- 1Department of Breast Cancer Center, Chongqing University Cancer Hospital, Chongqing, China
- 2Department of Breast Cancer Center, Chongqing University Cancer Hospital, School of Medicine, Chongqing University, Chongqing, China
- 3Department of Medical Record, Chongqing University Cancer Hospital, Chongqing, China
- 4Department of Radiation Oncology, Chongqing University Cancer Hospital, Chongqing, China
- 5Chongqing Key Laboratory of Translational Research for Cancer Metastasis and Individualized Treatment, Chongqing University Cancer Hospital, Chongqing, China
- 6Chongqing Key Laboratory for Intelligent Oncology in Breast Cancer (iCQBC), Chongqing University Cancer Hospital, Chongqing, China
Purpose: The present study aimed to identify clinicopathological characteristics of breast cancer liver metastasis (BCLM) as well as to characterize the risk and prognostic factors for the liver metastasis (LM) of breast cancer patients with de novo and relapsed distant metastasis in a Chinese population.
Materials and methods: Patients with metastatic breast cancer (MBC) who were hospitalized in the Breast Cancer Center at Chongqing University between January 2011 and December 2019 were included in the present study. Logistic regression analyses were conducted to identify risk factors for the presence of BCLM. Cox proportional hazard regression models were performed to determine the prognostic factors for the survival of BCLM patients. The correlation between LM and overall survival was assessed by the Kaplan–Meier method.
Results: In total, 1,228 eligible MBC patients, including 325 cases (26.5%) with de novo metastasis (cohort A) and 903 cases (73.5%) with relapsed metastasis (cohort B), were enrolled in the present study. In cohort A and cohort B, 81 (24.9%) and 226 (25.0%) patients had BCLM, respectively. Patients in these two cohorts had different clinicopathological features. Logistic regression analysis identified that the human epidermal growth factor receptor 2 (HER2) status in cohort A as well as the HER2 status and invasive ductal carcinoma histology in cohort B were risk factors for BCLM. The median OS of patients with LM was inferior to that of non-LM patients (17.1 vs. 37.7 months, P = 0.0004 and 47.6 vs. 84.0 months, P < 0.0001, respectively). Cox analysis identified that the primary T stage, Ki67 level, and breast surgery history were independent prognostic factors for cohorts A and B, respectively.
Conclusions: De novo and relapsed MBC patients have different risk and prognostic factors for LM. Patients with BCLM have an unfavorable prognosis.
Introduction
Breast cancer is the most common type of cancer worldwide (1). At the time of breast cancer diagnosis, approximately 5%–15% of patients have distant metastases (de novo metastatic disease) (2, 3). There are 20%–30% of early breast cancer patients who experience recurrence or relapse with distant metastases, such as bone, lung, liver, and/or brain metastases, after standard initial treatment (4, 5). Metastatic breast cancer (MBC) is incurable, and almost all fatalities due to breast cancer are caused by distant metastases (6–11).
It is worth noting that the liver is the third most common metastatic organ. Patients with breast cancer liver metastasis (BCLM) have a worse prognosis than those with bone or other organ metastases with 5-year survival rates of only 3.8%–12% (12), and BCLM patients have a median survival time of 2–3 years (6, 13). In addition, more than 20% of deaths from breast cancer are caused by liver metastasis (LM) (14, 15). BCLM is often asymptomatic or presents atypical symptoms, which tend to be ignored in the beginning stage of LM. Moreover, most patients with BLCM have extensive LM at the time of discovery as well as bone, lung, brain, or other organ metastases (16). Because every breast cancer patient has the risk of developing LM, it is important to identify the risk factors associated with the occurrence of BCLM to develop appropriate individualized treatment schemes to prolong survival time (17–21).
Breast cancer is a heterogeneous disease with multiple histological and molecular profiles related to different prognoses. Previous studies have demonstrated the association between clinicopathological factors and the predisposition to BCLM (6, 22). However, those studies did not differentiate de novo and recurrent distant metastasis in breast cancer patients. Many previous studies have indicated that patients with de novo MBC represent a distinct population of patients with recurrent MBC; changes in the tumor phenotype have been found between primary and recurrent breast cancer (3, 23–25). Their results suggest that de novo and relapsed MBC patients are distinct. Further, studies focusing on LM in Chinese individuals with breast cancer are lacking.
In the present study, we aimed to identify the clinicopathological characteristics of BCLM and explore the risk factors that affect the incidence and prognosis of LM in patients with de novo MBC and patients with relapsed MBC in the Chinese population.
Materials and methods
Patient selection
Between January 2011 and December 2019, patients with histologically proven breast cancer who were admitted to the Breast Cancer Center of Chongqing University Cancer Hospital (Chongqing, China) were screened. This cancer center is one of the largest in southwest China, covering an approximately 82,402.95 km2 area with a population of 32.05 million residents. The inclusion criteria were as follows: female patients with histologically diagnosed breast cancer with at least one distant site metastasis and with complete information on molecular typing. The exclusion criteria were as follows: patients with bilateral breast cancer; patients with other tumor disorders; patients with recurrence only in the regional lymph node and/or chest wall; and patients with unqualified medical records. This study was conducted in accordance with the Declaration of Helsinki and the principles of Good Clinical Practice, and it was approved by the Medical Ethics Committee of the Chongqing University Cancer Hospital (CZLS2022177-A).
Data collection
The following data were extracted from patient medical records: demographic information; clinicopathological factors, including age, menopause, and menstrual and reproductive history; hepatitis B infection information; the date of the breast cancer diagnosis; the date and location of first organ metastasis; height and weight at the diagnosis with distant metastasis; T, N, and M stages; histologic type and grades; estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) status; and treatment information. The body mass index (BMI) was subtyped based on the criteria of the World Health Organization (WHO) as follows: underweight (<18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (24.9–29.9 kg/m2), and obese (≥30 kg/m2). Menarche was defined as early menarche (≤12 years), normal menarche (13–14 years), and late menarche (≥15 years). The diagnosing and staging of breast cancer were in line with the tumor–node–metastasis (TNM) system based on the guidelines of the 7th Edition of American Joint Committee on Cancer (AJCC) (26). The WHO international histological classification was used to assess the final histopathological diagnosis (27). The primary tumor’s histologic grade was determined using the modified Scarff–Bloom–Richardson system (mSBR) (28). ER and PR were defined as positive if more than 1% of tumor cells showed positive nuclear staining by immunohistochemistry (IHC). IHC 3+ staining and IHC 2+ staining or ambiguous fluorescence in situ hybridization (FISH) results were regarded as positive. Some individuals with HER2 IHC 2+ but no FISH results were defined as HER2 negative.
The time to diagnose distant organ metastasis, including the bone, lung/pleura, liver, and central nervous system (CNS), was recorded. CNS metastatic patients were defined as those with either parenchymal brain metastasis and/or leptomeningeal metastasis. The evidence of organ metastasis was defined as the presence of clinical manifestations of metastases and confirmed with imaging/pathology examination and/or radiologic changes using a computed tomography scan with contrast or whole-body bone scan. Oligometastasis was defined as only one organ metastasis, and polymetastasis was defined as ≥2 organ metastasis, including LM.
Follow-up information
All patients were routinely followed up by telephone, outpatient visits, or hospitalization information. During the first 6 months after surgery, drug treatment, or radiotherapy, follow-up visits were scheduled at least once a month and every 6 months after that unless there were any unscheduled complications. Patient survival outcome information was collected through active and passive follow-ups. The fundamental cause of death was derived from medical records or information from immediate family members. The date of the last follow-up was 17 September 2021. Metastasis-free survival (MFS) was defined as the time interval from the breast cancer diagnosis to the first detection of distant metastasis, including LM, lung/pleura metastasis, bone metastasis, and/or CNS metastasis. Overall survival (OS) was calculated from the date of diagnosis with primary breast cancer to the date of death or last follow-up.
