- 1Department of Radiation Oncology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, China
- 2Guangdong Key Laboratory of Nasopharyngeal Carcinoma Diagnosis and Therapy, Guangzhou, China
- 3Department of Otorhinolaryngology Head and Neck Surgery, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China
Objectives: To develop a model that can predict the risk of hypothyroidism (HT) after intensity-modulated radiotherapy (IMRT) for nasopharyngeal carcinoma (NPC), and to accordingly recommend dose constraints.
Materials and Methods: NPC patients treated between 2011 and 2015 were retrospectively reviewed. HT was defined by an abnormally high level of thyrotropin. The dosimetry parameters Vx (percentage of thyroid volume receiving more than x Gy of radiation) and Va,b (percentage of thyroid volume receiving >a Gy, while ≤b Gy radiation) were calculated. The primary endpoint was the development of HT within the first 2 years after IMRT. The least absolute shrinkage and selection operator and multivariate logistic regression were used to identify predictors of HT.
Results: A total of 545 patients were included in the analyses, with a median follow-up of 36 months. Of the 545 patients, 138 developed HT within 2 years, and the 2-year incidence of HT was 25.3%. In patients with thyroid volume >20 cm3, the 2-year incidence of HT was 11.7% (16/137); in patients with thyroid volume ≤20 cm3 and V30,60 ≤ 80%, the 2-year HT incidence was 19.9% (33/166); in patients with thyroid volume ≤20 cm3 and V30,60 > 80%, the 2-year incidence of HT was 36.8% (89/242).
Conclusion: Thyroid volume and V30,60 could be reliable predictors of HT after IMRT for NPC. For patients with thyroid volume ≤20 cm3, thyroid V30,60 ≤ 80% might be a useful dose constraint to adopt during IMRT planning.
Introduction
Radiation-induced hypothyroidism (HT) is frequent after radiotherapy (RT) of the head and neck region, with an incidence of 20–50% (1). Nasopharyngeal carcinoma (NPC) tends to metastasize through the lymphatic network to the neck (2); hence, definitive or prophylactic RT of the neck region can affect the thyroid gland and result in HT. The survival of NPC patients has improved significantly after the introduction of chemotherapy and intensity-modulated radiotherapy (IMRT) during the last two decades (3). As quality of life is an important concern in treatment management, the prediction and prevention of HT after RT is critical for NPC patients.
With IMRT, it is possible to achieve sufficient dose coverage of the tumor and improve tumor control. Additionally, IMRT is enabled with precise dose estimates for organs at risk (OARs), as a result of which it is possible to alleviate normal tissue damage by setting dose constraints on OARs (4). The thyroid gland is composed of functional units, namely, follicles, which produce and release thyroid hormones independently. Radiation above a specific threshold dose will damage these follicles, and dysfunction of too many follicles results in HT. Therefore, dosimetry parameter depicting both the threshold dose of follicle and the number of influenced follicles will be a promising predictor for HT after RT, and that is the parameter Vx, defined as the percentage of thyroid volume receiving more than a to-be-determined threshold dose, x Gy. However, so far, there is no consensus on such a threshold dose, and the reported threshold dose ranges from 30 to 50 Gy (5–11). Previous studies have generally selected the best Vx for predicting HT based on multivariate regression analysis (5–8). However, the reported results are limited by the collinearity of the data caused by their overlap: that is, V10 would also include V20. In order to minimize the influence of collinearity, we have proposed a prediction model based on novel dosimetry parameters which are generated by subtractions between Vx.
The aim of this study is to determine the dosimetry predictors for HT after RT in NPC patients using a new prediction model, and to recommend the dose constraints on the thyroid gland accordingly.
Materials and Methods
Patient Selection
We retrospectively reviewed patients with newly diagnosed, biopsy-proven NPC treated at our institution between June 2011 and December 2015. Patients were included if they were identified as euthyroid before RT and were assessed for thyroid function regularly after RT. The exclusion criteria were previous thyroid surgery, pre-existing pituitary disorders, previous irradiation to the head and neck region or the whole body, and absence of thyroid function assessment after RT. The Institutional Review Board and the Ethics Committee of Sun Yat-sen University Cancer Center approved of this study. As this was a retrospective analysis of routine data, the need for written informed consent from the patients was waived. The key raw data used in this article have been uploaded to the Research Data Deposit public platform1.
Treatment
All patients underwent definitive IMRT. The prescribed doses were 66–72 Gy at 2.00–2.43 Gy per fraction to the nasopharynx gross tumor volume (GTVnx), 64–70 Gy to the malignant lymph nodes (GTVnd), 60–63 Gy to the high-risk clinical target volume (CTV1), and 54–56 Gy to the low-risk clinical target volume (CTV2). The prescribed doses were delivered in 28–33 fractions at the rate of 5 fractions per week. Details of the protocol for target volume delineation and dose constraints on OARs have been reported previously in a randomized controlled trial conducted at our institution (12). There was no dose constraint on the thyroid gland when the IMRT was planned. According to the guidelines of our center, RT alone was recommended for patients with stage I disease, and RT plus platinum-based concurrent chemotherapy with or without induction chemotherapy or adjuvant chemotherapy was recommended for patients with stage II–IVA disease.
