- 1Department of Urology, First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China
- 2Department of Urology, Baoji Central Hospital, Baoji, China
- 3Department of Pathology, First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China
- 4Department of Radiology, First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China
Metastases from prostate cancer (PCa) to the penis are extremely rare, and few case reports exist in the literature. Because most patients usually present with multiple distant metastases at diagnosis, the prognosis is very poor. With the wide application of prostate-specific membrane antigen (PSMA) PET/CT, penile metastases may be detected at an early stage. Thus, questions regarding whether early diagnosis and precise treatment will equate to a survival advantage have recently been raised. In the present study, we reported 3 cases of penile metastasis from castration-resistant PCa. Moreover, a patient with asymptomatic penile metastases was diagnosed by 18F-PSMA PET/CT followed by lesion biopsy, and the prognosis was very well, despite with an aggressive pathological feature and low treatment intensity. In addition, we performed a literature review and found 62.5% of asymptomatic penile metastases were diagnosed by PSMA PET/CT in past seven years. Thus, we believe that PSMA PET/CT may detect more asymptomatic penile metastases in future, which led to early diagnosis, treatment and survival advantage.
Introduction
The common sites of metastasis from prostate cancer (PCa), in order of decreasing frequency, are the bone, pelvic lymph nodes, lung and liver. Metastases from PCa to the penis are extremely rare, with a frequency of less than 0.3% (1, 2). The prognosis for these patients is very poor, as they usually present with disseminated disease. Kotake et al. reviewed the data of 25 PCa patients with penile metastasis and found that 41% of patients died within 6 months after diagnosis (1). However, the incidence of penile metastasis from PCa may be more frequent than previously thought, with wide application of prostate-specific membrane antigen (PSMA) PET/CT. In addition, penile metastasis from PCa may be detected at an early stage. Thus, questions regarding whether accurate diagnosis and appropriate treatment lead to better outcomes have recently been raised.
Case Description
Case 1
A 77-year-old male presented to our clinic in October 2009 with a 5-year history of dysuria. The serum prostate-specific antigen (PSA) level was 1145 ng/ml, and prostate biopsy was subsequently performed. The histopathology showed prostatic adenocarcinoma (Gleason score: 5 + 5 = 10). No bone metastases were observed by bone scan. Then, the patient received hormonal therapy (bilateral orchiectomy and 50 mg/day bicalutamide).
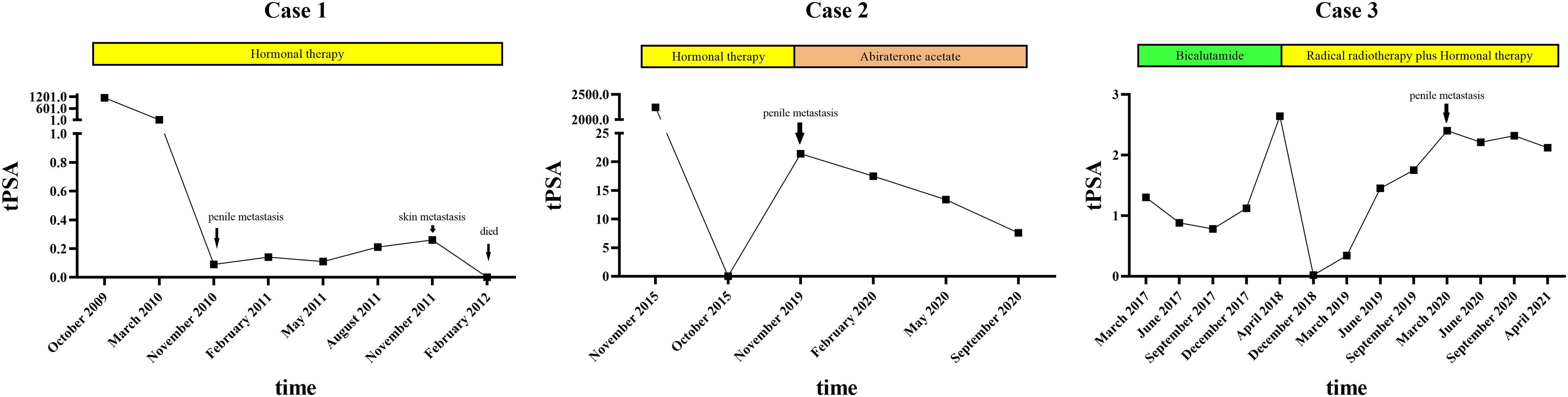
Regular follow-up showed no evidence of tumor recurrence until November 2010. He presented with a 2-month history of priapism, and the serum PSA level was found to be 0.09 ng/ml. Doppler ultrasonography revealed a high-resistance flow pattern in the penile artery. No lymph nodes and bone metastases were observed. Then, radical penectomy was performed, and the histopathologic examination of the surgical specimen revealed diffuse, poorly differentiated adenocarcinoma cells growing in the corpora cavernosa (Figure 1A). An immunostaining panel revealed that the tumor cells were positive for prostatic acid phosphatase but negative for PSA, which was consistent with metastatic prostatic adenocarcinoma. The patient refused radiotherapy and chemotherapy but continued hormonal therapy. After one year, multiple, subcutaneous, nontender, erythematous nodules over the right lower leg were found, and the PSA level increased to 0.26 ng/ml. Biopsy of a skin nodule revealed neoplastic cells with prominent atypical nucleoli, brisk mitotic activity, and clear cytoplasm, consistent with metastatic adenocarcinoma, and tumor cells were positive for prostatic acid phosphatase. These findings confirmed the diagnosis of metastatic PCa to the skin. The patient refused further active treatment and died due to progression of the disease 3 months after initial presentation with skin metastases. A timeline with relevant data of Case 1 was shown in Figure 3.
