- 1Department of Neurosurgery, The University of Oklahoma Health Sciences Center, Oklahoma University, Oklahoma City, OK, United States
- 2Faculty of Medicine, Pham Ngoc Thach University of Medicine, Ho Chi Minh City, Vietnam
- 3Department of Pathology, University of Medicine and Pharmacy at Ho Chi Minh City, Ho Chi Minh City, Vietnam
- 4Department of Neurosurgery, Division of Pediatric Neurosurgery, Oklahoma Children’s Hospital, The University of Oklahoma Health Sciences Center, Oklahoma University, Oklahoma City, OK, United States
- 5Department of Pediatrics, The University of Oklahoma Health Sciences Center, Oklahoma University, Oklahoma City, OK, United States
Introduction: Pediatric and adult H3K27M-mutant midline gliomas have variable clinical presentations, prognoses, and molecular backgrounds. In this study, we integrated data from published studies to investigate the differences between these two groups.
Methods: PubMed and Web of Science were searched for potential data. Studies were included if they had available individual participant data on patients age of H3K27M-mutant midline gliomas. For time-to-event analyses, Kaplan-Meier analysis and Cox regression models were carried out; corresponding hazard ratios (HR) and 95% confidence intervals (CI) were computed to analyze the impact of age and clinical covariates on progression-free survival (PFS) and overall survival (OS).
Results: We included 43 studies comprising 272 adults and 657 pediatric midline gliomas with H3K27M mutation for analyses. In adults, there was a male predilection whereas females were slightly more common than males in the pediatric group. Spinal cord tumors were more frequent in adults. The prevalence of H3.1 K27M mutation was significantly higher in the pediatric cohort. Compared to adult patients, pediatric H3K27M-mutant midline gliomas exhibited more aggressive features including higher rates of pathologic features of high-grade tumors and Ki67 proliferation index, and had a shorter PFS and OS. Genetically, ACVR1 mutations were more common whereas MGMT methylation, FGFR1, and NF1 mutations were less prevalent in the pediatric cohort.
Conclusion: Pediatric H3K27M-mutant midline gliomas were demographically, clinically, and molecularly distinct from adult patients, highlighting an opportunity to refine the risk stratification for these neoplasms.
Introduction
H3K27M-mutant glial tumors arise predominantly in midline structures such as the thalamus, brainstem, and spinal cord (1, 2). The H3K27M-mutant diffuse midline glioma is a relatively newly described entity, debuting in the revised 2016 World Health Organization classification of tumors of the central nervous system. These tumors are more commonly seen in pediatric patients and are associated with a poor prognosis of typically less than one year because of their infiltrative nature and difficulty in achieving complete surgical resection (3–6). H3 mutations could occur either on the HIST1H3B/C (H3.1), HIST2H3C (H3.2), or H3F3A (H3.3) genes with a lysine to methionine amino acid substitution at codon 27 (K27M) and are mutually exclusive with isocitrate dehydrogenase 1/2 (IDH1/2) mutations (7).
Despite aggressive therapeutic approaches and advances in chemoradiotherapy and targeted therapy regimens, there has been no survival improvement for patients with H3K27M mutations over the recent years (3, 8). Published studies have shown several differences in clinicopathological parameters between pediatric and adult patients with H3K27M-mutant gliomas (9, 10), but no significant differences in overall survival (11, 12). In this study, individual patient data from published studies were integrated to investigate the clinical and prognostic differences between pediatric and adult H3K27M-mutant midline gliomas.
Methods
Search Term and Literature Search
We accessed PubMed and Web of Science to search for relevant articles from inception to July 2021 using the following search term: Glioma AND (H3K27M OR H3-K27M OR H3 K27M OR H3F3A OR HIST1H3B OR HIST1H3C). The study protocol was strictly adherent to the recommendations of the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) statement (13).
Selection Criteria and Abstract Screening
Results from the two electronic databases were imported into EndNote (Clarivate, PA, USA) and duplicates were subsequently removed. Next, two reviewers (HGV and TNMN) independently screened the title and abstract of the articles using the following inclusion criteria: (i) studies providing individual participant data (IPD) of H3K27M-mutant gliomas and (ii) studies with patient age available. We excluded studies if they were: (i) not relevant to inclusion criteria; (ii) case reports; (iii) reviews, theses, or books; (iv) conference or proceeding papers; or (v) studies with duplicated populations.
Full-Text Screening and Data Extraction
Two reviewers (HGV and TNMN) independently reviewed the full text of potential studies and extracted data into a standardized worksheet. We also carefully reviewed the reference list of the included studies to find additional papers. The following IPD were extracted from the articles: authors, institution, country, year of publication, study period, patient identification number, H3 genotypes, detection methods, demographic information, tumor size, tumor location, histology grades, Ki67 index, treatments, PFS time, PFS status, OS time, OS status, and genetic alterations of H3K27M-mutant gliomas. Subsequently, we removed cases with missing data of patient age/tumor location or cases from non-midline locations.
Statistical Analyses
Patients were divided into pediatric (patients of 18 or less than 18 years of age) and adult groups (patients of more than 18 years of age). To avoid duplicated data between studies from the same institutions, we selected the study with the largest population. Categorical and continuous variables of pediatric and adult cohorts were compared utilizing Chi-square, Fisher’s exact test, and Wilcoxon rank-sum test, if applicable. Kaplan-Meier analysis and Cox proportional hazards model were conducted to assess the impact of various clinical parameters on survival of H3K27M-mutant gliomas. The deviance residuals and the dfbeta values were used to examine influential observations. Hazard ratios (HR) are presented as mean and 95% confidence interval (CI). A two-sided p-value of < 0.05 was considered statistically significant. The statistical analyses were performed using the R software, version 3.6.1 (The R Foundation, Vienna, Austria).
