- 1School of Pharmacy, Hangzhou Normal University, Hangzhou, China
- 2School of Public Health, Hangzhou Normal University, Zhejiang, China
- 3Scientific Research Department, Women’s Hospital School of Medicine Zhejiang University, Hangzhou, China
- 4Key Laboratory of Elemene Class Anti-Cancer Chinese Medicines, Engineering Laboratory of Development and Application of Traditional Chinese Medicines, Collaborative Innovation Center of Traditional Chinese Medicines of Zhejiang Province, Hangzhou Normal University, Hangzhou, China
Background: Genetic factors increase the individual risk of colorectal cancer (CRC); however, the extent to which a healthy lifestyle can offset increased genetic risk is unknown. This study investigated whether a healthy lifestyle is associated with lower CRC risk, regardless of genetic risk.
Methods: We recruited 390,365 participants without cancer at baseline (2006–2010) from the UK Biobank. The primary outcome was CRC incidence. A healthy lifestyle score constructed using 16 factors of six dimensions (smoking, drinking, body mass index, diet, exercise, and sleep) was categorized into three risk categories: favorable, intermediate, and unfavorable. To calculate the polygenic risk scores (PRSs) of UK Biobank participants, we extracted 454,678 single nucleotide polymorphisms (SNPs) from the UK Biobank and FinnGen Biobank after quality control. Cox proportional hazards regression was performed to evaluate the associations and was expressed as hazard ratios (HRs) with 95% confidence intervals (CIs).
Results: During a median follow-up of 10.90 years, 4,090 new CRC cases were reported in the UK Biobank. The “best-fit” PRSs were constructed using 59 SNPs based on the UK Biobank cohort and FinnGen genome-wide association study summary data (R2 = 0.23%) and were divided into low (lowest quintile), intermediate (including second–fourth quintile), and high (highest quintile) genetic risk categories. The multivariate-adjusted Cox model revealed that participants with favorable lifestyles had HRs of 0.66 (95% CI = 0.60–0.72) for developing CRC vs. those with unfavorable lifestyles; low genetic risk was associated with a decreased risk of CRC (HR = 0.67, 95% CI =0.61–0.74) compared with those with high genetic risk. The HRs for low genetic risk participants with favorable lifestyles were 0.44 (95% CI =0.36–0.55) vs. participants with high genetic risk and unfavorable lifestyles. Among the participants with low, intermediate, or high genetic risk, the HRs of favorable vs. unfavorable lifestyles were 0.74, 0.64, and 0.72 (all p< 0.05).
Conclusions: Low genetic risk and a favorable lifestyle were significantly associated with a decreased risk of CRC. A favorable lifestyle was associated with a lower CRC risk, regardless of genetic risk.
Introduction
Colorectal cancer (CRC) is the third most common cancer and the fourth leading cause of cancer-related death worldwide (1). As a complex disease, CRC is determined by the interplay between genetic and lifestyle factors (2). Genome-wide association studies (GWASs) with large sample sizes have revealed that hundreds of independent loci are associated with CRC risk (3–10). Although each genetic variant contributes little to CRC, when aggregated into a polygenic risk score (PRS), these associated alleles can provide an overall measure of genetic susceptibility to certain diseases. Therefore, research on the relationship between CRC risk and PRS has attracted widespread attention.
The American Cancer Society (ACS) has emphasized the critical role of lifestyle management in CRC prevention (11). However, previous studies on CRC prevention have concentrated on limited factors, such as smoking, alcohol consumption, exercise, sedentary behavior, and intake of fruits and vegetables, while emerging factors, such as sleep and intake of whole and refined grains, have been excluded from the lifestyle scores. Owing to the increasing number of people with sleep disorders (12, 13), and sleep disorders have been shown to increase the risk of CRC (14–16). Incorporating emerging factors such as sleep into lifestyle scores to illustrate the benefits of a healthy lifestyle in CRC prevention should be addressed.
Therefore, this study aimed to evaluate the genetic risk of CRC among UK Biobank participants (created PRS based on GWAS summary data from the FinnGen biobank) and to determine whether adherence to a favorable lifestyle (including emerging factors such as sleep) is associated with a lower risk of CRC, regardless of genetic risk.
Methods
Study population
We obtained data from the UK Biobank and FinnGen cohort in this study. A detailed introduction to the UK Biobank has been provided elsewhere (https://www.ukbiobank.ac.uk/). Briefly, the UK Biobank collected and stored blood samples of approximately half a million volunteers from 2006 to 2010 and continually followed up their health and medical information. We extracted 502,414 participants from the UK Biobank. Exclusion criteria were (1) participants with missing values or without genotype data (n = 37,959); (2) nonwhite ethnic background (n = 27,385); and (3) with cancer at baseline, were diagnosed with CRC or died within the first 2 years of follow-up, or were lost to follow-up (n = 40,551). The present analyses were restricted to individuals from white ethnic backgrounds with available genetic and lifestyle information. After exclusion, 396,519 potentially eligible participants remained for further analyses (Supplementary Figure S1).
The CRC GWAS summary statistics in this study were obtained from the FinnGen Biobank and included 215,770 controls and 3,022 CRC cases (https://finngen.gitbook.io/documentation/). FinnGen data are openly available online. The need for ethical approval and consent to participate was waived, for they have been obtained from the original studies. The UK Biobank obtained ethical approval from the North West Multicenter Research Ethics Committee (reference no. 16/NW/0274). All the UK Biobank participants signed an informed consent form. This study was approved by the UK Biobank (no. 78563).
Polygenic risk score
Polygenic risk score calculation requires separate base (GWAS summary statistics) and target data (individual-level genotype–phenotype data), as described previously (17, 18). For the base data, we used the FinnGen biobank GWAS summary data; for the target data, we used 396,519 individuals from the UK Biobank, which contains individual-level genotypes.
