing, China
- 6State Key Laboratory of Bioactive Substances and Functions of Natural Medicines, Neuroscience Center, Institute of Materia Medica, Chinese Academy of Medical Sciences, Peking Union Medical College, Beijing, China
- 7College of Pharmacy, Hunan University of Chinese Medicine, Changsha, China
Da-Bu-Yin-Wan (DBYW) is recorded originally in China over six centuries ago, and it is used to treat Parkinson’s disease (PD) clinically in recent decades. DJ-1 is a homodimeric protein linked to early-onset PD, and found in the mitochondria. In addition, DJ-1 could protect the cells by regulating gene transcription and modulating the Akt signal pathways. Therefore, in this research, we aimed to investigate the ameliorative effect of DBYW on mitochondria in the view of the DJ-1 and Akt signaling. Rat adrenal pheochromocytoma cell line PC-12 was transfected with the plasmid pcDNA3-Flag-DJ-1 (pDJ-1). Subsequently, PC-12 cells were exposed to the PD-related mitochondrial toxin (1-methyl-4-phenylpyridinium) without/with the DBYW. After transfected with the plasmid pDJ-1, the 1-methyl-4-phenylpyridinium-induced toxicity was decreased, and the DJ-1 expression in protein level was increased. DJ-1 overexpression not only increased the mitochondrial mass, but also improved the total ATP content. Moreover, Akt phosphorylation was augmented by DJ-1 overexpression. Additionally, DBYW enhanced the above effects. Conclusively, these findings indicate that DBYW promotes the ameliorative effects of DJ-1 on mitochondrial dysfunction at least through augmenting the Akt phosphorylation in 1-methyl-4-phenylpyridinium-treated PC-12 cells.
Introduction
Parkinson’s disease (PD) is a highly debilitating neurodegenerative disorder that induces body rigidity, tremor, bradykinesia, and postural instability (Kalia and Lang, 2015). The PD pathology is characterized by gradual loss of dopaminergic neurons in the substantia nigra (Pagonabarraga et al., 2015), the underlying mechanisms still need to be clarified even though the disease was first described 200 years ago (Przedborski, 2017). Compelling evidence from molecular studies and experimental animal models has demonstrated that mitochondrial dysfunction was associated with the pathogenesis of PD (Mattson et al., 2008). Given the critical role of mitochondria in cellular function, it is convincing that mitochondrial dysfunction has appeared as an important mechanism at the coincidence of genetic, environmental and neurotoxin threatens to PD (Dagda et al., 2009).
DJ-1, namely PARK7 (Parkinson protein 7), is a homodimeric protein highly conserved in divergent organisms and linked to early-onset PD (Bonifati et al., 2003). DJ-1 is detected in both the nucleus and cytoplasm, and found in the mitochondria (Zhang et al., 2005; Junn et al., 2009). DJ-1 could prevent the fragmentation of mitochondria (Blackinton et al., 2009), while DJ-1 mutations damage mitochondrial dynamics and lead to mitochondrial dysfunction (Wang et al., 2012). In addition, DJ-1 could protect the cells by regulating gene transcription and modulating cell signal pathways, e.g., Akt signaling (Wilson, 2011). Akt, a downstream protein of phosphoinositide 3-kinase (PI3K), is the essential mediator of neuron survival (Dudek et al., 1997). Akt exerts its neuroprotective effect on neuronal cells by phosphorylation (Franke et al., 2003), whereas Akt signaling defection has partly linked to the pathological process of PD (Burke, 2007; Levy et al., 2009). In addition, DJ-1 is important for Akt phosphorylation enhancement on oxidative stress in the models of PD (Aleyasin et al., 2010).
Da-Bu-Yin-Wan (DBYW) was originally interpreted in a traditional Chinese medicine (TCM) monograph Dan Xi Xin Fa authored by Dan-Xi Zhu, an outstanding TCM professionalist and physician during China Yuan Dynasty. DBYW is also recorded in the updated edition of Pharmacopeia of People’s Republic of China issued in the year of 2015 (Chinese Pharmacopoeia Commission, 2015). In China Ming Dynasty, Yi-Kui Sun (A.D. 1522–1619) firstly defined the disease dominated by body tremor as “Tremor Disease” in his literature Chi Shui Xuan Zhu. He considered by TCM theory that the tremor syndrome in the aged people resulted from multiple deficiencies in the human body, e.g., low Yin essence (Zhang et al., 2006). Accordingly, DBYW was employed as a TCM intervention to treat PD clinically in recent decades (Jia et al., 2010). Our previous studies demonstrate that DBYW increases the expression of tyrosine hydroxylase (TH) in SN, induces the ultrastructure change, and raises the level of monoamine neurotransmitters in the mice model of PD (He et al., 2010; Zhang et al., 2013). In addition, DBYW lessens the DNA damage of mitochondria, and increases the mitochondrial subunit NADH dehydrogenase 1 expression (Zhang et al., 2013). Moreover, DBYW up-regulates cellular adenosine 5′-triphosphate (ATP) content in the midbrain, and decreases the expression of ATP-sensitive potassium channel subunit (Gong et al., 2014). Additionally, DBYW could reduce the mitochondrial fragmentation induced by the PD-related mitochondrial toxin (1-methyl-4-phenylpyridinium) in human derived neuroblastoma cell line (Ma et al., 2015). However, the cellular mechanisms by which DBYW exerts its protective effect on mitochondria are not totally interpreted. Therefore, in this research, we examined the possible link between DBYW and mitochondria from DJ-1 and Akt signaling in the cellular model of PD.
Materials and Methods
Chemical Reagents and Antibodies
All reference standard chemicals were obtained from National Institutes for Food and Drug Control, China1, including berberine hydrochloride (C20H18ClNO4, PubChem CID: 12456, Lot No.: 111895–201504), mangiferin (C19 H18O11, PubChem CID: 5281647, Lot No.: 111607–201503), and phellodendrine chloride (C20H24ClNO4, PubChem CID: 59818, Lot No.: 110713–201212). Lipofectamine 2000 and MitoTracker Green (MTG) were purchased from Invitrogen (Grand Island, NY, United States). 1-methyl-4-phenylpyridinium (MPP+) were obtained from Sigma-Aldrich (St. Louis, MO, United States). The bicinchoninic acid kit, protease and phosphatase inhibitors, and enhanced chemiluminescence kit were bought from Applygen (Beijing, China). The used antibodies as the following: rabbit anti-DJ-1, rabbit anti-PI3K, rabbit anti-Akt, rabbit anti-Akt phosphorylationThr308, rabbit anti-Akt phosphorylationSer473 were obtained from Cell Signaling (Beverly, MA, United States). Mouse anti-beta-action primary antibody and all secondary antibodies were obtained from Zhong-Shan (Beijing, China).
Preparation and Analysis for the Decoction
All components of DBYW were listed as the following (with Pharmacopoeia and local names) and described previously (Zhang et al., 2013, 2016a): Amur corktree bark (Phellodendron chinense Cortex; Huang-Bai) 12 g, Common anemarrhena rhizome (Anemarrhenae Rhizoma; Zhi-Mu) 12 g, Prepared rehmannia root (Radix Rehmanniae Praeparata; Shu-Di-Huang) 18 g, and Tortoise shell (Carapax et Plastrum Testudinis; Gui-Jia) 18 g. All components were obtained from Beijing Tong-Ren-Tang Nature Pharmacy (Beijing, China) and authenticated by the pharmacognosy professionals in the pharmacy. Briefly, the preparation method for the decoction was described previously (Chan et al., 2012). After treatment, final dose of the decoction extract was condensed to 1 g/ml (equivalent to dry weight of the component raw materials) by water bath. The extract was passed through a 0.22 μm filter (Millipore, Billerica, MA, United States), then divided and stored as the stock solution at -70°C.
Identification and quantification of the marker compounds in DBYW decoction were performed according to the method described in the updated Pharmacopeia of People’s Republic of China (Chinese Pharmacopoeia Commission, 2015), with minor modifications relating to the instrument and chromatographic conditions. Briefly, the marker compounds were analyzed using high performance liquid chromatography (Agilent 1100) with the diode-array detection (HPLC-DAD, Agilent, Santa Clara, CA, United States), respectively. Chromatographic separations were carried out using a Diamonsil C18 column (250 mm × 4.6 mm, 5-μm particle size, Dikma, Beijing, China), and appropriate mixture of acetonitrile/phosphoric acid /HPLC-grade water as the mobile phase. The mobile phase was filtered through a membrane (0.45-μm pore size) and degassed by ultrasonication before use. All measurements were made at a flow-rate of 1 mL/min, and detector was set for various compounds at different wavelength according to the China Pharmacopeia, respectively. The injection volume was 1 μL with analyte concentrations of 10–100 μg/mL, respectively. Analyte concentrations were adjusted to avoid overload of the columns. The integration of the chromatograms was performed with the Clarity software (Version 2.6.3, DataApex, Prague, Czechia). Peak areas in the chromatograms of DBYW were quantitated by external standard technique using solutions of the relative reference standards as described previously (Zhang et al., 2013).
