The Roles of Epidemiologists, Laboratorians, and Public Health Agencies in Preventing Invasive Cronobacter Infection
- Jason and Jarvis Associates, LLC, Hilton Head Island, SC, USA
Background: Cronobacter can cause severe, invasive infection in very young infants. These bacteria can also colonize or cause insignificant infections in immunocompromised, elderly, and/or hospitalized adults.
Methods: This editorial review highlights key points addressed in the Frontiers Research Topic on Cronobacter, discusses the clinical presentation and epidemiology of Cronobacter infections, and examines the responses of public health agencies to this problem.
Results: Cronobacter is rarely isolated from hospitalized, immunocompromised and/or elderly adults and does not cause significant disease in those patients. Certain species and strains, especially of Cronobacter sakazakii, can cause invasive illness in previously healthy infants <2 months of age. Multilocus sequence type 4 and clonal complex 4 (ST4/MLST 4) C. sakazakii are the predominant cause of Cronobacter meningitis, which occurs only in infants. These infections and this strain type are strongly linked to powdered infant formulas (PIF), which can also be contaminated with other Cronobacter strains. End-product testing is not intended to guarantee the absence of these organisms. WHO has made recommendations that can help decrease but will not eliminate the risk of this infection.
Conclusion: To further define the spectrum of Cronobacter-associated disease, all isolates should be genetically typed using every currently available method, typing results should be linked to the associated epidemiologic and clinical data, and these data should be analyzed in a scientifically sound manner. Based on currently available information, more can be done now to prevent cause invasive infection in young infants. This includes encouragement of exclusive breastfeeding and/or use of commercially sterile ready-to-feed formulas in the first 2 months of life.
Background
Cronobacter are Gram-negative, non-spore-forming, enteric coliform bacteria defined as a new Enterobacter species in 1980 by Farmer et al. (1). Recently, polyphasic taxonomic analysis has determined that this group of organisms consists of several genomospecies, so these organisms have been reclassified as novel species and subspecies within a novel genus, Cronobacter gen. nov. (2). Cronobacter isolates vary in regard to enterotoxin production, virulence, and thermotolerance. These variations – and Cronobacter’s taxonomy, ecology, reservoirs in nature, and characteristics in general – are almost certainly of clinical importance but have not been adequately studied and are not fully understood. Of the strains examined, many are extremely heat tolerant and can survive for long periods of time in a dry state [e.g., Ref. (3, 4)]. At least some form biofilms and thus resist the effectiveness of disinfectants (3–5). Lag times and microbiologic incubation periods for some Cronobacter strains can be as low as a matter of hours (6–8). In one study, an initial concentration of only 1 colony forming unit (CFU)/ml of Enterobacter sakazakii in reconstituted PIF grew to 10,000,000 in 10 h, at room temperature (9). Of note, growth would be even faster at body temperature.
Cronobacter are rarely isolated from clinical specimens and, when isolated, are largely from the very young or the elderly. Some strains can be highly pathogenic in neonates, especially infants who are premature or otherwise immunocompromised. Cronobacter occasionally colonize immunocompromised and/or elderly adults who are infected with other, significant pathogens. In these adults, Cronobacter rarely, if ever, cause significant symptomatic disease. In neonates, some strains of Cronobacter (and, in particular, some strains of C. sakazakii) cause invasive infection, even though the initial isolate is often from the gastrointestinal tract. The presentations and outcomes of neonatal C. sakazakii infection include seizures, hydrocephalus, developmental delay, and death.
A number of gastrointestinal and immunologic characteristics can contribute to an infant’s susceptibility to invasive C. sakazakii infection. First, the stomach environment of newborns, especially of premature babies, is less acidic than that of adults. Gastric and pancreatic secretions, mucus, and inherent enzymes are not produced at the levels found in an adult’s gastrointestinal tract. This would more readily permit any C. sakazakii entering the body through the mouth to survive in a viable state as it moves from the infant’s stomach to his intestines. Second, although enteric neuronal development is generally completed at a gestational age of 32 weeks (10), ineffective, uncoordinated, erratic, or static gut motility (peristalsis) can persist at least intermittently in even full-term infants. Peristalsis is an important mechanism for moving pathogenic organisms through and out of the gut, so dysfunctional movement may be a factor in C. sakazakii breaching the intestinal wall. Third, a fetus’s gut is sterile but this changes with passage through the maternal birth canal and first feeding. A newborn infant’s gastrointestinal system remains functionally inert until it is activated by food intake and microbial colonization. This means that until feeding is stably established, the feedback system between an infant’s gut and gut organisms does not function properly. Until then, an infant’s gut has suboptimal bacterial-epithelial “cross-talk,” i.e., a relative inability to differentiate pathogens from symbiotic organisms and to deal with each appropriately (11). Fourth, even if C. sakazakii is recognized as an enemy, an infant’s relatively permeable mucosa make him less able to retain the organism in his gastrointestinal system and remove it from his body. Last and perhaps most important, gut immunity develops gradually between fetal life and adulthood. Premature newborns, full-term newborns (born at 37–42 weeks gestation), neonates (<28 days old), and older infants (more than 28 days but <1 year old) differ significantly from one another and from older children and adults in regard to adequacy, strength, pattern, components, and rapidity of their immune response (11–13). Neutrophils can disrupt the intestinal tight junctions by releasing excessive interleukin (IL)-8 (12). Innate immunity, as represented by Natural Killer Cell (NK) production of interferon (IFN)-γ, dominates in early life (13).
The reservoirs for Cronobacter species are unknown. The majority of E. sakazakii and C. sakazakii isolates have come from infants, powdered infant formulas (PIFs), and factories producing milk powder (14). Cronobacter species have also been isolated from a number of other food substances [e.g., Ref. (4, 15)], food factories, and environments, including households (16); however, to my knowledge, no non-formula source of E. sakazakii or C. sakazakii has yet been causally linked to a case of invasive infection, although other sources have been examined in epidemiologic investigations.
The Epidemiology of Cronobacter Infection
In Dr. Farmer’s first-hand review of Cronobacter’s microbiologic history (17), he appropriately credits Drs. Urmenyi and Franklin with the first clinical descriptions of invasive E. sakazakii disease, a description provided in a 1961 report of two 1958 cases of “neonatal death from pigmented coliform infection” (18). Those infants were born within months of each other, at a single United Kingdom hospital. Both developed hemorrhagic meningitis and died within 2 days of one another. One was full term, of normal birthweight, and had been discharged home. The other was premature, had low birthweight, and was still in the birth hospital when his symptoms began. Post mortem cultures from both infants grew identical “pigmented cloaca.” The infants had no known contact with one another and had not been on the same ward or nursery (18). In 1965, Jøker et al. reported a similar case, this time in Denmark. Dr. Farmer’s review article in this Frontiers Topic symposium describes how, over the following two decades, those isolates and similar pigmented coliforms were characterized, differentiated from other Enterobacter, and, in 1980, officially named Enterobacter sakazakii, according to the rules of the Bacteriological Code (17).