Thyroid Dosimetry Parameters and Function Test
Before treatment, a computed tomography (CT) simulation scan, including plain and enhanced CT scans, were taken (slice thickness = 3 mm); the imaging area extended from the top of the head to 2 cm below the sternoclavicular joint. Contouring of the thyroid gland on the CT simulation images was performed with the Monaco treatment planning system (version 5.0, Elekta AB, Stockholm, Sweden). Dose-volume histograms were retrieved from the planning system, including absolute thyroid volume and the percentage of thyroid volume receiving more than 10, 20, 30, 40, 50, and 60 Gy (V10, V20, V30, V40, V50, and V60) of radiation. The percentage of thyroid volume receiving 0–10 Gy (V0,10) was calculated as 100% minus V10; V10,20, as V10 minus V20; V20,30, as V20 minus V30; V30,40, as V30 minus V40; V40,50, as V40 minus V50; V50,60, as V50 minus V60.
After treatment was completed, the patients were followed up at least every 3 months during the first 2 years, every 6 months for at least the next 3 years, and annually thereafter. The thyroid function test was performed during the follow-up visit. HT was diagnosed if the thyrotropin (TSH) level was higher than the upper limit of our institutional reference range (0.27–4.20 μIU/mL) and the free thyroxine (fT4) level was not higher than the upper limit of our institutional reference range (12.00–22.00 pmol/L), regardless of symptoms.
Statistical Analysis
Time to HT was calculated from the end of RT to the first appearance of abnormally high levels of TSH, which is indicative of HT. Patients without HT were censored at the date of the last follow-up or death. Time to HT distribution was estimated using the Kaplan–Meier