- 1General Surgery and Organ Transplantation Unit, Sapienza, Rome, Italy
- 2Department of Medicine I, Innsbruck University, Innsbruck, Austria
- 3Institut de Recherche Expérimental et Clinique (IREC), Université Catholique de Louvain, Brussels, Belgium
- 4Department of Surgical, Oncological and Gastroenterological Sciences, Padua University, Padua, Italy
- 5UCL Institute for Liver and Digestive Health and Royal Free Sheila Sherlock Liver Centre, Royal Free Hospital, London, United Kingdom
- 6Klinik für Allgemein-, Viszeral- und Transplantationschirurgie, Mainz University, Mainz, Germany
- 7Division of General Surgery and Liver Transplantation, San Camillo Hospital, Rome, Italy
- 8Department of Transplant Surgery, PTV University, Rome, Italy
- 9Unit of Hepatobiliary Surgery and Transplantation, Marche Polytechnic University, Ancona, Italy
- 10Catholic University - Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome, Italy
Background: Long-term survival after liver transplantation (LT) for hepatocellular cancer (HCC) continues to increase along with the modification of inclusion criteria. This study aimed at identifying risk factors for 5- and 10-year overall and HCC-specific death after LT.
Methods: A total of 1,854 HCC transplant recipients from 10 European centers during the period 1987–2015 were analyzed. The population was divided in three eras, defined by landmark changes in HCC transplantability indications. Multivariable logistic regression analyses were used to evaluate the significance of independent risk factors for survival.
Results: Five- and 10-year overall survival (OS) rates were 68.1% and 54.4%, respectively. Two-hundred forty-two patients (13.1%) had HCC recurrence. Five- and 10-year recurrence rates were 16.2% and 20.3%. HCC-related deaths peaked at 2 years after LT (51.1% of all HCC-related deaths) and decreased to a high 30.8% in the interval of 6 to 10 years after LT. The risk factors for 10-year OS were macrovascular invasion (OR = 2.71; P = 0.001), poor grading (OR = 1.56; P = 0.001), HCV status (OR = 1.39; P = 0.001), diameter of the target lesion (OR = 1.09; P = 0.001), AFP slope (OR = 1.63; P = 0.006), and patient age (OR = 0.99; P = 0.01). The risk factor for 10-year HCC-related death were AFP slope (OR = 4.95; P < 0.0001), microvascular (OR = 2.13; P < 0.0001) and macrovascular invasion (OR = 2.32; P = 0.01), poor tumor grading (OR = 1.95; P = 0.001), total number of neo-adjuvant therapies (OR = 1.11; P = 0.001), diameter of the target lesion (OR = 1.11; P = 0.002), and patient age (OR = 0.97; P = 0.001). When analyzing survival rates in function of LT era, a progressive improvement of the results was observed, with patients transplanted during the period 2007–2015 showing 5- and 10-year death rates of 26.8% and 38.9% (vs. 1987–1996, P < 0.0001; vs. 1997–2006, P = 0.005).
Conclusions: LT generates long-term overall and disease-free survival rates superior to all other oncologic treatments of HCC. The role of LT in the modern treatment of HCC becomes even more valued when the follow-up period reaches at least 10 years. The results of LT continue to improve even when prudently widening the inclusion criteria for transplantation. Despite the incidence of HCC recurrence is highest during the first 5 years post-transplant, one-third of them occur later, indicating the importance of a life-long follow-up of these patients.
Introduction
Liver transplantation (LT) represents the gold-standard therapy to cure well-selected patients with hepatocellular cancer (HCC) (1). Before 1996, the absence of internationally recognized inclusion criteria explained the poor results of LT in patients with HCC (2). The introduction of the Milan criteria in clinical practice strongly modified the outcomes, resulting in 5-year survival rates similar to those obtained in non-HCC patients (3, 4). However, the rigorous adoption of these criteria significantly limits access to potentially successful treatment to a large number of patients, even slightly exceeding the selection criteria. Therefore, the transplant community widened in recent years the selection criteria for LT, thereby increasing the number of transplanted without impairing the expected results (5–7). Reporting of outcome is usually limited to 5-year survival rates. The impact of LT in the very long follow-up (i.e., ≥10 years) is still an unanswered question, especially when compared to other (curative) approaches such as liver resection (8).
In this light, it was hypothesized that LT should provide a beneficial 10-year survival impact. The study aimed at exploring the risk factors for 5- and 10-year death and HCC-specific death in a large international population of HCC liver patients.
Methods
Study Design
This is a retrospective international study carried out on prospectively maintained databases identifying adult (≥18 years) patients enlisted and transplanted with the primary diagnosis of HCC. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline (9). The institutional review board of Azienda Ospedaliero-Universitaria Policlinico Umberto I (coordinating center) approved the study.
Setting
Participants included 10 centers composing the EurHeCaLT Study Group. The centers participating in the study were as follows: Innsbruck University, Innsbruck, Austria (n = 296); Université Catholique de Louvain, Brussels, Belgium (n = 283); Padua University, Padua, Italy (n = 267); Sapienza University of Rome, Rome, Italy (n = 195); Royal Free Hospital, London, UK (n = 193); Mainz University, Mainz, Germany (n = 176); San Camillo Hospital, Rome, Italy (n = 142); PTV University Rome, Rome, Italy (n = 122); University of Marche, Ancona, Italy (n = 95); and Catholic University Rome, Rome, Italy (n = 85).
Population
The investigated population included consecutive adult (≥18 years) patients enlisted and transplanted with the primary diagnosis of HCC during the period 1987–2015. Patients with HCC diagnosed only at pathological examination (incidental HCC), mixed hepatocellular-cholangiocellular cancer, and cholangiocellular cancer misdiagnosed as HCC were not included in the study.
Variables and Data Collection
Collected patient-related data included the following: age and sex, cause of cirrhosis [hepatitis C virus (HCV), hepatitis B virus (HBV), alcohol, non-alcoholic steato-hepatitis (NASH), and other diseases], waiting time (WT) duration, model for end-stage liver disease (MELD), and period of LT (1987–1996, 1997–2006, and 2007–2015). Pre-LT available tumor-related data were morphologic HCC characteristics and alpha-fetoprotein (AFP) values evaluated at first referral and last pre-LT assessment, neo-adjuvant treatment(s), and subsequent modified Response Evaluation Criteria in Solid Tumors (mRECIST) status.
Tumor-related data obtained at pathological specimens were morphologic characteristics, multi-focality, bi-lobarity, poor grading, and micro- and macrovascular invasion. In all cases, morphologic HCC aspects referred to vital tumor tissue only.
Definitions
Patient death was defined as any death caused by tumor- and non-tumor–related causes observed during the entire post-transplant follow-up. Patient death time was calculated as the time from LT to death after LT during the follow-up.
HCC-specific death was defined as a death directly caused by a tumor recurrence observed during the follow-up.
HCC recurrence was defined as any hepatic and/or extra-hepatic reappearance of the tumor at any time from the LT. Tumor recurrence time was calculated as the time from LT to detect tumor recurrence after LT during the follow-up. The last follow-up date was December 31, 2021.
The periods of LT were defined according to the introduction of some innovation in the field of transplant oncology: period 1987–1996 corresponding to the pre-Milan criteria era (liberal approach); (2) period 1997–2006 to the Milan criteria era; (3,4) and period 2007–2015 corresponding to the expanded criteria era (safe enlargement of inclusion criteria). In detail, the Up-to-seven criteria or the UCSF criteria were adopted in the different centers, with the exception of the Padua center, adopting the HCC-MELD score based on benefit principles (5–7).
Statistical Analysis
Baseline characteristics of each data set were presented as medians and interquartile ranges (IQRs) for continuous variables and as numbers and percentages for discrete variables. Kruskal–Wallis test was adopted for comparing continuous variables. Chi-squared test was adopted for comparing dichotomous variables. Data missingness is detailed in Supplementary Table 1. In all the cases, covariates included in the analysis had missing data <10%. Missed data were handled with a single imputation method, and a median of nearby points was adopted (10).
