Estimate of the incidence of PANDAS and PANS in 3 primary care populations
- 1Department of Pediatrics, University of Wisconsin School of Medicine and Public Health, Madison, WI, United States
- 2Department of Biostatistics and Medical Informatics, University of Wisconsin School of Medicine and Public Health, Madison, WI, United States
- 3Medical Director Clinical Analytics and Reporting, University of Wisconsin, Madison, WI, United States
- 4Department of Pediatrics, Children’s Hospital at Dartmouth, Geisel School of Medicine at Dartmouth, Hanover, NH, United States
- 5Department of Pediatrics, University of Arkansas for Medical Sciences, Little Rock, AR, United States
- 6Department of Neurology, Geisel School of Medicine at Dartmouth, Hanover, NH, United States
- 7Department of Psychiatry, University of Arkansas for Medical Sciences, Little Rock, AR, United States
- 8Department of Psychiatry, Children’s Hospital at Dartmouth, Geisel School of Medicine at Dartmouth, Hanover, NH, United States
- 9Department of Epidemiology, Geisel School of Medicine at Dartmouth, Hanover, NH, United States
Objective: Pediatric Autoimmune Neuropsychiatric Disorder Associated with Streptococcal infection (PANDAS) and Pediatric Acute-Onset Neuropsychiatric syndrome (PANS) are presumed autoimmune complications of infection or other instigating events. To determine the incidence of these disorders, we performed a retrospective review for the years 2017–2019 at three academic medical centers.
Methods: We identified the population of children receiving well-child care at each institution. Potential cases of PANS and PANDAS were identified by including children age 3–12 years at the time they received one of five new diagnoses: avoidant/restrictive food intake disorder, other specified eating disorder, separation anxiety disorder of childhood, obsessive-compulsive disorder, or other specified disorders involving an immune mechanism, not elsewhere classified. Tic disorders was not used as a diagnostic code to identify cases. Data were abstracted; cases were classified as PANDAS or PANS if standard definitions were met.
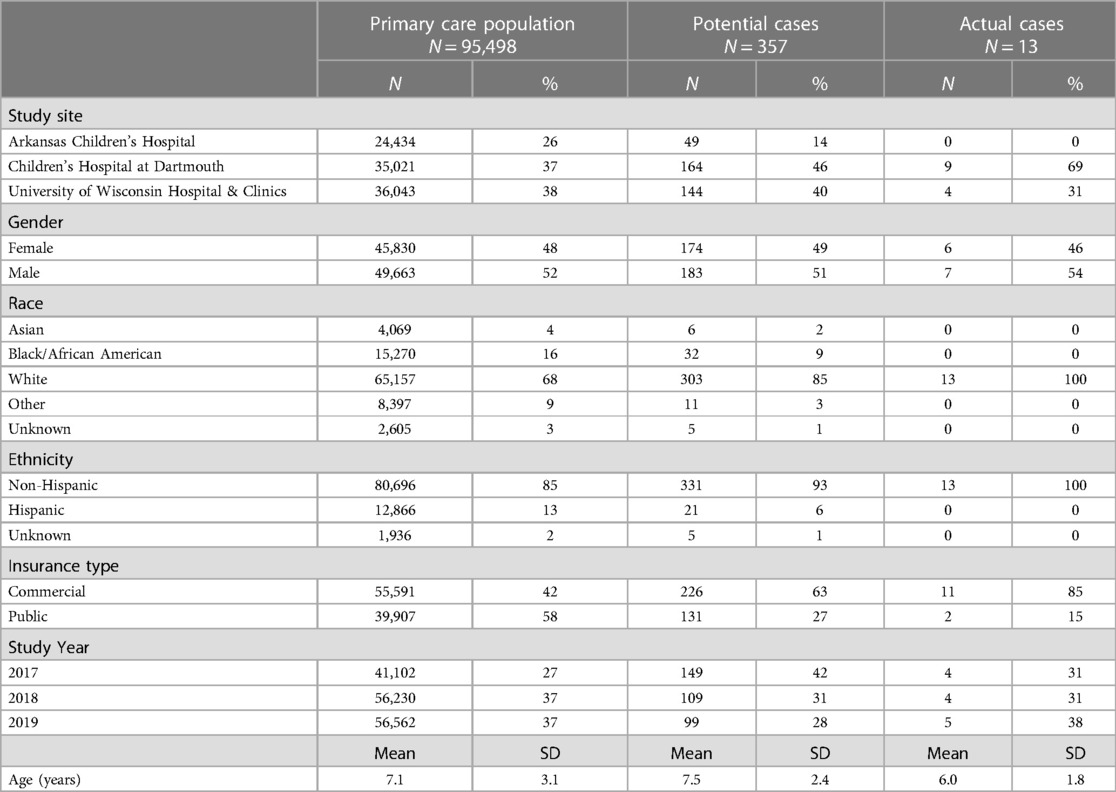
Results: The combined study population consisted of 95,498 individuals. The majority were non-Hispanic Caucasian (85%), 48% were female and the mean age was 7.1 (SD 3.1) years. Of 357 potential cases, there were 13 actual cases [mean age was 6.0 (SD 1.8) years, 46% female and 100% non-Hispanic Caucasian]. The estimated annual incidence of PANDAS/PANS was 1/11,765 for children between 3 and 12 years with some variation between different geographic areas.
