- 1Department of Hepatobiliary Surgery, Southwest Hospital, Third Military Medical University (Army Medical University), Chongqing, China
- 2Department of Hepatobiliary Surgery, Sichuan Provincial People’s Hospital, Chengdu, China
- 3Department of Hepatobiliary Surgery, Affiliated Hospital of Qinghai University, Xining, China
- 4Clinical Research Center of Oncology, Lishui Hospital of Zhejiang University, Lishui, China
- 5Department of General Surgery, 903rd Hospital of People’s Liberation Army, Hangzhou, China
- 6Department of Health Statistics, College of Military Preventive Medicine, Third Military Medical University (Army Medical University), Chongqing, China
Background & Aims: Tumor-associated chronic inflammation has been determined to play a crucial role in tumor progression, angiogenesis and immunosuppression. The objective of this study was to assess the prognostic value of the neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) in perihilar cholangiocarcinoma (pCCA) patients following curative resection.
Methods: Consecutive pCCA patients following curative resection at 3 Chinese hospitals between 2014 and 2018 were included. The NLR was defined as the ratio of neutrophil count to lymphocyte count. PLR was defined as the ratio of platelet count to lymphocyte count. The optimal cutoff values of preoperative NLR and PLR were determined according to receiver operating characteristic (ROC) curves for the prediction of 1-year overall survival (OS), and all patients were divided into high- and low-risk groups. Kaplan-Meier curves and Cox regression models were used to investigate the relationship between values of NLR and PLR and values of OS and recurrence-free survival (RFS) in pCCA patients. The usefulness of NLR and PLR in predicting OS and RFS was evaluated by time-dependent ROC curves.
Results: A total of 333 patients were included. According to the ROC curve for the prediction of 1-year OS, the optimal cutoff values of preoperative NLR and PLR were 1.68 and 113.1, respectively, and all patients were divided into high- and low-risk groups. The 5-year survival rates in the low-NLR (<1.68) and low-PLR groups (<113.1) were 30.1% and 29.4%, respectively, which were significantly higher than the rates of 14.9% and 3.3% in the high-NLR group (≥1.68) and high-PLR group (≥113.1), respectively. In multivariate analysis, high NLR and high PLR were independently associated with poor OS and RFS for pCCA patients. The time-dependent ROC curve revealed that both NLR and PLR were ideally useful in predicting OS and RFS for pCCA patients.
Conclusions: This study found that both NLR and PLR could be used to effectively predict long-term survival in patients with pCCA who underwent curative resection.
Introduction
Perihilar cholangiocarcinoma (pCCA) is a rare tumor that accounts for 50-70% of all biliary tract tumors and tends to occur at the site of biliary fusion or in the right or left liver duct (1, 2). Curative resection was the only treatment for achieving a potential cure, but long-term survival was poor (5-year survival ranged from 25% to 40%) (1, 3, 4). Accurate prediction of prognosis can better help surgeons develop personalized treatment strategies. However, the current prediction methods only include tumor markers and pathological tests. Assessment methods with more dimensions may enable more accurate prediction of patient prognosis.
Studies have shown that the tumor microenvironment and tumor-associated chronic inflammation play a crucial role in tumor progression (5, 6). During the development of pCCA, changes in inflammation levels further lead to immunosuppression and metabolic reprogramming and ultimately promote tumor progression. The condition of these patients could be reflected by complete blood count (CBC) markers (7), such as neutrophils, platelets, and lymphocytes. Specifically, neutrophils can directly promote tumor progression, metastasis and angiogenesis by releasing some enzymes (8–10). Platelets protect circulating tumor cells by encapsulating them in blood clots, protecting them from being lysed by natural killer cells or releasing thrombin to promote tumor proliferation and growth (11–13). Lymphocytes realize the tumor immune response through the recognition, killing and clearance of tumor cells, thereby playing a role in immunosuppression and antitumor immunity (14). Many studies have now confirmed that the inflammatory markers neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) (15–17) are associated with the prognosis of many cancers, such as hepatocellular carcinoma (HCC), intrahepatic cholangiocarcinoma, gallbladder carcinoma, and pancreatic cancer (18–22). However, the relationship between values of NLR and PLR and pCCA prognosis has not been studied.
The objective of this study was to assess the prognostic value of NLR and PLR in pCCA patients following curative resection. This was the first study conducted with data from a multicenter database on the long-term prognosis of NLR and PLR in pCCA patients undergoing curative resection.
Methods
Patient selection
Patients diagnosed with pCCA following curative resection between 2014 and 2018 at three hospitals in China (Southwest Hospital, Sichuan Provincial People’s Hospital, and Affiliated Hospital of Qinghai University) were included. The diagnosis of pCCA was confirmed by postoperative pathology. Patients who died within 30 days after surgery, those who had other autoimmune diseases, those who had inflammatory disease, or those whose data were missing important variables were excluded. Curative resection for this purpose included partial hepatic resection, cholangiotomy, biliary anastomosis, and lymph node dissection. If the tumor invaded the hepatic vein or hepatic artery, lateral vascular reconstruction was performed. Curative resection was defined as the resection of tissue with margins that were clear under the microscope without visible tumor cells. The study was approved by the Institutional Review Board of the Southwest Hospital of Chongqing, China (No. KY2021129). Because the study was retrospective and all data were anonymized, informed consent was not needed.
Clinicopathological variables
The demographic variables included age, sex, American Society of Anesthesiologists (ASA) grade, preoperative jaundice, cirrhosis, chronic hepatitis, and hepatolithiasis. The laboratory variables included alanine aminotransferase (ALT), aspartate transaminase (AST), carbohydrate antigen 19-9 (CA19-9), total bilirubin (TB) and preoperative neutrophils, platelets and lymphocytes. The pathological variables included cirrhosis, maximum tumor size, nerve invasion, the 8th American Joint Committee on Cancer (AJCC) stage (23), the Bismuth classification (24), tumor differentiation, macro- or microvascular invasion, lymph node metastasis, and peripheral nerve invasion. Both portal vein invasion and hepatic artery invasion were considered macrovascular invasion. The operative variables included the extent of hepatectomy (major vs. minor), intraoperative blood loss, and perioperative blood transfusion.
For laboratory parameters, patients were divided into normal and abnormal groups using the upper or lower limit of the normal values used in clinical practice, such as 35 g/L for albumin, 40 U/L for AST, 40 U/L for ALT, 1 mg/dL TB, and 1.15 for INR, as reported in a previous study (25–28). Cirrhosis was confirmed by histopathological examination. Major hepatectomy was defined as the resection of three or more segments of the Couinaud liver, and minor hepatectomy was defined as the resection of fewer than three segments (29). Preoperative jaundice was defined as a preoperative total bilirubin higher than 34 μmol/L.