method, and curves were compared using the log-rank test. The primary endpoint of our study was the development of HT within the first 2 years after RT. The least absolute shrinkage and selection operator (LASSO) was used to select dosimetry parameters with the glmnet package in R (13). The parameters that were selected by LASSO were entered into the multivariate logistic regression model through the backward elimination method. The cutoff value of the dosimetry parameter in the final multivariate model was determined using X-tile (14). SPSS version 22.0 (IBM Corporation, Armonk, NY, United States) and R version 3.6.02 were used for statistical analyses. A two-tailed P-value of <0.05 was considered to indicate statistical significance.
Results
Characteristics of the Patient Cohort
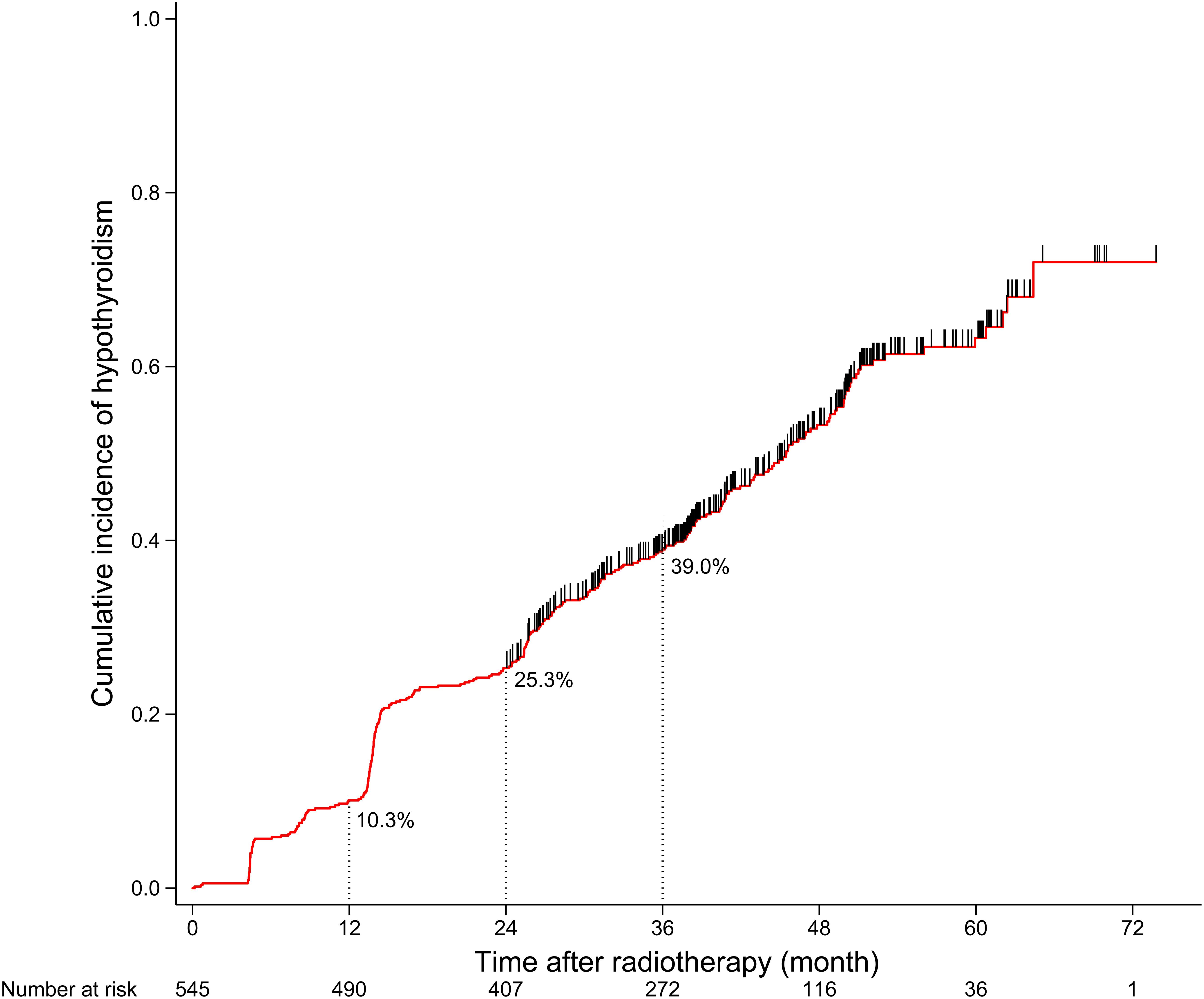
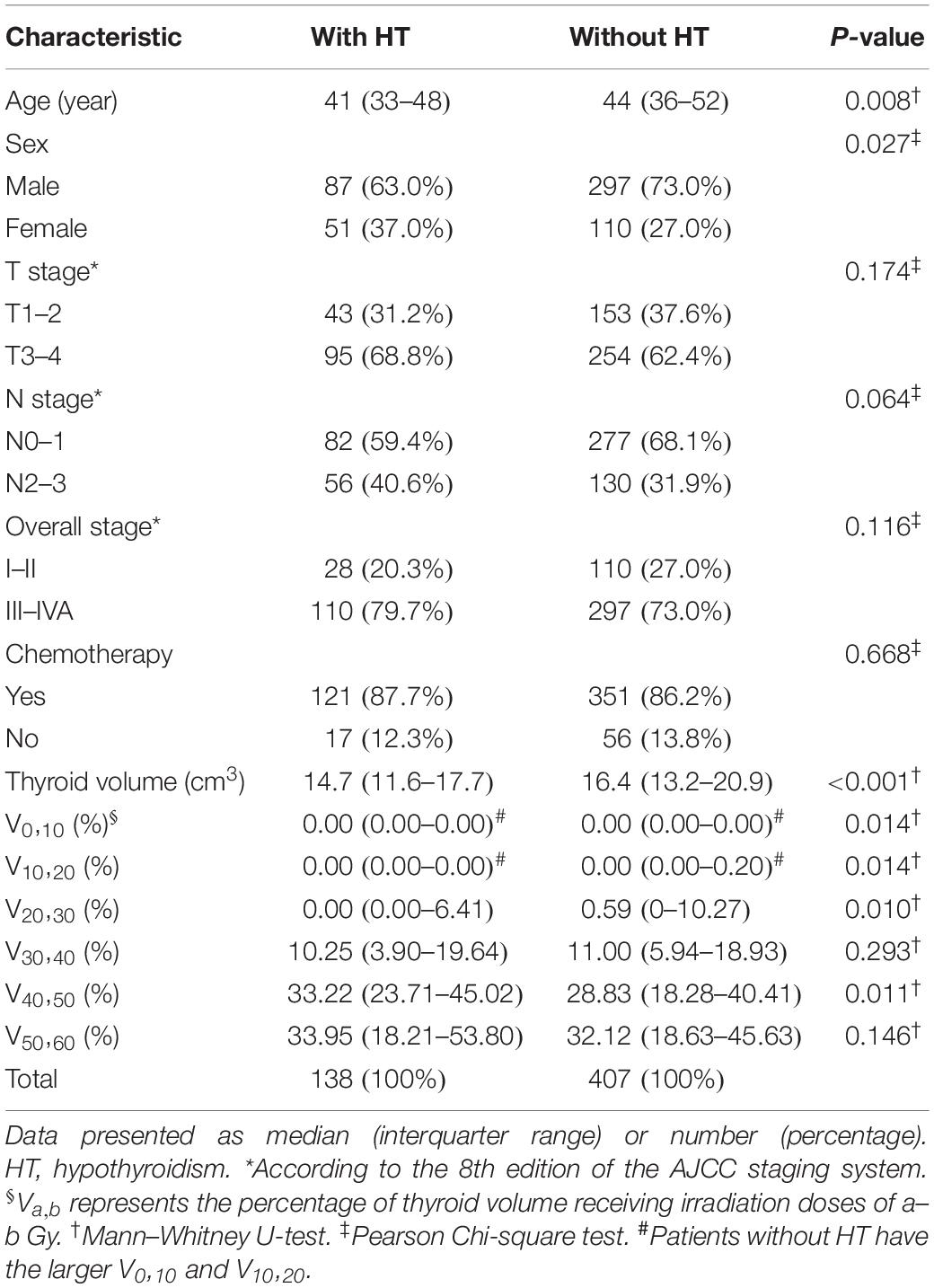
A total of 564 patients met the inclusion criteria, of which 19 patients who were censored within 2 years after RT were excluded from the next analysis. After a median follow-up duration of 36.0 months, 277 of the final cohort of 545 patients developed HT. The 1-, 2-, and 3-year cumulative incidence rates of HT were 10.3, 25.3, and 39.0%, respectively (Figure 1). For 277 patients who developed HT, the median time to HT was 24.2 months; and 138 patients developed HT within 2 years after RT. The clinical and dosimetry characteristics of patients with and without HT within 2 years after RT are summarized in Table 1. The data indicate that female and younger patients are more likely to develop HT within 2 years after RT. Compared to patients without HT, patients with HT had smaller thyroid volumes, higher V40,50 and lower V0,10, V10,20, and V20,30.
