- 1Division of Gastroenterology, Department of Internal Medicine, Incheon St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, South Korea
- 2Division of Gastroenterology, Yonsei University College of Medicine, Severance Hospital, Seoul, South Korea
- 3Center for Gastric Cancer, National Cancer Center, Goyang, South Korea
- 4Division of Gastroenterology, Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea
- 5Division of Gastroenterology, Department of Internal Medicine, Chungnam National University School of Medicine, Dajeon, South Korea
- 6Division of Gastroenterology, Department of Internal Medicine and Gastrointestinal Cancer Center, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea
- 7Division of Gastroenterology, Department of Internal Medicine, Pusan National University School of Medicine and Research Institute for Convergence of Biomedical Science and Technology, Pusan National University Yangsan Hospital, Yangsan, South Korea
- 8Division of Gastroenterology, Department of Internal Medicine, Korea University Guro Hospital, Korea University, College of Medicine, Seoul, South Korea
- 9Division of Gastroenterology, Department of Internal Medicine, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, South Korea
Background: Treatment recommendations for gastric mucosa-associated lymphoid tissue (MALT) lymphoma are based on case series and expert opinions. Only a few previous studies have focused on the long-term outcomes of gastric MALT lymphoma, especially according to stage.
Methods: Patients diagnosed with gastric MALT lymphoma from January 2000 to December 2018 at nine university hospitals in Korea were included. Clinical data of medical history, endoscopic features, histological diagnosis, results of Helicobacter pylori (H. pylori) testing, stage, treatment conditions, and outcomes were collected.
Results: A total of 1,163 patients was enrolled, and 97.6% (n=1,038) of patients were diagnosed as stage IE. 10-year overall survival (OS) for the entire population was 99.1% and was better for patients in stage IE compared with patients in stage III/IV (p=0.002). The 10-year OS for H. pylori-positive patients was better than that of H. pylori-negative patients (p=0.022). Multivariate analyses revealed initial stage III/IV as a prognostic factor associated with over-all survival.
Conclusion: The majority of gastric MALT lymphoma patients are diagnosed at an early localized stage in Korea. The overall survival rate of gastric MALT lymphoma is excellent and is associated with the initial stage of the disease.
Introduction
Extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue (MALT), also known as MALT lymphoma, arises from malignant transformation of B cells from the marginal zone of MALT (1). The gastrointestinal tract is the most common site of involvement in MALT lymphoma, with the stomach being the most common primary site (2, 3). Gastric MALT lymphoma is the most common type of gastric lymphoma, representing 38%-48% of primary gastric lymphoma cases (4–6). Gastric MALT lymphomas are generally low-grade lesions and are usually localized (7). The main risk of MALT lymphoma is its transformation to high-grade diffuse large B-cell lymphoma, although that is rare (8, 9).
Helicobacter pylori (H. pylori) plays a pivotal role in the pathogenesis of gastric MALT lymphoma. Epidemiologic studies show a close correlation between the prevalence of H. pylori infection and gastric lymphoma (10). H. pylori eradication is the treatment of choice in early stages, and successful eradication is sufficient for treatment in most cases (11, 12). Eradication therapy is recommended for early-stage disease, while radiation and surgery are reserved for advanced stages (13). Approximately 25% of patients do not respond to H. pylori eradication, and 20% of patients present as H. pylori negative (14, 15). The optimal management of these patients is not certain, and it is not clear if patients with more advanced disease should undergo eradication or additional treatment. However, the current treatment recommendations are based on case series and expert opinions (16, 17). Few studies have reported the treatment and survival of patients according to their different stages (18, 19). In addition, most previous studies that reported gastric MALT lymphoma survival rates included small population sizes. Understanding the survival rates of gastric MALT lymphoma patients according to stage should aid in improving therapeutic strategies. In this study, we evaluated treatments and long-term outcomes based on gastric MALT lymphoma stage in Korean patients. Specifically, we examined the survival rates of gastric MALT lymphoma patients according to stage and H. pylori infection status.
Methods
Patients
Patients diagnosed with gastric MALT lymphoma from January 2000 to December 2018, at nine university hospitals in Korea were enrolled. Medical records were reviewed retrospectively, and patients were excluded for any of the following conditions: synchronous cancer in another organ, no endoscopic follow-up, and/or incomplete medical records. The clinical data included medical history, endoscopic features, histological diagnosis, results of H. pylori testing, stage, treatment, and outcomes. The pathology reports were reviewed by an expert pathologist at each hospital, and the histological diagnosis was made according to the WHO Classification (20). The present study was conducted in accordance with the ethical guidelines of the Declaration of Helsinki and approved by the institutional review board of all participating hospitals.
Diagnosis and Staging
Initial gastric MALT lymphoma diagnosis was based on endoscopic examination and histologic assessment of biopsy specimens. Gastric MALT lymphoma was diagnosed histologically by the presence of a diffuse infiltrate of centrocyte-like B cells in the lamina propria with prominent lymphoepithelial lesions formed by invasion of individual glands by aggregates of lymphoma cells under histologic examination (21). Location of the dominant lesion was grouped into the upper, middle, or lower third of the stomach, and clinical stage was determined using the modified Ann Arbor staging system (22). The staging work-up included physical examination, computed tomography (CT) scans of the chest and abdomen, and biopsies. Endoscopic ultrasound sonography (EUS), bone marrow aspiration, and chromosomal translocation t(11;18)(q21;q21) were assessed by the physician.
