- 1Department of Gastroenterology and Hepatology, The Fourth Hospital of Hebei Medical University, Shijiazhuang, China
- 2Department of Rheumatology, The Fourth Hospital of Hebei Medical University, Shijiazhuang, China
- 3Department of Thoracic Surgery, The Fourth Hospital of Hebei Medical University, Shijiazhuang, China
Background: The impact of age on the efficacy and safety of immunotherapy remains controversial. The previous studies simply classified patients into younger and older groups, which might not reflect the real impact of young age on immunotherapy efficacy. The current study aimed to explore the efficacy and safety of immune checkpoint inhibitor (ICI) combined therapy in young (aged 18–44 years), middle-aged (aged 45–65 years), and old (aged >65 years) patients with metastatic gastrointestinal cancers (GICs), and further determine the role of immunotherapy in young patients.
Methods: Patients with metastatic GIC including esophageal cancer (EC), gastric cancer (GC), hepatocellular cancer (HCC), and biliary tract cancer (BTC) who received ICI combination therapy were enrolled, divided into young (aged 18–44 years), middle-aged (aged 45–65 years), and old (aged >65 years) groups. The clinical characteristics, objective response rate (ORR), disease control rate (DCR), progression-free survival (PFS), overall survival (OS), and immune-related adverse events (irAEs) were compared among three groups.
Results: A total of 254 patients were finally included, with 18, 139, and 97 cases in the young (aged 18–44 years), middle-aged (aged 45–65 years), and old (aged >65 years) groups, respectively. Compared to middle-aged and old patients, young patients had lower DCR (all p < 0.05) and also had inferior PFS (p < 0.001) and OS (p = 0.017). The multivariate analyses showed that young age was an independent prognostic factor for PFS [hazard ratio (HR) 3.474, 95% confidence interval (CI) 1.962–6.150, p < 0.001] and OS (HR 2.740, 95% CI 1.348–5.570, p = 0.005). Subsequent safety analyses referring to irAEs demonstrated no significant differences for distribution frequency among each age group (all p > 0.05), whereas patients with irAEs displayed better DCR (p = 0.035) and PFS (p = 0.037).
Conclusion: Younger GIC patients (aged 18–44 years) showed poor efficacy for ICI combined therapy, and irAEs could be used as a clinical biomarker to predict ICI efficacy in metastatic GIC patients.
Introduction
Gastrointestinal cancers (GIC), mostly composed of esophageal cancer (EC), gastric cancer (GC), colorectal cancer (CRC), pancreatic cancer (PC), hepatocellular cancer (HCC), and biliary tract cancer (BTC), account for 26% of global cancer incidence and 35% of all cancer-related deaths, which makes them one of the most common groups of malignancy worldwide (1). A large portion of patients have unresectable or metastatic disease at the time of diagnosis due to the late detection and high heterogeneity of these malignancies. Traditional chemotherapy, radiotherapy, and targeted therapy have been disappointing with a dismal 5-year survival in advanced stage disease (2). Over the last few decades, immune checkpoint inhibitors (ICIs) against programmed death receptor 1 (PD-1), programmed death receptor ligand 1 (PD-L1), and cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) have emerged as a revolutionary option for cancer treatment. However, unlike in lung cancer or melanoma, the response rates to immunotherapy in GIC are relatively low (3). More recently, ICIs plus other therapies like other ICIs, chemotherapy, radiotherapy, and targeted therapy have been shown to synergistically promote the efficacy of ICI monotherapy in GIC patients, which has been confirmed in several randomized controlled trials (RCTs) and systematic reviews (4–6). However, there is a lack of knowledge about predicting response to combining immunotherapies of GIC patients.
Currently, cancer is classically understood as a “disease of aging”, with a significantly higher incidence in patients aged ≥65 years (7). Compared with younger people, the immune system of older people can undergo a remodeling process during aging, which involves immune dysregulation in both cellular and humoral responses (8). It is speculated that older patients seem to obtain limited benefits from ICI treatment compared to younger patients. Some reports have demonstrated that ICI therapy is less effective in older people than in younger people (9, 10). However, a recent study found that young patients showed a worse response to immunotherapy than old patients. A further study reported that regulatory T cells (Tregs) are specifically increased within the tumor microenvironment of young patients. CD8+ T cells, which are the primarily activated cell type by anti-PD-1 checkpoint targeting as a tumor cell killer, are also decreased in melanoma tumors from younger patients (11). Meanwhile, many studies did not find ICI efficacy difference between young and elderly patients (12–14). Obviously, the definitions of young patients were quite inconsistent in previous studies and most studies simply classified patients into younger and older groups, which may not reflect the real impact of young age on immunotherapy efficacy. Cheng et al. indicated that age-specific impact on GC survival was possibly in a V-shaped distribution, i.e., the middle-aged subgroups showed better prognosis than the younger and oldest subgroups (15). However, it is not clear whether age will affect the immunotherapy efficacy like this pattern.
Therefore, in the present study, we divided GIC patients into three subgroups according to age of diagnosis: young (aged 18–44 years), middle-aged (aged 45–65 years), and old (aged >65 years), asutilized by previous studies (16–18), to compare the immunotherapy efficacy and safety of young age with that of middle age and old age, respectively, and hoped to further determine the role of immunotherapy in young GIC patients.
Methods
Patient screening and data collection
This study retrospectively analyzed the medical data of patients with EC, GC, HCC, and BTC who received ICI combination therapy in the Fourth Hospital of Hebei Medical University from November 2018 to December 2021. Inclusion criteria were as follows (1): imaging confirmed as metastatic GIC before treatment initiation; (2) patients who received ICI combination therapy; (3) detailed and complete clinical data; and (4) no relevant infection or acute or chronic inflammatory reaction before treatment initiation. The metastatic patterns were defined as any metastatic lesion in the liver, lung, bone, peritoneum, brain, distant lymph nodes, and other distant organs. Exclusion criteria were as follows: (1) patients treated with anti-PD-1 monotherapy; (2) combined with other tumor history, infection, or blood system disease; (3) patients who gave up treatment or refused to accept assessment; and (4) patients without complete medical records and laboratory results.
Study characteristics were extracted, including age, sex, performance status (PS) score, cancer type, therapy line, treatment modality, and immune-related adverse events (irAEs). Peripheral venous blood was collected from all patients on an empty stomach within 1 week before treatment initiation. The following laboratory indexes were collected: albumin level, neutrophil-to-lymphocyte ratio (NLR), lactate dehydrogenase (LDH), and creatinine clearance rate (Ccr). The study protocol was tested and approved by the ethics committee of The Fourth Hospital of Hebei Medical University (2020KS001), and due to the retrospective nature of this study, a waiver of informed consent was applied for these analyses.
