Effect of hypoalbuminemia on short-term outcomes after colorectal cancer surgery: A propensity score matching analysis
- 1Department of Gastrointestinal Surgery, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 2Department of Clinical Nutrition, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 3Department of General Surgery, Qijiang Hospital of the First Affiliated Hospital of Chongqing Medical University, Chongqing, China
Purpose: The purpose of our study was to investigate the effect of pre-operative hypoalbuminemia on the short-term outcomes after primary colorectal cancer (CRC) surgery.
Materials and methods: The retrospective study enrolled CRC patients who underwent primary surgery from January 2011 to December 2021 in a single teaching hospital. The short-term outcomes were compared between the hypoalbuminemia group and the normal group using propensity score matching (PSM). Univariate and multivariate logistic regression analyses were used for analyzing independent predictors of overall complications and major complications.
Results: A total of 7,072 patients from a single center were enrolled in this study. There were 1,078 (15.2%) patients in the pre-operative hypoalbuminemia group and 5,994 (84.8%) patients in the normal pre-operative albumin group. After 1:1 PSM, there were 1,028 patients in the hypoalbuminemia group and 1,028 patients in the normal group. No significant differences were found in baseline information between the two groups after PSM. In terms of short-term outcomes, the hypoalbuminemia group had a longer operation time (p = 0.003), greater volume of blood loss (p = 0.036), longer hospital stays (p < 0.01), higher proportion of overall complications (p = 0.003), major complications (p = 0.016), higher incidence of pneumonia and abdominal infection (p = 0.001) than the normal group after PSM. Furthermore, hypoalbuminemia was an independent predictor for overall complications (p = 0.008) and major complications (p = 0.016).
Conclusion: Pre-operative hypoalbuminemia increased overall complications and major complications after primary CRC surgery. Furthermore, hypoalbuminemia was an independent predictor for overall complications and major complications.
Introduction
Colorectal cancer (CRC) is the third most commonly occurring cancer and the second leading cause of cancer-related mortality worldwide (1–3). It is estimated that approximately 147,950 new CRC cases would be diagnosed, and 53,200 individuals would die of this disease in 2020 (4, 5). Although there have been many treatment methods for CRC including endoscopic mucosal resection (EMR), endoscopic submucosal dissection (ESD), surgery, cytotoxic chemotherapy, radiotherapy, and biologic therapy such as antibodies to cellular growth factors, immunotherapy, and combinations of methods (6–8), surgical resection remains the main curative option for both colon and rectal cancer (9–11).
Previous studies have shown that the rates of malnutrition in CRC patients ranged from 20 to 37%, depending on the tool used to assess nutritional status (12, 13). Pre-operative malnutrition is associated with higher post-operative morbidity, mortality, and length of hospital stay (14). Albumin has been used as a nutritional and inflammatory indicator (15) and pre-operative hypoalbuminemia was associated with post-operative complications, mortality, overall survival (OS), and cancer-specific survival (CSS) (16–18).
Hu et al. (19) previously reported an effect of pre-operative mild hypoalbuminemia on post-operative complications in CRC patients using propensity score matching (PSM), however, it depended on the American College of Surgeons-National Surgical Quality Improvement Program (ACS-NSQIP) database which lacks some important data such as tumor stage, operative time, the volume of blood loss, and length of hospital stay which are crucial in analyzing the effect of hypoalbuminemia on prognosis. Therefore, the purpose of our study was to investigate the effect of pre-operative hypoalbuminemia on short-term outcomes after primary CRC surgery using PSM.
Materials and methods
Setting
We retrospectively enrolled patients who underwent primary CRC surgery from January 2011 to December 2021 in a single teaching hospital. The study was approved by the ethics committee of the First Affiliated Hospital of Chongqing Medical University (2022-K205), and informed consent was obtained from all participants.
Study population selection
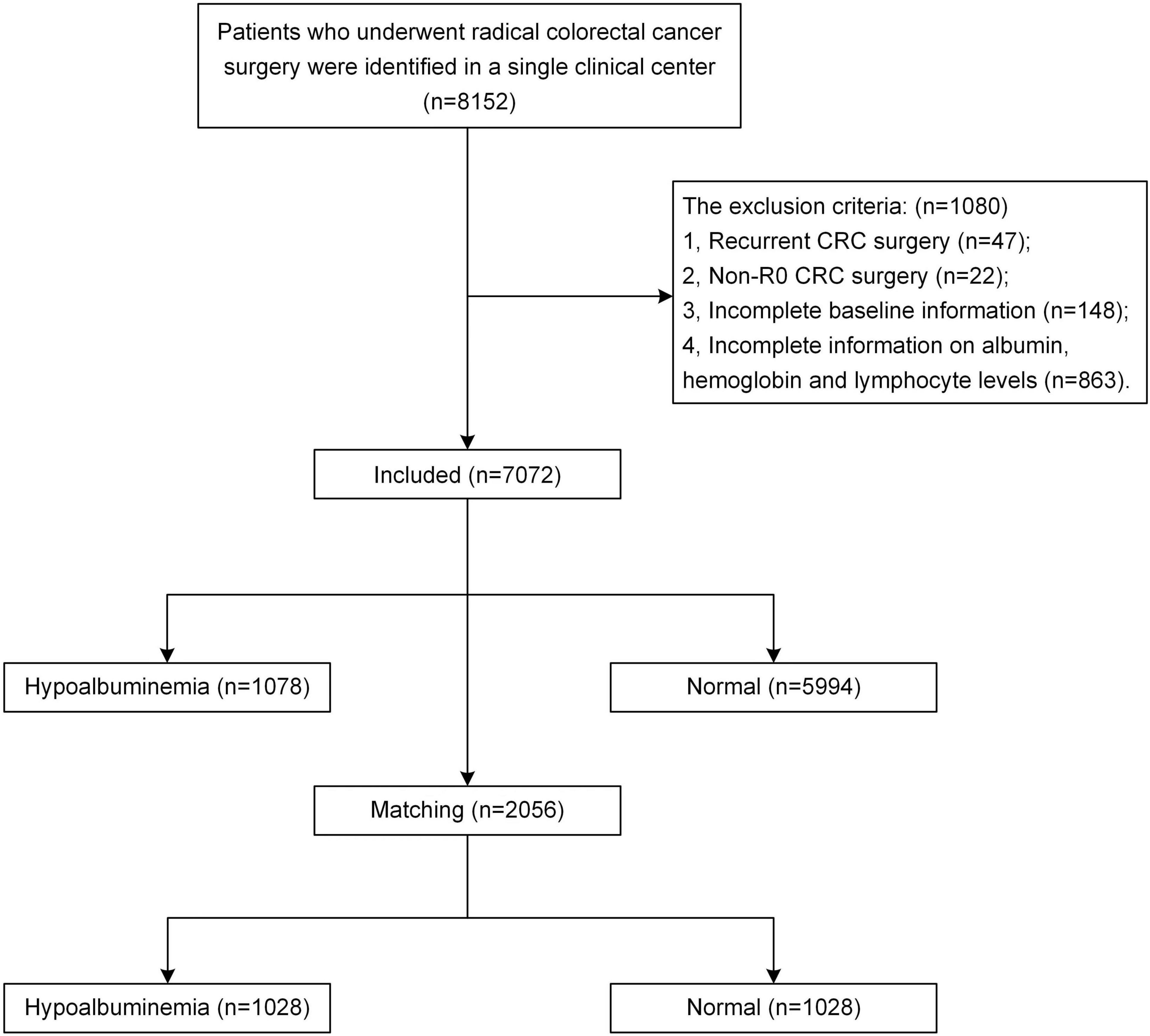
All enrolled patients underwent radical CRC resection (total mesorectal excision or complete mesocolic excision) by experienced surgeons according to the clinical guidelines, and the pathologic examination confirmed R0 resection. The exclusion criteria were as follows: (1) recurrent CRC surgery; (2) non-R0 CRC surgery, which was confirmed according to the pathologic examination; (3) incomplete baseline information; (4) incomplete information on albumin, hemoglobin, and lymphocyte levels.