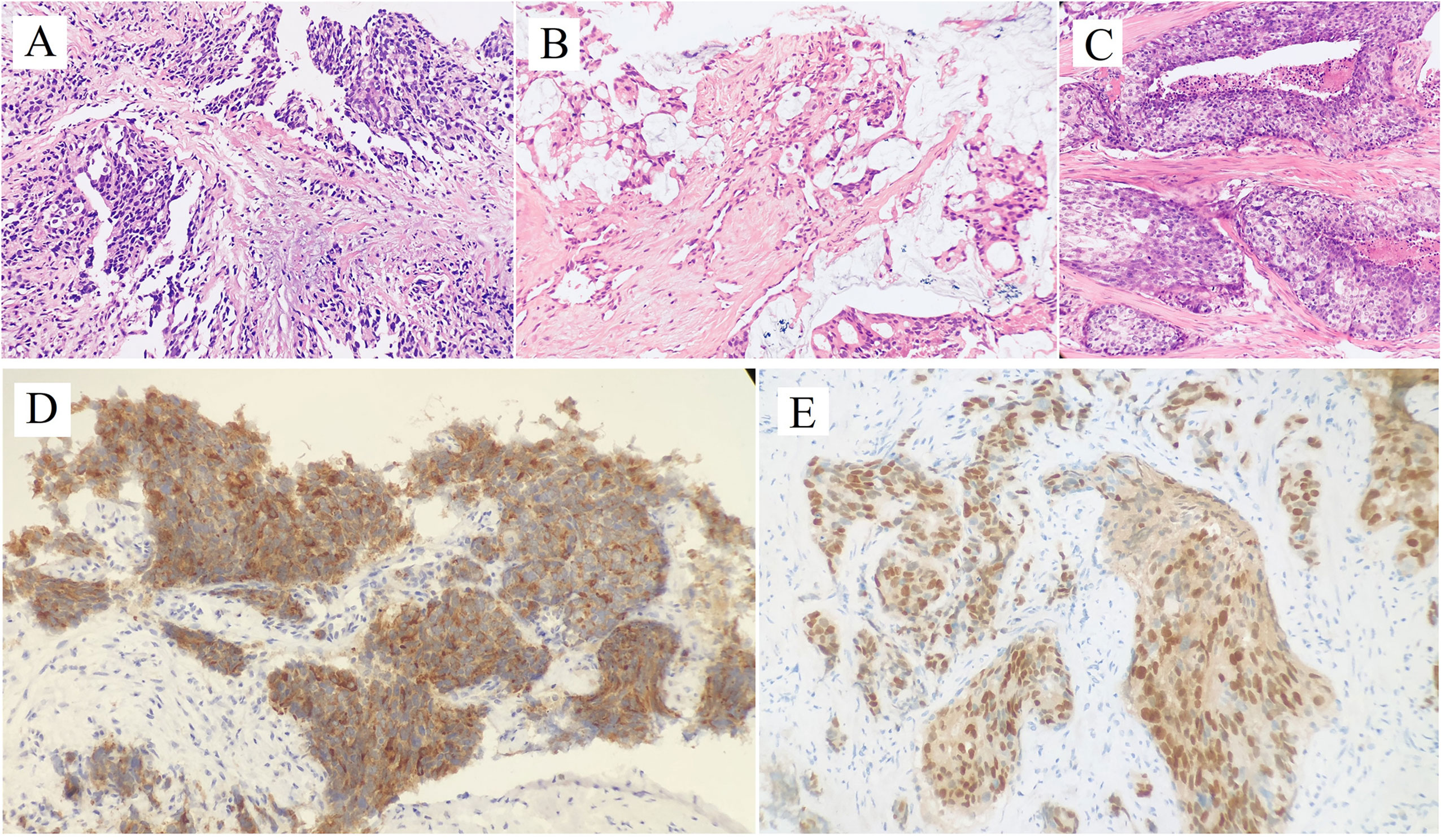
Figure 1 Hematoxylin and Eosin staining of penile metastatic lesions showed poorly differentiated prostatic adenocarcinoma (A: case 1; B: case 2; C: case 3). Two micrographs illustrate (D) PSA+, (E) NKX3.1+ tumor cells of the penile metastatic lesion in case 3.