Results
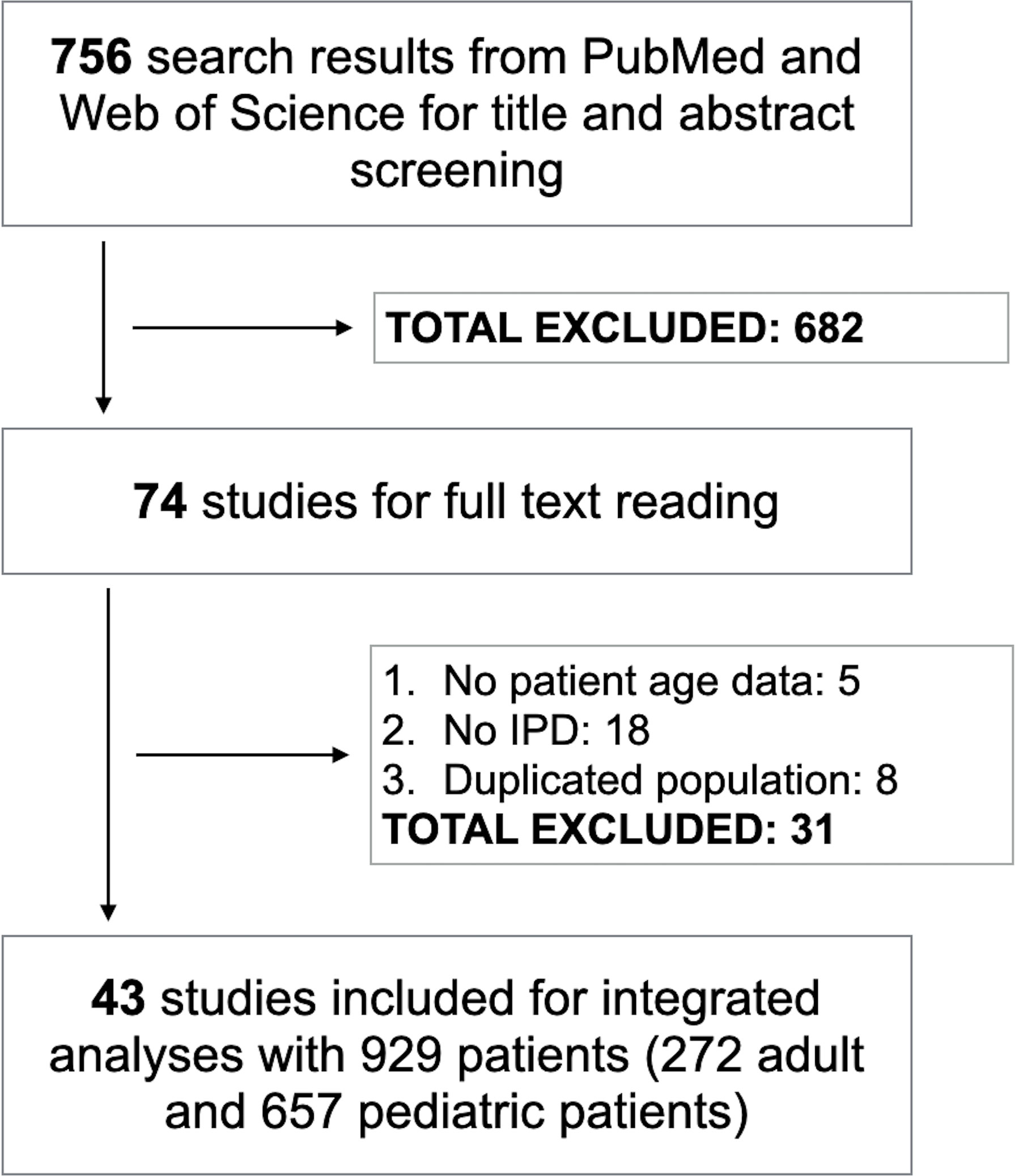
For the title and abstract screening, we identified 756 articles, and 74 of them were selected for full-text review. After reading full text, we included 43 studies comprised of 929 H3K27M-mutant midline gliomas for integrated analyses (Figure 1) (2, 4, 7, 8, 10–12, 14–44). The characteristics of all included studies are shown in Table S1. The median age of patients was 11.0 years (range, 1-82). The majority of tumors were found intracranially with brainstem or thalamus being the most frequent sites. The median OS was 11.3 months with 80% of patients expired at last follow-up. Stratified by age, 272 and 657 patients were separated into adult and pediatric groups, respectively.
The Differences Between Pediatric and Adult Midline Gliomas With H3K27M Mutations
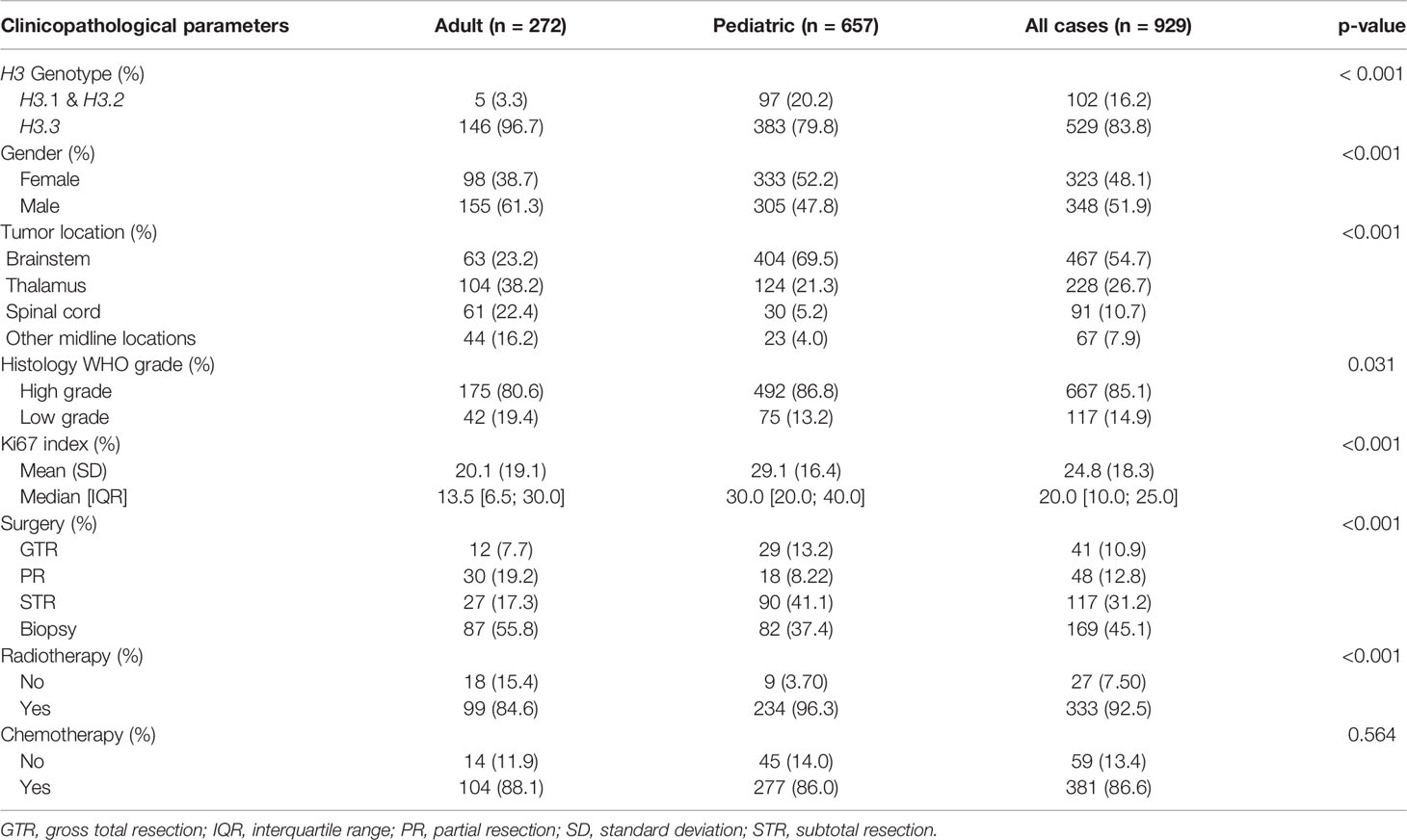
Table 1 presents the clinicopathological and therapeutic covariates of adult and pediatric H3K27M-mutant midline gliomas. In comparison to the adult counterpart, the K27M mutation in the H3.1 gene was more commonly identified in pediatric patients (p < 0.001). There was a male predilection in adult H3K27M-mutant gliomas whereas females were more common in the pediatric group (p < 0.001). In the pediatric population, H3K27M-mutant tumors were mostly seen in the brainstem whereas a higher proportion of thalamic and spinal lesions were identified in adult patients (p< 0.001). Adult tumors had a lower Ki67 index compared to the pediatric group (p < 0.001).