Patient follow-up
Patients were followed-up after curative resection regularly. The postoperative surveillance strategy involved physical examination, abdominal ultrasonography and laboratory control every 2 months in the first and second years after resection, then once every three months from the third to the fifth year and finally once every six months. At each visit, tumor markers such as carcinoembryonic antigen (CEA) and CA19-9 were included, and computed tomography and/or magnetic resonance cholangiopancreatography examinations were also performed. Overall survival (OS) was computed as the interval between the date of surgery and the date of death or the last follow-up. The recurrence-free survival (RFS) was computed as interval from the day of resection to the day of diagnosis of tumor recurrence for recurrent patients or from the day of resection to the day of death or date of last follow-up for patients without recurrence.
Statistical analysis
Continuous variables conforming to a normal distribution were expressed as the mean ± standard deviation and analyzed using t tests; those conforming to nonnormal distributions were expressed as the median (quartile) and tested with the Mann–Whitney U test. Categorical variables were expressed as numbers and percentages and compared between groups using the χ2 test or Fisher’s exact test. According to the ROC curve for the prediction of 1-year OS, the optimal cutoff values of preoperative NLR and PLR were calculated, and all patients were divided into high- and low-risk groups. RFS and OS were evaluated using the Kaplan–Meier method, and the differences between the two groups were examined by the log-rank test. Those variables with significance at P < 0.1 confirmed as noncollinearity by a variance inflation factor < 3 were entered into multivariable Cox proportional hazard models after univariable analyses, and 95% CI and hazard ratio values were calculated. The ability of NLR and PLR to predict OS and RFS was evaluated by time-dependent ROC curves. All data analyses were performed using SPSS software version 26.0 (IBM Corp., Armonk, NY, USA) and R software (version 3.5.1. http://www.r-project.org/). All P values reported were two-sided, and a P value < 0.05 was considered statistically significant.
Results
Clinicopathologic and operative variables of patients
Among the 404 patients who underwent curative resection for pCCA between January 2014 and January 2018, we excluded 24 patients who had recurrent pCCA, 18 patients for whom information was missing, 26 patients with incomplete treatment, and 4 patients who had other autoimmune diseases as shown in Figure 1. Thus, 333 pCCA patients were included in the final analytic cohort (213 male and 120 female patients), and the mean age was 57.03 ± 9.94 years.
ROC curves and cutoff values and groupings of NLR and PLR
According to the ROC curve, as shown in Figure 2, the optimal cutoff values of preoperative NLR and PLR for predicting 1-year OS were calculated to be 1.68 and 113.1, respectively. The ROC areas under the curve for NLR and PLR were 0.729 (95% CI: 0.663-0.795) and 0.786 (95% CI: 0.724-0.849), respectively. And according to their cutoff values, NLR < 1.68 was defined as low NLR (n = 155, 46.5%), NLR ≥ 1.68 was defined as high NLR (n =178, 53.5%), PLR < 113.1 (n = 231, 69.4%) was defined as low PLR, and PLR ≥ 113.1 was defined as high PLR (n = 102, 30.6%). The comparisons of patients’ clinicopathologic and operative variables between those with high and low NLR and PLR are shown in Table 1. High CA 19-9 level, poor differentiation and microvascular invasion were more commonly seen in high NLR patients (P < 0.05). Cirrhosis and lymph node metastasis were more commonly seen in high PLR patients (P < 0.05).
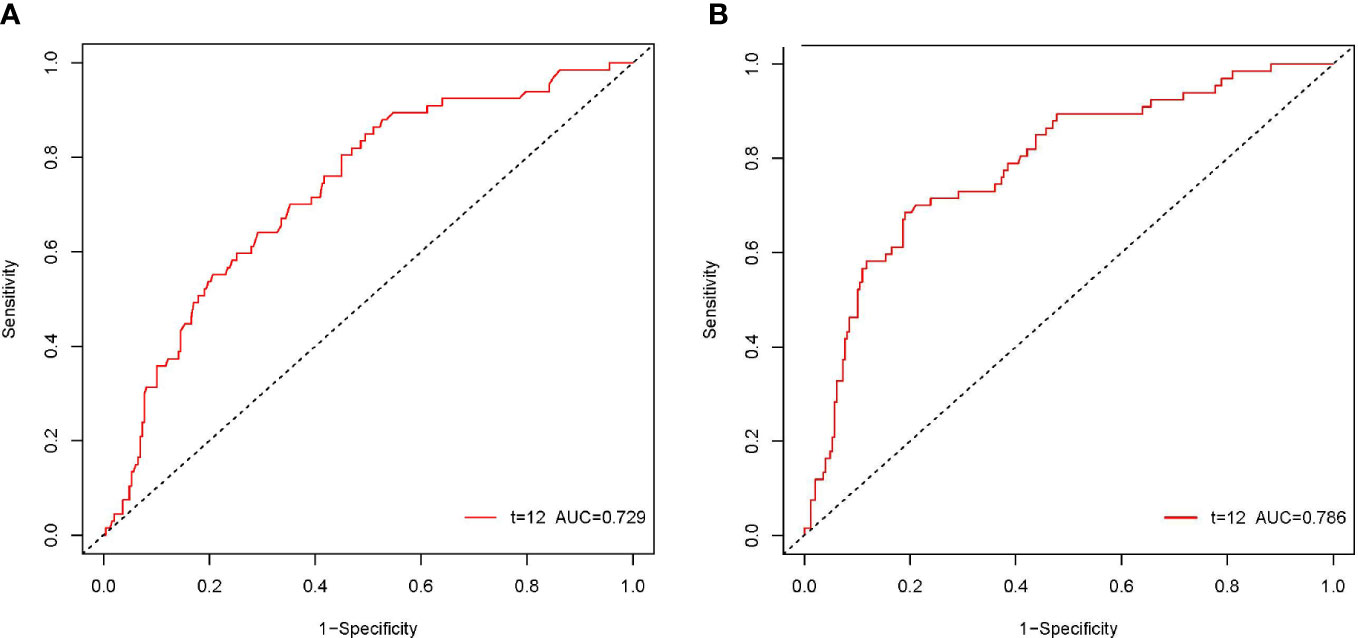
Figure 2 The ROC curves of the NLR and PLR in patients with pCCA. The ROC area of NLR was 0.729 (A). The ROC area of PLR was 0.786 (B).
