- Department of Nuclear Medicine, West China Hospital, Sichuan University, Chengdu, China
Background: Diagnosing the biochemical recurrence (BCR) of prostate cancer (PCa) is a clinical challenge, and early detection of BCR can help patients receive optimal treatment. We conducted a meta-analysis to define the diagnostic accuracy of PET/CT using 18F-labeled choline, fluciclovine, and prostate-specific membrane antigen (PSMA) in patients with BCR.
Methods: Multiple databases were searched until March 30, 2021. We included studies investigating the diagnostic accuracy of 18F-choline, 18F-fluciclovine, and 18F-PSMA PET/CT in patients with BCR. The pooled sensitivity, specificity, and detection rate of 18F-labeled tracers were calculated with a random-effects model.
Results: A total of 46 studies met the included criteria; 17, 16, and 13 studies focused on 18F-choline, fluciclovine, and PSMA, respectively. The pooled sensitivities of 18F-choline and 18F-fluciclovine were 0.93 (95% CI, 0.85–0.98) and 0.80 (95% CI, 0.65–0.897), and the specificities were 0.91 (95% CI, 0.73–0.97) and 0.66 (95% CI, 0.50–0.79), respectively. The pooled detection rates of 18F-labeled choline, fluciclovine and PSMA were 66, 74, and 83%, respectively. Moreover, the detection rates of 18F-labeled choline, fluciclovine, and PSMA were 35, 23, and 58% for a PSA level less than 0.5 ng/ml; 41, 46, and 75% for a PSA level of 0.5–0.99 ng/ml; 62, 57, and 86% for a PSA level of 1.0–1.99 ng/ml; 80, 92, and 94% for a PSA level more than 2.0 ng/ml.
Conclusion: These three 18F-labeled tracers are promising for detecting BCR in prostate cancer patients, with 18F-choline showing superior diagnostic accuracy. In addition, the much higher detection rates of 18F-PSMA showed its superiority over other tracers, particularly in low PSA levels.
Systematic Review Registration: PROSPERO, identifier CRD42020212531.
Introduction
Prostate cancer (PCa) is one of the most common malignancy in men worldwide and is also the fifth major cause of cancer-related death in men. It is estimated that over 300,000 PCa-related deaths occur in 2018 (1). In addition to its high morbidity and mortality, the recurrence and metastasis of prostate cancer are also troublesome in clinical practice (2, 3).
It is challenging to detect initial recurrence and metastasis after prior treatment because of few obvious characteristics on early recurrent or metastatic lesions. PCa recurrence is usually considered when observing a rise in the serum prostate-specific antigen (PSA) level. This is regarded as biochemical recurrence (BCR) of PCa, and the definition of BCR is a serum PSA level over a threshold of 0.2 ng/ml twice after radical prostatectomy (RP) or an absolute increase in PSA level of 2 ng/ml over the lowest posttreatment PSA level after radiation therapy (RT) (4, 5).
The key issue for patients with BCR is the early and correct identification of recurrent or metastatic disease, which is essential for further devising treatment strategies since treatment varies based on the presence of local recurrence, regional lymph node and distant viscera or bone metastasis (6). Conventional imaging modalities consisting of CT, bone scan, and MRI have been used for patients with advanced PCa, but their roles in detecting minimal or occult lesions are limited (7, 8). These conventional imaging modalities also have low sensitivity and specificity in detecting patients with BCR, especially those with a low PSA level. According to the 2020 American Society of Clinical Oncology (ASCO) guidelines, next-generation imaging (NGI) such as PET/CT, PET/MRI, and whole-body MRI is recommended for use in patients with rising PSA after prior treatment when conventional imaging findings are negative (9). Radioactive tracers such as choline and fluciclovine have been used for prostate cancer staging, restaging, and treatment response evaluation. Meanwhile, prostate-specific membrane antigen (PSMA), a new radiopharmaceutical that binds to prostate cancer-specific target, has demonstrated outstanding detection rate for recurrent or metastatic lesions among patients with BCR.
Choline is an essential element of phospholipids in the cellular wall, and the increased uptake of choline means increased metabolism of the cell membrane components of malignant tumors (10). 11C-choline was approved by the Food and Drug Administration (FDA) in 2012, but its short half-life limits its widespread use in PET/CT centers without onsite cyclotrons. Later, 18F-labeled choline was developed, and its longer half-life has solidified 18F-choline PET/CT as a significant imaging modality in patients with suspected PCa recurrence (11, 12). 18F-Fluciclovine (anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid, 18F-FACBC), as a synthetic amino acid that is upregulated in PCa, is an option for molecular imaging in patients with BCR, which was approved by the FDA in 2016 (13, 14). The main advantage of 18F-fluciclovine is its low urinary excretion, which allows for better detection and localization of PCa recurrence in patients with rising PSA level (15). PSMA is a type 2 transmembrane protein that is more highly expressed in the prostate cancer cell membrane than in normal tissues (16–18). Therefore, PSMA has become a promising target for imaging prostate cancer (19). 68Ga-PSMA PET/CT has been proven to improve the detection of metastatic disease and the monitoring of treatment effects in patients with PCa (20). Most recently, 68Ga-PSMA-11, as the first PSMA PET agent, has been approved by the FDA. 18F-labeled PSMA has also begun to be used in clinical practice, and its long half-life and high resolution in PET/CT images have further increased the detection rate of PSMA-targeted imaging in subtle or occult metastases (21, 22). Moreover, 18F-PSMA-1007 PET/CT can differentiate local recurrence from physical uptake in the urinary bladder or ureter due to non-urinary clearance (23, 24).
Some previous studies have compared the diagnostic roles of 11C-choline, 18F-fluciclovine, and 68Ga-PSMA PET/CT in patients with BCR, showing that 68Ga-PSMA PET/CT has a superior detection rate (25). Even so, there have been some clinical challenges existing in 68Ga-PSMA PET/CT due to certain shortcomings including its short half-life, non-ideal energies and the limited availability of 68Ga. Compared with 68Ga and 11C, 18F, as a longer half-life nuclide, has many advantages such centralized production in a cyclotron facility and more favorable positron energies for imaging, thereby motivating the development of 18F-labeled analogs. Currently, growing clinical experience has revealed the high diagnostic accuracy of some 18F-labeled tracers in PCa patients with BCR. However, the effectiveness of 18F-labeled choline, fluciclovine, and PSMA remains unclear because of limited number of studies. Herein, we aimed to perform a meta-analysis to review and compare the diagnostic value of 18F-labeled choline, fluciclovine, and PSMA PET/CT imaging for detecting BCR in patients with PCa, in order to provide better creditability for clinical practice.
Methods
The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines was used for our study (26). Our review has registered on the international prospective register of systematic reviews (PROSPERO) (CRD 42020198861).
Search Strategy
A literature research was conducted with scientific databases, including PubMed, EMBASE, and Web of Science, until March 30, 2021. A search algorithm was developed based on a combination of keywords (“choline” OR “fluciclovine” OR “FACBC” OR “PSMA” OR “DCFPyL” OR “DCFBC” OR “1007”) AND (“prostate cancer” OR “prostate neoplasm”) AND (“biochemical recurrence” OR “biochemical failure”) AND (“PET/CT” OR “positron emission tomography/computed tomography”) AND (“18F” OR “fluorine”).
Two authors independently screened and evaluated these studies. The reference lists of all relevant studies were further checked to find more suitable studies. A third author was responsible for disagreement and solved the controversy between two authors through discussion.
Selection of Studies
Studies using 18F-labeled tracers such as 18F-choline, 18F-fluciclovine, and 18F-PSMA were evaluated. Studies were included according to the following criteria: (a) sample size >10; (b) patients who had evidence of BCR underwent PET/CT; (c) studies evaluating the diagnostic accuracy of 18F-labeled tracers in prostate cancer patients with BCR; (d) histological results, imaging, or clinical follow-up as a reference standard. Studies on other tracers were not included. Abstracts, reviews, and case reports were also not included. If the studies included duplicate patients, we reviewed and included the study with the largest sample or the most recent study performed. The included studies were limited to those published in English.
Quality Assessment
The quality of included studies was critically assessed by two independent authors according to the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tool. This tool comprises four domains (patient selection, index test, reference standard, and flow and timing), and each domain was used to assess the risk of bias. Next, applicability was also considered according to patient selection, the index test, and the reference standard.
Data Extraction
Two authors collected various parameters and outcomes from each eligible study as follows: author, country, publication year, study design, number of patients, age, pre-PET PSA level, reference criteria, scanner model, ligands, and injection dose and the detection rate as well as true positive (TP), false positive (FP), false negative (FN), and true negative (TN) PET/CT with different tracers in patients with BCR. All discrepancies were resolved by consensus and ultimately based on the decision of the third author.
Statistical Analysis
For studies reporting the diagnostic performance of 18F-PSMA, 18F-choline, and 18F-fluciclovine PET/CT in patients with BCR, 2 × 2 table was used to calculate TP, FP, TN, and FN. The pooled sensitivity and specificity were calculated by a random-effects model. We developed a hierarchical summary receiver operating curve and calculated the area under the curve. We presented forest plots with 95% confidence intervals (CIs) for the sensitivity and specificity of each study. In addition, the detection rates of PET tracers were extracted and pooled using a random-effects model. If possible, subgroup analysis was considered based on different PSA serum values.
Heterogeneity within studies was evaluated using Cochran’s Q test and the I² statistic (27). An I² value greater than 50% was indicative of substantial heterogeneity. The funnel plot test and Egger’s test were used to assess the publication bias. All statistical analyses were performed using Stata 15.0 and RevMan 5.3. P-value <0.05 was considered to be statistically significant (28).