Covariates
Data were collected through the inpatient system, outpatient system, and telephone review. The baseline information included serum albumin level, age, sex, body mass index (BMI), smoking, drinking, hypertension, type 2 diabetes mellitus (T2DM), coronary heart disease (CHD), chronic kidney disease (CKD), chronic liver disease (CLD), surgical history, laparoscopy, serum hemoglobin and lymphocyte levels, tumor size, tumor location, and tumor stage. Blood for testing was collected in the morning after 8 h of fasting in the supine position on the first day after admission.
The short-term outcomes studies were operation time, the volume of blood loss, hospital stay, blood transfusion, and overall complications (including anastomotic leakage, pneumonia, wound infection, lymphatic fistula, intestinal obstruction, venous thrombosis, abdominal infection, 30-day death, and other complications), major complications. The tumor stage was diagnosed according to the AJCC 8th Edition (20). Pre-operative serum albumin < 35 g/L was considered hypoalbuminemia. The severity of post-operative complications was defined according to the Clavien-Dindo classification (21), and ≥III classification, including requiring surgical, endoscopic, or radiological intervention; life-threatening complications (central nervous system complications: brain hemorrhage, ischemic stroke, and subarachnoid bleeding, but excluding transient ischemic attacks); requiring intermediate care (IC)/intensive care unit (ICU) management and death were considered major complications (21, 22).
Statistical analysis
Propensity score matching was conducted between the hypoalbuminemia group and the normal group to minimize the bias of baseline information. Nearest neighbor matching was performed without replacement at a 1:1 ratio and a caliper width with a.01 standard deviation was specified. The baseline information was matched including age, sex, BMI, smoking, drinking, hypertension, T2DM, CHD, CKD, CLD, surgical history, laparoscopy, serum hemoglobin and lymphocyte levels, tumor size, tumor location, and tumor stage.
For statistical analysis, SPSS 22 analysis software was used. Continuous variables are expressed as the mean ± standard deviation (SD) and an independent-sample t-test was used to compare the difference between the hypoalbuminemia group and the normal group. Categorical variables are expressed as absolute values and percentages, and the Chi-square test or Fisher’s exact test was used to compare the differences between the hypoalbuminemia group and the normal group. Univariate and multivariate logistic regression analyses were performed to identify independent predictive factors for overall complications and major complications. A bilateral p-value < 0.05 was considered statistically significant.
Results
According to the inclusion and exclusion criteria, a total of 7,072 patients with CRC from a single center were enrolled. There were 1,078 (15.2%) patients in the pre-operative hypoalbuminemia group and 5,994 (84.8%) patients in the normal pre-operative albumin level group. After 1:1 PSM, 1028 patients with pre-operative hypoalbuminemia and 1,028 without pre-operative hypoalbuminemia were included in this study. The flow chart of patient selection is shown in Figure 1.