Treatment and assessment
Patients received ICI combination with chemotherapy or/and targeted therapy every 3 weeks in the clinical setting. Treatment continued until disease progression, clinical worsening, treatment-related adverse events, or patient refusal. Table S1 lists cancer type, treatment lines, number of patients in each therapeutic schedule, and types of immunotherapy drugs, targeted drugs, and chemotherapy drugs. Before starting the treatments, all patients received enhanced thoracic–abdominal–pelvic computed tomography (CT) scan. The CT image sets were retrieved and re-assessed according to the staging criteria for GIC, including EC, GC, HCC, and BTC. Treatment response was assessed with the Response Evaluation Criteria in Solid Tumours (RECIST) version 1.1 criteria (https://ctep.cancer.gov/protocolDevelopment/docs/recist_guideline.pdf#search=%22RECIST%201.1%22) and the modified RECIST 1.1 for immune-based therapeutic (iRECIST). Complete response (CR) was defined as the complete disappearance of the target lesion after chemotherapy. Partial response (PR) was defined as a reduction in the total diameter of each target lesion by 30% or more. Progressive disease (PD) was defined as at least a 20% increase in the sum of the long diameters of all target lesions and an absolute value for the increase of more than 5 mm, or the appearance of new lesions. Stable disease (SD) was defined as no change in target lesions. The objective response rate (ORR) was defined as the percentage of patients with a CR or PR among all the treated patients. The disease control rate (DCR) was defined as the percentage of patients who have achieved CR, PR, and SD. The toxicities of anti-PD-1 therapy were assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Event (NCI-CTCAE), version per protocol (https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40). Other adverse events including progression of malignancy and adverse events caused by chemotherapy drugs or targeted drugs were excluded.
Follow-up and study outcomes
Clinical information was obtained from patients’ medical records. All patients were followed up via re-hospitalization or re-examinations in the outpatient clinic or by telephone until mortality due to any reasons or loss of follow-up. Progression-free survival (PFS) was defined as the period from treatment initiation until the date of disease progression, death, or study cutoff, whichever occurred first. Overall survival (OS) was defined as time from treatment initiation to the date of death from any cause or study cutoff. The end point of follow-up was 1 May 2022 or the date of death.
Statistical analysis
All statistical analyses were performed using SPSS 24.0 software package (SPSS Inc., Chicago, IL, USA) and GraphPad Prism 9 (GraphPad Software, San Diego, CA). Qualitative variables and continuous variables were described as frequencies, percentages, mean, standard deviation, and median. Group comparison of qualitative variables was performed using Pearson’s chi-squared test or two-sided Fisher’s exact test, while continuous variables were compared with Student’s t-tests or Mann–Whitney U-test. Survival among different age groups was evaluated using the Kaplan–Meier method and log-rank tests. Univariate and multivariate Cox proportional hazards were performed to explore prognostic factors for survival. p < 0.05 was considered statistically significant, and all reported p-values are two-sided.
Results
The baseline clinical characteristics of patients
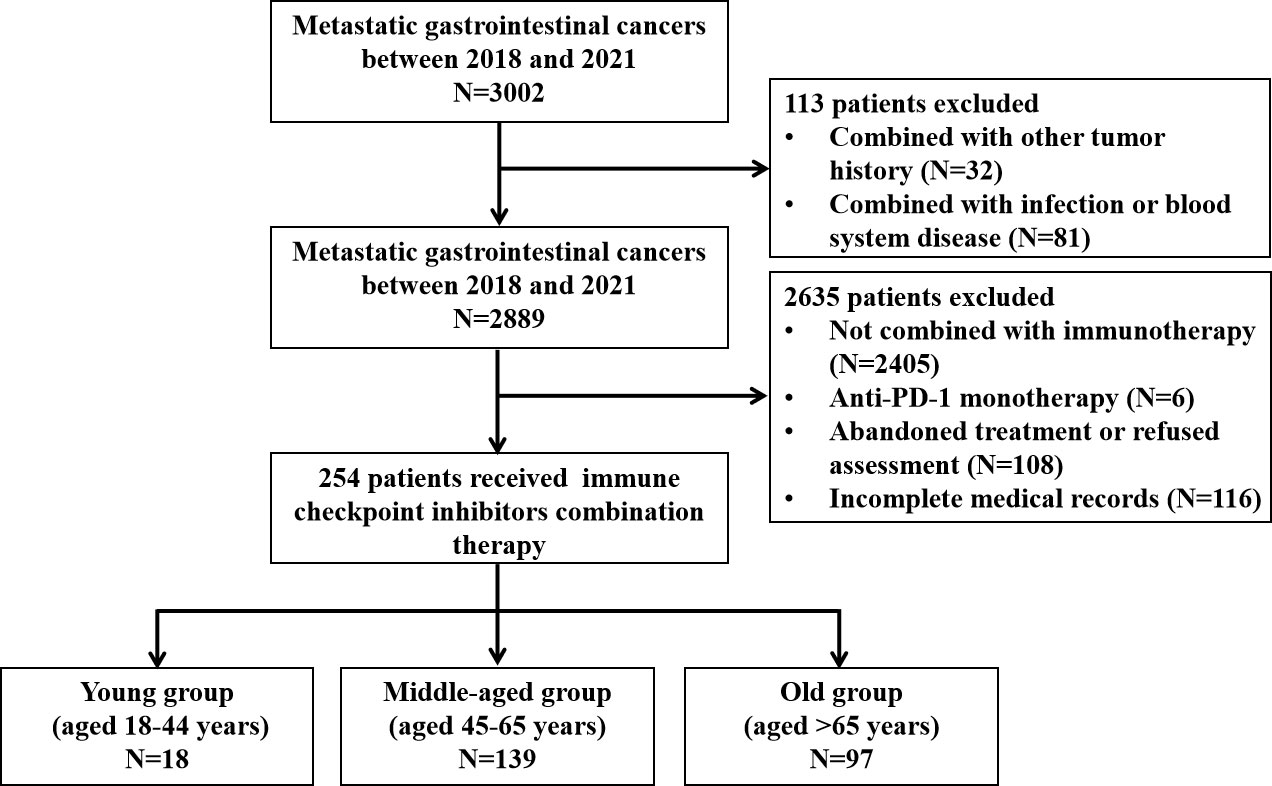
A total of 254 patients were enrolled in this study, with 18 (7.1%), 139 (54.7%), and 97 (38.2%) cases in the young (aged 18–44 years), middle-aged (aged 45–65 years), and old (aged >65 years) groups, respectively. Figure 1 shows the selection procedure of the study cohort based on inclusion and exclusion criteria. The median age of the three groups was 39 (range, 22–44 years), 58 (range, 45–65 years), and 70 (range, 66–88 years) years. Among them, there were 79 esophageal cancer patients who received anti-PD-1 antibodies in combination with chemotherapy or/and targeted therapy, 106 gastric cancer patients who received anti-PD-1 antibodies plus chemotherapy or/and targeted therapy, 51 hepatocellular cancer patients who received anti-PD-1 antibodies in combination with targeted therapy, and 18 biliary tract cancers patients who received anti-PD-1 antibodies in combination with targeted therapy or/and chemotherapy (Table S1).