In the subsequent two decades, Cronobacter microbiologic research progressed but epidemiologic studies came to the forefront, in the form of outbreak investigations of pediatric E. sakazakii infections in the Netherlands (1983), Greece (1987), Iceland (1989), the United States [U.S.] (1989 and 2001), Belgium (2001), Israel (2001), France (2004), and New Zealand (2004) (19–21). In the early investigations, no source was identified. Tellingly, nutrition was not examined in those outbreaks. The investigations of subsequent outbreaks repeatedly documented a strong statistical and microbiological link between PIF and invasive E. sakazakii infection. The outbreak studies were increasingly elegant and thorough. In all 10 outbreaks, at least some environmental testing had been done. In the eight outbreaks in which nutrition was examined, all the infected infants had received some type of PIF and there was a statistically powerful association between E. sakazakii infection and consumption of a specific PIF. In five outbreaks, E. sakazakii was isolated from PIF but not from any environmental samples; in two, E. sakazakii was isolated from PIF and the blender used in blending that specific PIF; and in one, E. sakazakii was isolated from PIF, a dish brush, and a stirring spoon. Four outbreak reports specified that previously unopened containers of PIF were cultured; of these, three were positive. In seven of the eight outbreaks where E. sakazakii was typed, a PIF isolate was indistinguishable from patient isolate(s), based on the results from one or another of the typing techniques summarized in Dr. Yan’s concise article in this Frontiers symposium (22). The formula consumed by infected infants in each of these outbreaks yielded E. sakazakii; in two outbreaks, formula from previously unopened cans from the same manufacturing batch also yielded E. sakazakii. In three outbreaks, investigators were able to show both statistical and microbiological associations between E. sakazakii infection and the consumption of PIF (23–25). In these investigations, there was no evidence of infant-to-infant or environmental transmission; all the infected infants had consumed the implicated formula (23–25).
At the time these investigations were done, epidemiologists had reason to suspect that E. sakazakii infections might be caused by a nutritional substance. Entero is derived from the Greek word for intestines. Enterobacter got that name because they have long been known to enter the body through the digestive system and reside in the intestines. There was also good reason to consider PIF as the potential nutritional source of these E. sakazakii infections. Infants are fed PIF and PIF is not sterile. Studies had already shown that strains of E. sakazakii could contaminate PIF components, become endemic in the post-pasteurization dry-processing areas of PIF processing plants, survive the dry-processing procedures, survive in a dry state for long periods of time, and become biologically active in the presence of moisture. Thus, it did not require great insight to include PIF as one of the independent variables studied in these outbreaks. However, these studies do demonstrate the elegant effectiveness of interactively applying epidemiologic, statistics, and microbiologic scientific techniques to outbreaks involving extremely few cases.
Concurrent with the above-described epidemiologic investigations, microbiologists and food scientists investigated the extent and nature of PIF contamination with E. sakazakii. Muytjens et al. examined 141 different powdered formulas on the market produced in 35 countries and found E. sakazakii and other Enterobacteriaceae were common contaminants (26). He isolated E. sakazakii at levels ranging from 0.36 to 66 CFU/100 g from 20 formula samples from 13 countries (27), even though all those formulas met the contemporaneous microbiological specifications for coliform counts in PIF (<3 CFU/g). A Canadian survey that investigated the incidence of E. sakazakii in PIF isolated the organism from eight of 120 cans on the market, from five different manufacturers (28).
In 2002, a Belgian infant who received PIF died from an E. sakazakii infection. The manufacturer retested the implicated batch (also referred to as a “lot”) and, although the prerelease testing of the product had been negative for E. sakazakii, the additional testing was positive. The company voluntarily recalled the product. Following the epidemiologic investigation of a 2001 U.S. newborn intensive care unit outbreak (23), a lot (also referred to as a “batch”) of the implicated product was recalled and the U.S. Food and Drug Administration (FDA) did a field survey of 10 U.S. powdered formula manufacturing plants run by various manufacturers (29). In that field survey, which included 22 finished product samples, five (22.7%) had a most probable number (MPN) above what was then the currently acceptable detection limit (0.003 MPN per gram of PIF). The positive samples included four of 14 (28.5%) formulas for full-term and one of four (25%) formulas for pre-term infants. The results were not related to product type (milk vs. soy) or manufacturing processing used (e.g., wet mixing-spray drying vs. dry blending) (29). Despite these findings, infant formula manufacturers did not readily accept that their products played any role in these infections or were of any risk to healthy, full-term infants (30). Public health and regulatory agencies did not fully agree with the manufacturers.
In 2002, the FDA released a protocol for isolation and enumeration of E. sakazakii from dehydrated PIF [formerly at http://www.fda.gov/Food/ScienceResearch/LaboratoryMethods/BacteriologicalAnalyticalManualBAM/ucm109656.htm. No longer available on at the FDA website.] The protocol was an improvement over formula companies’ standing protocols but had significant flaws and was worrisome in that it did not attempt to assure the absence of Cronobacter in end-product. Furthermore, the FDA did not require manufacturers to use the FDA protocol for PIF end-product testing, nor did the FDA address environmental testing.
Also in 2002, the FDA sent out a “Letter to Health Care Professionals,” informing them that PIF and powdered breast milk supplements are not sterile and warning that premature infants and infants with underlying medical conditions could become infected with E. sakazakii (31). In the letter, the FDA recommended PIF be avoided in newborn intensive care units unless there was no alternative.
Parents of newborn or even of newborn premature infants have never received similar information, although, even at that time (i.e., 2002–2003) invasive E. sakazakii infection had occurred in term and non-hospitalized infants (21). There is reason to believe infants’ caretakers were then and remain in need of this information: In a 2005–2006 nationally representative survey of U.S. mothers of 2-month-old infants, only 29.5% correctly answered that PIFs were “likely to contain germs,” while 31.1% incorrectly thought that commercially sterile, ready-to-feed formulas (RTFs) were likely to contain them (32). Of note, that survey was done after most, if not all, formula manufacturers had bowed to encouragement to educate the consumer by adding the statement “PIF is not sterile” to their package labels.
Based on the accumulating epidemiologic evidence and product-testing data, the World Health Organization (WHO) acknowledged there was a problem that needed to be addressed. In 2004–2008, the Food and Agriculture Organization of the United Nations (FAO) and World Health Organization (WHO) held a series of advisory meetings concerning E. sakazakii (33, 34). These resulted in a risk assessment model, as well as a series of reports and recommendations [FAO/WHO1]. In this Frontiers Topic symposium, Dr. Parra Flores applies the WHO’s risk assessment tool to explore the effect of temperature on Cronobacter growth (35). In 2006, WHO stated that “contaminated PIF has been convincingly shown, both epidemiologically and microbiologically, to be the vehicle and source of infection in infants” (36). However, WHO’s guidelines and model focus on decreasing the risk associated with contaminated PIF, not preventing the contamination.
