Curcumin - The Nutraceutical With Pleiotropic Effects? Which Cardiometabolic Subjects Might Benefit the Most?
- 1Faculty of Medical Sciences in Katowice, Medical University of Silesia, Katowice, Poland
- 2Club of Young Hypertensiologists, Polish Society of Hypertension, Gdańsk, Poland
- 3Biotechnology Research Center, Pharmaceutical Technology Institute, Mashhad University of Medical Sciences, Mashhad, Iran
- 4Neurogenic Inflammation Research Center, Mashhad University of Medical Sciences, Mashhad, Iran
- 5NomiBiotech Corporation, Złotniki, Poland
- 6Clinical Pharmacy and Therapeutics Research Group, School of Pharmacy and Biomolecular Sciences, Liverpool John Moores University, Liverpool, United Kingdom
- 7Liverpool Centre for Cardiovascular Science, Liverpool, United Kingdom
- 8Department of Preventive Cardiology and Lipidology, Medical University of Lodz, Łódź, Poland
- 9Cardiovascular Research Centre, University of Zielona Gora, Zielona Góra, Poland
- 10Department of Cardiology and Adult Congenital Heart Diseases, Polish Mother's Memorial Hospital Research Institute (PMMHRI), Łódź, Poland
Despite continuous advances in pharmacotherapy, atherosclerotic cardiovascular disease remains the world's leading killer. Atherosclerosis relates not only to an increased level of cholesterol, but involves the development of atherosclerotic plaques, which are formed as a result of processes including inflammation and oxidative stress. Therefore, in addition to the classical risk factors for ASCVD (such as type 2 diabetes, overweight, obesity, hypertension and metabolic syndrome), residual risk factors such as inflammation and oxidative stress should also be reduced. The most important intervention in ASCVD is prevention, which includes promoting a healthy diet based on products of natural origin. Curcumin, which is often present in the diet, has been demonstrate to confer several benefits to health. It has been shown in numerous clinical trials that curcumin exhibited anti-diabetic, lipid-lowering, antihypertensive, antioxidant and anti-inflammatory effects, as well as promoting weight loss. All this means that curcumin has a comprehensive impact on the most important risk factors of ASCVD and may be a beneficial support in the treatment of these diseases. Recently, it has also been shown that curcumin may have a beneficial effect on the course of SARS-CoV-2 infection and might be helpful in the prevention of long-COVID complications. The aim of this review is to summarize the current knowledge regarding the safety and efficacy of curcumin in the prevention and treatment of cardiometabolic diseases.
Introduction
The incidence of cardiovascular disease (CVD) in the world in 2019 was 523 million (95% uncertainty interval, UI: 497-550). The number of patients with CVD doubled between 1990 and 2019. The number of CVD deaths steadily increased from 12.1 million (95% UI: 11.4–12.6) in 1990 to 18.6 million (95% UI: 17.1–19.7) in 2019 (1). Of all CVDs, ischemic heart disease (IHD) was the most common cause of death (IHD; 49.2%). CVD causes twice as many deaths as cancers (2).
Such a high prevalence of CVD is a result of the high prevalence in the population of cardiovascular risk factors, such as carbohydrate disorders and diabetes, overweight and obesity, metabolic syndrome, hypertension, and lipid disorders. The worldwide prevalence of prediabetes (measured as impaired glucose tolerance) in 2019 was 7.5%, while it is estimated that by 2045 it will increase to 8.6% (3). The prevalence of diabetes in the world is also increasing. In 1990, the prevalence of this disease was 211.2 million (95% UI: 196.0–228.5), while in 2017 the number increased to 476.0 million (95% UI: 436.6–522.8) (4). The global prevalence of overweight and obesity is also significantly increasing. According to WHO (World Health Organization) Global Health Observatory data, in 2016, the prevalence of overweight and obesity was 1.9 billion and 650 million, respectively (5). It is estimated that in 2030 the number of overweight and obese patients will amount to 2.16 billion and 1.12 billion, respectively (6). The prevalence of the metabolic syndrome is also increasing, especially among young adults (7). Another important risk factor for CVD with an increasing prevalence is hypertension. The number of people diagnosed with hypertension doubled from 1990 to 2019, from 331 (95% CI: 306–359) million women and 317 (95% CI: 292–344) million men in 1990 to 626 (95% CI: 584–668) million women and 652 (95% CI: 604–698) million men in 2019 (8). Moreover, dyslipidemias are extremely common and contribute substantially to the occurrence of atherosclerotic CVD (ASCVD) (9). ASCVD is defined as coronary artery disease (CAD), cerebrovascular disease, or peripheral arterial disease (PAD) of atherosclerotic origin. ASCVD represents the most common cause of morbidity and mortality worldwide (10).
It should be mentioned that the current coronavirus disease 2019 (COVID-19) pandemic has been associated with an increasing burden of CVD risk factors in the population. This may result in future substantial increases in the incidence of CVD (11). Moreover, it is extremely important to control risk factors for CVD and to manage CVD effectively during the pandemic to improve the prognosis of COVID-19 patients (12).
Given the extremely unfavorable epidemiological data on CVD and its risk factors, new and effective therapies are being sought for the condition. Pharmacotherapy and lifestyle modifications remain the cornerstones of the prevention and treatment of CVD. However, additional interventions are needed to improve glycemic parameters, lipid parameters, blood pressure, and residual CVD risk factors, such as oxidative stress and chronic inflammation. The potential exploitation of non-traditional CVD risk reduction methods, such as use of curcumin (a very promising nutraceutical) is an area of growing scientific and clinical interest.
It is also very important to note that the prevalence of ASCVD is greatest in low- and middle-income countries, where access to pharmacological therapies can be limited (13). Moreover, in the United States, it has been observed that less affluent people are most likely to suffer from ASCVD (14). In this light, the availability of a cheap and commonly available food/nutraeutical such as curcumin could have an important role in the prevention of ASCVD.
This review summarizes current safety data for curcumin and its impact of on risk factors for ASCVD such as pre-diabetes, diabetes, obesity, metabolic syndrome, hypertension and hypercholesterolemia. Furthermore, data on the effect of curcumin on residual ASCVD risk factors such as inflammation and oxidative stress are provided.
In a position paper from an International Lipid Expert Panel (ILEP) (Table 1A), and the latest guidelines for the diagnosis and treatment of lipid disorders in Poland (Table 1B) as well as in the ILEP position paper on the anti-inflammatory effects of nutraceuticals (Table 1C) emphasis was made on the important role of curcumin in the prevention of ASCVD.
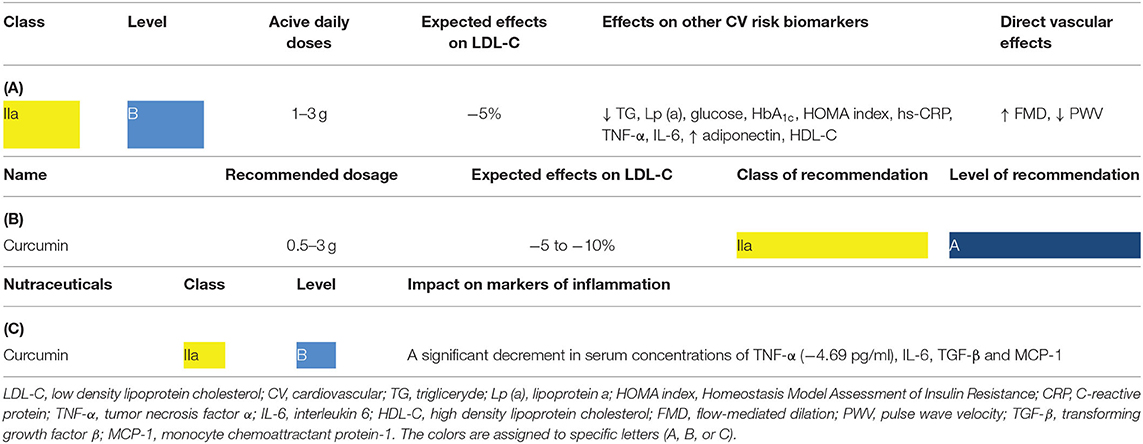
Table 1. Clinical relevance of curcumin based on existing recommendations: (A) lipid lowering properties based on the International Lipid Expert Panel (ILEP) position paper (15), (B) the place of curcumin in lipid-lowering therapy based on the Polish guidelines (16), (C) the role of curcumin in managing inflammatory parameters based on the ILEP position paper (17).
The use of nutraceuticals, including curcumin, may be useful in a range of clinical situations, including patients with mild to moderately elevated low density lipoprotein cholesterol (LDL-C) concentrations not treated with pharmacotherapy and with a low global risk of ASCVD; patients treated with statins who are unwilling or unable (as in the case of statin intolerance) to increase the dose or intensity of a statin (or add additional LDL-C lowering agents) regardless of the global risk of ASCVD and patients reluctant to take conventional LDL-C lowering pharmacotherapy (18, 19).
Another important justification for the topic under consideration is the fact that curcumin may be beneficial in supporting the treatment of COVID-19 (20), which is currently a challenge for healthcare systems worldwide. A recent systematic review by Vahedian-Azimi et al. showed that curcumin supplementation in patients with COVID-19 led to a significant decrease in common symptoms, duration of hospitalization and all-cause mortality. Moreover, a significant decrease in proinflammatory cytokines such as IL1β and IL6, with a concomitant significant increase in anti-inflammatory cytokines, including IL-10, IL-35 and TGF-α was observed. Thus, curcumin supplementation may offer an efficacious and safe option for improving COVID-19 disease outcomes and may be helpful in the prevention of long-term complications of COVID-19 (21, 22).
Summary and Take Home Message
Owing to its lipid-lowering and anti-inflammatory properties, curcumin is recommended for the prevention and treatment of ASCVD. Due to the widespread availability of curcumin, it can be an important component of the prevention of ASCVD (and may be used to support treatment) in low–and middle-income countries.
Curcumin–A Short Overview
Turmeric (Curcuma longa) is a plant related to the ginger family (Zingiberaceae), which originated from India and is currently grown in several other parts of the world, including Southeast Asia, China, and Latin America (23). Turmeric consists of ~70% carbohydrates, 13% water, 6% protein, 6% essential oils (phellandrene, sabinene, cineol, borneol, zingiberene, and sesquiterpenes), 5% fat, 3% mineral (potassium, calcium, phosphorus, iron, and sodium), 3–5% curcuminoids, and trace amounts of vitamins (B1, B2, C, and niacin). Among the curcuminoids, curcumin accounts for approximately 77%, demethoxycurcumin accounts for 17% and bisdemethoxycurcumin accounts for 3–6% (Figure 1) (23, 26, 27).
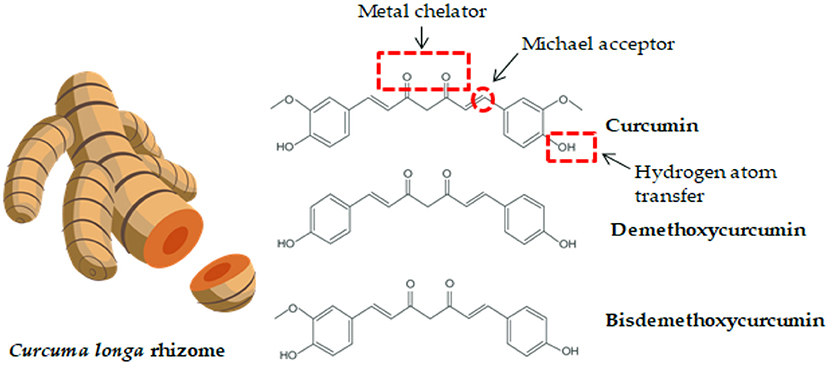
Figure 1. Curcuma longa rhizome and the chemical structure of curcumin and its derivatives. Functional groups in curcumin components which contribute to its activity and bioavailability are highlighted (24, 25).
The healing and health-promoting properties of turmeric were first reported in Middle Eastern medicine, or more precisely in the Indian Ayurverda (23).
