- 1School of Pharmacy, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong, Hong Kong
- 2Department of Clinical Oncology, Queen Elizabeth Hospital, Kowloon, Hong Kong
Lung cancer is the leading cause of cancer-related deaths worldwide. Immune checkpoint inhibitors, including monoclonal antibodies against programmed death-1 (PD-1) and programmed death ligand-1 (PD-L1), have dramatically improved the survival and quality of life of a subset of non-small cell lung cancer (NSCLC) patients. Multiple predictive biomarkers have been proposed to select the patients who may benefit from the immune checkpoint inhibitors. EGFR-mutant NSCLC is the most prevalent molecular subtype in Asian lung cancer patients. However, patients with EGFR-mutant NSCLC show poor response to anti-PD-1/PD-L1 treatment. While small-molecule EGFR tyrosine kinase inhibitors (TKIs) are the preferred initial treatment for EGFR-mutant NSCLC, acquired drug resistance is severely limiting the long-term efficacy. However, there is currently no further effective treatment option for TKIs-refractory EGFR-mutant NSCLC patients. The reasons mediating the poor response of EGFR-mutated NSCLC patients to immunotherapy are not clear. Initial investigations revealed that EGFR-mutated NSCLC has lower PD-L1 expression and a low tumor mutational burden, thus leading to weak immunogenicity. Moreover, the use of PD-1/PD-L1 blockade prior to or concurrent with osimertinib has been reported to increase the risk of pulmonary toxicity. Furthermore, emerging evidence shows that PD-1/PD-L1 blockade in NSCLC patients can lead to hyperprogressive disease associated with dismal prognosis. However, it is difficult to predict the treatment toxicity. New biomarkers are urgently needed to predict response and toxicity associated with the use of PD-1/PD-L1 immunotherapy in EGFR-mutated NSCLC. Recently, promising data have emerged to suggest the potentiation of PD-1/PD-L1 blockade therapy by anti-angiogenic agents and a few other novel therapeutic agents. This article reviews the current investigations about the poor response of EGFR-mutated NSCLC to anti-PD-1/PD-L1 therapy, and discusses the new strategies that may be adopted in the future.
Introduction
Lung cancer is the leading cause of cancer-related deaths worldwide (1). Non-small cell lung cancer (NSCLC) is the most common histological subtype which constitutes more than 85% of all lung cancer cases. The prognosis of advanced NSCLC is very poor. A few subsets of NSCLC patients harboring epidermal growth factor receptor (EGFR) mutation or anaplastic lymphoma kinase (ALK) rearrangement were known to respond well to the respective molecular targeted drugs with minimum adverse reaction (2). However, targeted therapies are ineffective in most NSCLC patients whose tumors lack the oncogenic driver alterations. On the other hand, despite excellent initial response to targeted therapies, essentially all EGFR-mutant NSCLC inevitably progress over time due to acquired drug resistance (3). There is currently no further effective therapeutic options for NSCLC who develop disease progression on EGFR tyrosine kinase inhibitors (TKIs) (4).
In recent years, immunotherapy has become integrated into the treatment plan of NSCLC patients, which tremendously improved survival and quality of life in some patients (5). Anti-CTLA-4 (e.g., ipilimumab) that changed the paradigm in melanoma treatment, when tested in clinical trials did not show the expected benefit in NSCLC patients (6, 7). On the other hand, monoclonal antibodies targeting programmed death-1 (PD-1) and programmed death ligand-1 (PD-L1) have demonstrated survival benefits, long lasting responses and good safety profile over chemotherapy in patients with advanced NSCLC in several recent Phase III trials (8–11). To date, four anti-PD-1/PD-L1 monoclonal antibodies (nivolumab, pembrolizumab (anti-PD-1); atezolimumab and durvalumab (anti-PD-L1)) have been approved as 1st or 2nd line therapy for NSCLC patients with metastatic and locally advanced NSCLC respectively (12, 13).
PD-1 is an inhibitory receptor expressed on activated T cells, B cells and natural killer cells, which normally function to blunt the immune response. The major ligand of PD-1, PD-L1, is expressed in tumor cells and infiltrating immune cells. When PD-L1 interacts with PD-1, they suppress the T cell-mediated cancer killing effect. Anti-PD-1/PD-L1 antibodies work by binding to inhibitory PD-1 receptor on tumor-reactive T cells and PD-L1 on tumor cells, respectively. The PD-1/PD-L1 interaction is then disrupted to reactivate the anti-tumor T cell-mediated cell cytotoxicity. Clinical benefit from anti-PD-1/PD-L1 therapy is associated with high tumor mutational load, high levels of pre-treatment tumor-infiltrating T cells, and high expression of pre-treatment PD-L1 on tumor cells and tumor-infiltrating immune cells (14).
EGFR-mutant NSCLC is the most prevalent molecular subtype in lung cancer patients. However, patients with EGFR-mutant NSCLC show poor response to anti-PD-1/PD-L1 treatment. The mechanisms mediating the poor response of EGFR-mutated NSCLC patients to immunotherapy are not clear. Initial investigations revealed that EGFR-mutated NSCLC has lower PD-L1 expression and a low tumor mutational burden (TMB), thus leading to weak immunogenicity.
This review recapitulates the underlying mechanisms contributing to the inferior clinical outcomes of anti-PD-1/PD-L1 immune-checkpoint inhibitors (ICIs) in NSCLC patients bearing EGFR mutations. Novel strategies to potentiate the use of PD-1 blockade therapy in EGFR mutant NSCLC are discussed.
Predictive Biomarkers for Selecting NSCLC Patients for Immunotherapy
Identification of predictive biomarkers to select NSCLC patients most likely responding to anti-PD-1/PD-L1 ICIs is currently an area of intensive research. The response rates of anti-PD-1/PD-L1 ICIs were estimated to be around 14-20% in unselected patients (15). The most established predictive biomarker is PD-L1 expression status of tumor cells from biopsy. It is now routinely used in clinical practice for treatment decision to select patients who may benefit the most. In fact, a number of clinical studies have reported the association between PD-L1 expression and clinical outcome in NSCLC patients [reviewed in (16)]. PD-L1 expression on tumor cells is considered not only a predictive biomarker for response to PD-1/PD-L1 ICIs but also a prognostic factor in NSCLC patients (17).
However, a recent study reported significant discrepancy in the assessment of PD-L1 tumor expression in NSCLC patients and its association with prognosis (17). Multiple PD-L1 immunohistochemical (IHC) assays with various scoring systems and cutoff values have been developed for companion diagnostic use (18, 19). Thus, appreciable differences in the correlation observed in different clinical trials may arise from the different IHC assays, the antibodies used for the assays, positivity cutoff, type of biopsies (primary versus metastasis) and staining of tumor versus immune cells (17). It will be important to standardize a universal assay to assess tumoral PD-L1 expression and also to define appropriate cut-off points (20).
On the other hand, PD-L1 is also known to be highly expressed in circulating immune cells, including dendritic cells (21) and myeloid-derived suppressor cells (22). They regulate T cell activation during antigen presentation or excessive inflammation (23, 24). It has been postulated that the baseline distribution of PD-L1 expression in systemically circulating immune cells could contribute to the therapeutic responses to PD-L1/PD-1 blockade immunotherapy. While tumoral PD-L1 expression was assessed in most clinical trials investigating anti-PD-1/PD-L1 ICIs, PD-L1 level of tumor-infiltrating immune cells was also evaluated in atezolizumab’s trials [POPLAR (25) and OAK (11)]. In both POPLAR and OAK trials, higher PD-L1 levels in both tumor cells and tumor-infiltrating immune cells were associated with improved patient survival after atezolizumab treatment. A more recent report also revealed that NSCLC patients with percentages of PD-L1+ CD11b+ myeloid cells above 30% before the start of anti-PD-L1 immunotherapy exhibited superior response rates of 50% (26). The data suggest that PD-L1 expression on myeloid cells in the systemic circulation could serve as a useful and accessible biomarker for patient stratification.
However, the utility of PD-L1 expression alone as an exclusive predictive biomarker for clinical efficacy of anti-PD-1/PD-L1 ICIs remains controversial (17). The determination of PD-L1 level alone is insufficient to understand the mechanisms of resistance to anti-PD-1/PD-L1 ICIs. It also does not explain why some PD-L1-negative patients can achieve response to treatment. PD-L2 is another ligand identified for PD-1 T cell receptor (TCR) (27). While PD-L1 is the predominant ligand for PD-1, PD-L2 could compete with PD-L1 with 2-6 fold higher affinity to PD-1 (28). However, the biological role of PD-L2 in the tumor microenvironment (TME) and as a predictive marker in NSCLC has not been definitively established. More investigations about the predictive and prognostic roles of PD-L2 are warranted.
Besides PD-L1 expression, TMB, DNA mismatch repair deficiency, extent of CD8+ cell infiltration, immune gene expression signatures and composition of the gut microbiome have also been proposed to correlate with clinical response to anti-PD-1/PD-L1 ICIs (29–32). NSCLC patients with high TMB and the smokers were found to respond better to anti-PD-1/PD-L1 ICIs (33). The potential use of TMB as a predictive marker of clinical response to anti-PD-1 therapy has been evaluated in the CheckMate026, CheckMate568 and CheckMate227 trials (34–37). NSCLC patients with high TMB showed prolonged clinical benefit and PFS to immunotherapy regardless of PD-L1 expression (38–40). It is noteworthy that lung cancer generally has higher TMB when compared with other tumor types (41). However, overall survival (OS) in anti-PD-1 ICI-treated NSCLC patients was not affected by TMB alone. Thus, further investigation about the role of TMB as a predictive biomarker is warranted before clinical implementation. Galectin-3 is a carbohydrate-binding lectin whose expression is associated with inflammatory cells including macrophage. Recently, NSCLC patients with negative or intermediate expression of galectin-3 in their tumor cells were found to demonstrate an early and durable response to pembrolizumab (42). A large multicenter clinical trial is underway to investigate the potential use of galectin-3 as a predictive marker for better patient selection for immunotherapy (42). Last but not least, NSCLC patients bearing EGFR mutations have been reported to show poor response to anti-PD-1/PD-L1 ICIs (43), which will be discussed in detail in the next section.
NSCLC Patients Harboring EGFR Mutations Show Poor Response to Anti-PD-1/PD-L1 Immunotherapy
Initial Enthusiasm About Using PD-1 ICIs in EGFR-Driven NSCLC According to Preclinical Studies
Early preclinical studies have reported that aberrant oncogenic EGFR signaling upregulates PD-L1 expression in NSCLC cell lines (44). PD-1 inhibitors were found to inhibit tumor cell proliferation in coculture systems of EGFR-mutant tumor and immune cells in vitro (44, 45). Moreover, PD-1 inhibitors were also shown to improve survival in EGFR-mutant mouse models (44). However, clinical studies have revealed an opposite result. NSCLC patients harboring EGFR mutation exhibited poorer response to PD-1/PD-L1 ICIs than those bearing wild-type EGFR (9, 11, 46, 47). More recently, a retrospective analysis conducted by Gainor et al. has revealed that EGFR mutations were associated with low clinical response to PD-1 blockade in NSCLC patients (48). The discrepancies between preclinical and clinical findings indicate a complex relationship among EGFR mutation, the immune microenvironment and therapeutic response from immunotherapy. Furthermore, EGFR TKI treatment in EGFR-driven NSCLC cell model was shown to cause PD-L1 downregulation (45), thus also deterring the utility of combining EGFR TKI with PD-1 inhibitor. In fact, the combination of EGFR TKI and PD-1 inhibitor did not lead to synergistic anticancer effect in EGFR-driven coculture system (45).
Key Clinical Trials Evaluating Anti-PD-1/PD-L1 ICIs in EGFR-Mutant NSCLC
Advanced NSCLC patients bearing EGFR mutations only account for about 5-14% of the total number of patients recruited in the major clinical trials investigating the four approved anti-PD-1/PD-L1 ICIs (Table 1) (8, 9, 11, 25, 46, 51, 52). Since these clinical trials were not designed solely to investigate the role of PD-1/PD-L1 blockade immunotherapy in EGFR mutant NSCLC patients, the efficacy in EGFR mutant patients was revealed by patient subgroup analysis. CheckMate-057 is the first Phase III trial to report the clinical efficacy of PD-1/PD-L1 inhibitors in NSCLC patients bearing EGFR mutant tumors. While this trial confirmed that patients with advanced non-squamous NSCLC and progress during or after platinum-based chemotherapy survived longer with nivolumab (an anti-PD-1 monoclonal antibody) than docetaxel, subgroup analysis revealed that there was no PFS or OS benefit in patients with activating EGFR mutation (9). Patient subgroup analysis in another Phase III trial (KEYNOTE-010) evaluating pembrolizumab (another PD-1 inhibitor) also indicated that EGFR mutant NSCLC did not achieve statistically significant OS benefit from immunotherapy over salvage chemotherapy (46). In another Phase III trial (OAK) evaluating atezolizumab (an anti-PD-L1 monoclonal antibody), NSCLC patients with EGFR-mutated tumor also did not achieve OS benefit from the immunotherapy over docetaxel (11). A pooled analysis evaluating data from 3 clinical trials (CheckMate-057, KEYNOTE-010 and POPLAR) confirmed that PD-1/PD-L1 ICIs did not enhance OS versus docetaxel in advanced NSCLC patients bearing EGFR mutation (n = 186, HR = 1.05, 95% CI: 0.70-1.55, P < 0.81) (47). Furthermore, another pooled analysis which covered 5 trials (CheckMate-017, CheckMate-057, KEYNOTE-010, OAK, and POPLAR) also verified that prolonged OS was only observed in the EGFR wild-type patient group but not in the EGFR-mutant subgroup (50).
