- Department of Laboratory Medicine, The Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan, China
Background: Circular RNAs (circRNAs) are receiving increasing attention as novel biomarkers. Our goal was to investigate the diagnostic, clinicopathological, and prognostic utility of circRNAs in prostate cancer (PCa).
Methods: Relevant literature was searched in PubMed, Web of Science, and EMBASE. Sensitivity, specificity, diagnostic odds ratio (DOR), negative likelihood ratio (NLR), positive likelihood ratio (PLR), and the area under the curve (AUC) were calculated to evaluate the diagnostic accuracy of circRNA expression. circRNAs’ clinical, pathological, and prognostic value was examined using pooled odds ratios (ORs) and hazard ratios (HRs).
Results: This meta-analysis included 23 studies, with 5 for diagnosis, 16 for clinicopathological parameters, and 10 for prognosis. For diagnostic value, the pooled sensitivity, pooled specificity, PLR, NLR, DOR, and AUC were 0.82, 0.62, 2.17, 0.29, 7.37, and 0.81, respectively. Upregulation of carcinogenic circRNAs was associated with poor clinical parameters (Gleason score: OR = 0.222, 95% CI: 0.145–0.340; T classification: OR = 0.274, 95% CI: 0.175–0.430; lymph node metastasis: OR = 0.353, 95% CI: 0.175–0.716; tumor size: OR = 0.226, 95% CI: 0.099–0.518) and could predict poor survival outcomes (HR = 2.408, 95% CI: 1.559–3.720, p < 0.001). Conversely, downregulation of tumor-suppressor circRNAs was also associated with poor clinical parameters (Gleason score: OR = 1.689, 95% CI: 1.144–2.493; T classification: OR = 2.586, 95% CI: 1.779–3.762) and worse prognosis (HR = 1.739, 95% CI: 1.147–2.576, p = 0.006).
Conclusion: Our results showed that circRNAs might be useful biomarkers for the diagnosis and prognosis of PCa.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42021284785.
Introduction
Prostate cancer (PCa) is one of the most common malignancies in men worldwide, accounting for approximately 27% of all cancer cases with a high mortality rate (1). According to the global cancer statistics, in 2018, there were about 1,276,106 new cases and 358,989 death cases every year (2). Although the morbidity of PCa is lower in China than in other countries, the annual incidence has been on the rise (3). Early-stage PCa can be cured by surgery and chemotherapy, with 5-year survival rates exceeding 90%. The inhibition of gonadal testosterone production with androgen deprivation therapy has been the cornerstone of PCa treatment (4). In addition, multiple drugs, including abiraterone, enzalutamide, apalutamide, darolutamide, and docetaxel, have been approved for advanced PCa (4, 5). However, most advanced PCa can turn into castration-resistant PCa after long-term castration treatments, with 5-year survival rates, were below 30% (5, 6). Therefore, biomarkers are needed urgently for early diagnosis and for assessing the prognostic status of patients with PCa.
In recent years, prostate-specific antigen (PSA) has been used for the early determination and staging of PCa. However, its accuracy in predicting prognosis and biochemical recurrence is still questionable; thus, PSA is not recommended by experts (7, 8). Therefore, there is an urgent need to explore new biomarkers with high sensitivity, specificity, and reproducibility in the diagnosis, prognosis, and treatment of PCa.
Circular RNAs (circRNAs) are endogenous non-coding RNAs that range from a few hundred to thousands of nucleotides (9). circRNAs were once thought to be splicing faults; however, their structure was discovered because of the rapid growth of whole-genome sequencing (10). The circRNAs’ 3′ and 5′ ends covalently form a loop, without the 5′ cap and 3′ poly(A) tails, making them structurally conserved and stable (11, 12). circRNAs are implicated in a variety of biological activities (13), including microRNA or protein sequestration, transcription regulation, splicing interference, and polypeptide translation (14). In addition, they play a role in physiological illnesses such as cancer cell proliferation, differentiation, apoptosis, and metastasis (15). Meanwhile, circRNAs are increasingly linked to the incidence and progression of PCa. Wang et al. (16) discovered that high levels of hsa_circ 0088233 increased the malignant phenotypes of PCa by sequestering miR-515-5p and inducing FKBP1A expression. Xia et al. explored the diagnostic value of circ_0057558 and circ_0062019 in PCa (17). Moreover, the prognostic role of circ_PSMC3 in PCa has also been explored in other studies (18). However, detailed discussions on the role of circRNAs in diagnostic and prognostic value are still lacking. In the present meta-analysis, we included papers on the involvement of circRNAs in PCa and investigated their potential diagnostic, clinicopathological, and prognostic relevance.
Materials and methods
Registration
The protocol was registered on the Prospective Register of Systematic Reviews (PROSPERO) with registration number CRD42021284785.
Data search strategy
Our literature search was guided by the recently published PRISMA statement (19, 20).
We searched all relevant articles through PubMed, Embase, and Web of Science online databases that were published before 8 October 2021.
To avoid omission, two independent researchers completed the retrieval process by combing Medical Subject Heading (MeSH) terms and text words. For literature retrieval, the following MeSH terms and text words were used: (1) “Prostatic Neoplasms”, “Prostate Cancer”, or “PCa”; (2) “RNA”, “Circular”, or “circRNAs”. The detailed search strategy is shown in Supplementary Material. The language was limited to English. In addition, the references of the identified studies were also searched for relevant documents. Other details are provided in the Supplementary Material. The authors of the included articles were contacted when deemed necessary.
Inclusion and exclusion criteria
Two independent investigators assessed the appropriate studies and extracted the imperative data, and disagreements were resolved by discussing with the third researcher. Studies were included if they assessed the accuracy of circRNA for differentiation between PCa and non-PCa patients. To be eligible, studies needed a clearly defined standard of reference. We defined PCa according to the guidelines of the European Association of Urology, the International Society of Geriatric Oncology (21), and the National Comprehensive Cancer Network (22).
The inclusion criteria were as follows: (1) patients with a pathological diagnosis of PCa; (2) expression level of circRNAs could be divided into high and low expression; (3) studies that included data to estimate the diagnostic, prognostic, and clinicopathologic features; and (4) cohort or case-control research.
The exclusion criteria are as follows: (1) duplicate studies; (2) reviews, meta-analyses, letters, conference abstracts, or case reports; (3) articles without complete information; (4) for diagnostic meta, those without clear tests and control group size, and those without true positive (TP), true negative (TN), false positive (FP), and false negative (FN) or sensitivity (SEN), specificity (SPC), and receiver operating characteristics (ROC); and (5) for the prognostic meta-analysis, those without clear information on the number of trial and control groups, survival information, and Kaplan-Meier (KM) plots.
Data extraction
Two researchers extracted the data independently. The extracted information was as follows: (1) first author, publication year, type of cancer, circRNAs, numbers of patients, detection methods, and outcomes; (2) follow-up time and outcomes; (3) sensitivity, specificity, and the areas under the curve (AUCs) of circRNAs for diagnosis; and (4) clinicopathological features including age, smoking, drinking, TNM stage, T classification, lymph node metastasis, distant metastasis, and Gleason score. If the HRs with 95% CIs for outcomes were not shown directly in the article, then the survival data were extracted from KM plots by Engauge Digitizer 4.1 software. The HRs and 95% CIs were calculated using the Excel program file provided by Tierney et al. (23). If the parameters of TP, TN, FP, and FN were not offered, then we assessed them according to sample size, SPC, SEN, and AUC.
Quality assessment
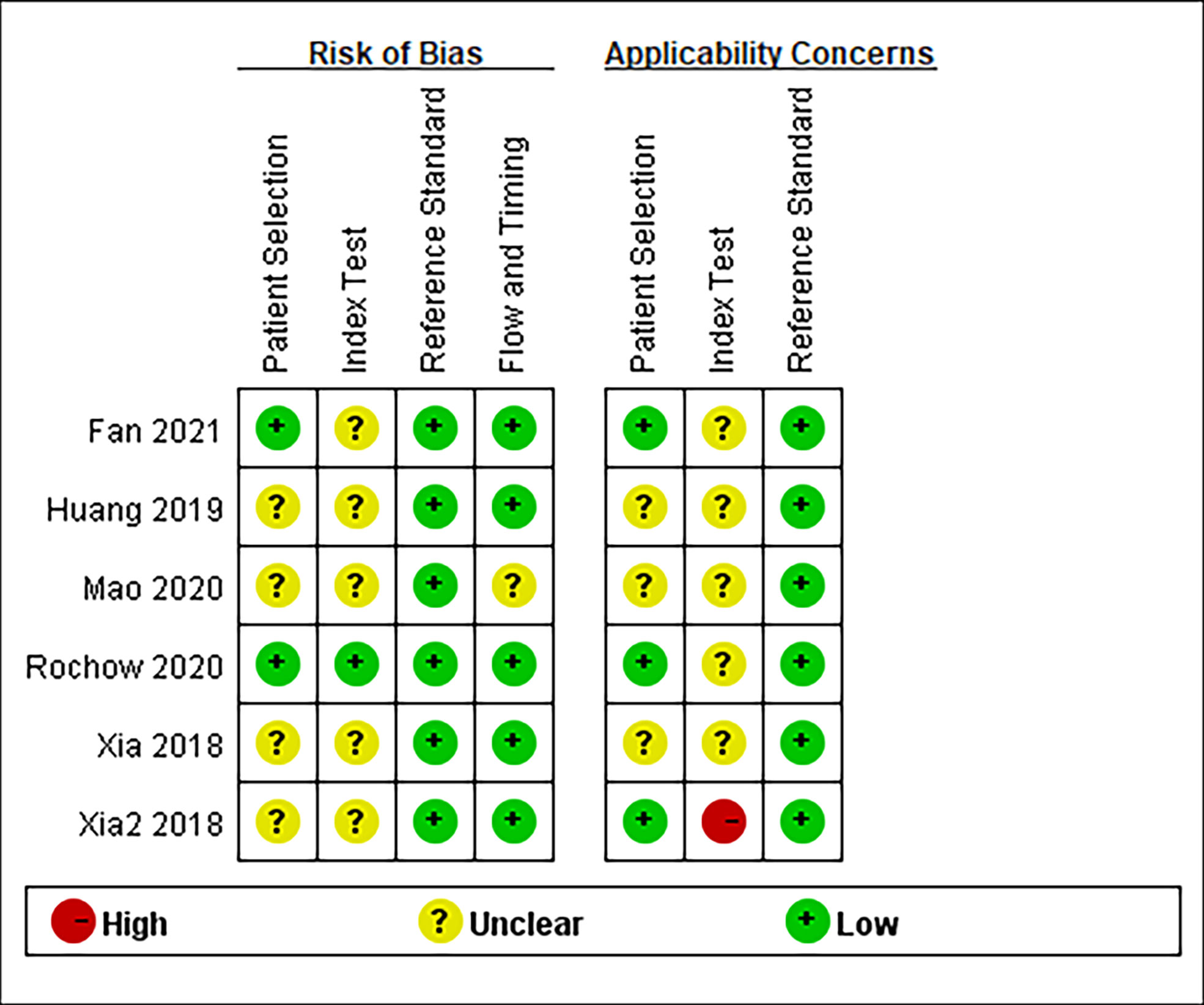
Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) and Newcastle Ottawa Scale (NOS) were used by two independent investigators to assess the quality of the studies for diagnosis and prognosis, respectively. The four domains of QUADAS-2 were as follows: patient selection, index test, reference standard, and flow and timing. Bias risk was graded as high (H), low (L), or unclear (U). The total scores of NOS ranged from 0 to 8, and the studies that were higher than 6 in NOS were considered high-quality studies.
