- 1Division of Spine, Department of Orthopedics, Tongji Hospital Affiliated to Tongji University School of Medicine, Shanghai, China
- 2Shanghai Bone Tumor Institution, Shanghai, China
- 3Department of Orthopedics, Shanghai General Hospital, School of Medicine, Shanghai Jiaotong University, Shanghai, China
- 4Department of Central Laboratory, Shanghai Tenth People's Hospital of Tongji University, School of Medicine, School of Life Sciences and Technology, Tongji University, Shanghai, China
- 5Beth Israel Deaconess Medical Center, BIDMC Cancer Center, Harvard Medical School, Cancer Research Institute, Boston, MA, United States
- 6Key Laboratory of Spine and Spinal Cord Injury Repair and Regeneration, Ministry of Education, Tongji University, Shanghai, China
Objectives: Chordoma is a rare bone malignancy that affects the spine and skull base. Treatment dilemma leads to a high rate of local relapse and distant metastases. Molecular targeted therapy (MTT) is an option for advanced chordoma, but its therapeutic efficacy and safety have not been investigated systematically. Therefore, a systematic review was conducted on studies reporting MTT regimens for chordoma.
Methods: Clinical trials, case series and case reports on chordoma MTT were identified using MEDLINE, Cochrane library and EMBASE, and systematically reviewed. Data on clinical outcomes, such as median overall survival, progression-free survival, response rate and adverse events (AEs) were extracted and analyzed.
Results: Thirty-three eligible studies were selected for the systematic review, which indicated that imatinib and erlotinib were the most frequently used molecular targeted inhibitors (MTIs) for chordoma. For PDGFR-positive and/or EGFR-positive chordoma, clinical benefits were achieved with acceptable AEs. Monotherapy is preferred as the first-line of treatment, and combined drug therapy as the second-line treatment. In addition, the brachyury vaccine has shown promising results.
Conclusions: The selection of MTIs for patients with advanced or relapsed chordoma should be based on gene mutation screening and immunohistochemistry (IHC). Monotherapy of TKIs is recommended as the first-line management, and combination therapy (two TKIs or TKI plus mTOR inhibitor) may be the choice for drug-resistant chordoma. Brachyury vaccine is a promising therapeutic strategy and requires more clinical trials to evaluate its safety and efficacy.
Introduction
Chordoma is a relatively rare malignant bone tumor with an incidence of 0.08 per 100,000 (1). It accounts for 1–4% of all bone malignancies, and ~20% of primary spine tumors (2). Although it can occur at any segment of the spine, the predominant site of chordoma are fused segments like clivus and sacrococcyx (3). It is an indolent malignancy that progresses slowly, but exhibits strong local aggressiveness and often grows into huge masses that compress vital nerves and blood vessels (4). In addition, since chordoma is usually unresponsive to the conventional radiotherapy and cytotoxic chemotherapy, surgery is the primary therapeutic option (1, 5). Large case series including our previous one have shown that a total resection of the tumor, with the goal of negative microscopic margins, is crucial for long-term positive outcomes (6). However, the complex anatomy of the spine and the relatively large tumor volume make a clean resection technically challenging, leading to a high rate of local relapse and distant metastases (7). Regarding to this advanced setting, conventional therapeutic methods were shown to be not highly effective (1). Therefore, novel therapeutic strategies are needed to prolong patients' survival and improve the quality of life.
Pathologically, chordoma arises from residual notochord cells within the vertebral body (8), as verified on the basis of genetic and immuno-phenotypic biomarkers (9). New insights into the molecular mechanism underlying chordoma have also identified novel therapeutic targets (5). Molecular targeted therapy (MTT) in chordoma includes (1) imatinib and dasatinib against platelet-derived growth factor receptors (PDGFR) and stem cell factor receptor (KIT) (10, 11); (2) erlotinib, lapatinib, gefitinib, and cetuximab against epidermal growth factor receptor (EGFR) and erbB-2/human epidermal growth factor receptor 2 (HER2/neu) (12, 13); (3) sorafenib, pazopanib, and sunitinib that target angiogenic factors like vascular endothelial growth factor receptor (VEGFR) (14–16); and (4) temsirolimus and sirolimus that target the phosphoinositide 3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) pathway (17).
The indications for MTTs in chordoma patients are largely based on a few prospective clinical trials, small retrospective studies, and even case reports (10–17). However, the efficacy and safety of MTT regimens in chordoma patients, as well as the underlying molecular mechanisms, lack systematic investigation. Therefore, we conducted a systematic review on MTT regimens in chordoma patients to determine the clinical outcomes and underlying molecular mechanisms.
Materials and Methods
Search Strategy
For this systematic review, we used standard procedures from PRISMA guidelines (18). A comprehensive, systematic search was performed using MEDLINE (via PubMed), Cochrane Library and EMBASE. To find appropriate studies in MEDLINE, we used a combination of terms related to the MeSH terms “Chordoma/drug therapy” OR the free-text searching “Chordoma” AND (“targeted therapy OR inhibitor OR inhibit OR inhibition”). This search was further modified as appropriate for Cochrane Library and EMBASE. Initial search was performed on January 17, 2018 and repeated on July 1, 2018.
Eligibility Criteria
Studies were deemed eligible for the assessment of MTTs in patients with chordoma, irrespective of previous and subsequent other treatment. Only English language publications were included. For clinical trials, case series and case reports published exclusively in abstract or news form, only those containing new data were analyzed. For literature reviews, new personal unpublished data is also included. Reference lists of selected studies and previous reviews associated with similar topics were screened manually. New clinical trials for chordoma were found from Chordoma Foundation, ClinicalTrials.gov, EU Clinical Trials Register and WHO International Clinical Trials Registry Platform. Although gray literature (such as unpublished reports, conference abstracts and dissertations) might provide some negative results and decrease the publication bias, we did not access them, because they were usually not peer reviewed and might be later published in peer-reviewed journals.
Data Extraction and Synthesis
After removal of duplicates, titles and abstracts of all identified publications were systematically screened by two independent reviewers (MT and YHB). Discrepancies between reviewers were resolved by discussion. When eligibility criteria seemed to be met, the two reviewers (MT and YHB) independently assessed retrieved full texts and extracted information. If disagreements were still remained, the third reviewer (SDW) helped to reach an agreement. We contacted with the Chordoma Foundation in order to get helpful information. Additionally, we corresponded with researchers clarify study eligibility if the published study was unclear, although responses were poor. Extracted data were study characteristics (study design, first author, year of publication), patient characteristics (total number, history of treatment) and tumor characteristics (gene mutation and immunohistochemistry), MTT information (type of agents, dosage, course of treatment and adverse events), evaluation criteria (Choi's criteria, the response evaluation criteria in solid tumor (RECIST), clinical and radiological or metabolic response), and survival (duration of follow-up, progression-free survival and overall survival).
Results
Search Results
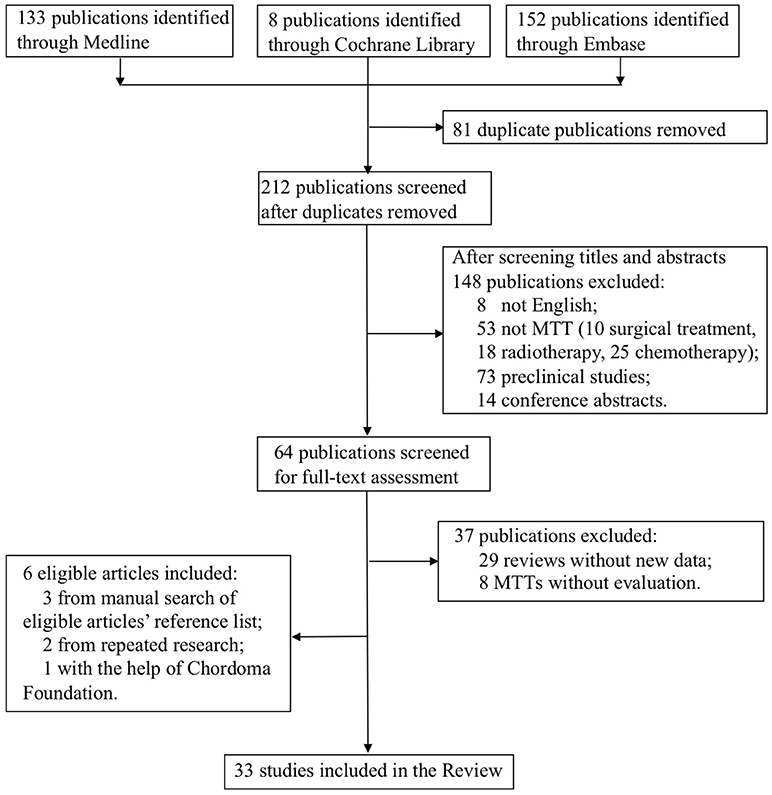
The flow-chart for the selection and exclusion of relevant publications is shown in Figure 1. We identified 293 studies in the initial screening, and after removing duplicates and papers based on their titles and abstracts, selected 64 publications for full-text assessment. Twenty-seven studies met our inclusion criteria, and six more were included—three from manually searching the reference list of the selected articles, two from repeated search and one with the help of the Chordoma Foundation. Finally, 33 studies were included in this systematic review.
