- 1Department of Ophthalmology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, Beijing, China
- 2Key Laboratory of Ocular Fundus Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
Purpose: To evaluate the clinical features, diagnostic techniques, various treatment strategies and prognosis of primary intraocular lymphoma (PIOL).
Methods: The databases PubMed, EMBASE, and Ovid were searched from inception to March 2021 to identify relevant studies. Statistical analyses were performed with R version 3.3.1.
Results: 87 studies involving 1484 patients (aged from 14 to 90 years old) were finally included. The pooling results indicated PIOL patients were female, elderly, binocular and B cell type dominated. About 19% have central nervous system (CNS) involvement at the first visit. During follow-up, the incidence of CNS involvement, death rate, 2-year and 5-year survival rate, 1-year and 2-year progression-free survival, and recurrence rate were 58%, 33%, 82%, 70%, 88%, 70%, 44%, respectively. The most common recurrent site was CNS. The delayed diagnosis rate was 85%, the misdiagnosed rate was 64%. The diagnostic technique with the highest positive rate was IL10:IL6>1 of aqueous (98%). The most common symptoms, signs, FFA and OCT features were blurring of vision (72%), vitreous inflammatory opacity (92%), FA/FAF reversal (91%) and hyper-reflective foci in posterior vitreous (53%), respectively. The prognosis of PIOL patients without CNS involvement was obviously better than those with CNS involvement. Overall, intravitreal injection of chemotherapy drug plus systemic chemotherapy (IV+CT) could achieve satisfactory prognosis, the combination of local radiotherapy (RT) could further decrease the recurrent and death rate.
Conclusion: PIOL patients with CNS involvement had significantly worse prognosis. The aqueous humor examination should be regarded as first-line and routine diagnostic technique. IV+CT could achieve satisfactory prognosis, the combination of RT was also beneficial.
Introduction
Primary intraocular lymphoma (PIOL) is an uncommon form of lymphoma disease, a subtype of primary central nervous system lymphoma (PCNSL) derived from intraocular tissue. PIOL differs from systemic and central nervous system (CNS) lymphoma which metastasize to the eye. It usually originates from vitreoretinal, known as primary vitreoretinal lymphoma (PVRL), and a few derives from uveal and optic nerve (1). PIOL is a life-threatening and blind-causing disease, especially when CNS is involved, resulting in higher mortality and worse prognosis (2). Therefore, accurate pre-CNS-involved diagnosis and effective therapy are of great significance to manage this fatal disease
PIOL usually masquerades as uveitis or other intraocular inflammation due to a wide variety of its manifestation, bringing great confusions to timely diagnosis (3). Some researchers even reported patients with misdiagnosis for more than 2 years (4), putting at risk of the prognosis of PIOL patients. Currently, diagnosis of PIOL mainly relies on the examination of intraocular fluid samples, including cytology, IL10, IL6, IL10/IL6 ratio, flow cytometry and immunoglobulin heavy chain gene rearrangement. The gold standard is still cytology (5, 6). Besides, the specific signs on optical coherence tomography (OCT), fundus fluorescein angiography (FFA) and other fundus imaging techniques also play an important role in the diagnosis and follow-up of PIOL (7, 8).
The treatment to prevent CNS involvement and recurrence is at great debate. The common treatments of PIOL included intravitreal injection (IV) or intrathecal injection (IT) of chemotherapy drug, systemic chemotherapy (CT) and local radiotherapy (RT). Combination therapy may serve as a prospective treatment to prevent or postpone CNS involvement (9–12).
Since delayed diagnosis of PIOL may lead to blindness and life-threatening complication, punctual and accurate diagnosis followed by appropriate treatment are of great significance. Numerous controversies in PIOL still existed. 1) The baseline clinical features of PIOL were reported inconsistently (3, 13–15), like the reported proportion of female cases ranged from 0% to 80% (16, 17), so were the proportion of patients over 60 years old (18, 19); 2) the reported CNS involvement rate at diagnosis varied significantly from 7% to 75.0% (20, 21), and expanded to 20% to 100% in the whole course of disease (22, 23); 3) the positive rate of various diagnostic techniques varied widely, thereby a comprehensive review of previous studies were urgently needed; 4) Many confounding factors, such as CNS involvement and different strategies of management, might affect the prognosis of PIOL, thus more precise evaluations were needed to perfect the treatment strategy. 5) Due to the small sample size of previous studies, the conclusions supported by previous studies are very limited, and their rating of the evidence level were also not high enough to be solid reference.
Until now, few systematic reviews have focused on these controversies of PIOL, a comprehensive and quantitative analysis of current evidence is urgently needed. Thus, our study is conducted to give a full picture of PIOL and evaluate the prognosis of different treatment strategies, providing credible reference for ophthalmologists.
Methods
This study is based on the standards of “Preferred Reporting Items for Systematic Reviews and Meta-analyses (the ‘PRISMA’ statement)” (24).
Search Strategy and Study Identification
Human studies in English were searched in PubMed, EMBASE, and Ovid to obtain articles that came out from inception to January 2021, searching by the keywords and their combination: (“Intraocular Lymphoma”[Mesh]) OR ((Primary Vitreoretinal Lymphoma [Title/Abstract]) OR (Primary Intraocular Lymphoma AND ((Humans [Filter]) AND (English [Filter]))) AND ((Humans [Filter]) AND (English [Filter]))). An extensive manual search strategy was also employed for related articles.
Inclusion Criteria and Exclusion Criteria
Inclusion criteria were: 1) research data on clinical manifestations, diagnosis and treatment of PIOL; 2) At least one of the following outcomes were reported: basic description of the characteristics of PIOL patients, delayed diagnosis rate, misdiagnosed rate, positive rate of various diagnostic techniques, symptoms and signs, features of multi-model imaging, prognosis of different interventions.
Exclusion criteria were: 1) researches about special objects, such as refractory and recurrent PIOL; 2) studies without distinguishing of primary and secondary intraocular lymphoma; 3) review articles; 4) animal experiments or mechanism description; 5) non-English or redundant publications.
Data Extraction and Assessment of Methodological Quality
Endnote was used to manage references. Two reviewers (X.-y.Z and T.-t.C.) read and screened the title and abstract of articles respectively after removing duplicates. Full texts were browsed for the following valuable information when relevant: first author, year of publication, type of article, cohort size, basic information of patients, diagnostic methods including aqueous humor and vitreous fluid examination, complaints of patients, fundus manifestations obtained by slit lamp, FFA and OCT, treatment strategies and outcomes, development of disease and prognosis, such as CNS involvement, recurrence and death. Authors were contacted for more details when information not available in the literature. A third reviewer (Y.-x.C.) intervened if any disagreement during data acquisition. Notably, the definition of PIOL in some studies was not accurate (no evidence of any other lymphoma at the first relevant visit), so manually identification and elimination was done. Besides, for updated publications with the same cohort of patients of the previous study, the data of the similar case was extracted synthetically and only once.
Statistical Methods
R version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria) was used to conduct statistical analysis. Freeman–Tukey variant of arcsine square was used to transform proportions with 95% confidence interval (CI) and statistical heterogeneity between studies was calculated with chi-square test and I2 statistics. For transformed proportion, a fixed-effect model was used if the heterogeneity was low (P>0.1, I2<50%), otherwise sensitivity analysis and subgroup analysis were utilized to find out the source of high heterogeneity (P<0.1, I2>50%), and a random-effect model was used if it could not be eliminated.
Statistical significance was measured with P<0.05. Publication bias was verified by funnel plots of the Egger test with statistically significant when P<0.05 (25).
Results
Study Characteristic
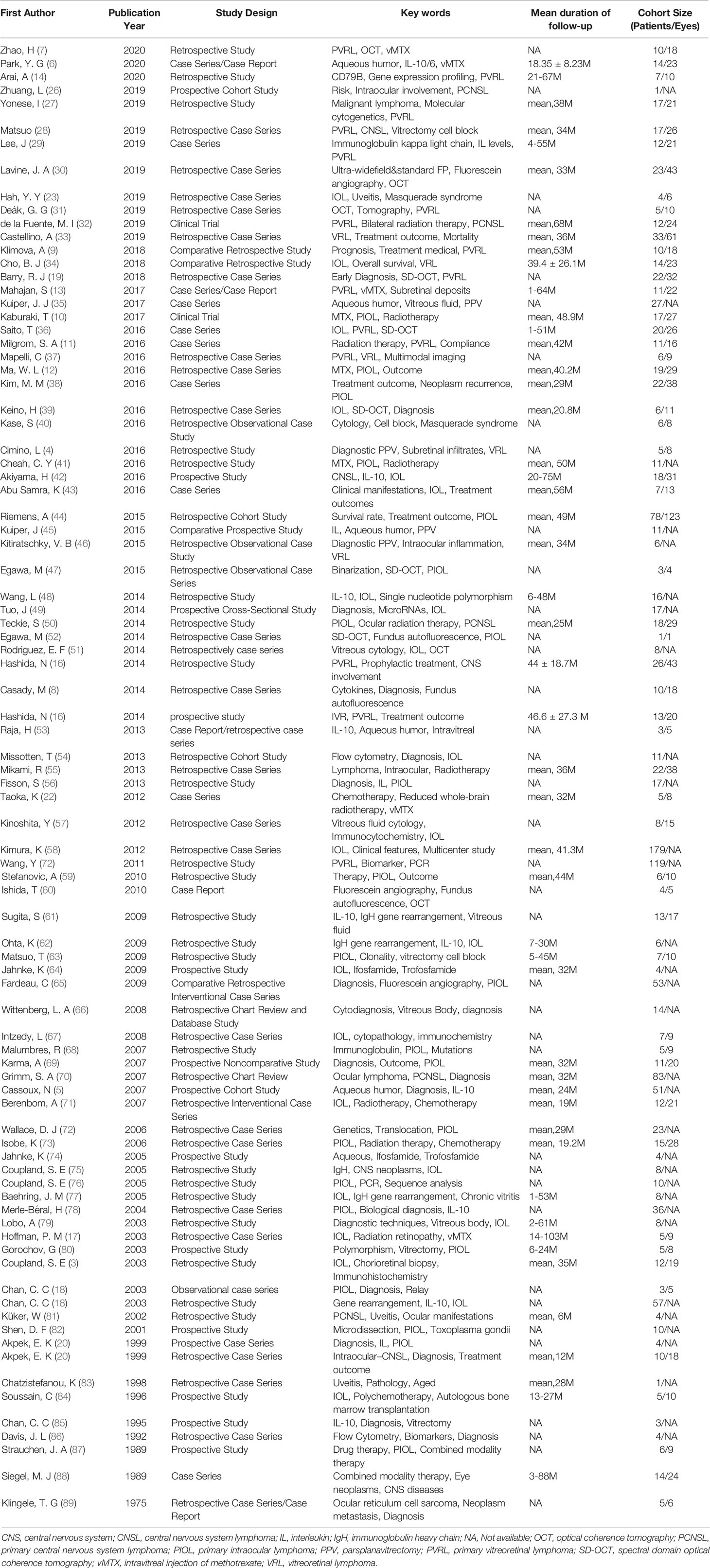
A total of 952 articles were included as possible related studies. The screening process is shown in Figure 1. After removing the repetition and reading abstracts, 123 articles were included for full details, in which 87 articles were finally included. The specific information of these articles is shown in Table 1, with 1483 patients in cohort (aged from 14 to 90 years old).