Results
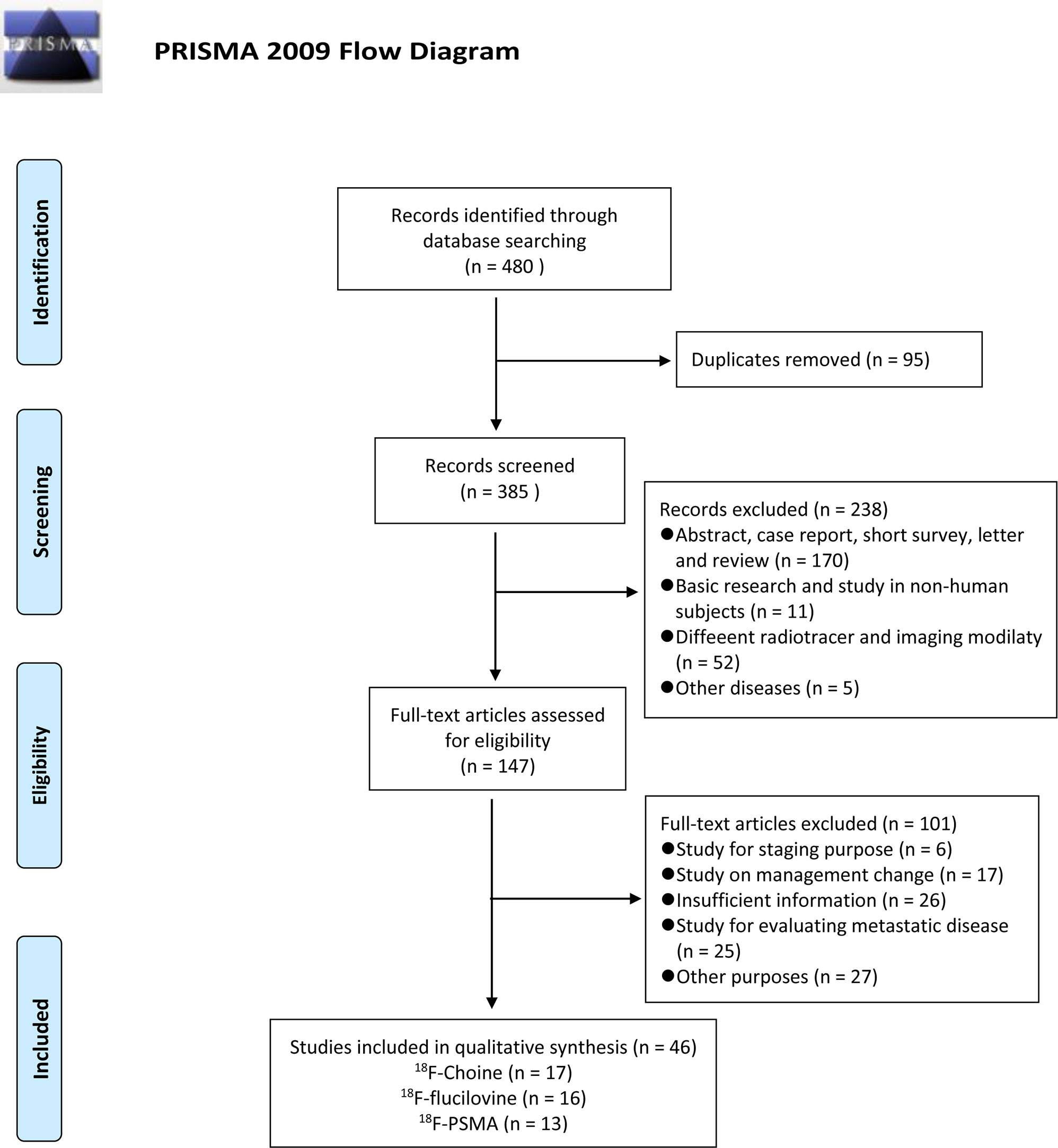
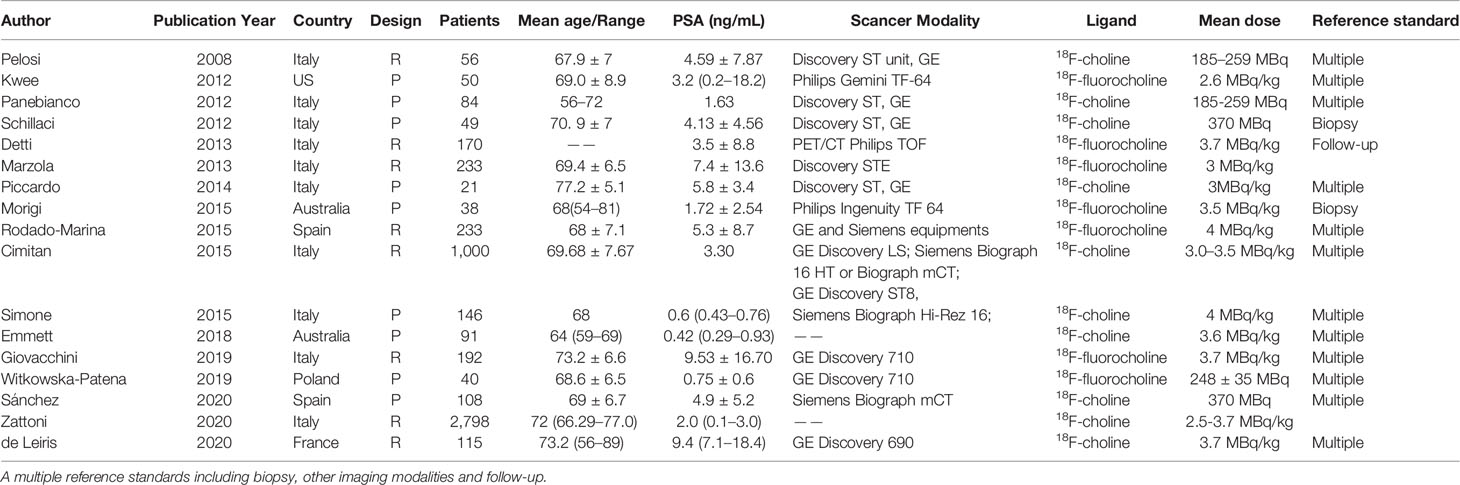
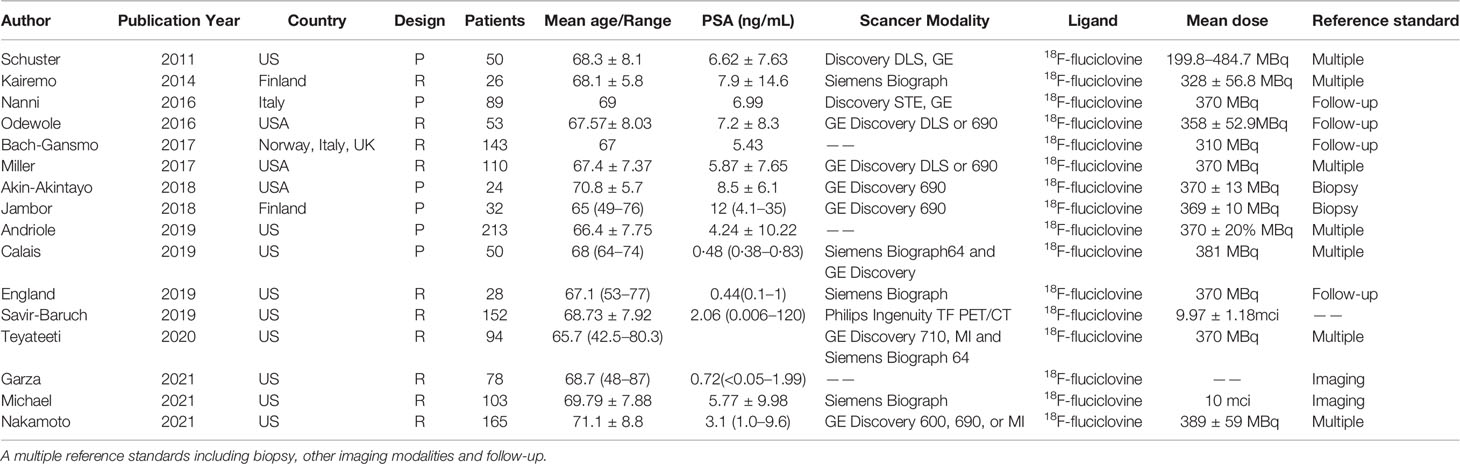
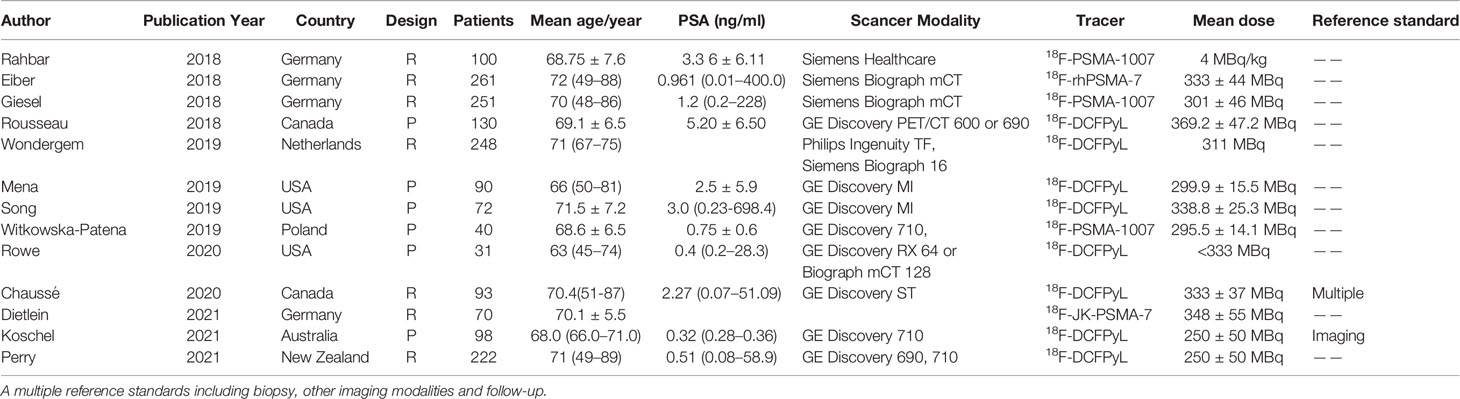
The flow chart demonstrates an overview of the search and selection process (Figure 1). The initial search yielded 480 studies, of which 95 were duplicates. Subsequently, after reviewing the titles and abstracts, we excluded 238 studies for the following reasons: 170 studies were case reports, reviews, and academic meeting abstracts, 11 were basic studies, five applications in other diseases, and 52 studies used different radiotracers and imaging modalities. Of the remaining studies, 75 studies were not relevant to our aims, and most of them investigated the impact of novel PET/CT tracers in treatment management for patients with PCa or focused on evaluating metastatic disease. In addition, 26 studies did not provide sufficient information and were excluded. Thus, only 46 studies were finally included. Of these, 17 studies focused on the role of 18F-choline PET/CT in prostate cancer patients with BCR (29–45). The numbers of included studies regarding 18F-fluciclovine and 18F-PSMA PET/CT were 16 and 13, respectively (46–74). Tables 1–3 outline the characteristics of each eligible study.
Quality Assessment
Figures 2A–C show the results of the quality assessment of each eligible study for 18F-choline, 18F-fluciclovine, and 18F-PSMA, respectively. Patient selection was not considered the source of bias because all studies had qualified patient selection criteria. For the index test and reference standard, some studies did not adopt the blinding method when interpreting the positive scan of the PET/CT findings, and we rated these studies as high or unclear levels regarding the risk of bias and applicability concern. Similarly, unclear or high levels were displayed on the applicability concern of flow and timing because of the different follow-up times and multiple reference standards.