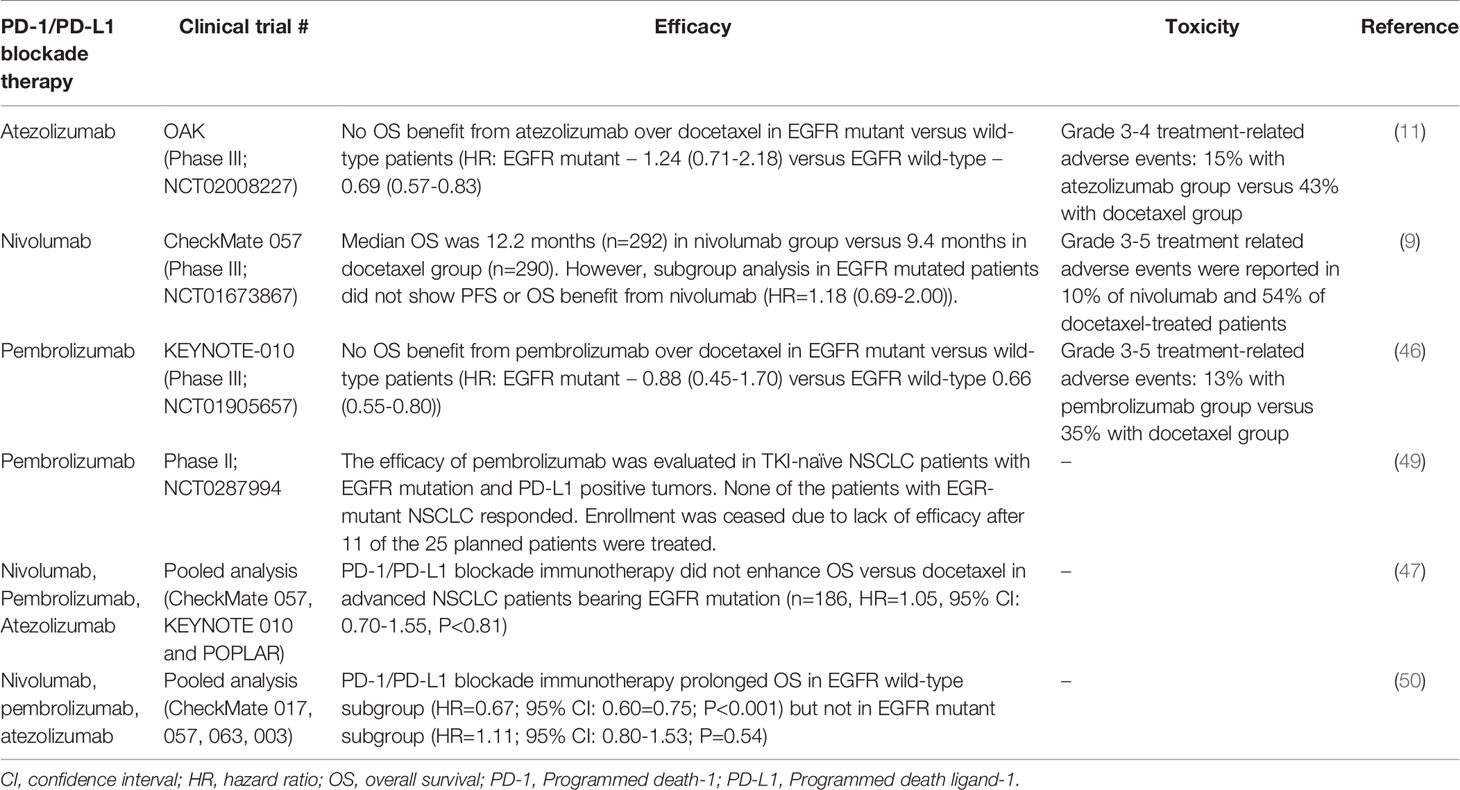
Table 1 Key clinical trials reporting efficacy and toxicity of PD-1/PD-L1 blockade immunotherapy in EGFR-mutant NSCLC patients.
A Phase II clinical trial (NCT0287994) was conducted to specifically evaluate the efficacy of pembrolizumab (anti-PD-1 monoclonal antibody) in TKI-naïve EGFR-mutant advanced NSCLC patients whose tumors have high PD-L1 expression (49). The trial enrolment was halted due to lack of efficacy after 11 of the 25 planned patients received the immunotherapy. Thus, the patient number is very small because of premature closure of the trial, none of the patients bearing EGFR mutations responded to pembrolizumab (49). Based on these clinical findings, the National Comprehensive Cancer Network (NCCN) clinical practice guidelines of NSCLC (version 4, 2021) did not recommend immunotherapy for the treatment of EGFR-mutant NSCLC patients.
Efficacy of Anti-PD-1/PD-L1 ICIs in NSCLC Bearing the Two Most Common EGFR Sensitizing Mutation Subtypes
Interestingly, the heterogeneity of EGFR mutation subtypes was found to cause variations in the therapeutic efficacy of anti-PD-1/PD-L1 ICIs. A multicenter retrospective study analyzed the clinical data of 171 EGFR mutant NSCLC patients on treatment with PD-1/PD-L1 ICI alone or in combination with CTLA4 inhibitor (53). While patients harboring EGFR exon 19 deletion or L858R mutation shown less benefit from immunotherapy than the EGFR wild-type group, the L858R group exhibited more favorable response than the exon 19 deletion group (ORR, 7% in EGFR 19 deletion subgroup versus 16% in L858R subgroup versus 22% in wild-type subgroup). However, EGFR T790M status and PD-L1 expression did not affect response and survival outcomes to anti-PD-1/PD-L1 ICIs. NSCLC tumors bearing EGFR exon 19 deletion was found to have a lower tumor mutation burden compared with the EGFR L858R subtype despite similar smoking history. Therefore, screening for EGFR mutation subtypes could be useful for personalized use of PD-1/PD-L1 ICIs in EGFR-mutant NSCLC patients. Further studies with larger patient cohorts are warranted.
Mechanisms Contributing to the Poor Efficacy of Anti-PD-1/PD-L1 Therapy in EGFR Mutant NSCLC
PD-L1 Expression
PD-L1 expression in tumor tissues is the most extensively studied predictive biomarker for clinical response to PD-1/PD-L1 inhibitors. Status of tumoral PD-L1 expression is now routinely used for selecting patients who could benefit the most. The contribution of PD-L1 expression to poor efficacy of anti-PD-1/PD-L1 therapy in EGFR mutant NSCLC is controversial. A number of studies have reported that high PD-L1 expression is found more frequently in EGFR-mutant than EGFR-wild type lung tumor tissues (44, 54–56). The activation of the PD-1 pathway is thus believed to contribute to immune escape in EGFR-driven NSCLC. In contrast, another study has found a decreased PD-L1 expression in tumor tissues from NSCLC patients bearing EGFR mutation (57). More recently, a pooled analysis of 15 clinical studies also revealed that patients with EGFR mutation have decreased PD-L1 expression (58). Analysis of The Cancer Genome Atlas (TCGA) and the Guangdong Lung Cancer Institute (GCLI) cohort have also confirmed an inverse correlation between EGFR mutation and PD-L1 expression in tumor tissues (58). On the other hand, other studies have reported a lack of correlation between the expression of PD-L1 and PD-L2 in patients with different EGFR mutation status (59). With these conflicting findings, the use of PD-L1 expression alone could not adequately predict and explain why EGFR-mutant NSCLC exhibits poor response to PD-1/PD-L1 inhibitors.
Tumor Mutational Burden (TMB)
In a recent study investigating the impact of TMB on clinical outcomes of NSCLC patients treated with EGFR TKIs, TMB was found to be remarkably lower in EGFR-mutated tumors (n = 153) than EGFR wild-type tumors (n = 1,849) (median 3.77 versus 6.12 mutations/Mb; P < 0.0001) (60). To this end, the association of higher TMB with tobacco smoking leading to better outcomes with PD-1/PD-L1 ICIs is well documented (9, 11, 34, 37, 46, 61). A recent meta-analysis also revealed that never smokers are less responsive to PD-1/PD-L1 inhibitors (62). Interestingly, EGFR mutant NSCLC is more enriched in the never smoking population (63).
Among the more common sensitizing EGFR mutations, TMB in the exon 19 deletion cohort was found to be lower than that in the L858R cohort (60). Hastings et al. has recently reported that clinical outcomes (OS and ORR) with PD-1 ICIs were worse in patients with exon 19 deletion than patients harboring L858R mutation (53). PD-L1 and smoking status were similar in the two patient subpopulations. Further TMB analyses of the two patient cohorts suggested that the higher TMB in EGFR L858R mutation could contribute to the differential responses to PD-1 ICIs.
In fact, specific subset of EGFR-mutant NSCLC patients have been shown to preferentially benefit from PD-1/PD-L1 ICIs to some extent (64–68). In a retrospective study evaluating NSCLC patients from the IMMUNOTARGET registry, the response of 125 pre-treated EGFR-mutated patients with ICI monotherapy was compared among patients with different EGFR mutation subgroups (69). The ORR was not notably affected by PD-L1 expression levels, smoking status or previous lines of treatment, but was significantly different in the various EGFR mutation subgroups (3.7%, 9.5%, 20.8% and 11.8%, respectively, in EGFR T790M, exon 19 deletion, L858R, and other EGFR mutation subgroups) (69). This is in line with the aforementioned findings of Hastings et al., demonstrating more favorable outcomes of L858R (coincident with higher TMB) than exon 19 deletion (coincident with lower TMB). The combination of nivolumab and erlotinib in 21 EGFR mutated NSCLC patients has been evaluated by Gettinger et al. (64). Intriguingly, patients bearing the EGFR L858R mutation were achieving longer survival benefit than those harboring other EGFR mutations (64). While TMB in the tumor tissues was not assessed in the study, the findings are consistent with the fact that TMB in EGFR L858R mutated NSCLC favors better response to PD-1 ICIs.
Immunosuppressive Tumor Microenvironment (TME) in EGFR-Mutant Tumors
A typical tumor mass consists of not only a heterogeneous population of cancer cells but also a variety of neighboring host cells, tumor-infiltrating lymphocytes, extracellular matrix proteins, and other secreted factors, which collectively referred to as the TME. Tumors have been classified into 4 different TME types according to the presence or absence of tumor-infiltrating lymphocytes (TIL) and PD-L1 expression (Type I: TIL+, PD-L1+; Type II: TIL-, PD-L1-; Type III: TIL-, PD-L1+; and Type IV: TIL+, PD-L1-) (70). Type I tumors were found to the only subtype that responds to PD-1/PD-L1 inhibitor (70). Therefore, besides PD-L1 expression, high level of TIL is critical to allow PD-1/PD-L1 inhibitors to work. Similarly, Chen et al. divided tumors into different immunity phenotypes (immune-desert phenotype; immune-excluded phenotype; and inflamed phenotype) according to a set of tumor, host, and environment factors (71). Using Chen’s classification, tumors with the immune-desert and immune-excluded phenotypes are resistant to PD-1/PD-L1 inhibitors (71). Importantly, there is a close correlation between EGFR mutations and an uninflamed TME with immunological tolerance and weak immunogenicity (48, 58). This correlation may explain the inferior response of EGFR-mutant NSCLC to PD-1 blockade therapy. The overexpression of CD73 has also been reported in EGFR mutant NSCLC, thus resulting an immunosuppressive TME and reduced IFN gamma signature (72). Figure 1 depicts the characteristic composition and function of the immunosuppressive TME in EGFR-mutated NSCLC cells.
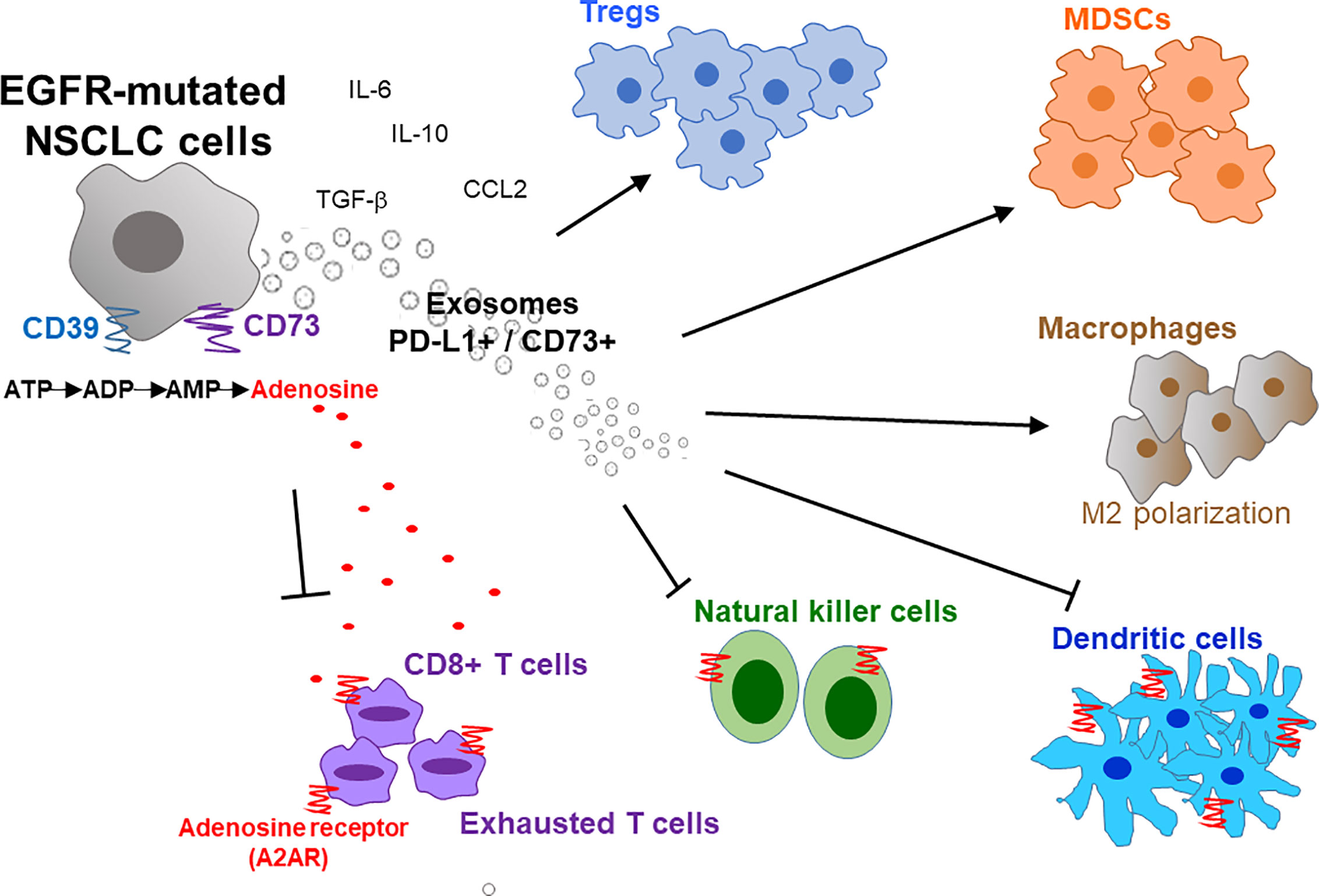
Figure 1 Immunosuppressive tumor microenvironment (TME) in EGFR-mutated NSCLC. EGFR mutations promote an immunosuppressive TME by interfering with several intracellular pathways and modulating immune accessory cells including tumor-infiltrating lymphocytes (TILs), natural killer cells (NK), T-regulatory cells (Tregs), myeloid-derived suppressor cells (MDSCs) and tumor-associated macrophages (TAMs). Overexpression of CD39/CD73 in EGFR-mutated NSCLC induces high extracellular production and release of adenosine that inhibit the activity of innate and adaptive immune system cells and endothelial cells in TME. Activation of CD39 triggers the de-phosphorylation of ATP to ADP, and subsequently to AMP. On the other hand, CD73 catalyzes the hydrolysis of AMP to adenosine and phosphate. The increased level of extracellular adenosine bind to A2A adenosine receptor (A2AR) expressed by both adaptive and innate immunity, thereby inhibiting the activity of various immune cells. Moreover, exosomes secreted from EGFR-mutated NSCLC cells also increase PD-L1+/CD73+ expression and extracellular adenosine release to promote immunosuppression. IL, interleukin; M2, macrophages 2; ATP, adenosine triphosphate; ADP, adenosine diphosphate; AMP, adenosine monophosphate; CCL2, C-C motif chemokine ligand 2.