Even as WHO was formulating a response to the Cronobacter problem, reports of intrinsic PIF contamination continued to be made to the European Rapid Alert System for Food and Feed (RASFF). Between 2002 and 2008, eleven lots of PIF were cleared and released for sale by their manufacturers, distributed in Europe, and subsequently reported by outside authorities to be contaminated with E. sakazakii (37). E. sakazakii infections and colonizations were reportedly associated with at least three of these contaminated products (37). At least one other lot was reported to RASFF, by France in 20092. Of note, the U.S. did not and does not have a reporting system for product contamination or infections with this organism. They are currently reportable in only one State – Minnesota (personal communication with Dr. A. Bowen, an epidemiologist at CDC, January 2012).
At the beginning of the twenty-first century, while health and regulatory agencies were developing models and guidelines, laboratorians were making striking advances. Their research followed two avenues: (a) typing and characterization of isolates and (b) investigating, characterizing, and statistically evaluating PIF contamination and end-product testing. These topics are addressed in this symposium’s articles by Dr. Yan and Dr. Kalyantanda (22, 38) but, in the following paragraphs, this editorial review will briefly discuss what are arguably the two most significant developments to date: identification of highly stable C. sakazakii clone with a high propensity for neonatal meningitis and determination that pre-market PIF testing cannot assure an absence of clinically significant contamination.
As noted in Dr. Farmer’s review article in this Frontiers Topic symposium (17), in 2007–2008, Iverson et al. proposed that Cronobacter be recognized as a new genus. That genus was to include organisms previously classified as E. sakazakii (2). As of this writing, 10 species and 3 subspecies of Cronobacter have been named and described. In their contribution to this symposium, Dr. Yan and Dr. Fanning succinctly describe the various and increasing number of laboratory techniques for identifying, tracking, comparing, and characterizing Cronobacter isolates (22, 38). Also in this Frontiers Topics symposium, Dr. Tall presents a pan genomic DNA microarray platform he developed and has used to document and characterize the genomic diversity among each member of the genus (39). That approach, as well as whole genome sequencing, will be powerful tools for genomic research on Cronobacter but one technique has already proved invaluable: a multi-locus sequence typing (MLST) scheme developed by Baldwin et al. (40). Application of that scheme to stored clinical, food, and environmental isolates has provided key information about the sources of various Cronobacter species and strains, the cause of Cronobacter meningitis, and the reasons why infants have severe clinical symptoms and adults do not (40–42).
Baldwin determined sequence types in relation to source and biotype, using 60 C. sakazakii and 16 C. malonaticus strains from clinical and non-clinical sources collected between 1951 and 2008 in the U.S., Canada, Europe, New Zealand, and the Far East. Twenty-two of 60 C. sakazakii isolates had sequence type 4 (ST4). Nine of those ST4 isolates were from clinical samples. Seven of the other 13 ST4 isolates were from infant formula (40). Joseph and Forsythe examined 41 C. sakazakii isolates, some of which appear to have been among those described by Baldwin (40, 41). Twenty of the 41 isolates were ST4; the remainder were one or another of nine other types. Of the 30 C. sakazakii isolates with known patient-source details, the only one from an adult patient was ST1, i.e., not ST4. Of the 20 ST4 strains, 10 were from neonates, seven from infants, one from a child, and two had no patient information. Although the seven housekeeping genes for MLST analysis are not virulence related, a large proportion of severe neonatal infections were caused by isolates with the ST4 sequence type: i.e., 9 of 12 isolates from meningitis cases were ST4 strains; the remaining ST4 strains were from a bacteremia case, necrotizing enterocolitis cases (two), and an undefined infection (one). The clinical disorders related to six of the ST4 strain isolates were unknown. Nine of 12 meningitis isolates were ST4. Joseph and Forsythe concluded that C. sakazakii ST4 and the related Clonal Complex 4 (CC4) represent a highly stable clone with a high propensity for neonatal meningitis (41, 42). They noted that C. sakazakii ST4 strains have been isolated from seven countries for >50 years and the earliest (1950) non-clinical isolate was from a can of dried milk. Joseph and Forsythe proposed that the relationships between genotypes and different age groups may reflect exposure to different genotypes of C. sakazakii according to age-related diet and lifestyle. In the context of Baldwin and Forsythe’s finding of a predominance of ST4 types in C. sakazakii isolates from infant formulas, Joseph and Forsythe’s results suggest that the key exposure in C. sakazakii-infected infants is intrinsically contaminated infant formula.
Of note, in an FDA investigation of 14 cases of community–acquired pediatric Cronobacter infections between May, 2010 and December, 2011, Dr. Tall et al. did ST typing. These isolates were not related to those previously studied by Dr. Baldwin et al. but they were also predominantly ST4 (43). MLST testing of isolates from numerous food and other sources from around the world is progressing at a rapid pace and indicates that MLST types vary by food and food-factory source, clinical source, and country. As noted by Dr. Kalyantanda in this Frontiers Topic symposium, results are being posted at an online database3 (38).
By far, the most significant development related to product safety and testing was a 2011 study, done in collaboration with a European branch a formula manufacturing company (44). In it, Jongenburger et al. examined a production “lot” (also referred to as a “batch”) of PIF. That batch had passed pre-market testing and been released to market, but it was subsequently found to be contaminated with Cronobacter. The researchers compared the contaminated batch to one recalled for non-microbiologic reasons and determined that: (a) both lots were actually positive for Cronobacter but a greater number of samples from the contaminated lot were positive, (b) the contamination was not homogeneous, and (c) the recalled lot had higher levels of contamination (44). The estimated degree of contamination varied among the positive samples and included clusters of 3–560 cells. The two largest clusters, of 123 and 560 cells, originated from two product bags only, consistent with clusters being present in a limited number of servings. Thus, an individual infant can be exposed to a significant inoculum, even if the remaining product in that can, the remaining product in that lot, and the product from that lot fed to other infants are free of Cronobacter. Dr. Jongenburger concluded that “finding such clusters is like looking for a needle in a haystack” and “when these clusters end up in one or a limited number of servings of an individual consumer, they may significantly impact public health” (44).
Dr. Jongenburger’s findings are especially meaningful because the sparse research on Cronobacter’s infectious dose suggests it can quite low, i.e., on the order of 1000 cells (3, 45) and quite possibly even lower for virulent strains. Extensive research has been done on related parameters, including lag time, generation time, and growth rate. Unfortunately, this research has not yet been done using specific isolates associated with proven, invasive clinical infections, e.g., C. sakazakii ST4 or CC4 isolates associated with Cronobacter meningitis in young infants. However, using the data that are available, albeit for non-virulent isolates, we can get a sense of how quickly an infectious dose of 1000 cells can be reached when contamination is clustered or clumped. Let us take a relatively small cluster of 10 cells. (Of note, in end-product testing, that cluster would appear as one CFU, even though all 10 cells could multiply independently.) Let us be conservative and round up the generation time determined by Dr. Parra Flores in his article in this Frontiers Topic symposium (35), which is consistent with the value found by others. Using a generation time of a half hour at 35°C (a temperature roughly comparable to the 36–37°C body temperature of an afebrile neonate) and assuming virulent Cronobacter strains thrive in the neonatal gastrointestinal tract, the 10-cell cluster would reach an infectious dose of 1,000 CFU within 3.5 h, a time interval consistent with the time it takes an infant to consume and digest a formula feeding.
