Testicular ischemia associated with IgA vasculitis in a child: a case report and literature review
- 1The First Clinical Medical College, Henan University of Chinese Medicine, Zhengzhou, China
- 2Department of Pediatrics, The First Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou, China
Testicular necrosis is a rare and severe complication of immunoglobulin A (IgA) vasculitis (IgAV). Herein, We report a case of a 10-year-old boy who was admitted to the hospital due to skin purpura and intermittent abdominal pain for 10 days and bilateral testicular pain for 2 days. Scrotal ultrasonography indicated right testicle ischemia, right epididymo-orchitis, and bilateral hydrocele of the testis. Scrotal surgical exploration revealed significant swelling and darkening of the right testicle. Conservative treatment led to improvement in his condition, and he was discharged. During 3 months of follow-up, there was no recurrence of skin purpura or pain, and the urine tests were normal. Color ultrasound indicated only partial blood flow signal to the right testicle tissue, which was slightly smaller than the left testicle. This case highlights the need for continuous attention from clinicians to the signs and symptoms of the reproductive system during the diagnosis and treatment of IgAV. Continuous monitoring with ultrasound can aid in early detection, diagnosis, and treatment of reproductive system lesions of IgA vasculitis.
1. Introduction
Immunoglobulin A vasculitis (IgAV), also known as Henoch–Schönlein purpura, is a systemic immune complex-mediated leukocytoclastic small vessel vasculitis frequently reported among children. Its primary clinical manifestations involve non-thrombocytopenic purpura, abdominal pain, joint swelling and pain, and renal disorders (1). The incidence of IgAV is reported to range from 10 to 20 cases per 100,000 children (2). In general, children with IgAV have a good prognosis, but serious complications such as myocarditis and disorders of the nervous system (such as intracranial hemorrhage), respiratory system (such as pulmonary hemorrhage), and reproductive system (such as scrotal edema, orchitis, testicular torsion) may occur owing to individual differences (3–6). Genital involvement in IgAV is infrequent, and testicular necrosis is extremely rare. To the best of our knowledge, only four cases of testicular necrosis have been reported worldwide (7–10). Recently, a boy with IgAV experiencing abdominal pain and testicular ischemia was treated in our hospital. The abdominal pain was relieved by treatment with glucocorticoid and immunoglobulin, but the partial absence of blood flow in the testicle persisted. In addition, we reviewed previously reported cases of IgAV with testicular ischemic necrosis.
2. Case report
The patient, a 10-year-old boy with bilateral testes measuring less than 2.5 cm in long axis and less than 4 mL in volume and no pubic hair, was classified as stage 1 according to the Tanner Stages (11). He was admitted to the Department of Pediatrics of our hospital and presented with a history of skin purpura accompanied by intermittent abdominal pain that persisted for 10 days without relief after treatment. He reported bilateral testicular pain that lasted for 2 days.