Baseline information
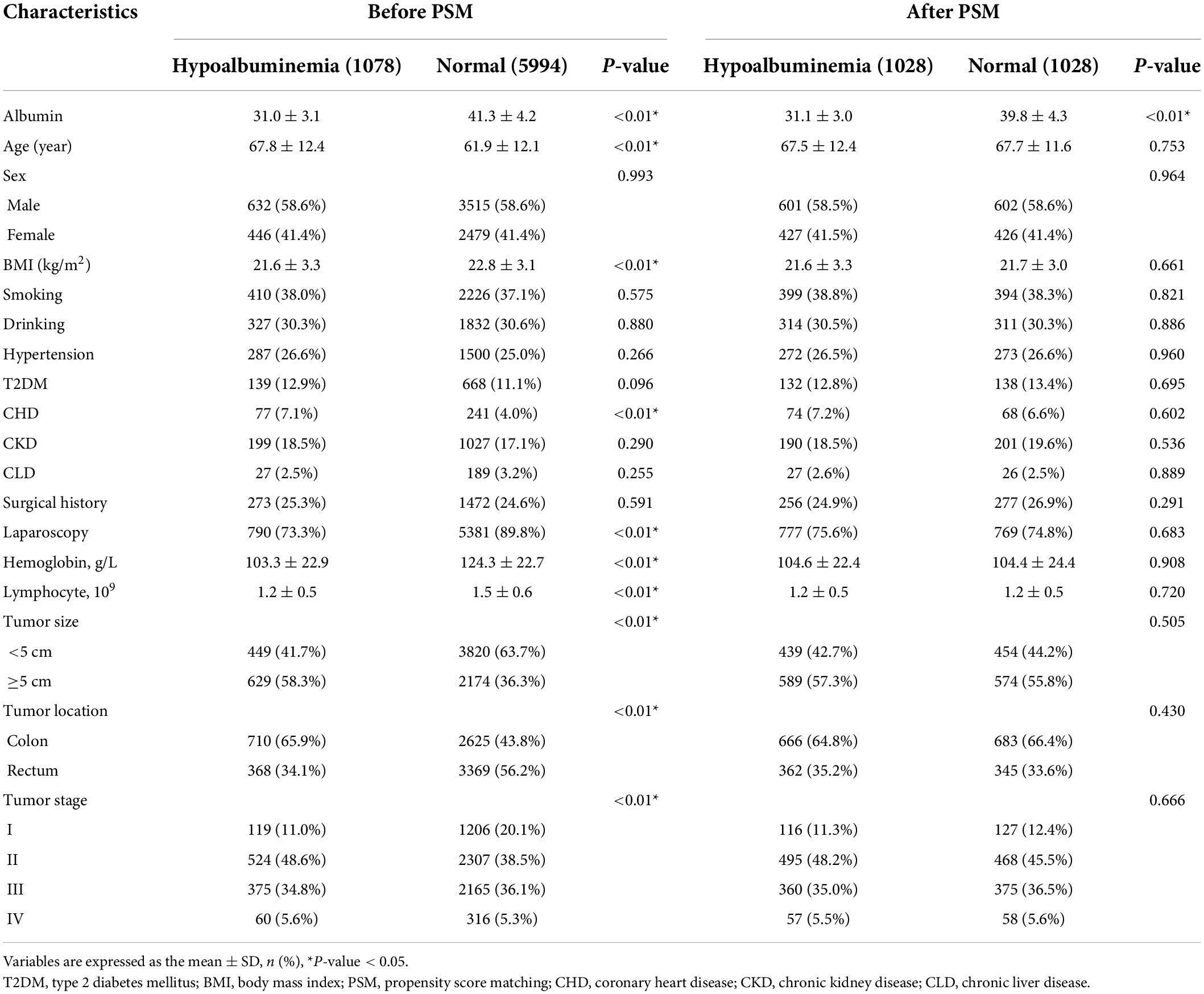
Baseline information was compared between the hypoalbuminemia group and the normal group. The hypoalbuminemia group had an older age (p < 0.01), lower BMI (p < 0.01), lower proportion of CHD (p < 0.01), lower proportion of laparoscopic CRC surgery (p < 0.01), lower serum hemoglobin level (p < 0.01), fewer serum lymphocyte (p < 0.01), larger tumor size (p < 0.01), and higher portion of colon cancer (p < 0.01) than the normal group before PSM. However, there were no significant differences in age, CHD, laparoscopy, hemoglobin, lymphocytes, tumor size, tumor location, or tumor stage between the two groups after PSM (p > 0.05) (Table 1).
Short-term outcomes
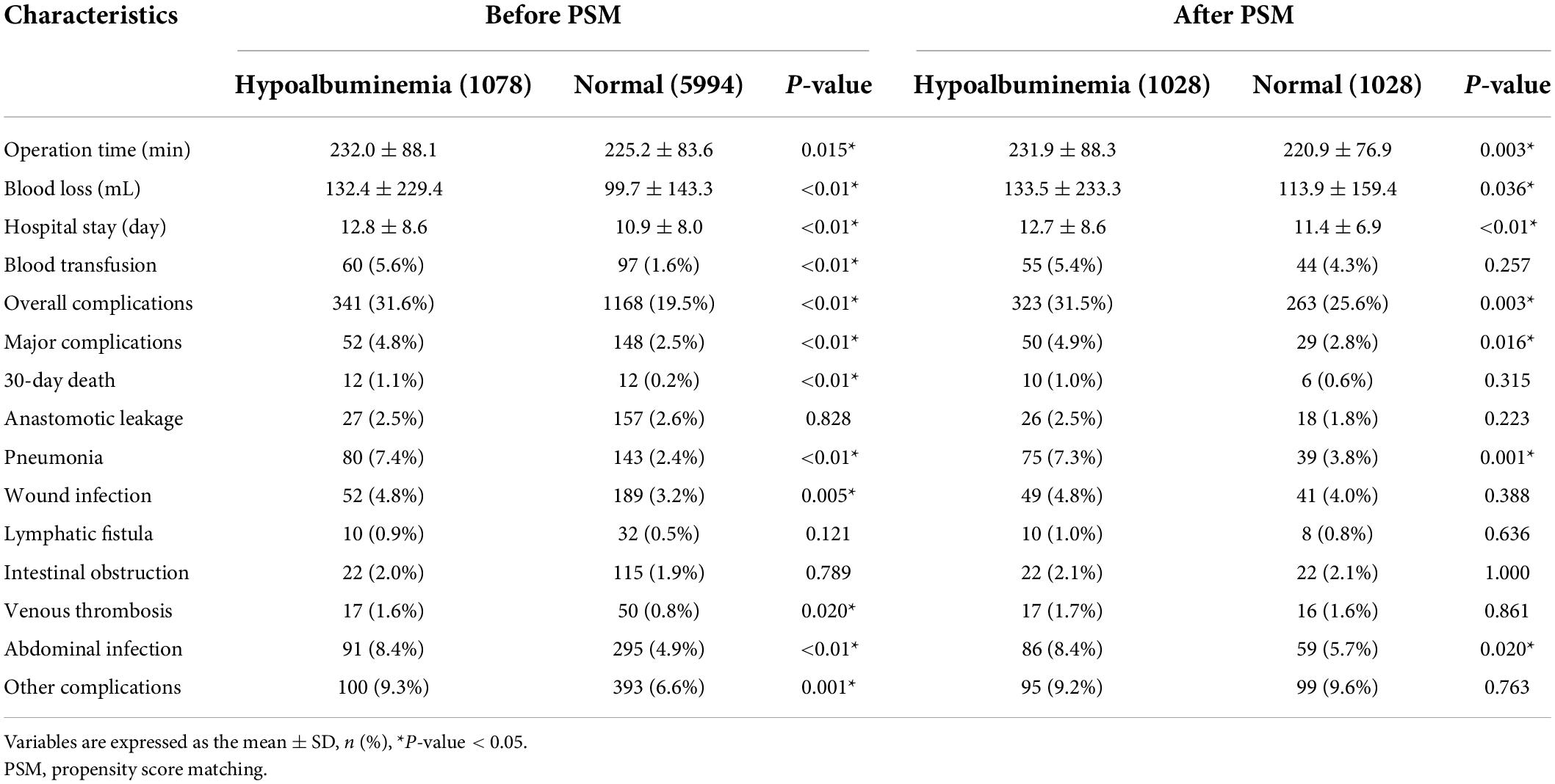
Table 2 shows the differences in short-term outcomes, including operation time, the volume of blood loss, hospital stay, blood transfusion, overall complications, major complications, 30-day death, and different types of post-operative complications between the hypoalbuminemia group and the normal group before and after PSM. The hypoalbuminemia group had a longer operation time (p = 0.015), greater volume of blood loss (p < 0.01), longer hospital stays (p < 0.01), higher proportion of blood transfusion (p < 0.01), overall complications (p < 0.01), major complications (p < 0.01), and 30-day death (p < 0.01) than the normal group before PSM. In terms of post-operative complications, the hypoalbuminemia group had more pneumonia (p < 0.01), wound infection (p = 0.005), venous thrombosis (p = 0.02), abdominal infection (p < 0.01), and other complications (p = 0.001) than the normal group. After PSM, the hypoalbuminemia group still had a longer operation time (p = 0.003); greater volume of blood loss (p = 0.036); longer hospital stay (p < 0.01); higher proportion of overall complications (p = 0.003) and major complications (p = 0.016); and a greater incidence of pneumonia (p = 0.001) and abdominal infection (p = -0.02) than the normal group.