To summarize, by the end of 2011, it had been determined that Cronobacter meningitis is associated with ST4 strains of C. sakazakii, these strains are found widely throughout PIF and PIF production facilities, and they appear to be stable clones present for many years in many countries. Other Cronobacter can be found in PIF and PIF factories but the evidence thus far suggests they do not cause meningitis. Dry-product Cronobacter contamination can be non-homogeneous, clustered, and clumped. End-product testing is inadequate for preventing that contamination and isolated contamination could be present even if the batch were negative to end-product testing and every other can in the batch was negative.
This laboratory progress was scientifically significant but, in late 2011, epidemiology again came to the forefront – if only in regard to the public media. Single reports of Cronobacter illness in infants in Missouri and Illinois caused the U.S. Centers for Disease Control and Prevention (CDC) to ask public health officials around the country to look for other cases of Cronobacter infection among infants. This generated reports of two additional cases, one in Oklahoma and one in Florida, bringing the 2011 U.S. case total to 13 (46). According to CDC’s Cronobacter website, CDC investigated the four cases occurring after November 1, 2011. All had been fed PIF, three had meningitis, and two died. The website indicates that CDC could not determine a source and DNA fingerprinting of isolates from two or the cases suggested they were unrelated. ST testing is not mentioned, nor if any of these cases were among those tested by Dr. Tall and found to be predominantly ST4, the strain type associated with both meningitis and PIF (see above) (43). The CDC website indicates that factory-testing was negative and notes that “Cronobacter bacteria are found in the environment and in hospitals and homes.” These statements ignore the wide variety in Cronobacter species and strains, the ST studies, and the extensive epidemiologic evidence supporting that PIF is the primary source of the specific ST type causing most cases of invasive Cronobacter meningitis.
At the time these 2011 cases were reported to the general public, I had reviewed the Cronobacter literature and material from formula manufacturers, as well as CDC, FDA, and WHO records and documents up to and through 2010, as well as the medical records of a number of Cronobacter cases that had not been investigated by those agencies. I was concerned that statements made in relation to these cases did not seem to match the material I had in hand. I therefore used that material to perform three epidemiologic analytic studies related to invasive E. sakazakii/Cronobacter infection. These studies were published as an article in Pediatrics, the peer-reviewed journal of the American Academy of Pediatrics (21). In this Frontiers editorial review i will now briefly describe those three epidemiologic studies and discuss how the findings and the current state of Cronobacter science are discordant with CDC’s current approach to epidemiologic surveillance and investigations, as well as with the FDA’s current PIF and PIF factory surveillance and testing protocols.
Two of these epidemiologic studies examined data on all reported cases of pediatric E. sakazakii/Cronobacter infection occurring worldwide from 1958 through 2010. The two studies also analyzed in greater depth all cases of invasive infection occurring in children who appeared to have been previously healthy, i.e., without any known underlying immunodeficiency or disorder. “Invasive disease” was defined as necrotizing enterocolitis, urinary tract infection, bacteremia, and/or meningitis, since this definition had been used in the previous Cronobacter literature [e.g., Ref. (19, 20)]. My first study was an epidemiologic characterization of all reported cases of invasive E. sakazakii or Cronobacter infection occurring in previously healthy children in 1958–2010. The second was a statistical comparison of cases occurring in 1958–2003 to those occurring in 2004–2010, i.e., before and after the dissemination of the FDA’s “2002 Letter/Revised Letter to Health Care Professionals,” in which the FDA recommended that “PIFs not be used in neonatal intensive care settings unless there is no alternative available” (31). As noted above, the FDA did not provide any guidance or recommendations to caretakers of infants living at home, even though cases had occurred in home settings by the time of the FDA’s Letter to Healthcare Professionals. The third study was a cross-sectional cost comparison of milk-based and soy-based: (a) PIF, (b) commercially sterile RTF, and (c) commercially sterile concentrates (which are to be diluted one-to-one with water prior to feeding). This study included data on a variety of brands of formula. It was done in response to statements made by the FDA, manufacturers, and others, in which they acknowledge that properly manufactured RTF cannot infect infants but insist that using RTF is not an option because it is significantly more expensive than PIF. These spokespersons provide only anecdotal statements and outdated references for their opinion [see, for example, Ref. (47)]. Therefore, my third study was intended to provide substantive, current data rather than anecdotes.
There were several striking findings in the first analytic study. First, for the entire time period examined, only one previously healthy infant who became invasively infected was older than 2 months of age at the time of symptom onset. Second, the majority of infected infants had meningitis and a third had documented bacteremia (Table 1). Third, 90% of invasively infected infants had received PIF at some point in time prior to onset of symptoms; this did not differ significantly between the two periods (Table 2). Only 4% of infants with invasive Cronobacter infection, worldwide, had been exclusively breastfed (EBF) (Figure 1). None of these breastfed infants resided in the U.S. but, for comparison: 46% of all U.S. neonates are EBF (48). Thus the proportion breastfed is much lower than would be expected, even if all the EBF infants had lived in the U.S.
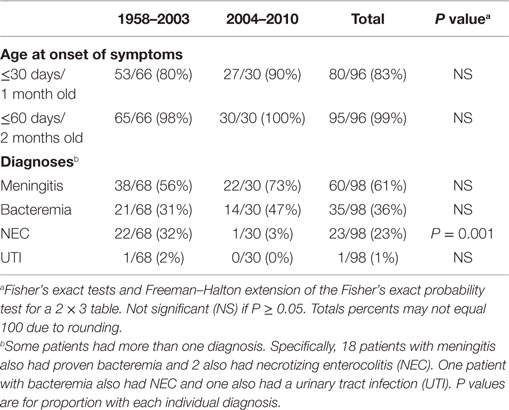
Table 1. Age and diagnoses of all reported infants without underlying disorders, invasively infected with Cronobacter, by time period.
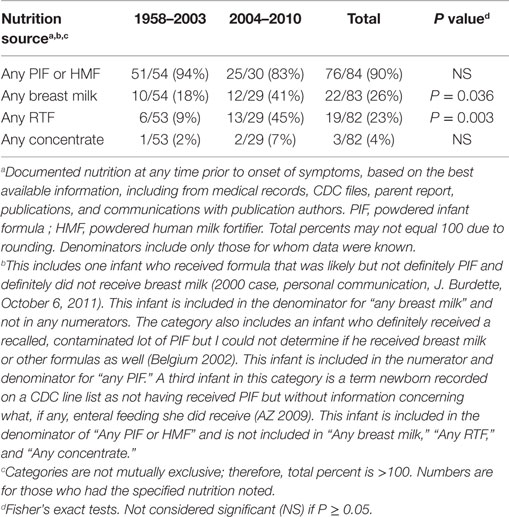
Table 2. Number and proportion of reported infants without underlying disorders, invasively infected with Cronobacter, by time period and nutrition source.