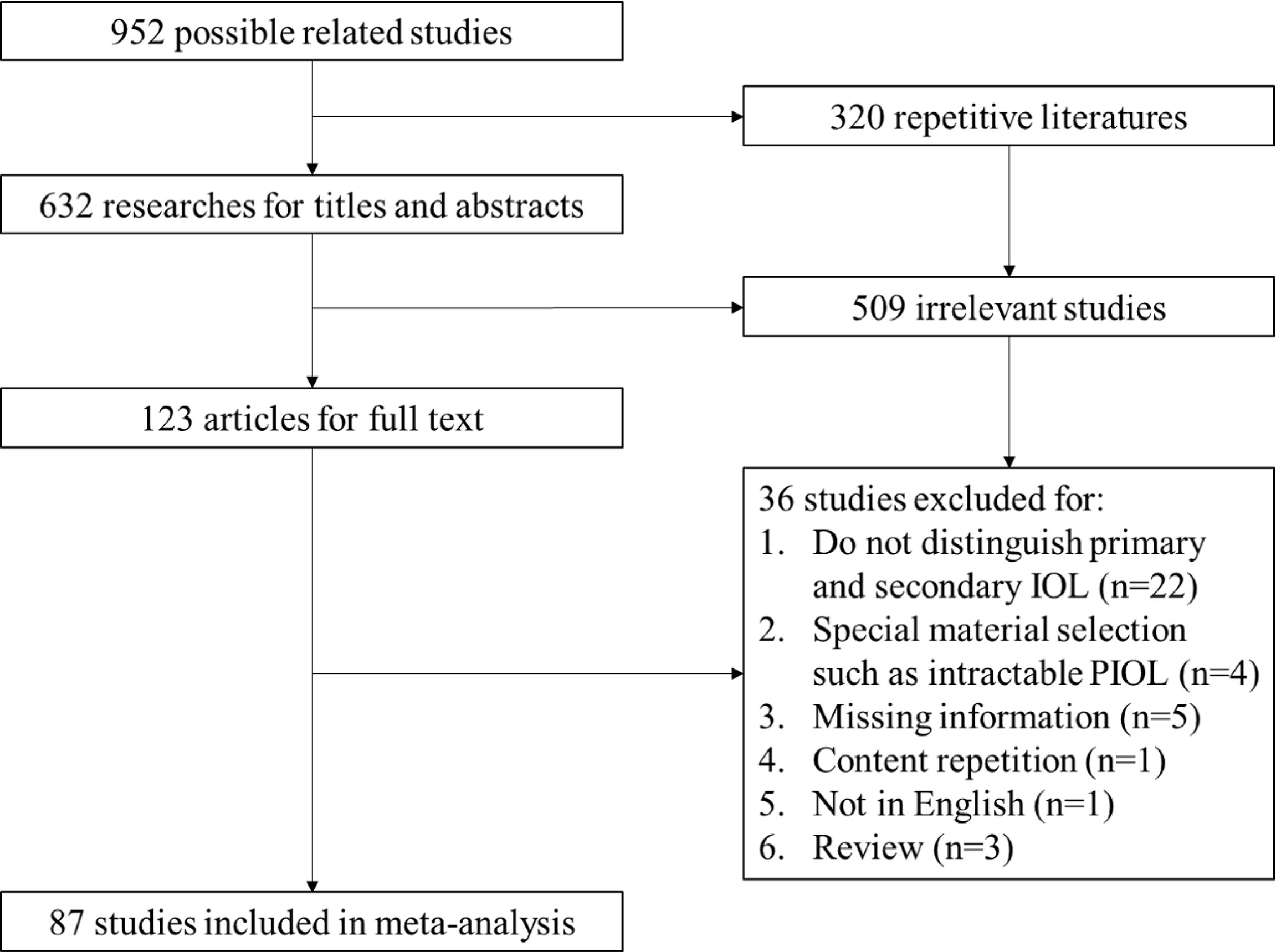
Figure 1 PRISMA flow of screening process. IOL, Intraocular Lymphoma; PIOL, Primary Intraocular Lymphoma.
Clinical Features for PIOL
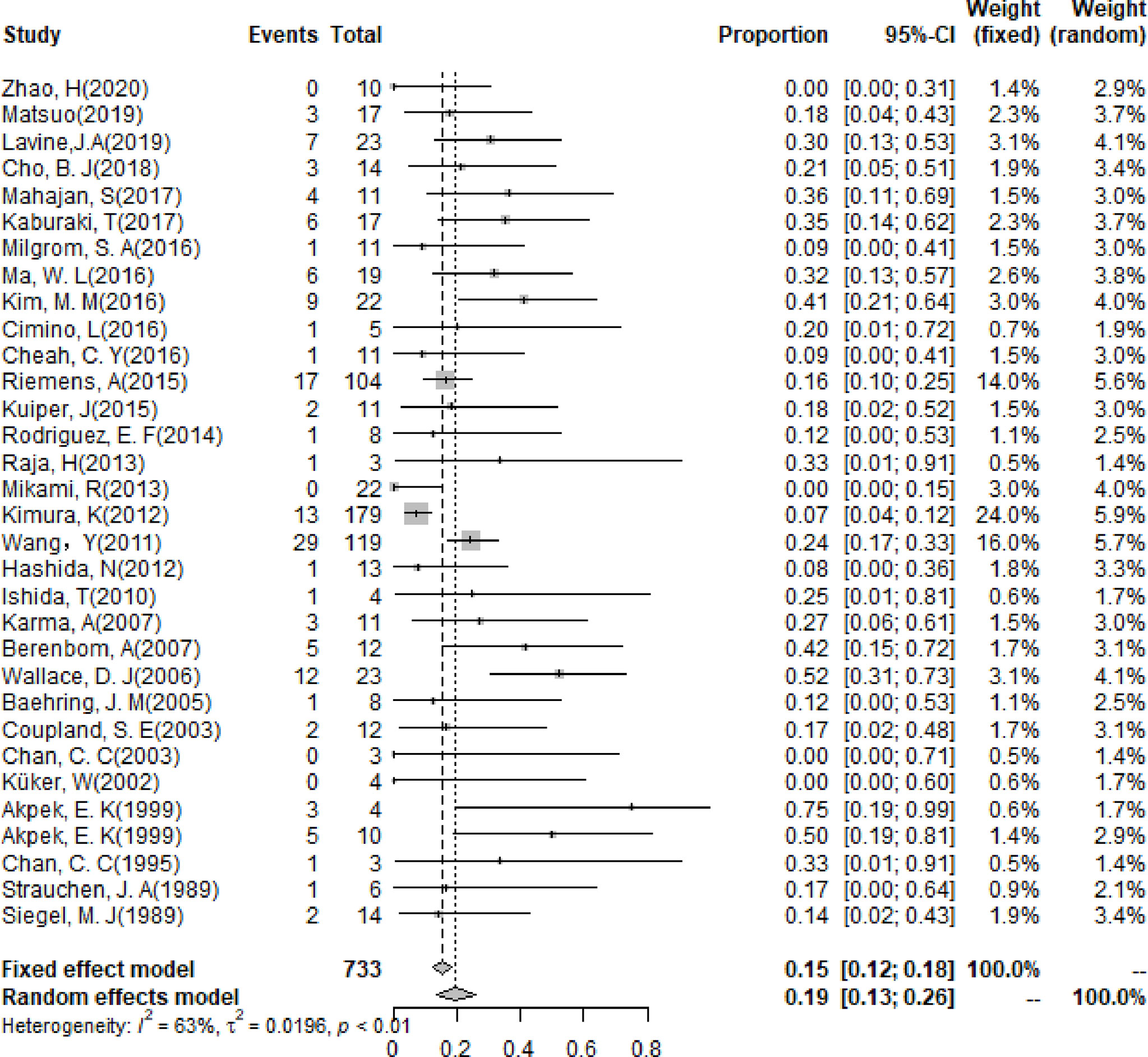
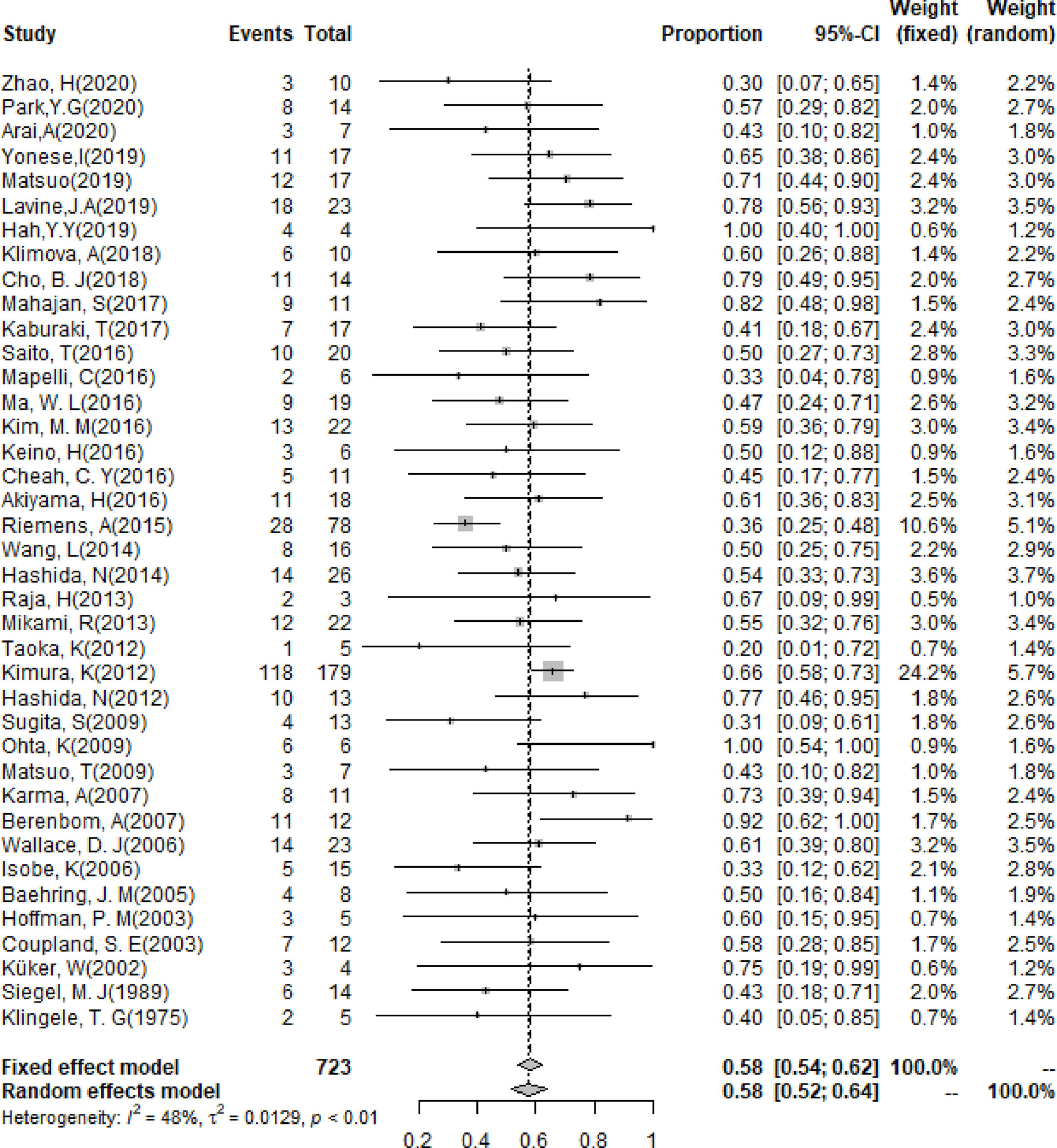
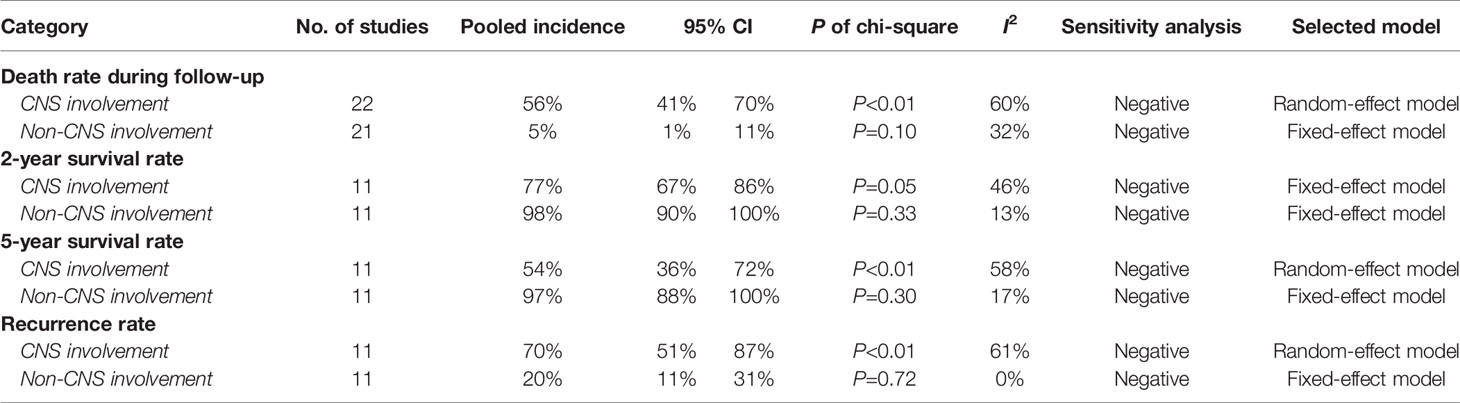
The pooling results of the baseline clinical features of PIOL patients were summarized in Table 2. Around 19% (95% CI [13%-26%]) of patients