Univariate and multivariate logistic regression analyses of the overall complications
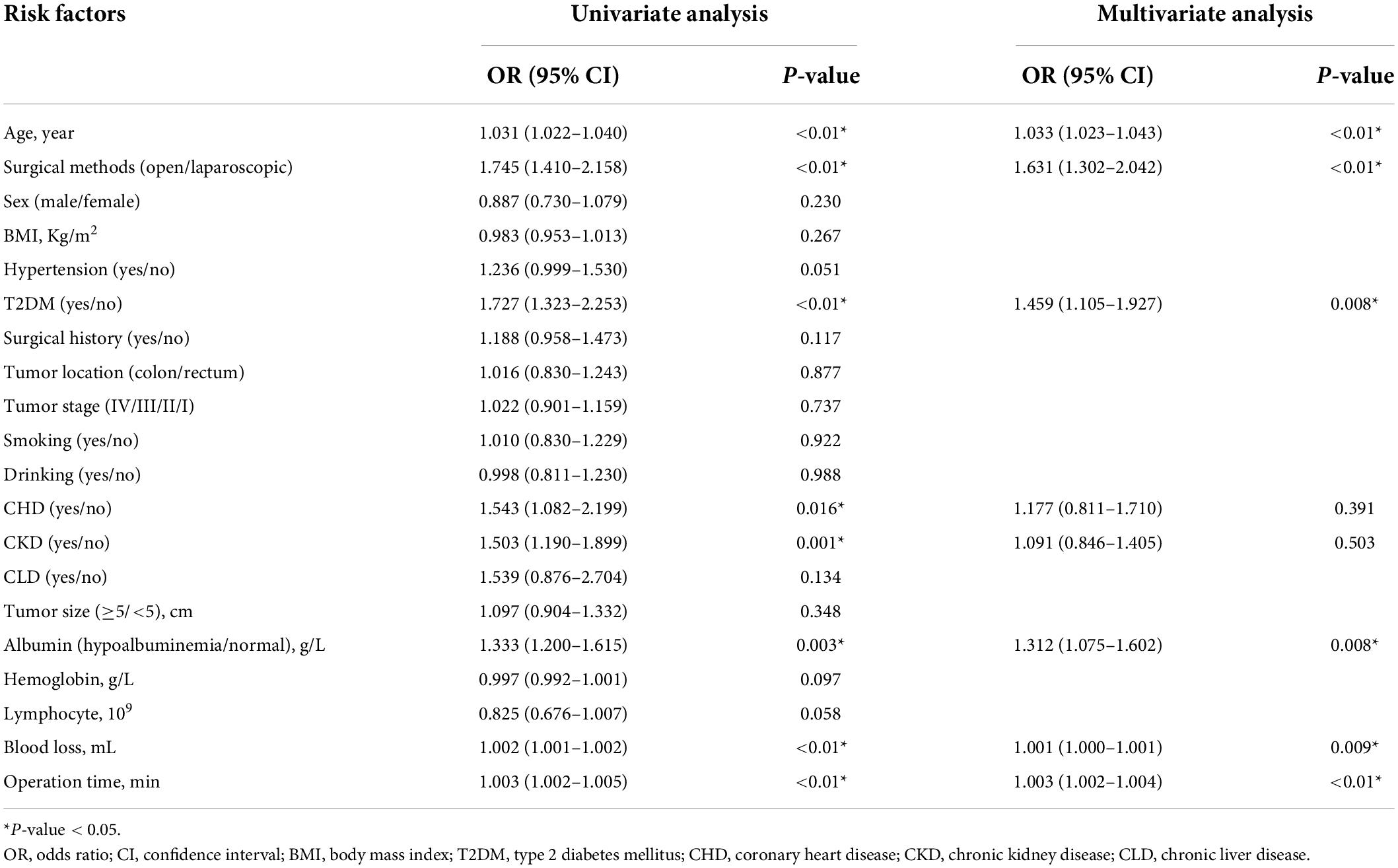
Age (p < 0.01, HR = 1.031, 95% CI = 1.022–1.04), surgical methods (p < 0.01, HR = 1.745, 95% CI = 1.41–2.158), T2DM (p < 0.01, HR = 1.727, 95% CI = 1.323–2.253), CHD (p = 0.016, HR = 1.543, 95% CI = 1.082–2.199), CKD (p = 0.001, HR = 1.503, 95% CI = 1.19–1.899), hypoalbuminemia (p = 0.003, HR = 1.333, 95% CI = 1.2–1.615), volume of blood loss (p < 0.01, HR = 1.002, 95% CI = 1.001–1.002), and operation time (p < 0.01, HR = 1.003, 95% CI = 1.002–1.005) were predictors in univariate analysis. In multivariate analysis, age (p < 0.01, HR = 1.033, 95% CI = 1.023–1.043), surgical methods (p < 0.01, HR = 1.631, 95% CI = 1.302–2.042), T2DM (p = 0.008, HR = 1.459, 95% CI = 1.105–1.927), hypoalbuminemia (p = 0.008, HR = 1.312, 95% CI = 1.075–1.602), blood loss (p = 0.009, HR = 1.001, 95% CI = 1–1.001), and operation time (p < 0.01, HR = 1.003, 95% CI = 1.002–1.004) were independent predictors for overall complications (Table 3).