Statistical analysis
Review Manager 5.3 and Stata 15.0 were used for statistical analysis. I2 statistics were used to perform the heterogeneity test. We determined that there was considerable heterogeneity among the included studies if the I2 value > 50% and the p-value < 0.05. Random-effects model was used to examine the pooled results. If there was no significant heterogeneity in the included studies, then a fixed-effects model was used. A p-value < 0.05 was used to determine statistical significance.
In the diagnostic meta-analysis, the number of TP, FP, FN, and TN was combined to calculate pooled results of sensitivity, specificity, diagnostic odds ratio (DOR), negative likelihood ratio (NLR), and positive likelihood ratio (PLR). The area under the summary ROC (SROC) was calculated to determine the diagnostic accuracy of circRNA expression. Deeks’ funnel plot asymmetry test was used to investigate potential publishing bias. If the p-value > 0.1, then we considered that there was no publishing bias. Pooled ORs and 95% CIs were used to explore the association between circRNA expression and clinicopathological features. For the prognostic meta-analysis, HRs and 95% CIs were used to assess the prognostic value of circRNAs. Egger’s tests were used to determine the possibility of publication bias. To determine the stability of the pooled HR, a sensitivity analysis was done. If the p-value > 0.1 for Egger’s tests, then we considered that there was no publication bias.
Results
Search results
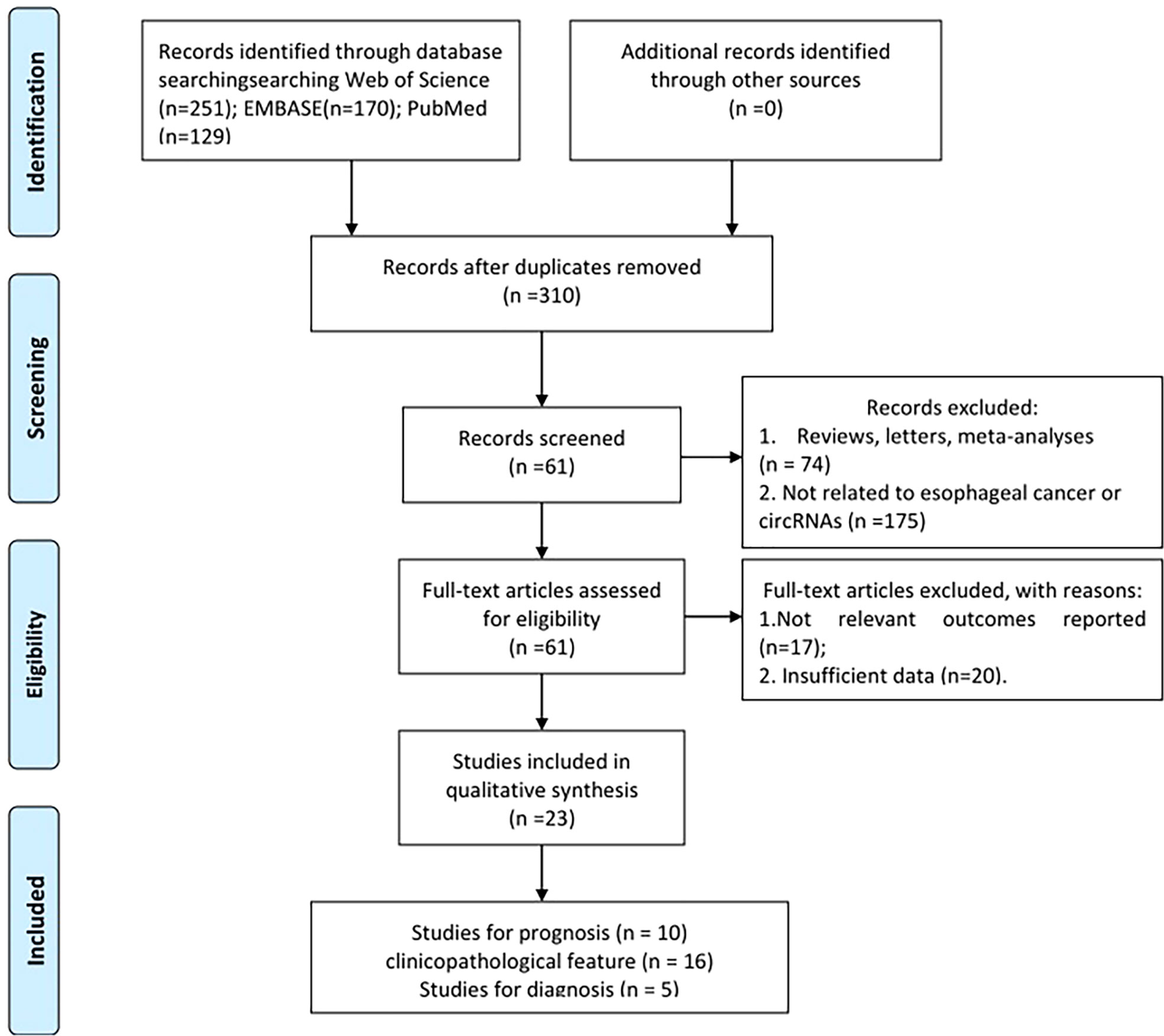
The flow diagram for study selection is given in Figure 1. A total of 550 relevant studies were found from PubMed (129 records), Embase (170 records), and Web of Science (251 records) from initial screening. After eliminating duplicate items, 310 articles were obtained. Furthermore, 249 articles were filtered out for inappropriate types (175 irrelevant articles and 74 reviews, letters, or meta-analyses). After the review of full-text articles, 37 articles were excluded for the following reasons: 17 did not include relevant outcomes and 20 did not report complete data. Finally, 23 studies ranging from 2018 to 2021 were screened for meta-analysis, including 5 for diagnosis, 16 for clinicopathological features, and 10 for prognosis (1, 14, 16–18, 24–40).
Study characteristics and quality assessment
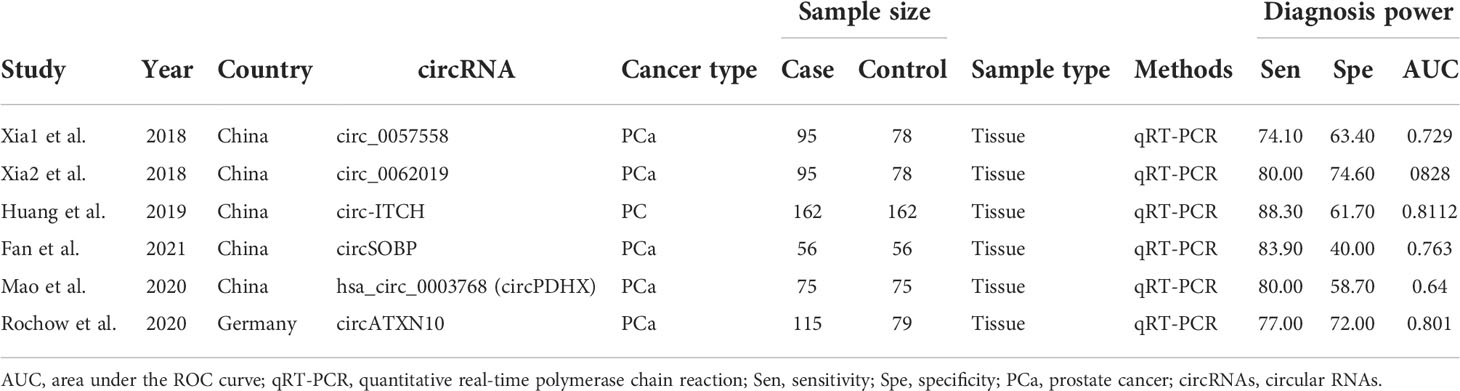
The essential characteristics of the included studies are shown in Tables 1–4. A total of 23 circRNAs were included and published between 2018 and 2021. Quantitative real-time reverse transcription PCR (qRT-PCR) was used to calibrate the expression of circRNAs. The majority of the included studies were from China. Among them, some elements employed in the diagnostic analysis, such as sensitivity, specificity, and AUC, are recorded in Table 1. The range of sample size was between 112 and 324. The quality of the contained literature was evaluated in terms of bias risk and applicability concerns. The results indicated that the quality of our included research was good (Figure 2).