already had CNS involvement when diagnosed (Figure 2), which progressed to 58% (95% CI [54%-62%]) during the whole disease course (Figure 3), around 33% (95% CI [26%-42%]) of PIOL patients died during follow-up. The most common recurrence site of PIOL was CNS alone (45%, 95% CI [32%-59%]).
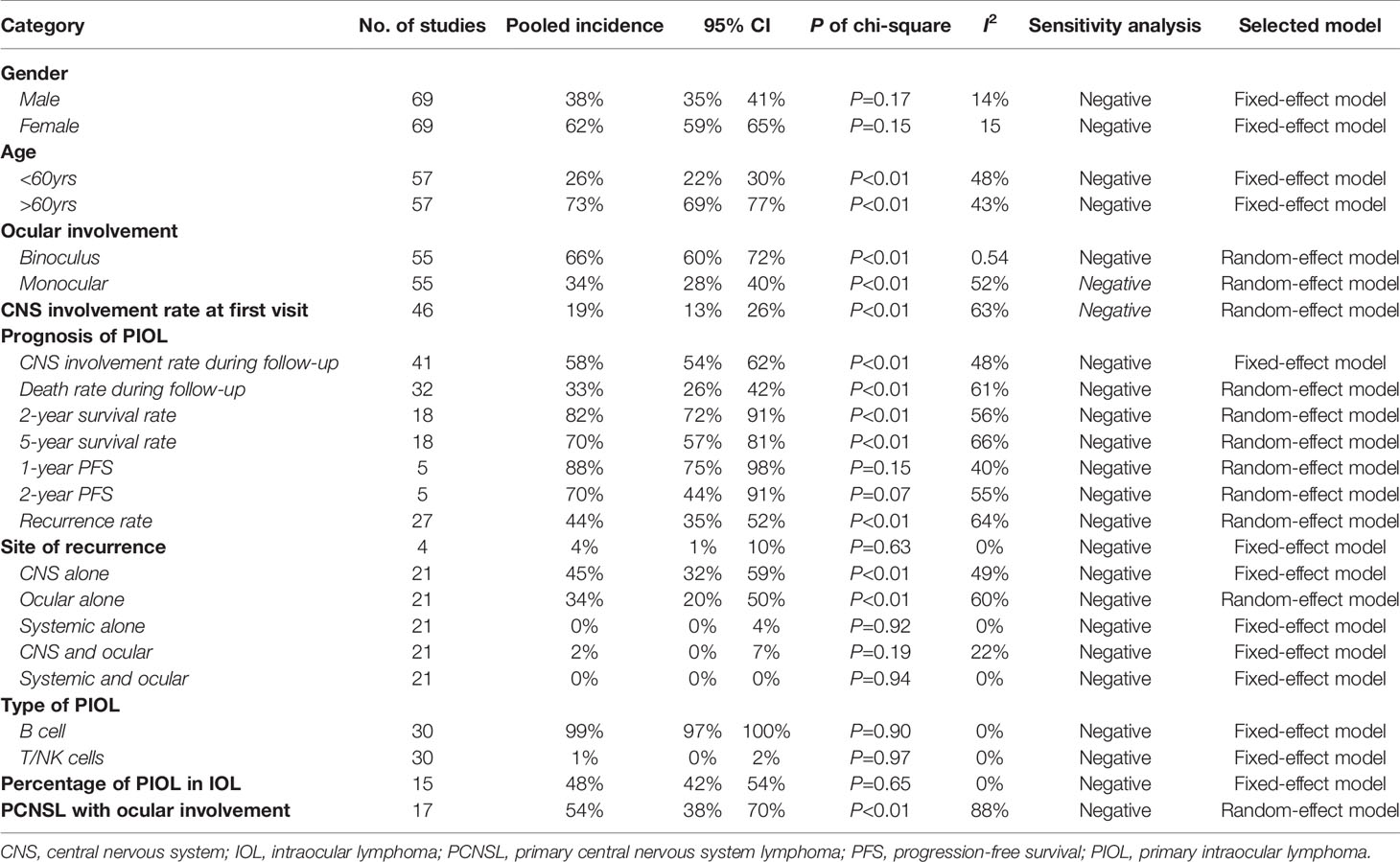
Table 2 Population distribution and clinical characteristics of primary intraocular lymphoma patients.