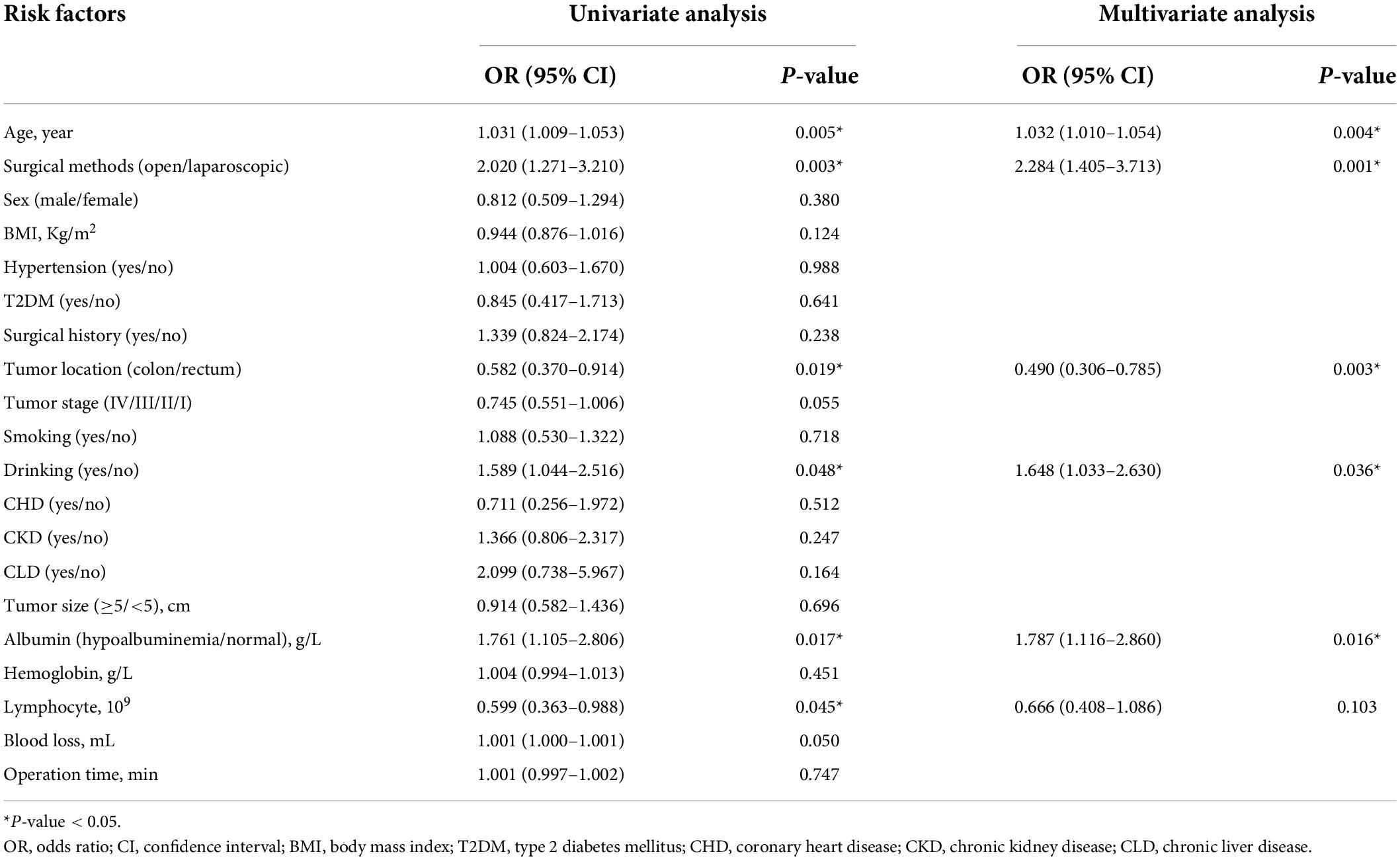
Univariate and multivariate logistic regression analyses of the major complications
In terms of the major complications, age (p = 0.005, HR = 1.031, 95% CI = 1.009–1.053), surgical methods (p = 0.003, HR = 2.02, 95% CI = 1.271–3.21), tumor location (p = 0.019, HR = 0.582, 95% CI = 0.37–0.914), drinking (p = 0.048, HR = 1.589, 95% CI = 1.044–2.516), hypoalbuminemia (p = 0.017, HR = 1.761, 95% CI = 1.105–2.806), and serum lymphocyte (p = 0.045, HR = 0.599, 95% CI = 0.363–0.988) were predictors in univariate analysis. In multivariate analysis, age (p = 0.004, HR = 1.032, 95% CI = 1.01–1.054), surgical methods (p = 0.001, HR = 2.284, 95% CI = 1.405–3.713), tumor location (p = 0.003, HR = 0.49, 95% CI = 0.306–0.785), drinking (p = 0.036, HR = 1.648, 95% CI = 1.033–2.63), and hypoalbuminemia (p = 0.016, HR = 1.787, 95% CI = 1.116–2.86) were independent predictors for major complications (Table 4).
Discussion
In this study, we found that the hypoalbuminemia group had a longer operation time and hospital stay, a greater volume of blood loss, a higher proportion of overall complications and major complications, and a greater incidence of pneumonia and abdominal infection than the normal group after 1:1 PSM. Hypoalbuminemia was an independent predictor for overall complications and major complications.