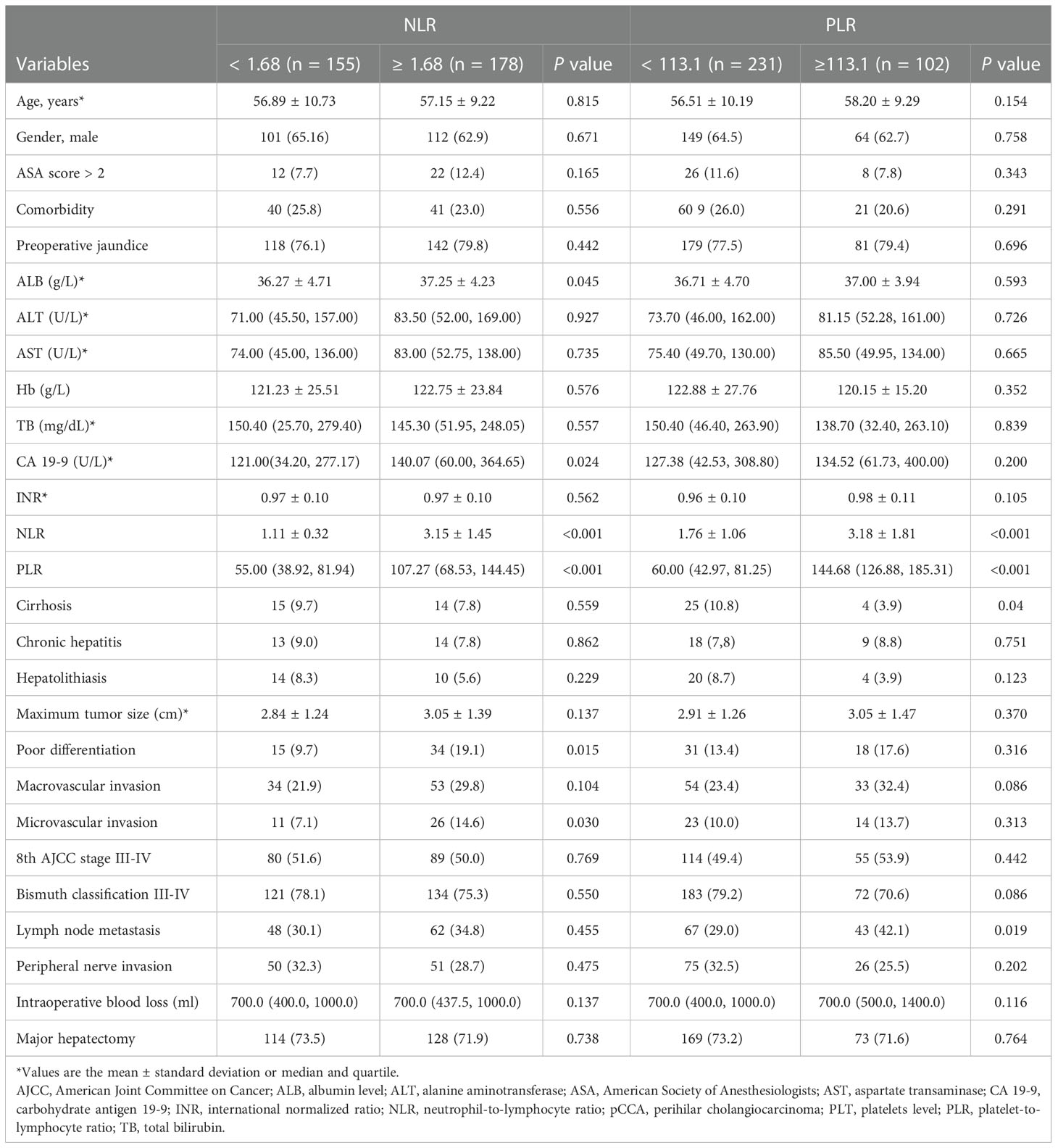
Table 1 Baseline for pCCA patients categorized by NLR, PLR and their clinical pathological characteristics.
Survival outcome
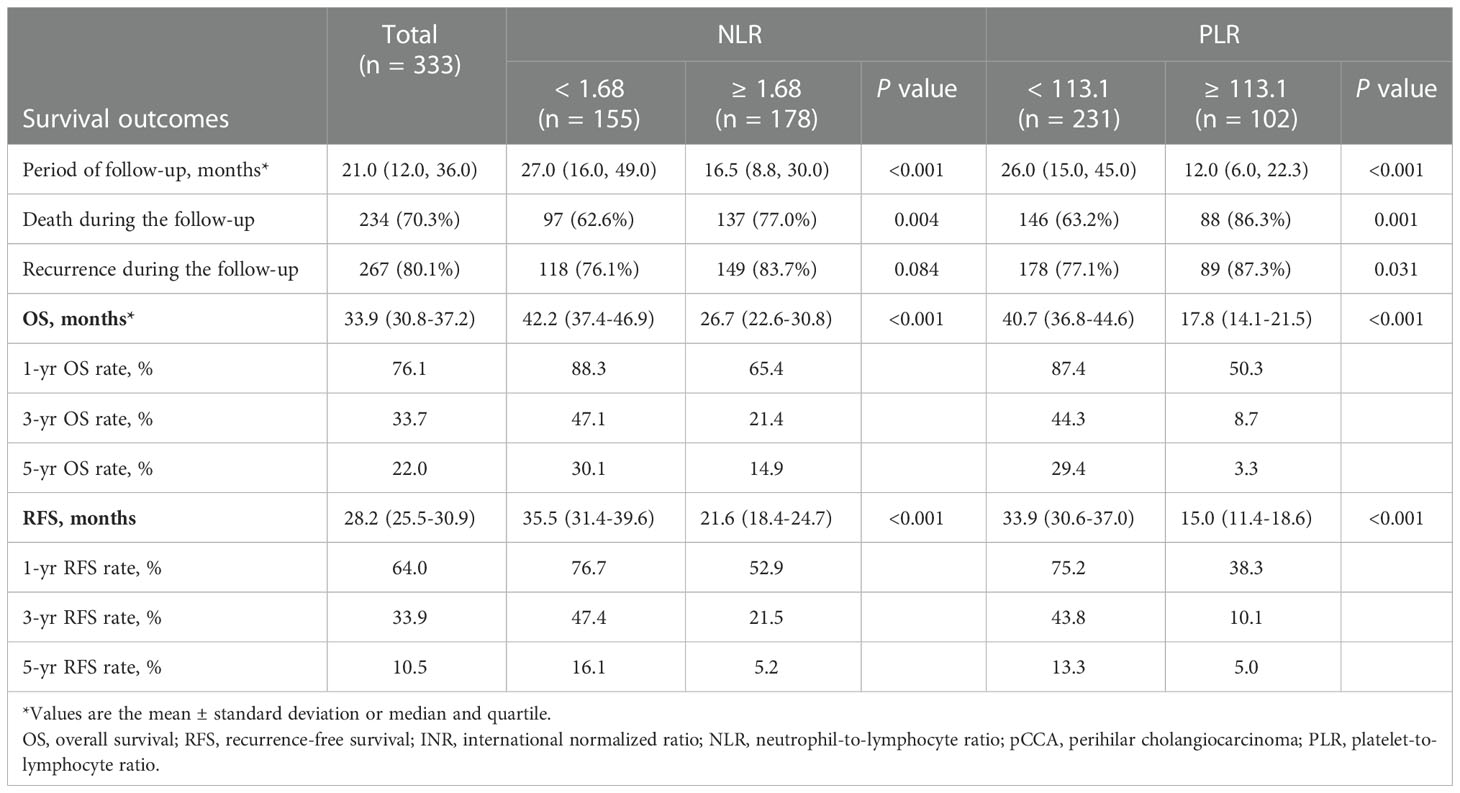
The median period of follow-up times, 5-year OS rates and 5-year RFS rates for all pCCA patients were 21.0 (12.0, 36.0) months, 22.0% and 10.5%, respectively. Regarding NLR, 5-year OS rates and 5-year RFS rates occurred for 14.9% and 5.2% in high NLR patients, respectively, and for 30.1% and 16.1% in low NLR patients, respectively. The rates of death and recurrence in high NLR patients were significantly lower in low NLR patients, as shown in Table 2 (death, P = 0.004; recurrence, P = 0.084). Regarding PLR, 5-year OS rates and 5-year RFS rates occurred for 3.3% and 5.0% in high PLR patients, respectively, and for 29.4% and 13.3% in low PLR patients, respectively. The rates of death and recurrence in high PLR patients were significantly lower in low PLR patients, as shown in Table 2 (death, P = 0.001; recurrence, P = 0.031). The survival and recurrence curves of high/low NLR patients and high/low PLR patients are shown in Figure 3.