Cell Line and Culture Conditions
Rat PC-12 cells (adrenal gland, pheochromocytoma) were obtained from Institute of Materia Medica, Chinese Academy of Medical Sciences and Peking Union Medical College. PC-12 cells were grown in a culture mixture of Dulbecco’s modified Eagle’s medium (HyClone, Logan, UT, United States) containing 6% horse serum (Invitrogen, Grand Island, NY, United States) and 6% fetal bovine serum (Sijiqing, Hangzhou, China), supplemented with 1% streptomycin/penicillin (Gibco, Grand Island, NY, United States), in 5% CO2 humidified chamber at 37°C. The culture procedures were in strict compliance with proper cell density for all the following experiments.
Transient Transfection and Treatments
The simplified structure of plasmid for expression of DJ-1 as described previously (Zhang et al., 2016b), pcDNA3-Flag-DJ-1 (pDJ-1), is displayed in Figure 1. The plasmid was validated by DNA sequencing and purified by the GoldHi plasmid kit (CoWin, Beijing, China) to remove endotoxin contamination. Cells were seeded at a density of 8 × 104/well 24 h prior to transfection. For each well in 6-well plate, cells were transfected with pDJ-1 by the polycationic liposome-mediated transfection method, using the optimum amount of Lipofectamine 2000. Twenty-four hours post transfection, cells were exposed to the medium containing MPP+ (1 mM) with/without different doses of DBYW for 48 h, respectively. Experimental treatments are shown in Table 1.
Cell Viability Determination
Cell viability was assessed by using the Cell Counting Kit-8 (CCK-8) colorimetric assay (Dojindo, Kumamoto, Japan) (Ishiyama et al., 1997). Briefly, 24 h after previous cell transfection, PC-12 cells were seeded at a density of 8 × 104 cells/mL in a 96-well plate and incubated for 24 h. Then various concentrations of DBYW were added with or without MPP+ (final concentrations mentioned in Table 1), respectively. Cells were incubated for a further 24 h, 10 μl of CCK-8 reagent was added to each well in a 96-well plate. After 1.5 h of incubation at 37°C, the absorbance was measured at a wavelength of 450 nm using the Safire2 microplate reader (Tecan, Männedorf, Switzerland). All results were expressed as compared to the control, which was defined as the baseline (100%).
Western Blot Analysis
Cells were washed with phosphate-buffered saline solution, followed by lysis with radioimmunoprecipitation assay buffer containing protease and phosphatase inhibitors. The extract of total protein was run and separate on sodium dodecyl sulfate polyacrylamide gel electrophoresis, then transferred onto polyvinylidene difluoride membrane (Millipore, Billerica, MA, United States). Different blots were incubated overnight with primary antibodies against DJ-1 (1:1000 dilution), PI3K (1:1000), Akt (1:1000), β-action (1:2000), Phospho-Akt (Thr308) (1:500), Phospho-Akt (Ser473) (1:500), respectively; followed by horseradish peroxidase-conjugated secondary antibodies (1:2000) for 1 h. Then complexes were visualized with enhanced chemiluminescence kit. Signals on the Flims were quantified by densitometry performed with the Bio-Rad Quantity One software, Version 4.62 (Hercules, CA, United States). Beta-actin served as an internal control for DJ-1, PI3K, and Akt, respectively; whereas the total Akt as loading control for the Akt phosphorylation.
Confocal Fluorescence Microscopy
To assess the mitochondrial mass, mitochondrial labeling was carried out using a cell-permeable fluorescent dye (MTG) based on the activity of mitochondria and involves minimal manipulation (Pendergrass et al., 2004). For visualization of mitochondria, cells were primarily treated with MTG (100 nM) for 15 min. Fluorescence was detected (490 nm/516 nm) by the confocal microscope FV1000 with the software Olympus FluoView Viewer, Version 3.1.2 (Olympus, Tokyo, Japan). Digital pictures were processed with the Image-Pro Plus software, Version 6.0 (Media Cybernetics, Bethesda, MD, United States).
Total ATP Content Detection
Total ATP content was detected by the Stay Brite ATP bioluminescence assay kit (BioVision, Milpitas, CA, United States) according to the manufacturer’s protocol, based on the measurement for the firefly luciferase bioluminescence (Crouch et al., 1993). Briefly, PC-12 cells were subcultured in 96-well plates at a density of 8 × 104 cells/ml. Twenty-four hours after various concentrations of DBYW treatment with or without MPP+ (1 mM), respectively. Then, 100 μl of ATP detection working solution was added to each well and incubated for 1 h at room temperature after lysed from the cells in the lysate buffer. The mixtures were centrifuged at 12,000 g for 30 s. The luminescence in the supernatant was recorded according to ATP-dependent luciferase activity, using the microplate reader Safire2 (Tecan, Männedorf, Switzerland). The bioluminescence value was normalized by the protein concentration that measured using bicinchoninic acid kit (Gong et al., 2014).
Statistical Analysis
All result data are expressed as the mean ± standard deviation. Statistically significant differences among means were determined by one-way analysis of variance followed by Newman–Keuls’ post hoc tests, using the GraphPad Prism software, Version 6.02 (GraphPad, San Diego, CA, United States). A typical level at which the threshold of P-value is taken at 0.05.
Results
Analysis of Marker Compounds of DBYW
The marker compounds in DBYW were analyzed with HPLC-DAD. By referring to reference standard chemicals, HPLC-DAD analysis indicated that the decoction contained the following marker compounds (n = 3): berberine hydrochloride (1.760 ± 0.033 mg/mL), mangiferin (0.501 ± 0.009 mg/mL), and phellodendrine chloride (0.476 ± 0.011 mg/mL). Chromatograms of the DBYW analyzed with relative reference standards are shown in Figure 2.
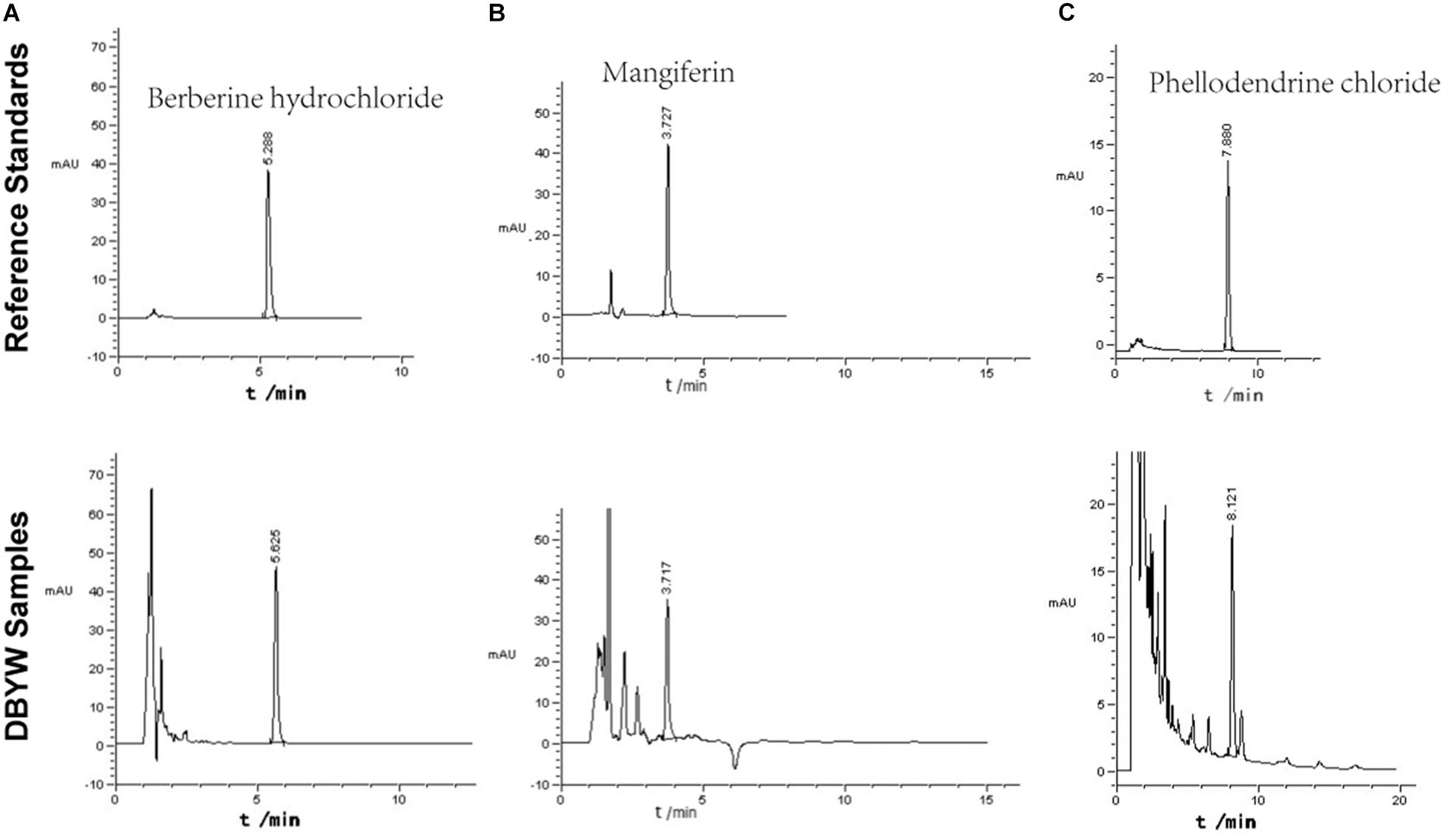
FIGURE 2. HPLC-DAD analysis of the DBYW decoction. Three reference standards were used for identifying and quantifying the marker compounds for DBYW. Panel (A) for berberine hydrochloride, panel (B) for mangiferin, and panel (C) for phellodendrine chloride.