Statistical analyses
The Pearson chi-square test, Fisher’s exact test, or Kruskal–Wallis test was used to examine the associations between LM and clinicopathologic parameters as appropriate. Multivariable logistic and Cox proportional hazard regression analyses were performed to explore risk and prognostic factors for BCLM based on the univariable regression results (P < 0.1). All confidence intervals (CIs) are shown at the 95% confidence level. The Kaplan–Meier method was used to plot survival curves, and the log-rank test was performed to compare the survival differences. Statistical analyses were performed using R software (version 3.4, http://www.r-project.org). A two-sided P-value of 0.05 or less was considered statistically significant.
Results
Patient characteristics
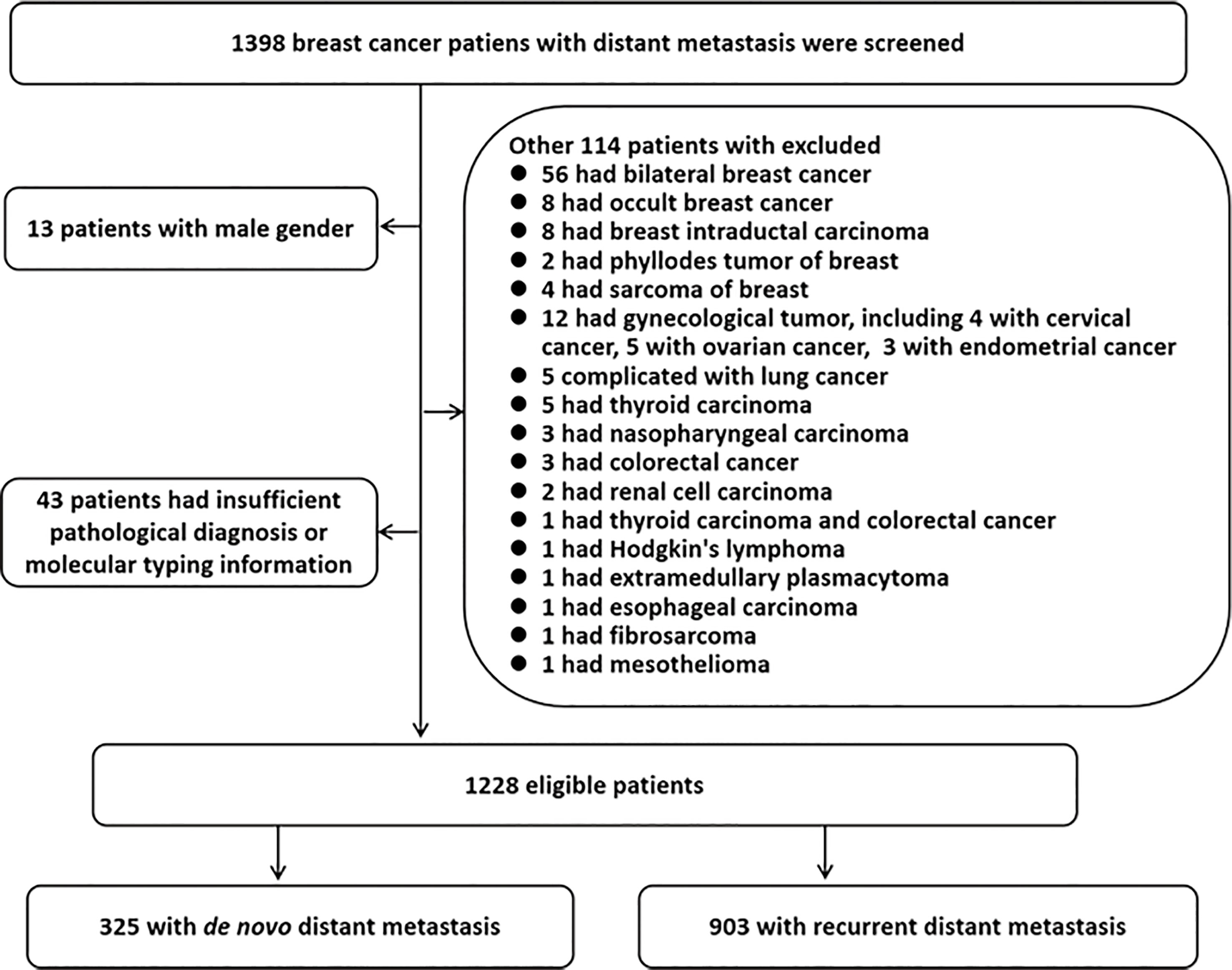
In total, 1,398 MBC patients were further screened. Of these, the following patients were excluded: 13 cases were excluded due to the male gender; 43 cases were eliminated due to incomplete information on the pathological diagnosis or molecular typing; and 114 cases were eliminated due to the presence of other primary cancers. Finally, 1,228 eligible patients were enrolled in the present study, including 325 (26.5%) patients with distant metastasis at diagnosis (termed cohort A), and 903 (73.5%) patients with relapsed distant metastasis (termed cohort B). A flowchart of the patient selection process is shown in Figure 1.
Hormone receptor (HR) positive and HER2 negative (HR+/HER2−) was the most common subtype among the MBC patients in both cohorts A and B followed by the HER2-positive and triple-negative breast cancer (TNBC) subtypes. The frequency of LM was 24.9% (81/325) and 25.0% (226/903) in cohorts A and B, respectively. The other sites of metastasis were the bone, lung/pleura, and CNS with percentages of 51.4% (167/325), 37.8% (123/325), and 9.8% (32/325) in cohort A as well as 46.3% (418/903), 42.8% (387/903), and 7.8% (71/903) in cohort B, respectively. The clinicopathological features of the patients stratified by LM in the two cohorts are summarized in Table 1. The mean age at diagnosis of breast cancer was 52.1 and 48.9 years in cohorts A and B, respectively (P < 0.0001), but no significant difference in age was found between the two cohorts (52.2 vs. 51.9 years). The mean age of patients with LM at the time of diagnosis of breast cancer in cohort A was older than that in cohort B (P = 0.026). The mean age at diagnosis of MBC in patients with LM was younger than that in patients without LM, but a significant difference was found only in cohort B (P = 0.015). In cohort B, patients with invasive ductal histology were more likely to have LM (P = 0.003). Patients with late menarche and the ER+ status tended to have a lower risk for LM, but there was no statistical difference. Regardless whether patients had de novo MBC or relapsed MBC after previous treatment, LM was more likely to occur in HER2+ tumors (P < 0.0001). Compared to patients in cohort A, patients in cohort B had lower T (P < 0.0001) and N staging (P = 0.011). Patients with LM in cohort A had a higher risk for CNS metastasis than those in cohort B (P = 0.015). Compared to patients without LM, the proportion of patients with distant lymph node metastasis and bone metastasis were lower in patients with LM (P < 0.0001). Among all patients, patients with LM had a higher risk of polymetastasis (P < 0.0001). In cohort B, the median time of MFS in MBC patients with LM was significantly shorter than that in those without LM (21.0 months vs. 27.0 months, P = 0.001), and the proportion of patients with MFS less than 1 year was higher in BCLM patients compared to non-BCLM patients (P < 0.0001).