Both the base and target data require quality control. First, we filtered the base data of the FinnGen cohort. From a total of 16,380,465 single nucleotide polymorphisms (SNPs), we excluded 7,279,657 SNPs due to a minor allele frequency (MAF)< 1% (18). Second, regarding the target data of 396,519 UK Biobank participants, we selected version 2 of the non-imputed genotype data (continued to be correct and current) for the subsequent analysis (https://biobank.ctsu.ox.ac.uk/showcase/label.cgi?id=100319). A total of 784,256 SNPs located in autosomal chromosomes were used to calculate the PRS, and X chromosomes were used for sex checks (X chromosome homozygosity estimate of female individuals must be<0.2, and that of male individuals must be >0.8). The exclusion criteria were as follows (19): (1) missing ratio for SNPs and individuals >2%; (2) sex discrepancy; (3) MAF<1%; (4) Hardy–Weinberg equilibrium (HWE) p-value< 1×10−6 for controls and p-value< 1×10−10 for cases; (5) heterozygosity rate deviating more than triple the standard deviation (SD) from the mean; and (6) 10 or more third-degree relatives among participants. After quality control, there were 390,365 participants and 538,634 SNPs (located on autosomal chromosomes) for further analysis. Third, SNPs must be harmonized by assigning the same effect allele across the base and target datasets. SNPs with palindrome structures were removed. The remaining 390,365 individuals with 454,678 SNPs were used for PRS calculations (Supplementary Figure S1). PRS calculations were performed using PLINK 1.90 and PRSice-2 software (20). PRSice calculates PRS at numerous p-value thresholds to provide the best-fit PRS (21). The PRS was calculated according to the formula (20):
where m is the number of SNPs under the corresponding p-value threshold, βi is ln(ORi)—natural logarithm transformed odds ratio of the i-th SNP obtained from the base data, and ωij indicates the probability of the genotype j (j = 0, 1, 2) for the i-th SNP.
Healthy lifestyle scores
The lifestyle scores were established based on the ACS guidelines for CRC prevention, including exercise, alcohol consumption, body mass index (BMI), and diet (intake of fruits, vegetables, fish, whole grains, processed meat, red meat, and refined grains) (11). In addition, we included sleep (sleep duration, chronotype, insomnia, snoring, and daytime dozing) and smoking in the lifestyle scores because of the growing interest in the association of sleep disorders and smoking with higher CRC risk (14, 15, 22, 23). Information on lifestyle factors was obtained at baseline by using a validated touchscreen questionnaire. Participants accumulated 1 point once in accordance with each of following six healthy lifestyle patterns based on ACS guidelines or national recommendations (Supplementary Table S1): 1) never smoked (24); 2) moderate alcohol consumption [women ≤ 14 g/day or men ≤ 28 g/day (25)]; 3) regular exercise (moderate physical activities ≥ 150 min/week, or ≥ 5 days/week, or vigorous exercise ≥ 75 min/week, or ≥ once a week, or an equivalent combination (26, 27); 4) healthy diet (individuals incorporating at least three of the following dietary behaviors: fruits ≥ 3 servings/day, vegetables ≥ 3 servings/day, fish ≥ twice a week, processed meats ≤ once a week, red meats ≤ twice a week, whole grains ≥ 3 servings/day, refined grains ≤ 1 serving/day) (28); 5) sleeping well (individuals having at least three of the following healthy sleep behaviors: sleep duration, 7–8 h/day, chronotype indicated as morning person, never or sometimes insomnia, no snoring, never or sometimes daytime dozing) (29); and 6) normal weight, 18.5 kg/m2 ≤ body mass index (BMI)< 25.0 kg/m2 is considered healthy weight (30). The total healthy lifestyle score ranges from 0 to 6, and the higher the score, the higher the tendency for a healthy lifestyle. Healthy lifestyle scores were categorized into three groups: unfavorable (scored 0–2 points), intermediate (scored 3–4 points), and favorable (scored 5-6 points).
Covariates
Covariate information was obtained at baseline, including the age when attending the assessment center; sex (female, male); ethnic background (British, Irish, any other white background); socioeconomic status as measured using the Townsend deprivation index (TDI), which combining information on non-homeownership, household overcrowding, non-car ownership, and unemployment at recruitment (31); and education (higher: college or university; middle: A levels/AS levels or equivalent, O levels/GCSEs or equivalent; lower: CSEs or equivalent, NVQ, HND, HNC, or equivalent; vocational: professional qualifications, e.g., nursing, teaching; other).
Ascertainment of CRC outcome
The primary outcome was the first incident CRC diagnosis or CRC first documented on the death certificates. Death information from the UK Biobank was obtained from the “death registry,” which is linked to the National Health Service (NHS) Central Register (Scotland participants) and NHS Digital Center (England/Wales participants) (32). Participants who died of CRC were recorded by the 10th revision of the International Classification of Diseases (ICD-10) (coding: C18, C19, and C20). The date of the “death registry” was updated to 31 October 2021 (for Scotland participants) or 30 September 2021 (for England/Wales participants).
The details of the first incident CRC diagnosis were obtained from the “cancer registry,” which is linked to the Information Services Division of the NHS Scotland (Scotland participants) and the Medical Research Information Service of the NHS (England/Wales participants) (33). CRC incidence was diagnosed using ICD-10 (C18, C19, and C20), the ICD-9 (153, 1540, and 1541), and self-reported cancer (1020, 1022, and 1023). The follow-up person-years of each participant were calculated from the baseline survey time to either the date of CRC outcome, death for any reason, 31 October 2015 (for Scotland participants), or 29 February 2020 (for England/Wales participants), whichever occurred first.
