- 1Key Lab of Chinese Medicine Resources Conservation, State Administration of Traditional Chinese Medicine of the People’s Republic of China, Institute of Medicinal Plant Development, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China
- 2College of Pharmaceutical Science, Dali University, Dali, China
More than 30 Ligularia Cass. (Asteraceae) species have long been used in folk medicine in China. Morphological features and common DNA regions are both not ideal to identify Ligularia species. As some Ligularia species contain pyrrolizidine alkaloids, which are hazardous to human and animal health and are involved in metabolic toxification in the liver, it is important to find a better way to distinguish these species. Here, we report complete chloroplast (CP) genomes of six Ligularia species, L. intermedia, L. jaluensis, L. mongolica, L. hodgsonii, L. veitchiana, and L. fischeri, obtained through high-throughput Illumina sequencing technology. These CP genomes showed typical circular tetramerous structure and their sizes range from 151,118 to 151,253 bp. The GC content of each CP genome is 37.5%. Every CP genome contains 134 genes, including 87 protein-coding genes, 37 tRNA genes, eight rRNA genes, and two pseudogenes (ycf1 and rps19). From the mVISTA, there were no potential coding or non-coding regions to distinguish these six Ligularia species, but the maximum likelihood tree of the six Ligularia species and other related species showed that the whole CP genome can be used as a super-barcode to identify these six Ligularia species. This study provides invaluable data for species identification, allowing for future studies on phylogenetic evolution and safe medical applications of Ligularia.
Introduction
Ligularia Cass., belonging to the Senecioneae tribe of Asteraceae, comprises about 140 species of perennial herbs. These species are distributed in Asia and Europe, with a total of 123 species distributed in China, 89 of which are endemic (Liu and Illarionova, 1989). In China, Ligularia species are mainly distributed in mountainous areas in the southwest (Liu and Illarionova, 1989) and more than 30 Ligularia species have long been used in folk medicine (Wang, 2007). The roots, stems, leaves, and flowers of them contain various chemical compounds, such as sesquiterpenes (Wang, 2007; Shimizu et al., 2014; Saito et al., 2017) and alkaloids (Asada et al., 1981; Feng, 2016). They are used as herbal medicines for the treatment of bronchitis, coughing, pulmonary tuberculosis, and hemoptysis. These herbal medicines are usually used as substitutes for Asteris Radix et Rhizoma which originates from Aster tataricus L. and is recorded in the Chinese Pharmacopoeia (Lin and Liu, 1989; Chinese Pharmacopoeia Commission, 2015). Approximately, 3% of flowering plants (as many as 6,000 species), including Ligularia species (Smith and Culvenor, 1981; Stegelmeier et al., 1999), contain pyrrolizidine alkaloids (PAs). It has been reported that various Ligularia species contain PAs, including L. japonica (Asada et al., 1981), L. wilsoniana (Xiong et al., 2016), L. duciformis, L. intermedia, L. hodgsonii, and L. veitchiana (Pu et al., 2004). PAs are phytoalexins that function in plant defense systems against herbivores, insects, and plant pathogens. However, they are harmful to human and animal health (Jank and Rath, 2017; Martinello et al., 2017), as they are involved in metabolic toxification in the liver caused by PA poisoning (Bull et al., 1968; Prakash et al., 1999). The German Federal Department of Health stated that the safe total daily dose of PA is less than 1 μg, and doctors do not allow continuous administration of drugs with PA for more than 6 weeks. In addition, all PA-containing products are banned in Australia (Wiedenfeld and Edgar, 2011).
Ligularia has been traditionally classified based on morphological structures, such as the arrangement of inflorescences, leaf shape, leaf veins, and phyllaries (Liu and Illarionova, 1989). Interspecific hybridization of Ligularia species is common and their morphological variation is complicated (Hanai et al., 2012; Yu et al., 2014; Saito et al., 2017), making it difficult to correctly identify species. Common DNA barcoding sequences (ITS, matK, psbA-trnH, and rbcL) are also not ideal for identifying Ligularia species (He and Pan, 2015). Recently, researchers have screened sequences from the whole chloroplast (CP) genome from numerous plant taxa, such as Juglans L. plants and bamboo (Zhang et al., 2011; Hu et al., 2016), or use CP genome as a super-barcode to distinguish species (Xia et al., 2016). The CP genome is highly conserved in plants regardless of the size, structure, or gene content (Tonti-Filippini et al., 2017), and the majority of the retained core genes are involved in the light reactions of photosynthesis or in functions related to transcription or translation (Sato et al., 1999). The CP genome map is a circular DNA molecule that includes a SSC region, a LSC region, and two inverted-repeat (IRa and IRb) regions (Sato et al., 1999). Several CP genomes from Asteraceae have previously been reported, including CP genomes from Aster (Choi and Park, 2015), Ambrosia (Nagy et al., 2017), Carthanus (Lu et al., 2015), and Taraxacum (Salih et al., 2017). However, only one CP genome from Ligularia, for L. fischeri, has previously been published (Lee et al., 2016). In this study, we report the CP genomes of six Ligularia species, L. intermedia, L. jaluensis, L. mongolica, L. hodgsonii, L. veitchiana, and L. fischeri, obtained through high-performance Illumina sequencing technology. Our aim is to use the CP genome as a super-barcode for the identification of Ligularia species to provide invaluable genetic information for future studies.
Materials and Methods
Plant Materials and DNA Extraction
Fresh leaves of L. intermedia and L. fischeri were collected from Baishan City and Tonghua City, Jilin Province, China, respectively. Fresh leaves of L. jaluensis and L. mongolica were collected from Yanbian Korean Autonomous Prefecture, Jilin Province. These four species were identified by Prof. Junlin Yu from Tonghua Normal University, Jilin. Fresh leaves of L. hodgsonii and L. veitchiana were collected from Enshi Tujia and Miao Autonomous Prefecture, Hubei Province, and the Qinling Mountains, Shaanxi Province, respectively. These two samples were identified by Prof. Yulin Lin from the Institute of Medicinal Plant Development (IMPLAD), Chinese Academy of Medical Sciences (CAMS), and Peking Union Medical College (PUMC). The exact GPS coordinates for the collection locations of six Ligularia species are listed in Supplementary Table S1. Voucher specimens were deposited in the herbarium at IMPLAD. Collected fresh leaves were stored in a -80°C freezer until further use. DNA extraction was performed using a DNeasy Plant Mini Kit (Qiagen Co., Germany) following the manufacturer’s protocol.
Illumina Sequencing and Genome Assembly
Approximately 5–10 μg of high-quality DNA were used to build shotgun libraries with insert sizes of 500 bp and were sequenced in accordance with the protocol for Illumina Hiseq X technology. The total raw data of the six species were produced with 150 bp paired-end read lengths. The software Trimmomatic (Bolger et al., 2014) was employed to filter low-quality reads from the raw data. After filtering for quality sequences, the remaining clean reads were used to assemble the CP genome sequences. The CP sequences of all plants downloaded from the National Center for Biotechnology Information (NCBI) were used to create a reference database. Then, the clean sequences were mapped to the database and the mapped reads were extracted on the basis of coverage and similarity. The extracted reads were assembled into contigs using SOAPdenovo2 (Luo et al., 2012). The scaffold of the CP genome was constructed using SSPACE (Boetzer et al., 2011), and the gaps were filled using GapFiller (Boetzer and Pirovano, 2012).