Regarding treatment modalities, pediatric H3K27M-mutant gliomas were associated with higher rates of gross total/subtotal removal and radiotherapy administration (p < 0.001). In comparison to the adult group, pediatric patients had a worse OS (median OS of 13.0 vs 14.7 months; HR = 1.630; 95% CI = 1.347-1.973; p < 0.001) (Figure 2) and PFS (median PFS of 7.0 vs 9.0 months; HR = 2.015; 95% CI = 1.132-3.587; p = 0.017) (Figure 3). When pediatric patients were further stratified into different subgroups of infants (0 < age ≤ 3), young children (3 < age ≤ 10), and adolescents (10 < age ≤ 18), there was a trend to better OS of infants (median OS of 14.5 months) as compared to young children (median OS of 11.3 months; p = 0.063) and adolescents (median OS of 10.8 months; p = 0.113) (Figure S1). We did not see any differences in survival patterns between young adults (19 < age ≤ 45), older adults (45 < age ≤ 60), and elderly (age > 60) (p = 0.67) (Figure S2).
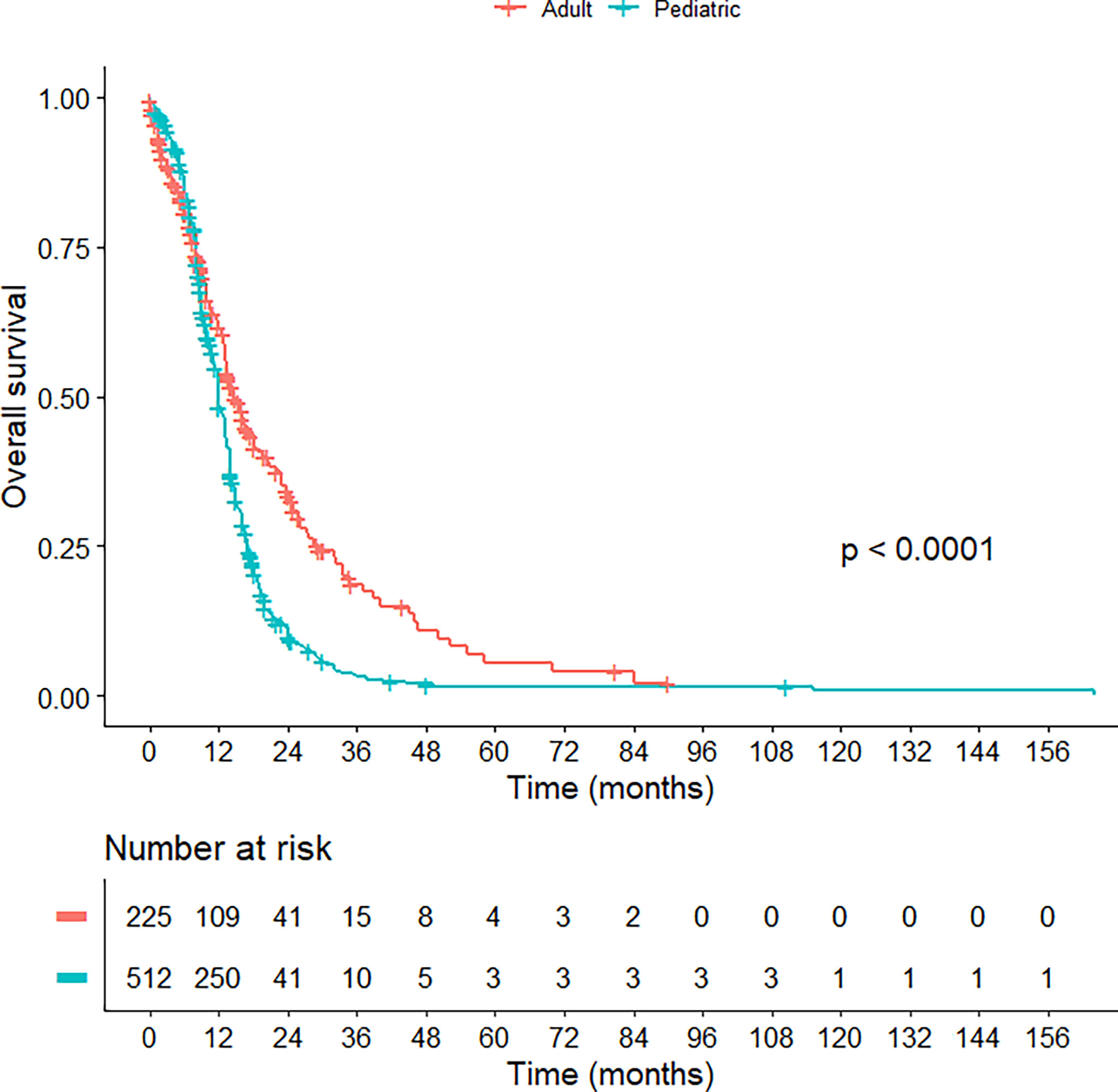
Figure 2 Kaplan-Meier curve illustrating the overall survival of pediatric versus adult H3K27M-mutant midline gliomas.