Statistical analysis
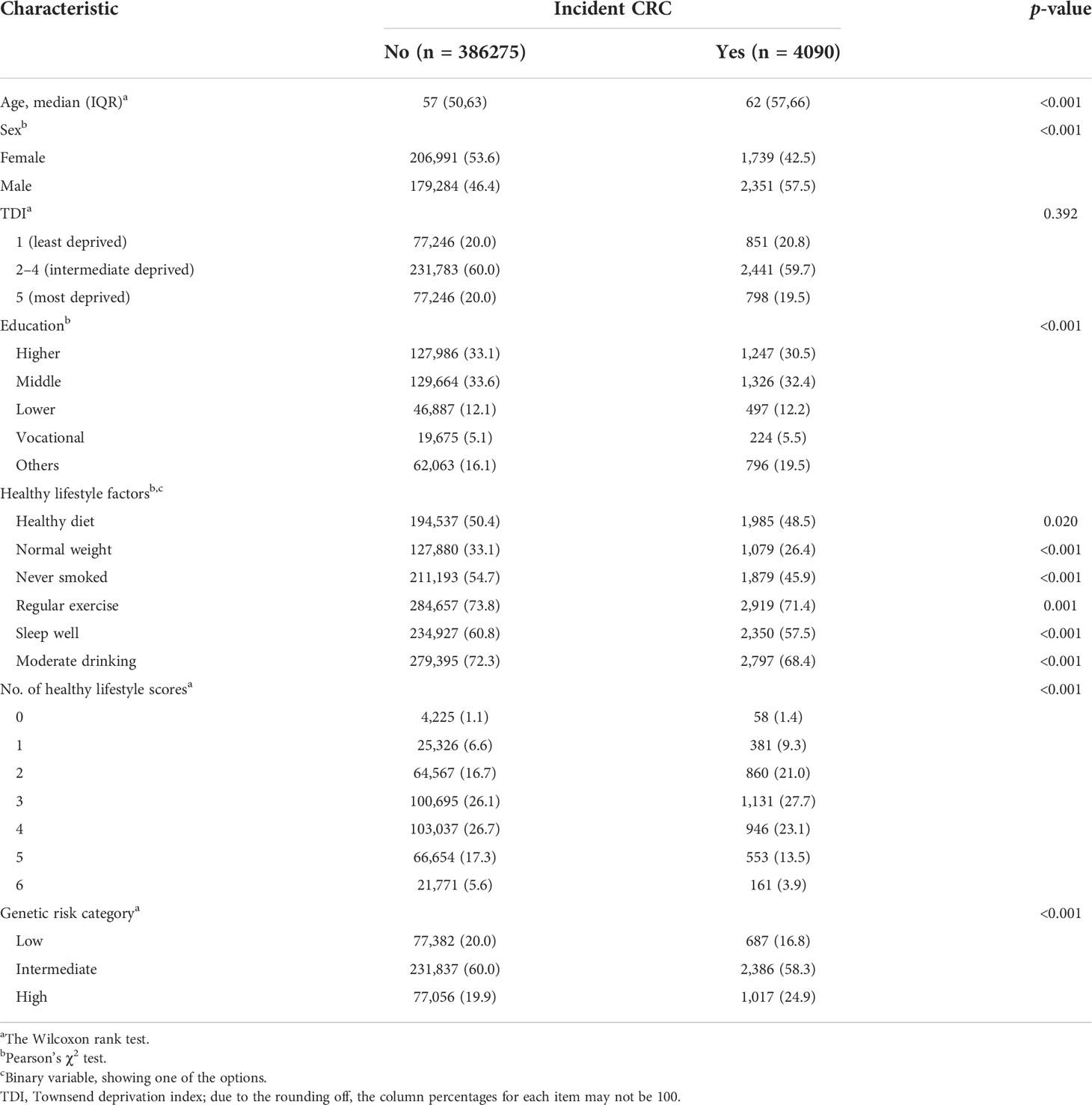
The baseline characteristics of each participant are presented by CRC status as frequencies (percentages) for categorical variables and median [(interquartile range) (IQR)] for non-normally distributed continuous variables. Pearson’s χ2 test was used to analyze unordered categorical variables, and the Wilcoxon rank test was used to analyze grade variables or non-normally distributed continuous variables. The reverse Kaplan–Meier method was used to calculate the median follow-up time.
Cox proportional hazards regression was used to evaluate the associations of genetic, lifestyle, and combined genetic–lifestyle factors with the risk of CRC; it was adjusted for potential confounding factors, and the results were expressed as adjusted hazard ratios (HRs) with 95% confidence intervals (CIs). Model 1 was adjusted for age (years), sex (male and female), education (higher, middle, lower, vocational, and other), Townsend deprivation index, and the first 10 genetic principal components (GPCs) at recruitment; Model 2 was additionally adjusted for healthy lifestyle scores (0-6), and Model 2b was additionally adjusted for the PRS based on Model 1. The proportionality of hazards assumption was examined using the Schoenfeld residuals; no evidence of non-proportionality was observed. Multiplicative interactions were calculated using Cox regression adjusted for Model 1. A restricted cubic spline (RCS) using three knots was used to examine dose–response associations. Additionally, absolute risks (ARs) were calculated using the CRC incidence in each risk group (34, 35).
Sensitivity analysis was conducted to examine the robustness of the findings. First, stratified analyses were conducted according to age at baseline, sex, socioeconomic status, and education. Second, weighted lifestyle scores were created and classified into three groups: unfavorable, intermediate, and favorable. Statistical analyses were performed using SAS version 9.1 (SAS Institute, Cary, NC, USA), and graphs were plotted using R [version 4.1.3; R Core Team (2022); Vienna, Austria]. All p-values were two-sided, and a p-value< 0.05 was considered statistically significant.
Results
The baseline characteristics of the 390,365 participants who were followed up for a median of 10.90 years (follow-up 4,135,798 total person-years) are presented by CRC status in Table 1. There were 4,090 cases of CRC. Participants without CRC were younger, more likely to be female, had a healthy diet, had normal weight, and never smoked (p< 0.05) (Supplementary Table S2). Among 454,678 SNPs for PRS calculation, we found that a p-value< 5×10−5 threshold, containing 59 SNPs, generated a “best-fit” PRS (R2 = 0.23%) (Supplementary Figure S2). The “best-fit” PRS and healthy lifestyle score (from 0 to 6 points) were all in accordance with a normal distribution (Supplementary Figure S3). The dose–response relationship suggested a positive association between PRS and CRC risk (Supplementary Figure S4). The PRS was then categorized into three genetic risk groups: low (lowest quintile), intermediate (including second–fourth quintile), and high (highest quintile). Notably, genetic risk categories were not associated with any lifestyle factors except for smoking status and exercise (Supplementary Table S3).
