- Department of Neurosurgery, Department of Oncology, Department of Postgraduate Students, West China School of Medicine, Sichuan University, Chengdu, China
Confusing masses constitute a challenging clinical problem for differentiating between cancer and tuberculosis diagnoses. This review summarizes the major theories designed to identify factors associated with misdiagnosis, such as imaging features, laboratory tests, and clinical characteristics. Then, the clinical experiences regarding the misdiagnosis of cancer and tuberculosis are summarized. Finally, the main diagnostic points and differential diagnostic criteria are explored, and the characteristics of multimodal imaging and radiomics are summarized.
Introduction
Cancer and tuberculosis are two of the most common diseases affecting health worldwide. According to a recent World Health Organization (WHO) report, tuberculosis has one of the highest mortalities among all infectious diseases worldwide, causing an estimated 1.5 million deaths in 2018 (1). In 2020, an estimated 1,806,590 new cancer cases and 606,520 cancer-related deaths were estimated to occur in the USA. Tuberculosis is a great mimicker and diagnostic chameleon and is prone to be diagnosed as cancer (2). Moreover, due to its unusual presentations and lack of specific diagnostic tests, patients with cancer have been misdiagnosed with tuberculosis (3, 4). Therefore, these confusing masses represent a substantial clinical problem in the differential diagnosis of cancer and tuberculosis. Although many reports have addressed the differences between tuberculosis and cancer, comprehensive summaries are very rare (5).
Multimodal imaging involves the combination of at least two imaging tools to obtain more detailed, accurate images for diagnosis. Radiomics is a newly emerging form of computational medical imaging which involves the analysis and translation of medical images into quantitative data and have been rapidly developed in recent years. The analysis begins with acquiring a sufficient number of multimodal images of good quality and diversity. After extracting imaging features using a computer, trained algorithms can provide diagnostic aid or exact quantitative information by calculating the extracted features (6–9). Thus, the combination use of multimodal imaging and radiomics may enable a more accurate differential diagnosis for confused masses.
This review summarizes major theories designed to identify the factors associated with misdiagnosis and highlights key findings related to these confusing features to enhance diagnostic accuracy in clinical practice. Additionally, the potential for using multimodal imaging and radiomics in differentiating cancer and tuberculosis was also discussed.
Methods
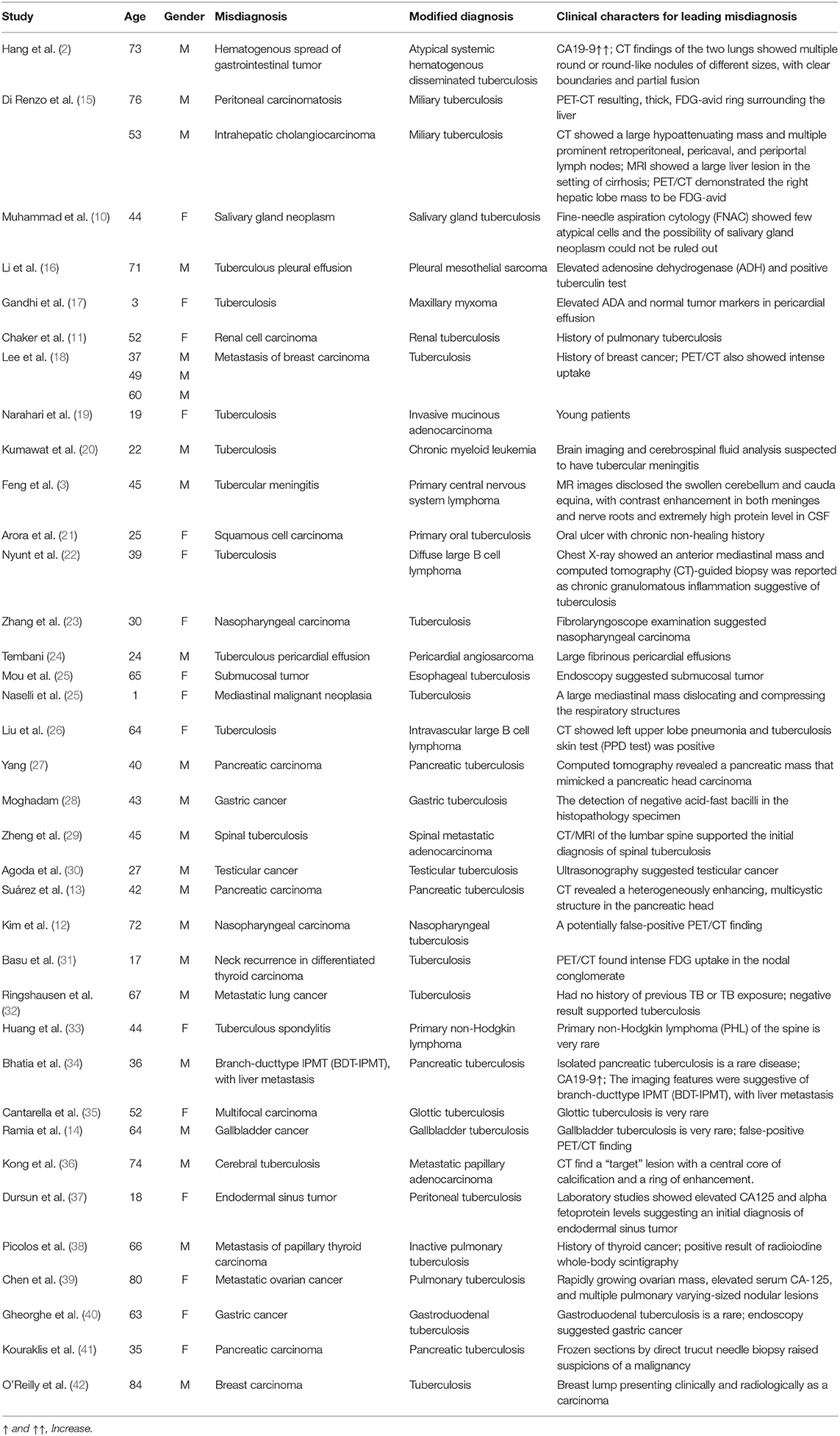
We searched PubMed for reported cases associated with the misdiagnosis of cancer and tuberculosis using the search strategy (“diagnostic errors” [Mesh]) AND (“tuberculosis” [Mesh])) AND (“neoplasms” [Mesh]) from 2000 to 2020. Among these patients, 11 of 37 were misdiagnosed with tuberculosis, and 26 of 37 were misdiagnosed with tuberculosis. Notably, these confusing diseases can involve any organ, such as the liver, salivary glands (10), kidneys (11), nasopharynx (12), pancreas (13), and gallbladder (14). The diagnoses of 33 of these patients were confirmed by biopsy of the lesion, while three were diagnosed based on their body fluid cultures, and one patient was diagnosed based on empiric antituberculosis treatment.