Case 2
A 76-year-old male presented to our clinic in November 2019 with a 2-month history of a painless lump at the base of the penis. Four years previously, he was diagnosed with metastatic prostatic adenocarcinoma based on a markedly elevated serum PSA level (2241 ng/ml), a left supraclavicular mass, imaging studies, and pathologic results of ultrasound-guided core needle biopsy of the supraclavicular mass and prostate. The histopathology results showed prostate adenocarcinoma (Gleason score: 4 + 4 = 8). No bone metastases were observed by bone scan. Then, he received intermittent hormonal therapy (1.2 mg/month goserelin and 50 mg/day bicalutamide).
After hormonal therapy, the left supraclavicular mass gradually disappeared, and the PSA level decreased to 0.01 ng/ml (October 2015). In November 2019, the patient found a painless lump at the base of the penis, and the serum PSA level was up to 21.4 ng/ml. Magnetic resonance imaging (MRI) showed prostate cancer invading the bladder neck and a heterogeneous mass of the bulb of the penis. Moreover, multiple bone metastases were observed by bone scan. Then, ultrasound-guided biopsy of the penile lump was performed, and the histopathology results of the specimen showed poorly differentiated prostatic adenocarcinoma (Figure 1B). Because of personal preference, the patient chose to receive abiraterone acetate (1000 mg/day) plus prednisone (10 mg/day). After treatment, the mass on the penis gradually disappeared, and serum PSA decreased to 7.6 ng/ml (September 2020). A timeline with relevant data of Case 2 was shown in Figure 3.
Case 3
A 72-year-old male who was presented in March 2017 with a 13-year history of worsening dysuria. The volume of his prostate was measured at 70 mL by transrectal ultrasound, and the PSA level was normal. Urinary flow rate assessment showed a Qmax was only 6 mL/s, and postvoid residual urine was 220 mL. Then he was diagnosed as benign prostatic hyperplasia and the transurethral resection of the prostate was performed. The histopathology examination of the surgical specimen showed poorly differentiated prostatic adenocarcinoma, and the serum PSA level after the operation was 1.3 ng/ml. Because of personal preference and economic burden, the patient chose to receive oral bicalutamide (50 mg/day) and was maintained under surveillance for serum PSA every 3 months.
In April 2018, the serum PSA level was found to be 2.64 ng/ml, and multiparametric MRI showed a heterogeneous mass in the prostate, extensively invading the bladder neck, bilateral seminal vesicles and anterior rectum wall. No bone or pelvic lymph node metastases were observed. Then, he received transrectal ultrasound-guided prostate biopsy, and histopathology verified prostate adenocarcinoma with focal intraductal carcinoma (Gleason score: 4 + 4 = 8). An immunostaining panel revealed that the tumor cells were positive for HCK, P63, CK5/6 and P53 but were negative for Syn and CD56. Subsequently, the patient underwent radical radiotherapy and hormonal therapy (1.2 mg/month goserelin and 50 mg/day bicalutamide).