Study Characteristics
Among 33 studies, nine studies were clinical trials (10–12, 14, 15, 19–22), with eight retrospective case series (16, 17, 23–28), and 16 case reports (13, 29–43). Imatinib was assessed in 18 studies with a total of 221 patients (10, 16, 17, 19, 23–28, 32, 34–36, 38, 39, 41, 42), erlotinib in 10 studies with 16 patients (13, 17, 22, 33, 35, 38, 40–42), cetuximab in five studies (seven patients) (13, 30, 31, 33, 41), sorafenib in four studies (65 patients) (15, 17, 21, 37), pazopanib in four studies with seven patients (16, 28, 41, 43) and sunitinib in three studies with 11 patients (14, 17, 28). Sirolimus, thalidomide, bevacizumab, gefitinib, linsitinib, and everolimus were accessed in two studies each (13, 22, 25, 28–31, 33, 34, 40–42), whereas dasatinib (32 patients) (11), lapatinib (18 patients) (12), rapamycin (one patients) (34), temosirolimus (one patients) (17) and yeast-brachyury (GI-6301) vaccine (11 patients) (20) were only analyzed in one study each (Figures 2 and 3). Monotherapy of MTTs was reported in 24 studies (10–12, 14–17, 20, 21, 23, 24, 26–28, 32, 34–39, 41, 43, 44) with combination therapy in 13 studies (13, 19, 22, 25, 28–31, 33, 39–42).
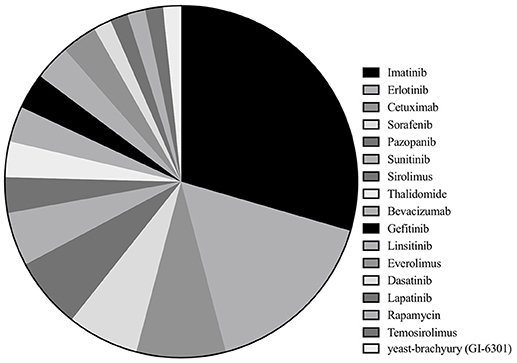
Figure 2. Of the included studies, the proportion of studies reporting each molecular targeted inhibitor.
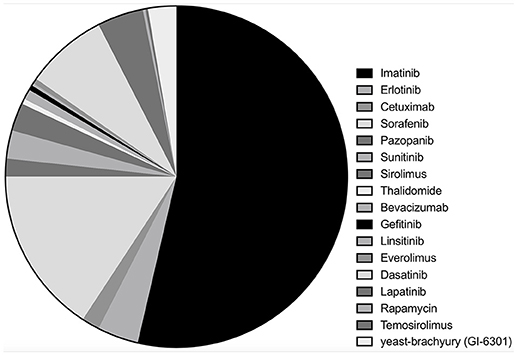
Figure 3. Of the included chordoma patients, the proportion of patients received each molecular targeted inhibitor.
RECIST evaluation criteria was used in 19 studies (10–15, 17, 19, 20, 22, 25–28, 32, 40, 42, 43, 45) and Choi's criteria is applied in three studies (11, 12, 25). Twenty-one studies were evaluated by clinical/radiological or metabolic responses (16, 23–27, 29–43). Adverse events (AEs) were reported in 25 studies, including hematological anomalies like anemia, thrombocytopenia, as well as non-hematological AEs like fatigue, fever, anorexia, QTc prolongation, abnormal liver function, nausea, and vomiting (10–15, 19, 22, 23, 26–38, 40, 43, 45).
Efficacy and Safety of MTT Regimens in Chordoma Patients
PDGFR Inhibitors (Table 1)
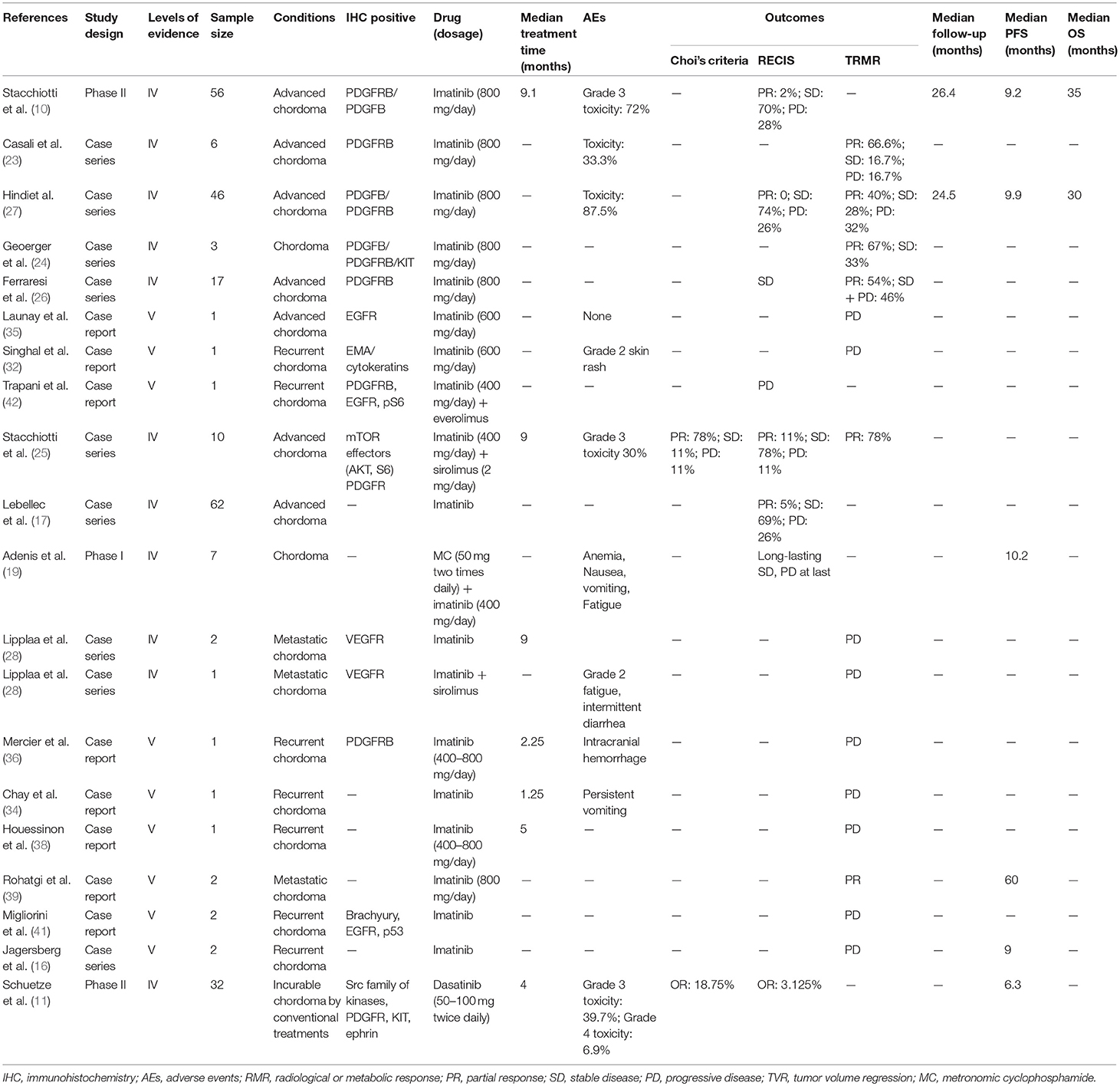
Imatinib mesylate (IM), a specific tyrosine kinase inhibitor (TKI) targeting PDGFR and KIT (10, 46), was the most frequently-used MTT in chordoma patients. Eighteen studies investigated the therapeutic efficacy of IM on 221 patients (10, 16, 17, 19, 23–28, 32, 34–36, 38, 39, 41, 42), including three clinical trials (10, 19, 28), seven retrospective case series (16, 17, 23–27), and eight case reports (32, 34–36, 38, 39, 41, 42). Fourteen studies (204 patients) analyzed the efficacy of imatinib as monotherapy (10, 16, 17, 23, 24, 26–28, 32, 34–36, 39, 41), of which four studies (181 patients) used RECIST and 3 were focused on PDGFRβ-expressing chordoma. In these four studies, four patients achieved partial response (PR) (2.2%), 133 cases sustained stable disease (SD) (73.5%) and 44 cases experienced progressive disease (PD) (24.3%) (10, 17, 26, 27, 32). Clinical/radiological or metabolic responses were evaluated in 13 studies (85 patients), with 33 patients achieving PR (38.8%), 23 patients sustaining SD (27.1%) and 29 patients experiencing PD (34.1%) (16, 23, 24, 26–28, 32, 34–36, 38, 39, 41). Five of the above studies (73 patients) focused on PDGFRβ-expressing chordoma, with 45.2% PR, 31.5% SD, and 23.3% PD cases (23, 24, 26, 27, 36), and eight studies included 12 patients that experienced PD within a short period of time.