In summary, the clinical manifestations of these cases are non-specific. The main characteristics leading to the misdiagnosis of tuberculosis as cancer included false-positive positron emission tomography/computerized tomography (PET/CT) findings, an oncologic history and elevated carbohydrate antigen 125 (CA 125) or carbohydrate antigen 19-9 (CA 19-9) levels, while the main reasons for misdiagnosing cancer as tuberculosis were a history of tuberculosis, a positive tuberculin test, and a history of a rare cancer. The features associated with the chosen cases are summarized in Table 1.
Results and Discussion
Imaging Modalities
Computerized Tomography (CT)
Computerized tomography (CT) is commonly used in clinical practice to initially assess whether a mass is a malignant tumor or benign nodule according to the imaging features such as the size, shape, tumor border, and enhancement characteristics. A previous study reported a misdiagnosis case based on a head and neck CT scan showing a target sign with a ring enhancement around a central nidus of calcification, which led to the misdiagnosis of metastatic papillary adenocarcinoma (originating from primary lung carcinoma) as cerebral tuberculosis (36). The target sign of cerebral tuberculosis was first described in 1979 (43) and became a pathognomonic requirement for a cerebral tuberculosis diagnosis in 1988 (44). As a non-specific radiologic finding, the target sign most commonly indicated cerebral tuberculoma or metastatic adenocarcinoma, and recent clinical evidence suggested its specificity for tuberculosis (45, 46). Cerebral tuberculosis also shows a solid enhancing mass (47). Therefore, in the different clinical contexts, this target sign confuses clinicians (36).
Chest CT scans lead to higher rates of misdiagnosis between tuberculosis and cancer than head and neck CT and abdominal CT. The CT scan features of pulmonary tuberculosis include irregular linear opacity, discrete miliary nodules, calcified nodules or consolidation, parenchymal bands, and pericicatricial emphysema (18). The most common findings of CT scans in patients with early bronchogenic spread of primary tuberculosis are 2–4 mm centrilobular nodules and branching linear lesions presenting as intrabronchiolar and peribronchiolar caseation necrosis. As the disease progresses, 2–4 mm centrilobular nodules may coalesce and become lobular and consolidated or expand to 5–8 mm nodules (48). After antituberculous chemotherapy, resolution of the lesion occurs, resulting in bronchovascular distortion, bronchiectasis, emphysema, and fibrosis (49). CT scans of early miliary dissemination commonly feature ground-glass opacification with barely discernible nodules, followed by discrete miliary nodules representing round intrapulmonary lesions of <3 cm (50). Multiple pulmonary nodular lesions of varying sizes usually lead to misdiagnosis, especially when complemented by non-specific symptoms (2, 26, 32). In some tuberculosis cases, chest CTs showed multiple round nodules of different sizes, with clear boundaries and partial fusion (2). In addition, chest CT scans have also shown pneumonia in patients with cancer. A previous study reported that chest CT scans of a patient with large B cell non-Hodgkin's lymphoma showed pneumonia throughout the entire lung and diffuse ground glass opacities in both lung fields (26). Furthermore, in patients with histories of tuberculosis and cancer, the imaging manifestations are so similar that definitive diagnoses are difficult to make, and errors occur more frequently than in patients without histories of these diseases (38, 39).
The common features of abdominal CT scans related to misdiagnosis are the pancreatic head (13, 27) and peritoneum (15). The incidence of pancreatic tuberculosis is rare, and this disease may present as a heterogeneously enhanced structure in the pancreatic head with multiple enlarged lymph nodes surrounding the head of the pancreas (13). These findings lead to errors in the diagnosis of patients suspected of having a pancreatic neoplasm with multiple lymph node metastases (27). Regarding miliary tuberculosis of the abdomen, abdominal CT scans may show a solitary liver mass with an irregular enhancing rim and progressive enhancement, which likely leads to a radiographic diagnosis of intrahepatic cholangiocarcinoma (15).
In general, the CT imaging features of spinal tuberculosis include irregular lytic lesions and sclerosis, narrowing of the intervertebral disk space, disc collapse with eventual progression to kyphotic deformity, destruction of the anterior parts of adjacent vertebrae, formation of a large paravertebral abscess, and calcifications or sequestra within the paravertebral abscess (51). The clinical characteristics of spinal tuberculosis and cancer metastasis are non-specific. The imaging presentations of spinal metastatic adenocarcinoma are highly consistent with spinal tuberculosis, and misdiagnosis occurs (29). On the other hand, radiotherapy is often used for suspected malignant spinal lesions without histologic confirmation, and a definitive diagnosis of spinal tuberculosis was finally made (52). Therefore, in cases of spinal lesions of unknown origin, tuberculosis should be taken into consideration despite a previous diagnosis of cancer.