Multivariable logistic regression analyses were used to evaluate the significance of independent risk factors for survival as independent prognostic factors for observed 5- and 10-year overall survival (OS) and for HCC-specific 5- and 10-year survival. The investigated variables were initially introduced using a “full model” approach, and then, the most relevant ones were selected using a backward Wald method with the intent to develop more parsimonious models. Odds ratios (ORs) and 95.0% confidence intervals (95.0% CIs) were reported.
Kaplan–Meier survival estimates were used to calculate survival curves. Log-rank test was used for comparing the survival distributions of different groups. A p-value <0.05 was considered statistically significant. Statistical analyses were conducted using SPSS 27.0 (SPSS Inc., Chicago, IL, USA).
Results
Patient and Tumor Characteristics
Patient and tumor characteristics are reported in Table 1. A total of 1,854 patients were enrolled for the present study. The median follow-up was 46.4 months (IQR: 16.4–90.0). A total of 751 (40.5%) and 256 (13.8%) patients overpassed the 5 and 10 years of follow-up, respectively.
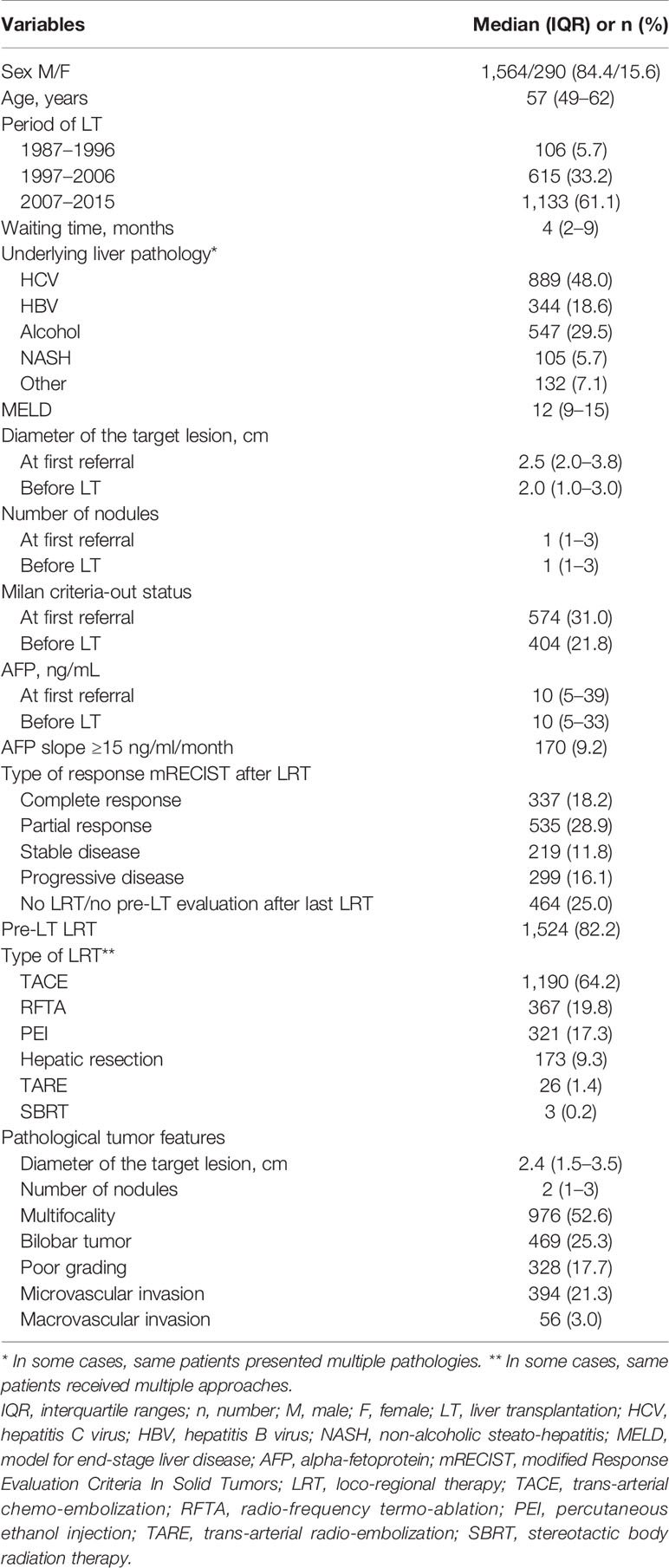
Table 1 Patient demographic data and tumor features at first referral, last radiological assessment before LT, and pathological examination.
The median age of the patients was 57 years (IQR = 49–62), males (n = 1,564, 84.4%) largely outnumbered female patients. The main underlying liver disease was HCV, followed by alcoholic-related cirrhosis. The median MELD value was 12 (IQR = 9–15). The median duration of the waiting time was 4 months (IQR = 2–9).
Median diameter of the target lesion at time of LT was 2.0 cm, with a higher prevalence of single lesions. The median pre-LT AFP value was 10 ng/ml; 170 (9.2%) patients presented an AFP-slope >15 ng/ml/month during the waiting time. Neo-adjuvant treatment was applied in 82.2% of cases. Trans-arterial chemo-embolization (TACE) was the most commonly adopted loco-regional therapy (LRT), followed by radio-frequency ablation. Salvage LT after resection was carried out in 173 (9.3%) cases. A complete radiological tumor response was obtained in 337 (18.2%) of patients, and 299 (16.1%) patients had a progressive disease.
At pathological examination of the hepatectomy specimen, the median diameter of the target lesion was 2.4 cm, and the median number of lesions was 2. A poor tumor grading was observed in 328 (17.7%) cases. Micro- and macrovascular invasions were present in 394 (21.3%) and 56 (3.0%) patients, respectively.
Patient Survival, HCC-Related Death, and Recurrence Estimates
During the follow-up period, 651 of 1,854 (35.1%) liver patients died: 512 (27.6%) patients died within the first 5 years post-LT, 104 (5.6%) between 6 and 10 years, and 35 (1.9%) more than 10 years after LT. In Table 2, different measures of survival were reported. The 5- and 10-year Kaplan–Meier OS estimates were 68.1% and 54.4%, respectively (Figure 1).
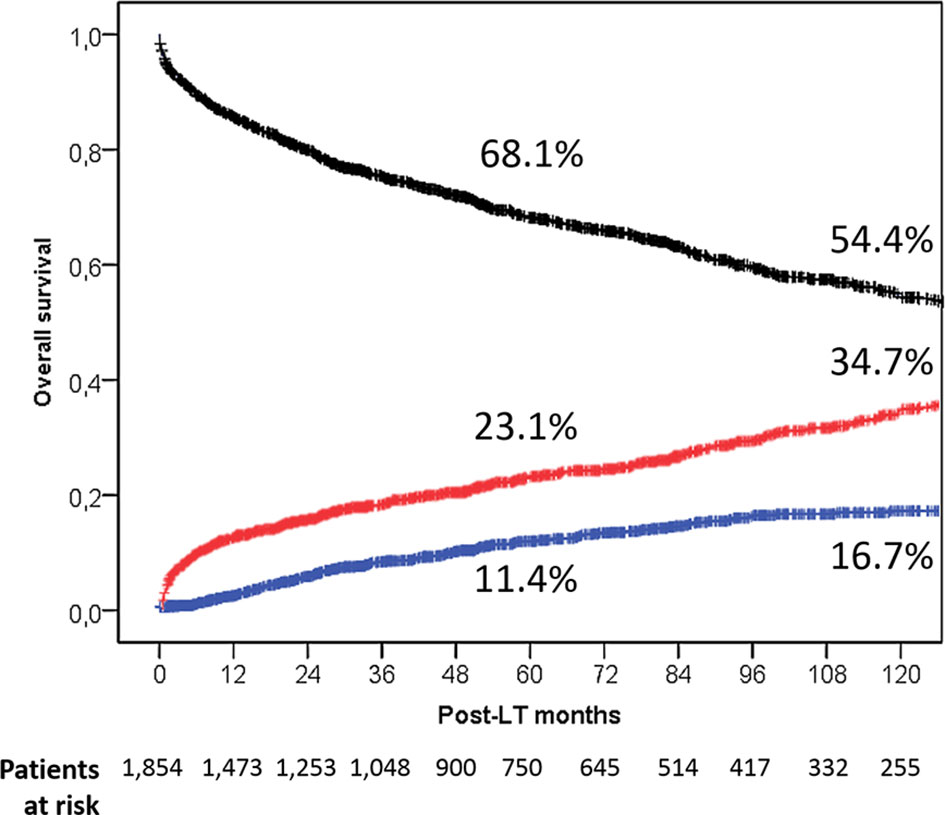
Figure 1 Overall patient survival rates in the entire population (black line). Death rates caused by tumor (blue line) and caused by other causes (red line) are also reported.
