- 1Department of Radiation Oncology, State Key Laboratory of Respiratory Disease, Affiliated Cancer Hospital & Institute of Guangzhou Medical University, Guangzhou Medical University, Guangzhou, China
- 2Department of Oncology, First affiliated Hospital of Anhui Medical University, Hefei, China
- 3Department of Biochemistry, University of Sialkot, Sialkot, Pakistan
- 4Department of Radiation Oncology, Sun Yat-sen University Cancer Center, Guangzhou, China
Randomized controlled trials have failed to report any survival advantage for WBRT combined with SRS in the management of brain metastases, despite the enhanced local and distant control in comparison to each treatment alone. Literature review have revealed important role of primary histology of the tumor when dealing with brain metastases. NSCLC responds better to combined approach even when there was only single brain metastasis present while breast cancer has registered better survival with SRS alone probably due to better response of primary tumor to advancement in surgical and chemotherapeutic agents. Furthermore, mutation status (EGFR/ALK) in lung cancer and receptor status (ER/PR/HER2) in breast cancer also exhibit diversity in their response to radiotherapy. Radioresistant tumors like renal cell carcinoma and melanoma brain metastases have achieved better results when treated with SRS alone. Secondly, single brain metastasis may benefit from local and distant brain control achieved with combined treatment. These diverse outcomes suggest a primary histology-based analysis of the radiotherapy regimens (WBRT, SRS, or their combination) would more ideally establish the role of radiotherapy in the management of brain metastases. Molecularly targeted therapeutic and immunotherapeutic agents have revealed synergism with radiation therapy particularly SRS in treating cancer patients with brain metastases. Clinical updates in this regard have also been reviewed.
Rationale
Brain metastasis is associated with worst prognosis and considered a major cause of cancer morbidity and mortality (1). Radiation therapy has long been the mainstay of treatment for brain metastases and is still contributing to this group of patients (2). A number of randomized controlled trials were conducted comparing different types of radiation therapies. Whole brain radiotherapy alone or in combination with stereotactic radiosurgery or stereotactic radiosurgery alone were the main components of investigation in these trials. These modes of treatments were compared for their safety and efficacy in terms of control and overall survival (Table 1) (3–8). Combined approach has revealed better brain control but no survival advantage (56). However, a survival advantage was demonstrated for combined approach when secondary analyses were restricted to patients with favorable prognosis (13, 14). Interesting point to note here is, these analyses were restricted to NSCLC primary histology (13, 14). Another aspect is if this benefit in control achieved with combined treatment could also lead to survival advantage in patients with solitary brain metastases. In fact, surge in survival with combined approach was reported when single brain metastases were considered only (4). These observations may make one think that the better local and distant control associated with combined approach might lead to survival advantage if a more dynamic selection of patient is exercised. Here, we report some of the related evidence highlighting these two points: a possible miscalculation in designing randomized controlled trial for this group of patients. The scope of this paper is restricted to the three radiotherapeutic treatment regimens (WBRT alone, SRS alone and WBRT plus SRS) in the treatment of brain metastases. In addition, relevant advancements in the field of targeted therapy and immunotherapy alone or in combination with radiotherapy have also been reviewed.
Primary Cancer Histology
Brain metastases originate from various primary cancers with different frequency and propensity. Lung cancer (40–50%) being the most frequent followed by breast (15–25%), or melanoma (5–20%), and to a smaller extent from renal cell carcinoma, colorectal cancer and sarcoma (Figure 1) (57). It has been suggested that primary cancer histology may play an important role in determining the survival due to its response to different treatments (radiation or chemotherapeutic), propensity to metastasize and aggressiveness (11).
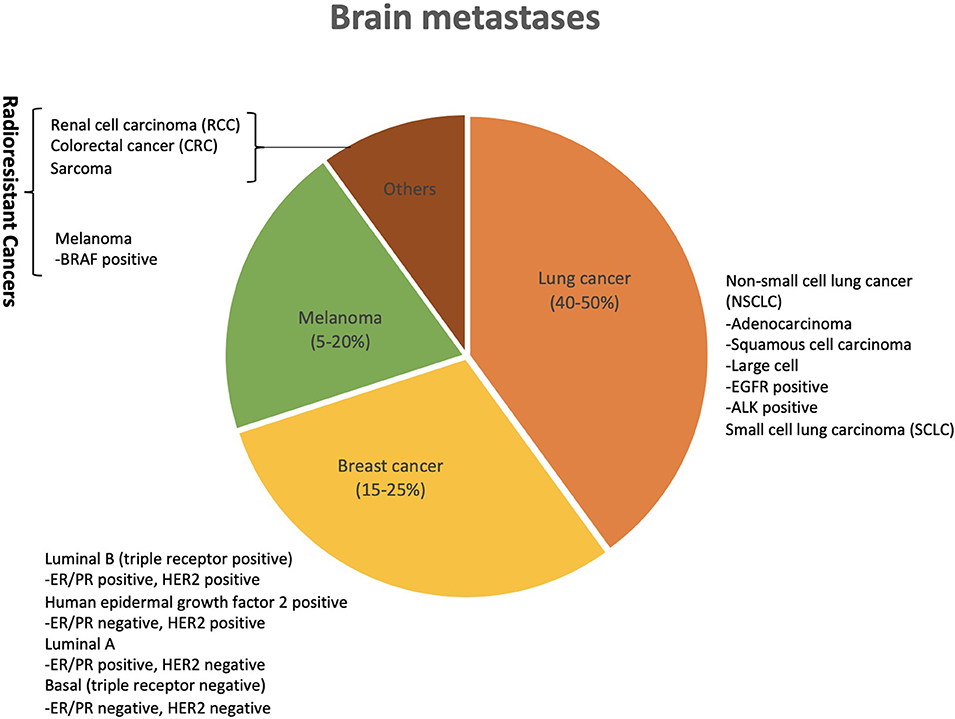
Figure 1. Primary cancer sites with corresponding frequencies of causing brain metastases. Lung cancer is the most frequent to cause brain metastases followed by breast, melanoma and renal cell carcinoma. Histology subtypes and mutation status (EGFR/ALK) in lung cancer, and receptor status (+/–) in breast cancer have also shown relevance when it comes to their response in terms of brain control and survival to radiation therapy in the form of WBRT (whole brain radiotherapy), SRS (stereotactic radiosurgery) and combination of both (WBRT plus SRS).
Lung Cancer
Primary site and histology subtypes have been regarded essential in deriving survival advantage from radiation therapy in these patients (11, 12). Lung cancer histology is broadly divided into two main types; non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC). NSCLC constitute 80–85% of overall lung cancer cases. Further subclasses of NSCLC include adenocarcinoma (50%), squamous cell carcinomas (30%) and large cell carcinomas (58, 59). So far, the trials as well as several other retrospective studies had pooled together patients with brain metastases originating from different primary sites and histology including the ones resistant to radiation therapy such as malignant renal cell carcinoma and melanoma (3–12). Lung cancer has been the most frequent histology in these trials (3–8). Andrew et al.'s RCT of 331 brain metastatic patients revealed superior survival in patients with squamous NSCLC for the treatment difference favoring WBRT plus SRS (p = 0.0508). This result was not reciprocated for adenocarcinoma subtype (4). Secondary analysis of this trial which included 252 patients evaluated by GPA (graded prognostic assessment) also revealed survival benefit in patients (primarily lung cancer 211/252) with favorable prognosis (DS-GPA 3.5–4.0) assessed by diagnosis specific graded post-stratification assessment (median survival time [MST] for WBRT + SRS vs. WBRT alone was 21.0 vs. 10.3 months, P = 0.05) (13). Secondary analysis of the JROSG 99-1 Randomized Clinical Trial, which compare WBRT plus SRS to SRS alone in patients with NSCLC and prognostic score of DS-GPA 2.5–4.0 (n = 47) demonstrated better survival with combined treatment (median survival time [MST] for WBRT + SRS vs. SRS alone was 16.7 vs. 10.6 months, P = 0.04) (14). Brain tumor recurrence rates were different between the two groups, suggesting additive WBRT had a more significant impact in the DS-GPA 2.5–4.0 group (HR, 8.31; 95% CI, 3.05–29.13) (P < 0.001) vs. the DS-GPA 0.5–2.0 group (HR, 3.57; 95% CI, 1.02–16.49) (P = 0.04). A similar secondary analysis of another trial, the NCCTG N0574 (Alliance) Randomized Controlled Trial, however, didn't reveal such improvement in a similar categorized group (DS-GPA 2.5–4.0) of recipients (n = 29) (15). It is noteworthy that of the 29 patients in this prognostic group of DS-GPA 2.5–4.0, 26 had achieved a score of DS-GPA 2.5–3.0, comparatively fewer than Aoyama et al. secondary analysis which included 37 patients (14, 15). The DS-GPA group (3.5–4.0) only had 3 patients and that too in SRS group only whereas Aoyama et al. contained 10 patients (SRS; 3 vs. WBRT+SRS; 7) (14, 15). Overall low number of participants and also the lack of patients achieving higher prognostic score in WBRT + SRS group make the Churilla et al. analysis less effectual (15). It's unfortunate that not much is available for analysis in this regard from the primary RCTs (3–8).