Magnetic Resonance Imaging
Although the lung parenchyma was considered difficult to evaluate by magnetic resonance imaging (MRI) due to low proton density in the pulmonary tissue, susceptibility artifacts and respiratory motion artifacts (53), MRI would be helpful for discriminating pulmonary lesions because of its higher contrast resolution, absence of radiation, and multiple-parameter imaging (54). MRI has found relevant applications in the diagnosis of chest diseases and the differential diagnosis of benign and malignant lung lesions (55, 56). Qi et al. (57) investigated the differences in the imaging features of mass-like tuberculosis and lung cancer on conventional MR sequences and found that most tuberculosis lesions showed low signal intensity on T2-weighted images while lung cancer showed high signal intensity; the signal of tuberculosis lesions was mostly uneven on T2-weighted images, but the signal of lung cancer was mostly uniform; most tuberculosis lesions showed high signal intensity on T1-weighted images while lung cancer showed low signal intensity. Besides, benign mediastinal lymph nodes in tuberculosis lesions showed a variety of signals on T2-weighted images, whereas the majority of metastatic mediastinal lymph nodes displayed slight homogeneous hyperintensity.
Radiomics in Chest Imaging
Non-invasive and computer-aided alternatives have gradually been used in the differentiation of tuberculosis and lung cancer. In recent years, radiomics has attracted more and more attention due to its high-throughput extraction and distinguishing features from medical images, and to construct radiomics nomogram model to assist physicians to make the most accurate diagnosis (58–60). Cui et al. (61) developed and validated radiomics methods for distinguishing pulmonary tuberculosis from lung cancer based on CT images; they found the radiomics nomogram model exhibited good discrimination, with an AUC of 0.914 in the training cohort, and 0.900 in the validation cohort, showing that proposed radiomic methods can be used as a non-invasive tool for discrimination of tuberculosis and lung cancer on the basis of preoperative CT data. Another study (62) investigated the preoperative differential diagnostic performance of a radiomics nomogram in tuberculous granuloma and lung adenocarcinoma appearing as solitary pulmonary solid nodules and found that the radiomics nomogram showed better diagnostic accuracy than any single model with the AUC 0.9660, 0.9342, and 0.9064 for the training, internal validation, and external validation cohorts, respectively, which similarly indicated the radiomics nomogram could preoperatively distinguish between lung cancer and tuberculosis.
False-Positive PET/CT Findings
PET/CT is a powerful diagnostic method for characterizing masses, and it can more accurately assess mediastinal lymph nodes stages in cancer than CT (63). Many clinical conditions are now well-known to be responsible for false positives in oncological PET/CT scanning and are often related to uptake because of inflammation or infection processes. Infectious diseases, post-operative surgical conditions and radiation pneumonitis show as high fludeoxyglucose (FDG) uptake on PET/CT scans (64). Overexpression of glucose transporter-1 (GLUT-1) receptors in human macrophages, neutrophils, and lymphocytes following stimulation with cytokines or mutagens has been implicated in the intense uptake of FDG in inflammatory conditions. This highlights that standard uptake value (SUV) alone is an unreliable parameter for characterizing lesions in such a setting and should be used with caution and adequate correlation (31). A previous study showed that tuberculosis was mostly responsible (50%) for false positives in PET/CT (65). Tuberculosis at various locations, such as the gallbladder, nasopharynx, and peritoneum, is usually misdiagnosed due to false-positive findings on PET/CT scans (14, 15). In a case of nasopharyngeal tuberculosis, PET/CT revealed intense activity in soft tissue masses in the nasopharynx and cervical lymph nodes (12). Combined with those of fibrolaryngoscope examination, the results may lead to misdiagnosis as nasopharyngeal carcinoma (23). In lymph node tuberculosis, PET/CT also showed intense uptake. Patients previously diagnosed with cancer with multifocal hypermetabolic lesions may confuse clinicians, leading to misdiagnosis (66). Thus, when PET/CT findings show increased FDG uptake in patients with cancer as well as suspected metastatic lesions, tuberculous lymphadenopathy should be considered a differential diagnosis.
Laboratory Tests
Non-Specificity of CA 19-9 Levels in Serum
CA 19-9 is synthesized by pancreatic, gastric, colon, biliary ductal, and endometrial tissues, and its serum levels are extremely low (67). As a tumor marker for pancreatic, hepatobiliary, and gastrointestinal cancer, high CA 19-9 levels are indicative of advanced disease and a poor prognosis (CA 19-9 level of >100 U/ml usually suggests unresectable or metastatic disease) (68, 69). In addition to cancer, overexpression of CA 19-9 has also been observed in some benign conditions, including gastrointestinal disorders, hepatobiliary system diseases, pneumonia, pleural effusion, renal failure, and systemic lupus erythematosus (SLE), and this phenomenon may be associated with glycan-mediated cell-cell interactions in mucosal immunity (67). Hence, this indicator has low specificity. In the misdiagnosis of cancer and tuberculosis, sharply increased CA 19-9 levels also play a key role (2). CA 19-9 was reportedly elevated in cases of pulmonary tuberculosis (70), hematogenous disseminated tuberculosis (2), and pancreatic tuberculosis (34, 71). In pulmonary disease, the median CA 19-9 level in patients with pulmonary tuberculosis (median CA 19-9 level: 5.85 U/ml) was significantly lower than that in patients with pulmonary non-tuberculous mycobacterial disease (median CA 19-9 level: 13.80 U/ml, p < 0.001). Moreover, the CA 19-9 levels tended to decrease when pulmonary non-tuberculous mycobacterial disease was successfully treated, but this was not observed in pulmonary tuberculosis (70). Although the serum levels of CA 19-9 are commonly significantly lower in non-malignant diseases than in malignant diseases, CA 19-9 was reported to reach concentrations of 165 U/ml in hematogenous disseminated tuberculosis (2) and 66.84 U/ml in pancreatic tuberculosis (27). Therefore, although serum CA 19-9 levels lack specificity, CA 19-9 is the only marker of pancreatic cancer used in the clinic (69). When patients with increased CA 19-9 levels present atypically, tuberculosis may be the definitive diagnosis.
Non-Specificity of Serum CA 125 Levels
Since the report on CA 125 expression in ovarian tumors published in the early 1980s, serum CA 125 levels have been utilized as a biomarker for the differential diagnosis of pelvic masses and have been widely used to monitor patients with ovarian cancer (72–74). Although the CA 125 levels in serum are elevated in over 80% of patients with ovarian cancer at the time of initial diagnosis, using this parameter as a diagnostic marker can lead to clinical mistakes (75). In regard to peritoneal tuberculosis, patients with rapidly growing ovarian masses and elevated serum CA 125 levels usually lead to an initial diagnosis of ovarian cancer (37). Furthermore, tuberculosis is misdiagnosed as a metastatic ovarian cancer when patients show elevated serum CA 125 levels and multiple pulmonary nodular lesions (39). Thus, infectious diseases can mimic metastatic diseases and therefore increase the difficulty of diagnosis.