Conclusion: Our results indicate that PANDAS/PANS is a rare disorder with substantial heterogeneity across geography and time. A prospective investigation of the same question is warranted.
1. Introduction
Pediatric autoimmune neuropsychiatric disorder associated with streptococcal infection (PANDAS), believed to be a non-suppurative complication of infection with Group A streptococcus (GAS), was first described in 1998 (1). The existence of PANDAS as a clinical entity distinct from Sydenham chorea, childhood onset obsessive-compulsive disorder (OCD) or tic disorder of childhood has been controversial since its initial description (2, 3). The controversy has focused, at least in part, on the inclusion of tics as a presenting symptom and the relationship of the syndrome to GAS as an instigator event and a cause of exacerbations of neuropsychiatric symptoms. A decade later, the concept of pediatric acute-onset neuropsychiatric syndrome (PANS) was introduced to broaden the scope of this disorder and to allow for provocative events other than GAS (4).
Neither the incidence nor natural history of PANDAS or PANS has been systematically described. In one prospective evaluation performed in a large private pediatric practice in Rochester, NY, children with the acute presentation of neuropsychiatric or behavioral symptoms suggestive of PANDAS were evaluated for evidence of streptococcal infection (5). In this study, 12 children were identified during a 3-year period. The primary care population base was approximately 10,000 children with an estimated 3,200 children between 3 and 12 years (personal communication Michael Pichichero, MD). The incidence of PANDAS can be estimated to be 4 per 3,200 children/year or approximately 1 per thousand from this study.
The overall incidence of childhood onset OCD in the population of children less than 10 years of age varies from approximately 2%–4% (6). Swedo has speculated that as many as 25% of these cases may be related to GAS, for an incidence of 5–10 per 1,000 children (7). In contrast, a study by Jaspers-Fayer et al, reported that 5% of their population of children with OCD fit criteria for PANDAS or PANS (8). On a common website for parents, it is estimated that PANDAS affects 1 in 200 children (9).
Although a prospective study examining the incidence of PANDAS and PANS would be most desirable, a prospective strategy may not provide representative results during the current COVID pandemic and the immediate post-COVID period; masking and distancing may alter the natural history of those infections and other triggers that precede the onset of these syndromes. Accordingly, we embarked on a retrospective review for the calendar years 2017–2019 at three different academic medical centers. Our goal was to determine the incidence of PANDAS and PANS in several geographically and socioeconomically diverse primary care populations of children.
2. Methods
A retrospective investigation was undertaken within the primary care populations at the University of Wisconsin Hospital and Clinics (Madison, Wisconsin and the surround), Arkansas Children's Hospital (Little Rock, Arkansas and the surrounding geographic area) and the Children's Hospital at Dartmouth (Lebanon, New Hampshire and the surrounding geographic area). To identify cohorts of children who received primary care at each health care system for each of the study years, a proxy definition was used based on children who had completed at least one well-child visit any time during the study period. For the University of Wisconsin and Dartmouth locations, the study period was 1/1/2017–12/31/2019, while for the Arkansas location, the study period was defined as 1/1/2018–12/31/2019 due to limited data access for the calendar year 1/1/2017–12/31/2017. Institutional review board approval was obtained for each institution.
2.1. Target population
Each institution extracted data from their respective electronic health record of all children aged 3–12 years who had received at least one well-child visit at the institution at any time during the study period. A well-child visit was defined as either an encounter for routine child health examination with abnormal findings (Z00.121) or an encounter for routine child health examination without abnormal findings (Z00.129).
For each patient in the primary care population, the data collected included date of birth, sex, first (listed) race, ethnicity, zip code, and state. Well-child visit information included visit date and visit primary payer financial class.
2.2. Potential cases
To facilitate the review of patient charts, potential cases of PANS and PANDAS were identified from the overall primary care population. A case could be identified in primary care clinic, specialty clinic, emergency department or urgent care. Potential cases were defined as children who were aged 3–12 years old at the time they received one of five new diagnoses during the study period: avoidant/restrictive food intake disorder (ICD-10-CM: F50.82), other specified eating disorder (ICD-10-F50.89), separation anxiety disorder of childhood (ICD-10-CM:F93.0), obsessive-compulsive disorder (ICD-10-CM:F42.*), or other specified disorders involving the immune mechanism, not elsewhere classified (ICD-10-CM: D89.89). Potential cases could not have any of the five diagnoses from January 2007 to the start of the organization's study period, as the intent was to identify first presentation. The diagnosis of tic disorder was not used to identify potential cases because it is very common and usually self-limited. Separation anxiety of childhood was included to cast a slightly broader net as it often accompanies OCD and is a common supporting criterion in patients with PANS.
For each potential case, the first and last dates of any of the five diagnoses were collected. The diagnosis could have been entered by the primary care physician, neurologist, psychiatrist or other health care provider. The medical record of each potential case was reviewed from the time of the first presentation which was recorded as the age at presentation. The abstracted information included demographics (age, sex, location), date of onset of symptoms and whether criteria for PANS or PANDAS were fulfilled as defined in the next paragraph.