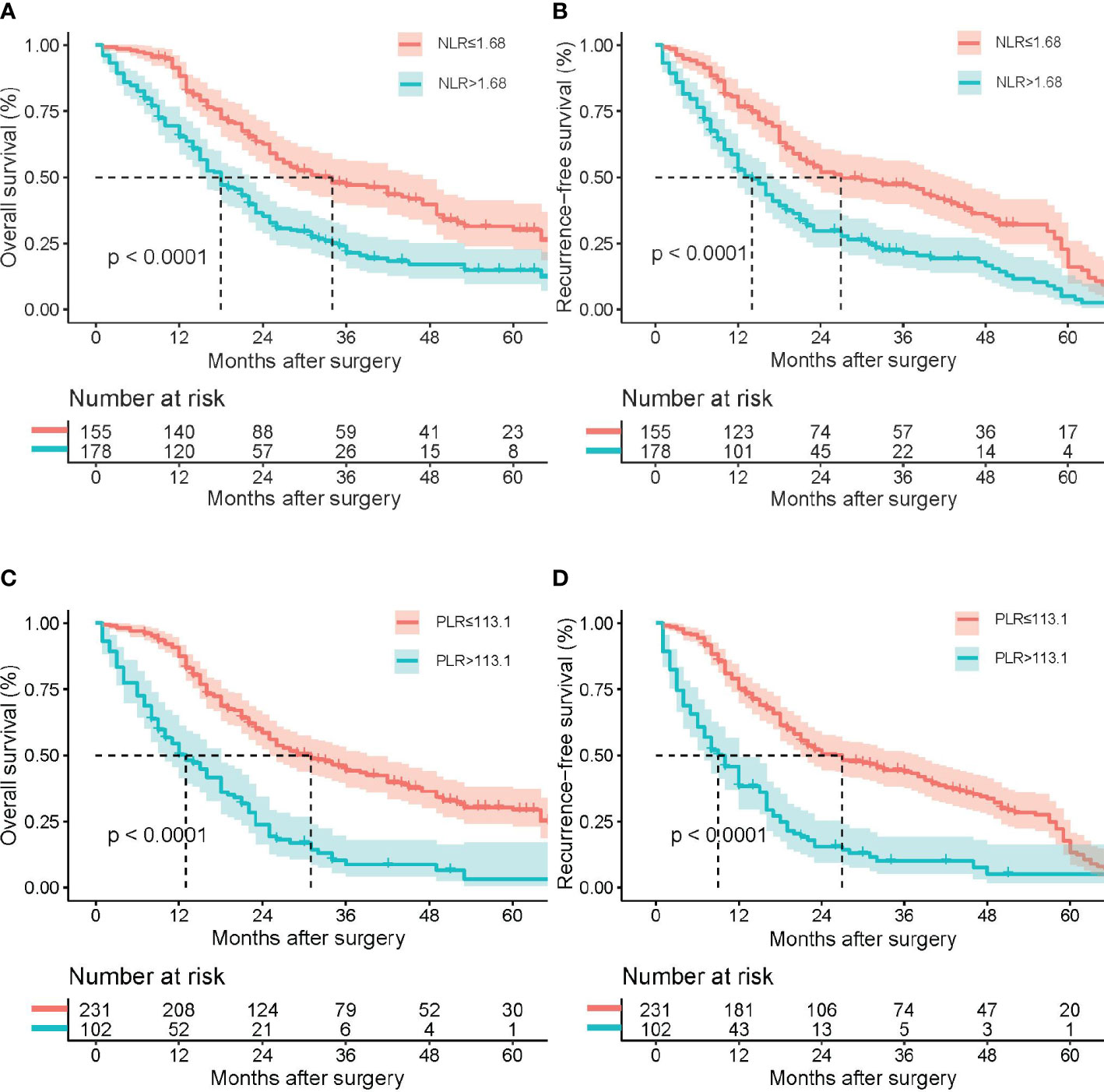
Figure 3 Kaplan–Meier survival analysis indicated that patients with NLR ≥ 1.68 had a shorter RFS and OS; patients with PLR ≥ 113.1 had a shorter RFS and OS. Overall survival (A) and recurrence-free survival (B) curve comparisons between patients with NLR ≥ 1.68 and NLR < 1.68; overall survival (C) and recurrence-free survival (D) curve comparisons between patients with PLR ≥ 113.1 and PLR < 113.1.