H. pylori Infection and Eradication
H. pylori infection status was determined by histology, rapid urease test, or urea breath test, and H. pylori infection was regarded as positive when at least one of these tests yielded positive results. Eradication regimens consisted of standard triple therapy, sequential therapy, concomitant therapy, bismuth quadruple therapy, or levofloxacin-based triple therapy. The specific medication and dose for each regimen have been reported elsewhere (23). The outcomes of eradication therapy were determined using a urea breath test or a rapid urease test performed at least 4 weeks after completion of antibiotic therapy.
Outcomes
The primary aim of this study was to examine the 10-year overall survival (OS) of gastric MALT lymphoma patients. We also examined the 10-year OS by stage, H. pylori infection status, and treatment. Clinical factors associated with OS also were assessed. OS was defined as the interval between the date of diagnosis and the final date of follow up and was censored at that date. We also calculated the median follow-up as the median time between diagnosis to the time the last subject has an event or is censored.
Statistical Analysis
The baseline patient characteristics are summarized using descriptive statistics. The continuous data are presented as mean (standard deviation) or median (interquartile range) and categorical data as quantity and proportion. Comparison of categorical variables was performed using the χ2 test or Fisher’s exact test. The OS rates were calculated as the median time to censoring or death using Kaplan-Meier (KM) analysis and were compared using log-rank tests. The Cox proportional hazards model was applied to perform univariate and multivariate analyses to identify risk factors for mortality. Clinically relevant factors were included in the univariate model, whereas factors with p value less than 0.05 were considered for multivariate analyses. All statistical analyses were performed using SPSS software version 18.0 (SPSS Inc., Chicago, IL), and a p value less than 0.05 was considered statistically significant.