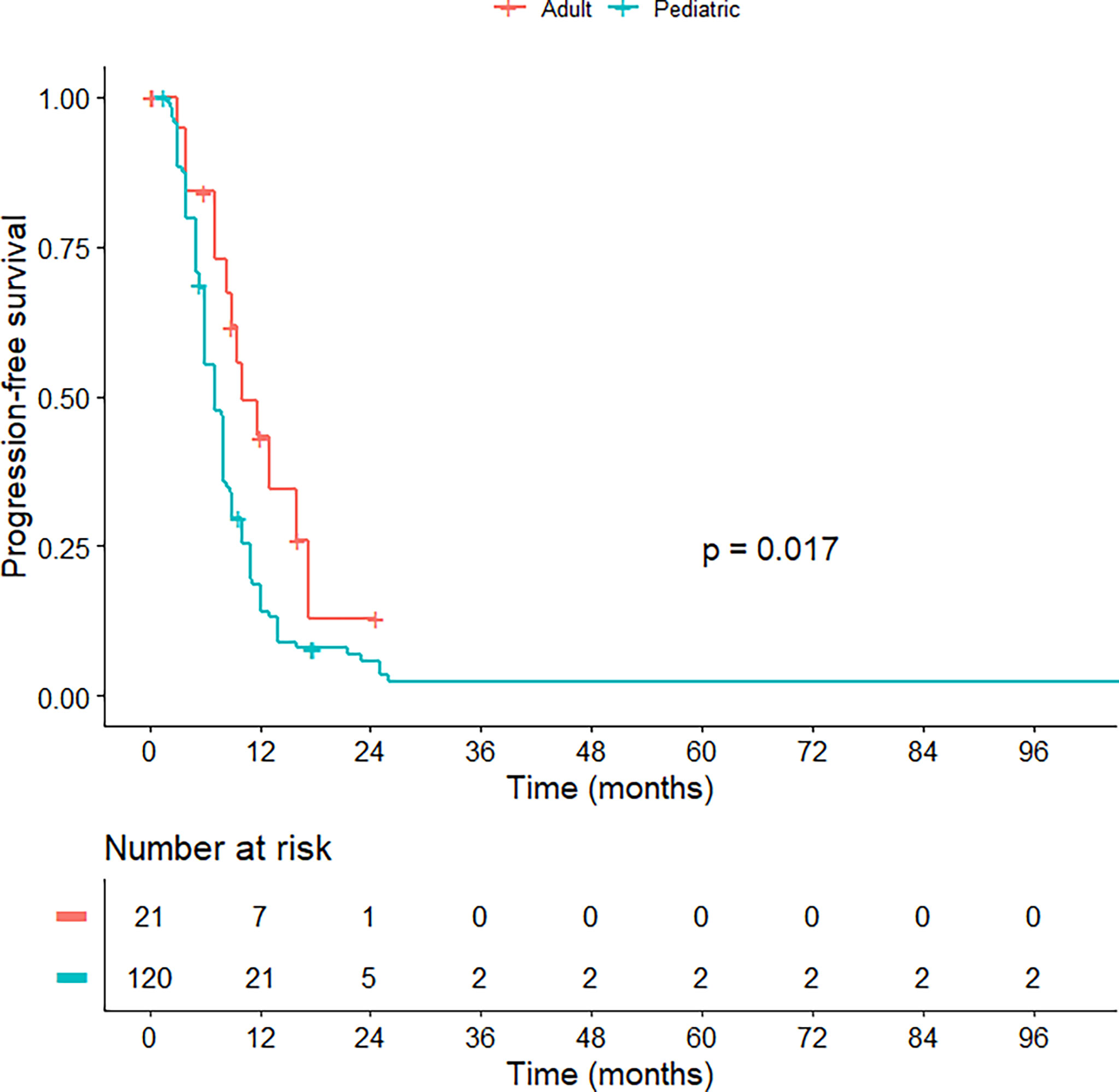
Figure 3 Kaplan-Meier curve illustrating the progression-free survival of pediatric versus adult H3K27M-mutant midline gliomas.
Among intracranial tumors, OS of pediatric H3K27M-mutant gliomas remained significantly shorter (HR = 1.430; 95% CI = 1.162-1.759; p < 0.001) (Figure S3). We also observed a similar OS trend for spinal H3K27M-mutant midline gliomas, but the difference did not reach statistical significance (HR = 2.035; 95% CI = 0.914-4.534; p = 0.082) (Figure S4). Diffuse intrinsic pontine gliomas (DIPG) were seen in 33.6% and 5.5% of pediatric and adult H3K27M-mutant midline gliomas. We still observed a significant difference in overall survival between pediatric and adult patients after excluding them from the analysis (Figure S5). Because of missing data, we could not stratify PFS analysis into subgroups.
Prognostic Factors for OS of H3K27M-Mutant Midline Gliomas
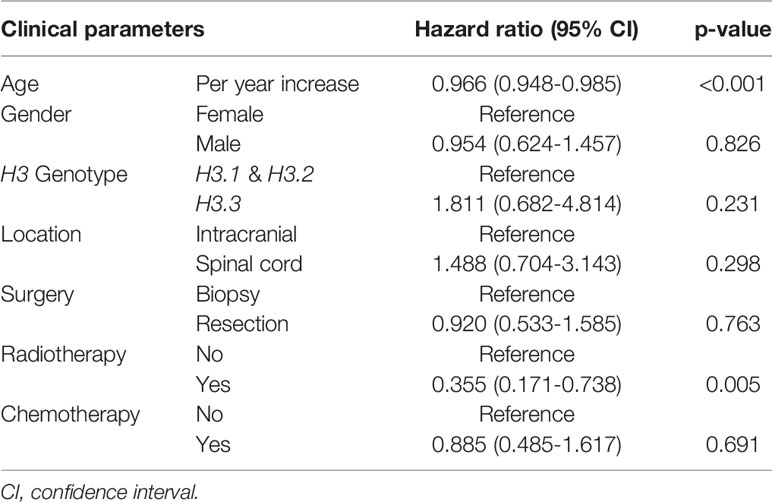
In a multivariate Cox regression model for OS of H3K27M-mutant midline gliomas adjusted for age, gender, H3 genotype, tumor location, the extent of surgical resection, radiation, and chemotherapy; advanced age and radiotherapy administration were positive prognostic factors for overall survival. Other parameters were not associated with patient survival (Table 2).
Genetic Alterations of Pediatric Versus Adult H3K27M-Mutant Midline Gliomas
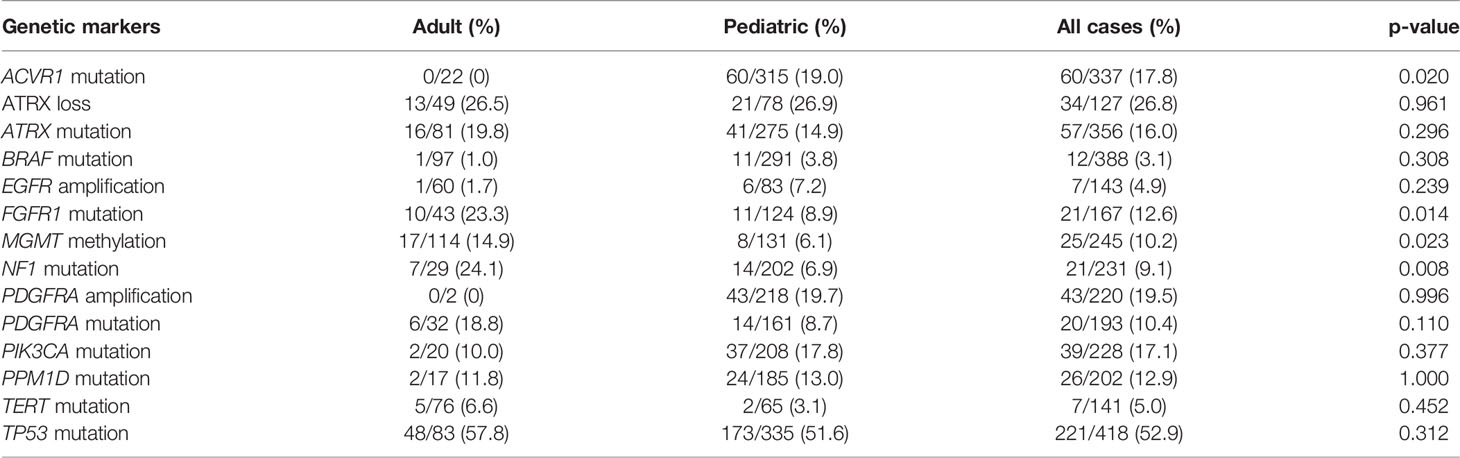
We observed a significantly higher prevalence of MGMT methylation, NF1, and FGFR1 mutations in adults in comparison to pediatric patients whereas ACVR1 mutations were solely detected in children. The frequencies of EGFR and PDGFRA amplifications, ATRX, BRAF, PDGFRA, PIK3CA, PPM1D, TERT promoter, and TP53 mutations were not statistically different between these two groups (Table 3).