NLR and PLR as prognostic markers
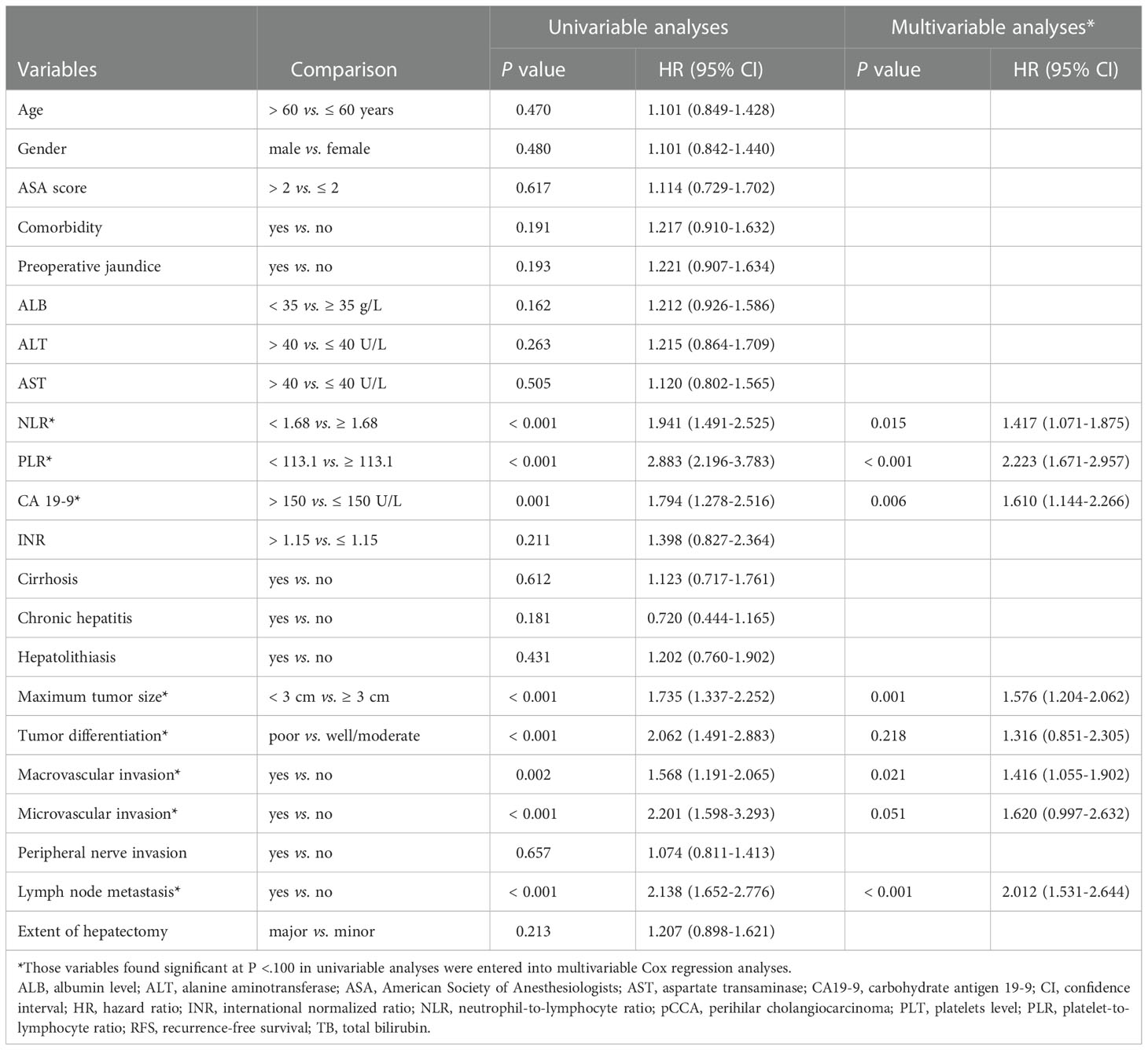
On multivariable Cox regression analyses, six variables were independently associated with OS in pCCA patients as shown in Table 3, including NLR < 1.68 vs. ≥ 1.68) (HR: 1.417, 95% CI: 1.071-1.875, P=); PLR (< 113.1 vs. ≥ 113.1) (HR: 2.223, 95% CI: 1.671-2.957); CA 19-9 (> 150 vs. ≤ 150 U/L) (HR: 1.610, 95% CI: 1.144-2.266); maximum tumor size (< 3 cm vs. ≥ 3 cm) (HR: 1.576, 95% CI: 1.204-2.062); macrovascular invasion (Yes vs. No) (HR: 1.416, 95% CI: 1.055-1.902); and lymph node metastasis (Yes vs. No) (HR: 2.012, 95% CI: 1.531-2.644). There were five independent variables associated with RFS in pCCA patients as shown in Table 4, including NLR (< 1.68 vs. ≥ 1.68) (HR: 1.598, 95% CI: 1.224-2.088); PLR (< 113.1 vs. ≥ 113.1) (HR: 2.138, 95% CI: 1.613-2.833), maximum tumor size (< 3 cm vs. ≥ 3 cm) (HR: 1.398, 95% CI: 1.080-1.812); macrovascular invasion (yes vs. no) (HR: 1.367, 95% CI: 1.030-1.815); and lymph node metastasis (yes vs. no) (HR: 1.638, 95% CI: 1.652-2.776). Moreover, NLR and PLR were found to be useful in effectively predicting OS and RFS through the result of the time-dependent ROC analysis, as shown in Figure 4.
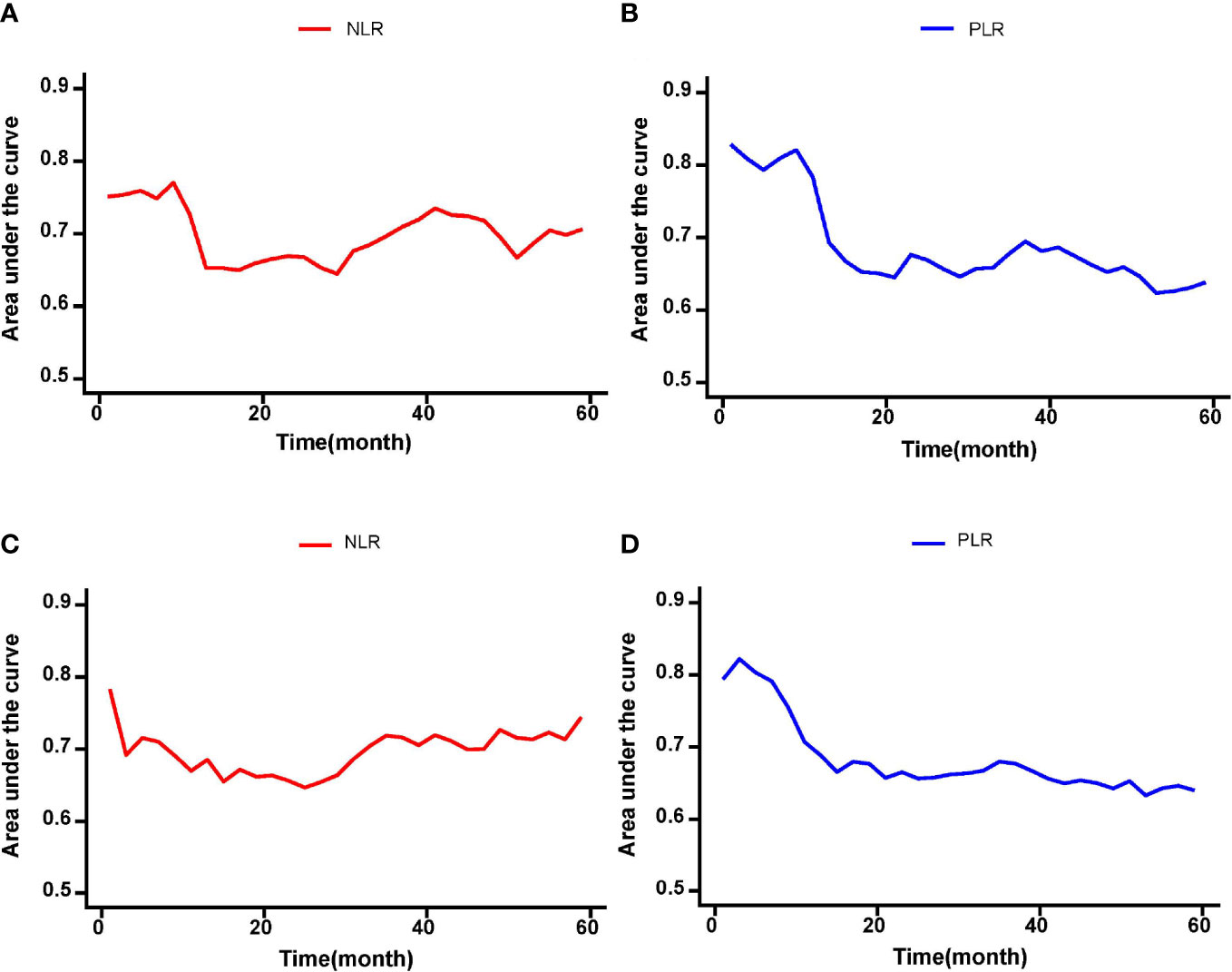
Figure 4 The time-dependent ROC curves of the NLR and PLR for OS and RFS in pCCA patients. (A-D) NLR and PLR were useful in effectively predicting long-term outcomes such as OS and RFS according to the results of time-dependent ROC analysis.