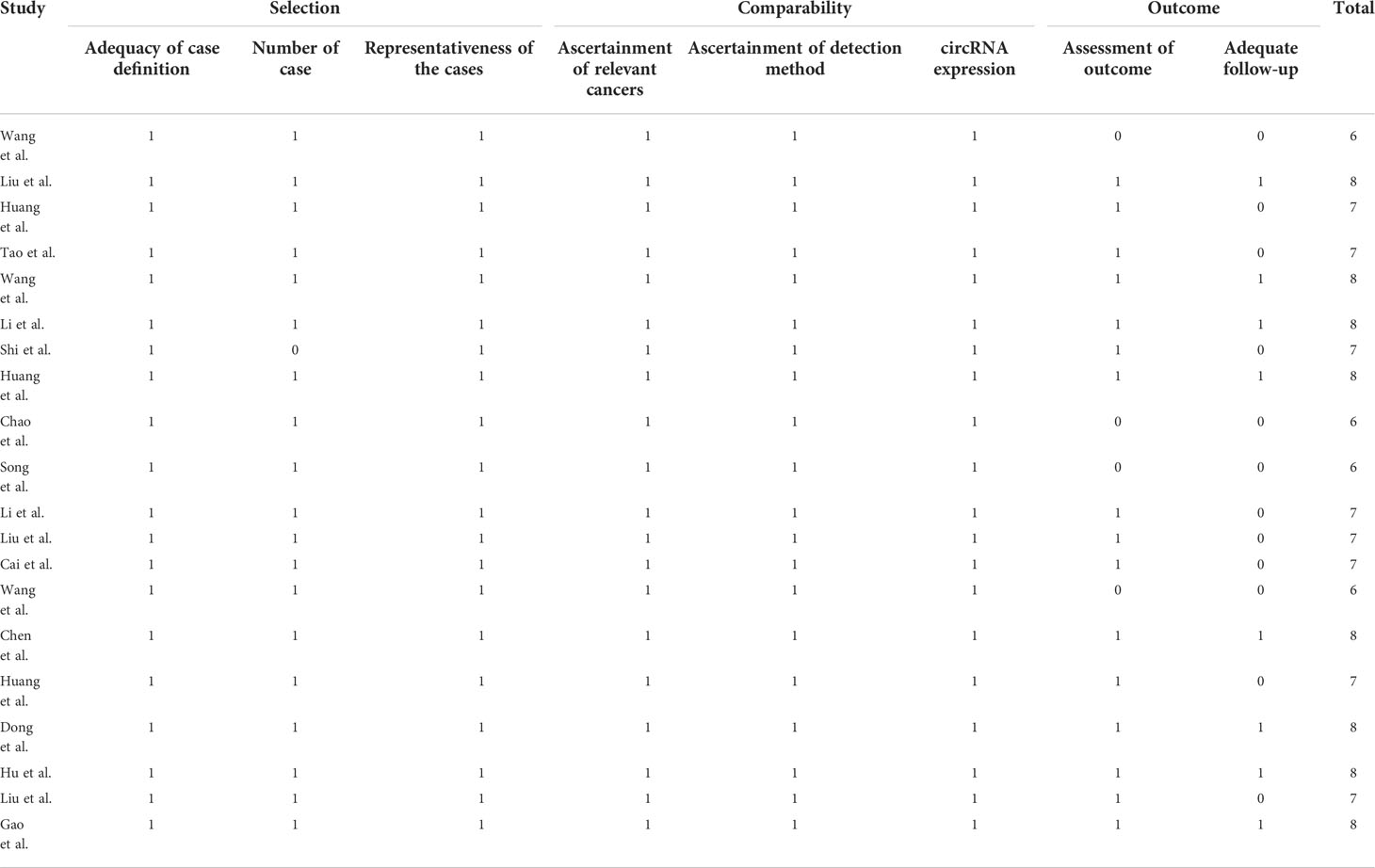
Table 4 Quality assessment of eligible studies for clinical parameter analysis and prognosis analysis according to the Newcastle-Ottawa Scale.
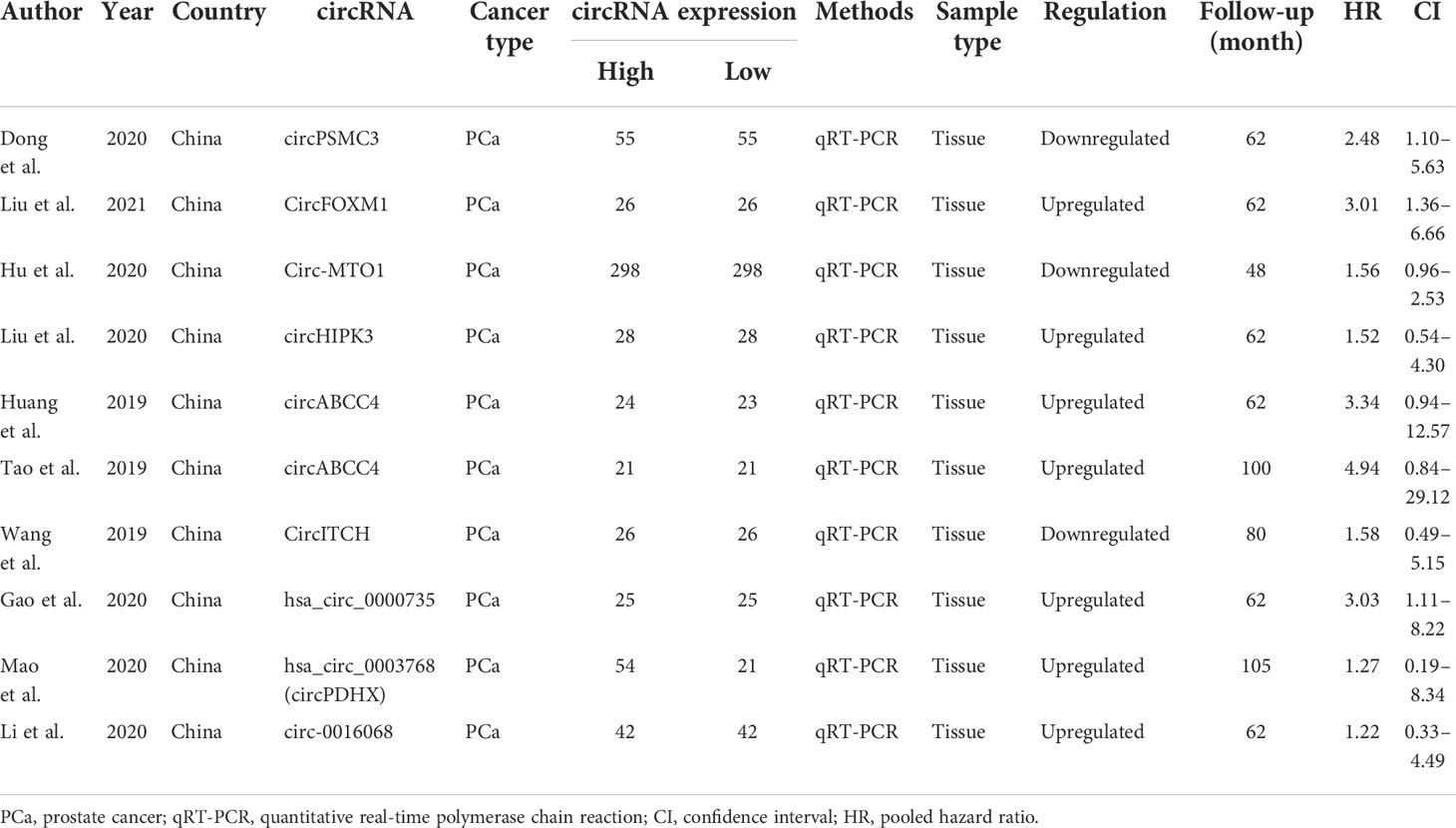
The relationship between clinicopathological characteristics and circRNAs is given in Table 2. As indicated in Table 3, a total of 10 circRNAs were used in 10 investigations, providing some basic information about the prognostic analysis. The patients’ follow-up time ranged from 48 to 105 months, and the number of samples collected ranged from 42 to 596. The NOS scores indicated the excellent quality of studies that were included for clinical parameter analysis and prognostic analysis on each of the eight dimensions (Table 4).
Diagnosis analysis
The diagnostic meta-analysis included 1,024 patients from five studies that were all qualified. The pooled SEN and SPC were calculated to examine the diagnostic utility of circRNAs, and the findings are shown in Figure 3. A random-effects model was used because of the observable heterogeneity (I2 = 67.42% and I2 = 77.73%). As the results showed, the pooled SPC was 0.82 (95% CI: 0.76–0.86), whereas the pooled SEN was 0.62 (95% CI: 0.53–0.71). Moreover, the AUC was 0.81 (95% CI: 0.77–0.84) according to the SROC curve analysis (Figure 4). Thus, it could be seen that these circRNAs had excellent diagnostic efficacy in distinguishing patients with PCa from the healthy population. As shown in the bivariate boxplot and Galbraith plot (Figures 5A, B), one study fell outside the game and Galbraith diagrams, respectively, which suggested that there was heterogeneity in the analysis. Because of the limited inclusion of articles, a subgroup analysis could not be performed to find the sources of heterogeneity. In addition, the DOR was 7.37 (95% CI: 4.87–11.14). The pooled PLR and NLR were 2.17 (95% CI: 1.73–2.71) and 0.29 (95% CI: 0.23–0.38), respectively (Figures 6A–C). These results also demonstrated the excellent diagnostic ability of circRNAs in patients with PCa. These findings suggested that circRNAs have good diagnostic accuracy for PCa when taken combined.