A total of 180 (9.7%) and 471 (25.4%) deaths were HCC-related and no HCC-related, respectively. Five- and 10-year HCC-related and non-HCC–related death estimates were 11.4% and 16.7% vs. 23.1% and 37.4%, respectively (Figure 1).
In relation to the timeline of post-LT deaths, a fast increase of the tumor-related deaths was seen with a peak during the second post-LT year (51.1% of death causes). Later on, a slight decline was observed (third year = 46.4%; fourth year = 43.2; fifth year = 35.6%). The percentage of cancer-related deaths between 6 and 10 years post-LT was surprisingly high (30.8%). The risk of dying from an HCC-related cause lowered to 9.1% and 8.3%, respectively, during the post-LT periods of 11–15 and >15 years (Figure 2).
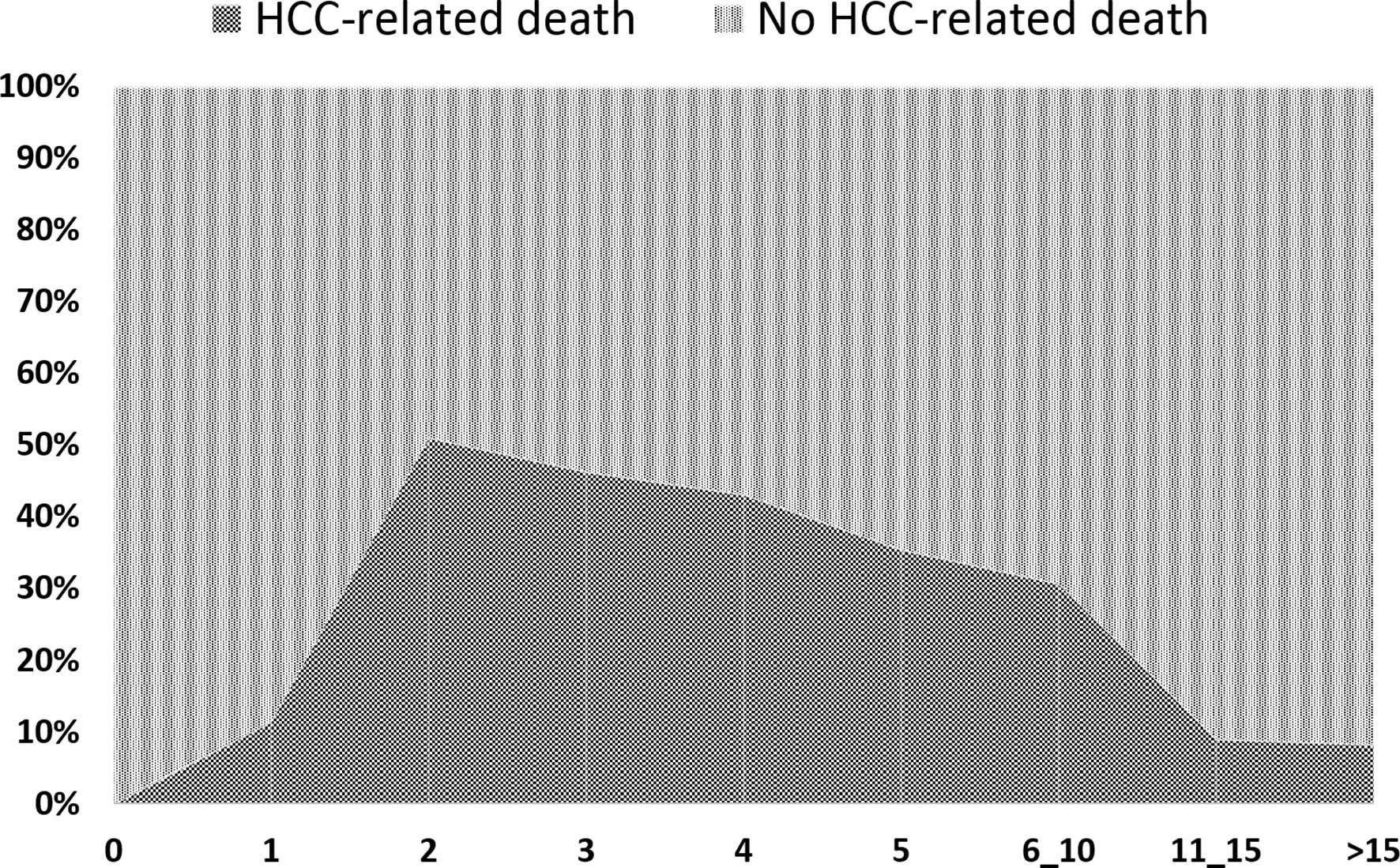
Figure 2 Causes of death expressed in percentages on the total number of cases at different time point of the follow-up.
Two hundred forty-two (13.1%) recurrences were reported; 62 (3.4%) of these patients were still alive at the last follow-up. The 5- and 10-year Kaplan–Meier recurrence rates were 16.2% and 20.3%.
Risk Factors for Overall Patient Death
Two separate multivariable logistic regression analyses were performed to explore the features connected with increased odds for the risk of 5- and 10-year death for any cause (Table 3). Observing the independent risk factors for 5-year death, macrovascular invasion showed the highest OR of 3.60 (P < 0.0001), followed by the diameter of the target lesion (OR = 1.12; P < 0.0001), poor grading (OR = 1.40; P = 0.01), AFP slope > 15 ng/ml/month (OR = 1.52; P = 0.02), and MELD score (OR = 1.02; P = 0.02).