The chemical structure of curcumin was described in 1910 by Polish chemists (28). Curcumin [1,7-bis (4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione] is a beta-diketone and consists of two feruloyl residues linked by a carbon atom1. The compound owes its characteristic yellow color to the presence of phenolic rings. Curcumin is a phenolic molecule that can exist in two different forms containing the same number of these same atoms in the molecule, but in different inter-convertible isomers (so-called tautomers). Curcumin tautomers exist are in the keto-enol form (23, 26). Phenol groups in the structure of curcumin explain its ability to eliminate oxygen free radicals such as: hydrogen peroxide (H2O2), superoxide anion () and nitric oxide (NO) (29). Curcumin metabolites are excreted from the body primarily in the feces, and, to a lesser extent, in the urine (29).
Curcumin exhibits a range of pharmacological and biological actions that have been demonstrated in both in vitro and in vivo studies, and include antioxidant, cardio-protective, anti-inflammatory, anti-microbial, nephro-protective, anti-neoplastic, hepato-protective, immunomodulatory, hypoglycaemic and anti-rheumatic effects (29).
Summary and Take Home Message
Curcumin is the most common curcuminoid found in turmeric which is widely used in natural medicine.
Bioavailability of Curcumin
Turmeric (and therefore curcumin) has been used for thousands of years in traditional Ayurvedic medicine and in the medical systems of countries in which this rhizome occurs naturally and forms part of the regular diet. It has been used as an anti-inflammatory drug and as a remedy for a number of diseases and health conditions (29). However, there are no clinical data that clearly confirms its effectiveness in this regard. Taking into account the growing body of research results, it can be concluded with a high degree of certainty that oral administration of curcumin as a dietary component in a naturally occurring form, (contained in both fresh and dried turmeric used as a seasoning and food ingredient, and administration of turmeric in the form of standardized curcuminoid extract from the turmeric rhizome) does not result in reproducible health-enhancing effects, probably because of the low bioavailability of curcumin delivered in this manner (24).
This issue has been discussed in numerous reviews, the general conclusion of which is that the basic obstacle in conducting structured clinical trials, resulting from the chemical structure of the curcumin molecule, is two major and related problems–low water solubility and subsequent low bioavailability (24, 30, 31). Moreover, most of the published studies results refer to the different combinations of various doses of curcumin administered together with substances referred to as “bioavailability enhancers” such as piperine. Piperine can enhance permeation of circumin through the epithelial barrier by inducing changes in membrane dynamics, however, due to its biological activity it might interfere not only with the curcumin molecule, but also exert some additional effects (25). The biological activity of piperine has been reviewed in Gorgani et al. (32). It is also worth mentioning that piperine, in addition to its role in increasing the bioavailability of curcumin, also directly affects ASCVD risk factors. Piperine has been found to have antidiabetic, anti-inflammatory, antioxidant and lipid-lowering effects (33). This means that in some studies where curcumin was used together with piperine, a synergistic effect may have occurred. As a result, curcumin is included in the so-called PAINS (pan-assay interference compounds), i.e., molecules with a structure that makes it impossible to study because of artifacts that prevent elucidation of a clear clinical picture (34).
The chemical structure of curcumin makes this compound practically insoluble in water–with solubility estimated at 3.12 mg/l at 25°C2 and insoluble in oil (35). Curcumin is soluble in some organic solvents such as methanol (4.44 mg/mL), 2-butanone (2.17 mg/mL), ethanol (5.6 mg/mL), isopropanol (3.93 mg/mL), acetone (7.75 mg/mL), 1,2-dichloroethane (0.5125 mg/mL) and dimethyl solphooxide (DMSO) (20 mg/mL) (36). Due to its very low solubility in water and fats, curcumin has a very low bioavailability. The absorption of this compound is too low for it to exhibit significant biological effects.
Curcumin is considered to be a non-toxic substance (37). A comprehensive review of the subject has been published by Soleimani et al. It has been clearly demonstrated that taking doses of up to 6 g of curcumin daily in patients with breast cancer (38) or 2 x 500 mg of curcumin with increased bioavailability were safe. In patients with fatty liver disease, curcumin does not cause side effects (39). According to EFSA recommendations, a safe dose of curcumin is up to 3 mg/kg of body weight3. It is worth bearing in mind that this dose refers to curcuminoid extract and does not take into account the potentially increased bioavailability of some formulations.
To solve the problem of low bioavailability of native curcumin, without resorting to the use of biologically active compounds such as piperine, in recent years a number of pharmaceutical strategies have been proposed. The approaches include dispersion, nano- and micro- formulations, encapsulation with polysaccharides and lipids, micellization and others. The formulations improve bioavailability by increasing absorption, stabilizing curcumin, or delaying its release.
It has been shown that dispersion of curcuminoids in an aqueous environment significantly improves their bioavailability (40). A common way to obtain curcuminoids dispersible in water is to dissolve them in 70–80% ethanol together with β-cyclodextrin, followed by heating and mixing for at least 4 h at a temperature of 70°C (41, 42). Solvents (ethanol and water) are usually removed by spray drying, vacuum evaporation or freeze drying. This method of obtaining curcuminoids dispersible in water has several disadvantages. Unfavorable chemical transformations of curcuminoids occur in the in the water-ethanol environment as a result of thermal decomposition. Research has demonstrated that in an aqueous environment when temperature is elevated temperature, even at the most favorable pH (curcuminoids are most stable at a pH between 3 and 6) curcuminoids undergo decomposition (43). The preparation of curcuminoids dispersible in water should be carried out at the lowest possible temperatures.
Other formulations of curcuminoids dispersible in water include curcumin adsorbed on silicates, e.g., silicon dioxide (44) or on microcrystalline cellulose with the addition of soy lecithin (45). Several approaches to prepare formulation based on curcuminoid extract suspended in fat particles have been proposed with the addition of phosphatidylcholine (46) or in short chain triacylglycerols with the addition of free fatty acids and polyglycerol esters, stabilized with hydroxypromyl methylcellulose, sodium alginate and microcrystalline cellulose (47) or curcumin powder dispersed in triacetin (glycerol triacetate) and a mixture of lipids obtained from the seeds of soybean, oil palm and rapeseed (48).
Other approaches include 20–28% of turmeric extract (a mixture of three curcuminoids), suspended in a mixture comprised of 63–75% of polyvinylpyrrolidone (E 1201), 10–40% cellulose derivatives with the addition of 1–3% of natural antioxidants (40), or essential oil consisting of 45% ar- turmerone and curcuminoids (49) or curcuminoids in combination with γ-cyclodextrin (50). Nano-colloidal preparation described by Sasaki et al. comprises up to 30% of curcuminoids with glycerin and Ghatti gum (51).
It should be emphasized that the data on the bioavailability of curcumin (curcuminides) in the form of complex formulations described above are inconclusive. Up to date, no comprehensive compilation of the data on bioavailability of a number of leading preparations, made in a uniform experimental system based on the use of a unified method, has been published. This applies to both the experimental model (data are mainly based on animal models, including mice and rats, with a small number of tests in humans), the method of administration, the dose per kilogram of body weight, and the method of determining the level of curcuminoids (taking into account the curcuminoid profile) in plasma, the time points of measurement, including the point where the concentration peak is reached, allowing an approximation of the absorption kinetics. The published review summaries are not based on a uniform baseline, due to the use of standardized but curcuminoid (not curcumin) extracts obtained by various extraction methods, and their very low solubility in water can significantly disturb the actual level of growth bioavailability, therefore the data and claims regarding the bioavailability must be considered as non-conclusive. Studies have demonstrated that the form of curcumin administration and the composition of the food in which it is found can significantly affect absorption (31).
Regardless of the research model and the preparation, curcumin reaches its peak concentration within 45–120 min after oral administration, and its level gradually decreases, returning to the base value. within 6–10 h after consumption of the preparation. Depending on the administered dose and the experimental model and physiological state, the maximum concentration of curcumin in the blood plasma is observed in a very wide range, and can be as high as 3,200 ng/ml (52–54).
Summary and Take Home Message
Due to its chemical structure, curcumin is characterized by low bioavailability from normal foods. Several methods have been developed to increase the bioavailability of curcumin, including nano-curcumin formulation.
Curcumin in Patients With Diabetes
The effectiveness of curcumin in the prevention of diabetes and its treatment support has been the subject of several randomized controlled clinical trials (Table 2).
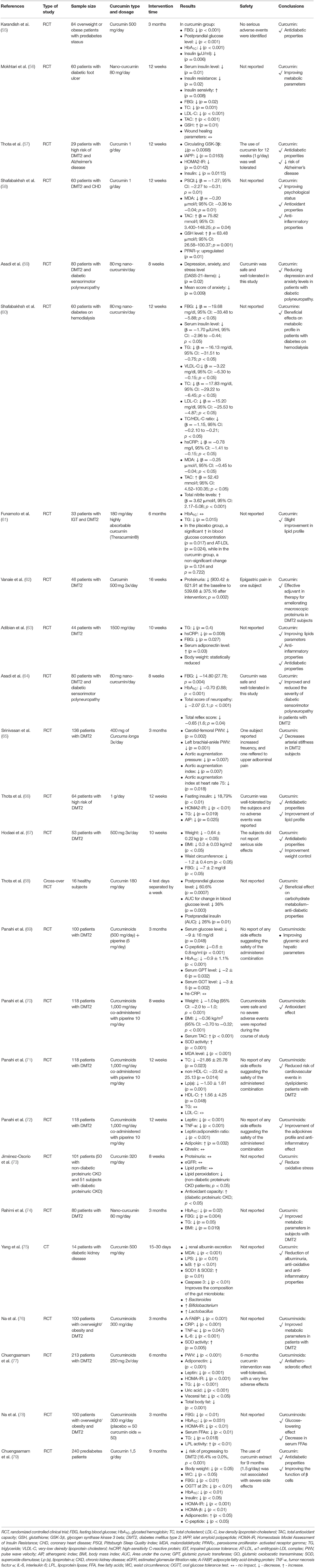
Table 2. Summary of studies on the effects of curcumin on the prevention and control of type 2 diabetes.