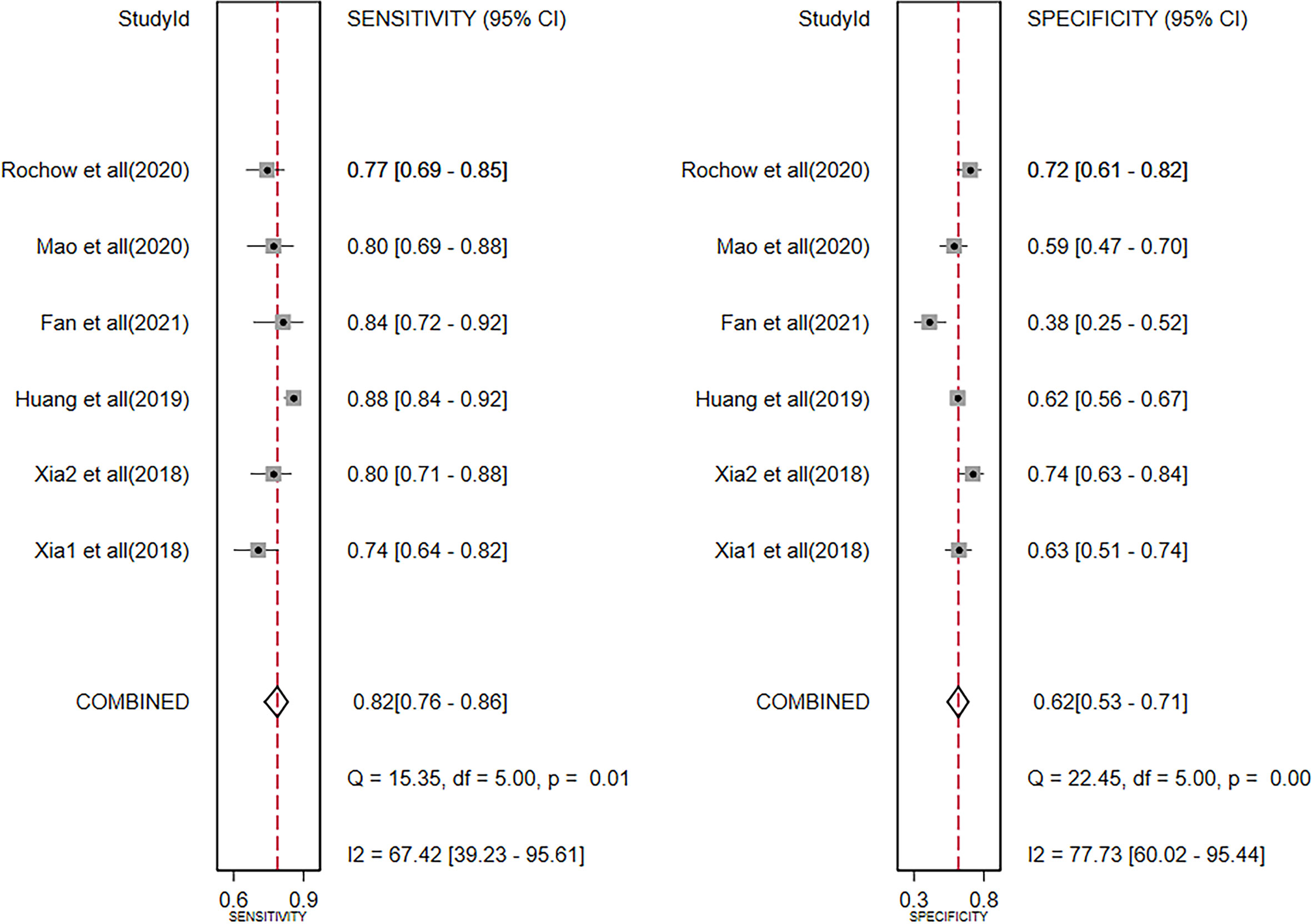
Figure 3 Forest plots of summary sensitivity and specificity to illustrate the diagnostic value of circRNAs for PCa. circRNAs, circular RNAs; PCa, prostate cancer.
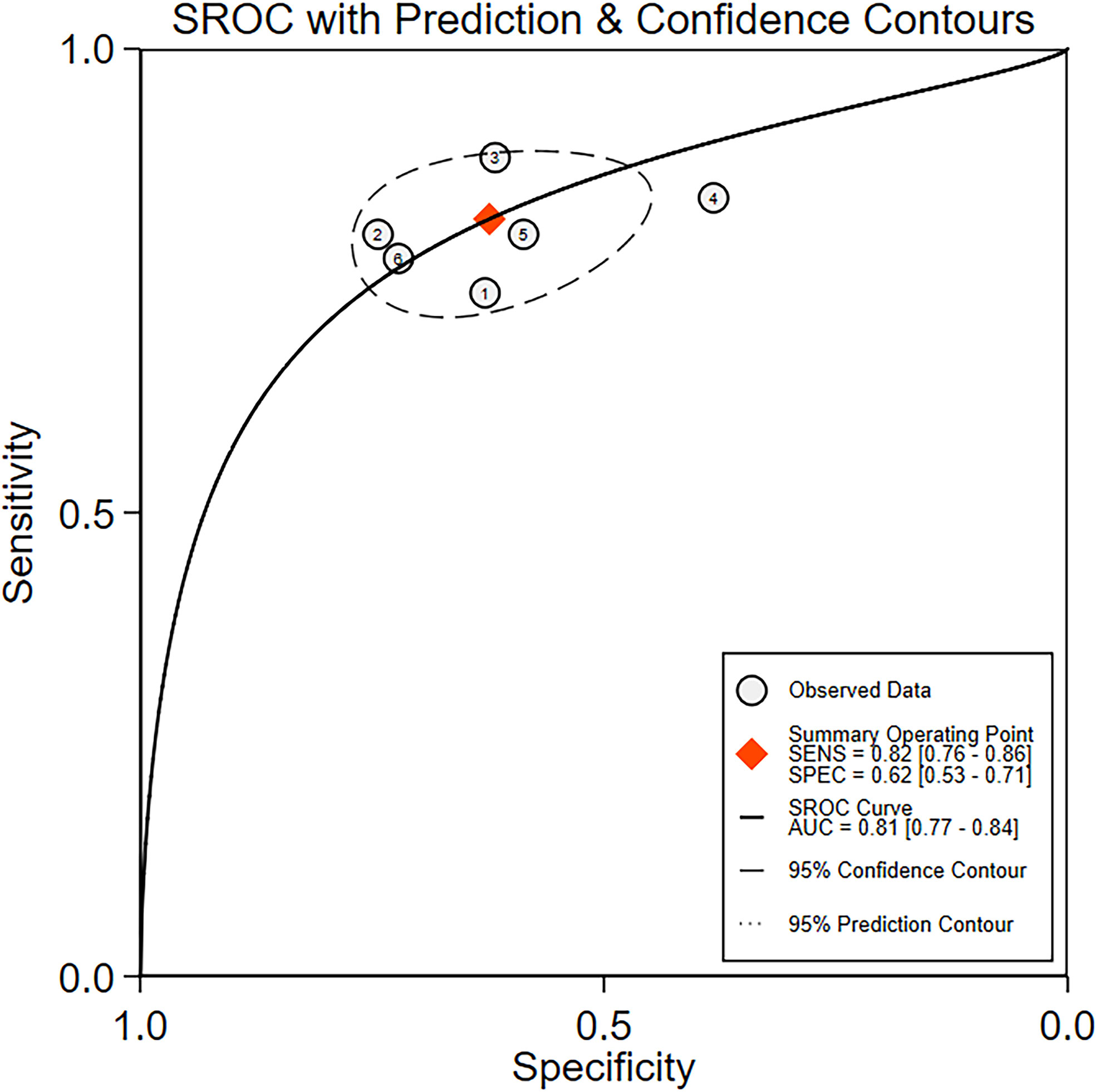
Figure 4 The summary receiver operating characteristic (SROC) curve based on circRNAs for diagnosis analysis. ROC, receiver operator characteristic.
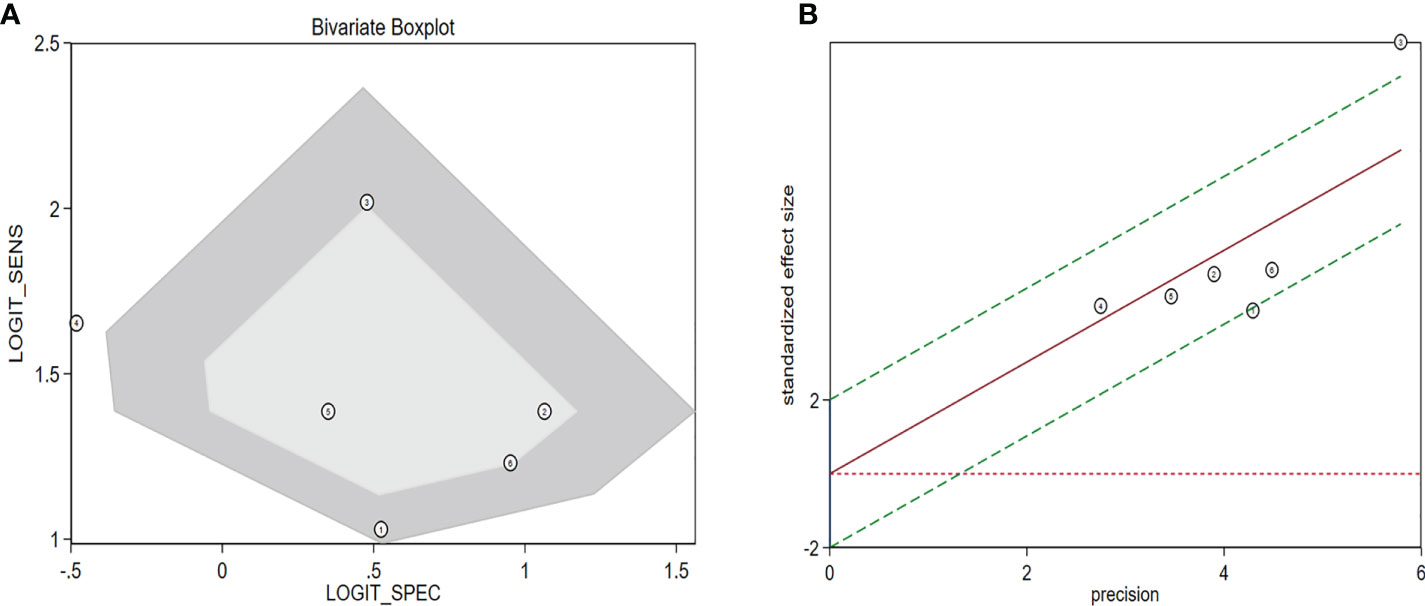
Figure 5 Assessment of the diagnostic accuracy of circRNAs in PCa. (A) Bivariate boxplot. (B) Galbraith plot. circRNAs, circular RNAs; PCa, prostate cancer.