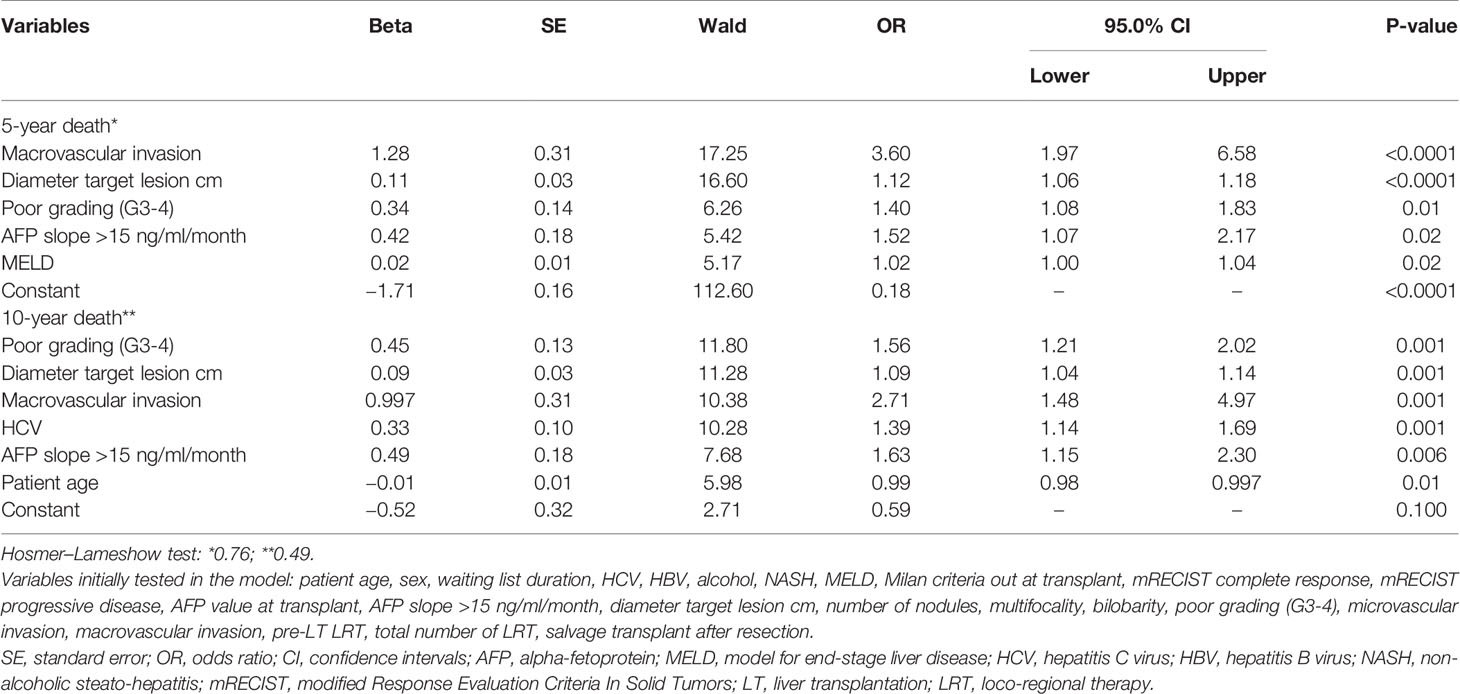
Table 3 Multivariable logistic regression analysis for the risk of 5- and 10-year death after LT (backward Wald method).
Recalculating the odds with a time horizon of 10 years, the following variables confirmed their negative prognostic impact: macrovascular invasion (OR = 2.71; P = 0.001), diameter of the target lesion (OR = 1.09; P = 0.001), poor grading (OR = 1.56; P = 0.001), and AFP slope (OR = 1.63; P = 0.006). In contrast, MELD score lost its relevance. HCV status (OR = 1.39; P = 0.001) and patient age (OR = 0.99; P = 0.01) reported statistically relevant odds in this long-term analysis.
Risk Factors for HCCRelated Death
Two separate multivariable logistic regression analyses were utilized to explore the features connected with increased odds for the risk of 5- and 10-year HCC-related death (Table 4).
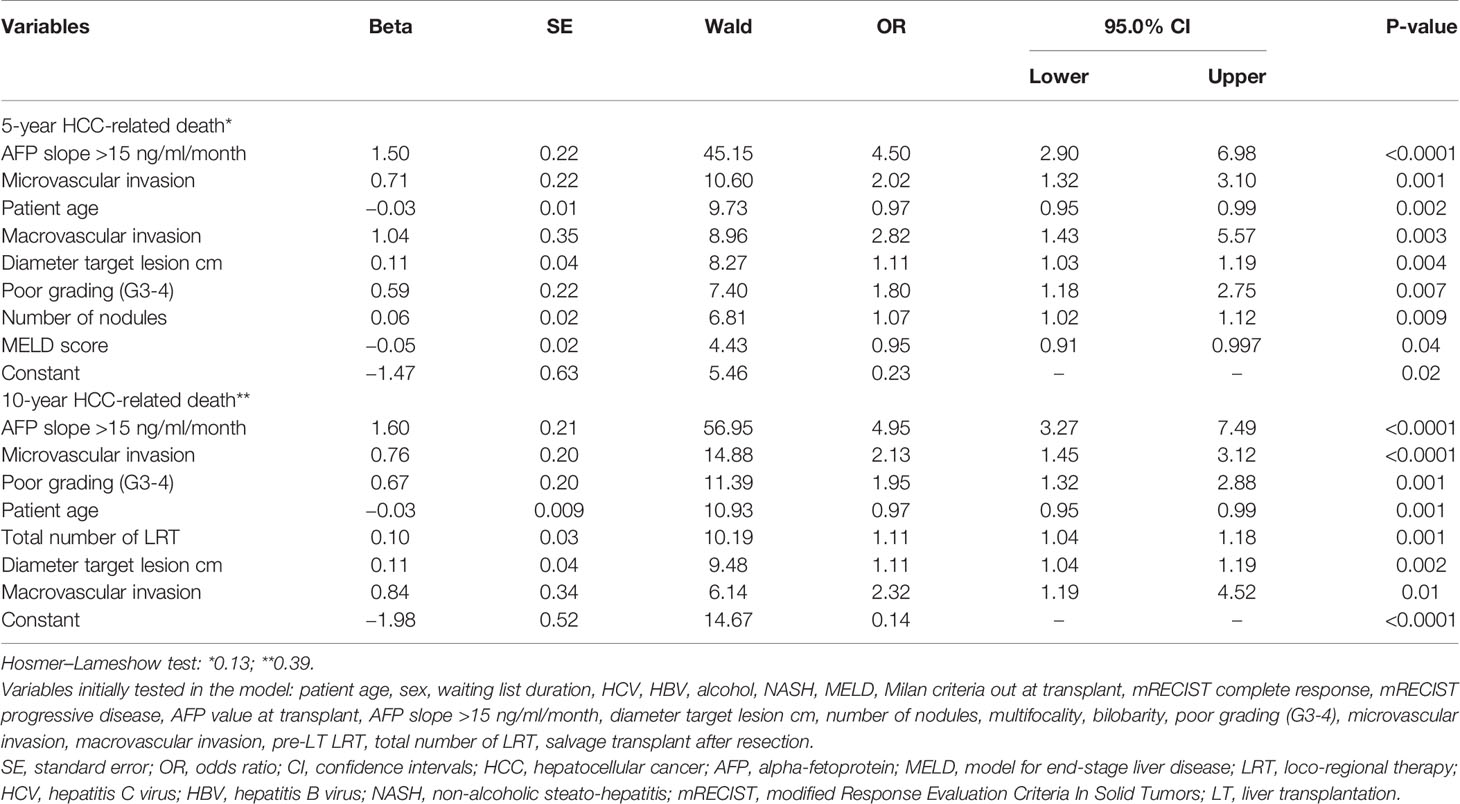
Table 4 Multivariable logistic regression analysis for the risk of 5- and 10-year HCC-related death after LT (backward Wald method).
Again, similar variables were observable in the two models. As for the risk of 5-year HCC-specific death, AFP slope had the highest OR of 4.50 (P < 0.0001), followed by microvascular invasion (OR = 2.02; P = 0.001), macrovascular invasion (OR = 2.82; P = 0.003), diameter of the target lesion (OR = 1.11; P = 0.004), poor grading (OR = 1.80; P = 0.007), and number of nodules (OR = 1.07; P = 0.009). Patient age (OR = 0.97; P = 0.002) and MELD score (OR = 0.95; P = 0.04) were protective for the risk of HCC-specific death.
When the time horizon was set at to 10 years, the relevant role of AFP slope was confirmed (OR = 4.95; P < 0.0001), followed by microvascular (OR = 2.13; P < 0.0001) and macrovascular invasion (OR = 2.32; P = 0.01), poor tumor grading (OR = 1.95; P = 0.001), total number of neo-adjuvant therapies (OR = 1.11; P = 0.001), and diameter of the target lesion (OR = 1.11; P = 0.002). Again, patient age was a protective factor (OR = 0.97; P = 0.001).