Validation, Annotation, and Sequence Submission
The accuracy of the assembly of the four boundaries (SSC, LSC, IRa, and IRb regions) of the CP sequences was confirmed through PCR and Sanger sequencing using validated primers (Supplementary Table S2). The thermocycler conditions for the PCR were as follows: 94°C for 5 min; 94°C for 30 s, 56°C for 30 s, 72°C for 1.5 min, and 32 cycles; 72°C for 10 min. The online programs Dual Organellar GenoMe Annotator (DOGMA) (Wyman et al., 2004) and CPGAVAS (Liu et al., 2012) were used for the initial annotation of the CP genomes of the six species, followed by manual correction. The complete data from the study were submitted to NCBI under the BioProject ID PRJNA400300 and BioSample ID SAMN07562669. The assembled complete CP genome sequences of the six Ligularia species were submitted to NCBI GenBank with the accession numbers MF539929-MF539933, and MG729822.
Genome Structure Analysis
The software tRNAscan-SE (Schattner et al., 2005) and DOGMA (Wyman et al., 2004) were used to identify tRNA genes. Gene maps were generated using Organellar Genome DRAW v1.2 (Lohse et al., 2007) with default settings and then the gene maps were checked manually. MEGA 6.0 was used to calculate the GC content (Tamura et al., 2013). REPuter (University of Bielefeld, Bielefeld, Germany) (Kurtz et al., 2001) was used to identify the size and location of repeat sequences in the CP genomes of the six Ligularia species. We used the MISA software Misa-Microsatellite Identification Tool, 20171 to detect SSRs with the parameter settings the same as those described in Li et al. (2013). All the repeated sequences were manual verified and excess data were removed. The distribution of codon usage was studied using CodonW with the relative synonymous codon usage (RSCU) ratio (Sharp and Li, 1987). The online program Predictive RNA Editor for Plants suite (Mower, 2009) with a cutoff value of 0.8 were used to predict RNA editing sites in the six CP genomes of Ligularia species.
Phylogenetic Analysis
For identification purposes and to further phylogenetic research on this genus, we used mVISTA (Thompson et al., 1994) to compare six Ligularia species with L. hodgsonii as the reference genome. MEGA 6.0 was used to construct the phylogenetic tree with Platycodon grandiflorus and Adenophora remotiflora included as outgroups based on ML analysis. The details of the selected species (excluding the six Ligularia species) are presented in Supplementary Table S3.
Results and Discussion
CP Genome Structure of Six Ligularia Species
The raw data from the six Ligularia species is 9.1 Gb for L. intermedia, 7.2 Gb for L. hodgsonii, 7.4 Gb for L. jaluensis, 6.4 Gb for L. mongolica, 6.3 Gb for L. veitchiana, and 6.2 Gb for L. fischeri. The sizes of the six CP genomes range from 151,118 bp for L. mongolica to 151,253 bp for L. veitchiana, which are similar to other Asteraceae CP genomes (Liu et al., 2013; Salih et al., 2017; Wang et al., 2017; Zhang et al., 2017). The investigated genomes showed typical circular tetramerous structure, including an SSC region and an LSC region, separated by two IR regions (Figure 1). The corresponding lengths of the four regions from the six species are similar: the SSC lengths range from 18,214 to 18,247 bp, the LSC lengths range from 83,244 to 83,330 bp, and the IR lengths range from 24,830 to 24,838 bp (Table 1). The size of the previously published L. fischeri CP genome is 151,133 bp, and included an SSC region (18,233 bp), an LSC region (83,238 bp), and two IR regions (24,831 bp apart) (Lee et al., 2016). Our results showed that all six of the newly sequenced CP genomes have a GC content of 37.5%, which is lower than some Asteraceae species (Liu et al., 2013; Salih et al., 2017; Wang et al., 2017; Zhang et al., 2017). The GC content of four homologous regions of the six CP genomes is the same. However, the distribution of the GC content in each region is uneven. The GC content in the IR region is the largest (43.0%), followed by the LSC region (35.6%), and the region with the lowest GC content is the SSC region (30.7%). Our analysis showed that the high GC content in the IR region is attributed to four rRNA genes (rrn16, rrn23, rrn4.5, and rrn5). The AT content of the first, second, and third position of protein-coding genes in the six CP genomes are 54.5–54.6%, 61.9–62.0%, and 70.1%, respectively. The higher AT content in the third site has also been observed in other plants (Yi and Kim, 2012; He et al., 2017; Zhou et al., 2017) and is usually used to distinguish DNA of CP, nucleus, and mitochondria origin (Clegg et al., 1994).
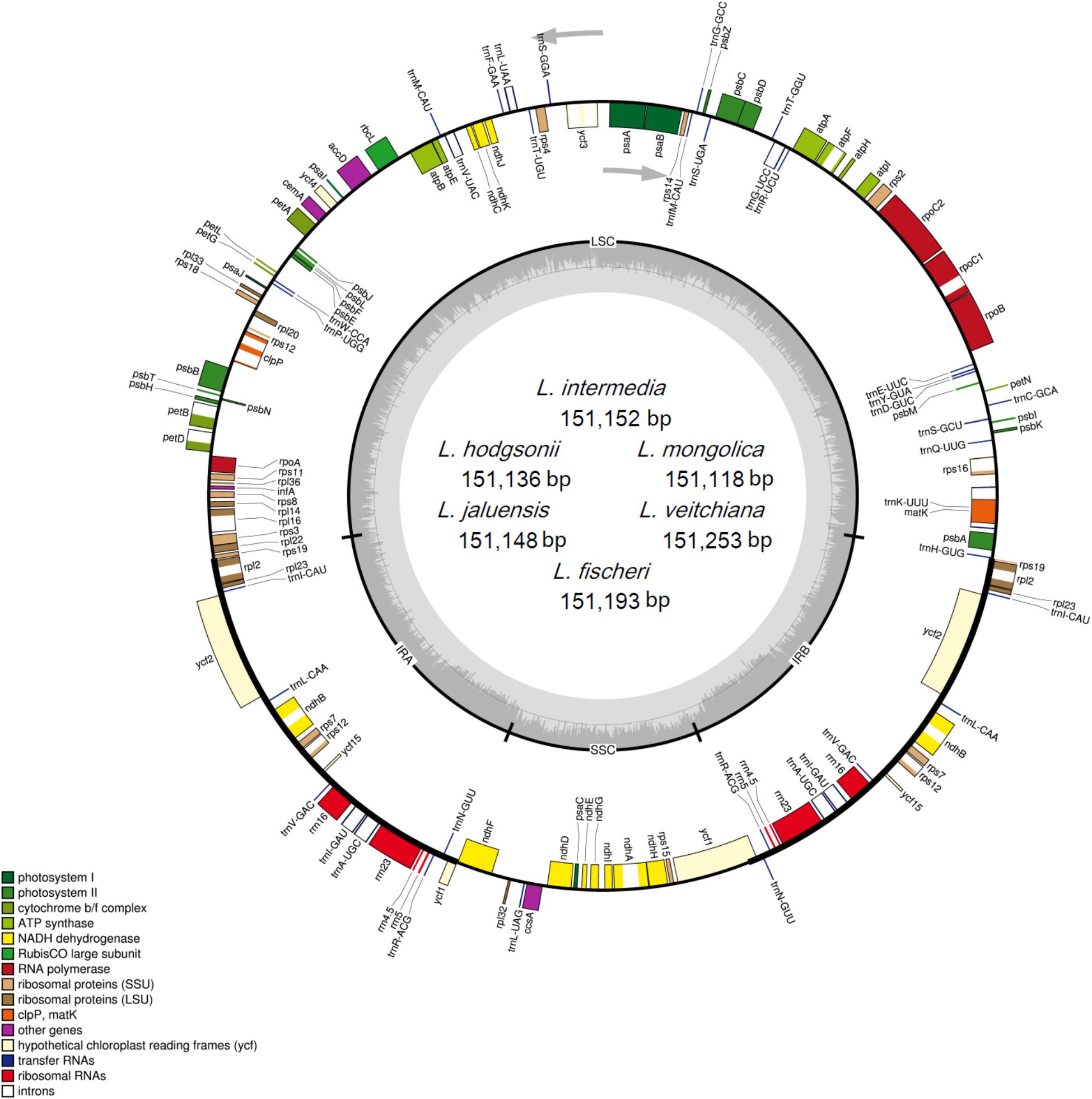
FIGURE 1. Gene map of the complete CP genomes of the six Ligularia species. Genes on the inside of the circle are transcribed clockwise, while those outside are transcribed counter clockwise. The darker gray in the inner circle corresponds to GC content, whereas the lighter gray corresponds to AT content.