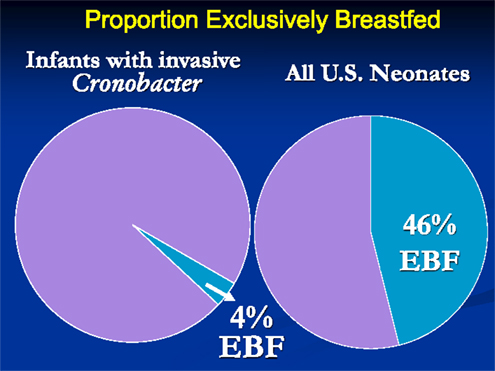
Figure 1. Proportion of infants with invasive Cronobacter infection and proportion of all U.S. neonates who are exclusively breastfed.
The second set of analyses documented several significant differences between cases occurring in 2004–2010 compared to 1958–2003. The proportion with necrotizing enterocolitis, a disease that occurs in hospitalized premature infants, was significantly lower in 2004–2010 (Table 1). This decrease is consistent with the following findings: in 2004–2010, a significantly higher proportion of invasively infected infants were full term, normal birthweight, and living at home when symptoms began (Table 3). Also, in 2004–2010, a significantly higher proportion of infected infants had been given multiple types of nutrition prior to onset of symptoms (Table 2). This was largely due to their receiving RTF in their birth hospital and being switched to PIF at home (data not shown). This combination of findings supports that invasive infections can occur in healthy newborns and the proportion of cases in this group increased in the years following the FDA recommendation to avoid feeding PIF to hospitalized infants.
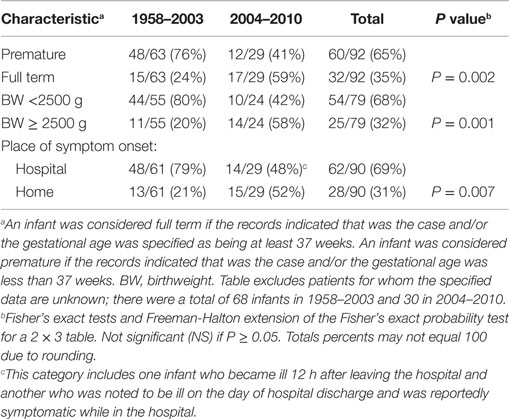
Table 3. Characteristics of all reported infants without underlying disorders, invasively infected with Cronobacter, by time period.
The third study addressed the relative costs two alternative forms of infant formula, RTF and concentrates, compared to PIF. A cross-sectional survey was done in September 2011. It compared on-line-prices for six formulas commonly used from birth to 6 or 12 months of age and available in PIF, RTF, and concentrate formulations. Prices varied relatively widely within and among brands, products, formulations, and stores. The approximate daily (four ounces of formula every 4 h) costs for feeding a neonate the least expensive formula of each type were calculated and compared. Details are provided in the footnotes to Table 4. The results support that if an infant’s caretaker was not brand-committed, the cost of RTF did not differ meaningfully from the cost of PIF. Specifically, milk-based RTF costs only 84 cents more a day than milk-based PIF and soy-based RTF costs 24 cents less a day than soy-based PIF (Table 4).
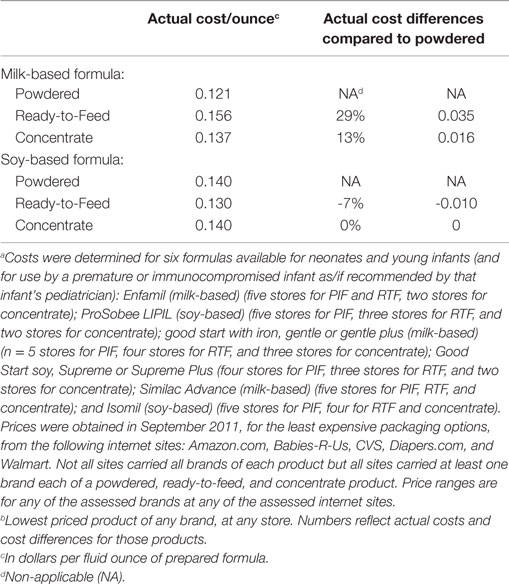
Table 4. Per ounce price and price difference, by type and form of infant formula, comparing least-expensive available product in stated categorya,b.
The following consumer recommendation appeared on CDC’s Cronobacter webpage in December 2012: “If your baby gets formula, choose infant formula sold in liquid form, especially when your baby is a newborn or very young”4. Unfortunately, CDC’s one epidemiologic contribution to the recent Cronobacter literature, a 2014 article by Patrick et al. published in CDC’s journal Emerging Infectious Diseases, not as insightful (49). It is seriously flawed and misleading in a number of respects but this editorial review will focus on a few key issues.
The 2014 Patrick/CDC study examined data collected through CDC’s FoodNet Program [Foodborne Diseases Active Surveillance Network (FoodNet)] (50), an excellent laboratory isolate surveillance program but one that does not collect detailed patient information. The FoodNet data support what was previously known: with rare exception, clinical Cronobacter isolates come from the very young and the very old. Despite that bimodal distribution, Patrick et al. stated that the median age for Cronobacter disease is 59 years, a number that has been widely quoted by the lay press and formula manufacturers’ representatives. As the authors surely knew, a median misrepresents a bimodal distribution. In the case of Cronobacter, it implies that middle-aged people are at risk of Cronobacter infection. That is precisely not the case – but it is a “statistic” which the CDC authors provided in their article and which PIF manufacturers’ lawyers can now use as evidence that late-middle-aged adults may transmit Cronobacter to their grandchildren.
Cronobacter infections are rare in both infants and adults but the similarity stops there. An equally egregious flaw in this often-cited article is that it obfuscates at least three distinct differences between Cronobacter infections in children and Cronobacter infections in adults. First, evidence increasingly supports that they are caused by different Cronobacter species and strains and the sources of these strains are different. Evidence to date strongly indicates that PIF is the primary source of invasive infections in young infants. The sources of infection/colonization in adults are unknown but these infections are likely transmitted nosocomially (i.e., through exposures related to their hospitalization and hospital care) and/or through contaminated nutrition these adults are receiving. Second, Cronobacter infections occur in previously healthy infants. Virtually all Cronobacter infection and colonization of adults occur in immunocompromised, elderly, and/or hospitalized adults who are ill with more significant infections and/or illnesses. Third, in infants, Cronobacter can cause meningitis, sepsis, and death. In adults, Cronobacter is an opportunistic organism that merely colonizes the patient or causes symptoms that are insignificant in comparison to that adult’s underlying medical problem and other infections. The authors compare apples to oranges when they state, “the highest rates occurred among persons ≥80 years of age, followed by persons 70–79 years of age and infants” (49).
Thanks to this article and CDC’s related website verbiage (51), the press, other authors, and public health agencies now parrot the phrase “Cronobacter infection occurs more frequently in adults than children.” This ignores the elephant-in-the-room, an elephant that is reflected in the very name of the genus: i.e., Cronobacter was named after Cronos, the Greek god who devoured his own children (Figure 2). Patrick et al.’s final recommendation concerning Cronobacter disease in adults is that “routine, systematic surveillance and special studies will be essential for understanding these findings, identifying reservoirs of infection and vehicles of transmission, and developing effective prevention and control measures.” Cronobacter is a rare infection; realistically, special studies would take decades and be expensive at a time when research dollars are scarce.