Discussion
Diffuse midline gliomas are mostly high-grade tumors and have a lethal outcome. These neoplasms can occur at any age from infants to elderly patients but are most commonly found in children (35, 45, 46). About 80-90% of these tumors harbor mutations in H3 genes, most of which are H3K27M genotypes (45). H3K27M-mutant midline gliomas are associated with a worse outcome compared to H3-wild-type tumors (4). Because midline gliomas with H3K27M mutations are clinically different from H3K27M-mutated cortical high-grade gliomas (47), we only focused on midline tumors to avoid the risk of bias. Given its rarity in adults, it is challenging to investigate the differences in clinical manifestations and prognosis between pediatric and adult groups (11). In this study, we conducted an integrated analysis of more than 900 H3K27M-mutated midline gliomas to examine the clinicopathological parameters and prognosis between adults versus pediatrics. Our results showed that pediatric tumors were not only clinically and prognostically different but also had distinct molecular profiles as compared to the adult group.
In this study of H3K27M-mutant midline tumors, young age portended an adverse prognosis with shorter PFS and OS as compared with adult patients. While earlier reports suggested that OS of pediatric and adult H3K27M-mutant gliomas are not different (8.9 vs 9.3 months, respectively), only a small number of patients were compared (11). Schulte et al. (48) reported a higher survival rate of adult H3K27M midline gliomas compared to children in published case series and hypothesized that adults might have a better survival. Notably, our results revealed that pediatric patients had a higher rate of tumor resection and radiation administration than adult patients, substantiating that pediatric H3K27M-mutant midline gliomas carry worse outcome despite more aggressive treatment attempts than in adult patients. Our findings showed that tumors in pediatric patients had more aggressive pathologic features including a significantly advanced histological grade and Ki67 proliferation index, which may explain the poor survival of the pediatric cohort. Hoffmann et al. reported that pediatric DIPG with age of less than 3 and more than 10 years were associated with longer-term survival compared to those aged 3-10 (49). In this study, although the p-value of the log-rank test demonstrated significant differences between these three age subgroups, pairwise comparison showed that the differences between infants vs young children and infants vs adolescents did not reach statistical significance.
While earlier studies reported a high frequency of H3 K27M mutation in both adult and pediatric high-grade spinal gliomas (50), we found a significantly higher frequency of adult H3 K27M spinal cord tumors as compared to the pediatric population. These results were consistent with published population-level data on high-grade gliomas and glioblastomas of the spinal cord that these neoplasms are most commonly seen in adults (51, 52), as opposed to DIPGs or brainstem gliomas which are more likely to occur in the pediatric population (3, 8). Several reports indicated the aggressiveness of H3K27M-mutant diffuse midline gliomas is independent of their anatomical locations (1, 53). However, other published data reported that patient survival may be influenced by different anatomical locations (42, 54). When we stratified cases into subgroups of intracranial and spinal tumors, we still observed the same result for pediatric intracranial tumors and a similar trend for pediatric spinal gliomas, possibly due to the relatively small sample size of spinal cord gliomas. These results confirmed the uniformly short survival of H3K27M midline gliomas in the pediatric population, regardless of anatomical location.
Compared to pediatric tumors, adult H3K27M-mutant midline gliomas were also genetically different. We found that ACVR1 mutations were exclusively seen in pediatric patients, particularly in children less than six years of age. On the other hand, the incidence of FGFR1, NF1 mutations, and MGMT methylation were more significantly prevalent in adult patients. The lower frequency of MGMT hypermethylation in pediatric patients may contribute to the poor response to temozolomide and a worse PFS/OS as compared to adult cohort. FGFR1 mutations are associated with prolonged survival in H3K27M-mutant gliomas (55), which may influence the more favorable outcome in adult patients. Other genetic alterations such as EGFR alterations, TERT promoter mutations, and mutations in the tumor suppressor PTEN are less likely to exist in high-grade gliomas in children (56, 57). However, we observed an insignificant difference in the prevalence of these genetic markers between pediatric and adult H3K27M-mutated midline gliomas. Hopefully, these observations may help understand the molecular profile of midline gliomas as treatment.
This study is the first study to demonstrate the differences in demographics, clinical manifestations, prognoses, as well as molecular backgrounds of pediatric versus adult H3K27M-mutant midline gliomas. Because of the rarity of H3K27M-mutant midline gliomas in adult patients, it is challenging to observe the significant differences between pediatric and adult cohorts in institutional studies (11). Our results are of clinical interest as they may aid in refining natural history expectations and in tailoring therapeutic approaches. However, this study has certain limitations. First, we could not avoid selection bias originating from the included datasets because most of them were retrospective studies. In the multivariate Cox regression model adjusted for multiple clinical covariates, the prognostic implication of age remained significant, validating the independent role of this parameter in the risk stratification of H3K27M-mutant midline gliomas. Additionally, there are important clinical factors that we could not incorporate into the multivariate analysis due to missing data, such as the Karnofsky Performance Scale, tumor size, and disease stage. Pediatric patients may have limited self-care ability resulting in lower KPS and advanced disease stages compared with adults. Future prospective studies are needed to confirm the results of this study. Another bias could occur due to differences in treatment protocols across included institutions. Finally, although we strictly screened the included patients using patient identification code, demographic information, and clinical data to avoid duplicating patients, there are still possibilities of overlap among the dataset which may affect the analyses.
In conclusion, the present study demonstrated that pediatric H3K27M-mutant midline gliomas are demographically, clinically, prognostically distinct from adult tumors. H3K27- mutant midline gliomas in pediatric patients were associated with a significantly shorter PFS and OS. Additionally, the underlying molecular backgrounds of these two cohorts were also different. Our results may improve our biological understanding of H3K27M-mutant midline gliomas.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author Contributions
HV, conceptualization, data curation, formal analysis, investigation, methodology, project administration, software, validation, writing original, review, and editing. TN, HL, AJ, MH, JB, and RM-K, data curation, formal analysis, investigation, methodology, review, and editing. IFD, data curation, formal analysis, investigation, methodology, project administration, validation, review, editing, and supervision. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.858148/full#supplementary-material
Abbreviations
CI, confidence interval; H3.1, HIST1H3B/C; H3.2, HIST2H3C; H3.3, H3F3A; HR, hazard ratio; IDH, isocitrate dehydrogenase; IPD, individual participant data; OS, overall survival; PFS, progression-free survival.