DBYW Affects the Cell Viability
The cells were exposed to MPP+ (1 mM) with/without different doses of DBYW, respectively. As illustrated in Figures 3A,B, MPP+ significantly inhibited the cell viability (P < 0.05). However, cytotoxic effect of MPP+ was ameliorated in PC-12 cells transfected with pDJ-1. Moreover, this effect was promoted by DBYW dose-dependently (P < 0.05; Figures 3A,B).
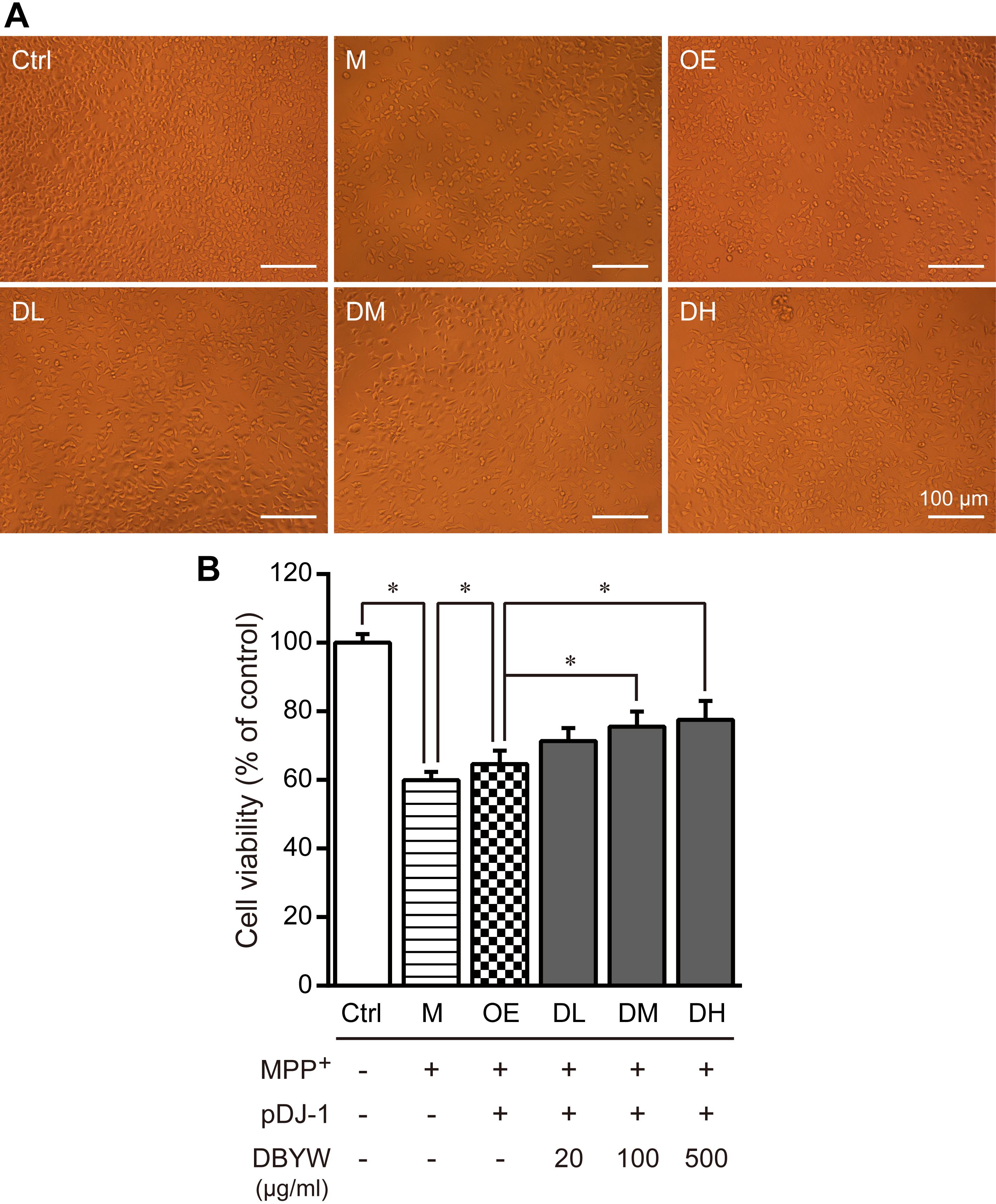
FIGURE 3. Cell viability detection. (A) Representative images showed treatment with MPP+ (1 mM) with/without DBYW in the PC-12 cells transfected with pDJ-1. (B) Cell viability was detected by CCK-8 assay. Ctrl, the control group; M, the MPP+-treated group; OE, the DJ-1 overexpression group; DL/DM/DH, DBYW low/medium/high dose groups; pDJ-1, the plasmid pDJ-1 transfection group. Analysis of variance, P < 0.05, post hoc ∗P < 0.05 versus compared group.
DBYW Affects the DJ-1 Expression
To examine the effect of DBYW on the DJ-1 expression, western blot was performed. The results displayed that MPP+ (1 mM) treatment decreased the DJ-1 expression (Figure 4). The plasmid pDJ-1 transfection inhibited the MPP+-induced DJ-1 decreased expression in PC-12 cells. Similarly, DBYW at various concentrations attenuated the MPP+-induced decrease of DJ-1 in PC-12 cells with pDJ-1 transfected, in comparison with only pDJ-1 transfection (P < 0.05; Figure 4). The combined results demonstrated that pDJ-1 could overexpress the protein of DJ-1, and DBYW could promote the DJ-1 expression.
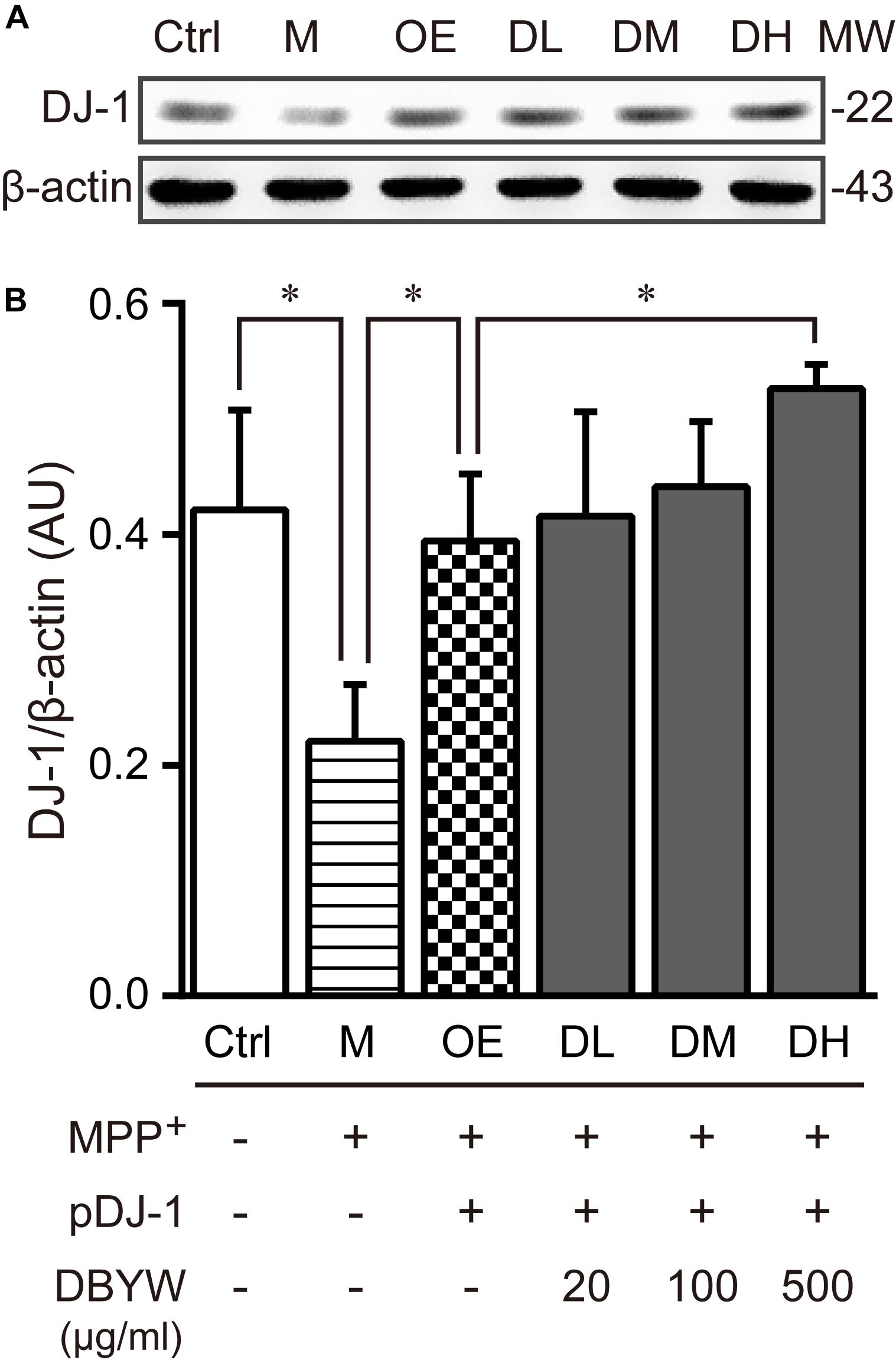
FIGURE 4. DJ-1 expression detection. (A) Representative expressions for DJ-1/internal control are demonstrated. (B) The graph displays the analysis from three independent bolts for the expression ratio of DJ-1/beta-actin. AU, arbitrary unit; Ctrl, the control group; M, the MPP+-treated group; MW, molecular weight (kDa); OE, the DJ-1 overexpression group; DL/DM/DH, DBYW low/medium/high dose groups; pDJ-1, the plasmid pDJ-1 transfection group. Analysis of variance, P < 0.05, post hoc ∗P < 0.05 versus compared group.