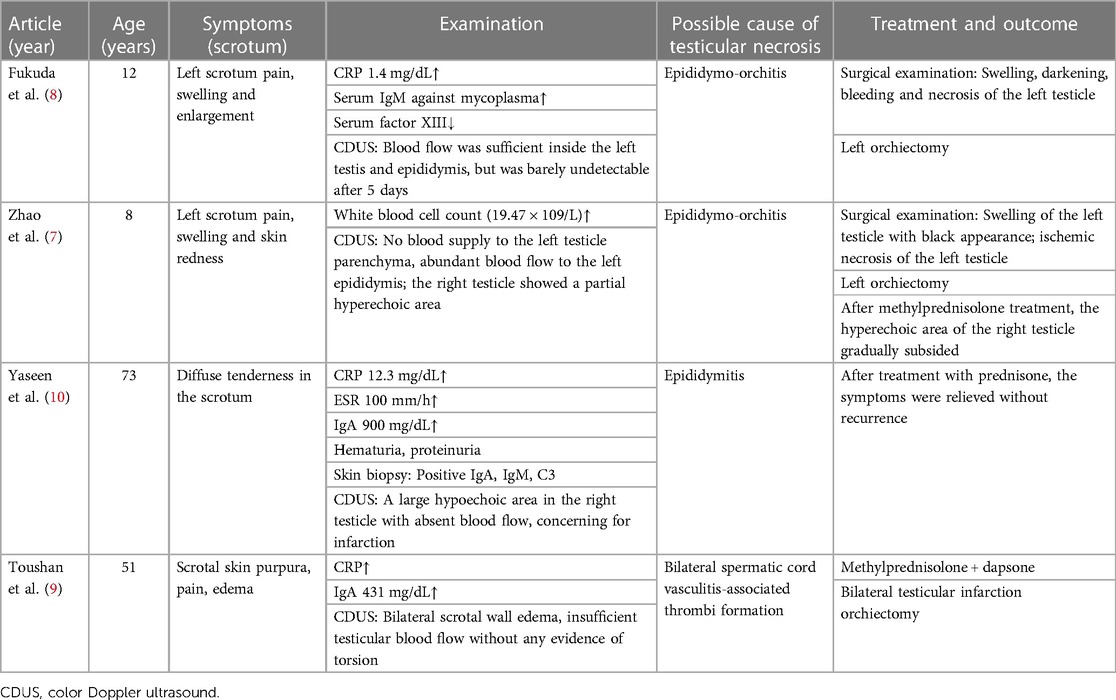
The child developed several skin purpuric lesions on both lower limbs without inducement, accompanied by abdominal pain and knee joint pain 10 days prior to hospital admission. He had normal blood and urine test results and was diagnosed with Henoch–Schönlein purpura. The patient was treated with methylprednisolone 40 mg intravenous drip for 7 days; however, he was discharged despite no relief from abdominal pain . On day 8, he developed bilateral testicular pain that was more severe on the right side. He was transferred to our hospital for treatment on day 10. Physical examination revealed millet-like purpura in bilateral auriculae and both lower limbs, slight abdominal tenderness, and obvious bilateral testicular tenderness. Auxiliary examination showed increased white blood cell (14.3 × 109/L) and neutrophil (10.45 × 109/L) counts. Routine urine and 24-h urine protein results were normal. The patient’s IgA level had increased to 3.15 g/L, but all other laboratory tests were within the normal range. Scrotal color Doppler ultrasound showed that the left testicle was 15 mm × 13 mm × 9 mm in size, exhibiting uniform echo and normal blood flow signal. The right testicle was 16 mm × 10 mm × 8 mm in size, displaying uneven echo and no obvious blood flow signal (Figure 1). The blood flow signal to the right epididymis had increased, along with the presence of a bilateral testicular hydrocele. Combined with clinical practice, testicular torsion was not excluded, and further examination was recommended.
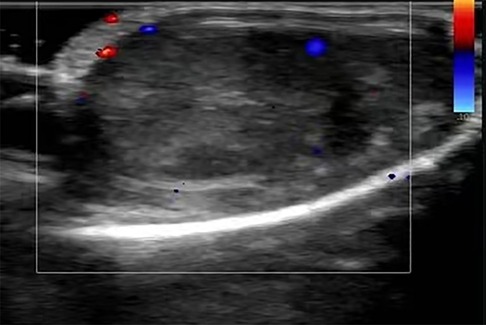
Figure 1. Ultrasound image of the right testis on hospital admission. The color Doppler ultrasound image revealed the uneven echo of the right testis, which showed no significant blood flow signal.
On the day of admission, surgical exploration of testicular torsion was performed under general anesthesia. The surgery revealed a significantly swollen, dark right testicle and no obvious torsion. Testicular ischemia was thought to be caused by vascular occlusion and poor blood supply. Therefore, a warm saline gauze was applied for hot compression. Given the absence of any improvement after half an hour, a lidocaine blockade test was performed. There was no improvement after 20 min of observation (Figure 2). Intraoperative ultrasonic exploration reaffirmed that the echo of the right testicle was slightly uneven and there was no significant blood flow signal. The disease condition was communicated to the family, and it was advised to remove the right testicle. As the family refused this suggestion, the testicle was fixed in the fleshy membrane of the scrotum in its natural position and a rubber drainage strip was placed inside the incision. During the surgery, a grayish-white soft mass of approximately 0.3 cm × 0.3 cm size was detected within the tunica vaginalis of the testis. Subsequent pathological examination under an objective microscope revealed that the lump was a fibrous tissue nodule with a large amount of inflammatory exudation at the center, which contained a small number of fungal spores.