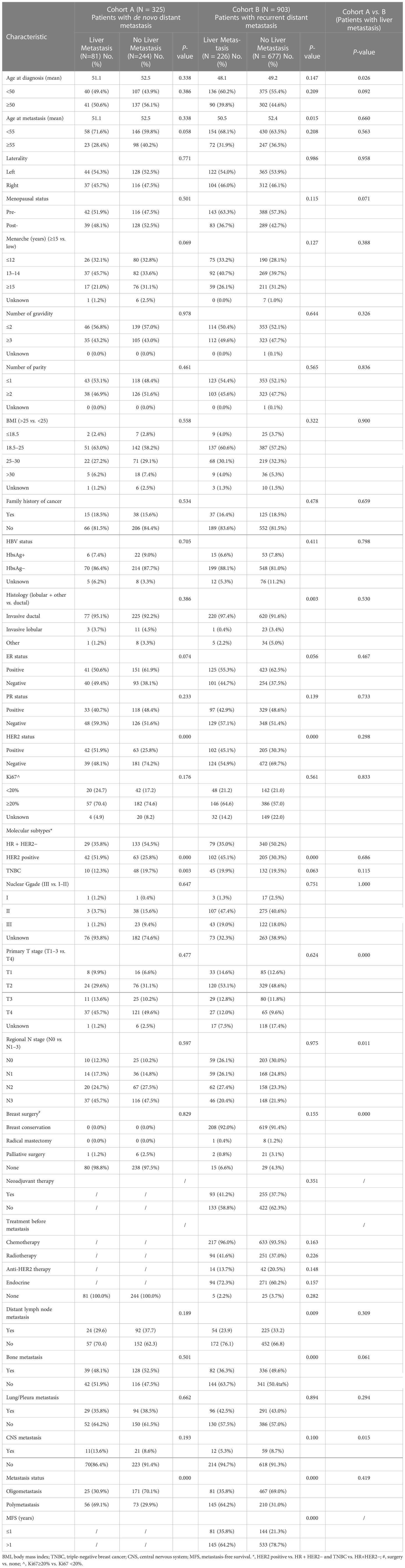
Table 1 Patient characteristics by the liver metastasis (LM) status in de novo and relapsed metastatic breast cancer.
Univariable and multivariable logistic regression analyses for breast cancer liver metastasis presence
The univariable logistic regression analysis results for the presence of LM in cohorts A and B are shown in Table 2. In cohort A, patients with a younger age at the diagnosis of MBC (≥55 vs. <55 years, OR = 0.591, 95% CI = 0.342–1.020, P = 0.059), ER− status (positive vs. negative, OR = 0.631, 95% CI = 0.380–1.048, P = 0.075), and HER2+ status (positive vs. negative, OR = 3.094, 95% CI = 1.836–5.213, P = 0.000) were more likely to present with LM. In cohort B, patients with the ER− status (positive vs. negative, OR = 0.743, 95% CI = 0.548–1.008, P = 0.056), HER2+ status (positive vs. negative, OR = 1.907, 95% CI = 1.400–2.598, P = 0.000), HbsAg− status (HbsAg + vs. HbsAg−, OR = 0.681, 95% CI = 0.513–0.903, P = 0.008) and invasive ductal carcinoma (IDC) histology (invasive lobular + other vs. invasive ductal, OR = 0.297, 95% CI = 0.126-0.698, P = 0.005) had a higher risk to present with LM. Multivariable logistic regression analyses indicated that the HER2 status (positive vs. negative, OR = 2.845, 95% CI = 1.618–5.000, P = 0.000) in cohort A as well as HER2 status (positive vs. negative, OR = 1.689, 95% CI = 1.217–2.343, P = 0.001) and histology (invasive lobular + other vs. invasive ductal, OR = 0.349, 95% CI = 0.147–0.831, P = 0.017) in cohort B were independent predictors for the presence of BCLM (Table 3).
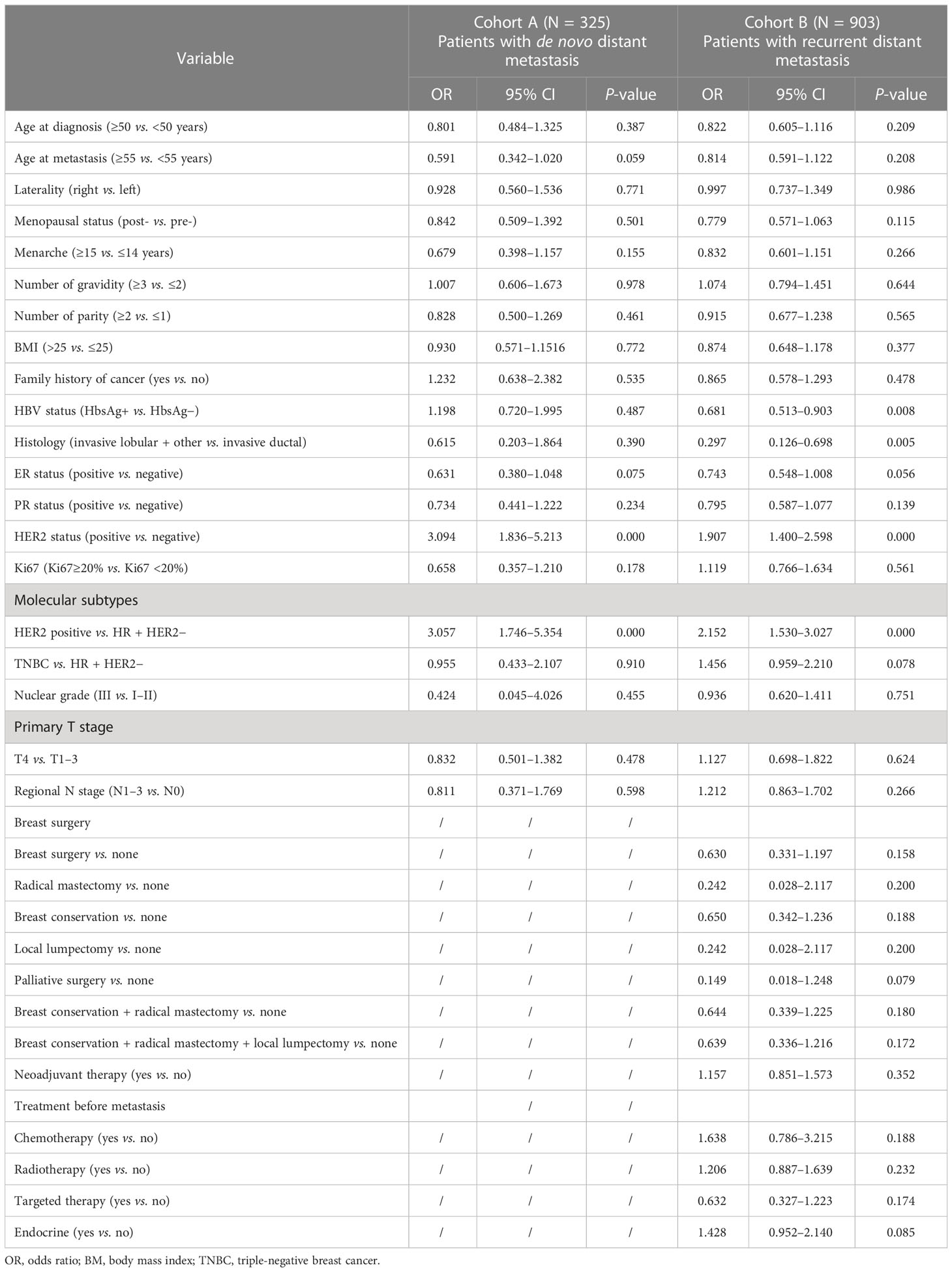
Table 2 Univariable logistic regression analysis for LM in breast cancer patients with de novo and recurrent distant metastasis.