DBYW Ameliorated the Mitochondrial Dysfunction
Confocal fluorescence images evidenced that treatment with MPP+ (1 mM) has decreased mitochondrial mass significantly, while pDJ-1 transfection prevented the loss of mitochondrial mass (P < 0.05; Figure 5). Moreover, different doses of DBYW promoted the mitochondrial mass dose-dependently in the PC-12 cells transfected with pDJ-1, compared to the cells only transfected with pDJ-1 (P < 0.05; Figure 5).
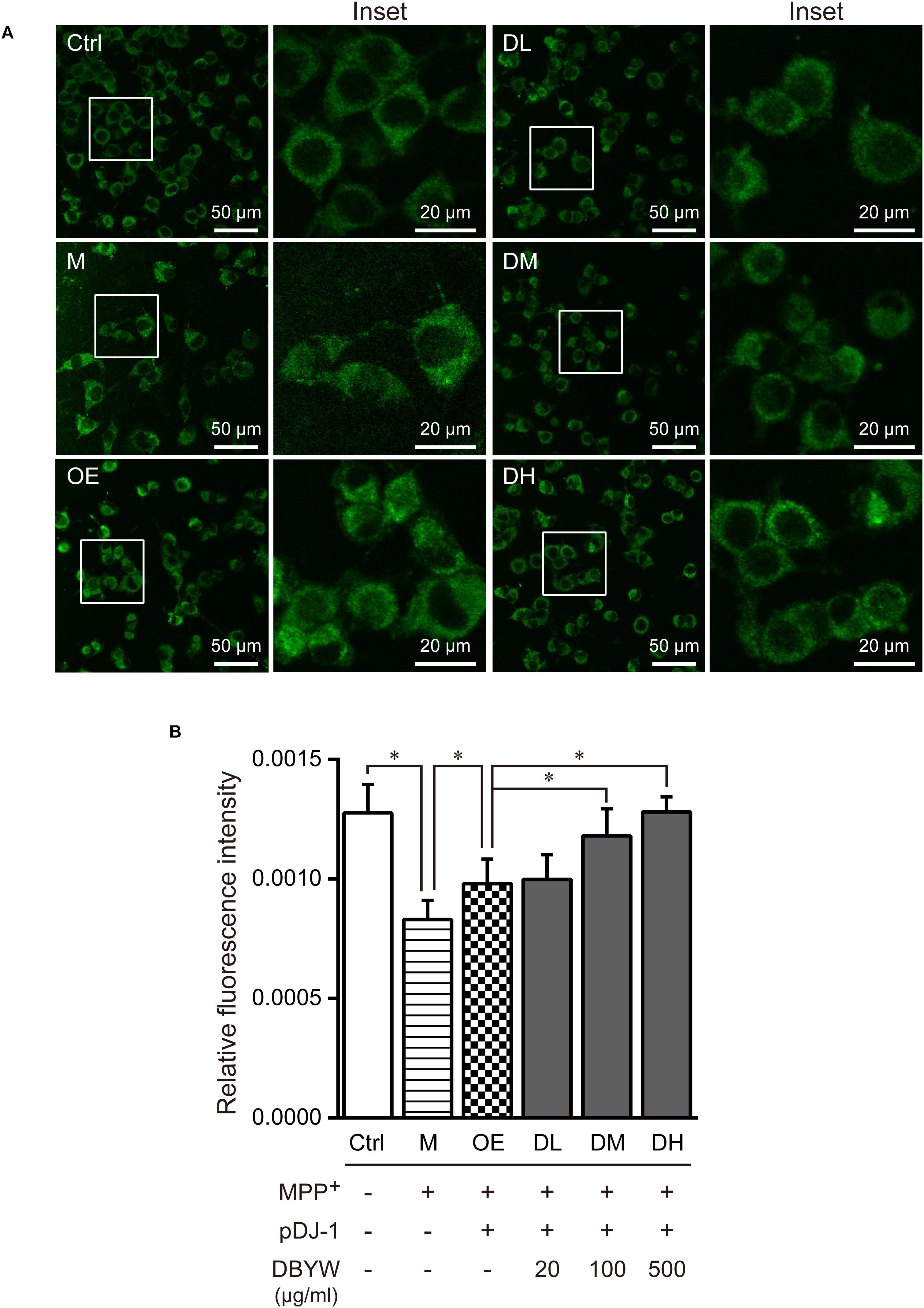
FIGURE 5. Mitochondrial mass assessment. (A) Representative images taken by the confocal microscopy in the different groups. (B) Results are the mean ± standard deviation of ten replicates. Ctrl, the control group; M, the MPP+-treated group; OE, the DJ-1 overexpression group; DL/DM/DH, DBYW low/medium/high dose groups; pDJ-1, the plasmid pDJ-1 transfection group. Analysis of variance, P < 0.05, post hoc ∗P < 0.05 versus compared group.
Subsequently, we measured changes in the total ATP content. The data displayed that MPP+ (1 mM) treatment significantly reduced the level of total ATP. Similarly, pDJ-1 transfection reversed the reduction in MPP+-treated PC-12 cells. Additionally, DBYW at different doses considerably increased total ATP content in a dose-dependent manner in the PC-12 cells transfected with pDJ-1, compared to the cells only transfected with pDJ-1 (P < 0.05; Figure 6).
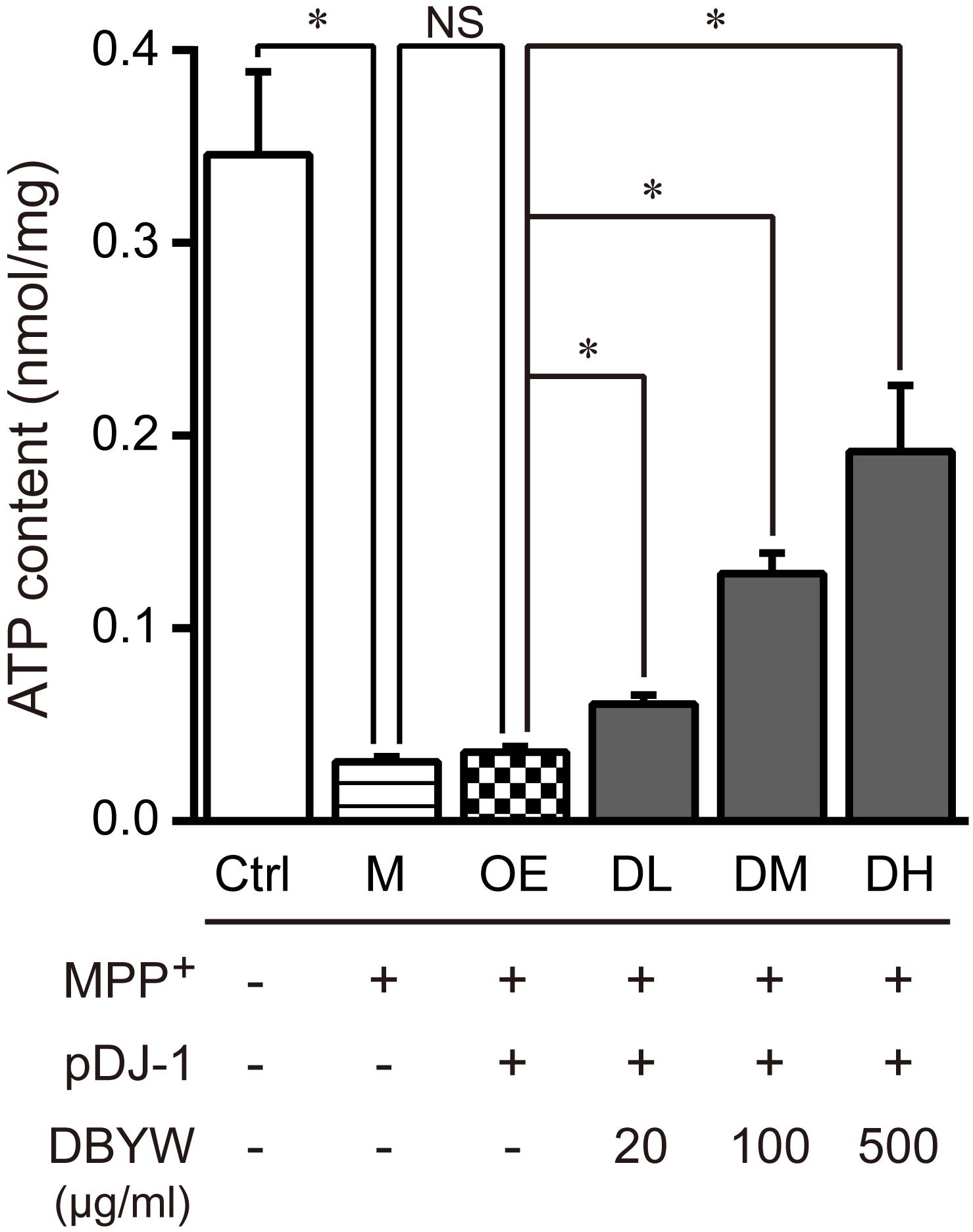
FIGURE 6. Total ATP content detection. Ctrl, the control group; M, the MPP+-treated group; OE, the DJ-1 overexpression group; DL/DM/DH, DBYW low/medium/high dose groups; pDJ-1, the plasmid pDJ-1 transfection group. Analysis of variance, P < 0.05, post hoc ∗P < 0.05 versus compared group.