Results
Characteristics of Patients With Gastric MALT Lymphoma
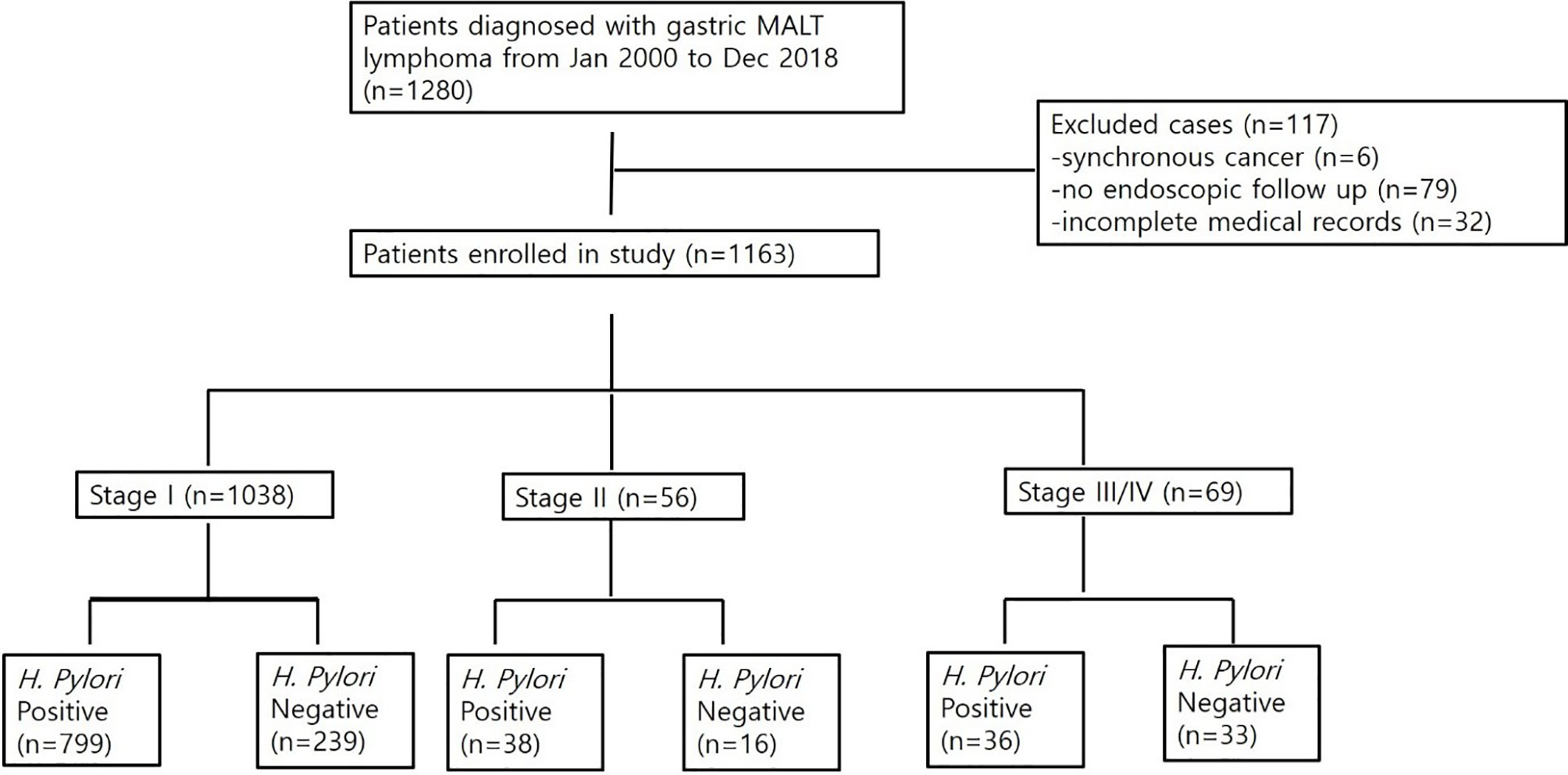
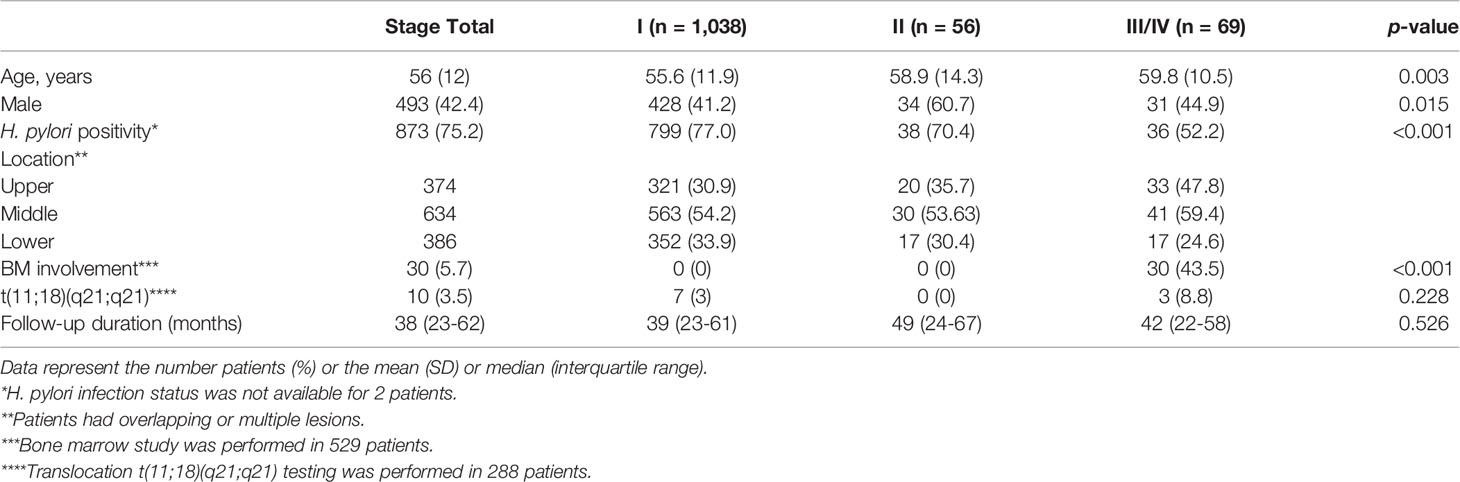
A total of 1,280 patients was diagnosed with gastric MALT lymphoma during the study period (Figure 1). Of these, 6 patients were excluded due to the presence of synchronous cancer, 79 patients were excluded due to lack of endoscopic follow up, and 32 patients were excluded due to incomplete medical records. Thus, 1,163 patients were included in the final analyses. The baseline characteristics of these patients are summarized in Table 1. The mean age at the time of diagnosis was 56 ± 12 years, and the proportion of males was 42.4%. The median follow-up for all patients was 102 months (range, 1.5 to 211 months). A total of 1,038 patients (97.6%) was diagnosed as stage IE, 56 patients (4.8%) were diagnosed as stage IIE, and 69 patients (5.9%) were diagnosed as stage III/IV. There was a significant difference in age, prevalence of H. pylori, and bone marrow involvement between the three groups. However, there was no difference in follow-up duration. Transformation into diffuse large B-cell lymphoma occurred in one patient diagnosed with stage IE and two patients diagnosed with stage II.
Treatment of Patients With Gastric MALT Lymphoma
Table 2 shows the various treatments the patients received according to stage. H. pylori eradication was performed in 72.4% of stage IE patients and was the most frequent treatment method in this group. However, eradication was performed in 25.0% and 20.3% of stage IIE and stage III/IV patients, respectively. The most frequent treatment for stage IIE patients was H. pylori eradication, followed by radiotherapy, accounting for 35.7% of patients. The most frequent treatment for stage III/IV patients was chemotherapy, accounting for 29.0% of patients. However, H. pylori eradication followed by radiotherapy or chemotherapy also was frequent and accounted for 23.2% and 13.0% of patients, respectively.