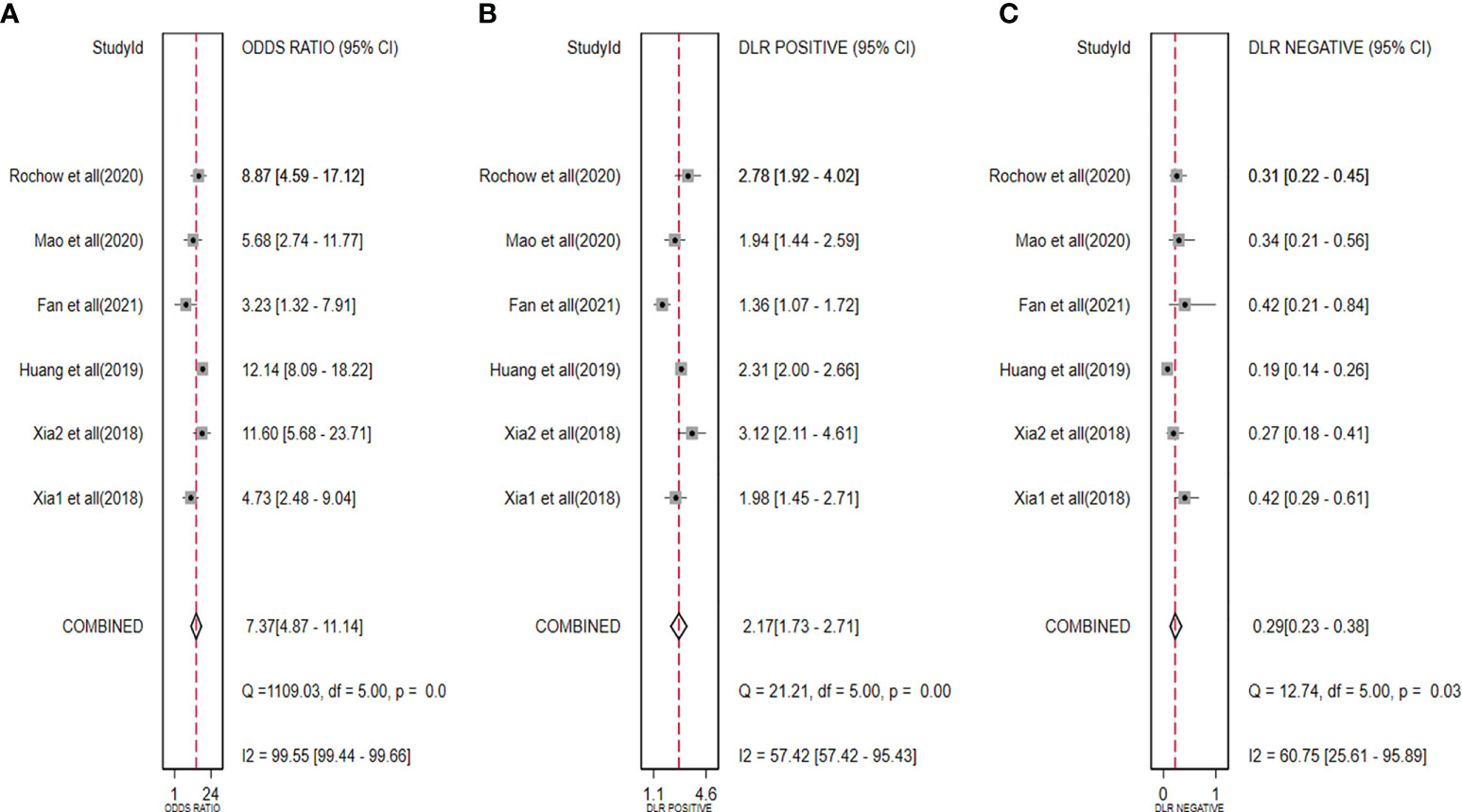
Figure 6 Forest plots for pooled DOR (A), PLR (B), and NLR (C) of circRNAs for PCa. DOR, diagnostic odds radio; PLR, positive likelihood ratio; NLR, negative likelihood ratio; circRNAs, circular RNAs; PCa, prostate cancer.
Clinicopathological parameters
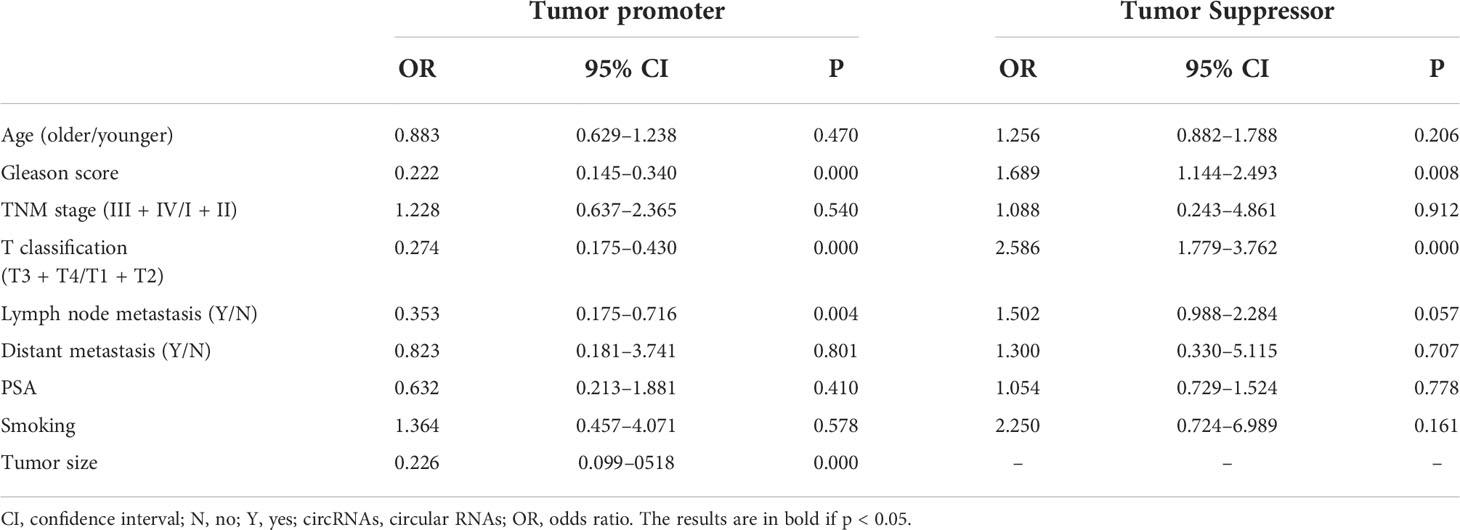
The association between circRNAs and clinicopathological features is shown in Table 3. For clinicopathological features, 16 studies were enrolled in our meta-analysis with a total of 1,153 patients. Upregulation of carcinogenic circRNAs was linked to adverse clinical characteristics (Gleason score: OR = 0.222, 95% CI: 0.145–0.340; T classification: OR = 0.274, 95% CI: 0.175–0.430; lymph node metastasis: OR = 0.353, 95% CI: 0.175–0.716; tumor size: OR = 0.226, 95% CI: 0.099–0518). Furthermore, decreased expression of tumor-suppressor circRNAs was also linked to worse clinical outcomes (Gleason score: OR = 1.689, 95% CI: 1.144–2.493; T classification: OR = 2.586, 95% CI: 1.779–3.762);. Remarkably, there was no correlation between circRNA expression and other clinicopathologic factors such as age, TNM stage, distant metastasis, expression of PSA, and smoking.
Prognosis analysis
In our work, the prognosis meta-analysis included 1,164 participants from 10 investigations, all of which were eligible. The role of upregulated circRNAs in PCa prognosis was estimated using fixed-effect models (I2 = 0.0%, p = 0.782), and the results demonstrated that upregulation of carcinogenic circRNAs was linked to a poor prognosis (HR = 2.408, 95% CI: 1.559–3.720, p < 0.001) (Figure 7A). At the same time, downregulation of tumor-suppressor circRNAs was significantly associated with worse PCa prognosis (HR = 1.739, 95% CI: 1.147–2.576, p = 0.006). Because there was no heterogeneity between studies (I2 = 0%, p = 0.624), a fixed-effects model was used (Figure 7B).
