- 1Departments of Gastrointestinal Surgery and Clinical Nutrition, Beijing Shijitan Hospital, Beijing, China
- 2Department of Oncology, Capital Medical University/Ninth Clinical Medical College, Peking University, Beijing, China
- 3Department of Clinical Nutrition, Daping Hospital, Army Medical University, Chongqing, China
- 4Comprehensive Oncology Department, National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 5Department of Medical Oncology, Fujian Cancer Hospital, Fujian Medical University Cancer Hospital, Fuzhou, China
Background: Malnutrition and systemic inflammation are common in patients with nasopharyngeal carcinoma (NPC). The Patient-Generated Subjective Global Assessment (PG-SGA) score and neutrophil-to-lymphocyte ratio (NLR) reflect the integrated nutritional status and inflammatory level of patients with NPC, respectively. We performed this study to identify whether NLR and PG-SGA score are associated with outcome and survival time for patients with NPC undergoing chemoradiotherapy.
Methods: The multicenter cohort study included 1,102 patients with NPC between June 2012 and December 2019. The associations of all-cause mortality with NLR and PG-SGA score were calculated using the Kaplan–Meier method and the log-rank test. We also did a multivariate-adjusted Cox regression analysis to identify the independent significance of different parameters. Restricted cubic spline regression was carried out to evaluate the association between NLR and overall survival (OS). A nomogram was established using the independent prognostic variables. Interaction terms were used to investigate whether there was an interactive association between NLR and PG-SGA.
Results: A total of 923 patients with NPC undergoing chemoradiotherapy were included in this study: 672 (72.8%) were males and 251 (27.2%) were females, with a mean age of 49.3 ± 11.5 years. The Kaplan–Meier curves revealed that patients with malnutrition (PG-SGA score >3) had worse survival than patients who were in the well-nourished group (PG-SGA score ≤3) (p < 0.0001). In addition, patients in the high NLR group (NLR ≥ 3) had worse survival than those in the low NLR group (NLR < 3) (p < 0.0001). Patients with high PG-SGA and high NLR had the worst survival (p < 0.0001). An increase in NLR had an inverted L-shaped dose–response association with all-cause mortality. A nomogram was developed by incorporating domains of NLR and PG-SGA score to accurately predict OS 12–60 months for patients [the C-index for OS prediction of nomogram was 0.75 (95% CI, 0.70–0.80)]. The interaction of PG-SGA with NLR was significant (p = 0.009). Patients with high PG-SGA and high NLR had a nearly 4.5-fold increased risk of death (HR = 4.43, 95% CI = 2.60–7.56) as compared with patients with low PG-SGA and low NLR.
Conclusions: Our study provided clear evidence that high PG-SGA score and high NLR adversely and interactively affects the OS of patients with NPC undergoing chemoradiotherapy.
Introduction
Nasopharyngeal carcinoma (NPC) is a specific head and neck epithelial malignant tumor with obvious endemic and racial distribution differences, especially in southern China, with the incidence ranging from 20 to 30 per 100,000 (1).
Due to the complex anatomical location, high sensitivity of irradiation, and a locoregional advanced presentation at diagnosis, the primary treatment for non-disseminated NPC is not radical surgery but radiotherapy (RT) alone or concurrent chemoradiotherapy (CCRT), which is recognized as the most effective and reasonable strategy to control local recurrence and prolong survival time (2, 3). However, the therapeutic effect is unsatisfactory (4). More than 20% of the patients with advanced disease still develop local recurrence and distant metastases even after radical RT and systemic chemotherapy (5). Recurrent or metastatic NPC is associated with poor prognosis, with a median survival period of approximately 17 months (6). Therefore, improving the treatment effect and survival time of NPC patients remains challenging.
In clinical practice, malnutrition is one of major problems that can affect the curative effect and prognosis for people with NPC; 35% of NPC patients lose more than 5% of their weight (7). Even for newly diagnosed patients with NPC, through ideal weight percentage and serum albumin level to evaluate the nutritional status, malnutrition has been estimated in 36.5% and 34.6%, respectively (8). RT will cause further aggravation of malnutrition via mucositis, reaching a peak in 3 weeks. A clinical observational study reported that 20.19% of patients with NPC under RT were able to lose 10% of their body weight within 2 months (9). Furthermore, side effect, fatigue, anorexia, etc. often lead to malnutrition during the process of chemotherapy for NPC (10).
Malnutrition and weight loss may impair immune function, restrict vitality, reduce resistance to the disease, and decrease wound healing, which lead to complications, treatment-related toxicities, and resistance to cancer treatment (11). In addition, the effectiveness of the chemotherapy and RT will be significantly reduced, if nutritional status of patients becomes so bad (12). The treatments had to be suspended even and terminated early because of intolerance. Therefore, it is not surprising that malnutrition or weight loss during treatments has been found to be significantly associated with poorer survival outcomes and impaired the quality of life (QoL) in NPC. Furthermore, an increase in 20% weight reduction over the course of treatments was significantly associated with toxicity and mortality during RT in NPC (13).
The Patient-Generated Subjective Global Assessment (PG-SGA) was proposed in 1996 and developed by the European Society for Clinical Nutrition and Metabolism (ESPEN), which is a tool that enables evaluation of the nutritional status for patients with cancer (14). This scoring system has two main parts: one questionnaire for the patient with four questions and one questionnaire for the physician. The questionnaire for the patient includes weight change, symptoms, daily diet, and daily activities; the questionnaire for the physician includes diagnosis, physical examinations, metabolic evaluation, and nutrition-related complications. Finally, the grades for nutritional assessment are divided into the following categories: good nutritional status, suspicious malnutrition, moderate malnutrition, and severe malnutrition. A patient with over 3 points would be regarded as relatively malnourished (15). Thus, PG-SGA has been used for guiding nutritional treatment, making clinical decisions, and predicting outcomes in various cancers, including NPC (16).
In recent years, an increasing number of studies have focused on the influence of nutrition and systemic inflammation on the prognosis of patients with cancer (17, 18). Systemic inflammatory response has been shown to be closely associated with weight loss and malnutrition in NPC (4, 9). Neutrophil-to-lymphocyte ratio (NLR), which is calculated based on the absolute neutrophil count divided by the absolute lymphocyte count, is known to be an indicator of both the immune system and systemic inflammation of cancer patients. NLR and other systemic inflammation-related biomarkers [such as prognostic nutritional index (PNI) (19), platelet-to-lymphocyte ratio (PLR) (20), C-reactive protein (CRP) (21)] are commonly used to predict clinical outcomes for patients with various malignancies (17, 18).
The clinical predictive value of a combination of PG-SGA score and NLR has rarely been investigated in patients with NPC undergoing chemoradiotherapy. Therefore, we performed this paper to identify which prognostic factors may help select patients who will benefit from more aggressive treatment and to evaluate the relationship between nutritional status and systemic inflammation in patients with NPC undergoing chemoradiotherapy.
Subjects and Methods
Study Design and Participants
The retrospective data were collected from a multicenter, prospective, observational cohort study named the Investigation on Nutrition Status and its Clinical Outcome of Common Cancers (INSCOC) project of China (chictr.org.cn, registration number: ChiCTR1800020329). In the present study, a total of 1,102 patients with NPC were recruited from 40 clinical centers throughout the China from June 2012 to December 2019, who had to meet the following inclusion criteria: 1) aged 18 to 80 years; 2) with pathological diagnosis of NPC; 3) received radical RT, chemotherapy, or both; 4) without liver and kidney dysfunction; and 5) obtained complete baseline clinical information and laboratory data and complete follow-up data.