Correlation Between Death and Period of Transplant
Relevant differences existed among the different periods in terms of patient and tumor characteristics and clinical management, as reported in Table 5. In light of these aspects, a sub-analysis was done focused on the different risk factors for 10-year HCC-related death in the three different periods (Table 6). In detail, the slope of AFP was always the most relevant risk factors in all the different periods. The number of LRT emerged as a detrimental factor only in the last two periods, in which the pre-LT management with multiple LRT has raised as a routine approach in HCC transplant candidates.
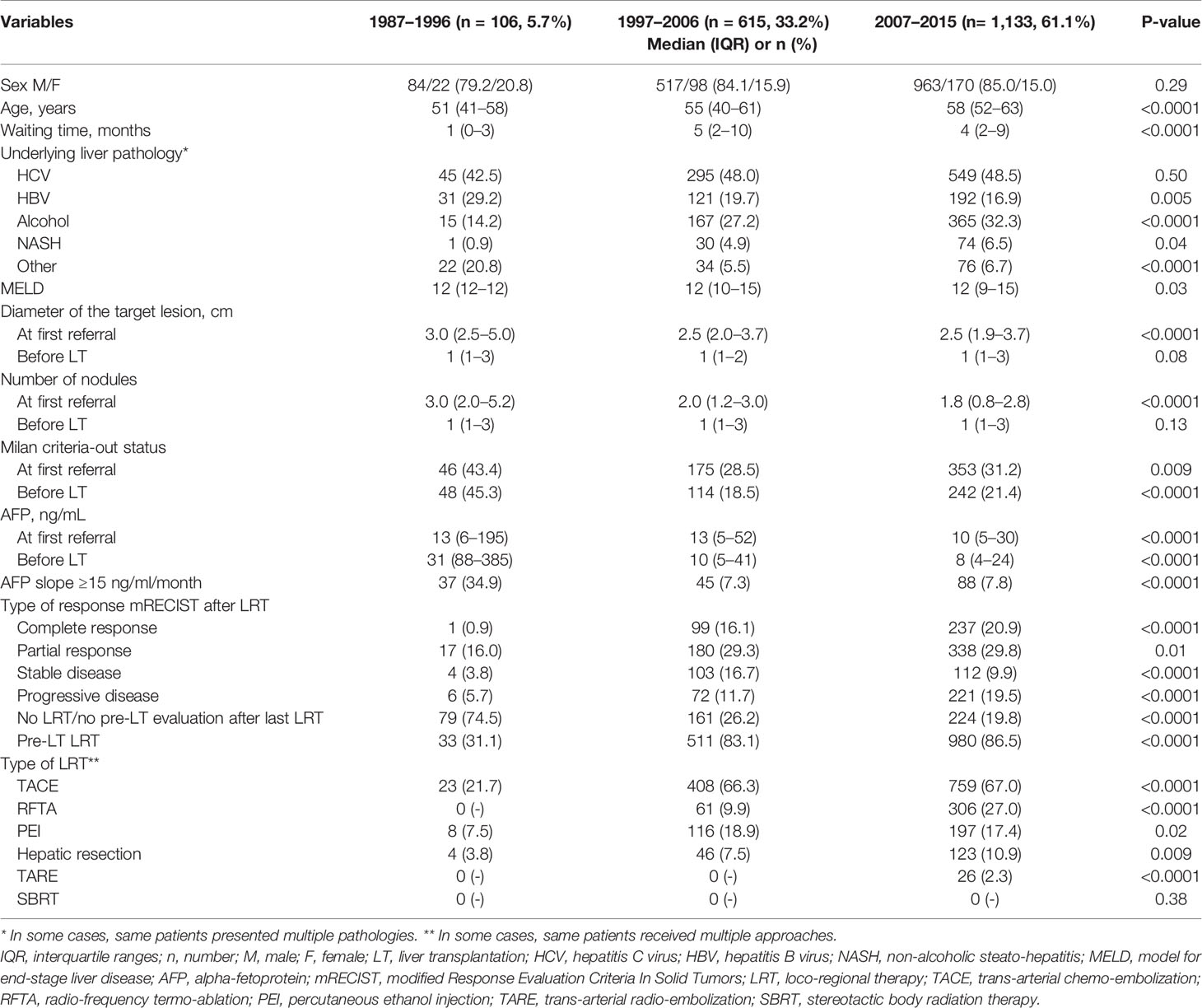
Table 5 Patient demographic data and tumor features at first referral and last radiological assessment before LT in the three different periods.
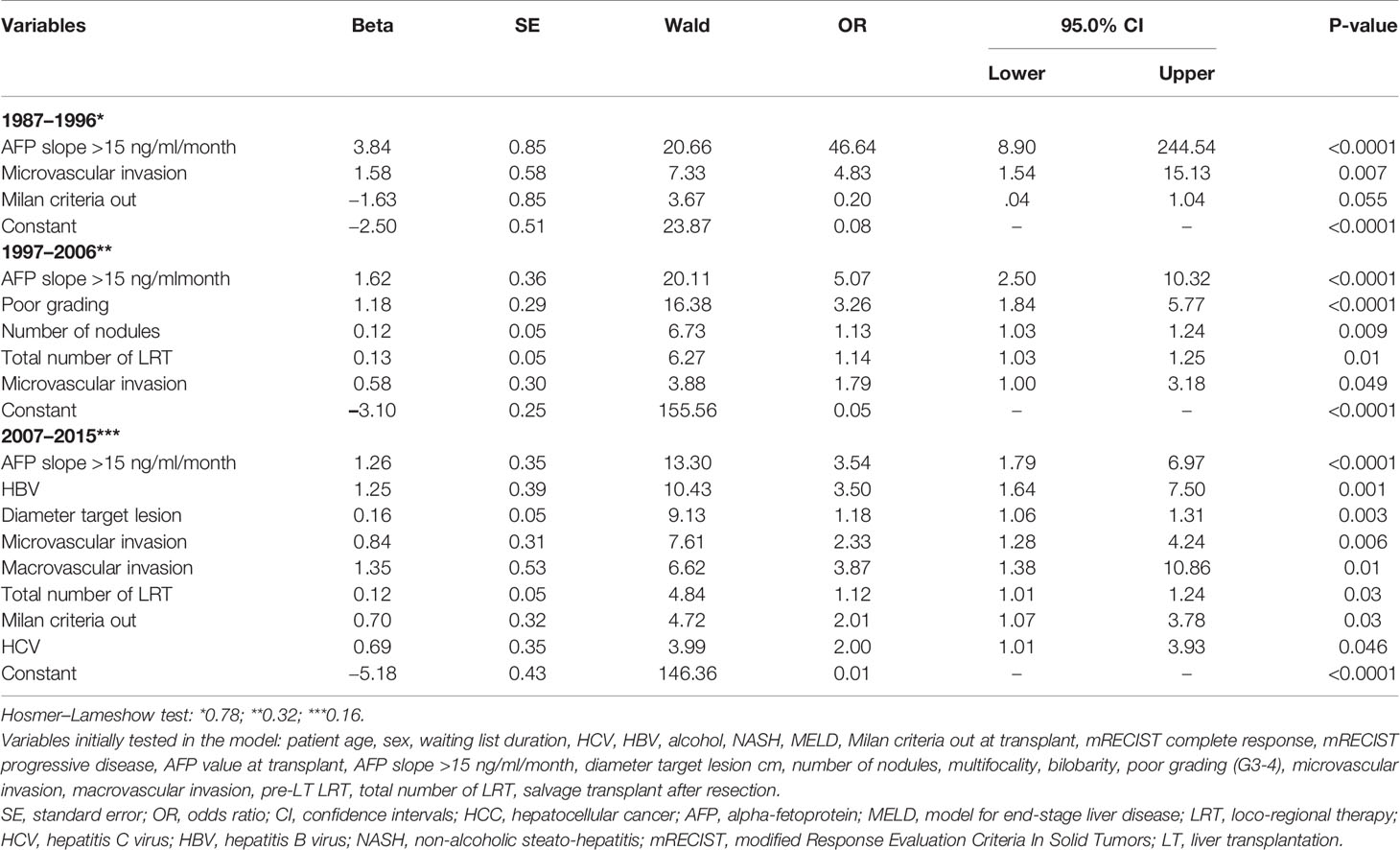
Table 6 Multivariable logistic regression analysis for the risk of 10-year HCC-related death after LT (backward Wald method) in the three different periods.