Adenosine Deaminase Activity
Adenosine deaminase activity (ADA) activity is widely distributed in human tissues and is the highest in lymphoid tissues, and two isozymes of ADA exist, ADA1 and ADA2 (76). The serum concentrations of ADA1 are high in patients with tuberculosis, and previous evidence suggests that the increased ADA1 is the results of T cell lymphocyte stimulation by mycobacterial antigens (77). ADA has been developed and widely used for the diagnosis of tuberculosis. However, it is not a specific index for differentiating between tuberculosis and cancer diagnoses. Although elevated ADA levels usually indicate tuberculosis, the non-specific characteristics of cancer patients presenting with increased ADA levels and a positive tuberculin test can lead to misdiagnosis (16, 17).
Carcinoembryonic Antigen and Cytokeratin Fraction 21-1 in Serum
Both carcinoembryonic antigen (CEA) and cytokeratin fraction 21-1 (CYFRA 21-1) are widely used as tumor markers and are not considered to be confusing indexes based on the reviewed literature above. What is more, Jia et al. report that the CEA and CYFRA 21-1 levels are very valuable for distinguishing between lung cancer and pulmonary tuberculosis (78).
Fine-Needle Aspiration Cytology
The diagnosis of cancer is usually made based on histological analyses of excisional biopsies, fine-needle aspiration cytology, and liquid biopsy (16). Fine-needle aspiration cytology, a commonly used method that is minimally invasive, quick, and accurate, involves the use of a thin needle to acquire cellular material from a bodily lesion or mass for diagnostic purposes. However, fine-needle aspiration cytometry is less sensitive and specific for some malignant tumors, such as those of the parotid gland (6), pancreas (41), and malignant lymphoma (79). In the parotid gland, the sensitivity and specificity of fine-needle aspiration cytology are 79 and 96%, respectively (80). In lymphoma, the median rate at which fine-needle aspiration cytology yields a subtype-specific diagnosis of lymphoma is 74% (77). Several factors are related to the specificity of fine-needle aspiration cytometry. First, fine-needle aspiration is unsatisfactory, and only the inflammatory necrosis area is obtainable in cases of small amounts of tissue, deep locations, and hard masses. Determining whether necrosis is caused by tumor disease or simple inflammation is almost impossible (81). Second, during pathological examination, seriously degenerated tumor cells are poorly stained, leading to fuzzy chromatin staining and structures. In particular, it is easy to make a misdiagnosis of tuberculosis when some carcinoma cells are spindle shaped, when nuclei exhibit vacuolar changes, and when lymphoma cells have lightly stained chromatin with obvious nucleoli and inflammatory necrosis (82). In addition, granulomatous inflammation often occurs in infectious diseases such as tuberculosis and mycosis and can also occur as a local inflammatory reaction in malignant tumors (83–85). Hence, although biopsy pathology is crucial for differentiating between cancer and tuberculosis, the findings of this examination may be a key factor underlying misdiagnosis.
Other Characteristics
History of Previous Disease
Tuberculosis and cancer have a complex and dangerous liaison relationship (86). On the one hand, long-term chemotherapy, radiotherapy, and surgery will weaken the immune system in patients with cancer, which increases the risk of tuberculosis infection (87, 88). On the other hand, a large cohort study reported that pulmonary tuberculosis is associated with an increased risk of developing lung cancers (89). In clinical practice, without specific information, patients that have been previously diagnosed with cancer or tuberculosis may be misdiagnosed (38, 66).
Definitive Diagnoses of Rare Diseases
Physicians usually do not apt to consider diagnosis of patients as rare diseases at first time, such as primary non-Hodgkin lymphoma of the spine (33), glottic tuberculosis (35), isolated pancreatic tuberculosis (34), and gallbladder tuberculosis (14). The limited knowledge and preconceived professional ideas may lead to errors in the diagnoses of these rare cancers and tuberculosis.
Conclusions
CT is commonly used in clinical practice to initially assess diseases. However, the shortcomings of this technology also cause confusion. Fine-needle aspiration cytology is a commonly used biopsy method but not reliable. Excisional biopsies may be the best definitive diagnostic method for confusing masses. When PET/CT findings show cancer patients with increased FDG uptake, tuberculosis should be considered a differential diagnosis, except in suspected cases of metastatic lesions. ADA and tumor markers are not specific indexes in the differential diagnosis of tuberculosis and metastasis. Thus, the following features are summarized to differentiate tuberculosis from cancer: (1) Fine-needle aspiration cytology has low sensitivity and specificity. (2) High FDG uptake on PET/CT scans can occur in cases of tuberculosis. (3) Tumor markers, including CA 19-9 and CA125, could be increased in tuberculosis patients. (4) ADA can be upregulated in in cancer patients. (5) Histopathological examination and tuberculosis cultures are still the gold standards for diagnosis.
In the diagnosis of tuberculosis, multimodal imaging could offer high spatial resolution, soft tissue contrast, and biological information at the molecular level with high sensitivity. Radiomics technology use computers to extract numerous imaging features, especially the ones that cannot be distinguished by eyes directly, which may enable the ability of automatically distinguishing complex images and offer quantitative information. However, the characteristics and quality of the images cannot be automatically assessed, which means the extracted features should be calculated by using a computational-aided diagnostic algorithms before they can be used practically (7, 90). With well-trained algorithms, confusing masses can be identified based on the extracted features. Beig et al. provided an excellent example of using radiomic features on lung CT images to distinguish adenocarcinomas from granulomas (8).
In summary, although clinicians should conduct more studies on the special features of these diseases, a comprehensive review of misdiagnosis characteristics could also guide the plan for use of multimodal imaging and radiomics-based algorithms.