Data Collection and Variable Definition
Within the first 48 h after hospital admission, demographic and clinicopathological data were collected by trained investigators, including gender, age at diagnosis, body mass index (BMI), smoking status, comorbidities, alcohol consumption, tea consumption, family history of cancer, tumor stage, NLR, and PG-SGA score. The nutritional status of all patients was evaluated using the PG-SGA: a patient with over 3 points would be regarded as relatively malnourished (15); in contrast, less than or equal to 3 points was classified into the well-nourished group. BMI was calculated by body weight in kilograms divided by the height in squared meters (kg/m2). NLR was used as a continuous variable, and widely accepted values were used to group patients. The optimal cutoff values (“normal” (<3) and “moderate and high” (≥3) inflammation) for NLR were generated according to reference (17). The tumor stage in all patients was confirmed by pathological examination. NPC patients were staged according to the 8th edition of the American Joint Committee on Cancer/Union for International Cancer Control (AJCC/UICC) TNM staging system. The types of chemotherapy or RT included curative, neoadjuvant, adjuvant, maintenance, and palliative types in the study. All patients were regularly followed up via outpatient visits or telephone interviews until death, last contact on March 31, 2020. The study was approved by the research ethical committees of all participating institutions and was designed in accordance with the Declaration of Helsinki, and all participants signed the consent before study entry.
Statistical Analysis
Categorical variables were expressed as whole numbers and percentages and were compared by the chi‐square test or Fisher’s exact test, as appropriate. Quantitative variables were reported as mean ± standard deviation (SD), and differences were analyzed using Student’s t-test or the Mann–Whitney U test for groups without a normal distribution. Overall survival (OS) was calculated using the Kaplan–Meier method and the log-rank test, and hazard ratios (HRs) together with 95% CIs were calculated using a univariate Cox regression analysis to investigate the association between potential predictors and mortality. We also did a multivariate-adjusted Cox regression analysis using backward selection to identify the independent significance of different parameters. Restricted cubic spline regression was carried out to evaluate the association between NLR and OS. Based on the results of multivariable analysis, we formulated a nomogram using the “rms” package in R to predict the probability of 12-, 24-, 36-, 48-, and 60-month OS rates for NPC patients after chemoradiotherapy. Harrell’s C-index was used to evaluate discernibility ability of the nomogram. The area under the receiver operating characteristic (ROC) curve (AUC) was used to evaluate the predictive accuracy of the 12-, 24-, 36-, and 48-month OS. Interaction terms were used to investigate whether there was an interactive association between NLR and PG-SGA score. All statistical analyses were performed with R software (version 3.6.2, http://www.rproject.org). p < 0.05 from the two-sided test was considered statistically significant.
Results
Clinical Characteristics of the Investigated Nasopharyngeal Carcinoma Patients
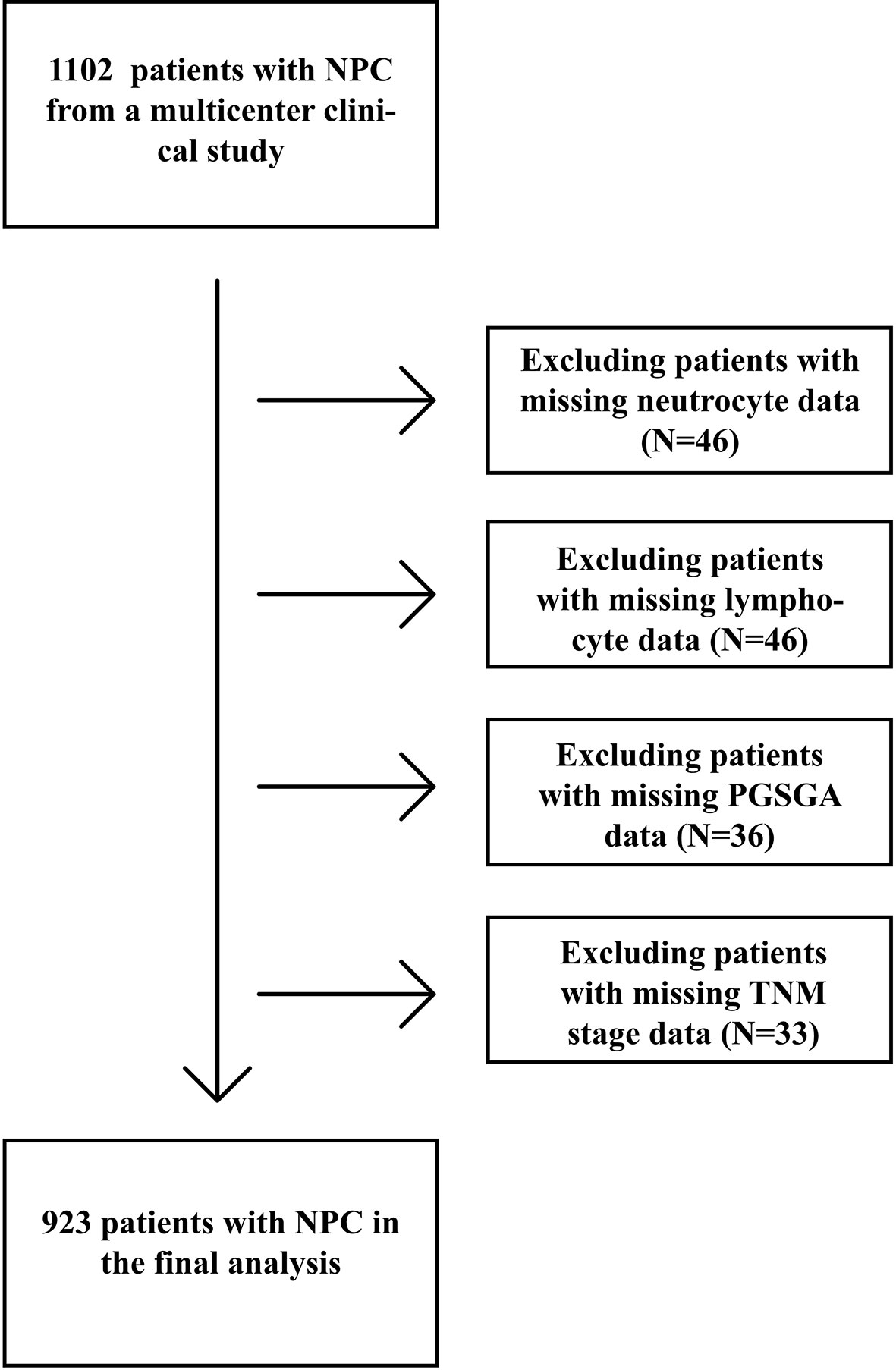
A total of 923 patients with NPC undergoing chemoradiotherapy were included in this study; the flowchart of screening process is shown in Figure 1. One hundred sixty-one patients were excluded due to missing key data or variables in our analysis. A total of 18 cases were lost to follow-up. The follow-up rate was 98.4%. Among the remaining cases, the average follow-up time was 28.3 ± 16.3 months, during which there were 99 deaths. The general characteristics of all participants with NPC undergoing chemoradiotherapy by category of PG-SGA score are shown in Table 1. There were 672 (72.8%) males and 251 (27.2%) females, with a mean age of 49.3 ± 11.5 years. Among all NPC patients, the PG-SGA-diagnosed severe malnutrition rate was 16.4% (151 patients, determined by the PG-SGA score >3). The TNM stage and BMI between patients with PG-SGA score >3 and PG-SGA score ≤3 had significant difference (p < 0.001).