Diagnosis of PIOL
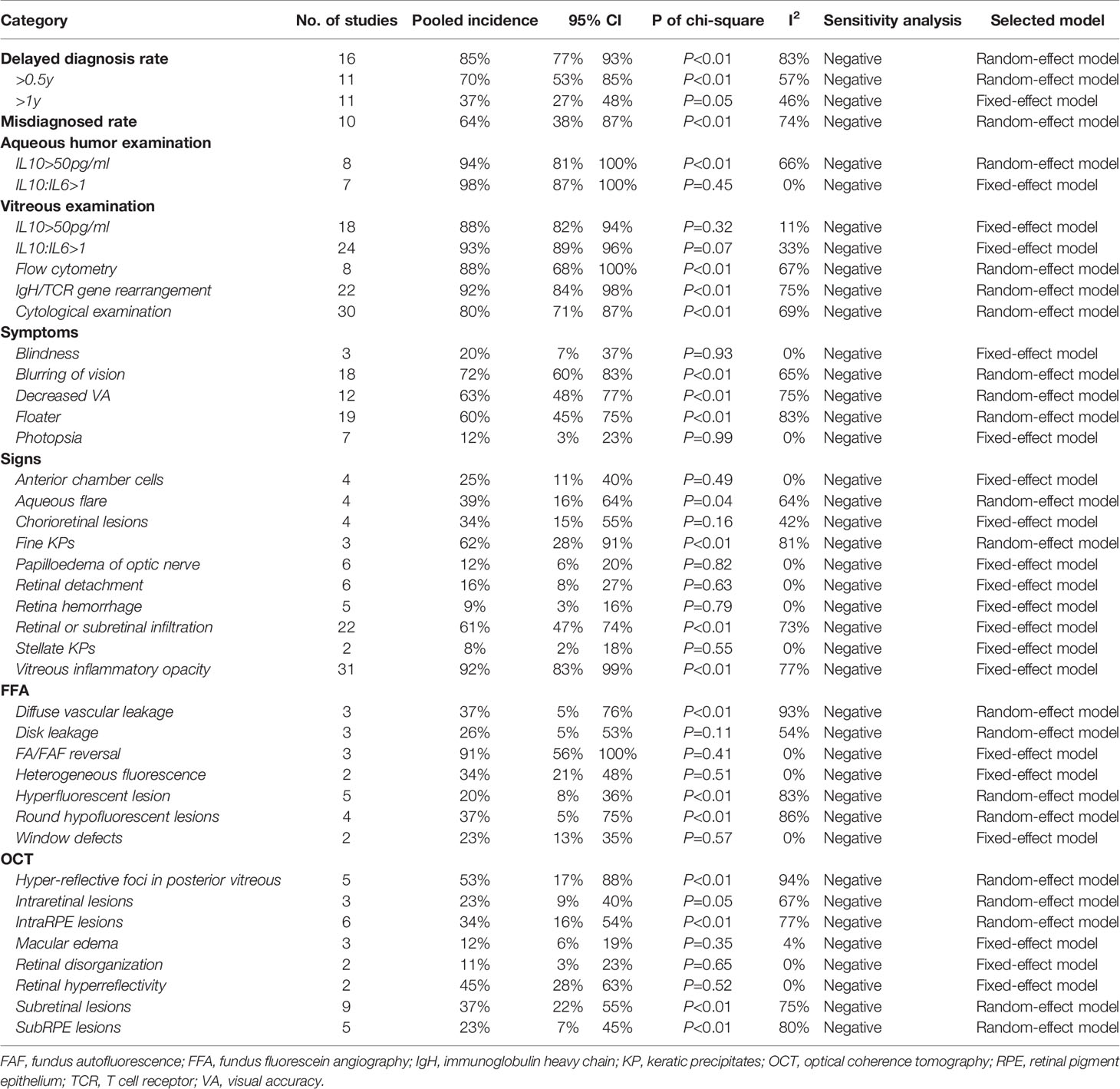
Diagnosis delay of PIOL is defined as the time from onset of ocular symptoms to diagnosis of PIOL. The pooling results indicated that the general delayed diagnosis rate was 85% (95% CI [77% - 93%]), with around 70% (95% CI [53% - 85%]) of PIOL patients having a delayed diagnosis longer than half a year, 37% (95% CI [27% - 48%]) longer than a year. Meanwhile, the misdiagnosed rate was around 64% (95% CI [38% - 70%]). The pooling results of the common symptoms, the positive rate of various diagnostic techniques and the multi-model imaging features were summarized in Table 3. The positive rate of aqueous and vitreous examination ranged from 80% to 98%. The most common symptoms, signs, FFA and OCT features were blurring of vision (72%, 95% CI [60% - 83%]), vitreous inflammatory opacity (92%, 95% CI [83% - 99%]), FA/FAF reversal (91%, 95% CI [56% - 100%]) and hyper-reflective foci in posterior vitreous (53%, 95% CI [17% - 88%]), respectively.
Subgroup Analysis of Prognosis and Complications of Treatments for PIOL
Subgroup analysis of the prognosis of PIOL was carried out between patients with and without CNS involvement. The detailed results were shown in Table 4.
The subgroup of various treatments was analyzed, including CT, RT, IV and IT, to figure out their corresponding efficacies and prognosis, especially the involvement of CNS, recurrence rate and mortality. Then, more detailed interventions were evaluated, including CT+RT, CT+RT+IT, IV+CT, IV+CT+RT, IV+IT and IV+ RT, to determine which can give a better prognosis of PIOL (Table 5).
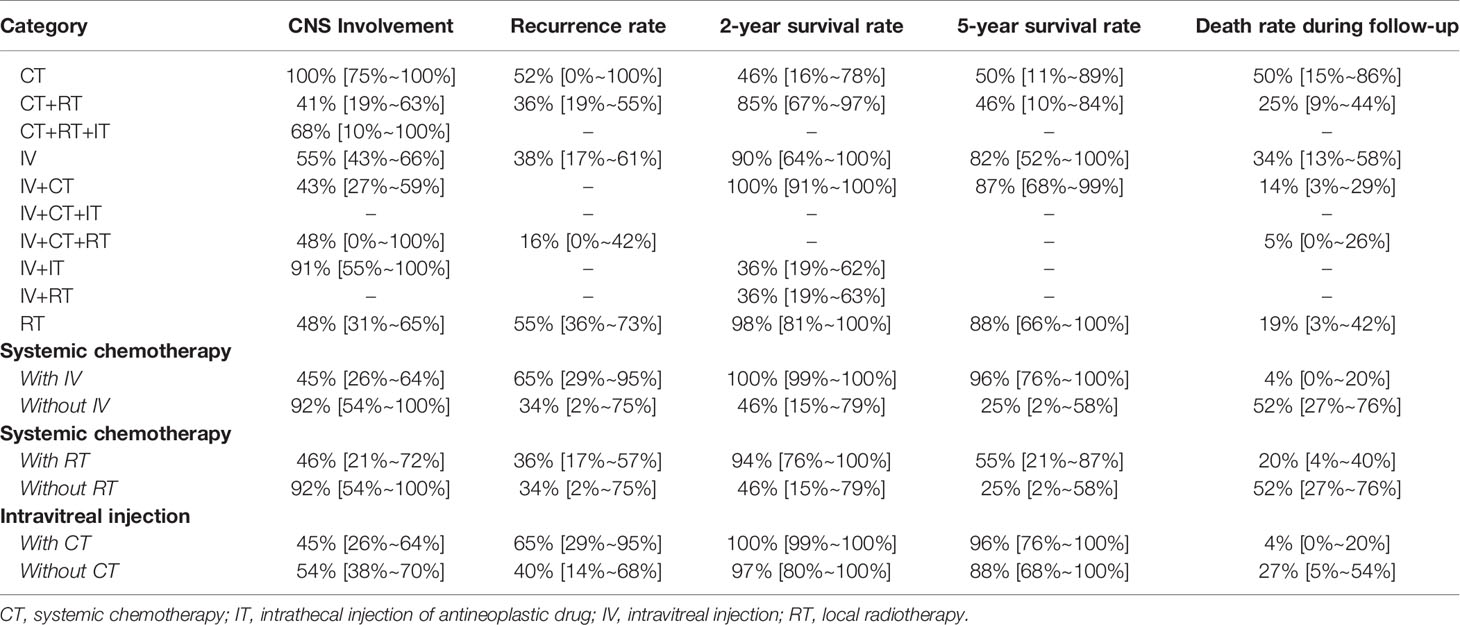
Table 5 Pooling results about treatment strategy and efficacy for PIOL patients without CNS involvement at first.
Publication Bias
Using the Egger test (P =0.133), no evidence of publication bias was found.
Discussion
PIOL is an eyesight-damaging and life-threatening disease. Based on currently available research, our study suggested that PIOL patients are female, elderly, binocular and B-cell type dominated. About 19% are CNS-involved when diagnosed. During the follow-up period, the incidence of CNS involvement, death rate, 2-year and 5-year survival rate, 1-year and 2-year PSF, and recurrence rate were 58%, 33%, 82%, 70%, 88%, 70%, 44%, respectively. The most common recurrent site was CNS. About half of IOL was PIOL, as well as PCNSL with ocular involvement. The delayed diagnosis rate of PIOL was extremely high, so was the misdiagnosed rate. IL10:IL6>1 of the aqueous had the highest positive rate among laboratory examinations (98%, 95% CI [87% - 100%]). The most common symptoms, signs, FFA and OCT features were blurring of vision, vitreous inflammatory opacity, FA/FAF reversal and hyper-reflective foci in posterior vitreous, respectively. Overall, the prognosis of PIOL patients without CNS involvement was much better than those with CNS involvement, such as death rate (5% versus 56%), 2-year survival rate (98% versus 77%), 5-year survival rate (97% versus 54%) and recurrence rate (20% versus 70%). IV+CT was recommended as a satisfactory treatment strategy with less burden and side effects, the combination of RT might further benefit in decreasing the recurrent and death rate.