2.3. Actual cases
All actual cases were reviewed by two investigators. An actual case was identified as being consistent with PANDAS if the following criteria were met: (1) presence of OCD and/or tic disorder, (2) prepubertal onset, (3) acute and severe onset and dramatic exacerbations of symptoms, (4) presence of neurologic abnormalities during symptom exacerbations and (5) a temporal relationship between GAS infections and exacerbations of symptoms (positive culture, rapid test or serology) (1). Tics were included in the actual cases found in our study but were not included as a diagnostic code to identify cases in the incidence data and analysis. An actual case was identified as being consistent with PANS if the following criteria were met: abrupt, dramatic onset (within 48 h) of (1) OCD or (2) severely restricted food intake and (3) concurrent abrupt onset of additional severe neuropsychiatric symptoms from at least 2 of the following 7 categories: (1) anxiety; (2) emotional lability and/or depression; (3) irritability, aggression, and/or severe oppositional behaviors; (4) developmental regression; (5) deterioration in school performance; (6) sensory or motor abnormalities, including heightened sensitivity to sensory stimuli, hallucinations, dysgraphia, complex motor, and/or vocal tics; (7) somatic signs and symptoms, including sleep disturbances, enuresis or urinary frequency and the requirement that symptoms are not better explained by a known neurologic or medical disorder (4).
2.4. Statistical analysis
Demographic characteristics of the study population (overall and stratified by study site) and cases were summarized in terms of frequencies and percentages or means and standard deviations. Comparisons of demographic distributions between study sites were conducted using chi-square analysis. Frequencies and percentages of diagnostic symptoms of the cases were reported. Overall estimated incidence rates of PANDAS/PANS cases (per 100,000) were reported and stratified by demographic characteristics, along with the corresponding two-sided 95% confidence intervals (CIs) which were constructed using the Clopper-Pearson method. Comparisons of incidence rates between study sites, gender and study year were performed by calculating ratios of predicted probabilities derived from fitting corresponding logistic regression models. Due to the small number of actual cases, Fisher's exact test was used to compare incidence rates of actual cases between study sites, gender and study year. Pooled estimates of incidence rates of potential and actual cases and corresponding 95% CIs across the three study sites and across the three study years were obtained using a fixed effects model or random effects model with the inverse variance weighting method (10). Heterogeneity of incidence rates among sites was quantified by calculating the I2 statistics. All reported p-values are two-sided and P < 0.05 was used to define statistical significance. Statistical analyses were conducted using SAS software (SAS Institute, Cary NC), version 9.4. Calculation of pooled rates were conducted using R software (Foundation for Statistical Computing, Vienna, Austria, version 3.5.1) meta package.
3. Results
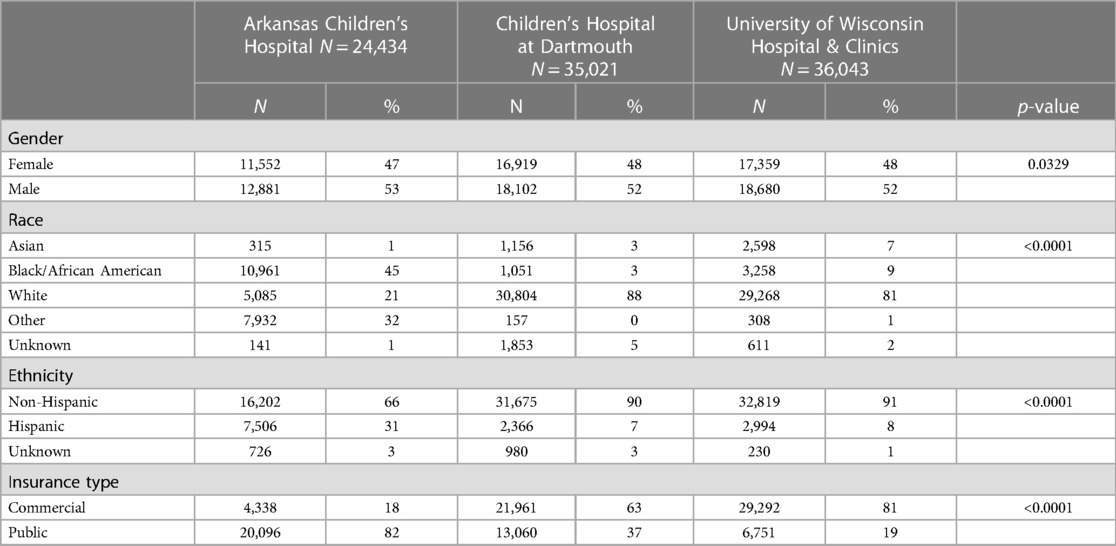
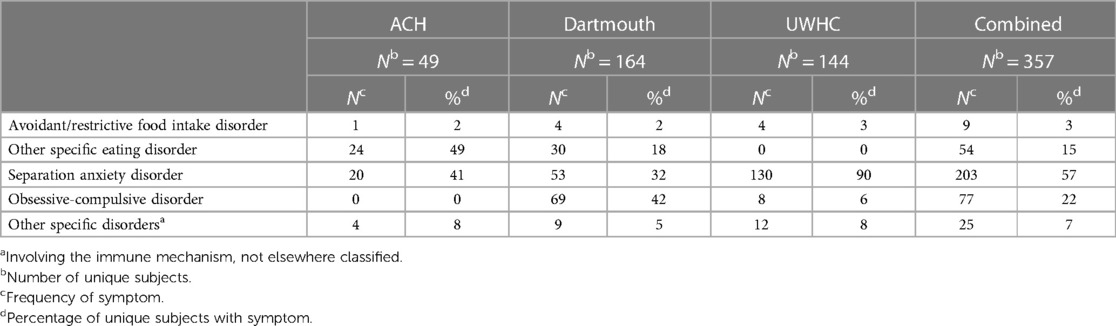
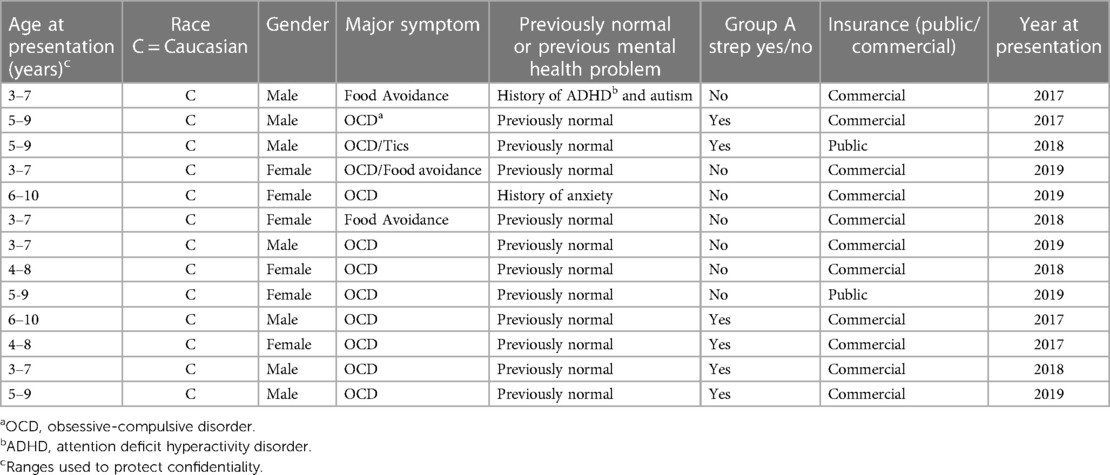