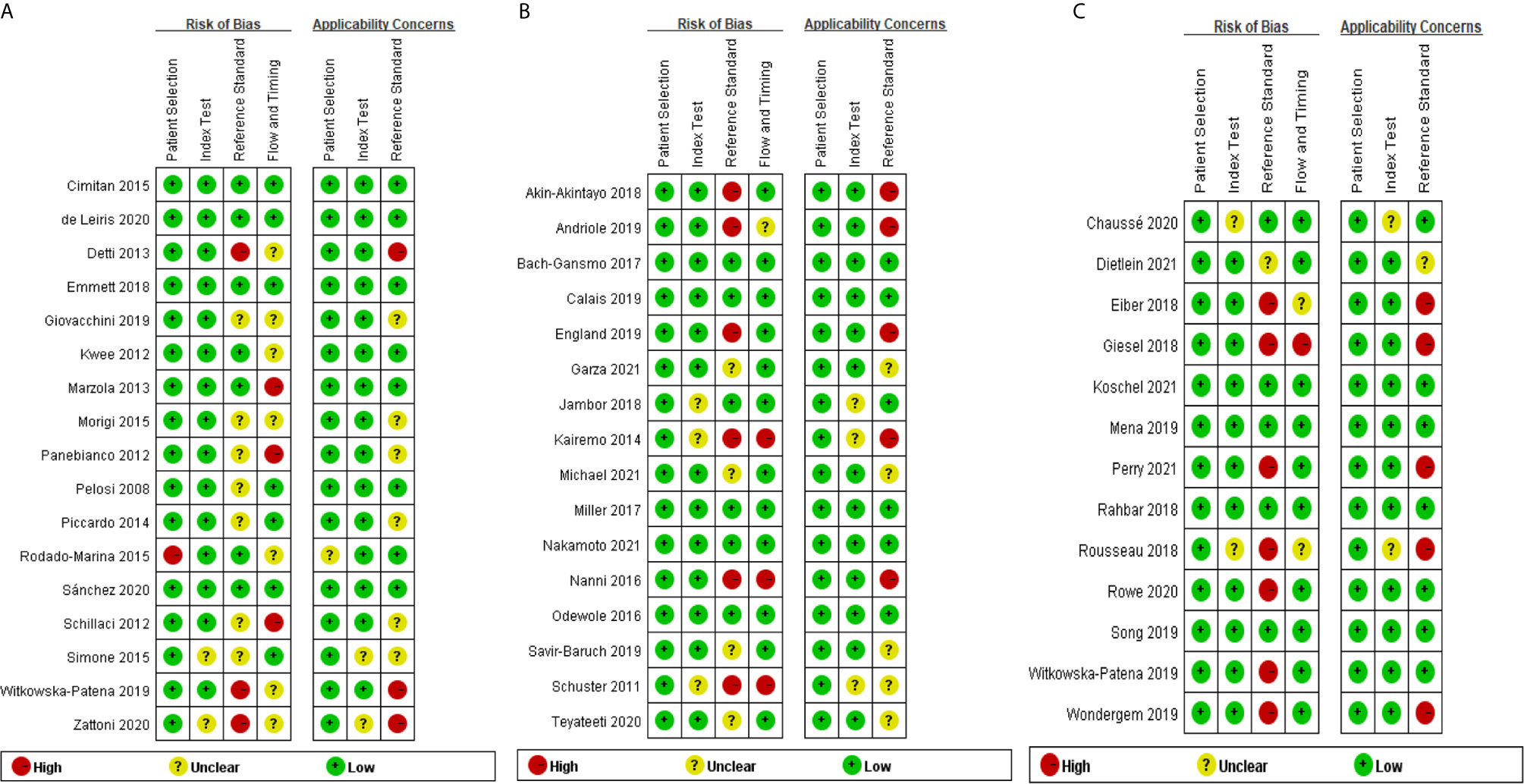
Figure 2 (A) Quality Assessment of Diagnostic Accuracy Studies-2 analysis of study bias in 18F-choline cohort. (B) Quality Assessment of Diagnostic Accuracy Studies-2 analysis of study bias in 18F-fluciclovine cohort. (C) Quality Assessment of Diagnostic Accuracy Studies-2 analysis of study bias in 18F prostate-specific membrane antigen (PSMA) cohort.
Diagnostic Performance of 18F-Choline and 18F-Fluciclovine PET/CT
Seventeen studies reported the diagnostic performance of 18F-choline, and the summary sensitivity and specificity of 18F-choline PET/CT in patients with BCR were 0.93 (95% CI, 0.85–0.96) and 0.91 (95% CI, 0.73–0.97), respectively (Figure 3). The summary sensitivity and specificity drawn from studies on 18F-fluciclovine were 0.80 (95% CI, 0.65–0.89) and 0.66 (95% CI, 0.50–0.79), respectively (Figure 4). However, the summary sensitivity and specificity were not constructed for 18F-PSMA PET/CT imaging because these studies mostly focused on the detection rate in patients with BCR. Summary receiver operating characteristic (SROC) curves of 18F-choline and 18F-fluciclovine were demonstrated in Figures 5A, B.
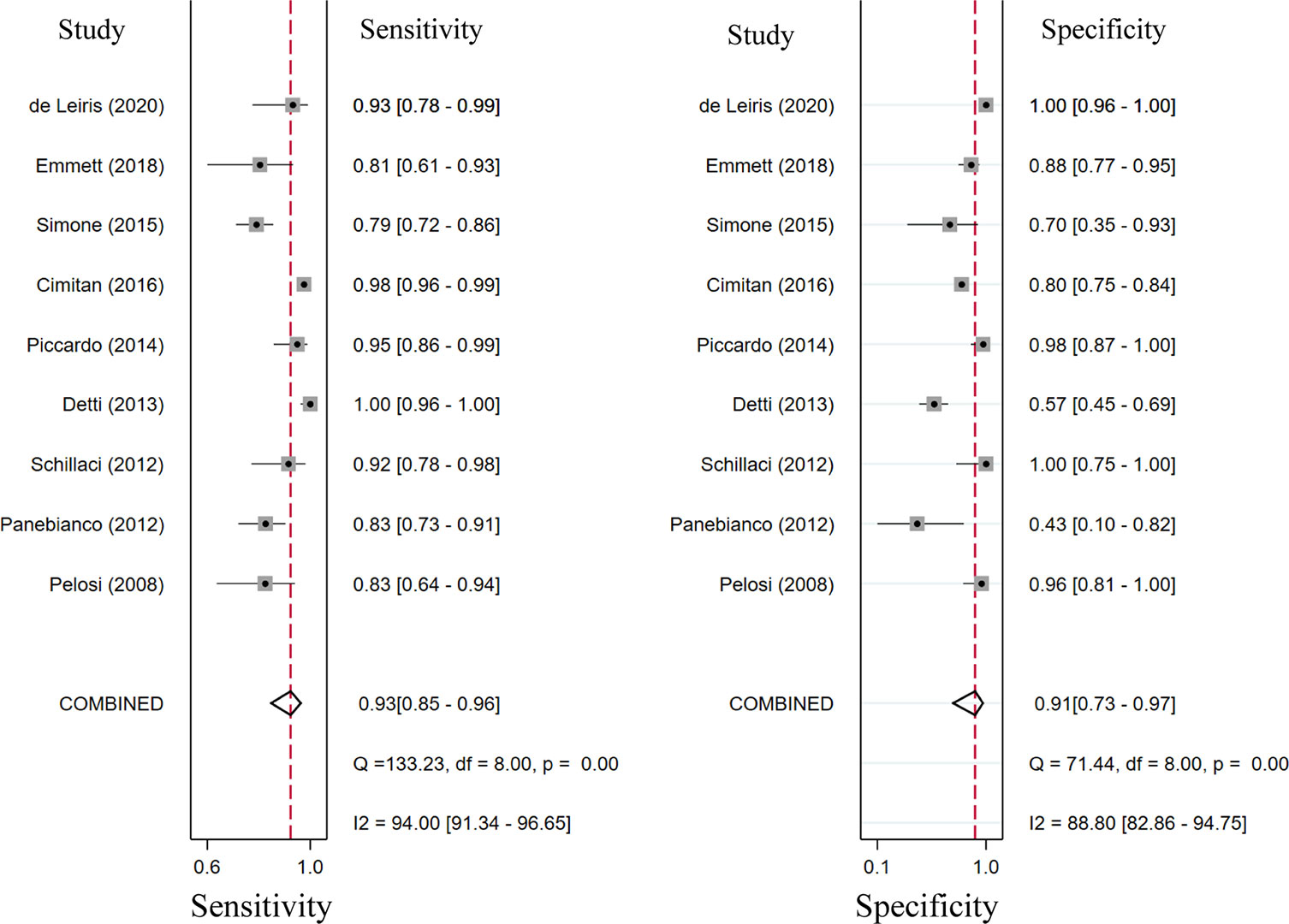
Figure 3 Forest plot of the proportion of 18F-choline positron emission tomography/computed tomography (PET/CT) sensitivity and specificity in prostate cancer patients with biochemical recurrence.
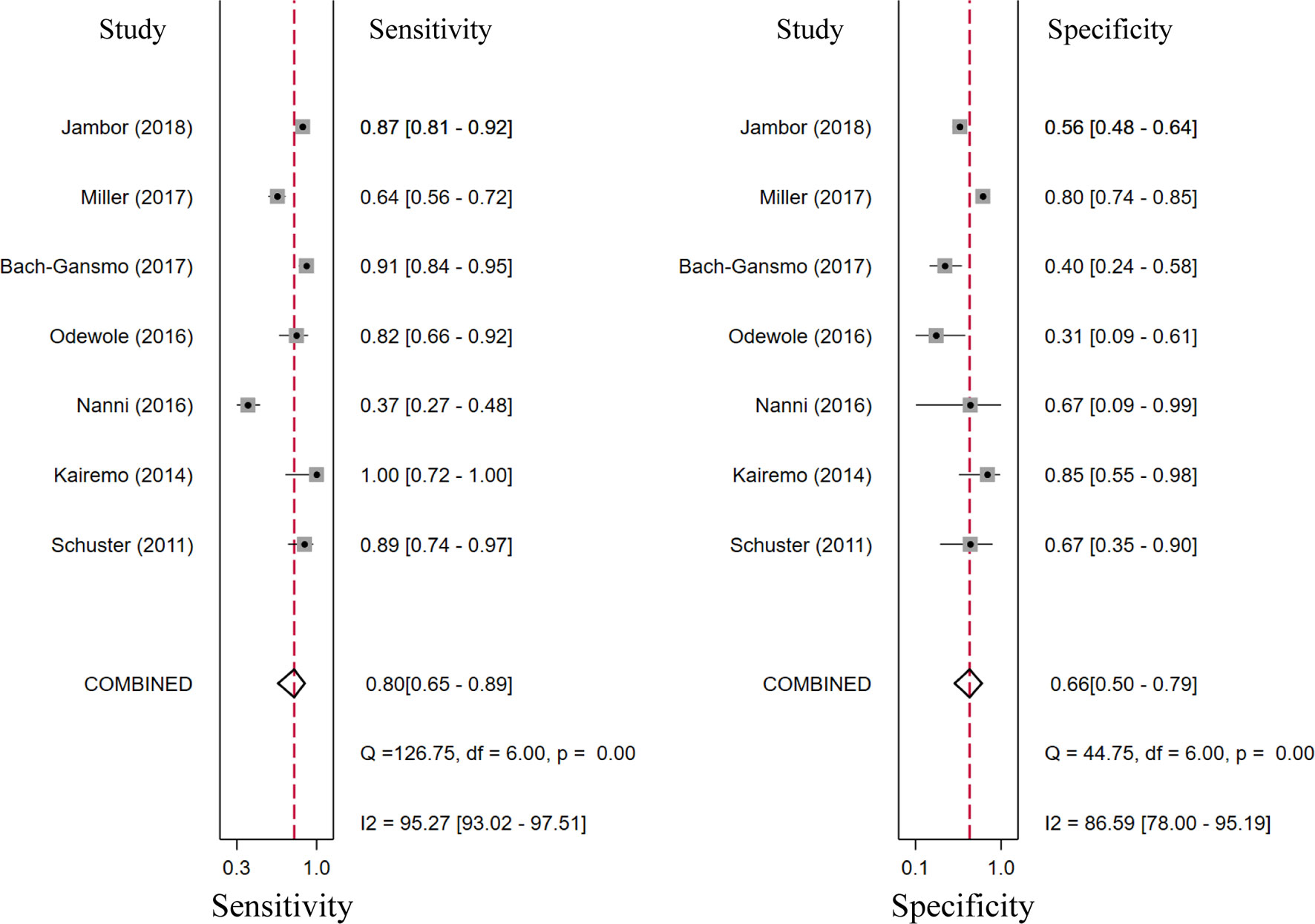
Figure 4 Forest plot of the proportion of 18F-fluciclovine PETCT sensitivity and specificity in prostate cancer patients with biochemical recurrence.