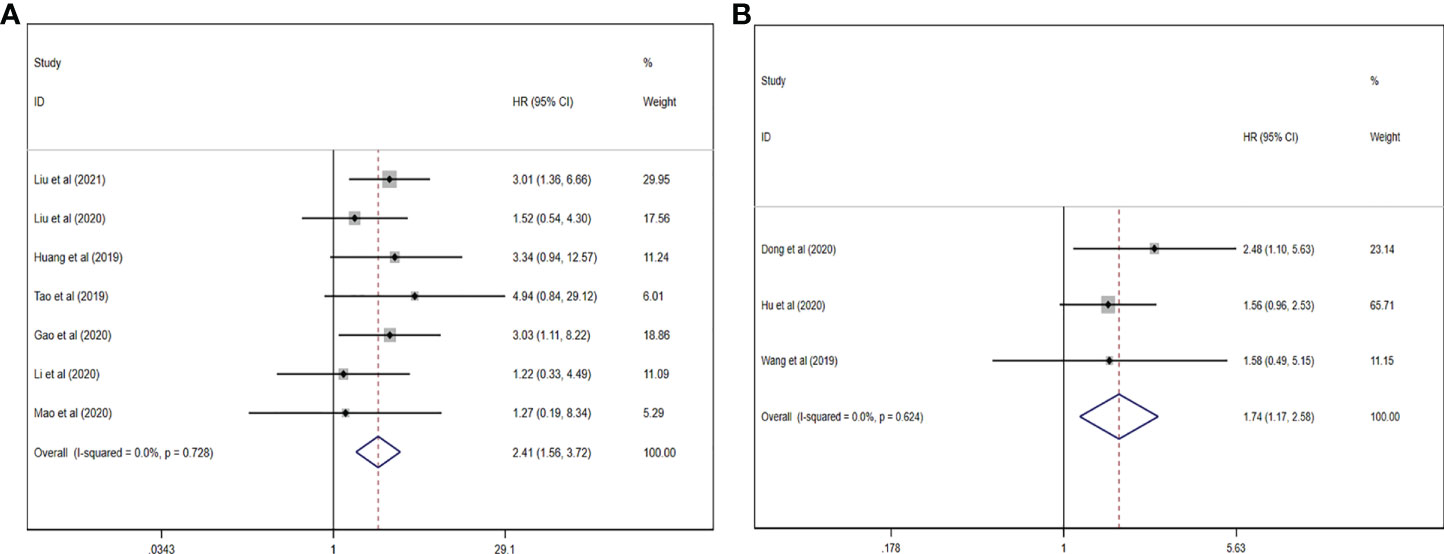
Figure 7 Forest plots for the association between circRNAs and estimating HR in PCa. (A) Upregulated circRNAs. (B) Downregulated circRNAs. HR, hazard ratios; PCa, prostate cancer; circRNAs, circular RNAs.
Publication bias and sensitivity analysis
We used the Deeks’ funnel plot asymmetry test to analyze the potential publication bias of diagnostic meta-analysis and found that there was no clear publication bias (p = 0.07) (Figure 8A). Furthermore, the potential publication bias of prognostic meta-analysis was investigated, which showed no publication bias in the included studies (Figure 8B). In addition, the Egger’s test also supported the conclusion that there was no publication bias (p = 0:85, Figure 8C). At the same time, in our research, sensitivity analysis revealed that the pooled results in the prognostic meta-analysis were stable (Figure 8D).
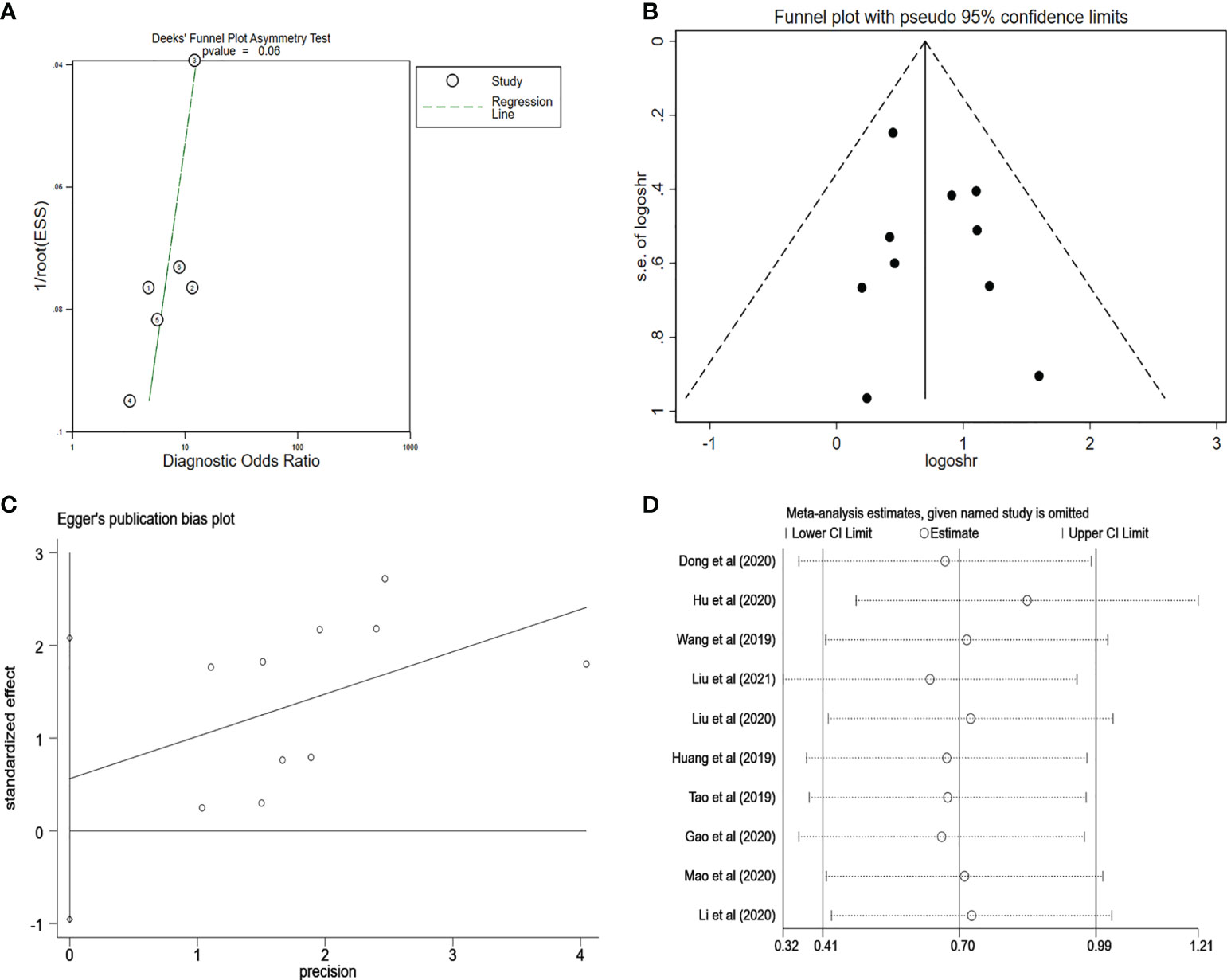
Figure 8 (A) Deeks’ funnel plot for evaluating the publication bias of the included study on the diagnosis analysis. (B) Funnel plot for evaluating the publication bias of the included study on the prognosis analysis. (C) Egger’s funnel plot for evaluating the publication bias of the included study on the prognosis analysis. (D) Sensitivity analysis for evaluating the influence of the omitted study on the pooled HR. PCa, prostate cancer; HR, hazard ratio.
Discussion
In recent years, the high incidence and mortality of PCa have caused worldwide concern (41, 42). The diagnosis and prognosis of PCa are currently determined by tissue biopsy and PSA levels (42). However, tissue biopsy is invasive, and the predicted accuracy of PSA should be furthermore improved. Therefore, PSA has been steadily discouraged from being advised in recent years (43, 44).
Many researchers are looking for new biomarkers to determine the diagnosis and prognosis of patients with PCa to enhance their survival (45). circRNAs are non-coding RNAs playing an important role in the development and progression of cancer (46). At the same time, circRNAs were thought to have promising prospects and advantages as perfect biomarkers for human cancer diagnosis and prognosis (47).
Therefore, as a novel biomarker, circRNAs have several advantages for clinical applications. First, traditional puncture biopsy entails a risk of injuries and is more difficult for the operator to perform. In contrast, circRNAs in plasma are more readily available and harmless. Second, circRNAs are resistant to denaturation because of their stable structures and conservative sequences. Third, circRNAs outperform standard markers in terms of diagnostic and prognostic value, as well as accuracy.
The aberrant expression of circRNAs has been shown in numerous investigations in patients with PCa; with a few studies, few meta-analyses have been published on the role of circRNAs in PCa diagnosis or prognosis. Our study is the first to discuss the link between circRNA expression and diagnostic performance, prognostic value, and clinical characteristics in PCa.
In our meta-analysis, the aggregated data revealed an AUC of 0.81, with a sensitivity of 0.82 and a specificity of 0.62 for diagnostic value, indicating that circRNAs could be employed as diagnostic biomarkers for PCa. In terms of clinical and prognostic importance, abnormal expression of circRNAs was connected to clinical parameters and prognosis.
The sensitivity and specificity results also demonstrated that circRNAs could discriminate between healthy people and patients, but the specificity needs to be enhanced. However, the bivariate boxplot and Galbraith plot results indicated that the studies included in the diagnostic analysis were heterogeneous. Because the number of included papers was insufficient to allow additional subgroup analysis to identify sources of heterogeneity, further research is warranted.
In clinical practice, the PLR and NLR signify diagnostic ability. The PLR > 10 and NLR < 0.1 are considered to indicate good diagnostic ability (47). However, in our study, PLR was 2.17 and NLR was 0.29, which meant that circRNAs’ diagnostic accuracy is currently limited. In other words, circRNAs would give a higher rate of FN and FP in clinical applications.
DOR is a measurement index for diagnostic performance that incorporates the advantages of sensitivity and specificity. The higher the value of DOR, the better it can identify test performance (48). In our study, the pooled DOR was 7.37, which supported the use of circRNAs as a viable diagnostic tool for PCa.