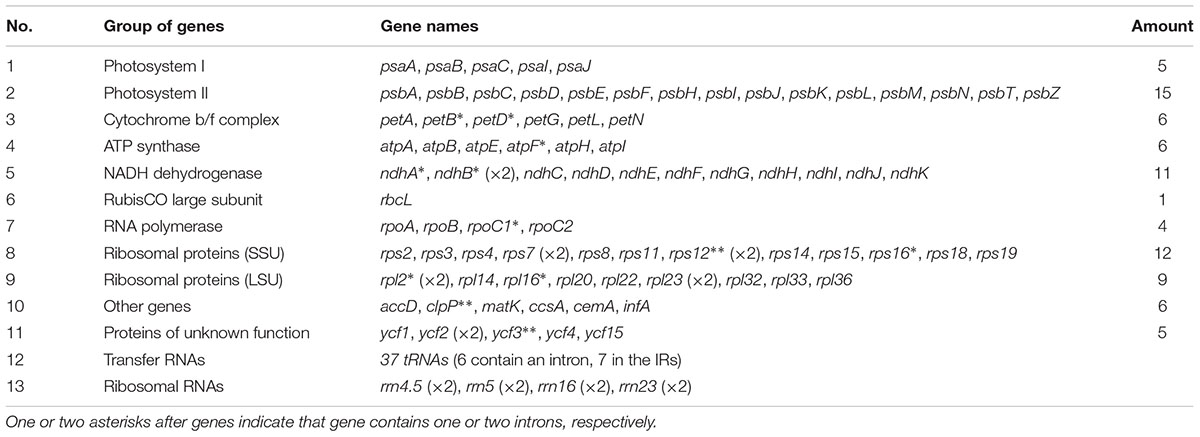
Each of the six CP genomes contains 134 genes, including 87 protein-coding genes, 37 tRNA genes, eight rRNA genes, and two pseudogenes (ycf1 and rps19; Table 2). Seven protein-coding genes (ndhB, rp12, rp123, rps12, rps7, ycf15, and ycf2), seven tRNAs (trnA-UGC, trnI-CAU, trnI-GAU, trnL-CAA, trnN-GUU, trnR-ACG, and trnV-GAC), and all of the rRNAs (rrn16, rrn23, rrn4.5, and rrn5) are duplicated in the IR regions, which is similar to Artemisia annua (Shen et al., 2017) and Artemisia frigida (Liu et al., 2013). The CP genomes of the six Ligularia species contain a small 3.4 kb inversion within a large 23 kb inversion in the LSC region, which is a unique feature in Asteraceae (Kim et al., 2005; Liu et al., 2013). The LSC region included 62 protein-coding genes and 22 tRNA genes. The SSC region included 11 protein-coding genes and one tRNA gene (trnL-UAG). The CP genomes of each of these six Ligularia species did not have an inverted SSC region, which has also been found in the CP genomes of A. frigida (Liu et al., 2013), Scutellaria baicalensis (Jiang et al., 2017), Carthamus tinctorius (Lu et al., 2015), and Juglans L. (Hu et al., 2016). In contrast, the SSC regions of Helianthus annuus, Lactuca sativa (Timme et al., 2007), and Aster spathulifolius (Choi and Park, 2015) are inverted. The functional ycf1 copy is located in the IRb-SSC boundary and the pseudogene ycf1 copy is located in the IRa region. The functional rps19 copy is on the boundary of LSC and IRa and the pseudogene rps19 copy is on the IRb region. The coding region occupied 59.67–59.72% of the CP genomes of six Ligularia species, including protein-coding genes, tRNA genes, and rRNA genes. Meanwhile, non-coding regions, including introns, pseudogenes, and intergenic spacers occupied 40.28–40.33% of the CP genomes of the six Ligularia species.
Codon Usage and RNA Editing Sites
All protein-coding genes in the six Ligularia CP genomes are composed of 26,136–26,138 codons. The most and least universal amino acids of the CP genomes of the six Ligularia species are leucine (10.8%) and cysteine (1.1%), respectively (Figure 2). This is also similar to the CP genome from artichoke (Curci et al., 2015). However, the most universal amino acid from A. frigida is isoleucine (Liu et al., 2013). The most and the least abundant amino acids in the Taraxacum obtusifrons and Taraxacum amplum CP genomes are serine and methionine (Salih et al., 2017), respectively. Figure 2 shows that with the increase of specific amino acid codes the RSCU increases accordingly. Most of the amino acid codons have preferences, except for methionine and tryptophan. Potential RNA editing sites were predicted for 35 genes from the CP genomes of the six Ligularia species. Forty-eight RNA editing sites were identified. S to L of amino acid change appeared most frequently, while R to W and T to I occurred least. Each corresponding gene from the RNA editing sites of the six Ligularia species is at the same nucleotide position (Supplementary Table S4).
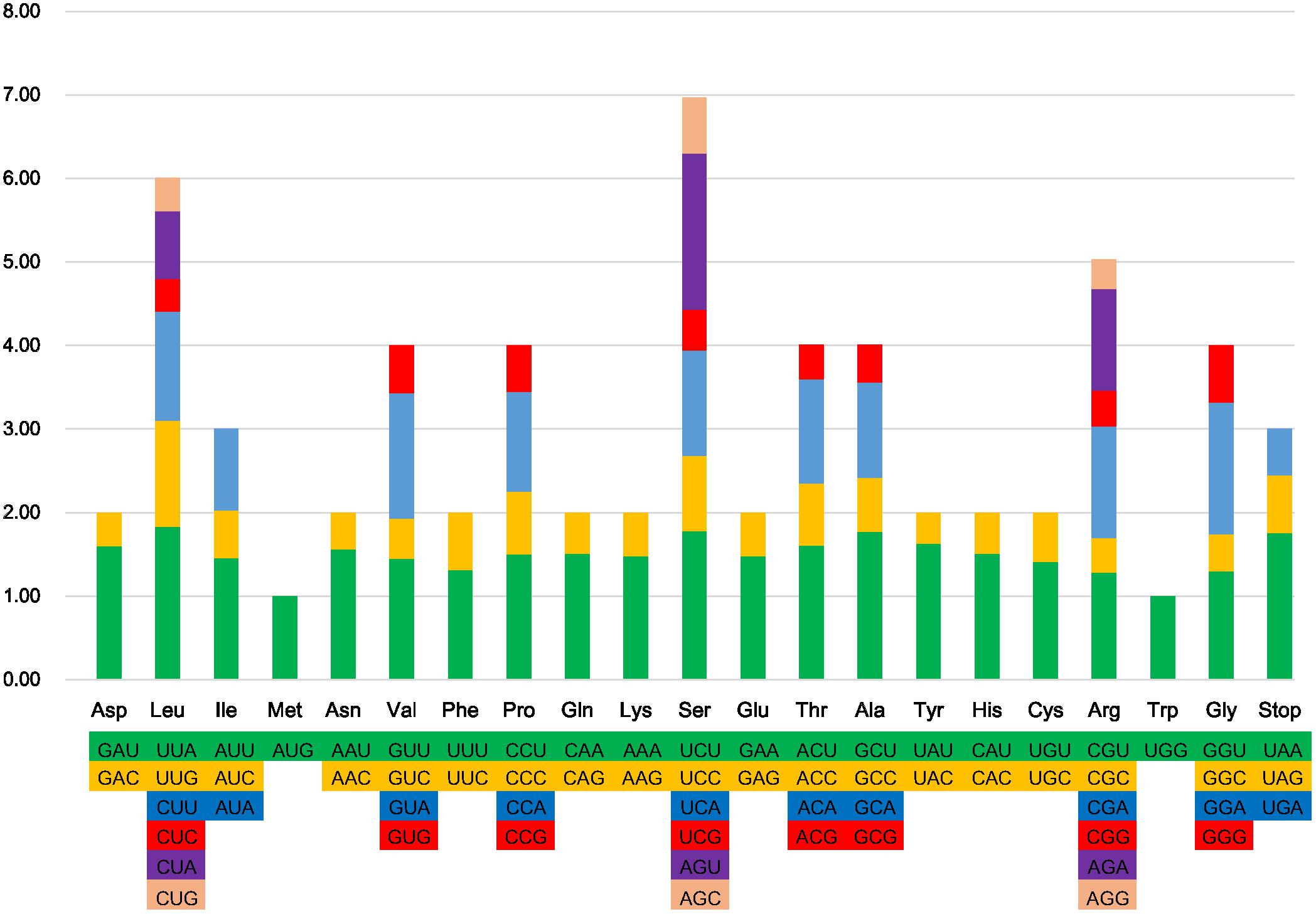
FIGURE 2. Codon content of 20 amino acid and stop codons in all protein-coding genes of the CP genome of L. hodgsonii.