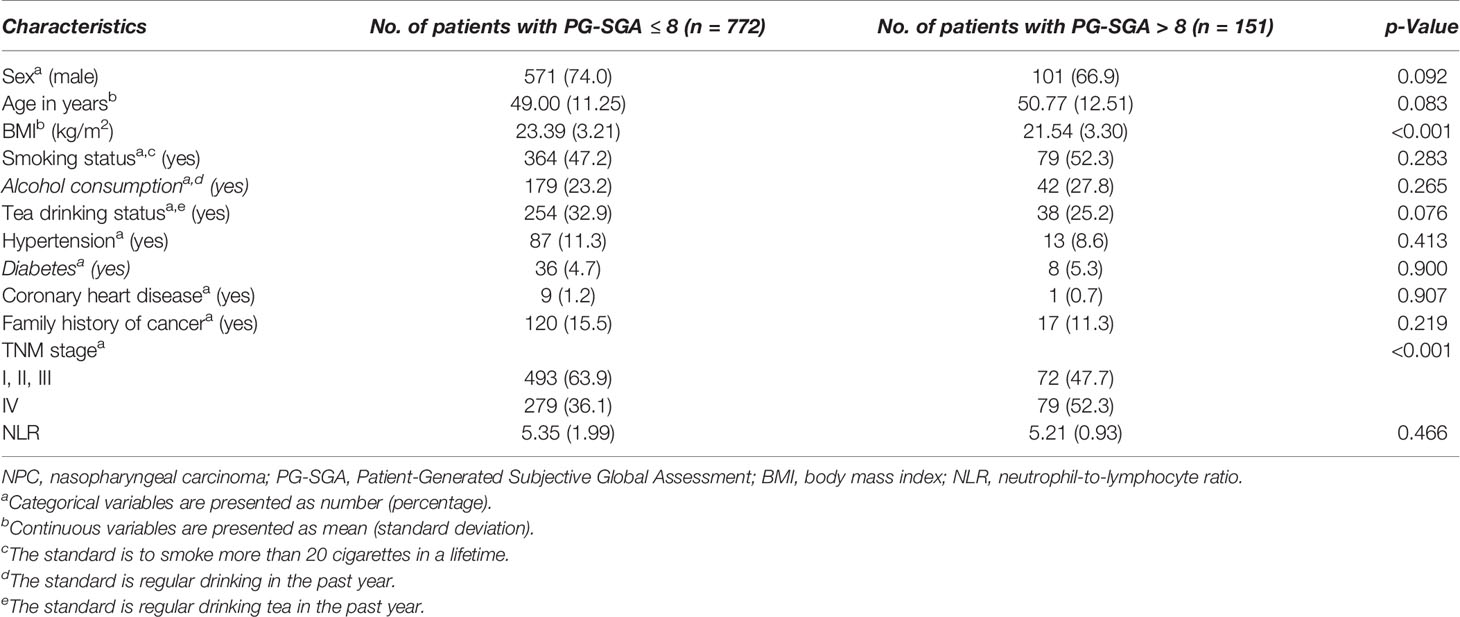
Table 1 Demographic and clinical characteristics of patients with NPC undergoing chemoradiotherapy stratified by PG-SGA.
Risk Factors of All-Cause Mortality for Patients With Nasopharyngeal Carcinoma Undergoing Chemoradiotherapy Were Determined Using Univariate and Multivariate Cox Regression Analyses
The Kaplan–Meier curves revealed that patients with NPC undergoing chemoradiotherapy, with malnutrition (PG-SGA score >3), had worse survival (HR = 1.441, 95% CI = 0.90–2.31, median OS [MOS] = 21.32 months) than patients with NPC who were in the well-nourished group (PG-SGA score ≤3) (MOS = 24.51 months, p < 0.0001, Figure 2A). In addition, patients in the high NLR group (NLR ≥ 3) had worse survival (HR = 2.36, 95% CI = 1.56–3.56, MOS = 21.16 months) than those in the low NLR group (NLR < 3) (MOS = 24.86 months, p < 0.0001, Figure 2B). After the study population was further stratified into four subgroups, patients with high PG-SGA (PG-SGA score >3) and high NLR (NLR ≥ 3) had the worst survival (HR = 4.43, 95% CI = 2.60–7.56, p < 0.0001, Figure 2C), compared with those in the high PG-SGA and low NLR group, the low PG-SGA and high NLR group, and the low PG-SGA and low NLR group.
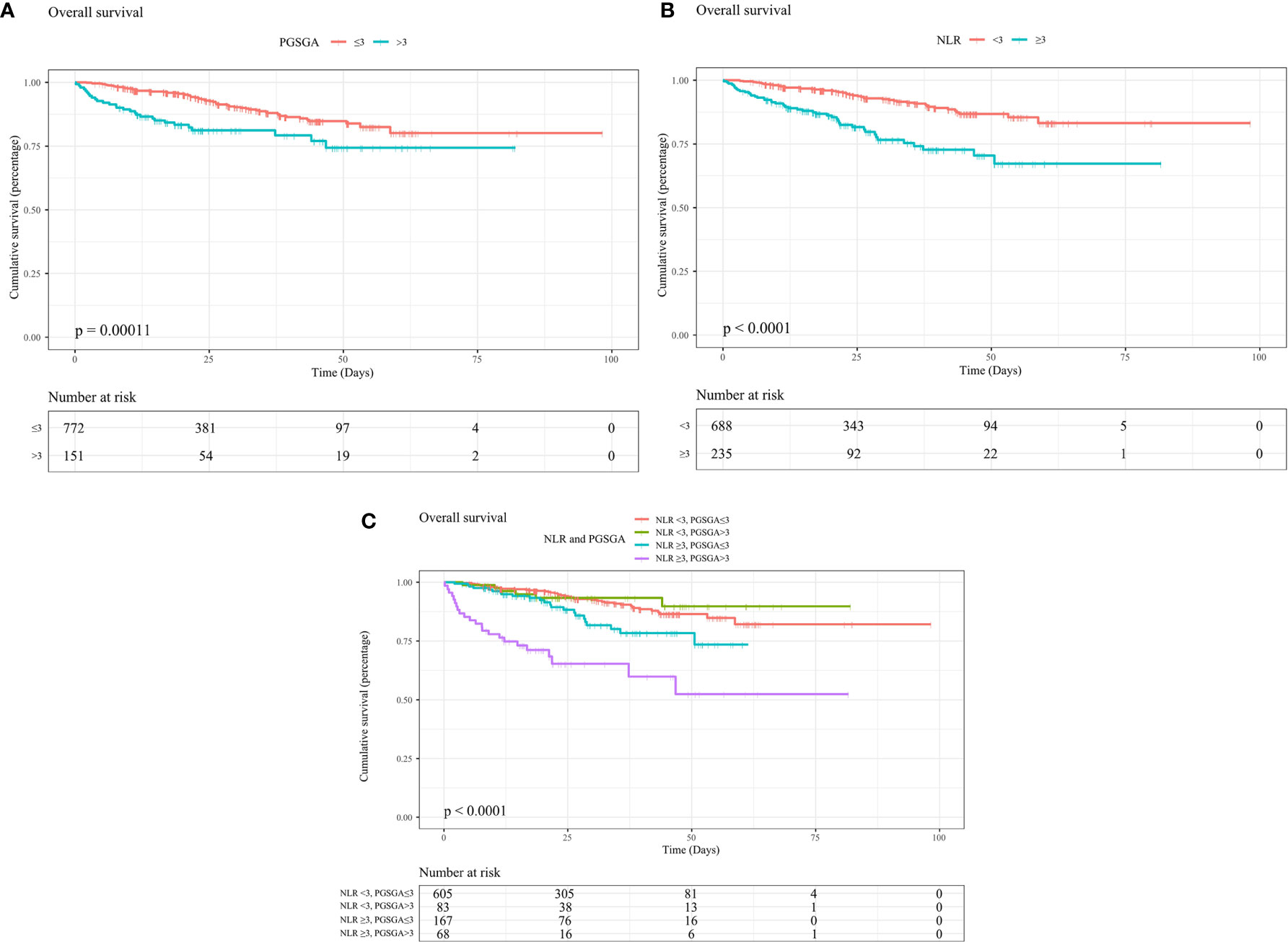
Figure 2 Kaplan–Meier curves show survival rates for patients with NPC undergoing chemoradiotherapy with (A) PG-SGA (PG-SGA score >3, ≤3), with (B) NLR (NLR ≥ 3, < 3), with (C) NLR and PG-SGA (high PG-SGA and high NLR group, high PG-SGA and low NLR group, low PG-SGA and high NLR group, and low PG-SGA and low NLR group). NPC, nasopharyngeal carcinoma; PG-SGA, Patient-Generated Subjective Global Assessment; NLR, neutrophil-to-lymphocyte ratio.