Selection of Dosimetry Parameters
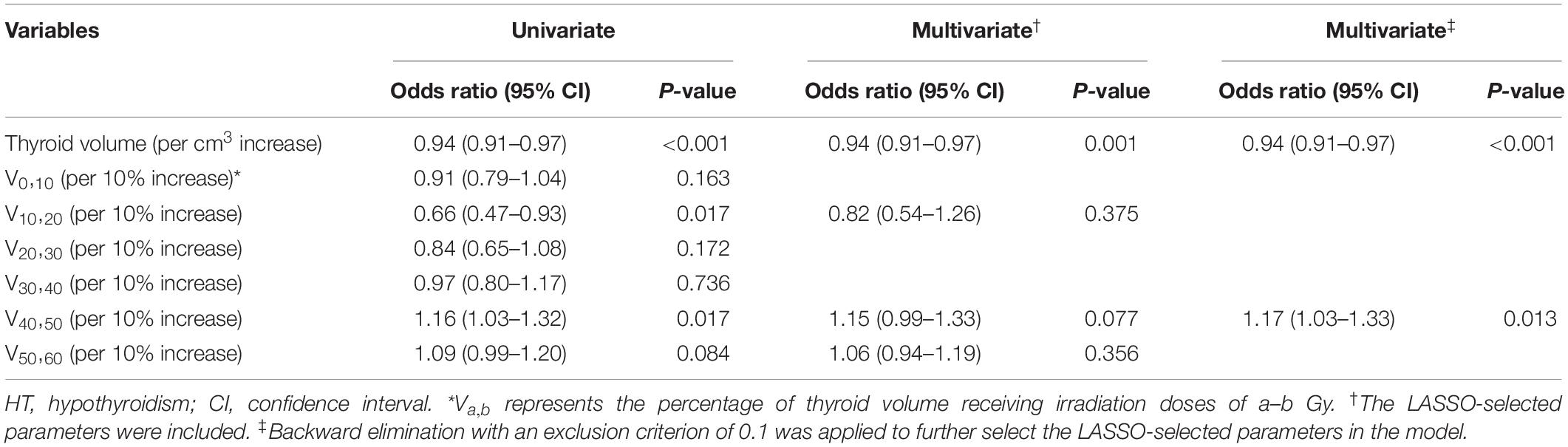
The results of unadjusted univariate logistic regression for dosimetry parameters associated with HT within 2 years after RT are shown in Table 2. Of these dosimetry parameters, thyroid volume, V10,20 and V40,50 were statistically significant predictors of HT within 2 years after RT. With the LASSO method, thyroid volume, V10,20, V40,50, and V50,60 were identified, based on logistic regression, as predictors of HT within 2 years after RT. These LASSO-selected parameters were entered into the multivariate logistic regression, with which only thyroid volume was found to be a significant predictor of HT (Table 2). The LASSO-selected parameters were further selected by backward elimination in the multivariate logistic regression model, and thyroid volume and V40,50 remained as significant predictors (Table 2).
Inferring the Threshold Dose for the Thyroid
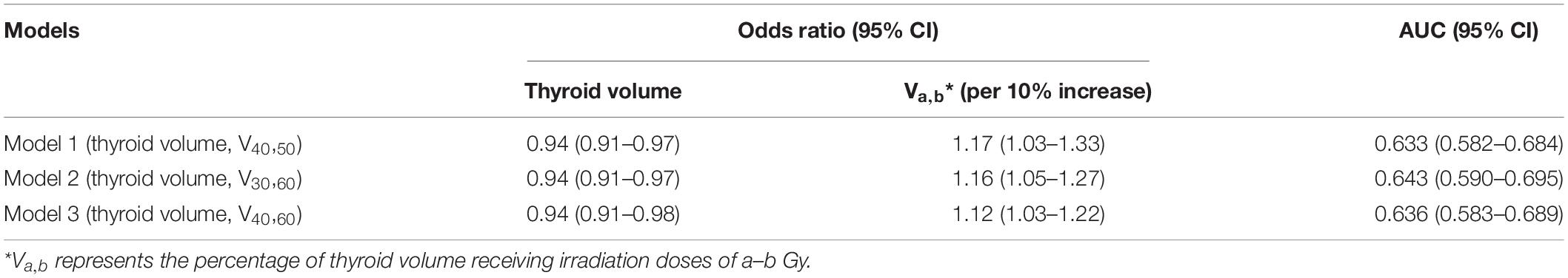
Although LASSO was adopted to reduce the effects of collinearity among dosimetry parameters, the correlation among the LASSO-selected parameters cannot be neglected (Figure 2). Obviously, the thyroid volume receiving higher doses of irradiation (V40,50, V50,60) would increase if the thyroid volume receiving lower doses (V0,10, V10,20, V20,30) decreased. From the univariate logistic regression analysis shown in Table 2, it was inferred that irradiation doses of 40–50 Gy and above would damage the thyroid gland. This means that V40,50 and V50,60 were positive predictors for HT. V50,60 might not have achieved statistical significance (P = 0.084) due to its correlation with the other predictors. Similarly, it was inferred that irradiation doses of 20–30 Gy and below would not have destructive effect on the thyroid gland. However, it was unclear whether doses of 30–40 Gy would damage the thyroid gland. Under the presumption that doses of 30–40 Gy would damage the thyroid gland, V10,20 and V50,60 (selected by LASSO) might have been excluded by backward elimination because V10,20 (negative predictor for HT) correlated positively with V30,40 (positive predictor for HT), and V50,60 (positive predictor for HT) correlated negatively with V30,40 (positive predictor for HT) (Figure 2). To further validate this presumption that the threshold dose that causes damage to the thyroid gland was 30 Gy rather than 40 Gy, we substituted V30,60 (percentage of thyroid volume receiving doses of 30–60 Gy) or V40,60 (percentage of thyroid volume receiving doses of 40–60 Gy) with V40,50 in the final model. Different models were evaluated for their prediction performance based on the area under the curve (AUC). The model that included thyroid volume and V30,60 had the largest AUC (Table 3).