Effect of DBYW on the PI3K/Akt Signaling
Western blot results showed that expressions of PI3K or Akt were not affected by MPP+ treatment or transfection with pDJ-1. Additionally, DBYW at different doses (20, 100, and 500 μg/ml) could not statistically change the expressions of PI3K or total Akt in the transfected PC-12 cells (P > 0.05; Figure 7), suggesting that DJ-1 or DBYW could not affect the PI3K and total Akt expressions in MPP+-treated PC-12 cells.
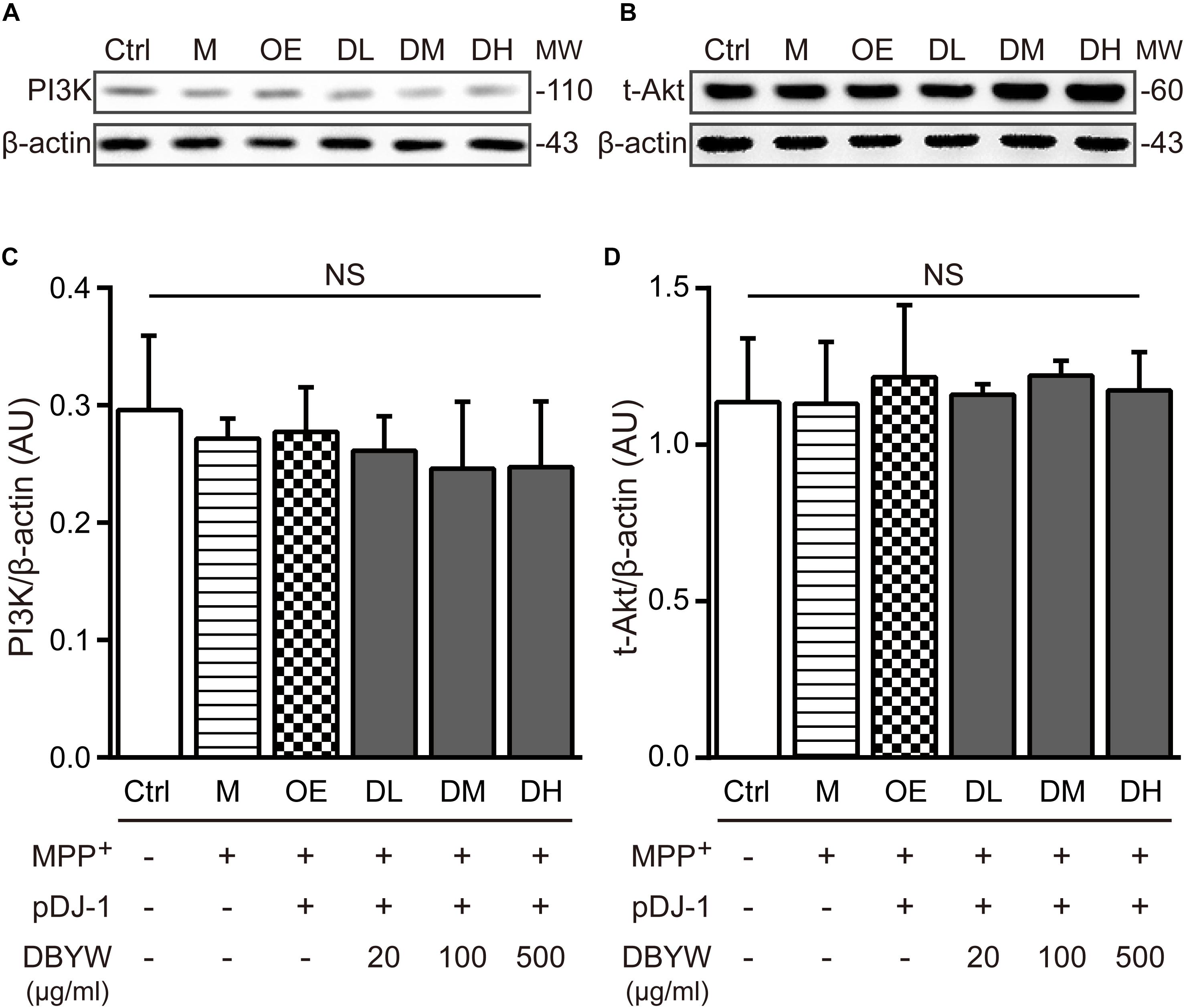
FIGURE 7. PI3K and total Akt expressions detection. (A,B) Representative expressions for PI3K, total Akt, and beta-actin are demonstrated. (C,D) The graph shows the analysis in from three independent blots for the expression ratios of PI3K or total Akt, normalized to beta-actin. AU, arbitrary unit; Ctrl, the control group; M, the MPP+-treated group; MW, molecular weight (kDa); OE, the DJ-1 overexpression group; DL/DM/DH, DBYW low/medium/high dose groups; pDJ-1, the plasmid pDJ-1 transfection group; NS, not significant statistically.
Subsequently, threonine 308 (Thr308) and serine 473 (Ser473), two residues of Akt phosphorylation (Sarbassov et al., 2005), were investigated by western blot. MPP+ (1 mM) treatment significantly decreased the expression of Akt phosphorylation on these two residuses, while pDJ-1 transfection reversed the decrease. Furthermore, different doses of DBYW enhanced the Akt phosphorylation on these two residues dose-dependently, respectively, in the PC-12 cells transfected with pDJ-1 (P < 0.05; Figure 8), compared to the cells only transfected with pDJ-1. The results displayed that DJ-1 could augment the Akt phosphorylation; and the treatment with DBYW enhanced the effects.
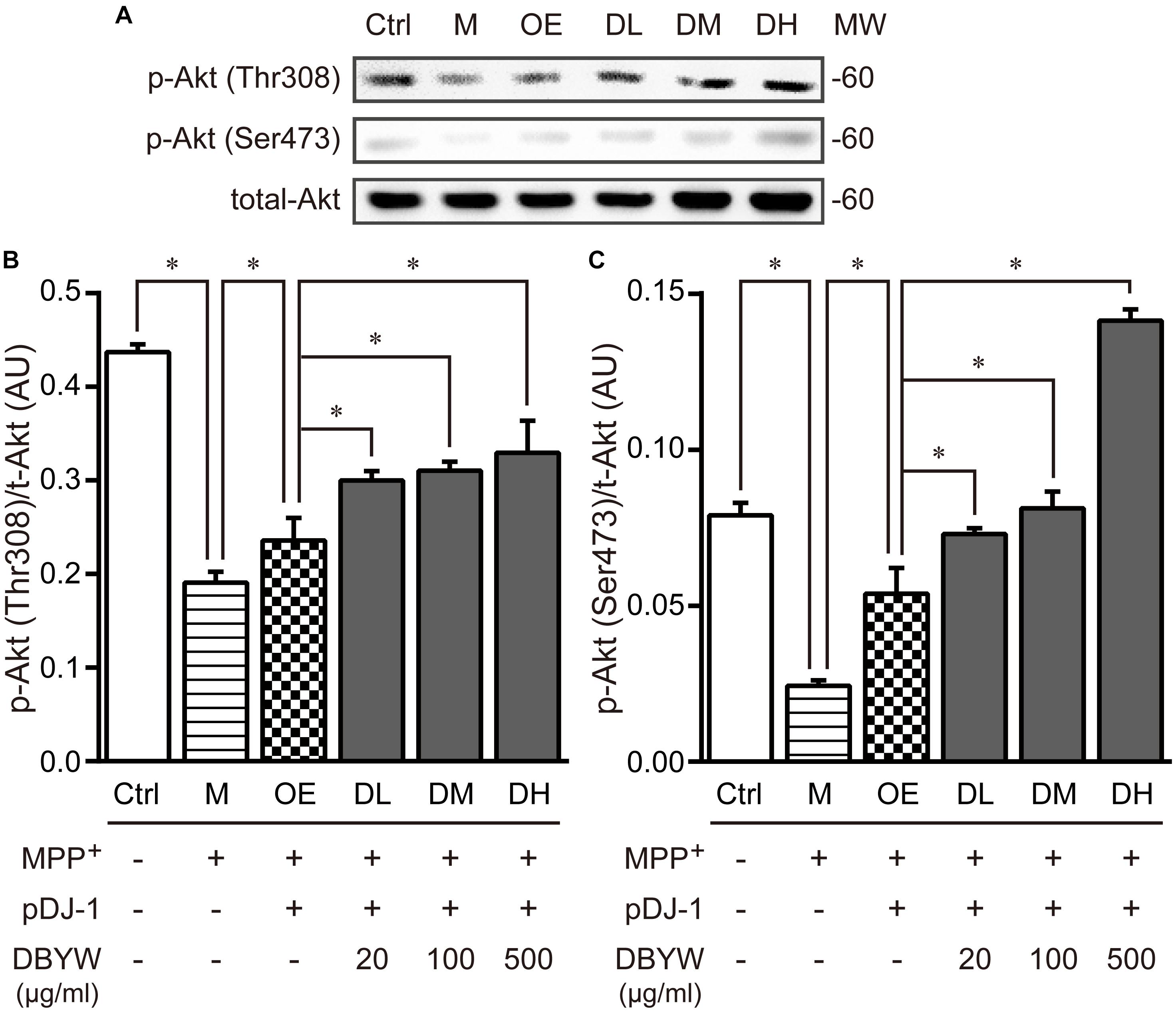
FIGURE 8. Akt phosphorylation detection. (A) Representative expressions for p-AktThr308, p-AktSer473, and total Akt are demonstrated. (B,C) The graph demonstrates the analysis from three independent blots for the expression ratios of p-AktThr308 or p-AktSer473, normalized to total Akt. AU, arbitrary unit; Ctrl, the control group; M, the MPP+-treated group; MW, molecular weight (kDa); OE, the DJ-1 overexpression group; DL/DM/DH, DBYW low/medium/high dose groups; pDJ-1, the plasmid pDJ-1 transfection group. Analysis of variance, P < 0.05, post hoc ∗P < 0.05 versus compared group.