Figure 5 (A) SROC curve for the diagnostic accuracy of 18F-choline PET/CT in prostate cancer patients with biochemical recurrence. (B) SROC curve for the diagnostic accuracy of 18F-fluciclovine PET/CT in prostate cancer patients with biochemical recurrence. SROC, summary receiver operating characteristic.
Detection Rate of 18F-Choline, 18F-Fluciclovine, and 18F-PSMA PET/CT
The pooled detection rate of 18F-choline PET/CT was 66%, lower than 74% of 18F-fluciclovine PET/CT. In addition, the pooled detection rate of 18F-PSMA PET/CT was 83% (Figure 6). Meanwhile, the detection rates of 18F-labeled choline, fluciclovine, and PSMA were 35, 23, and 58% for a PSA level less than 0.5 ng/ml (Figure 7); 41, 46, and 75% for a PSA level of 0.5–0.99 ng/ml (Figure 8); 62, 57, and 86% for a PSA level of 1.0–1.99 ng/ml (Figure 9); 80, 92, and 94% for a PSA level more than 2.0 ng/ml (Figure 10).
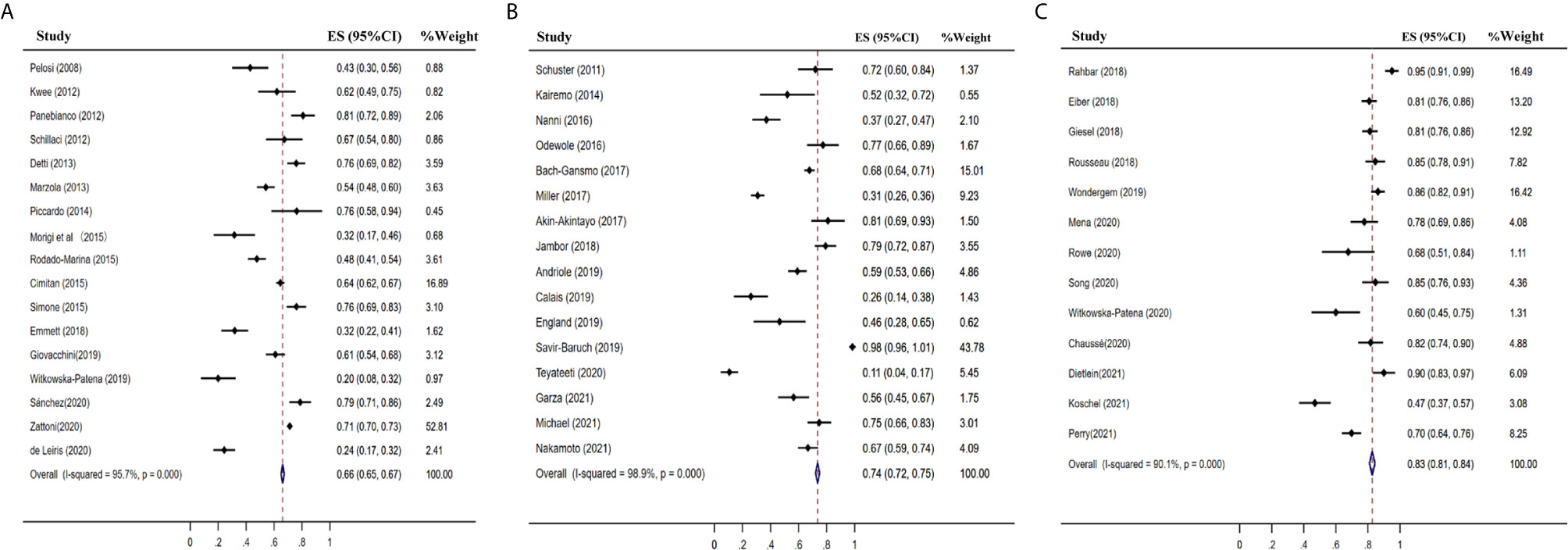
Figure 6 Forest plot of the proportion of 18F-labeled choline (A), fluciclovine (B) and PSMA (C) PET/CT positivity of prostate cancer patients with biochemical recurrence.
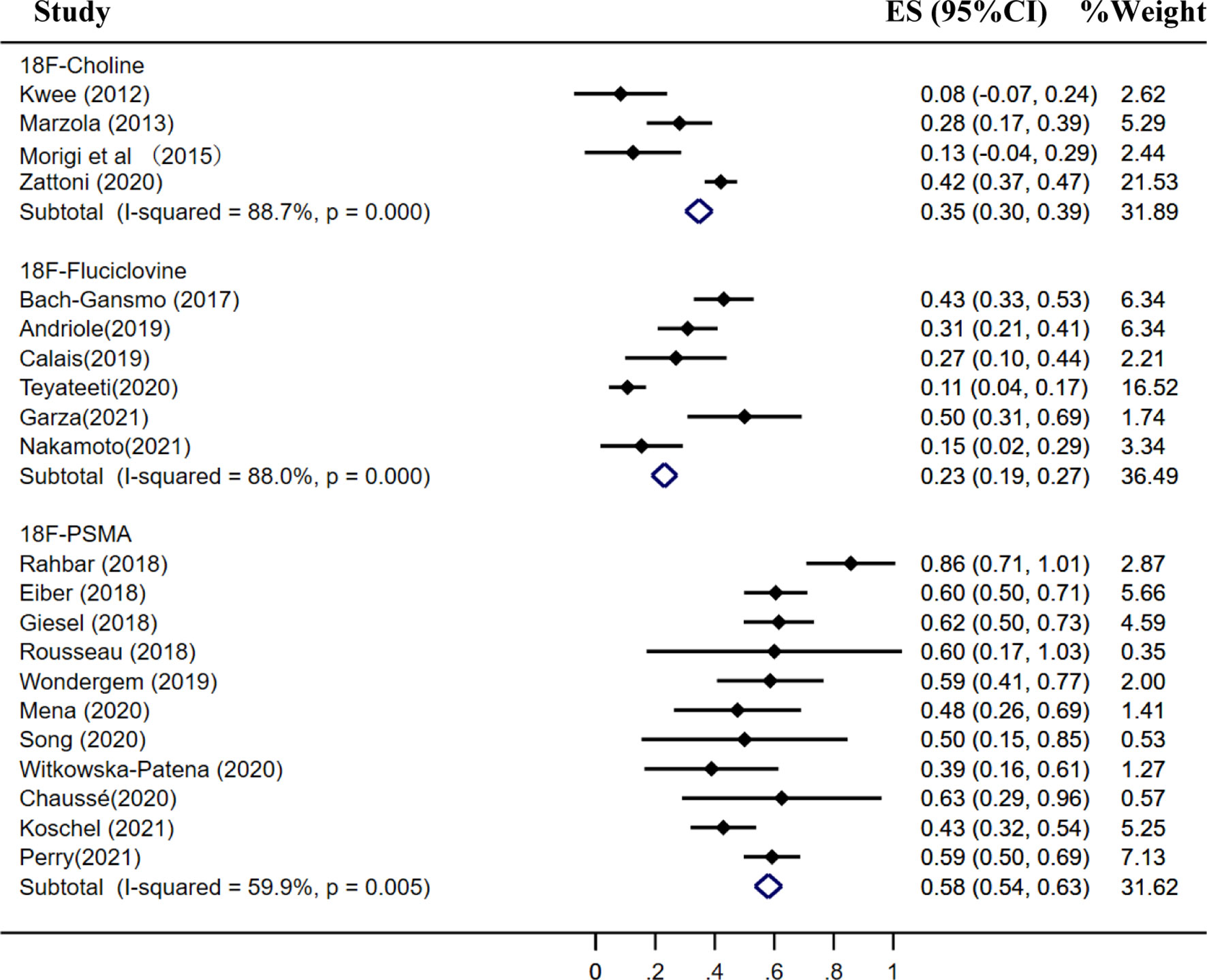
Figure 7 Forest plot of the proportion of 18F-labeled choline, fluciclovine and PSMA positivity of prostate cancer patients with BCR for PSA less than 0.5 ng/ml.
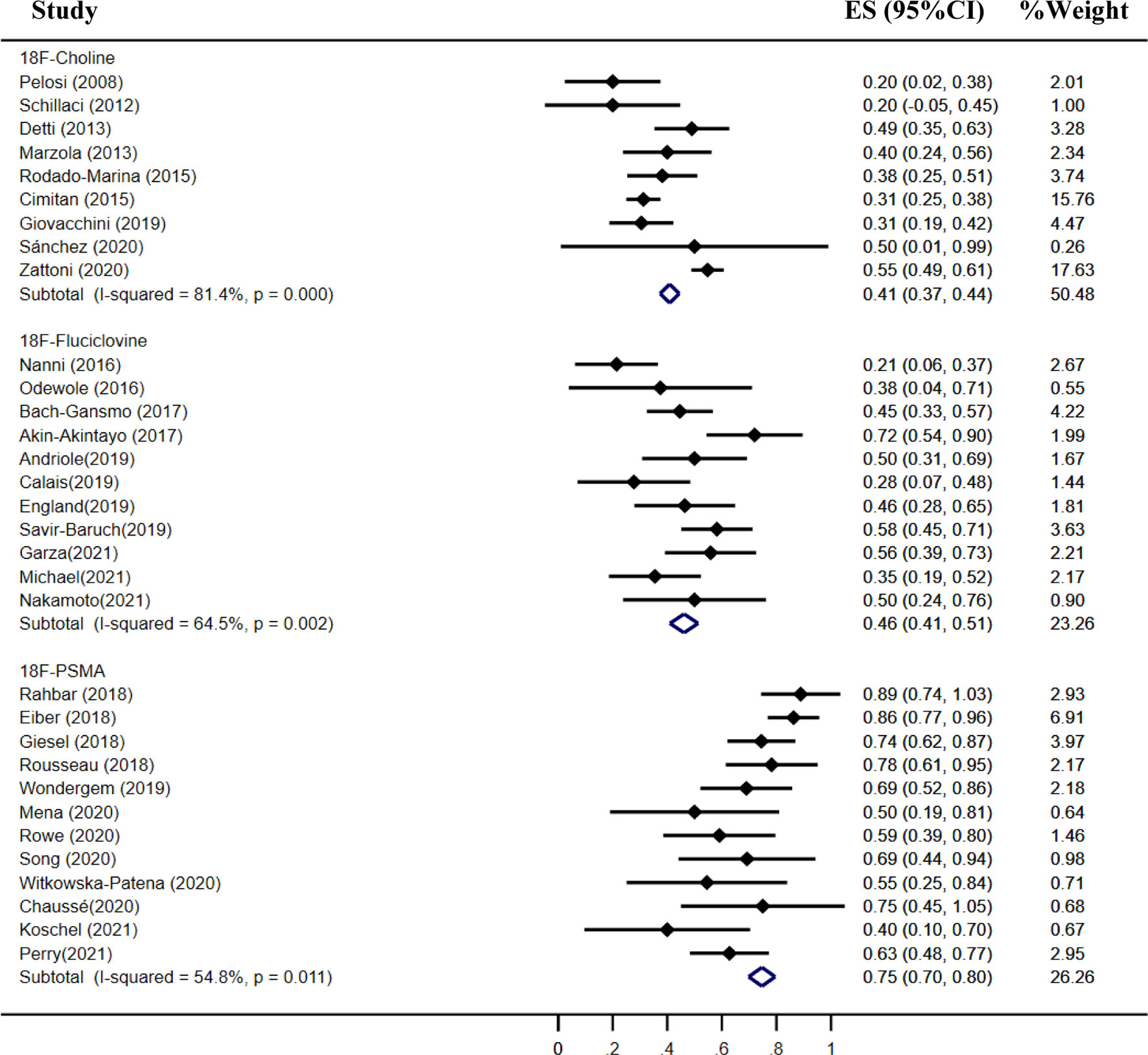
Figure 8 Forest plot of the proportion of 18F-labeled choline, fluciclovine, and PSMA positivity of prostate cancer patients with BCR for PSA 0.5–0.99 ng/ml.