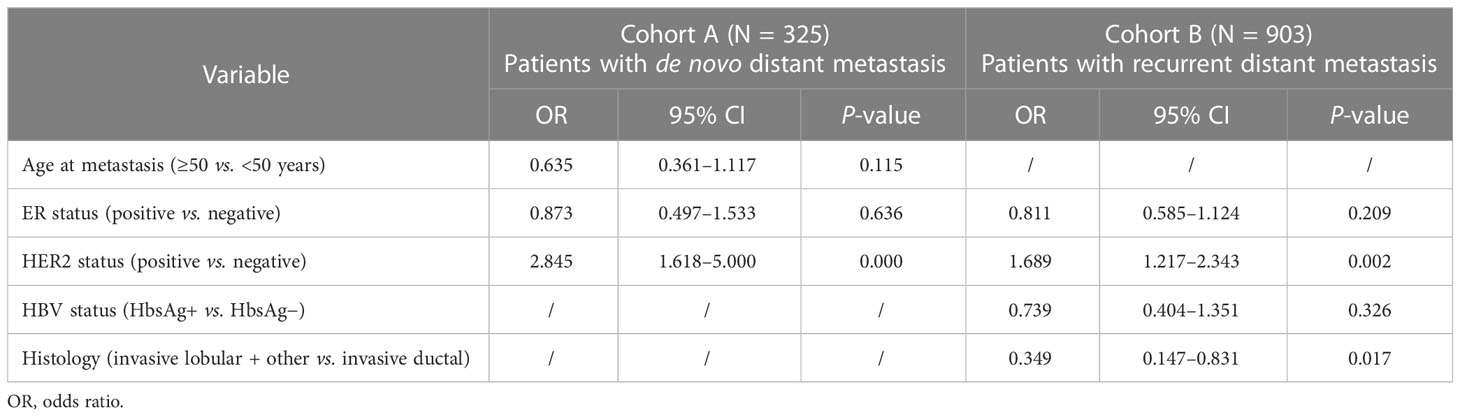
Table 3 Multivariable logistic regression analysis for LM in breast cancer patients with de novo and recurrent distant metastasis.
Survival analysis
In total, 503 (55.7%) patients with recurrent MBC and 184 (56.6%) patients with de novo MBC died by the date of this analysis (17 September 2021). The median OS of the de novo and relapsed MBC populations was 30.4 and 80.1 months, respectively. Figures 2A, B show that BCLM patients had a significantly worse prognosis than non-BCLM patients both with de novo (17.1 vs. 37.7 months, P = 0.0004) and relapsed (47.6 vs. 84.0 months, P < 0.0001) MBC.
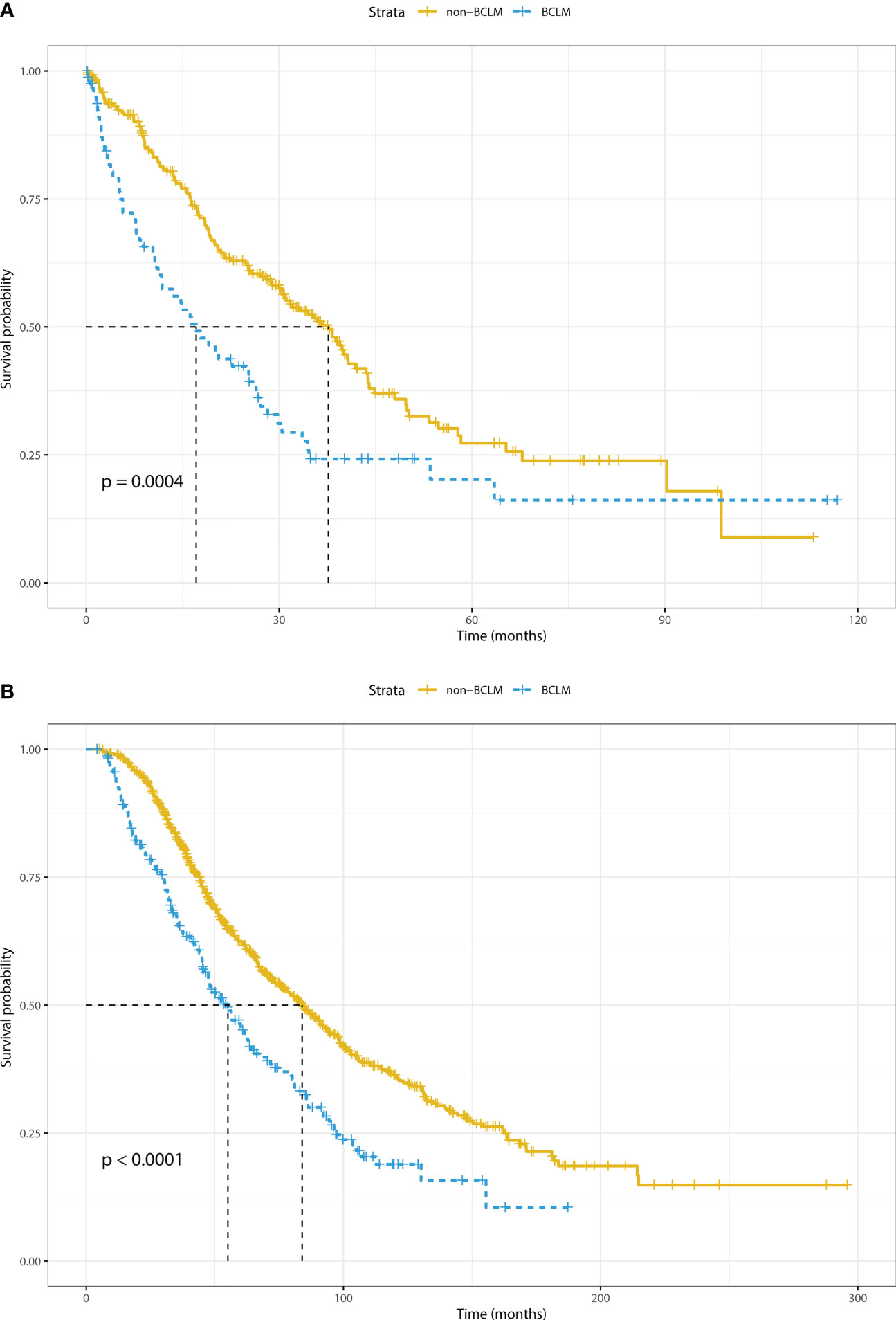
Figure 2 Overall survival of breast cancer liver metastasis (BCLM) and non-BCLM patients with (A) de novo and (B) relapsed distant metastasis. BCLM, breast cancer liver metastasis.