References
1. Mosaab A, El-Ayadi M, Khorshed EN, Amer N, Refaat A, El-Beltagy M, et al. Histone H3K27M Mutation Overrides Histological Grading in Pediatric Gliomas. Sci Rep (2020) 10(1):8368. doi: 10.1038/s41598-020-65272-x
2. Korshunov A, Ryzhova M, Hovestadt V, Bender S, Sturm D, Capper D, et al. Integrated Analysis of Pediatric Glioblastoma Reveals a Subset of Biologically Favorable Tumors With Associated Molecular Prognostic Markers. Acta Neuropathol (2015) 129(5):669–78. doi: 10.1007/s00401-015-1405-4
3. Khuong-Quang DA, Buczkowicz P, Rakopoulos P, Liu XY, Fontebasso AM, Bouffet E, et al. K27M Mutation in Histone H3.3 Defines Clinically and Biologically Distinct Subgroups of Pediatric Diffuse Intrinsic Pontine Gliomas. Acta Neuropathol (2012) 124(3):439–47. doi: 10.1007/s00401-012-0998-0
4. Sturm D, Witt H, Hovestadt V, Khuong-Quang DA, Jones DT, Konermann C, et al. Hotspot Mutations in H3F3A and IDH1 Define Distinct Epigenetic and Biological Subgroups of Glioblastoma. Cancer Cell (2012) 22(4):425–37. doi: 10.1016/j.ccr.2012.08.024
5. Vuong HG, Tran TTK, Ngo HTT, Pham TQ, Nakazawa T, Fung KM, et al. Prognostic Significance of Genetic Biomarkers in Isocitrate Dehydrogenase-Wild-Type Lower-Grade Glioma: The Need to Further Stratify This Tumor Entity - A Meta-Analysis. Eur J Neurol (2019) 26(3):379–87. doi: 10.1111/ene.13826
6. Cole BL. Neuropathology of Pediatric Brain Tumors: A Concise Review. Neurosurgery (2022) 90(1):7–15. doi: 10.1093/neuros/nyab182
7. Castel D, Philippe C, Calmon R, Le Dret L, Truffaux N, Boddaert N, et al. Histone H3F3A and HIST1H3B K27M Mutations Define Two Subgroups of Diffuse Intrinsic Pontine Gliomas With Different Prognosis and Phenotypes. Acta Neuropathol (2015) 130(6):815–27. doi: 10.1007/s00401-015-1478-0
8. Schwartzentruber J, Korshunov A, Liu XY, Jones DT, Pfaff E, Jacob K, et al. Driver Mutations in Histone H3.3 and Chromatin Remodelling Genes in Paediatric Glioblastoma. Nature (2012) 482(7384):226–31. doi: 10.1038/nature10833
9. Roux A, Pallud J, Saffroy R, Edjlali-Goujon M, Debily M-A, Boddaert N, et al. High-Grade Gliomas in Adolescents and Young Adults Highlight Histomolecular Differences From Their Adult and Pediatric Counterparts. Neuro-Oncology (2020) 22(8):1190–202. doi: 10.1093/neuonc/noaa024
10. Meyronet D, Esteban-Mader M, Bonnet C, Joly MO, Uro-Coste E, Amiel-Benouaich A, et al. Characteristics of H3 K27M-Mutant Gliomas in Adults. Neuro-Oncology (2017) 19(8):1127–34. doi: 10.1093/neuonc/now274
11. Kleinschmidt-DeMasters BK, Mulcahy Levy JM. H3 K27M-Mutant Gliomas in Adults vs. Children Share Similar Histological Features and Adverse Prognosis. Clin Neuropathol (2018) 37(2):53–63. doi: 10.5414/NP301085
12. Wang Y, Feng LL, Ji PG, Liu JH, Guo SC, Zhai YL, et al. Clinical Features and Molecular Markers on Diffuse Midline Gliomas With H3K27M Mutations: A 43 Cases Retrospective Cohort Study. Front Oncol (2020) 10:602553. doi: 10.3389/fonc.2020.602553
13. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PloS Med (2009) 6(7):e1000097. doi: 10.1371/journal.pmed.1000097
14. Aihara K, Mukasa A, Gotoh K, Saito K, Nagae G, Tsuji S, et al. H3F3A K27M Mutations in Thalamic Gliomas From Young Adult Patients. Neuro-Oncol (2014) 16(1):140–6. doi: 10.1093/neuonc/not144
15. Alvi MA, Ida CM, Paolini MA, Kerezoudis P, Meyer J, Barr Fritcher EG, et al. Spinal Cord High-Grade Infiltrating Gliomas in Adults: Clinico-Pathological and Molecular Evaluation. Modern Pathol: An Off J United States Can Acad Pathol Inc (2019) 32(9):1236–43. doi: 10.1038/s41379-019-0271-3
16. Bruzek AK, Ravi K, Muruganand A, Wadden J, Babila CM, Cantor E, et al. Electronic DNA Analysis of CSF Cell-Free Tumor DNA to Quantify Multi-Gene Molecular Response in Pediatric High-Grade Glioma. Clin Cancer Res: An Off J Am Assoc Cancer Res (2020) 26(23):6266–76. doi: 10.1158/1078-0432.CCR-20-2066
17. Buczkowicz P, Hoeman C, Rakopoulos P, Pajovic S, Letourneau L, Dzamba M, et al. Genomic Analysis of Diffuse Intrinsic Pontine Gliomas Identifies Three Molecular Subgroups and Recurrent Activating ACVR1 Mutations. Nat Genet (2014) 46(5):451–6. doi: 10.1038/ng.2936
18. Chiba K, Aihara Y, Masui K, Abe K, Komori T, Kawamata T. Pulvinar Locus is Highly Relevant to Patients’ Outcomes in Surgically Resected Thalamic Gliomas in Children. World Neurosurg (2020) 134:e530–e9. doi: 10.1016/j.wneu.2019.10.116
19. Crotty EE, Leary SES, Geyer JR, Olson JM, Millard NE, Sato AA, et al. Children With DIPG and High-Grade Glioma Treated With Temozolomide, Irinotecan, and Bevacizumab: The Seattle Children’s Hospital Experience. J Neuro-Oncology (2020) 148(3):607–17. doi: 10.1007/s11060-020-03558-w
20. Daoud EV, Rajaram V, Cai C, Oberle RJ, Martin GR, Raisanen JM, et al. Adult Brainstem Gliomas With H3K27M Mutation: Radiology, Pathology, and Prognosis. J Neuropathol Exp Neurol (2018) 77(4):302–11. doi: 10.1093/jnen/nly006
21. Dono A, Takayasu T, Ballester LY, Esquenazi Y. Adult Diffuse Midline Gliomas: Clinical, Radiological, and Genetic Characteristics. J Clin Neurosci: Off J Neurosurg Soc Australasia (2020) 82(Pt A):1–8. doi: 10.1016/j.jocn.2020.10.005