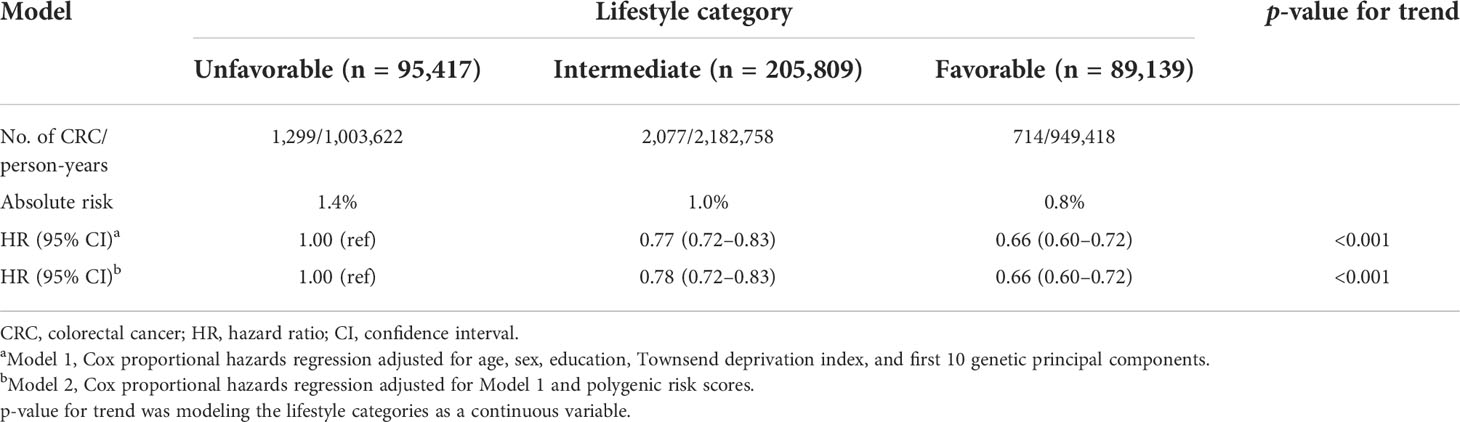
The risk of CRC decreased monotonically across the healthy lifestyle groups (Table 2). Participants with a favorable lifestyle had an HR of 0.66 (95% CI = 0.60–0.72), and those with an intermediate lifestyle had an HR of 0.77 (95% CI =0.72–0.83) for developing CRC vs. those with an unfavorable lifestyle in Model 1. The results remained significant after additional adjustment for PRS in Model 2. The HRs for favorable and intermediate lifestyles were 0.66 (95% CI = 0.60–0.72) and 0.78 (95% CI = 0.72–0.83), respectively. A similar pattern of effects was observed when healthy lifestyle scores were used as continuous variables (Supplementary Table S4).
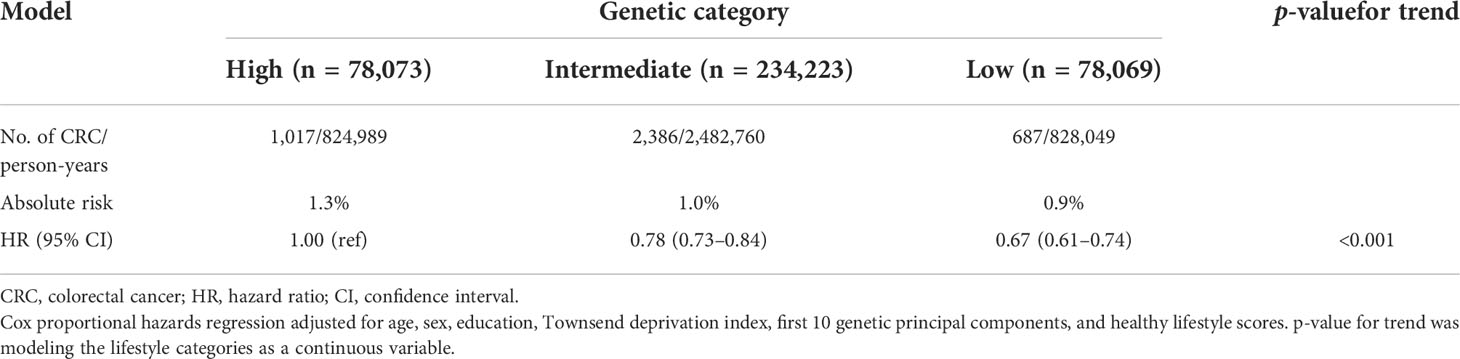
A decreased monotonically gradient risk of CRC was also observed across low genetic risk groups (Table 3). Participants with low genetic risk had HRs of 0.67 (95% CI = 0.61–0.74), and intermediate genetic risk participants had HRs of 0.78 (95% CI =0.73–0.84) for developing CRC vs. high genetic risk participants. The AR for CRC was 1.3% in the high-risk genetic group and 0.9% in the low-risk genetic group. In addition, the higher PRS quintiles were also associated with a monotonically increased risk of CRC compared with the lowest quintile (Supplementary Table S5).
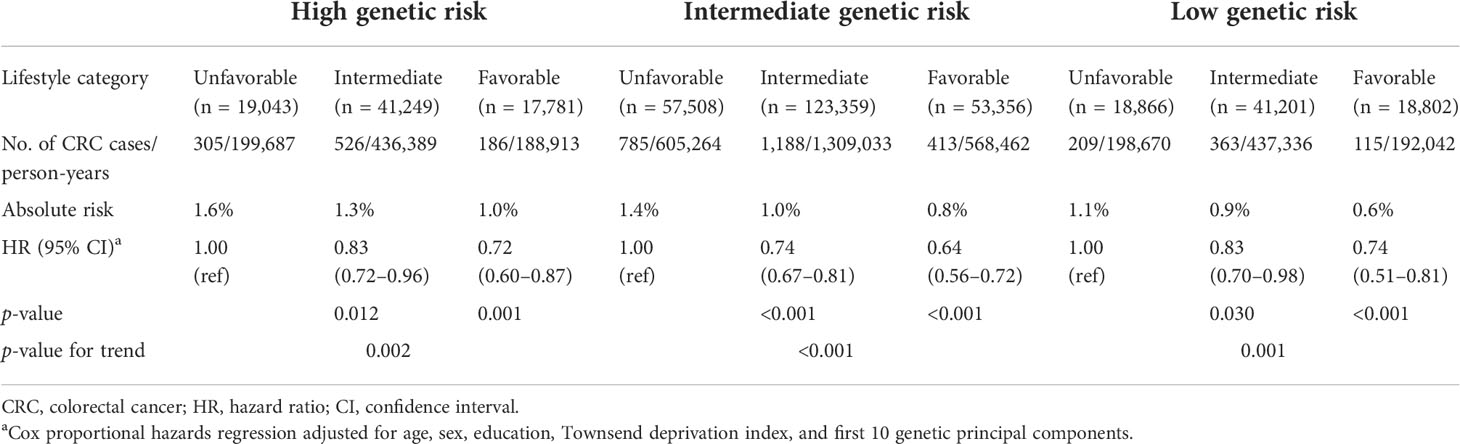
To explore the impact of lifestyle on CRC risk according to different genetic risk categories, we stratified lifestyles by genetic risk category using unfavorable lifestyles as the reference group and found that favorable lifestyles were associated with a lower risk of CRC in any genetic risk group (Table 4). In the low genetic risk category, participants with a favorable lifestyle had an HR of 0.74 (95% CI = 0.51–0.81) for developing CRC vs. participants with an unfavorable lifestyle. The HRs of favorable lifestyles vs. unfavorable lifestyles among participants in intermediate and high genetic risk category were 0.64 (95% CI = 0.56–0.72) and 0.72 (95% CI = 0.60–0.87), respectively.