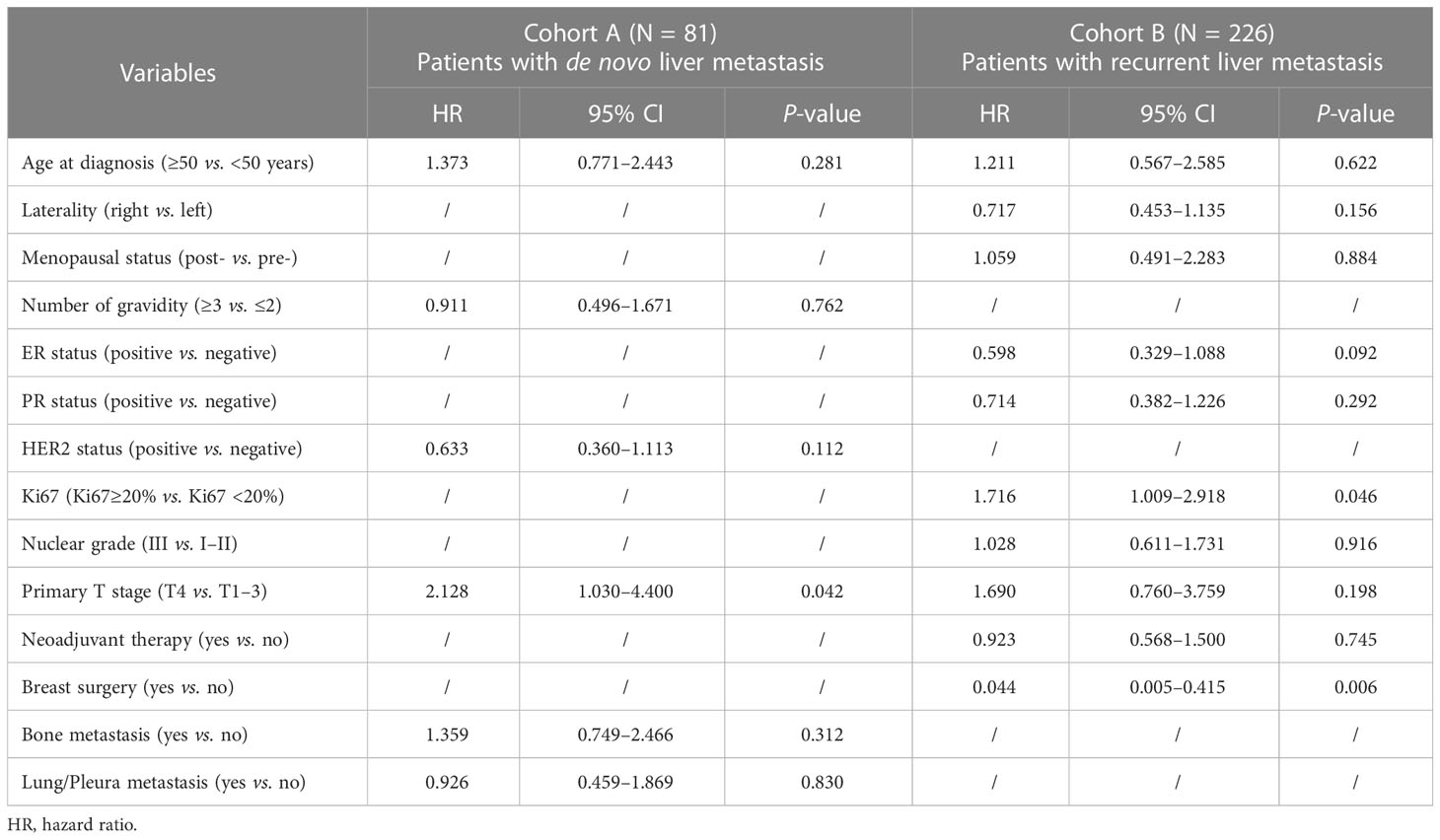
Univariable and multivariable Cox proportional hazard regression analyses were performed to assess the prognostic factors of BCLM patients. According to the univariable analysis (Table 4), the following variables were incorporated into the multivariable regression analysis: age at diagnosis, number of gravidity, HER2 status, primary T stage, bone metastasis, and lung/pleura metastasis for cohort A; and age at diagnosis, laterality, menopausal status, ER status, PR status, Ki67 level, nuclear grade, primary T stage, and history of neoadjuvant therapy and breast surgery for cohort B. As shown in Table 5, only the primary T stage (T4 vs. T1-3, HR = 2.128, 95% CI = 1.030-4.400, P = 0.042) was significantly associated with increased mortality for BCLM patients in cohort A. Regarding cohort B, Ki67 level (Ki67≥20% vs. Ki67 <20%, HR = 1.716, 95% CI = 1.009–2.918, P = 0.046) significantly increased mortality risk and breast surgery (yes vs. no, HR = 0.044, 95% CI = 0.005-0.415, P = 0.006) significantly reduced the mortality risk of BCLM patients.
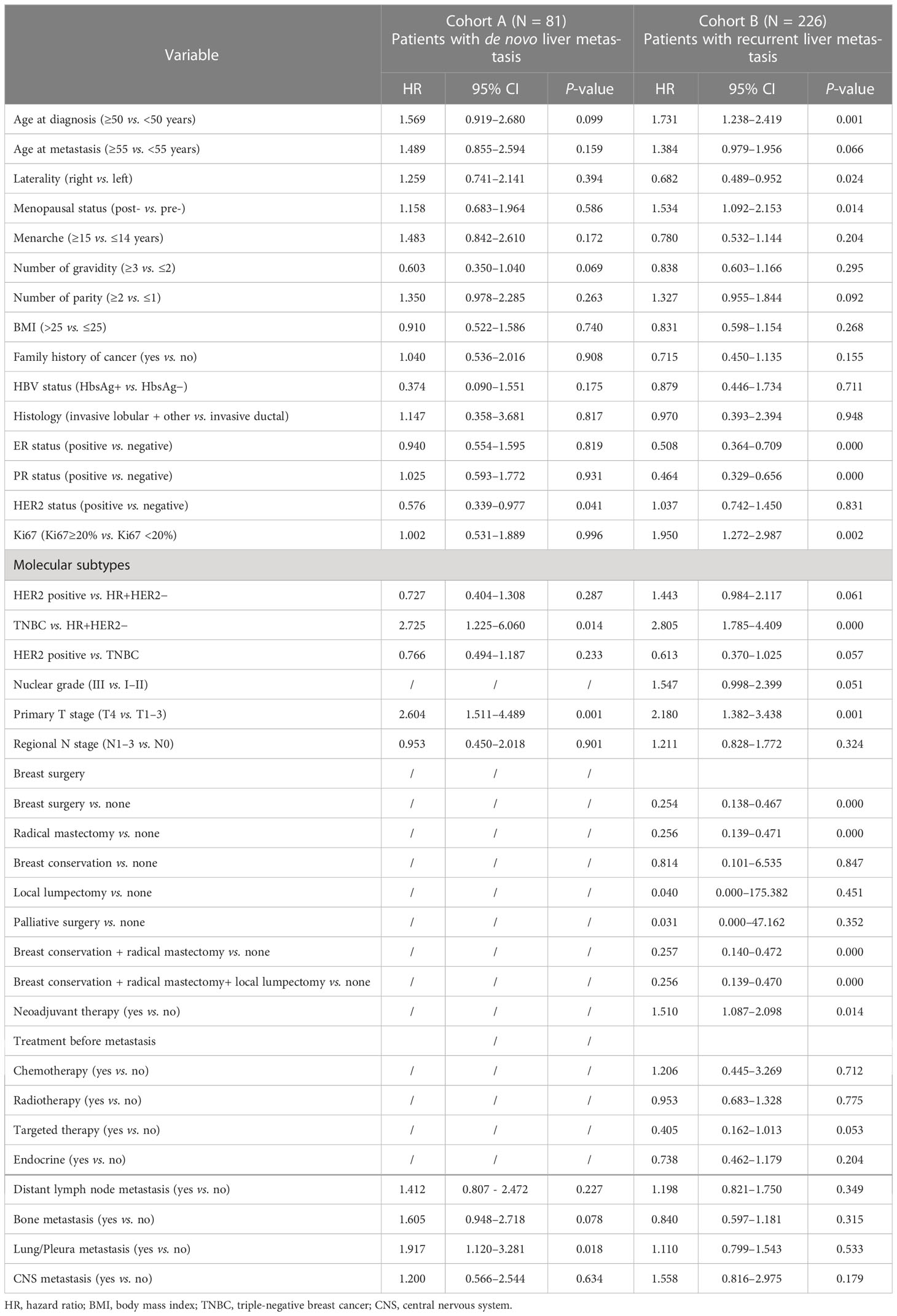
Table 4 Univariable Cox regression analysis of overall survival (OS) in breast cancer patients with LM.