22. Dorfer C, Czech T, Gojo J, Hosmann A, Peyrl A, Azizi AA, et al. Infiltrative Gliomas of the Thalamus in Children: The Role of Surgery in the Era of H3 K27M Mutant Midline Gliomas. Acta Neurochirurgica (2021) 163(7):2025–35. doi: 10.1007/s00701-020-04589-y
23. Ebrahimi A, Skardelly M, Schuhmann MU, Ebinger M, Reuss D, Neumann M, et al. High Frequency of H3 K27M Mutations in Adult Midline Gliomas. J Cancer Res Clin Oncol (2019) 145(4):839–50. doi: 10.1007/s00432-018-02836-5
24. Eschbacher KL, Ida CM, Johnson DR, Alvi MA, Jenkins SM, Ruff MW, et al. Diffuse Gliomas of the Brainstem and Cerebellum in Adults Show Molecular Heterogeneity. Am J Surg Pathol (2021) 45(8):1082–90. doi: 10.1097/PAS.0000000000001690
25. Fukami S, Nakajima N, Okada H, Akimoto J, Miki T, Fukuhara H, et al. Pathologic Findings and Clinical Course of Midline Paraventricular Gliomas Diagnosed Using a Neuroendoscope. World Neurosurg (2018) 114:e366–e77. doi: 10.1016/j.wneu.2018.02.185
26. Garibotto F, Madia F, Milanaccio C, Verrico A, Piccardo A, Tortora D, et al. Pediatric Diffuse Midline Gliomas H3 K27M-Mutant and non-Histone Mutant Midline High-Grade Gliomas in Neurofibromatosis Type 1 in Comparison With Non-Syndromic Children: A Single-Center Pilot Study. Front Oncol (2020) 10:795. doi: 10.3389/fonc.2020.00795
27. Giagnacovo M, Antonelli M, Biassoni V, Schiavello E, Warmuth-Metz M, Buttarelli FR, et al. Retrospective Analysis on the Consistency of MRI Features With Histological and Molecular Markers in Diffuse Intrinsic Pontine Glioma (DIPG). Childs Nervous Syst (2020) 36(4):697–704. doi: 10.1007/s00381-019-04463-y
28. Gojo J, Pavelka Z, Zapletalova D, Schmook MT, Mayr L, Madlener S, et al. Personalized Treatment of H3K27M-Mutant Pediatric Diffuse Gliomas Provides Improved Therapeutic Opportunities. Front Oncol (2020) 9. doi: 10.3389/fonc.2019.01436
29. Hoffman LM, DeWire M, Ryall S, Buczkowicz P, Leach J, Miles L, et al. Spatial Genomic Heterogeneity in Diffuse Intrinsic Pontine and Midline High-Grade Glioma: Implications for Diagnostic Biopsy and Targeted Therapeutics. Acta Neuropathol Commun (2016) 4. doi: 10.1186/s40478-015-0269-0
30. Karlowee V, Amatya VJ, Takayasu T, Takano M, Yonezawa U, Takeshima Y, et al. Immunostaining of Increased Expression of Enhancer of Zeste Homolog 2 (EZH2) in Diffuse Midline Glioma H3K27M-Mutant Patients With Poor Survival. Pathobiol: J Immunopathol Mol Cell Biol (2019) 86(2-3):152–61. doi: 10.1159/000496691
31. Liu Y, Zhang Y, Hua W, Li Z, Wu B, Liu W. Clinical and Molecular Characteristics of Thalamic Gliomas: Retrospective Report of 26 Cases. World Neurosurg (2019) 126:e1169–e82. doi: 10.1016/j.wneu.2019.03.061
32. Mackay A, Burford A, Molinari V, Jones DTW, Izquierdo E, Brouwer-Visser J, et al. Molecular, Pathological, Radiological, and Immune Profiling of non-Brainstem Pediatric High-Grade Glioma From the HERBY Phase II Randomized Trial. Cancer Cell (2018) 33(5):829–42.e5. doi: 10.1016/j.ccell.2018.04.004
33. Mueller S, Jain P, Liang WS, Kilburn L, Kline C, Gupta N, et al. A Pilot Precision Medicine Trial for Children With Diffuse Intrinsic Pontine Glioma-PNOC003: A Report From the Pacific Pediatric Neuro-Oncology Consortium. Int J Cancer (2019) 145(7):1889–901. doi: 10.1002/ijc.32258
34. Nomura M, Mukasa A, Nagae G, Yamamoto S, Tatsuno K, Ueda H, et al. Distinct Molecular Profile of Diffuse Cerebellar Gliomas. Acta Neuropathol (2017) 134(6):941–56. doi: 10.1007/s00401-017-1771-1
35. Pan C, Diplas BH, Chen X, Wu Y, Xiao X, Jiang L, et al. Molecular Profiling of Tumors of the Brainstem by Sequencing of CSF-Derived Circulating Tumor DNA. Acta Neuropathol (2019) 137(2):297–306. doi: 10.1007/s00401-018-1936-6
36. Picca A, Berzero G, Bielle F, Touat M, Savatovsky J, Polivka M, et al. FGFR1 Actionable Mutations, Molecular Specificities, and Outcome of Adult Midline Gliomas. Neurology (2018) 90(23):e2086–e94. doi: 10.1212/WNL.0000000000005658
37. Reinhardt A, Stichel D, Schrimpf D, Koelsche C, Wefers AK, Ebrahimi A, et al. Tumors Diagnosed as Cerebellar Glioblastoma Comprise Distinct Molecular Entities. Acta Neuropathol Commun (2019) 7(1). doi: 10.1186/s40478-019-0801-8
38. Reuss DE, Kratz A, Sahm F, Capper D, Schrimpf D, Koelsche C, et al. Adult IDH Wild Type Astrocytomas Biologically and Clinically Resolve Into Other Tumor Entities. Acta Neuropathol (2015) 130(3):407–17. doi: 10.1007/s00401-015-1454-8
39. Ryall S, Krishnatry R, Arnoldo A, Buczkowicz P, Mistry M, Siddaway R, et al. Targeted Detection of Genetic Alterations Reveal the Prognostic Impact of H3K27M and MAPK Pathway Aberrations in Paediatric Thalamic Glioma. Acta Neuropathol Commun (2016) 4(1):93. doi: 10.1186/s40478-016-0353-0
40. Sievers P, Sill M, Schrimpf D, Stichel D, Reuss DE, Sturm D, et al. A Subset of Pediatric-Type Thalamic Gliomas Share a Distinct DNA Methylation Profile, H3k27me3 Loss and Frequent Alteration of EGFR. Neuro-Oncology (2021) 23(1):34–43. doi: 10.1093/neuonc/noaa251
41. Taylor KR, Mackay A, Truffaux N, Butterfield Y, Morozova O, Philippe C, et al. Recurrent Activating ACVR1 Mutations in Diffuse Intrinsic Pontine Glioma. Nat Genet (2014) 46(5):457–61. doi: 10.1038/ng.2925