Discussion
Cholangiocarcinoma represent a class of malignant tumors that originate in epithelial cells, of which hilar cholangiocarcinoma is the most common type, occurring at the site of biliary fusion or in the right or left liver duct (30) and accounting for approximately 50% of all cases. Resection is the only treatment with a potential of curing it. However, even after curative resection, the 5-year survival rate of patients is only 20% to 40% (1–3). Therefore, it is of great significance to actively identify prognostic factors that affect the long-term prognosis of pCCA. Many studies have demonstrated that chronic inflammation is associated with the occurrence and progression of tumors (5, 31). Studies have shown that cancer originates from chronic inflammatory sites, and there are a large number of inflammatory cells in tumor biopsies. Chronic inflammation provides a preferred microenvironment for the occurrence, progression and metastasis of tumors (5). During chronic inflammation, inflammatory cells and cytokines may act as tumor promoters, promoting cell survival, proliferation, invasion, and angiogenesis (5). Specific markers in the CBC panel of tests can be used as an accurate reflection of patients’ inflammatory levels, via generation of parameters such as NLR and PLR, and, thus, potentially assist clinicians in better predicting long-term prognosis in pCCA patients.
In previous studies, NLR and PLR have been shown to be important markers of long-term prognosis in patients with other digestive system tumors (18, 22). Hsiang et al. analyzed the long-term prognosis of 239 patients with hepatocellular carcinoma who underwent curative resection and found that the median OS of patients with NLR < 2.4 was significantly better than the median OS of patients with NLR ≥ 2.4 (median OS: 28.5 vs. 6.0 mo., P < 0.001). Sha et al. conducted a long-term prognostic analysis of 285 patients with gallbladder cancer who underwent cholecystectomy and found that the median OS of patients with NLR < 3.13 was significantly better than that of patients with NLR ≥ 3.13 (median OS: 13.0 vs. 8.27 mo., P < 0.001); the median OS of patients with PLR < 143.77 was significantly better than that of patients with PLR ≥ 143.77 (median OS: 10.80 vs. 10.27 mo., P > 0.05). However, the value of NLR and PLR in assessing long-term prognosis after curative resection of pCCA has not been demonstrated. To our knowledge, this is the first multicenter study to evaluate the usefulness of inflammatory markers NLR and PLR as indicators of OS and RFS after curative resection of pCCA.
In this multicenter study, a total of 333 patients underwent curative resection of pCCA. Moreover, the cutoff values obtained by NLR and PLR for predicting 1-year OS were used to group all patients, namely, the NLR cutoff values was 1.68, and the PLR cutoff values was 113.1. There were 178 patients (53.5%) in the high NLR group, 155 patients (46.5%) in the low NLR group, 102 patients (30.6%) in the high PLR group and 231 patients (69.4%) in the low PLR group. In the univariate analysis, the median OS and RFS of patients with a low NLR were significantly better than those with a high NLR (42.2 vs. 26.7 mo., P < 0.001; 35.5 vs. 21.6 mo., P < 0.001), and the median OS and RFS of patients with a low PLR were significantly better than those with a high PLR (40.7 vs. 17.8 mo., P < 0.001; 33.9 vs. 15.0 mo., P < 0.001). In multivariate Cox regression analysis, NLR and PLR were confirmed as independent risk factors for predicting OS and RFS in patients after curative resection of pCCA.
The mechanism of these phenomena has been confirmed and explained in previous studies (8, 9, 12, 13, 32–34). Neutrophils are the first responders to cell damage, and neutrophil infiltration marks persistent inflammation, which not only causes tissue damage but also, more importantly, promotes tumor progression, metastasis, and angiogenesis. In addition, reactive oxygen species and reactive nitrogen species produced by neutrophils can produce proto-oncogenes, leading to oxidative DNA damage and increasing genetic instability (8). It cannot be ignored that the enzymes produced and released by neutrophils, such as myeloperoxidase, neutrophil elastase, and matrix metalloproteinases, can also promote tumor progression (8). Neutrophils typically produce NETs (neutrophil-extracellular traps) during inflammation. NETs can capture circulating cancer cells (CTCs), and when they are released into the tumor microenvironment, they stimulate tumor cell migration and invasion (8, 9). Platelets also play an important role in promoting tumorigenesis and development. Platelets have been confirmed to promote tumor angiogenesis by releasing p-selectin and vascular endothelial growth factor, and they provide a suitable tumor microenvironment for tumor cell metastasis (12, 13). Platelets can also protect CTCs from being lysed by natural killer cells by encasing tumor cells in thrombi (32). However, as a type of immune cell, lymphocytes can play an important antitumor role. When the number of lymphocytes decreases, the body’s resistance to tumor cells decreases accordingly. Previous studies have reported that tumor-infiltrating lymphocytes play a positive role in resisting various advanced malignant tumors (33, 34).
In recent studies, there have been significant differences between the high NLR group and the low NLR group and between the high PLR group and the low PLR group. Before comparative analysis between the two groups, it may not be appropriate to use propensity score matching to examine the relationship between NLR, PLR and long-term oncology results to balance the baseline characteristics because this may lead to an increase in selection biases between the two groups. Therefore, univariate and multivariate Cox regression analyses were used in this study to determine whether high NLR and PLR were independently related to worse OS and RFS after curative resection of pCCA and to adjust for other prognostic risk factors.
With the advent of the era of immunotherapy, immune checkpoint inhibitors have achieved great success in the treatment of almost all solid tumors, bringing hope for tumor patients. Similarly, in related studies on biliary tumors, some clinical drug trials have achieved exciting progress (clinical trial information: NCT03875235 and NCT03875235) (35). In recent years, a PD-L1 receptor inhibitor (EnvafoLimab) has been approved by the FDA for the treatment of biliary tumors. Since both NLR and PLR can reflect immune function, clarifying the relationship between these two markers and prognosis will provide strong support for further exploring the formulation of personalized immunotherapy programs in patients with hilar cholangiocarcinoma.