The combined primary care study population consisted of 95,498 unique subjects across the three study sites. The majority of the study population was non-Hispanic Caucasian (85%), 48% was female and the mean age was 7.1 (SD 3.1) years (Table 1). There was a total of 357 potential cases and 13 actual cases across the three geographic areas. The mean age of the potential cases was 7.5 (SD 2.4) years, 93% were non-Hispanic Caucasian and 49% were female. Significant differences in the distribution of gender, race, ethnicity and insurance type were detected among the three study sites (Table 2). Arkansas Children's Hospital serves a much larger population of underrepresented minority children (Black and Hispanic) who are publicly insured than either the Children's Hospital at Dartmouth or the University of Wisconsin. There was substantial heterogeneity in the symptom distributions of potential cases between study sites (Table 3). Across all three sites, the most common symptom was separation anxiety disorder of childhood (57%), 22% OCD, other specific eating disorder (15%), other specific disorders involving the immune system (7%) and avoidant/restrictive food intake disorders (3%). The most common symptom of the actual cases was OCD which was identified as the major symptom in 11 out of 13 subjects (85%). The mean age of the 13 actual cases was 6.0 (SD 1.8) years, 46% were female; 100% were non-Hispanic Caucasians, 85% had a commercial health insurance and 6 of 13 (46%) had a definite relationship to GAS. Details of the 13 actual cases are shown in Table 4.
The incidence rates of potential cases across the three geographic areas in the US ranged from 201 (95% CI: 152–265) to 468 (95% CI: 402–545) per 100,000 (Table 5). The heterogeneity of incidence rates of potential cases between sites was large with an I2 of 93%. The pooled estimate of the cumulative incidence rate of potential cases across the three geographic areas over the 3-year surveillance period was 340 (95% CI: 206–547) per 100,000. Incidence rates of potential cases were similar between females vs. males (RR 1.0, 95% CI: 0.8–1.3) and health insurance type (commercial vs. public, RR = 1.2, 95% CI: 1.0–1.5). The annual incidence rate of potential cases was significantly higher in 2017 when compared to 2019 (RR 2.1, 95% CI: 1.6–2.7, p < 0.0001). The pooled estimate of the cumulative incidence rate of actual cases across the three sites over the 3-year surveillance period was 14 (95% CI: 6–37) per 100,000 with an I2 of 55%, indicating a substantial amount of heterogeneity between geographic areas (Table 5). There were no statistically significant differences in the incidence rates of actual cases observed between males vs. females, insurance type or study year. However, all actual cases were
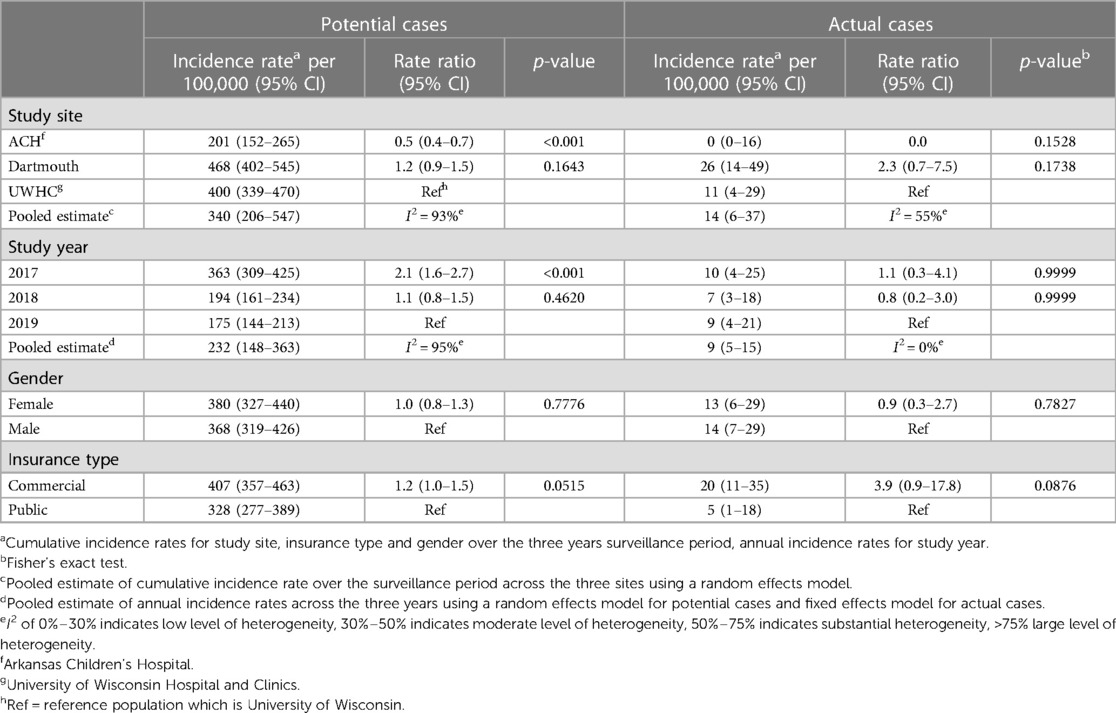
Table 5. Incidence rates of potential cases and actual cases, stratified by demographic characteristics and pooled estimates across the three study sites (geographic areas).
4. Discussion
This retrospective study performed in three geographically and demographically diverse sections of the US showed that the estimated annual incidence of PANDAS/PANS was 1 in 11,765 children between 3 and 12 years of age with considerable place-to-place variation. Tic disorders was not used as a diagnostic code to identify cases of PANDAS. The mean age was 6.0 years; 46% of cases were related to GAS and the most common presenting symptom complex was with OCD.