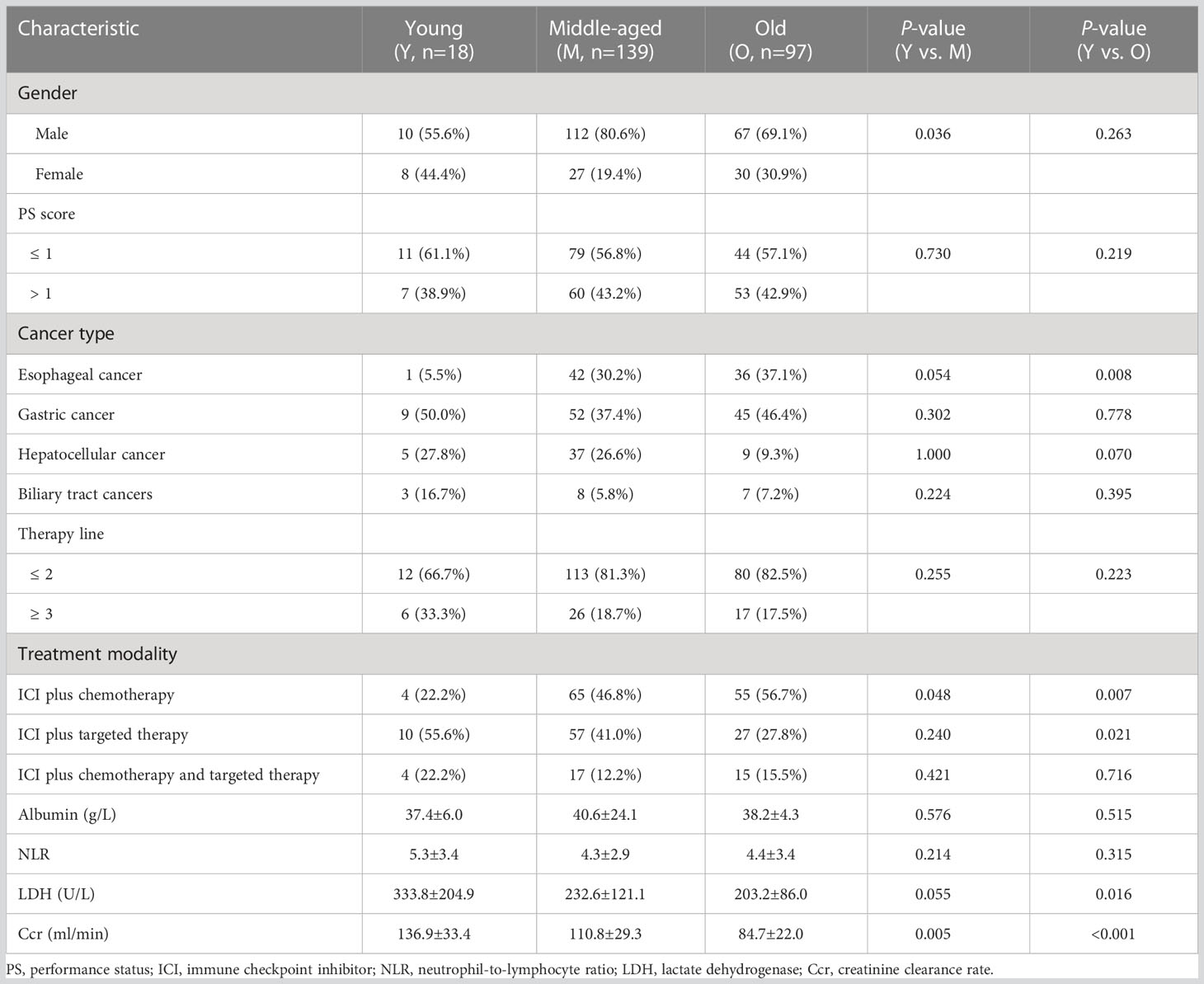
The detailed baseline clinical characteristics of different age groups are summarized in Table 1. Compared with the middle-aged and old groups, the proportions of ICI plus chemotherapy were significantly lower in the young group (all p < 0.05). Young patients also have higher baseline Ccr level than those of middle-aged and old patients (all p < 0.05). Meanwhile, young patients were different from the middle-aged group in the proportion of female patients (p = 0.036). However, the young group shared some features with the middle-aged group rather than with the old group, including the proportion of esophageal cancer (p = 0.008), ICI plus targeted therapy (p = 0.021), and the baseline LDH level (p = 0.016).
Treatment efficacy
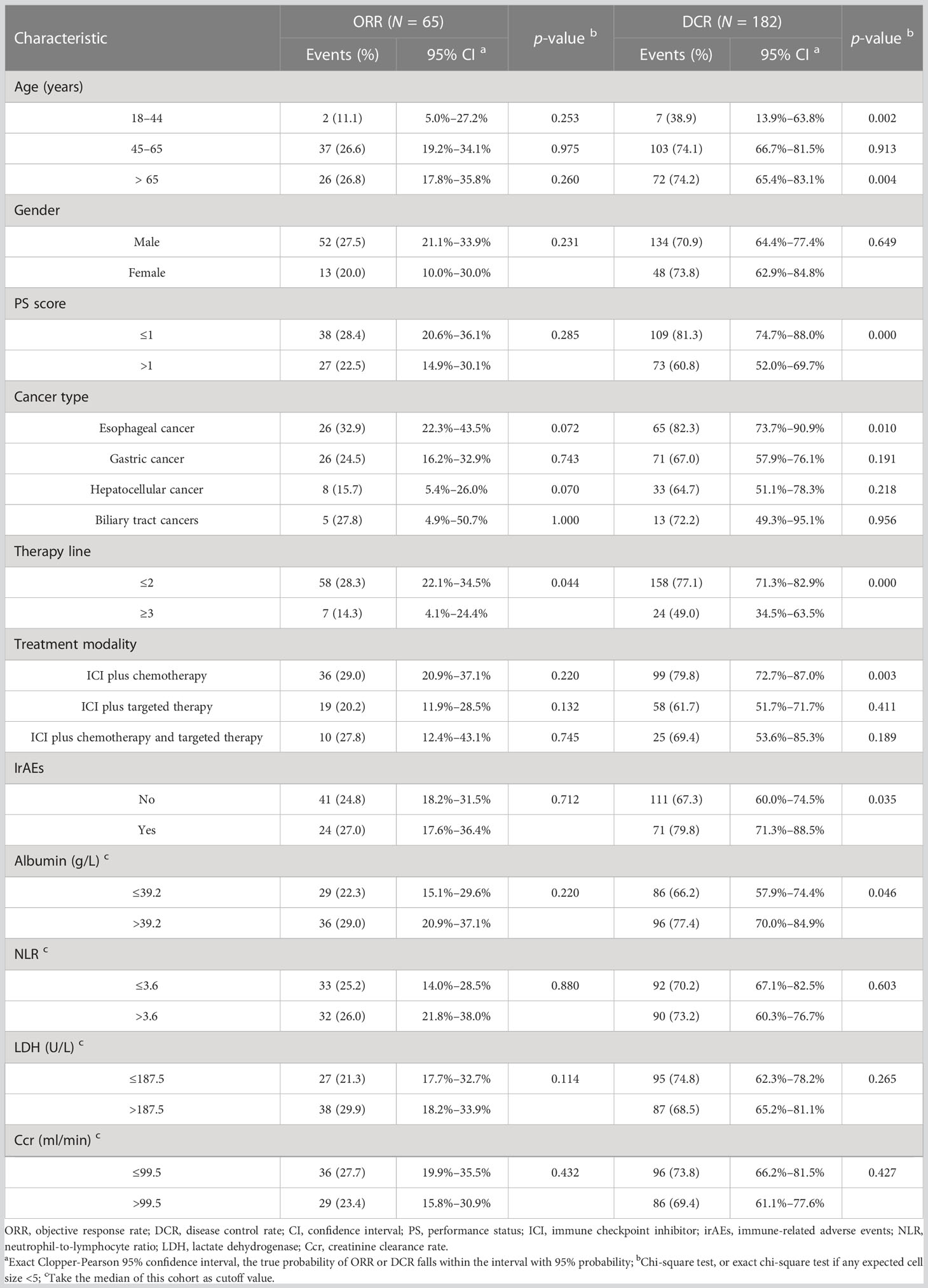
The patients with PD were all patients with icPD (immunity confirmed PD). As shown in Table 2, the ORR of the three age groups was 11.1% (2/18), 26.6% (37/139), and 26.8% (26/97), with no significant difference for each age group (all p > 0.05). Stratified by therapy line, ICI combined therapy in the first-line or second-line setting was associated with higher ORR than that in later line treatment (p = 0.044). The DCR of the three age groups was 38.9% (7/18), 74.1% (103/139), and 74.2% (72/97), respectively. We did not find a significant difference between the middle-aged and old groups (p = 0.913), but the young group showed a significantly lower DCR (all p < 0.05). We also observed that the patients with good PS, esophageal cancer, ICI combined therapy in the first-line or second-line setting, an occurrence of irAEs, and baseline albumin level > 39.2 g/L displayed higher DCR (all p < 0.05). Moreover, ICI plus chemotherapy had higher DCR than ICI combined with other therapies (p = 0.003).