A meta-analysis by Zhang et al., which included the results of 4 randomized clinical trials (n = 453 subjects) assessed the efficacy and safety of curcumin on glycemic control in patients with diabetes mellitus type 2 (DMT2). In the studies which were eligible for inclusion, curcuminoids (doses: 300–1,000 mg) or curcumin (doses: 1,000–1,500 mg) or nanocurcumin (doses: 80 mg) were used for a period of 8–24 weeks. The obtained results differed depending on the origin of the respondents. The use of curcumin was associated with a lower: HOMA-IR (Middle East: MD = −0.60; 95% CI: −0.74 to −0.46 and Asia Pacific: MD = −2.41; 95% CI: −4.44 to −0.39), glycated hemoglobin (HbA1C) (MD = −0.70; 95% CI: −0.87 to −0.54), TC, TG, and LDL-C (Asia Pacific only: MD = −23.45; 95% CI: −40.06 to −6.84 and MD = −54.14; 95% CI: −95.71 to −12.57 and MD = −20.85; 95% CI: −28.78 to −12.92), FBG (Asia Pacific subgroup only: −0.57; 95% CI: −0.79 to −0.36). Moreover, curcumin led to a significant increase in adiponectin levels (MD = 0.50; 95% CI: 0.16–0.83). There were no serious side effects. Based on the results of this meta-analysis, researchers concluded that curcumin may assist in improving insulin resistance, glycemic control, and may result in as decrease in TG and TC in patients with DMT2 (80). A very interesting meta-analysis by Huang et al. including the results of 14 randomized clinical trials (n = 1,277 subjects) also assessed the anti-diabetic properties of curcuminoids. Only curcumin (compared to curcuminoids and turmeric) was shown to significantly reduce FBG (SMD −0.42; 95% CI: −0.76 to −0.07, p = 0.049). Moreover, the beneficial effect of curcuminoids was dose-dependent–only the use of ≥300 mg/day led to a significant reduction in FBG (SMD −0.58; 95% CI: −1.04 to −0.11, p = 0.000). The duration of curcuminoid therapy was also very important - only their use for ≥12 weeks led to a significant reduction in FBG (SMD −0.55; 95% CI: −0.97 to −0.13, p = 0.000). The observed effects of curcuminoids on FBG were significant in people with diabetes but not with metabolic syndrome. This meta-analysis also assessed the effect of curcuminoids on HbA1C levels. In the case of HbA1C, only the use of curcuminoids (vs curcumin) led to a significant decrease in it (SMD −0.414; 95% CI: −0.665 to −0.164, p = 0.001). Only the use of curcuminoids in a dose of ≥300 mg / day led to a significant decrease in HbA1C (SMD −0.322; 95% CI: −0.588 to −0.056, p = 0.018). Only the duration of the intervention for ≥12 weeks led to a significant reduction in HbA1C (SMD −0.488; 95% CI: −0.790 to −0.185, p = 0.002) (81). Similar results were obtained by de Melo et al. in a meta-analysis including the results of 11 randomized clinical trials. These researchers found that the use of curcuminoids or curcumin led to a decrease in FBG (MD −8.88 mg/dL; 95% CI: −5.04 to −2.72, p = 0.005), with isolated curcumin being more potent than curcuminoids (MD −16.02; 95% CI: −29.57 to −2.48 vs. MD = −8.82; 95% CI: −17.30 to −0.34) and this beneficial effect was significant only in people with pre-diabetes or DMT2. Moreover, use of curcumin led to a decrease in the concentrations of HbA1C (MD −0.54%; 95% CI: −1.09 to −0.002, p = 0.049) (82). A meta-analysis by Altobelli et al. evaluated the efficacy of curcumin in patients with uncomplicated DMT2. The meta-analysis included the results of 7 randomized clinical trials (n = 552 subjects). Curcumin use was been shown to be associated with reductions in: HbA1C (effect size: ES −0.42; 95% CI: −0.72 to −0.11, p = 0.008), HOMA-IR (ES −0.41; 95% CI: −0.66 to −0.22, p < 0.001), LDL-C (ES −0.28; 95% CI: −0.5 to −0.04, p = 0.021), TG (ES −0.57; 95% CI: −0.83 to −0.31, p < 0.001), TC (ES −0.30; 95% CI: −0.53 to −0.07, p = 0.01). The researchers concluded that the use of daily supplements of curcumin could improve some metabolic parameters in individuals with uncomplicated DMT2 (83).
A systematic review of the literature by Marton et al. of 16 studies found that the antidiabetic properties of curcumin are due to: (1) suppression of oxidative stress and inflammatory process; (2) reduction of fasting blood glucose, glycated hemoglobin, and body mass index; and (3) reduction in triglycerides, very-low density lipoprotein cholesterol (VLDL-C), TC, LDL-C, HDL-C, serum CRP, and plasma malonaldehyde (MDA) (84). Another systematic review of 11 studies (n = 1,131 participants) conducted by Mahdavi et al. assessed the efficacy of curcumin in supporting the treatment of type 2 diabetes. It was found that curcumin supplementation led to a reduction in FBS and HbA1C. Curcumin also tended to lower HOMA-IR. Patients who had used curcumin for ≥ 12 weeks showed a significant reduction in glycemic indices. Thus, as the authors of this review point out, curcumin may be a promising addition to therapies used to manage DMT2 (85). Another systematic review in which the anti-diabetic properties of curcumin was assessed was conducted by Pivari et al. who summarized the results of numerous in vitro and in vivo studies. They found strong evidence suggestive of the efficacy of curcumin in DMT2. It has been shown in clinical trials that curcumin is particularly effective in lowering HbA1C. The authors of this systematic review also concluded that curcumin has poor bioavailability (86).
In a new review of the literature by Mohammadi et al. the effect of curcumin on glucagon-like peptide-1 (GLP-1), dipeptidyl peptidase-4 (DPP-4), glucose transporters, α-glycosidase, α-amylase, and peroxisome proliferator -activated gamma receptor (PPARγ) were investigated in addition to the standard biomarkers of diabetes described above. These are novel signaling pathways involved in the potential beneficial effects of curcumin for the treatment of diabetes (87). A potential explanation for the effects of curcumin on signaling pathways may be the recently postulated action of curcumin and its structural derivatives, such as C0818 [3,5-(E)-bis(3-methoxy-4-hydroxybenzal)-4-piperidinonehydrochloride], as inhibitors of Hsp90 heat shock protein (88). This is consistent with the results on the role of curcumin in the inhibition of adenovirus replication by disruption of E1A protein via Hsp90 dependant pathway (89, 90) and the results on the role of curcumin rescuing the nuclear localization and transactivation activity of mutated PHOX2B carrying the largest expansion of polyAla in CCHS, where curcumin exerts and effect analogous to canonical Hsp90 inhibitors such as 17-AAG (91). Given the role of the Hsp90 protein, the use of curcumin as a low-toxic inhibitor of Hsp90 may be of great importance not only in diabetes, but also in CVD, and this topic certainly deserves further in-depth research.
Summary and Take Home Message
The use of curcumin in patients at high risk of diabetes and in patients with existing diabetes (with and without complications) is characterized by hypoglycemic, lipid-lowering, anti-inflammatory and antioxidant effects. This is likely to translate into a reduction in cardiovascular risk in this group of patients.
Curcumin in Patients With Obesity/Metabolic Syndrome
The effectiveness of curcumin in the prevention of diabetes and its treatment support has been the subject of several randomized controlled clinical trials (Table 3).
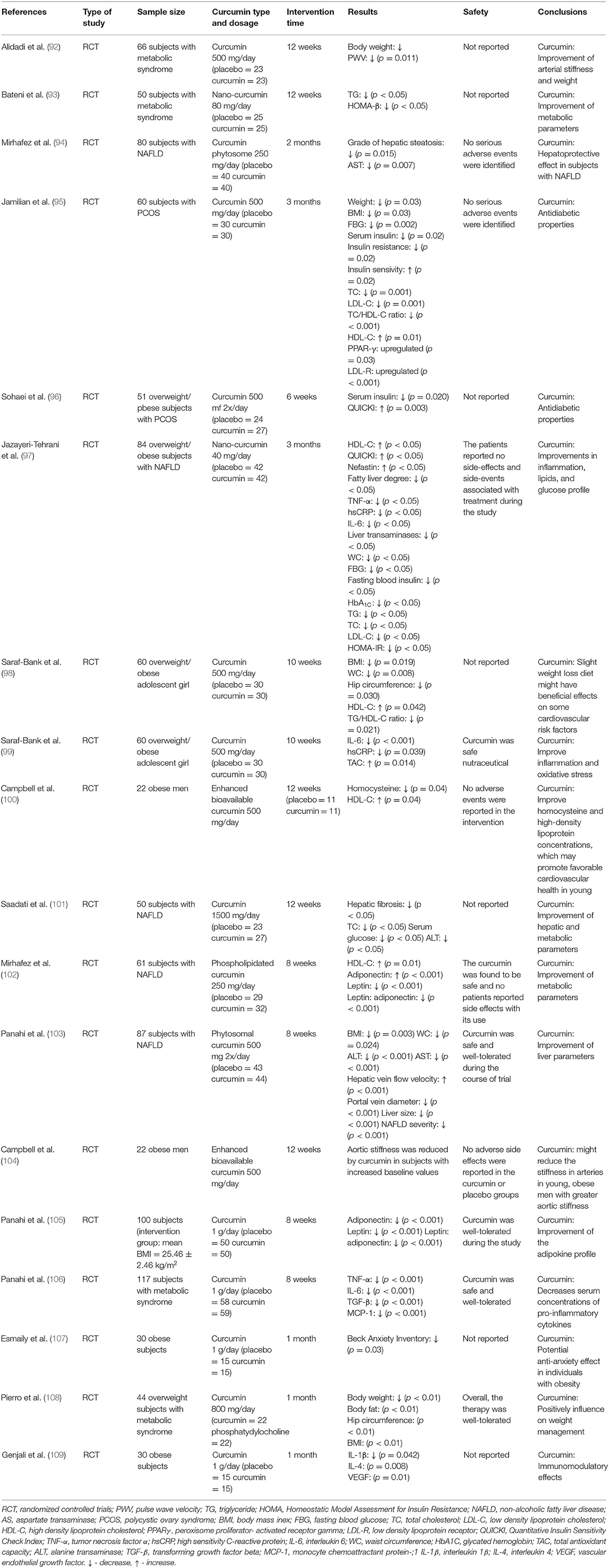
Table 3. Summary of studies on the effects of curcumin on the obesity, metabolic syndrome, and obesity-related diseases.
The beneficial properties of curcumin have also been confirmed in numerous meta-analyzes and systematic reviews. In a meta-analysis of the results of 4 studies conducted by Atkin et al., the effect of curcuminoid supplementation on the concentration of leptin was assessed. The duration of the intervention ranged from 4 weeks to 6 months, and the dose of curcuminoids was 250–1,000 mg/day. It was shown that curcuminoids led to a significant decrease in leptin concentration (SMD = −0.695; 95% CI: −1.162 to −0.229, p = 0.003) (110). Furthermore, a meta-analysis of 6 randomized clinical trials conducted by Clark et al. found that curcumin supplementation (duration of intervention: 6–39 weeks, dose 200–1,500 mg/day curcumin/curcuminoids) led to a significant increase in adiponectin concentration (WMD: 0.82; 95% CI: 0.33–1.30, p < 0.001) (111). A meta-analysis by Azhdari et al., including the results of 7 randomized clinical trials, assessed the efficacy of curcumin in people with metabolic syndrome. The duration of the intervention ranged from 6 to 12 weeks, while curcumin or curcuminoids were used at a dose of 800–2,400 mg/day (up to 20 mg curcumin/kg body weight). Curcumin use was associated with a significant decrease in FBG (WMD = −9.18; 95% CI: −16.70 to −1.66, p = 0.000), TG (WMD = −33.65; 95% CI: −51.27 to −16.03, p < 0.001) and a significant increase in HDL -C (WMD = 4.31; 95% CI: 1.50–7.11, p < 0.001) (112). The anti-inflammatory efficacy of curcumin in subjects with metabolic syndrome was assessed in a meta-analysis conducted by Panahi et al., which included the results of 8 randomized clinical trials. The duration of supplementation in the included studied ranged between 2 and 12 weeks and the administered doses of curcuminoids ranged between 80 mg/day and 6 g/day. It was found that curcumin significantly decreased CRP concentration (WMD = −2.20 mg/l; 95% CI: −3.96 to −0.44, p = 0.01) (113). The beneficial effect of curcumin was also confirmed in a meta-analysis conducted by Baziar and Parohan. This meta-analysis assessed the effectiveness of curcumin in patients with non-alcoholic fatty liver disease (NAFLD), which is often a complication of obesity. The meta-analysis included 8 randomized clinical trials with a total of 520 participants. The dose of the supplement ranged from 70 to 3,000 mg/day and the intervention was given for between 8 and 12 weeks. It was found that curcumin led to a significant decrease in BMI (WMD = −0.34 kg/m2; 95% CI: −0.64 to −0.04, p < 0.05) and waist circumference (WMD = −2.12 cm; 95% CI: −3.26 to −0.98, p < 0.001) (114). A very interesting meta-analysis of the results of 11 randomized clinical trials conducted by Mousavi et al. assessed the effect of curcumin on body weight. These studies used curcumin or nanocurcumin or curcuminoids at a dose of 80–1,900 mg/day for 4–13 weeks. A significant effect of curcumin administration on body weight was demonstrated (WMD = −1.14 kg; 95% CI: −2.16 to −0.12, p = 0.02) and BMI (WMD = −0.48 kg/m2; 95% CI: −0.78 to −0.17, p = 0.002). Furthermore, the effect of curcumin on WC was significant in studies that prescribed ≥1,000 mg/day curcumin (p ≤ 0.001), those with the intervention duration of ≥ 8 weeks (p ≤ 0.001), and those that was performed on overweight subjects (p ≤ 0.001) (115). A meta-analysis of the results of 26 randomized clinical trials conducted by Tabrizi et al. summarized the effect of curcumin supplementation on glycemic control and lipid profile in patients with metabolic syndrome. Curcumin supplementation was shown to lead to reduced fasting glucose levels (SMD = −0.78; 95% CI: −1.20, −0.37, p < 0.001), HOMA-IR (SMD = −0.91; 95% CI: −1.52, −0.31, p = 0.003) and HbA1C (SMD = −0.92; 95% CI: −1.37, −0.47, p < 0.001). Moreover, curcumin supplementation was significantly associated with reduced triglyceride concentration (SMD = −1.21; 95% CI: −1.78, −0.65, p < 0.001) and total cholesterol reduction (SMD = −0.73; 95% CI: −1.32, −0.13, p = 0.01) (116).