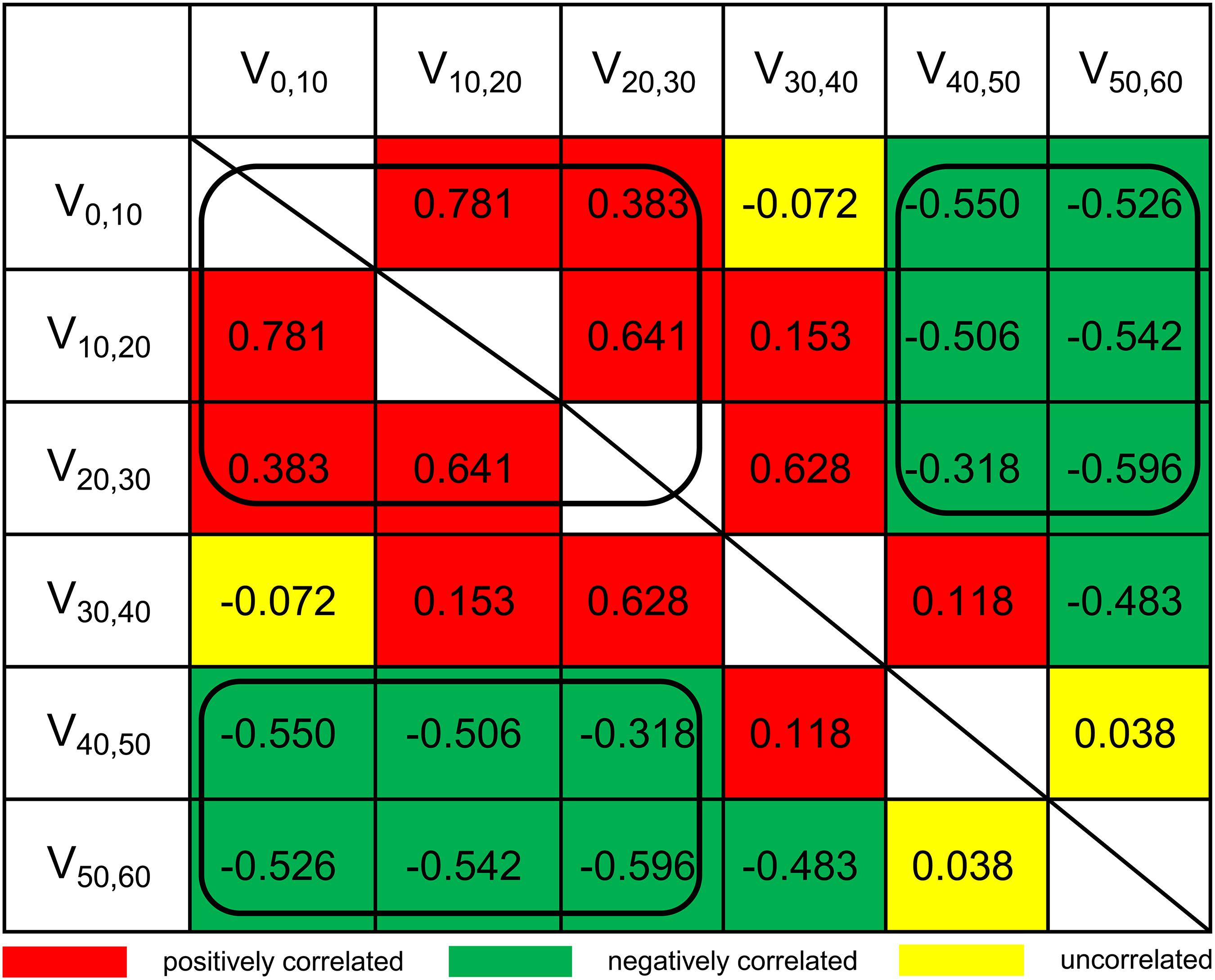
Figure 2. Pairwise Spearman correlation coefficients between dosimetry parameters. Va,b, the percentage of thyroid volume receiving irradiation doses of a–b Gy.
Prediction Model of HT
Based on the analyses above, a dose constraint on thyroid was set based on thyroid volume and V30,60. The dichotomous cutoff values determined by X-tile were 20 cm3 for thyroid volume and 80% for V30,60. Patients were grouped into four cohorts, and the incidence rates of HT within 2 years after RT were calculated for each cohort. In cohort 1 comprising patients with thyroid volume >20 cm3 and V30,60 ≤ 80%, the HT incidence rate was 8.0% (4/50). In cohort 2 comprising patients with thyroid volume >20 cm3 and V30,60 > 80%, the HT incidence was 13.8% (12/87). In cohort 3 comprising patients with thyroid volume ≤20 cm3 and V30,60 ≤ 80%, the HT incidence was 19.9% (33/166). Finally, in cohort 4 comprising patients with thyroid volume ≤20 cm3 and V30,60 > 80%, the HT incidence rate was 36.8% (89/242). Cohort 1 and 2 were combined because of the similar incidence rates of HT within 2 years after RT. The cumulative incidence curves of HT in different cohorts during the entire follow-up period are shown in Figure 3.