We further combined the genetic and lifestyle categories to explore the joint effects of genetic–lifestyle on CRC risk. As shown in Figure 1, participants with low genetic risk and favorable lifestyles had an HR of 0.44 (95% CI =0.36–0.55) for developing CRC vs. participants with high genetic risk and unfavorable lifestyles. The HRs for low genetic risk participants with unfavorable lifestyles were 0.69 (95% CI =0.58–0.82) vs. participants with high genetic risk and unfavorable lifestyles.
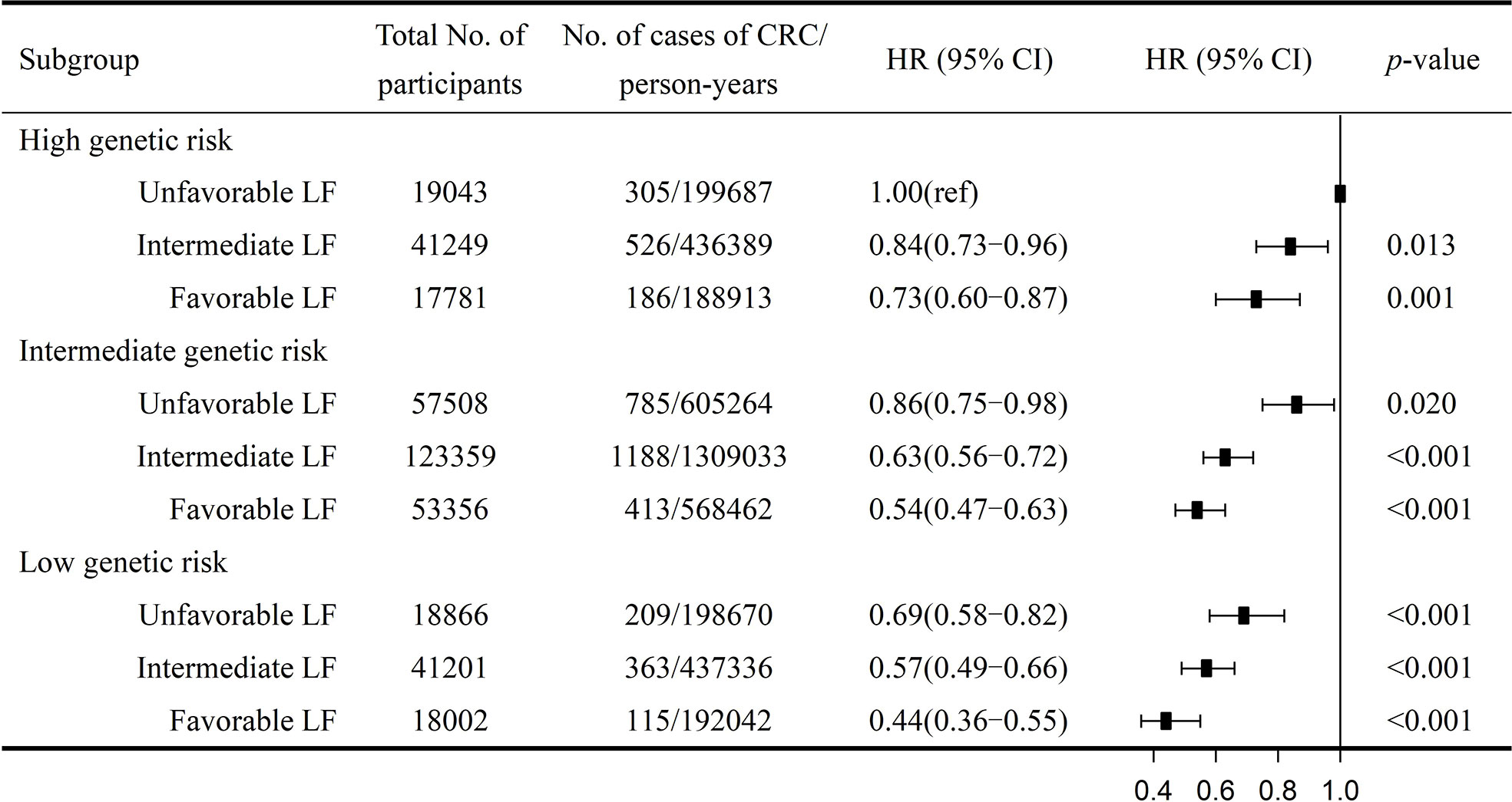
Figure 1 Risk of CRC according to genetic and lifestyle risk. LF, lifestyle; HR, hazard ratio; CI, confidence interval. Cox proportional hazards regression adjusted for age, sex, education, Townsend deprivation index, and the first 10 principal components of ancestry. p for multiplicative interaction< 0.001.
The sensitivity analysis indicated that the results were robust. First, low genetic risk combined with a healthy lifestyle has been observed to be associated with lower CRC risk when stratified by age, sex, socioeconomic status, and education (Supplementary Tables S6-S9). Second, the results did not change when using the weighted lifestyle scores (Supplementary Figures S5, S6).
Discussion
This study presented the quantitative effects of genetic risk, lifestyle, and their joint effects on CRC risk using data from the UK Biobank and FinnGen. The results indicated that the high genetic risk was significantly correlated with an increased CRC risk. If individuals were at high genetic risk for CRC, the susceptibility of CRC may be enhanced but may also be modified through a healthy lifestyle, namely, as a result of their combined effects. Notably, adherence to a favorable lifestyle was correlated with a lower CRC risk, regardless of the genetic risk category.
Numerous studies have reported that decreased CRC risk is related to a healthy lifestyle, which is consistent with the results of this study (36–38). For instance, a Danish cohort study involving 55,487 participants constructed a lifestyle score for factors such as smoking, exercise, drinking, waist circumference, and diet and found that a healthy lifestyle was associated with reduced CRC risk (RR = 0.89) (39). Compared with past studies, our research scale was an order of magnitude larger, and genetic risk was considered. We selected 59 SNPs from 454,678 SNPs to construct the “best-fit” PRS, which can effectively evaluate the genetic susceptibility of CRC and help save costs. Although individuals may think that they are powerless against their CRC genetic susceptibility, our research shows that favorable lifestyles can still significantly lower the risk of CRC, regardless of genetic background. Within any genetic risk background, adherence to a favorable lifestyle may modify CRC risk and, to a certain extent, help to prevent CRC.