A recent systematic review of the results of 28 randomized clinical trials conducted by Safari et al. summarized the knowledge about the effectiveness of curcumin in overweight or obese people. The authors indicate that available studies indicate that curcumin has beneficial impacts on various anthropometric indices in these group of patients (117).
From a clinical point of view, it is important to note that curcumin improves the endocrine function of adipose tissue. A meta-analysis of the results of 6 clinical trials conducted by Simental-Mendía et al. showed that curcuminoid supplementation led to a significant increase in adiponectin levels (WMD = 6.47 ng/mL, 95% CI: 1.85–11.10, p = 0.010) (118).
Summary and Take Home Message
The use of curcumin in patients with overweight/obesity, metabolic syndrome, PCOS, who are objectively characterized by an increased cardiovascular risk, led to the improvement of a number of metabolic parameters affecting this risk.
Anti-Hypertensive, Lipid-Lowering, Anti-Inflammatory, and Antioxidant Properties of Curcumin
Curcumin has well-documented antihypertensive properties. In a meta-analysis by Hadi et al. including the results of 11 clinical trials, the antihypertensive properties of curcumin were assessed. The duration of intervention was different among studies and ranged between 6 and 24 weeks. The dose of curcumin/turmeric administration ranged from 150 to 2,400 mg/day. A significant reduction in SBP (ES = −1.24 mmHg; 95% CI: −2.26 to −0.22) was demonstrated in studies with ≥12-week curcumin supplementation (119). Interestingly, in an experimental study by Lee et al. it was shown that co-administration of amlodipine and curcumin had a stronger vasorelaxant effect than amlodipine alone. Thus, the use of curcumin and amlodipine may be an effective antihypertensive combination (120).
Curcumin is characterized by lipid-lowering properties, as reflected in the ILEP position paper from (Table 1A) (15), and in the latest guidelines for the diagnosis and treatment of lipid disorders in Poland (Table 1B) (16). A meta-analysis of the results of 20 randomized clinical trials conducted by Simental-Mendía et al. showed that curcuminoid supplementation led to a significant reduction in plasma triglycerides (WMD = −21.36 mg/dL; 95% CI: −32.18, −10.53, p < 0.001), and an elevation in plasma HDL-C levels (WMD = 1.42 mg/dL; 95% CI: 0.03–2.81, p = 0.046). Importantly, the effects of curcuminoids on lipids were not found to be dependent on the duration of supplementation (121). Similar results were obtained by Yuan et al. in a meta-analysis of the results of 12 randomized clinical trials involving adults with metabolic diseases. Turmeric and curcuminoids supplementation was found to be associated with a reduction in: TG by −19.1 mg/dL (95% CI: −31.7, −6.46 mg/dL, p = 0.003), TC by −11.4 mg/dL (95% CI: −17.1, −5.74 mg/dL, p < 0.0001), and LDL cholesterol by −9.83 mg/dL (95% CI: −15.9, −3.74 mg/dL, p = 0.002). HDL-C was increased by 1.9 mg/dL (95% CI: 0.31–3.49 mg/dL, p = 0.02). The beneficial effect of turmeric and curcuminoids supplementation depended on the intervention time (more than 8 weeks) and dose (higher doses) (122). A meta-analysis of the results of 7 randomized clinical trials conducted by Qin et al. also demonstrated lipid-lowering properties of turmeric and curcumin. It was found that turmeric and curcumin significantly reduced serum LDL-C (SMD = −0.340; 95% CI: −0.530, −0.150, p < 0.0001) and TG (SMD = −0.214; 95% CI: −0.369, −0.059, p = 0.007) (123). In a randomized study by Ferguson et al. involving 70 hypercholesterolaemic individuals, a significant lipid-lowering effect of curcumin was demonstrated, especially when combined with phytosterols (124).
In the ILEP position paper on the anti-inflammatory effects of nutraceuticals (Table 1C), the very favorable effects of curcumin in this area were discussed (17). A meta-analysis of the results of 9 randomized clinical trials conducted by Derosa et al. showed that curcuminoid supplementation significantly reduced circulating IL-6 concentrations (WMD = −0.60pg/mL; 95% CI: −1.06, −0.14, p = 0.011). The observed effect was independent of the dose or duration of intervention (125). Moreover, a meta-analysis of the results of 32 randomized clinical trials conducted by Gorabi et al. showed that curcumin supplementation significantly decreased serum levels of IL-1 (WMD = −2.33 pg/mL; 95% CI: −3.33, −1.34, p < 0.001) and TNF-α (WMD = −1.61 pg/mL; 95% CI: −2.72, −0.51, p < 0.001) compared to the placebo group following treatment (126). A meta-analysis of the results of 32 randomized clinical trials conducted by Ferguson et al. found that curcumin supplementation led to reduction in CRP (WMD = −1.55 mg/L; 95% CI: −1.81, −1.30), IL-6 (WMD = −1.69 pg/mL; 95% CI: −2.56, −0.82), TNF-α (WMD = −3.13 pg/mL; 95% CI: −4.62, −1.64), IL-8 (WMD = −0.54 pg/mL; 95% CI: −0.82, −0.28), MCP-1 (WMD = −2.48 pg/mL; 95% CI: −3.96, −1.00). An increase in concentrations of the anti-inflammatory cytokine IL-10 (WMD = 0.49 pg/mL; 95% CI: 0.10–0.88) was observed (127). The beneficial effect of curcumin supplementation on the reduction of inflammation was confirmed in a meta-analysis of the results of 23 randomized clinical trials by Gorabi et al. It was found that curcumin significantly reduced the level of CRP compared to placebo (WMD = −3.67 mg/L; 95% CI: −6.96, −0.38, p = 0.02). The reduction in CRP levels was greatest when a dose of ≤1,000 mg/day was used for > 10 weeks (128). In an interesting meta-analysis of the results of 15 randomized clinical trials conducted by Tabrizi et al. the antioxidant activity of curcumin was assessed in addition to its anti-inflammatory effects. It was demonstrated that the use of supplements containing curcumin resulted in a decrease in IL-6 levels (SMD = −2.08; 95% CI: −3.90, −0.25, p = 0.02), hs-CRP (SMD = −0.65; 95% CI: −1.20, −0.10, p = 0.02), and malondialdehyde (MDA) concentrations (SMD = −3.14; 95% CI: −4.76, −1.53, p < 0.001) (129). The antioxidant properties of curcumin were confirmed by Qin et al. in a meta-analysis of the results of 8 randomized clinical trials. These researchers showed that curcumin supplementation led to reduction in circulating MDA concentrations (SMD = −0.769; 95% CI: −1.059, −0.478) and a significant increase in SOD activity (SMD = 1.084; 95% CI: 0.487–1.680). The antioxidant effect was most pronounced when using curcumin at a dose ≥ 600 mg/day (130). Interestingly, as demonstrated by Panahi et al. supplementation of curcumin (1 g/day for 8 weeks) led to a decrease in uric acid levels (p < 0.001), which is also a significant risk factor for CVD (131).
Summary and Take Home Message
Curcumin use is characterized by general antihypertensive, lipid-lowering, anti-inflammatory and antioxidant effects, which may be used in reducing cardiovascular risk in patients with these risk factors.
Curcumin as a Support in the Treatment of ASCVD
It has been demonstrated that curcumin is a natural product with anti-atherosclerotic properties (132). The pleiotropic effects of curcumin described above have a very beneficial effect as a support for the treatment of patients with ASCVD. In a study by Mirzabeigi et al. Thirty-three patients with CAD received curcumin (500 mg/day for 8 weeks) or placebo. Curcumin was shown to significantly reduce triglycerides (p = 0.01), LDL cholesterol (p = 0.03) and VLDL cholesterol (p = 0.04) (133). Curcumin is also effective in patients with ASCVD. In a study by Alwi et al. involving 75 patients with acute coronary syndrome, it was shown that the administration of curcumin led to a reduction in total cholesterol level and LDL cholesterol level in these patients (134). In a study by Wongcharoen et al. which included 121 patients after coronary bypass surgery, the effect of curcuminoid supplementation (4 g/day started 3 days before the scheduled surgery and continued until 5 days after surgery) on the risk of MI was assessed. It was shown that incidence of in-hospital MI was decreased from 30.0% in the placebo group to 13.1% in the curcuminoid group (aHR = 0.35; 95% CI: 0.13–0.95, p = 0.038). Moreover, a reduction in the level of CRP, MDA, and N-terminal pro-B-type natriuretic peptide was found (135). Curcumin is also effective in controlling the lipid and glycaemic profiles in patients with acute MI. In a randomized clinical trial by Tabaee et al. involving 72 patients with acute MI, curcumin supplementation (500 mg/day, 95% curcuminoids for 8 weeks) led to a reduction in LDL levels (−10.3 ± 20.7 vs. +0.2 ± 22.5, p = 0.039), an increase in HDL level (+4.5 ± 8.9 vs. −1.6 ± 7.7, p = 0.002) and a decrease in HbA1C (-0.3 ± 2.2 vs. + 1.1 ± 1.3, p = 0.002) (136). From a clinical point of view, it is relevant that curcumin can reduce muscle pain and limit muscle damage associated with statin use, making curcumin a very important supplement in patients with statin intolerance (137). An important group of patients at high risk of ASCVD are hemodialysis patients with ESRD. In a study by Afshar et al. which included 54 hemodialysis patients, the effect of curcumin supplementation (nano-curcumin at a dose of 120 mg over 12 weeks) or placebo on inflammation and biomarkers of atherosclerosis was analyzed. A significant reduction in the level of hs-CRP (p < 0.001), VCAM-1 (p < 0.001) and ICAM-1 (p < 0.05) has been demonstrated, which means that curcumin may reduce the inflammation and progression of atherosclerosis in these patients (138). Another group of patients who may benefit from curcumin supplementation are those after PCI. In a study by Silalahi et al., 50 patients with stable CAD after PCI were supplemented with curcumin (5 mg/day was given 7 days prior to PCI until 2 days after PCI) or placebo. It was shown that curcumin significantly reduce the serum hsCRP (p = 0.006) and sCD40L (p = 0.002) 7 days before PCI to 48 h post-PCI. The decrement of hsCRP (−14.2 vs. −7.4%) and sCD40L (−24.3 vs. −13.2%) from 24 to 48 h post-PCI was higher in the curcumin group than placebo group. Thus, curcumin supplementation may be effective in reducing inflammation in patients with stable CAD after PCI (139).
Summary and Take Home Message
Curcumin has a several beneficial effects relevant to the supportive management of patients with ASCVD.
Conclusions and Perspectives
The impressive efficiency of curcumin in the prevention of ASCVD is highlighted by a recent recent meta-analysis conducted by Ashtary-Larky et al. (140), which included the results of 9 randomized clinical trials (n = 510 participants). In the analyzed studies, nano-curcumin was used at a dose of 40–120 mg/day for a period of 6–12 weeks (140). The results of this important meta-analysis are summarized in Table 4.