After radiotherapy, the PSA level decreased to 0.018 mg/L (December 2018). The patient remained asymptomatic until March 2020, and serum PSA was found to be 2.4 ng/ml. 18F-prostate specific membrane antigen (18F-PSMA) PET/CT was performed and revealed suspicious tracer uptake in the base of the penis as well as multiple bone metastases (Figure 2). Ultrasound-guided core needle biopsy was performed, and the histopathology results showed poorly differentiated prostatic adenocarcinoma (Gleason score: 5 + 5 = 10) (Figure 1C). An immunostaining panel revealed that the tumor cells were positive for PSA (Figure 1D) and NKX3.1 (Figure 1E). The patient refused radical penectomy and chemotherapy while opting for continuing hormonal therapy (1.2 mg/month goserelin and 50 mg/day bicalutamide). Until now, there has been no evidence of tumor recurrence or progression, and the recent serum PSA level was 2.12 ng/ml (April 2021). A timeline with relevant data of Case 3 was shown in Figure 3.
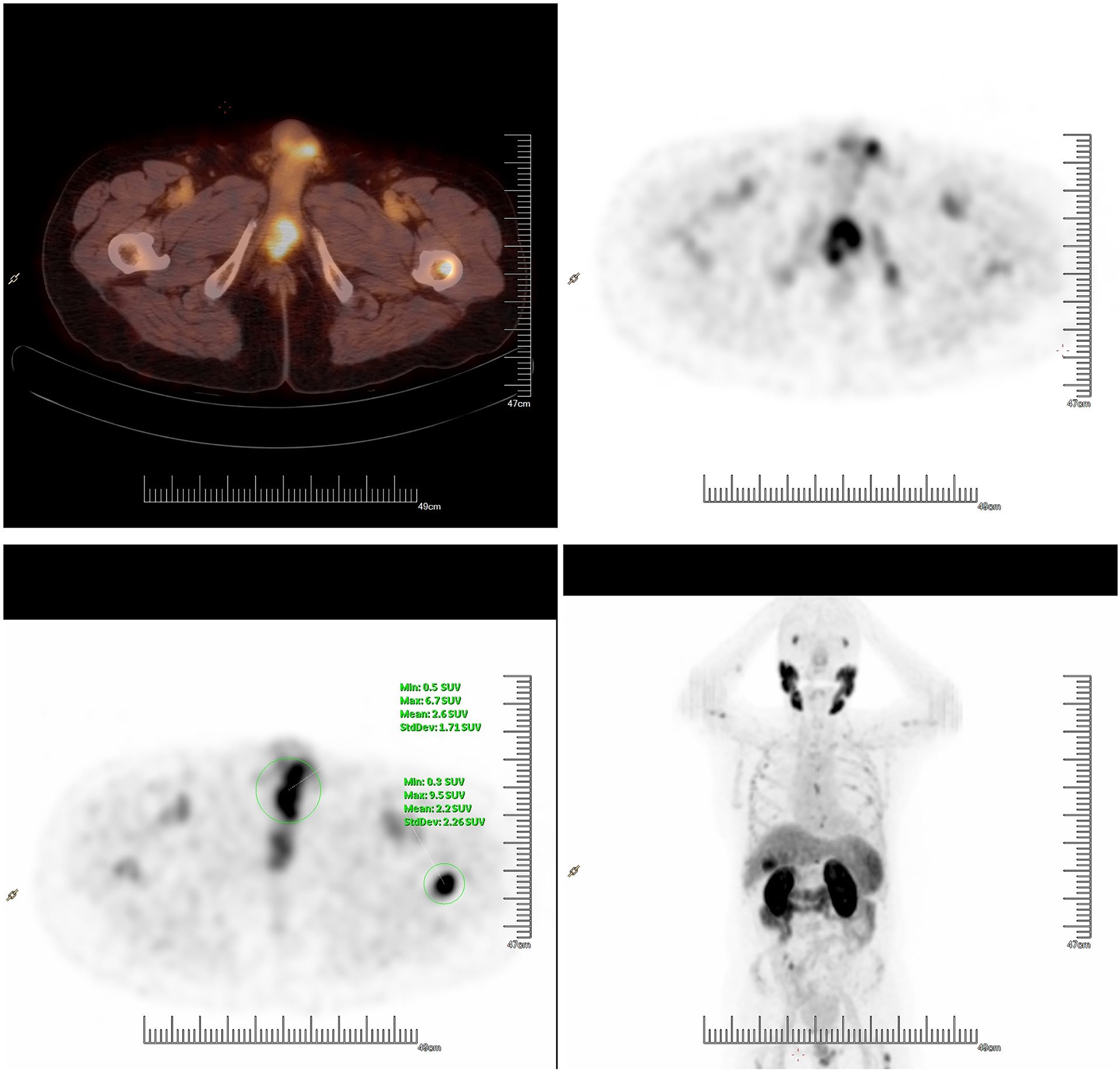
Figure 2 18F prostate-specific membrane antigen PET/CT showed suspicious tracer uptake in the base of the penis and multiple bone metastases.