As for clinicopathological parameters, T classification and Gleason score were found to be linked with both upregulated and downregulated circRNAs. The aberrant expression of circRNAs in colorectal carcinoma and esophageal cancer was also linked to T classification in the studies by Yuan et al. (49) and Lin et al. (50). Together, this could imply that circRNAs are vital for tumor staging.
The Gleason score is a widely used method for histological grading of PCa and a useful tool for making plans for PCa treatment (51). Our findings revealed that circRNAs were highly linked to a low Gleason score, indirectly reflecting the status of PCa.
In terms of prognostic values, our recent meta-analysis found that aberrant circRNA expression was strongly linked to poor overall survival. As a result, prompt monitoring of circRNA alterations in patients with PCa can aid clinical decision-making and help patients live longer.
Some studies focusing on experimental research rather than cohort or case-control research also demonstrated that circRNA does play a role in the diagnosis and prognosis of PCa. As described by Vo et al., multiple upregulated or downregulated circRNAs were shown to be associated with PCa progression (52). Their results are consistent with previous studies showing that circRNAs generally do not directly contribute to the growth of cancer cells but instead do so indirectly through controlling mRNAs (53–55). These studies support the value of circRNA in PCa; however, we did not include them in our meta-analysis because most of them are clustered in experiments that lack data from entire cohort studies.
Numerous studies about the effect of circRNAs on various cancers have been conducted. Some meta-analyses had demonstrated that circRNA had good diagnostic and prognostic value for patients with colorectal carcinoma (49), solid cancers (56), and osteosarcoma (57). However, only several meta-analyses exploring the diagnostic and prognostic value of circRNA in PCa have been conducted to date. Yuan et al. performed a meta-analysis that included only articles from 2015 to 2019 (49). However, in the last 3 years, more studies have focused on the differences in circRNA expression between patients with cancer patients and healthy subjects. Our study fills this gap and covers a wider range of data. In addition, Li et al. included only five studies in their prognosis meta-analysis (56). This meta-analysis was based on a small number of studies, which may have introduced some bias. The present study has been quantitatively supplemented to make the results of the meta-analysis more convincing. In addition, the source of specimens was indicated in our meta-analysis. Compared with previous meta-analyses, we excluded the effect of heterogeneity due to specimen source. Thus, our study is an update and addition to similar meta-analyses evaluating the role of circRNA in PCa and provides a grounding for related research.
There are still limitations in our current meta-analysis. First, we only included five papers in our diagnostic meta-analysis, and the small number of research has limited the popularization of circRNAs in clinical applications. Second, because of the restricted amount of data from included studies, we were unable to run a subgroup analysis to analyze the source of heterogeneity in the diagnostic meta-analysis. Furthermore, some research did not provide clear HR data. We retrieved necessary data from provided KM curves, which might have caused some bias. Finally, because the majority of the research examined was from China, the external application of our findings across diverse areas may be hampered. To ensure the accuracy and relevance of the findings, a comprehensive research is required.
Conclusion
Together, our meta-analysis revealed that circRNAs have moderately high diagnostic accuracy for PCa. The results of the prognostic meta-analysis revealed that aberrant expression of circRNAs is significantly associated with poor prognostic outcomes and clinicopathological values. Therefore, circRNAs can be useful indicators for the diagnosis and prognosis of PCa. However, further studies with more multicenter data, as well as high-quality studies, are needed to demonstrate the role of circRNAs in PCa.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
JX and HJ contributed to conceive this study, performed quality assessment of included studies, analyze the data, and write the manuscript. YZ analyzed the data and performed quality assessment of included studies. LZ and ZZ reviewed the manuscript. JL provided the financial support. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This manuscript was supported by the grants from Sichuan Science and Technology Program (2021YFS0332), and Southwest Medical University Science Program (00031839).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.945143/full#supplementary-material
References
1. Liu GX, Zheng T, Zhang Y, Hao P. Circfoxm1 silencing represses cell proliferation, migration and invasion by regulating mir-515-5p/Adam10 axis in prostate cancer. Anticancer Drugs (2022) 33(1):e573–83. doi: 10.1097/cad.0000000000001183
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
3. Wu C, Li M, Meng H, Liu Y, Niu W, Zhou Y, et al. Analysis of status and countermeasures of cancer incidence and mortality in China. Sci China Life Sci (2019) 62(5):640–7. doi: 10.1007/s11427-018-9461-5
4. Swami U, McFarland TR, Nussenzveig R, Agarwal N. Advanced prostate cancer: Treatment advances and future directions. Trends Cancer (2020) 6(8):702–15. doi: 10.1016/j.trecan.2020.04.010
5. Teo MY, Rathkopf DE, Kantoff P. Treatment of advanced prostate cancer. Annu Rev Med (2019) 70:479–99. doi: 10.1146/annurev-med-051517-011947
6. Sternberg CN. Novel hormonal therapy for castration-resistant prostate cancer. Ann Oncol (2012) 23 Suppl 10:x259–63. doi: 10.1093/annonc/mds362
7. Hayes JH, Barry MJ. Screening for prostate cancer with the prostate-specific antigen test: A review of current evidence. Jama (2014) 311(11):1143–9. doi: 10.1001/jama.2014.2085
8. Zhang H, Shen T, Zhang Z, Li Y, Pan Z. Expression of Kif18a is associated with increased tumor stage and cell proliferation in prostate cancer. Med Sci Monit (2019) 25:6418–28. doi: 10.12659/msm.917352
9. Li P, Chen S, Chen H, Mo X, Li T, Shao Y, et al. Using circular rna as a novel type of biomarker in the screening of gastric cancer. Clin Chim Acta (2015) 444:132–6. doi: 10.1016/j.cca.2015.02.018
10. Nigro JM, Cho KR, Fearon ER, Kern SE, Ruppert JM, Oliner JD, et al. Scrambled exons. Cell (1991) 64(3):607–13. doi: 10.1016/0092-8674(91)90244-s
11. Gong GH, An FM, Wang Y, Bian M, Wang D, Wei CX. Comprehensive circular rna profiling reveals the regulatory role of the circrna-0067835/Mir-155 pathway in temporal lobe epilepsy. Cell Physiol Biochem (2018) 51(3):1399–409. doi: 10.1159/000495589
12. Kristensen LS, Hansen TB, Venø MT, Kjems J. Circular rnas in cancer: Opportunities and challenges in the field. Oncogene (2018) 37(5):555–65. doi: 10.1038/onc.2017.361
13. Rybak-Wolf A, Stottmeister C, Glažar P, Jens M, Pino N, Giusti S, et al. Circular rnas in the mammalian brain are highly abundant, conserved, and dynamically expressed. Mol Cell (2015) 58(5):870–85. doi: 10.1016/j.molcel.2015.03.027
14. Li Q, Wang W, Zhang M, Sun W, Shi W, Li F. Circular rna circ-0016068 promotes the growth, migration, and invasion of prostate cancer cells by regulating the mir-330-3p/Bmi-1 axis as a competing endogenous rna. Front Cell Dev Biol (2020) 8:827. doi: 10.3389/fcell.2020.00827
15. Hsiao KY, Sun HS, Tsai SJ. Circular rna - new member of noncoding rna with novel functions. Exp Biol Med (Maywood) (2017) 242(11):1136–41. doi: 10.1177/1535370217708978
16. Wang G, Zhao H, Duan X, Ren Z. Circrna pappalysin 1 facilitates prostate cancer development through mir-515-5p/Fkbp1a axis. Andrologia (2021) 53(11):e14227. doi: 10.1111/and.14227
17. Xia Q, Ding T, Zhang G, Li Z, Zeng L, Zhu Y, et al. Circular rna expression profiling identifies prostate cancer- specific circrnas in prostate cancer. Cell Physiol Biochem (2018) 50(5):1903–15. doi: 10.1159/000494870
18. Dong JS, Wu B, Chen XH. Circ Psmc3 inhibits prostate cancer cell proliferation by downregulating Dgcr8. Eur Rev Med Pharmacol Sci (2020) 24(5):2264–70. doi: 10.26355/eurrev_202003_20492
19. Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (Prisma-p) 2015: Elaboration and explanation. Bmj (2015) 350:g7647. doi: 10.1136/bmj.g7647
20. Stewart LA, Clarke M, Rovers M, Riley RD, Simmonds M, Stewart G, et al. Preferred reporting items for systematic review and meta-analyses of individual participant data: The prisma-ipd statement. Jama (2015) 313(16):1657–65. doi: 10.1001/jama.2015.3656
21. Cornford P, van den Bergh RCN, Briers E, Van den Broeck T, Cumberbatch MG, De Santis M, et al. Eau-Eanm-Estro-Esur-Siog guidelines on prostate cancer. part ii-2020 update: Treatment of relapsing and metastatic prostate cancer. Eur Urol (2021) 79(2):263–82. doi: 10.1016/j.eururo.2020.09.046