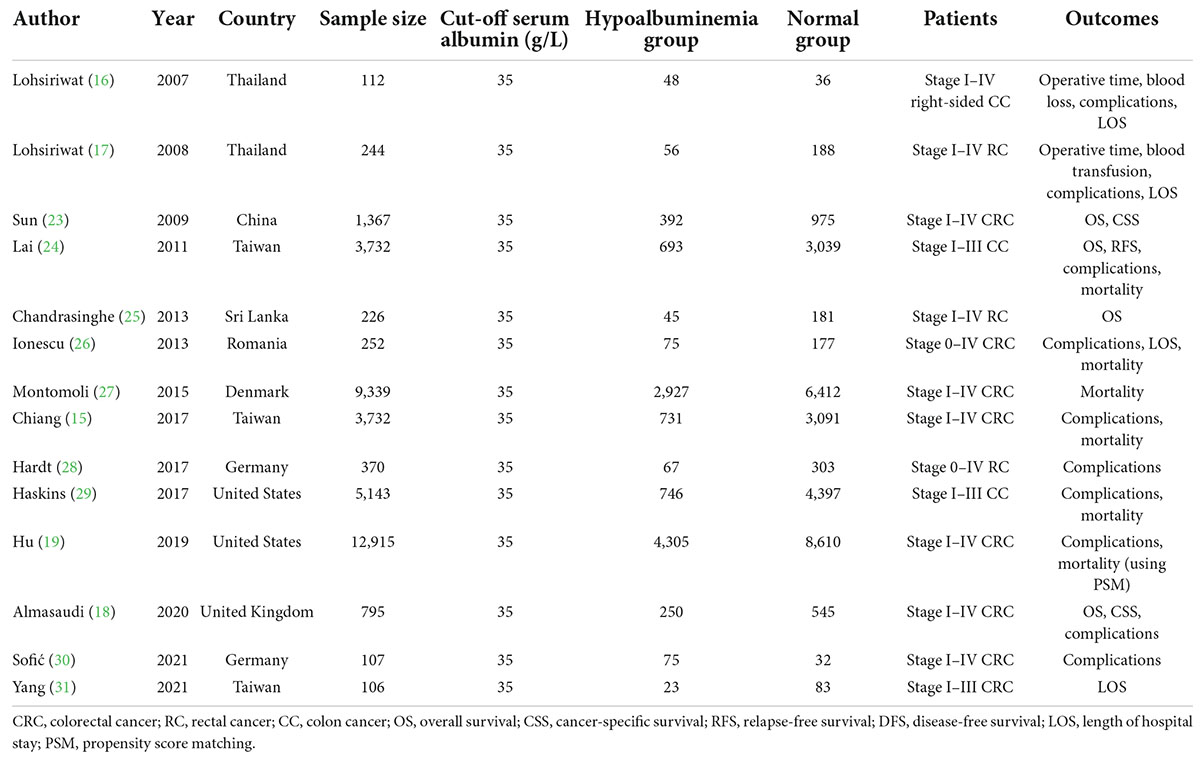
Some studies strengthened the correlation of pre-operative hypoalbuminemia with post-operative outcomes in CRC patients (15–19, 23–31), and we summarize them in Table 5. Some of these studies highlighted the prognosis, such as OS (18, 23–25), CSS (18, 23), and relapse-free survival (RFS) (24), and found that hypoalbuminemia was a prognostic factor for the poorer long-term survival of colon and rectal cancer after curative surgery.
Regarding short-term outcomes, some studies reported that pre-operative hypoalbuminemia was associated with post-operative outcomes following CRC surgery including prolonged length of hospital stay (16, 17, 26, 31), more complications (15, 17–19, 24, 26, 28, 29), and higher incidence of mortality (15, 19, 27, 29). However, most of these studies had small sample sizes, and all of them lacked the matching of confounding factors in basic data which may interfere with the results. Hu et al. (19) previously reported that pre-operative mild hypoalbuminemia affected post-operative complications for CRC patients using PSM, however, it depended on the ACS-NSQIP database which lacked some important data such as tumor stage, operative time, the volume of blood loss, and length of hospital stay which were crucial in analyzing the effect of hypoalbuminemia on prognosis. Interestingly, the study of Sofić et al. (30) showed that pre-operatively measured levels of serum albumin cannot serve as predictors for post-operative complications. Therefore, whether pre-operative hypoalbuminemia can affect short-term outcomes or be an independent predictor for overall complications and major complications after primary radical CRC surgery should be analyzed in a large sample size with less confounding baseline information.
In this study, we analyzed the effect of pre-operative hypoalbuminemia on the short-term outcomes of CRC surgery, furthermore, PSM was conducted to minimize the bias of baseline information. To avoid affecting pre-operative serum albumin levels, commodities, including CKD and CLD were analyzed by PSM between the two groups. We found that pre-operative hypoalbuminemia prolonged the operation time and hospital stays and increased blood loss and the incidence of major complications and pneumonia. Albumin is the most abundant protein in human blood and increasing capillary permeability during inflammation promotes albumin transfer to the interstitial space (32, 33). Hypoalbuminemia was associated with inflammation (32, 34, 35), indicating that in patients with pre-operative inflammation (36), the inflammation might induce more blood loss, increase the difficulty of the operation and prolong the operation time. Furthermore, hypoalbuminemia affects tissue damage and wound healing post-operatively, impairing gastrointestinal function and mobility, and slowing recovery (35, 36), which might lead to a longer hospital stay.
After 1:1 PSM, we found that the hypoalbuminemia group had more overall complications than the normal group. In one study that enrolled 75 patients in the hypoalbuminemia group and 32 patients in the normal group pre-operatively measured levels of serum albumin could not serve as predictors for post-operative complications (30). The sample size of this German study was small, and some bias might exist. In the present study, hypoalbuminemia was found to be an independent predictive factor of overall complications, which was consistent with a previous study (17). A decrease in pre-operative albumin might be associated with inflammation which increases tissue catabolism (37), and patients with colorectal cancer might have accelerated loss of albumin from the gastrointestinal tract (32, 37, 38). Therefore, hypoalbuminemia might increase the incidence of post-operative overall complications.
Regarding major complications, the hypoalbuminemia group had more major complications than the normal group after PSM in this study. Many factors might affect major complications after primary CRC surgery, such as age, pre-operative morbidities, surgical methods, and tumor stage (39). A previous study showed that hypoalbuminemia was an independent risk factor for post-operative high-grade morbidity (28), and Montomoli et al. (27) found that pre-operative hypoalbuminemia increased 30-day mortality following CRC surgery. Our study was consistent with these studies, and we also found that hypoalbuminemia was an independent predictive factor of major complications. Large samples and randomized controlled trials are needed to clarify the mechanisms of this correlation.