In recent years, studies on the genetic and lifestyle risks of CRC have begun. A recent study of 346,297 UK Biobank participants constructed PRS and a healthy lifestyle score including eight lifestyle factors (BMI, waist-to-hip ratio, exercise, sedentary time, intake of processed meat, red meat, vegetables, and fruits) and found that the PRS and healthy lifestyle had a significant additive interaction (p< 0.05) (40). However, the base data (containing 95 SNPs) used in that study were derived from two published articles, partially involving East Asians and African Americans (35, 41), and the lifestyle factors included in the analysis were not comprehensive enough. In our study, we used the entire FinnGen cohort (containing 16,380,465 SNPs) as the base data; the Finnish and UK populations are genetically closer compared to the UK population and East Asians and African Americans, and the SNPs selected to construct the PRS are also different. In addition, we included sleep duration; sleep chronotype; insomnia; snoring; daytime dozing; and intake of fish, whole grains, and refined grains in the construction of a healthy lifestyle score. Our study provides evidence of a healthy lifestyle for lower CRC risk, regardless of genetic background. Our findings are consistent with a case–control analysis using 7,558 German participants, which investigated the absolute CRC risk according to genetics, diet, BMI, smoking, alcohol consumption, exercise, and colonoscopy history, and found that the OR for high genetic risk was about 2.20 vs. that for the low genetic risk, and the OR for a favorable lifestyle was about 0.50 vs. that for an unfavorable lifestyle (3). Our research included six dimensions of 16 lifestyle factors and provided quantitative results to complement the joint effects of genetic risk and lifestyle factors on CRC. These results revealed a strong association of genetic and lifestyle factors with the risk of CRC. Our study supports the long-held view that genetic variants identifiable at birth change the risk of CRC (42–44), which is beneficial for the primary prevention of CRC.
Numerous biological mechanisms have been proposed to explain the association of genetics and lifestyle with the risk of CRC. Insufficient exercise affects the body’s immune system and depresses immune function in CRC, while nicotine may partially promote colorectal tumor growth and angiogenesis (45). Excess drinking causes acetaldehyde to accumulate in the colorectum, which may cause DNA damage (46). Inadequate sleep may affect the release of prolactin, growth hormone, and melatonin and may also depress the function of proinflammatory cytokine genes (47). These unhealthy lifestyles may lead to obesity, systemic inflammation, insulin resistance, and type 2 diabetes and may impact CRC risk to a certain extent. The genetic risk of CRC may be related to mutated oncogenes, such as APC, TP53, KRAS, PI3KCA, BRAF, and NRAS (48, 49). Our study found that there is a high risk of CRC in high genetic risk categories with unfavorable lifestyles, which may better alert the high-risk CRC population to adopt a healthy lifestyle.
This is the largest prospective cohort study to date that has investigated the association of genetic factors and lifestyles, including six dimensions, with CRC risk. It focuses on the risk of integrated lifestyles and genetic susceptibility to CRC. Compared to modeling a single factor alone, it can better capture the complex nature and multiple dimensions of lifestyle behaviors. However, this study has some limitations. First, lifestyle scores use a dichotomy, and the selection of cutoff points is mainly based on public health recommendations, which are general rather than specific for certain risks. Second, several participants were excluded because of missing values, which may have significantly reduced the data. Third, the lifestyle factors that we constructed did not include all possible lifestyle factors such as aspirin medication history, family history of CRC, and other possible influencing factors. Incorporating them into the model may help improve the accuracy of CRC risk assessment. Finally, this study mainly focused on participants from a white ethnic background. Future studies should evaluate the generalizability of these research results to other populations.
Conclusions
In conclusion, our findings validate that low genetic risk and a favorable lifestyle are significantly associated with a decreased CRC risk. Additionally, we found that a favorable lifestyle was associated with a lower CRC risk in any genetic background, indicating the potential of modifiable lifestyle factors in CRC prevention.
Data availability statement
Publicly available datasets were analyzed in this study. The data can be found here: The FinnGen cohort Genome-wide association study (GWAS) summary data are openly available from the website https://finngen.gitbook.io/documentation/(R5). The UK Biobank datasets are openly available by submitting a data request proposal from https://www.ukbiobank.ac.uk/. We are able to access the UK Biobank database through the Access Management System (AMS). The application number is 78563.
Author contributions
EW performed the statistical analysis and wrote the manuscript. J-TN designed the study. XC, Z-HZ, and H-QX were responsible for technical support. TX and LT revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by the General Projects of National Natural Science Foundation of China (81973635) (to TX) and the Start-up Grant of HZNU (4125C5021720421) (to LT). The funding sources had no role in the study design; in the collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Acknowledgments
We thank the participants and staff of the UK Biobank for their dedication and contribution to the research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer XC declared a shared parent affiliation with the author JN to the handling editor at the time of review.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.894086/full#supplementary-material