Apart from these primary trials, there are a number of retrospective studies in which combined treatment have resulted in better survival for patients with lung cancer brain metastases in comparison to WBRT alone (16–19, 22, 24). Li et al. showed significant improvement in survival for combined treatment in comparison to WBRT alone but not SRS alone for patients with single NSCLC brain metastasis (16). In a large retrospective study of patients with newly diagnosed brain metastases (n = 4,259) from various primary tumors reported significant survival benefit for SRS alone (HR: 0.62; 0.51–0.75, p < 0.0001) and WBRT plus SRS (HR: 0.53; 0.45–0.63, p < 0.0001) against WBRT alone for patients with brain metastases from NSCLC (n = 1,888) (17). Lin et al. concluded that GK radiosurgery combined with WBRT increased the survival of NSCLC patients with brain metastases (18). Minniti et al. also reported WBRT plus SRS to be a safe, minimally invasive and well-tolerated treatment for patients with up to three brain metastases from NSCLC resulting in longer survival and better disease control in comparison with WBRT alone (19). Marko et al. revealed numerically better survival (Kaplan–Meier survival means for the SRS only, WBRT only, WBRT plus SRS groups were 14.3, 14.8, and 19.1 months, respectively), however, statistically not significant (P = 0.143–0.159) (20). In multivariate analysis of a study of 100 patients and 184 brain metastases from NSCLC evaluating GKRS and prognostic factors for overall survival, adenocarcinoma histology revealed to be prognostic for survival. Addition of WBRT had no impact on survival in this study (21). Overall, SRS boost to WBRT reveals general superiority over WBRT alone but not SRS alone. In fact, omission of WBRT had no impact on survival (HR: 1.06; 0.90–1.26, p = 0.8084) for NSCLC patients with brain metastases in a comparative trial of WBRT plus optimum standard care (dexamethasone) compared to optimum standard care alone (60).
Small cell lung carcinoma constitutes a rather small group of lung cancer patients (15–20%) (58). Andrews et al. demonstrated survival benefit for patients with WBRT plus SRS in comparison to WBRT alone when analyses were restricted to 24 SCLC patients (n = 24) with 1 to 3 brain metastases (p = 0.039) (4). Sun et al. also revealed that WBRT plus a radiation boost (Cyberknife) was significantly associated with improved OS in patients with 1–3 SCLC brain metastases when compared to WBRT-alone (13.4 vs. 9.6 months, p = 0.022) (22). The 6-, 12-, and 24 month survival rates were also comparatively higher for combined treatment (84.5%, 62.7%, and 21.5 vs. 59.8%, 29.9%, 9.6%, p = 0.004). Similar survival rates (84.5, 62.7, and 21.5%) were repeated in study of 36 patients with SCLC brain metastases who had received WBRT boost (23). In comparison to SRS alone, WBRT with SRS boost had also shown survival efficacy as revealed in a study of 44 SCLC patients (WBRT + SRS vs. SRS; 14 vs. 6 m, p = 0.04). However, number of patients in combined group were comparatively smaller (n = 6) while patients in the SRS alone group had also received prior WBRT, PCI (prophylactic irradiation), or both (24). Sperduto et al. (17) retrospective analysis also contained a total of 299 patients with brain metastases from SCLC. A significant surge in survival (HR: 0.29; 0.13–0.66, p = 0.003) was derived with SRS boost to WBRT (n = 21) in contrast to WBRT alone (n = 247) (17). Elaimy et al. retrospective study disclosed patients with different primary histology responded differently to treatment. In univariate analysis, NSCLC was favored statistically in terms of survival than SCLC, and classified in the other primary histology group. Moreover, NSCLC patients derived significant survival benefit when compared to the combined melanoma and renal-cell carcinoma group on multivariate analysis (12). Overall, Lung cancer brain metastases seems to respond better to combined treatment and maybe recommended in patients with better prognostic standings.
There is considerable amount of progress in targeted therapy aimed at the mutation carrying by NSCLC patients such as EGFR (epidermal growth factor receptor) and ALK. Gefitinib and erlotinib (first generation EGFR targeting agents) have shown greater efficacy in the brain (61, 62). Studies that included randomized controlled trials, prospective trials and retrospective studies have reported conflicting results in terms of OS outcome for addition of radiation therapy to EGFR-TKIs in these patients (18, 61–77). However, Meta-analyses comprising most of these studies have suggested a significantly better survival associated with receiving additional radiation therapy (78–80). WBRT addition to EGFR-TKIs (gefitinib, erlotinib, and icotinib) have significantly increased survival for brain metastatic NSCLC patients with unknown EGFR status or without molecular selection (61, 64, 65, 71–73). Evidence included a randomized controlled trial revealing a median survival of 13.3 vs. 12.7 months (P < 0.05) (64). This advantage in OS has also been demonstrated in EGFR-mutated NSCLC patients (74–77). SRS plus EGFR-TKIs were reported to be equally effective in treating EGFR-mutated NSCLC patients against EGFR-TKIs alone in a retrospective study (70). However, Magnuson et al. study identified significant improvement for such patients either receiving upfront WBRT or SRS as compared to upfront EGFR-TKIs alone (76). Despite the fact that low prognostic patients were allocated to WBRT group, upfront WBRT followed by EGFR-TKIs produced better survival outcome (HR: 0.70; 0.50–0.98, p = 0.039) in comparison to upfront EGFR-TKIs. In the same study, SRS followed by EGFR-TKIs also derived better survival benefit as opposed to upfront EGFR-TKIs (HR: 0.39; 0.26–0.58, p = 0.001) (76). An RCT by Sperduto et al. revealed WBRT plus SRS in combination showed slight superior median survival, however not significant, for molecularly unselected NSCLC patients as opposed to addition of EGFR-TKIs to WBRT and SRS (13.4 vs. 6.1, p = NS) (81). Nonetheless, a retrospective study demonstrated efficacy of adding gefitinib to WBRT or WBRT plus SRS (18). In fact, efficacy was greater when WBRT plus SRS were applied. Addition of WBRT or WBRT + SRS to gefitinib reported significant improvement in survival for brain metastatic NSCLC patients opposite to WBRT alone (p < 0.0001). Furthermore, results of WBRT + SRS + gefitinib were superior to WBRT + SRS as well as WBRT + gefitinib (p < 0.001) (18). ALK inhibitors, particularly the second-generation agents alectinib and brigatinib, have also shown promising responses in the treatment of brain metastases (82). However, there is no study done for comparison of the combination with radiation therapy to ALK inhibitors alone. A prior RT delivery was shown to have a positive impact on brain efficacy of these agents. Immunotherapy agents like nivolumab and pembrolizumab are also being used rigorously making the treatment paradigm diverse (83). Furthermore, combination of these agents with WBRT plus SRS or WBRT alone or SRS alone would clearly establish the role of each radiation therapy for this group of patients.
Breast Cancer
Like NSCLC histology, breast cancer as primary site for brain metastases has also demonstrated a distinct behavior in response to radiation therapy. A control rate between 90 and 94%, and median survival between 10 and 16 months, prognosis for breast cancer brain metastases appears to be superior in comparison to other histologic groups (84). Multivariate analysis of Elaimy et al. retrospective study showed breast cancer group was statistically better in term of survival to patients with NSCLC brain metastases (12). Frazier et al. identified primary breast cancer site to be prognostic of survival, which also included several other primary histologic sites including NSCLC, melanoma and renal cell carcinoma (11). Sperduto et al. retrospective analysis revealed superior survival for WBRT plus SRS vs. WBRT alone (HR: 0.72; 0.53–0.98, p = 0.035) in breast cancer patients (n = 642) (17). Radiosurgery as salvage therapy for tumor recurrence after fractionated WBRT has been helpful in prolonging the median survival from 3–5 to 10.3–14 months suggesting a better result for combined treatment compare to WBRT alone (84). Moreover, a longer interval from WBRT to SRS was identified on multivariate analyses as prognostic for breast cancer patients in a study of salvage SRS for BM after prior WBRT (25). Studies have shown breast cancer patients responding well to SRS alone and contribution of adding WBRT in this group of patients has not been determined (11, 12, 17, 26–28). Addition of WBRT together with SRS as well as prior intervention of WBRT followed by SRS as salvage therapy have not been superior to SRS alone (17, 25–29, 67–84). Perez et al. has registered survival boost for SRS alone in comparison to combined approach (30). Univariate analysis showed negative correlation for prior WBRT (HR: 1.58; 1.12–2.22, p = 0.0087) while WBRT after SRS was shown to impact survival negatively on multivariate analysis (HR: 1.78; 1.06–2.99, p = 0.030) (30). It has been suggested that the better response might have been due to advancement of surgery and chemotherapeutic care of breast cancer patients (26, 85). Breast cancer, not only as the primary site has behaved distinctly, in fact, breast cancer histologic subtypes as primary histology for brain metastases have also been revealed to be distinct entities when it comes to their response to radiation therapy. The prognosis for basal subtype (triple negative), luminal A (ER/PR positive/HER2 negative), HER2 positive/ER/PR negative and luminal B (triple positive) subtype were different in terms of median survival as 7.3, 10, 17.9, and 22.9 months (p < 0.01), respectively (31). In a retrospective study of 131 patients who received SRS for breast cancer brain metastases between 2001 and 2013 revealed a median overall survival of 16, 26, 23, and 7 months for ER positive/HER2 negative, ER positive/HER2 positive, ER negative/HER2 positive, and TNBC (triple negative breast cancer), respectively (p < 0.001) (32). HER2 positive breast cancer brain metastases responded to SRS better as compared to HER2 negative breast cancer patients (median survival of 31.3 vs. 14.1 months (p < 0.01) (33). Patients with TNBC had the shortest time to retreatment with WBRT or SRS or death with hazard ratio of 3.12 (p < 0.001) compared to ER positive/HER2 negative (32). Triple negative subtype is associated with worst prognosis after SRS treatment (median survival of TN vs. Non-TN: 6 vs. 16 months) (34). Breast cancer brain metastases, as a whole have good response to SRS alone except for some histology subtypes and the role of WBRT plus SRS could be contested for survival outcome. Control of the primary disease with advancements in surgical and chemotherapeutic interventions might have something to do with the better outcome.