EGFR-mutant NSCLC is characterized by its aberrant activation of the EGFR signaling pathway. To this end, activation of EGFR signaling has been reported in numerous studies to participate in immunosuppression and immune escape. Regulatory T cells (Tregs) play a critical role in suppressing the immune response to self and foreign particles, which help prevent autoimmune disease. They generally suppress the induction and proliferation of effector T cells. Amphiregulin (AREG) is an EGF-like growth factor and it is frequently upregulated in tumors (73). AREG is a known ligand of EGFR (74). Importantly, AREG is critical for Treg function in vivo, thus providing a mechanistic link between the EGFR signaling and regulation of the immune system (75). Wang et al. reported that AREG maintains the suppressive function of Tregs via the EGFR/GSK-3β/Foxp3 machinery in vitro and in vivo, thus confirming the importance of EGFR signaling in the regulation of Tregs (76). Recently, a long noncoding RNA lnc-EGFR has been shown to stimulate Treg differentiation and promote immune invasion of hepatocellular carcinoma via an EGFR-dependent signaling pathway (77). Moreover, the inhibition of EGFR signaling by gefitinib has been shown to alter the immune environment of the targeted cancer in vitro and in vivo, probably by reducing the number of Tregs in the tumors (78).
Myeloid-derived suppressor cells (MDSCs) are immature myeloid cells that suppress immune responses. MDSCs expand during cancer, infection and inflammatory diseases. A recent study reported that EGFR TKI therapy alters the TME in EGFR mutant NSCLC and elevates the level of mononuclear MDSCs (79). The serum level of inflammatory factors IL-10 and CCL-2 was also found to be increased in vivo after EGFR TKI treatment (79). The increase in MDSC and inflammatory factors associated with EGFR TKI treatment has been proposed to explain why most EGFR TKI-resistant NSCLC patients are also refractory to anti-PD-1/PD-L1 ICIs (79). Moreover, MDSCs are known to inhibit IL-2 and anti-CD3/CD28 mAb-induced T cell amplification and Th1 polarization but induce apoptosis in T cells in an IDO-dependent manner (80). To this end, the activation of STAT3 (an important downstream signaling molecule of the EGFR pathway) is required for IDO expression (80). Therefore, STAT3 activation is essential for immune suppression of MDSCs. In fact, persistent activation of STAT3 has been shown to promote MDSC-mediated immune suppression in lung cancer (81).
Yes-Associated Protein (YAP)
YAP is a major mediator of the Hippo pathway and it has been shown to promote cancer progression, drug resistance and metastasis in NSCLC (82). Accumulating evidence suggests that YAP also plays critical role in cancer immunity. YAP interacts with interferon regulatory factor 3 to negatively regulate innate immunity (83). YAP has also been reported to regulate tumor–associated immune cells (including MDSCs, macrophages, and Tregs) in the TME (83–85). In NSCLC tumor specimens, high nuclear YAP staining is associated with positive PD-L1 expression (86). Genetic knockdown or chemical inhibition of YAP was shown to reduce mRNA and protein expression of PD-L1 in NSCLC cell lines (86). On the other hand, forced expression of YAP was shown to increase PD-L1 protein expression in NSCLC A549 cells (87). Recently, a gefitinib-resistant PC9 cell line has been shown to express higher protein level of YAP and PD-L1 than the parental cells (88). Importantly, YAP knockdown could reduce PD-L1 expression in gefitinib-resistant PC9 cells (88). It is also noteworthy that the EGFR signaling pathway has crosstalk with the Hippo/YAP pathway, which positively regulating the YAP oncogenic function in various cancers including NSCLC (89).
Preferential Response of NSCLC Patients With Uncommon EGFR Mutations to PD-1 Blockade Therapy
Emerging evidence from a few recent studies suggest that the efficacy of PD-1 blockade therapy is relatively more favorable in NSCLC patients bearing uncommon EGFR mutations compared to those with the classical mutations (46, 69, 90). Approximately 10% of EGFR mutant NSCLC is classified as the uncommon subtypes (91), including G719X, L861Q, S768I, and exon 20 insertion, which have different clinicopathological characteristics and response to EGFR TKIs (92–96). Most recently, Chen et al. investigated the clinical response of Chinese NSCLC patients harboring uncommon EGFR mutations to PD-1/PD-L1 inhibitors and the underlying mechanisms (90). They tied the favorable response of the NSCLC patients with uncommon EGFR mutations to the high incidence of concomitant PD-L1 expression and CD8+ tumor-infiltrating lymphocytes within TME (90). In a retrospective efficacy analysis of PD-1 inhibitors conducted by a Japanese group, NSCLC patients harboring uncommon EGFR mutations and without T790M mutations were associated with significantly longer PFS than those with common EGFR mutations or with T790M mutation (97). This retrospective study was limited by the small sample size (n = 27). Further investigations are warranted to identify the clinical biomarkers useful for predicting the ICI responders with EGFR mutations.
Toxicity Experienced on Combination Treatment With EGFR TKI and Immunotherapy
Immunotherapy generally has a lower incidence of adverse reactions than chemotherapy. However, ICIs are known to mediate inflammatory side effects commonly referred to as immune-related adverse events (irAEs) (98). The etiology leading to irAEs is largely unknown but it is believed to be caused by the disruption of immunologic homeostasis (99). The occurrence of irAEs generally predicts treatment efficacy of ICIs in NSCLC (100, 101), and it also triggers treatment discontinuation and premature termination of clinical trials. A Phase 1b TATTON trial (NCT02143466) investigating osimertinib (3rd generation EGFR TKI) plus nivolumab was stopped early because of the high incidence (38%) of interstitial lung disease (102). Another Phase III open-label CAURAL trial (NCT02454933) evaluating the combination of osimertinib and durvalumab in EGFR T790M positive NSCLC patients was also prematurely terminated due to safety concerns (103). irAEs can affect one or multiple organ systems. The incidence of grade 3 or above toxicities is around 7-13% in NSCLC patients treated with anti-PD-1/PD-L1 ICIs (104). It is noteworthy that toxicity experienced upon combination treatment with immunotherapy and EGFR TKIs can be severe, difficult to predict and with unusual forms of presentation. Specific laboratory tests and regular physical examinations should be conducted to facilitate early detection of irAEs and their effective management (105, 106).
Hyperprogressive Disease (HPD) Associated With PD-1/PD-L1 Blockade in NSCLC Patients
Several recent studies have reported a paradoxical deleterious effect of anti-PD-1/PD-L1 immunotherapy, which is described as “hyperprogressive disease (HPD)”, in a subset of patients (106, 107). HPD is characterized as an unexpected and fast progression in tumor volume and rate, poor survival of patients and early fatality (108).
HPD has been defined in different studies using 5 different criteria (109). Various parameters including tumor growth rate, tumor growth kinetics and time to treatment failure were used to define and quantify the incidence of HPD (110). In a recent retrospective cohort study of NSCLC patients, these 5 definitions of HPD were found to be associated with different tumoral behaviors. Kas et al. proposed a new definition of HPD, which is based on ΔTGR (tumor growth rate) greater than 100 (110). This new definition appeared to be more closely associated with the expected characteristics for HPD (i.e., rapid increase in tumor kinetics and poor patient survival). Numerous biomarkers associated with HPD have been proposed, which may be used to stratify patients for anti-PD-1/PD-L1 immunotherapy. Among them, EGFR mutation represents an important tumor cell biomarker linked with HPD after immunotherapy (108). HPD has been reported in 20% of patients with EGFR mutations and it was associated with worse clinical outcome. EGFR mutations were known to upregulate cell surface inhibitory receptors (e.g., PD-1/PD-L1 and CTLA-4), cytokines and immunosuppressive cells, subsequently driving innate immune resistance (44). The precise role of EGFR mutations in HPD warrants further investigation.
Novel Strategies to Potentiate PD-1/PD-L1 Blockade Immunotherapy in EGFR Mutant NSCLC
A promising therapeutic approach is to combine a PD-1/PD-L1 ICI with chemotherapy, EGFR TKI, or other type of ICI with an aim to increase the immunogenicity of tumor cells, or to inhibit immunosuppressive signaling in the TME (111).
Combination of PD-1/PD-L1 Blockade Therapy and Conventional Chemotherapy
In lung cancer patients without targetable mutations, the addition of PD-1/PD-L1 blockade therapy to standard chemotherapy has been shown to give rise to significantly longer OS and PFS than chemotherapy alone (10). Therefore, it is also speculated that addition of PD-1/PD-L1 immunotherapy to chemotherapy in NSCLC patients with EGFR mutation could also achieve desirable clinical outcomes.
Antitumor effect from conventional chemotherapy is not solely attributed to the direct tumor cell cytotoxicity, but it is also mediated by the restoration of immunosurveillance. To this end, antitumor immune response and re-establishment of immunosurveillance can be primed by immunogenic cell death (ICD). ICD comprises the release of damage-associated molecular patterns (DAMPs) from dying tumor cells that result in activation of tumor-specific immune responses (112). This can trigger long-term efficacy of anticancer drugs by combining direct cancer cell killing and antitumor immunity. ICD-induced DAMPs include surface-exposed calreticulin (CALR) and secreted ATP, annexin A1, type I interferon, and high mobility group box 1 (113). A number of classical chemotherapeutic drugs, including anthracyclines, cyclophosphamide, oxaliplatin, and paclitaxel, are known to elicit ICD (114). In mice bearing KRAS-positive and TP53-negative NSCLC tumor xenograft, two immunogenic chemotherapeutic drugs (oxaliplatin and cyclophosphamide) were shown to strongly enhance T cell infiltration of the tumors and sensitize them to subsequent checkpoint inhibition targeting both CTLA-4 and PD-1 (115).
A few recent clinical reports have corrobated the hypothesis that pretreatment with ICD-inducing anthracyclines or irradiation could potentiate the efficacy of ICIs in various tumor types including NSCLC (116) (Table 2). The PACIFIC study is a Phase III trial that evaluated durvalumab (PD-L1 antibody) as consolidation therapy in stage III NSCLC patients (51). The enrolled subjects did not present disease progression after 2 or more cycles of platinum-based chemotherapy (51). The study showed a significantly longer median PFS in the durvalumab cohort (16.8 months) than that in the placebo cohort (5.6 months). Importantly, in subgroup analysis, patients with EGFR-mutations demonstrated slightly more clinical benefit from durvalumab after chemoradiotherapy (platinum doublet chemotherapy administered with definitive-dose radiotherapy) (51). An open-label Phase III trial (Checkmate 722; NCT02864251) has also been conducted to compare the efficacy of nivolumab plus chemotherapy and nivolumab plus ipilimumab with chemotherapy alone (117). The Checkmate 722 study enrolled patients with EGFR-mutated metastatic or recurrent NSCLC who progressed on 1st or 2nd line EGFR TKIs (117). The final result has not been reported yet. Another ongoing Phase III trial (KEYNOTE-789; NCT03515837) is evaluating the efficacy of pembrolizumab in combination with chemotherapy (pemetrexed, carboplatin or cisplatin). Unlike the CheckMate 722 trial, KEYNOTE-789 will also recruit NSCLC patients bearing EGFR T790M who have acquired resistance to osimertinib (118).
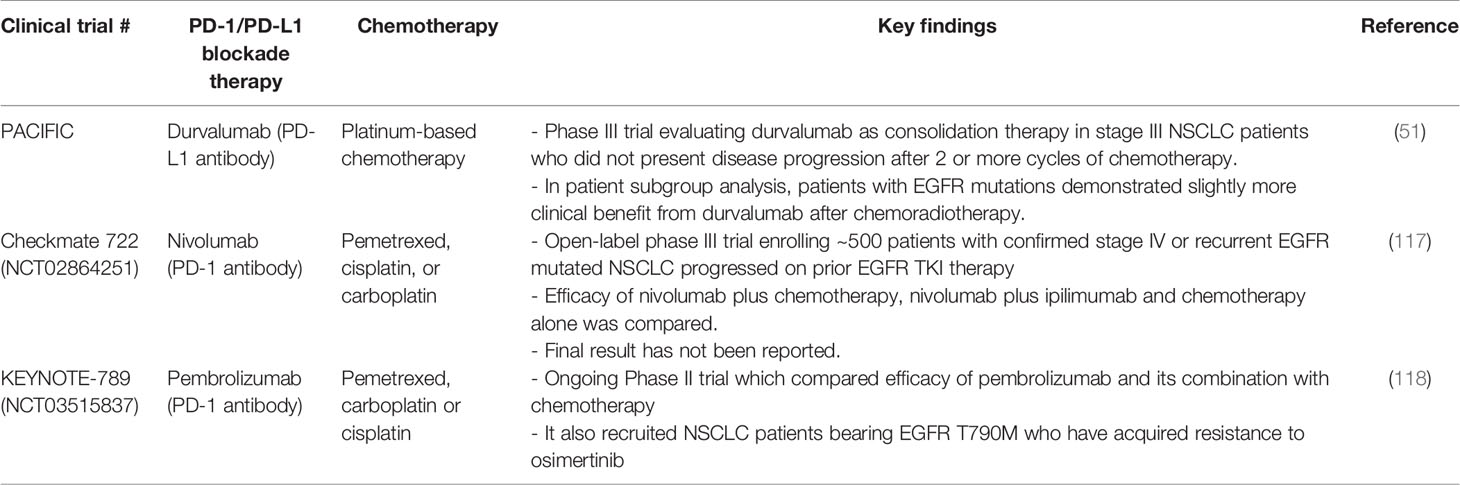
Table 2 Representative clinical trials evaluating the combination of PD-1/PD-L1 blockade immunotherapy and conventional chemotherapy in EGFR-mutant NSCLC patients.
Combination of PD-1/PD-L1 Immunotherapy With Targeted Therapy
High levels of intra-tumoral T cells and tumor antigenicity have been shown to correlate with favorable response from immunotherapy (119). Thus, therapies capable of increasing these factors may be combined effectively with ICIs to enhance the treatment outcome. In addition to the specific effects on oncogenic signaling pathways, a few targeted therapeutic agents are also known to increase tumor antigen presentation (120, 121), promote intra-tumoral T cell infiltration (122), or upregulate PD-1/PD-L1 expression (123). Therefore, it is logical to combine these targeted therapies with immunotherapies.
EGFR TKIs
EGFR TKIs have been reported to cause immunogenic apoptosis of tumor cells (124), and subsequently releasing aberrant intracellular antigens and recruiting T cells via interferon-γ-induced major histocompatibility complex class I presentation (120). A few clinical studies have been conducted to evaluate the combination of PD-1-based immunotherapy and EGFR targeted therapy in EGFR TKI-naïve and/or pretreated EGFR-mutant NSCLC patients (Table 3).