Abbreviations
CRC, colorectal cancer; SNP, single nucleotide polymorphism; PRS, polygenic risk scores; HR, hazard ratio; CI, confidence interval; GWAS, genome-wide association study; GPC, genetic principal component; MAF, minor allele frequency; HWE, Hardy–Weinberg equilibrium; SD, standard deviation; BMI, body mass index; TDI, Townsend deprivation index.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
2. Huyghe JR, Bien SA, Harrison TA, Kang HM, Chen S, Schmit SL, et al. Discovery of common and rare genetic risk variants for colorectal cancer. Nat Genet (2019) 51(1):76–87. doi: 10.1038/s41588-018-0286-6
3. Carr PR, Weigl K, Edelmann D, Jansen L, Chang-Claude J, Brenner H, et al. Estimation of absolute risk of colorectal cancer based on healthy lifestyle, genetic risk, and colonoscopy status in a population-based study - ScienceDirect. Gastroenterology (2020) 159(1):129–38. doi: 10.1053/j.gastro.2020.03.016
4. Fujita M, Liu X, Iwasaki Y, Terao C, Mizukami K, Kawakami E, et al. Population-based screening for hereditary colorectal cancer variants in Japan. Clin Gastroenterol Hepatol (2022) 20(9):2132–2141.e9. doi: 10.1016/j.cgh.2020.12.007
5. Law PJ, Timofeeva M, Fernandez-Rozadilla C, Broderick P, Thibodeau SN. Association analyses identify 31 new risk loci for colorectal cancer susceptibility. Nat Commun (2019) 10(1):2154. doi: 10.1038/s41467-019-09775-w
6. Lu Y, Kweon SS, Tanikawa C, Jia WH, Xiang YB, Cai Q, et al. Large-Scale genome-wide association study of East asians identifies loci associated with risk for colorectal cancer. Gastroenterology (2019) 156(5):1455–66. doi: 10.1053/j.gastro.2018.11.066
7. Thomas M, Sakoda LC, Hoffmeister M, Rosenthal EA, Li H. Genome-wide modeling of polygenic risk score in colorectal cancer risk. Am J Hum Genet (2020) 107(3):432–44. doi: 10.1016/j.ajhg.2020.07.006
8. Jeon J, Du M, Schoen RE, Hoffmeister M, Newcomb PA, Berndt SI, et al. Determining risk of colorectal cancer and starting age of screening based on lifestyle, environmental, and genetic factors. Gastroenterology (2018) 154(8):2152–64. doi: 10.1053/j.gastro.2018.02.021
9. Burnett-Hartman AN, Newcomb PA, Peters U. Challenges with colorectal cancer family history assessment-motivation to translate polygenic risk scores into practice. Gastroenterology (2019) 158(2):433–5. doi: 10.1053/j.gastro.2019.10.030
10. Tanskanen T, van den Berg L, Välimäki N, Aavikko M, Ness-Jensen E, Hveem K, et al. Genome-wide association study and meta-analysis in northern European populations replicate multiple colorectal cancer risk loci. Int J Cancer (2018) 142(3):540–6. doi: 10.1002/ijc.31076
11. Rock CL, Thomson C, Gansler T, Gapstur SM, McCullough ML, Patel AV, et al. American Cancer society guideline for diet and physical activity for cancer prevention. CA Cancer J Clin (2020) 70(4):245–71. doi: 10.3322/caac.21591
12. Zheng B, Yu C, Lv J, Guo Y, Bian Z, Zhou M, et al. Insomnia symptoms and risk of cardiovascular diseases among 0.5 million adults: A 10-year cohort. Neurology (2019) 93(23):e2110–20. doi: 10.1212/wnl.0000000000008581
13. Kripke DF, Simons RN, Garfinkel L, Hammond EC. Short and long sleep and sleeping pills. Is increased mortality associated? Arch Gen Psychiatry (1979) 36(1):103–16. doi: 10.1001/archpsyc.1979.01780010109014
14. Erren TC, Morfeld P, Foster RG, Reiter RJ, Gross JV, Westermann IK. Sleep and cancer: Synthesis of experimental data and meta-analyses of cancer incidence among some 1,500,000 study individuals in 13 countries. Chronobiol Int (2016) 33(4):325–50. doi: 10.3109/07420528.2016.1149486
15. Zhang X, Giovannucci EL, Wu K, Gao X, Hu F, Ogino S, et al. Associations of self-reported sleep duration and snoring with colorectal cancer risk in men and women. Sleep (2013) 36(5):681–8. doi: 10.5665/sleep.2626
16. Zhao H, Yin JY, Yang WS, Qin Q, Li TT, Shi Y, et al. Sleep duration and cancer risk: a systematic review and meta-analysis of prospective studies. Asian Pac J Cancer Prev (2013) 14(12):7509–15. doi: 10.7314/apjcp.2013.14.12.7509
17. International Schizophrenia Consortium, Purcell SM, Wray NR, Stone JL, Visscher PM, O'Donovan MC, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature (2009) 460(7256):748–52. doi: 10.1038/nature08185
18. Choi SW, Mak TS, O'Reilly PF. Tutorial: a guide to performing polygenic risk score analyses. Nat Protoc (2020) 15(9):2759–72. doi: 10.1038/s41596-020-0353-1
19. Marees AT, de Kluiver H, Stringer S, Vorspan F, Curis E, Marie-Claire C, et al. A tutorial on conducting genome-wide association studies: Quality control and statistical analysis. Int J Methods Psychiatr Res (2018) 27(2):e1608. doi: 10.1002/mpr.1608
20. Choi SW, O'Reilly PF. PRSice-2: Polygenic risk score software for biobank-scale data. GigaScience (2019) 8(7):giz082. doi: 10.1093/gigascience/giz082
21. Euesden J, Lewis CM, O'Reilly PF. PRSice: Polygenic risk score software. Bioinf (Oxford England) (2015) 31(9):1466–8. doi: 10.1093/bioinformatics/btu848
22. Lin CL, Liu TC, Wang YN, Chung CH, Chien WC. The association between sleep disorders and the risk of colorectal cancer in patients: A population-based nested case-control study. In Vivo (2019) 33(2):573–9. doi: 10.21873/invivo.11513
23. Gu WJ, Pei JP, Lyu J, Akimoto N, Haruki K, Ogino S, et al. The burden of early-onset colorectal cancer and its risk factors from 1990 to 2019: A systematic analysis for the global burden of disease study 2019. Cancers (Basel) (2022) 14(14):3502. doi: 10.3390/cancers14143502
24. Peppone LJ, Mahoney MC, Cummings KM, Michalek AM, Reid ME, Moysich KB, et al. Colorectal cancer occurs earlier in those exposed to tobacco smoke: implications for screening. J Cancer Res Clin Oncol (2008) 134(7):743–51. doi: 10.1007/s00432-007-0332-8
25. Klarich D, Brasser SM, Hong MY. Moderate alcohol consumption and colorectal cancer risk. Alcoholism Clin Exp Res (2015) 39(8):1280–91. doi: 10.1111/acer.12778