Survival of Patients With Gastric MALT Lymphoma
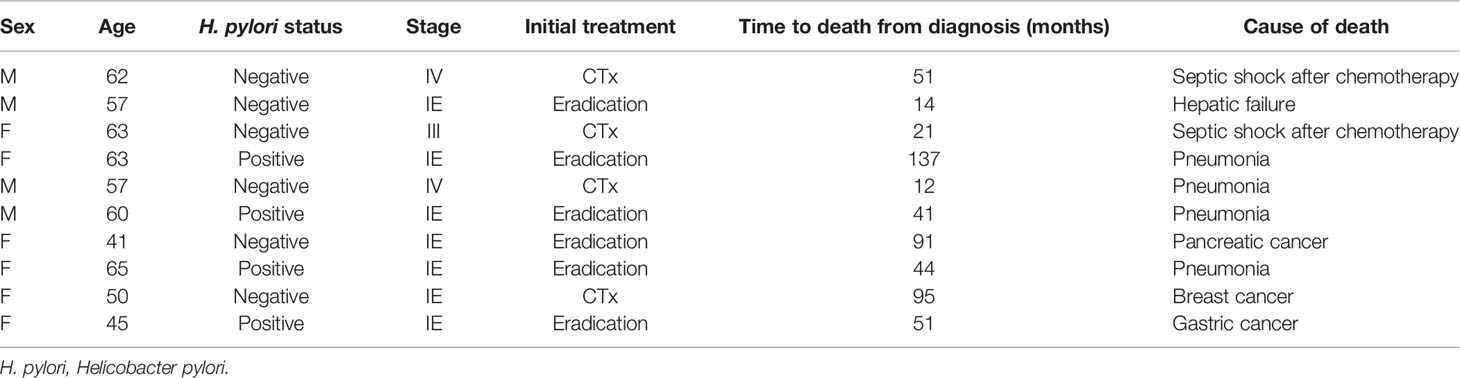
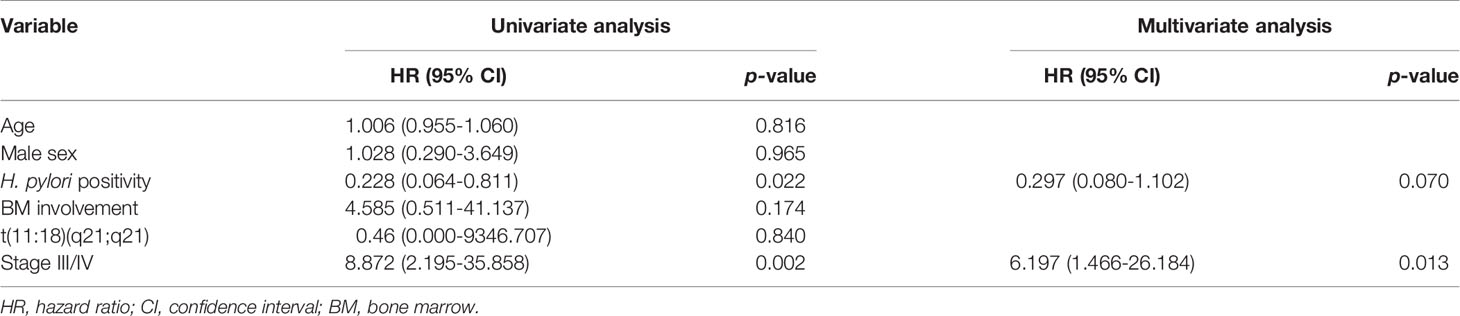
During the follow-up period, 10 of the 1,163 patients died. The clinical features of the 10 patients who expired are shown in Table 3. The KM curves for 10-year OS are presented in Figure 2. The 10-year OS for the entire population was 99.1% (Figure 2A), and 10-year OS for stage IE, IIE, and III/IV patients were 99.3%, 100%, and 94.6%, respectively (Figure 2B). 10-year OS was better for stage IE patients compared to stage III/IV patients (p=0.002). The 10-year OS for patients who were H. pylori positive was 99.5%, which was better than the 97.9% for H. pylori-negative patients (p=0.022, Figure 3). The 10-year OS rates of patients who received only H. pylori therapy was 99.5% and was better than the 96.6% of that of patients who received additional treatment after eradication or other treatment (p=0.007). The multivariate model adjusted for treatment showed initial stage III/IV as a poor prognostic factor (Table 4).
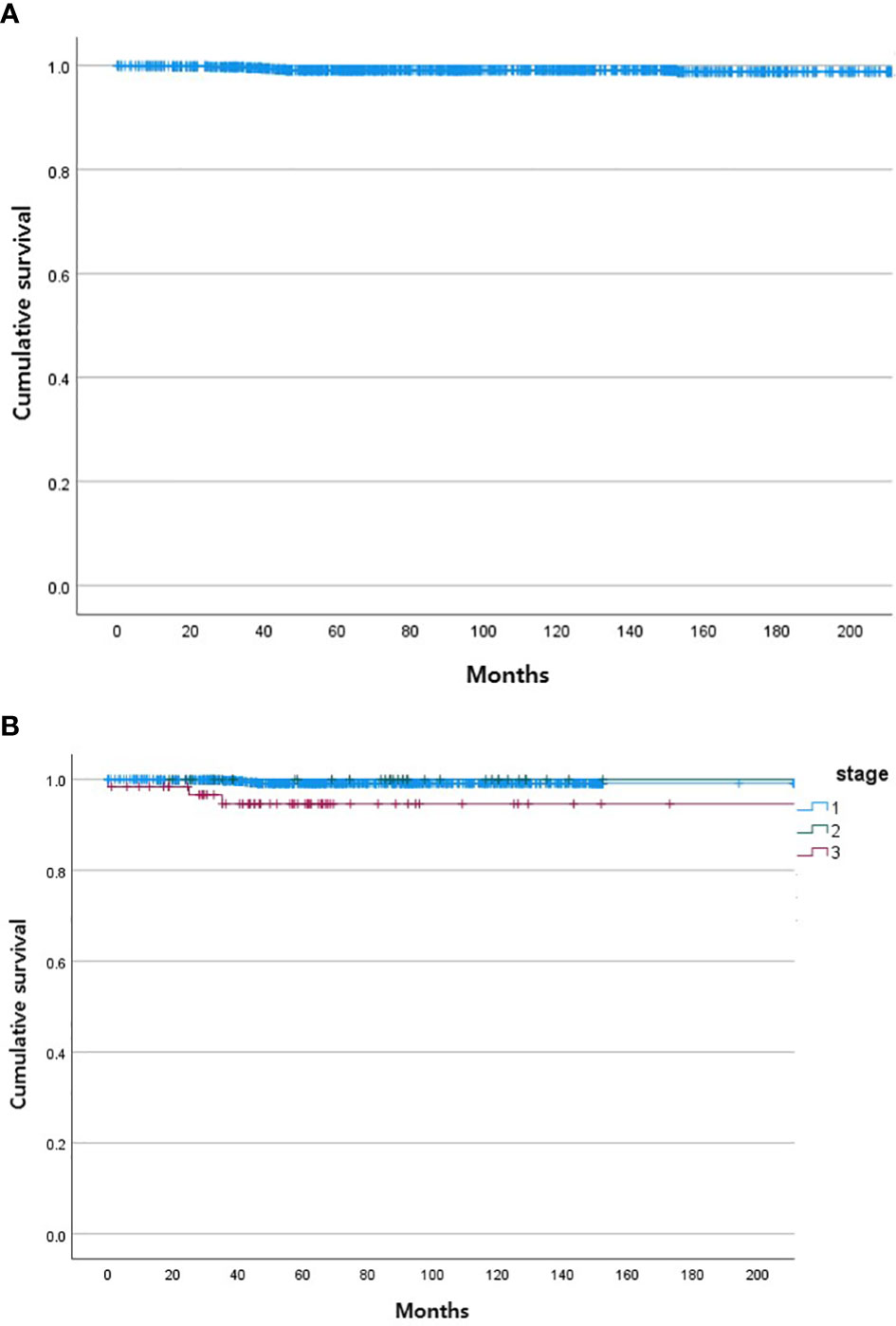
Figure 2 Kaplan Meier curves of gastric MALT lymphoma patient survival. (A) Total. (B) According to stage of gastric MALT lymphoma (stage I, stage II, stage III/IV).
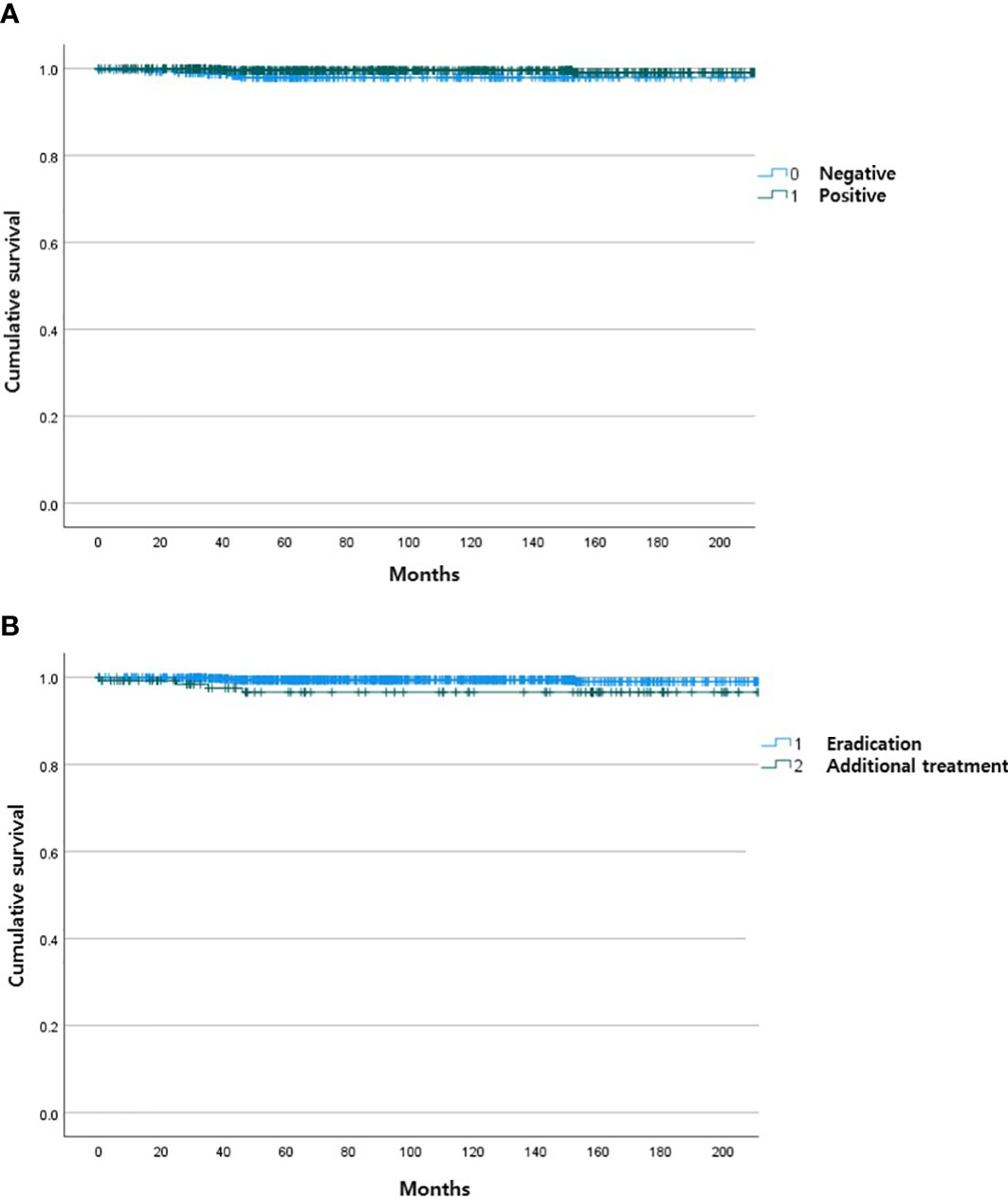
Figure 3 Kaplan Meier curves of gastric MALT lymphoma patient survival according to H pylori status (A) and type of treatment (B).