Rather than start from scratch, it would behoove CDC to identify and consolidate the many Cronobacter isolates it has obtained over the last 60+ years. One would expect that these isolates and their associated epidemiologic data have been safely stored. Patrick’s stated goals would be better and more efficiently served by testing those isolates using Baldwin’s MLST scheme and Tall’s pan genomic DNA microarray platform and analyzing the results.
U.S. Centers for Disease Control and Prevention appears to have thus far ignored the recent Cronobacter research related to strain typing and contamination distribution in PIF products. What, then, has been the response of other health agencies? This Frontiers Topic editorial review will end with an examination of the related current policies of three institutions: the FDA, the U.S. Special Supplemental Nutrition Program for Women, Infants, and Children (WIC), and the WHO.
By legal mandate, the FDA must oversee manufacturers of infant formulas and help ensure that these products are safe and support healthy growth in infants who consume them. On June 9, 2014 it finalized a rule that set standards for manufacturers of infant formula. Manufacturers had to comply with that rule as of September 8, 2014 (47). The new rules have, in general, eased FDA oversight of PIF manufacturing but the FDA has updated its protocol for testing end-products, including changing its statistical sampling plan to be in accordance with Dr. Jongenburger’s recommendations (44). The FDA notes that, with this sampling plan, “when the production aggregate (FDA’s new term for a lot or batch) is sampled and the composite is tested, if the pathogen is not detected, the manufacturer has a 95% level of confidence that there would be <1 CFU Cronobacter spp. in 100 g powder” (52). That, of course, continues to ignore the practical significance of Cronobacter’s clumping. Also, it merits noting that a 95% level of confidence means that, assuming the confidence interval/hypothesis test is one-sided, there is up to approximately a 5% chance of a type I error, i.e., that a production aggregate testing negative with FDA’s proposed testing scheme might actually contain ≥1 CFU Cronobacter spp. in 100 g powder but be released to market. This probability is indeed very low but since thousands of production aggregates are released to market each year, this risk is not inconsequential. If a released aggregate contains one or more contaminating clumps or clusters of a strain or sequence type that can produce invasive infection (e.g., ST4 or a virulent serogroup) and these clumps are fed to a young, susceptible infant, the consequences can be devastating.
Despite data to the contrary, on its “Consumer Update Page,” the FDA places RTF third on its list of three formula options and provides the following description of that option: “Ready-to-feed – the most expensive form of formula that requires no mixing” (47). Following the 2011 Cronobacter cases, the WIC Program reviewed its policies and decided to continue to preferentially support PIF over RTF, even in the first 2 months of life. WIC provides Federal grants to States for supplemental foods for approximately half of all U.S. infants. Parents on WIC cannot comparison shop; they must take what WIC provides them. WIC purchases infant formula at a discount, through a state-by-state exclusive contract bidding process, and provides that formula to non-breastfeeding or breast milk-supplementing mothers. WIC provides PIF to these mothers (53) and WIC programs instructs physicians to prescribe RTF only under certain, specific conditions, for their clients on WIC [e.g., Ref. (54)5].
The World Health Organization’s current recommendations related to Cronobacter are that PIF be reconstituted with water that has been boiled and then cooled to 70°C, to inactivate any Cronobacter contaminating the PIF (55, 56). There are at least three problems with this recommendation, one of which was highlighted in Dr. Parra Flores’ Frontiers Topic manuscript (35). First, it is unlikely that this recommendation will be accurately followed in a home setting. Few caretakers will routinely measure the temperature of boiled water before they mix it with PIF. The time required will vary with ambient temperature. If the water is cooled too long, the previously boiled, now warm water will incubate contaminating Cronobacter, not kill it. Second, some organizations disagree with this recommendation because of concerns that it may destroy heat-sensitive nutrients, change some formulas’ physical characteristics, cannot be accurately followed in a real-world setting, and/or because the hot water could injure personnel preparing the formula (21). Third, the rehydration instructions on some PIF labels do not comply with WHO guidelines.
Conclusion
In an ideal world, medical, laboratory, and epidemiologic researchers and health agencies work hand-in-hand to further scientific knowledge and apply that knowledge to benefit society. In the past, in regard to Cronobacter, this interaction was more or less ideal and led to significant progress. In recent years, laboratorians have been doing more than their fair share. It is time for epidemiologists and health agencies to view this issue with fresh eyes and step up to the plate.
Author Contributions
JJ was responsible for the concept, literature review, and manuscript preparation.
Conflict of Interest Statement
The author has not received any funding for this study. She has acted as an expert witness in legal cases related to Cronobacter infection; therefore, if this study leads to preventive action, it is, in a practical sense, contrary to her personal financial interests.
Footnotes
- ^ftp://ftp.fao.org/docrep/fao/009/a0707e/a0707e00.pdf and http://www.fstools.org/esakmodel/ESAKRAModelWizard.aspx
- ^http://ec.europa.eu/food/food/rapidalert/reports/week8-2009_en.pdf
- ^http://pubmlst.org/Cronobacter/
- ^http://www.cdc.gov/cronobacter/investigation.html#advice
- ^https://www1.maine.gov/dhhs/mecdc/health-equity/wic/documents/health-RTFricestarchformulas.pdf
References
1. Farmer JJ III, Asbury MA, Hickman FW, Brenner DJ, The Enterobacteriaceae Study Group (USA). Enterobacter sakazakii: a new species of “Enterobacteriaceae” isolated from clinical specimens. Int J Syst Bacteriol (1980) 30:569–84. doi:10.1099/00207713-30-3-569
2. Iversen C, Mullane N, McCardell B, Tall BD, Lehner A, Fanning S, et al. Cronobacter gen. nov., a new genus to accommodate the biogroups of Enterobacter sakazakii, and proposal of Cronobacter sakazakii gen. nov., comb. nov., Cronobacter malonaticus sp. nov., Cronobacter turicensis sp. nov., Cronobacter muytjensii sp. nov., Cronobacter dublinensis sp. nov., Cronobacter genomospecies 1, and of three subspecies, Cronobacter dublinensis subsp. dublinensis subsp. nov., Cronobacter dublinensis subsp. lausannensis subsp. nov. and Cronobacter dublinensis subsp. lactaridi subsp. nov. Int J Syst Evol Microbiol (2008) 58:1442–7. doi:10.1099/ijs.0.65577-0
3. Iversen C, Lane M, Forsythe S. The growth profile, thermotolerance and biofilm formation of Enterobacter sakazakii grown in infant formula milk. Lett Appl Microbiol (2004) 38:378–82. doi:10.1111/j.1472-765X.2004.01507.x
4. Kucerova E, Joseph S, Forsythe S. The Cronobacter genus: ubiquity and diversity. Qual Assur Saf Crops Foods (2011) 3:104–22. doi:10.1111/j.1757-837X.2011.00104.x
5. Lehner A, Riedel K, Eberl L, Breeuwer P, Diep B, Stephan R. Biofilm formation, extracellular polysaccharide production, and cell-to-cell signaling in various Enterobacter sakazakii strains: aspects promoting environmental persistence. J Food Prot (2005) 68:2287–94.