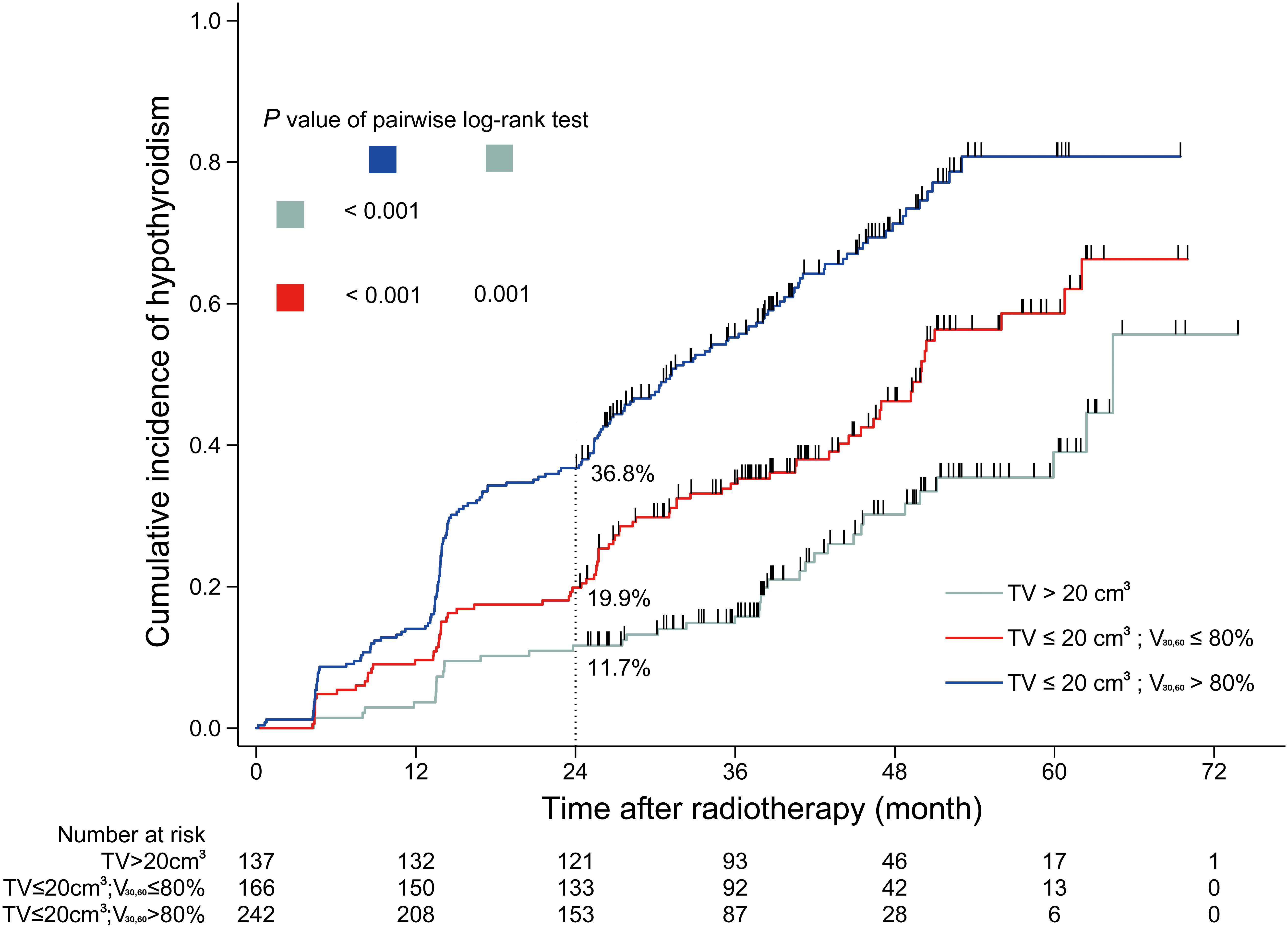
Figure 3. Cumulative incidence curve of hypothyroidism for sub-cohorts. TV, thyroid volume; V30,60, the percentage of thyroid volume receiving irradiation doses of 30–60 Gy.
Discussion
In the current study, we have investigated the incidence of radiation-induced HT in a large cohort of NPC patients who underwent IMRT, with the aim of determining the dosimetry parameters that could be used as predictors of HT. The 2-year incidence of HT in the present study cohort was 25.3%. The HT defined in this study was actually biochemical HT or subclinical HT, which was diagnosed based on the thyroid function test, regardless of symptoms (15). Although only 2.4–4.3% of patients with subclinical HT progress to overt HT each year (16), subclinical HT is associated with an increased risk of cardiovascular and cerebrovascular morbidity and mortality in younger patients (under the age of 65 years) and in those with high TSH levels (17, 18). For high-risk patients with subclinical HT, the guidelines recommend treatment with levothyroxine (19). Thus, it is of clinical importance to identify the predictors of radiation-induced HT and to reduce its incidence in NPC patients receiving RT.
Although the parameter Vx can depict the dose distribution of thyroid well, there is repeated information among different Vx. For example, V10 consists of V20 and V10,20, which would result in the problem of collinearity during statistical analysis. In the current study, we were able to reduce collinearity among dosimetry parameters to some extent by new dosimetry parameter Va,b (the percentage of thyroid volume receiving a–b Gy) which was generated from subtractions between Vx. Based on our analysis, we propose 30 Gy to be the threshold dose, and dose above 30 Gy is likely to cause damage to the thyroid follicles and lead to deficiency in thyroid function.