Discussion
The main stay of treatment for low-grade gastric MALT lymphoma is H. pylori eradication. A systematic review of 1,408 patients with low-grade lymphoma at an early stage found that H. pylori eradication achieved remission in 77.5% of patients (12). Earlier studies reported that 5-year OS and disease-free survival (DFS) rates were as high as 90% and 75%, respectively, when lymphoma was treated at an early stage (24). However, a recent population-based study in France reported 5-year OS rates of 79%, which is considerably lower than that of previous studies (18). This study reported 25% of patients diagnosed at an advanced stage (III/IV), which is in contrast to most previous clinical studies that reported greater than 90% of patients with gastric MALT lymphoma diagnosed at a localized stage (25, 26). The larger percentage of patients in stage III/IV might have resulted in a lower survival rate in this study compared to previous studies.
The 10-year OS of gastric MALT lymphoma patients in our study was 99.1% and considerably higher than that reported previously. Korea has a high prevalence of H. pylori and shows the highest age-standardized incidence rate of gastric cancer worldwide (27, 28). A National Cancer Screening Program (NCSP) for gastric cancer invites every Korean individual 40 years of age or older to undergo endoscopy or an upper gastrointestinal series every 2 years. Participation rates for the NCSP increased from 7.5% in 2002 to 47.3% in 2012 (29). Gastric MALT lymphoma frequently is found incidentally at an early stage during screening endoscopy for gastric cancer in Korea. This is evidenced by the high rates of stage IE disease in our study.
Approximately 25% of patients do not respond to H. pylori eradication, and 20% of patients present with H. pylori-negative gastric MALT lymphoma (14, 15). The optimal management of these patients is not certain, and there is no consensus regarding optimal management of advanced gastric MALT lymphoma that has spread to lymph nodes or other extranodal sites. Current guidelines recommend H. pylori eradication as the first-line treatment of choice for all gastric MALT lymphomas (30). Our study showed great heterogeneity in treatment modalities and nonadherence to current guidelines. Surprisingly, some patients with localized stages received overtreatment with systematic therapy. Heterogeneity was the greatest in patients with advanced stage gastric MALT lymphoma. H. pylori eradication was performed in only 58% of stage III/IV patients. Chemotherapy and radiation was performed in 29.0% and 4.3% of patients. Despite the various treatments, the OS of advanced stage patients was still high in our study. Future studies are warranted to determine the best treatment modality for this group of patients. Efforts should be made to increase general awareness and adherence to current practice guidelines (16, 17, 31).
Recently, a MALT lymphoma prognostic index was developed from a Cox regression analysis of 401 patients (32). The authors reported age, Ann Arbor stage III or IV, and an elevated lactate dehydrogenase level as the main factors associated with survival. This prognostic index was derived from a heterogeneous group of patients including both gastric and non-gastric lymphomas. In our study, we examined the factors associated with survival in only gastric MALT lymphoma patients and found stage III/IV of the disease as the only factor associated with OS.
This study has limitations, most of which are inherent to its retrospective nature. First, there was a wide variation in treatments among patients, which only allowed us to estimate the OS of patients according to stage. The different types of treatments and their possible implications in the survival of patients need to be assessed in the future. H. pylori infection may have been underestimated in our study as the diagnostic methods were not predetermined. We also cannot exclude that the results from our study are influenced by confounding factors due to the observational nature of our study design; furthermore, risk of selection bias cannot be overlooked. Our study reported a high rate of 10-year OS compared to previous studies, even for advanced-stage gastric MALT lymphomas. We also did not find any disease related deaths associated with gastric MALT lymphomas which is contrary to previous reports (18). However, our results are consistent with those of a recent study that examined the survival rates of MALT lymphoma from the Korean Center Cancer Registry (33). This study reported survival rates of 97.9% for unknown stages of MALT-lymphoma during the 2013-2017 period. To the best of our knowledge, our study examined the largest number of gastric MALT lymphoma patients over a long follow-up period. We argue that our multi-center study is a fairly accurate illustration of routine clinical practice and outcomes of gastric MALT lymphoma despite its retrospective design.
In conclusion, the majority of gastric MALT lymphoma patients is diagnosed at an early stage in Korea. The OS of gastric MALT lymphoma is excellent and is associated with the initial stage of the disease. Future studies are warranted to determine the best treatment modality in patients with advanced-stage disease.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by IRB of Catholic University of Korea. The ethics committee waived the requirement of written informed consent for participation.
Author Contributions
JK and JCP: planned the study. JK, JCP, JL, and JA wrote the manuscript. All authors interpreted the date, revised the manuscript for intellectual content, and approved the final manuscript.