The basic hypothesis underlying the concept of PANDAS by Swedo is that it represents a nonsuppurative complication of infection with GAS (on the basis of molecular mimicry) similar to, but different than, acute rheumatic fever (1). However, it is the very existence of Sydenham chorea, accompanied by severe OCD in some cases, that provides the biologic plausibility for the entity of PANDAS, i.e., why not OCD without the movement disorder of chorea as a manifestation of a presumed autoimmune disorder (11, 12). This hypothesis, which includes molecular mimicry and anti-neuronal autoantibodies, is supported by the fact that Sydenham chorea and PANDAS are strikingly similar with overlapping symptoms. The two diseases can be identified by testing for a group of cross-reactive anti-neuronal autoantibodies which are elevated when symptoms are present and decrease during improvement (13).
The controversy surrounding PANDAS/PANS has not served the primary care provider very well, leaving them confused as to its importance and existence as a diagnostic entity. Studies intended to clarify the role of GAS as an antecedent event in cases of PANDAS have resulted in differing results, with some supporting its role (14–16) and others not (17–19). Although the relationship of GAS pharyngitis as an antecedent event to acute rheumatic fever is well-established it is qualified by at least two factors. Not all GAS cause acute rheumatic fever and not all individuals have the genetic predisposition to develop acute rheumatic fever even when infected with a rheumatogenic strain of streptococcus (20, 21). These factors may explain, at least in part, the discrepant results observed by investigators when attempting to discern the relationship between GAS and PANDAS/PANS. The definitive diagnosis of PANS and/or PANDAS requires a comprehensive gathering of historical information supplemented by a variable laboratory evaluation for potential infectious triggers at the time of presentation. If acuity of onset of neuropsychiatric symptoms and potential infectious triggers are not explored the diagnosis may be missed. This information will guide treatment that may differ if symptoms are not post-infectious.
Although we elected not to study the incidence of PANDAS\PANS during COVID, there have been multiple reports of SARS-COV-2 related cases of PANS as well as other post-infectious COVID-19 related neurologic syndromes (22–25). This is not surprising as there are many infectious disease agents that have been associated with the development of PANS (26).
In our study, 7 of 13 (54%) children with PANS/PANDAS were male which was not significantly different than the incidence in girls. However, the numbers of actual cases are small, and 5 of 6 children with PANDAS were boys, similar to the report by Swedo et al. (1). It is of interest that when Cox et al., were exploring the prevalence of antineuronal antibodies in a large group of children with a history of streptococcal infection and tic and/or OCD they found the highest association with a history of GAS in children with both disorders compared to either tics or OCD alone (27). The demonstration of antineuronal antibodies was similar to their previous demonstration of antibodies in both Sydenham chorea and PANDAS (28, 29).