Prognostic analysis
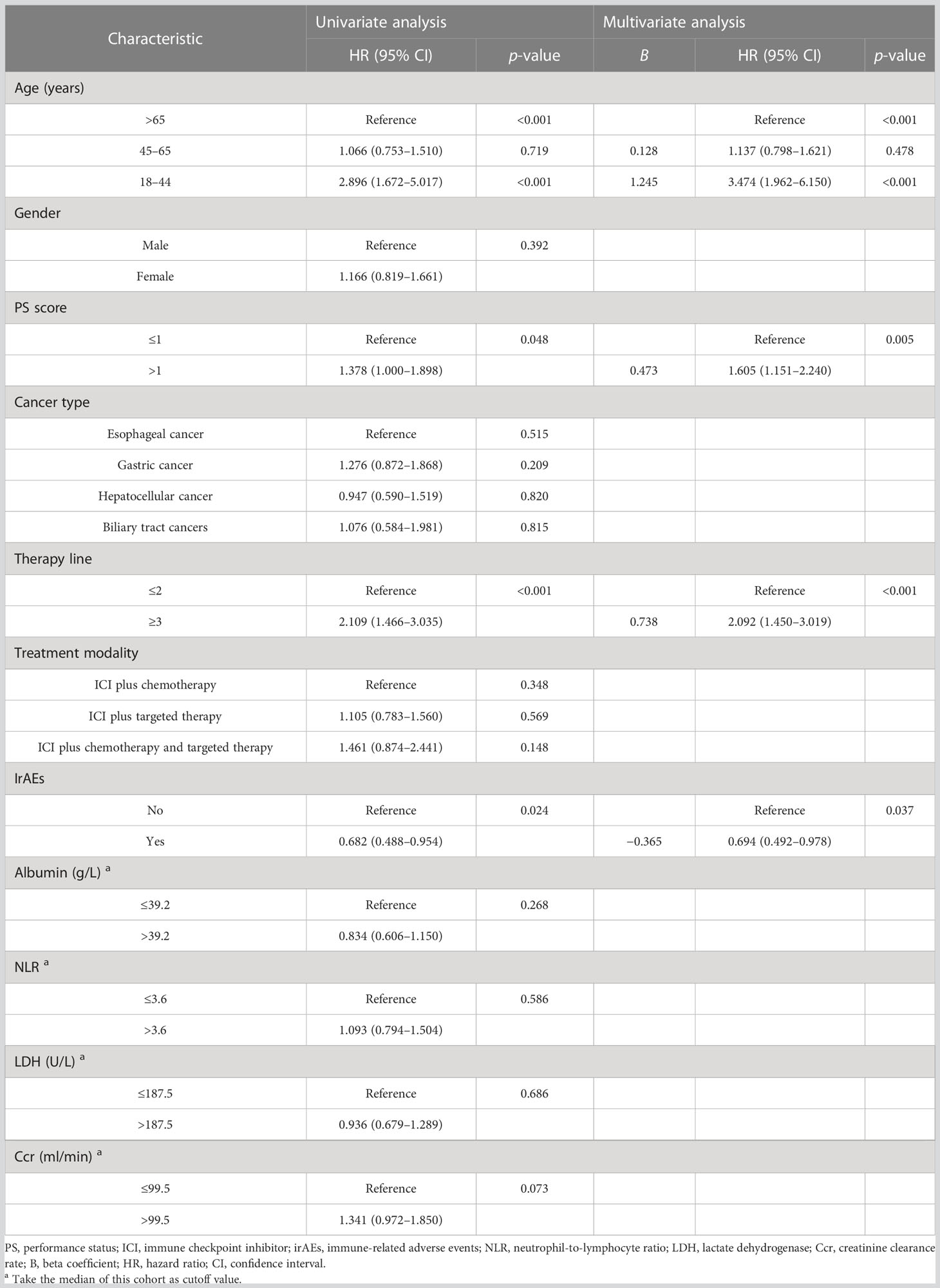
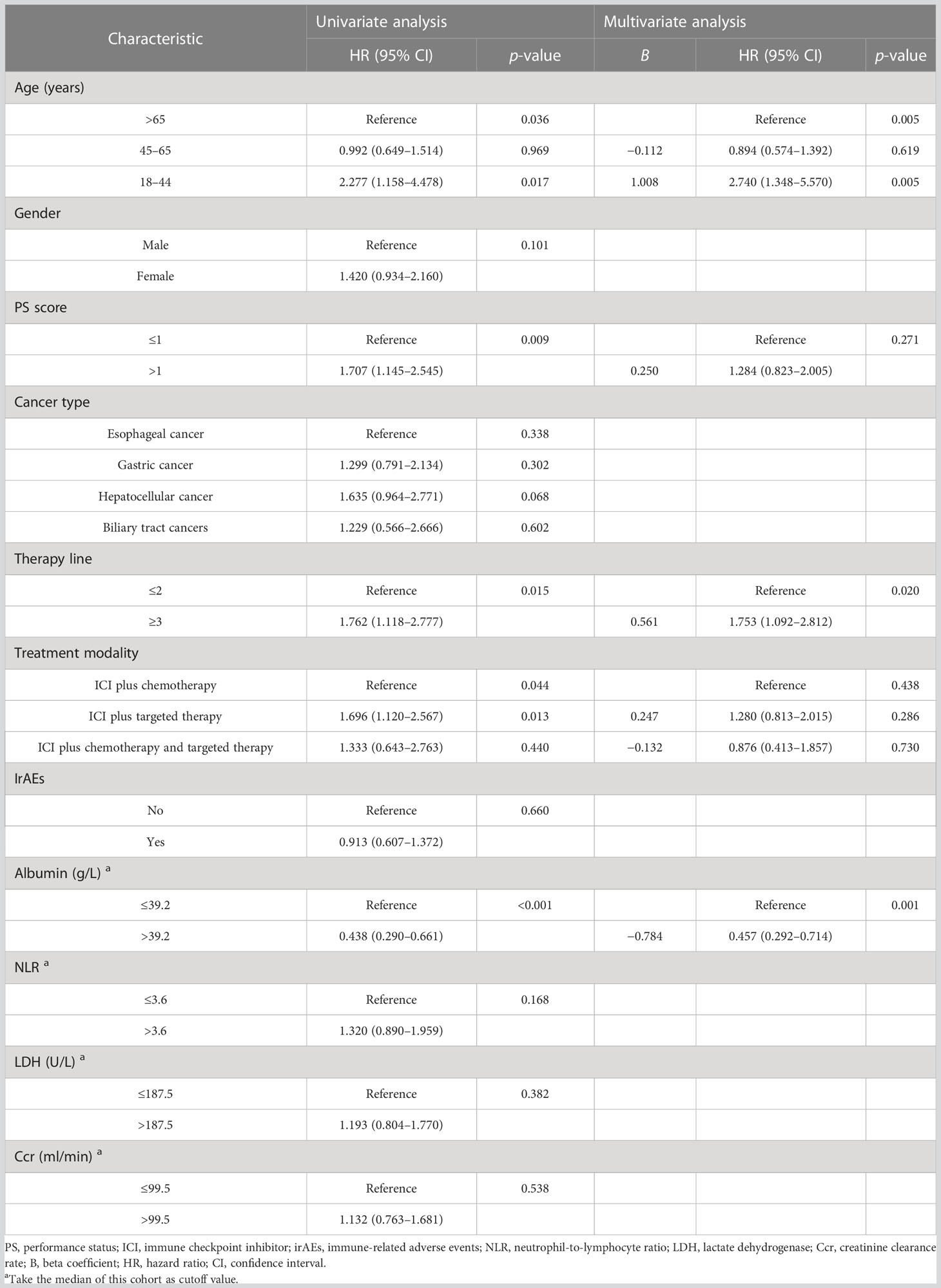
To determine the potential role of age on PFS, Kaplan–Meier plots were applied. The median PFS was 1.8, 7.3, and 9.8 months in the young, middle-aged, and old groups. We did not find significant PFS difference between the middle-aged and old groups (p = 0.719), but young patients had the worst PFS (p < 0.001) (Figure 2A). Stratified by PS score and therapy line, we found that the patients with good PS and who received ICI combination therapy in the first-line or second-line setting were associated with better PFS (all p < 0.05). Moreover, we also observed an improvement in terms of PFS in patients who developed irAEs (p = 0.024) (Figure 2B). Next, we performed multivariate analyses to identify independent prognostic factors for PFS. As the Cox-proportional hazard model in Table 3 shows, age was an independent prognostic factor for PFS, with the hazard ratio (HR) of 3.474 [95% confidence interval (CI) 1.962–6.150, p < 0.001] for those aged <45 years and 1.137 (95% CI 0.798–1.621, p = 0.478) for 45–65 years. Other independent prognostic factors for PFS were PS score (HR 1.605, 95% CI 1.151–2.240, p = 0.005), therapy line (HR 2.092, 95% CI 1.450–3.019, p < 0.001), and irAEs (HR 0.694, 95% CI 0.492–0.978, p = 0.037).
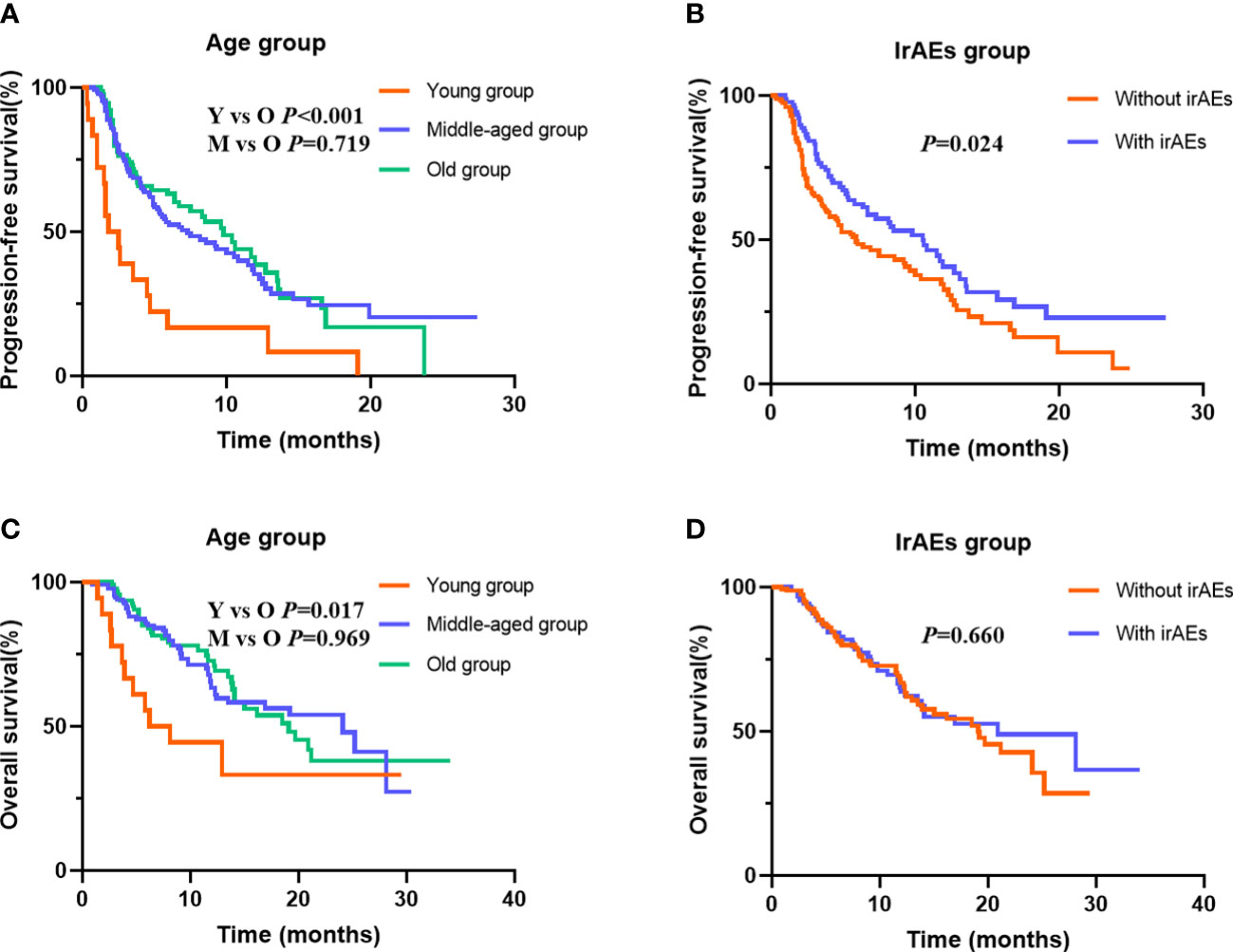