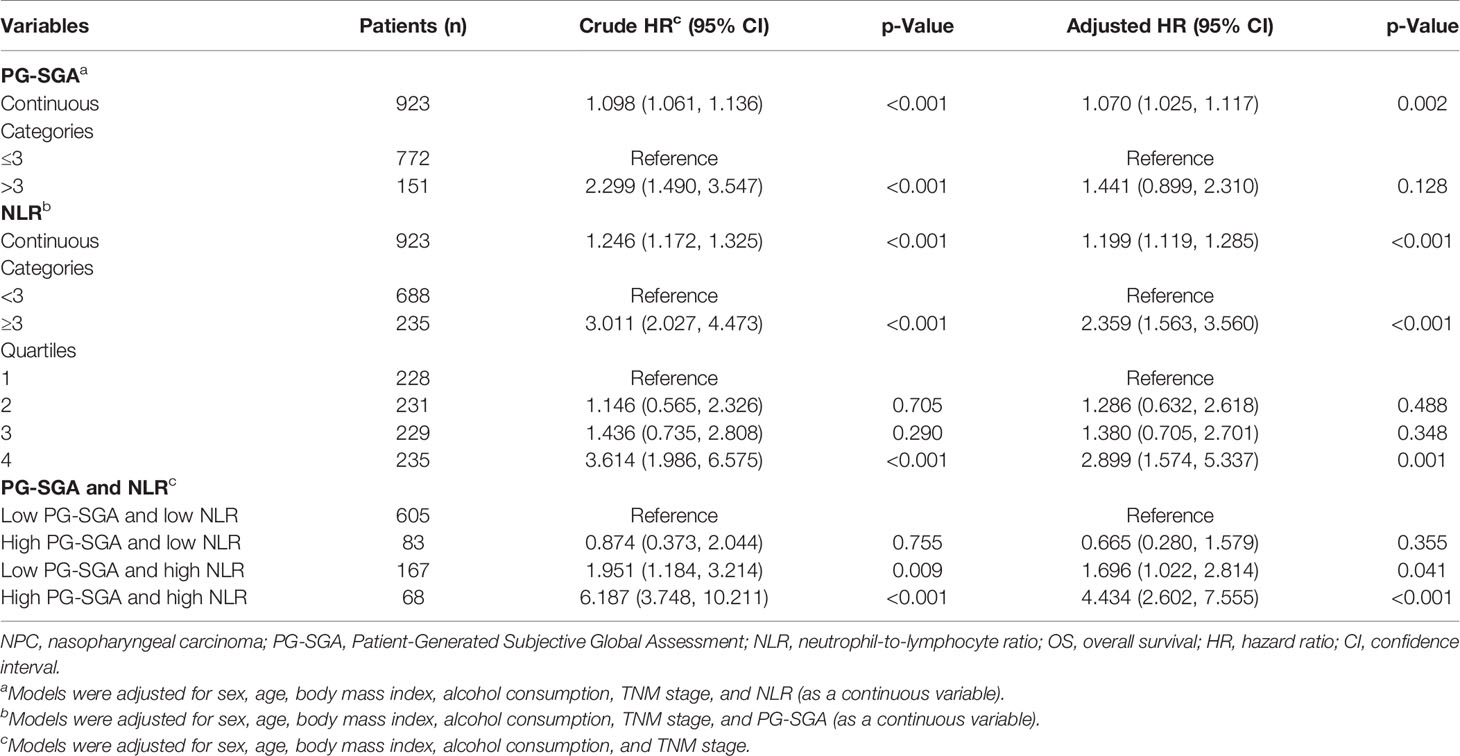
We carried out multivariate analyses that included patient age, sex, BMI, alcohol consumption, TNM stage, PG-SGA, and NLR. As shown in Table 2, age (HR, 1.040, 95% CI 1.022–1.059; p < 0.001), PG-SGA (HR, 1.070, 95% CI 1.025–1.117; p = 0.002) and NLR (HR, 1.199, 95% CI 1.119–1.285; p < 0.001) were significantly associated with OS. In multivariable Cox proportional hazards models (Table 3), high NLR (NLR ≥ 3) was associated with a 135.9% (HR, 2.36; 95% CI, 1.56–3.56) greater risk of death, as compared with those with low NLR (NLR < 3). An increase in 1 score of PG-SGA was associated with a 7% increased risk of death. Additionally, NLR was divided into quartiles; the fourth quartile (≥3) was positively correlated with a worse prognosis (p < 0.001), compared with the first (< 1.61), second (≥1.61, <2.15), and third (≥2.15, <3) quartiles. After the confounding factors were adjusted, HRs of all-cause mortality (HR, 95% CI) were 1.29 (0.63–2.62), 1.38 (0.71–2.70), and 2.90 (1.57–5.34) for the second, third, and fourth quartiles, respectively, showing an increasing trend in the risk of death.
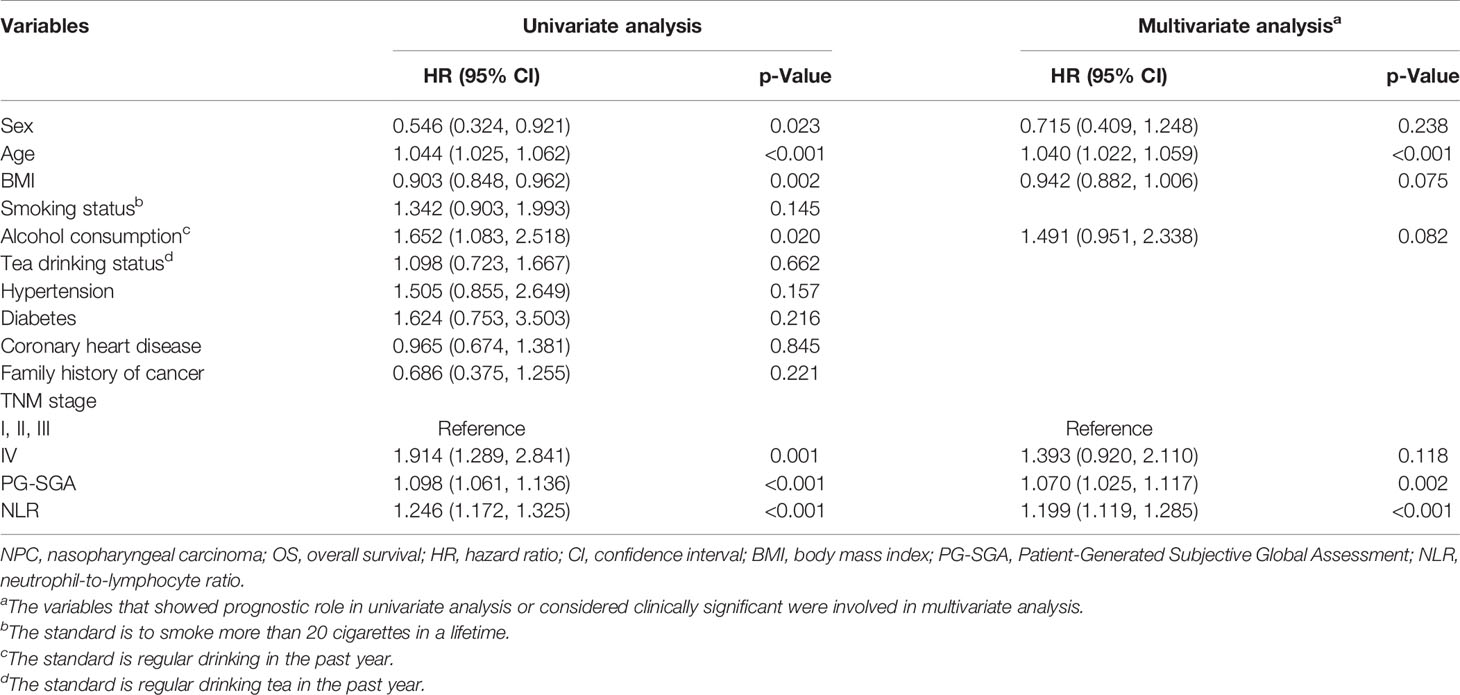
Table 2 Association between clinical variables and OS in patients with NPC undergoing chemoradiotherapy.