Apart from V30,60, the absolute thyroid volume was also identified as an independent predictor of HT in our analysis. This is probably because the absolute thyroid volume represents the functional reserve of thyroid. The thyroid volume data of this cohort follows normal distribution (Supplementary Figure 1), with a mean value similar to a Korea normal cohort (20). Considering above, thyroid volume data in this study should not be biased. However, the thyroid volume is influenced by sex, age, and body size, and may differ across different populations (20, 21). When generalizing our model to a different population, the cut-off value of thyroid volume should be reconsidered. The thyroid damage model proposed by our study is simple as it only considers the direct radiation-induced damage to independent thyroid functional units. However, radiation-induced damage to thyroid vessels and immune-mediated damage may also influence the thyroid (22). We are aware that a prediction model generated from clinical data may not be able to explain all the biological processes perfectly. It is its clinical utility that makes it a valuable prediction model, as patients at different levels of risk of HT can be well differentiated based on thyroid volume and V30,60.
We determined the cut-off value of thyroid volume and V30,60 using X-tile method, which is based on choosing the lowest P-value, which might exaggerate the performance of our model. Thus, the performance of our model should be further validated in another cohort.
This study is limited by a potential selection bias, as only patients who underwent regularly thyroid function tests were included: that is, patients who had a better prognosis and stayed alive for a longer time were more likely to be included. To rule out the possibility of severe selection bias, we compared the characteristics of NPC patients treated during the same period in our institution and characteristics of patients included in our study (Supplementary Table 1). We found that cohort of our study had younger age than the reference cohort. Considering above, age should be taken into consideration when generalizing our prediction model. Finally, in order to overcome the follow-up bias that patients with longer follow-up had more chances of developing HT, the primary endpoint was defined as the development of HT within the first 2 years after RT, and patients who were censored within 2 years were excluded from analysis.
Conclusion
In conclusion, the results of this study indicate that the combination of thyroid volume and V30,60 could be useful as a predictor of the risk of HT within 2 years after RT in NPC patients who undergo IMRT. In patients with a greater thyroid volume (>20 cm3), the radiation dose distribution did not seem to have a considerable influence on the thyroid gland (the 2-year incidence of HT was as low as about 10%). However, for patients with smaller thyroid volume (≤20 cm3), a dose constraint of V30,60 on ≤80% of the thyroid volume should be applied without compromising tumor coverage. Besides, further validation studies using an independent cohort are warranted to compare the performance of our model and other similar models.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: www.researchdata. org.cn (approval number RDDA2019001022).
Ethics Statement
The studies involving human participants were reviewed and approved by the Institutional Review Board and the Ethics Committee of Sun Yat-sen University Cancer Center. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
L-LT, LP, and C-LH: conceptualization. LP, Y-PM, and C-LH: methodology, investigation, and data curation. LP and C-LH: software and formal analysis. LP, C-LH, and RG: validation. L-LT and JM: resources. LP, C-LH, RG, and Y-PM: writing – original draft preparation. W-PW, Y-PM, JM, and L-LT: writing – review and editing. L-LT: supervision, project administration, and funding acquisition. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by grants from the Planned Science and Technology Project of Guangdong Province (2019B020230002), Natural Science Foundation of Guangdong Province (2017A030312003), Health & Medical Collaborative Innovation Project of Guangzhou City, China (201803040003), Innovation Team Development Plan of the Ministry of Education (No. IRT_17R110), and Overseas Expertise Introduction Project for Discipline Innovation (111 Project, B14035).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Dr. Fo-ping Chen from Sun Yat-sen University Cancer Center for supporting the dosimetry data extraction.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.551255/full#supplementary-material
Supplementary Figure 1 | The distribution of thyroid volume of the cohort.
Footnotes
- ^ www.researchdata.org.cn, approval number RDDA2019001022
- ^ http://www.rproject.org/
References
1. Boomsma MJ, Bijl HP, Langendijk JA. Radiation-induced hypothyroidism in head and neck cancer patients: a systematic review. Radiother Oncol. (2011) 99:1–5. doi: 10.1016/j.radonc.2011.03.002
2. Lin L, Lu Y, Wang XJ, Chen H, Yu S, Tian J, et al. Delineation of neck clinical target volume specific to nasopharyngeal carcinoma based on lymph node distribution and the international consensus guidelines. Int J Radiat Oncol Biol Phys. (2018) 100:891–902. doi: 10.1016/j.ijrobp.2017.11.004