In context of molecular targeted therapy for BM with breast cancer histology, a number of agents have been approved and others are being under investigations. However, agents like transtuzumab (a monoclonal humanized antibody approved for the treatment of HER2-positive breast cancer) has limited intracranial efficacy due to its lack of BBB crossing ability (86). Lapatinib (a dual HER2 and EGFR inhibitor) has also revealed a mere CNS ORR of 6% in a phase II trial of 242 patients that had previously received transtuzumab and radiation therapy (WBRT or SRS) (87). However, in a separate study, patients with concurrent lapatinib had higher rates of complete response (35 vs. 11%, P = 0.008) in comparison to SRS alone (88). In patients with HER2-amlified lesions who also had undergone prior radiation therapies (WBRT, SRS and surgery), both HER2 antibodies (17.9 vs. 15.1 m; p < 0.04) as well as lapatinib (21.1 vs. 15.4 months; P < 5.03) were associated with improved median survival (89). WBRT was associated with better local [LC (SRS+/–WBRT); 6.9 vs. 11.0%, p < 0.02] and distant control [DF (WBRT+/–); 17.4 vs. 28.4%, p < 0.01] in combination with SRS or other targeted agents (transtuzumab, lapatinib), respectively (90). Concurrent lapatinib and transtuzumab with SRS compared to SRS alone had also significantly improved local control among HER2-amplified lesions [LF (lapatinib); 15.1 vs. 5.7%, p < 0.001, and (transtuzumab); 18.4 vs. 10.2%, p < 0.003] (90, 91). Yomo et al. also revealed significant 1-year local control in lapatinib group as per lesion analysis but not patient analysis (LC; 86 vs. 69%, p < 0.001) (92). Though concurrent use of lapatinib with SRS vs. SRS alone has not yield survival advantage, patients ever using lapatinib were associated with improved median survival (88–91). In retrospective study of 126 BM patients with HER2+ breast cancer undergoing SRS had also received lapatinib (n = 47) during the treatment. Use of lapatinib with SRS resulted in significant survival improvement (27.3 vs. 19.5 months, p = 0.03) for these patients as opposed to SRS alone (89). A similar result was reciprocated in a separate study with patients ever (n = 43) or never (n = 41) using lapatinib increased median survival from 23.6 months to 33.3 months (p = 0.009) (88). Currently, lapatinib in combination with WBRT or SRS is being investigated (NCT01622868, NCT00470847) which will more clearly establish the role of either radiation therapy in the treatment of HER2 positive breast cancer patients. Lapatinib in combination with capecitabine has also reported a 66% CNS ORR in a study that included 45 RT naïve patients (92). Trastuzumab emtansine (T-DM1) had also reported an intracranial clinical efficacy in 5 out of 10 (50%) patients, suggesting a similar response rate observed with lapatinib plus capecitabine (93). This result is also supported by the retrospective analysis of EMILIA trial that revealed similar PFS (HR = 1.00; P = 1.000; median PFS, 5.9 vs. 5.7 months) for patients with baseline brain metastases comparing the two treatments (94). Other agents like afatinib and neratinib had also been tried with mere CNS responses (95, 96). Furthermore, immunotherapy has also entered clinical trial (NCT03449238) in this group of patients in combination with SRS in optimism for synergistic responses.
Radioresistant Tumors
Melanoma and renal cell carcinoma brain metastases are considered radioresistant, however, their response to SRS have been encouraging (35). Adjuvant WBRT in addition to SRS, in a study of 31 patients with brain metastases from renal cell carcinoma, melanoma, or sarcoma, resulted in 6 month actuarial local control and distant brain failure rate (DBF) of 100 and 17% as compared with 85 and 64% in patients with no WBRT addition (P = 0.018 and P = 0.0027), respectively. This suggests a role of WBRT in controlling distant failure for these radioresistant histology (36). A Phase II trial of radiosurgery for one to three newly diagnosed brain metastases (n = 36) from renal cell carcinoma, melanoma, and sarcoma reported high degree of failures within the brain (~50% of patients by 6 months) with the omission of WBRT (37). Outcome variation among “radioresistant” brain metastases treated with stereotactic radiosurgery was also reported in retrospective study of 189 patients. Survival after SRS was significantly (P = 0.0354) worse for patients with melanoma (n = 103) and sarcoma (n = 9) brain metastases compared to patients with renal cell carcinoma (n = 77). The actuarial freedom from progression after 1 year was 64% for renal cell carcinoma patients, 47% for melanoma patients, and 0% for sarcoma patients (P < 0.001) (38). Of the radioresistant tumors brain metastases, renal cell carcinoma has resulted in better survival. Overall, a role of WBRT is observed in control of the disease and hence a trial is warranted to identify which primary histology would derive better survival out of the better control.
Renal Cell Carcinoma
WBRT has achieved survival of only 3.0–4.4 months in RCC brain metastases (39) while a better survival from 9.5 months to as high as 17.8 months have been reported with SRS (36, 40, 41). Primary renal cell carcinoma was identified as predictor of longer survival in a study which also had contained melanoma and sarcoma (36). Addition of WBRT (upfront or as salvage therapy) to SRS was not associated with local control or survival in patients with RCC brain metastases (42). There was no significant difference in distant control either with upfront WBRT [n = 2/8 (25%) vs. n = 3/9 (33%)] as well (42). A similar scenario of additive WBRT failure in prolonging survival and distant control (46 vs. 50%) was reported in another study (43). Ippen, et al. found significant association (p = 0.0097) between prior WBRT with poor overall survival (44). Foakas et al., however, identified that the addition of WBRT to the SRS improved LC (p = 0.032) but not OS (p = 0.703) (45). Bates et al. also revealed no difference for SRS alone, WBRT alone or WBRT plus SRS in treating RCC patients with BM [8.3 vs. 2.8 m (p = 0.82) vs. 8.5 m (p = 0.65), respectively] (46). Renal cell carcinoma, though, has derived better survival compared to melanoma, the role of WBRT is not clear. RCC related medical evidence is distinct because of no brain control with WBRT addition.
Though several targeted agents for RCC have been approved so far, their efficacy data in regard to the RCC brain metastases is limited. Sorafenib in combination with radiation therapy used after surgery in a case report of a patient with brain metastasis from RCC revealed a 4-year recurrence free survival (97). Sunitinib has shown safety and efficacy in metastatic RCC in a number of case reports and trials (98–103). Pazopanib and cabozatinib have also demonstrated intracranial activity in this group of patients (104–107). Concurrent multi-kinase inhibitors (mKIs) with SRS have revealed superior median survival (108–110). Significant local control (LF: 93 vs. 60%, p = 0.01) and improved median survival (16.6 vs. 7.2 m, p = 0.04) was reported in patients with receiving targeted agents in addition to SRS (108). A similar result was achieved in a separate study as well (16.8 vs. 7.3 m, p < 0.001) (109). Verma et al. identified TKIs use after BM development was highly significant for deriving survival benefit (23.6 vs. 4.41 m, p = 0.0001) (110). Bates et al., however, failed to report such advantage with concurrent TKIs. Nevertheless, radiation therapy in this study comprised of WBRT, SRS or both (46). Sunitinib or other targeted agents mentioned above or immunotherapeutic agents combined with radiotherapy could be evaluated in clinical trials particularly the SRS as suggested by some authors in order to achieve a more potent response as observed in the case of metastatic melanoma (111).
Melanoma
Use of WBRT alone in treating melanoma brain metastases has merely achieved a median survival of 3.5 months (47). Melanoma brain metastases responds better to SRS, however, median survival reported in the range of 5.3 to 10.6 months is comparatively lower to that of renal cell carcinoma (36, 48–52, 97–101). Up-front WBRT omission was associated with worse overall survival (multivariate HR 2.56, p = 0.08), and distant intracranial progression (multivariate HR 2.24, p = 0.005) (53). On the other hand, in a study of 185 patients, the addition of WBRT was shown to lack a LC, OS or PFS advantage in these patients (54). Initial WBRT was associated with no survival advantage (p = 0.88) but a delay in distant progression was observed; however, not significant (P = 0.13; n = 6) (55). Role of WBRT cannot be assessed clearly from these studies due to their retrospective nature and the fact that WBRT is usually administered in aggressive disease or in patients with multiple brain metastases (38, 52).