When analyzed as a continuous variable, restricted cubic spline plot showed multivariable-adjusted HRs (95% CI) for NLR, which had an inverted L-shaped dose–response association with the all-cause mortality risk in NPC patients (Figure 3A).
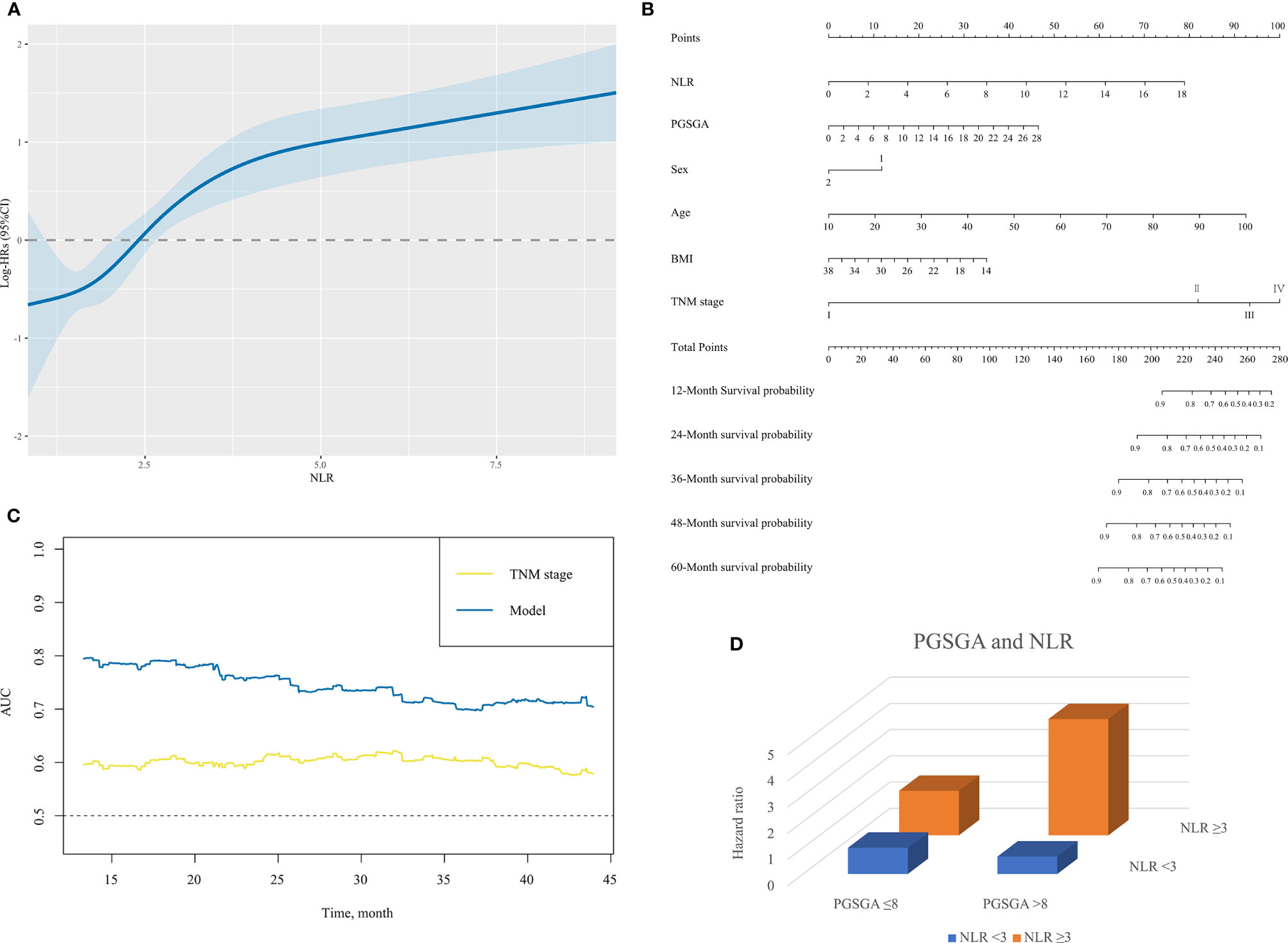
Figure 3 (A) The association between NLR (continuous) and hazard ratio of overall survival for patients with NPC undergoing chemoradiotherapy. Splines are adjusted by sex, age, body mass index, alcohol consumption, TNM stage and PG-SGA. (B) The nomogram for predicting the 12- to 60-month overall survival time of patients with NPC undergoing chemoradiotherapy. In the nomogram, an individual patient’s value is located on each variable axis. Based on the number of points received for each corresponding value, the sum of these numbers is located on the total point axis to determine the likelihood of 12- to 60-month survival in the matched survival axes. (C) A summary chart merged the AUC for prediction of survival at 12, 24, 36, or 48 months by nomogram (model) and TNM stage. The predictive capacity of model had a significant improvement as compared with TNM stage. (D) The interactive association of NLR and PG-SGA for patients with NPC undergoing chemoradiotherapy, presented by three-dimensional bar diagram. NLR, neutrophil-to-lymphocyte ratio; NPC, nasopharyngeal carcinoma; PG-SGA, Patient-Generated Subjective Global Assessment; AUC, area under the receiver operating characteristic curve.
The Nomogram for Predicting the 12–60 Months’ Overall Survival Time of Patients With Nasopharyngeal Carcinoma Undergoing Chemoradiotherapy
A nomogram for the 12-, 24-, 36-, 48-, and 60-month OS based on those factors (NLR, PG-SGA, sex, age, BMI, and TNM stage) was developed for predicting OS in patients with NPC undergoing chemoradiotherapy (Figure 3B). Scales of the nomogram reflected coefficients from the Cox model converted to a practical 100-point range, and OS was accurately predicted by the nomogram (model) with an AUC of 0.803, 0.759, 0.700, and 0.727 for prediction of mortality in comparison with TNM stage (0.593, 0.603, 0.603, 0.591, respectively) at 12, 24, 36, and 48 months (Figures 3C and Supplementary Figure S1). The C-index for OS prediction of nomogram was 0.75 (95% CI, 0.70–0.80), better than that of TNM stage 0.60 (0.54–0.65).