Clinical Features of PIOL
The basic clinical characteristics about PIOL were quite controversial, and the limited sample size and inconsistent data of previous studies could not offer us a reliable impression. For example, previous studies demonstrated a large variation in the proportion of female in PIOL patients (20% to 100%) (16, 17), binocular involvement (22% to 100%) (32, 67), and CNS involvement rate when diagnosed (7% to 75%) (20, 21). By pooling the data of all currently available studies, our study gave a solid reference when explaining the clinical features of PIOL to patients.
Generally, PIOL began as monocular onset, and more than half progressed to binocular, for the destruction of blood-eye barrier might stimulate the fellow eye. It occurred mainly in the elderly due to weak immune system and mutation accumulation (1). About 19% of PIOL patients are already CNS-involved when diagnosed, defined as the ocular symptoms occurred before the diagnosis of CNS involvement, which further progressed to 58% during the whole disease course. When giving a PIOL diagnosis, we must clarify whether CNS were involved, as their prognosis was very different. Thus, routine head magnetic resonance imaging (MRI) and even cerebrospinal fluid (CSF) examination should be conducted at baseline.
Most PIOL were reported to be B cell types (99%, 95% CI [97% - 100%]). Two hypotheses were proposed to explain its origin. Chan et al. (18) detected B-cell receptors (CXCR4 and CXCR5) on the surface of lymphoma cells, and found the corresponding ligand B-lymphocytic chemoattractant (BLC) on RPE, indicating the abnormal expression of BLC on lymphoma cells are attracted to eye tissues through chemotaxis. Shen et al. (82) found that infections such as HHV8 virus and Toxoplasma may be associated with some PIOL, triggering B cell monoclonal proliferation and thereby lymphoma. T cell-derived PIOL was very rare, which mainly involves vitreoretinal. Its population distribution and ocular manifestations were generally similar to B cells types, which could only be differentiated by laboratory examinations (90).
Signs and Imaging Findings
Our study suggested that the leading three complaints of PIOL patients were blurred vision, decreased vision acuity and floater, the most common signs were vitreous opacity, fine KPS and retinal or subretinal infiltration. These were mainly caused by the aggressive destruction of the retinal photosensitive structure by lymphoma cells’ invasion or the production of space-occupying turbidity in the vitreous. Besides, PIOL could not only be manifested as fine KPs, but also as stellate KPs.
In OCT, more than half of the patients had hyper-reflective foci in posterior vitreous, combined with retinal hyperreflectivity, subretinal lesions and intra-RPE lesions, representing the functional abnormality and structural interruption caused by the infiltration of different degrees of lymphoma cells in different layers of the retina, which would not appear in eye inflammatory diseases (7). OCT could show more detailed features of lymphoma infiltration and have definite diagnostic significance for PIOL, especially when dense vitritis occurred or the lesions were small. Zhao et al. also suggested that OCT could be used as a non-invasive method to reflect the therapeutic effect and progress of PIOL (7).
The most frequent finding of FFA was FA/FAF reverse (91%, 95% CI [56% - 100%]), defining as high autofluorescence spot on FAF corresponding to a low autofluorescence spot without leakage in this region on FFA. The incidence of other signs was relatively lower than 50%, such as diffuse vascular leakage corresponding to lesion and surrounding small blood vessels, which might be an earlier sign of PIOL before the formation of subretinal lesions.
Laboratory Examination and Diagnosis
The examination of aqueous humor and vitreous fluid was of great significance for the diagnosis of PIOL. However, the positive rate of each index reported in previous studies fluctuated greatly, making it difficult to launch parallel comparison (5, 6, 16). Our study evaluated and reported the utility of different laboratory examinations, providing reference for diagnosis of PIOL.
Pars plana vitrectomy (PPV) was the last resort for the diagnosis of PIOL with a reliable positive rate due to its damaging operation. Our study indicated that the positive rate of gene rearrangement of IgH and T cell receptor (TCR) was 92% (95% CI [84%-98%]). For cytokine test, positive rate of IL10>50pg/ml (29) was 88% (95% CI [82%-94%]), and the sensitive of IL10/IL6>1 was higher as 93% (95% CI [89%-96%]). Besides, flow cytometry could be used to detect markers on cell surface if samples were enough, with the positive rate of 88% (95% CI [68%-100%]). In addition, observing lymphoma cells in cytological examination directly was still the gold standard with the highest specificity, but the sensitivity was only around 80% (95% CI [71%-87%]), resulting from the fragility of tumor cells with the degeneration caused by untimely inspection. Resultantly, multiple inspections or other markers were needed.
Aqueous humor tests might be an ideal technique due to minimal trauma and valid positive rate compared to vitreous fluid. The most promising one was IL10/IL6>1 with a positive rate near 98% (95% CI [87%-100%]) in PIOL patients. The positive rate of IL10>50pg/ml (5) was also as high as 94% (95% CI [81%-100%]). IL10 was expressed by malignant tumor cells, inhibiting various immune-related cell populations to achieve immune escape, while the rise of IL6 occurred in inflammation indicated stronger immune response. Thus, the levels of IL10 and IL6 have a great potential to distinguish PIOL from ocular inflammation to recognize camouflage syndrome.
According to clinical expression, the positive rate of vitreous fluid examination should be the highest. Interestingly, our analysis showed that of aqueous humor tests was higher. The possible reasons could be as follows: 1) The collection of aqueous humor was so convenient that most operation errors and detection delay were avoided; 2) Cytokines level aqueous humor and vitreous fluid might be generally equivalent because cytokines produced by tumor cells in vitreous cavity could smoothly diffuse into aqueous humor with an ideal concentration; 3) The absence of microenvironment effect of tumor cells reduced the difficulty of cytokines detection in aqueous humor. Meanwhile, the possible reasons for the relatively low positive rate of diagnostic vitrectomy specimens included difficulty of sample acquisition, inspection delay, and sample dilution et al. Besides, multiple PPV may increase the risk of lymphoma spread through the sclerotomy port to the epibulbar space (23). Therefore, we suggest that for patients with clinical manifestations and imaging characteristics supporting PIOL, the aqueous humor test should be the first choice for confirming the diagnosis, diagnostic PPV should be the last resort.
For accurate diagnosis, when a patient experience vision loss, fine KPs, retinal or subretinal infiltration and vitreous inflammatory opacity, hyper-reflective foci in posterior vitreous, retinal hyperreflectivity on OCT, FA/FAF reversal on FFA, ophthalmologists should consider the possibility of PIOL, especially in the elderly. Aqueous humor test and cranial MRI should be routinely conducted to prompt the need for further invasive procedures to make timely diagnosis and treatment.
Treatment and Prognosis
Subgroup analysis indicated that the prognosis of PIOL patients without CNS involvement was much better than those with CNS involvement, such as death rate during follow-up (5% versus 56%), 2-year survival rate (98% versus 77%), 5-year survival rate (97% versus 54%) and recurrence rate (20% versus 70%). Thus, clarification of CNS involvement is of great significance for the prognosis to PIOL patients.
As for the treatment of PIOL, many controversies still existed due to small sample size, and thereby expanding the size of study is the key. Therefore, our study integrated all the available data to provide a solid reference for the management of PIOL.
According to Table 5, IV+CT could achieve a satisfactory prognosis regarding CNS involvement, 2-year and 5-year survival rate, and death rate during follow-up. IV+CT+RT could achieve the lowest recurrent rate, while survival and death rate during follow-up could not be evaluated due to limited data. For CT+RT, the CNS involvement rate was the lowest, while the 2-year and 5-year survival rates were not satisfactory. Subgroup analysis indicated that the prognosis of CT combined with IV was significantly better than that of CT alone considering CNS involvement (45% versus 92%), death rate (4% versus 52%), 2-year survival rate (100% versus 46%) and 5-year rate (96% versus 25%). Similarly, CT combined with RT could improve prognosis more than CT alone. Therefore, we believe that the current evidence supports CT+IV be used as the first-line treatment for the PIOL patients. If possible, the combination of RT could further decrease the recurrence rate and death rate (14% versus 5%) during follow-up. Other more aggressive treatment showed limited efficacy in studies and were not recommended.
Strengths and Limitations
To the best of our knowledge, this is the first meta-analysis evaluating all available evidence of PIOL from different angles. This study characterized with the largest sample size and the highest level of evidence, which may provide solid references for ophthalmologists, contribute to a better understanding of the disease course and facilitate smoother communication with patients.
However, some limitations still existed. Firstly, the included studies span a wide period time from 1975 to 2020. Although the heterogeneity and publication bias were properly controlled, a difference existed in baseline features of patients (institutional referral bias) and medical techniques, might influence the results of our study. Besides, due to the lack of data in IV+CT+IT or IV+CT+RT, the efficacy of some strategies could not be comprehensively evaluated. To evaluate the prognosis of different treatments, we only focused on major treatment strategy, giving an overall direction and propose that IV + CT could achieve satisfactory prognosis. More large-scale clinical trials were needed to further explore the specific dose and refinement method in the future. We really hope our study could service as directional reference for future clinical trials and research. Secondly, genetic features of PIOL were of great value for the diagnosis and prognosis. Yonese et al. (27) detected that CD79B(Y196) in vitreous DNA might contribute to the confirmation of the diagnosis, and Arai et al. (14) regarded that CD79B mutations showed potential to serve as prognostic markers for CNS progression. Another study conducted by Wallace et al. (72) described that the bcl-2 t(14,18) translocations, the bcl-10 gene, and expression of bcl-6 mRNA in PIOL when compared with other systemic lymphomas, providing useful adjuncts to the pathologic diagnosis of this complex disease. But the number of articles were far too small for conducting a reliable meta-analysis, we did not report them in our study. Genetic features were indeed a potential diagnostic and prognostic marker for PIOL. Thus, we should pay more attention on it in future study. Thirdly, the visual outcome was also of great importance for the surviving PIOL patients. While the current data of visual outcomes was too limited for conducting a reliable meta-analysis, so we did not report it in our study. Thus, we suggest that further study should not only focus on the survival of the PIOL patients, but also pay more attention to the visual outcomes.