Funding
This research was supported by the 2020 Research Fund from the Korean College of Helicobacter and Upper Gastrointestinal Research. (KCHUGR-20200250).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer EJG declared a shared affiliation, with one of the authors JA to the handling editor at the time of the review.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Isaacson P, Wright DH. Malignant Lymphoma of Mucosa-Associated Lymphoid Tissue. A Distinctive Type of B-Cell Lymphoma. Cancer (1983) 52:1410–6. doi: 10.1002/1097-0142(19831015)52:8<1410::AID-CNCR2820520813>3.0.CO;2-3
2. Wotherspoon AC, Doglioni C, Diss TC, Pan L, Moschini A, de Boni M, et al. Regression of Primary Low-Grade B-Cell Gastric Lymphoma of Mucosa-Associated Lymphoid Tissue Type After Eradication of Helicobacter Pylori. Lancet (1993) 342:575–7. doi: 10.1016/0140-6736(93)91409-F
3. Khalil MO, Morton LM, Devesa SS, Check DP, Curtis RE, Weisenburger DD, et al. Incidence of Marginal Zone Lymphoma in the United States, 2001-2009 With a Focus on Primary Anatomic Site. Br J Haematol (2014) 165:67–77. doi: 10.1111/bjh.12730
4. Nakamura S, Matsumoto T, Iida M, Yao T, Tsuneyoshi M. Primary Gastrointestinal Lymphoma in Japan: A Clinicopathologic Analysis of 455 Patients With Special Reference to its Time Trends. Cancer (2003) 97:2462–73. doi: 10.1002/cncr.11415
5. Ferrucci PF, Zucca E. Primary Gastric Lymphoma Pathogenesis and Treatment: What has Changed Over the Past 10 Years? Br J Haematol (2007) 136:521–38. doi: 10.1111/j.1365-2141.2006.06444.x
6. Psyrri A, Papageorgiou S, Economopoulos T. Primary Extranodal Lymphomas of Stomach: Clinical Presentation, Diagnostic Pitfalls and Management. Ann Oncol (2008) 19:1992–9. doi: 10.1093/annonc/mdn525
7. Wang YG, Zhao LY, Liu CQ, Pan SC, Chen XL, Liu K, et al. Clinical Characteristics and Prognostic Factors of Primary Gastric Lymphoma: A Retrospective Study With 165 Cases. Med (Baltimore) (2016) 95:e4250. doi: 10.1097/MD.0000000000004250
8. Fischbach W, Goebeler ME, Ruskone-Fourmestraux A, Wündisch T, Neubauer A, Raderer M, et al. Most Patients With Minimal Histological Residuals of Gastric MALT Lymphoma After Successful Eradication of Helicobacter Pylori Can Be Managed Safely by a Watch and Wait Strategy: Experience From a Large International Series. Gut (2007) 56:1685–7. doi: 10.1136/gut.2006.096420
9. Stathis A, Chini C, Bertoni F, Proserpio I, Capella C, Mazzucchelli L, et al. Long-Term Outcome Following Helicobacter Pylori Eradication in a Retrospective Study of 105 Patients With Localized Gastric Marginal Zone B-Cell Lymphoma of MALT Type. Ann Oncol (2009) 20:1086–93. doi: 10.1093/annonc/mdn760
10. Zaki M, Schubert ML. Helicobacter Pylori and Gastric Lymphoma. Gastroenterology (1995) 108:610–2. doi: 10.1016/0016-5085(95)90098-5
11. Du MQ, Isaccson PG. Gastric MALT Lymphoma: From Aetiology to Treatment. Lancet Oncol (2002) 3:97–104. doi: 10.1016/S1470-2045(02)00651-4
12. Zullo A, Hassan C, Cristofari F, Andriani A, De Francesco V, Ierardi E, et al. Effects of Helicobacter Pylori Eradication on Early Stage Gastric Mucosa-Associated Lymphoid Tissue Lymphoma. Clin Gastroenterol Hepatol (2010) 8:105–10. doi: 10.1016/j.cgh.2009.07.017
13. Nakamura S, Matsumoto T, Suekane H, Nakamura S, Matsumoto H, Esaki M, et al. Long-Term Clinical Outcome of Helicobacter Pylori Eradication for Gastric Mucosa-Associated Lymphoid Tissue Lymphoma With a Reference to Second-Line Treatment. Cancer (2005) 104:532–40. doi: 10.1002/cncr.21152
14. Malfertheiner P, Megraud F, O'Morain C, Bazzoli F, El-Omar E, Graham D, et al. Current Concepts in the Management of Helicobacter Pylori Infection: The Maastricht III Consensus Report. Gut (2007) 56:772–81. doi: 10.1136/gut.2006.101634
15. Park HS, Kim YJ, Yang WI, Suh CO, Lee YC. Treatment Outcome of Localized Helicobacter Pylori-Negative Low-Grade Gastric MALT Lymphoma. World J Gastroenterol (2010) 16:2158–62. doi: 10.3748/wjg.v16.i17.2158
16. Ruskoné-Fourmestraux A, Fischbach W, Aleman BM, Boot H, Du MQ, Megraud F, et al. EGILS Consensus Report. Gastric Extranodal Marginal Zone B-Cell Lymphoma of MALT. Gut (2011) 60:747–58. doi: 10.1136/gut.2010.224949
17. Zucca E, Copie-Bergman C, Ricardi U, Thieblemont C, Raderer M, Ladetto M. Gastric Marginal Zone Lymphoma of MALT Type: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann Oncol (2013) 24 Suppl 6:vi144–8. doi: 10.1093/annonc/mdt343
18. Matysiak-Budnik T, Jamet P, Ruskoné-Fourmestraux A, de Mascarel A, Velten M, Maynadié M, et al. Gastric MALT Lymphoma in a Population-Based Study in France: Clinical Features, Treatments and Survival. Aliment Pharmacol Ther (2019) 50:654–63. doi: 10.1111/apt.15409