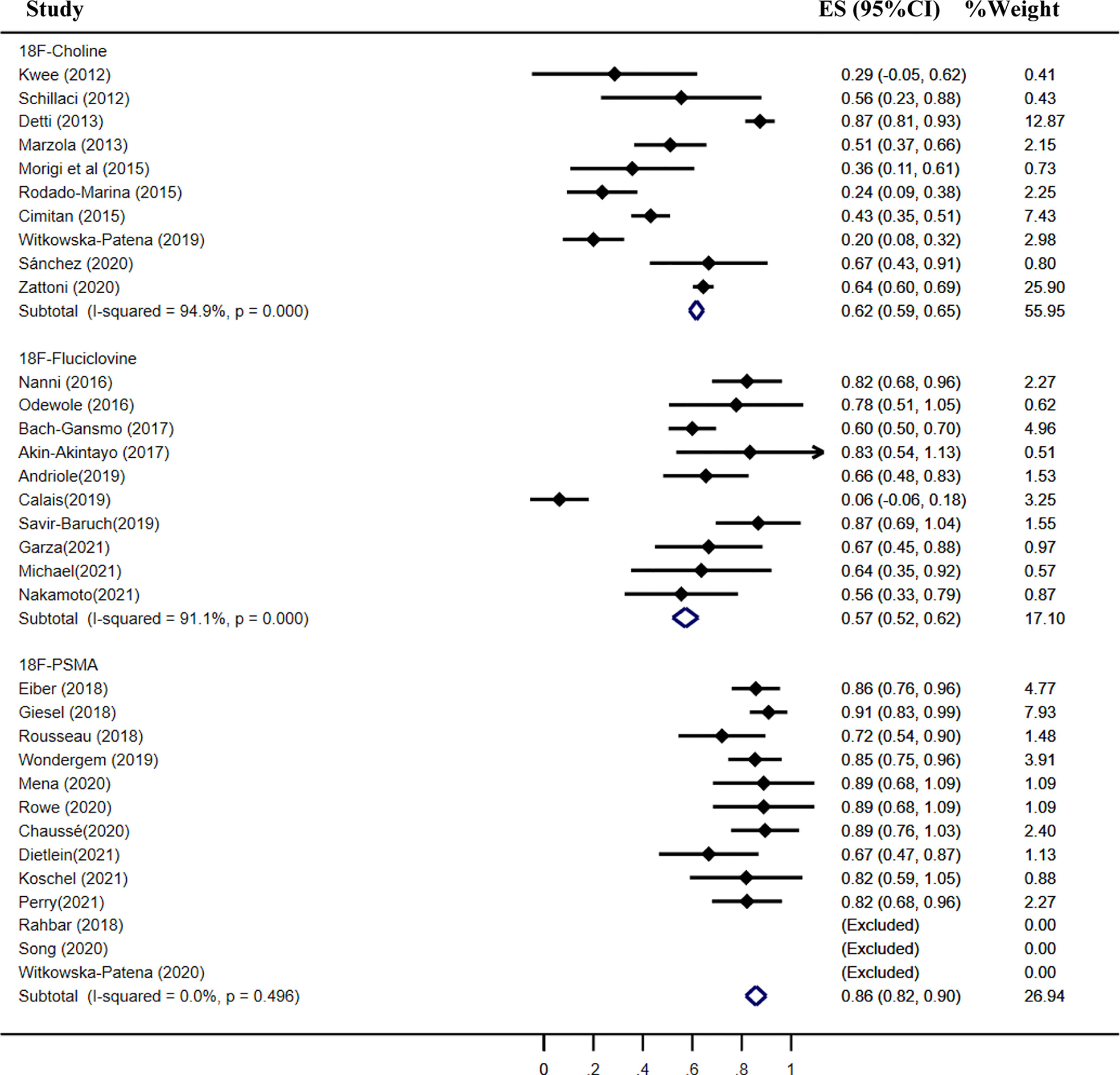
Figure 9 Forest plot of the proportion of 18F-labeled choline, fluciclovine, and PSMA positivity of prostate cancer patients with BCR for PSA 1.0–1.99 ng/ml.
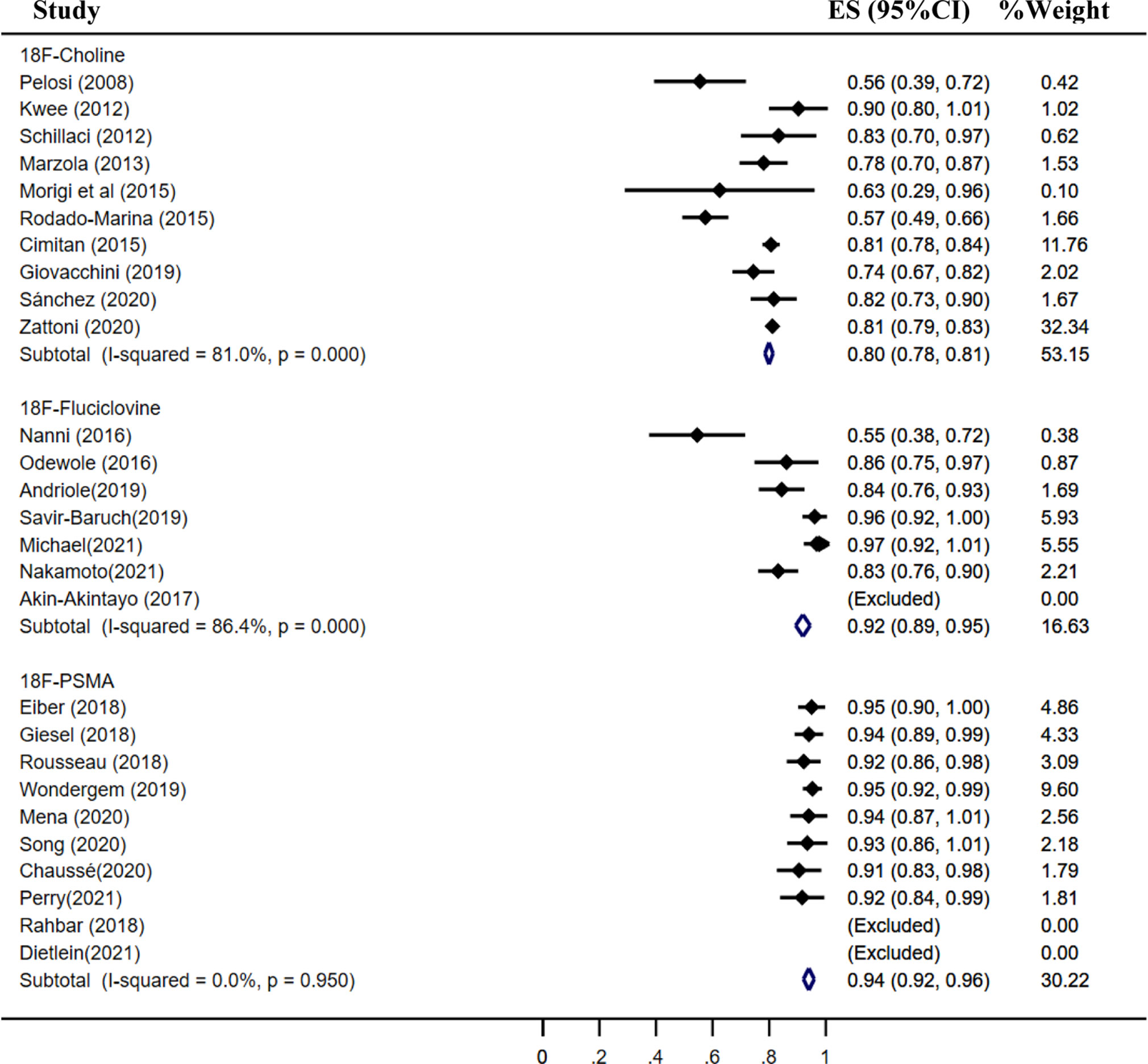
Figure 10 Forest plot of the proportion of 18F-labeled choline, fluciclovine, and PSMA positivity of prostate cancer patients with BCR for PSA more than 2.0 ng/ml.
Discussion
Our meta-analysis included studies investigating the diagnostic roles of three novel 18F-labeled tracers applied in prostate cancer patients with BCR. From our study, the summary sensitivity and specificity of 18F-choline and 18F-fluciclovine PET/CT were 0.93 and 0.91, and 0.80 and 0.66, respectively. For the detection rate, the pooled detection rates of 18F-labeled choline, fluciclovine, and PSMA were 66, 74, and 83%, respectively. Meanwhile, we observed a higher detection rate of biochemically recurrent PCa with 18F-PSMA compared with choline and fluciclovine PET/CT for the different PSA level subgroups.
Multiple PET/CT radiotracers have been developed and experimented in recent years, motivating the wide use of PET/CT or PET/MRI in patients with PCa for staging, restaging, and response evaluation (75, 76). 18F-fluciclovine PET/CT showed a superior advantage over 11C-choline PET/CT in patients with BCR and further aid guiding decision-making in regard to patients’ treatment strategy (48, 77). In addition, PSMA PET/CT has shown superior diagnostic accuracy for recurrence and metastases of prostate cancer than fluciclovine and choline. A meta-analysis defined the diagnostic accuracy of PET/CT imaging using 11C-choline, 18F-fluciclovine, or 68Ga-PSMA, showing that 68Ga-PSMA PET/CT has a nearly equal sensitivity but the highest specificity among these tracers for PET/CT imaging in detecting biochemically recurrent PCa (25).
In contrast, our meta-analysis focused on only long-half radionuclides as 18F-labeled tracers and summarized the diagnostic accuracy of 18F-labeled choline, fluciclovine, and PSMA in detecting patients with BCR. Our study revealed that 18F-PSMA had the highest detection rate at different PSA levels, and the detection rate was related to the PSA level. These results were consistent with another meta-analysis that compared the detection rate of biochemically recurrent PCa between PSMA-targeted radiotracers and 18F-fluciclovine, finding that PSMA-targeted radiotracers demonstrate a greater detection rate than 18F-fluciclovine (78). A study compared prospectively paired 18F-fluciclovine and PSMA PET/CT scans for localizing recurrence of PCa after prostatectomy in patients with a PSA level <2.0 ng/ml (55). They found that PSMA PET/CT showed higher detection rates and should be the tracer choice when PET/CT imaging is considered for patients with biochemical recurrence after radical prostatectomy with low PSA concentrations (≤2.0 ng/ml). The same conclusion was drawn from another prospective study paired that compared 18F-PSMA and 18F-fluorocholine PET/CT in patients with BCR (42). The advantage of PSMA-targeted PET/CT imaging could be attributed to the high expression of PSMA in PCa and its metastases.