Interactive Association of Exposures With Mortality Between Patient-Generated Subjective Global Assessment and Neutrophil-to-Lymphocyte Ratio
Table 3 and Figure 3D illustrate the HR for each condition of exposure combinations compared with the referent: low PG-SGA (PG-SGA score ≤3) and low NLR (NLR < 3) group. The interaction of PG-SGA with NLR was significant (p = 0.009). Patients in NPC with high PG-SGA and high NLR had a nearly 4.5-fold increased risk of death (HR = 4.43, 95% CI = 2.60–7.56) as compared with patients with low PG-SGA and low NLR. PG-SGA and NLR showed synergism with each other.
Discussion
This was a large multicenter study comprising 923 patients with NPC undergoing chemoradiotherapy across several provinces in China, which investigated the relationships between the nutritional status and patients’ prognosis through a long-term follow-up. Malnutrition is commonly seen in patients with NPC undergoing chemoradiotherapy (9, 16, 22). Patients with NPC suffer from malnutrition caused by complex factors, including the following: 1) the tumor invades the basis cranii, causing paralysis of the glossopharyngeal nerve, which leads to dysphagia (23). 2) As a consequence of injuries to normal tissues, such as the oral mucosa, taste buds, and salivary glands, radiation-induced oral mucositis and other complications are very common in patients with NPC undergoing RT, causing difficulty in chewing and swallowing (24, 25). 3) The concurrent chemotherapy often cause severe side effects of gastrointestinal tract such as asitia, nausea, and vomiting, which lead to decreased appetite and gastrointestinal dysfunction (26). 4) Patients with NPC also suffer from emotional disorders such as fear, anxiety, and depression, which further impair the patient’s ability to eat and digestive function (19, 27). 5) The insufficient nutritional supply or unreasonable ingredients was found in the nursing care of patients with NPC, due to patients’ and their relatives’ poor nutritional knowledge. However, few data are available regarding the nutritional status of patients with NPC undergoing chemoradiotherapy. This study investigated the nutritional status of patients with NPC who received RT, chemotherapy, or both, on the basis of PG-SGA score and BMI. In addition, an NLR was established to reflect patients’ systemic inflammation (28), and the correlation between PG-SGA and NLR on survival time was explored.
Based on our results, we found that PG-SGA score and NLR at admission were associated with poor prognosis of NPC undergoing chemoradiotherapy (Figures 2A–C and Tables 2, 3). NLR had an inverted L-shape relationship with mortality risk among patients with NPC undergoing chemoradiotherapy (Figure 3A). Furthermore, we established a nomogram model based on domains of NLR, PG-SGA, sex, age, BMI, and tumor stage to predict the OS rate associated with NPC patients undergoing chemoradiotherapy. The nomogram was able to predict an individual’s risk of 12-, 24-, 36-, 48-, and 60-month OS rates with relatively good accuracy, especially for predicting the 12-month OS rates (Figures 3B, C and Supplementary Figure S1). Finally, the co-occurrence of high NLR and high PG-SGA score was associated with a nearly 4.5-fold increased risk of mortality among patients with NPC undergoing chemoradiotherapy (Table 3 and Figure 3D). To sum up, there was a potential synergistic effect of systemic inflammation and severe malnutrition with mortality risks for patients with NPC who received RT, chemotherapy, or both.
In recent years, an increasing number of studies have demonstrated that measures of inflammation and malnutrition are each independently powerful prognostic indicator in patients with cancer (28). A prospective cohort (17) of 2,470 patients with stage I to III CRC revealed that pre-diagnosis inflammation was associated with at-diagnosis sarcopenia. OS was worse in the group with simultaneous existence of sarcopenia and high NLR, which seem to be consistent with our results. In previous studies (18), the level of NLR in patients with cancer cachexia is generally higher than that in patients without cancer cachexia, and the high NLR level was related to poorer survival of cancer patients, indicating that NLR could be used as an independent prognostic factor for patients with cachexia. Wang et al. reported that in patients with NPC, weight loss makes them more vulnerable to recurrent pneumonia. Notably, our results suggest likely synergistic effects between inflammation and malnutrition for patients with NPC undergoing chemoradiotherapy, which explains why nutritional therapy for patients with NPC could be beneficial to reduce systemic inflammation and improve the immunity of patients.
The close connections of nutrition, inflammation, and cancer have been attracting our attentions for many years. Our findings also indicated the effect of chemoradiotherapy on the nutritional status of patients with NPC. In addition to malnutrition, chemoradiotherapy may induce mucositis, tissue damage, and acute toxicities, which may cause systemic inflammatory response (9, 29). Thus, malnutrition and systemic inflammation may have a prognostic role in the process of chemoradiotherapy. Malnutrition expended massive protein, which further disturbs metabolic function, impairs immunity, increases susceptibility to infection, compromises the response to antitumor treatments, and aggravates chemoradiotherapy toxicity, hence severely affecting survival and outcomes (4, 22, 25, 30). Additionally, protein–energy malnutrition may induce a high level of systemic inflammatory factors, such as tumor necrosis factor (TNF)-α and interleukin-6, can facilitate tumor cell proliferation invasion and metastasis, and can enhance malignant properties (31). As a consequence, malnutrition was also an indicator of a systemic inflammatory response. In turn, pro-inflammatory cytokines and tumor factors are involved in high catabolism in the inflammatory state, which consumes a large amount of the patient’s skeletal muscle mass and caloric intake (17). This metabolic disorder will eventually lead to continuous weight loss and deterioration of nutritional status, damage to tissue and organ function, increase in the incidence of complications, and decrease in survival time. More importantly, inflammation plays an important role in the occurrence and progression of malignant tumors. Malnutrition, inflammation, and cancer really have become a vicious circle. Therefore, it is particularly important for cancer patients with malnutrition to control systemic inflammatory; and for cancer patients with high levels of inflammation, it is necessary to pay more attention to early nutritional intervention to better improve the prognosis of cancer patients. Interestingly, now that the survival rates of patients with high NLR may potentially benefit from nutritional therapy in our results, some special nutritional support with anti-inflammatory function should be given more to the patients with NPC undergoing chemoradiotherapy, such as ω-3 polyunsaturated fatty acid (ω-3 PUFA)-enriched and β-hydroxy-β-methylbutyrate (HMB)-enriched supplement. ω-3 PUFA has already been widely recognized as one of the anti-inflammatory nutrients (32). HMB, an intermediate metabolite of leucine that exists in human muscle cells, has been shown to inhibit protein degradation, attenuate the decrease in protein synthesis, and improve nutritional status (33). Moreover, one study (34) found that HMB attenuated the inflammatory effect of lipopolysaccharide (LPS), TNF, interferon (IFN), and angiotensin II (ANG II). A randomized trial (35) examined the effects of HMB supplementation on inflammation, protein metabolism, and pulmonary function in patients with chronic obstructive pulmonary disease, requiring mechanical ventilation. After 7 days of intervention, patients who were treated with HMB showed consistent reductions in CRP and in white blood cell counts from baseline, suggesting that HMB plays a positive role in the treatment of chronic inflammation.
To our knowledge, this is one of the meaningful studies on NPC to examine the relationship between malnutrition and biomarkers of systemic inflammation and the only study to examine whether high NLR and high PG-SGA score have a synergetic interaction on the NPC patient’s survival risk. The survival difference among the four groups (different combinations of PG-SGA score and NLR) indicates that prolonged survival needs not only a better nutrition status but also low-level systemic inflammation, which is a significant issue that should not be underestimated. Similar to all clinical research, our study was subject to several limitations: first, we could not examine other markers of systemic inflammation, such as PNI (19), PLR (20), and CRP levels (21). Because the related data are so insufficient or inappropriate, we had to stop analyzing them. Second, we did not study whether dynamic NLR could modify the prognostic value. Moreover, other covariates were collected using electronic medical record, questionnaire, or a one-on-one interview; and errors in measurement were inevitable. Finally, different diagnostic and therapeutic levels in the various hospitals might have masked the true associations. Further data are required to confirm these findings and to increase the precision of the effect for these exposures.