Conclusion
PIOL is an eyesight-damaging and life-threatening disease, patients with CNS involvement had a significantly worse prognosis. The aqueous humor examination should be regarded as a first-line and routine diagnostic technique. IV+CT could achieve a satisfactory prognosis with less burden and side effects, the combination of RT could further decrease the recurrent and death rate during follow-up.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author Contributions
X-yZ designed this subject, carried out statistical analysis and revised the manuscript. T-tC conducted literature searching and data extraction, then drafted manuscripts. L-hM and W-fZ helped revised the manuscript. Y-xC coordinated and participated in the entire process of composing the manuscript. All authors read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
X-yZ wants to thank, in particular, the invaluable support received from Shengzhi Liu over the years.
References
1. Touhami S, Audo I, Terrada C, Gaudric A, LeHoang P, Touitou V, et al. Neoplasia and Intraocular Inflammation: From Masquerade Syndromes to Immunotherapy-Induced Uveitis. Prog Retin Eye Res (2019) 72:100761. doi: 10.1016/j.preteyeres.2019.05.002
2. Hormigo A, DeAngelis LM. Primary Ocular Lymphoma: Clinical Features, Diagnosis, and Treatment. Clin Lymphoma (2003) 4(1):22–9. doi: 10.3816/clm.2003.n.010
3. Coupland SE, Bechrakis NE, Anastassiou G, Foerster AM, Heiligenhaus A, Pleyer U, et al. Evaluation of Vitrectomy Specimens and Chorioretinal Biopsies in the Diagnosis of Primary Intraocular Lymphoma in Patients With Masquerade Syndrome. Graefes Arch Clin Exp Ophthalmol (2003) 241(10):860–70. doi: 10.1007/s00417-003-0749-y
4. Cimino L, Coassin M, Chan CC, Marchi S, Belpoliti M, Fanti A, et al. Vitreoretinal Lymphomas Misdiagnosed as Uveitis: Lessons Learned From a Case Series. Indian J Ophthalmol (2016) 64(5):369–75. doi: 10.4103/0301-4738.185600
5. Cassoux N, Giron A, Bodaghi B, Tran TH, Baudet S, Davy F, et al. IL-10 Measurement in Aqueous Humor for Screening Patients With Suspicion of Primary Intraocular Lymphoma. Invest Ophthalmol Vis Sci (2007) 48(7):3253–9. doi: 10.1167/iovs.06-0031
6. Park YG, Park WK, Kim RY, Kim M, Park YH. Serial Changes in the Aqueous IL-10 Level After Intravitreal Methotrexate Injection as an Indicator of Primary Vitreoretinal Lymphoma Recurrence. Sci Rep (2020) 10(1):15992. doi: 10.1038/s41598-020-73111-2
7. Zhao H, Wang X, Mao Y, Peng X. Longitudinal Observation of OCT Imaging is a Valuable Tool to Monitor Primary Vitreoretinal Lymphoma Treated With Intravitreal Injections of Methotrexate. BMC Ophthalmol (2020) 20(1):10. doi: 10.1186/s12886-019-1300-1
8. Casady M, Faia L, Nazemzadeh M, Nussenblatt R, Chan CC, Sen HN. Fundus Autofluorescence Patterns in Primary Intraocular Lymphoma. Retina (2014) 34(2):366–72. doi: 10.1097/IAE.0b013e31829977fa
9. Klimova A, Heissigerova J, Rihova E, Brichova M, Pytlik R, Spicka I, et al. Combined Treatment of Primary Vitreoretinal Lymphomas Significantly Prolongs the Time to First Relapse. Br J Ophthalmol (2018) 102(11):1579–85. doi: 10.1136/bjophthalmol-2017-311574
10. Kaburaki T, Taoka K, Matsuda J, Yamashita H, Matsuda I, Tsuji H, et al. Combined Intravitreal Methotrexate and Immunochemotherapy Followed by Reduced-Dose Whole-Brain Radiotherapy for Newly Diagnosed B-Cell Primary Intraocular Lymphoma. Br J Haematol (2017) 179(2):246–55. doi: 10.1111/bjh.14848
11. Milgrom SA, Cheah CY, Pinnix CC, Smith GL, Dabaja BS, Horace P, et al. Acute and Late Toxicity of Bilateral Orbital Irradiation in the Management of Primary Intraocular Lymphoma. Leuk Lymphoma (2016) 57(11):2612–8. doi: 10.3109/10428194.2016.1166490
12. Ma WL, Hou HA, Hsu YJ, Chen YK, Tang JL, Tsay W, et al. Clinical Outcomes of Primary Intraocular Lymphoma Patients Treated With Front-Line Systemic High-Dose Methotrexate and Intravitreal Methotrexate Injection. Ann Hematol (2016) 95(4):593–601. doi: 10.1007/s00277-015-2582-x
13. Mahajan S, Nijhawan R, Rajwanshi A, Karkhur S, Mulkutar S, Dogra M, et al. Clinical Characteristics of Primary Vitreoretinal Lymphoma in an Indian Population. Ocul Immunol Inflammation (2017) 25(5):633–8. doi: 10.3109/09273948.2016.1139731
14. Arai A, Takase H, Yoshimori M, Yamamoto K, Mochizuki M, Miura O. Gene Expression Profiling of Primary Vitreoretinal Lymphoma. Cancer Sci (2020) 111(4):1417–21. doi: 10.1111/cas.14347
15. Dunn JP. Interleukins in the Diagnosis of Intraocular Lymphoma: Do We Still Need Histologic Confirmation? Retina (2018) 38(4):647–9. doi: 10.1097/iae.0000000000002157
16. Hashida N, Nakai K, Saitoh N, Nishida K. Association Between Ocular Findings and Preventive Therapy With Onset of Central Nervous System Involvement in Patients With Primary Vitreoretinal Lymphoma. Graefes Arch Clin Exp Ophthalmol (2014) 252(4):687–93. doi: 10.1007/s00417-014-2584-8
17. Hoffman PM, McKelvie P, Hall AJ, Stawell RJ, Santamaria JD. Intraocular Lymphoma: A Series of 14 Patients With Clinicopathological Features and Treatment Outcomes. Eye (Lond) (2003) 17(4):513–21. doi: 10.1038/sj.eye.6700378
18. Chan CC, Shen D, Hackett JJ, Buggage RR, Tuaillon N. Expression of Chemokine Receptors, CXCR4 and CXCR5, and Chemokines, BLC and SDF-1, in the Eyes of Patients With Primary Intraocular Lymphoma. Ophthalmology (2003) 110(2):421–6. doi: 10.1016/s0161-6420(02)01737-2
19. Barry RJ, Tasiopoulou A, Murray PI, Patel PJ, Sagoo MS, Denniston AK, et al. Characteristic Optical Coherence Tomography Findings in Patients With Primary Vitreoretinal Lymphoma: A Novel Aid to Early Diagnosis. Br J Ophthalmol (2018) 102(10):1362–6. doi: 10.1136/bjophthalmol-2017-311612
20. Akpek EK, Maca SM, Christen WG, Foster CS. Elevated Vitreous Interleukin-10 Level is Not Diagnostic of Intraocular-Central Nervous System Lymphoma. Ophthalmology (1999) 106(12):2291–5. doi: 10.1016/s0161-6420(99)90528-6
21. Kimura K, Usui Y, Goto H. Clinical Features and Diagnostic Significance of the Intraocular Fluid of 217 Patients With Intraocular Lymphoma. Jpn J Ophthalmol (2012) 56(4):383–9. doi: 10.1007/s10384-012-0150-7
22. Taoka K, Yamamoto G, Kaburaki T, Takahashi T, Araie M, Kurokawa M. Treatment of Primary Intraocular Lymphoma With Rituximab, High Dose Methotrexate, Procarbazine, and Vincristine Chemotherapy, Reduced Whole-Brain Radiotherapy, and Local Ocular Therapy. Br J Haematol (2012) 157(2):252–4. doi: 10.1111/j.1365-2141.2011.08938.x
23. Hah YY, Ho SL, Beng SC, Agrawal R. Epidemiology and Clinical Features of Intraocular Lymphoma in Singapore. Nepal> J Ophthalmol (2019) 11(22):158–66. doi: 10.3126/nepjoph.v11i2.27822
24. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PloS Med (2009) 6(7). doi: 10.1371/journal.pmed.1000097
25. Egger M, Davey Smith G, Schneider M, Minder C. Bias in Meta-Analysis Detected by a Simple, Graphical Test. BMJ (1997) 315(7109):629–34. doi: 10.1136/bmj.315.7109.629
26. Zhuang L, Lai J, Chen K, Ding T, Yuan Y, Ma Y, et al. Intraocular Involvement Is Associated With a High Risk of Disease Relapse in Primary Central Nervous System Lymphoma. Oncol Rep (2019) 41(1):397–404. doi: 10.3892/or.2018.6781
27. Yonese I, Takase H, Yoshimori M, Onozawa E, Tsuzura A, Miki T, et al. CD79B Mutations in Primary Vitreoretinal Lymphoma: Diagnostic and Prognostic Potential. Eur J Haematol (2019) 102(2):191–6. doi: 10.1111/ejh.13191
28. Matsuo T, Tanaka T. Are There Primary Intraocular Lymphomas That Do Not Develop Into Central Nervous System Lymphomas? J Clin Exp Hematop (2019) 59(4):168–74. doi: 10.3960/jslrt.19019