In late 2020, 68Ga-PSMA-11 became the first PSMA PET tracer to be approved by the FDA, which may facilitate widespread adaptation. Despite this, there also have been some limitations related to 68Ga-PSMA PET/CT because of the short half-life, non-ideal energies, and limited availability of 68Ga, limiting its clinical application in detecting occult or metastatic lesions in the prostate bed (62, 79). However, 18F-PSMA analogs seemed to be more favorable due to their longer half-life and a higher physical spatial resolution (23), and 18F-PSMA-1007, as a second-generation 18F-labeled PSMA tracer, demonstrated high labeling yields, better tumor uptake, and hepatobiliary excretion, making it an ideal PSMA-target tracer for diagnostic imaging in patients with BCR (21, 23). Our meta-analysis found the pooled detection rate with 18F-PSMA of 58% for a PSA level of less than 0.5 ng/ml, 75% for a PSA level of 0.5 to 0.99 ng/ml, and 86% for a PSA level of 1.0 to 1.99 ng/ml. These detection rates are equal or higher than those in recent studies involving 68Ga PSMA PET/CT (80, 81).
Compared with FDA approval of 68Ga-PSMA-11 in late 2020, 11C-choline and 18F-fluciclovine PET/CT have one temporary advantage as they have been granted FDA approval early. They were more accessible and used in the US and Europe. Many studies compared the diagnostic utility of 18F-fluciclovine with 11C-choline PET/CT imaging, showing a better performance in terms of lesion detection rate (48, 82). A recent meta-analysis demonstrated that 18F-fluciclovine had the similar sensitivity and detection rate compared with 11C-choline, but lower specificity than 11C-choline (83). Unlike the short physical half-life of 11C-choline, the radiofluorine of 18F-choline provides a long physical half-life (109.8 min), allowing for centralized manufacture and distribution. These intrinsic advantages of 18F labeling has made 18F-choline PET/CT valuable in staging patients with PCa and detecting recurrently PCa metastases after initial treatment (33, 84). There were limited studies in comparing directly to determine which imaging modality has a better diagnostic efficiency between 18F-choline and 18F-fluciclovine. In our meta-analysis, 18F-choline had a higher sensitivity and specificity than 18F-fluciclovine through assessing the summary sensitivity and specificity. 18F-choline also has better detection rates than 18F-fluciclovine at PSA levels under 0.5 ng/ml and 1.0–1.99 ng/ml, but the pooled detection rate of 18F-fluciclovine was higher than that of 18F-choline in biochemically recurrent PCa. This difference could be interpreted by different biological processes between amino acid transport and choline expression.
Limitations
There were several limitations to this study that should be mentioned. First, we only evaluated the diagnostic accuracy of both 18F-choline and 18F-fluciclovine PET/CT in patients with BCR, and the pooled sensitivity and specificity for 18F-PSMA PET/CT were not feasible because of insufficient published data. Second, there were significant heterogeneities among institutions, PET/CT scanners, radiotracers, and prior treatment of patients, which increased the risk of bias and led to significant heterogeneity among 18F-choline, 18F-fluciclovine and 18F-PSMA PET/CT. Third, most of the included studies were retrospective analyses, had small sample sizes, had limited reference standards, and lacked prospective, large sample, and interagent comparison studies. Fourth, there was publication bias according to Egger’s test regarding the included studies of 18F-choline, 18F-flocilovine, and 18F-PSMA PET/CT, limiting the interpretation of the data to some degree.
Conclusion
PET/CT imaging with 18F-choline, 18F-fluciclovine, and 18F-PSMA is promising in detecting prostate cancer patients with BCR. 18F-PSMA PET/CT demonstrated a significantly higher detection rate over 18F-choline and 18F-fluciclovine for different PSA levels, particularly in PSA level less than 2.0 ng/ml.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author Contributions
Conceived and designed the experiments: RW, GS, and RT. Performed the experiments: GS, RW, and MH. Analyzed the data: RW, GS, and RT. Analyzed tools: GS and MH. Wrote the paper: RW and RT. Provided critical input into the design and drafting of the manuscript: RW, GS, and RT. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (81971653).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2018) 68:394–424. doi: 10.3322/caac.21492
2. Hull GW, Rabbani F, Abbas F, Wheeler TM, Kattan MW, Scardino PT. Cancer Control With Radical Prostatectomy Alone in 1,000 Consecutive Patients. J Urol (2002) 167:528–34. doi: 10.1097/00005392-200202000-00018
3. Freedland SJ, Humphreys EB, Mangold LA, Eisenberger M, Dorey FJ, Walsh PC, et al. Risk of Prostate Cancer-Specific Mortality Following Biochemical Recurrence After Radical Prostatectomy. JAMA (2005) 294:433–9. doi: 10.1001/jama.294.4.433
4. Kuban DA, Levy LB, Potters L, Beyer DC, Blasko JC, Moran BJ, et al. Comparison of Biochemical Failure Definitions for Permanent Prostate Brachytherapy. Int J Radiat Oncol Biol Phys (2006) 65:1487–93. doi: 10.1016/j.ijrobp.2006.03.027
5. Stephenson AJ, Scardino PT, Kattan MW, Pisansky TM, Slawin KM, Klein EA, et al. Predicting the Outcome of Salvage Radiation Therapy for Recurrent Prostate Cancer After Radical Prostatectomy. J Clin Oncol (2007) 25:2035–41. doi: 10.1200/JCO.2006.08.9607
6. Fanti S, Minozzi S, Antoch G, Banks I, Briganti A, Carrio I, et al. Consensus on Molecular Imaging and Theranostics in Prostate Cancer. Lancet Oncol (2018) 19:e696–708. doi: 10.1016/S1470-2045(18)30604-1
7. Hovels AM, Heesakkers RAM, Adang EM, Jager GJ, Strum S, Hoogeveen YL, et al. The Diagnostic Accuracy of CT and MRI in the Staging of Pelvic Lymph Nodes in Patients With Prostate Cancer: A Meta-Analysis. Clin Radiol (2008) 63:387–95. doi: 10.1016/j.crad.2007.05.022
8. Filson CP, Natarajan S, Margolis DJ, Huang J, Lieu P, Dorey FJ, et al. Prostate Cancer Detection With Magnetic Resonance-Ultrasound Fusion Biopsy: The Role of Systematic and Targeted Biopsies. Cancer (2016) 122:884–92. doi: 10.1002/cncr.29874
9. Trabulsi EJ, Rumble RB, Jadvar H, Hope T, Pomper M, Turkbey B, et al. Optimum Imaging Strategies for Advanced Prostate Cancer: ASCO Guideline. J Clin Oncol (2020) 38:1963–96. doi: 10.1200/JCO.19.02757
10. Zeisel SH. Dietary Choline: Biochemistry, Physiology, and Pharmacology. Annu Rev Nutr (1981) 1:95–121. doi: 10.1146/annurev.nu.01.070181.000523
11. Evangelista L, Zattoni F, Guttilla A, Saladini G, Zattoni F, Colletti PM, et al. Choline PET or PET/CT and Biochemical Relapse of Prostate Cancer: A Systematic Review and Meta-Analysis. Clin Nucl Med (2013) 38:305–14. doi: 10.1097/RLU.0b013e3182867f3c
12. Krause BJ, Souvatzoglou M, Treiber U. Imaging of Prostate Cancer With PET/CT and Radioactively Labeled Choline Derivates. Urol Oncol-Seminars Orig Investigations (2013) 31:427–35. doi: 10.1016/j.urolonc.2010.08.008
13. Oka S, Okudaira H, Yoshida Y, Schuster DM, Goodman MM, Shirakami Y. Transport Mechanisms of Trans-1-Amino-3-Fluoro[1-(14)C]Cyclobutanecarboxylic Acid in Prostate Cancer Cells. Nucl Med Biol (2012) 39:109–19. doi: 10.1016/j.nucmedbio.2011.06.008
14. Parent EE, Schuster DM. Update on (18)F-Fluciclovine PET for Prostate Cancer Imaging. J Nucl Med (2018) 59:733–9. doi: 10.2967/jnumed.117.204032
15. Bach-Gansmo T, Nanni C, Nieh PT, Zanoni L, Bogsrud TV, Sletten H, et al. Multisite Experience of the Safety, Detection Rate and Diagnostic Performance of Fluciclovine (F-18) Positron Emission Tomography/Computerized Tomography Imaging in the Staging of Biochemically Recurrent Prostate Cancer. J Urol (2017) 197:676–82. doi: 10.1016/j.juro.2016.09.117
16. Wright GL Jr, Haley C, Beckett ML, Schellhammer PF. Expression of Prostate-Specific Membrane Antigen in Normal, Benign, and Malignant Prostate Tissues. Urol Oncol (1995) 1:18–28. doi: 10.1016/1078-1439(95)00002-y
17. Wright GL Jr., Grob BM, Haley C, Grossman K, Newhall K, Petrylak D, et al. Upregulation of Prostate-Specific Membrane Antigen After Androgen-Deprivation Therapy. Urology (1996) 48:326–34. doi: 10.1016/s0090-4295(96)00184-7
18. Perner S, Hofer MD, Kim R, Shah RB, Li H, Möller P, et al. Prostate-Specific Membrane Antigen Expression as a Predictor of Prostate Cancer Progression. Hum Pathol (2007) 38:696–701. doi: 10.1016/j.humpath.2006.11.012
19. Eder M, Schafer M, Bauder-Wust U, Hull WE, Wangler C, Mier W, et al. 68Ga-Complex Lipophilicity and the Targeting Property of a Urea-Based PSMA Inhibitor for PET Imaging. Bioconjug Chem (2012) 23:688–97. doi: 10.1021/bc200279b
20. Afshar-Oromieh A, Avtzi E, Giesel FL, Holland-Letz T, Linhart HG, Eder M, et al. The Diagnostic Value of PET/CT Imaging With the (68)Ga-Labelled PSMA Ligand HBED-CC in the Diagnosis of Recurrent Prostate Cancer. Eur J Nucl Med Mol Imaging (2015) 42:197–209. doi: 10.1007/s00259-014-2949-6
21. Rahbar KA-O, Weckesser M, Ahmadzadehfar H, Schäfers M, Stegger L, Bögemann M. Advantage of (18)F-PSMA-1007 Over (68)Ga-PSMA-11 PET Imaging for Differentiation of Local Recurrence vs. Urinary Tracer Excretion. Eur J Nucl Med Mol Imaging (2018) 45:1076–7. doi: 10.1007/s00259-018-3952-0