This study has several limitations. First, it is a retrospective study, which will inevitably lead to bias in data collection. Second, the patients’ NLR and PLR values were calculated by a single measurement at admission, imposing some uncertainty. Third, the patients in this study were all from China, so data from Western patients was lacking. Therefore, the applicability of this conclusion to Western patients needs to be verified. Fourth, the number of patients included in this study was small; although it was a multicenter study, there were only 333 patients, possibly related to the relatively low incidence of pCCA. In the future, we will work with individual centers to provide a higher level of evidence.
In conclusion, our study suggests that NLR and PLR are potential prognostic factors for long-term prognosis in pCCA patients undergoing curative resection. After curative resection, these ratios are strongly correlated with survival, readily available, and economically determined. This study has strong clinical implications: higher NLR and PLR indicate a poor prognosis, so more attention should be given to patients with such values of NLR and PLR. It may be possible to minimize the levels of these two markers to improve the prognosis of patients, but further confirmation is needed. Once these findings have been validated in a larger prospective cohort, NLR and PLR markers could be used to help guide the clinical management of patients with pCCA.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Southwest Hospital of Chongqing, China (No. KY2021129). The patients/participants provided their written informed consent to participate in this study.
Author contributions
Conception—Z-YC, YJ, YG. Study design—M-YG, Z-PL, H-SD. Administrative support—Z-YC, YG. Data collection and acquisition—S-YG, S-YZ, X-YC, J-HZ, Y-HL, X-CL, H-NF, W-YC, Z-RW, X-YY, JB. Data analysis—M-YG, Z-PL, Y-QZ. Manuscript preparation—M-YG, Z-PL, YP, J-YW, XW. Critical revision—Z-YC, YJ, YG. Final approval of manuscript—All authors. M-YG, Z-PL, YP, J-YW, and XW contributed equally to this work. All authors contributed to the article and approved the submitted version.
Funding
This work was supported in part by the National Natural Science Foundation of China (No. 81874211) and Chongqing Technology Innovation and Application Development Special Key Project (No. CSTC2021jscx-gksb-N0009).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AJCC, American Joint Committee on Cancer; ALB, albumin; ALT, alanine aminotransferase; ASA, American Society of Anesthesiologists; AST, aspartate transaminase; CA 19-9, carbohydrate antigen 19-9; CBC, complete blood count; CEA, carcinoembryonic antigen; CI, confidence interval; CTC, circulating cancer cells; HR, hazard ratio; INR, international normalized ratio; NLR, neutrophil-to-lymphocyte ratio; pCCA, perihilar cholangiocarcinoma; OR, odds ratio; OS, overall survival; PLR, platelet-to-lymphocyte ratio; RFS, recurrence free survival; ROC, receiver operating characteristic; TB, total bilirubin.
References
1. Ito F, Cho CS, Rikkers LF, Weber SM. Hilar cholangiocarcinoma: Current management. Ann Surg (2009) 250(2):210–8. doi: 10.1097/SLA.0b013e3181afe0ab
2. Soares KC, Jarnagin WR. The landmark series: Hilar cholangiocarcinoma. Ann Surg Oncol (2021) 28(8):4158–70. doi: 10.1245/s10434-021-09871-6
3. Soares KC, Kamel I, Cosgrove DP, Herman JM, Pawlik TM. Hilar cholangiocarcinoma: Diagnosis, treatment options, and management. Hepatobiliary Surg Nutr (2014) 3(1):18–34. doi: 10.3978/j.issn.2304-3881.2014.02.05
4. Liu ZP, Zhang QY, Chen WY, Huang YY, Zhang YQ, Gong Y, et al. Evaluation of four lymph node classifications for the prediction of survival in hilar cholangiocarcinoma. J Gastrointest Surg (2022) 26:1030–40. doi: 10.1007/S11605-021-05211-X
5. Zhao H, Wu L, Yan G, Chen Y, Zhou M, Wu Y, Li Y, et al. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transd Target Ther (2021) 6(1):263. doi: 10.1038/s41392-021-00658-5
6. Zhou J, Valentini E, Boutros M. Microenvironmental innate immune signaling and cell mechanical responses promote tumor growth. Dev Cell (2021) 56(13):1884–1899.E5. doi: 10.1016/j.devcel.2021.06.007
7. Rojko L, Megyesfalvi Z, Czibula E, Reiniger L, Teglasi V, Szegedi Z, et al. Longitudinal analysis of complete blood count parameters in advanced-stage lung cancer patients. Thorac Cancer (2020) 11:3193–204. doi: 10.1111/1759-7714.13642
8. Giese MA, Hind LE, Huttenlocher A. Neutrophil plasticity in the tumor microenvironment. Blood (2019) 133(20):2159–67. doi: 10.1182/blood-2018-11-844548
9. Masucci MT, Minopoli M, Del Vecchio S, Carriero MV. The emerging role of neutrophil extracellular traps (Nets) in tumor progression and metastasis. Front Immunol (2020) 11:1749. doi: 10.3389/fimmu.2020.01749
10. Hedrick CC, Malanchi I. Neutrophils in cancer: Heterogeneous and multifaceted. Nat Rev Immunol (2022) 22(3):173–87. doi: 10.1038/s41577-021-00571-6
11. Menter DG, Tucker SC, Kopetz S, Sood AK, Crissman JD, Honn KV. Platelets and cancer: A casual or causal relationship: Revisited. Cancer Metastasis Rev (2014) 33(1):231–69. doi: 10.1007/s10555-014-9498-0
12. Schlesinger M. Role of platelets and platelet receptors in cancer metastasis. J Hematol Oncol (2018) 11(1):125. doi: 10.1186/s13045-018-0669-2
13. Haemmerle M, Stone RL, Menter DG, Afshar-Kharghan V, Sood AK. The platelet lifeline to cancer: Challenges and opportunities. Cancer Cell (2018) 33(6):965–83. doi: 10.1016/j.ccell.2018.03.002
14. Meyer CE, Key PN, Zhu T, Shabsovich M, Ni A, Tripathy SK. Expression of the inhibitory receptor Nkg2a correlates with increased liver and splenic nk cell response to activating receptor engagement. Immun Inflammation Dis (2017) 5(2):177–89. doi: 10.1002/iid3.156