The current study analyzed the effect of hypoalbuminemia on short-term outcomes after CRC surgery in relatively big data using PSM. To our knowledge, this was the first study to analyze the effect of pre-operative hypoalbuminemia on short-term outcomes after primary radical CRC surgery in southwestern China.
There are some limitations of our study. First, this was a retrospective single-center study that inevitably had some selection bias. Second, the important inflammation parameter C-reactive protein (CRP) was not a routine test in our patients; we did not include CRP in this study and did not judge the degree of inflammation. Third, we defined hypoalbuminemia according to the lower limit of reference values and did not consider it a continuous variable or classify it into multiple categories, which might affect the results. Multicenter and multigroup (according to different serum albumin levels) trials with long-term prognosis could be conducted further.
In conclusion, pre-operative hypoalbuminemia increases overall complications and major complications after primary CRC surgery. Furthermore, hypoalbuminemia is an independent predictor for overall complications and major complications.
Data availability statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by The First Affiliated Hospital of Chongqing Medical University, 2022-K205. The patients/participants provided their written informed consent to participate in this study.
Author contributions
BK, X-YL, and DP contributed to the conception and design of the study. Y-XC and WT organized the database. DP finished the statistical analysis. BK and X-YL wrote the first draft of the manuscript. All authors contributed to revising the manuscript, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Funding
This study was supported by Chongqing’s Key Diseases Research and Application Demonstration Program [Colorectal Cancer Prevention and Treatment Technology Research and Application Demonstration (No. 2019ZX003)].
Acknowledgments
We acknowledge all the authors whose publications are referred to in our manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Baidoun F, Elshiwy K, Elkeraie Y, Merjaneh Z, Khoudari G, Sarmini MT, et al. Colorectal cancer epidemiology: recent trends and impact on outcomes. Curr Drug Targets. (2021) 22:998–1009. doi: 10.2174/1389450121999201117115717
2. Shaukat A, Kahi CJ, Burke CA, Rabeneck L, Sauer BG, Rex DK. ACG clinical guidelines: colorectal cancer screening 2021. Am J Gastroenterol. (2021) 116:458–79. doi: 10.14309/ajg.0000000000001122
3. Peng D, Liu XY, Cheng YX, Tao W, Cheng Y. Improvement of diabetes mellitus after colorectal cancer surgery: a retrospective study of predictive factors for type 2 diabetes mellitus remission and overall survival. Front Oncol. (2021) 11:694997. doi: 10.3389/fonc.2021.694997
4. Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. (2020) 70:145–64. doi: 10.3322/caac.21601
5. Liu XY, Kang B, Cheng YX, Yuan C, Tao W, Zhang B, et al. The short-term and oncologic outcomes of younger VS older colorectal cancer patients undergoing primary surgery: a propensity score matching analysis. BMC Cancer. (2022) 22:153. doi: 10.1186/s12885-022-09246-4
6. Biller LH, Schrag D. Diagnosis and treatment of metastatic colorectal cancer: a review. JAMA. (2021) 325:669–85. doi: 10.1001/jama.2021.0106
7. Tanaka S, Kashida H, Saito Y, Yahagi N, Yamano H, Saito S, et al. JGES guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc. (2015) 27:417–34. doi: 10.1111/den.12456
8. Ferlitsch M, Moss A, Hassan C, Bhandari P, Dumonceau JM, Paspatis G, et al. Colorectal polypectomy and endoscopic mucosal resection (EMR): european society of gastrointestinal endoscopy (ESGE) Clinical Guideline. Endoscopy. (2017) 49:270–97. doi: 10.1055/s-0043-102569
9. Watanabe T, Momosaki R, Suzuki S, Abo M. Preoperative rehabilitation for patients undergoing colorectal cancer surgery: a retrospective cohort study. Support Care Cancer. (2020) 28:2293–7. doi: 10.1007/s00520-019-05061-z
10. Thanikachalam K, Khan G. Colorectal cancer and nutrition. Nutrients. (2019) 11:164. doi: 10.3390/nu11010164
11. Liu XY, Yuan C, Kang B, Cheng YX, Tao W, Zhang B, et al. Predictors associated with planned and unplanned admission to intensive care units after colorectal cancer surgery: a retrospective study. Support Care Cancer. (2022) 28:5099–105. doi: 10.1007/s00520-022-06939-1
12. Gillis C, Richer L, Fenton TR, Gramlich L, Keller H, Culos-Reed SN, et al. Colorectal cancer patients with malnutrition suffer poor physical and mental health before surgery. Surgery. (2021) 170:841–7. doi: 10.1016/j.surg.2021.04.003
13. Gillis C, Nguyen TH, Liberman AS, Carli F. Nutrition adequacy in enhanced recovery after surgery: a single academic center experience. Nutr Clin Pract. (2015) 30:414–9. doi: 10.1177/0884533614562840
14. Burden ST, Hill J, Shaffer JL, Todd C. Nutritional status of preoperative colorectal cancer patients. J Hum Nutr Diet. (2010) 23:402–7. doi: 10.1111/j.1365-277X.2010.01070.x
15. Chiang JM, Chang CJ, Jiang SF, Yeh CY, You JF, Hsieh PS, et al. Pre-operative serum albumin level substantially predicts post-operative morbidity and mortality among patients with colorectal cancer who undergo elective colectomy. Eur J Cancer Care (Engl). (2017) 26:e12403. doi: 10.1111/ecc.12403
16. Lohsiriwat V, Chinswangwatanakul V, Lohsiriwat S, Akaraviputh T, Boonnuch W, Methasade A, et al. Hypoalbuminemia is a predictor of delayed postoperative bowel function and poor surgical outcomes in right-sided colon cancer patients. Asia Pac J Clin Nutr. (2007) 16:213–7.