Discussion
In our previous research, we exposed PC-12 cells to various doses of MPP+ for different time periods, respectively. We found a significant loss of PC-12 cells treated with 1 mM MPP+ for 48 h. Therefore, we used this condition for PC-12 cells in present research. Additionally, PC-12 cells have been widely served as a cellular model system for investigating PD (Hatanaka, 1981; Westerink and Ewing, 2008), because they have the enzymes for dopamine synthesis, metabolism and transportation (Hatanaka, 1981; Tuler et al., 1989). Our recent research demonstrated that DJ-1 could protect the mitochondria through enhancing the phosphorylation of Akt in MPP+-untreated PC-12 cells (Zhang et al., 2016b).
Traditional Chinese medicine formulas or other natural medicines are complex mixtures of many chemical compounds that have diverse pharmacological properties (Zhang Y. et al., 2014). Anemarrhena asphodeloides Bge., native to China, Korea, and Mongolia (Wang et al., 2014), is one component of DBYW. Mangiferin is a natural C-glucoside xanthone commonly encountered in Anemarrhena asphodeloides Bge. (Vyas et al., 2012). Mangiferin also increases the superoxide dismutase activity and glutathione levels, and prevents depletion of dopamine and its metabolites (3-methoxy-4-hydroxy-phenylacetic acid and homovanillic acid) in the striatum of MPTP-induced mice (Kavitha et al., 2013; Feng et al., 2014). Catalpol, an ingredient abundant in the Radix Rehmanniae Praeparata, exerts protective effects on PC-12 cells injured by L-glutamate and Aβ25-35 (Wang et al., 2008). In addition, catalpol reverses intracellular calcium level, mitochondrial membrane potential, and reactive oxygen species (ROS) accumulation in MPTP-treated mesencephalic neuron-astrocyte cultures and inhibits the activity of monoamine oxidase B in MPP+-treated astrocytes (Bi et al., 2008). The antioxidant α-tocopherol (vitamin E) also significantly increases the synthesis rate and the levels of monoaminergic neurotransmitters in the hippocampus and striatum, brain regions involved in memory processing and motor coordination (Ramis et al., 2016). Additionally, the aqueous extract of Harpagophytum procumbens could reduce amyloid β-peptide stimulation of malondialdehyde and 3-hydroxykynurenine and blunt the decrease of dopamine, norepinephrine, and serotonin, in the cortex (Ferrante et al., 2017). Catalpol protects dopaminergic neurons against lipopolysaccharide-induced neurotoxicity dose-dependently, through reducing the release of ROS, nitric oxide, and tumor necrosis factor-α, and attenuating the expression of inducible nitric-oxide synthase in mesencephalic neuron-glia cultures (Tian et al., 2006). Moreover, catalpol could protect mitochondrial function through inhibiting ROS production and nitric oxide synthase activity, increasing activities of mitochondrial complexes and level of mitochondrial membrane potential in the cortex and hippocampus mitochondria of D-galactose injected mouse (Zhang et al., 2010). Furthermore, catalpol improves the locomotor ability dose-dependently and raises the TH neuron number in SN, the density of striatal dopamine transporter, and the protein level of striatal glial cell derived neurotrophic factor in MPTP-treated mice (Xu et al., 2010). Catalpol also attenuates chronic cerebral hypoperfusion-induced white matter lesions by promoting oligodendrocyte survival and oligodendrocyte progenitor differentiation through the Akt signaling in Wistar rats (Cai et al., 2014). Additionally, tetrahydroberberine, an alkaloid isolated from Phellodendron chinense Schneid., protects neurons against degeneration through blocking neuronal ATP-sensitive potassium channels in SN of rat (Wu et al., 2010). The extracted decoction of Plastrum Testudinis (Tortoise shell), another component of DBYW, could substantially reduce the rotational behavior (Li et al., 2004; Deng et al., 2008) and increase the TH-positive neurons in the compact zone of substantia nigra (Deng et al., 2008), and also increase the levels of dopamine, 3,4-dihydroxyphenylacetic acid (DOPAC), and homovanillic acid (HVA) in the striatum of the PD model rats (Li et al., 2004). In addition, treatment with the combination of Plastrum Testudinis and β-asarone could improve the behavior of PD rats and increased TH-positive neurons, while decrease α-synuclein level in the corpus striatum (Zhang S. et al., 2014). Additionally, Plastrum Testudinis is one of the main components of Gui-Ling-Pa-An Capsule (GLPAC), a TCM formula that is used in the treatment for PD clinically. A multicenter, randomized, double-blind, controlled clinical trial has demonstrated that GLPAC shows obvious effects in improving the motor syndrome and quality of life of PD patients, and also reduces the required dosage of levodopa (Zhao et al., 2009).
Akt signaling defection has partly involved in the neurodegenerative progression of Alzheimer’s and Huntington’s diseases (Colin et al., 2005; Griffin et al., 2005). In addition, Akt signaling has a crucial role in mediating the dopaminergic receptor (Beaulieu et al., 2007) and redistributing the dopaminergic transporters (Garcia, 2005). Moreover, some PD treatment drugs (e.g., bromocriptine and ropinirole) that targeting the dopaminergic system had demonstrated the neuroprotective effects via Akt signaling (Lim et al., 2008; Nair and Olanow, 2008). Akt activation could induce various biological responses, such as insulin metabolic function, oncogenic signal transduction, higher brain function linked to cognition (Franke, 2008). Phosphorylation is a crucial modulatory mechanism that occurs both in prokaryotic and eukaryotic organisms (Barford et al., 1998), and the significant post-translational determinant of Akt activity (Sarbassov et al., 2005). Rasagiline, a selective monoamine oxidase B inhibitor, could up-regulate the Akt phosphorylation on Ser473 in midbrain dopamine neurons in MPTP-induced mice (Sagi et al., 2007). Furthermore, both gain-of-function and loss-of-function experiments have demonstrated that DJ-1 promotes cell survival via Akt phosphorylation on Ser473 in Drosophila (Kim et al., 2005). Additionally, using the DJ-1 null mouse model of PD, reduced Akt phosphorylation on Ser473 is associated with gradual loss of neurons in SN (Aleyasin et al., 2010). Further studies in vivo should be performed to better understand these in vitro findings.
In summary, these results demonstrate that DJ-1 could ameliorate the mitochondrial dysfunction at least through medicating the Akt phosphorylation in the rat adrenal pheochromocytoma PC-12 cells treated with MPP+. Additionally, our findings also suggest that DBYW promotes the ameliorative effect of DJ-1 in the MPP+-treated PC-12 cells.
Author Contributions
YZ and HMS conceptualized the study. YZ, XGG, ZZW, and HMS analyzed the data. YZ, XGG, ZYG, JHH, YYW, and WDF performed the experiments. YZ drafted and finalized the paper. ZZW, HMS, LL, PL, and NHC were the contributors in writing and revising the manuscript.
Funding
This study was supported by grants from the National Natural Science Foundation of China (Nos. 81473376, 81573773, and 81774110), Follow-Up Research Project of Beijing University of Chinese Medicine (No. 81202939), and Open Program of Key Laboratory of Neurodegenerative Diseases of Ministry of Education (Capital Medical University) (No. 2016SJBX03).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank everyone who contributed to this manuscript.