Progression-free survival (PFS) and overall survival (OS) are important indices of clinical outcome, and they were reported in two large case-studies (10, 27). Stacchiotti et al. conducted a phase II trial in 56 patients with chordoma, and the median PFS and OS were 9 and 35 months, respectively (10). A retrospective study on 46 chordoma patients reported a median PFS of 9.9 months (27).
AEs were reported in eight studies (10, 23, 27, 32, 34, 36), with skin rash being the most common, followed by oedema, chronic anemia, fatigue and fluid retention (10, 26). Subacute intraventricular hemorrhage was seen in one case of clivus chordoma treated with imatinib (36).
Dasatinib, an inhibitor of PDGFR and Src, was evaluated in a phase II study (NCT00464620) (11) on 32 patients. The median PFS and 6 months PFS rate were 6.3 months and 54%, respectively. The 2- and 5-years OS rate were 43 and 18%, respectively. Six patients had an objective response (OR) according to Choi criteria and one for RECIST. Fatigue, fever, anorexia, nausea, and vomiting occurred in more than 5% of the patients.
EGFR Inhibitors (Table 2)
Erlotinib was the most commonly used anti-EGFR agent and was analyzed in 10 studies (16 patients) for the treatment of chordoma (13, 17, 22, 32, 33, 35, 38, 40–42), including one clinical trials (22), one retrospective case study (17) and eight case reports (13, 17, 22, 32, 33, 35, 38, 40–42). Monotherapy with erlotinib was used in five studies (nine patients) (17, 32, 35, 38, 42), three (seven patients) of which were evaluated by RECIST (17, 32, 42), reporting PR in two patients and SD in five patients. Three case reports were evaluated by clinical/radiological or metabolic responses (32, 35, 38). All achieved PR and significant tumor bulk reduction was seen in two patients (70 and 46%, respectively). Skin rashes were commonly seen in the erlotinib-treated patients.
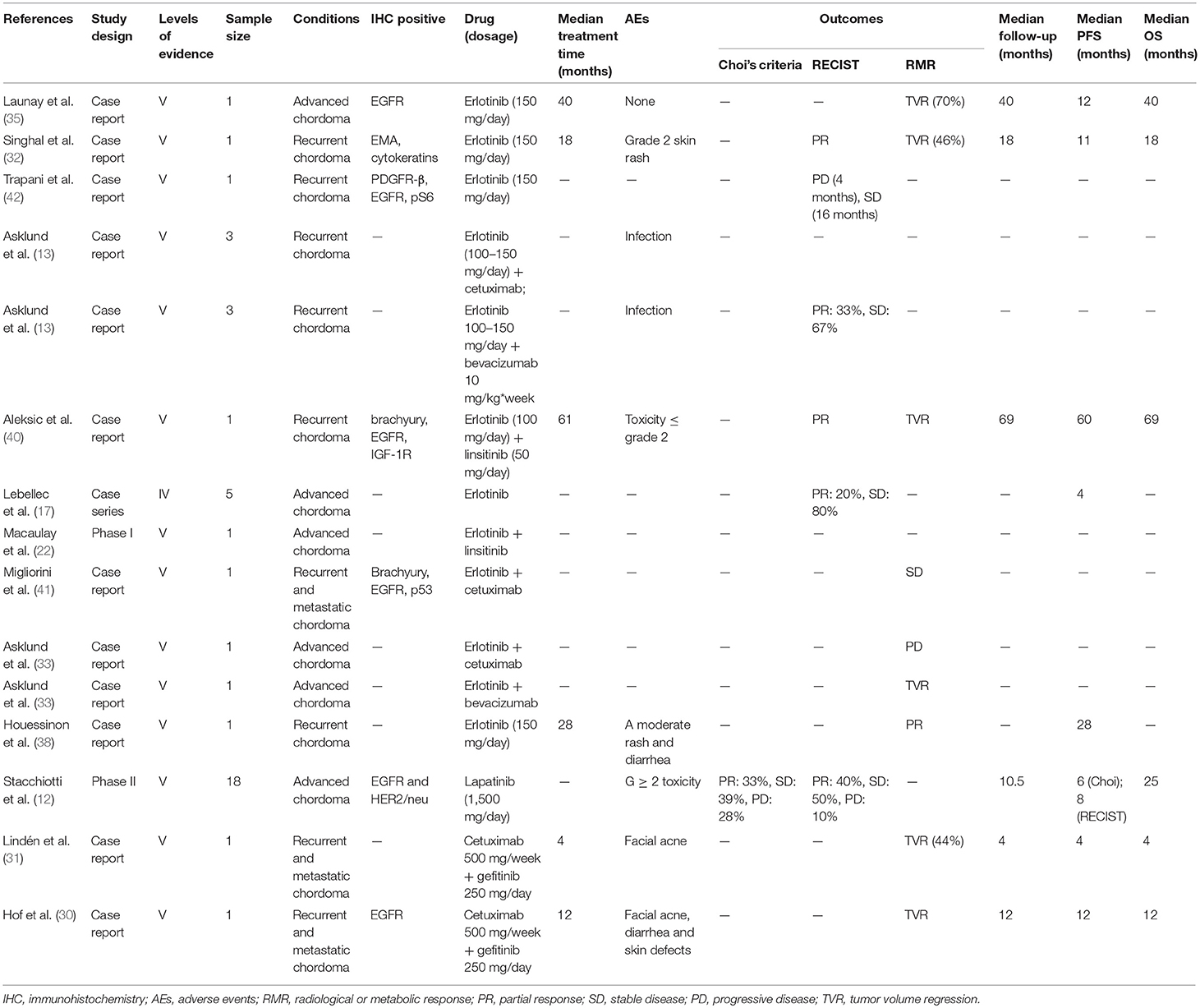
Table 2. Molecular targeted therapy of chordomas with EGFR inhibitors (erlotinib, linsitinib, cetuximab, lapatinib, gefitinib).
Lapatinib monotherapy was evaluated in a phase II clinical trial on 18 patients with EGFR-positive chordoma (12). Six patients achieved PR and seven sustained SD, with the median PFS of 6 months according to the Choi criteria. In contrast, all patients had SD by RECIST criteria with the median PFS of 8 months. Most patients experienced G ≥ 2 AEs.
Combined therapy with EGFR inhibitors was used in seven studies (eight patients) (13, 22, 30, 31, 33, 40, 41). Erlotinib was also the most common agents used in the combined MTT regimens (five studies, seven patients) (13, 22, 33, 40, 41).
Linsitinib, an inhibitor of IGF-1R/insulin receptor (INSR), was evaluated in a phase I study in combination with erlotinib (NCT00739453) (22). One patient with chordoma achieved PR for 18 months according to RECIST, with a PFS of 5 years. AEs included QTc prolongation, abnormal liver function, hyperglycemia and anorexia (22, 40).
The anti-EGFR monoclonal antibody (mAb) cetuximab was applied in combination with erlotinib in one patient with EGFR-positive chordoma, and he had a SD for 6 months (41). However, four patients with EGFR-negative chordoma experienced PD after receiving the same regimen. The treatment failure prompted a switch to bevacizumab, an anti-VEGF mAb (13, 33). Following this change, two patients achieved PR and another two presented SD. Treatment-related fatigue was observed in one patient (13, 33). Combined regimen of cetuximab and gefitinib was also effective in two cases of EGFR-positive chordoma (30, 31), where one achieved a PR for 9 months and the other had a 44% reduction in tumor bulk. Pronounced AEs, such as rash, acne, diarrhea, and skin defects, were reported in both cases (30, 31).
VEGFR Inhibitors (Table 3)
Sorafenib, a TKI against VEGFR and PDGFR, was assessed in four studies (15, 17, 21, 37). A phase II trial was conducted on 27 patients with chordomas (NCT00874874) (15), and OR was observed in one patient as per RECIST. The 12 months PFS and OS rates were 73.0 and 86.5%, respectively. In a study on 11 patients treated with sorafenib, PR was obtained in one patient, with SD in nine patients and PD in one patient according to RECIST (17). Another study assessing sorafenib reported a PFS of 12 months (37). However, sorafenib was limited by severe AEs like thrombocytopenia and diarrhea, and the rates of grade 3 and 4 toxicity were 77.8 and 14.8%, respectively.