Discussion
Distant metastasis is a common sequela of advanced tumor stage that suggests unfavorable outcomes for breast cancer patients. In the present study, LM occurred in approximately 25% of patients with MBC, and it was ranked after bone and lung/pleura metastasis, which was consistent with previous studies (12, 29, 30). However, compared to recent data from the China National Cancer Center and Fudan University Shanghai Cancer Center, the proportion of de novo MBC patients was higher (26.5% vs. 9.4%, 26.5% vs. 20.4%, and 17.6%, respectively) (29–31), and the frequencies of specific organ metastasis were also higher than those previously reported (29, 31). These differences may be attributed to the following factors: differences or imbalances in patient groups; inclusion and exclusion criteria for the study population; medical levels; and socioeconomic factors. These differences emphasize the importance of early diagnosis and standardized treatment.
Specific standard-of-care therapeutic strategies for BCLM are lacking (6). However, in addition to systemic therapies, including endocrine therapies, anti-HER2-targeted therapies and chemotherapy, local therapies have application prospects for the treatment of BCLM. It has been reported that liver resection (LR) has favorable clinical outcomes in a selective population of BCLM, especially in patients with isolated LM or oligometastatic disease (17). Radiofrequency ablation may be utilized in patients with BCLM who do not benefit from LR (18). In addition, radical radiation therapy, radioembolization, transarterial chemoembolization, and radioembolization can be used in the management of some cases with BCLM (6, 32, 33). There is growing evidence that the incorporation of local intervention with systemic therapy is the most promising scheme for BCLM treatment (19–21). Thus, risk factors that identify BCLM patients in a timely manner will help clinicians make appropriate medical decisions for these patients.
Several studies have investigated risk factors for the development of BCLM (22, 34), but the majority of the previous research has focused on a specific population or analyzed MBC patients as a whole population. To our knowledge, the present study is the first and largest study to focus on LM patients with de novo or recurrent MBC in China, representing different MBC populations. Importantly, we found that patients with de novo and recurrent MBC had both similar and different risk factors involving LM, indicating that the risk odds for LM should be distinguished and evaluated between de novo and relapsed MBC populations.
In concordance with previous studies (34–36), the present study demonstrated that younger patients were more susceptible to LM than elderly patients, especially in posttreatment MBC patients. Compared to elderly patients, younger patients usually have tumors with higher malignancy or more aggressive behavior, which may eventually result in a greater tendency for LM or other organs of distant metastases (36–40). Although there were differences in distant metastasis patterns between these two study cohorts, BCLM patients were more likely to have synchronous polymetastasis. Furthermore, patients with MFS ≤1 year had a higher risk of LM in patients with relapsed MBC. These factors are indicators of aggressive tumors and a poor prognosis (29, 41).
Breast cancer is stratified into different subtypes, namely, histology and molecular. Previous studies have noted clear organ-specific patterns of metastatic colonization that are unique to each subtype (6). In the present study, HER2+ breast cancers aggressively spread to the liver in the pretreatment and posttreatment MBC population. These findings were validated by univariable and multivariable logistic regression analyses. This relationship between HER2+ breast cancers and LM has also been observed in other population-based studies in addition to those utilizing SEER data (13, 30, 42–48). Anti-HER2 agents improve disease outcomes for HER2+ breast cancer patients. Although patients in the present study who received anti-HER2-targeted therapy were limited, the proportion of BCLM patients with bone metastasis and CNS metastasis decreased compared to de novo MBC patients. Moreover, the HER2 status was not a predictor for the prognosis of the de novo or relapsed MBC population according to the Cox proportional hazard regression analyses. Thus, these results suggested that treatment changes the malignant behavior of some tumors. Furthermore, IDC breast cancer is the most common pathological type, and invasive lobular carcinoma (ILC) accounts for approximately 10%. A previous study reported that IDC most frequently metastasizes to the bone, lung, and liver, whereas ILC preferentially metastasizes to the gastrointestinal tract (41). In the present study, IDC was an independent risk factor of LM for patients with relapsed MBC, which was consistent with previous studies (28, 42). However, this association was not found in patients with de novo MBC.
The median OS of BCLM patients in the de novo and recurrent MBC populations was 17.1 and 47.6 months, respectively, which was inferior to that of non-BCLM patients (median OS was 36.7 and 84.0 months in de novo and recurrent MBC populations, respectively). The survival of BCLM patients with de novo MBC in the present study was consistent with that reported in previous retrospective studies, ranging from 12 to 31.4 months (13, 30, 49, 50). In addition, the survival of patients with recurrent BCLM was more prolonged than that of de novo BCLM. These differences may be partly attributed to the distinction of different MBC populations. In the Cox proportional hazard regression analyses, the primary T4 stage significantly increased the mortality risk for BCLM patients in the de novo MBC population. Patients with a higher Ki67 level were associated with increased mortality risk, and breast surgery reduced the mortality risk of BCLM patients in the relapsed MBC population. These results are, in general, consistent with previous findings. Moreover, it is noteworthy that the OS of newly diagnosed MBC patients was significantly worse than that of relapsed MBC patients. This may boil down to the aggressive behavior of the newly diagnosed metastatic tumor and the early diagnosis and early treatment of the relapsed tumor. These results emphasized the importance of early screening, diagnosis, and prompt treatment.
The present study had several limitations. Firstly, the present study was a retrospective study at a single institution, resulting in potential referral bias, and some parameters had missing values. Primarily due to the long time span of this study, the mode of treatment also changed during the course of the study. Moreover, the palliative treatment regime also varied over time or limited to treatment conditions at that time. All these factors would affect the survival of patients. In the present study, we preliminary analyzed the effects of radiotherapy, chemotherapy, endocrine therapy, and targeted therapy before metastasis on the survival of patients but did not analyze the impact of palliative treatment on the prognosis for these patients. Therefore, it was difficult to analyze the accurate impact of a particular treatment on the outcome of patients with MBC due to the complexity and variability of the treatments. Moreover, we only analyzed patients who presented distant organ metastasis at the initial diagnosis of MBC, while patients who developed LM later in the course of the disease were not included, which may have led to incomplete patient information. Meanwhile, we also did not analyze the correlation of the liver distribution of lesions with the metastatic pattern of the liver, which should be further illustrated based on large-sample prospective clinical study research. Finally, other parameters (e.g., other clinicopathological and molecular biomarkers or biochemical factors) that were not included in the present study may provide valuable predictive information, which should be included in future studies.
Conclusions
The present study provided insight into the incidence and prognosis of LM at the initial diagnosis of MBC, including treatment-naïve and posttreatment relapsed cases in China. We identified different risk and prognostic factors for the LM of breast cancer patients with de novo and relapsed distant metastasis. These parameters may help to identify MBC patients with a high risk of LM and evaluate the prognosis of BCLM patients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Chongqing University Cancer Hospital. Written informed consent for participation was not required for this studyin accordance with the national legislation and the institutional requirements.