Author Contributions
QZ conceived and designed the experiments and revised the manuscript. YX, CH, and YH contributed reagents and materials and helped with the analysis. YX and CH wrote the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Harding E. WHO global progress report on tuberculosis elimination. Lancet Respir Med. (2020) 8:19. doi: 10.1016/S2213-2600(19)30418-7
2. Hang TX, Fang G, Huang Y, Hu CM, Chen W. Misdiagnosis of a multi-organ involvement hematogenous disseminated tuberculosis as metastasis: a case report and literature review. Infect Dis Poverty. (2020) 9:66. doi: 10.1186/s40249-020-00681-8
3. Feng M, Yang X, Ma Q, He Y. Retrospective analysis for the false positive diagnosis of PET-CT scan in lung cancer patients. Medicine. (2017) 96:e7415. doi: 10.1097/MD.0000000000007415
4. Khurse BB, Kumar S, Deo S, Malik P, Kumar V, Kumar R, et al. Mediastinal nodal staging of non-small cell lung cancer using PET-CT in a tuberculosis-endemic country. Ann Oncol. (2017) 28:ii14. doi: 10.1093/annonc/mdx086.002
5. Singh SK, Ahmad Z, Bhargava R, Pandey DK, Gupta V, Garg PK. Coincidence of tuberculosis and malignancy: a diagnostic dilemma. South Med J. (2009) 102:113. doi: 10.1097/SMJ.0b013e318188e32c
6. Lee SY, Jeon SI, Jung S, Chung IJ, Ahn CH. Targeted multimodal imaging modalities. Adv Drug Deliv Rev. (2014) 76:60–78. doi: 10.1016/j.addr.2014.07.009
7. Hosny A, Parmar C, Quackenbush J, Schwartz LH, Aerts HJWL. Artificial intelligence in radiology. Nat Rev Cancer. (2018) 18:500–10. doi: 10.1038/s41568-018-0016-5
8. Beig N, Khorrami M, Alilou M, Prasanna P, Braman N, Orooji M, et al. Perinodular and intranodular radiomic features on lung CT images distinguish adenocarcinomas from granulomas. Radiology. (2019) 290:783–92. doi: 10.1148/radiol.2018180910
9. Schwalbe N, Wahl B. Artificial intelligence and the future of global health. Lancet. (2020) 395:1579–86. doi: 10.1016/S0140-6736(20)30226-9
10. Muhammad T, Iftikhar H, Ikram M. Intraparotid tuberculosis misdiagnosed as neoplasm on FNAC. J Coll Physicians Surg Pakistan. (2019) 29:593–4. doi: 10.29271/jcpsp.2019.06.593
11. Chaker K, Chakroun M, Gharbi M, Chebil M. Renal tuberculosis mimicking renal cell carcinoma: a case report. J Med Case Rep. (2019) 13:139. doi: 10.1186/s13256-019-2073-0
12. Kim KS. Primary nasopharyngeal tuberculosis mimicking carcinoma: a potentially false-positive PET/CT finding. Clin Nucl Med. (2010) 35:346–8. doi: 10.1097/RLU.0b013e3181d624ff
13. Suárez-Moreno RM, Hernández-Ramirez DA, Madrazo-Navarro M, Salazar-Lozano CR, García-Álvarez KG, Espinoza-Álvarez A. Tuberculosis of the pancreas: an unsuspected cause of abdominal pain and fever. Cir Cir. (2010) 78:352–6. doi: 10.1016/j.ciresp.2009.12.024
14. Ramia JM, Muffak K, Fernández A, Villar J, Garrote D, Ferron JA. Gallbladder tuberculosis: False-positive PET diagnosis of gallbladder cancer. World J Gastroenterol. (2006) 12:6559–60. doi: 10.3748/wjg.v12.i40.6559
15. Di Renzo C, Tabrizian P, Kozuch DE, Fiel MI, Schwartz ME. Abdominal tuberculosis mimicking cancer clinically and on fluorodeoxyglucose (FDG)-positron emission tomography (PET) imaging: a two-case series. Am J Case Rep. (2020) 21:e918901. doi: 10.12659/AJCR.918901
16. Li WQ, Fu AS, Shao DF, Zhang Q, Wang MH, Wang HY, et al. Elevated adenosine dehydrogenase (ADH) and positive tuberculin test firstly misdiagnosed as tuberculous pleural effusion finally proved as pleural mesothelial sarcoma by thoracoscopic biopsy pathology: A case report and literature review. Clin Lab. (2019) 65:190323. doi: 10.7754/Clin.Lab.2019.190323
17. Gandhi D, Pawar V, Chandane P, Shah I. Maxillary myxoma misdiagnosed as tuberculosis. Trop Doct. (2019) 49:227–9. doi: 10.1177/0049475519829599
18. Lee JY, Lee KS, Jung KJ, Han J, Kwon OJ, Kim J, et al. Pulmonary tuberculosis: CT and pathologic correlation. J Comput Assist Tomogr. (2000) 24:691–8. doi: 10.1097/00004728-200009000-00005
19. Narahari NK, Uppin SG, Kapoor A, Stalin BJ, Paramjyothi GK. Invasive mucinous adenocarcinoma of the lung in a 19-year-old female. Asian Cardiovasc Thorac Ann. (2018) 26:635–9. doi: 10.1177/0218492318804951
20. Kumawat BL, Sharma CM, Saini PK, Garg A. Central nervous system blast crisis of chronic myeloid leukaemia misdiagnosed as tubercular meningitis. BMJ Case Rep. (2018) 2018:223923. doi: 10.1136/bcr-2017-223923
21. Arora KS, Garg S, Kaur P, Mohapatra S. Primary oral tuberculosis on the tongue mimicking squamous cell carcinoma. Indian J Tuberc. (2018) 65:84–6. doi: 10.1016/j.ijtb.2016.09.019
22. Nyunt WWT, Wong YP, Wan Jamaludin WF, Abdul Wahid SFS. Diffuse large B cell lymphoma with chronic granulomatous inflammation. Malays J Pathol. (2016) 38:55–9.