Discussion
Although the corpora cavernosum of the penis is rich in blood supply, the incidence of penile metastases from PCa is extremely low. A plausible explanation for its presumed low incidence may be that imaging for PCa does not typically include the penis. PSMA is a type II transmembrane protein that is overexpressed on the cell membrane of nearly all prostatic cancer cells, especially in advanced-stage and castration-resistant PCa (3). Thus, PSMA is a promising and specific target for PCa imaging and shows great promise for improving the management of patients with PCa.
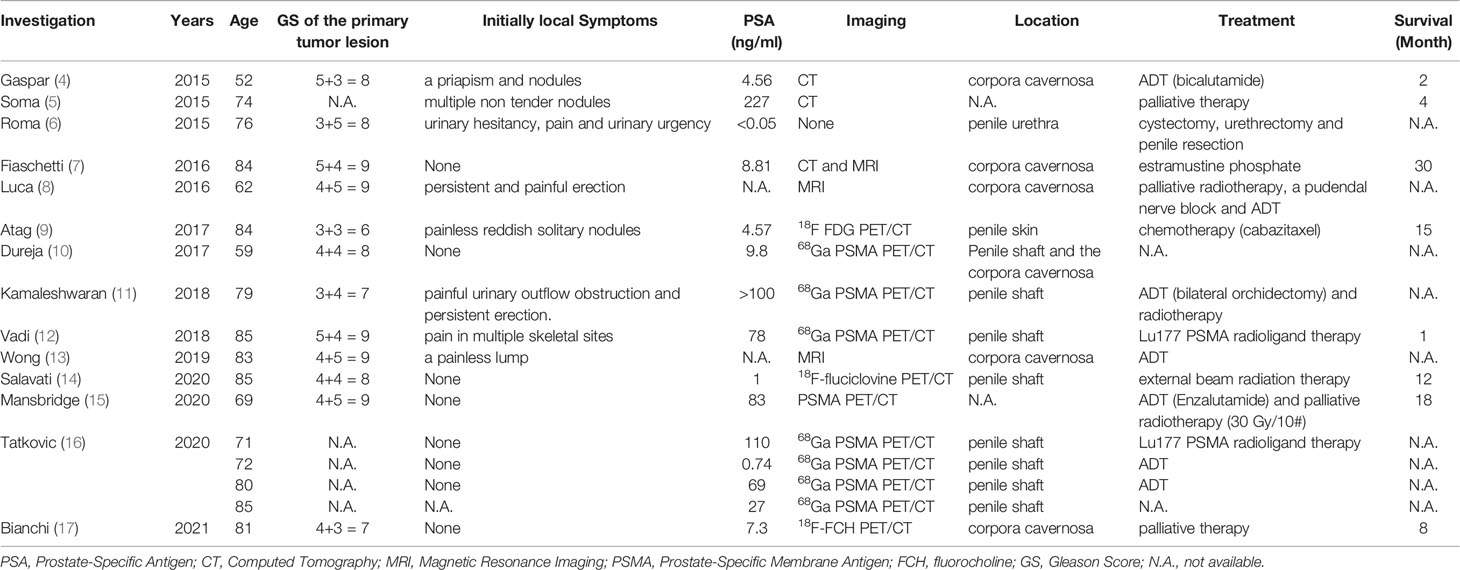
Comparing conversation imaging (i.e., bone scan, CT and MRI), PSMA PET/CT does not increase the accuracy of primary staging but identifies distant uncommon site metastases, such as those in the brain and penis. To provide a summary list of case reports, we performed a search of PubMed-indexed biomedical journals. We identified 17 individual cases with penile metastases from PCa reported between 2015 and January 2021, and PET/CT had gradually become the main imaging diagnoses of penile metastases (Table 1). Of 8 cases with asymptomatic penile metastases, 5 cases (62.5%) were diagnosed by PSMA PET/CT, especially for 68Ga-PSMA PET/CT. Vadi et al. reported that a patient with PCa was diagnosed with penile metastases by 68Ga-PSMA PET/CT and underwent 177Lu-labeled PSMA radioligand therapy (12). However, patient clinical course information or patient outcomes were not detailed. Recently, Mansbridge et al. outlined a history of castration-resistant PCa patients with asymptomatic penile metastases found on PSMA PET/CT (15). Currently, 68Ga-PSMA is a widely used tracer for PET imaging applications in the detection and staging of PCa. However, the disadvantage of 68Ga-PSMA PET/CT is that it has more bladder activity, which may influence the uptake evaluation of the prostate bed. 18F-PSMA is a novel PSMA-based radiopharmaceutical that has several advantages over 68Ga-PSMA, such as high labeling yields, outstanding tumor uptake and fast, nonurinary background clearance. Hence, it may have better diagnostic performance than 68Ga-PSMA in local recurrence and micrometastases. To our knowledge, this is the first report of asymptomatic penile metastases detected by 18F-PSMA PET/CT. In our report, 18F-PSMA PET/CT revealed unusual tracer uptake in the base of the penis, and the lesion was subsequently proven to be metastatic adenocarcinoma from the prostate by biopsy. Thus, we assumed that PSMA PET/CT, especially using 18F-labeled tracers, may lead to more reports of incidental cases in the future and broaden our understanding of the pathogenesis of penile metastases.