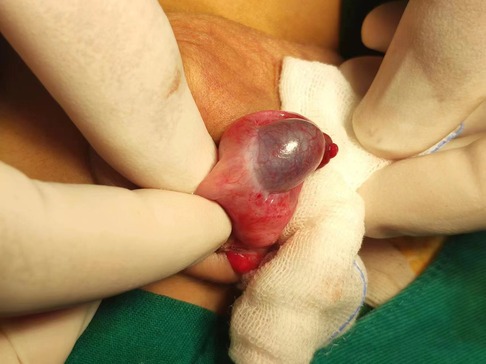
Figure 2. Obvious enlargement and darkening of the right testis due to testicle ischemia owing to vascular occlusion and poor blood supply.
The right scrotum was slightly swollen after surgery, but the left side was normal. The patient was intravenously administered with ceftriaxone sodium, levornidazole, low-molecular-weight heparin, 5 mg/(kg·d) methylprednisolone, and 10 g of human immunoglobulin daily for 2 days. After treatment, the skin purpura gradually subsided, the abdominal pain reduced, and the swelling of the right scrotum was alleviated. Three days after surgery, a color Doppler ultrasound revealed the presence of patchy hypoechoic regions in the right testicle where no significant blood flow signal was detected. The blood flow signals were observed in the surrounding testicular tissue of hypoechogenicity (Figure 3A). Edema of the right scrotal wall and thickening of the right spermatic cord were evident. Ten days after surgery, a color Doppler ultrasound showed areas of patchy hypoechogenicity (the range was smaller than before) in the right testicle, slightly sparse internal blood flow signal, and visible blood flow signal in the surrounding testicular tissue with low echoes along with right scrotal wall edema and right spermatic cord thickening. A color Doppler ultrasound conducted 2 weeks after surgery showed that the internal echo of the right testicle was normal without any abnormal echo areas and the blood flow signal was better than that observed before (Figure 3B). Testicular ultrasound microvascular imaging showed a short-line blood flow signal in the right testicular tissue (Figure 3C).
Figure 3. Ultrasound images of the right testicle during treatment and follow-up. From postoperative day 3 (A) to postoperative week 2 (B), the ultrasound showed two patchy hypoechoic areas (arrow) in the right testicle, which gradually narrowed and almost returned to normalcy. Two weeks after surgery, testicular ultrasound microvascular imaging (C) showed a short lines-like blood flow signal in the right testicular tissue. The ultrasound follow-up conducted 3 months after surgery showed that the right testicle was slightly smaller than the left testicle (D).
The patient has been diligently followed up for 3 months after surgery. Throughout this monitoring period, neither recurrence of skin purpura nor pain was observed. Furthermore, urinalysis revealed normal results. Notably, the color Doppler ultrasound highlighted a partial absence of the blood flow signal to the right testicular tissue, which was 13 mm × 8 mm × 6 mm in size and approximately 0.44 mL in volume. The left testicle, on the other hand, was 16 mm × 11 mm × 7 mm in size and approximately 0.87 mL in volume. The right testicle was slightly smaller than the left testicle (Figure 3D). Importantly, the patient has not reported experiencing any discomfort or symptoms of the scrotum.
3. Discussion
IgAV is the most common vasculitis encountered in children. The pathogenesis of IgAV is thought to involve humoral immunity abnormalities triggered by infection and other factors, which result in excessive production of galactose-deficient IgA1 (Gd-IgA1). Gd-IgA1 binds to specific antibodies and forms immune complexes that get deposited in the walls of small blood vessels and cause autoinflammation and tissue damage. IgAV mainly affects capillaries but may also involve arterioles and venules, which may increase the permeability and brittleness of blood vessels. There is exudation of serous fluid and red blood cells around blood vessels, infiltration of inflammatory cells, and intimal hyperplasia of small blood vessels, consistent with transparent degeneration and necrosis. This phenomenon causes narrowing of the vascular lumen and infarction and may lead to necrosis of small arteritis (12). The deposition of the immune complex stimulates vascular endothelial damage, activates the coagulation system, and thus mediates a hypercoagulable state, which can lead to ischemia, hypoxia, and tissue damage of the reproductive organs. Therefore, IgAV can be associated with acute lesions of the genitals.