23. Zhang Y, Chen Y, Huang Z, Cai L, Wu J. Nasopharyngeal tuberculosis mimicking nasopharyngeal carcinoma on 18F-FDG PET/CT in a young patient. Clin Nucl Med. (2015) 40:518–20. doi: 10.1097/RLU.0000000000000656
24. Tembani-Munyandu NC, Katsidzira L, Makunike-Mutasa R, Chinogureyi A. An unusual cause of a large fibrinous pericardial effusion. Cardiovasc J Afr. (2015) 26:e7–e10. doi: 10.5830/CVJA-2014-075
25. Naselli A, Granata C, Garaventa A, Conte M, Losurdo G, Castagnola E. Tuberculosis diagnosed after chemotherapy for presumed mediastinal malignant neoplasia. Pediatr Blood Cancer. (2014) 61:1897–8. doi: 10.1002/pbc.25053
26. Liu C, Lai N, Zhou Y, Li S, Chen R, Zhang N. Intravascular large B-cell lymphoma confirmed by lung biopsy. Int J Clin Exp Pathol. (2014) 7:6301–6.
27. Yang Y-J. Pancreatic tuberculosis mimicking pancreatic carcinoma during anti-tuberculosis therapy: a case report. World J Clin Cases. (2014) 2:167–9. doi: 10.12998/wjcc.v2.i5.167
28. Moghadam AG, Alborzi A, Pouladfar G, Monabati A. Primary gastric tuberculosis mimicking gastric cancer: a case report. J Infect Dev Ctries (2013) 7(04):355–7. doi: 10.3855/jidc.2598
29. Zheng CY, Liu DX, Luo SW, Du SX. Imaging presentation highly manifested as tuberculosis in a case of spinal metastatic carcinoma. Orthopedics. (2011) 34:e436–8. doi: 10.3928/01477447-20110627-32
30. Agoda-Koussema L, Tchaou M, Adjénou V, Sonhaye L, Anoukoum T, Tengué K, et al. Heterogeneous testicle on ultrasonography: consider tuberculosis after cancer in endemic zone. Med Trop. (2011) 71:100.
31. Basu S, Menon S. FDG avid supraclavicular neck adenopathy of tubercular etiology masquerading as neck recurrence in differentiated thyroid carcinoma: potential source of false positive FDG-PET study. Int J Oral Maxillofac Surg. (2010) 39:628–9. doi: 10.1016/j.ijom.2010.01.021
32. Ringshausen FC, Tannapfel A, Nicolas V, Weber A, Duchna HW, Schultze-Werninghaus G, et al. A fatal case of spinal tuberculosis mistaken for metastatic lung cancer: recalling ancient Pott's disease. Ann Clin Microbiol Antimicrob. (2009) 8:32. doi: 10.1186/1476-0711-8-32
33. Huang B, Li CQ, Liu T, Zhou Y. Primary non-Hodgkin's lymphoma of the lumbar vertebrae mimicking tuberculous spondylitis: a case report. Arch Orthop Trauma Surg. (2009) 129:1621–5. doi: 10.1007/s00402-009-0835-7
34. Bhatia V, Garg PK, Arora VK, Sharma R. Isolated pancreatic tuberculosis mimicking intraductal pancreatic mucinous tumor. Gastrointest Endosc. (2008) 68:610–1. doi: 10.1016/j.gie.2007.12.022
35. Cantarella G, Pagani D, Fasano V, Scaramellini G. Glottic tuberculosis masquerading as early multifocal carcinoma. Tumori J. (2007) 93:302–4. doi: 10.1177/030089160709300315
36. Kong A, Koukourou A, Boyd M, Crowe G. Metastatic adenocarcinoma mimicking “target sign” of cerebral tuberculosis. J Clin Neurosci. (2006) 13:955–8. doi: 10.1016/j.jocn.2005.11.039
37. Dursun P, Ersoz S, Gultekin M, Aksan G, Yüce K, Ayhan A. Disseminated peritoneal tuberculosis mimicking advanced-stage endodermal sinus tumor: a case report. Int J Gynecol Cancer. (2006) 1:303–7. doi: 10.1111/j.1525-1438.2006.00205.x
38. Picolos MK, Habra M, Safdar A, Sarlis NJ. Inactive pulmonary tuberculosis mimicking metastasis from papillary thyroid carcinoma in diagnostic radioiodine whole-body scintigraphy. Thyroid. (2005) 15:1105–6. doi: 10.1089/thy.2005.15.1105
39. Chen CH, Huang CY, Chow SN. Early-stage ovarian carcinoma combined with pulmonary tuberculosis mimicking advanced ovarian cancer: a case report. Int J Gynecol Cancer. (2004) 14:1007–11. doi: 10.1111/j.1048-891X.2004.014543.x
40. Gheorghe L, Bǎncilǎ I, Gheorghe C, Herlea V, Vasilescu C, Aposteanu G. Antro-duodenal tuberculosis causing gastric outlet obstruction–a rare presentation of a protean disease. Rom J Gastroenterol. (2002) 11:149–52.
41. Kouraklis G, Glinavou A, Karayiannakis A, Karatzas G. Primary tuberculosis of the pancreas mimicking a pancreatic tumor. Int J Pancreatol. (2001) 29:151–3. doi: 10.1385/ijgc:29:3:151
42. O'Reilly M, Patel K, Cummins R. Tuberculosis of the breast presenting as carcinoma. Mil Med. (2000) 165:800–2. doi: 10.1159/000054256
43. Welchman JM. Computerised tomography of intracranial tuberculomata. Clin Radiol. (1979) 30:567–73. doi: 10.1016/S0009-9260(79)80199-3
44. van Dyk A. CT of intracranial tuberculomas with specific reference to the “target sign". Neuroradiology. (1988) 30:329–36. doi: 10.1007/BF00328184
45. Schaller MA, Wicke F, Foerch C, Weidauer S. Central nervous system tuberculosis: etiology, clinical manifestations and neuroradiological features. Clin Neuroradiol. (2019) 29:3–18. doi: 10.1007/s00062-018-0726-9
46. Bernaerts A, Vanhoenacker FM, Parizel PM, Van Goethem JWM, van Altena R, Laridon A, et al. Tuberculosis of the central nervous system: overview of neuroradiological findings. Eur Radiol. (2003) 13:1876–90. doi: 10.1007/s00330-002-1608-7
47. Yanardag H, Uygun S, Yumuk V, Caner M, Canbaz B. Cerebral tuberculosis mimicking intracranial tumour. Singapore Med J. (2005) 46:731–3.