42. Wang L, Li Z, Zhang M, Piao Y, Chen L, Liang H, et al. H3 K27M-Mutant Diffuse Midline Gliomas in Different Anatomical Locations. Hum Pathol (2018) 78:89–96. doi: 10.1016/j.humpath.2018.04.015
43. Yi S, Choi S, Shin DA, Kim DS, Choi J, Ha Y, et al. Impact of H3.3 K27M Mutation on Prognosis and Survival of Grade IV Spinal Cord Glioma on the Basis of New 2016 World Health Organization Classification of the Central Nervous System. Neurosurgery (2019) 84(5):1072–81. doi: 10.1093/neuros/nyy150
44. Zhou C, Zhao H, Yang F, Huangfu L, Dong C, Wang S, et al. Clinical and Genetic Features of Brainstem Glioma in Adults: A Report of 50 Cases in a Single Center. J Clin Neurol (Seoul Korea) (2021) 17(2):220–8. doi: 10.3988/jcn.2021.17.2.220
45. Buczkowicz P, Bartels U, Bouffet E, Becher O, Hawkins C. Histopathological Spectrum of Paediatric Diffuse Intrinsic Pontine Glioma: Diagnostic and Therapeutic Implications. Acta Neuropathol (2014) 128(4):573–81. doi: 10.1007/s00401-014-1319-6
46. Alzoubi H, Maraqa B, Hasasna N, Giangaspero F, Antonelli M, Gianno F, et al. Diffuse Midline Glioma H3 K27M-Mutant in Adults: A Report of Six Cases and Literature Review. Clin Neuropathol (2021) 40(2):108–17. doi: 10.5414/NP301331
47. Louis DN, Giannini C, Capper D, Paulus W, Figarella-Branger D, Lopes MB, et al. Cimpact-NOW Update 2: Diagnostic Clarifications for Diffuse Midline Glioma, H3 K27M-Mutant and Diffuse Astrocytoma/Anaplastic Astrocytoma, IDH-Mutant. Acta Neuropathol (2018) 135(4):639–42. doi: 10.1007/s00401-018-1826-y
48. Schulte JD, Buerki RA, Lapointe S, Molinaro AM, Zhang Y, Villanueva-Meyer JE, et al. Clinical, Radiologic, and Genetic Characteristics of Histone H3 K27M-Mutant Diffuse Midline Gliomas in Adults. Neuro-Oncol Adv (2020) 2(1):vdaa142. doi: 10.1093/noajnl/vdaa142
49. Hoffman LM, Veldhuijzen van Zanten SEM, Colditz N, Baugh J, Chaney B, Hoffmann M, et al. Clinical, Radiologic, Pathologic, and Molecular Characteristics of Long-Term Survivors of Diffuse Intrinsic Pontine Glioma (DIPG): A Collaborative Report From the International and European Society for Pediatric Oncology DIPG Registries. J Clin Oncol: Off J Am Soc Clin Oncol (2018) 36(19):1963–72. doi: 10.1200/JCO.2017.75.9308
50. Gessi M, Gielen GH, Dreschmann V, Waha A, Pietsch T. High Frequency of H3F3A (K27M) Mutations Characterizes Pediatric and Adult High-Grade Gliomas of the Spinal Cord. Acta Neuropathol (2015) 130(3):435–7. doi: 10.1007/s00401-015-1463-7
51. Cheng L, Yao Q, Ma L, Duan W, Guan J, Zhang C, et al. Predictors of Mortality in Patients With Primary Spinal Cord Glioblastoma. Eur Spine J: Off Publ Eur Spine Society Eur Spinal Deformity Soc Eur Section Cervical Spine Res Soc (2020) 29(12):3203–13. doi: 10.1007/s00586-020-06515-3
52. Liu J, Zheng M, Yang W, Lo SL, Huang J. Impact of Surgery and Radiation Therapy on Spinal High-Grade Gliomas: A Population-Based Study. J Neuro-Oncol (2018) 139(3):609–16. doi: 10.1007/s11060-018-2904-7
53. Karremann M, Gielen GH, Hoffmann M, Wiese M, Colditz N, Warmuth-Metz M, et al. Diffuse High-Grade Gliomas With H3 K27M Mutations Carry a Dismal Prognosis Independent of Tumor Location. Neuro-Oncol (2018) 20(1):123–31. doi: 10.1093/neuonc/nox149
54. Yao JJ, Wang LM, Ge HJ, Yin HF, Piao YS. Diffuse Midline Glioma With H3 K27M Mutation in the Spinal Cord: A Series of 33 Cases. Neuropathol: Off J Jpn Soc Neuropathol (2021) 41(3):183–90. doi: 10.1111/neup.12714
55. Schüller U, Iglauer P, Dorostkar MM, Mawrin C, Herms J, Giese A, et al. Mutations Within FGFR1 Are Associated With Superior Outcome in a Series of 83 Diffuse Midline Gliomas With H3F3A K27M Mutations. Acta Neuropathol (2021) 141(2):323–5. doi: 10.1007/s00401-020-02259-y
56. Nakamura M, Shimada K, Ishida E, Higuchi T, Nakase H, Sakaki T, et al. Molecular Pathogenesis of Pediatric Astrocytic Tumors. Neuro-Oncology (2007) 9(2):113–23. doi: 10.1215/15228517-2006-036
57. Vuong HG, Altibi AMA, Duong UNP, Ngo HTT, Pham TQ, Chan AK, et al. TERT Promoter Mutation and its Interaction With IDH Mutations in Glioma: Combined TERT Promoter and IDH Mutations Stratifies Lower-Grade Glioma Into Distinct Survival Subgroups-A Meta-Analysis of Aggregate Data. Crit Rev Oncol/Hematol (2017) 120:1–9. doi: 10.1016/j.critrevonc.2017.09.013
Keywords: H3K27M, midline glioma, H3F3A, HIST1H3B/C, pediatric, adult, overall survival, progression-free survival
Citation: Vuong HG, Ngo TNM, Le HT, Jea A, Hrachova M, Battiste J, McNall-Knapp R and Dunn IF (2022) Prognostic Implication of Patient Age in H3K27M-Mutant Midline Gliomas. Front. Oncol. 12:858148. doi: 10.3389/fonc.2022.858148
Received: 19 January 2022; Accepted: 28 February 2022;
Published: 18 March 2022.
Edited by:
André O. von Bueren, Hôpitaux Universitaires de Genève, SwitzerlandReviewed by:
Michael Karremann, University of Heidelberg, GermanyMoatasem El-Ayadi, Cairo University, Egypt
Copyright © 2022 Vuong, Ngo, Le, Jea, Hrachova, Battiste, McNall-Knapp and Dunn. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ian F. Dunn, Ian-Dunn@ouhsc.edu
 Huy Gia Vuong
Huy Gia Vuong Tam N. M. Ngo
Tam N. M. Ngo Hieu Trong Le3
Hieu Trong Le3 Ian F. Dunn
Ian F. Dunn