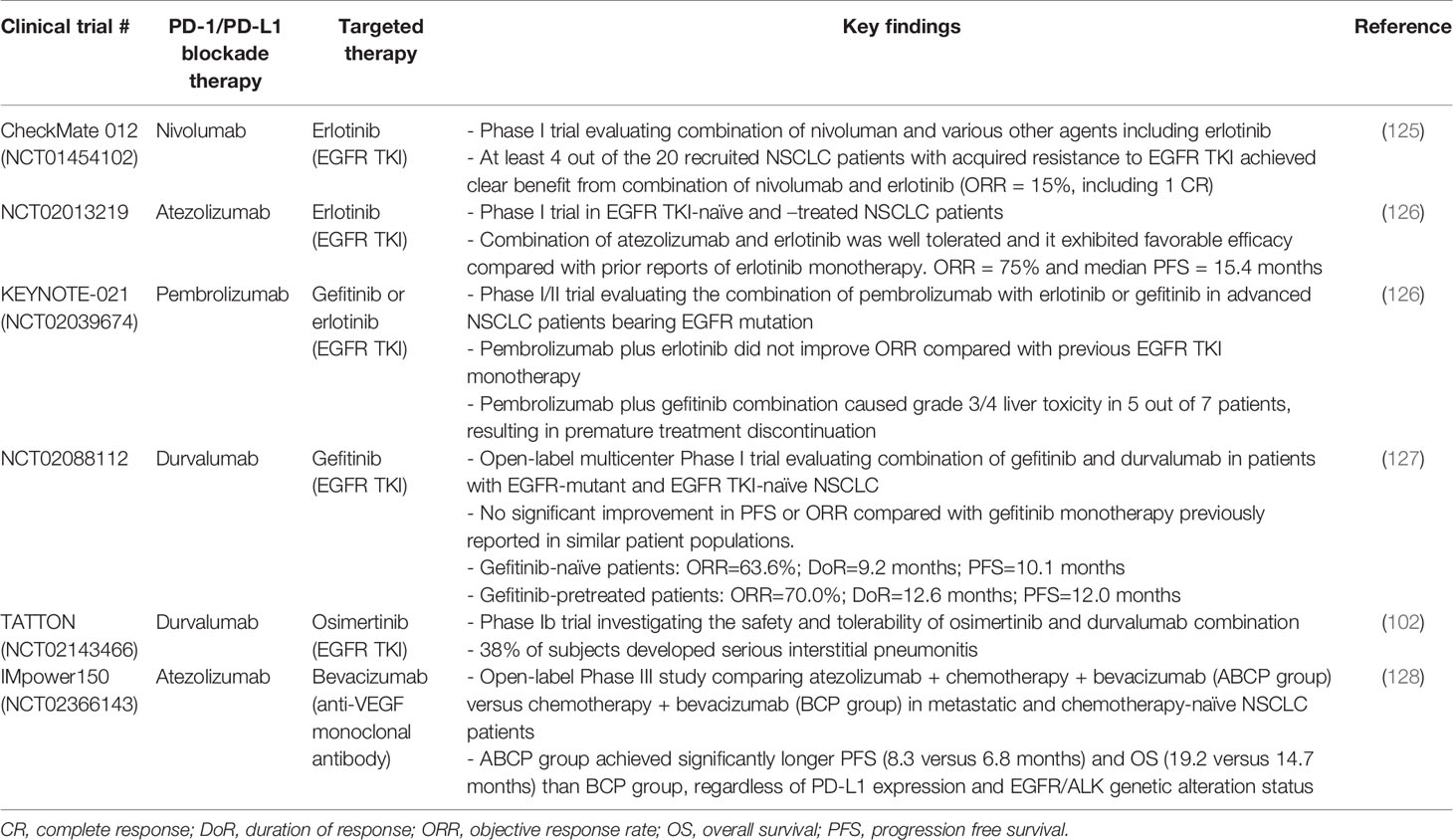
Table 3 Representative clinical trials evaluating the combination of PD-1/PD-L1 blockade immunotherapy and targeted therapy in EGFR-mutant NSCLC patients.
CheckMate 012 (NCT01454102) is a multi-arm Phase 1 study evaluating the combination of nivolumab and different agents including erlotinib in advanced NSCLC with small sample size (n = 20) (125). At least 4 out of the 20 patients with acquired resistance to EGFR TKI were shown to achieve clear benefit from the addition of nivolumab to EGFR TKI therapy [objective response rate (ORR) = 15%, including 1 complete response (CR)] (125). The combination of atezolizumab and erlotinib has been evaluated in another Phase I clinical trial in EGFR TKI-naïve and –treated NSCLC patients (NCT02013219; n = 28) (126). Atezolizumab plus erlotinib was shown to demonstrate a tolerable safety profile and favorable efficacy compared with prior reports of erlotinib monotherapy. ORR was 75% and median PFS was 15.4 months (126).
On the other hand, the combination of EGFR TKIs with PD-1/PD-L1 inhibitors did not demonstrate favorable clinical efficacy in EGFR-mutated NSCLC patients in a few other trials. In the Phase I/II KEYNOTE-021 trial (NCT02039674), the combination of erlotinib (n = 12) or gefitinib (n = 7) with pembrolizumab was evaluated in EGFR-mutant advanced NSCLC patients (129). While pembrolizumab plus erlotinib produced similar adverse events as erlotinib monotherapy, pembrolizumab plus gefitinib combination caused grade 3/4 liver toxicity in five out of seven patients and resulting in premature treatment discontinuation. Disappointingly, pembrolizumab plus erlotinib did not improve ORR compared with previous EGFR TKI monotherapy studies (129). Another open-label multicenter Phase I trial (NCT02088112) has also been recently conducted to evaluate the combination of gefitinib and duvalumab in patients with EGFR-mutant and EGFR TKI-naïve NSCLC (127). Durvalumab and gefitinib in combination was shown to produce higher toxicity than either drug alone. There was no significant improvement in PFS or ORR compared with gefitinib monotherapy previously reported in similar patient populations. To the best of our knowledge, no other Phase III trials investigating the combination EGFR TKI and PD-1 inhibitors in EGFR TKI-naïve patients are currently planned or actively recruiting.
In preclinical studies, the 3rd generation EGFR TKI, osimertinib, has been shown to enhance the antitumor efficacy of PD-1/PD-L1 blockade therapy by increasing CD8+ T cell infiltration in tumors (130). However, in a Phase Ib trial TATTON (NCT02143466) investigating the safety and tolerability of combining osimertinib and durvalumab, 38% of patients developed serious interstitial pneumonitis and thus the poor safety profile renders the combination not feasible (102). In an animal study using EGFR mutated tumor-bearing mouse model, osimertinib (but not gefitinib) combined with anti-PD-L1 therapy was shown to cause pneumonitis and injury to lung tissues (124).
Vascular Endothelial Growth Factor Receptor (VEGFR) TKI
IMpower150 was an open-label Phase III study (NCT02366143) comparing atezolizumab ± chemotherapy + bevacizumab (ABCP group) versus chemotherapy + bevacizumab (BCP group) in metastatic NSCLC patients who had not previously received chemotherapy (128). Bevacizumab is an anti-VEGF monoclonal antibody and its combination with chemotherapy has been approved for the treatment of metastatic NSCLC (131). Apart from the well-known antiangiogenic effects of bevacizumab (132), the inhibition of VEGF has also been shown to mediate immunomodulatory effects (14, 133, 134). Thus, the efficacy of atezolizumab may be enhanced by the addition of bevacizumab to reverse VEGF-mediated immunosuppression (134, 135). Encouragingly, the ABCP group was shown to achieve significantly longer PFS (8.3 months versus 6.8 months) and OS (19.2 months versus 14.7 months) than the BCP group, regardless of PD-L1 expression and EGFR/ALK genetic alteration status (128) (Table 3).
Combination of PD-1/PD-L1 ICIs With Other Immunotherapies
Both CTLA-4 and PD-1 ICIs demonstrated impressive durable antitumor response and they had a manageable safety profile. However, benefits of monotherapy were limited by low response rates and only a fraction of patients were found to be responsive (136). Combination of CTLA-4 and PD-1/PD-L1 blockade was proposed to increase the response rates and patient survival rates. It was thought that blockade of CTLA-4 (primarily involved in regulation of T cell activation in lymph nodes and in suppression of DC activity via Treg cells) could act synergistically with blockade of PD-1 (mainly involved in inhibition of effect T cells and NK cell activation in peripheral tissues and in induction of Treg cell differentiation) (137).
Multiple studies have investigated the combination of PD-1/PD-L1 plus CTLA-4 antibodies in treating NSCLC (Table 4). The first study is a phase Ib trial that evaluated the safety and efficacy of durvalumab (anti-PD-L1) and tremelimumab (anti-CTLA-4) combination. Encouraging clinical activity was observed in NSCLC patients with PD-L1 positive as well as PD-L1 negative tumors, with investigator assessed confirmed ORR in 23% patients (138). Importantly, this study revealed that the antitumor effect of the combination does not depend on PD-L1 expression. Thus, this might provide a new treatment option for patients with negative PD-L1 expression (138). In a phase III trial evaluating chemotherapy-naïve stage IV or recurrent NSCLC patients, the combination of nivolumab and ipilimumab achieved ORR of 45.3%, 1-year progressive free survival rate of 42.6% and median PFS of 7.2 months (139). The relative incidence of disease progression or death was significantly lower in nivolumab plus ipilimumab combination compared to chemotherapy alone group (HR for disease progression or death, 0.58, p < 0.001). In another phase II study, the efficacy and safety of nivolumab plus “low dose” ipilimumab as first line treatment for metastatic NSCLC was investigated (140). The association of efficacy with PD-L1 expression and TMB was also assessed. The ORR achieved by the combination was found to be higher in patients with TMB of at least 10 mutations per megabase and it was not dependent on PD-L1 expression (140).
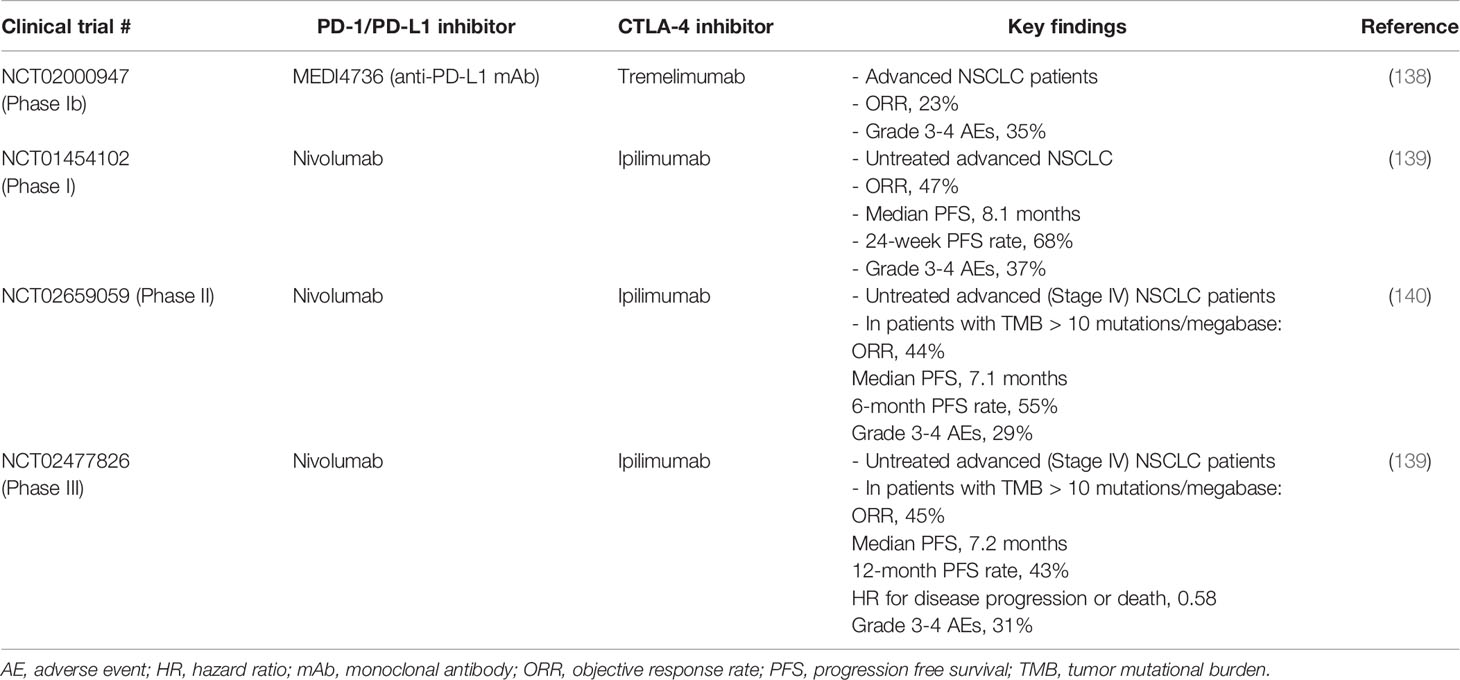
Table 4 Representative clinical trials investigating the combination of PD-1/PD-L1 and CTLA-4 blockade immunotherapies in NSCLC.
Combination of PD-1/PD-L1 Blockade Immunotherapy With Other Miscellaneous Therapies
Interleukin-10 (IL-10) is known to possess anti-inflammatory and CD8+ T cell stimulating activities (141). Pegilodecakin (pegylated IL-10) is a first-in-class long-acting IL-10 receptor agonist that induces oligoclonal T cell expansion (142). It has demonstrated single-agent activity in advanced solid tumors (143) (Table 5). IVY is a multicenter, multicohort, open-label, phase Ib trial (NCT02009449) evaluating the combination of pegilodecakin and pembrolizumab or nivolumab for patients with advanced solid tumors (144). The combination of pegilodecakin with anti-PD-1 monoclonal antibodies demonstrated a manageable toxicity profile and promising antitumor activity. Th ORR was relatively higher for NSCLC (43%), than that in renal cell carcinoma (40%) and melanoma (10%) (144). The favorable responses were also observed when PD-1/PD-L1 blockade immunotherapy only produced limited benefit, such as low PD-L1 expression, low TMB and liver metastasis. Since subgroup analysis focusing on EGFR-mutant patients were not available from the study, further investigation is needed to verify its clinical usefulness for NSCLC patients with EGFR mutations.