There are no previous examinations of the estimated incidence of PANDAS/PANS in the US or any other population. Popular estimates relate to the epidemiology of OCD and what proportion may be attributable to GAS as an instigating event (8). Other estimates are not based on obvious sources of information (9). GARD (Genetic and rare diseases information center) is a program of the National institutes of Health that provides free access to reliable and easy to understand information about genetic and rare diseases. They list PANDAS and state that the population estimate section is “in development” (30). For comparison, the estimated incidence of autoimmune encephalitis in Olmstead County, Minnesota in 2014 was shown to be 137/100,000 or 1 in 7,299 (31).
We attempted to identify potential cases of PANDAS and PANS by searching for new diagnoses of OCD, food avoidance/restriction, other specified eating disorder, separation anxiety of childhood and other specified disorders of the immune system not otherwise classified in our primary care population. The proportion of the diagnoses shown at each institution were surprisingly different (Table 3). Arkansas had no cases of obsessive-compulsive disorder perhaps contributing, at least in part, to these differences, which otherwise remain unexplained but portend the substantial differences in estimated incidence at the three centers. Often potential cases identified on the basis of a new diagnosis of separation anxiety were missing key criteria for the diagnosis of PANDAS/PANS. Many patients with food restriction/avoidance or other specified eating disorders were categorized as anorexia nervosa or pica. Ten patients with OCD were lacking a description of the acuity of onset of symptoms and could not be classified as PANS or PANDAS. The complete absence of cases at Arkansas was unexpected but perhaps reflects either the absence of “pandogenic” streptococci during the study period, or potential differences in risk of disease related to underrepresented minority status. The demographics of the population studied at Arkansas was also different from the others with respect to insurance and race.
Although our results indicate that PANDAS/PANS is a rare disorder with substantial heterogeneity across geography and time, we must consider potential causes of underdiagnosis or how our methods may have missed cases. First, there are physicians who are unaware of this diagnosis and therefore do not associate any of the symptoms which commonly signal the disorder such as anxiety, food restriction or other specified eating disorders or OCD as possibly triggered by infectious agents. On some occasions, the physician may simply not inquire about a particular symptom. Since our search for cases is dependent on finding these codes for symptoms, their absence from the chart does not assure that the question was asked. When physicians do identify a symptom, they may not elicit a thorough history which indicates that the onset of the symptom was uncommonly abrupt. They will be unlikely to search for triggers in a child who presents with debilitating urinary frequency and a negative urinalysis; this highlights the impossibility of retrospectively determining whether symptom onset was preceded by an asymptomatic GAS infection. Physicians may fail to recognize other behaviors as consistent with OCD, thereby again raising the important question of the ability to study the incidence of these conditions retrospectively. In addition, there are some practitioners who view the published evidence supporting PANDAS/PANS as unconvincing and who therefore may be less likely to pursue any supporting evidence such as cultures or serology in children with the acute onset of neuropsychiatric symptoms.
We decided not to use the diagnostic code for tics to identify cases of PANDAS despite the fact that tics are a major criterion upon which this diagnosis is based. The decision was predicated on the very common occurrence of tics, almost all of which have their onset in childhood, which, it is safe to say, is upward of a least 10%–20% of children depending on geography, age and study methods (32). We were concerned that including tics would introduce excessive data which was likely to be incomplete and increase the noise/signal ratio in an unhelpful way. Some patients detected through diagnostic code D89.89 acquired this code, which is used for patients with PANDAS, by their presentation with tics and otherwise fulfilled diagnostic criteria. We recognize that not using the code for tics may have resulted in an underestimate of the cases of PANDAS and be a limitation of this study. Several other limitations should be noted. Our proxy definition for determining the cohort of children receiving primary care at each institution in each study year may have overestimated or underestimated the number of person-years in our denominator(s) which in turn may have resulted in biased year-specific estimates of incident rates. Given the large data base, we doubt this resulted in a significant difference. In addition, the age group studied, 3–12 years of age, was selected to avoid missing younger children with PANDAS. However, there is a slight skewness in the age distribution with over-representation of the younger age groups which may bias results in favor of a lower estimate. In addition, not extending the upper bound of age to 18 years may miss patients with PANS.
What we have learned from this pilot study is that PANS and PANDAS appear to be uncommon diagnoses in children in these areas of Wisconsin, Arkansas and New Hampshire who seek care at academic medical centers. In addition, we learned from careful review of our potential cases that some patients who ultimately came to hold a diagnosis of PANS/PANDAS did not qualify for inclusion as a “case” in this study because of an initial lack of evaluation or incomplete evaluation. While we await additional prospective studies to help determine the precise incidence of these diagnoses and their natural history, although they are likely rare, the clinician should be prepared to contemplate and pursue a PANS/PANDAS diagnosis when clinical symptoms are suggestive of a post-infectious potentially autoimmune neuropsychiatric syndrome.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: existing medical records from three different academic medical centers were abstracted, reviewed and analyzed. Requests to access these datasets should be directed to Jens Eickhoff Eickhoff@biostat.wisc.edu.