Footnotes
References
Aleyasin, H., Rousseaux, M. W., Marcogliese, P. C., Hewitt, S. J., Irrcher, I., Joselin, A. P., et al. (2010). DJ-1 protects the nigrostriatal axis from the neurotoxin MPTP by modulation of the AKT pathway. Proc. Natl. Acad. Sci. U.S.A. 107, 3186–3191. doi: 10.1073/pnas.0914876107
Barford, D., Das, A. K., and Egloff, M. (1998). The structure and mechanism of protein phosphatases: insights into catalysis and regulation. Annu. Rev. Biophys. Biomol. 27, 133–164. doi: 10.1146/annurev.biophys.27.1.133
Beaulieu, J., Gainetdinov, R. R., and Caron, M. G. (2007). The Akt–GSK-3 signaling cascade in the actions of dopamine. Trends Pharmacol. Sci. 28, 166–172. doi: 10.1016/j.tips.2007.02.006
Bi, J., Wang, X., Chen, L., Hao, S., An, L., Jiang, B., et al. (2008). Catalpol protects mesencephalic neurons against MPTP induced neurotoxicity via attenuation of mitochondrial dysfunction and MAO-B activity. Toxicol. Vitro 22, 1883–1889. doi: 10.1016/j.tiv.2008.09.007
Blackinton, J., Lakshminarasimhan, M., Thomas, K. J., Ahmad, R., Greggio, E., Raza, A. S., et al. (2009). Formation of a stabilized cysteine sulfinic acid is critical for the mitochondrial function of the Parkinsonism protein DJ-1. J. Biol. Chem. 284, 6476–6485. doi: 10.1074/jbc.M806599200
Bonifati, V., Rizzu, P., van Baren, M. J., Schaap, O., Breedveld, G. J., Krieger, E., et al. (2003). Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science 299, 256–259. doi: 10.1126/science.1077209
Burke, R. (2007). Inhibition of mitogen-activated protein kinase and stimulation of Akt kinase signaling pathways: two approaches with therapeutic potential in the treatment of neurodegenerative disease. Pharmacol. Ther. 114, 261–277. doi: 10.1016/j.pharmthera.2007.02.002
Cai, Q., Yao, Z., and Li, H. (2014). Catalpol promotes oligodendrocyte survival and oligodendrocyte progenitor differentiation via the Akt signaling pathway in rats with chronic cerebral hypoperfusion. Brain Res. 1560, 27–35. doi: 10.1016/j.brainres.2014.03.001
Chan, K., Shaw, D., Simmonds, M. S., Leon, C. J., Xu, Q., Lu, A., et al. (2012). Good practice in reviewing and publishing studies on herbal medicine, with special emphasis on traditional Chinese medicine and Chinese materia medica. J. Ethnopharmacol. 140, 469–475. doi: 10.1016/j.jep.2012.01.038
Chinese Pharmacopoeia Commission (2015). “Da-Bu-Yin-Wan,” in Pharmacopoeia of the People’s Republic of China (Part 1). Beijing: Chinese Medical Science Press, 498.
Colin, E., Régulier, E., Perrin, V., Dürr, A., Brice, A., Aebischer, P., et al. (2005). Akt is altered in an animal model of Huntington’s disease and in patients. Eur. J. Neurosci. 21, 1478–1488. doi: 10.1111/j.1460-9568.2005.03985.x
Crouch, S. P., Kozlowski, R., Slater, K. J., and Fletcher, J. (1993). The use of ATP bioluminescence as a measure of cell proliferation and cytotoxicity. J. Immunol. Methods 160, 81–88. doi: 10.1016/0022-1759(93)90011-U
Dagda, R. K., Zhu, J., and Chu, C. T. (2009). Mitochondrial kinases in Parkinson’s disease: converging insights from neurotoxin and genetic models. Mitochondrion 9, 289–298. doi: 10.1016/j.mito.2009.06.001
Deng, R. D., Li, Y. W., Chen, D. F., Li, H., Zhang, S. X., Zhao, D., et al. (2008). Protective effect of Plastrum testudinis on the apoptosis of dopamine neurons of rats with Parkinson’s disease. Chin. J. Neuroanat. 3, 301–306.
Dudek, H., Datta, S. R., Franke, T. F., Birnbaum, M. J., Yao, R., Cooper, G. M., et al. (1997). Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science 275, 661–665. doi: 10.1126/science.275.5300.661
Feng, G., Zhang, Z., Bao, Q., Zhang, Z., Zhou, L., Jiang, J., et al. (2014). Protective effect of chinonin in MPTP-induced C57BL/6 mouse model of Parkinson’s disease. Biol. Pharm. Bull. 37, 1301–1307. doi: 10.1248/bpb.b14-00128
Ferrante, C., Recinella, L., Locatelli, M., Guglielmi, P., Secci, D., Leporini, L., et al. (2017). Protective effects induced by microwave-assisted aqueous harpagophytum extract on rat cortex synaptosomes challenged with amyloid beta-peptide. Phytother. Res. 31, 1257–1264. doi: 10.1002/ptr.5850
Franke, T. F. (2008). PI3K/Akt: getting it right matters. Oncogene 27, 6473–6488. doi: 10.1038/onc.2008.313
Franke, T. F., Hornik, C. P., Segev, L., Shostak, G. A., and Sugimoto, C. (2003). PI3K/Akt and apoptosis: size matters. Oncogene 22, 8983–8998. doi: 10.1038/sj.onc.1207115
Garcia, B. (2005). Akt is essential for insulin modulation of amphetamine-induced human dopamine transporter cell-surface redistribution. Mol. Pharmacol. 68, 102–109. doi: 10.1124/mol.104.009092
Gong, X. G., Sun, H. M., Zhang, Y., Zhang, S. J., Gao, Y. S., Feng, J., et al. (2014). Da-Bu-Yin-Wan and Qian-Zheng-San to neuroprotect the mouse model of Parkinson’s disease. Evid. Based Complement. Alternat. Med. 2014:729195. doi: 10.1155/2014/729195
Griffin, R. J., Moloney, A., Kelliher, M., Johnston, J. A., Ravid, R., Dockery, P., et al. (2005). Activation of Akt/PKB, increased phosphorylation of Akt substrates and loss and altered distribution of Akt and PTEN are features of Alzheimer’s disease pathology. J. Neurochem. 93, 105–117. doi: 10.1111/j.1471-4159.2004.02949.x
Hatanaka, H. (1981). Nerve growth factor-mediated stimulation of tyrosine hydroxylase activity in a clonal rat pheochromocytoma cell line. Brain Res. 222, 225–233. doi: 10.1016/0006-8993(81)91029-5
He, X., Sun, H. M., Wu, H. X., Xu, H., Wang, Y. Y., Gao, Y. S., et al. (2010). Neuroprotective effect of Dabuyin Pill, Qianzheng powder and combined formula on Parkinson’s disease mice brain. Liaoning J. Tradit. Chin. Med. 37, 2098–2101.
Ishiyama, M., Miyazono, Y., Sasamoto, K., Ohkura, Y., and Ueno, K. (1997). A highly water-soluble disulfonated tetrazolium salt as a chromogenic indicator for NADH as well as cell viability. Talanta 44, 1299–1305. doi: 10.1016/S0039-9140(97)00017-9
Jia, Y. Q., Zhang, L. Z., Feng, L. Y., and Zhen, W. L. (2010). Overview of Chinese medicine treatment for Parkinson’s disease. Chin. J. Tradit. Med. Sci. Technol. 17, 183–184.
Junn, E., Jang, W. H., Zhao, X., Jeong, B. S., and Mouradian, M. M. (2009). Mitochondrial localization of DJ-1 leads to enhanced neuroprotection. J. Neurosci. Res. 87, 123–129. doi: 10.1002/jnr.21831
Kalia, L. V., and Lang, A. E. (2015). Parkinson’s disease. Lancet 386, 896–912. doi: 10.1016/S0140-6736(14)61393-3
Kavitha, M., Nataraj, J., Essa, M. M., Memon, M. A., and Manivasagam, T. (2013). Mangiferin attenuates MPTP induced dopaminergic neurodegeneration and improves motor impairment, redox balance and Bcl-2/Bax expression in experimental Parkinson’s disease mice. Chem. Biol. Interact. 206, 239–247. doi: 10.1016/j.cbi.2013.09.016
Kim, R. H., Peters, M., Jang, Y., Shi, W., Pintilie, M., Fletcher, G. C., et al. (2005). DJ-1, a novel regulator of the tumor suppressor PTEN. Cancer Cell 7, 263–273. doi: 10.1016/j.ccr.2005.02.010
Levy, O. A., Malagelada, C., and Greene, L. A. (2009). Cell death pathways in Parkinson’s disease: proximal triggers, distal effectors, and final steps. Apoptosis 14, 478–500. doi: 10.1007/s10495-008-0309-3
Li, Y. W., Zhou, J. H., Chen, D. F., Li, H., and Du, S. H. (2004). Effect of tortoises shell on behavior and striatum levels of dopamine in rat model of Parkinson’s disease. Anat. Res. 26, 17–21.
Lim, J. H., Kim, K., Kim, S. W., Hwang, O., and Choi, H. J. (2008). Bromocriptine activates NQO1 via Nrf2-PI3K/Akt signaling: novel cytoprotective mechanism against oxidative damage. Pharmacol. Res. 57, 325–331. doi: 10.1016/j.phrs.2008.03.004
Ma, L., Sun, H. M., Gong, X. G., He, X., Gao, Y. S., Zhang, Y., et al. (2015). Protective effect of Qianzheng san plus dabuyin wan on mitochondria in SH-SY5Y cells in Parkinson’s disease model induced by MPP +. World J. Integr. Tradit. West. Med. 10, 487–492.