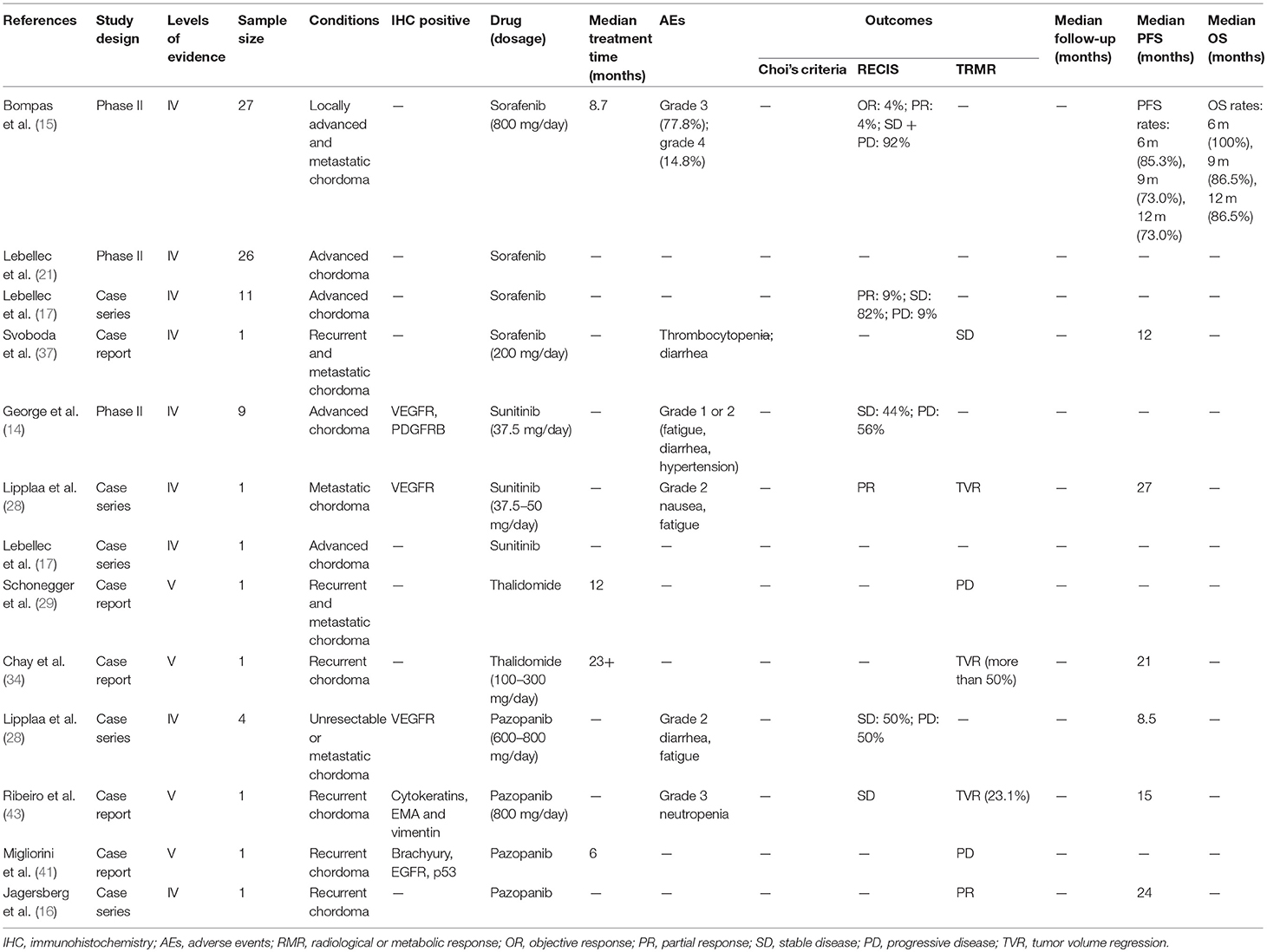
Table 3. Molecular targeted therapy with VEGF and VEGFR inhibitors (sorafenib, sunitinib, thalidomide, pazopanib).
Sunitinib, a multi-targeting TKI against VEGFR and PDGFR, was assessed in three studies (14, 17, 28). A phase II trial on sunitinib was conducted on nine patients (14), four of which achieved SD according to RECIST, concurrent to a qualitative decrease in tumor density, along with a median PFS of 12 months (14). Two patients treated with sunitinib had at least SD according to RECIST (17, 28), and one achieved a PR after a 27 months SD (28). The major toxicities were of grade 1 or 2 (14).
Pazopanib, another VEGFR inhibitor, was analyzed in seven patients (16, 28, 41, 43), of which four sustained SD with the median PFS of 15 months and the remaining three experienced PD. Thalidomide, an inhibitor of VEGF, was used as a second-line treatment for chordoma after failure of imatinib, rapamycin and other chemotherapy (29, 34). While one patient achieved a 50% tumor reduction, another experienced a PD (29, 34). In addition, severe toxicities of grade 3 and 4 were reported in both cases.
Other Molecular Targeted Inhibitors (MTIs) (Table 4)
Monotherapy with the mTOR inhibitors rapamycin and everolimus were ineffective in chordoma patients (34, 41). The combined MTT regimen of everolimus and imatinib resulted in sustained SD in one patient, with a PFS of 16 months (42). In addition, IM plus sirolimus was used in 10 patients with IM-refractory chordoma and activated mTOR (25). Nine patients were assessed, of which one achieved PR, seven sustained SD and one experienced PD according to RECIST. According to Choi criteria, seven patients achieved PR, and one sustained SD and one experienced PD. The same MTT regimen was also used against IM- and sunitinib-refractory chordoma but was not effective due to short of the mTOR expression (28). A phase I trial evaluated the effect of IM plus metronomic cyclophosphamide (MC)-based chemotherapy on 7 IM- and sunitinib-refractory chordoma patients (19). The median PFS was 10.2 months, and the 12 months PFS and OS rates were 42.9 and 85.7%, respectively according to RECIST. No dose-limiting toxicity and drug pharmacokinetic interactions were observed.
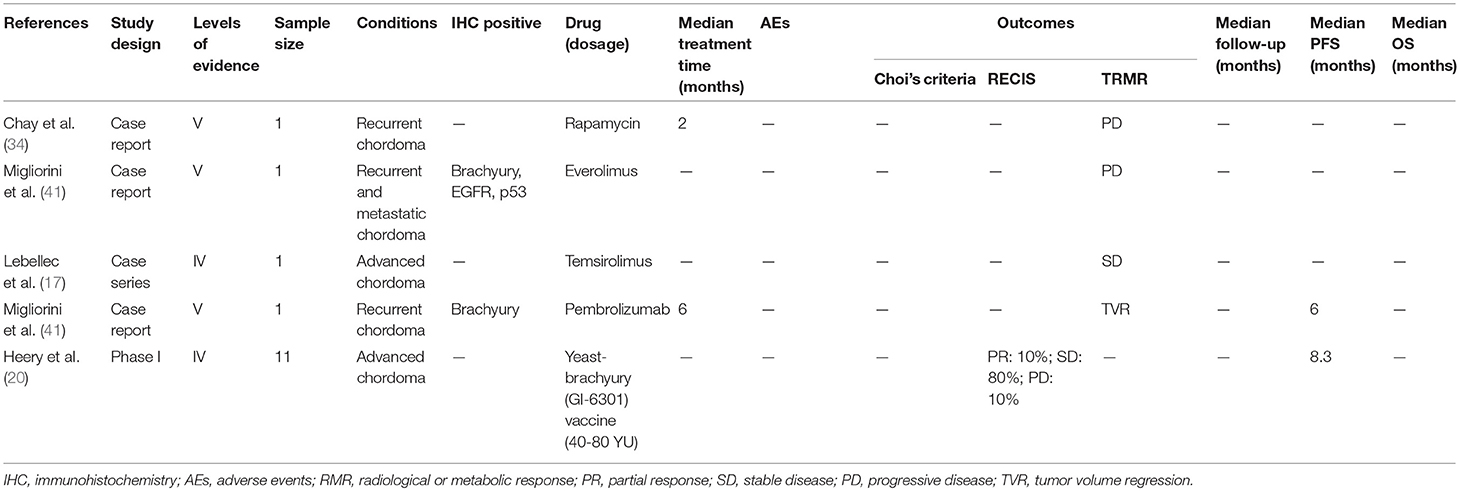
Table 4. Molecular targeted therapy of chordomas with other inhibitors (rapamycin, temsirolimus, yeast brachyury vaccine).
Brachyury Vaccine (Table 4)
A phase I dose-escalation trial using a recombinant Saccharomyces cerevisiae (yeast) vaccine encoding brachyury (GI-6301) was conducted on 11 patients (20), and 10 evaluable patients showed a median PFS of 8.3 months. One patient achieved PR, with eight sustaining SD and one experiencing PD at 3 months according to RECIST. Seven patients had no evidence of PD, giving a clinical benefit rate of 70% at 5 months. The most common AEs were injection site reactions.