Immunotherapy in the treatment of brain metastases has mainly been assessed in melanoma patients. Recent studies have suggested role of immunotherapy (anti-CTLA-4/anti-PD-1 agents) and targeted therapy (BRAFi/MEKi) in combination with SRS leading to improved survival in comparison to SRS alone (112, 113). SRS followed by immunotherapy or targeted therapy have shown better local control (1-year LC; 100 vs. 83.3%, p = 0.023) as well as improvement in survival (MST; 10.95 vs. 2.29 m, p < 0.001) (114, 115). A met-analysis of ipilimumab plus SRS revealed significantly better survival over SRS alone in melanoma BMs (112). As well, this benefit from SRS plus Ipilimumab seems to be comparatively superior to ipilimumab combination with WBRT (116). Furthermore, there is theoretical and clinical evidence of this combination leading to brain control other than the therapeutic target area that is also termed as abscopal effect (117, 118). Targeted therapies in melanoma patients aimed at mutation such as BRAF inhibitors (50% of malignant melanomas) and MEK inhibitors have also been studied extensively. Vemurafenib and dabrafenib (BRAF inhibitors) alone or dabrafenib plus trametinib have shown excellent intracranial responses (119–125). Comparative studies have suggested combination of SRS and BRAF inhibitors resulting in better outcome for BRAF-mutant melanoma patients (126–130). Improved local control as well as superior median survival with BRAFi with SRS particularly when used concurrently or initiated after SRS (126–130). Side effects remains a concern as intracranial hemorrhage and radionecrosis has been associated with the SRS in conjunction with BRAF inhibitors (126, 131). These results suggest that this group of patients respond better with SRS in comparison to WBRT even in combination with immunotherapy and targeted molecular agents such as BRAF inhibitors.
It can be suggested that primary histology and subtypes may have a role in defining the outcome in these patients. Hence, pooling them together might have compromised the outcome of the aforementioned primary trials (3–8). In fact, the diverse prognostic factors associated with each histology had led to the creation of diagnosis specific graded prognostic assessment (DS-GPA), which associates different sets of prognostic factors to each histology (13).
Single Brain Metastases
Previously, Kondziolka et al., suggested omission of WBRT for single brain metastases while denying the existence of micrometastases (not apparent on high-resolution imaging) or diffuse brain disease based on the result of one study (132). However, there is Class I evidence showing a clear role of addition of WBRT reducing the local and distant failure significantly with subsequent improvement in survival. Andrew et al. reported a significant survival for single brain metastatic patients for treatment comparison (WBRT plus SRS vs. WBRT alone) (4). In the overall analysis, SRS boost was not received in some patients (single BM = 15% and 2–3 BM = 24%; 19% overall), which might have also influenced overall survival analysis (4, 13). Post-stratification by DS-GPA of Andrew et al. trial revealed significant survival advantage for favorable recipients (DS-GPA 3.5–4.0) which included single brain metastasis at large (13). Secondary Analysis of the JROSG 99-1 Randomized Clinical Trial revealed significantly improved survival from combined treatment for prognostically better placed recipients (DS-GPA 2.5–4.0) in comparison to SRS alone. Participants were single brain metastases at majority, however, 2–3 and even 4 brain metastases were also present (14). Li et al. reported SRS alone and SRS+WBRT to be better in prolonging life and improving quality of life than WBRT alone for patients with single brain metastasis from lung cancer (16). It is unfortunate that an overall assessment of the single to multiple (2, 3) brain metastases was reported in primary trials but no assessment of treatment comparison for single brain metastasis was carried out except for Andrew et al. The addition of WBRT to SRS seems to essential to treat single brain metastases where a clear advantage in survival is achieved with this approach particularly in comparison to WBRT alone. The significant intracranial control in the majority of the studies and reported better survival for single brain metastasis suggest a diffuse disease state which leads to distant failure when SRS alone is used. SRS alone has been associated with significant local and particularly distant cranial failure and requires high salvage therapy as compared to combined treatment, thereby increasing the number of hospital visits which can increase psychological burden for patients.
Future Perspective
Treatment paradigms for brain metastases are shifting with the entries of molecular targeted agents and immune checkpoint inhibitors. However, radiation therapy continues to play a role in this group of patients. SRS is taking a more robust role in combination with new agents. However, the local and distant control achieved with combined approach in selected patients might reveal a superior alternative. Or, would the abscopal effect of immunotherapy provide distant brain control associated with additive WBRT? As absence of immunotherapy after radiosurgery (HR: 0.380, p = 0.002) increased the odds of developing new brain metastases (50). Moreover, radiation therapy and immunotherapy are still the only options for cancer patients with no harboring mutations. Our study reports a slight clinical benefit for each primary histology with either WBRT or SRS, for example, WBRT plus SRS for NSCLC, SRS for breast cancer and radioresistant tumors like RCC and melanoma. It's encouraging to see that EGFR inhibitors were combined with WBRT for comparison in NSCLC patients with brain metastases and SRS combined with targeted or immunotherapeutic agents is mainly investigated in melanoma and RCC patients. Therefore, to clearly validate the role of each radiotherapeutic approach (WBRT, SRS, or WBRT + SRS) for patients with brain metastases would be essential through a clinical trial with a much more precise selection design based on primary histology along with other influencing factors.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 81572964, 81773354, 81803170, and 81872195), China Postdoctoral Science Foundation (No. 2017M622664), and Guangzhou Key Medical Discipline Construction Project Fund.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Nathoo N, Chahlavi A, Barnett GH, Toms SA. Pathobiology of brain metastases. J Clin Pathol. (2005) 58:237–42. doi: 10.1136/jcp.2003.013623
2. Gemici C, Yaprak G. Whole-brain radiation therapy for brain metastases: detrimental or beneficial? Radiat Oncol. (2015) 10:153. doi: 10.1186/s13014-015-0466-9
3. Kondziolka D, Patel A, Lunsford LD, Kassam A, Flickinger JC. Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int J Radiat Oncol Biol Phys. (1999) 45:427–34. doi: 10.1016/S0360-3016(99)00198-4
4. Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. (2004) 363:1665–72. doi: 10.1016/S0140-6736(04)16250-8
5. Aoyama H, Shirato H, Tago M, Nakagawa K, Toyoda T, Hatano K, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. (2006) 295:2483–91. doi: 10.1001/jama.295.21.2483
6. El Gantery MM, Abd El Baky HM, El Hossieny HA, Mahmoud M, Youssef O. Management of brain metastases with stereotactic radiosurgery alone versus whole brain irradiation alone versus both. Radiat Oncol. (2014) 9:116. doi: 10.1186/1748-717X-9-116
7. Brown PD, Jaeckle K, Ballman KV, Farace E, Cerhan JH, Anderson SK, et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. JAMA. (2016) 316:401–9. doi: 10.1001/jama.2016.9839
8. Chougule PB, Burton-Williams M, Saris S, Zheng Z, Ponte B, Noren G, et al. Randomized treatment of brain metastasis with gamma knife radiosurgery, whole brain radiotherapy or both. Int J Radiat Oncol Biol Phys. (2000) 48(Suppl. 1):114. doi: 10.1016/S0360-3016(00)80024-3
9. Sanghavi SN, Miranpuri SS, Chappell R, Buatti JM, Sneed PK, Suh JH, et al. Radiosurgery for patients with brain metastases: a multi-institutional analysis, stratified by the RTOG recursive partitioning analysis method. Int J Radiat Oncol Biol Phys. (2001) 51:426–34. doi: 10.1016/S0360-3016(01)01622-4
10. Sneed PK, Suh JH, Goetsch SJ, Sanghavi SN, Chappell R, Buatti JM, et al. A multi-institutional review of radiosurgery alone vs. radiosurgery with whole brain radiotherapy as the initial management of brain metastases. Int J Radiat Oncol Biol Phys. (2002) 53:519–26. doi: 10.1016/S0360-3016(02)02770-0
11. Frazier JL, Batra S, Kapor S, Vellimana A, Gandhi R, Carson KA, et al. Stereotactic radiosurgery in the management of brain metastases: an institutional retrospective analysis of survival. Int J Radiat Oncol Biol Phys. (2010) 76:1486–92. doi: 10.1016/j.ijrobp.2009.03.028
12. Elaimy AL, Mackay AR, Lamoreaux WT, Fairbanks RK, Demakas JJ, Cooke BS, et al. Multimodality treatment of brain metastases: an institutional survival analysis of 275 patients. World J Surg Oncol. (2011) 9:69. doi: 10.1186/1477-7819-9-69
13. Sperduto PW, Shanley R, Luo X, Andrews D, Werner-Wasik M, Valicenti R, et al. Secondary analysis of RTOG 9508, a phase 3 randomized trial of whole-brain radiation therapy versus WBRT plus stereotactic radiosurgery in patients with 1–3 brain metastases; poststratified by the graded prognostic assessment (GPA). Int J Radiat Oncol Biol Phys. (2014) 90:526–31. doi: 10.1016/j.ijrobp.2014.07.002
14. Aoyama H, Tago M, Shirato H. Stereotactic radiosurgery with or without whole-brain radiotherapy for brain metastases: secondary analysis of the JROSG 99–1 randomized clinical trial. JAMA Oncol. (2015) 1:457–64. doi: 10.1001/jamaoncol.2015.1145
15. Churilla TM, Ballman KV, Brown PD, Twohy EL, Jaeckle K, Farace E, et al. Stereotactic radiosurgery with or without whole-brain radiation therapy for limited brain metastases: a secondary analysis of the North Central Cancer Treatment Group N0574 (Alliance) randomized controlled trial. Int J Radiat Oncol Biol Phys. (2017) 99:1173–8. doi: 10.1016/j.ijrobp.2017.07.045
16. Li B, Yu J, Suntharalingam M, Kennedy AS, Amin PP, Chen Z, et al. Comparison of three treatment options for single brain metastasis from lung cancer. Int J Cancer. (2000) 90:37–45. doi: 10.1002/(SICI)1097-0215(20000220)90:1<37::AID-IJC5>3.0.CO;2-7
17. Sperduto PW, Chao ST, Sneed PK, Luo X, Suh J, Roberge D, et al. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys. (2010) 77:655–61. doi: 10.1016/j.ijrobp.2009.08.025
18. Lin CH, Hsu KH, Chang SN, Tsou HK, Sheehan J, Sheu ML, et al. Increased survival with the combination of stereotactic radiosurgery and gefitinib for non-small cell lung cancer brain metastasis patients: a nationwide study in Taiwan. Radiat Oncol. (2015) 10:127. doi: 10.1186/s13014-015-0431-7
19. Minniti G, Salvati M, Muni R, Lanzetta G, Osti MF, Clarke E, et al. Stereotactic radiosurgery plus whole-brain radiotherapy for treatment of multiple metastases from non-small cell lung cancer. Anticancer Res. (2010) 30:3055–61.