29. Lee J, Kim SW, Kim H, Lee CS, Kim M, Lee SC. Differential Diagnosis for Vitreoretinal Lymphoma With Vitreoretinal Findings, Immunoglobulin Clonality Tests, and Interleukin Levels. Retina (2019) 39(6):1165–76. doi: 10.1097/iae.0000000000002127
30. Lavine JA, Singh AD, Sharma S, Baynes K, Lowder CY, Srivastava SK. Ultra- Widefield Multimodal Imaging of Primary Vitreoretinal Lymphoma. Retina (2019) 39(10):1861–71. doi: 10.1097/iae.0000000000002260
31. Deák GG, Goldstein DA, Zhou M, Fawzi AA, Jampol LM. Vertical Hyperreflective Lesions on Optical Coherence Tomography in Vitreoretinal Lymphoma. JAMA Ophthalmol (2019) 137(2):194–8. doi: 10.1001/jamaophthalmol.2018.5835
32. de la Fuente MI, Alderuccio JP, Reis IM, Omuro A, Markoe A, Echegaray JJ, et al. Bilateral Radiation Therapy Followed by Methotrexate-Based Chemotherapy for Primary Vitreoretinal Lymphoma. Am J Hematol (2019) 94(4):455–60. doi: 10.1002/ajh.25414
33. Castellino A, Pulido JS, Johnston PB, Ristow KM, Nora Bennani N, Inwards DJ, et al. Role of Systemic High-Dose Methotrexate and Combined Approaches in the Management of Vitreoretinal Lymphoma: A Single Center Experience 1990-2018. Am J Hematol (2019) 94(3):291–8. doi: 10.1002/ajh.25350
34. Cho BJ, Kim DY, Park UC, Lee JY, Yoon YH, Yu HG. Clinical Features and Treatment Outcomes of Vitreoretinal Lymphoma According to Its Association With CNS Lymphoma. Ocul Immunol Inflam (2018) 26(3):365–71. doi: 10.1080/09273948.2017.1421669
35. Kuiper JJ, Beretta L, Nierkens S, van Leeuwen R, Ten Dam-van Loon NH, Ossewaarde-van Norel J, et al. An Ocular Protein Triad Can Classify Four Complex Retinal Diseases. Sci Rep (2017) 7:41595. doi: 10.1038/srep41595
36. Saito T, Ohguro N, Iwahashi C, Hashida N. Optical Coherence Tomography Manifestations of Primary Vitreoretinal Lymphoma. Graefes Arch Clin Exp Ophthalmol (2016) 254(12):2319–26. doi: 10.1007/s00417-016-3395-x
37. Mapelli C, Invernizzi A, Barteselli G, Pellegrini M, Tabacchi E, Staurenghi G, et al. Multimodal Imaging of Vitreoretinal Lymphoma. Ophthalmologica (2016) 236(3):166–74. doi: 10.1159/000447412
38. Kim MM, Dabaja BS, Medeiros J, Kim S, Allen P, Chevez-Barrios P, et al. Survival Outcomes of Primary Intraocular Lymphoma: A Single-Institution Experience. Am J Clin Oncol (2016) 39(2):109–13. doi: 10.1097/coc.0000000000000028
39. Keino H, Okada AA, Watanabe T, Echizen N, Inoue M, Takayama N, et al. Spectral-Domain Optical Coherence Tomography Patterns in Intraocular Lymphoma. Ocul Immunol Inflammation (2016) 24(3):268–73. doi: 10.3109/09273948.2014.1002568
40. Kase S, Namba K, Iwata D, Mizuuchi K, Kitaichi N, Tagawa Y, et al. Diagnostic Efficacy of Cell Block Method for Vitreoretinal Lymphoma. Diagn Pathol (2016) 11:29. doi: 10.1186/s13000-016-0479-1
41. Cheah CY, Milgrom S, Chihara D, Gombos DS, Pinnix CC, Dabaja BS, et al. Intensive Chemoimmunotherapy and Bilateral Globe Irradiation as Initial Therapy for Primary Intraocular Lymphoma. Neuro Oncol (2016) 18(4):575–81. doi: 10.1093/neuonc/nov253
42. Akiyama H, Takase H, Kubo F, Miki T, Yamamoto M, Tomita M, et al. High-Dose Methotrexate Following Intravitreal Methotrexate Administration in Preventing Central Nervous System Involvement of Primary Intraocular Lymphoma. Cancer Sci (2016) 107(10):1458–64. doi: 10.1111/cas.13012
43. Abu Samra K, Oray M, Ebrahimiadib N, Lee S, Anesi S, Foster CS. Intraocular Lymphoma: Descriptive Data of 26 Patients Including Clinico-Pathologic Features, Vitreous Findings, and Treatment Outcomes. Ocul Immunol Inflammation (2016) 26(3):347–52. doi: 10.1080/09273948.2016.1193206
44. Riemens A, Bromberg J, Touitou V, Sobolewska B, Missotten T, Baarsma S, et al. Treatment Strategies in Primary Vitreoretinal Lymphoma: A 17-Center European Collaborative Study. JAMA Ophthalmol (2015) 133(2):191–7. doi: 10.1001/jamaophthalmol.2014.4755
45. Kuiper J, Ten Dam-van Loon N, Domanian A, Schellekens P, Nierkens S, Radstake T, et al. Correlation Between Measurement of IL-10 and IL-6 in Paired Aqueous Humour and Vitreous Fluid in Primary Vitreoretinal Lymphoma. Acta Ophthalmol (2015) 93(8):e680–1. doi: 10.1111/aos.12733
46. Kitiratschky VB, Deuter C, Beck R, Schulte B, Müller H, Blumenstock G, et al. Relationship Between Suspected Reasons of Intraocular Inflammation and the Results of Diagnostic Vitrectomy: An Observational Study. Ocul Immunol Inflammation (2015) 23(1):59–66. doi: 10.3109/09273948.2013.870212
47. Egawa M, Mitamura Y, Sano H, Akaiwa K, Niki M, Semba K, et al. Changes of Choroidal Structure After Treatment for Primary Intraocular Lymphoma: Retrospective, Observational Case Series. BMC Ophthalmol (2015) 15:136. doi: 10.1186/s12886-015-0127-7
48. Wang L, Sato-Otsubo A, Sugita S, Takase H, Mochizuki M, Usui Y, et al. High-Resolution Genomic Copy Number Profiling of Primary Intraocular Lymphoma by Single Nucleotide Polymorphism Microarrays. Cancer Sci (2014) 105(5):592–9. doi: 10.1111/cas.12388
49. Tuo J, Shen D, Yang HH, Chan CC. Distinct microRNA-155 Expression in the Vitreous of Patients With Primary Vitreoretinal Lymphoma and Uveitis. Am J Ophthalmol (2014) 157(3):728–34. doi: 10.1016/j.ajo.2013.12.014
50. Teckie S, Yahalom J. Primary Intraocular Lymphoma: Treatment Outcomes With Ocular Radiation Therapy Alone. Leuk Lymphoma (2014) 55(4):795–801. doi: 10.3109/10428194.2013.819576
51. Rodriguez EF, Sepah YJ, Jang HS, Ibrahim M, Nguyen QD, Rodriguez FJ. Cytologic Features in Vitreous Preparations of Patients With Suspicion of Intraocular Lymphoma. Diagn Cytopathol (2014) 42(1):37–44. doi: 10.1002/dc.23059
52. Egawa M, Mitamura Y, Hayashi Y, Naito T. Spectral-Domain Optical Coherence Tomographic and Fundus Autofluorescence Findings in Eyes With Primary Intraocular Lymphoma. Clin Ophthalmol (2014) 8:335–41. doi: 10.2147/OPTH.S58114
53. Raja H, Snyder MR, Johnston PB, O'Neill BP, Caraballo JN, Balsanek JG, et al. Effect of Intravitreal Methotrexate and Rituximab on Interleukin-10 Levels in Aqueous Humor of Treated Eyes With Vitreoretinal Lymphoma. PloS One (2013) 8(6):e65627. doi: 10.1371/journal.pone.0065627
54. Missotten T, Tielemans D, Bromberg JE, van Hagen PM, van Lochem EG, van Dongen JJ, et al. Multicolor Flowcytometric Immunophenotyping Is a Valuable Tool for Detection of Intraocular Lymphoma. Ophthalmology (2013) 120(5):991–6. doi: 10.1016/j.ophtha.2012.11.007
55. Mikami R, Nakayama H, Goto H, Kimura K, Usui Y, Nogi S, et al. Preliminary Results of Radiotherapy for Primary Intraocular Non-Hodgkin Lymphoma. Leuk Lymphoma (2013) 54(10):2181–4. doi: 10.3109/10428194.2013.769216
56. Fisson S, Ouakrim H, Touitou V, Baudet S, Ben Abdelwahed R, Donnou S, et al. Cytokine Profile in Human Eyes: Contribution of a New Cytokine Combination for Differential Diagnosis Between Intraocular Lymphoma or Uveitis. PloS One (2013) 8(2):e52385. doi: 10.1371/journal.pone.0052385
57. Kinoshita Y, Takasu K, Adachi Y, Yuri T, Nagumo S, Shikata N. Retrospective Cytological Study of Intraocular Lymphoma Using Vitreous and Intraocular Perfusion Fluid. Diagn Cytopathol (2012) 40(7):604–7. doi: 10.1002/dc.21596
58. Wang Y, Shen D, Wang VM, Sen HN, Chan CC. Molecular Biomarkers for the Diagnosis of Primary Vitreoretinal Lymphoma. Int J Mol Sci (2011) 12(9):5684–97. doi: 10.3390/ijms12095684
59. Stefanovic A, Davis J, Murray T, Markoe A, Lossos IS. Treatment of Isolated Primary Intraocular Lymphoma With High-Dose Methotrexate-Based Chemotherapy and Binocular Radiation Therapy: A Single-Institution Experience. Br J Haematol (2010) 151(1):103–6. doi: 10.1111/j.1365-2141.2010.08321.x
60. Ishida T, Ohno-Matsui K, Kaneko Y, Tobita H, Shimada N, Takase H, et al. Fundus Autofluorescence Patterns in Eyes With Primary Intraocular Lymphoma. Retina (2010) 30(1):23–32. doi: 10.1097/IAE.0b013e3181b408a2