A total of 18 genes containing introns, including 12 protein-coding genes (atpF, clpP, ndhA, ndhB, petB, petD, rpl16, rpl2, rpoC1, rps12, rps16, and ycf3), and six tRNA genes (trnA-UGC, trnG-UCC, trnI-GAU, trnK-UUU, trnL-UAA, and trnV-UAC; Supplementary Table S5), were identified in this study. Nine protein-coding genes contain only one intron and three protein-coding genes (clpP, rps12, and ycf3) contain two introns. All six tRNAs contain only one intron. TrnK-UUU has the longest intron (2,556 bp), which contains matK. The clpP gene and ycf3 gene are both located in the LSC region. The rps12 gene is a special trans-splicing gene with the 5′ exon located in the LSC region, but the 3′ exon located in the IR region. This condition exists in many species, such as A. frigida (Liu et al., 2013), artichoke (Curci et al., 2015), and Aster spathulifolius (Choi and Park, 2015).
Long Repeats and SSRs in the CP Genomes From the Six Ligularia Species
Repeat sequences, which are related to plastome organization (Salih et al., 2017), are mostly distributed in intergenic regions and intron regions, and only a small fraction is present in the genetic region. Four types of long repeats were observed in the CP genomes of the six Ligularia species, including forward, palindromic, reverse, and complement repeats (Figure 3). The length of the repeat unit ranged from 30 to 48 bp. Ligularia intermedia and L. jaluensis both had 19 forward and 20 palindromic repeats. Ligularia hodgsonii had the following repeats: 18 forward, 20 palindromic, and one reverse. Ligularia mongolica had 18 forward and 20 palindromic repeats. Ligularia veitchiana had 20 forward and 21 palindromic repeats. Ligularia fischeri had the following repeats: 19 forward, 19 palindromic, and one complement. The long repeat sequences observed in the CP genomes of the six Ligularia species, with L. hodgsonii as the reference, are presented in Supplementary Table S6.
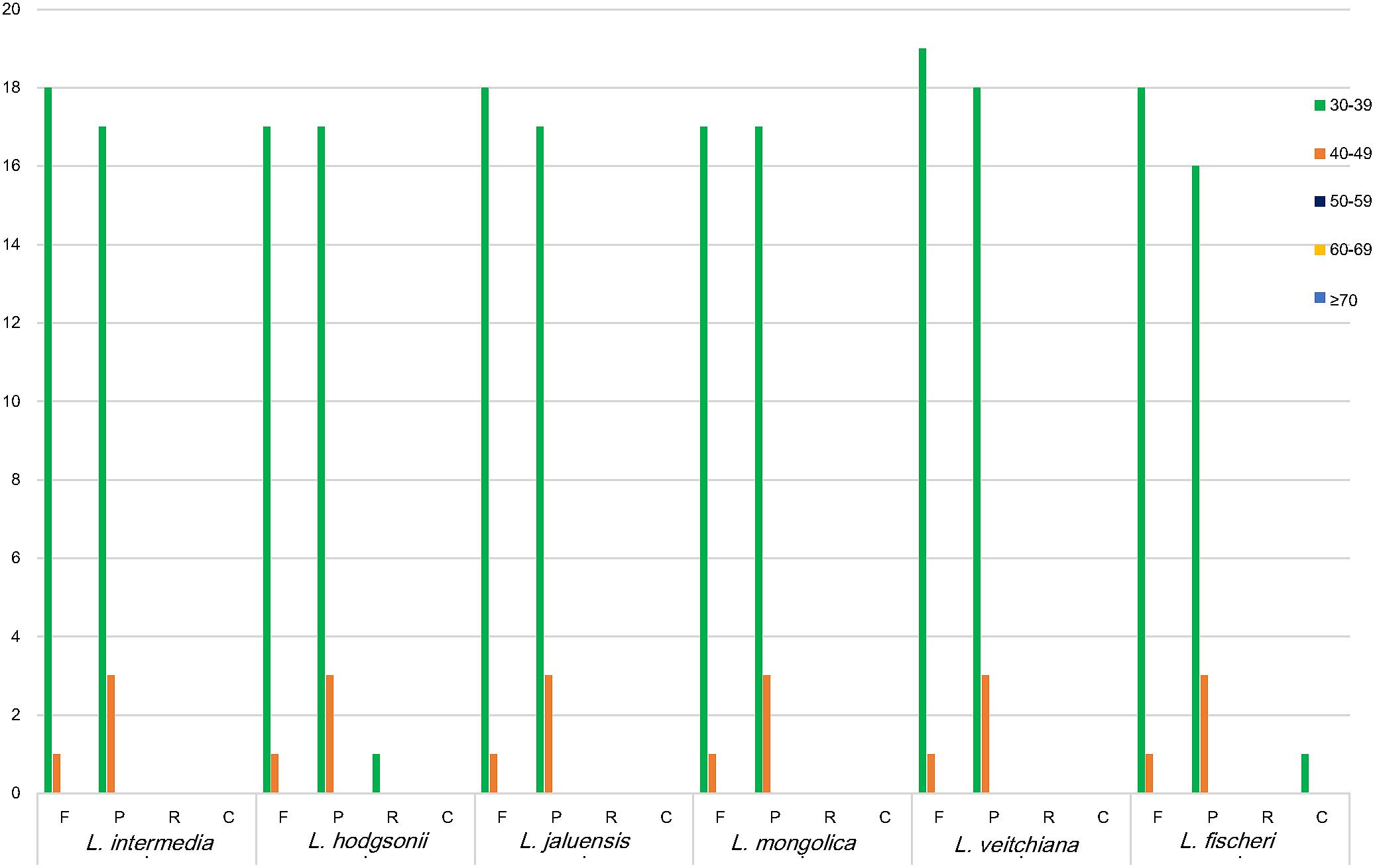
FIGURE 3. Repeat sequences in six CP genomes. REPuter was used to identify repeat sequences with length ≥30 bp and sequences identified ≥90% in the CP genomes. F, P, R, and C indicate the repeat types F (forward), P (palindrome), R (reverse), and C (complement). Repeats with different lengths are indicated in different colors.