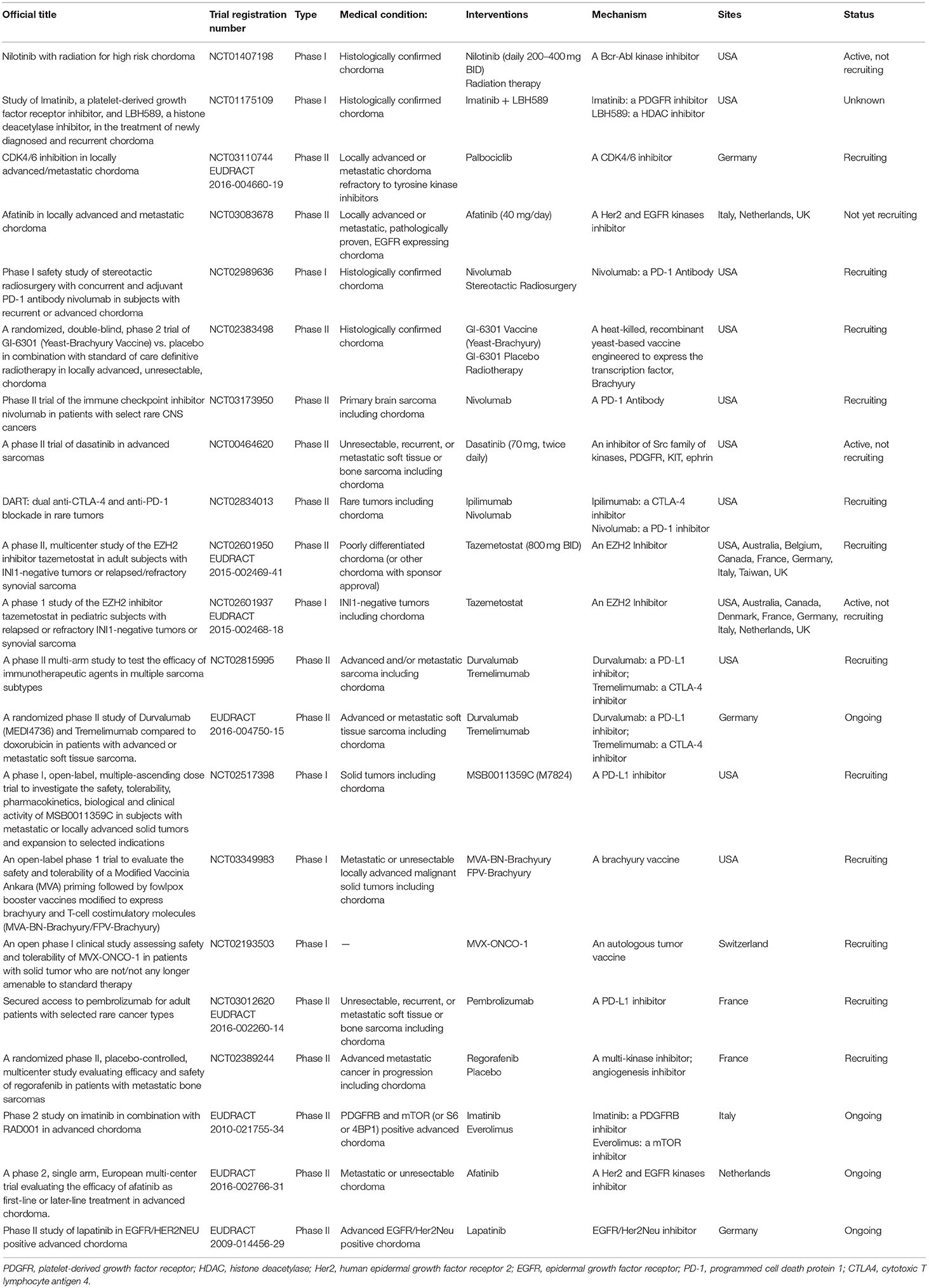
Ongoing and planned clinical trials on chordoma MTT are listed in Table 5.
Discussion
Novel therapeutic strategies against chordoma are urgently needed to prolong the overall survival and relieve symptoms. Elucidation of the underlying molecular mechanisms of chordoma have helped identify numerous potential therapeutic targets (47, 48), and several anti-chordoma agents are currently being tested in animal models and clinical trials. This systematic review is focused on the pharmacological management of chordoma patients and the clinical outcomes. Furthermore, the molecular mechanisms of MTT action have also been assessed.
Molecular Targets
Chordoma is a genetically heterogeneous tumor with frequent imbalances of large chromosomal regions. Somatic duplications of the notochordal transcription factor brachyury (47, 48), chromosomal copy loss of phosphatase and tensin homolog (PTEN) (49), tuberous sclerosis complex (TSC) (50), cyclin-dependent kinase inhibitor 2A and 2B (CDKN2A and CDKN2B) (51), SMARCB1 (49), and PIK3CA (9) mutations are key aspects of chordoma pathogenesis, and therefore potential targets.
RTKs are the key players in the development and progression of chordoma, and their mutated forms can activate signaling cascades resulting in dysregulation of many essential proteins. Therefore, mutational analyses and IHC can greatly assist oncologists to determine the optimal inhibitors (52–56). It needs to be emphasized that mutations in the molecular targets are clinically more relevant than their immunoreactivity, since target overexpression is not always driven by the activation of the corresponding signaling pathway. For example, high levels of EGFR in the chordoma cell line JHC7 was not accompanied by activated EGFR signaling (57).
Indications and Evaluation Criteria for MTTs
MTTs are not the first treatment options for chordoma, and only recommended for advanced or recurrent chordoma that are unresponsive to either surgical resection or radiotherapy.
The outcomes of MTTs is often difficult to evaluate in chordoma. Choi's criteria is based on changes in tumor size and density following contrast administration in CT or MRI (58). A radiological PR is defined as ≥10% decrease in tumor size or ≥15% decrease in tumor density/contrast enhancement in CT/MRI. RECIST defines PR as ≥20% decrease in tumor growth, which occurs later than that required for Choi criteria. Therefore, RECIST is not fully adequate to evaluate the clinical response in chordoma (59). Clinical/radiological and metabolic responses include symptom relief, anti-tumor effects (such as liquefaction) and changes in tumor density in the CT scan, reduction in contrast enhancement in MR, and maximum standardized uptake (SUVmax) in PET (23). However, typical tumor tissue characteristics like component and scirrhosity may also affect tumor-related symptoms, even in the absence of any changes in tumor size, resulting in incorrect readings.
MTTs for Chordoma
Imatinib was the first effective agent tested against chordoma, and is currently the most commonly used MTIs (23). Most patients with PDGFRβ-positive chordoma benefited from imatinib treatment and avoided rapid PD, likely due to tumor necrosis and intra-tumoral subacute bleeding that manifest as liquefaction (36). A dosage of 800 mg/day is recommended, except in cases of high toxicity. The major AEs associated with imatinib include oedema, chronic anemia, fatigue and even subacute intraventricular hemorrhage (36).
Several trials have also reported the ineffectiveness of imatinib in chordoma (19, 28, 32, 35, 38, 42). In such cases, EGFR inhibitor is the second line of treatment, since PDGFRβ activation can also stimulate EGFR, given an EGFR gene copy number gain (CNG) or strong intra-tumoral EGFR staining is detected. Around 40% of chordoma patients show CNG of the chromosome band 7p12, where EGFR is located. Erlotinib has shown a good clinical effect EGFR-positive chordoma, and could serve as the second choice for imatinib-refractory chordoma (32, 35). The combination of gefitinib and cetuximab, two other inhibitors of EGFR, showed improved clinical benefits and decreased AEs (30, 31).
HER2/neu is involved in EGFR dimer formation, and the possibility of heterodimerization increases the sensitivity of EGFR-positive chordoma to 54% (60). Lapatinib, a bi-specific inhibitor blocking both EGFR and HER2/neu, achieved 33.3% PR and 38.9% SD as per Choi criteria and 100% SD according to RECIST in EGFR-positive chordoma (12). Afatinib, another bi-specific inhibitor of EGFR and HER2/neu, was the only agent which showed cytotoxic effects across multiple chordoma cell lines in a drug sensitivity assessment (57). On this basis, a new clinical trial on the effects of afatinib is currently enrolling patients (NCT03083678).
IGF signaling is also important in chordoma tumorigenesis, since IGF-1 and IGF-1R have been detected in 92 and 76% of chordoma tissues (61), and are absent in benign notochordal cell tumor and fetal notochord (52). Linsitinib, an IGF-1R inhibitor, was assessed in two studies (22, 40), and effectively controlled chordoma progression in combination with erlotinib (22, 40).
VEGF levels are significantly higher in chordoma tissues and associated with angiogenesis (62). Five VEGFR or VEGF inhibitors (sorafenib, sunitinib, pazopanib, thalidomide, bevacizumab) were evaluated in this systematic review. Although occasional severe AEs were observed occasionally, sorafenib, sunitinib, and pazopanib monotherapy resulted in substantial clinical effects. Although thalidomide was effective against drug-resistant chordomas, severe toxicities limit its clinical application. Bevacizumab can be used as a supplement for erlotinib in drug-resistant chordomas, and their combination showed good clinical effect and high tolerance. A new phase II trial evaluating the efficacy and safety of regorafenib, a multi-kinase inhibitor of VEGFR, is ongoing in France for metastatic bone sarcoma (NCT02389244).