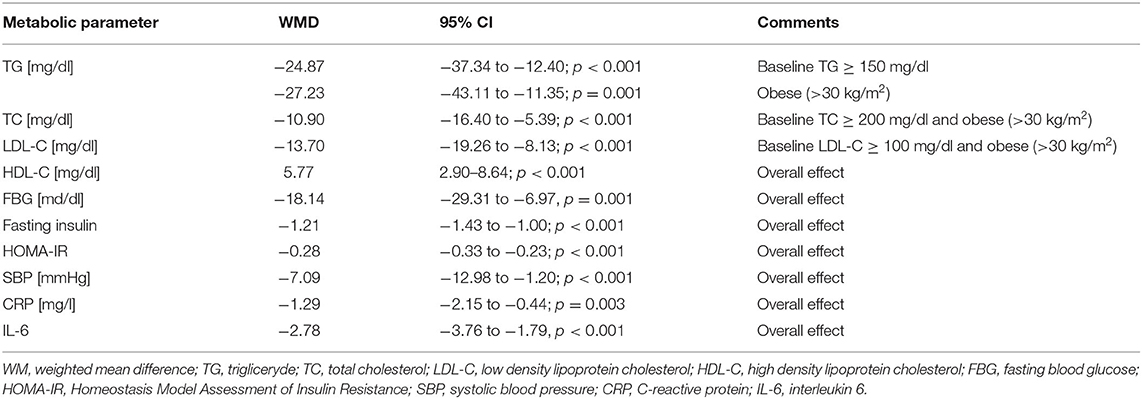
Table 4. The effect of nano-curcumin supplementation on the control of cardiovascular parameters. Based on (140).
Thus, in this meta-analysis, it was found that nano-curcumin supplementation was associated with an improvement in the glycemic profile by reducing fasting blood glucose (FBG), fasting insulin, and Homeostasis Model Assessment of Insulin Resistance (HOMA-IR). In addition, nano-curcumin supplementation was shown to increase high density lipoprotein cholesterol (HDL-C). Hypolipidemic effects (reduction of TG, TC and LDL-C) of this compound have been demonstrated in patients with dyslipidemia (triglyceride [TG] > 150 mg/dl; total cholesterol [TC] > 200 mg/dl; and low-density lipoprotein cholesterol [LDL-C] > 100 mg/dl). Declines in C-reactive protein (CRP), interleukin 6 (IL-6) and systolic blood pressure (SBP) were also found, which show a beneficial anti-inflammatory and hypotensive effect of nano-curcumin supplementation (140). It is also worth mentioning that curcumin has other effects as well, such as anti-cancer properties. It is therefore a nutraceutical with great prophylactic and therapeutic potential, not only in the management of ASCVD (141).
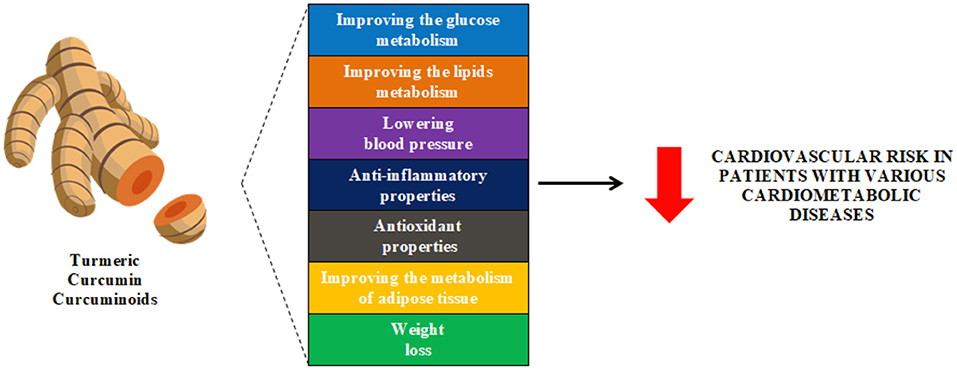
Thus, curcumin has pleiotropic effects that as an adjuvant supplement can comprehensively reduce CVD risk factors and support the treatment of ASCVD in a wide variety of patient groups (Figure 2).
Author Contributions
SS and MB contributed significantly to analysis and manuscript preparation. SS, MB, JU, AS, and PP supported valuable discussion and revised the whole manuscript. All authors collated papers, wrote the manuscript, read, and approved the final manuscript.
Conflict of Interest
JU is CSO at Nomi Biotech Corporation. PP owns four shares in AstraZeneca PLC and has received honoraria and/or travel reimbursement for events sponsored by AKCEA, Amgen, AMRYT, Link Medical, Mylan, Napp, Sanofi. MB: speakers bureau: Amgen, Herbapol, Kogen, KRKA, Polpharma, Mylan/Viatris, Novartis, Novo-Nordisk, Sanofi-Aventis, Teva, Zentiva; consultant to Amgen, Daichii Sankyo, Esperion, Freia Pharmaceuticals, Novartis, Novo-Nordisk, Polfarmex, Sanofi-Aventis; Grants from Amgen, Mylan/Viatris, Sanofi and Valeant; CMO at Nomi Biotech Corporation.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
1. ^https://pubchem.ncbi.nlm.nih.gov/compound/969516 (accessed January 29, 2022).
2. ^https://www.epa.gov/oppt/exposure/pubs/episuitedl.htm (accessed January 29, 2022).
3. ^https://www.efsa.europa.eu/en/efsajournal/pub/1679 (accessed January 29, 2022).
References
1. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J Am Coll Cardiol. (2020) 76:2982–3021. doi: 10.1016/j.jacc.2020.11.010
2. Li Z, Lin L, Wu H, Yan L, Wang H, Yang H, et al. Global, regional, and national death, and disability-adjusted life-years (DALYs) for cardiovascular disease in 2017 and trends and risk analysis from 1990 to 2017 using the global burden of disease study and implications for prevention. Front Public Health. (2021) 9:559751. doi: 10.3389/fpubh.2021.559751
3. Williams R, Karuranga S, Malanda B, Saeedi P, Basit A, Besançon Besançon S, et al. Global and regional estimates and projections of diabetes-related health expenditure: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. (2020) 162:108072. doi: 10.1016/j.diabres.2020.108072
4. Lin X, Xu Y, Pan X, Xu J, Ding Y, Sun X, et al. Global, regional, and national burden and trend of diabetes in 195 countries and territories: an analysis from 1990 to 2025. Sci Rep. (2020) 10:14790. doi: 10.1038/s41598-020-71908-9
5. The Lancet Gastroenterology Hepatology. Obesity: another ongoing pandemic. Lancet Gastroenterol Hepatol. (2021) 6:411. doi: 10.1016/S2468-1253(21)00143-6
6. Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes. (2008) 32:1431–37. doi: 10.1038/ijo.2008.102
7. Hirode G, Wong RJ. Trends in the prevalence of metabolic syndrome in the United States, 2011-2016. JAMA. (2020) 323:2526–8. doi: 10.1001/jama.2020.4501
8. Zhou B, Carrillo-Larco RM, Danaei G, Riley LM, Paciorek CJ, Stevens GA, et al. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet. (2021) 398:957–80. doi: 10.1016/S0140-6736(21)01330-1
9. Pirillo A, Casula M, Olmastroni E, Norata GD, Catapano AL. Global epidemiology of dyslipidaemias. Nat Rev Cardiol. (2021) 18:689–700. doi: 10.1038/s41569-021-00541-4
10. Sathiyakumar V, Kapoor K, Jones SR, Banach M, Martin SS. Novel therapeutic targets for managing dyslipidemia. Trends Pharmacol Sci. (2018) 39:733–47. doi: 10.1016/j.tips.2018.06.001
11. Komiyama M, Hasegawa K. Coronavirus disease 2019: psychological stress and cardiovascular diseases. Eur Cardiol. (2021) 16:e33. doi: 10.15420/ecr.2021.10
12. Zheng Z, Peng F, Xu B, Zhao J, Liu H, Peng J, et al. Risk factors of critical and mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect. (2020) 81:16–25. doi: 10.1016/j.jinf.2020.04.021
13. Rosengren A, Smyth A, Rangarajan S, Ramasundarahettige C, Bangdiwala SI, AlHabib KF, et al. Socioeconomic status and risk of cardiovascular disease in 20 low-income, middle-income, and high-income countries: the prospective urban rural epidemiologic (PURE) study. Lancet Glob Health. (2019) 7:748–60. doi: 10.1016/S2214-109X(19)30045-2
14. Abdalla SM, Yu S, Galea S. Trends in cardiovascular disease prevalence by income level in the United States. JAMA Netw Open. (2020) 3:e2018150. doi: 10.1001/jamanetworkopen.2020.18150
15. Cicero AFG, Colletti A, Bajraktari G, Descamps O, Djuric DM, Ezhov M, et al. Lipid lowering nutraceuticals in clinical practice: position paper from an international lipid expert panel. Arch Med Sci. (2017) 13:965–1005. doi: 10.5114/aoms.2017.69326
16. Banach M, Burchardt P, Chlebus K, Dobrowolski P, Dudek D, Dyrbuś K, et al. PoLA/CFPiP/PCS/PSLD/PSD/PSH guidelines on diagnosis and therapy of lipid disorders in Poland 2021. Arch Med Sci. (2021) 17:1447–547. doi: 10.5114/aoms/141941
17. Ruscica M, Penson PE, Ferri N, Sirtori CR, Pirro M, Mancini GBJ, et al. Impact of nutraceuticals on markers of systemic inflammation: potential relevance to cardiovascular diseases - a position paper from the International Lipid Expert Panel (ILEP). Prog Cardiovasc Dis. (2021) 67:40–52. doi: 10.1016/j.pcad.2021.06.010
18. Banach M, Bruckert E, Descamps OS, Ellegård L, Ezhov M, Föger B, et al. The role of red yeast rice (RYR) supplementation in plasma cholesterol control: a review and expert opinion. Atheroscler Suppl. (2019) 39:1–8. doi: 10.1016/j.atherosclerosissup.2019.08.023
19. Averna M, Banach M, Bruckert E, Drexel H, Farnier M, Gaita D, et al. Practical guidance for combination lipid-modifying therapy in high- and very-high-risk patients: a statement from a European atherosclerosis society task force. Atherosclerosis. (2021) 325:99–109. doi: 10.1016/j.atherosclerosis.2021.03.039
20. Zahedipour F, Hosseini SA, Sathyapalan T, Majeed M, Jamialahmadi T, Al-Rasadi K, et al. Potential effects of curcumin in the treatment of COVID-19 infection. Phytother Res. (2020) 34:2911–20. doi: 10.1002/ptr.6738
21. Vahedian-Azimi A, Abbasifard M, Rahimi-Bashar F, Guest PC, Majeed M, Mohammadi A, et al. Effectiveness of curcumin on outcomes of hospitalized COVID-19 patients: a systematic review of clinical trials. Nutrients. (2022) 14:256. doi: 10.3390/nu14020256
22. Lewek J, Jatczak-Pawlik I, Maciejewski M, Jankowski P, Banach M. COVID-19 and cardiovascular complications - preliminary results of the LATE-COVID study. Arch Med Sci. (2021) 17:818–22. doi: 10.5114/aoms/134211
23. Kotha RR, Luthria DL. Curcumin: biological, pharmaceutical, nutraceutical, and analytical aspects. Molecules. (2019) 24:2930. doi: 10.3390/molecules24162930
24. Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. (2007) 4:807–18. doi: 10.1021/mp700113r
25. Khajuria A, Zutshi U, Bedi K. Permeability characteristics of piperine on oral absorption: an active alkaloid from peppers and a bioavailability enhancer. Ind J Exp Biol. (1998) 36:46–50.