The possible mechanisms of penile metastasis from PCa have been explained as follows: 1) direct invasion; 2) implantation (from prior instrumentation); 3) retrograde venous flow; and 4) arterial or lymphatic dissemination. A review conducted by Mearini et al. pointed out that the communication between the prostatic plexus of Santorini and the deep dorsal vein of the penis is the main reason for the higher incidence of penile secondary tumors from PCa (18). Moreover, they also found that most penile metastases were located in the corpora cavernosa. Subsequently, we also documented that penile shaft and corpora cavernosa were the mostly location of metastases (Table 1). Furthermore, one patient in our report received radical penectomy, and the histopathology results of the surgical specimen revealed the tumor emboli in the vein of the penile cavernosa. Therefore, retrograde venous flow was deemed the most common pathway of penile metastasis from the prostate.
Previous reports have shown that the prognosis of metastatic penile cancer is very poor, and few patients have been reported to survive for 2 years or more (19). Moreover, the average survival is reported to be 6 months upon presentation of penile metastases from PCa (20). In our report, the average survival after diagnosing penile metastases was 10 months, which was better than that previously reported. Early diagnosis and treatment were the major reasons. The choice of treatment generally depends on patient clinical status and disease burden, and treatments include chemotherapy, radiotherapy and hormonal therapy. Partial or radical penectomy may be performed for patients with priapism or uncontrollable pain (20). However, for metastatic PCa, the main treatment is androgen deprivation therapy (ADT). After developing to the lethal castration-resistant PCa (CRPC), new hormonal therapy (i.e., abiraterone, enzalutamide) and chemotherapy (i.e., docetaxel) have shown efficacy in improving overall survival for these patients. In addition, local radiotherapy will improve the control of locoregional tumor and relieve the local symptoms (uncontrollable pain), which also improve the prognosis and quality of life. Moreover, 177Lu-labeled PSMA radioligand therapy has also shown encouraging results.
PSMA PET/CT may identify asymptomatic penile metastases and has important implications for disease management. In a case report by Mansbridge et al., asymptomatic penile metastases were found by PSMA PET/CT, and then this patient received local palliative radiotherapy (15). Fortunately, after 18 months, this patient was still alive and was being prepared for chemotherapy. Furthermore, Davidson et al. recently reported the characteristics of 24 patients with penile metastases by reviewed 18F-FDG and 68Ga-PSMA PET/CT records in their institution (21). Subsequently, they pointed that prostate was the most common primary site of penile metastases, and 12 (50%) patients with penile metastases from PCa, which was higher than previous reported. All the penile metastases of these patients were detected by PSMA PET/CT, which was also consistent with our observation. This indicates that the incidence of penile metastasis from PCa may be more frequent than previous knowledge with wide application of PSMA PET/CT. Also, the median survival was 11.5 months in 6 cases with PCa, which was better than previous reports. The reason of this phenomena may be due to an early diagnosis and more effective treatment. However, the initial local symptoms of these patients were not detailed in their study. In our report, a patient with asymptomatic penile metastases was also diagnosed by 18F-PSMA PET/CT followed by lesion biopsy and at the stage of castration-sensitive. PSMA PET/CT have a better ability to identify asymptomatic penile metastases, when comparing with conventional imaging [ultrasonography (Case 1), MRI (Case 2)] and provide the accuracy staging of PCa. Based on the accurate staging of PCa, we will perform more effective treatment in advance (i.e., local radiotherapy, chemotherapy, abiraterone and enzalutamide), which will be helpful to improve survival. Despite an aggressive pathological feature (Gleason score 5 + 5 = 10) and low treatment intensity (hormonal therapy), there was no sign of tumor recurrence or progression after 13 months, which was better than previous reports. However, due to the limitation of cases, it is still too soon to assume that an early diagnosis through this technique leads to a survival advantage, but rather to an increase in the time of disease consciousness. PSMA PET/CT is a novel imaging technique, which can not only increase the accuracy of PCa staging, but also help to identify some distant uncommon site metastases (i.e, brain, penis) when comparing to other conventional imaging. Subsequently, it will lead to a more accurate diagnosis and effective treatment for patients. Early detection at the stage of hormone-sensitive PCa and more active treatment may contribute to a survival advantage. However, this conclusion still needs to be confirmed by more studies to rule out the effects of time bias in future.