19. Moleiro J, Ferreira S, Lage P, Dias Pereira A. Gastric Malt Lymphoma: Analysis of a Series of Consecutive Patients Over 20 Years. United Eur Gastroenterol J (2016) 4:395–402. doi: 10.1177/2050640615612934
20. Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 Revision of the World Health Organization Classification of Lymphoid Neoplasms. Blood (2016) 127:2375–90. doi: 10.1182/blood-2016-01-643569
21. Sandmeier D, Benhattar J, Bouzourene H. The Natural History of a Gastric Low Grade B Cell MALT Lymphoma Followed During 11 Years Without Treatment. J Clin Pathol (2002) 55:548–50. doi: 10.1136/jcp.55.7.548
22. Rohatiner A, d'Amore F, Coiffier B, Crowther D, Gospodarowicz M, Isaacson P, et al. Report on a Workshop Convened to Discuss the Pathological and Staging Classifications of Gastrointestinal Tract Lymphoma. Ann Oncol (1994) 5:397–400. doi: 10.1093/oxfordjournals.annonc.a058869
23. Malfertheiner P, Megraud F, O'Morain CA, Gisbert JP, Kuipers EJ, Axon AT, et al. Management of Helicobacter Pylori Infection-The Maastricht V/Florence Consensus Report. Gut (2017) 66:6–30. doi: 10.1136/gutjnl-2016-312288
24. Stolte M, Bayerdörffer E, Morgner A, Alpen B, Wündisch T, Thiede C, et al. Helicobacter and Gastric MALT Lymphoma. Gut (2002) 50 Suppl 3:Iii19–24. doi: 10.1136/gut.50.suppl_3.iii19
25. Fischbach W, Dragosics B, Kolve-Goebeler ME, Ohmann C, Greiner A, Yang Q, et al. Primary Gastric B-Cell Lymphoma: Results of a Prospective Multicenter Study. The German-Austrian Gastrointestinal Lymphoma Study Group. Gastroenterology (2000) 119:1191–202. doi: 10.1053/gast.2000.19579
26. Montalbán C, Castrillo JM, Abraira V, Serrano M, Bellas C, Piris A, et al. Gastric B-Cell Mucosa-Associated Lymphoid Tissue (MALT) Lymphoma. Clinicopathological Study and Evaluation of the Prognostic Factors in 143 Patients. Ann Oncol (1995) 6:355–62. doi: 10.1093/oxfordjournals.annonc.a059184
27. Lee JH, Choi KD, Jung HY, Baik GH, Park JK, Kim SS, et al. Seroprevalence of Helicobacter Pylori in Korea: A Multicenter, Nationwide Study Conducted in 2015 and 2016. Helicobacter (2018) 23:e12463. doi: 10.1111/hel.12463
28. Arnold M, Park JY, Camargo MC, Lunet N, Forman D, Soerjomataram I, et al. Is Gastric Cancer Becoming a Rare Disease? A Global Assessment of Predicted Incidence Trends to 2035. Gut (2020) 69:823–9. doi: 10.1136/gutjnl-2019-320234
29. Suh M, Song S, Cho HN, Park B, Jun JK, Choi E, et al. Trends in Participation Rates for the National Cancer Screening Program in Korea, 2002-2012. Cancer Res Treat (2017) 49:798–806. doi: 10.4143/crt.2016.186
30. Matysiak-Budnik T, Fabiani B, Hennequin C, Thieblemont C, Malamut G, Cadiot G, et al. Gastrointestinal Lymphomas: French Intergroup Clinical Practice Recommendations for Diagnosis, Treatment and Follow-Up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, SFH). Dig Liver Dis (2018) 50:124–31. doi: 10.1016/j.dld.2017.12.006
31. Zucca E, Arcaini L, Buske C, Johnson PW, Ponzoni M, Raderer M, et al. Marginal Zone Lymphomas: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann Oncol (2020) 31:17–29. doi: 10.1016/j.annonc.2019.10.010
32. Thieblemont C, Cascione L, Conconi A, Kiesewetter B, Raderer M, Gaidano G, et al. A MALT Lymphoma Prognostic Index. Blood (2017) 130:1409–17. doi: 10.1182/blood-2017-03-771915
Keywords: endoscopy, lymphoma, Helicobacter pylori, MALT, stomach
Citation: Kim JS, Park JC, Lee JY, Ahn JY, Kang SH, Yang H-J, Kim SJ, Joo MK and Park JM (2021) Long-Term Clinical Outcomes of Gastric MALT Lymphoma: A Nationwide Multicenter Study in Korea. Front. Oncol. 11:681689. doi: 10.3389/fonc.2021.681689
Received: 17 March 2021; Accepted: 28 September 2021;
Published: 14 October 2021.
Edited by:
Simona Gurzu, George Emil Palade University of Medicine, Pharmacy, Sciences and Technology of Târgu Mureş, RomaniaReviewed by:
Eun Jeong Gong, University of Ulsan, South KoreaXueliang Wu, First Affiliated Hospital of Hebei North University, China
Emanuele Zucca, Oncology Institute of Southern Switzerland (IOSI), Switzerland
Copyright © 2021 Kim, Park, Lee, Ahn, Kang, Yang, Kim, Joo and Park. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jong Yeul Lee, jylee@ncc.re.kr; Ji Yong Ahn, ji110@hanmail.net
†These authors have contributed equally to this work and share first authorship
 Joon Sung Kim
Joon Sung Kim Jun Chul Park
Jun Chul Park Jong Yeul Lee
Jong Yeul Lee Ji Yong Ahn4*
Ji Yong Ahn4* Sun Hyung Kang
Sun Hyung Kang Moon Kyung Joo
Moon Kyung Joo