6. Palcich G, de Moraes Gillio C, Aragon-Alegro LC, Pagotto FJ, Farber JM, Landgraf M, et al. Enterobacter sakazakii in dried infant formulas and milk kitchens of maternity wards in São Paulo, Brazil. J Food Prot (2009) 72:37–42.
7. Lenati RF, O’Connor DL, Hébert KC, Farber JM, Pagotto FJ. Growth and survival of Enterobacter sakazakii in human breast milk with and without fortifiers as compared to powdered infant formula. Int J Food Microbiol (2007) 22:171–9. doi:10.1016/j.ijfoodmicro.2007.11.084
8. Kandhai MC, Reij MW, Grognou C, van Schothorst M, Gorris LGM, Zwietering MH. Effects of preculturing conditions on lag time and specific growth rate of Enterobacter sakazakii in reconstituted powdered infant formula. Appl Environ Microbiol (2006) 72:2721–9. doi:10.1128/AEM.72.4.2721-2729.2006
9. Pagotto FJ, Nazarowec-White M, Bidawid S, Farber JM. Enterobacter sakazakii: infectivity and enterotoxin production in vitro and in vivo. J Food Prot (2003) 66:370–5.
10. Rolle U, Nemeth L, Puri P. Nitrergic innervation of the normal gut and in motility disorders of childhood. J Pediatr Surg (2002) 37:551–67. doi:10.1053/jpsu.2002.31610
11. Newburg DS, Walker WA. Protection of the neonate by the innate immune system of developing gut and of human milk. Pediatr Res (2007) 61:2–8. doi:10.1203/01.pdr.0000250274.68571.18
12. Forchielli ML, Walker WA. The effect of protective nutrients on mucosal defense in the immature intestine. Acta Paediatr (2005) 94(Suppl 449):74–83. doi:10.1111/j.1651-2227.2005.tb02159.x
13. Pettiford JN, Jason J, Nwanyanwu OC, Archibald LK, Kazembe PN, Dobbie H, et al. Age-related differences in cell-specific cytokine production by acutely ill Malawian patients. Clin Exp Immunol (2002) 128:110–7. doi:10.1046/j.1365-2249.2002.01813.x
14. Iversen C, Forsythe S. Isolation of Enterobacter sakazakii and other Enterobacteriaceae from powdered infant formula milk and related products. Food Microbiol (2004) 21:771–6. doi:10.1016/j.fm.2004.01.009
15. Friedemann M. Enterobacter sakazakii in food and beverages other than infant formula and milk powder. Int J Food Microbiol (2007) 116:1–10. doi:10.1016/j.ijfoodmicro.2006.12.018
16. Kandhai MC, Reij MW, Gorris LG, Guillaume-Gentil O, van Schothorst M. Occurrence of Enterobacter sakazakii in food production environments and households. Lancet (2004) 363:39–40. doi:10.1016/S0140-6736(03)15169-0
17. Farmer JJ III. My 40-year history with Cronobacter/Enterobacter sakazakii – lessons learned, myths debunked, and recommendations. Front Pediatr (2015) 3:84. doi:10.3389/fped.2015.00084
18. Urmenyi A, Franklin A. Neonatal death from pigmented coliform infection. Lancet (1961) 1(7172):313–5. doi:10.1016/S0140-6736(61)91481-7
19. Drudy D, Mullane NR, Quinn T, Wall PG, Fanning S. Enterobacter sakazakii: an emerging pathogen in powdered infant formula. Clin Infect Dis (2006) 42(7):996–1002. doi:10.1086/501019
20. Bowen AB, Braden CR. Invasive Enterobacter sakazakii disease in infants. Emerg Infect Dis (2006) 12(8):1185–9. doi:10.3201/eid1208.051509
21. Jason J. Prevention of invasive Cronobacter infections in young infants fed powdered infant formulas. Pediatrics (2012) 130(5):e1076–84. doi:10.1542/peds.2011-3855
22. Yan Q, Fanning S. Strategies for the identification and tracking of Cronobacter species: an opportunistic pathogen of concern to neonatal health. Front Pediatr (2015) 3:38. doi:10.3389/fped.2015.00038
23. Centers for Disease Control and Prevention (CDC). Enterobacter sakazakii infections associated with the use of powdered infant formula – Tennessee, 2001. MMWR Morb Mortal Wkly Rep (2002) 51:297–300.
24. Simmons BP, Gelfand MS, Haas M, Metts L, Ferguson J. Enterobacter sakazakii infections in neonates associated with intrinsic contamination of a powdered infant formula. Infect Control Hosp Epidemiol (1989) 10:398–401. doi:10.2307/30144207
25. van Acker J, de Smet F, Muyldermans G, Bougatef A, Naessens A, Lauwers S. Outbreak of necrotizing enterocolitis associated with Enterobacter sakazakii in powdered milk formula. J Clin Microbiol (2001) 39:293–7. doi:10.1128/JCM.39.1.293-297.2001
26. Muytjens HL, Zanen HC, Sonderkamp HJ, Kollee LA, Wachsmuth IK, Farmer JJ. Analysis of eight cases of neonatal meningitis and sepsis due to Enterobacter sakazakii. J Clin Microbiol (1988) 18:115–20.
27. Muytjens HL, Roelofs-Willemse H, Jaspar GH. Quality of powdered substitutes for breast milk with regard to members of the family Enterobacteriaceae. J Clin Microbiol (1983) 26:743–6.
28. Nazarowec-White M, Farber JM. Incidence, survival, and growth of Enterobacter sakazakii in infant formula. J Food Prot (1997) 60:226–30.
29. Zink DL. FDA Field Survey of Powdered Formula Manufacturing. U.S. FDA Document Obtained Through Freedom of Information Act. US Freedom of Information Act (2011).