Chordomas with indication of anti-RTK agents may also relapse or progress early. In TKI-resistant chordomas, p-AKT is a relative reliable indicator and its persistent expression following tyrphostin treatment resulted in relapse and progression (54). AKT is activated by mTOR, its downstream molecules (RPS6 and eIF4E), and Stat3. The combination of the antagonists of upstream RTKs and downstream mTOR/PI3K/MAPK/Stat not only synergistically reduced chordoma growth by avoiding the negative feedback loop (63) and PI3K-dependent feedback loop (64), but also significantly decreased the cytotoxicity of either agent (65). For example, monotherapy of rapamycin or everolimus was ineffective against tumor progression (34, 41), while combining imatinib with everolimus or sirolimus induced good clinical effects in 3 studies (12 patients) (25, 28, 42). Therefore, the combined therapy can be considered for drug-resistant chordoma.
Mutations in the downstream effectors of RTKs, like PTEN and PIK3CA, also impair TKI response (66, 67). PTEN deficient chordoma cell lines exhibit increased proliferation, reduced apoptosis and enhanced migration in chordoma cell lines (68). Reintroduction of PTEN in tumor cells increased their therapeutic sensitivity to PDGFR inhibitors, and the combination of histone deacetylase (HDAC) and PDGFR inhibitors effectively reduced the growth and invasion of chordoma cells, irrespective of PTEN status (69). On this basis, a new phase I trial of Imatinib and LBH589 (a HDAC inhibitor) is ongoing in chordoma patients (NCT01175109).
Chordomas frequently show deletions in the SMARCB1 locus (49). SMARCB1 directly antagonizes the histone methyltransferase EZH2 and regulates the cell-cycle by activating CDKN2A (45). A phase I trial on the EZH2 inhibitor tazemetostat, confirmed complete or partial responses were observed in two children with chordoma according to RECIST (NCT02601937) (45). Therefore, another phase II clinical trial on tazemetostat is ongoing in patients with SMARCB1/INI1 deleted chordoma (NCT02601950).
The loss of chromosome 9 or 9p region, which contains CDKN2A, has been reported in some chordoma patients (51). The inactivation of CDKN2A universally activates the CDK4/6 and Rb pathways (70), which are highly expressed in the chordoma tissues (71). The CDK4/6 inhibitors palbociclib and LY2835219 inhibited chordoma cell growth and proliferation in vitro efficiently (72, 73). A phase II clinical trial on palbociclib is currently enrolling patients with chordoma (NCT03110744).
Somatic duplications of the notochordal transcription factor brachyury was demonstrated in chordoma, and enhanced tumor growth by activating YAP (9, 47, 48). Preclinical studies have shown that a recombinant Saccharomyces cerevisiae (yeast) vaccine encoding brachyury (GI-6301) activates human T cells in vitro. A phase II GI-6301 dose-escalation trial showed a 70% clinical benefit rate in chordoma patients (20). A phase II clinical trial on the combination of GI-6301 and radiotherapy is currently enrolling chordoma patients in the United States (NCT02383498). Additionally, a phase I trial of a Modified Vaccinia Ankara (MVA)-brachyury and a fowlpox (FPV)-brachyury vaccines is currently ongoing in patients with solid tumors, including chordoma (NCT03349983).
Limitations
In order to decrease the selection bias, this systematic review screened all published studies enrolling chordoma patients treated with MTT, including clinical trials, case series and even case reports, and provides the most detailed information. However, there were some limitations that need to be addressed. We included case reports on account of the rarity of chordoma and the paucity of available studies. However, a case report might overemphasize the final results due to lack of strong results. In addition, we only included English language publications which can also increase the selection bias. Furthermore, the baseline conditions of the patients and the evaluation criteria were not consistent across studies which is another factor contributing to selection bias. Therefore, large prospective randomized clinical trials are warranted to help clinicians determine the optimum treatment modality for chordoma patients.
Conclusions
The selection of MTIs for patients with advanced or relapsed chordoma should be based on gene mutation screening and immunohistochemistry (IHC). Monotherapy of TKIs is recommended as the first-line treatment. Combined therapy (two TKIs or TKI plus mTOR inhibitor) may be the choice for drug-resistant chordoma. Brachyury vaccine is a promising therapeutic strategy and requires more clinical trials to evaluate its safety and efficacy.
Author Contributions
TM did the literature search, data and data analysis, and led the writing of the review. HY, JJ, and RH contributed to the design, data collection, and analysis. HY, DS, and LC contributed their experience of clinical practice in chordoma to ensure the relevance of findings. JJ and CJ contributed their experience in the discussion of the molecular mechanism underlying chordoma and drug interaction.
Funding
This work was supported in part by the National Natural Science Foundation of China [grant numbers 81702659, 81772856] and National Key R&D Program of China [grant numbers 2016YFA0100800].
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We acknowledge the contributions of Josh Sommer of the Chordoma Foundation, who assisted with the revision of concept and the final manuscript.
References
1. Walcott BP, Nahed BV, Mohyeldin A, Coumans JV, Kahle KT, Ferreira MJ. Chordoma: current concepts, management, and future directions. Lancet Oncol. (2012) 13:e69–76. doi: 10.1016/S1470-2045(11)70337-0
2. Mcmaster ML, Goldstein AM, Bromley CM, Ishibe N, Parry DM. Chordoma: incidence and survival patterns in the United States, 1973–1995. Cancer Causes Control. (2001) 12:1–11. doi: 10.1023/A:1008947301735
3. Chugh R, Tawbi H, Lucas DR, Biermann JS, Schuetze SM, Baker LH. Chordoma: the nonsarcoma primary bone tumor. Oncologist (2007) 12:1344–50. doi: 10.1634/theoncologist.12-11-1344
4. Casali PG, Stacchiotti S, Sangalli C, Olmi P, Gronchi A. Chordoma. Curr Opin Oncol. (2007) 19:367–70. doi: 10.1097/CCO.0b013e3281214448
5. Stacchiotti S, Sommer J. Building a global consensus approach to chordoma: a position paper from the medical and patient community. Lancet Oncol. (2015) 16:e71–83. doi: 10.1016/S1470-2045(14)71190-8
6. Meng T, Yin H, Li B, Li Z, Xu W, Zhou W, et al. Clinical features and prognostic factors of patients with chordoma in the spine: a retrospective analysis of 153 patients in a single center. Neuro Oncol. (2015) 17:725–32. doi: 10.1093/neuonc/nou331
7. Stacchiotti S, Casali PG, Lo Vullo S, Mariani L, Palassini E, Mercuri M, et al. Chordoma of the mobile spine and sacrum: a retrospective analysis of a series of patients surgically treated at two referral centers. Ann Surg Oncol. (2010) 17:211–9. doi: 10.1245/s10434-009-0740-x
8. Arain A, Hornicek FJ, Schwab JH, Chebib I, Damron TA. Chordoma arising from benign multifocal notochordal tumors. Skeletal Radiol. (2017) 46:1745–52. doi: 10.1007/s00256-017-2727-1
9. Tarpey PS, Behjati S, Young MD, Martincorena I, Alexandrov LB, Farndon SJ, et al. The driver landscape of sporadic chordoma. Nat Commun. (2017) 8:890. doi: 10.1038/s41467-017-01026-0