Conclusion
Penile metastases from PCa are extremely rare and typically indicate an advanced stage disease and a poor prognosis. PSMA PET/CT may detect more asymptomatic penile metastases in the future. In addition, early diagnosis and treatment can result in survival advantages.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics Statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
JF: data collection, literature search, and manuscript writing. HL: literature searching, pathological specimens reviewing, and manuscript writing. XZ: data collection and manuscript writing. XC: perform biopsy and manuscript editing. XD: PET/CT data collection and manuscript writing. LL: operation performance and manuscript editing. DH: project development and manuscript editing. KW: project development, operation performance, and manuscript editing. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Clinical Application Research Grant of the First Affiliated Hospital of Xi’an Jiaotong University (to LL).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors appreciate Prof. Xinyang Wang from the Department of Urology, the First Affiliated Hospital of Xi’an Jiaotong University for editing this paper.
References
1. Kotake Y, Gohji K, Suzuki T, Watsuji T, Kusaka M, Takahara K, et al. Metastases to the Penis From Carcinoma of the Prostate. Int J Urol (2001) 8(2):83–6. doi: 10.1046/j.1442-2042.2001.00245.x
2. Tu SM, Reyes A, Maa A, Bhowmick D, Pisters LL, Pettaway CA, et al. Prostate Carcinoma With Testicular or Penile Metastases. Clinical, Pathologic, and Immunohistochemical Features. Cancer (2002) 94(10):2610–7. doi: 10.1002/cncr.10546
3. Maurer T, Eiber M, Schwaiger M, Gschwend JE. Current Use of PSMA-PET in Prostate Cancer Management. Nat Rev Urol (2016) 13(4):226–35. doi: 10.1038/nrurol.2016.26
4. da Silva Gaspar SR, Nunes A, Dias JS, Lopes T. Malignant Priapism: Penile Metastasis Originating on a Primary Prostate Adenocarcinoma. Urol Ann (2015) 7(3):391–5. doi: 10.4103/0974-7796.152030
5. Soma S, Reddy PC, Bhat R, Prabhu S. Penile Metastases From Prostate Adenocarcinoma: A Rare Presentation. J Clin Diagn Res (2015) 9(9):PD03–4. doi: 10.7860/JCDR/2015/14775.6421
6. Roma AA, Magi-Galluzzi C, Wood H, Fergany A, McKenney JK. Metastatic Prostate Adenocarcinoma to the Penis Presenting as Pagetoid Carcinoma: A Phenomenon Not Previously Reported. Am J Surg Pathol (2015) 39(5):724–6. doi: 10.1097/PAS.0000000000000417
7. Fiaschetti V, Liberto V, Claroni G, Loreni G, Formica V, Roselli M, et al. Relevance of Computed Tomography and Magnetic Resonance Imaging for Penile Metastasis After Prostatectomy: Uncommon Case Report and Brief Review of the Literature. Radiol Case Rep (2016) 11(3):255–9. doi: 10.1016/j.radcr.2016.04.003
8. De Luca F, Zacharakis E, Shabbir M, Maurizi A, Manzi E, Zanghi A, et al. Malignant Priapism Due to Penile Metastases: Case Series and Literature Review. Arch Ital Urol Androl (2016) 88(2):150–2. doi: 10.4081/aiua.2016.2.150
9. Atag E, Semiz HS, Kazaz SN, Tuna EB, Ozdogan O, Bozkurt O, et al. Response to Cabazitaxel Beyond 20 Cycles in A Patient With Penile Metastasis of Prostate Cancer: A Case Report. Urol J (2017) 14(1):2985–8. doi: 10.22037/uj.v14i1.3697