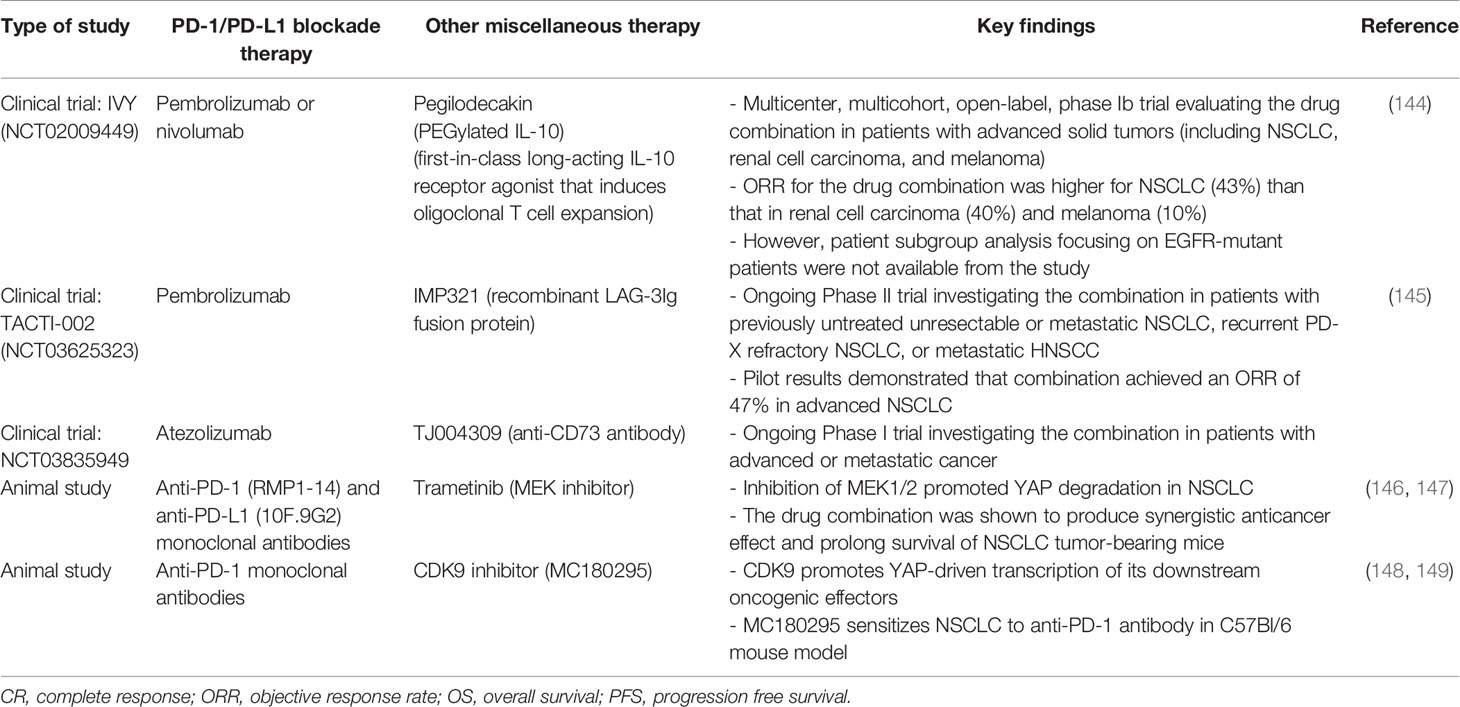
Table 5 Representative studies (clinical trials and animal studies) evaluating the combination of PD-1/PD-L1 blockade immunotherapy and other miscellaneous therapies in EGFR-mutant NSCLC.
As discussed above, the major Hippo regulator YAP plays critical role in regulating tumor immunity and PD-L1 expression (83–88). It follows that therapies targeting YAP may potentially enhance the efficacy of anti-PD-1/PD-L1 ICIs in EGFR-mutant NSCLC. A few small molecule compounds or drugs, including dasatinib, JQ1, norcantharidin, MLN8237 and dobutamine, have been shown to inhibit YAP (150). Further investigation is needed to verify the beneficial effect of combining YAP inhibitors with anti-PD-1/PD-L1 ICIs for treating EGFR TKI resistant NSCLC. Apart from inhibiting YAP, the modulation of YAP-related oncogenic pathways may also be evaluated. Inhibition of MEK1/2 is known to promote YAP degradation in NSCLC (146) (Table 5). Recently, the combination of MEK inhibitor and anti-PD-1/PD-L1 antibodies have been shown to produce synergistic anticancer effect and prolong survival of NSCLC tumor-bearing mice (147). On the other hand, cyclin-dependent kinase 9 (CDK9) is a key mediator promoting YAP-driven transcription of its downstream oncogenic effectors (148). Therefore, CDK9 inhibitors, such as dinaciclib and seliciclib, may be evaluated for potentiation of PD-1/PD-L1 blockade therapy. In fact, a recent animal study has shown that a highly selective CDK9 inhibitor (MC180295) sensitizes cancer cells to anti-PD-1 antibodies (149).
The combination of a few novel immune modulating agents and PD-1/PD-L1 ICIs have also been investigated. Eftilagimod alpha (IMP321) is a recombinant LAG-3Ig fusion protein that binds to MHC class II to activate antigen presenting cell and CD8 T-cell. The increase in activated T cells by IMP321 could potentially reduce the number of non-responders to pembrolizumab. Pilot results from the TACTI-002 trial showed that the combination of IMP321 and pembrolizumab achieved an ORR of 47% in advanced NSCLC (145) (Table 5). Activation of EGFR is associated with overactivation of Tregs. To this end, CD36 is known to make Tregs more adaptable to TME by serving as a metabolic modulator (151). Forced expression of CD63 by genetic approach in Tregs was shown to suppress tumor growth and enhance the antitumor efficacy of PD-1 therapy (151). CD73 is a cell surface nucleotidase, which catalyzes the hydrolysis of AMP into adenosine and phosphate. CD73-generated adenosine plays a critical role in tumor immunoescape (152). In an ongoing clinical trial (NCT03835949), the combination of an anti-CD73 drug (TJ004309) and atezolizumab is investigated in patients with advanced or metastatic cancer (153).
Local Co-Treatments
Besides the aforementioned systemic combination treatment for potentiating immunotherapy, a few local treatment options have also been investigated. These include thermal therapies, radiotherapy and minimal invasive intratumoral therapy.
Immunomodulation by Local Thermal Ablation of Cancer
Thermal ablation has been used for the management of localized tumors for patients not eligible for surgical resection. A growing body of evidence suggests that thermoablation could modulate both adaptive and innate immunity (154). However, the induced immune responses are mostly weak and not sufficient for the eradication of tumors or durable prevention of disease progression. In recent years, the combination of thermal ablation and ICIs therapy have been evaluated with promising results. Shi et al. reported that radiofrequency ablation (RFA) treatment of liver metastases increased not only T cell infiltration but also PD-L1 expression in primary human colorectal tumors (155). Using mouse tumor models, RFA treatment of one tumor was found to initially enhance a strong T cells mediated immune response in tumor. However, the tumor quickly overcame the immune responses by inhibiting CD8 and CD4 T cell function, subsequently driving a shift to higher regulatory T cell to Teff ratio (155). Importantly, the combination of RFA and anti-PD-1 antibodies was found to significantly enhance T cell immune responses and lead to prolonged animal survival (155).
Radiotherapy to Induce ICD
Radiotherapy is commonly used in cancer therapy to achieve a local control of the irradiated target tumor lesions regardless of clinical stage. Numerous preclinical studies have demonstrated that radiotherapy could activate anti-tumor immune response. Irradiation is known to activate host immunity by triggering ICD, which is characterized by the release of DAMPs to activate dendritic cells and to prime antigen-specific T cells in a dose-dependent manner (156). Procureur et al. has recently published an excellent review about the enhancement of ICIs by radiotherapy-induced immunogenic cell death (157). Radiotherapy-induced systemic immune activation could cause shrinkage of distant tumor lesions outside the irradiated field, a phenomenon known as abscopal effect (158). In the past, abscopal effect was believed to be a very rare phenomenon. However, recent clinical data revealed that the combination of radiotherapy and anti-PD-1/PD-L1 ICIs could induce the abscopal effect (159).
On the other hand, the induction of immunosuppressive cytokines and chemokines by radiotherapy contribute to immunosuppressive reactions (160). The PD-1/PD-L1 axis is one of the key factors in cancer immune escape induced by radiotherapy. Moreover, upregulation of PD-L1 expression has been reported in NSCLC patients who have undergone radiotherapy with or without chemotherapy as preoperative treatment (161). In addition, EGFR signaling after irradiation leads to PD-L1 upregulation via the IL-6/JAK/STAT3 pathway (162). As anti-PD-1/PD-L1 antibodies could relieve this immunosuppression, it makes sense to combine PD-1/PD-L1 ICIs and radiotherapy (161).
The phase III PACIFIC trial (NCT02125461) provided the most remarkable clinical data to support the combination of radiotherapy and anti-PD-1/PD-L1 ICIs. In this study, progression-free survival was significantly prolonged by prescribing durvalumab as a consolidation therapy after concurrent chemoradiotherapy as compared with placebo (51). Based on this trial, durvalumab following chemoradiotherapy has been approved for the treatment of NSCLC by the US Food and Drug Administration in 2018.
Minimal Invasive Intratumoral Injection of ICD Inducer
Local immunotherapies such as the intratumoral injection of oncolytic compounds have been used to reinstate and enhance systemic anticancer immune responses. A recent animal study reported the use of local immunotherapy to sensitize the tumor to subsequent immune checkpoint blockade (163). LTX-401 is an oncolytic peptide designed for local immunotherapy. The sequential LTX-401 treatment combined with double checkpoint inhibition of PD-1 and CTLA-4 exhibited strong antineoplastic effects on both the primary lesions and distant tumors (163).
Conclusion
In this review, we summarize the recent investigations about the use of PD-1/PD-L1 blockade therapy in EGFR-mutated NSCLC. The underlying mechanisms leading to the inferior clinical efficacy of PD-1/PD-L1 inhibitors in EGFR-mutated NSCLC and new strategies for its potentiation are discussed. Currently, the NCCN guidelines do not recommend immunotherapy for treating NSCLC patients carrying EGFR mutations. The combinations of PD-1/PD-L1-based immunotherapy and several other treatment modalities are under active investigation in clinical trials. While outcomes of these trials are immature, the optimal sequence, schedule and dosing remain to be determined. Moreover, the possible risk of combined toxicity pose a major challenge for the drug combinations. Therefore, a thorough investigation about the mechanism of action and risks associated with drug combinations is needed. It will help identify specific patient population that can benefit from the drug combination, predict the likelihood of toxicities, and guide dosing/administration sequencing and clinical monitoring consideration.
Author Contributions
All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We acknowledge the researchers who have contributed to the development of novel treatment of EGFR-mutant lung cancer by immunotherapy and whose works have not been cited here because of space limitation.
References
1. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2020. CA Cancer J Clin (2020) 70:7–30. doi: 10.3322/caac.21590
2. Jiang W, Cai G, Hu PC, Wang Y. Personalized Medicine in non-Small Cell Lung Cancer: A Review From a Pharmacogenomics Perspective. Acta Pharm Sin B (2018) 8:530–8. doi: 10.1016/j.apsb.2018.04.005
3. Gao J, Li HR, Jiang JH, Ding JY. Strategies to Overcome Acquired Resistance to EGFR TKI in the Treatment of non-Small Cell Lung Cancer. Clin Transl Oncol (2019) 21:1287–301. doi: 10.1007/s12094-019-02075-1
4. Yuan M, Huang LL, Chen JH, Wu J, Xu Q. The Emerging Treatment Landscape of Targeted Therapy in non-Small-Cell Lung Cancer. Signal Tranduct Targeting Ther (2019) 4:61. doi: 10.1038/s41392-019-0099-9
5. Passaro A, Attili I, Morganti S, Del Signore E, Gianoncelli L, Spitaleri G, et al. Clinical Features Affecting Survival in Metastatic NSCLC Treated With Immunotherapy: A Critical Review of Published Data. Cancer Treat Rev (2020) 89:102085. doi: 10.1016/j.ctrv.2020.102085
6. Govindan R, Szczesna A, Ahn MJ, Schneider CP, Gonzalez Mella PF, Barlesi F, et al. Phase III Trial of Ipilimumab Combined With Paclitaxel and Carboplatin in Advanced Squamous non-Small-Cell Lung Cancer. J Clin Oncol (2017) 35:3449–57. doi: 10.1200/JCO.2016.71.7629
7. Mooradian MJ, Gainor JF. Putting the Brakes on CTLA-4 Inhibition in Lung Cancer? Transl Lung Cancer Res (2018) 7(Suppl 1):S35–8. doi: 10.21037/tlcr.2018.01.05
8. Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, et al. Nivolumab Versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med (2015) 373:123–35. doi: 10.1056/NEJMoa1504627
9. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab Versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med (2015) 373:1627–39. doi: 10.1056/NEJMoa1507643
10. Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab Plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med (2018) 378:2078–92. doi: 10.1056/NEJMoa1801005
11. Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab Versus Docetaxel in Patients With Previously Treated Non-Small-Cell Lung Cancer (OAK): A Phase 3, Open-Label, Multicenter Randomized Controlled Trial. Lancet (2017) 389:255–65. doi: 10.1016/S0140-6736(16)32517-X
12. Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. Pembrolizumab Versus Chemotherapy for PD-L1-positive Non-Small-Cell Lung Cancer. N Engl J Med (2016) 375:1823–33. doi: 10.1056/NEJMoa1606774
13. Langer CJ, Gadgeel SM, Borghaei H, Papadimitrakopoulou VA, Patnaik A, Powell SF, et al. Carboplatin and Pemetrexed With or Without Pembrolizumab for Advanced, non-Squamous Non-Small-Cell Lung Cancer: A Randomized, Phase 2 Cohort of the Open-Label KETNOTE-021 Study. Lancet Oncol (2016) 17:1497–508. doi: 10.1016/S1470-2045(16)30498-3
14. Kim JM, Chen DS. Immune Escape to PD-L1/PD-1 Blockade: Seven Steps to Success (or Failure). Ann Oncol (2016) 27:1492–504. doi: 10.1093/annonc/mdw217
15. Xia L, Liu Y, Wang Y. Pd-1/Pd-L1 Blockade Therapy in Advanced Non-Small-Cell Lung Cancer: Current Status and Future Directions. Oncologist (2019) 1:31–41. doi: 10.1634/theoncologist.2019-IO-S1-s05
16. Takada K, Okamoto T, Toyokawa G, Kozuma Y, Matsubara T, Haratake N, et al. The Expression of PD-L1 Protein as a Prognostic Factor in Lung Squamous Cell Carcinoma. Lung Cancer (2017) 104:7–15. doi: 10.1016/j.lungcan.2016.12.006