Ethics statement
Ethical review and approval was not required for the study of human participants in accordance with the local legislation and institutional requirements. Written informed consent from the patients/ participants OR patients/participants legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
EW: Conceptualization, Methods, Investigation, Supervision, Data curation, Writing, Review. JE: Conceptualization, Methods, Formal analysis, Review. GF: Methods, Data curation, Review. MH: Methods, Investigation, Data curation, Review. DL: Methods, Investigation, Data curation, Review. AA: Methods, Data curation, Review. RM: Methods, Data curation, Review. VR: Conceptualization, Methods, Data curation, Writing, Review. AV: Conceptualization, Data curation, Review. JM: Conceptualization, Methods, Investigation, Supervision Data curation, Writing, Review. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Swedo SE, Leonard HL, Garvey M, Mittleman B, Allen AJ, Perlmutter S, et al. Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections: clinical description of the first 50 cases. Am J Psychiatry. (1998) 155:264–71. doi: 10.1176/ajp.155.2.264
2. Gilbert DL, Mink JW, Singer HS. A pediatric neurology perspective on pediatric autoimmune neuropsychiatric disorder associated with streptococcal infection and pediatric acute-onset neuropsychiatric syndrome. J Pediatr. (2018) 199:243–51. doi: 10.1016/j.jpeds.2018.04.035
3. Wald ER. (2019). A pediatric infectious disease perspective on pediatric autoimmune neuropsychiatric disorder associated with streptococcal infection and pediatric acute onset neuropsychiatric syndrome. (2019). Pediat Infect Dis J. 38:706–9. doi: 10.1097/INF.0000000000002295
4. Swedo SE, Leckman JF, Rose NR. From research subgroup to clinical syndrome: modifying the PANDAS criteria to describe PANS (pediatric acute-onset neuropsychiatric syndrome). Pediatr Therapeut. (2012) 2:113. doi: 10.4172/2161-0665.1000113
5. Murphy ML, Pichichero ME. Prospective identification and treatment of children with pediatric autoimmune neuropsychiatric disorder associated with group A streptococcal infection (PANDAS). Arch Pediatr Adolesc Med. (2002) 156:356–61. doi: 10.1001/archpedi.156.4.356
6. Geller DA. Obsessive-compulsive and spectrum disorders in children and adolescents. Psychiat Clin N Am. (2006) 29:353–70. doi: 10.1016/j.psc.2006.02.012
8. Jaspers-Fayer F, Han SHJ, Chan E, McKenney K, Simpson A, Boyle A, et al. Prevalence of acute-onset subtypes in pediatric obsessive-compulsive disorder. J Child Adolesc Psychopharmacol. (2017) 27:332–41. doi: 10.1089/cap.2016.0031
9. PANDAS network. Available at: https://pandasnetwork.org (Accessed February 10, 2023).
10. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2
11. Swedo SE. Sydenham’s chorea: a model for childhood autoimmune neuropsychiatric disorders. JAMA. (1994) 272:1788–91. doi: 10.1001/jama.1994.03520220082035
12. Swedo SE, Rapoport JL, Cheslow DL, Leonard HL, Ayoub EB, Hosier DM, et al. High prevalence of obsessive-compulsive symptoms in patients with sydenham’s chorea. Am J Psychiatry. (1989) 146:246–9. doi: 10.1176/ajp.146.2.246
13. Chain JL, Alvarez K, Mascaro-Blanco A, Reim S, Bentley R, Hommer R, et al. Autoantibody biomarkers for basal ganglia encephalitis in Sydenham chorea and pediatric autoimmune neuropsychiatric disorder associated with streptococcal infections. Front Psychiatry. (2020) 11:564. doi: 10.3389/fpsyt.2020.00564.eXollwxrion.2020
14. Cardona F, Orifici G. Group A streptococcal infections in an Italian pediatric population. J Pediatr. (2001) 138:71–5. doi: 10.1067/mpd.2001.110325
15. Kurlan R, Johnson D, Kaplan EL and the Tourette Syndrome Study Group. Streptococcal infection and exacerbations of childhood tics and obsessive-compulsivesymptoms: a prospective blinded cohort study. Pediatrics. (2008) 121:1188–97. doi: 10.1542/peds.2007-2657
16. Mell LK, David RL, Owens D. Association between streptococcal infection and obsessive-compulsive disorder, Tourette’s syndrome and tic disorder. Pediatrics. (2005) 116:5660. doi: 10.1542/peds.2004-2058
17. Leckman JF, King RA, Gilbert DL, Coffey BJ, Singer HS, Dure L 5th, et al.. Streptococcal upper respiratory tract infections and exacerbations of tic and obsessive-compulsive symptoms: a prospective longitudinal study. J Am Acad Child Adolesc Psychiatry. (2011) 50:108–18.e3. doi: 10.1016/j.jaac.2010.10.011