20. Marko NF, Suh JH, Chao ST, Barnett GH, Vogelbaum MA, Toms S, et al. Gamma knife stereotactic radiosurgery for the management of incidentally-identified brain metastasis from non-small cell lung cancer. J Neurooncol. (2011) 104:817–24. doi: 10.1007/s11060-011-0553-1
21. Abacioglu U, Caglar H, Atasoy BM, Abdulloev T, Akgun Z, Kilic T. Gamma knife radiosurgery in non small cell lung cancer patients with brain metastases: treatment results and prognostic factors. J BUON. (2010) 15:274–80.
22. Sun H, Xu L, Wang Y, Zhao J, Xu K, Qi J, et al. Additional radiation boost to whole brain radiation therapy may improve the survival of patients with brain metastases in small cell lung cancer. Radiat Oncol. (2018) 13:250. doi: 10.1186/s13014-018-1198-4
23. Mansour W, Shawky H. Additional radiation boost to whole brain radiation therapy for brain metastases in small cell lung cancer-a phase II study. Cancer Biol. (2019) 9:1–8. doi: 10.7537/marscbj090419.01
24. Wegner RE, Olson AC, Kondziolka D, Niranjan A, Lundsford LD, Flickinger JC. Stereotactic radiosurgery for patients with brain metastases from small cell lung cancer. Int J Radiat Oncol Biol Phys. (2011) 81:e21–7. doi: 10.1016/j.ijrobp.2011.01.001
25. Caballero JA, Sneed PK, Lamborn KR, Ma L, Denduluri S, Nakamura JL, et al. Prognostic factors for survival in patients treated with stereotactic radiosurgery for recurrent brain metastases after prior whole brain radiotherapy. Int J Radiat Oncol Biol Phys. (2012) 83:303–9. doi: 10.1016/j.ijrobp.2011.06.1987
26. Firlik KS, Kondziolka D, Flickinger JC, Lunsford LD. Stereotactic radiosurgery for brain metastases from breast cancer. Ann Surg Oncol. (2000) 7:333–8. doi: 10.1007/s10434-000-0333-1
27. Muacevic A, Kreth FW, Tonn J-C, Wowra B. Stereotactic radiosurgery for multiple brain metastases from breast carcinoma. Cancer. (2004) 100:1705–11. doi: 10.1002/cncr.20167
28. Kased N, Binder DK, McDermott MW, Nakamura JL, Huang K, Berger MS, et al. Gamma Knife radiosurgery for brain metastases from primary breast cancer. Int J Radiat Oncol Biol Phys. (2009) 75:1132–40. doi: 10.1016/j.ijrobp.2008.12.031
29. Jaboin JJ, Ferraro DJ, DeWees TA, Rich KM, Chicoine MR, Dowling JL, et al. Survival following gamma knife radiosurgery for brain metastasis from breast cancer. Radiat Oncology. (2013) 8:131. doi: 10.1186/1748-717X-8-131
30. Perez JL, Ozpinar A, Kano H, Phan B, Niranjan A, Lunsford LD. Salvage stereotactic radiosurgery in breast cancer patients with multiple brain metastases. World Neurosurg. (2019) 125:e479–86. doi: 10.1016/j.wneu.2019.01.108
31. Sperduto PW, Kased N, Roberge D, Chao ST, Shanley R, Luo X, et al. The effect of tumor subtype on the time from primary diagnosis to development of brain metastases and survival in patients with breast cancer. J Neurooncol. (2013) 112:467–72. doi: 10.1007/s11060-013-1083-9
32. Cho E, Rubinstein L, Stevenson P, Gooley T, Philips M, Halasz LM, et al. The use of stereotactic radiosurgery for brain metastases from breast cancer: who benefits most? Breast Cancer Res Treat. (2015) 149:743–9. doi: 10.1007/s10549-014-3242-x
33. Xu Z, Marko NF, Chao ST, Angelov L, Vogelbaum MA, Suh JH, et al. Relationship between HER2 status and prognosis in women with brain metastases from breast cancer. Int J Radiat Oncol Biol Phys. (2012) 82:e739–47. doi: 10.1016/j.ijrobp.2011.06.1968
34. Xu Z, Schlesinger D, Toulmin S, Rich T, Sheehan J. Impact of triple-negative phenotype on prognosis of patients with breast cancer brain metastases. Int J Radiat Oncol Biol Phys. (2012) 84:612–8. doi: 10.1016/j.ijrobp.2011.12.054
35. Lwu S, Goetz P, Monsalves E, Aryaee M, Ebinu J, Laperriere N, et al. Stereotactic radiosurgery for the treatment of melanoma and renal cell carcinoma brain metastases. Oncol Rep. (2013) 29:407–12. doi: 10.3892/or.2012.2139
36. Brown PD, Brown CA, Pollock BE, Gorman DA, Foote RL. Stereotactic radiosurgery for patients with “radioresistant” brain metastases. Neurosurgery. (2008) 62(Suppl. 2):790–801. doi: 10.1227/01.neu.0000316283.45242.e1
37. Manon R, O'Neill A, Knisely J, Werner-Wasik M, Lazarus HM, Wagner H, et al. Phase II trial of radiosurgery for one to three newly diagnosed brain metastases from renal cell carcinoma, melanoma, and sarcoma: an Eastern Cooperative Oncology Group study (E 6397). J Clin Oncol. (2005) 23:8870–6. doi: 10.1200/JCO.2005.01.8747
38. Chang EL, Selek U, Hassenbusch SJ III, Maor MH, Allen PK, Mahajan A, et al. Outcome variation among “radioresistant” brain metastases treated with stereotactic radiosurgery. Neurosurgery. (2005) 56:936–45; discussion−45. doi: 10.1227/01.NEU.0000158324.20757.AC
39. Wronski M, Maor MH, Davis BJ, Sawaya R, Levin VA. External radiation of brain metastases from renal carcinoma: a retrospective study of 119 patients from the M. D. Anderson Cancer Center. Int J Radiat Oncol Biol Phys. (1997) 37:753–9. doi: 10.1016/S0360-3016(97)00006-0
40. Takashi S, Shigeo I, Hideyo F, Hisato N. Gamma Knife surgery for metastatic brain tumors from renal cell carcinoma. J Neurosurg. (2006) 105:555–60. doi: 10.3171/jns.2006.105.4.555
41. Jason PS, Ming-Hsi S, Douglas K, John F, Lunsford LD. Radiosurgery in patients with renal cell carcinoma metastasis to the brain: long-term outcomes and prognostic factors influencing survival and local tumor control. J Neurosurg. (2003) 98:342–9. doi: 10.3171/jns.2003.98.2.0342
42. Goyal LK, Suh JH, Reddy CA, Barnett GH. The role of whole brain radiotherapy and stereotactic radiosurgery on brain metastases from renal cell carcinoma. Int J Radiat Oncol Biol Phys. (2000) 47:1007–12. doi: 10.1016/S0360-3016(00)00536-8
43. Mori Y, Kondziolka D, Flickinger JC, Logan T, Lunsford LD. Stereotactic radiosurgery for brain metastasis from renal cell carcinoma. Cancer. (1998) 83:344–53.