17. Takada K, Toyokawa G, Shoji F, Okamoto T, Maehara Y. The Significance of the PD-L1 Expression in non-Small-Cell Lung Cancer: Trenchant Double Swords as Predictive and Prognostic Markers. Clin Lung Cancer (2017) 19:120–9. doi: 10.1016/j.cllc.2017.10.014
18. Kerr K, Hirsch F. Programmed Death Ligand-1 Immunohistochemistry. Friend or Foe? Arch Pathol Lab Med (2016) 140:326–31. doi: 10.5858/arpa.2015-0522-SA
19. Ratcliffe MJ, Sharpe A, Midha A, Barker C, Scott M, Scorer P, et al. Agreement Between Programmed Cell Death Ligand-1 Diagnostic Assays Across Multiple Protein Expression Cutoffs in Non-Small Cell Lung Cancer. Clin Cancer Res (2017) 23:3585–91. doi: 10.1158/1078-0432.CCR-16-2375
20. Carbognin L, Pilotto S, Milella M, Vaccaro V, Brunelli M, Calio A, et al. Differential Activity of Nivolumab, Pembrolizumab and MPDL3280A According to the Tumor Expression of Programmed Death-Ligand-1 (PD-L1): Sensitivity Analysis of Trials in Melanoma, Lung and Genitourinary Cancers. PloS One (2015) 10:e0130142. doi: 10.1371/journal.pone.0130142
21. Karwacz K, Bricogne C, Macdonald D, Arce F, Bennett CL, Collins M, et al. Pd-L1 Co-Stimulation Contributes to Ligand-Induced T Cell Receptor Down-Modulation on CD8(+) T Cells. EMBO Mol Med (2011) 3:581–92. doi: 10.1002/emmm.201100165
22. Gato-Canas M, Martinez de Morentin X, Blanco-Luquin I, Fernandez-Irigoyen J, Zudaire I, Liechtenstein T, et al. A Core of Kinase-Regulated Interactomes Defines the Neoplastic MDSC Lineage. Oncotarget (2015) 6:27160–75. doi: 10.18632/oncotarget.4746
23. Ibanez-Vea M, Zuazo M, Gato M, Arasanz H, Fernandez-Hinojal G, Escors D, et al. Myeloid-Derived Suppressor Cells in the Tumor Microenvironment: Current Knowledge and Future Perspectives. Arch Immunol Ther Exp (2018) 66:113–23. doi: 10.1007/s00005-017-0492-4
24. Karwacz K, Arce F, Bricogne C, Kochan G, Escors D. Pd-L1 Co-Stimulation, Ligand-Induced TCR Down-Regulation and Anti-Tumor Immunotherapy. Oncoimmunology (2012) 1:86–8. doi: 10.4161/onci.1.1.17824
25. Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, et al. Atezolizumab Versus Docetaxel for Patients With Previously Treated Non-Small-Cell Lung Cancer (POPLAR): A Multicentre, Open-Label, Phase 2 Randomized Controlled Trial. Lancet (2016) 387:P1837–1846. doi: 10.1016/S0140-6736(16)00587-0
26. Bocanegra A, Fernandez-Hinojal G, Zuazo-Ibarra M, Arasanz H, Garcia-Granda MJ, Hernandez C, et al. Pd-L1 Expression in Systemic Immune Cell Populations as a Potential Predictive Biomarker of Responses to PD-L1/PD-1 Blockade Therapy in Lung Cancer. Int J Mol Sci (2019) 20:1631. doi: 10.3390/ijms20071631
27. Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, et al. Pd-L2 Is a Second Ligand for PD-1 and Inhibits T Cell Activation. Nat Immunol (2001) 2:261–8. doi: 10.1038/85330
28. Tang S, Kim PS. A High-Affinity Human PD-1/PD-L2 Complex Informs Avenues for Small-Molecule Immune Checkpoint Drug Discovery. Proc Natl Acad Sci USA (2019) 116:24500–6. doi: 10.1073/pnas.1916916116
29. Kim JH, Kim HS, Kim BJ. Prognostic Value of Smoking Status in Non-Small-Lung Cancer Patients Treated With Immune Checkpoint Inhibitors: A Meta-Analysis. Oncotarget (2017) 8:93149–55. doi: 10.18632/oncotarget.18703
30. Cristescu R, Mogg R, Ayers M, Albright A, Murphy E, Yearley J, et al. Pan-Tumor Genomic Biomarkers for PD-1 Checkpoint Blockade-Based Immunotherapy. Science (2018) 362:eaar3593. doi: 10.1126/science.aar3593
31. Chan TA, Yarchoan M, Jaffee E, Swanton C, Quezada SA, Stenzinger A, et al. Development of Tumor Mutation Burden as an Immunotherapy Biomarker: Utility for the Oncology Clinic. Ann Oncol (2019) 30:44–56. doi: 10.1093/annonc/mdy495
32. Maung T, Ergin H, Javed M, Inga EE, Khan S. Immune Checkpoint Inhibitors in Lung Cancer: Role of Biomarkers and Combination Therapies. Cureus (2020) 12:e8095. doi: 10.7759/cureus.8095
33. Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Mutational Landscape Determines Sensitivity to PD-1 Blockade in Non-Small Cell Lung Cancer. Science (2015) 348:124–8. doi: 10.1126/science.aaa1348
34. Peters S, Creelan B, Hellmann MD, Socinski MA, Reck M, Bhagavatheeswaran P, et al. Abstract Ct082: Impact of Tumor Mutation Burden on the Efficacy of First-Line Nivolumab in Stage IV or Recurrent non-Small Cell Lung Cancer: An Exploratory Analysis of Checkmate 026. Cancer Res (2017) 77:CT082. doi: 10.1158/1538-7445.AM2017-CT082
35. Chang H, Sasson A, Srinivasan S, Golhar R, Greenawalt DM, Geese WJ, et al. Bioinformatic Methods and Bridging of Assay Results for Reliable Tumor Mutational Burden Assessment in Non-Small-Cell Lung Cancer. Mol Diagn Ther (2019) 23:507–20. doi: 10.1007/s40291-019-00408-y
36. Ramalingam SS, Hellmann MD, Awad MM, Borghaei H, Gainor J, Brahmer J, et al. Abstract Ct078: Tumor Mutational Burden (Tmb) as a Biomarker for Clinical Benefit From Dual Immune Checkpoint Blockade With Nivolumab (Nivo) + Ipilimumab (Ipi) in First-Line (1l) Non-Small Cell Lung Cancer (Nsclc): Identification of Tmb Cutoff From Checkmate 568. Cancer Res (2018) 78:CT078. doi: 10.1158/1538-7445.AM2018-CT078
37. Hellmann MD, Ciuleanu TE, Pluzanski A, Lee JS, Otterson GA, Audigier-Valette C, et al. Nivolumab Plus Ipilimumab in Lung Cancer With a High Tumor Mutational Burden. N Engl J Med (2018) 378:2093–104. doi: 10.1056/NEJMoa1801946
38. Wang L, Zhao D, Qin K, Rehman FU, Zhang X. Effect and Biomarker of Nivolumab for Non-Small-Cell Lung Cancer. BioMed Pharmacother (2019) 117:109199. doi: 10.1016/j.biopha.2019.109199
39. Duma N, Santana-Davila R, Molina JR. Non-Small Cell Lung Cancer: Epidemiology, Screening, Diagnosis, and Treatment. Mayo Clin Proc (2019) 94:1623–40. doi: 10.1016/j.mayocp.2019.01.013
40. Hirsch FR, Kerr KM, Bunn PA Jr, Kim ES, Obasaju C, Perol M, et al. Molecular and Immune Biomarker Testing in Squamous-Cell Lung Cancer: Effect of Current and Future Therapies and Technologies. Clin Lung Cancer (2018) 19:331–9. doi: 10.1016/j.cllc.2018.03.014
41. Pu X, Wu L, Su D, Mao W, Fang B. Immunotherapy for non-Small-Cell Lung Cancers: Biomarkers for Predicting Responses and Strategies to Overcome Resistance. BMC Cancer (2018) 1186:1082. doi: 10.1186/s12885-018-4990-5
42. Capalbo C, Scafetta G, Filetti M, Marchetti P, Bartolazzi A. Predictive Biomarkers for Checkpoint Inhibitor-Based Immunotherapy: The Galectin-3 Signature in Nsclcs. Int J Mol Sci (2019) 20:E1607. doi: 10.3390/ijms20071607
43. Proto C, Ferrara R, Signorelli D, Lo Russo G, Galli G, Imbimbo M, et al. Choosing Wisely First-Line Immunotherapy in Non-Small Cell Lung Cancer (NSCLC): What to Add and What to Leave Out. Cancer Treat Rev (2019) 75:39–51. doi: 10.1016/j.ctrv.2019.03.004
44. Akbay EA, Koyama S, Carretero J, Altabef A, Tchaicha JH, Christensen CL, et al. Activation of the PD-1 Pathway Contributes to Immune Escape in EGFR-Driven Lung Tumors. Cancer Discovery (2013) 3:1355–63. doi: 10.1158/1535-7163.TARG-13-B290
45. Chen N, Fang W, Zhan J, Hong S, Tang Y, Kang S, et al. Upregulation of PD-L1 by EGFR Activation Mediates the Immune Escape in EGFR-driven NSCLC: Implication for Optional Immune Targeted Therapy for NSCLC Patients With EGFR Mutations. J Thorac Oncol (2015) 10:910–23. doi: 10.1097/JTO.0000000000000500
46. Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, et al. Pembrolizumab Versus Docetaxel for Previously Treated, PD-L1-Positive, Advanced Non-Small-Cell Lung Cancer (KEYNOTE-010): A Randomized Controlled Trial. Lancet (2016) 387:1540–50. doi: 10.1016/S0140-6736(15)01281-7
47. Lee CK, Man J, Lord S, Links M, Gebski V, Mok T, et al. Checkpoint Inhibitors in Metastatic EGFR-mutated Non-Small Cell Lung Cancer- a Meta-Analysis. J Thorac Oncol (2017) 12:403–7. doi: 10.1016/j.jtho.2016.10.007
48. Gainor JF, Shaw AT, Sequist LV, Fu X, Azzoli CG, Piotrowska Z, et al. EGFR Mutations and ALK Rearrangements are Associated With Low Response Rates to PD-1 Pathway Blockade in Non-Small Cell Lung Cancer: A Retrospective Analysis. Clin Cancer Res (2016) 22:4585–93. doi: 10.1158/1078-0432.CCR-15-3101
49. Lisberg A, Cummings A, Goldman JW, Bornazyan K, Reese N, Wang T, et al. A Phase II Study of Pembrolizumab in EGFR-Mutant, Pd-L1+, Tyrosine Kinase Inhibitor Naïve Patients With Advanced NSCLC. J Thorac Oncol (2018) 13:1138–45. doi: 10.1016/j.jtho.2018.03.035
50. Lee CK, Man J, Lord S, Cooper W, Links M, Gebski V, et al. Clinical and Molecular Characteristics Associated With Survival Among Patients Treated With Checkpoint Inhibitors for Advanced Non-Small Cell Lung Carcinoma: A Systematic Review and Meta-Analysis. JAMA Oncol (2018) 4:210–6. doi: 10.1001/jamaoncol.2017.4427
51. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab After Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med (2017) 377:1919–29. doi: 10.1056/NEJMoa1709937
52. Antonia SJ, Borghaei H, Ramalingam SS, Horn L, De Castro Carpeno J, Pluzanski A, et al. Four-Year Survival With Nivolumab in Patients With Previously Treated Advanced Non-Small-Cell Lung Cancer: A Pooled Analysis. Lancet Oncol (2019) 20:1395–408. doi: 10.1016/S1470-2045(19)30407-3
53. Hastings K, Yu HA, Wei W, Sanchez-Vega F, DeVeaux M, Choi J, et al. EGFR Mutation Subtypes and Response to Immune Checkpoint Blockade Treatment in Non-Small-Cell Lung Cancer. Ann Oncol (2019) 30:1311–20. doi: 10.1093/annonc/mdz141
54. D’Incecco A, Andreozzi M, Ludovini V, Rossi E, Capodanno A, Landi L, et al. PD-1 and PD-L1 Expression in Moleculely Selected Non-Small-Cell Lung Cancer Patients. Br J Cancer (2015) 112:95–102. doi: 10.1038/bjc.2014.555
55. Azuma K, Ota K, Kawahara A, Hattori S, Iwama E, Harada T, et al. Association of PD-L1 Overexpression With Activating EGFR Mutations in Surgically Resected Nonsmall-Cell Lung Cancer. Ann Oncol (2014) 25:1935–40. doi: 10.1093/annonc/mdu242
56. Tang Y, Fang W, Zhang Y, Hong S, Kang S, Yan Y, et al. The Association Between PD-L1 and EGFR Status and the Prognostic Value of PD-L1 in Advanced Non-Small Cell Lung Cancer Patients Treated With EGFR-Tkis. Oncotarget (2015) 6:14209–19. doi: 10.18632/oncotarget.3694
57. Ji M, Liu Y, Li Q, Ning Z, Zhao W, Shi H, et al. Pd-1/Pd-L1 Expression in non-Small-Cell Lung Cancer and its Correlation With EGFR/KRAS Mutations. Cancer Biol Ther (2016) 17:407–13. doi: 10.1080/15384047.2016.1156256
58. Dong ZY, Zhang JT, Liu SY, Su J, Zhang C, Xie Z, et al. EGFR Mutation Correlates With Uninflamed Phenotype and Weak Immunogenicity, Causing Impaired Response to PD-1 Blockade in Non-Small Cell Lung Cancer. Oncoimmunology (2017) 6:e1356145. doi: 10.1080/2162402X.2017.1356145
59. Koh J, Go H, Keam B, Kim MY, Nam SJ, Kim TM, et al. Clinicopathologic Analysis of Programmed Cell Death-1 and Programmed Cell Death-Ligand 1 and 2 Expressions in Pulmonary Adenocarcinoma: Comparison With Histology and Driver Oncogenic Alteration Status. Mod Pathol (2015) 28:1154–66. doi: 10.1038/modpathol.2015.63
60. Offin M, Rizvi H, Tenet M, Ni A, Sanchez-Vega F, Li BT, et al. Tumor Mutation Burden and Efficacy of EGFR-tyrosine Kinase Inhibitors in Patients With EGFR-mutant Lung Cancers. Clin Cancer Res (2019) 25:1063–9. doi: 10.1158/1078-0432.CCR-18-1102
61. Rizvi H, Plodkowski AJ, Tenet M, Halpenny D, Long N, Sauter JL, et al. Clinical and Molecular Features Predicting Long-Term Response (LTR) to anti-PD-(L)-1 Based Therapy in Patients With NSCLC. J Clin Oncol (2018) 36(Suppl 15):9022. doi: 10.1200/JCO.2018.36.15_suppl.9022
62. Mo J, Hu X, Gu L, Chen B, Khadaroo PA, Shen Z, et al. Smokers or non-Smokers: Who Benefits More From Immune Checkpoint Inhibitors in Treatment of Malignancies? An Up-to-Date Meta-Analysis. World J Surg Oncol (2020) 18:15. doi: 10.1186/s12957-020-1792-4
63. Yarchoan M, Hopkins A, Jaffee EM. Tumor Mutational Burden and Response Rate to PD-1 Inhibition. N Engl J Med (2017) 377:2500–1. doi: 10.1056/NEJMc1713444