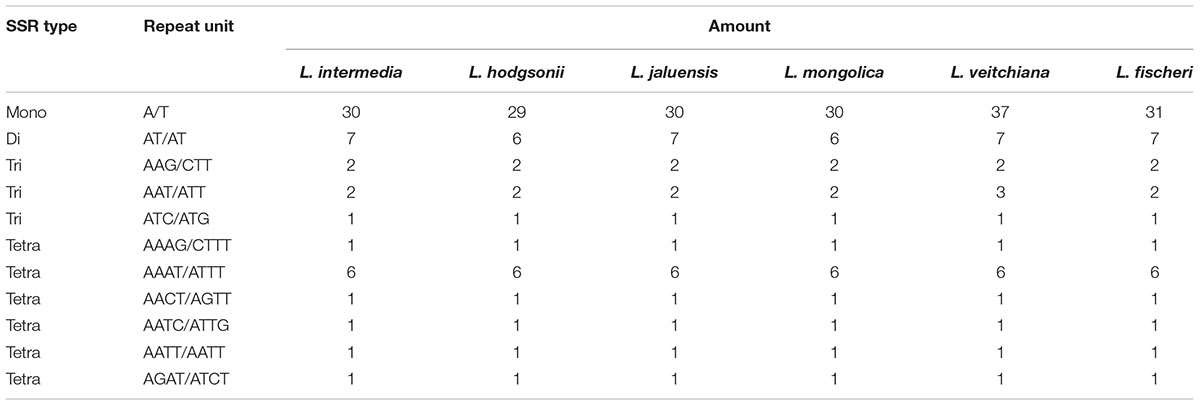
Simple sequence repeats, also called microsatellites, exist widely in the genome, and the sequences consist of one to six nucleotide repeat units (Powell et al., 1995). SSRs are widely used in studies on species identification, population genetics, and phylogenetic studies based on polymorphisms (Yang et al., 2011; Jiao et al., 2012; Xue et al., 2012). Four types of SSRs were found in the CP genomes from the six Ligularia species: mononucleotide (56.6–60.7%), dinucleotide (11.5–13.2%), trinucleotide (9.3–9.8%), and tetranucleotide (18.0–21.6%); the SSRs were mainly distributed in the non-coding region of the LSC and SSC. Of all these SSRs, the number of mononucleotide SSRs (A/T) is the largest, ranging from 29 in L. hodgsonii to 37 in L. veitchiana, enriching A and T in the CP genomes. The next most common SSR is dinucleotide (AT/AT), six dinucleotide SSRs in CP genomes of L. hodgsonii and L. mongolica and seven dinucleotide SSRs in other four CP genomes. All of the CP genomes from the six species have two trinucleotide AAG/CTT SSRs, one ATC/ATG trinucleotide SSR, and 11 tetranucleotide SSRs (Table 3). The CP genome of L. veitchiana has three AAT/ATT trinucleotide SSRs, while the other five species only have two trinucleotide SSRs.
Identification and Phylogenetic Analysis of Ligularia Species
The CP genomes from the six Ligularia species are highly similar. Among the few variations, non-coding regions exhibited higher levels of variability than the coding regions. The largest change in gene length occurred in pseudogene ycf1, with 5,097 bp in L. mongolica, 5,100 bp in L. hodgsonii and L. veitchiana, and 5,094 bp in the other three species. This difference led to a divergence in the length of the coding regions of the six species. The IR regions of the six CP genomes are conservative regardless of the number and order of the genes. Previous research screened highly variable region from CP genomes as the potential DNA barcodes for authenticating species, such as Dioscorea (Ma et al., 2018) and Fritillaria species (Li et al., 2016).
Sequence homology was investigated compared with the reference CP genome from L. hodgsonii using the mVISTA software (Figure 4). Our results showed high similarity among all sequences. Differences were observed in the intergenic regions of matK-trnK and ndhG-ndhI (Figure 4). There was only one variable site in the matK-trnK region and five variable sites in ndhG-ndhI region, but this is not enough to distinguish among the six Ligularia species. Because of the highly conservative sequences, the structure, and size of the CP genomes of Ligularia species, no obvious hypervariable region was screened. Thus, the complete CP genomes were considered to distinguish Ligularia species.
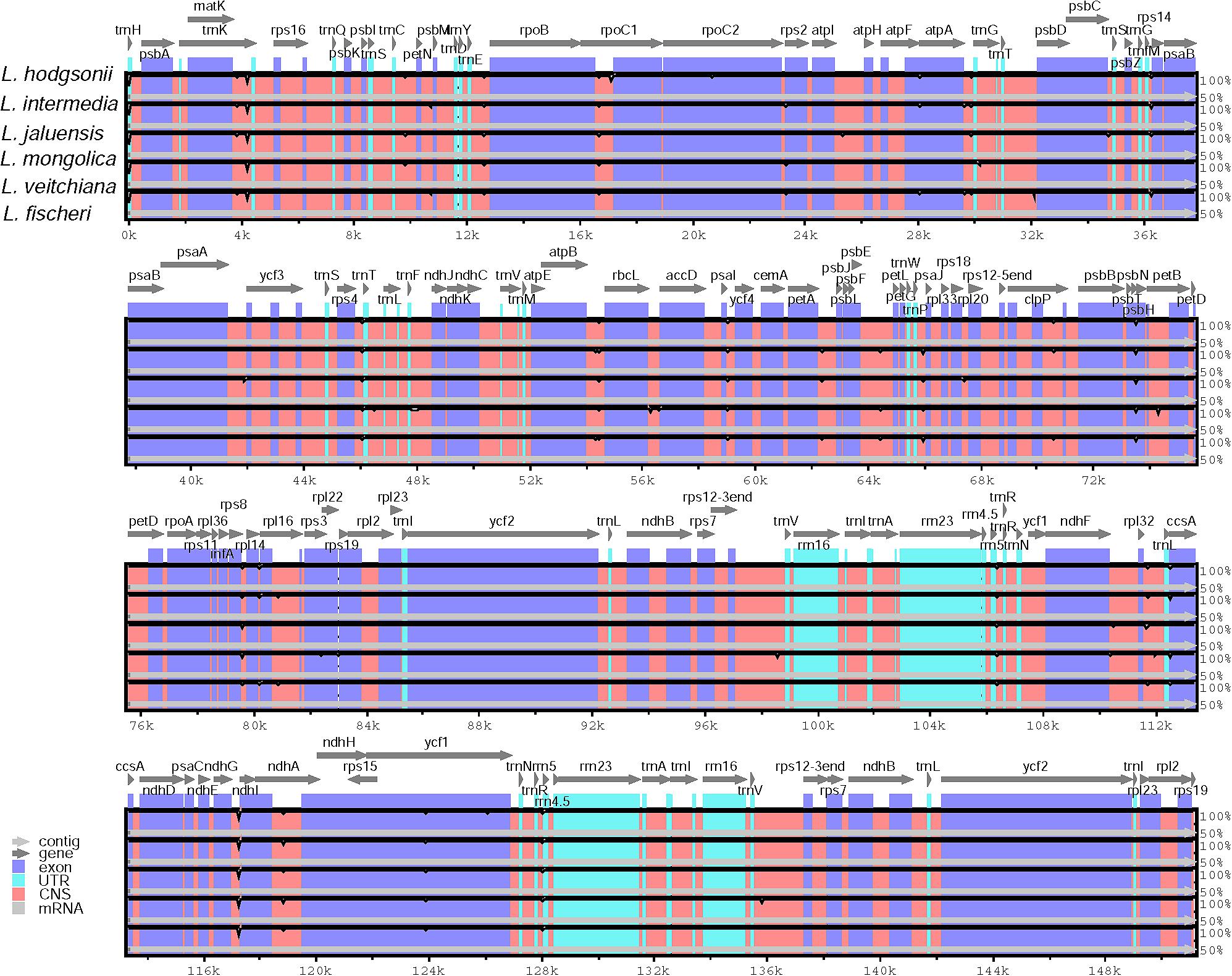
FIGURE 4. Sequence identity plot comparing six CP genomes with L. hodgsonii as a reference using mVISTA. Gray arrows and thick black lines above the alignment indicate genes with their orientation and the position of the IRs, respectively. A cutoff of 70% identity was used for the plots, and the Y-scale represents the percent identity ranging from 50 to 100%.