10. Stacchiotti S, Longhi A, Ferraresi V, Grignani G, Comandone A, Stupp R, et al. Phase II study of imatinib in advanced chordoma. J Clin Oncol. (2012) 30:914–20. doi: 10.1200/JCO.2011.35.3656
11. Schuetze SM, Bolejack V, Choy E, Ganjoo KN, Staddon AP, Chow WA, et al. Phase 2 study of dasatinib in patients with alveolar soft part sarcoma, chondrosarcoma, chordoma, epithelioid sarcoma, or solitary fibrous tumor. Cancer (2017) 123:90–7. doi: 10.1002/cncr.30379
12. Stacchiotti S, Tamborini E, Lo Vullo S, Bozzi F, Messina A, Morosi C, et al. Phase II study on lapatinib in advanced EGFR-positive chordoma. Ann Oncol. (2013) 24:1931–6. doi: 10.1093/annonc/mdt117
13. Asklund T, Sandstrom M, Shahidi S, Riklund K, Henriksson R. Durable stabilization of three chordoma cases by bevacizumab and erlotinib. Acta Oncol. (2014) 53:980–4. doi: 10.3109/0284186X.2013.878472
14. George S, Merriam P, Maki RG, Van Den Abbeele AD, Yap JT, Akhurst T, et al. Multicenter phase II trial of sunitinib in the treatment of nongastrointestinal stromal tumor sarcomas. J Clin Oncol. (2009) 27:3154–60. doi: 10.1200/JCO.2008.20.9890
15. Bompas E, Le Cesne A, Tresch-Bruneel E, Lebellec L, Laurence V, Collard O, et al. Sorafenib in patients with locally advanced and metastatic chordomas: a phase II trial of the French Sarcoma Group (GSF/GETO). Ann Oncol. (2015) 26:2168–73. doi: 10.1093/annonc/mdv300
16. Jagersberg M, El Rahal A, Dammann P, Merkler D, Weber DC, Schaller K. Clival chordoma: a single-centre outcome analysis. Acta Neurochir (Wien). (2017) 159:1815–23. doi: 10.1007/s00701-017-3163-7
17. Lebellec L, Chauffert B, Blay JY, Le Cesne A, Chevreau C, Bompas E, et al. Advanced chordoma treated by first-line molecular targeted therapies: outcomes and prognostic factors. A retrospective study of the French Sarcoma Group (GSF/GETO) and the Association des Neuro-Oncologues d'Expression Francaise (ANOCEF). Eur J Cancer (2017) 79:119–28. doi: 10.1016/j.ejca.2017.03.037
18. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ (2009) 339:b2700. doi: 10.1136/bmj.b2700
19. Adenis A, Ray-Coquard I, Italiano A, Chauzit E, Bui-Nguyen B, Blay JY, et al. A dose-escalating phase I of imatinib mesylate with fixed dose of metronomic cyclophosphamide in targeted solid tumours. Br J Cancer (2013) 109:2574–8. doi: 10.1038/bjc.2013.648
20. Heery CR, Singh BH, Rauckhorst M, Marte JL, Donahue RN, Grenga I, et al. Phase I trial of a yeast-based therapeutic cancer vaccine (GI-6301) targeting the transcription factor brachyury. Cancer Immunol Res. (2015) 3:1248–56. doi: 10.1158/2326-6066.CIR-15-0119
21. Lebellec L, Bertucci F, Tresch-Bruneel E, Bompas E, Toiron Y, Camoin L, et al. Circulating vascular endothelial growth factor (VEGF) as predictive factor of progression-free survival in patients with advanced chordoma receiving sorafenib: an analysis from a phase II trial of the french sarcoma group (GSF/GETO). Oncotarget (2016) 7:73984–94. doi: 10.18632/oncotarget.12172
22. Macaulay VM, Middleton MR, Eckhardt SG, Rudin CM, Juergens RA, Gedrich R, et al. Phase I dose-escalation study of linsitinib (OSI-906) and erlotinib in patients with advanced solid tumors. Clin Cancer Res. (2016) 22:2897–907. doi: 10.1158/1078-0432.CCR-15-2218
23. Casali PG, Messina A, Stacchiotti S, Tamborini E, Crippa F, Gronchi A, et al. Imatinib mesylate in chordoma. Cancer (2004) 101:2086–97. doi: 10.1002/cncr.20618
24. Geoerger B, Morland B, Ndiaye A, Doz F, Kalifa G, Geoffray A, et al. Target-driven exploratory study of imatinib mesylate in children with solid malignancies by the Innovative Therapies for Children with Cancer (ITCC) European Consortium. Eur J Cancer (2009) 45:2342–51. doi: 10.1016/j.ejca.2009.03.007
25. Stacchiotti S, Marrari A, Tamborini E, Palassini E, Virdis E, Messina A, et al. Response to imatinib plus sirolimus in advanced chordoma. Ann Oncol. (2009) 20:1886–94. doi: 10.1093/annonc/mdp210
26. Ferraresi V, Nuzzo C, Zoccali C, Marandino F, Vidiri A, Salducca N, et al. Chordoma: clinical characteristics, management and prognosis of a case series of 25 patients. BMC Cancer (2010) 10:22. doi: 10.1186/1471-2407-10-22
27. Hindi N, Casali PG, Morosi C, Messina A, Palassini E, Pilotti S, et al. Imatinib in advanced chordoma: a retrospective case series analysis. Eur J Cancer (2015) 51:2609–14. doi: 10.1016/j.ejca.2015.07.038
28. Lipplaa A, Dijkstra S, Gelderblom H. Efficacy of pazopanib and sunitinib in advanced axial chordoma: a single reference centre case series. Clin Sarcoma Res. (2016) 6:19. doi: 10.1186/s13569-016-0059-x
29. Schonegger K, Gelpi E, Prayer D, Dieckmann K, Matula C, Hassler M, et al. Recurrent and metastatic clivus chordoma: systemic palliative therapy retards disease progression. Anticancer Drugs (2005) 16:1139–43. doi: 10.1097/00001813-200511000-00015
30. Hof H, Welzel T, Debus J. Effectiveness of cetuximab/gefitinib in the therapy of a sacral chordoma. Onkologie (2006) 29:572–4. doi: 10.1159/000096283
31. Linden O, Stenberg L, Kjellen E. Regression of cervical spinal cord compression in a patient with chordoma following treatment with cetuximab and gefitinib. Acta Oncol. (2009) 48:158–9. doi: 10.1080/02841860802266672
32. Singhal N, Kotasek D, Parnis FX. Response to erlotinib in a patient with treatment refractory chordoma. Anticancer Drugs (2009) 20:953–5. doi: 10.1097/CAD.0b013e328330c7f0
33. Asklund T, Danfors T, Henriksson R. PET response and tumor stabilization under erlotinib and bevacizumab treatment of an intracranial lesion non-invasively diagnosed as likely chordoma. Clin Neuropathol. (2011) 30:242–6. doi: 10.5414/NP300371
34. Chay WY, Teo M, Sittampalam K, Toh HC. Effective use of thalidomide in the treatment of recurrent metastatic chordoma. J Clin Oncol. (2011) 29:e477–80. doi: 10.1200/JCO.2010.34.2139
35. Launay SG, Chetaille B, Medina F, Perrot D, Nazarian S, Guiramand J, et al. Efficacy of epidermal growth factor receptor targeting in advanced chordoma: case report and literature review. BMC Cancer (2011) 11:423. doi: 10.1186/1471-2407-11-423
36. Mercier F, Guiot MC, Bojanowski MW. Treatment of chordoma with imatinib complicated by intracranial hemorrhage: a case showing dissociation between biological effect and therapeutic outcome. J Neurooncol. (2012) 107:435–7. doi: 10.1007/s11060-011-0767-2
37. Svoboda RM, Mackay D, Welsch MJ, Anderson BE. Multiple cutaneous metastatic chordomas from the sacrum. J Am Acad Dermatol. (2012) 66:e246–7. doi: 10.1016/j.jaad.2011.07.037
38. Houessinon A, Boone M, Constans JM, Toussaint P, Chauffert B. Sustained response of a clivus chordoma to erlotinib after imatinib failure. Case Rep Oncol. (2015) 8:25–9. doi: 10.1159/000371843
39. Rohatgi S, Ramaiya NH, Jagannathan JP, Howard SA, Shinagare AB, Krajewski KM. Metastatic chordoma: report of the two cases and review of the literature. Eurasian J Med. (2015) 47:151–4. doi: 10.5152/eurasianjmed.2015.52
40. Aleksic T, Browning L, Woodward M, Phillips R, Page S, Henderson S, et al. Durable response of spinal chordoma to combined inhibition of IGF-1R and EGFR. Front Oncol. (2016) 6:98. doi: 10.3389/fonc.2016.00098
41. Migliorini D, Mach N, Aguiar D, Vernet R, Landis BN, Becker M, et al. First report of clinical responses to immunotherapy in 3 relapsing cases of chordoma after failure of standard therapies. Oncoimmunology (2017) 6:e1338235. doi: 10.1080/2162402X.2017.1338235
42. Trapani D, Conforti F, De Pas T. EGFR Inhibition in a pretreated sacral chordoma: a role for erlotinib? Case Report and a Brief Review of Literature. Transl Med UniSa (2017) 16:30–3.