64. Gettinger S, Horn L, Jackman D, Spigel D, Antonia S, Hellmann M, et al. Five-Year Follow-Up of Nivolumab in Previously Treated Advanced Non-Small-Cell Lung Cancer: Results From the CA209-003 Study. J Clin Oncol (2018) 36:1675–84. doi: 10.1200/JCO.2017.77.0412
65. Hui R, Garon EB, Goldman JW, Leighl NB, Hellmann MD, Patnaik A, et al. Pembrolizumab as First-Line Therapy for Patients With PD-L1-positive Advanced Non-Small Cell Lung Cancer: A Phase I Trial. Ann Oncol (2017) 28:874–81. doi: 10.1093/annonc/mdx008
66. Garassino MC, Cho BC, Kim JH, Mazieres J, Vansteenkiste J, Lena H, et al. Durvalumab as Third-Line or Later Treatment for Advanced Non-Small-Cell Lung Cancer (ATLANTIC): An Open-Label, Single-Arm, Phase 2 Study. Lancet Oncol (2018) 19:521–36. doi: 10.1016/S1470-2045(18)30144-X
67. Leighl NB, Hellmann MD, Hui R, Carcereny E, Felip E, Ahn MJ, et al. Pembrolizumab in Patients With Advanced Non-Small-Cell Lung Cancer (KEYNOTE-001): 3-Year Results From an Open-Label, Phase I Study. Lancet Respir Med (2019) 7:347–57. doi: 10.1016/S2213-2600(18)30500-9
68. von Pawel J, Bordoni R, Satouchi M, Fehrenbacher L, Cobo M, Han JY, et al. Long-Term Survival in Patients With Advanced Non-Small-Cell Lung Cancer Treated With Atezolizumab Versus Docetaxel: Results From the Randomized Phase III OAK Study. Eur J Cancer (2019) 107:124–32. doi: 10.1016/j.ejca.2018.11.020
69. Mazieres J, Drilon A, Lusque A, Mhanna L, Cortot AB, Mezquita L, et al. Immune Checkpoint Inhibitors for Patients With Advanced Lung Cancer and Oncogenic Driver Alterations: Results From the Global IMMUNOTARGET Registry. Ann Oncol (2019) 30:1321–8. doi: 10.1093/annonc/mdz167
70. Teng MW, Ngiow SF, Ribas A, Smyth MJ. Classifying Cancers Based on T-Cell Infiltration and PD-L1. Cancer Res (2015) 75:2139–45. doi: 10.1158/0008-5472.CAN-15-0255
71. Chen DS, Mellan I. Elements of Cancer Immunity and the Cancer-Immune Set Point. Nature (2017) 541:321–30. doi: 10.1038/nature21349
72. Streicher K, Higgs BW, Wu S, Coffman K, Damera G, Durham N, et al. Increased CD73 and Reduced IFNG Signature Expression in Relation to Response Rates to Anti-PD-1(L1) Therapies in EGFR-mutant Nsclc. J Clin Oncol (2017) 35:abstr 11505. doi: 10.1200/JCO.2017.35.15_suppl.11505
73. Busser B, Sancey L, Brambilla E, Coll JL, Hurbin A. The Multiple Roles of Amphiregulin in Human Cancer. Biochim Biophys Acta (2011) 1816:119–31. doi: 10.1016/j.bbcan.2011.05.003
74. Singh B, Carpenter G, Coffey RJ. EGF Receptor Ligands: Recent Advances. F1000Res (2016) 5:F1000 Faculty Rev–2270. doi: 10.12688/f1000research.9025.1
75. Zaiss DM, van Loosdregt J, Gorlani A, Bekker CP, Grone A, Sibilia M, et al. Amphiregulin Enhances Regulatory T Cell-Suppressive Function Via the Epidermal Growth Factor Receptor. Immunity (2013) 38:275–84. doi: 10.1016/j.immuni.2012.09.023
76. Wang S, Zhang Y, Wang Y, Ye P, Li J, Li H, et al. Amphiregulin Confers Regulatory T Cell Suppressive Function and Tumor Invasion Via the EGFR/GSK-3beta/Foxp3 Axis. J Biol Chem (2016) 291:21085–95. doi: 10.1074/jbc.M116.717892
77. Jiang R, Tang J, Chen Y, Deng L, Ji J, Xie Y, et al. The Long Noncoding RNA lnc-EGFR Stimulates T-Regulatory Cells Differentiation Thus Promoting Hepatocellular Carcinoma Immune Evasion. Nat Commun (2017) 8:15129. doi: 10.1038/ncomms15129
78. Mascia F, Schloemann DT, Cataisson C, McKinnon KM, Krymskaya L, Wolcott KM, et al. Cell Autonomous or Systemic EGFR Blockade Alters the Immune-Environment in Squamous Cell Carcinomas. Int J Cancer (2016) 139:2593–7. doi: 10.1002/ijc.30376
79. Jia J, Li X, Jiang T, Zhao S, Zhao C, Zhang L, et al. EGFR-Targeted Therapy Alters the Tumor Microenvironment in EGFR-Driven Lung Tumors: Implications for Combination Therapies. Int J Cancer (2019) 145:1432–44. doi: 10.1002/ijc.32191
80. Yu J, Du W, Yan F, Wang Y, Li H, Cao S, et al. Myeloid-Derived Suppressor Cells Suppress Antitumor Immune Responses Through IDO Expression and Correlate With Lymph Node Metastasis in Patients With Breast Cancer. J Immunol (2013) 190:3783–97. doi: 10.4049/jimmunol.1201449
81. Wu L, Du H, Li Y, Qu P, Yan C. Signal Transducer and Activator of Transcription 3 (STAT3C) Promotes Myeloid-Derived Suppressor Cell Expansion and Immune Suppression During Lung Tumorigenesis. Am J Pathol (2011) 179:2131–41. doi: 10.1016/j.ajpath.2011.06.028
82. Lo Sardo F, Strano S, Blandino G. YAP and TAZ in Lung Cancer: Oncogenic Role and Clinical Targeting. Cancers (Basel) (2018) 10:137. doi: 10.3390/cancers10050137
83. Wang G, Lu X, Dey P, Deng P, Wu CC, Jiang S, et al. Targeting YAP-dependent MDSC Infiltration Impairs Tumor Progression. Cancer Discovery (2016) 6:80–95. doi: 10.1158/2159-8290.CD-15-0224
84. Murakami S, Shahbazian D, Surana R, Zhang W, Chen H, Graham GT, et al. Yes-Associated Protein Mediates Immune Reprogramming in Pancreatic Ductal Adenocarcinoma. Oncogene (2017) 36:1232–44. doi: 10.1038/onc.2016.288
85. Ni X, Tao J, Barbi J, Chen Q, Park BV, Li Z, et al. YAP is Essential for Treg-Mediated Suppression of Antitumor Immunity. Cancer Discovery (2018) 8:1026–43. doi: 10.1158/2159-8290.CD-17-1124
86. Miao J, Hsu PC, Yang YL, Xu Z, Dai Y, Wang Y, et al. YAP Regulates PD-L1 Expression in Human NSCLC Expression in Human NSCLC Cells. Oncotarget (2017) 8:114576–87. doi: 10.18632/oncotarget.23051
87. Hsu PC, Miao J, Wang YC, Zhang WQ, Yang YL, Wang CW, et al. Inhibition of Yes-Associated Protein Down-Regulates PD-L1 (CD274) Expression in Human Malignant Pleural Mesothelioma. J Cell Mol Med (2018) 22:3139–48. doi: 10.1111/jcmm.13593
88. Lee BS, Park DI, Lee DH, Lee JE, Yeo MK, Park YH, et al. Hippo Effector YAP Directly Regulates the Expression of PD-L1 Transcripts in EGFR-TKI-Resistant Lung Adenocarcinoma. Biochem Biophys Res Commun (2017) 491:493–9. doi: 10.1016/j.bbrc.2017.07.007
89. McGowan M, Kleinberg L, Halvorsen AR, Helland A, Brustugun OT. NSCLC Depend Upon YAP Expression and Nuclear Localization After Acquiring Resistance to EGFR Inhibitors. Genes Cancer (2017) 8:497–504. doi: 10.18632/genesandcancer.136
90. Chen K, Cheng G, Zhang F, Zhu G, Xu Y, Yu X, et al. Pd-L1 Expression and T Cells Infiltration in Patients With Uncommon EGFR-Mutant Non-Small Cell Lung Cancer and the Response to Immunotherapy. Lung Cancer (2020) 142:98–105. doi: 10.1016/j.lungcan.2020.02.010
91. Brindel A, Althakfi W, Barritault M, Watkin E, Maury JM, Bringuier PP, et al. Uncommon EGFR Mutations in Lung Adenocarcinoma: Features and Response to Tyrosine Kinase Inhibitors. J Thorac Dis (2020) 12:4643–50. doi: 10.21037/jtd-19-3790
92. De Pas T, Toffalorio F, Manzotti M, Fumagalli C, Spitaleri G, Catania C, et al. Activity of Epidermal Growth Factor Receptor-Tyrosine Kinase Inhibitors in Patients With Non-Small Cell Lung Cancer Harboring Rare Epidermal Growth Factor Receptor Mutations. J Thorac Oncol (2011) 6:1895–901. doi: 10.1097/JTO.0b013e318227e8c6
93. Yang JC, Sequist LV, Geater SL, Tsai CM, Mok TS, Schuler M, et al. Clinical Activity of Afatinib in Patients With Advanced Non-Small-Cell Lung Cancer Harbouring Uncommon EGFR Mutations: A Combined Post-Hoc Analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol (2015) 16:830–8. doi: 10.1016/S1470-2045(15)00026-1
94. Lund-Iversen M, Kleinberg L, Fjellbirkeland L, Helland A, Brustugun OT. Clinicopathological Characteristics of 11 NSCLC Patients With EGFR-Exon 20 Mutations. J Thorac Oncol (2012) 7:1471–3. doi: 10.1097/JTO.0b013e3182614a9d
95. Meseure D, Vacher S, Alsibai KD, Nicolas A, Chemlali W, Caly R, et al. Expression of ANRIL-polycomb complexes-CDKN2A/B/ARF Genes in Breast Tumors: Identification of a Two-Gene (EZH2/CBX7) Signature With Independent Prognostic Value. Mol Cancer Res (2016) 14:623–33. doi: 10.1158/1541-7786.MCR-15-0418
96. Massarelli E, Johnson FM, Erickson HS, Wistuba II, Papadimitrakopoulou V. Uncommon Epidermal Growth Factor Receptor Mutations in Non-Small Cell Lung Cancer and Their Mechanisms of EGFR Tyrosine Kinase Inhibitors Sensitivity and Resistance. Lung Cancer (2013) 80:235–41. doi: 10.1016/j.lungcan.2013.01.018
97. Yamada T, Hirai S, Katayama Y, Yoshimura A, Shiotsu S, Watanabe S, et al. Retrospective Efficacy Analysis of Immune Checkpoint Inhibitors in Patients With EGFR-Mutated Non-Small Cell Lung Cancer. Cancer Med (2019) 8:1521–9. doi: 10.1002/cam4.2037
98. Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated With Immune Checkpoint Blockade. N Engl J Med (2018) 378:158–68. doi: 10.1056/NEJMra1703481
99. Das S, Johnson DB. Immune-Related Adverse Events and Anti-Tumor Efficacy of Immune Checkpoint Inhibitors. J Immunother Cancer (2019) 7:306. doi: 10.1186/s40425-019-0805-8
100. Haratani K, Hayashi H, Chiba Y, Kudo K, Yonesaka K, Kato R, et al. Association of Immune-Related Adverse Events With Nivolumab Efficacy in non-Small-Cell Lung Cancer. JAMA Oncol (2018) 4:374–8. doi: 10.1001/jamaoncol.2017.2925
101. Terraoka S, Fujimoto D, Morimoto T, Kawachi H, Ito M, Sato Y, et al. Early Immune-Related Adverse Events and Association With Outcome in Advanced Non-Small Cell Lung Cancer Patients Treated With Nivolumab: A Prospective Cohort Study. J Thorac Oncol (2017) 12:1798–805. doi: 10.1016/j.jtho.2017.08.022
102. Oxnard GR, Yang JC, Yu H, Kim SW, Saka H, Horn L, et al. TATTON: A Multi-Arm, Phase Ib Trial of Osimertinib Combined With Selumetinib, Savolitinib, or Durvalumab in EGFR-Mutant Lung Cancer. Ann Oncol (2020) 31:507–16. doi: 10.1016/j.annonc.2020.01.013
103. Yang JC, Shepherd FA, Kim DW, Lee GW, Lee JS, Chang GC, et al. Osimertinib Plus Durvalumab Versus Osimertinib Monotherapy in EGFR T790M-Positive NSCLC Following Previous EGFR TKI Therapy: CAURAL Brief Report. J Thorac Oncol (2019) 14:933–9. doi: 10.1016/j.jtho.2019.02.001
104. Puzanov I, Diab A, Abdallah K, Bingham CO3, Brogdon C, Dadu R, et al. Managing Toxicities Associated With Immune Checkpoint Inhibitors: Consensus Recommendations From the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer (2017) 5:95. doi: 10.1186/s40425-017-0300-z
105. Michot JM, Bigenwald C, Champiat S, Collins M, Carbonnel F, Postel-Vinay S, et al. Immune-Related Adverse Events With Immune Checkpoint Blockade: A Comprehensive Review. Eur J Cancer (2016) 54:139–48. doi: 10.1016/j.ejca.2015.11.016
106. Champiat S, Lambotte O, Barreau E, Belkhir R, Berdelou A, Carbonnel F, et al. Management of Immune Checkpoint Blockade Dysimmune Toxicities: A Collaborative Position Paper. Ann Oncol (2016) 27:559–74. doi: 10.1093/annonc/mdv623
107. Ferrara R, Mezquita L, Texier M, Lahmar J, Audigier-Valette C, Tessonnier L, et al. Hyperprogressive Disease in Patients With Advanced non-Small Cell Lung Cancer Treated With PD-1/PD-L1 Inhibitors or With Single-Agent Chemotherapy. JAMA Oncol (2018) 4:1543–52. doi: 10.1001/jamaoncol.2018.3676
108. Wang X, Wang F, Zhong M, Yarden Y, Fu L. The Biomarkers of Hyperprogressive Disease in PD-1/PD-L1 Blockage Therapy. Mol Cancer (2020) 19:81. doi: 10.1186/s12943-020-01200-x
109. Fuentes-Antras J, Provencio M, Diaz-Rubio E. Hyperprogression as a Distinct Outcome After Immunotherapy. Cancer Treat Rev (2018) 70:16–21. doi: 10.1016/j.ctrv.2018.07.006
110. Kas B, Talbot H, Ferrara R, Richard C, Lamarque JP, Pitre-Champagnat S, et al. Clarification of Definitions of Hyperprogressive Disease During Immunotherapy for Non-Small Cell Lung Cancer. JAMA Oncol (2020) 6:1039–46. doi: 10.1001/jamaoncol.2020.1634
111. Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Immunological Effects of Conventional Chemotherapy and Targeted Anticancer Agents. Cancer Cell (2015) 28:690–714. doi: 10.1016/j.ccell.2015.10.012
112. Ahmed A, Tait SWG. Targeting Immunogenic Cell Death in Cancer. Mol Oncol (2020) 14:2994–3006. doi: 10.1002/1878-0261.12851
113. Fucikova J, Kepp O, Kasikova L, Petroni G, Yamazaki T, Liu P, et al. Detection of Immunogenic Cell Death and its Relevance of Cancer Therapy. Cell Death Dis (2020) 11:1013. doi: 10.1038/s41419-020-03221-2