26. Li H, Sureda A, Devkota HP, Pittalà V, Barreca D, Silva AS, et al. Curcumin, the golden spice in treating cardiovascular diseases. Biotechnol Adv. (2020) 38:107343. doi: 10.1016/j.biotechadv.2019.01.010
27. Ganjani S, Blesso CN, Banach M, Pirro M, Majeed M, Sahebkar A. Effects of curcumin on HDL functionality. Pharmacol Res. (2017) 119:208–18. doi: 10.1016/j.phrs.2017.02.008
28. Miłobedzka J, Kostanecki S, Lampe V. Zur kenntnis des curcumins. Berichte der deutschen chemischen Gesellschaft. (1910) 43:2163–70. doi: 10.1002/cber.191004302168
29. Pulido-Moran M, Moreno-Fernandez J, Ramirez-Tortosa C, Ramirez-Tortosa M. Curcumin and health. Molecules. (2016) 21:264. doi: 10.3390/molecules21030264
30. Liu W, Zhai Y, Heng X, Che FY, Chen W, Sun D, et al. Oral bioavailability of curcumin: problems and advancements. J Drug Target. (2016) 24:694–702. doi: 10.3109/1061186X.2016.1157883
31. Dei Cas M, Ghidoni R. Dietary curcumin: correlation between bioavailability and health potential. Nutrients. (2019) 11:2147. doi: 10.3390/nu11092147
32. Gorgani L, Mohammadi M, Najafpour GD, Nikzad M. Piperine-the bioactive compound of black pepper: from isolation to medicinal formulations. Compr Rev Food Sci Food Saf. (2016) 16:124–40. doi: 10.1111/1541-4337.12246
33. Stojanović-Radić Z, Pejčić M, Dimitrijević M, Aleksić A, Kumar KVA, Salehi B, et al. Piperine-a major principle of black pepper: a review of its bioactivity and studies. Appl Sci. (2019) 9:4270. doi: 10.3390/app9204270
34. Baell J, Walters M. Chemistry: chemical con artists foil drug discovery. Nature. (2014) 513:481–3. doi: 10.1038/513481a
35. Furia TE. CRC Handbook of Food Additives. 2nd ed. Volume 2. Boca Raton, Florida: CRC Press, Inc., (1980).
36. Ma Z, Wang N, He H, Tang X. Pharmaceutical strategies of improving oral systemic bioavailability of curcumin for clinical application. J Control Release. (2019) 316:359–80. doi: 10.1016/j.jconrel.2019.10.053
37. Soleimani V, Sahebkar A, Hosseinzadeh H. Turmeric (Curcuma longa) and its major constituent (curcumin) as nontoxic and safe substances: review. Phytother Res. (2018) 32:985–95. doi: 10.1002/ptr.6054
38. Ryan JL, Heckler CE, Ling M, Katz A, Williams JP, Pentland AP, et al. Curcumin for radiation dermatitis: a randomized, double-blind, placebo-controlled clinical trial of thirty breast cancer patients. Radiat Res. (2013) 180:34–43. doi: 10.1667/RR3255.1
39. Rahmani S, Asgary S, Askari G, Keshvari M, Hatamipour M, Feizi A, et al. Treatment of non-alcoholic fatty liver disease with curcumin: a randomized placebo-controlled trial. Phytother Res. (2016) 30:1540–8. doi: 10.1002/ptr.5659
40. Jäger R, Lowery RP, Calvanese AV, Joy JM, Purpura M, Wilson JM. Comparative absorption of curcumin formulations. Nutr J. (2014) 13:11. doi: 10.1186/1475-2891-13-11
41. Mangolim CS, Moriwaki C, Nogueira AC, Sato F, Baesso LM, Neto AM, et al. Curcumin–β-cyclodextrin inclusion complex: stability, solubility, characterisation by FT-IR, FT- Raman, X-ray diffraction and photoacoustic spectroscopy, and food application. Food Chem. (2014) 153:361–70. doi: 10.1016/j.foodchem.2013.12.067
42. Marcolino VA, Zanin GM, Durrant LR, Benassi MT, Matioli G. Interaction of curcumin and bixin with β-cyclodextrin: complexation methods, stability, and applications in food. J Agric Food Chem. (2011) 59:3348–57 doi: 10.1021/jf104223k
43. Wang YJ, Pan MH, Cheng AL, Lin LI, Ho YS, Hsieh CY, et al. Stability of curcumin in buffer solutions and characterization of its degradation products. J Pharm Biomed Anal. (1997) 15:1867–76. doi: 10.1016/S0731-7085(96)02024-9
44. Martins RM, Pereira SV, Siqueira S, Salomão WF, Freitas LAP. Curcuminoid content and antioxidant activity in spray dried microparticles containing turmeric extract. Food Res Int. (2013) 50:657–63. doi: 10.1016/j.foodres.2011.06.030
45. Cuomo J, Appendino G, Dern AS, Schneider E, McKinnon TP, Brown MJ. Comparative absorption of a standardized curcuminoid mixture and its lecithin formulation. J Nat Prod. (2011) 74:664–9. doi: 10.1021/np1007262
46. Gota VS, Maru GB, Soni TG, Gandhi TR, Kochar N, Agarwal MG. Safety and pharmacokinetics of a solid lipid curcumin particle formulation in osteosarcoma patients and healthy volunteers. J Agric Food Chem. (2010) 58:2095–9. doi: 10.1021/jf9024807
47. Madhavi D, Kagan D. Bioavailability of a sustained release formulation of curcumin. Integr Med. (2014) 13:24–30.
48. Schiborr C, Kocher A, Behnam D, Jandasek J, Toelstede S, Frank J. The oral bioavailability of curcumin from micronized powder and liquid micelles is significantly increased in healthy humans and differs between sexes. Mol Nutr Food Res. (2014) 58:516–27. doi: 10.1002/mnfr.201300724
49. Antony B, Merina B, Iyer VS, Judy N, Lennertz K, Joyal S, et al. pilot cross-over study to evaluate human oral bioavailability of BCM-95CG (Biocurcumax), a novel bioenhanced preparation of curcumin. Indian J Pharm Sci. (2008) 70:445–9. doi: 10.4103/0250-474X.44591
50. Purpura M, Lowery RP, Wilson JM, Mannan H, Münch G, Razmovski-Naumovski V. Analysis of different innovative formulations of curcumin for improved relative oral bioavailability in human subjects. Eur J Nutr. (2018) 57:929–38. doi: 10.1007/s00394-016-1376-9
51. Sasaki H, Sunagawa Y, Takahashi K, Imaizumi A, Fakuda H, Hashimoto T, et al. Innovative preparation of curcumin for improved oral bioavailability. Biol Pharm Bull. (2011) 34:660–5. doi: 10.1248/bpb.34.660
52. Chen AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Sheh TS, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. (2001) 21:e2900.
53. Lao CD, Ruffin MT IV, Normolle D, Heath DD, Murray SI, Bailey JM, et al. Dose escalation of a curcuminoid formulation. BMC Complement Altern Med. (2006) 6:4–7. doi: 10.1186/1472-6882-6-10
54. Vareed SK, Kakarala M, Ruffin MT, Crowell JA, Normolle DP, Djuric Z, et al. Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects. Cancer Epidemiol Biomark Prev. (2008) 17:1411–7. doi: 10.1158/1055-9965.EPI-07-2693
55. Karandish M, Mozaffari-Khosravi H, Mohammadi SM, Cheraghian B, Azhdari M. The effect of curcumin and zinc co-supplementation on glycemic parameters in overweight or obese prediabetic subjects: a phase 2 randomized, placebo-controlled trial with a multi-arm, parallel-group design. Phytother Res. (2021) 35:4377–87. doi: 10.1002/ptr.7136
56. Mokhtari M, Razzaghi R, Momen-Heravi M. The effects of curcumin intake on wound healing and metabolic status in patients with diabetic foot ulcer: a randomized, double-blind, placebo-controlled trial. Phytother Res. (2021) 35:2099–107. doi: 10.1002/ptr.6957
57. Thota RN, Rosato JI, Dias CB, Burrows TL, Martins RN, Garg ML. Dietary supplementation with curcumin reduce circulating levels of glycogen synthase kinase-3β and islet amyloid polypeptide in adults with high risk of type 2 diabetes and Alzheimer's disease. Nutrients. (2020) 12:1032. doi: 10.3390/nu12041032
58. Shafabakhsh R, Mobini M, Raygan F, Aghadavod E, Ostadmohammadi V, Amirani E, et al. Curcumin administration and the effects on psychological status and markers of inflammation and oxidative damage in patients with type 2 diabetes and coronary heart disease. Clin Nutr ESPEN. (2020) 40:77–82. doi: 10.1016/j.clnesp.2020.09.029
59. Asadi S, Gholami MS, Siassi F, Qorbani M, Sotoudeh G. Beneficial effects of nano-curcumin supplement on depression and anxiety in diabetic patients with peripheral neuropathy: a randomized, double-blind, placebo-controlled clinical trial. Phytother Res. (2020) 34:896–903. doi: 10.1002/ptr.6571
60. Shafabakhsh R, Asemi Z, Reiner Z, Soleimani A, Aghadavod E, Bahmani F. The effects of nano-curcumin on metabolic status in patients with diabetes on hemodialysis, a randomized, double blind, placebo-controlled trial. Iran J Kidney Dis. (2020) 14:290–9.
61. Funamoto M, Shimizu K, Sunagawa Y, Katanasaka Y, Miyazaki Y, Kakeya H, et al. Effects of highly absorbable curcumin in patients with impaired glucose tolerance and non-insulin-dependent diabetes mellitus. J Diabetes Res. (2019) 2019:8208237. doi: 10.1155/2019/8208237
62. Vanaie A, Shahidi S, Iraj B, Siadat ZD, Kabirzade M, Shakiba F, et al. Curcumin as a major active component of turmeric attenuates proteinuria in patients with overt diabetic nephropathy. J Res Med Sci. (2019) 24:77. doi: 10.4103/jrms.JRMS_1055_18
63. Adibian M, Hodaei H, Nikpayam O, Sohrab G, Hekmatdoost A, Hedayati M. The effects of curcumin supplementation on high-sensitivity C-reactive protein, serum adiponectin, and lipid profile in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial. Phytother Res. (2019) 33:1374–83. doi: 10.1002/ptr.6328
64. Asadi S, Gholami MS, Siassi F, Qorbani M, Khamoshian K, Sotoudeh G. Nano curcumin supplementation reduced the severity of diabetic sensorimotor polyneuropathy in patients with type 2 diabetes mellitus: a randomized double-blind placebo- controlled clinical trial. Complement Ther Med. (2019) 43:253–60. doi: 10.1016/j.ctim.2019.02.014
65. Srinivasan A, Selvarajan S, Kamalanathan S, Kadhiravan T, Lakshmi NCP, Adithan S. Effect of Curcuma longa on vascular function in native Tamilians with type 2 diabetes mellitus: a randomized, double-blind, parallel arm, placebo-controlled trial. Phytother Res. (2019) 33:1898–911. doi: 10.1002/ptr.6381
66. Tohta RN, Acharya SA, Garg ML. Curcumin and/or omega-3 polyunsaturated fatty acids supplementation reduces insulin resistance and blood lipids in individuals with high risk of type 2 diabetes: a randomised controlled trial. Lipids Health Dis. (2019) 18:31. doi: 10.1186/s12944-019-0967-x
67. Hodaei H, Adibian M, Nikpayam O, Hedayati M, Sohrab G. The effect of curcumin supplementation on anthropometric indices, insulin resistance and oxidative stress in patients with type 2 diabetes: a randomized, double-blind clinical trial. Diabetol Metab Syndr. (2019) 11:41. doi: 10.1186/s13098-019-0437-7
68. Thota RN, Dias CB, Abbott KA, Acharya SH, Garg ML. Curcumin alleviates postprandial glycaemic response in healthy subjects: a cross-over, randomized controlled study. Sci Rep. (2018) 8:13679. doi: 10.1038/s41598-018-32032-x
69. Panahi Y, Khalili N, Sahebi E, Namazi S, Simental-Mendía LE, Majeed M, et al. Effects of curcuminoids plus piperine on glycemic, hepatic and inflammatory biomarkers in patients with type 2 diabetes mellitus: a randomized double-blind placebo-controlled trial. Drug Res. (2018) 68:403–9. doi: 10.1055/s-0044-101752
70. Panahi Y, Khalili N, Sahebi E, Namazi S, Karimian MS, Majeed M, et al. Antioxidant effects of curcuminoids in patients with type 2 diabetes mellitus: a randomized controlled trial. Inflammopharmacology. (2017) 25:25–31. doi: 10.1007/s10787-016-0301-4
71. Panahi Y, Khalili N, Sahebi E, Namazi S, Reiner Z, Majeed M, et al. Curcuminoids modify lipid profile in type 2 diabetes mellitus: a randomized controlled trial. Complement Ther Med. (2017) 33:1–5. doi: 10.1016/j.ctim.2017.05.006
72. Panahi Y, Khalili N, Sahebi E, Namazi S, Atkin SL, Majeed M, et al. Curcuminoids plus piperine modulate adipokines in type 2 diabetes mellitus. Curr Clin Pharmacol. (2017) 12:253–8. doi: 10.2174/1574884713666180104095641
73. Jiménez-Osorio AS, García-Niño WR, González-Reyes S, Álvarez-Mejía AE, Guerra-León S, Salazar-Segovia J, et al. The effect of dietary supplementation with curcumin on redox status and Nrf2 activation in patients with nondiabetic or diabetic proteinuric chronic kidney disease: a pilot study. J Ren Nutr. (2016) 26:237–44. doi: 10.1053/j.jrn.2016.01.013
74. Rahimi HR, Mohammadpour AH, Dastani M, Jaafari MR, Abnous K, Mobarhan MG, et al. The effect of nano-curcumin on HbA1c, fasting blood glucose, and lipid profile in diabetic subjects: a randomized clinical trial. Avicenna J Phytomed. (2016) 6:567–77.