Mattson, M. P., Gleichmann, M., and Cheng, A. (2008). Mitochondria in neuroplasticity and neurological disorders. Neuron 60, 748–766. doi: 10.1016/j.neuron.2008.10.010
Nair, V. D., and Olanow, C. W. (2008). Differential modulation of Akt/glycogen synthase kinase-3 pathway regulates apoptotic and cytoprotective signaling responses. J. Biol. Chem. 283, 15469–15478. doi: 10.1074/jbc.M707238200
Pagonabarraga, J., Kulisevsky, J., Strafella, A. P., and Krack, P. (2015). Apathy in Parkinson’s disease: clinical features, neural substrates, diagnosis, and treatment. Lancet Neurol. 14, 518–531. doi: 10.1016/S1474-4422(15)00019-8
Pendergrass, W., Wolf, N., and Poot, M. (2004). Efficacy of mitotracker green and CMXrosamine to measure changes in mitochondrial membrane potentials in living cells and tissues. Cytometry A 61, 162–169. doi: 10.1002/cyto.a.20033
Przedborski, S. (2017). The two-century journey of Parkinson disease research. Nat. Rev. Neurosci. 18, 251–259. doi: 10.1038/nrn.2017.25
Ramis, M. R., Sarubbo, F., Terrasa, J. L., Moranta, D., Aparicio, S., Miralles, A., et al. (2016). Chronic alpha-tocopherol increases central monoamines synthesis and improves cognitive and motor abilities in old rats. Rejuvenation Res. 19, 159–171. doi: 10.1089/rej.2015.1685
Sagi, Y., Mandel, S., Amit, T., and Youdim, M. B. H. (2007). Activation of tyrosine kinase receptor signaling pathway by rasagiline facilitates neurorescue and restoration of nigrostriatal dopamine neurons in post-MPTP-induced parkinsonism. Neurobiol. Dis. 25, 35–44. doi: 10.1016/j.nbd.2006.07.020
Sarbassov, D. D., Guertin, D. A., Ali, S. M., and Sabatini, D. M. (2005). Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307, 1098–1101. doi: 10.1126/science.1106148
Tian, Y., An, L., Jiang, L., Duan, Y., Chen, J., and Jiang, B. (2006). Catalpol protects dopaminergic neurons from LPS-induced neurotoxicity in mesencephalic neuron-glia cultures. Life Sci. 80, 193–199. doi: 10.1016/j.lfs.2006.09.010
Tuler, S. M., Hazen, A. A., and Bowen, J. M. (1989). Release and metabolism of dopamine in a clonal line of pheochromocytoma (PC12) cells exposed to fenthion. Fundam. Appl. Toxicol. 13, 484–492. doi: 10.1016/0272-0590(89)90284-4
Vyas, A., Syeda, K., Ahmad, A., Padhye, S., and Sarkar, F. H. (2012). Perspectives on medicinal properties of mangiferin. Mini Rev. Med. Chem. 12, 412–425. doi: 10.2174/138955712800493870
Wang, J. H., Zhu, S. H., Sun, S. M., Zhong, Y. M., and Qu, H. M. (2008). Compare of the protective effect of catalpol on PC12 cells injury induced by L-glutamate and Aβ25-35. Chin. Prac. Med. 3, 25–26.
Wang, X., Petrie, T. G., Liu, Y., Liu, J., Fujioka, H., and Zhu, X. (2012). Parkinson’s disease-associated DJ-1 mutations impair mitochondrial dynamics and cause mitochondrial dysfunction. J. Neurochem. 121, 830–839. doi: 10.1111/j.1471-4159.2012.07734.x
Wang, Y., Dan, Y., Yang, D., Hu, Y., Zhang, L., Zhang, C., et al. (2014). The genus Anemarrhena Bunge: a review on ethnopharmacology, phytochemistry and pharmacology. J. Ethnopharmacol. 153, 42–60. doi: 10.1016/j.jep.2014.02.013
Westerink, R. H. S., and Ewing, A. G. (2008). The PC12 cell as model for neurosecretion. Acta Physiol. 192, 273–285. doi: 10.1111/j.1748-1716.2007.01805.x
Wilson, M. A. (2011). The role of cysteine oxidation in DJ-1 function and dysfunction. Antioxid. Redox Signal. 15, 111–122. doi: 10.1089/ars.2010.3481
Wu, C., Yang, K., Liu, Q., Wakui, M., Jin, G. Z., Zhen, X., et al. (2010). Tetrahydroberberine blocks ATP-sensitive potassium channels in dopamine neurons acutely-dissociated from rat substantia nigra pars compacta. Neuropharmacology 59, 567–572. doi: 10.1016/j.neuropharm.2010.08.018
Xu, G., Xiong, Z., Yong, Y., Wang, Z., Ke, Z., Xia, Z., et al. (2010). Catalpol attenuates MPTP induced neuronal degeneration of nigral-striatal dopaminergic pathway in mice through elevating glial cell derived neurotrophic factor in striatum. Neuroscience 167, 174–184. doi: 10.1016/j.neuroscience.2010.01.048
Zhang, L., Shimoji, M., Thomas, B., Moore, D. J., Yu, S. W., Marupudi, N. I., et al. (2005). Mitochondrial localization of the Parkinson’s disease related protein DJ-1: implications for pathogenesis. Hum. Mol. Genet. 14, 2063–2073. doi: 10.1093/hmg/ddi211
Zhang, S., Gui, X. H., Huang, L. P., Deng, M. Z., Li, L., and Fang, Y. Q. (2014). Neuroprotective effect and mechanism of Carapax et Plastrum testudinis combined with β-asarone against rat model of Parkinson’s disease. Tradit. Chin. Drug Res. Clin. Pharmacol. 25, 264–268.
Zhang, X., Liu, W., Niu, X., and An, L. (2010). Systemic administration of catalpol prevents D-galactose induced mitochondrial dysfunction in mice. Neurosci. Lett. 473, 224–228. doi: 10.1016/j.neulet.2010.02.054
Zhang, Y., Gong, X. G., Wang, Z. Z., Sun, H. M., Guo, Z. Y., Gai, C., et al. (2016a). Protective effects of DJ-1 medicated Akt phosphorylation on mitochondrial function are promoted by Da-Bu-Yin-Wan in 1-methyl-4-phenylpyridinium-treated human neuroblastoma SH-SY5Y cells. J. Ethnopharmacol. 187, 83–93. doi: 10.1016/j.jep.2016.04.029
Zhang, Y., Gong, X. G., Wang, Z. Z., Sun, H. M., Guo, Z. Y., Hu, J. H., et al. (2016b). Overexpression of DJ-1/PARK7, the Parkinson’s disease-related protein, improves mitochondrial function via Akt phosphorylation on threonine 308 in dopaminergic neuron-like cells. Eur. J. Neurosci. 43, 1379–1388. doi: 10.1111/ejn.13216
Zhang, Y., Sun, H. M., He, X., Wang, Y. Y., Gao, Y. S., Wu, H. X., et al. (2013). Da-Bu-Yin-Wan and Qian-Zheng-San, two traditional Chinese herbal formulas, up-regulate the expression of mitochondrial subunit NADH dehydrogenase 1 synergistically in the mice model of Parkinson’s disease. J. Ethnopharmacol. 146, 363–371. doi: 10.1016/j.jep.2013.01.005
Zhang, Y., Wang, Z. Z., Sun, H. M., Li, P., Li, Y. F., and Chen, N. H. (2014). Systematic review of traditional Chinese medicine for depression in Parkinson’s disease. Am. J. Chin. Med. 42, 1035–1051. doi: 10.1142/S0192415X14500657
Keywords: Da-Bu-Yin-Wan, Parkinson’s disease, DJ-1, Akt, mitochondrial function
Citation: Zhang Y, Gong X-G, Sun H-M, Guo Z-Y, Hu J-H, Wang Y-Y, Feng W-D, Li L, Li P, Wang Z-Z and Chen N-H (2018) Da-Bu-Yin-Wan Improves the Ameliorative Effect of DJ-1 on Mitochondrial Dysfunction Through Augmenting the Akt Phosphorylation in a Cellular Model of Parkinson’s Disease. Front. Pharmacol. 9:1206. doi: 10.3389/fphar.2018.01206
Received: 07 November 2017; Accepted: 02 October 2018;
Published: 18 October 2018.
Edited by:
Marcello Locatelli, Università degli Studi “G. d’Annunzio” Chieti – Pescara, ItalyReviewed by:
Luigi Menghini, Università degli Studi “G. d’Annunzio” Chieti – Pescara, ItalyGokhan Zengin, Selçuk University, Turkey
Copyright © 2018 Zhang, Gong, Sun, Guo, Hu, Wang, Feng, Li, Li, Wang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Zhang, zy_dalian@yahoo.com; yzhang@bucm.edu.cn Hong-Mei Sun, hm.sun@yahoo.com
†These authors have contributed equally to this work
 Yi Zhang
Yi Zhang Xiao-Gang Gong
Xiao-Gang Gong Hong-Mei Sun1*
Hong-Mei Sun1* Lin Li
Lin Li Ping Li
Ping Li Zhen-Zhen Wang
Zhen-Zhen Wang Nai-Hong Chen
Nai-Hong Chen