Conclusions
Malnutrition and systemic inflammation are common in patients with NPC. The PG-SGA score and NLR reflect the integrated nutrition status and inflammatory level of NPC patients, respectively. Our study provided clear evidence that high PG-SGA score and high NLR adversely affect the OS of patients with NPC undergoing chemoradiotherapy, suggesting that PG-SGA score and NLR were independent prognostic indicators for patients with NPC undergoing chemoradiotherapy. We also found that PG-SGA and NLR were interactive with each other. The co-occurrence of malnutrition and systemic inflammation at admission identified patients with a nearly 4.5-fold increased risk of mortality as compared with patients with neither condition. Therefore, on the one hand, it is particularly important for cancer patients with malnutrition to control systemic inflammation; on the other hand, for cancer patients with high levels of inflammation, it is necessary to offer nutritional intervention as early as possible to better improve the prognosis of cancer patients. Future studies are required to dynamically explore specific relationships and exact mechanisms between nutrition status and systemic inflammation and could assist to develop better strategies to increase the survival time and QoL of patients with NPC undergoing chemoradiotherapy.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
HS and XW are responsible for the study design, conceptualization, systematic review, project administration, the decision to publish, and manuscript preparation. MY, YG, and MT helped conduct the statistical analyses. BR and YC revised and edited the manuscript. ZG, HX, and MC provided data support from INSCOC project. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by a National Science and Technology Major Project from Ministry of Science and Technology of the People’s Republic of China, the National Key Research and Development Program (No: 2017YFC1309200; Title: The key technology of palliative care and nursing for cancer patients).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.766398/full#supplementary-material
Supplementary Figure 1 | In ROC curve analysis, comparisons of the AUC for prediction of survival at 12- or 24- or 36- or 48- month by nomogram and TNM stage. NPC, Nasopharyngeal carcinoma; PGSGA, Patient-Generated Subjective Global Assessment; NLR, neutrocyte to lymphocyte ratio; HR, hazard ratio; CI, confidence interval; BMI, body mass index; ROC, receiver operator characteristic; AUC, area under curve.
References
1. Almomani MH, Zulfiqar H, Nagalli S. Nasopharyngeal Carcinoma (NPC, Lymphoepithelioma). In: StatPearls. Treasure Island (FL): StatPearls Publishing (2021).
2. He Q, Huang Y, Wan G, Feng M, Zeng H, Liu M, et al. A Novel Prognostic Marker Based on Risk Stratification With Prognostic Nutritional Index and Age for Nasopharyngeal Carcinoma Patients Who Received Neoadjuvant Chemotherapy. Biomark Med (2019) 13:1013–23. doi: 10.2217/bmm-2018-0401
3. Pignon JP, Bourhis J, Domenge C, Designe L. Chemotherapy Added to Locoregional Treatment for Head and Neck Squamous-Cell Carcinoma: Three Meta-Analyses of Updated Individual Data. MACH-NC Collaborative Group. Meta-Analysis of Chemotherapy on Head and Neck Cancer. Lancet (2000) 355:949–55. doi: 10.1016/S0140-6736(00)90011-4
4. Tang M, Jia Z, Zhang J. The Prognostic Role of Prognostic Nutritional Index in Nasopharyngeal Carcinoma: A Systematic Review and Meta-Analysis. Int J Clin Oncol (2021) 26:66–77. doi: 10.1007/s10147-020-01791-x
5. Lee AW, Lin JC, Ng WT. Current Management of Nasopharyngeal Cancer. Semin Radiat Oncol (2012) 22:233–44. doi: 10.1016/j.semradonc.2012.03.008
6. Hsu CL, Chang KP, Lin CY, Chang HK, Wang CH, Lin TL, et al. Plasma Epstein-Barr Virus DNA Concentration and Clearance Rate as Novel Prognostic Factors for Metastatic Nasopharyngeal Carcinoma. Head Neck (2012) 34:1064–70. doi: 10.1002/hed.21890
7. Irungu CW, Oburra HO, Ochola B. Prevalence and Predictors of Malnutrition in Nasopharyngeal Carcinoma. Clin Med Insights Ear Nose Throat (2015) 8:19–22. doi: 10.4137/CMENT.S12119
8. Li G, Gao J, Liu ZG, Tao YL, Xu BQ, Tu ZW, et al. Influence of Pretreatment Ideal Body Weight Percentile and Albumin on Prognosis of Nasopharyngeal Carcinoma: Long-Term Outcomes of 512 Patients From a Single Institution. Head Neck (2014) 36:660–6. doi: 10.1002/hed.23357
9. Hong JS, Wu LH, Su L, Zhang HR, Lv WL, Zhang WJ, et al. Effect of Chemoradiotherapy on Nutrition Status of Patients With Nasopharyngeal Cancer. Nutr Cancer (2016) 68:63–9. doi: 10.1080/01635581.2016.1115099
10. Qiu C, Yang N, Tian G, Liu H. Weight Loss During Radiotherapy for Nasopharyngeal Carcinoma: A Prospective Study From Northern China. Nutr Cancer (2011) 63:873–9. doi: 10.1080/01635581.2011.582223
11. Larsson M, Hedelin B, Johansson I, Athlin E. Eating Problems and Weight Loss for Patients With Head and Neck Cancer: A Chart Review From Diagnosis Until One Year After Treatment. Cancer Nurs (2005) 28:425–35. doi: 10.1097/00002820-200511000-00004
12. Li G, Jiang XY, Qiu B, Shen LJ, Chen C, Xia YF. Vicious Circle of Acute Radiation Toxicities and Weight Loss Predicts Poor Prognosis for Nasopharyngeal Carcinoma Patients Receiving Intensity Modulated Radiotherapy. J Cancer (2017) 8:832–8. doi: 10.7150/jca.17458
13. Colasanto JM, Prasad P, Nash MA, Decker RH, Wilson LD. Nutritional Support of Patients Undergoing Radiation Therapy for Head and Neck Cancer. Oncol (Williston Park) (2005) 19:371–9. discussion 380-372, 387.