48. Skoura E, Zumla A, Bomanji J. Imaging in tuberculosis. Int J Infect Dis. (2015) 32:87–93. doi: 10.1016/j.ijid.2014.12.007
49. Im JG, Itoh H, Shim YS, Lee JH, Ahn J, Han MC, et al. Pulmonary tuberculosis: CT findings - Early active disease and sequential change with antituberculous therapy. Radiology. (1993) 186:653–60. doi: 10.1148/radiology.186.3.8430169
50. Setio AAA, Traverso A, de Bel T, Berens MSN, Bogaard C van den, Cerello P, et al. Validation, comparison, and combination of algorithms for automatic detection of pulmonary nodules in computed tomography images: the LUNA16 challenge. Med Image Anal. (2017) 42:1–13. doi: 10.1016/j.media.2017.06.015
51. Ansari S, Amanullah M, Ahmad K, Rauniyar RK. Pott's spine: diagnostic imaging modalities and technology advancements. N Am J Med Sci. (2013) 5:404–11. doi: 10.4103/1947-2714.115775
52. Jutte PC, van Altena R, Pras E, Thijn CJP. Causes of misdiagnosis and mistreatment of spinal tuberculosis with radiotherapy in nonendemic areas: a pitfall in diagnosis and treatment: hazards of radiotherapy on the tuberculous lesion. Spine. (2005) 30:E300–4. doi: 10.1097/01.brs.0000163886.20464.02
53. Kapur S, Bhalla A S, Jana M. Pediatric chest MRI: a review. Indian J Pediatr. (2019) 86:842–53. doi: 10.1007/s12098-018-02852-w
54. Rizzi EB, Schinina' V, Cristofaro M, Goletti D, Palmieri F, Bevilacqua N, et al. Detection of pulmonary tuberculosis: comparing MR imaging with HRCT. BMC Infect Dis. (2011) 11:243. doi: 10.1186/1471-2334-11-243
55. Schaefer J F, Vollmar J, Schick F, Vonthein R, Seemann MD, Aebert H, et al. Solitary pulmonary nodules: dynamic contrast-enhanced MR imaging—perfusion differences in malignant and benign lesions. Radiology. (2004) 232:544–53. doi: 10.1148/radiol.2322030515
56. Fujimoto K. Usefulness of contrast-enhanced magnetic resonance imaging for evaluating solitary pulmonary nodules. Cancer imaging. (2008) 8:36–44. doi: 10.1102/1470-7330.2008.0009
57. Qi LP, Chen KN, Zhou XJ, Tang L, Liu YL, Li XT, et al. Conventional MRI to detect the differences between mass-like tuberculosis and lung cancer. J Thorac Dis. (2018) 10:5673–84. doi: 10.21037/jtd.2018.09.125
58. Aerts HJWL, Velazquez ER, Leijenaar RTH, Parmar C, Grossmann P, Carvalho S, et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun. (2014) 5:4006. doi: 10.1038/ncomms5006
59. Lambin P, Rios-Velazquez E, Leijenaar R, Carvalho S, van Stiphout RGPM, Granton P, et al. Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer. (2012) 48:441–6. doi: 10.1016/j.ejca.2011.11.036
60. Zhou H, Dong D, Chen B, Fang M, Cheng Y, Gan Y, et al. Diagnosis of distant metastasis of lung cancer: based on clinical and radiomic features. Transl Oncol. (2018) 11:31–6. doi: 10.1016/j.tranon.2017.10.010
61. Cui EN, Yu T, Shang SJ, Wang XY, Jin YL, Dong Y, et al. Radiomics model for distinguishing tuberculosis and lung cancer on computed tomography scans. World J Clin Cases. (2020) 8:5203–12. doi: 10.12998/wjcc.v8.i21.5203
62. Feng B, Chen X, Chen Y, Liu K, Li K, Liu X, et al. Radiomics nomogram for preoperative differentiation of lung tuberculoma from adenocarcinoma in solitary pulmonary solid nodule. Eur J Radiol. (2020) 128:109022. doi: 10.1016/j.ejrad.2020.109022
63. Boland GWL, Dwamena BA, Sangwaiya MJ, Goehler AG, Blake MA, Hahn PF, et al. Characterization of adrenal masses by using FDG PET: a systematic review and meta-analysis of diagnostic test performance. Radiology. (2011) 259:117–26. doi: 10.1148/radiol.11100569
64. Chang JM, Lee HJ, Goo JM, Lee HY, Lee JJ, Chung JK, et al. False positive and false negative FDG-PET scans in various thoracic diseases. Korean J Radiol. (2006) 7:57–69. doi: 10.3348/kjr.2006.7.1.57
65. Yen RF, Chen KC, Lee JM, Chang YC, Wang J, Cheng MF, et al. 18F-FDG PET for the lymph node staging of non-small cell lung cancer in a tuberculosis-endemic country: Is dual time point imaging worth the effort? Eur J Nucl Med Mol Imaging. (2008) 35:1305–15. doi: 10.1007/s00259-008-0733-1
66. Lee S, Woo SU, Kim WY, Lee JB, Eo JS. Lymphadenopathy by tuberculosis seemed like metastasis on FDG PET/CT in patients with breast carcinoma. Breast J. (2019) 25:723–5. doi: 10.1111/tbj.13248