10. Dureja S, Thakral P, Pant V, Sen I. Rare Sites of Metastases in Prostate Cancer Detected on Ga-68 Psma PET/CT Scan-a Case Series. Indian J Nucl Med (2017) 32(1):13–5. doi: 10.4103/0972-3919.198450
11. Kamaleshwaran KK, Balasundararaj BKP, Jose R, Shinto AS. Penile Metastasis From Prostate Cancer Presenting as Malignant Priapism Detected Using Gallium-68 Prostate-Specific Membrane Antigen Positron Emission Tomography/Computed Tomography. Indian J Nucl Med (2018) 33(1):57–8. doi: 10.4103/ijnm.IJNM_107_17
12. Vadi SK, Kumar R, Mittal BR, Parihar AS, Singh SK. Unusual Case of Diffuse Penile Metastasis of Prostate Cancer on 68Ga PSMA Pet/Ct Imaging and 177Lu PSMA Posttherapy Scintigraphy. Clin Nucl Med (2018) 43(4):276–8. doi: 10.1097/RLU.0000000000002001
13. Wong HL, Shi H, Koh LT. Solitary Metastasis to the Penis From Prostate Adenocarcinoma - a Case Report. J Radiol Case Rep (2019) 13(12):20–8. doi: 10.3941/jrcr.v13i12.3846
14. Salavati A, Schik AN, Koksel Y, Gencturk M, Froelich JW. Solitary Penile Metastasis of Prostate Cancer on 18F-Fluciclovine PET/CT Imaging in a Patient With PSA of 1 Ng/Ml. Clin Nucl Med (2020) 45(5):389–91. doi: 10.1097/RLU.0000000000002987
15. Mansbridge MM, Strahan A, Parker J, Rhee H. Psma-PET/CT-avid Metastatic Prostate Cancer to the Penis. BMJ Case Rep (2020) 13(3):e233522. doi: 10.1136/bcr-2019-233522
16. Tatkovic A, McBean R, Schoeman J, Wong D. Prostate Penile Metastasis: Incidence and Imaging Pattern on (68) Ga-PSMA Pet/Ct. J Med Imaging Radiat Oncol (2020) 64(4):499–504. doi: 10.1111/1754-9485.13052
17. Bianchi D, Rizzo A, Bonacina M, Zaniboni A, Savelli G. Penile Metastasis From Prostate Cancer Detected by 18F-Fluorocholine Pet/Ct. Clin Nucl Med (2021) 46(1):e38–9. doi: 10.1097/RLU.0000000000003249
18. Mearini L, Colella R, Zucchi A, Nunzi E, Porrozzi C, Porena M. A Review of Penile Metastasis. Oncol Rev (2012) 6(1):e10. doi: 10.4081/oncol.2012.e10
19. Chaux A, Amin M, Cubilla AL, Young RH. Metastatic Tumors to the Penis: A Report of 17 Cases and Review of the Literature. Int J Surg Pathol (2011) 19(5):597–606. doi: 10.1177/1066896909350468
20. Philip J, Mathew J. Penile Metastasis of Prostatic Adenocarcinoma: Report of Two Cases and Review of Literature. World J Surg Oncol (2003) 1(1):16. doi: 10.1186/1477-7819-1-16
Keywords: PSMA PET/CT, prostate cancer, penile metastases, early diagnosis, survival advantage
Citation: Fan J, Liang H, Zhang X, Chen X, Duan X, Li L, He D and Wu K (2021) Case Report: 18F-PSMA PET/CT May Improve the Clinical Management of Penile Metastases From Prostate Cancer. Front. Oncol. 11:683343. doi: 10.3389/fonc.2021.683343
Received: 20 March 2021; Accepted: 26 April 2021;
Published: 13 May 2021.
Edited by:
Gianluca Ingrosso, University of Perugia, ItalyReviewed by:
Alessandro Antonelli, University of Verona, ItalyAmin Nassar, Brigham and Women’s Hospital and Harvard Medical School, United States
Copyright © 2021 Fan, Liang, Zhang, Chen, Duan, Li, He and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kaijie Wu, kaijie_wu@163.com
†These authors have contributed equally to this work and share first authorship
 Junjie Fan
Junjie Fan Hua Liang3†
Hua Liang3† Lei Li
Lei Li Kaijie Wu
Kaijie Wu