114. Wang YJ, Fletcher R, Yu J, Zhang L. Immunogenic Effects of Chemotherapy-Induced Tumor Cell Death. Genes Dis (2018) 5:194–203. doi: 10.1016/j.gendis.2018.05.003
115. Pfirschke C, Engblom C, Rickelt S, Cortez-Retamozo V, Garris C, Pucci F, et al. Immunogenic Chemotherapy Sensities Tumors to Checkpoint Blockade Therapy. Immunity (2016) 44:343–54. doi: 10.1016/j.immuni.2015.11.024
116. Kepp O, Zitvogel L, Kroemer G. Clinical Evidence That Immunogenic Cell Death Sensitizes to PD-1/PD-L1 Blockade. Oncoimmunology (2019) 8:1637188. doi: 10.1080/2162402X.2019.1637188
117. Park K, Yang JC, Girard N, Mok T, Gainor J, Nakagawa K, et al. P1-182 – Nivolumab + Chemotherapy vs Chemotherapy in EGFR-mutated NSCLC After 1L or 2L EGFR Tkis (CheckMate 722). Ann Oncol (2019) 30(suppl_6):vi126. doi: 10.1093/annonc/mdz343.039
118. Riely G, Hui R, Carbone D, Park K, Carrigan M, Xu X, et al. P1.01-81 Phase 3 Study of Pemetrexed-Platinum With or Without Pembrolizumab for TKI-Resistant / EGFR-mutated Advanced NSCLC: Keynote-789. J Thorac Oncol (2018) 13(10):Supp S494. doi: 10.1016/j.jtho.2018.08.637
119. Plesca I, Tunger A, Muller L, Wehner R, Lai X, Grimm M-L, et al. Characteristics of Tumor-Infiltrating Lymphocytes Prior to and During Immune Checkpoint Inhibitor Therapy. Front Immunol (2020) 11:364. doi: 10.3389/fimmu.2020.00364
120. Li X, Lian Z, Wang S, Xing L, Yu J. Interactions Between EGFRand Pd-1/Pd-L1 Pathway: Implications for Treatment of NSCLC. Cancer Lett (2018) 418:1–9. doi: 10.1016/j.canlet.2018.01.005
121. Lizotte PH, Hong RL, Luster TA, Cavanaugh ME, Taus LJ, Wang S, et al. A High-Throughput Immune-Oncology Screen Identifies EGFR Inhibitors as Potent Enhancers of Antigen-Specific Cytotoxic T-lymphocyte Tumor Cell Killing. Cancer Immunol Res (2018) 6:1511–23. doi: 10.1158/1538-7445.AM2018-4935
122. Hughes PE, Caenepeel S, Wu LC. Targeted Therapy and Checkpoint Immunotherapy Combinations for the Treatment of Cancer. Trends Immunol (2016) 37:462–76. doi: 10.1016/j.it.2016.04.010
123. Moya-Horno I, Viteri S, Karachaliou N, Rosell R. Combination of Immunotherapy With Targeted Therapies in Advanced non-Small Cell Lung Cancer (NSCLC). Ther Adv Med Oncol (2018) 10:1758834017745012. doi: 10.1177/1758834017745012
124. Jia Y, Zhao S, Jiang T, Li X, Zhao C, Liu Y, et al. Impact of EGFR-TKIs Combined With PD-L1 Antibody on the Lung Tissue of EGFR-Driven Tumor-Bearing Mice. Lung Cancer (2019) 137:85–93. doi: 10.1016/j.lungcan.2019.09.016
125. Gettinger S, Hellmann M, Chow L, Borghaei H, Antonia S, Brahmer J, et al. Nivolumab Plus Erlotinib in Patients With EGFR-Mutant Advanced NSCLC. J Thorac Oncol (2018) 13:1363–72. doi: 10.1016/j.jtho.2018.05.015
126. Rudin C, Cervanters A, Dowlati A, Besse B, Ma B, Costa D, et al. Abstract MA15.02 Long-Term Safety and Clinical Activity Results From a Phase IB Study of Erlotinib Plus Atezolizumab in Advanced NSCLC. J Thorac Oncol (2018) 13:MA15.02. doi: 10.1016/j.jtho.2018.08.440
127. Creelan BC, Yeh TC, Kim SW, Nogami N, Kim DW, Chow LQ, et al. A Phase 1 Study of Gefitinib Combined With Durvalumab in EGFR TKI-Naïve Patients With EGFR Mutation-Positive Locally Advanced/Metastatic non-Small-Cell Lung Cancer. Br J Cancer (2021) 124:383–90. doi: 10.1038/s41416-020-01099-7
128. Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med (2018) 378:2288–301. doi: 10.1056/NEJMoa1716948
129. Yang J, Gadgeel S, Sequist L, Wu C, Papadimitrakopoulou V, Su W, et al. Pembrolizumab in Combination With Erlotinib or Gefitinib as First-Line Therapy for Advanced NSCLC With Sensitizing EGFR Mutation. J Thorac Oncol (2019) 14:553–9. doi: 10.1016/j.jtho.2018.11.028
130. Thress KS, Jacobs V, Angell HK, Yang JC, Sequist LV, Blackhall F, et al. Modulation of Biomarker Expression by Osimertinib: Results of the Paired Tumor Biopsy Cohorts of the AURA Phase I Trial. J Thorsc Oncol (2017) 12:1588–94. doi: 10.1016/j.jtho.2017.07.011
131. Reck M, von Pawel J, Zatloukal P, Ramlau R, Gorbounova V, Hirsh V, et al. Overall Survival With Cisplatin-Gemcitabine and Bevacizumab or Placebo as First-Line Therapy for Nonsquamous Non-Small-Cell Lung Cancer: Results From a Randomized Phase III Trial (Avail). Ann Oncol (2010) 21:1804–9. doi: 10.1093/annonc/mdq020
132. Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and Development of Bevacizumab, an anti-VEGF Antibody for Treating Cancer. Nat Rev Drug Discovery (2004) 3:391–400. doi: 10.1038/nrd1381
133. Voron T, Colussi O, Marcheteau E, Pernot S, Nizard M, Pointet AL, et al. VEGF-a Modulates Expression of Inhibitory Checkpoints on CD8+ T Cells in Tumors. J Exp Med (2015) 212:139–48. doi: 10.1084/jem.20140559
134. Hedge PS, Wallin JJ, Mancao C. Predictive Markers of anti-VEGF and Emerging Role of Angiogenesis Inhibitors as Immunotherapeutics. Semin Cancer Biol (2018) 52:117–24. doi: 10.1016/j.semcancer.2017.12.002
135. Chen DS, Mellman I. Oncology Meets Immunology: The Cancer-Immunity Cycle. Immunity (2013) 39:1–10. doi: 10.1016/j.immuni.2013.07.012
136. Rotte A, Jin JY, Lemaire V. Mechanistic Overview of Immune Checkpoints to Support the Rational Design of Their Combinations in Cancer Immunotherapy. Ann Oncol (2018) 29:71–83. doi: 10.1093/annonc/mdx686
137. Rotte A. Combination of CTLA-4 and PD-1 Blockers for Treatment of Cancer. J Exp Clin Cancer Res (2019) 38:255. doi: 10.1186/s13046-019-1259-z
138. Antonia S, Goldberg SB, Balmanoukian A, Chaft JE, Sanborn RE, Gupta A, et al. Safety and Antitumor Activity of Durvalumab Plus Tremelimumab in Non-Small Cell Lung Cancer: A Multicentre, Phase 1B Study. Lancet Oncol (2016) 17:299–308. doi: 10.1016/S1470-2045(15)00544-6
139. Hellmann MD, Ciuleanu TE, Pluzanski A, Lee JS, Otterson GA, Audigier-Valette C, et al. Nivolumab Plus Ipilimumab in Lung Cancer With a High Tumor Mutational Burden. N Engl J Med (2018) 378:2093–104. doi: 10.1056/NEJMoa1801946
140. Ready N, Hellmann MD, Awad MM, Otterson GA, Gutierrez M, Gainor JF, et al. First-Line Nivolumab Plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer (CheckMate 568): Outcomes by Programmed Death Ligand 1 and Tumor Mutational Burden as Biomarkers. J Clin Oncol (2019) 37:992–1000. doi: 10.1200/JCO.18.01042
141. Mumm JB, Emmerich J, Zhang X, Chan I, Wu L, Mauze S, et al. Il-10 Elicits IFNgamma-dependent Tumor Immune Surveillance. Cancer Cell (2011) 20:781–96. doi: 10.1016/j.ccr.2011.11.003
142. Naing A, Infante JR, Papadopoulos KP, Chan IH, Shen C, Ratti NP, et al. Pegylated IL-10 (Pegilodecakin) Induces Systemic Immune Activation, CD8(+) T Cell Invigoration and Polyclonal T Cell Expansion in Cancer Patients. Cancer Cell (2018) 34:775–91. doi: 10.1016/j.ccell.2018.10.007
143. Oft M. Immune Regulation and Cytotoxic T Cell Activation of IL-10 Agonists – Preclinical and Clinical Experience. Semin Immunol (2019) 44:101325. doi: 10.1016/j.smim.2019.101325
144. Naing A, Wong DJ, Infante JR, Korn WM, Aljumaily R, Papadopoulos KP, et al. Pegilodecakin Combined With Pembrolizumab or Nivolumab for Patients With Advanced Solid Tumors (IVY): A Multicenter, Multicohort, Open-Label, Phase Ib Trial. Lancet Oncol (2019) 20:1544–55. doi: 10.1016/S1470-2045(19)30514-5
145. Peguero JA, Bajaj P, Carcereny E, Clay TD, Doger B, Felip E, et al. A Multicentre, Phase II Study of Soluble LAG-3 (Eftilagimod Alpha) in Combination With Pembrolizumab (TACTI-002) in Patients With Advanced non-Small Cell Lung Cancer (NSCLC) or Head and Neck Squamous Cell Carcinoma (HNSCC). J Clin Oncol (2019) 3715_suppl:TPS2667–TPS2667. doi: 10.1200/JCO.2019.37.15_suppl.TPS2667
146. You B, Yang YL, Xu Z, Dai Y, Liu S, Mao JH, et al. Inhibition of ERK1/2 Down-Regulates the Hippo/YAP Signalling Pathway in Human NSCLC Cells. Oncotarget (2015) 6:4357–68. doi: 10.18632/oncotarget.2974
147. Lee JW, Zhang Y, Eoh KJ, Sharma R, Sanmamed MF, Wu J, et al. The Combination of MEK Inhibitor With Immunomodulatory Antibodies Targeting Programmed Death 1 and Programmed Death Ligand 1 Results in Prolonged Survival in Kras/p53-driven Lung Cancer. J Thorac Oncol (2019) 14:1046–60. doi: 10.1016/j.jtho.2019.02.004
148. Galli GG, Carrara M, Yuan WC, Valdes-Quezada C, Gurung B, Pepe-Mooney B, et al. YAP Drives Growth by Controlling Transcriptional Pause Release From Dynamic Enhancers. Mol Cell (2015) 60:328–37. doi: 10.1016/j.molcel.2015.09.001
149. Zhang H, Pandey S, Travers M, Sun H, Morton G, Madzo J, et al. Targeting CDK9 Reactivates Epigeneitically Silenced Genes in Cancer. Cell (2018) 175:1244–58. doi: 10.1016/j.cell.2018.09.051
150. Tang Z, Ma Q, Wang L, Liu C, Gao H, Yang Z, et al. A Brief Review: Some Compounds Targeting YAP Againist Malignancies. Future Oncol (2019) 15:1535–43. doi: 10.2217/fon-2019-0035
151. Wang H, Franco F, Tsui YC, Xie X, Trefny MP, Zappasodi R, et al. CD36-Mediated Metabolic Adaptation Supports Regulatory T Cell Survival and Function in Tumors. Nat Immunol (2020) 21:298–308. doi: 10.1038/s41590-019-0589-5
152. Ghiringhelli F, Bruchard M, Chalmin F, Rebe C. Production of Adenosine by Ectonucleotides: A Key Factor in Tumor Immunoescape. J BioMed Biotech (2012) 2012:473712. doi: 10.1155/2012/473712
153. ClinicalTrials.gov. Study of TJ004309 in Combination With Atezolizumab (Tecentriq) in Patients With Advanced or Metastatic Cancer. Available at: https://clinicaltrials.gov/ct2/show/NCT03835949 (Accessed 15 Apr 2021).
154. Haen SP, Pereira PL, Salih HR, Rammensee HG, Gouttefangeas C. More Than Just Tumor Destruction: Immunomodulation by Thermal Ablation of Cancer. Clin Dev Immunol (2011) 2011:160250. doi: 10.1155/2011/160250
155. Shi L, Chen L, Wu C, Zhu Y, Xu B, Zheng X, et al. PD-1 Blockade Boosts Radiofrequency Ablation-Elicited Adaptive Immune Responses Against Tumor. Clin Cancer Res (2016) 22:1173–84. doi: 10.1158/1078-0432.CCR-15-1352
156. Golden EB, Frances D, Pellicciotta I, Demaria S, Barcellos-Hoff MH, Formenti SC. Radiation Fosters Dose-Dependent and Chemotherapy-Induced Immunogenic Cell Death. Oncoimmunology (2014) 3:28518. doi: 10.4161/onci.28518
157. Procureur A, Simonaggio A, Bibault JE, Oudard S, Vano YA. Enhance the Immune Checkpoint Inhibitors Efficacy With Radiotherapy Induced Immunogenic Cell Death: A Comprehensive Review and Latest Developments. Cancers (2021) 13:678. doi: 10.3390/cancers13040678
158. Yilmaz MT, Elmali A, Yazici G. Abscopal Effect, From Myth to Reality: From Radiation Oncologists’ Perspective. Cureus (2019) 11:e3860. doi: 10.7759/cureus.3860
159. Ashrafizadeh M, Farhood B, Musa AE, Taeb S, Rezaeyan A, Najafi M. Abscopal Effect in Radioimmunotherapy. Int Immunopharmacol (2020) 85:106663. doi: 10.1016/j.intimp.2020.106663
160. Shevtsov M, Sato H, Multhoff G, Shibata A. Novel Approaches to Improve the Efficacy of Immune-Radiotherapy. Front Oncol (2019) 9:156. doi: 10.3389/fonc.2019.00156
161. Sato H, Okonogi N, Nakano T. Rationale of Combination of Anti-PD-1/PD-L1 Antibody Therapy and Radiotherapy for Cancer Treatment. Int J Clin Oncol (2020) 25:801–9. doi: 10.1007/s10147-020-01666-1
162. Zhang N, Zeng YY, Du WW, Zhu JJ, Shen D, Liu Z, et al. The EGFR Pathway is Involved in the Regulation of PD-L1 Expression Via the IL-6/JAK/STAT3 Signaling Pathway in EGFR-mutated Non-Small Cell Lung Cancer. Int J Oncol (2016) 49:1360–8. doi: 10.3892/ijo.2016.3632
Keywords: targeted therapy, non-small cell lung cancer, immunotherapy, PD-1, PD-L1, EGFR mutation, tyrosine kinase inhibitor
Citation: To KKW, Fong W and Cho WCS (2021) Immunotherapy in Treating EGFR-Mutant Lung Cancer: Current Challenges and New Strategies. Front. Oncol. 11:635007. doi: 10.3389/fonc.2021.635007
Received: 29 November 2020; Accepted: 30 April 2021;
Published: 25 May 2021.
Edited by:
Yaxiong Zhang, Sun Yat-sen University Cancer Center (SYSUCC), ChinaCopyright © 2021 To, Fong and Cho. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kenneth K. W. To, kennethto@cuhk.edu.hk; William C. S. Cho, chocs@ha.org.hk
 Kenneth K. W. To
Kenneth K. W. To Winnie Fong1
Winnie Fong1 William C. S. Cho
William C. S. Cho