Figure 2 Kaplan–Meier curves for progression-free survival (PFS) and overall survival (OS) stratified by age and immune-related adverse events (irAEs). (A) for PFS in different age group; (B) for PFS in patients with or without irAEs; (C) for OS in different age group; (D) for OS in patients with or without irAEs.
At data cutoff, 99 (40.0%) deaths had occurred, 146 (57.5%) patients were alive, and 9 (3.5%) cases were lost to follow-up. The median OS of the three age groups was 6.2, 24.1, and 19.1 months, respectively. Figure 2C shows that old age was not inferior to middle age in predicting OS (p = 0.969), but the young age group had the worst OS (p = 0.017). In addition, Table 4 shows that the patients with good PS, who received ICI combination therapy in the first-line or second-line setting and ICI plus chemotherapy, and with baseline albumin level > 39.2 g/L were associated with better OS (all p < 0.05). Moreover, patients with irAEs showed superior OS compared with patients without irAEs, but without statistical difference (p = 0.660) (Figure 2D). Multivariate Cox analysis indicated that young age was an independent prognostic factor for OS (HR 2.740, 95% CI 1.348–5.570, p = 0.005). Moreover, therapy line (HR 1.753, 95% CI 1.092–2.812, p = 0.020) and baseline albumin level (HR 0.457, 95% CI 0.292–0.714, p = 0.001) remained as independent prognostic factors for OS (Table 4).