75. Yang H, Xu W, Zhou Z, Liu J, Li X, Chen L, et al. Curcumin attenuates urinary excretion of albumin in type II diabetic patients with enhancing nuclear factor erythroid-derived 2-like 2 (Nrf2) system and repressing inflammatory signaling efficacies. Exp Clin Endocrinol Diabetes. (2015) 123:360–7. doi: 10.1055/s-0035-1545345
76. Na LX, Yan BL, Jiang S, Cui HL Li Y, Sun CH. Curcuminoids target decreasing serum adipocyte-fatty acid binding protein levels in their glucose-lowering effect in patients with type 2 diabetes. Biomed Environ Sci. (2014) 27:902–6. doi: 10.3967/bes2014.127
77. Chuengsamarn S, Rattanamongkolgul S, Phonrat B, Tungtrongchitr R, Jirawatnotai S. Reduction of atherogenic risk in patients with type 2 diabetes by curcuminoid extract: a randomized controlled trial. J Nutr Biochem. (2014) 25:144–50. doi: 10.1016/j.jnutbio.2013.09.013
78. Na LX, Li Y, Pan HZ, Zhou XL, Sun DJ, Meng M, et al. Curcuminoids exert glucose-lowering effect in type 2 diabetes by decreasing serum free fatty acids: a double-blind, placebo-controlled trial. Mol Nutr Food Res. (2013) 57:1569–77. doi: 10.1002/mnfr.201200131
79. Chuengsamarn S, Rattanamongkolgul S, Luechapudiporn R, Phisalaphong C, Jirawatnotai S. Curcumin extract for prevention of type 2 diabetes. Diabetes Care. (2012) 35:2121–7. doi: 10.2337/dc12-0116
80. Zhang T, He Q, Liu Y, Chen Z, Hu H. Efficacy and safety of curcumin supplement on improvement of insulin resistance in people with type 2 diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med. (2021) 2021:4471944. doi: 10.1155/2021/4471944
81. Huang J, Qin S, Huang L, Tang Y, Ren H, Hu H. Efficacy and safety of Rhizoma curcumea longae with respect to improving the glucose metabolism of patients at risk for cardiovascular disease: a meta-analysis of randomised controlled trials. J Hum Nutr Diet. (2019) 32:591–606. doi: 10.1111/jhn.12648
82. De Melo ISV, Dos Santos AF, Bueno NB. Curcumin or combined curcuminoids are effective in lowering the fasting blood glucose concentrations of individuals with dysglycemia: systematic review and meta-analysis of randomized controlled trials. Pharmacol Res. (2018) 128:137–44. doi: 10.1016/j.phrs.2017.09.010
83. Altobelli E, Angeletti PM, Marziliano C, Mastrodomenico M, Giuliani AR, Petrocelli R. Potential therapeutic effects of curcumin on glycemic and lipid profile in uncomplicated type 2 diabetes - a meta-analysis of randomized controlled trial. Nutrients. (2021) 13:404. doi: 10.3390/nu13020404
84. Morton LT, Pescinini-E-Salzedas LM, Camargo MEC, Barbalho SM, Dos Santos Haber JF, et al. The effects of curcumin on diabetes mellitus: a systematic review. Front Endocrinol. (2021) 12:669448. doi: 10.3389/fendo.2021.669448
85. Mahdavi A, Moradi S, Askari G, Iraj B, Sathyapalan T, Guest PC, et al. Effect of curcumin on glycemic control in patients with type 2 diabetes: a systematic review of randomized clinical trials. Adv Exp Med Biol. (2021) 1291:139–49. doi: 10.1007/978-3-030-56153-6_8
86. Pivari F, Mingione A, Brasacchio C, Soldati L. Curcumin and type 2 diabetes mellitus: prevention and treatment. Nutrients. (2019) 11:1837. doi: 10.3390/nu11081837
87. Mohammadi E, Behnam B, Mohammadinejad R, Guest PC, Simental-Mendía LE, Sahebkar A. Antidiabetic properties of curcumin: insights on new mechanisms. Adv Exp Med Biol. (2021) 1291:151–64. doi: 10.1007/978-3-030-56153-6_9
88. Abdelmoaty AAA, Zhang P, Lin W, Fan Y, Ye S, Xu J. C0818, a novel curcumin derivative, induces ROS-dependent cytotoxicity in human hepatocellular carcinoma cells in vitro via disruption of Hsp90 function. Acta Pharmacol Sin. (2022) 43:446–56. doi: 10.1038/s41401-021-00642-3
89. Dalidowska I, Gazi O, Sulejczak D, Przybylski M, Bieganowski P. Heat shock protein 90 chaperones E1A early protein of adenovirus 5 and is essential for replication of the virus. Int J Mol Sci. (2021) 22:2020. doi: 10.3390/ijms22042020
90. Jennings MR, Parks RJ. Antiviral effects of curcumin on adenovirus replication. Microorganisms. (2020) 8:1524. doi: 10.3390/microorganisms8101524
91. Di Zanni E, Bachetti T, Parodi S, Bocca P, Prigione I, Di Lascio S, et al. In vitro drug treatments reduce the deleterious effects of aggregates containing polyAla expanded PHOX2B proteins. Neurobiol Dis. (2012) 45:508–18. doi: 10.1016/j.nbd.2011.09.007
92. Alidadi M, Sahebkar A, Eslami S, Vakilian F, Jarahi L, Alinezhad-Namaghi M, et al. The effect of curcumin supplementation on pulse wave velocity in patients with metabolic syndrome: a randomized, double-blind, placebo-controlled trial. Adv Exp Med Biol. (2021) 1308:1–11. doi: 10.1007/978-3-030-64872-5_1
93. Bateni Z, Rahimi HR, Hedayati M, Afsharian S, Goudarzi R, Sohrab G. The effects of nano-curcumin supplementation on glycemic control, blood pressure, lipid profile, and insulin resistance in patients with the metabolic syndrome: a randomized, double-blind clinical trial. Phytother Res. (2021) 35:3945–53. doi: 10.1002/ptr.7109
94. Mirhafez SR, Azimi-Nezhad M, Dehabeh M, Hriri M, Naderan RD, Movahedi A, et al. The effect of curcumin phytosome on the treatment of patients with non-alcoholic fatty liver disease: a double-blind, randomized, placebo-controlled trial. Adv Exp Med Biol. (2021) 1308:25–35. doi: 10.1007/978-3-030-64872-5_3
95. Jamilian M, Foroozanfard F, Kavossian E, Aghadavod E, Shafabakhsh R, Hoseini A, et al. Effects of curcumin on body weight, glycemic control and serum lipids in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Clin Nutr ESPEN. (2020) 36:128–33. doi: 10.1016/j.clnesp.2020.01.005
96. Sohaei S, Amani R, Tarrahi MJ, Ghasemi-Tehrani H. The effects of curcumin supplementation on glycemic status, lipid profile and hs-CRP levels in overweight/obese women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled clinical trial. Complement Ther Med. (2019) 47:102201. doi: 10.1016/j.ctim.2019.102201
97. Jazayeri-Tehrani SA, Rezayat SM, Mansouri S, Qorbani M, Alavian SM, Daneshi-Maskooni M, et al. Nano-curcumin improves glucose indices, lipids, inflammation, and Nesfatin in overweight and obese patients with non-alcoholic fatty liver disease (NAFLD): a double-blind randomized placebo-controlled clinical trial. Nutr Metab. (2019) 16:8. doi: 10.1186/s12986-019-0331-1
98. Saraf-Bank S, Ahmadi A, Paknahad Z, Maracy M, Nourian M. Effects of curcumin on cardiovascular risk factors in obese and overweight adolescent girls: a randomized clinical trial. São Paulo Med J. (2019) 137:414–22. doi: 10.1590/1516-3180.2018.0454120419
99. Saraf-Bank S, Ahmadi A, Paknahad Z, Maracy M, Nourian M. Effects of curcumin supplementation on markers of inflammation and oxidative stress among healthy overweight and obese girl adolescents: a randomized placebo-controlled clinical trial. Phytother Res. (2019) 33:2015–22. doi: 10.1002/ptr.6370
100. Campbell MS, Ouyang A, Krishnakumar IM, Charnigo RJ, Westgate PM, Fleenor BS. Influence of enhanced bioavailable curcumin on obesity-associated cardiovascular disease risk factors and arterial function: a double-blinded, randomized, controlled trial. Nutrition. (2019) 62:135–9. doi: 10.1016/j.nut.2019.01.002
101. Saadati S, Hatami B, Yari Z, Shahrbaf MA, Eghtesad S, Mansour A, et al. The effects of curcumin supplementation on liver enzymes, lipid profile, glucose homeostasis, and hepatic steatosis and fibrosis in patients with non-alcoholic fatty liver disease. Eur J Clin Nutr. (2019) 73:441–9. doi: 10.1038/s41430-018-0382-9
102. Mirhafez SR, Farimani AR, Dehhabe M, Bidkhori M, Hariri M, Ghouchani BF, et al. Effect of Phytosomal curcumin on circulating levels of adiponectin and leptin in patients with non-alcoholic fatty liver disease: a randomized, double-blind, placebo-controlled clinical trial. J Gastrointestin Liver Dis. (2019) 28:183–9. doi: 10.15403/jgld-179
103. Panahi Y, Kianpour P, Mohtashami R, Jafari R, Simental-Mendía LE, Sahebkar A. Efficacy and safety of phytosomal curcumin in non-alcoholic fatty liver disease: a randomized controlled trial. Drug Res. (2017) 67:244–51. doi: 10.1055/s-0043-100019
104. Campbell MS, Berrones AJ, Krishnakumar IM, Charnigo RJ, Westgate PM, Fleenor BS. Responsiveness to curcumin intervention is associated with reduced aortic stiffness in young, obese men with higher initial stiffness. J Funct Foods. (2017) 29:154–60. doi: 10.1016/j.jff.2016.12.013
105. Panahi Y, Hosseini MS, Khalili N, Naimi E, Soflaei SS, Majeed M, et al. Effects of supplementation with curcumin on serum adipokine concentrations: a randomized controlled trial. Nutrition. (2016) 32:1116–22. doi: 10.1016/j.nut.2016.03.018
106. Panahi Y, Hosseini MS, Khalili N, Naimi E, Simental-Mendía LE, Majeed M, et al. Effects of curcumin on serum cytokine concentrations in subjects with metabolic syndrome: a post-hoc analysis of a randomized controlled trial. Biomed Pharmacother. (2016) 82:578–82. doi: 10.1016/j.biopha.2016.05.037
107. Esmaily H, Sahebkar A, Iranshahi M, Ganjali S, Mohammadi A, Ferns G, et al. An investigation of the effects of curcumin on anxiety and depression in obese individuals: a randomized controlled trial. Chin J Integr Med. (2015) 21:332–8. doi: 10.1007/s11655-015-2160-z
108. Di Pierro F, Bressan A, Ranaldi D, Rapacioli G, Giacomelli L, Bertuccioli A. Potential role of bioavailable curcumin in weight loss and omental adipose tissue decrease: preliminary data of a randomized, controlled trial in overweight people with metabolic syndrome. Preliminary study. Eur Rev Med Pharmacol Sci. (2015) 19:4195–202.