17. Lohsiriwat V, Lohsiriwat D, Boonnuch W, Chinswangwatanakul V, Akaraviputh T, Lert-Akayamanee N. Pre-operative hypoalbuminemia is a major risk factor for postoperative complications following rectal cancer surgery. World J Gastroenterol. (2008) 14:1248–51. doi: 10.3748/wjg.14.1248
18. Almasaudi AS, Dolan RD, Edwards CA, McMillan DC. Hypoalbuminemia reflects nutritional risk, body composition and systemic inflammation and is independently associated with survival in patients with colorectal cancer. Cancers (Basel). (2020) 12:1986. doi: 10.3390/cancers12071986
19. Hu WH, Eisenstein S, Parry L, Ramamoorthy S. Preoperative malnutrition with mild hypoalbuminemia associated with postoperative mortality and morbidity of colorectal cancer: a propensity score matching study. Nutr J. (2019) 18:33. doi: 10.1186/s12937-019-0458-y
20. Weiser MR. AJCC 8th edition: colorectal cancer. Ann Surg Oncol. (2018) 25:1454–5. doi: 10.1245/s10434-018-6462-1
21. Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. (2009) 250:187–96. doi: 10.1097/SLA.0b013e3181b13ca2
22. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. (2004) 240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae
23. Sun LC, Chu KS, Cheng SC, Lu CY, Kuo CH, Hsieh JS, et al. Preoperative serum carcinoembryonic antigen, albumin and age are supplementary to UICC staging systems in predicting survival for colorectal cancer patients undergoing surgical treatment. BMC Cancer. (2009) 9:288. doi: 10.1186/1471-2407-9-288
24. Lai CC, You JF, Yeh CY, Chen JS, Tang R, Wang JY, et al. Low preoperative serum albumin in colon cancer: a risk factor for poor outcome. Int J Colorectal Dis. (2011) 26:473–81. doi: 10.1007/s00384-010-1113-4
25. Chandrasinghe PC, Ediriweera DS, Kumarage SK, Deen KI. Pre-operative hypoalbuminaemia predicts poor overall survival in rectal cancer: a retrospective cohort analysis. BMC Clin Pathol. (2013) 13:12. doi: 10.1186/1472-6890-13-12
26. Ionescu D, Tibrea C, Puia C. Pre-operative hypoalbuminemia in colorectal cancer patients undergoing elective surgery - a major risk factor for postoperative outcome. Chirurgia (Bucur). (2013) 108:822–8.
27. Montomoli J, Erichsen R, Antonsen S, Nilsson T, Sørensen HT. Impact of preoperative serum albumin on 30-day mortality following surgery for colorectal cancer: a population-based cohort study. BMJ Open Gastroenterol. (2015) 2:e000047. doi: 10.1136/bmjgast-2015-000047
28. Hardt J, Pilz L, Magdeburg J, Kienle P, Post S, Magdeburg R. Preoperative hypoalbuminemia is an independent risk factor for increased high-grade morbidity after elective rectal cancer resection. Int J Colorectal Dis. (2017) 32:1439–46. doi: 10.1007/s00384-017-2884-7
29. Haskins IN, Baginsky M, Amdur RL, Agarwal S. Preoperative hypoalbuminemia is associated with worse outcomes in colon cancer patients. Clin Nutr. (2017) 36:1333–8. doi: 10.1016/j.clnu.2016.08.023
30. Sofić A, Rašić I, Halilović E, Mujić A, Muslić D. Is preoperative hypoproteinemia associated with colorectal cancer stage and postoperative complications? Med Glas (Zenica). (2021) 18:450–5. doi: 10.17392/1353-21
31. Yang SP, Wang TJ, Huang CC, Chang SC, Liang SY, Yu CH. Influence of albumin and physical activity on postoperative recovery in patients with colorectal cancer: an observational study. Eur J Oncol Nurs. (2021) 54:102027. doi: 10.1016/j.ejon.2021.102027
32. Soeters PB, Wolfe RR, Shenkin A. Hypoalbuminemia: pathogenesis and clinical significance. JPEN J Parenter Enteral Nutr. (2019) 43:181–93. doi: 10.1002/jpen.1451
33. Jensen GL, Bistrian B, Roubenoff R, Heimburger DC. Malnutrition syndromes: a conundrum vs continuum. JPEN J Parenter Enteral Nutr. (2009) 33:710–6. doi: 10.1177/0148607109344724
34. Sleep D. Albumin and its application in drug delivery. Expert Opin Drug Deliv. (2015) 12:793–812. doi: 10.1517/17425247.2015.993313
35. Evans DC, Corkins MR, Malone A, Miller S, Mogensen KM, Guenter P, et al. The use of visceral proteins as nutrition markers: an ASPEN position paper. Nutr Clin Pract. (2021) 36:22–8. doi: 10.1002/ncp.10588
36. Mazzaferro EM, Edwards T. Update on albumin therapy in critical illness. Vet Clin North Am Small Anim Pract. (2020) 50:1289–305. doi: 10.1016/j.cvsm.2020.07.005
37. Kim S, McClave SA, Martindale RG, Miller KR, Hurt RT. Hypoalbuminemia and clinical outcomes: what is the mechanism behind the relationship? Am Surg. (2017) 83:1220–7. doi: 10.1177/000313481708301123
38. Fulks M, Stout RL, Dolan VF. Albumin and all-cause mortality risk in insurance applicants. J Insur Med. (2010) 42:11–7.
Keywords: colorectal cancer, hypoalbuminemia, outcomes, complications, surgery
Citation: Kang B, Zhao Z-Q, Liu X-Y, Cheng Y-X, Tao W, Wei Z-Q and Peng D (2022) Effect of hypoalbuminemia on short-term outcomes after colorectal cancer surgery: A propensity score matching analysis. Front. Nutr. 9:925086. doi: 10.3389/fnut.2022.925086
Received: 21 April 2022; Accepted: 29 July 2022;
Published: 29 August 2022.
Edited by:
Clelia Madeddu, University of Cagliari, ItalyReviewed by:
Jonathan Montomoli, Ospedale Infermi di Rimini, ItalyMasaichi Ohira, Osaka City University, Japan
Copyright © 2022 Kang, Zhao, Liu, Cheng, Tao, Wei and Peng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dong Peng, carry_dong@126.com
†These authors have contributed equally to this work
 Bing Kang
Bing Kang Zhi-Qiang Zhao
Zhi-Qiang Zhao Xiao-Yu Liu
Xiao-Yu Liu Yu-Xi Cheng
Yu-Xi Cheng Wei Tao
Wei Tao Zheng-Qiang Wei
Zheng-Qiang Wei Dong Peng
Dong Peng