15. Chen Y, Yang Y, Zhang XY, Fan QS, Li X, Xin YJ, et al. Nomogram based on neutrophil-To-Lymphocyte ratio and platelet-To-Lymphocyte ratio to predict recurrence in patients with hepatocellular carcinoma after radiofrequency ablation. Cardiovasc Intervent Radiol (2021) 44(10):1551–60. doi: 10.1007/s00270-021-02872-8
16. Diem S, Schmid S, Krapf M, Flatz L, Born D, Jochum W, et al. Neutrophil-To-Lymphocyte ratio (Nlr) and platelet-To-Lymphocyte ratio (Plr) as prognostic markers in patients with non-small cell lung cancer (Nsclc) treated with nivolumab. Lung Cancer (2017) 111:176–81. doi: 10.1016/J.Lungcan.2017.07.024
17. Fang T, Wang Y, Yin X, Zhai Z, Zhang Y, Yang Y, et al. Diagnostic sensitivity of nlr and plr in early diagnosis of gastric cancer. J Immunol Res (2020) 2020:9146042. doi: 10.1155/2020/9146042
18. Zhu S, Yang J, Cui X, Zhao Y, Tao Z, Xia F, et al. Preoperative platelet-To-Lymphocyte ratio and neutrophil-To-Lymphocyte ratio as predictors of clinical outcome in patients with gallbladder cancer. Sci Rep (2019) 9(1):1823. doi: 10.1038/s41598-018-38396-4
19. Toledano-Fonseca M, Cano MT, Inga E, Gómez-España A, Guil-Luna S, García-Ortiz MV, et al. The combination of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio with liquid biopsy biomarkers improves prognosis prediction in metastatic pancreatic cancer. Cancers (Basel) (2021) 13(6):5. doi: 10.3390/cancers13061210
20. Muhammed A, Fulgenzi C, Dharmapuri S, Pinter M, Balcar L, Scheiner B, et al. The systemic inflammatory response identifies patients with adverse clinical outcome from immunotherapy in hepatocellular carcinoma. Cancers (Basel) (2021) 14(1):6–11. doi: 10.3390/cancers14010186
21. Sellers CM, Uhlig J, Ludwig JM, Stein SM, Kim HS. Inflammatory markers in intrahepatic cholangiocarcinoma: Effects of advanced liver disease. Cancer Med (2019) 8(13):5916–29. doi: 10.1002/cam4.2373
22. Hsiang CW, Huang WY, Yang JF, Shen PC, Dai YH, Wang YF, et al. Dynamic changes in neutrophil-To-Lymphocyte ratio are associated with survival and liver toxicity following stereotactic body radiotherapy for hepatocellular carcinoma. J Hepatocell Carcinoma (2021) 8:1299–309. doi: 10.2147/JHC.S334933
23. Chun YS, Pawlik TM, Vauthey JN. 8th edition of the ajcc cancer staging manual: Pancreas and hepatobiliary cancers. Ann Surg Oncol (2018) 25(4):845–7. doi: 10.1245/s10434-017-6025-x
24. Bismuth H, Nakache R, Diamond T. Management strategies in resection for hilar cholangiocarcinoma. Ann Surg (1992) 215(1):31–8. doi: 10.1097/00000658-199201000-00005
25. Liu ZP, Chen WY, Wang ZR, Liu XC, Fan HN, Xu L, et al. Development and validation of a prognostic model to predict recurrence-free survival after curative resection for perihilar cholangiocarcinoma: A multicenter study. Front Oncol (2022) 12:849053. doi: 10.3389/fonc.2022.849053
26. Liu ZP, Chen WY, Zhang YQ, Jiang Y, Bai J, Pan Y, et al. Postoperative morbidity adversely impacts oncological prognosis after curative resection for hilar cholangiocarcinoma. World J Gastroenterol (2022) 28(9):948–60. doi: 10.3748/wjg.v28.i9.948
27. Liu ZP, Cheng ZJ, Dai HS, Zhong SY, Zhao DC, Gong Y, et al. Impact of perioperative blood transfusion on long-term survival in patients with different stages of perihilar cholangiocarcinoma treated with curative resection: A multicentre propensity score matching study. Front Oncol (2022) 12:1059581. doi: 10.3389/Fonc.2022.1059581
28. Liu ZP, Yao LQ, Diao YK, Chen ZX, Feng ZH, Gu WM, et al. Association of preoperative body mass index with surgical textbook outcomes following hepatectomy for hepatocellular carcinoma: A multicenter study of 1206 patients. Ann Surg Oncol (2022) 3-4. doi: 10.1245/S10434-022-11721-Y
29. Adam R, Chiche L, Aloia T, Elias D, Salmon R, Rivoire M, et al. Hepatic resection for noncolorectal nonendocrine liver metastases: Analysis of 1,452 patients and development of a prognostic model. Ann Surg (2006) 244:524–35. doi: 10.1097/01.Sla.0000239036.46827.5f
30. Valle JW, Kelley RK, Nervi B, Oh DY, Zhu AX. Biliary tract cancer. Lancet (2021) 397(10272):428–44. doi: 10.1016/S0140-6736(21)00153-7
31. Coussens LM, Werb Z. Inflammation and cancer. Nature (2002) 420(6917):860–7. doi: 10.1038/nature01322
32. Nieswandt B, Hafner M, Echtenacher B, Männel DN. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res (1999) 59(6):1295–300.
33. Topalian SL, Solomon D, Avis FP, Chang AE, Freerksen DL, Linehan WM, et al. Immunotherapy of patients with advanced cancer using tumor-infiltrating lymphocytes and recombinant interleukin-2: A pilot study. J Clin Oncol (1988) 6(5):839–53. doi: 10.1200/JCO.1988.6.5.839
34. Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: Harnessing the T cell response. Nat Rev Immunol (2012) 12(4):269–81. doi: 10.1038/nri3191
Keywords: neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, perihilar cholangiocarcinoma, hepatectomy, survical
Citation: Ge M-Y, Liu Z-P, Pan Y, Wang J-Y, Wang X, Dai H-S, Gao S-Y, Zhong S-Y, Che X-Y, Zuo J-H, Liu Y-H, Liu X-C, Fan H-N, Chen W-Y, Wang Z-R, Yin X-Y, Bai J, Zhang Y-Q, Jiang Y, Gong Y and Chen Z-Y (2023) Assessment of the prognostic value of the neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in perihilar cholangiocarcinoma patients following curative resection: A multicenter study of 333 patients. Front. Oncol. 12:1104810. doi: 10.3389/fonc.2022.1104810
Received: 22 November 2022; Accepted: 12 December 2022;
Published: 04 January 2023.
Edited by:
Qizhao Huang, Southern Medical University, ChinaReviewed by:
Yunjie Lu, First People’s Hospital of Changzhou, ChinaWei Chen, The First Affiliated Hospital of Sun Yat-sen University, China
Copyright © 2023 Ge, Liu, Pan, Wang, Wang, Dai, Gao, Zhong, Che, Zuo, Liu, Liu, Fan, Chen, Wang, Yin, Bai, Zhang, Jiang, Gong and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhi-Yu Chen, chenzhiyu_umn@163.com; Yi Gong, gongyiitmmu@163.com; Yan Jiang, jiangyantmmu@163.com
†These authors have contributed equally to this work
 Ming-Yang Ge1†
Ming-Yang Ge1† Hai-Su Dai
Hai-Su Dai Zhi-Yu Chen
Zhi-Yu Chen