30. Food Advisory Committee (FAC)/FDA. Transcript of the Meeting of the Contaminants and Natural Toxicants Subcommittee of the Food Advisory Committee, FDA. (2003). Available from: http://www.fda.gov/ohrms/dockets/ac/03/transcripts/3939t1.htm
31. FDA. Health Professionals Letter on Enterobacter sakazakii Infections Associated with Use of Powdered (Dry) Infant Formulas in Neonatal Intensive Care Units. 2002. (2002). Formerly Available from: http://www.fda.gov/Food/FoodSafety/Product-SpecificInformation/InfantFormula/AlertsSafetyInformation/ucm111299.htm
32. CDC and the U.S. Food and Drug Administration (FDA). Infant Feeding Practices Study II. (2008). Available from: http://www.cdc.gov/ifps/results/index.htm
33. Food and Agriculture Organization of the United Nations/World Health Organization (FAO/WHO). (0000) Enterobacter sakazakii and Other Microorganisms in Powdered Infant Formula. Meeting report. [FAO/WHO, Geneva, 2004 February 2–5; Microbiological Risk Assessment Series, No. 6, ISBN 92 4 156262 5 (WHO)]; Enterobacter sakazakii in Powdered Infant Formula (INFOSAN Information Note No. 1/2005, 2005 January 13); Update of the Expert Meeting, February 23rd, 2007 (The Food and Agriculture Organization of the United Nations and the World Health Organization). Available from: http://www.fao.org/3/a-y5502e.pdf
34. FAO/WHO (2008). Enterobacter sakazakii (Cronobacter spp.) in Powdered Follow-Up Formulae, Annex 1. (Meeting held in Washington DC, USA, on 2008 July 15–18; Microbiological Risk Assessment Series No. 15, Rome. 90pp). Available from: http://www.fao.org/3/a-i0453e.pdf
35. Parra-Flores J, Rodriguez A, Riffo F, Arvizu-Medrano SM, Arias-Rios EV, Aguirre J. Investigation on the factors affecting Cronobacter sakazakii contamination levels in reconstituted powdered infant formula. Front Pediatr (2015) 3:72. doi:10.3389/fped.2015.00072
36. FAO/WHO. Enterobacter sakazakii and Salmonella in Powdered Infant Formula. Meeting report. [FAO/WHO, Geneva, 2006 January 16–20; Microbiological Risk Assessment Series, No. 10, ISBN 92-5-105574-2(WHO)]. (2006). Available from: http://www.who.int/foodsafety/publications/micro/mra10/en/
37. Friedemann M. Epidemiology off invasive neonatal Cronobacter (Enterobacter sakazakii) infections. Eur J Clin Microbiol Infect Dis (2009) 28:1297–304. doi:10.1007/s10096-009-0779-4
38. Kalyantanda G, Shumyak L, Archibald LK. Cronobacter species contamination of powdered infant formula and the implications for neonatal health. Front Pediatr (2015) 3:56. doi:10.3389/fped.2015.00056
39. Tall BD, Gangiredla J, Gopinath GR, Yan Q, Chase HR, Lee B, et al. Development of a custom-designed, pan genomic DNA microarray to characterize strain-level diversity among Cronobacter spp. Front Pediatr (2015) 3:36. doi:10.3389/fped.2015.00036
40. Baldwin A, Loughlin M, Caubilla-Barron J, Kucerova E, Manning G, Dowson C, et al. Multilocus sequence typing of Cronobacter sakazakii and Cronobacter malonaticus reveals stable clonal structures with clinical significance which do not correlate with biotypes. BMC Microbiol (2009) 9:223. doi:10.1186/1471-2180-9-223
41. Joseph S, Forsythe SJ. Predominance of Cronobacter sakazakii sequence type 4 in neonatal infections. Emerg Infect Dis (2011) 17(9):1713–5. doi:10.3201/eid1709.110260
42. Forsythe SJ, Dickins B, Jolley KA. Cronobacter, the emergent bacterial pathogen Enterobacter sakazakii comes of age; MLST and whole genome sequence analysis. BMC Genomics (2014) 2014(15):1121. doi:10.1186/1471-2164-15-1121
43. Tall BD, Franco AA, Jarvis KG, et al. Plasmidotyping, LPS Determination, Optical Map, PFGE and Whole Genome Sequencing Analyses of Cronobacter Isolates Associated with Community-Acquired Infantile Disease in the USA. (2012). Poster Board P2-07.
44. Jongenburger I, Reij MW, Boer EPJ, Gorris LGM, Zwietering MH. Actual distribution of Cronobacter spp. in industrial batches of powdered infant formula and consequences for performance of sampling strategies. Int J Food Microbiol (2011) 151:62–9. doi:10.1016/j.ijfoodmicro.2011.08.003
45. Richardson A, Lambert S, Smith MA. Neonatal mice as models for Cronobacter sakazakii infection in infants. J Food Prot (2009) 72:2363–7.
46. CDC. (2011) Media Statement for Immediate Release: December 30, 2011, 6 p.m.: FDA and CDC Update: Investigation of Cronobacter Bacteria Illness in Infants. [Available from: http://www.cdc.gov/media/releases/2011/s1230_Cronobacter.html]; CDC Update: Investigation of Cronobacter Infections Among Infants in the United States. 2012 January 13. [Available from: http://www.cdc.gov/foodsafety/diseases/cronobacter/investigation.html]
47. FDA. FDA Takes Final Step on Infant Formula Protection. FDA Consumer Health Information/U. S. Food and Drug Administration. (Vol. 9). (2014). Available from: http://www.fda.gov/forconsumers/consumerupdates/ucm048694.htm
48. National Center for Health Statistics (NCHS). Breastfeeding Among U.S. Children Born 2000–2008, CDC National Immunization Survey. (2015). Available from: http://www.cdc.gov/breastfeeding/data/NIS_data/index.htm
49. Patrick ME, Mahon BE, Greene SA, Rounds J, Cronquist A, Wymore K, et al. Incidence of Cronobacter spp. infections, United States, 2003-2009. Emerg Infect Dis (2014) 20(9):1520–3. doi:10.3201/eid2009.140545
50. CDC. Foodborne Diseases Active Surveillance Network (FoodNet). (2015). Available from: http://www.cdc.gov/foodnet/index.html
51. CDC. Cronobacter: People at Risk. (2015). Available from: http://www.cdc.gov/cronobacter/
52. FDA. Current Good Manufacturing Practices, Quality Control Procedures, Quality Factors, Notification Requirements, and Records and Reports, for Infant Formula A Rule by the Food and Drug Administration on 02/10/2014. (2014). Available from: https://www.federalregister.gov/articles/2014/02/10/2014-02148/current-good-manufacturing-practices-quality-control-procedures-quality-factors-notification
53. United States Department of Agriculture (USDA). FDA Takes Final Step on Infant Formula Protections. (2015). Available from: http://www.fns.usda.gov/sites/default/files/03-04-14_WIC-Food-Packages-Final-Rule.pdf
54. State of Maine. WIC Provision of Ready to Feed Formulas. (2015). Available from: https://www1.maine.gov/dhhs/mecdc/health-equity/wic/documents/health-RTFricestarchformulas.pdf.
55. FAO/WHO. Safe Preparation, Storage and Handling of Powdered Infant Formula: Guidelines. Geneva: WHO Press, World Health Organization (2007). Available from: http://www.who.int/foodsafety/publications/micro/pif_guidelines.pdf
56. FAO/WHO/Food Safety Authority of Ireland. How to Prepare Formula for Bottle Feeding at Home. (2007). Available from: http://www.who.int/foodsafety/publications/micro/PIF_Bottle_en.pdf
Keywords: Cronobacter, sakazakii, infant formula, neonatal infection, ST4
Citation: Jason J (2015) The Roles of Epidemiologists, Laboratorians, and Public Health Agencies in Preventing Invasive Cronobacter Infection. Front. Pediatr. 3:110. doi: 10.3389/fped.2015.00110
Received: 24 September 2015; Accepted: 04 December 2015;
Published: 24 December 2015
Edited by:
Zeehaida Mohamed, Universiti Sains Malaysia, MalaysiaReviewed by:
AbdelRahman Zueter, Universiti Sains Malaysia, MalaysiaNurulhasanah Othman, Universiti Sains Malaysia, Malaysia
Copyright: © 2015 Jason. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Janine Jason, jjason@post.harvard.edu
 Janine Jason
Janine Jason