Author contributions
XZ, DT and NZ conceived and designed the study. NZ, YX, DT, QS, JW, YL, HL and XZ collected and analyzed the data. NZ wrote the manuscript. XZ and DT reviewed and edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Discipline Construction and Upgrading Project of National Key Clinical Specialty Construction Project, Chongqing Research Institute Performance Incentive Guide Special Project, Chongqing Science and Health Joint Medical Research Project (Grant Nos. 2021MSXM291 and 2022MSXM004); Talent Program of Chongqing (Grant No. CQYC20200303137); Chongqing Municipal Health and Health Commission (Grant No. 2019NLTS005); and Clinical Research Special Fund of Wu Jieping Medical Foundation (320.6750.2020-20-13).
Acknowledgments
We would like to thank all of the patients and authors who contributed to this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin (2021) 71:7–33. doi: 10.3322/caac.21654
2. Den Brok WD, Speers CH, Gondara L, Baxter E, Tyldesley SK, Lohrisch CA. Survival with metastatic breast cancer based on initial presentation, de novo versus relapsed. Breast Cancer Res Treatment (2017) 161(3):549–56. doi: 10.1007/s10549-016-4080-9
3. Dawood S, Broglio K, Ensor J, Hortobagyi GN, Giordano SH. Survival differences among women with de novo stage IV and relapsed breast cancer. Ann Oncol (2010) 21:2169–74. doi: 10.1093/annonc/mdq220
4. Wang R, Zhu Y, Liu X, Liao X, He J, Niu L. The clinicopathological features and survival outcomes of patients with different metastatic sites in stage IV breast cancer. BMC Cancer (2019) 19:1091. doi: 10.1186/s12885-019-6311-z
5. Burguin A, Diorio C, Durocher F. Breast cancer treatments: Updates and new challenges. J Pers Med (2021) 11:808. doi: 10.3390/jpm11080808
6. Rashid NS, Grible JM, Clevenger CV, Harrell JC. Breast cancer liver metastasis: current and future treatment approaches. Clin Exp Metastasis (2021) 38(3):263–77. doi: 10.1007/s10585-021-10080-4
7. Gao JJ, Cheng J, Bloomquist E, Sanchez J, Wedam SB, Singh H, et al. CDK4/6 inhibitor treatment for patients with hormone receptor-positive, HER2-negative, advanced or metastatic breast cancer: a US food and drug administration pooled analysis. Lancet Oncol (2020) 21:250–60. doi: 10.1016/S1470-2045(19)30804-6
8. Swain SM, Miles D, Kim SB, Im YH, Im SA, Semiglazov V, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol (2020) 21:519–30. doi: 10.1016/S1470-2045(19)30863-0
9. Bardia A, Hurvitz SA, Tolaney SM, Loirat D, Punie K, Oliveira M, et al. Sacituzumab govitecan in metastatic triple-negative breast cancer. N Engl J Med (2021) 384:1529–41. doi: 10.1056/NEJMoa2028485
10. Murthy RK, Loi S, Okines A, Paplomata E, Hamilton E, Hurvitz SA, et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N Engl J Med (2020) 382:597–609. doi: 10.1056/NEJMoa1914609
11. Cardoso F, Spence D, Mertz S, Corneliussen-James D, Sabelko K, Gralow J, et al. Global analysis of advanced/metastatic breast cancer: Decade report (2005-2015). Breast (2018) 39:131–8. doi: 10.1016/j.breast.2018.03.002
12. Ruiz A, Wicherts DA, Sebagh M, Giacchetti S, Castro-Benitez C, van Hillegersberg R, et al. Predictive profile-nomogram for liver resection for breast cancer metastases: An aggressive approach with promising results. Ann Surg Oncol (2017) 24:535–45. doi: 10.1245/s10434-016-5522-7
13. Zhao HY, Gong Y, Ye FG, Ling H, Hu X. Incidence and prognostic factors of patients with synchronous liver metastases upon initial diagnosis of breast cancer: a population-based study. Cancer Manag Res (2018) 10:5937–50. doi: 10.2147/CMAR.S178395
14. Hagemeister FB Jr., Buzdar AU, Luna MA, Blumenschein GR. Causes of death in breast cancer: a clinicopathologic study. Cancer (1980) 46:162–7. doi: 10.1002/1097-0142(19800701)46:1<162::AID-CNCR2820460127>3.0.CO;2-B
15. Soni A, Ren Z, Hameed O, Chanda D, Morgan CJ, Siegal GP, et al. Breast cancer subtypes predispose the site of distant metastases. Am J Clin Pathol (2015) 143:471–8. doi: 10.1309/AJCPYO5FSV3UPEXS
16. Wyld L, Gutteridge E, Pinder SE, James JJ, Chan SY, Cheung KL, et al. Prognostic factors for patients with hepatic metastases from breast cancer. Br J Cancer (2003) 89:284–90. doi: 10.1038/sj.bjc.6601038
17. Tasleem S, Bolger JC, Kelly ME, Boland MR, Bowden D, Sweeney KJ, et al. The role of liver resection in patients with metastatic breast cancer: a systematic review examining the survival impact. Ir J Med Sci (2018) 187:1009–20. doi: 10.1007/s11845-018-1746-9
18. Xiao YB, Zhang B, Wu YL. Radiofrequency ablation versus hepatic resection for breast cancer liver metastasis: a systematic review and meta-analysis. J Zhejiang Univ Sci B (2018) 19:829–43. doi: 10.1631/jzus.B1700516
19. Adam R, Aloia T, Krissat J, Bralet MP, Paule B, Giacchetti S, et al. Is liver resection justified for patients with hepatic metastases from breast cancer? Ann Surg (2006) 244:897–907. doi: 10.1097/01.sla.0000246847.02058.1b
20. Mariani P, Servois V, De Rycke Y, Bennett SP, Feron JG, Almubarak MM, et al. Liver metastases from breast cancer: Surgical resection or not? a case-matched control study in highly selected patients. Eur J Surg Oncol (2013) 39:1377–83. doi: 10.1016/j.ejso.2013.09.021
21. Ruiz A, van Hillegersberg R, Siesling S, Castro-Benitez C, Sebagh M, Wicherts DA, et al. Surgical resection versus systemic therapy for breast cancer liver metastases: Results of a European case matched comparison. Eur J Cancer (2018) 95:1–10. doi: 10.1016/j.ejca.2018.02.024
22. Lin Z, Yan S, Zhang J, Pan Q. A nomogram for distinction and potential prediction of liver metastasis in breast cancer patients. J Cancer (2018) 9:2098–106. doi: 10.7150/jca.24445
23. Lobbezoo DJ, van Kampen RJ, Voogd AC, Dercksen MW, van den Berkmortel F, Smilde TJ, et al. Prognosis of metastatic breast cancer: are there differences between patients with de novo and recurrent metastatic breast cancer? Br J Cancer (2015) 112:1445–51. doi: 10.1038/bjc.2015.127
24. Zhao W, Wu L, Zhao A, Zhang M, Tian Q, Shen Y, et al. A nomogram for predicting survival in patients with de novo metastatic breast cancer: a population-based study. BMC Cancer (2020) 20:982. doi: 10.1186/s12885-020-07449-1
25. Dieci MV, Barbieri E, Piacentini F, Ficarra G, Bettelli S, Dominici M, et al. Discordance in receptor status between primary and recurrent breast cancer has a prognostic impact: a single-institution analysis. Ann Oncol (2013) 24:101–8. doi: 10.1093/annonc/mds248
26. Edge SB, Compton CC. The American joint committee on cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol (2010) 17:1471–4. doi: 10.1245/s10434-010-0985-4
27. Sinn HP, Kreipe H. A brief overview of the WHO classification of breast tumors, 4th edition, focusing on issues and updates from the 3rd edition. Breast Care (Basel) (2013) 8:149–54. doi: 10.1159/000350774
28. Genestie C, Zafrani B, Asselain B, Fourquet A, Rozan S, Validire P, et al. Comparison of the prognostic value of scarff-Bloom-Richardson and Nottingham histological grades in a series of 825 cases of breast cancer: major importance of the mitotic count as a component of both grading systems. Anticancer Res (1998) 18:571–6.