3. Au KH, Ngan RKC, Ng AWY, Poon DMC, Ng WT, Yuen KT, et al. Treatment outcomes of nasopharyngeal carcinoma in modern era after intensity modulated radiotherapy (IMRT) in Hong Kong: a report of 3328 patients (HKNPCSG 1301 study). Oral Oncol. (2018) 77:16–21. doi: 10.1016/j.oraloncology.2017.12.004
4. Chen L, Zhang Y, Lai SZ, Li WF, Hu WH, Sun R, et al. 10-year results of therapeutic ratio by intensity-modulated radiotherapy versus two-dimensional radiotherapy in patients with nasopharyngeal carcinoma. Oncologist. (2019) 24:e38–45. doi: 10.1634/theoncologist.2017-0577
5. Sommat K, Ong WS, Hussain A, Soong YL, Tan T, Wee J, et al. Thyroid V40 predicts primary hypothyroidism after intensity modulated radiation therapy for nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. (2017) 98:574–80. doi: 10.1016/j.ijrobp.2017.03.007
6. Luo R, Li M, Yang Z, Zhan Y, Huang B, Lu J, et al. Nomogram for radiation-induced hypothyroidism prediction in nasopharyngeal carcinoma after treatment. Br J Radiol. (2017) 90:20160686. doi: 10.1259/bjr.20160686
7. Zhai RP, Kong FF, Du CR, Hu CS, Ying HM. Radiation-induced hypothyroidism after IMRT for nasopharyngeal carcinoma: clinical and dosimetric predictors in a prospective cohort study. Oral Oncol. (2017) 68:44–9. doi: 10.1016/j.oraloncology.2017.03.005
8. Luo R, Wu VWC, He B, Gao X, Xu Z, Wang D, et al. Development of a normal tissue complication probability (NTCP) model for radiation-induced hypothyroidism in nasopharyngeal carcinoma patients. BMC Cancer. (2018) 18:575. doi: 10.1186/s12885-018-4348-z
9. Xu Y, Shao Z, Tang T, Liu G, Yao Y, Wang J, et al. A dosimetric study on radiation-induced hypothyroidism following intensity-modulated radiotherapy in patients with nasopharyngeal carcinoma. Oncol Lett. (2018) 16:6126–32. doi: 10.3892/ol.2018.9332
10. Kim MY, Yu T, Wu HG. Dose-volumetric parameters for predicting hypothyroidism after radiotherapy for head and neck cancer. Jpn J Clin Oncol. (2014) 44:331–7. doi: 10.1093/jjco/hyt235
11. Akgun Z, Atasoy BM, Ozen Z, Yavuz D, Gulluoglu B, Sengoz M, et al. V30 as a predictor for radiation-induced hypothyroidism: a dosimetric analysis in patients who received radiotherapy to the neck. Radiat Oncol. (2014) 9:104. doi: 10.1186/1748-717x-9-104
12. Sun Y, Li WF, Chen NY, Zhang N, Hu GQ, Xie FY, et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol. (2016) 17:1509–20. doi: 10.1016/s1470-2045(16)30410-7
13. Engebretsen S, Bohlin J. Statistical predictions with glmnet. Clin Epigenet. (2019) 11:123. doi: 10.1186/s13148-019-0730-1
14. Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. (2004) 10:7252–9. doi: 10.1158/1078-0432.ccr-04-0713
15. Ronjom MF, Brink C, Bentzen SM, Hegedus L, Overgaard J, Johansen J. Hypothyroidism after primary radiotherapy for head and neck squamous cell carcinoma: normal tissue complication probability modeling with latent time correction. Radiother Oncol. (2013) 109:317–22. doi: 10.1016/j.radonc.2013.06.029
16. Vanderpump MP, Tunbridge WM, French JM, Appleton D, Bates D, Clark F, et al. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham Survey. Clin Endocrinol. (1995) 43:55–68. doi: 10.1111/j.1365-2265.1995.tb01894.x
17. Razvi S, Shakoor A, Vanderpump M, Weaver JU, Pearce SH. The influence of age on the relationship between subclinical hypothyroidism and ischemic heart disease: a metaanalysis. J Clin Endocrinol Metab. (2008) 93:2998–3007. doi: 10.1210/jc.2008-0167
18. Chaker L, Baumgartner C, den Elzen WP, Ikram MA, Blum MR, Collet TH, et al. Subclinical hypothyroidism and the risk of stroke events and fatal stroke: an individual participant data analysis. J Clin Endocrinol Metab. (2015) 100:2181–91. doi: 10.1210/jc.2015-1438
19. Garber JR, Cobin RH, Gharib H, Hennessey JV, Klein I, Mechanick JI, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid. (2012) 22:1200–35. doi: 10.1089/thy.2012.0205
20. Lee DH, Cho KJ, Sun DI, Hwang SJ, Kim DK, Kim MS, et al. Thyroid dimensions of Korean adults on routine neck computed tomography and its relationship to age, sex, and body size. Surg Radiol Anat. (2006) 28:25–32. doi: 10.1007/s00276-005-0042-3
21. Veres C, Garsi JP, Rubino C, Pouzoulet F, Bidault F, Chavaudra J, et al. Thyroid volume measurement in external beam radiotherapy patients using CT imaging: correlation with clinical and anthropometric characteristics. Phys Med Biol. (2010) 55:N507–19. doi: 10.1088/0031-9155/55/21/n02
Keywords: nasopharyngeal carcinoma, hypothyroidism, intensity-modulated radiotherapy, dosimetry parameters, predicting model
Citation: Peng L, Mao Y-P, Huang C-L, Guo R, Ma J, Wen W-P and Tang L-L (2020) A New Model for Predicting Hypothyroidism After Intensity-Modulated Radiotherapy for Nasopharyngeal Carcinoma. Front. Oncol. 10:551255. doi: 10.3389/fonc.2020.551255
Received: 12 April 2020; Accepted: 02 September 2020;
Published: 25 September 2020.
Edited by:
Lidia Strigari, Regina Elena National Cancer Institute (IRCCS), ItalyReviewed by:
Edgar K. Selzer, Medical University of Vienna, AustriaRaffaele Liuzzi, Institute of Biostructure and Bioimaging (IBB-CNR), Italy
Copyright © 2020 Peng, Mao, Huang, Guo, Ma, Wen and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ling-Long Tang, tangll@sysucc.org.cn
†These authors have contributed equally to this work and share first authorship
 Liang Peng
Liang Peng Yan-Ping Mao
Yan-Ping Mao Cheng-Long Huang1,2†
Cheng-Long Huang1,2† Rui Guo
Rui Guo Jun Ma
Jun Ma Wei-Ping Wen
Wei-Ping Wen Ling-Long Tang
Ling-Long Tang