Safety analysis
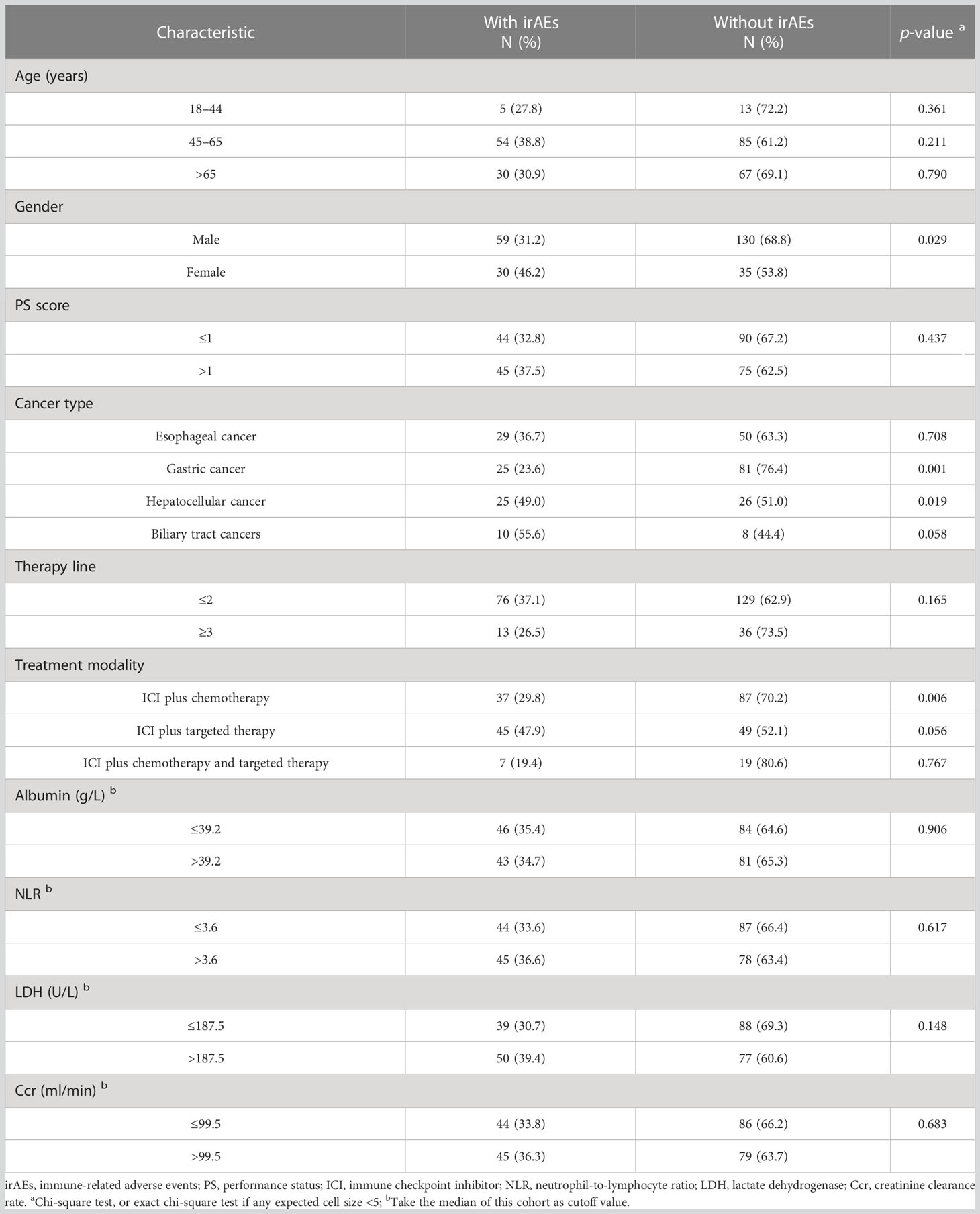
The overall incidence of irAEs was 35.0% (89/254) with grade 3 or higher irAEs of 3.9% (10/254). The most common irAEs were thyroid disorders (50/89, 56.2%), skin disorders (16/89, 18.0%), and elevated cardiac enzymes (10/89, 11.2%). Table 5 shows the relationship between characteristics of patients and irAEs. The results indicated no significant differences in the incidence of irAEs for each age group (all p > 0.05). IrAEs occurred in 25 patients with hepatocellular cancer (25/51, 49.0%), which was significantly higher than those with other GICs (p = 0.019). However, compared with other GICs, the incidence of irAEs in gastric cancer (25/106, 23.6%) is significantly lower (p = 0.001). Meanwhile, when irAEs were evaluated according to sex and treatment modality, women were more likely to experience irAEs than men (p = 0.029), and ICI plus chemotherapy significantly decreased the rate of irAEs than ICI combined with other therapies (p = 0.006).
Discussion
Despite the fact that ICI immunotherapies have changed the landscape of cancer treatment for several advanced solid tumors, little is known about the impact of age on clinical efficacy and safety of ICI combined therapy in GIC patients. A meta-analysis performed indicated comparable efficacy of ICI-based combination therapy in younger and older patients with non-small cell lung cancer (NSCLC) with a cutoff age of 65 years (19). Another meta-analysis also did not find a difference in survival benefit of immunotherapy in older (≥65 years) vs. younger (<65 years) patients including 37 phase 2 or 3 randomized clinical trials of 23,760 patients (20). Patients were commonly classified into young and old groups based on the different cutoff value of age (65 or 70 years) (13, 19), which may partly lead to the failure of identifying the role of immunotherapy in young patients. However, we believe that the immune function of people of different ages is different, and the analysis of only older than or younger than an age point may mask the response of different age groups to ICIs. In the current study, we compared the clinical efficacy and safety of ICIs combined therapy of young age with that of middle age and old age. Patients aged 18–44 years showed a lower DCR and poorer survival, but there was no significant difference on the efficacy of ICI combination therapy between middle-aged (aged 45–65 years) and old (aged >65 years) patients, which indicated that young GIC patients showed poor efficacy for ICI combined therapy.
Several meta-analyses of clinical trials in multiple cancer types treated with ICI demonstrated similar results to our study and found that older patients can benefit more from immunotherapy than younger patients (21–24). However, the cause of low immunotherapy response in young patients is still unclear and several reasons may explain this. Castro et al. presented evidence that younger patients showed the strongest effects of major histocompatibility complex (MHC)-based driver mutation selection, which may influence the availability of mutant peptides capable of driving effective response to ICI therapy (25). A recent study by Kugel et al. indicated that intratumoral CD8+ T cells:Treg ratios of younger melanoma patients treated with PD-1 inhibitors significantly decreased compared to older patients and a similar result was observed in young mice (11). Therefore, young GIC patients may not be good candidates for immunotherapy, and further studies with evidence of high level are needed.