26. Lourida I, Hannon E, Littlejohns TJ, Langa KM, Llewellyn DJ. Association of lifestyle and genetic risk with incidence of dementia. JAMA: J Am Med Assoc (2019) 322(5):430–7. doi: 10.1001/jama.2019.9879
27. Oru Z, Kaplan MA. Effect of exercise on colorectal cancer prevention and treatment. World J Gastroint Oncol (2019) 11(5):348–66. doi: 10.4251/wjgo.v11.i5.348
28. Mozaffarian D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity a comprehensive review. Circulation: Off J Am Heart Assoc (2016) 133(2):187–225. doi: 10.1161/CIRCULATIONAHA.115.018585
29. Li X, Xue Q, Wang M, Zhou T, Qi L. Adherence to a healthy sleep pattern and incident heart failure: A prospective study of 408802 UK biobank participants. Circulation (2020) 143(1):97–9. doi: 10.1161/CIRCULATIONAHA
30. Lin XJ, Wang CP, Liu XD, Yan KK, Li S, Bao HH, et al. Body mass index and risk of gastric cancer: a meta-analysis. Japanese J Clin Oncol (2014) 44(9):783–91. doi: 10.1093/jjco/hyu082
31. Pei YF, Zhang L. Is the Townsend deprivation index a reliable predictor of psychiatric disorders? Biol Psychiatry (2021) 89(9):839–41. doi: 10.1016/j.biopsych.2021.02.006
32. Wu E, Ni J-T, Zhu Z-H, Xu H-Q, Tao L, Xie T. Association of a healthy lifestyle with all-cause, cause-specific mortality and incident cancer among individuals with metabolic syndrome: A prospective cohort study in UK biobank. Int J Environ Res Public Health (2022) 19(16):9936. doi: 10.3390/ijerph19169936
33. Knuppel A, Fensom GK, Watts EL, Gunter MJ, Murphy N, Papier K, et al. Circulating insulin-like growth factor-I concentrations and risk of 30 cancers: Prospective analyses in UK biobank. Cancer Res (2020) 80(18):4014–21. doi: 10.1158/0008-5472.Can-20-1281
34. Wen W, Shu XO, Guo X, Cai Q, Long J, Bolla MK, et al. Prediction of breast cancer risk based on common genetic variants in women of East Asian ancestry. Breast Cancer research: BCR (2016) 18(1):124. doi: 10.1186/s13058-016-0786-1
35. Jia G, Lu Y, Wen W, Long J, Liu Y, Tao R, et al. Evaluating the utility of polygenic risk scores in identifying high-risk individuals for eight common cancers. JNCI Cancer Spectr (2020) 4(3):pkaa021. doi: 10.1093/jncics/pkaa021
36. Carr PR, Weigl K, Jansen L, Walter V, Erben V, Chang-Claude J, et al. Healthy lifestyle factors associated with lower risk of colorectal cancer irrespective of genetic risk. Gastroenterology (2018) 155(6):1805–1815.e5. doi: 10.1053/j.gastro.2018.08.044
37. Aleksandrova K, Pischon T, Jenab M, Bueno-de-Mesquita HB, Fedirko V, Norat T. Combined impact of healthy lifestyle factors on colorectal cancer: a large European cohort study. BMC Med (2014) 12(1):168. doi: 10.1186/s12916-014-0168-4
38. Hang J, Cai B, Xue P, Wang L, Hu H, Zhou Y, et al. The joint effects of lifestyle factors and comorbidities on the risk of colorectal cancer: A Large Chinese retrospective case-control study. PloS One (2015) 10(12):e0143696. doi: 10.1371/journal.pone.0143696
39. Kirkegaard H, Johnsen NF, Christensen J, Frederiksen K, Overvad K, Tjønneland A. Association of adherence to lifestyle recommendations and risk of colorectal cancer: a prospective Danish cohort study. Bmj (2010) 341:c5504. doi: 10.1136/bmj.c5504
40. Choi J, Jia G, Wen W, Shu XO, Zheng W. Healthy lifestyles, genetic modifiers, and colorectal cancer risk: a prospective cohort study in the UK biobank. Am J Clin Nutr (2021) 113(4):810–20. doi: 10.1093/ajcn/nqaa404
41. Schmit SL, Edlund CK, Schumacher FR, Gong J, Harrison TA, Huyghe JR, et al. Novel common genetic susceptibility loci for colorectal cancer. J Natl Cancer Inst (2019) 111(2):146–57. doi: 10.1093/jnci/djy099
42. Bustoros M, Sklavenitis-Pistofidis R, Park J, Redd R, Zhitomirsky B, Dunford AJ, et al. Genomic profiling of smoldering multiple myeloma identifies patients at a high risk of disease progression. J Clin Oncol (2020) 38(21):2380–9. doi: 10.1200/JCO.20.00437
43. Khera AV, Emdin CA, Drake I, Natarajan P, Bick AG, Cook NR, et al. Genetic risk, adherence to a healthy lifestyle, and coronary disease. N Engl J Med (2016) 375(24):2349–58. doi: 10.1056/NEJMoa1605086
44. Chen X, Jansen L, Guo F, Hoffmeister M, Chang-Claude J, Brenner H. Smoking, genetic predisposition, and colorectal cancer risk. Clin Transl Gastroenterol (2021) 12(3):e00317. doi: 10.14309/ctg.0000000000000317
45. Wong HP, Yu L, Lam EK, Tai EK, Wu WK, Cho CH. Nicotine promotes cell proliferation via alpha7-nicotinic acetylcholine receptor and catecholamine-synthesizing enzymes-mediated pathway in human colon adenocarcinoma HT-29 cells. Toxicol Appl Pharmacol (2007) 221(3):261–7. doi: 10.1016/j.taap.2007.04.002
46. Seitz HK, Stickel F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nat Rev Cancer (2007) 7(8):599–612. doi: 10.1038/nrc2191
47. Savvidis C, Koutsilieris M. Circadian rhythm disruption in cancer biology. Mol Med (2012) 18:1249–60. doi: 10.2119/molmed.2012.00077
48. Zhang L, Shay JW. Multiple roles of APC and its therapeutic implications in colorectal cancer. J Natl Cancer Inst (2017) 109(8):djw332. doi: 10.1093/jnci/djw332
Keywords: colorectal cancer, polygenic risk scores, lifestyle factors, epidemiology, prevention
Citation: Wu E, Ni J-T, Chen X, Zhu Z-H, Xu H-Q, Tao L and Xie T (2022) Genetic risk, incident colorectal cancer, and the benefits of adhering to a healthy lifestyle: A prospective study using data from UK Biobank and FinnGen. Front. Oncol. 12:894086. doi: 10.3389/fonc.2022.894086
Received: 11 March 2022; Accepted: 13 September 2022;
Published: 06 October 2022.
Edited by:
Tianhui Chen, University of Chinese Academy of Sciences, ChinaReviewed by:
Xin Chen, Zhejiang University School of Medicine, ChinaKailin Tang, Tongji University, China
Copyright © 2022 Wu, Ni, Chen, Zhu, Xu, Tao and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lin Tao, taolin@hznu.edu.cn; Tian Xie, xbs@hznu.edu.cn
†These authors share first authorship
 E. Wu1,2†
E. Wu1,2† Xin Chen
Xin Chen Tian Xie
Tian Xie