44. Ippen FM, Mahadevan A, Wong ET, Uhlmann EJ, Sengupta S, Kasper EM. Stereotactic radiosurgery for renal cancer brain metastasis: prognostic factors and the role of whole-brain radiation and surgical resection. J Oncol. (2015) 2015:636918. doi: 10.1155/2015/636918
45. Fokas E, Henzel M, Hamm K, Surber G, Kleinert G, Engenhart-Cabillic R. Radiotherapy for brain metastases from renal cell cancer: should whole-brain radiotherapy be added to stereotactic radiosurgery?: analysis of 88 patients. Strahlenther Onkol Org Deutschen Rontgengesellschaft. (2010) 186:210–7. doi: 10.1007/s00066-010-2055-z
46. Bates J, Youn P, Peterson C, Usuki K, Walter K, Okunieff P, et al. Radiotherapy for brain metastases from renal cell carcinoma in the targeted therapy era. Am J Clin Oncol. (2015) 40:439–43. doi: 10.1097/COC.0000000000000186
47. Hauswald H, Dittmar J-O, Habermehl D, Rieken S, Sterzing F, Debus J, et al. Efficacy and toxicity of whole brain radiotherapy in patients with multiple cerebral metastases from malignant melanoma. Radiat Oncol. (2012) 7:130. doi: 10.1186/1748-717X-7-130
48. Noël G, Simon JM, Valery CA, Cornu P, Boisserie G, Ledu D, et al. Linac radiosurgery for brain metastasis of melanoma. Stereotactic Funct Neurosurg. (2002) 79:245–55. doi: 10.1159/000070838
49. Seung SK, Sneed PK, McDermott MW, Shu HK, Leong SP, Chang S, et al. Gamma knife radiosurgery for malignant melanoma brain metastases. Cancer J Sci Am. (1998) 4:103–9.
50. Mathieu D, Kondziolka D, Cooper PB, Flickinger JC, Niranjan A, Agarwala S, et al. Gamma knife radiosurgery for malignant melanoma brain metastases. Clin Neurosurg. (2007) 54:241–7.
51. Yu C, Chen JC, Apuzzo ML, O'Day S, Giannotta SL, Weber JS, et al. Metastatic melanoma to the brain: prognostic factors after gamma knife radiosurgery. Int J Radiat Oncol Biol Phys. (2002) 52:1277–87. doi: 10.1016/S0360-3016(01)02772-9
52. Selek U, Chang EL, Hassenbusch SJ III, Shiu AS, Lang FF, Allen P, et al. Stereotactic radiosurgical treatment in 103 patients for 153 cerebral melanoma metastases. Int J Radiat Oncol Biol Phys. (2004) 59:1097–106. doi: 10.1016/j.ijrobp.2003.12.037
53. Dyer MA, Arvold ND, Chen Y-H, Pinnell NE, Mitin T, Lee EQ, et al. The role of whole brain radiation therapy in the management of melanoma brain metastases. Radiat Oncology. (2014) 9:143. doi: 10.1186/1748-717X-9-143
54. Bagshaw HP, Ly D, Suneja G, Jensen RL, Shrieve DC. Local control of melanoma brain metastases treated with stereotactic radiosurgery. J Radiosurg SBRT. (2016) 4:181–90.
55. Radbill AE, Fiveash JF, Falkenberg ET, Guthrie BL, Young PE, Meleth S, et al. Initial treatment of melanoma brain metastases using gamma knife radiosurgery. Cancer. (2004) 101:825–33. doi: 10.1002/cncr.20447
56. Khan M, Lin J, Liao G, Li R, Wang B, Xie G, et al. Comparison of WBRT alone, SRS alone, and their combination in the treatment of one or more brain metastases: review and meta-analysis. Tumour Biol. (2017) 39:1010428317702903. doi: 10.1177/1010428317702903
57. Eichler AF, Chung E, Kodack DP, Loeffler JS, Fukumura D, Jain RK. The biology of brain metastases-translation to new therapies. Nat Rev Clin Oncol. (2011) 8:344–56. doi: 10.1038/nrclinonc.2011.58
58. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. (2019) 69:7–34. doi: 10.3322/caac.21551
59. Petersen I. The morphological and molecular diagnosis of lung cancer. Dtsch Arztebl Int. (2011) 108:525–31. doi: 10.3238/arztebl.2011.0525
60. Mulvenna P, Nankivell M, Barton R, Faivre-Finn C, Wilson P, McColl E, et al. Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): results from a phase 3, non-inferiority, randomised trial. Lancet. (2016) 388:2004–14. doi: 10.1016/S0140-6736(16)30825-X
61. Welsh JW, Komaki R, Amini A, Munsell MF, Unger W, Allen PK, et al. Phase II trial of erlotinib plus concurrent whole-brain radiation therapy for patients with brain metastases from non–small-cell lung cancer. J Clin Oncol. (2013) 31:895–902. doi: 10.1200/JCO.2011.40.1174
62. Lee S, Lewanski C, Counsell N, Ottensmeier C, Bates A, Patel N, et al. Randomized trial of erlotinib plus whole-brain radiotherapy for NSCLC patients with multiple brain metastases. J Natl Cancer Inst. (2014) 106:dju151. doi: 10.1093/jnci/dju151
63. Pesce GA, Klingbiel D, Ribi K, Zouhair A, von Moos R, Schlaeppi M, et al. Outcome, quality of life and cognitive function of patients with brain metastases from non-small cell lung cancer treated with whole brain radiotherapy combined with gefitinib or temozolomide. A randomised phase II trial of the Swiss Group for Clinical Cancer Research (SAKK 70/03). Eur J Cancer. (2012) 48:377–84. doi: 10.1016/j.ejca.2011.10.016
64. Wang F, Ning F, Liu C, Hao Y, Li L, Yu Z, et al. Comparison of gefitinib versus VMP in the combination with radiotherapy for multiple brain metastases from non-small cell lung cancer. Cell Biochem Biophys. (2014) 71:1261–5. doi: 10.1007/s12013-014-0286-9
65. Zhuang H, Yuan Z, Wang J, Zhao L, Pang Q, Wang P. Phase II study of whole brain radiotherapy with or without erlotinib in patients with multiple brain metastases from lung adenocarcinoma. Drug Design Dev Ther. (2013) 7:1179–86. doi: 10.2147/DDDT.S53011
66. Lind JSW, Lagerwaard FJ, Smit EF, Senan S. Phase I study of concurrent whole brain radiotherapy and erlotinib for multiple brain metastases from non–small-cell lung cancer. Int J Radiat Oncol Biol Phys. (2009) 74:1391–6. doi: 10.1016/j.ijrobp.2008.10.026
67. Zeng Y-D, Liao H, Qin T, Zhang L, Wei W-D, Liang J-Z, et al. Blood-brain barrier permeability of gefitinib in patients with brain metastases from non-small-cell lung cancer before and during whole brain radiation therapy. Oncotarget. (2015) 6:8366–76. doi: 10.18632/oncotarget.3187
68. Ma S, Xu Y, Deng Q, Yu X. Treatment of brain metastasis from non-small cell lung cancer with whole brain radiotherapy and Gefitinib in a Chinese population. Lung Cancer. (2009) 65:198–203. doi: 10.1016/j.lungcan.2008.10.028
69. Olmez I, Donahue B, Butler J, Huang Y, Rubin P, Xu Y. Clinical outcomes in extracranial tumor sites and unusual toxicities with concurrent whole brain Radiat (WBRT) and Erlotinib treatment in patients with non-small cell lung cancer (NSCLC) with brain metastasis. Lung Cancer. (2010) 70:174–9. doi: 10.1016/j.lungcan.2010.01.018
70. Kim HJ, Kim W, Kwon D, Cho Y, Choi C-M. Effects of an epithelial growth factor receptor-tyrosine kinase inhibitor add-on in stereotactic radiosurgery for brain metastases originating from non-small-cell lung cancer. J Kor Neurosurg Soc. (2015) 58:205–10. doi: 10.3340/jkns.2015.58.3.205
71. Zeng Y-D, Zhang L, Liao H, Liang Y, Xu F, Liu J-L, et al. Gefitinib alone or with concomitant whole brain radiotherapy for patients with brain metastasis from non-small-cell lung cancer: a retrospective study. Asian Pacific J Cancer Prev. (2012) 13:909–14. doi: 10.7314/APJCP.2012.13.3.909
72. Cai Y, Wang J-Y, Liu H. Clinical observation of whole brain radiotherapy concomitant with targeted therapy for brain metastasis in non-small cell lung cancer patients with chemotherapy failure. Asian Pacific J Cancer Prev. (2013) 14:5699–703. doi: 10.7314/APJCP.2013.14.10.5699