67. Pavai S, Yap SF. The clinical significance of elevated levels of serum CA 19-9. Med J Malaysia. (2003) 58:667–72.
68. Ballehaninna UK, Chamberlain RS. Serum CA 19-9 as a biomarker for pancreatic cancer-a comprehensive review. Indian J Surg Oncol. (2011) 2:88–100. doi: 10.1007/s13193-011-0042-1
69. Scarà S, Bottoni P, Scatena R. CA 19-9: biochemical and clinical aspects. Adv Exp Med Biol. (2015) 867:247–60. doi: 10.1007/978-94-017-7215-0_15
70. Hong JY, Jang SH, Kim SY, Chung KS, Song JH, Park MS, et al. Elevated serum CA 19-9 levels in patients with pulmonary nontuberculous mycobacterial disease. Brazil J Infect Dis. (2016) 20:26–32. doi: 10.1016/j.bjid.2015.09.005
71. Falkowski AL, Graber J, Haack HG, Tarr PE, Rasch H. Isolated pancreatic tuberculosis: a case report and radiological comparison with cystic pancreatic lesions radiological comparison with cystic pancreatic lesions. J Radiol Case Rep. (2013) 7:1–11. doi: 10.3941/jrcr.v7i1.1292
72. Karam AK, Karlan BY. Ovarian cancer: the duplicity of CA125 measurement. Nat Rev Clin Oncol. (2010) 7:335–9. doi: 10.1038/nrclinonc.2010.44
73. Felder M, Kapur A, Gonzalez-Bosquet J, Horibata S, Heintz J, Albrecht R, et al. MUC16 (CA125): tumor biomarker to cancer therapy, a work in progress. Mol Cancer. (2014) 13:129. doi: 10.1186/1476-4598-13-129
74. Duffy MJ, Bonfrer JM, Kulpa J, Rustin GJS, Soletormos G, Torre GC, et al. CA125 in ovarian cancer: European Group on Tumor Markers guidelines for clinical use. Int J Gynecol Cancer. (2005) 15:679–91. doi: 10.1111/j.1525-1438.2005.00130.x
75. Dochez V, Caillon H, Vaucel E, Dimet J, Winer N, Ducarme G. Biomarkers and algorithms for diagnosis of ovarian cancer: CA125, HE4, RMI and ROMA, a review. J Ovarian Res. (2019) 12:28. doi: 10.1186/s13048-019-0503-7
77. Barua R, Hossain M. Adenosine deaminase in diagnosis of tuberculosis: a review. Anwer Khan Mod Med Coll J. (2014) 52:43–8 doi: 10.3329/akmmcj.v5i2.21132
78. Jia H, Zhang L, Wang B. The value of combination analysis of tumor biomarkers for early differentiating diagnosis of lung cancer and pulmonary tuberculosis. Ann Clin Lab Sci. (2019) 49:645–9.
79. Frederiksen JK, Sharma M, Casulo C, Burack WR. Systematic review of the effectiveness of fine-needle aspiration and/or core needle biopsy for subclassifying lymphoma. Arch Pathol Lab Med. (2015) 139:245–51. doi: 10.5858/arpa.2013-0674-RA
80. Kuan EC, Mallen-St. Clair J, St. John MA. Evaluation of parotid lesions. Otolaryngol Clin North Am. (2016) 49:313–25. doi: 10.1016/j.otc.2015.10.004
81. Monaco SE. Fine needle aspiration cytology. Pathobiol Hum Dis. (2014) 3379–98. doi: 10.1016/B978-0-12-386456-7.06504-7
82. Garg SK, Tiwari RP, Tiwari D, Singh R, Malhotra D, Ramnani VK, et al. Diagnosis of tuberculosis: available technologies, limitations, and possibilities. J Clin Lab Anal. (2003) 17:155–63. doi: 10.1002/jcla.10086
83. Trinchieri G. Cancer and inflammation: an old intuition with rapidly evolving new concepts. Annu Rev Immunol. (2012) 30:677–706. doi: 10.1146/annurev-immunol-020711-075008
84. Candido J, Hagemann T. Cancer-related inflammation. J Clin Immunol. (2013) 33(Suppl. 1):S79–84. doi: 10.1007/s10875-012-9847-0
85. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. (2010) 140:883–99. doi: 10.1016/j.cell.2010.01.025
86. Vento S, Lanzafame M. Tuberculosis and cancer: a complex and dangerous liaison. Lancet Oncol. (2011) 12:520–2. doi: 10.1016/S1470-2045(11)70105-X
87. Cheng MP, Chakra CNA, Yansouni CP, Cnossen S, Shrier I, Menzies D, et al. Risk of active tuberculosis in patients with cancer: a systematic review and metaanalysis. Clin Infect Dis. (2017) 64:635–44. doi: 10.1093/cid/ciw838
88. Simonsen DF, Farkas DK, Horsburgh CR, Thomsen RW, Sørensen HT. Increased risk of active tuberculosis after cancer diagnosis. J Infect. (2017) 74:590–8. doi: 10.1016/j.jinf.2017.03.012
89. Yu YH, Liao CC, Hsu WH, Chen HJ, Liao WC, Muo CH, et al. Increased lung cancer risk among patients with pulmonary tuberculosis: a population cohort study. J Thorac Oncol. (2011) 11:1899–906. doi: 10.1097/JTO.0b013e3181fb4fcc
Keywords: clinical diagnosis, cancer, tuberculosis, clinical feature, imaging feature, multimodal imaging, radiomics
Citation: Xiang Y, Huang C, He Y and Zhang Q (2021) Cancer or Tuberculosis: A Comprehensive Review of the Clinical and Imaging Features in Diagnosis of the Confusing Mass. Front. Oncol. 11:644150. doi: 10.3389/fonc.2021.644150
Received: 20 December 2020; Accepted: 23 February 2021;
Published: 28 April 2021.
Edited by:
Changqiang Wu, North Sichuan Medical College, ChinaReviewed by:
Ke Xie, Sichuan Academy of Medical Sciences and Sichuan Provincial People's Hospital, ChinaPeng Zhou, Sichuan Cancer Hospital, China
Copyright © 2021 Xiang, Huang, He and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qin Zhang, 76690830@qq.com
†These authors have contributed equally to this work
 Yufan Xiang†
Yufan Xiang† Chen Huang
Chen Huang Yan He
Yan He