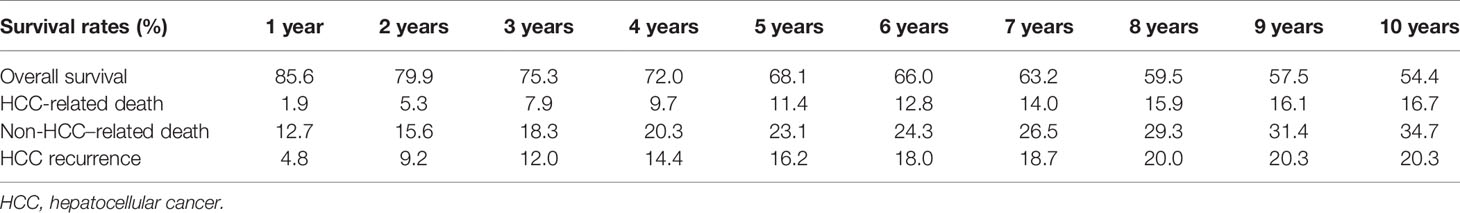
When analyzing survival rates in function of LT era, a progressive improvement of the results was observed (Figure 3). Patients transplanted during the 1987–1996 “liberal era”, characterized by the absence of any recognized inclusion criterion, had exceedingly high 5- and 10-year overall death rates of 59.4% and 68.0%. As expected, the results improved significantly during the 1997–2006 “Milan criteria era”, with 5- and 10-year death rates declining to of 33.8% and 47.7%. Log-rank test showed a statistically relevant difference between these two eras (P < 0.0001). Last, the results further improved during the 2007–2015 “safe criteria enlargement era”, with 5- and 10-year death rates of 26.8% and 38.9%. During this latter period, Milan criteria were progressively expanded by introducing San Francisco and Up-to-seven criteria. Interestingly, log-rank analysis survival rates were significantly improved when compared to those obtained during the first (P < 0.0001) and second era (P = 0.005).
Five- and 10-year HCC-related death rates were 35.6% and 41.7%, 12.6% and 18.0%, and 8.0% and 11.3% during the periods 1987–1996, 1997–2006, and 2007–2015, respectively. It was interesting to note that the latter period showed better results despite a slight enlargement of the criteria was adopted during this period respect to the previous one (P = 0.005).
Discussion
In the present study, the 5- and 10-year survival rates of 68.1% and 54.4% observed in a large European cohort containing 1,854 patients with HCC compared favorably with the widely accepted lower limit for 5-year patient survival after LT of 50% (11).. These results are in line with findings reported in large international databases such as the European Liver Transplant Registry (ELTR), which reported, in a cohort of 18,349 HCC liver patients, 5- and 10-year survival rates of 66% and 51%, respectively (12).
Compared to all other therapeutic modalities, the long-term superiority of LT does not disserve sufficient attention within the medical community, although well known since long time (13).
A recent Chinese study including 1,255 patients with HCC compared the 10-year survival outcomes from three different first-line treatments, namely, radiofrequency ablation, liver resection, and transplantation. LT was clearly superior in terms of 10-year survival, even after adjustment for confounders and balancing of the compared cohorts using inverse probability weighting (8). A meta-analysis comparing LT and resection as the treatment options in small HCC meeting the Milan criteria reported that the 5-year OS rates were similar, whereas the 10-year rates were significantly higher in patients who underwent LT than resection (50.0 vs. 29.8%; P < 0.001) (14).
These findings were also confirmed when the concept of “transplant benefit” was investigated. Exploring the data of 1,028 HCC cirrhotic patients coming from one Eastern and two Western surgical units, the 10-year scenario increased drastically the transplant benefit in all subgroups of resectable patients, and LT became an effective therapy for all patients without microvascular invasion independent of tumor extension and for oligo-nodular HCC with microvascular invasion meeting the conventional Milan and San Francisco criteria (15).
The present study confirms that a combination of morphological and biological tumor variables is linked to risk of death and HCC recurrence. A large US experience including 3,276 patients validated the Risk Estimation of Tumor Recurrence After Transplant (RETREAT) score, consisting of AFP value at LT, microvascular invasion, and the sum of the largest viable tumor and number of tumors in the total hepatectomy specimen (16). Interestingly, all these variables were statistically relevant risk factors for 10-year HCC-related death in our series.
Moreover, we explored the AFP dynamics during the waiting time instead of looking at the last available value before LT. Several studies assigned a relevant role to the AFP slope as a predictor for recurrence and death (17–19).
Macrovascular invasion is another relevant variable that has been recently explored in large international series. A retrospective study analyzing 45 patients with macrovascular patients before LT reported a very high risk of recurrence especially if the AFP value at LT was >10 ng/ml (5-year disease-free survival rates 27.8 vs. 71.8%; P = 0.008) (20). A ELTR study (n = 9,324) reported that vascular invasion overruled as prognostic indicator all criteria based on size and number of nodules; 5-year OS rates reached 39.6%, 58.8%, and 73.2% in patients with macrovascular invasion, microvascular invasion, or absent invasion (21). All these experiences are in line with our findings. Both micro- and macrovascular invasion at pathological examination of the hepatectomy specimen correlated with poor tumor-related survival. The growing role of advanced locoregional therapies like the radio-embolization is showing promising results in terms of efficacious downstaging of macrovascular invasion using “superdownstaging” protocols (22).
In relation to the total number of neo-adjuvant treatments, several studies explored the negative effect of repeated therapies as a surrogate of a more aggressive tumor behavior. A large US experience including 789 Milan criteria-out HCC patients reported a detrimental effect of LRT in patients failing to be successfully downstaged when compared to directly transplanted patients (HCC recurrence: 34.1% vs. 26.1%; p < 0.001) (23). A European experience based on the analysis of 1,083 Milan criteria-in patients reported that up to three LRTs are beneficial for success in intention-to-treat LT patients, but, if patients need more LRT, this benefit is lost (24). Our series confirmed that the risk for long-term tumor-related death was increased in patients requiring more LRT, supporting the hypothesis that the need for more LRT is equivalent to higher tumor aggressiveness.
Our study explored the impact of risk factors available at the time of LT only, whereas other relevant aspects such as the role of immunosuppressive treatment were not. Despite there is some recent evidence that immunosuppression either as maintenance or anti-rejection treatment may play a role as another risk factor for HCC recurrence after transplantation, it was not explored because intending to investigate only variables that are available at the time of LT (25, 26). The investigation of variables obtainable after LT in fact introduces an “immortal bias” into the analysis. This potential risk was avoided excluding all the post-LT variables.
Our analysis confirmed the negative role of HCV infection on the long-term survival. During the studied time period, early viral allograft reinfection was universal. Nowadays, direct-acting antiviral agents have almost eliminated this risk for death. Therefore, it has to be foreseen that HCV infection will lose its role as a relevant risk factor for long-term death (27).
In the future, the prevalence of NASH will become the main reason to LT in patients with HCC, and this underlying disease will replace very soon HCV as a risk factor for delayed death after LT (28). In our series, the impact of NASH appears to be relatively limited, but its raising role is clearly reported observing the growing number of cases observed in the different LT periods.
Interestingly, in our series many patients (71 of 242; 29.3%) recurred very late (>5 years). Unfortunately, it was impossible to analyze more in detail if these recurrences were “real” ones or de novo HCCs in the transplanted graft (29, 30). The very late detection of HCC in this series suggests that one should be very cautious when declaring a patient cured from HCC if no recurrence has been diagnosed within 5 years after LT and also underlines the importance of a long-life oncologic follow-up (31).