22. Mohler JL, Antonarakis ES. Nccn guidelines updates: Management of prostate cancer. J Natl Compr Canc Netw (2019) 17(5.5):583–6. doi: 10.6004/jnccn.2019.5011
23. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-Event data into meta-analysis. Trials (2007) 8:16. doi: 10.1186/1745-6215-8-16
24. Wang K, Fan Y, Sun J, Zhao L, Yu Y, Li G. Circ_0061140 stimulates the malignant development of prostate cancer by targeting mir-1193. Transl Androl Urol (2021) 10(5):1928–38. doi: 10.21037/tau-20-1477
25. Huang C, Deng H, Wang Y, Jiang H, Xu R, Zhu X, et al. Circular rna Circabcc4 as the cerna of mir-1182 facilitates prostate cancer progression by promoting Foxp4 expression. J Cell Mol Med (2019) 23(9):6112–9. doi: 10.1111/jcmm.14477
26. Tao LJ, Pan XY, Wang JW, Zhang L, Tao LS, Liang CZ. Circular rna Circanks1b acts as a sponge for mir-152-3p and promotes prostate cancer progression by upregulating tgf-A expression. Prostate (2021) 81(5):271–8. doi: 10.1002/pros.24102
27. Wang X, Wang R, Wu Z, Bai P. Circular rna itch suppressed prostate cancer progression by increasing Hoxb13 expression Via spongy mir-17-5p. Cancer Cell Int (2019) 19:328. doi: 10.1186/s12935-019-0994-8
28. Shi J, Liu C, Chen C, Guo K, Tang Z, Luo Y, et al. Circular rna Circmboat2 promotes prostate cancer progression Via a mir-1271-5p/Mtor axis. Aging (Albany NY) (2020) 12(13):13255–80. doi: 10.18632/aging.103432
29. Huang E, Chen X, Yuan Y. Downregulated circular rna itchy E3 ubiquitin protein ligase correlates with advanced pathologic T stage, high lymph node metastasis risk and poor survivals in prostate cancer patients. Cancer Biomark (2019) 26(1):41–50. doi: 10.3233/cbm-182111
30. Chao F, Song Z, Wang S, Ma Z, Zhuo Z, Meng T, et al. Novel circular rna circsobp governs amoeboid migration through the regulation of the mir-141-3p/Mypt1/P-Mlc2 axis in prostate cancer. Clin Transl Med (2021) 11(3):e360. doi: 10.1002/ctm2.360
31. Song Z, Zhuo Z, Ma Z, Hou C, Chen G, Xu G. Hsa_Circ_0001206 is downregulated and inhibits cell proliferation, migration and invasion in prostate cancer. Artif Cells Nanomed Biotechnol (2019) 47(1):2449–64. doi: 10.1080/21691401.2019.1626866
32. Li H, Zhi Y, Ma C, Shen Q, Sun F, Cai C. Circ_0062020 knockdown strengthens the radiosensitivity of prostate cancer cells. Cancer Manag Res (2020) 12:11701–12. doi: 10.2147/cmar.S273826
33. Liu F, Fan Y, Ou L, Li T, Fan J, Duan L, et al. Circhipk3 facilitates the G2/M transition in prostate cancer cells by sponging mir-338-3p. Onco Targets Ther (2020) 13:4545–58. doi: 10.2147/ott.S242482
34. Cai C, Zhi Y, Wang K, Zhang P, Ji Z, Xie C, et al. Circhipk3 overexpression accelerates the proliferation and invasion of prostate cancer cells through regulating mirna-338-3p. Onco Targets Ther (2019) 12:3363–72. doi: 10.2147/ott.S196931
35. Chen W, Cen S, Zhou X, Yang T, Wu K, Zou L, et al. Circular rna Circnolc1, upregulated by nf-kappab, promotes the progression of prostate cancer Via mir-647/Paqr4 axis. Front Cell Dev Biol (2020) 8:624764. doi: 10.3389/fcell.2020.624764
36. Huang B, Zhou D, Huang X, Xu X, Xu Z. Silencing Circslc19a1 inhibits prostate cancer cell proliferation, migration and invasion through regulating mir-326/Mapk1 axis. Cancer Manag Res (2020) 12:11883–95. doi: 10.2147/cmar.S267927
37. Hu Y, Guo B. Circ-Mto1 correlates with favorable prognosis and inhibits cell proliferation, invasion as well as mir-17-5p expression in prostate cancer. J Clin Lab Anal (2020) 34(3):e23086. doi: 10.1002/jcla.23086
38. Liu Y, Xia L, Dong L, Wang J, Xiao Q, Yu X, et al. Circhipk3 promotes gemcitabine (Gem) resistance in pancreatic cancer cells by sponging mir-330-5p and targets Rassf1. Cancer Manag Res (2020) 12:921–9. doi: 10.2147/cmar.S239326
39. Gao Y, Liu J, Huan J, Che F. Downregulation of circular rna Hsa_Circ_0000735 boosts prostate cancer sensitivity to docetaxel Via sponging mir-7. Cancer Cell Int (2020) 20:334. doi: 10.1186/s12935-020-01421-6
40. Rochow H, Jung M, Weickmann S, Ralla B, Stephan C, Elezkurtaj S, et al. Circular rnas and their linear transcripts as diagnostic and prognostic tissue biomarkers in prostate cancer after prostatectomy in combination with clinicopathological factors. Int J Mol Sci (2020) 21(21):7812. doi: 10.3390/ijms21217812
41. Fort RS, Mathó C, Oliveira-Rizzo C, Garat B, Sotelo-Silveira JR, Duhagon MA. An integrated view of the role of mir-130b/301b mirna cluster in prostate cancer. Exp Hematol Oncol (2018) 7:10. doi: 10.1186/s40164-018-0102-0
42. Cieślikowski WA, Antczak A, Nowicki M, Zabel M, Budna-Tukan J. Clinical relevance of circulating tumor cells in prostate cancer management. Biomedicines (2021) 9(9):1179. doi: 10.3390/biomedicines9091179
43. Roma-Rodrigues C, Fernandes AR, Baptista PV. Exosome in tumour microenvironment: Overview of the crosstalk between normal and cancer cells. BioMed Res Int (2014) 2014:179486. doi: 10.1155/2014/179486
44. De Luca S, Passera R, Sottile A, Fiori C, Scarpa RM, Porpiglia F. [-2]Propsa versus ultrasensitive psa fluctuations over time in the first year from radical prostatectomy, in an high-risk prostate cancer population: A first report. BMC Urol (2016) 16:14. doi: 10.1186/s12894-016-0131-0
45. Sun Y, Chen G, He J, Huang ZG, Li SH, Yang YP, et al. Clinical significance and potential molecular mechanism of mirna-222-3p in metastatic prostate cancer. Bioengineered (2021) 12(1):325–40. doi: 10.1080/21655979.2020.1867405
46. Fabris L, Ceder Y, Chinnaiyan AM, Jenster GW, Sorensen KD, Tomlins S, et al. The potential of micrornas as prostate cancer biomarkers. Eur Urol (2016) 70(2):312–22. doi: 10.1016/j.eururo.2015.12.054
47. Shen L, Li Y, Li N, Zhao Y, Zhou Q, Li Z. Clinical utility of contrast-enhanced ultrasonography in the diagnosis of benign and malignant small renal masses among Asian population. Cancer Med (2019) 8(18):7532–41. doi: 10.1002/cam4.2635
48. Gismervik S, Drogset JO, Granviken F, Rø M, Leivseth G. Physical examination tests of the shoulder: A systematic review and meta-analysis of diagnostic test performance. BMC Musculoskelet Disord (2017) 18(1):41. doi: 10.1186/s12891-017-1400-0
49. Yuan J, Guo D, Li X, Chen J. Prognostic and diagnostic value of circrna expression in colorectal carcinoma: A meta-analysis. BMC Cancer (2020) 20(1):448. doi: 10.1186/s12885-020-06932-z
50. Lin H, Yuan J, Liang G, Wu Y, Chen L. Prognostic and diagnostic significance of circrna expression in esophageal cancer: A meta-analysis. Gastroenterol Res Pract (2020) 2020:8437250. doi: 10.1155/2020/8437250
51. Srigley JR, Delahunt B, Samaratunga H, Billis A, Cheng L, Clouston D, et al. Controversial issues in Gleason and international society of urological pathology (Isup) prostate cancer grading: Proposed recommendations for international implementation. Pathology (2019) 51(5):463–73. doi: 10.1016/j.pathol.2019.05.001
52. Vo JN, Cieslik M, Zhang Y, Shukla S, Xiao L, Zhang Y, et al. The landscape of circular rna in cancer. Cell (2019) 176(4):869–81.e13. doi: 10.1016/j.cell.2018.12.021
53. Cuzick J, Swanson GP, Fisher G, Brothman AR, Berney DM, Reid JE, et al. Prognostic value of an rna expression signature derived from cell cycle proliferation genes in patients with prostate cancer: A retrospective study. Lancet Oncol (2011) 12(3):245–55. doi: 10.1016/s1470-2045(10)70295-3
54. Rubin MA, Zhou M, Dhanasekaran SM, Varambally S, Barrette TR, Sanda MG, et al. Alpha-methylacyl coenzyme a racemase as a tissue biomarker for prostate cancer. Jama (2002) 287(13):1662–70. doi: 10.1001/jama.287.13.1662
55. Dong C, Fan B, Ren Z, Liu B, Wang Y. Circsmarca5 facilitates the progression of prostate cancer through mir-432/Pdcd10 axis. Cancer Biother Radiopharm (2021) 36(1):70–83. doi: 10.1089/cbr.2019.3490
56. Li F, Huang Q, Gong Z, Wang H, Chen J. Diagnostic and prognostic roles of circ-shprh for solid cancers: A meta-analysis. Onco Targets Ther (2019) 12:4351–7. doi: 10.2147/ott.S200755
Keywords: circular RNAs, prostate cancer, diagnosis, prognosis, clinicopathological features
Citation: Xie J, Jiang H, Zhao Y, Jin Xr, Li B, Zhu Z, Zhang L and Liu J (2022) Prognostic and diagnostic value of circRNA expression in prostate cancer: A systematic review and meta-analysis. Front. Oncol. 12:945143. doi: 10.3389/fonc.2022.945143
Received: 31 May 2022; Accepted: 10 October 2022;
Published: 07 November 2022.
Edited by:
Gianluca Ingrosso, University of Perugia, ItalyReviewed by:
Annika Fendler, Francis Crick Institute, United KingdomPushpinder Bawa, Boston University, United States
Copyright © 2022 Xie, Jiang, Zhao, Jin, Li, Zhu, Zhang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinbo Liu, liujb7203@swmu.edu.cn
†These authors have contributed equally to this work and share first authorship
 Jingling Xie
Jingling Xie Hui Jiang†
Hui Jiang† Zixin Zhu
Zixin Zhu