In addition to the six CP genomes sequenced in this study, 25 other CP genomes from Asteraceae were chosen to construct the phylogenetic tree, and P. grandiflorus and A. remotiflora (Campanulaceae) were included as outgroups (Figure 5). In the ML tree, we identified two main clades (clade A and B) excluding outgroup species. Six species of Ligularia were a monophyly with well-supported (100%). The support values in clade A were not less than 60%, and L. fischeri and L. jaluensis have a close relationship. Ligularia is most closely related to L. sativa, Saussurea involucrata, Centaurea diffusa, and Carthamus tinctorius. The results showed that the CP genomes can be used to identify the six Ligularia species.
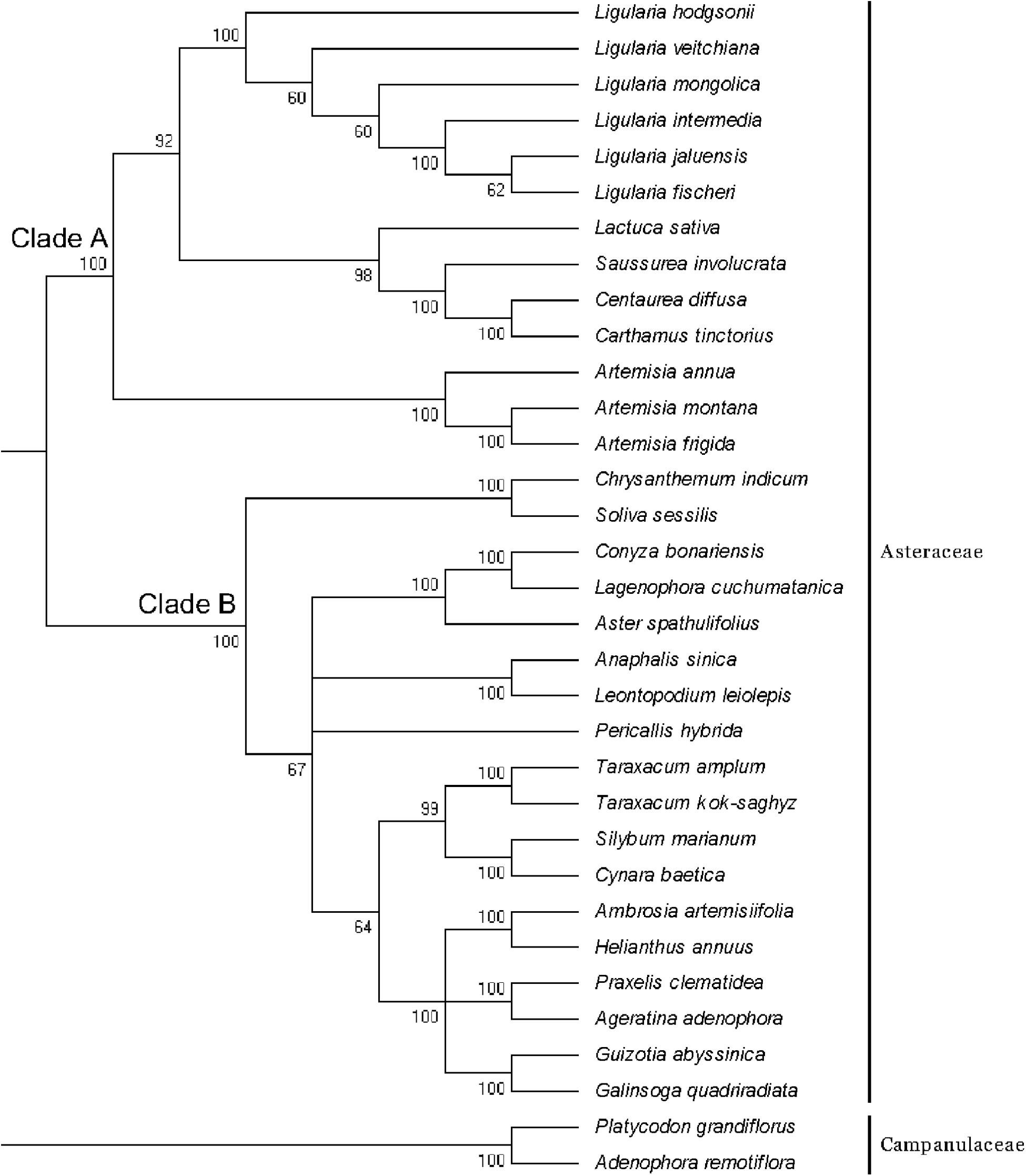
FIGURE 5. Phylogenetic tree constructed using ML based on complete CP genomes of six Ligularia and other 25 species. Numbers above the branches are bootstrap support values.
Conclusion
This study reported the CP genomes from six Ligularia species, and the structure and composition of the CP genomes are highly similar. Like most Asteraceae species, the CP genomes of the six Ligularia species had a small 3.4 kb inversion within a large 23 kb inversion in the LSC region. The ML tree showed that the CP genome can be used to identify the six Ligularia species and is expected to become a super-barcode for the identification of Ligularia species.
Author Contributions
HY conceived the study and acquired the funding. XC, YW, and BD collected samples and conducted the experiment. JZ and YC performed the genome assembly and analysis on the data. XC and JZ wrote the manuscript. All authors have read and approved the final manuscript.
Funding
This work was supported by CAMS Innovation Fund for Medical Sciences (CIFMS) (No. 2016-I2M-3-016) and Major Scientific and Technological Special Project for “Significant New Drugs Creation” (No. 2014ZX09304307001).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2018.00695/full#supplementary-material
Abbreviations
IR, inverted repeat; LSC, large single-copy; ML, maximum likelihood; SSC, small single-copy; SSRs, simple sequence repeats.
Footnotes
References
Asada, Y., Furuya, T., and Murakami, N. (1981). Pyrrolizidine alkaloids from Ligularia japonica. Planta Med. 42, 202–203. doi: 10.1055/s-2007-971628
Boetzer, M., Henkel, C. V., Jansen, H. J., Butler, D., and Pirovano, W. (2011). Scaffolding pre-assembled contigs using SSPACE. Bioinformatics 27, 578–579. doi: 10.1093/bioinformatics/btq683
Boetzer, M., and Pirovano, W. (2012). Toward almost closed genomes with GapFiller. Genome Biol. 13:R56. doi: 10.1186/gb-2012-13-6-r56
Bolger, A. M., Lohse, M., and Usadel, B. (2014). Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120. doi: 10.1093/bioinformatics/btu170
Bull, L. B., Culvenor, C. C. J., and Dick, A. T. (1968). The Pyrrolizidine Alkaloids. Amsterdam: North Holland Publishing Co.
Chinese Pharmacopoeia Commission (2015). Pharmacopoeia of the Peoples Republic of China. China Medical. Ottawa: Science Press, 342–343.