73. Fan Y, Huang Z, Fang L, Miao L, Gong L, Yu H, et al. A phase II study of icotinib and whole-brain radiotherapy in Chinese patients with brain metastases from non-small cell lung cancer. Cancer Chemother Pharmacol. (2015) 76:517–23. doi: 10.1007/s00280-015-2760-5
74. Lu Y, Fan Y. Combined action of EGFR tyrosine kinase inhibitors and whole-brain radiotherapy on EGFR-mutated non-small-cell lung cancer patients with brain metastasis. Oncotargets Ther. (2016) 9:1135. doi: 10.2147/OTT.S95871
75. Zhou L, He J, Xiong W, Liu Y, Xiang J, Yu Q, et al. Impact of whole brain radiation therapy on CSF penetration ability of Icotinib in EGFR-mutated non-small cell lung cancer patients with brain metastases: results of phase I dose-escalation study. Lung Cancer. (2016) 96:93–100. doi: 10.1016/j.lungcan.2016.04.003
76. Magnuson WJ, Lester-Coll NH, Wu AJ, Yang TJ, Lockney NA, Gerber NK, et al. Management of brain metastases in tyrosine kinase inhibitor-naive epidermal growth factor receptor-mutant non-small-cell lung cancer: a retrospective multi-institutional analysis. J Clin Oncol. (2017) 35:1070–7. doi: 10.1200/JCO.2016.69.7144
77. Dong K, Liang W, Zhao S, Guo M, He Q, Li C, et al. EGFR-TKI plus brain radiotherapy versus EGFR-TKI alone in the management of EGFR-mutated NSCLC patients with brain metastases. Transl Lung Cancer Res. (2019) 8:268–79. doi: 10.21037/tlcr.2019.06.12
78. Luo S, Chen L, Chen X, Xie X. Evaluation on efficacy and safety of tyrosine kinase inhibitors plus radiotherapy in NSCLC patients with brain metastases. Oncotarget. (2015) 6:16725–34. doi: 10.18632/oncotarget.4264
79. Jiang T, Min W, Li Y, Yue Z, Wu C, Zhou C. Radiotherapy plus EGFR TKIs in non-small cell lung cancer patients with brain metastases: an update meta-analysis. Cancer Med. (2016) 5:1055–65. doi: 10.1002/cam4.673
80. Wang C, Lu X, Lyu Z, Bi N, Wang L. Comparison of up-front radiotherapy and TKI with TKI alone for NSCLC with brain metastases and EGFR mutation: a meta-analysis. Lung Cancer. (2018) 122:94–9. doi: 10.1016/j.lungcan.2018.05.014
81. Sperduto PW, Wang M, Robins HI, Schell MC, Werner-Wasik M, Komaki R, et al. A phase 3 trial of whole brain radiation therapy and stereotactic radiosurgery alone versus WBRT and SRS with temozolomide or erlotinib for non-small cell lung cancer and 1 to 3 brain metastases: Radiation Therapy Oncology Group 0320. Int J Radiat Oncol Biol Phys. (2013) 85:1312–8. doi: 10.1016/j.ijrobp.2012.11.042
82. Khan M, Lin J, Liao G, Tian Y, Liang Y, Li R, et al. ALK Inhibitors in the Treatment of ALK Positive NSCLC. Front Oncol. (2019) 8:557. doi: 10.3389/fonc.2018.00557
83. Khan M, Lin J, Liao G, Tian Y, Liang Y, Li R, et al. Comparative analysis of immune checkpoint inhibitors and chemotherapy in the treatment of advanced non-small cell lung cancer: a meta-analysis of randomized controlled trials. Medicine. (2018) 97:e11936. doi: 10.1097/MD.0000000000011936
84. Lippitz B, Lindquist C, Paddick I, Peterson D, O'Neill K, Beaney R. Stereotactic radiosurgery in the treatment of brain metastases: the current evidence. Cancer Treat Rev. (2014) 40:48–59. doi: 10.1016/j.ctrv.2013.05.002
85. Akyurek S, Chang EL, Mahajan A, Hassenbusch SJ, Allen PK, Mathews LA, et al. Stereotactic radiosurgical treatment of cerebral metastases arising from breast cancer. American J Clin Oncol. (2007) 30:310–4. doi: 10.1097/01.coc.0000258365.50975.f6
86. Stemmler HJ, Schmitt M, Willems A, Bernhard H, Harbeck N, Heinemann V. Ratio of trastuzumab levels in serum and cerebrospinal fluid is altered in HER2-positive breast cancer patients with brain metastases and impairment of blood-brain barrier. Anti Cancer Drugs. (2007) 18:23–8. doi: 10.1097/01.cad.0000236313.50833.ee
87. Lin NU, Dieras V, Paul D, Lossignol D, Christodoulou C, Stemmler HJ, et al. Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clin Cancer Res. (2009) 15:1452–9. doi: 10.1158/1078-0432.CCR-08-1080
88. Kim JM, Miller JA, Kotecha R, Chao ST, Ahluwalia MS, Peereboom DM, et al. Stereotactic radiosurgery with concurrent HER2-directed therapy is associated with improved objective response for breast cancer brain metastasis. Neuro Oncol. (2019) 21:659–68. doi: 10.1093/neuonc/noz006
89. Shireen P, Jacob AM, Aditya J, Samuel TC, Rupesh K, Alireza MM, et al. Stereotactic radiosurgery with concurrent lapatinib is associated with improved local control for HER2-positive breast cancer brain metastases. J Neurosurg. (2019) 132:503–11. doi: 10.3171/2018.10.JNS182340
90. Miller JA, Kotecha R, Ahluwalia MS, Mohammadi AM, Chao ST, Barnett GH, et al. Overall survival and the response to radiotherapy among molecular subtypes of breast cancer brain metastases treated with targeted therapies. Cancer. (2017) 123:2283–93. doi: 10.1002/cncr.30616
91. Yomo S, Hayashi M, Cho N. Impacts of HER2-overexpression and molecular targeting therapy on the efficacy of stereotactic radiosurgery for brain metastases from breast cancer. J Neurooncol. (2013) 112:199–207. doi: 10.1007/s11060-013-1046-1
92. Bachelot T, Romieu G, Campone M, Dieras V, Cropet C, Dalenc F, et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. Lancet Oncol. (2013) 14:64–71. doi: 10.1016/S1470-2045(12)70432-1
93. Bartsch R, Berghoff AS, Vogl U, Rudas M, Bergen E, Dubsky P, et al. Activity of T-DM1 in Her2-positive breast cancer brain metastases. Clin Exp Metastasis. (2015) 32:729–37. doi: 10.1007/s10585-015-9740-3
94. Krop IE, Lin NU, Blackwell K, Guardino E, Huober J, Lu M, et al. Trastuzumab emtansine (T-DM1) versus lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer and central nervous system metastases: a retrospective, exploratory analysis in EMILIA. Ann Oncol. (2015) 26:113–9. doi: 10.1093/annonc/mdu486
95. Cortes J, Dieras V, Ro J, Barriere J, Bachelot T, Hurvitz S, et al. Afatinib alone or afatinib plus vinorelbine versus investigator's choice of treatment for HER2-positive breast cancer with progressive brain metastases after trastuzumab, lapatinib, or both (LUX-Breast 3): a randomised, open-label, multicentre, phase 2 trial. Lancet Oncol. (2015) 16:1700–10. doi: 10.1016/S1470-2045(15)00373-3
96. Freedman RA, Gelman RS, Wefel JS, Melisko ME, Hess KR, Connolly RM, et al. Translational Breast Cancer Research Consortium (TBCRC) 022: a phase II trial of neratinib for patients with human epidermal growth factor receptor 2-positive breast cancer and brain metastases. J Clin Oncol. (2016) 34:945–52. doi: 10.1200/JCO.2015.63.0343
97. Kolsi F, Mechergui H, Kammoun B, Mellouli M, Khrifech M, Zaher Boudawara M. Delayed brain metastasis from renal cell carcinoma. Urol Case Rep. (2018) 22:54–6. doi: 10.1016/j.eucr.2018.10.020
98. Négrier S. Case report 3. Sunitinib malate in patients with cerebellar metastases. Eur J Cancer Suppl. (2007) 5:32–4. doi: 10.1016/S1359-6349(07)70114-3
99. Medioni J, Cojocarasu O, Belcaceres JL, Halimi P, Oudard S. Complete cerebral response with sunitinib for metastatic renal cell carcinoma. Ann Oncol. (2007) 18:1282–3. doi: 10.1093/annonc/mdm275
100. Koutras KA, Krikelis D, Alexandrou N, Starakis I, Kalofonos PH. Brain metastasis in renal cell cancer responding to sunitinib. Anticancer Res. (2007) 27:4255–7.