The incidence of IgAV combined with genital involvement has been reported to range from 2% to 38% (13). The vast majority of the cases have been reported in men and involved the scrotum, penis, epididymis, testicles, and spermatic cord. Cases involving testicular blood vessels may be complicated with epididymitis or epididymo-orchitis (7, 8, 10), testicular torsion (14), and spermatic vein thrombosis (9, 15), all of which are risk factors for testicular necrosis that can lead to testicular ischemia and necrosis if not timely treated.
This boy had no history of trauma or intense activity; hence, his testicular ischemia was related to IgAV. Color ultrasound and scrotal exploration revealed no signs of spermatic vein thrombosis and testicular torsion. Given the increased blood flow signal in the right epididymis, edema of the right scrotal wall, thickening of the spermatic cord, and bilateral testicular hydrocele, histopathological examination indicated that the mass in the tunica vaginalis was a fibrous tissue nodule with a large amount of inflammatory exudation visible in its center. Testicular ischemia might be caused by the development of epididymo-orchitis. Inflammatory exudation and edema contribute to the thickening of the spermatic cord sheath and formation of a narrow ring at the transition of the spermatic cord sheath and the testicular sheath. This process leads to the compression of the testicular artery within the spermatic cord and, consequently, to testicular ischemia. Epididymitis or epididymo-orchitis in children with IgAV is related to the involvement of epididymal and testicular blood vessels and may also be associated with infection or compromised immunity due to the use of glucocorticoids. The pathogens can cause epididymitis or epididymo-orchitis by reaching the epididymis through the blood, lymphatic vessels, or vas deferens.
A literature review was conducted using the China National Knowledge Network database and PubMed database based on the terms “Henoch-Schönlein purpura” or “IgA vasculitis,” “testicular ischemia,” “testicular infarction,” and “testicular necrosis.” Publications describing cases of IgAV combined with testicular necrosis were selected. Four case reports described the symptoms, examinations, diagnoses, treatments, and outcomes of IgA vasculitis with testicular necrosis (Table 1).
Among three patients with epididymitis or epididymo-orchitis, one presented a large hypoechoic area in the right testicle without blood flow in a scrotal ultrasound examination, which was related to infarction. The symptoms were alleviated following treatment with prednisone (10). The other two cases developed left testicular necrosis and underwent testicular resection (7, 8). Bilateral testicular necrosis occurred in one patient with bilateral spermatic cord thrombosis, which was resolved by orchiectomy (9).
While IgAV is more frequent in children, it is clinically more severe in adults, who often exhibit more severe clinical manifestations and poor prognosis, including severe kidney damage. However, the reasons underlying this difference in clinical presentation related to age of onset are unclear. One hypothesis centers around the combined effects of comorbidities and aging. Potential precipitating factors such as infection are more common in children than in adults, and the susceptibility to upper respiratory tract infections in children may be associated with the increased incidence of IgAV in pediatric patients (16). Conversely, testicular necrosis is infrequent, and there has been limited investigation comparing adults and children in this regard.
Previous findings have recommended early treatment with glucocorticoids in children with IgAV who experience gastrointestinal symptoms, nephritis, arthritis, pulmonary hemorrhage, and acute vasculitis of the reproductive organs (17). IgAV complicated with epididymitis or orchitis is mostly a self-limiting disease and is effectively treated with glucocorticoids and antibiotics (18). The acute phase is often accompanied by a hypercoagulable state characterized by abnormal levels of various coagulation factors and can be managed by the addition of anticoagulants. Diana et al. (15) reported an 8-year-old boy with IgAV who experienced acute pain and swelling of the left scrotum. Exploratory surgery revealed extensive thrombosis of the spermatic vein. The patient was treated with low molecular weight heparin that alleviated thrombosis, as observed in the ultrasound examination.