Choi, K. S., and Park, S. (2015). The complete chloroplast genome sequence of Aster spathulifolius (Asteraceae); genomic features and relationship with Asteraceae. Gene 572, 214–221. doi: 10.1016/j.gene.2015.07.020
Clegg, M. T., Gaut, B. S., Learn, G. H., and Morton, B. R. (1994). Rates and patterns of chloroplast DNA evolution. Proc. Natl. Acad. Sci. U.S.A. 91, 6795–6801. doi: 10.1073/pnas.91.15.6795
Curci, P. L., De, P. D., Danzi, D., Vendramin, G. G., and Sonnante, G. (2015). Complete chloroplast genome of the multifunctional crop globe artichoke and comparison with other Asteraceae. PLoS One 10:e0120589. doi: 10.1371/journal.pone.0120589
Feng, K. (2016). Analysis of Alkaloid Component and Toxicity Test in the Aerial Parts of Ligularia rumicifolia. Ph.D. thesis, Northwest A&F University, Yangling.
Hanai, R., Yamada, H., Suzuki, Y., Nagano, H., Kawahara, T., Yu, J. J., et al. (2012). Chemical constituents of Ligularia nelumbifolia and L. subspicata hybrid collected in Shangrila County, Yunnan province of China. Nat. Prod. Commun. 7, 1565–1568.
He, L., Qian, J., Li, X., Sun, Z., Xu, X., and Chen, S. (2017). Complete chloroplast genome of medicinal plant Lonicera japonica: genome rearrangement, intron gain and loss, and implications for phylogenetic studies. Molecules 22, 249–260. doi: 10.3390/molecules22020249
He, W., and Pan, Y. (2015). Study on the DNA barcoding of genus Ligularia Cass.(Asteraceae). Plant Divers. 37, 693–703.
Hu, Y., Woeste, K. E., and Zhao, P. (2016). Completion of the chloroplast genomes of five Chinese juglans and their contribution to chloroplast phylogeny. Front. Plant Sci. 7:1955. doi: 10.3389/fpls.2016.01955
Jank, B., and Rath, J. (2017). The risk of pyrrolizidine alkaloids in human food and animal feed. Trends Plant Sci. 22, 191–193. doi: 10.1016/j.tplants.2017.01.002
Jiang, D., Zhao, Z., Zhang, T., Zhong, W., Liu, C., Yuan, Q., et al. (2017). The chloroplast genome sequence of Scutellaria baicalensis provides insight into intraspecific and interspecific chloroplast genome diversity in Scutellaria. Genes 8:E227. doi: 10.3390/genes8090227
Jiao, Y., Jia, H., Li, X., Chai, M., Jia, H., Chen, Z., et al. (2012). Development of simple sequence repeat (SSR) markers from a genome survey of Chinese bayberry (Myrica rubra). BMC Genomics 13:201. doi: 10.1186/1471-2164-13-201
Kim, K. J., Choi, K. S., and Jansen, R. K. (2005). Two chloroplast DNA inversions originated simultaneously during the early evolution of the sunflower family (Asteraceae). Mol. Biol. Evol. 22, 1783–1792. doi: 10.1093/molbev/msi174
Kurtz, S., Choudhuri, J. V., Ohlebusch, E., Schleiermacher, C., Stoye, J., and Giegerich, R. (2001). Reputer: the manifold applications of repeat analysis on a genomic scale. Nucleic Acids Res. 29, 4633–4642. doi: 10.1093/nar/29.22.4633
Lee, J., Lee, H., Lee, S. C., Sang, H. S., Kang, J. H., Lee, T. J., et al. (2016). The complete chloroplast genome sequence of Ligularia fischeri (Ledeb.) Turcz. (Asteraceae). Mitochondrial DNA B 1, 4–5. doi: 10.1080/23802359.2015.1137793
Li, X., Gao, H., Wang, Y., Song, J., Henry, R., Wu, H., et al. (2013). Complete chloroplast genome sequence of Magnolia grandiflora and comparative analysis with related species. Sci. China Life Sci. 56, 189–198. doi: 10.1007/s11427-012-4430-8
Li, Y., Yao, H., Song, J., Ren, F., Li, X., and Sun, C. (2016). Screening Fritillaria genus-specific DNA barcodes based on complete chloroplast genome sequences. Mod. Tradit. Chin. Med. Mater. Med. World Sci. Technol. 18, 24–28. doi: 10.11842/wst.2016.01.004
Liu, C., Shi, L., Zhu, Y., Chen, H., Zhang, J., Lin, X., et al. (2012). CpGAVAS, an integrated web server for the annotation, visualization, analysis, and GenBank submission of completely sequenced chloroplast genome sequences. BMC Genomics 13:715. doi: 10.1186/1471-2164-13-715
Liu, Y., Huo, N., Dong, L., Wang, Y., Zhang, S., Young, H. A., et al. (2013). Complete chloroplast genome sequences of Mongolia medicine Artemisia frigida and phylogenetic relationships with other plants. PLoS One 8:e57533. doi: 10.1371/journal.pone.0057533
Lohse, M., Drechsel, O., and Bock, R. (2007). OrganellarGenomeDRAW (OGDRAW): a tool for the easy generation of high-quality custom graphical maps of plastid and mitochondrial genomes. Curr. Genet. 52, 267–274. doi: 10.1007/s00294-007-0161-y
Lu, C., Shen, Q., Yang, J., Wang, B., and Song, C. (2015). The complete chloroplast genome sequence of safflower (Carthamus tinctorius L.). Mitochondrial DNA 27, 1–3. doi: 10.3109/19401736.2015.1018217
Luo, R., Liu, B., Xie, Y., Li, Z., Huang, W., Yuan, J., et al. (2012). SOAPdenovo2: an empirically improved memory-efficient short-read de novo assembler. Gigascience 1, 18–23. doi: 10.1186/2047-217X-1-18
Ma, S., Zhou, J., Li, Y., Chen, X., Wu, M., Sun, W., et al. (2018). Study on complete chloroplast genome of Dioscorea opposite and D. collettii and screening specific DNA barcodes (in Chinese). Sci. China Life Sci. 2018, 48–59. doi: 10.1360/N052017-00160
Martinello, M., Borin, A., Stella, R., Bovo, D., Biancotto, G., Gallina, A., et al. (2017). Development and validation of a QuEChERS method coupled to liquid chromatography and high resolution mass spectrometry to determine pyrrolizidine and tropane alkaloids in honey. Food Chem 295–302. doi: 10.1016/j.foodchem.2017.04.186
Misa-Microsatellite Identification Tool (2017). Available at: http://pgrc.ipk-gatersleben.de/misa/ [accessed 16 September 2017]
Mower, J. P. (2009). The PREP suite: predictive RNA editors for plant mitochondrial genes, chloroplast genes and user-defined alignments. Nucleic Acids Res. 37, 253–259. doi: 10.1093/nar/gkp337
Nagy, E., Hegedûs, G., Taller, J., Kutasy, B., and Virág, E. (2017). Illumina sequencing of the chloroplast genome of common ragweed (Ambrosia artemisiifolia L.). Data Brief. 15, 606–611. doi: 10.1016/j.dib.2017.10.009
Powell, W., Morgante, M., Mcdevitt, R., Vendramin, G. G., and Rafalski, J. A. (1995). Polymorphic simple sequence repeat regions in chloroplast genomes: applications to the population genetics of pines. Proc. Natl. Acad. Sci. U.S.A. 92, 7759–7763. doi: 10.1073/pnas.92.17.7759
Prakash, A. S., Pereira, T. N., Reilly, P. E., and Seawright, A. A. (1999). Pyrrolizidine alkaloids in human diet. Mutat. Res. 443, 53–67. doi: 10.1016/S1383-5742(99)00010-1