In addition, young patients have higher baseline LDH levels than old patients in our cohort. Elevated LDH is a negative prognostic biomarker not only because it is a key enzyme involved in cancer metabolism, but also because it alters the tumor microenvironment in hematologic and solid neoplasms, allowing tumor cells to suppress and evade the immune system (26). Its increase exhibited a negative effect for ipilimumab on metastatic melanoma in clinical applications (27, 28). However, we did not find elevated LDH to be associated with the immunotherapy efficacy and survival of patients in our study. The difference from other studies may be explained by cutoff value variations. Nonetheless, this might explain at least partly the poor ICI response in young patients in our study, and prospective new studies are needed to provide more information on this subject. Moreover, with the improvement of health habits, there are fewer and fewer opportunities to contact pathogenic microorganisms in childhood so as to obtain a sound immune system. Patients older than 45 years old may have much stronger immune systems to initiate immune response for this reason. Nevertheless, our results suggested that a detailed age stratification should be carried out when evaluating the efficacy of immunotherapy to deal with an aging society.
We also explored the safety of ICI combined therapy in young, middle-aged, and old patients. The overall incidence of irAE was 35.0% with grade 3 or higher irAEs of 3.9% in GIC, which was comparable with the previously reported incidence (29, 30). The most common adverse events were thyroid disorders, skin disorders, and elevated cardiac enzymes. Although the creatinine clearance rate significantly decreased with age, we found no significant differences in the incidence of irAEs for each age group. Several retrospective reviews focused on this issue also did not find statistically significant differences in irAEs based on age (31–33), so did another retrospective case–control study of patients involving melanoma, renal cell carcinoma, or NSCLC with three age groups: <65 years old, 65–74 years old, and ≥75 years old (34). These results suggest that ICIs could be safely used in older patients. Our study also observed significantly better DCR and a higher PFS in irAE patients, which confirmed the suggestions that irAE onset might become the first clinical biomarker for anti-PD-1 antibody response in patients with indications to receive ICIs (35–37). Our study also showed that women were more likely to develop irAEs than men, and ICI plus chemotherapy significantly decreased the rate of irAEs than ICI combined with other therapies. However, our series did not explore the relationships for different irAE symptoms and grades with different characteristics and immunotherapy response due to the relatively small sample size, and further research is necessary to explore this further.
The present study had some limitations, namely, its retrospective nature, patients having different treatment backgrounds, patients coming from one center, and the small sample size (n = 18) of those <45 years. However, to our knowledge, this was the first study that determined the role of ICI combined therapy in GIC patients with detailed age stratification. Findings from this pilot project would build the foundation for future research with more patients and more centers. Given this, there is an urgent need for a larger, multicenter prospective study to explore the predictive power of the age for ICI combined therapy to consolidate our findings.
Conclusions
In summary, younger GIC patients (aged 18–44 years) showed poor efficacy for ICI combined therapy, and irAEs could be used as a clinical biomarker to predict ICI efficacy in GIC patients. Further studies are warranted to figure out the age-specific mechanisms of ICI combined therapy.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
All procedures were supervised and approved by the ethics committee of the Fourth Hospital of Hebei Medical University (2020KS001). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
YW designed the experiments, collected the data of patients, and wrote the paper. SZ, FZ and LW followed up the patients. CW, XZ and RZ performed statistical analysis and collected the data. ZG designed the experiments, conceived the concept, and wrote the paper. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the Key Research and Development Projects of Hebei Province (Grant No. 21377759D) and the Foundation of Hebei Provincial Department of Science and Technology and Hebei Medical University (Grant No. 2020TXZH03).
Acknowledgments
The authors would like to thank the patients who consented to participate and release their personal data for the scientific purposes of this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1155019/full#supplementary-material
References
1. Arnold M, Abnet CC, Neale RE, Vignat J, Giovannucci EL, McGlynn KA, et al. Global burden of 5 major types of gastrointestinal cancer. Gastroenterology (2020) 159(1):335–49. doi: 10.1053/j.gastro.2020.02.068
2. Vedeld HM, Goel A, Lind GE. Epigenetic biomarkers in gastrointestinal cancers: the current state and clinical perspectives. Semin Cancer Biol (2018) 51:36–49. doi: 10.1016/j.semcancer.2017.12.004
3. Hsu A, Mendelson L, Almhanna K. Immune checkpoint inhibitors in the treatment of gastrointestinal malignancies: a review of current and future therapies. R I Med J (2013) (2020) 103(3):33–7.
4. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med (2020) 382(20):1894–905. doi: 10.1056/NEJMoa1915745
5. Kato K, Shah MA, Enzinger P, Bennouna J, Shen L, Adenis A, et al. KEYNOTE-590: phase III study of first-line chemotherapy with or without pembrolizumab for advanced esophageal cancer. Future Oncol (2019) 15(10):1057–66. doi: 10.2217/fon-2018-0609
6. Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet (2021) 398(10294):27–40. doi: 10.1016/S0140-6736(21)00797-2
7. National Cancer Institute. The surveillance, epidemiology and EndResults (2020). Available at: https://seer.cancer.gov/.
8. Prcina M, Novak M, Cigankova V, Kontsekova E. Immunosenescence-the role in the immunotherapy of older population. Bratisl Lek Listy (2018) 119(4):217–20. doi: 10.4149/BLL_2018_040
9. Popescu T, Karlsson U, Vinh-Hung V, Trigo L, Thariat J, Vuong T, et al. Challenges facing radiation oncologists in the management of older cancer patients: consensus of the international geriatric radiotherapy group. Cancers (Basel) (2019) 11(3):371. doi: 10.3390/cancers11030371
10. Salminen A, Kaarniranta K, Kauppinen A. Phytochemicals inhibit the immunosuppressive functions of myeloid-derived suppressor cells (MDSC): impact on cancer and age-related chronic inflammatory disorders. Int Immunopharmacol (2018) 61:231–40. doi: 10.1016/j.intimp.2018.06.005
11. Kugel CH 3rd, Douglass SM, Webster MR, Kaur A, Liu Q, Yin X, et al. Age correlates with response to anti-PD1, reflecting age-related differences in intratumoral effector and regulatory T-cell populations. Clin Cancer Res (2018) 24(21):5347–56. doi: 10.1158/1078-0432.CCR-18-1116