43. Ribeiro M, De Sousa MC, Hanna SA, Maldaun MVC, Kurimori CO, De Lima L, et al. Tumor reduction with pazopanib in a patient with recurrent lumbar chordoma. Case Rep Oncol Med. (2018) 2018:4290131. doi: 10.1155/2018/4290131
44. Mcpherson CM, Suki D, Mccutcheon IE, Gokaslan ZL, Rhines LD, Mendel E. Metastatic disease from spinal chordoma: a 10-year experience. J Neurosurg Spine (2006) 5:277–80. doi: 10.3171/spi.2006.5.4.277
45. Chi S, Fouladi M, Shukla N, Bourdeaut F, Margol A, Makin G, et al. Phase 1 study of the EZH2 inhibitor, tazemetostat, in children with relapsed or refractory INI1-negative tumors including rhabdoid tumors, epithelioid sarcoma, chordoma, and synovial sarcoma. Mol Cancer Ther. (2018) 17:1. doi: 10.1158/1535-7163
46. Tamborini E, Miselli F, Negri T, Lagonigro MS, Staurengo S, Dagrada GP, et al. Molecular and biochemical analyses of platelet-derived growth factor receptor (PDGFR) B, PDGFRA, and KIT receptors in chordomas. Clin Cancer Res. (2006) 12:6920–8. doi: 10.1158/1078-0432.CCR-06-1584
47. Presneau N, Shalaby A, Ye H, Pillay N, Halai D, Idowu B, et al. Role of the transcription factor T (brachyury) in the pathogenesis of sporadic chordoma: a genetic and functional-based study. J Pathol. (2011) 223:327–35. doi: 10.1002/path.2816
48. Shah SR, David JM, Tippens ND, Mohyeldin A, Martinez-Gutierrez JC, Ganaha S, et al. Brachyury-YAP regulatory axis drives stemness and growth in cancer. Cell Rep. (2017) 21:495–507. doi: 10.1016/j.celrep.2017.09.057
49. Choy E, Macconaill LE, Cote GM, Le LP, Shen JK, Nielsen GP, et al. Genotyping cancer-associated genes in chordoma identifies mutations in oncogenes and areas of chromosomal loss involving CDKN2A, PTEN, and SMARCB1. PLoS ONE (2014) 9:e101283. doi: 10.1371/journal.pone.0101283
50. Lee-Jones L, Aligianis I, Davies PA, Puga A, Farndon PA, Stemmer-Rachamimov A, et al. Sacrococcygeal chordomas in patients with tuberous sclerosis complex show somatic loss of TSC1 or TSC2. Genes Chromosomes Cancer (2004) 41:80–5. doi: 10.1002/gcc.20052
51. Hallor KH, Staaf J, Jonsson G, Heidenblad M, Vult Von Steyern F, Bauer HC, et al. Frequent deletion of the CDKN2A locus in chordoma: analysis of chromosomal imbalances using array comparative genomic hybridisation. Br J Cancer (2008) 98:434–42. doi: 10.1038/sj.bjc.6604130
52. Sommer J, Itani DM, Homlar KC, Keedy VL, Halpern JL, Holt GE, et al. Methylthioadenosine phosphorylase and activated insulin-like growth factor-1 receptor/insulin receptor: potential therapeutic targets in chordoma. J Pathol. (2010) 220:608–17. doi: 10.1002/path.2679
53. Tamborini E, Virdis E, Negri T, Orsenigo M, Brich S, Conca E, et al. Analysis of receptor tyrosine kinases (RTKs) and downstream pathways in chordomas. Neuro Oncol. (2010) 12:776–89. doi: 10.1093/neuonc/noq003
54. De Castro CV, Guimaraes G, Aguiar S Jr Lopes A, Baiocchi G, Da Cunha IW, et al. Tyrosine kinase receptor expression in chordomas: phosphorylated AKT correlates inversely with outcome. Hum Pathol. (2013) 44:1747–55. doi: 10.1016/j.humpath.2012.11.024
55. Akhavan-Sigari R, Gaab MR, Rohde V, Abili M, Ostertag H. Prognostic significance of immunohistochemical expression of VEGFR2 and iNOS in spinal chordoma. Eur Spine J. (2014) 23:2416–22. doi: 10.1007/s00586-014-3417-5
56. Hu Y, Mintz A, Shah SR, Quinones-Hinojosa A, Hsu W. The FGFR/MEK/ERK/brachyury pathway is critical for chordoma cell growth and survival. Carcinogenesis (2014) 35:1491–9. doi: 10.1093/carcin/bgu014
57. Magnaghi P, Salom B, Cozzi L, Amboldi N, Ballinari D, Tamborini E, et al. Afatinib is a new therapeutic approach in chordoma with a unique ability to target EGFR and brachyury. Mol Cancer Ther. (2018) 17:603–13. doi: 10.1158/1535-7163.MCT-17-0324
58. Choi H, Charnsangavej C, Faria SC, Macapinlac HA, Burgess MA, Patel SR, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. (2007) 25:1753–9. doi: 10.1200/JCO.2006.07.3049
59. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer (2009) 45:228–47. doi: 10.1016/j.ejca.2008.10.026
60. Stommel JM, Kimmelman AC, Ying H, Nabioullin R, Ponugoti AH, Wiedemeyer R, et al. Coactivation of receptor tyrosine kinases affects the response of tumor cells to targeted therapies. Science (2007) 318:287–90. doi: 10.1126/science.1142946
61. Scheipl S, Froehlich EV, Leithner A, Beham A, Quehenberger F, Mokry M, et al. Does insulin-like growth factor 1 receptor (IGF-1R) targeting provide new treatment options for chordomas? A retrospective clinical and immunohistochemical study. Histopathology (2012) 60:999–1003. doi: 10.1111/j.1365-2559.2012.04186.x
62. Akhavan-Sigari R, Gaab MR, Rohde V, Brandis A, Tezval H, Abili M, et al. Expression of vascular endothelial growth factor receptor 2 (VEGFR-2), inducible nitric oxide synthase (iNOS), and Ki-M1P in skull base chordoma: a series of 145 tumors. Neurosurg Rev. (2014) 37:79–88. doi: 10.1007/s10143-013-0495-5
63. Roux PP, Shahbazian D, Vu H, Holz MK, Cohen MS, Taunton J, et al. RAS/ERK signaling promotes site-specific ribosomal protein S6 phosphorylation via RSK and stimulates cap-dependent translation. J Biol Chem. (2007) 282:14056–64. doi: 10.1074/jbc.M700906200
64. Carracedo A, Ma L, Teruya-Feldstein J, Rojo F, Salmena L, Alimonti A, et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest. (2008) 118:3065–74. doi: 10.1172/JCI34739
65. Yang C, Schwab JH, Schoenfeld AJ, Hornicek FJ, Wood KB, Nielsen GP, et al. A novel target for treatment of chordoma: signal transducers and activators of transcription 3. Mol Cancer Ther. (2009) 8:2597–605. doi: 10.1158/1535-7163.MCT-09-0504
66. Ohashi K, Maruvka YE, Michor F, Pao W. Epidermal growth factor receptor tyrosine kinase inhibitor-resistant disease. J Clin Oncol. (2013) 31:1070–80. doi: 10.1200/JCO.2012.43.3912
67. Chen K, Mo J, Zhou M, Wang G, Wu G, Chen H, et al. Expression of PTEN and mTOR in sacral chordoma and association with poor prognosis. Med Oncol. (2014) 31:886. doi: 10.1007/s12032-014-0886-7
68. Han S, Polizzano C, Nielsen GP, Hornicek FJ, Rosenberg AE, Ramesh V. Aberrant hyperactivation of akt and Mammalian target of rapamycin complex 1 signaling in sporadic chordomas. Clin Cancer Res. (2009) 15:1940–6. doi: 10.1158/1078-0432.CCR-08-2364
69. Lee DH, Zhang Y, Kassam AB, Park MJ, Gardner P, Prevedello D, et al. Combined PDGFR and HDAC inhibition overcomes PTEN disruption in chordoma. PLoS ONE (2015) 10:e0134426. doi: 10.1371/journal.pone.0134426
70. Wang L, Zehir A, Nafa K, Zhou N, Berger MF, Casanova J, et al. Genomic aberrations frequently alter chromatin regulatory genes in chordoma. Genes Chromosomes Cancer (2016) 55:591–600. doi: 10.1002/gcc.22362
71. Bosotti R, Magnaghi P, Di Bella S, Cozzi L, Cusi C, Bozzi F, et al. Establishment and genomic characterization of the new chordoma cell line Chor-IN-1. Sci Rep. (2017) 7:9226. doi: 10.1038/s41598-017-10044-3
72. Liu T, Shen JK, Choy E, Zhang Y, Mankin HJ, Hornicek FJ, et al. CDK4 Expression in chordoma: a potential therapeutic target running title: CDK4 expression in chordoma. J Orthop Res. (2018) 36:1581–9. doi: 10.1002/jor.23819
73. Levy J. Chordoma Foundation In Vivo Drug Screening Program. Chordoma Foundation (2018). Available online at: https://figshare.com/projects/Chordoma_Foundation_In_Vivo_Drug_Screening_Program/25948 (accessed January 25, 2018).
Keywords: molecular targeted therapy, bone tumor, chordoma, systematic review, imatinib, erlotinib
Citation: Meng T, Jin J, Jiang C, Huang R, Yin H, Song D and Cheng L (2019) Molecular Targeted Therapy in the Treatment of Chordoma: A Systematic Review. Front. Oncol. 9:30. doi: 10.3389/fonc.2019.00030
Received: 05 November 2018; Accepted: 10 January 2019;
Published: 01 February 2019.
Edited by:
Jian-ye Zhang, Guangzhou Medical University, ChinaReviewed by:
Claudia Palena, National Cancer Institute (NCI), United StatesVictor C. Kok, Asia University, Taiwan
Copyright © 2019 Meng, Jin, Jiang, Huang, Yin, Song and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huabin Yin, yinhuabin@aliyun.com
Dianwen Song, osongdianwen@126.com
Liming Cheng, limingcheng@tongji.edu.cn
†These authors have contributed equally to this work and share first authorship
 Tong Meng
Tong Meng Jiali Jin
Jiali Jin Cong Jiang
Cong Jiang Runzhi Huang
Runzhi Huang Huabin Yin
Huabin Yin Dianwen Song
Dianwen Song Liming Cheng
Liming Cheng