14. Bauer J, Capra S, Ferguson M. Use of the Scored Patient-Generated Subjective Global Assessment (PG-SGA) as a Nutrition Assessment Tool in Patients With Cancer. Eur J Clin Nutr (2002) 56:779–85. doi: 10.1038/sj.ejcn.1601412
15. Cao J, Xu H, Li W, Guo Z, Lin Y, Shi Y, et al. Nutritional Assessment and Risk Factors Associated to Malnutrition in Patients With Esophageal Cancer. Curr Probl Cancer (2021) 45:100638. doi: 10.1016/j.currproblcancer.2020.100638
16. Ding H, Dou S, Ling Y, Zhu G, Wang Q, Wu Y, et al. Longitudinal Body Composition Changes and the Importance of Fat-Free Mass Index in Locally Advanced Nasopharyngeal Carcinoma Patients Undergoing Concurrent Chemoradiotherapy. Integr Cancer Ther (2018) 17:1125–31. doi: 10.1177/1534735418807969
17. Feliciano EMC, Kroenke CH, Meyerhardt JA, Prado CM, Bradshaw PT, Kwan ML, et al. Association of Systemic Inflammation and Sarcopenia With Survival in Nonmetastatic Colorectal Cancer: Results From the C SCANS Study. JAMA Oncol (2017) 3:e172319. doi: 10.1001/jamaoncol.2017.2319
18. Zhang Q, Song MM, Zhang X, Ding JS, Ruan GT, Zhang XW, et al. Association of Systemic Inflammation With Survival in Patients With Cancer Cachexia: Results From a Multicentre Cohort Study. J Cachexia Sarcopenia Muscle (2021). doi: 10.1002/jcsm.12761
19. Du XJ, Tang LL, Mao YP, Guo R, Sun Y, Lin AH, et al. Value of the Prognostic Nutritional Index and Weight Loss in Predicting Metastasis and Long-Term Mortality in Nasopharyngeal Carcinoma. J Transl Med (2015) 13:364. doi: 10.1186/s12967-015-0729-0
20. Yang L, Xia L, Wang Y, Hong S, Chen H, Liang S, et al. Low Prognostic Nutritional Index (PNI) Predicts Unfavorable Distant Metastasis-Free Survival in Nasopharyngeal Carcinoma: A Propensity Score-Matched Analysis. PloS One (2016) 11:e0158853. doi: 10.1371/journal.pone.0158853
21. Tang LQ, Hu DP, Chen QY, Zhang L, Lai XP, He Y, et al. Elevated High-Sensitivity C-Reactive Protein Levels Predict Decreased Survival for Nasopharyngeal Carcinoma Patients in the Intensity-Modulated Radiotherapy Era. PloS One (2015) 10:e0122965. doi: 10.1371/journal.pone.0122965
22. Miao J, Xiao W, Wang L, Han F, Wu H, Deng X, et al. The Value of the Prognostic Nutritional Index (PNI) in Predicting Outcomes and Guiding the Treatment Strategy of Nasopharyngeal Carcinoma (NPC) Patients Receiving Intensity-Modulated Radiotherapy (IMRT) With or Without Chemotherapy. J Cancer Res Clin Oncol (2017) 143:1263–73. doi: 10.1007/s00432-017-2360-3
23. Lengyel E, Baricza K, Somogyi A, Olajos J, Papai Z, Godeny M, et al. Reirradiation of Locally Recurrent Nasopharyngeal Carcinoma. Strahlenther Onkol (2003) 179:298–305. doi: 10.1007/s00066-003-1048-6
24. Deng J, He Y, Sun XS, Li JM, Xin MZ, Li WQ, et al. Construction of a Comprehensive Nutritional Index and its Correlation With Quality of Life and Survival in Patients With Nasopharyngeal Carcinoma Undergoing IMRT: A Prospective Study. Oral Oncol (2019) 98:62–8. doi: 10.1016/j.oraloncology.2019.09.014
25. Peng H, Chen BB, Tang LL, Chen L, Li WF, Zhang Y, et al. Et Al: Prognostic Value of Nutritional Risk Screening 2002 Scale in Nasopharyngeal Carcinoma: A Large-Scale Cohort Study. Cancer Sci (2018) 109:1909–19. doi: 10.1111/cas.13603
26. Pan X, Wang C, Li R, Su L, Zhang M, Cai C, et al. Applicability of the Nutrition Risk Screening 2002 Combined With a Patient-Generated Subjective Global Assessment in Patients With Nasopharyngeal Carcinoma. Cancer Manag Res (2020) 12:8221–7. doi: 10.2147/CMAR.S261945
27. McDowell LJ, Rock K, Xu W, Chan B, Waldron J, Lu L, et al. Long-Term Late Toxicity, Quality of Life, and Emotional Distress in Patients With Nasopharyngeal Carcinoma Treated With Intensity Modulated Radiation Therapy. Int J Radiat Oncol Biol Phys (2018) 102:340–52. doi: 10.1016/j.ijrobp.2018.05.060
28. Cupp MA, Cariolou M, Tzoulaki I, Aune D, Evangelou E, Berlanga-Taylor AJ. Neutrophil to Lymphocyte Ratio and Cancer Prognosis: An Umbrella Review of Systematic Reviews and Meta-Analyses of Observational Studies. BMC Med (2020) 18:360. doi: 10.1186/s12916-020-01817-1
29. Ehrsson YT, Hellstrom PM, Brismar K, Sharp L, Langius-Eklof A, Laurell G. Explorative Study on the Predictive Value of Systematic Inflammatory and Metabolic Markers on Weight Loss in Head and Neck Cancer Patients Undergoing Radiotherapy. Support Care Cancer (2010) 18:1385–91. doi: 10.1007/s00520-009-0758-4
30. Hong JS, Hua YJ, Su L, Zhang HR, Lv WL, Chen XY, et al. Modified-Nutrition Index Is a Significant Prognostic Factor for the Overall Survival of the Nasopharyngeal Carcinoma Patients Who Undergo Intensity-Modulated Radiotherapy. Nutr Cancer (2017) 69:1011–8. doi: 10.1080/01635581.2017.1359311
31. OuYang PY, Zhang LN, Tang J, Lan XW, Xiao Y, Gao YH, et al. Evaluation of Body Mass Index and Survival of Nasopharyngeal Carcinoma by Propensity-Matched Analysis: An Observational Case-Control Study. Med (Baltimore) (2016) 95:e2380. doi: 10.1097/MD.0000000000002380
32. Lavriv DS, Neves PM, Ravasco P. Should Omega-3 Fatty Acids be Used for Adjuvant Treatment of Cancer Cachexia? Clin Nutr ESPEN (2018) 25:18–25. doi: 10.1016/j.clnesp.2018.02.006
33. Mirza KA, Pereira SL, Voss AC, Tisdale MJ. Comparison of the Anticatabolic Effects of Leucine and Ca-Beta-Hydroxy-Beta-Methylbutyrate in Experimental Models of Cancer Cachexia. Nutrition (2014) 30:807–13. doi: 10.1016/j.nut.2013.11.012
34. Eley HL, Russell ST, Tisdale MJ. Attenuation of Depression of Muscle Protein Synthesis Induced by Lipopolysaccharide, Tumor Necrosis Factor, and Angiotensin II by Beta-Hydroxy-Beta-Methylbutyrate. Am J Physiol Endocrinol Metab (2008) 295:E1409–1416. doi: 10.1152/ajpendo.90530.2008
35. Hsieh LC, Chien SL, Huang MS, Tseng HF, Chang CK. Anti-Inflammatory and Anticatabolic Effects of Short-Term Beta-Hydroxy-Beta-Methylbutyrate Supplementation on Chronic Obstructive Pulmonary Disease Patients in Intensive Care Unit. Asia Pac J Clin Nutr (2006) 15:544–50. doi: 10.1016/j.appet.2005.10.003
Keywords: nasopharyngeal carcinoma, PG-SGA, neutrophil-to-lymphocyte ratio (NLR), malnutrition, systemic inflammation, chemoradiotherapy
Citation: Wang X, Yang M, Ge Y, Tang M, Rao B, Chen Y, Xu H, Cong M, Guo Z and Shi H (2021) Association of Systemic Inflammation and Malnutrition With Survival in Nasopharyngeal Carcinoma Undergoing Chemoradiotherapy: Results From a Multicenter Cohort Study. Front. Oncol. 11:766398. doi: 10.3389/fonc.2021.766398
Received: 29 August 2021; Accepted: 06 October 2021;
Published: 26 October 2021.
Edited by:
Xiaobiao Zhang, Fudan University, ChinaReviewed by:
Tianwen Gao, Nanjing Medical University, ChinaYongzhen Mo, Central South University, China
Copyright © 2021 Wang, Yang, Ge, Tang, Rao, Chen, Xu, Cong, Guo and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hanping Shi, shihp@ccmu.edu.cn
 Xin Wang1,2
Xin Wang1,2 Yizhong Ge
Yizhong Ge Meng Tang
Meng Tang Hongxia Xu
Hongxia Xu Zengqing Guo
Zengqing Guo Hanping Shi
Hanping Shi