12. Huang XZ, Gao P, Song YX, Sun JX, Chen XW, Zhao JH, et al. Efficacy of immune checkpoint inhibitors and age in cancer patients. Immunotherapy (2020) 12(8):587–603. doi: 10.2217/imt-2019-0124
13. Archibald WJ, Victor AI, Strawderman MS, Maggiore RJ. Immune checkpoint inhibitors in older adults with melanoma or cutaneous malignancies: the wilmot cancer institute experience. J Geriatr Oncol (2020) 11(3):496–502. doi: 10.1016/j.jgo.2019.07.005
14. Fox B, de Toro Carmena M, Álvarez Carmina R, Calles Blanco A, López Carmina C, Pérez Ramírez S, et al. Efficacy and safety of immune checkpoint inhibitor immunotherapy in elderly cancer patients. Clin Transl Oncol (2020) 22(4):555–62. doi: 10.1007/s12094-019-02161-4
15. Cheng L, Chen S, Wu W, Kuo ZC, Wei Z, Meng S, et al. Gastric cancer in young patients: a separate entity with aggressive features and poor prognosis. J Cancer Res Clin Oncol (2020) 146(11):2937–47. doi: 10.1007/s00432-020-03268-w
16. Puccini A, Lenz HJ, Marshall JL, Arguello D, Raghavan D, Korn WM, et al. Impact of patient age on molecular alterations of left-sided colorectal tumors. Oncologist (2019) 24(3):319–26. doi: 10.1634/theoncologist.2018-0117
17. van Lier MG, Westerman AM, Wagner A, Looman CW, Wilson JH, de Rooij FW, et al. High cancer risk and increased mortality in patients with peutz-jeghers syndrome. Gut (2011) 60(2):141–7. doi: 10.1136/gut.2010.223750
18. Li D, Sun CL, Kim H, Soto-Perez-de-Celis E, Chung V, Koczywas M, et al. Geriatric assessment-driven intervention (GAIN) on chemotherapy-related toxic effects in older adults with cancer: a randomized clinical trial. JAMA Oncol (2021) 7(11):e214158. doi: 10.1001/jamaoncol.2021.4158
19. Yan X, Tian X, Wu Z, Han W. Impact of age on the efficacy of immune checkpoint inhibitor-based combination therapy for non-small-Cell lung cancer: a systematic review and meta-analysis. Front Oncol (2020) 10:1671. doi: 10.3389/fonc.2020.01671
20. Yang F, Markovic SN, Molina JR, Halfdanarson TR, Pagliaro LC, Chintakuntlawar AV, et al. Association of sex, age, and Eastern cooperative oncology group performance status with survival benefit of cancer immunotherapy in randomized clinical trials: a systematic review and meta-analysis. JAMA Netw Open (2020) 3(8):e2012534. doi: 10.1001/jamanetworkopen.2020.12534
21. Wu Q, Wang Q, Tang X, Xu R, Zhang L, Chen X, et al. Correlation between patients' age and cancer immunotherapy efficacy. Oncoimmunology (2019) 8(4):e1568810. doi: 10.1080/2162402X.2019.1568810
22. Jain V, Hwang WT, Venigalla S, Nead KT, Lukens JN, Mitchell TC, et al. Association of age with efficacy of immunotherapy in metastatic melanoma. Oncologist (2020) 25(2):e381–5. doi: 10.1634/theoncologist.2019-0377
23. Zhang Y, Lin A, Li Y, Ding W, Meng H, Luo P, et al. Age and mutations as predictors of the response to immunotherapy in head and neck squamous cell cancer. Front Cell Dev Biol (2020) 8:608969. doi: 10.3389/fcell.2020.608969
24. Perier-Muzet M, Gatt E, Péron J, Falandry C, Amini-Adlé M, Thomas L, et al. Association of immunotherapy with overall survival in elderly patients with melanoma. JAMA Dermatol (2018) 154(1):82–7. doi: 10.1001/jamadermatol.2017.4584
25. Castro A, Pyke RM, Zhang X, Thompson WK, Day CP, Alexandrov LB, et al. Strength of immune selection in tumors varies with sex and age. Nat Commun (2020) 11(1):4128. doi: 10.1038/s41467-020-17981-0
26. Ding J, Karp JE, Emadi A. Elevated lactate dehydrogenase (LDH) can be a marker of immune suppression in cancer: interplay between hematologic and solid neoplastic clones and their microenvironments. Cancer Biomark (2017) 19(4):353–63. doi: 10.3233/CBM-160336
27. Kelderman S, Heemskerk B, van Tinteren H, van den Brom RR, Hospers GA, van den Eertwegh AJ, et al. Lactate dehydrogenase as a selection criterion for ipilimumab treatment in metastatic melanoma. Cancer Immunol Immunother (2014) 63(5):449–58. doi: 10.1007/s00262-014-1528-9
28. Dick J, Lang N, Slynko A, Kopp-Schneider A, Schulz C, Dimitrakopoulou-Strauss A, et al. Use of LDH and autoimmune side effects to predict response to ipilimumab treatment. Immunotherapy (2016) 8(9):1033–44. doi: 10.2217/imt-2016-0083
29. Rogado J, Sánchez-Torres JM, Romero-Laorden N, Ballesteros AI, Pacheco-Barcia V, Ramos-Leví A, et al. Immune-related adverse events predict the therapeutic efficacy of anti-PD-1 antibodies in cancer patients. Eur J Cancer (2019) 109:21–7. doi: 10.1016/j.ejca.2018.10.014
30. Sato K, Akamatsu H, Murakami E, Sasaki S, Kanai K, Hayata A, et al. Correlation between immune-related adverse events and efficacy in non-small cell lung cancer treated with nivolumab. Lung Cancer (2018) 115:71–4. doi: 10.1016/j.lungcan.2017.11.019
31. Schulz GB, Rodler S, Szabados B, Graser A, Buchner A, Stief C, et al. Safety, efficacy and prognostic impact of immune checkpoint inhibitors in older patients with genitourinary cancers. J Geriatr Oncol (2020) 11(7):1061–6. doi: 10.1016/j.jgo.2020.06.012
32. Sattar J, Kartolo A, Hopman WM, Lakoff JM, Baetz T. The efficacy and toxicity of immune checkpoint inhibitors in a real-world older patient population. J Geriatr Oncol (2019) 10(3):411–4. doi: 10.1016/j.jgo.2018.07.015
33. Corbaux P, Maillet D, Boespflug A, Locatelli-Sanchez M, Perier-Muzet M, Duruisseaux M, et al. Older and younger patients treated with immune checkpoint inhibitors have similar outcomes in real-life setting. Eur J Cancer (2019) 121:192–201. doi: 10.1016/j.ejca.2019.08.027
34. Samani A, Zhang S, Spiers L, Mohamed AA, Merrick S, Tippu Z, et al. Impact of age on the toxicity of immune checkpoint inhibition. J Immunother Cancer (2020) 8(2):e000871. doi: 10.1136/jitc-2020-000871
35. Kawai T, Sato Y, Makino K, Yamada Y, Nomiya A, Nakamura M, et al. Immune-related adverse events predict the therapeutic efficacy of pembrolizumab in urothelial cancer patients. Eur J Cancer (2019) 116:114–5. doi: 10.1016/j.ejca.2019.05.017
36. Xu S, Lai R, Zhao Q, Zhao P, Zhao R, Guo Z. Correlation between immune-related adverse events and prognosis in hepatocellular carcinoma patients treated with immune checkpoint inhibitors. Front Immunol (2021) 12:794099. doi: 10.3389/fimmu.2021.794099
Keywords: gastrointestinal cancers, ICIs combined therapy, young, prognosis, irAEs
Citation: Wang Y, Zhang S, Zhang F, Wang L, Wu C, Zhang X, Zhang R and Guo Z (2023) Young patients show poor efficacy for immune checkpoint inhibitor combined therapy in metastatic gastrointestinal cancers. Front. Oncol. 13:1155019. doi: 10.3389/fonc.2023.1155019
Received: 31 January 2023; Accepted: 04 April 2023;
Published: 03 May 2023.
Edited by:
Zequn Li, The Affiliated Hospital of Qingdao University, ChinaReviewed by:
Wenhong Deng, Renmin Hospital of Wuhan University, ChinaYuqi Sun, Qingdao University, China
Zhengdong Zhang, First Affiliated Hospital of Chengdu Medical College, China
Copyright © 2023 Wang, Zhang, Zhang, Wang, Wu, Zhang, Zhang and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhanjun Guo, zjguo5886@aliyun.com
 Yingnan Wang
Yingnan Wang Shasha Zhang2
Shasha Zhang2 Fengbin Zhang
Fengbin Zhang Lei Wang
Lei Wang Zhanjun Guo
Zhanjun Guo