18. Perrin EM, Murphy ML, Casey JR, Pichichero ME, Runyan DK, Miller WC, et al. Does group A beta-hemolytic streptococcal infection increase risk for behavioral and neuropsychiatric symptoms in children? Arch Pediatri Adolesc Med. (2004) 158:848–56. doi: 10.1001/archpedi.158.9.848
19. Schrag A-E, Martino D, Wang H, Ambler G, Benaroya-Milstein N, Buttiglione M, et al. Lack of association of group A streptococcal infection and onset of tics: European multicenter tics in children study. Neurology. (2022) 98:e1175–83. doi: 10.1212/WNL.0000000000013298
20. Bryant PA, Smyth GK, Gooding T, Oshlack A, Harrington Z, Currie B, et al. Susceptibility to acute rheumatic fever based on differential expression or genes involved in cytotoxicity, chemotaxis, apoptosis. Infect Immun. (2014) 82:753–61. doi: 10.1128/IAI.01152-13
21. de Crombrugghe G, Baroux N, Botteaux A, Moreland NJ, Williamson DA, Steer AC, et al. Limitations of the rheumatogenic concept for group A streptococcus: systematic review and genetic analysis. Clin Infect Dis. (2020) 70:1453–60. doi: 10.1093/cid/ciz425
22. Bartley CM, Johns C, Ngo TT, Dandekar R, Loudermilk RL, Alvarenga BD, et al. Anti-SARS-CoV-2 and autoantibody profiles in the cerebropspinal fluid of 3 teenaged patients with COVID-19 and subacute neuropsychiatric symptoms. JAMA Neurol. (2021) 78(12):1503–9. doi: 10.1001/jamaneurol.2021.3821
23. Berloffa S, Salvati A, Pantalone G, Falcioni L, Rizza MA, Naldini F, et al. Steroid treatment response to post SARS-CoV-2 PANS symptoms: case series. Front Neurol. (2023) 14. doi: 10.3389/fneur.2023.1085948
24. Efe A. SARS-CoV-2/COVID-19 associated pediatric acute-onset neuropsychiatric syndrome a case report of female twin adolescents. Psychiatry Res Case Rep. (2022) 1(2):100074. doi: 10.1016/j.psycr.2022.100074
25. Pavone P, Ceccarelli M, Marino S, Caruso D, Falsaperla R, Berretta M, et al. SARS-CoV-2 related paediatric acute-onset neuropsychiatric syndrome. Lancet Child Adolesc Health. (2021) 5:e19–21. doi: 10.1016/S2352-4642(21)00135-8
26. Chang K, Frankovich J, Cooperstock M, Cunningham MW, Latimer ME, Murphy TK, et al. Clinical evaluation of youth with pediatric acute-onset neuropsychiatric syndrome (PANS): recommendations from the 2013 PANS consensus conference. J Child Adol Psychopharmacol. (2015) 25:3–13. doi: 10.10.1089/cap.2014.0084
27. Cox CJ, Zuccola AJ, Edwards EV, Mascaro-Blanco A, Alvarez K, Stoner J, et al. Antineuronal antibodies in a heterogeneous group of youth and young adults with tics and obsessive-compulsive disorder. J Child Adol Psychopharmacol. (2015) 25:76–85. doi: 10.1089/cap.2014.0048
28. Kirvan CA, Swedo SE, Snider LA, Cunningham MW. Mimicry and autoantibody-mediated neuronal cell signaling in Sydenham chorea. Nat Med. (2006) 9:914–20. doi: 10.1038/nm892
29. Kirvan CA, Swedo SE, Snider LA, Cunningham MW. Antibody-mediated neuronal cell signaling in behavior and movement disorders. J Neuroimmunol. (2006) 179:173–9. doi: 10.1016/j.jneuroim.2006.06.017
30. GARD Genetic and Rare Diseases Information Center. National center for advancing translational science at National Institutes of Health. Available at: Rarediseases.info.nih.gov (Accessed February 10, 2023).
31. Dubey D, Pittock SJ, Kelly CR, McKeon A, Lopez-Chiriboga AS, Lennon VA, et al. Autoimmune encephalitis epidemiology and a comparison to infectious encephalitis. Ann Neurol. (2018) 83:166–77. doi: 10.1002/1n125131
Keywords: PANS, PANDAS, group A streptococcus, obsessive-compulsive disorder, food restriction, epidemiology
Citation: Wald ER, Eickhoff J, Flood GE, Heinz MV, Liu D, Agrawal A, Morse RP, Raney VM, Veerapandiyan A and Madan JC (2023) Estimate of the incidence of PANDAS and PANS in 3 primary care populations. Front. Pediatr. 11:1170379. doi: 10.3389/fped.2023.1170379
Received: 23 February 2023; Accepted: 6 September 2023;
Published: 21 September 2023.
Edited by:
Madeleine W. Cunningham, University of Oklahoma Health Sciences Center, United StatesReviewed by:
Margo Thienemann, Stanford University, United StatesPiero Pavone, University of Catania, Italy
© 2023 Wald, Eickhoff, Flood, Heinz, Liu, Agrawal, Morse, Raney, Veerapandiyan and Madan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ellen R. Wald erwald@wisc.edu
 Ellen R. Wald
Ellen R. Wald Jens Eickhoff
Jens Eickhoff Grace E. Flood3
Grace E. Flood3  Michael V. Heinz
Michael V. Heinz Alisha Agrawal
Alisha Agrawal Richard P. Morse
Richard P. Morse Veronica M. Raney
Veronica M. Raney Aravindhan Veerapandiyan
Aravindhan Veerapandiyan Juliette C. Madan
Juliette C. Madan