101. Zeng H, Li X, Yao J, Zhu Y, Liu J, Yang Y, et al. Multifocal brain metastases in clear cell renal cell carcinoma with complete response to sunitinib. Urol Int. (2009) 83:482–5. doi: 10.1159/000251193
102. Gore ME, Szczylik C, Porta C, Bracarda S, Bjarnason GA, Oudard S, et al. Safety and efficacy of sunitinib for metastatic renal-cell carcinoma: an expanded-access trial. Lancet Oncol. (2009) 10:757–63. doi: 10.1016/S1470-2045(09)70162-7
103. Chevreau C, Ravaud A, Escudier B, Amela E, Delva R, Rolland F, et al. A phase II trial of sunitinib in patients with renal cell cancer and untreated brain metastases. Clin Genitourin Cancer. (2014) 12:50–4. doi: 10.1016/j.clgc.2013.09.008
104. Hingorani M, Dixit S, Maraveyas A. Pazopanib-induced regression of brain metastasis after whole brain palliative radiotherapy in metastatic renal cell cancer progressing on first-line sunitinib: a case report. World J Oncol. (2014) 5:223–7. doi: 10.14740/wjon843w
105. Jacobs C, Kim DW, Straka C, Timmerman RD, Brugarolas J. Prolonged survival of a patient with papillary renal cell carcinoma and brain metastases using pazopanib. J Clin Oncol. (2013) 31:e114–7. doi: 10.1200/JCO.2012.46.0501
106. Gooch ME, Nader K, Kubicek GJ, Somer RA. Brain metastasis responsive to pazopanib in renal cell carcinoma: a case report and review of the literature. Clin Genitourin Cancer. (2016) 14:e401–4. doi: 10.1016/j.clgc.2016.01.005
107. Uche A, Sila C, Tanoura T, Yeh J, Bhowmick N, Posadas E, et al. Brain complete response to cabozantinib prior to radiation therapy in metastatic renal cell carcinoma. Case Rep Urol. (2019) 2019:4. doi: 10.1155/2019/6769017
108. Cochran DC, Chan MD, Aklilu M, Lovato JF, Alphonse NK, Bourland JD, et al. The effect of targeted agents on outcomes in patients with brain metastases from renal cell carcinoma treated with Gamma Knife surgery. J Neurosurg. (2012) 116:978–83. doi: 10.3171/2012.2.JNS111353
109. Juloori A, Miller J, Parsai S, Kotecha R, Ahluwalia M, Mohammadi A, et al. Overall survival and response to radiation and targeted therapies among patients with renal cell carcinoma brain metastases. J Neurosurg. (2019) 132:1–9. doi: 10.3171/2018.8.JNS182100
110. Verma J, Jonasch E, Allen PK, Weinberg JS, Tannir N, Chang EL, et al. The impact of tyrosine kinase inhibitors on the multimodality treatment of brain metastases from renal cell carcinoma. Am J Clin Oncol. (2013) 36:620–4. doi: 10.1097/COC.0b013e31825d59db
111. Kim YH, Kim JW, Chung HT, Paek SH, Kim DG, Jung HW. Brain metastasis from renal cell carcinoma. Prog Neurol Surg. (2012) 25:163–75. doi: 10.1159/000331190
112. Khan M, Lin J, Liao G, Tian Y, Liang Y, Li R, et al. SRS in combination with ipilimumab: a promising new dimension for treating melanoma brain metastases. Technol Cancer Res Treat. (2018) 17:1533033818798792. doi: 10.1177/1533033818798792
113. Weaver BD, Goodman JR, Jensen R. Concurrent radiosurgery and systemic therapies for melanoma brain metastases: a systematic review. Cureus. (2019) 11:e6147. doi: 10.7759/cureus.6147
114. Hadi I, Roengvoraphoj O, Bodensohn R, Hofmaier J, Niyazi M, Belka C, et al. Stereotactic radiosurgery combined with targeted/ immunotherapy in patients with melanoma brain metastasis. Radiat Oncol. (2020) 15:37. doi: 10.1186/s13014-020-1485-8
115. Gaudy-Marqueste C, Dussouil AS, Carron R, Troin L, Malissen N, Loundou A, et al. Survival of melanoma patients treated with targeted therapy and immunotherapy after systematic upfront control of brain metastases by radiosurgery. Eur J Cancer. (2017) 84:44–54. doi: 10.1016/j.ejca.2017.07.017
116. Gerber NK, Young RJ, Barker CA, Wolchok JD, Chan TA, Yamada Y, et al. Ipilimumab and whole brain radiation therapy for melanoma brain metastases. J Neurooncol. (2015) 121:159–65. doi: 10.1007/s11060-014-1617-9
117. Kalbasi A, June CH, Haas N, Vapiwala N. Radiation and immunotherapy: a synergistic combination. J Clin Investig. (2013) 123:2756–63. doi: 10.1172/JCI69219
118. Choong ES, Lo S, Drummond M, Fogarty GB, Menzies AM, Guminski A, et al. Survival of patients with melanoma brain metastasis treated with stereotactic radiosurgery and active systemic drug therapies. Eur J Cancer. (2017) 75:169–78. doi: 10.1016/j.ejca.2017.01.007
119. Dummer R, Goldinger SM, Turtschi CP, Eggmann NB, Michielin O, Mitchell L, et al. Vemurafenib in patients with BRAF(V600) mutation-positive melanoma with symptomatic brain metastases: final results of an open-label pilot study. Eur J Cancer. (2014) 50:611–21. doi: 10.1016/j.ejca.2013.11.002
120. Dzienis MR, Atkinson VG. Response rate to vemurafenib in patients with B-RAF-positive melanoma brain metastases: a retrospective review. Melanoma Res. (2014) 24:349–53. doi: 10.1097/CMR.0000000000000068
121. Gibney GT, Gauthier G, Ayas C, Galebach P, Wu EQ, Abhyankar S, et al. Treatment patterns and outcomes in BRAF V600E-mutant melanoma patients with brain metastases receiving vemurafenib in the real-world setting. Cancer Med. (2015) 4:1205–13. doi: 10.1002/cam4.475
122. Harding JJ, Catalanotti F, Munhoz RR, Cheng DT, Yaqubie A, Kelly N, et al. A Retrospective evaluation of vemurafenib as treatment for BRAF-mutant melanoma brain metastases. Oncologist. (2015) 20:789–97. doi: 10.1634/theoncologist.2014-0012
123. McArthur GA, Maio M, Arance A, Nathan P, Blank C, Avril MF, et al. Vemurafenib in metastatic melanoma patients with brain metastases: an open-label, single-arm, phase 2, multicentre study. Ann Oncol. (2017) 28:634–41 doi: 10.1093/annonc/mdw641
124. Falchook GS, Long GV, Kurzrock R, Kim KB, Arkenau TH, Brown MP, et al. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a phase 1 dose-escalation trial. Lancet. (2012) 379:1893–901. doi: 10.1016/S0140-6736(12)60398-5
125. Long GV, Trefzer U, Davies MA, Kefford RF, Ascierto PA, Chapman PB, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol. (2012) 13:1087–95. doi: 10.1016/S1470-2045(12)70431-X
126. Ly D, Bagshaw HP, Anker CJ, Tward JD, Grossmann KF, Jensen RL, et al. Local control after stereotactic radiosurgery for brain metastases in patients with melanoma with and without BRAF mutation and treatment. J Neurosurg. (2015) 123:395–401. doi: 10.3171/2014.9.Jns141425
127. Wolf A, Zia S, Verma R, Pavlick A, Wilson M, Golfinos JG, et al. Impact on overall survival of the combination of BRAF inhibitors and stereotactic radiosurgery in patients with melanoma brain metastases. J Neurooncol. (2016) 127:607–15. doi: 10.1007/s11060-016-2072-6
128. Xu Z, Lee CC, Ramesh A, Mueller AC, Schlesinger D, Cohen-Inbar O, et al. BRAF V600E mutation and BRAF kinase inhibitors in conjunction with stereotactic radiosurgery for intracranial melanoma metastases. J Neurosurg. (2017) 126:726–34. doi: 10.3171/2016.2.JNS1633
129. Kotecha R, Miller JA, Venur VA, Mohammadi AM, Chao ST, Suh JH, et al. Melanoma brain metastasis: the impact of stereotactic radiosurgery, BRAF mutational status, and targeted and/or immune-based therapies on treatment outcome. J Neurosurg. (2018) 129:50–9. doi: 10.3171/2017.1.JNS162797
130. Mastorakos P, Xu Z, Yu J, Hess J, Qian J, Chatrath A, et al. BRAF V600 Mutation and BRAF kinase inhibitors in conjunction with stereotactic radiosurgery for intracranial melanoma metastases: a multicenter retrospective study. Neurosurgery. (2019) 84:868–80. doi: 10.1093/neuros/nyy203
131. Patel KR, Chowdhary M, Switchenko JM, Kudchadkar R, Lawson DH, Cassidy RJ, et al. BRAF inhibitor and stereotactic radiosurgery is associated with an increased risk of radiation necrosis. Melanoma Res. (2016) 26:387–94. doi: 10.1097/CMR.0000000000000268
Keywords: whole brain radiotherapy (WBRT), stereotactic radiosurgery (SRS), brain metastases (BM), tumor control (TC), overall survival (OS), primary histology
Citation: Khan M, Arooj S, Li R, Tian Y, Zhang J, Lin J, Liang Y, Xu A, Zheng R, Liu M and Yuan Y (2020) Tumor Primary Site and Histology Subtypes Role in Radiotherapeutic Management of Brain Metastases. Front. Oncol. 10:781. doi: 10.3389/fonc.2020.00781
Received: 14 August 2019; Accepted: 22 April 2020;
Published: 07 July 2020.
Edited by:
Sonali Rudra, MedStar Georgetown University Hospital, United StatesReviewed by:
Michael Charles Repka, Winthrop University Hospital, United StatesJohn E. Mignano, Tufts University School of Medicine, United States
Copyright © 2020 Khan, Arooj, Li, Tian, Zhang, Lin, Liang, Xu, Zheng, Liu and Yuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yawei Yuan, yuanyawei@gzhmu.edu.cn; Mengzhong Liu, liumengzhong@126.com; Ronghui Zheng, 27819000@qq.com
 Muhammad Khan
Muhammad Khan Sumbal Arooj
Sumbal Arooj Rong Li
Rong Li Yunhong Tian
Yunhong Tian Jian Zhang1
Jian Zhang1 Jie Lin
Jie Lin Yingying Liang
Yingying Liang Mengzhong Liu
Mengzhong Liu Yawei Yuan
Yawei Yuan