Pu, S., Xu, D., Zhang, M., Zhou, H., Wang, Z., and Yu, G. (2004). Detection of hepatotoxic pyrrolizidine alkaloids in Ligularia Cass. with LC/MSn. Acta Pham. Sin. 39, 831–835. doi: 10.3321/j.issn:0513-4870.2004.10.014
Saito, Y., Ichihara, M., Takashima, Y., Okamoto, Y., Gong, X., Hanai, R., et al. (2017). Chemical constituents of hybrids of Ligularia cyathiceps and L. lamarum/L. subspicata collected in china: structures of subspicatins M, N, O1, and O2, and related compounds. Phytochemistry 140, 69–76. doi: 10.1016/j.phytochem.2017.04.015
Salih, R. H. M., Majeský, L’., Schwarzacher, T., Gornall, R., and Heslop-Harrison, P. (2017). Complete chloroplast genomes from apomictic Taraxacum (Asteraceae): identity and variation between three microspecies. PLoS One 12:e0168008. doi: 10.1371/journal.pone.0168008
Sato, S., Nakamura, Y., Kaneko, T., Asamizu, E., and Tabata, S. (1999). Complete structure of the chloroplast genome of Arabidopsis thaliana. DNA Res. 6, 283–290. doi: 10.1093/dnares/6.5.283
Schattner, P., Brooks, A. N., and Lowe, T. M. (2005). The tRNAscan-SE, snoscan and snoGPS web servers for the detection of tRNAs and snoRNAs. Nucleic Acids Res. 33, 686–689. doi: 10.1093/nar/gki366
Sharp, P. M., and Li, W. H. (1987). The codon Adaptation Index–a measure of directional synonymous codon usage bias, and its potential applications. Nucleic Acids Res. 15, 1281–1295. doi: 10.1093/nar/15.3.1281
Shen, X., Wu, M., Liao, B., Liu, Z., Bai, R., Xiao, S., et al. (2017). Complete chloroplast genome sequence and phylogenetic analysis of the medicinal plant Artemisia annua. Molecules 22, 1330–1343. doi: 10.3390/molecules22081330
Shimizu, A., Suzuki, Y., Hanai, R., Okamoto, Y., Tori, M., Gong, X., et al. (2014). Chemical and genetic similarity and diversity of Ligularia anoleuca and L. fischeri collected in the Hengduan Mountains of China. Phytochemistry 102, 137–144. doi: 10.1016/j.phytochem.2014.03.019
Smith, L. W., and Culvenor, C. C. J. (1981). Plant sources of hepatotoxic pyrrolizidine alkaloids. J. Nat. Prod. 44, 129–152. doi: 10.1021/np50014a001
Stegelmeier, B. L., Edgar, J. A., Colegate, S. M., Gardner, D. L., Schoch, T. K., Coulombe, R. A., et al. (1999). Pyrrolizidine alkaloids plants, metabolism and toxicity. J. Nat. Toxins 8, 95–116.
Tamura, K., Stecher, G., Peterson, D., Filipski, A., and Kumar, S. (2013). MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 30, 2725–2729. doi: 10.1093/molbev/mst197
Thompson, J. D., Higgins, D. G., and Gibson, T. J. (1994). CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22, 4673–4680. doi: 10.1093/nar/22.22.4673
Timme, R., Kuehl, J., Boore, J., and Jansen, R. (2007). A comparative analysis of the Lactuca and Helianthus (Asteraceae) plastid genomes: Identification of divergent regions and categorization of shared repeats. Am. J. Bot. 94, 302–312. doi: 10.3732/ajb.94.3.302
Tonti-Filippini, J., Nevill, P. G., Dixon, K., and Small, I. (2017). What can we do with 1000 plastid genomes? Plant J. 90, 808–818. doi: 10.1111/tpj.13491
Wang, Q. (2007). Cytotoxic Chemical Constituents of Three Ligularia Medicinal Plants. Ph. D. thesis, Fudan University, Shanghai.
Wang, X., Zhou, Z., Liu, G., and Qian, Z. (2017). Characterization of the complete chloroplast genome of the invasive weed Galinsoga quadriradiata, (Asterales: Asteraceae). Conserv. Genet. Resour. 10, 89–92. doi: 10.1007/s12686-017-0771-8
Wiedenfeld, H., and Edgar, J. (2011). Toxicity of pyrrolizidine alkaloids to humans and ruminants. Phytochem. Rev. 10, 137–151. doi: 10.1007/s11101-010-9174-0
Wyman, S. K., Jansen, R. K., and Boore, J. L. (2004). Automatic annotation of organellar genomes with DOGMA. Bioinformatics 20, 3252–3255. doi: 10.1093/bioinformatics/bth352
Xia, Y., Hu, Z., Li, X., Wang, P., Zhang, X., Li, Q., et al. (2016). The complete chloroplast genome sequence of Chrysanthemum indicum. Mitochondrial DNA A 27, 4668–4669. doi: 10.3109/19401736.2015.1106494
Xiong, A., Yan, A. L., Bi, C. W., Lam, K. Y., Chan, G. K., Lau, K. K., et al. (2016). Clivorine, an otonecine pyrrolizidine alkaloid from Ligularia species, impairs neuronal differentiation via NGF-induced signaling pathway in cultured PC12 cells. Phytomedicine 23, 931–938. doi: 10.1016/j.phymed.2016.06.006
Xue, J., Wang, S., and Zhou, S. (2012). Polymorphic chloroplast microsatellite loci in Nelumbo (Nelumbonaceae). Am. J. Bot. 99, 240–244. doi: 10.3732/ajb.1100547
Yang, A., Zhang, J., Yao, X., and Huang, H. (2011). Chloroplast microsatellite markers in Liriodendron tulipifera (Magnoliaceae) and cross-species amplification in L. chinense. Am. J. Bot. 98, e123–e126. doi: 10.3732/ajb.1000532
Yi, D. K., and Kim, K. J. (2012). Complete chloroplast genome sequences of important oilseed crop Sesamum indicum L. PLoS One 7:e35872. doi: 10.1371/journal.pone.0035872
Yu, J., Kuroda, C., and Gong, X. (2014). Natural hybridization and introgression between Ligularia cymbulifera and L. tongolensis (Asteraceae, Senecioneae) in four different locations. PLoS One 9:e115167. doi: 10.1371/journal.pone.0115167
Zhang, Y., Iaffaldano, B. J., Zhuang, X., Cardina, J., and Cornish, K. (2017). Chloroplast genome resources and molecular markers differentiate rubber dandelion species from weedy relatives. BMC Plant Biol. 17:34. doi: 10.1186/s12870-016-0967-1
Zhang, Y., Ma, P., and Li, D. (2011). High-throughput sequencing of six bamboo chloroplast genomes: phylogenetic implications for temperate woody bamboos (Poaceae: Bambusoideae). PLoS One 6:e20596. doi: 10.1371/journal.pone.0020596
Keywords: Ligularia Cass., chloroplast genome, identification, super-barcode, Illumina sequencing
Citation: Chen X, Zhou J, Cui Y, Wang Y, Duan B and Yao H (2018) Identification of Ligularia Herbs Using the Complete Chloroplast Genome as a Super-Barcode. Front. Pharmacol. 9:695. doi: 10.3389/fphar.2018.00695
Received: 27 April 2018; Accepted: 08 June 2018;
Published: 03 July 2018.
Edited by:
Jiang Xu, China Academy of Chinese Medical Sciences, ChinaReviewed by:
Zhi Chao, Southern Medical University, ChinaEszter Virag, University of Pannonia, Hungary
Copyright © 2018 Chen, Zhou, Cui, Wang, Duan and Yao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Yao, scauyaoh@sina.com
†These authors have contributed equally to this work.
 Xinlian Chen1†
Xinlian Chen1† Hui Yao
Hui Yao