61. Sugita S, Takase H, Sugamoto Y, Arai A, Miura O, Mochizuki M. Diagnosis of Intraocular Lymphoma by Polymerase Chain Reaction Analysis and Cytokine Profiling of the Vitreous Fluid. Jpn J Ophthalmol (2009) 53(3):209–14. doi: 10.1007/s10384-009-0662-y
62. Ohta K, Sano K, Imai H, Kikuchi T. Cytokine and Molecular Analyses of Intraocular Lymphoma. Ocul Immunol Inflammation (2009) 17(3):142–7. doi: 10.1080/09273940802702553
63. Matsuo T, Ichimura K, Ichikawa T, Okumura Y, Kaji M, Yoshino T. Positron Emission Tomography/Computed Tomography After Immunocytochemical and Clonal Diagnosis of Intraocular Lymphoma With Vitrectomy Cell Blocks. J Clin Exp Hematop (2009) 49(2):77–87. doi: 10.3960/jslrt.49.77
64. Jahnke K, Thiel E, Bechrakis NE, Willerding G, Kraemer DF, Fischer L, et al. Ifosfamide or Trofosfamide in Patients With Intraocular Lymphoma. J Neurooncol (2009) 93(2):213–7. doi: 10.1007/s11060-008-9761-8
65. Fardeau C, Lee CP, Merle-Béral H, Cassoux N, Bodaghi B, Davi F, et al. Retinal Fluorescein, Indocyanine Green Angiography, and Optic Coherence Tomography in non-Hodgkin Primary Intraocular Lymphoma. Am J Ophthalmol (2009) 147(5):886–94, 94.e1. doi: 10.1016/j.ajo.2008.12.025
66. Wittenberg LA, Maberley DA, Ma PE, Wade NK, Gill H, White VA. Contribution of Vitreous Cytology to Final Clinical Diagnosis Fifteen-Year Review of Vitreous Cytology Specimens From One Institution. Ophthalmology (2008) 115(11):1944–50. doi: 10.1016/j.ophtha.2008.05.022
67. Intzedy L, Teoh SC, Hogan A, Mangwana S, Mayer EJ, Dick AD, et al. Cytopathological Analysis of Vitreous in Intraocular Lymphoma. Eye (Lond) (2008) 22(2):289–93. doi: 10.1038/sj.eye.6702965
68. Malumbres R, Davis J, Ruiz P, Lossos IS. Somatically Mutated Immunoglobulin IGHV@ Genes Without Intraclonal Heterogeneity Indicate a Postgerminal Centre Origin of Primary Intraocular Diffuse Large B-Cell Lymphomas. Br J Haematol (2007) 138(6):749–55. doi: 10.1111/j.1365-2141.2007.06744.x
69. Karma A, von Willebrand EO, Tommila PV, Paetau AE, Oskala PS, Immonen IJ. Primary Intraocular Lymphoma: Improving the Diagnostic Procedure. Ophthalmology (2007) 114(7):1372–7. doi: 10.1016/j.ophtha.2006.11.009
70. Grimm SA, Pulido JS, Jahnke K, Schiff D, Hall AJ, Shenkier TN, et al. Primary Intraocular Lymphoma: An International Primary Central Nervous System Lymphoma Collaborative Group Report. Ann Oncol (2007) 18(11):1851–5. doi: 10.1093/annonc/mdm340
71. Berenbom A, Davila RM, Lin HS, Harbour JW. Treatment Outcomes for Primary Intraocular Lymphoma: Implications for External Beam Radiotherapy. Eye (Lond) (2007) 21(9):1198–201. doi: 10.1038/sj.eye.6702437
72. Wallace DJ, Shen D, Reed GF, Miyanaga M, Mochizuki M, Sen HN, et al. Detection of the Bcl-2 T (14,18) Translocation and Proto-Oncogene Expression in Primary Intraocular Lymphoma. Invest Ophthalmol Vis Sci (2006) 47(7):2750–6. doi: 10.1167/iovs.05-1312
73. Isobe K, Ejima Y, Tokumaru S, Shikama N, Suzuki G, Takemoto M, et al. Treatment of Primary Intraocular Lymphoma With Radiation Therapy: A Multi-Institutional Survey in Japan. Leuk Lymphoma (2006) 47(9):1800–5. doi: 10.1080/10428190600632881
74. Jahnke K, Wagner T, Bechrakis NE, Willerding G, Coupland SE, Fischer L, et al. Pharmacokinetics and Efficacy of Ifosfamide or Trofosfamide in Patients With Intraocular Lymphoma. Ann Oncol (2005) 16(12):1974–8. doi: 10.1093/annonc/mdi409
75. Coupland SE, Loddenkemper C, Smith JR, Braziel RM, Charlotte F, Anagnostopoulos I, et al. Expression of Immunoglobulin Transcription Factors in Primary Intraocular Lymphoma and Primary Central Nervous System Lymphoma. Invest Ophthalmol Vis Sci (2005) 46(11):3957–64. doi: 10.1167/iovs.05-0318
76. Coupland SE, Hummel M, Müller HH, Stein H. Molecular Analysis of Immunoglobulin Genes in Primary Intraocular Lymphoma. Invest Ophthalmol Vis Sci (2005) 46(10):3507–14. doi: 10.1167/iovs.05-0401
77. Baehring JM, Androudi S, Longtine JJ, Betensky RA, Sklar J, Foster CS, et al. Analysis of Clonal Immunoglobulin Heavy Chain Rearrangements in Ocular Lymphoma. Cancer (2005) 104(3):591–7. doi: 10.1002/cncr.21191
78. Merle-Béral H, Davi F, Cassoux N, Baudet S, Colin C, Gourdet T, et al. Biological Diagnosis of Primary Intraocular Lymphoma. Br J Haematol (2004) 124(4):469–73. doi: 10.1046/j.1365-2141.2003.04800.x
79. Lobo A, Lightman S. Vitreous Aspiration Needle Tap in the Diagnosis of Intraocular Inflammation. Ophthalmology (2003) 110(3):595–9. doi: 10.1016/s0161-6420(02)01895-x
80. Gorochov G, Parizot C, Bodaghi B, Raphael D, Cassoux N, Merle-Beral H, et al. Characterization of Vitreous B-Cell Infiltrates in Patients With Primary Ocular Lymphoma, Using CDR3 Size Polymorphism Analysis of Antibody Transcripts. Invest Ophthalmol Vis Sci (2003) 44(12):5235–41. doi: 10.1167/iovs.03-0035
81. Küker W, Herrlinger U, Grönewäller E, Rohrbach JM, Weller M. Ocular Manifestation of Primary Nervous System Lymphoma: What can be Expected FromImaging? JNeurol (2002) 249(12):1713–6. doi: 10.1007/s00415-002-0919-6
82. Shen DF, Herbort CP, Tuaillon N, Buggage RR, Egwuagu CE, Chan CC. Detection of Toxoplasma Gondii DNA in Primary Intraocular B-Cell Lymphoma. Mod Pathol (2001) 14(10):995–9. doi: 10.1038/modpathol.3880424
83. Chatzistefanou K, Markomichelakis NN, Christen W, Soheilian M, Foster CS. Characteristics of Uveitis Presenting for the First Time in the Elderly. Ophthalmology (1998) 105(2):347–52. doi: 10.1016/s0161-6420(98)93523-0
84. Soussain C, Merle-Béral H, Reux I, Sutton L, Fardeau C, Gerber S, et al. A Single-Center Study of 11 Patients With Intraocular Lymphoma Treated With Conventional Chemotherapy Followed by High-Dose Chemotherapy and Autologous Bone Marrow Transplantation in 5 Cases. Leuk> Lymphoma (1996) 23(3-4):339–45. doi: 10.3109/10428199609054837
85. Chan CC, Whitcup SM, Solomon D, Nussenblatt RB. Interleukin-10 in the Vitreous of Patients With Primary Intraocular Lymphoma. Am J Ophthalmol (1995) 120(5):671–3. doi: 10.1016/s0002-9394(14)72217-2
86. Davis JL, Solomon D, Nussenblatt RB, Palestine AG, Chan CC. Immunocytochemical Staining of Vitreous Cells. Indications, Techniques, and Results. Ophthalmol (1992) 99(2):250–6. doi: 10.1016/s0161-6420(92)31984-0
87. Strauchen JA, Dalton J, Friedman AH. Chemotherapy in the Management of Intraocular Lymphoma. Cancer (1989) 63(10):1918–21. doi: 10.1002/1097-0142(19890515)63:10<1918::aid-cncr2820631008>3.0.co;2-d
88. Siegel MJ, Dalton J, Friedman AH, Strauchen J, Watson C. Ten-Year Experience With Primary Ocular 'Reticulum Cell Sarcoma' (Large Cell non- Hodgkin's Lymphoma). Br J Ophthalmol (1989) 73(5):342–6. doi: 10.1136/bjo.73.5.342
89. Klingele TG, Hogan MJ. Ocular Reticulum Cell Sarcoma. Am J Ophthalmol (1975) 79(1):39–47. doi: 10.1016/0002-9394(75)90453-5
Keywords: clinical features, diagnosis, meta-analysis, primary intraocular lymphoma, treatment
Citation: Zhao X-y, Cheng T-t, Meng L-h, Zhang W-f and Chen Y-x (2022) Clinical Features, Diagnosis, Management and Prognosis of Primary Intraocular Lymphoma. Front. Oncol. 12:808511. doi: 10.3389/fonc.2022.808511
Received: 04 November 2021; Accepted: 11 January 2022;
Published: 03 February 2022.
Edited by:
David Walker, University of Nottingham, United KingdomReviewed by:
Lyndon Kim, Mount Sinai Hospital, United StatesYi Miao, Nanjing Medical University, China
Copyright © 2022 Zhao, Cheng, Meng, Zhang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: You-xin Chen, 478252553@qq.com; chenyx@pumch.cn
†These authors have contributed equally to this work
 Xin-yu Zhao1,2†
Xin-yu Zhao1,2†