29. Li Y, Li Q, Mo H, Guan X, Lin S, Wang Z, et al. Incidence, risk factors and survival of patients with brain metastases at initial metastatic breast cancer diagnosis in China. Breast (2021) 55:30–6. doi: 10.1016/j.breast.2020.11.021
30. Lin S, Mo H, Li Y, Guan X, Chen Y, Wang Z, et al. Risk factors and survival of patients with liver metastases at initial metastatic breast cancer diagnosis in han population. Front Oncol (2021) 11:670723. doi: 10.3389/fonc.2021.670723
31. Ji L, Fan L, Zhu X, Gao Y, Wang Z. A prognostic model for breast cancer with liver metastasis. Front Oncol (2020) 10:1342. doi: 10.3389/fonc.2020.01342
32. Trovo M, Furlan C, Polesel J, Fiorica F, Arcangeli S, Giaj-Levra N, et al. Radical radiation therapy for oligometastatic breast cancer: Results of a prospective phase II trial. Radiother Oncol (2018) 126:177–80. doi: 10.1016/j.radonc.2017.08.032
33. Liberchuk AN, Deipolyi AR. Hepatic metastasis from breast cancer. Semin Intervent Radiol (2020) 37:518–26. doi: 10.1055/s-0040-1720949
34. Ji L, Cheng L, Zhu X, Gao Y, Fan L, Wang Z. Risk and prognostic factors of breast cancer with liver metastases. BMC Cancer (2021) 21:238. doi: 10.1186/s12885-021-07968-5
35. Cummings MC, Simpson PT, Reid LE, Jayanthan J, Skerman J, Song S, et al. Metastatic progression of breast cancer: insights from 50 years of autopsies. J Pathol (2014) 232:23–31. doi: 10.1002/path.4288
36. Purushotham A, Shamil E, Cariati M, Agbaje O, Muhidin A, Gillett C, et al. Age at diagnosis and distant metastasis in breast cancer–a surprising inverse relationship. Eur J Cancer (2014) 50:1697–705. doi: 10.1016/j.ejca.2014.04.002
37. Kataoka A, Iwamoto T, Tokunaga E, Tomotaki A, Kumamaru H, Miyata H, et al. Young adult breast cancer patients have a poor prognosis independent of prognostic clinicopathological factors: a study from the Japanese breast cancer registry. Breast Cancer Res Treat (2016) 160:163–72. doi: 10.1007/s10549-016-3984-8
38. Cheng SH, Tsou MH, Liu MC, Jian JJ, Cheng JC, Leu SY, et al. Unique features of breast cancer in Taiwan. Breast Cancer Res Treat (2000) 63:213–23. doi: 10.1023/A:1006468514396
39. Copson E, Eccles B, Maishman T, Gerty S, Stanton L, Cutress RI, et al. Prospective observational study of breast cancer treatment outcomes for UK women aged 18-40 years at diagnosis: the POSH study. J Natl Cancer Inst (2013) 105:978–88. doi: 10.1093/jnci/djt134
40. van de Water W, Markopoulos C, van de Velde CJ, Seynaeve C, Hasenburg A, Rea D, et al. Association between age at diagnosis and disease-specific mortality among postmenopausal women with hormone receptor-positive breast cancer. JAMA (2012) 307:590–7. doi: 10.1001/jama.2012.84
41. Bale R, Putzer D, Schullian P. Local treatment of breast cancer liver metastasis. Cancers (Basel) (2019) 11:1341. doi: 10.3390/cancers11091341
42. Harrell JC, Prat A, Parker JS, Fan C, He X, Carey L, et al. Genomic analysis identifies unique signatures predictive of brain, lung, and liver relapse. Breast Cancer Res Treat (2012) 132:523–35. doi: 10.1007/s10549-011-1619-7
43. Smid M, Wang Y, Zhang Y, Sieuwerts AM, Yu J, Klijn JG, et al. Subtypes of breast cancer show preferential site of relapse. Cancer Res (2008) 68:3108–14. doi: 10.1158/0008-5472.CAN-07-5644
44. Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH, et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol (2010) 28:3271–7. doi: 10.1200/JCO.2009.25.9820
45. Kaplan MA, Arslan UY, Isikdogan A, Dane F, Oksuzoglu B, Inanc M, et al. Biological subtypes and distant relapse pattern in breast cancer patients after curative surgery (Study of Anatolian society of medical oncology). Breast Care (Basel) (2016) 11:248–52. doi: 10.1159/000448186
46. Ekpe E, Shaikh AJ, Shah J, Jacobson JS, Sayed S. Metastatic breast cancer in Kenya: Presentation, pathologic characteristics, and patterns-findings from a tertiary cancer center. J Glob Oncol (2019) 5:1–11. doi: 10.1200/JGO.19.00036
47. Arciero CA, Guo Y, Jiang R, Behera M, O'Regan R, Peng L, et al. ER(+)/HER2(+) breast cancer has different metastatic patterns and better survival than ER(-)/HER2(+) breast cancer. Clin Breast Cancer (2019) 19:236–45. doi: 10.1016/j.clbc.2019.02.001
48. Sihto H, Lundin J, Lundin M, Lehtimaki T, Ristimaki A, Holli K, et al. Breast cancer biological subtypes and protein expression predict for the preferential distant metastasis sites: a nationwide cohort study. Breast Cancer Res (2011) 13:R87. doi: 10.1186/bcr2944
49. Largillier R, Ferrero JM, Doyen J, Barriere J, Namer M, Mari V, et al. Prognostic factors in 1,038 women with metastatic breast cancer. Ann Oncol (2008) 19:2012–9. doi: 10.1093/annonc/mdn424
Keywords: breast cancer, risk factors, liver metastasis, de novo distant metastasis, relapsed distant metastasis, prognosis
Citation: Zhang N, Xiang Y, Shao Q, Wu J, Liu Y, Long H, Tao D and Zeng X (2023) Different risk and prognostic factors for liver metastasis of breast cancer patients with de novo and relapsed distant metastasis in a Chinese population. Front. Oncol. 13:1102853. doi: 10.3389/fonc.2023.1102853
Received: 19 November 2022; Accepted: 27 March 2023;
Published: 14 April 2023.
Edited by:
Xin Zhang, Jiangmen Central Hospital, ChinaReviewed by:
Riccardo Boetto, University of Padua, ItalySan-Gang Wu, First Affiliated Hospital of Xiamen University, China
Copyright © 2023 Zhang, Xiang, Shao, Wu, Liu, Long, Tao and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dan Tao, taodan2003@126.com; Xiaohua Zeng, zxiaohuacqu@126.com
 Ningning Zhang
Ningning Zhang Yimei Xiang2
Yimei Xiang2 Qing Shao
Qing Shao Dan Tao
Dan Tao Xiaohua Zeng
Xiaohua Zeng