22. Treglia G, Annunziata S, Pizzuto DA, Giovanella L, Prior JO, Ceriani L. Detection Rate of (18)F-Labeled PSMA PET/CT in Biochemical Recurrent Prostate Cancer: A Systematic Review and a Meta-Analysis. Cancers (Basel) (2019) 11:710. doi: 10.3390/cancers11050710
23. Giesel FL, Hadaschik B, Cardinale J, Radtke J, Vinsensia M, Lehnert W, et al. F-18 Labelled PSMA-1007: Biodistribution, Radiation Dosimetry and Histopathological Validation of Tumor Lesions in Prostate Cancer Patients. Eur J Nucl Med Mol Imaging (2017) 44:678–88. doi: 10.1007/s00259-016-3573-4
24. Rahbar KA-O, Afshar-Oromieh A, Bögemann M, Wagner S, Schäfers M, Stegger L, et al. (18)F-PSMA-1007 PET/CT at 60 and 120 Minutes in Patients With Prostate Cancer: Biodistribution, Tumour Detection and Activity Kinetics. Eur J Nucl Med Mol Imaging (2018) 45:1329–34. doi: 10.1007/s00259-018-3989-0
25. Sathianathen NJ, Butaney M, Konety BR. The Utility of PET-Based Imaging for Prostate Cancer Biochemical Recurrence: A Systematic Review and Meta-Analysis. World J Urol (2019) 37:1239–49. doi: 10.1007/s00345-018-2403-7
26. Liberati A, Altman Dg Fau - Tetzlaff J, Tetzlaff J Fau - Mulrow C, Mulrow C Fau - Gøtzsche PC, Gøtzsche Pc Fau - Ioannidis JPA, Ioannidis Jp Fau - Clarke M, et al. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. J Clin Epidemiol (2009) 62:e1–34. doi: 10.1016/j.jclinepi.2009.06.006
27. Higgins JP, Thompson SG. Quantifying Heterogeneity in a Meta-Analysis. Stat Med (2002) 21:1539–58. doi: 10.1002/sim.1186
28. Moses LE, Shapiro D Fau - Littenberg B, Littenberg B. Combining Independent Studies of a Diagnostic Test Into a Summary ROC Curve: Data-Analytic Approaches and Some Additional Considerations. Stat Med (1993) 12:1293–316. doi: 10.1002/sim.4780121403
29. Pelosi E, Arena V, Skanjeti A, Pirro V, Douroukas A, Pupi A, et al. Role of Whole-Body 18F-Choline PET/CT in Disease Detection in Patients With Biochemical Relapse After Radical Treatment for Prostate Cancer. Radiol Med (2008) 113:895–904. doi: 10.1007/s11547-008-0263-8
30. Kwee SA, Coel MN, Lim J. DR–Detection of Recurrent Prostate Cancer With 18F-Fluorocholine PET/CT in Relation to PSA Level at the Time of Imaging. Ann Nucl Med (2012) 26:501–7. doi: 10.1007/s12149-012-0601-8
31. Panebianco V, Sciarra A, Lisi D, Galati F, Buonocore V, Catalano C, et al. Prostate Cancer: 1HMRS-DCEMR at 3T Versus [(18)F]Choline PET/CT in the Detection of Local Prostate Cancer Recurrence in Men With Biochemical Progression After Radical Retropubic Prostatectomy (RRP). Eur J Radiol (2012) 81:700–8. doi: 10.1016/j.ejrad.2011.01.095
32. Schillaci O, Calabria F, Tavolozza M, Caracciolo CR, Finazzi Agro E, Miano R, et al. Influence of PSA, PSA Velocity and PSA Doubling Time on Contrast-Enhanced 18F-Choline PET/CT Detection Rate in Patients With Rising PSA After Radical Prostatectomy. Eur J Nucl Med Mol Imaging (2012) 39:589–96. doi: 10.1007/s00259-011-2030-7
33. Detti B, Scoccianti S, Franceschini D, Cipressi S, Cassani S, Villari D, et al. Predictive Factors of 18F -Choline PET/CT in 170 Patients With Increasing PSA After Primary Radical Treatment. J Cancer Res Clin Oncol (2013) 139:521–8. doi: 10.1007/s00432-012-1354-4
34. Marzola MC, Chondrogiannis S, Ferretti A, Grassetto G, Rampin L, Massaro A, et al. DR–Role of 18F-Choline PET/CT in Biochemically Relapsed Prostate Cancer After Radical Prostatectomy: Correlation With PSA Doubling Time, and Metastatic Distribution. Clin Nucl Med (2013) 38:e26–32. doi: 10.1097/RLU.0b013e318266cc38
35. Piccardo A, Paparo F, Piccazzo R, Naseri M, Ricci P, Marziano A, et al. Value of Fused 18F-Choline-PET/MRI to Evaluate Prostate Cancer Relapse in Patients Showing Biochemical Recurrence After EBRT: Preliminary Results. BioMed Res Int (2014) 2014:103718. doi: 10.1155/2014/103718
36. Cimitan M, Evangelista L, Hodolič M, Mariani G, Baseric T, Bodanza V, et al. Gleason Score at Diagnosis Predicts the Rate of Detection of 18F-Choline PET/CT Performed When Biochemical Evidence Indicates Recurrence of Prostate Cancer: Experience With 1,000 Patients. J Nucl Med (2015) 56:209–15. doi: 10.2967/jnumed.114.141887
37. Morigi JJ, Stricker PD, van Leeuwen PJ, Tang R, Ho B, Quoc N, et al. DR–Prospective Comparison of F-18-Fluoromethylcholine Versus Ga-68-PSMA PET/CT in Prostate Cancer Patients Who Have Rising PSA After Curative Treatment and are Being Considered for Targeted Therapy. J Nucl Med (2015) 56:1185–90. doi: 10.2967/jnumed.115.160382
38. Rodado-Marina S, Coronado-Poggio M, García-Vicente AM, García-Garzón JR, Alonso-Farto JC, de la Jara AC, et al. Clinical Utility of (18)F-Fluorocholine Positron-Emission Tomography/Computed Tomography (PET/CT) in Biochemical Relapse of Prostate Cancer After Radical Treatment: Results of a Multicentre Study. BJU Int (2015) 115:874–83. doi: 10.1111/bju.12953
39. Simone G, Di Pierro GB, Papalia R, Sciuto R, Rea S, Ferriero M, et al. Significant Increase in Detection of Prostate Cancer Recurrence Following Radical Prostatectomy With an Early Imaging Acquisition Protocol With 18F-Fluorocholine Positron Emission Tomography/Computed Tomography. World J Urol (2015) 33:1511–8. doi: 10.1007/s00345-015-1481-z
40. Emmett L, Metser U, Bauman G, Hicks RJ, Weickhardt A, Davis ID, et al. Prospective, Multisite, International Comparison of F-18-Fluoromethylcholine PET/CTand Ga-68-HBED-CC PSMA-11 PET/CT in Men With High-Risk Features and Biochemical Failure After Radical Prostatectomy: Clinical Performance and Patient Outcomes. J Nucl Med (2019) 60:794–800. doi: 10.2967/jnumed.118.220103
41. Giovacchini G, Giovannini E, Borsò E, Lazzeri P, Riondato M, Leoncini R, et al. Sensitivity of Fluorine-18-Fluoromethylcholine PET/CT to Prostate-Specific Antigen Over Different Plasma Levels: A Retrospective Study in a Cohort of 192 Patients With Prostate Cancer. Nucl Med Commun (2019) 40:258–63. doi: 10.1097/mnm.0000000000000959
42. Witkowska-Patena E, Giżewska A, Dziuk M, Miśko J, Budzyńska A, Walęcka-Mazur A. Head-to-Head Comparison of 18F-Prostate-Specific Membrane Antigen-1007 and 18F-Fluorocholine PET/CT in Biochemically Relapsed Prostate Cancer. Clin Nucl Med (2019) 44:e629–e33. doi: 10.1097/rlu.0000000000002794
43. de Leiris N, Leenhardt J, Boussat B, Montemagno C, Seiller A, Phan Sy O, et al. Does Whole-Body Bone SPECT/CT Provide Additional Diagnostic Information Over [18F]-FCH PET/CT for the Detection of Bone Metastases in the Setting of Prostate Cancer Biochemical Recurrence? Cancer Imaging (2020) 20:58. doi: 10.1186/s40644-020-00333-y
44. Sánchez N, Valduvieco I, Ribal MJ, Campos F, Casas F, Nicolau C, et al. Diagnostic Utility and Therapeutic Impact of PET/CT 18F-Fluoromethylcholine -Choline in the Biochemical Recurrence of Prostate Cancer. Rev Esp Med Nucl Imagen Mol (2020) 39:284–91. doi: 10.1016/j.remn.2020.03.010
45. Zattoni F, Ravelli I, Rensi M, Capobianco D, Borsatti E, Baresic T, et al. 10-Year Clinical Experience With 18F-Choline PET/CT: An Italian Multicenter Retrospective Assessment of 3343 Patients. Clin Nucl Med (2020) 45:594–603. doi: 10.1097/rlu.0000000000003125
46. Schuster DM, Savir-Baruch B, Nieh PT, Master VA, Halkar RK, Rossi PJ, et al. Detection of Recurrent Prostate Carcinoma With Anti-1-Amino-3-F-18-Fluorocyclobutane-1-Carboxylic Acid PET/CT and in-111-Capromab Pendetide SPECT/CT. Radiology (2011) 259:852–61. doi: 10.1148/radiol.11102023
47. Kairemo K, Rasulova N, Partanen K, Joensuu T. Preliminary Clinical Experience of Trans-1-Amino-3-(18)F-Fluorocyclobutanecarboxylic Acid (Anti-(18)F-FACBC) PET/CT Imaging in Prostate Cancer Patients. BioMed Res Int (2014) 2014:305182. doi: 10.1155/2014/305182
48. Nanni C, Zanoni L, Pultrone C, Schiavina R, Brunocilla E, Lodi F, et al. (18)F-FACBC (Anti1-Amino-3-(18)F-Fluorocyclobutane-1-Carboxylic Acid) Versus (11)C-Choline PET/CT in Prostate Cancer Relapse: Results of a Prospective Trial. Eur J Nucl Med Mol Imaging (2016) 43:1601–10. doi: 10.1007/s00259-016-3329-1
49. Odewole OA, Tade FI, Nieh PT, Savir-Baruch B, Jani AB, Master VA, et al. Recurrent Prostate Cancer Detection With Anti-3-[18F]FACBC PET/CT: Comparison With CT. Eur J Nucl Med Mol Imaging (2016) 43:1773–83. doi: 10.1007/s00259-016-3383-8
50. Akin-Akintayo OO, Jani AB, Odewole O, Tade FI, Nieh PT, Master VA, et al. Change in Salvage Radiotherapy Management Based on(Fluciclovine) PET/CT in Postprostatectomy Recurrent Prostate Cancer. Clin Nucl Med (2017) 42:e22–e8. doi: 10.1097/RLU.0000000000001379
51. Bach-Gansmo T, Nanni C, Nieh PT, Zanoni L, Bogsrud TV, Sletten H, et al. Multisite Experience of the Safety, Detection Rate and Diagnostic Performance of Fluciclovine ((18)F) Positron Emission Tomography/Computerized Tomography Imaging in the Staging of Biochemically Recurrent Prostate Cancer. J Urol (2017) 197:676–83. doi: 10.1016/j.juro.2016.09.117
52. Miller MP, Kostakoglu L, Pryma D, Yu JQ, Chau A, Perlman E, et al. Reader Training for the Restaging of Biochemically Recurrent Prostate Cancer Using (18)F-Fluciclovine PET/CT. J Nucl Med (2017) 58:1596–602. doi: 10.2967/jnumed.116.188375
53. Jambor I, Kuisma A, Kahkonen E, Kemppainen J, Merisaari H, Eskola O, et al. Prospective Evaluation of (18)F-FACBC PET/CT and PET/MRI Versus Multiparametric MRI in Intermediate- to High-Risk Prostate Cancer Patients (FLUCIPRO Trial). Eur J Nucl Med Mol Imaging (2018) 45:355–64. doi: 10.1007/s00259-017-3875-1