Another interesting aspect to highlight is the fact that a high number of patients with HCC with recurrence were still alive at the time of last follow-up. This finding further underlines the role of screening protocols, which represent the only way to early diagnose and, whenever possible, aggressively treat the recurrence (32). In this setting, the beneficial role of the new systemic therapies is unexplored. However, the potential ability of these drugs to prevent or to manage mid- and long-term recurrence requires further attention (33, 34).
The study presents some limitations. First, this is a retrospective analysis, but the great majority of studies focusing on transplant oncology derive from retrospective cohorts. Second, this study is based on a large European experience with an extended enrolment period (1987–2015). Such a long-time span leads to several potential biases linked to a modified and improved tumor and patient management. The enrolment of patients transplanted during the earlier periods was necessary to document long-term oncologic results and patient survivals post-LT. To mitigate potential biases, the variable “era of LT” was introduced in the mathematical models, and several sub-analyses focused on the different periods were performed. The multicenter nature of the study is likely to add another bias due to some differences in relation to HCC policies in the different centers. The enrollment of large patient numbers should mitigate a “center-related” effect, minimizing the potential impairment caused by different waiting times, neo-adjuvant strategies, and center volumes. Moreover, the composition of this European collaborative group was based on a similar interest and approach toward patients with HCC selected for a potential liver transplantation (LT). Last, the inclusion criteria of patients with HCC for LT changed during the study period, moving from a “liberal” approach via the exclusive use of the Milan criteria to the more recent use of the expanded criteria. Therefore, the variable “LT era” was introduced in the mathematical models and a LT period–oriented analysis was also performed to look at the effect of changes in the treatment of HCC in potential liver patients.
In conclusion, LT generates long-term overall and disease-free survival rates which are superior to all other oncologic treatments of HCC. The role of LT in the modern treatment of HCC becomes even more valued when the follow-up period reaches at least 10 years. The results of LT continue to improve even when prudently widening the inclusion criteria for transplantation. Despite the fact that the incidence of HCC recurrence is highest during the first 5 years post-transplant, one-third of them occur later on, indicating the importance of a life-long follow-up of these patients.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by AOU Policlinico Umberto I Rome. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
QL and JL contributed to conception and design of the study. QL, AnV, SI, AlV, GM, SO, MH-L, MC, TM, FM, and GS contributed to acquisition of data. QL analyzed and interpreted the data. QL and JL drafted the article. SA, MV, GT, GM, JM, ET, MR, UC, BS, and JL critically revised the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.877107/full#supplementary-material
Abbreviations
AFP, alpha-fetoprotein; CI, confidence interval; ELTR, European Liver Transplant Registry; HBV, hepatitis B virus; HCC, hepatocellular cancer; HCV, hepatitis C virus; IQR, interquartile range; LRT, loco-regional therapy; LT, liver transplantation; MELD, model for end-stage liver disease; mRECIST, modified Response Evaluation Criteria in Solid Tumors; NASH, non-alcoholic steato-hepatitis; OR, odds ratio; OS, overall survival; RETREAT, Risk Estimation of Tumor Recurrence After Transplant; STROBE, Strengthening the Reporting of Observational Studies in Epidemiology; TACE, trans-arterial chemo-embolization; WT, waiting time.
References
1. Mehta N, Bhangui P, Yao FY, Mazzaferro V, Toso C, Akamatsu N, et al. Liver Transplantation for Hepatocellular Carcinoma. Working Group Report From the ILTS Transplant Oncology Consensus Conference. Transplantation (2020) 104:1136–42. doi: 10.1097/TP.0000000000003174
2. Yokoyama I, Todo S, Iwatsuki S, Starzl TE. Liver Transplantation in the Treatment of Primary Liver Cancer. Hepatogastroenterology (1990) 37:188–93.
3. Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver Transplantation for the Treatment of Small Hepatocellular Carcinomas in Patients With Cirrhosis. N Engl J Med (1996) 334:693–9. doi: 10.1056/NEJM199603143341104
4. Mazzaferro V, Bhoori S, Sposito C, Bongini M, Langer M, Miceli R, et al. Milan Criteria in Liver Transplantation for Hepatocellular Carcinoma: An Evidence-Based Analysis of 15 Years of Experience. Liver Transpl (2011) 17:S44–57. doi: 10.1002/lt.22365
5. Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, et al. Liver Transplantation for Hepatocellular Carcinoma: Expansion of the Tumor Size Limits Does Not Adversely Impact Survival. Hepatology (2001) 33:1394–403. doi: 10.1053/jhep.2001.24563
6. Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, et al. Predicting Survival After Liver Transplantation in Patients With Hepatocellular Carcinoma Beyond the Milan Criteria: A Retrospective, Exploratory Analysis. Lancet Oncol (2009) 10:35–43. doi: 10.1016/S1470-2045(08)70284-5
7. Lerut J, Foguenne M, Lai Q. Hepatocellular Cancer Selection Systems and Liver Transplantation: From the Tower of Babel to an Ideal Comprehensive Score. Updates Surg (2021) 73:1599–614. doi: 10.1007/s13304-021-01078-4
8. Meng F, Zhang H, Peng H, Lu S. Comparison of 10-Year Survival Outcomes for Early Single Hepatocellular Carcinoma Following Different Treatments. BioMed Res Int (2021) 2021:6638117. doi: 10.1155/2021/6638117
9. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Lancet (2007) 370:1453–7. doi: 10.1016/S0140-6736(07)61602-X
10. Kang H. The Prevention and Handling of the Missing Data. Korean J Anesthesiol (2013) 64(5):402–6. doi: 10.4097/kjae.2013.64.5.402
11. Jain A, Reyes J, Kashyap R, Dodson SF, Demetris AJ, Ruppert K, et al. Long-Term Survival After Liver Transplantation in 4,000 Consecutive Patients at a Single Center. Ann Surg (2000) 232:490–500. doi: 10.1097/00000658-200010000-00004
12. Adam R, Karam V, Cailliez V, O Grady JG, Mirza D, Cherqui D, et al. All the Other 126 Contributing Centers (Www.Eltr.Org) and the European Liver and Intestine Transplant Association (ELITA). Annual Report of the European Liver Transplant Registry (ELTR) - 50-Year Evolution of Liver Transplantation. Transpl Int (2018) 31:1293–317. doi: 10.1111/tri.13358
13. Iwatsuki S, Starzl TE, Sheahan DG, Yokoyama I, Demetris AJ, Todo S, et al. Hepatic Resection Versus Transplantation for Hepatocellular Carcinoma. Ann Surg (1991) 214:221–8. doi: 10.1097/00000658-199109000-00005