For male children with IgAV, attention should be paid to conducting genital examinations combined with dynamic observation of the color Doppler ultrasound. In the case of testicular torsion, it is necessary to obtain time for surgical reduction as soon as possible and strive to complete the operation within 6 h of the appearance of symptoms. Kajitani et al. (14) reported a case of a child with testicular torsion who had decreased blood flow in the center of the testis. Testicular torsion reduction fixation was performed in time to avoid any occurrence of testicular necrosis. Testicular necrosis is a surgical emergency. The ischemic necrosis of the testicle is accompanied by the destruction of the blood–testis barrier, which may lead to the production of antisperm antibodies and the triggering of autoimmune reactions, and even involve the healthy testicle. The consequences can result in changes in sperm quality or immune infertility. Therefore, early surgical removal of the affected testicles is warranted (19).
In this particular case, there was no obvious blood flow signal in the testis in the acute stage neither was any improvement in the condition after half an hour of hot compression with warm saline gauze. The parents refused orchiectomy. After medical treatment, the symptoms were relieved and partial restoration of testicular blood flow was observed. Future studies are needed to determine the optimal timing and indication for testicular resection in such cases. In addition, follow-up is necessary to evaluate the long-term prognosis of children with IgAV involving the reproductive system and its potential impact on future fertility.
In conclusion, IgAV can affect not only the skin, gastrointestinal tract, joints, and kidneys but also the reproductive system. Nevertheless, the presence of testicular involvement has been largely underestimated in a large series of children with IgA vasculitis (20). Genital involvement in children with IgAV may lead to testicular necrosis if not diagnosed and treated in time, suggesting that clinicians should pay more attention to the occurrence of such rare complications in conjunction with ultrasonography during the diagnosis and treatment of IgAV. It is important to consider the potential risk of this complication in boys with IgA vasculitis. Consequently, a thorough clinical evaluation should be performed to exclude testicular ischemia in these patients.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
QG and SZ contributed to the conception, participated in the study design, and reviewed the manuscript. SZ wrote the manuscript and conducted the literature search, while QW and ZL organized data collection and follow-up. All authors contributed to the article and approved the submitted version.
Funding
This study acknowledges the financial support provided by the Natural Science Foundation of Henan Province (212300410369), the Project of Henan Provincial Administration of Traditional Chinese Medicine (20-21zy1048), and the Traditional Chinese Medicine “Double First Class” Scientific Research Project in Henan Province (HSRP-DFCTCM-2023-1-17 and HSRP-DFCTCM-2023-4-07).
Acknowledgments
The authors are thankful to the staff of the hospitals for their support of data collecting.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 Revised international Chapel Hill consensus conference nomenclature of vasculitides. Arthritis Rheum. (2013) 65(1):1–11. doi: 10.1002/art.37715
2. Song Y, Huang X, Yu G, Qiao J, Cheng J, Wu J, et al. Pathogenesis of IgA vasculitis: an up-to-date review. Front Immunol. (2021) 12:771619. doi: 10.3389/fimmu.2021.771619
3. Buscatti IM, Abrão HM, Kozu K, Marques VLS, Gomes RC, Sallum AME, et al. Characterization of scrotal involvement in children and adolescents with IgA vasculitis. Adv Rheumatol. (2018) 58(1):38. doi: 10.1186/s42358-018-0039-3