54. Andriole GL, Kostakoglu L, Chau A, Duan F, Mahmood U, Mankoff DA, et al. The Impact of Positron Emission Tomography With 18F-Fluciclovine on the Treatment of Biochemical Recurrence of Prostate Cancer: Results From the LOCATE Trial. J Urol (2019) 201:322–31. doi: 10.1016/j.juro.2018.08.050
55. Calais J, Ceci F, Eiber M, Hope TA, Hofman MS, Rischpler C, et al. Head to Head-FACBC vs PSMA-Restaging-DR 18F-Fluciclovine PET-CT and 68Ga-PSMA-11 PET-CT in Patients With Early Biochemical Recurrence After Prostatectomy: A Prospective, Single-Centre, Single-Arm, Comparative Imaging Trial. Lancet Oncol (2019) 20:1286–94. doi: 10.1016/s1470-2045(19)30415-2
56. England JR, Paluch J, Ballas LK, Jadvar H. 18F-Fluciclovine PET/CT Detection of Recurrent Prostate Carcinoma in Patients With Serum PSA </= 1 Ng/Ml After Definitive Primary Treatment. Clin Nucl Med (2019) 44:e128–e32. doi: 10.1097/RLU.0000000000002432
57. Savir-Baruch B, Lovrec P, Solanki AA, Adams WH, Yonover PM, Gupta G, et al. Fluorine-18-Labeled Fluciclovine PET/CT in Clinical Practice: Factors Affecting the Rate of Detection of Recurrent Prostate Cancer. AJR Am J Roentgenol (2019) 213:851–8. doi: 10.2214/ajr.19.21153
58. Teyateeti A, Khan B, Teyateeti A, Chen B, Bridhikitti J, Pan T, et al. Diagnostic Performance of F-18 Fluciclovine PET/CT in Post-Radical Prostatectomy Prostate Cancer Patients With Rising Prostate-Specific Antigen Level </=0.5 Ng/Ml. Nucl Med Commun (2020) 41:906–15. doi: 10.1097/MNM.0000000000001228
59. Garza D, Kandathil A, Xi Y, Subramaniam RM. 18F-Fluciclovine PET/CT Detection of Biochemical Recurrent Prostate Cancer in Patients With PSA Levels <2.00 Ng/Ml. Nucl Med Commun (2021). doi: 10.1097/MNM.0000000000001412
60. Michael J, Khandani AH, Basak R, Tan HJ, Royce TJ, Wallen E, et al. Patterns of Recurrence, Detection Rates, and Impact of 18-F Fluciclovine PET/CT on the Management of Men With Recurrent Prostate Cancer. Urology (2021) S0090-4295:00110–2. doi: 10.1016/j.urology.2021.01.038
61. Nakamoto R, Harrison C, Song H, Guja KE, Hatami N, Nguyen J, et al. The Clinical Utility of (18)F-Fluciclovine PET/CT in Biochemically Recurrent Prostate Cancer: An Academic Center Experience Post FDA Approval. Mol Imaging Biol (2021). doi: 10.1007/s11307-021-01583-3
62. Rahbar K, Afshar-Oromieh A, Seifert R, Wagner S, Schäfers M, Bögemann M, et al. Diagnostic Performance of (18)F-PSMA-1007 PET/CT in Patients With Biochemical Recurrent Prostate Cancer. Eur J Nucl Med Mol Imaging (2018) 45:2055–61. doi: 10.1007/s00259-018-4089-x
63. Eiber M, Kroenke M, Wurzer A, Ulbrich L, Jooß L, Maurer T, et al. 18F-Rhpsma-7 Positron Emission Tomography (PET) for the Detection of Biochemical Recurrence of Prostate Cancer Following Radical Prostatectomy. J Nucl Med (2019) 61:696–701. doi: 10.2967/jnumed.119.234914
64. Giesel FL, Knorr K, Spohn F, Will L, Maurer T, Flechsig PDetection Efficacy of (18)F-PSMA-1007 PET/CT in 251 Patients With Biochemical Recurrence of Prostate Cancer After Radical Prostatectomy. J Nucl Med 2019) 6460:362–8. doi: 10.2967/jnumed.118.212233
65. Rousseau E, Wilson D, Lacroix-Poisson F, Krauze A, Chi K, Gleave M, et al. A Prospective Study on F-18-Dcfpyl PSMA PET/CT Imaging in Biochemical Recurrence of Prostate Cancer. J Nucl Med (2019) 60:1587–93. doi: 10.2967/jnumed.119.226381
66. Wondergem M, Jansen BHE, van der Zant FM, van der Sluis TM, Knol RJJ, van Kalmthout LWM, et al. Early Lesion Detection With (18)F-Dcfpyl PET/CT in 248 Patients With Biochemically Recurrent Prostate Cancer. Eur J Nucl Med Mol Imaging (2019) 46:1911–8. doi: 10.1007/s00259-019-04385-6
67. Chausse G, Ben-Ezra N, Stoopler M, Levett JY, Niazi T, Anidjar M, et al. Diagnostic Performance of (18)F-Dcfpyl Positron Emission Tomography/Computed Tomography for Biochemically Recurrent Prostate Cancer and Change-of-Management Analysis. Can Urol Assoc J (2020) 15. doi: 10.5489/cuaj.6817
68. Mena E, Lindenberg ML, Turkbey IB, Shih JH, Harmon SA, Lim I, et al. F-18-Dcfpyl PET/CT Imaging in Patients With Biochemically Recurrent Prostate Cancer After Primary Local Therapy. J Nucl Med (2020) 61:881–9. doi: 10.2967/jnumed.119.234799
69. Rowe SP, Campbell SP, Mana-Ay M, Szabo Z, Allaf ME, Pienta KJ, et al. Prospective Evaluation of PSMA-Targeted F-18-Dcfpyl PET/CT in Men With Biochemical Failure After Radical Prostatectomy for Prostate Cancer. J Nucl Med (2020) 61:58–61. doi: 10.2967/jnumed.119.226514
70. Song H, Harrison C, Duan H, Guja K, Hatami N, Franc BL, et al. Prospective Evaluation of (18)F-Dcfpyl PET/CT in Biochemically Recurrent Prostate Cancer in an Academic Center: A Focus on Disease Localization and Changes in Management. J Nucl Med (2020) 61:546–51. doi: 10.2967/jnumed.119.231654
71. Witkowska-Patena E, Giżewska A, Dziuk M, Miśko J, Budzyńska A, Walęcka-Mazur A. Diagnostic Performance of 18F-PSMA-1007 PET/CT in Biochemically Relapsed Patients With Prostate Cancer With PSA Levels ≤ 2.0 Ng/Ml. Prostate Cancer Prostatic Dis (2020) 23:343–8. doi: 10.1038/s41391-019-0194-6
72. Dietlein F, Mueller P, Kobe C, Endepols H, Hohberg M, Zlatopolskiy BD, et al. [(18)F]-JK-PSMA-7 PET/CT Under Androgen Deprivation Therapy in Advanced Prostate Cancer. Mol Imaging Biol (2021) 23:277–86. doi: 10.1007/s11307-020-01546-0
73. Koschel S, Taubman K, Sutherland T, Yap K, Chao M, Guerrieri M, et al. Patterns of Disease Detection Using [(18)F]Dcfpyl PET/CT Imaging in Patients With Detectable PSA Post Prostatectomy Being Considered for Salvage Radiotherapy: A Prospective Trial. Eur J Nucl Med Mol Imaging (2021). doi: 10.1007/s00259-021-05354-8
74. Perry E, Talwar A, Taubman K, Ng M, Wong LM, Booth R, et al. [(18)F]Dcfpyl PET/CT in Detection and Localization of Recurrent Prostate Cancer Following Prostatectomy Including Low PSA < 0.5 Ng/Ml. Eur J Nucl Med Mol Imaging (2021) 48:2038–46. doi: 10.1007/s00259-020-05143-9
75. Ghafoor S, Burger IA, Vargas AH. Multimodality Imaging of Prostate Cancer. J Nucl Med (2019) 60:1350–8. doi: 10.2967/jnumed.119.228320
76. Wang R, Shen G, Yang R, Ma X, Tian R. (68)Ga-PSMA PET/MRI for the Diagnosis of Primary and Biochemically Recurrent Prostate Cancer: A Meta-Analysis. Eur J Radiol (2020) 130:109131. doi: 10.1016/j.ejrad.2020.109131
77. Glaser ZA, Rais-Bahrami S. Fluciclovine Positron Emission Tomography in the Setting of Biochemical Recurrence Following Local Therapy of Prostate Cancer. Transl Androl Urol (2018) 7:824–30. doi: 10.21037/tau.2018.07.17
78. Tan N, Oyoyo U, Bavadian N, Ferguson N, Mukkamala A, Calais J, et al. PSMA-Targeted Radiotracers Versus F-18 Fluciclovine for the Detection of Prostate Cancer Biochemical Recurrence After Definitive Therapy: A Systematic Review and Meta-Analysis. Radiology (2020) 296:44–55. doi: 10.1148/radiol.2020191689
79. Giesel FL, Kesch C, Yun M, Cardinale J, Haberkorn U, Kopka K, et al. 18F-PSMA-1007 PET/CT Detects Micrometastases in a Patient With Biochemically Recurrent Prostate Cancer. Clin Genitourin Cancer (2017) 15:e497–e9. doi: 10.1016/j.clgc.2016.12.029
80. Fendler WP, Calais J, Eiber M, Flavell RR, Mishoe A, Feng FY, et al. Assessment of 68Ga-PSMA-11 PET Accuracy in Localizing Recurrent Prostate Cancer: A Prospective Single-Arm Clinical Trial. JAMA Oncol (2019) 5:856–63. doi: 10.1001/jamaoncol.2019.0096
81. Lawhn-Heath C, Flavell RR, Behr SC, Yohannan T, Greene KL, Feng F, et al. Single-Center Prospective Evaluation of (68)Ga-PSMA-11 PET in Biochemical Recurrence of Prostate Cancer. AJR Am J Roentgenol (2019) 213:266–74. doi: 10.2214/AJR.18.20699
82. Nanni C, Schiavina R, Brunocilla E, Boschi S, Borghesi M, Zanoni L, et al. 18F-Fluciclovine PET/CT for the Detection of Prostate Cancer Relapse: A Comparison to 11C-Choline PET/CT. Clin Nucl Med (2015) 40:e386–91. doi: 10.1097/rlu.0000000000000849
83. Abiodun-Ojo OA, Akintayo AA, Akin-Akintayo OO, Tade FI, Nieh PT, Master VA, et al. (18)F-Fluciclovine Parameters on Targeted Prostate Biopsy Associated With True Positivity in Recurrent Prostate Cancer. J Nucl Med (2019) 60:1531–6. doi: 10.2967/jnumed.119.227033
84. Gauvin S, Cerantola Y, Haberer E, Pelsser V, Probst S, Bladou F, et al. Initial Single-Centre Canadian Experience With 18F-Fluoromethylcholine Positron Emission Tomography-Computed Tomography (18F-FCH PET/CT) for Biochemical Recurrence in Prostate Cancer Patients Initially Treated With Curative Intent. Can Urol Assoc J (2017) 11:47–52. doi: 10.5489/cuaj.4068
Keywords: PET/CT, 18F, choline, fluciclovine, prostate-specific membrane antigen, prostate cancer
Citation: Wang R, Shen G, Huang M and Tian R (2021) The Diagnostic Role of 18F-Choline, 18F-Fluciclovine and 18F-PSMA PET/CT in the Detection of Prostate Cancer With Biochemical Recurrence: A Meta-Analysis. Front. Oncol. 11:684629. doi: 10.3389/fonc.2021.684629
Received: 23 March 2021; Accepted: 31 May 2021;
Published: 17 June 2021.
Edited by:
Harun Ilhan, Hospital of the University of Munich, GermanyReviewed by:
Matthias Miederer, Johannes Gutenberg University Mainz, GermanyMurat Tuncel, Hacettepe University, Turkey
Copyright © 2021 Wang, Shen, Huang and Tian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rong Tian, rongtiannuclear@126.com
†These authors have contributed equally to this work
 Rang Wang
Rang Wang Guohua Shen†
Guohua Shen† Mingxing Huang
Mingxing Huang Rong Tian
Rong Tian