14. Menahem B, Lubrano J, Duvoux C, Mulliri A, Alves A, Costentin C, et al. Liver Transplantation Versus Liver Resection for Hepatocellular Carcinoma in Intention to Treat: An Attempt to Perform an Ideal Meta-Analysis. Liver Transpl (2017) 23:836–44. doi: 10.1002/lt.24758
15. Vitale A, Cucchetti A, Qiao GL, Cescon M, Li J, Ramirez Morales R, et al. Is Resectable Hepatocellular Carcinoma a Contraindication to Liver Transplantation? A Novel Decision Model Based on Number of Patients Needed to Transplant as Measure of Transplant Benefit. J Hepatol (2014) 60:1165–71. doi: 10.1016/j.jhep.2014.01.022
16. Mehta N, Dodge JL, Roberts JP, Yao FY. Validation of the Prognostic Power of the RETREAT Score for Hepatocellular Carcinoma Recurrence Using the UNOS Database. Am J Transplant (2018) 18:1206–13. doi: 10.1111/ajt.14549
17. Giard JM, Mehta N, Dodge JL, Roberts JP, Yao FY. Alpha-Fetoprotein Slope >7.5 Ng/mL Per Month Predicts Microvascular Invasion and Tumor Recurrence After Liver Transplantation for Hepatocellular Carcinoma. Transplantation (2018) 102:816–22. doi: 10.1097/TP.0000000000002094
18. Vibert E, Azoulay D, Hoti E, Iacopinelli S, Samuel D, Salloum C, et al. Progression of Alphafetoprotein Before Liver Transplantation for Hepatocellular Carcinoma in Cirrhotic Patients: A Critical Factor. Am J Transplant (2010) 10:129–37. doi: 10.1111/j.1600-6143.2009.02750.x
19. Lai Q, Nicolini D, Inostroza Nunez M, Iesari S, Goffette P, Agostini A, et al. A Novel Prognostic Index in Patients With Hepatocellular Cancer Waiting for Liver Transplantation: Time-Radiological-Response-Alpha-Fetoprotein-INflammation (TRAIN) Score. Ann Surg (2016) 264:787–96. doi: 10.1097/SLA.0000000000001881
20. Assalino M, Terraz S, Grat M, Lai Q, Vachharajani N, Gringeri E, et al. Liver Transplantation for Hepatocellular Carcinoma After Successful Treatment of Macrovascular Invasion - A Multi-Center Retrospective Cohort Study. Transpl Int (2020) 33:567–75. doi: 10.1111/tri.13586
21. Pommergaard HC, Rostved AA, Adam R, Thygesen LC, Salizzoni M, Gómez Bravo MA, et al. Vascular Invasion and Survival After Liver Transplantation for Hepatocellular Carcinoma: A Study From the European Liver Transplant Registry. HPB (Oxford) (2018) 20:768–75. doi: 10.1111/tri.13123
22. Serenari M, Cappelli A, Cucchetti A, Mosconi C, Strigari L, Monari F, et al. Deceased Donor Liver Transplantation After Radioembolization for Hepatocellular Carcinoma and Portal Vein Tumoral Thrombosis: A Pilot Study. Liver Transpl (2021) 27:1758–66. doi: 10.1002/lt.26257
23. Kardashian A, Florman SS, Haydel B, Ruiz RM, Klintmalm GB, Lee DD, et al. Liver Transplantation Outcomes in a U.S. Multicenter Cohort of 789 Patients With Hepatocellular Carcinoma Presenting Beyond Milan Criteria. Hepatology (2020) 72:2014–28. doi: 10.1002/hep.31210
24. Lai Q, Vitale A, Iesari S, Finkenstedt A, Mennini G, Onali S, et al. The Intention-To-Treat Effect of Bridging Treatments in the Setting of Milan Criteria-In Patients Waiting for Liver Transplantation. Liver Transpl (2019) 25:1023–33. doi: 10.1002/lt.25492
25. Lerut J, Iesari S, Foguenne M, Lai Q. Hepatocellular Cancer and Recurrence After Liver Transplantation: What About the Impact of Immunosuppression? Transl Gastroenterol Hepatol (2017) 2:80. doi: 10.21037/tgh.2017.09.06
26. Lai Q, Iesari S, Finkenstedt A, Hoppe-Lotichius M, Foguenne M, Lehner K, et al. Hepatocellular Carcinoma Recurrence After Acute Liver Allograft Rejection Treatment: A Multicenter European Experience. Hepatobil Pancreat Dis Int (2019) 18:517–24. doi: 10.1016/j.hbpd.2019.05.006
27. Guarino M, Viganò L, Ponziani FR, Giannini EG, Lai Q, Morisco F. Special Interest Group on Hepatocellular Carcinoma and New Anti-HCV Therapies” of the Italian Association for the Study of the Liver. Recurrence of Hepatocellular Carcinoma After Direct Acting Antiviral Treatment for Hepatitis C Virus Infection: Literature Review and Risk Analysis. Dig Liver Dis (2018) 50:1105–14. doi: 10.1016/j.dld.2018.08.001
28. Vitale A, Svegliati-Baroni G, Ortolani A, Cucco M, Dalla Riva GV, Giannini EG, et al. Epidemiological Trends and Trajectories of MAFLD-Associated Hepatocellular Carcinoma 2002-2033: The ITA.LI.CA Database. Gut (2021), gutjnl–2021-324915. doi: 10.1136/gutjnl-2021-324915
29. Flemming P, Tillmann HL, Barg-Hock H, Kleeberger W, Manns MP, Klempnauer J, et al. Donor Origin of De Novo Hepatocellular Carcinoma in Hepatic Allografts. Transplantation (2003) 76:871–3. doi: 10.1097/01.TP.0000086341.57778.D9
30. Trevisani F, Garuti F, Cucchetti A, Lenzi B, Bernardi M. De Novo Hepatocellular Carcinoma of Liver Allograft: A Neglected Issue. Cancer Lett (2015) 357:47–54. doi: 10.1016/j.canlet.2014.11.032
31. Park MS, Lee KW, Yi NJ, Choi YR, Kim H, Hong G, et al. Optimal Tailored Screening Protocol After Living Donor Liver Transplantation for Hepatocellular Carcinoma. J Korean Med Sci (2014) 29:1360–6. doi: 10.3346/jkms.2014.29.10.1360
32. Lee DD, Sapisochin G, Mehta N, Gorgen A, Musto KR, Hajda H, et al. Surveillance for HCC After Liver Transplantation: Increased Monitoring May Yield Aggressive Treatment Options and Improved Postrecurrence Survival. Transplantation (2020) 104:2105–12. doi: 10.1097/TP.0000000000003117
33. Giannini EG, Aglitti A, Borzio M, Gambato M, Guarino M, Iavarone M, et al. Overview of Immune Checkpoint Inhibitors Therapy for Hepatocellular Carcinoma, and The ITA.LI.CA Cohort Derived Estimate of Amenability Rate to Immune Checkpoint Inhibitors in Clinical Practice. Cancers (Basel) (2019) 11:1689. doi: 10.3390/cancers11111689
Keywords: recurrence, alpha-fetoprotein, radiological response, Milan criteria, expanded criteria
Citation: Lai Q, Viveiros A, Iesari S, Vitale A, Mennini G, Onali S, Hoppe-Lotichius M, Colasanti M, Manzia TM, Mocchegiani F, Spoletini G, Agnes S, Vivarelli M, Tisone G, Ettorre GM, Mittler J, Tsochatzis E, Rossi M, Cillo U, Schaefer B and Lerut JP (2022) Prognostic Factors for 10-Year Survival in Patients With Hepatocellular Cancer Receiving Liver Transplantation. Front. Oncol. 12:877107. doi: 10.3389/fonc.2022.877107
Received: 16 February 2022; Accepted: 29 March 2022;
Published: 27 April 2022.
Edited by:
Fabio Melandro, Pisana University Hospital, ItalyReviewed by:
Anna Mrzljak, University of Zagreb, CroatiaMatteo Ravaioli, University of Bologna, Italy
Copyright © 2022 Lai, Viveiros, Iesari, Vitale, Mennini, Onali, Hoppe-Lotichius, Colasanti, Manzia, Mocchegiani, Spoletini, Agnes, Vivarelli, Tisone, Ettorre, Mittler, Tsochatzis, Rossi, Cillo, Schaefer and Lerut. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Quirino Lai, quirino.lai@uniroma1.it
 Quirino Lai
Quirino Lai Andre Viveiros2
Andre Viveiros2 Samuele Iesari
Samuele Iesari Alessandro Vitale
Alessandro Vitale Gianluca Mennini
Gianluca Mennini Simona Onali
Simona Onali Maria Hoppe-Lotichius
Maria Hoppe-Lotichius Tommaso M. Manzia
Tommaso M. Manzia Federico Mocchegiani
Federico Mocchegiani Gabriele Spoletini
Gabriele Spoletini Giuseppe M. Ettorre
Giuseppe M. Ettorre Emmanuel Tsochatzis
Emmanuel Tsochatzis Umberto Cillo
Umberto Cillo