109. Ganjali S, Sahebkar A, Mahdipour E, Jamialahmadi K, Torabi S, Akhlaghi S, et al. Investigation of the effects of curcumin on serum cytokines in obese individuals: a randomized controlled trial. Sci World J. (2014) 2014:898361. doi: 10.1155/2014/898361
110. Atkin SL, Kastsiki N, Derosa G, Maffioli P, Sahebkar A. Curcuminoids lower plasma leptin concentrations: a meta-analysis. Phytother Res. (2017) 31:1836–41. doi: 10.1002/ptr.5905
111. Clark CCT, Ghaedi E, Arab A, Pourmasoumi M, Hadi A. The effect of curcumin supplementation on circulating adiponectin: a systematic review and meta-analysis of randomized controlled trials. Diabetes Metab Syndr. (2019) 13:2819–25. doi: 10.1016/j.dsx.2019.07.045
112. Azhdari M, Karandish M, Mansoori A. Metabolic benefits of curcumin supplementation in patients with metabolic syndrome: a systematic review and meta-analysis of randomized controlled trials. Phytother Res. (2019) 33:1289–301. doi: 10.1002/ptr.6323
113. Panahi Y, Hosseini MS, Khalili N, Naimi E, Majeed M, Sahebkar A. Antioxidant and anti-inflammatory effects of curcuminoid-piperine combination in subjects with metabolic syndrome: a randomized controlled trial and an updated meta-analysis. Clin Nutr. (2015) 34:1101–8. doi: 10.1016/j.clnu.2014.12.019
114. Baziar N, Parohan M. The effects of curcumin supplementation on body mass index, body weight, and waist circumference in patients with nonalcoholic fatty liver disease: a systematic review and dose-response meta-analysis of randomized controlled trials. Phytother Res. (2020) 34:464–74. doi: 10.1002/ptr.6542
115. Mousavi SM, Milajerdi A, Varkaneh HK, Gorjipour MM, Esmaillzadeh A. The effects of curcumin supplementation on body weight, body mass index and waist circumference: a systematic review and dose-response meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr. (2020) 60:171–80. doi: 10.1080/10408398.2018.1517724
116. Tabrizi R, Vakili S, Lankarani KB, Akbari M, Mirhosseini N, Ghayour-Mobarhan M, et al. The effects of curcumin on glycemic control and lipid profiles among patients with metabolic syndrome and related disorders: a systematic review and metaanalysis of randomized controlled trials. Curr Pharm Des. (2018) 24:3184–99. doi: 10.2174/1381612824666180828162053
117. Safari Z, Bagherniya M, Askari G, Sathyapalan T, Sahebkar A. The effect of curcumin supplemsentation on anthropometric indices in overweight and obese individuals: a systematic review of randomized controlled trials. Adv Exp Med Biol. (2021) 1291:121–37. doi: 10.1007/978-3-030-56153-6_7
118. Simental-Mendía LE, Cicero AFG, Atkin SL, Majeed M, Sahebkar A. A systematic review and meta-analysis of the effect of curcuminoids on adiponectin levels. Obes Res Clin Pract. (2019) 13:340–4. doi: 10.1016/j.orcp.2019.04.003
119. Hadi A, Pourmasoumi M, Ghaedi E, Sahebkar A. The effect of curcumin/turmeric on blood pressure modulation: a systematic review and meta-analysis. Pharmacol Res. (2019) 150:104505. doi: 10.1016/j.phrs.2019.104505
120. Lee S, Jo C, Choi HY, Lee K. Effect of co-administration of curcumin with amlodipine in hypertension. Nutrients. (2021) 13:2797. doi: 10.3390/nu13082797
121. Simental-Mendía LE, Pirro M, Gotto AM Jr, Banach M, Atkin SL, Majeed M, et al. Lipid-modifying activity of curcuminoids: a systematic review and meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr. (2019) 59:1178–87. doi: 10.1080/10408398.2017.1396201
122. Yuan F, Dong H, Gong J, Wang D, Hu M, Huang W, et al. A systematic review and meta-analysis of randomized controlled trials on the effects of turmeric and curcuminoids on blood lipids in adults with metabolic diseases. Adv Nutr. (2019) 10:791–802. doi: 10.1093/advances/nmz021
123. Qin S, Huang L, Gong J, Shen S, Huang J, Ren H, et al. Efficacy and safety of turmeric and curcumin in lowering blood lipid levels in patients with cardiovascular risk factors: a meta-analysis of randomized controlled trials. Nutr J. (2017) 16:68. doi: 10.1186/s12937-017-0293-y
124. Ferguson JJA, Stojanovski E, MacDonald-Wicks L, Garg ML. Curcumin potentiates cholesterol-lowering effects of phytosterols in hypercholesterolaemic individuals. A randomised controlled trial. Metabolism. (2018) 82:22–35. doi: 10.1016/j.metabol.2017.12.009
125. Derosa G, Maffioli P, Simental-Mendía LE, Bo S, Sahebkar A. Effect of curcumin on circulating interleukin-6 concentrations: a systematic review and meta-analysis of randomized controlled trials. Pharmacol Res. (2016) 111:394–404. doi: 10.1016/j.phrs.2016.07.004
126. Gorabi AM, Razi B, Aslani S, Abbasifard M, Imani D, Sathyapalan T, et al. Effect of curcumin on proinflammatory cytokines: a meta-analysis of randomized controlled trials. Cytokine. (2021) 143:155541. doi: 10.1016/j.cyto.2021.155541
127. Ferguson JJA, Abbott KA, Garg ML. Anti-inflammatory effects of oral supplementation with curcumin: a systematic review and meta-analysis of randomized controlled trials. Nutr Rev. (2021) 79:1043–66. doi: 10.1093/nutrit/nuaa114
128. Gorabi AM, Abbasifard M, Imani D, Aslani S, Razi B, Alizadeh S, et al. Effect of curcumin on C-reactive protein as a biomarker of systemic inflammation: an updated meta-analysis of randomized controlled trials. Phytother Res. (2022) 36:85–97. doi: 10.1002/ptr.7284
129. Tabrizi R, Vakili S, Akbari M, Mirhosseini N, Lankarani KB, Rahimi M, et al. The effects of curcumin-containing supplements on biomarkers of inflammation and oxidative stress: a systematic review and meta-analysis of randomized controlled trials. Phytother Res. (2019) 33:253–62. doi: 10.1002/ptr.6226
130. Qin S, Huang L, Gong J, Shen S, Huang J, Tang Y, et al. Meta-analysis of randomized controlled trials of 4 weeks or longer suggest that curcumin may afford some protection against oxidative stress. Nutr Res. (2018) 60:1–12. doi: 10.1016/j.nutres.2018.08.003
131. Panahi Y, Kianpour P, Mohtashami R, Jafari R, Simental-Mendía LE, Sahebkar A. Curcumin lowers serum lipids and uric acid in subjects with nonalcoholic fatty liver disease: a randomized controlled trial. J Cardiovasc Pharmacol. (2016) 68:223–9. doi: 10.1097/FJC.0000000000000406
132. Singh L, Sharma S, Xu S, Tewari D, Fang J. Curcumin as a natural remedy for atherosclerosis: a pharmacological review. Molecules. (2021) 26:4036. doi: 10.3390/molecules26134036
133. Mirzabeigi P, Mohammadpour AH, Salarifar M, Gholami K, Mojtahedzadeh M, Javadi MR. The effect of curcumin on some of traditional and non-traditional cardiovascular risk factors: a pilot randomized, double-blind, placebo-controlled trial. Iran J Pharm Res. (2015) 14:479–86.
134. Alwi I, Santoso T, Suyono S, Sutrisna B, Suyatna FD, Kresno SB, et al. The effect of curcumin on lipid level in patients with acute coronary syndrome. Acta Med Indones. (2008) 40:201–10.
135. Wongcharoen W, Jai-Aue S, Phrommintikul A, Nawarawong W, Woragidpoonpol S, Tepsuwan T, et al. Effects of curcuminoids on frequency of acute myocardial infarction after coronary artery bypass grafting. Am J Cardiol. (2012) 110:40–4. doi: 10.1016/j.amjcard.2012.02.043
136. Tabaee S, Sahebkar A, Aghamohammadi T, Pakdel M, Dehabeh M, Sobhani R, et al. The effects of curcumin plus piperine supplementation in patients with acute myocardial infarction: a randomized, double-blind, and placebo-controlled trial. Adv Exp Med Biol. (2021) 1328:199–211. doi: 10.1007/978-3-030-73234-9_13
137. Sahebkar A, Saboni N, Pirro M, Banach M. Curcumin: an effective adjunct in patients with statin-associated muscle symptoms? J Cachexia Sarcopenia Muscle. (2017) 8:19–24. doi: 10.1002/jcsm.12140
138. Vafadar Afshar G, Rasmi Y, Yaghmaei P, Khadem-Ansari MH, Makhdomii K, Rasooli J. The effects of nano-curcumin supplementation on serum level of hs-CRP, adhesion molecules, and lipid profiles in hemodialysis patients, a randomized controlled clinical trial. Iran J Kidney Dis. (2020) 14:52–61.
139. Silalahi T, Alwi I, Suyatna F, Sartika KD. Curcumin's effect on inflammatory response following percutaneous coronary intervention in adult patients with stable coronary heart disease. Int J Angiol. (2021) 30:132–8. doi: 10.1055/s-0040-1720969
140. Ashtary-Larky D, Kelishadi MR, Bagheri R, Moosavian SP, Wong A, Davoodi SH, et al. The Effects of nano-curcumin supplementation on risk factors for cardiovascular disease: a GRADE-assessed systematic review and meta-analysis of clinical trials. Antioxidants. (2021) 10:1015. doi: 10.3390/antiox10071015
Keywords: curcumin, cardiovascular risk, cardiovascular disease, treatment, prevention
Citation: Surma S, Sahebkar A, Urbański J, Penson PE and Banach M (2022) Curcumin - The Nutraceutical With Pleiotropic Effects? Which Cardiometabolic Subjects Might Benefit the Most? Front. Nutr. 9:865497. doi: 10.3389/fnut.2022.865497
Received: 29 January 2022; Accepted: 07 April 2022;
Published: 17 May 2022.
Edited by:
Luca Rastrelli, University of Salerno, ItalyReviewed by:
Neftali Eduardo Antonio-Villa, Universidad Nacional Autónoma de México, MexicoYounes Zaid, Université de Montréal, Canada
Copyright © 2022 Surma, Sahebkar, Urbański, Penson and Banach. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maciej Banach, maciej.banach@umed.lodz.pl
 Stanisław Surma
Stanisław Surma Amirhossein Sahebkar3,4
Amirhossein Sahebkar3,4  Jakub Urbański
Jakub Urbański Maciej Banach
Maciej Banach