4. Yılmaz N, Yüksel S, Becerir T, Girişgen İ, Ufuk F, Gürses D, et al. Myocarditis and intracardiac thrombus due to Henoch–Schönlein purpura: case report and literature review. Clin Rheumatol. (2021) 40(4):1635–44. doi: 10.1007/s10067-020-05317-8
5. Wen YK, Yang Y, Chang CC. Cerebral vasculitis and intracerebral hemorrhage in Henoch–Schonlein purpura treated with plasmapheresis. Pediatr Nephrol. (2005) 20(2):223–5. doi: 10.1007/s00467-004-1673-z
6. Di Pietro GM, Castellazzi ML, Mastrangelo A, Montini G, Marchisio P, Tagliabue C. Henoch–Schönlein Purpura in children: not only kidney but also lung. Pediatr Rheumatol. (2019) 17(1):75. doi: 10.1186/s12969-019-0381-y
7. Zhao L, Zheng S, Ma X, Yan W. Henoch–Schönlein purpura with testicular necrosis: sonographic findings at the onset, during treatment, and at follow-up. Urology. (2017) 107:223–5. doi: 10.1016/j.urology.2017.05.005
8. Fukuda S, Takahashi T, Kumori K, Takahashi Y, Yasuda K, Kasai T, et al. Idiopathic testicular infarction in a boy initially suspected to have acute epididymo-orchitis associated with mycoplasma infection and Henoch–Schönlein purpura. J Pediatr Urol. (2009) 5(1):68–71. doi: 10.1016/j.jpurol.2008.07.003
9. Toushan M, Atodaria A, Lynch SD, Kanaan HD, Yu L, Amin MB, et al. Bilateral testicular infarction from IgA vasculitis of the spermatic cords. Case Rep Nephrol. (2017) 2017: 9437965. doi: 10.1155/2017/9437965
10. Yaseen K. Testicular infarction in an adult patient with systemic IgA vasculitis. Clin Rheumatol. (2023) 42(4):1213–4. doi: 10.1007/s10067-023-06515-w
12. Heineke MH, Ballering AV, Jamin A, Ben Mkaddem S, Monteiro RC, Van Egmond M. New insights in the pathogenesis of immunoglobulin A vasculitis (Henoch-Schönlein purpura). Autoimmun Rev. (2017) 16(12):1246–53. doi: 10.1016/j.autrev.2017.10.009
13. Ha TS, Lee JS. Scrotal involvement in childhood Henoch-Schönlein purpura. Acta Paediatr. (2007) 96(4):552–5. doi: 10.1111/j.1651-2227.2006.00173.x
14. Kajitani S, Miyamoto M, Tokura Y, Mizuno T, Kambara T, Ichikawa G, et al. Testicular torsion associated with Henoch–Schönlein purpura. J Pediatr. (2022) 243:231–2. doi: 10.1016/j.jpeds.2021.12.001
15. Diana A, Gaze H, Laubscher B, De Meuron G, Tschantz P. A case of pediatric Henoch–Schönlein purpura and thrombosis of spermatic veins. J Pediatr Surg. (2000) 35(12):1843. doi: 10.1053/jpsu.2000.9293
16. González-Gay MA, Blanco R, Castañeda S. Henoch–Schönlein purpura (IgA vasculitis): the paradox of the different incidence and clinical spectrum in children and adults. Clin Exp Rheumatol. (2017) 35 Suppl 103(1):3–4.
17. Ozen S, Marks SD, Brogan P, Groot N, De Graeff N, Avcin T, et al. European consensus-based recommendations for diagnosis and treatment of immunoglobulin a vasculitis—the SHARE initiative. Rheumatology. (2019) 58(9):1607–16. doi: 10.1093/rheumatology/kez041
18. Ma Y, Zhang S, Chen J, Kong H, Diao J. Henoch-Schönlein purpura with scrotal involvement: a case report and literature review. J Pediatr Hematol Oncol. (2021) 43(6):211–5. doi: 10.1097/MPH.0000000000002161
19. Rodriguez MG, Rival C, Theas MS, Lustig L. Immunohistopathology of the contralateral testis of rats undergoing experimental torsion of the spermatic cord. Asian J Androl. (2006) 8(5):576–83. doi: 10.1111/j.1745-7262.2006.00146.x
Keywords: IgA vasculitis, Henoch–Schönlein purpura, children, epididymo-orchitis, testicular ischemia, testicular necrosis, case report, literature review
Citation: Zhang S, Wang Q, Li Z and Guo Q (2023) Testicular ischemia associated with IgA vasculitis in a child: a case report and literature review. Front. Pediatr. 11:1219878. doi: 10.3389/fped.2023.1219878
Received: 9 May 2023; Accepted: 26 July 2023;
Published: 11 August 2023.
Edited by:
Daniel E. Nassau, Nicklaus Children’s Health System, United StatesReviewed by:
Miguel Angel González-Gay, University of Cantabria, SpainSantos Castañeda, Hospital de La Princesa, Spain
© 2023 Zhang, Wang, Li and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qingyin Guo docgqy@126.com
 Shuya Zhang
Shuya Zhang Qingwen Wang1
Qingwen Wang1